Abstract
Manual micropipettes are the most heavily used liquid handling devices in biological and chemical laboratories; however, they suffer from low precision for volumes under 1 μl and inevitable human errors. For a manual device, the human errors introduced pose potential risks of failed experiments, inaccurate results, and financial costs. Meanwhile, low precision under 1 μl can cause severe quantification errors and high heterogeneity of outcomes, becoming a bottleneck of reaction miniaturization for quantitative research in biochemical labs. Here, we report Dotette, a programmable, plug-and-play microfluidic pipetting device based on nanoliter liquid printing. With automated control, protocols designed on computers can be directly downloaded into Dotette, enabling programmable operation processes. Utilizing continuous nanoliter droplet dispensing, the precision of the volume control has been successfully improved from traditional 20%–50% to less than 5% in the range of 100 nl to 1000 nl. Such a highly automated, plug-and-play add-on to existing pipetting devices not only improves precise quantification in low-volume liquid handling and reduces chemical consumptions but also facilitates and automates a variety of biochemical and biological operations.
I. INTRODUCTION
Micropipettes have been widely deployed as a volumetric quantitation apparatus in many fields of research, including biochemistry, immunology, and molecular biology, where convenience-to-operate, reliability, and precision have to be considered altogether. However, inevitable operational human errors and inaccuracy in low volume quantitation have been two standing challenges for the handheld manual micropipettes, the most frequently used in laboratories.1–4 Dominantly operated under a classic piston-driven air displacement mechanism, the micropipettes target at handling liquid volumes between 0.1 μl and 1000 μl (1 ml), with various range configurations. Although these pipettes have demonstrated their capacity of being reliable for most applications above 1 μl, they are unfortunately subject to inaccuracies caused by the temperature, tip residues, surface contact, and user experiences due to limitations of the air-displacement working principle.5–8
Specifically, the inaccuracies of the quantitative volume handling have been found with a significant increase to more than 20% in the ranges below 1 μl, according to the literature,1,2,9,10 leading to a severely negative impact on the experimental outcomes. On the other hand, as a manual device, the handheld micropipette operations inevitably involve human errors, which become more difficult to predict and manage.11 Human pipetting errors constitute a substantial source of lost productivity in clinical and research laboratories. In a typical molecular biology laboratory setting, it is typical to involve several hundred pipetting steps to fulfill a single 96-well plate. Also, it is sometime inadvertently for one to lose track on the workflow in between dispensing steps, which requires additional repeats.11 As such, without an effective means to monitor each operational step, many experimental results may become unreliable, even when multiple repeats with the extra expense of costly labor and reagents are conducted to validate the findings.11–13 In addition, the contact nature of liquid dispensing using a micropipette could contribute to accumulated systematic errors introduced by not changing tips during a typical procedure, such as series dilution.14 Thus, an automatic pipetting with pre-loaded workflow would provide a way to track work flow efficiently and reduce unnecessary repeats of the experiments.
The continuous development of the robotic-based liquid handling system has addressed the aforementioned issues of human-induced and systematic errors to a large extent, while the accuracy of the volume handling below 1 μl is still limited by the capillary and adhesive forces due to the nature of the current pipetting dispensing mechanism. Syringe-based automatic dispensers (SGE, Eprep) improve the accuracy in submicroliter; however, the dispensing process is in the contact mode, causing contamination for biological experiments. To address these issues in low-volume ranges, liquid jetting and droplet generation have shown promises to remove undesired surface interactions during volume transfer.15 Recently, the micropipette industries have witnessed an array of new equipment releases to reflect such an unmet demand, such as Labcyte™ Echo® Liquid Handlers, BioFluidix PipeJet® NanoDispensers, Tecan D300e, and Biodot™ Dispense systems, all of which have claimed high reliability and accuracy. However, high price-tag, bulky equipment design, expensive operations and maintenance, and complicated, unfriendly user interface make them less attractive for routine operations in a typical research laboratory environment, particularly, for those applications currently conducted by handheld micropettes.2 Therefore, an ideal replacement for the handheld micropipette is highly sought-after, which would keep its existing features of easiness-to-operate and small form factor, while offering high accuracy and automation from the latest development and being compatible with existing protocols, in addition to affordability for research laboratories.1,3,16
