Abstract
Objective:
Hippocampus avoidance in whole brain radiotherapy (HA-WBRT) offers the feasibility of less-impaired cognitive function than conventional WBRT. The study aims to assess the radiological distribution of brain metastases (BMs) with relation to the hippocampus and peri-hippocampus region as defined by the RTOG 0933 for better understanding of margin definition in HA-WBRT treatment planning.
Methods:
Consecutive patients with diagnosis of BM from enhanced MRI between March 2011 and July 2016 were analysed. The pre-treatment T1 weighted, T2 weighted, T2 flair, three-dimensional spoiled gradient axial and contrast-enhanced axial cranial MR images of 226 patients are examined. The closest distances between the edge of hippocampus and the margin of tumours on different planes were measured.
Results:
A total of 226 patients with 1080 visible metastatic sites were reviewed. The origin of the primary tumors was in 72.6% lung (n = 164), in 45 cases (19.9%) breast cancer and in 7.5% other malignancies (n = 17). There were 758 (70.2%) lesions situated beyond the tentorium. The median size of single lesion was 13.9 ± 14.7 mm. Impossible, it seems that more of the patients are with only one lesion, to verify. The hippocampus involvement was found in 3.1% (n = 7, 95% CI 0.01–0.05) within 5 mm, 5.7% (n = 13, 95% CI 0.03–0.09) within 10mm and 8.4% (n = 19, 95% CI 0.05–0.12) within 20 mm. In multivariate analysis, the number 6 BM or higher was found to be an independent risk factor for hippocampal involvement (HI) (OR: 5.2, 5.38 and 3.84 in 5, 10 and 20 mm).
Conclusion:
This radiological study found that the incidence of hippocampus involvement is low in patients with BM. HA-WBRT can be delivered under the context of complete radiological diagnosis after careful delineation, proper margin definition and individual planning optimization.
Advances in knowledge:
The incidence of HI in patients with initial diagnosis of BM from solid tumours impacts the radiotherapeutic decision. Our radiological data analysed the incidence of HI not only to the conventional 5 mm margin definition, but also expanded to wider margins as 10 and 20 mm from hippocampus, which will help the treatment planning optimization with different technique.
Introduction
It is estimated that 200,000–400,000 patients are presented with brain metastases (BM) from different kinds of primary malignant tumours in China every year. In most of these, patients are diagnosed with lung or breast cancer.1 Radiotherapy remains as one of the treatments of choice for BM, which includes two different approaches: whole brain radiotherapy (WBRT) and stereotactic radiosurgery (SRS).2 Large prospective studies have found that for patients with 1 to 3 metastases, SRS alone had similar effect on overall survival as SRS+WBRT.3–5 On the other hand, higher toxicity was observed in patients treated with WBRT,6–8 with symptoms as neurocognitive decline significantly affecting the quality of life. Thus, it is necessary to improve the technique of WBRT so as to lower the rate of complication and remain the same in cranial local control. Hippocampus avoidance in WBRT (HA-WBRT) has become a treatment approach with increasing interest in recent years.
Hippocampus dysfunction is associated with memory problems.9 The neural progenitor cells residing in the subventricular zone as well as the hippocampus are very sensitive to radiation. Even doses of 2 Gy could be very toxic.10 Considering the damage of neurocognitive function and quality of life, hippocampus avoidance in case of WBRT offers better tolerance. Results from RTOG 0933 study have shown that HA-WBRT could result in decreased rate of side effects.11 At the same time, the oncological safety is also an important point.
The purpose of this study is to assess the radiological distribution of BMs with relation to the hippocampus and peri-hippocampus (PH) regions as defined by the RTOG 0933 and to better define the hippocampal avoidance zone.
Methods
We studied all patients diagnosed with BM using MRI in the period between March 2011 and July 2016 in Ruijin Hospital. The eligibility of this study included: pre-treatment T1 weighted, T2 weighted, T2 flair, three-dimensional spoiled gradient (3D-SPGR) axial and contrast-enhanced axial cranial MR images, pathological proofs of primary tumours available from hospitalization system. The clinical characteristics including age at diagnosis of BM, sex, histology of primary tumours, BM status (synchronous or metachronous), extracranial metastases, number and distribution of BMs were collected. Number of BMs was calculated at the time of diagnosis. In total, 437 patients were screened and 226 of them were enrolled. The remaing 211 patients were excluded because of the following reasons: lack of histology of the primary disease, skull metastases or meningeal metastasis without visible BM, the diagnosis of BM unclear, etc.