Besides the above-mentioned industrial attempts, a number of researchers from the emerging microfluidic community have extended various alternative approaches to tackle liquid-handling and small-volume operations in laboratories. For instance, Qin's group has reported a convenient means to combine a micropipette with microfluidic-designed tips to isolate single cells for transcriptome analysis in submicroliter volumes.17,18 In another effort, Garstecki's group has adopted micropipettes as a replacement of the sophisticated flow sources, which is typically used to generate libraries of nanoliter droplets.19 Moreover, Kim's group has developed a disposable plug-and-play nanoliter dispensing system for rapid and reliable dispensing of minute volume droplets into multi-well plates.16 Gelinsky's group has adopted a piezoelectric nanoliter pipette for in situ functionalization of scaffolds during extrusion-based 3D plotting.20 Recently, to enable quantitative control in high-throughput screening, Neild's group incorporated volume pipetting functions in droplet microfluidic chips via surface acoustic waves.21 Despite the rapid development of microfluidics and nanofluidics, microfluidic devices have not, up-to-date, yet achieved the same level of flexibility and user experience as the conventional micropipettes. Furthermore, the interfaces between the microscopic world and mesoscale liquid handling system still serve as a bottleneck to the wide spread of the technology into laboratory research.22
Therefore, in this article, we have first proposed a programmable, handheld, and microfluidic dispensing add-on to the existing pipette device with nanoliter resolution, disposable microfluidic tips, and protocol-programmable capacity to solve both precision and human error issues for micropipettes. This device, referred to as Dotette, utilizes the principle of the microfluidic impact printing to dispense consistent nanoliter droplets, configured with an interchangeable microfluidic printing tip and non-contact low-cost actuators,23–25 from which desired liquid volumes are reached by the exact number of droplet ejection. Markedly, the droplet volumes generated by the Dotette technology have shown a small coefficient of variation (2.0%) in the droplet diameter and further reducing random errors (<5%) in low-volume dispensing (<1 μl). Meanwhile, the programmable dispensing function provides a seamless way to turn desired protocols into automated operational procedures, reducing human errors during pipetting. Overall, such an implementation of the Dotette device can improve quantification precision in low-volume liquid handling, reduce sample consumption, and eliminate human errors and, moreover, facilitate interfaces between the conventional experiments and micro total analysis systems.
II. DESIGN
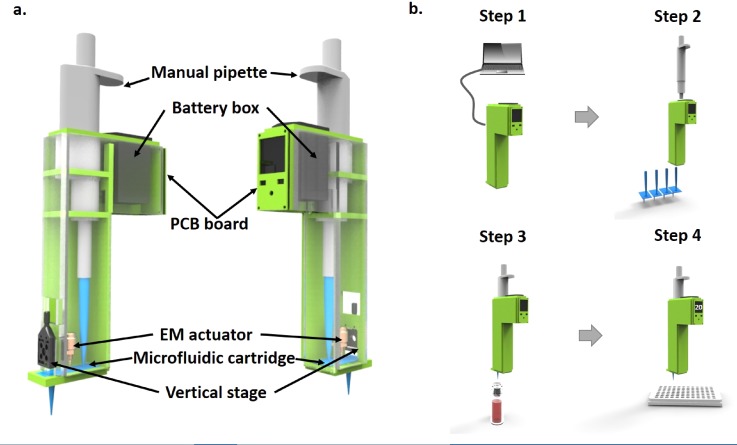
A handheld droplet dispensing device, Dotette, has been designed to be amounted onto a standard micropipette. It is composed of an electronic control, an electromagnetic (EM) actuator, and a disposable microfluidic cartridge containing a reservoir and a printing tip, along with some mechanical adjustment for printing, as shown in Fig. 1(a). As the core element, the interchangeable microfluidic cartridge is designed to replace the regular pipette tip and compatibly fit onto the micropipette. The adjacent adjustment stage is used to tune the actuation distance between the EM actuator and the microfluidic cartridge to implement droplet size control. A solenoid electromechanical actuator (DSTL-0216–05, Delta Electronics) has been adopted for its low voltage operational requirement (5.4 V, 1.1 W) and large stroke distance (2.70 mm maximum). This actuator provides a force of 0.39 N at zero stroke length, which decreases with increasing the stroke distance at a linear rate of 0.11 N/mm. The EM actuator is driven by pulsed wave with 10 ms width, 100 ms period and 5.4 V amplitude. We are aware of the fact that the stroke force and distance can impact the droplet size in a small range (e.g., 5 nl–10 nl). The adjacent adjustment stage is incorporated as a pre-characterization tool. For example, we will tune the stage continuously to achieve a pre-specified volume (e.g., 5 nl) of a single water droplet per stroke before dispensing. Then the distance is fixed for further dispensing. The characterization of droplet size has been elaborated in Sec. III. Moreover, the control board contains a small printed circuit board (PCB), amounted with a microcontroller, an input panel, a small LCD display, along with peripheral components, which grants computer access through either wired or wireless link for downloading the program of the operational protocols. The major distinction between Dotette and a regular micropipette is that Dotette is based on the non-contact microfluidic droplet generation to achieve precision at low volume and reduce variance by accumulation.14
FIG. 1.
Design and operation principles. (a) Systematic diagram of Dotette, with a microfluidic cartridge containing fluidic inlet and printing outlet, a holding case to amount onto a micropipette, a PCB board, an electromagnetic actuator, and an adjustment stage. (b) Operation procedure of the Dotette, illustrated in four steps: (1) Downloading protocols from a computer. (2) Attaching the microfluidic cartridge. (3) Liquid aspiration from a vial. (4) Digital dispensing, following the instruction displayed on the control panel.
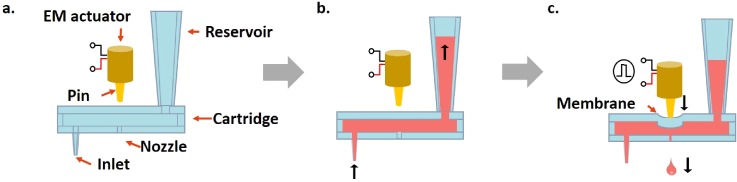
Dotette is operated based on the microfluidic impact printing principle, under which the actuation membrane of the separable microfluidic cartridge is deformed by the external EM actuator, and the mechanical deformation accelerates the fluid inside the microfluidic channel, and ejects part of it from the bottom nozzle (in Fig. 2). The EM actuator is driven by a pulsed wave with the 10 ms width, 100 ms period, and 5.4 V amplitude. To make the microfluidic cartridge compatible with standard micropipette operations, we have merged the pipette structure into a planar microfluidic chip. In particular,24,26,27 we have attached a pipette tip of 1 μl onto the microchip inlet and connected the outlet to another pipette reservoir.
FIG. 2.
Design and working principle of a microfluidic cartridge. (a) Microfluidic cartridge before use. (b) Cartridge filled with liquid (red). (c) When the electromagnetic actuator is actuated, the pin strikes on the membrane and droplets are dispensed.
III. METHODS AND MATERIALS
The Dotette body was designed using SOLIDWORKS. Polymethyl methacrylate (PMMA) and aluminum sheets (McMaster-Carr) were machined using a Kern Micro 24 laser cutter and a CNC milling machine, respectively, to fabricate parts of the Dotette body. After machining, the Dotette body was then assembled with its mortise and tenon joints. A PCB circuit board driving the electromagnetic actuator with an operation panel is customized and ordered from TacSense, Inc. A metal pin (Keystone Electronics 4–1411-1) was attached to the bottom of the electromagnetic actuator column (Digi-Key 1144–1279-ND) for striking the deformable actuation membrane. The EM (electromagnetic) actuator was mounted onto a miniature linear stage with a travel distance of 4 mm (NEWPORT M-MR1.4). The brand name of the micropipette inserted in the slots was Eppendorf Research 100–1000 μl.
For microfluidic cartridge fabrication, two 100 μm-thickness hydrophilic polyethylene terephthalate (PET) films (3M™ 9984 Diagnostic Microfluidic Surfactant Free Fluid Transport film) were cut using a CO2 laser machine (Universal Laser Systems, VersaLaser 2.30) and used as a top elastic layer connected to the reservoir and a bottom layer connected to the inlet tip and a small hole as a nozzle. The details of nozzle fabrication are elaborated in a previous publication.34 Diameters of the small holes on the nozzle layer were in the range of 60 μm–120 μm. A 150 μm-thickness PET film with a double-sided adhesive film (3M 467 200MP) was cut using a CO2 laser machine as a channel layer with a circular chamber. After laser machining, the PET layer with double-sided adhesive is sandwiched in between two hydrophilic PET layers under a mask aligner, with the chamber centered to the nozzle. The inlet tip (10 μl) and reservoir tip (1 ml) were mounted onto a PMMA ring and glued to the top and bottom PET layers with epoxy (Super Glue Corp. SY-QS), respectively. The entire channel can be exhausted; however, we estimate the dead volume to be around 1 μl, as it is preferred to keep the micro-channel filled throughout the printing process.