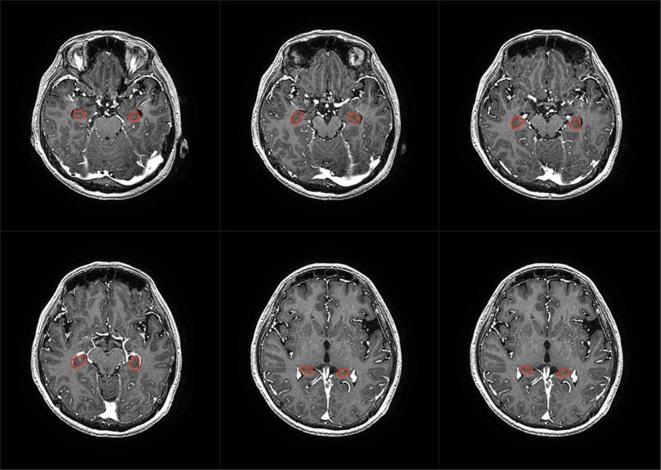
For all eligible patients, coronal and sagittal images were reconstructed. Slice thickness of 1.3 mm was used to contour the hippocampus region. A senior resident performed the hippocampus contouring according to the contouring atlas of RTOG 0933, and validated by a senior radiologist,12 focusing on the subgranular zone. Hippocampus was delineated on 3D-T1W1 axial sequences in which BM could be better visualized than T1weighted sequence.13 Figures 1 and 2 show the contours made by the DICOM (Digital Imaging and Communications in Medicine) system in our centre.
Figure 1.
Example for the hippocampal contouring of our hospital by DICOM system at the positon of axial. The 3D-T1W1 axial sequences was used for better visualization than T1 weighted sequence.
Figure 2.
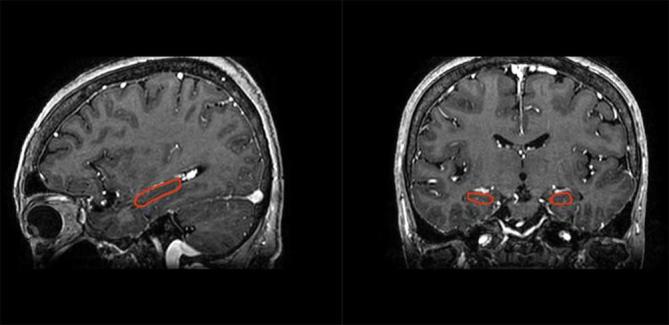
Coronal and sagittal reconstructed images are useful in localizing the hippocampus.
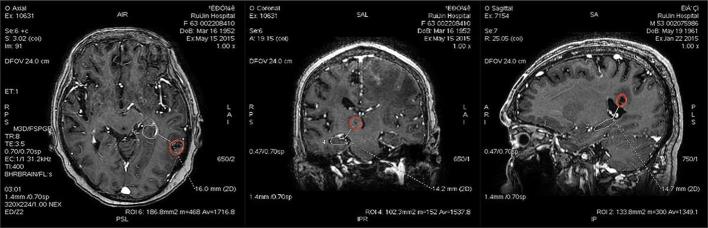
All the metastatic lesions were identified and measured by the same senior resident and validated by the senior radiologist. The lesions close to the hippocampus was contoured in order to precisely measure the closest distance.Tumours larger than 2 mm on the graph was included. The distance between the edge of hippocampus and the lesions (oedema was excluded) on different slices were measured on the DICOM server (Figure 3). In case of multiple metastases, the closest distance to hippocampus was calculated.
Figure 3.
Example for the measure of the closest distances between the edge of hippocampus and the margin of tumours (oedema was excluded) on different plane.
All data collected were analysed using the SPSS statistical software package (v. 19.0, IBM, Armonk, New York, United States). Synchronous BM were defined as BM diagnosed before or no more in 2 months from the diagnosis of the primary malignancy, and a metachronous BM is defined as a BM which was diagnosed 2 months or later after the diagnosis of the primary malignancy.14 Student's t-test was used to compare the latency period between the diagnosis of the primary and the diagnosis BMs in patients with lung and breast cancer. Binary logistic regression analysis was used to determine the relationship between clinical characteristics and the involvement of PH. Multivariate regression analysis was used to identify the risk factors of PH involvement and the cut-off value. All significant results were tested two-side with a p value of <0.05.