Two methods have been used for characterizing droplet sizes: one is an imaging characterization method and the other is a gravitation-based approach. Before being printed into the multi-well plates, the droplet volume was calibrated by printing into 2 mm-thick silicone oil (Sigma-Aldrich) and measuring the diameters of suspended droplets under a bright field microscope (Life Technologies EVOS XL). In the imaging characterization method, 400–700 droplets were first printed into a Petri dish (Corning, 60 mm diameter) containing 2 mm-thick silicone oil. Then, pictures were taken under a bright field microscope with 4× magnification. Diameters were analyzed using a batch macro by the particle analysis function in ImageJ, based on which volume distribution was calculated. In the gravitational method, a microbalance (Mettler Toledo AX26 DeltaRange, 0.001 mg resolution) was used to characterize the volume linearity and systematic and random errors in the range of 100 nl to 1000 nl. Droplets were also printed into a container with 2 mm-thick silicone oil. Micropipettes were calibrated and operated according to the user manual provided by the manufacturer. The accuracy and precision of micropipettes in a sub-microliter range were from the published data of Eppendorf Research® plus micropipettes 0.1–2.5 μl, following the test procedure of EN ISO 8655–2 and 8655–6.
IV. RESULTS AND DISCUSSION
A. Dotette device
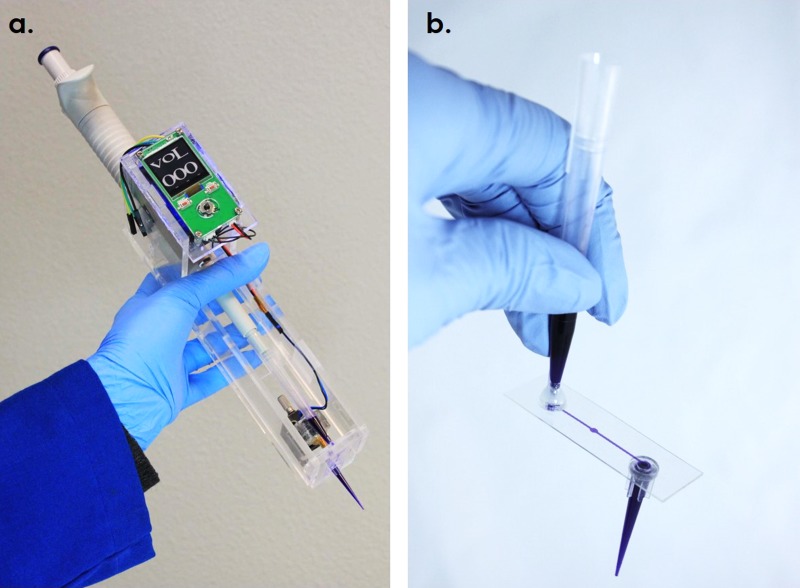
The photographs of the Dotette prototype and the microfluidic tip are shown in Fig. 3. As can be seen, the prototype system contains a custom-made case to reversibly fit with a standard micropipette, a microfluidic cartridge, a control circuit and interface with power, an electromagnetic actuator, and a vertical adjustment stage. The Dotette is self-contained, powered using a 9 V battery. Disposable cartridges are made from low-cost polymers with simple fabrication steps, with easy mounting to the micropipette. The loading volume is between 20 μl and 1 ml. A power button, a dispense button, and a toggle switch used to manually adjust the droplet number are included on the front panel. After loading the sample liquid, according to the protocol, users can click the dispense button to start dispensing into each well in a pre-loaded volume sequence. The PCB board contains a control circuit that outputs 6 V pulse waves for each printing. With an actuation current of 40 mA, a 500 mAh 9 V battery can sustain the power of printing for 1.35 × 106 droplets. The LCD screen exhibits the number of droplets to be printed into current well in real time. The whole prototype is a 310-g light-weight device, including the battery.
FIG. 3.
Photographs of (a) a Dotette prototype and (b) a microfluidic tip with both the pipette inlet and outlet attached.
B. Automated operations
To reduce human errors, a pre-programming function is incorporated in Dotette to guide the pipetting operations and reduce the cost of unnecessary repeating. As aforementioned, the detailed pipetting protocols, including the number of dispensing and the volume in each well, are pre-programmed on a computer and downloaded to the Dotette device, as illustrated in Fig. 1(b). Easy pre-programming on a laptop and automated array dispensing in multi-well plates have been demonstrated in the supplementary material video.
The operational procedure of Dotette is illustrated in Fig. 1(b). Initially, the detailed pipetting protocols, including the number of dispensing and the volume in each well, are pre-programmed in a computer (Step 1), which can be downloaded to the Dotette device either wired or wirelessly. Alternatively, the dispensing droplet number can be manually adjusted using the toggle from 0 to 999. Subsequently, the microfluidic cartridge can be easily attached to the micropipette through its outlet pipette tip fixture (Step 2). During the loading process, the pipette tip is immersed into the sample as usual, and the air displacement mechanism built into the micropipette will drive the liquid into the microfluidic cartridge (Step 3). The printing nozzle is located in the center of the chip. Once the dispensing button is clicked, the EM actuator can be driven by electrical pulses on demand, from which the metallic actuator pillar would move up and down and generate pulsed impact forces onto the microfluidic membrane, leading to physical displacement of liquid inside the channel and subsequent ejection of droplets from the nozzle (Step 4). A 500 ms delay between button clicking and dispensing is designed to prevent instability caused by hand movement due to the button clicking. The number of pulses is determined by the volume needed in the protocol. After finishing all dispensing steps in the protocol, the microfluidic tip can be detached and disposed of as a regular consumable pipette tip. Droplets are ejected into the air and dropped into silicone oil. The modeling of droplet ejection and mathematical derivation of striking velocity and droplet volume control have been elaborated in detail in our previous publications.23–25 In our practice, the reservoir connecting to the micro-pipette is detached after the filling step to prevent negative pressure. Since the reservoir is fitted on the acrylic holder, the detaching step will not affect printing. The existence of the aspiration tip indeed limits the dimension of microplates we can work on. Future work includes a second version to fully remove the extra tip and merge the nozzle and tip into one hole, making it usable on current standard biological assay platforms.
C. Characterization studies of droplet dispensing
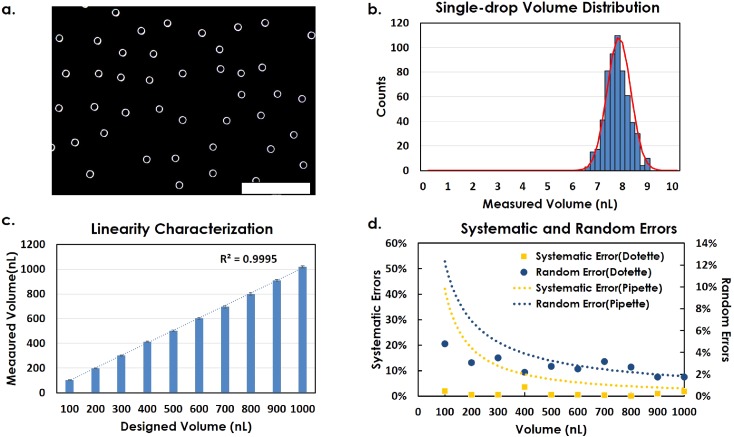
Characterization studies of Droplet Dispensing are performed by both the imaging-based and gravitational approaches as aforementioned. In these experiments, we consider the coefficients of variation (CV) as the random errors, while we use the differences between the measured mean and target values as the systematic errors. We have first conducted the imaging-based method to evaluate the single-droplet dispensing from a Dotette device, from which the droplet volume distribution can be analyzed.
Figure 4(a) shows a microscopic image of an array of DI water droplets (with a viscosity of 0.890 cP and a surface tension of 72 dyn/cm at about 25 °C) printed into an oil bath, while Fig. 4(b) exhibits the volume distribution histogram obtained using ImageJ from the printed droplet array, from which a mean value of 7.76 nl and a diameter CV of 2.0% are shown [Fig. 4(a)]. As a reference, the CV of diameters generated by Microfab droplet dispensers is in the range of 2.6%–3.5%.26 However, in recent publications, where the high voltage-driven piezo-stacks as actuators were used, the CV of diameters fell in the range of 1.4%–2.5%.27 Therefore, our results indicate that the ejected droplet uniformity and repeatability of the handheld Dotette device are comparable to those of the complex and large-footprint equipment driven by expensive integrated piezoelectric actuators and operated under a high voltage (110 V–200 V).