Results
A total of 226 patients with 1080 visible metastatic lesions were collected and analysed. There were 115 male patients (50.9%). The median age at the diagnosis of BM was 61 years (range, 20–84). The primary tumour site was lung in 164 patients (72.6%), followed by breast cancer in 45 patients (19.9%) and other malignancies in 17 cases (7.5%). The demographic and clinical characteristics of all 226 patients are given in Table 1.
Table 1.
Demographic and clinical characteristics in 226 patients
| Parameters | Numbers of patients | Percentage (%) | |
|---|---|---|---|
| Sex | |||
| Male | 115 | 50.9 | |
| Female | 111 | 49.1 | |
| Age in years | |||
| <60 | 116 | ||
| ≥60 | 100 | ||
| Median | 61 | ||
| Range | 20–84 | ||
| Primary tumours | |||
| Lung cancer | 164 | 72.6 | |
| Breast cancer | 45 | 19.9 | |
| Other primaries | 17 | 7.5 | |
| Number of BMs | |||
| 1–3 | 160 | 70.8 | |
| 3–9 | 47 | 20.8 | |
| >9 | 19 | 8.4 | |
| Extracranial metastases | |||
| Yes | 129 | 57.1 | |
| No | 97 | 42.9 | |
| BM status | |||
| Metachronous | 155 | 68.6 | |
| Synchronous | 71 | 31.4 | |
| Extracranial tumour | |||
| Stable | 106 | 46.9 | |
| Active | 120 | 53.1 |
BM, brain metastase.
There were 71 patients diagnosed with synchronous BMs, and 64 of them presented primary lung cancer. In 155 patients, we observed metachronous BMs, with mean latency time of 27.5 ± 40.9 months, of them 14.2 ± 13.9 months in patients with lung cancer (n = 100) and 59.0 ± 61.4 months in patients with breast cancer (n = 42), respectively (p < 0.01).
The mean lesion size was 13.9 ± 14.7 mm (95% CI 11.9–15.8). The distribution of BMs in different intracranial substructure is shown in Table 2.
Table 2.
The distribution of metastatic lesions by intracranial location (n = 1080)
| Intracranial location | Number of lesions | Percentage (%) |
|---|---|---|
| Cerebellum lobe | 322 | 29.8 |
| Frontal lobe | 268 | 24.8 |
| Temporal lobe | 168 | 15.6 |
| Parietal lobe | 128 | 11.9 |
| Occipital lobe | 131 | 12.1 |
| Thalamus | 45 | 4.2 |
| Brainstems | 18 | 1.6 |
| Total | 1080 | 100 |
70% of the lesions (n = 758) were situated beyond the tentorium.
After measuring the distance of the closest margin from BMs to the hippocampus border in each patient, the frequency of hippocampal involvement (HI) was classifed into three categories: 3.1% (7/226, 95% CI 0.01–0.05) within 5 mm, 5.7% (13/226, 95% CI 0.03–0.09) within 10 mm and 8.4% (19/226, 95% CI 0.05–0.12) within 20 mm. The details of incidence of PH involvement are described in Table 3.
Table 3.
The incidence of hippocampal involvement with different peri-hippocampal margin definition
| ≤5 mm |
≤10 mm (%) |
≤20 mm (%) |
||||
|---|---|---|---|---|---|---|
| No (%) | Yes (%) | No (%) | Yes (%) | No (%) | Yes (%) | |
| Histology of primary tumour | ||||||
| Lung | ||||||
| Non-small cell lung cancer | 141 (96.6) | 5 (3.4) | 139 (95.2) | 7 (4.8) | 134 (91.8) | 12 (9.2) |
| Small cell lung cancer | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Breast | ||||||
| Luminal | 16 (94.1) | 1 (5.9) | 15 (88.2) | 2 (11.8) | 15 (88.2) | 2 (11.8) |
| HER 2 over-expressed | 12 (92.3) | 1 (7.7) | 11 (84.6) | 2 (15.4) | 11 (84.6) | 2 (15.4) |
| Triple-negative | 13(100) | 0 (0) | 12 (92.3) | 1 (7.7) | 11 (84.6) | 2 (15.4) |
| Unknown | ||||||
| Other | 17(100) | 0 (0) | 16 (94.1) | 1 (5.9) | 16 (94.1) | 1 (5.9) |
Univarite analyses was performed to identify the risk factors affecting HI with three different margin categories. The following variables were evaluated: age, sex, extracranial metastases, BM status and number of BM. Number of BM was calculated both as continuous variable and categorical variable. The risk of HI was significantly increased with the increased number of BM. As categorical variable, the risk of HI is significantly increased in patients with 5 or more BMs. The details of univariated analysis are presented in Table 4. In multivariate analysis, 5 or more BMs are independent risk factors for HI except for margin category of 5 mm, and 6 or more BMs are independent risk factors for HI with all the margin categories (Table 5).