FIG. 4.
(a) Sample image of individual droplets printed into oil. Scale bar: 2 mm. (b) Single-drop volume distribution histogram characterized by an imaging method. (c) Linearity of accumulated droplets from 100 nl to 1000 nl, characterized by a gravitational method. (d) Decreasing trend of systematic error and random error with the increasing volume range, calculated from (c).
The CV of volume can be further reduced with increased dispensing volume (100 nl–1000 nl). According to the theory of the sum of standard deviations, when the number of sampled droplets increases by n, the CV of total volume will decrease by 1/√n, cvsum = (1/√n)cv,28,29 where cvsum means the coefficient of variation of the total volume, cv means the coefficient of variation of the single droplet, and n means the number of droplets. In our case, the number of droplets is dependent on total volume required divided by single-droplet volume, n = V/Vs, where Vs is the volume of the single droplet and V is the required total volume in a well. Thus, in theory, cvsum =(√Vs/√V)cv, which means that the CV of total dispensed volume will decrease by 1/√V, which is proved by the following gravitational volume characterization.
In the following gravitational evaluation of the Dotette system, droplets are accumulated to reach the desired volume in the range of 100 nl to 1000 nl. The weights of these droplet pools are measured and plotted in Fig. 4(c), where the x-axis represents the targeted volumes and the y axis represents the corresponding volumes measured by the gravitational method, from which the trend is fitted by a linear curve, indicating a high linearity of R2 = 0.9995. 7 repeats of each experiment have been conducted, exhibiting high repeatability. Using the same group of data in Fig. 4(c), the random and systematic errors from Dotette are calculated and plotted in Fig. 4(d), whereas reference error values are provided by the micropipette vendor as dotted lines for comparison. From our calculation, droplet dispensing can control the random errors to be within 5%, compared with 4%–12% random errors of micropipettes in the range of 100 nl to 500 nl.1,10,30–33 As we expected, the random errors of volume using Dotette have a decreasing trend with increasing dispensing volumes [Fig. 4(d)]. Similarly, in terms of systematic error, the Dotette shows apparent advantages over the traditional pipette, where the former one only shows marginal offsets of below 5% from the designated volume (square) and the latter has significantly larger systematic error (dashed line, mostly above 9%–48%) in the volume range from 100 nl to 500 nl. This is highly possibly due to the fact that the traditional pipette has a fixed mechanical tolerance, and therefore, when the volume becomes smaller, the systematic error becomes larger.
Liquid with different viscosities or surface tensions will induce changes in single-droplet size, but the accuracy remains similar since the consistency of droplets for the same liquid is ensured by the microfluidic printing technology. Liquid with higher viscosity or surface tension requires a large force for dispensing; therefore, the dispensable liquid is limited by the impact force. This device is capable of dispensing glycerol solution in water up to 30% volume concentration and cell media with a surfactant less than 0.5%. With a higher concentration, the droplet will not be able to break up the surface tension and electrostatic force from the nozzle, which will cause air trap and block the channel. Dilution with non-surfactant cell culture media helps in these cases. As mentioned by an previous art,35 pipette dispensing is controlled by air pressure, capillary force, and electrostatic force. The electrostatic charge amount, depending on molecular contents and the size of the droplet, may reduce the surface tension of the droplet and cause errors in experiments where surface tension plays a key role. In our case, the interaction between surface tension, membrane displacement, gravity, and electrostatic force determines the critical speed for a successful breakup of the droplet from the reservoir. Further investigation of how the electrostatic force will influence the biochemical contents is needed to ensure the proper application in biochemical assays.
Using the gravitational method, we prove that, with the same targeted volume, both systematic errors and random errors of droplet dispensing are significantly lower than conventional manual pipette dispensing. Therefore, Dotette provides an automated, high-accuracy, and high-precision alternative for ultra-low volume dispensing and digital droplet experiments. In addition, this device is promising in biological or chemical applications such as single-cell isolation and digital PCR where compartmentalization of the sample into thousands of units is needed. For example, for digital PCR, highly diluted sample solution mixed with PCR reagents can be printed into oil, forming massive nanoliter droplets. Then, the droplets are incubated, followed by calculation of the percentage of fluorescent drops.
V. CONCLUSIONS
In this article, we have reported the design and characterization of Dotette, a handheld, programmable, and high-precision microfluidic pipetting device. It is operated by a microfluidic impact printing principle and dispenses the fluid into the digital nanoliter droplet stream. As such, it addresses two main issues in manual pipetting devices, human errors and precision under 1 μl. In particular, the human errors are eliminated by a computer programmed protocol to automate the pipetting process, while precision can be improved by high consistency in the generated droplet volume from the microfluidic impact printing and can be substantially decreased by further reduced droplet volume and increased droplet number. Utilizing nanoliter droplet accumulating, the precision of dispensed volume is successfully improved from traditional 20%–50% to under 5% in the range of 100 nl to 1000 nl. It is particularly useful for Dotette in precise low-volume dispensing, precious sample handling, and the interface with cutting-edge lab-on-a-chip operations and micro total analysis systems.
VI. SUPPLEMENTARY MATERIAL
See supplementary material for the demonstration of Dotette operation and the droplet dispensed from the nozzle into oil in a video.
ACKNOWLEDGMENTS
This research work was supported in part by a National Science Foundation Award (No. DBI-1256193) and National Institutes of Health Awards (No. P42ES004699). B.L. acknowledges the grant support from the National Natural Science Foundation of China (No. 51675505) and the Joint Research Fund for Overseas Chinese, Hong Kong, and Macao Young Scientists of the National Natural Science Foundation of China (Grant No. 51628502). The authors would like to acknowledge Xibi Chen, Ray Lin, Aaron Cohen, and Hong Ye for their assistance in circuit board design and Tonya Kuhl and Amanda Dang for their assistance in the gravitational measurement.
Contributor Information
Baoqing Li, Email: .
Tingrui Pan, Email: .
References
- 1. Berg M., Undisz K., Thiericke R., Zimmermann P., Moore T., and Posten C., J. Biomol. Screening 6(1), 47–56 (2001). 10.1177/108705710100600107 [DOI] [PubMed] [Google Scholar]
- 2. Oosterbroek R. E. and Berg A. v d., ( Elsevier B.V., Amsterdam, 2003). [Google Scholar]
- 3. Kong F., Yuan L., Zheng Y. F., and Chen W. D., JALA 17(3), 169–185 (2012). 10.1177/2211068211435302 [DOI] [PubMed] [Google Scholar]
- 4. Miller J. S., Sass M. E., Wong S. J., and Nienhuis J., Am. Biol. Teach. 66, 291–296 (2004). 10.1662/0002-7685(2004)066[0291:MAILSF]2.0.CO;2 [DOI] [Google Scholar]
- 5. Extrand C. W. and Kumagai Y., J. Colloid Interface Sci. 184, 191–200 (1996). 10.1006/jcis.1996.0611 [DOI] [PubMed] [Google Scholar]
- 6. Feldmann R. and Lochner K. H., Accredit. Qual. Assur. 21, 69–82 (2016). 10.1007/s00769-015-1171-y [DOI] [Google Scholar]
- 7. Rodrigues G. and Curtis R., ( John Wiley & Sons, Inc., New York, NY, 2010). [Google Scholar]
- 8. Rakhmankulova M., Stavrou S. W., Yuen A. P., Zhou R., Kessler P., and Pevsner P. H., Rapid Commun. Mass Spectrom.: RCM 22, 2349–2354 (2008). 10.1002/rcm.3620 [DOI] [PubMed] [Google Scholar]
- 9. Mortimer D., Shu M. A., Tan R., and Mortimer S. T., Human Reprod. 4, 166–168 (1989). 10.1093/oxfordjournals.humrep.a136865 [DOI] [PubMed] [Google Scholar]
- 10. Beroz J. and Hart A. J., Rev. Sci. Instrum. 87, 115112 (2016). 10.1063/1.4966989 [DOI] [PubMed] [Google Scholar]
- 11. Kyle Lyman D. F., Han Y., and Chetkovich D. M., J. Med. Lab. Diagn. 6, 36–40 (2015). 10.5897/JMLD2015.0110 [DOI] [Google Scholar]
- 12. Blow N., Nat. Methods 5, 109–111 (2008). 10.1038/nmeth0108-109 [DOI] [PubMed] [Google Scholar]
- 13. Procop G. W., Yerian L. M., Wyllie R., Harrison A. M., and Kottke-Marchant K., Am. J. Clin. Pathol. 141, 718–723 (2014). 10.1309/AJCPOWHOIZBZ3FRW [DOI] [PubMed] [Google Scholar]
- 14. Hindson C. M., Chevillet J. R., Briggs H. A., Gallichotte E. N., Ruf I. K., Hindson B. J., Vessella R. L., and Tewari M., Nat. Methods 10(10), 1003 (2013). 10.1038/nmeth.2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Streule W., Lindemann T., Birkle G., Zengerle R., and Koltay P., J. Assoc. Lab. Autom. 9, 300–306 (2004). 10.1016/j.jala.2004.08.008 [DOI] [Google Scholar]
- 16. Choi I. H., Kim H., Lee S., Baek S., and Kim J., Biomicrofluidics 9, 064102 (2015). 10.1063/1.4935937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang K., Gao M., Chong Z. C., Li Y., Han X., Chen R., and Qin L. D., Lab Chip 16, 4742–4748 (2016). 10.1039/C6LC01241H [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang K., Han X., Li Y., Li S. Y., Zu Y. L., Wang Z. Q., and Qin L. D., J. Am. Chem. Soc. 136, 10858–10861 (2014). 10.1021/ja5053279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dutka F., Opalski A. S., and Garstecki P., Lab Chip 16, 2044–2049 (2016). 10.1039/C6LC00265J [DOI] [PubMed] [Google Scholar]
- 20. Giron S., Lode A., and Gelinsky M., J. 3D Print. Med. 1(1), 25–29 (2016). 10.2217/3dp-2016-0003 [DOI] [Google Scholar]
- 21. Sesen M., Devendran C., Malikides S., Alan T., and Neild A., Lab Chip 17, 438–447 (2017). 10.1039/C6LC01318J [DOI] [PubMed] [Google Scholar]
- 22. Chung S. E., Kim J., Oh D. Y., Song Y., Lee S. H., Min S., and Kwon S., Nat. Commun. 5, 3468 (2014). [DOI] [PubMed] [Google Scholar]
- 23. Ding Y. Z., Li J. N., Xiao W. W., Xiao K., Lee J., Bhardwaj U., Zhu Z. J., Digiglio P., Yang G. M., Lam K. S., and Pan T. R., Anal. Chem. 87, 10166–10171 (2015). 10.1021/acs.analchem.5b00826 [DOI] [PubMed] [Google Scholar]
- 24. Li B., Fan J., Li J., Chu J., and Pan T., Biomicrofluidics 9, 054101 (2015). 10.1063/1.4928298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ding Y. Z., Huang E., Lam K. S., and Pan T. R., Lab Chip 13, 1902–1910 (2013). 10.1039/c3lc41372a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bsoul A., Pan S., Cretu E., Stoeber B., and Walus K., Lab Chip 16, 3351–3361 (2016). 10.1039/C6LC00636A [DOI] [PubMed] [Google Scholar]
- 27. Schoendube J., Wright D., Zengerle R., and Koltay P., Biomicrofluidics 9, 014117 (2015). 10.1063/1.4907896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eisenberg B. and Sullivan R., Math. Mag. 81, 362–366 (2008). 10.1080/0025570X.2008.11953577 [DOI] [Google Scholar]
- 29. Dally J. W., ( Springer, 2008), pp. 259–280. [Google Scholar]
- 30. Hedges A. J., Int. J. Food Microbiol. 76, 207–214 (2002). 10.1016/S0168-1605(02)00022-3 [DOI] [PubMed] [Google Scholar]
- 31. Hayashi Y. and Matsuda R., Anal. Sci. 10, 881–888 (1994). 10.2116/analsci.10.881 [DOI] [Google Scholar]
- 32. Grgicak C. M., Urban Z. M., and Cotton R. W., J. Forensic Sci. 55, 1331–1339 (2010). 10.1111/j.1556-4029.2010.01460.x [DOI] [PubMed] [Google Scholar]
- 33.See https://online-shop.eppendorf.us/eshopdownload/downloadbykey/62903_186 for “Eppendorf Research® plus–Technical Datas.”
- 34. Fan J., Villarreal F., Weyers B., Ding Y., Tseng K. H., Li J., Li B., Tan C., and Pan T., Lab Chip 17, 2198–2207 (2017). 10.1039/C7LC00382J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Choi D., Lee H., Im D. J., Kang I. S., Lim G., Kim D. S., and Kang K. H., Sci. Rep. 3, 2037 (2013). 10.1038/srep02037 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See supplementary material for the demonstration of Dotette operation and the droplet dispensed from the nozzle into oil in a video.