Table 4.
Univariate analysis of the risk factors of HI with different margin definition
| |
The expanded margin of peri-hippocampus |
|||||
|---|---|---|---|---|---|---|
| 5 mm | 10 mm | 20 mm | ||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Age | ||||||
| ≥60 vs <60 | 0.99 (0.93–1.06) | 0.83 | 0.99 (0.95–1.04) | 0.78 | 0.99 (0.95–1.03) | 0.73 |
| Sex | ||||||
| Male vs female | 0.71 (0.16–3.27) | 0.67 | 0.41 (0.12–1.367) | 0.14 | 0.68 (0.2633–1.76) | 0.42 |
| Extracranial tumour | ||||||
| Active vs stable | 3.75 (0.27–51.37) | 0.32 | 0.4 (0.03–6.18) | 0.51 | 0.54 (0.96–2.99) | 0.48 |
| Extracranial metastases | ||||||
| No vs yes | 1.95 (0.36–10.89) | 0.43 | 0.87 (0.28–2.68) | 0.81 | 0.65 (0.25–1.67) | 0.37 |
| BM status | ||||||
| Synchronous vs metachronous | 0.35 (0..04–2.98) | 0.34 | 0.38 (0.81–1.75) | 0.21 | 0.76 (0.26–2.19) | 0.61 |
| Number of BM | ||||||
| Continuous variable | 1.08 (1.03–1.12) | 0.002 | 1.08 (1.03–1.13) | 0.001 | 1.08 (1.03–1.12) | 0.001 |
| >3 vs 1–3 | 3.38 (0.73–15.52) | 0.12 | 4.28 (1.34–13.60) | 0.14 | 3.00 (1.16–7.76) | 0.24 |
| >4 vs 1–4 | 2.90 (0.63–13.43) | 0.29 | 3.49 (1.11–10.93) | 0.15 | 3.04 (1.15–8.04) | 0.10 |
| >5 vs 1–5 | 3.81 (0.82–17.77) | 0.09 | 4.68 (1.48–14.79) | 0.01 | 4.13 (1.54–11.08) | 0.01 |
| >6 vs 1–6 | 4.91 (1.05–23.01) | 0.04 | 4.31 (1.31–14.13) | 0.02 | 3.21 (1.12–9.19) | 0.03 |
| Primary tumour | 0.83 (0.22–3.51) | 0.78 | 0.88 (0.34–2.31) | 0.80 | 0.61 (0.23–1.59) | 0.31 |
BM, brain metastase.
Table 5.
Multivariate analysis of the risk factors of HI with different margin definition
| Variables |
The expanded margin of peri-hippocampus |
|||||
|---|---|---|---|---|---|---|
| 5 mm | 10 mm | 20 mm | ||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Age | ||||||
| ≥60 vs <60 | 0.71 (0.14–3.48) | 0.67 | 0.98 (0.30–3.21) | 0.97 | 1.04 (0.39–2.80) | 0.94 |
| BM status | ||||||
| Synchronous vs metachronous | 0.33 (0.04–2.97) | 0.32 | 0.30 (0.06–1.46) | 0.53 (0.20–1.45) | 0.22 | |
| Number of BM | ||||||
| >5 vs 1-5 | 2.55 (0.52–12.40) | 0.25 | 3.84 (1.15–12.76) | 0.03 | 3.60 (1.29–10.05) | 0.01 |
| >6 vs 1–6 | 5.20 (1.048–25.75) | 0.04 | 5.38 (1.54–18.86) | 0.01 | 3.84 (1.28–11.50) | 0.02 |
BM, brain metastase.
45 of 226 patients who received WBRT after the BM diagnosis had complete radiological follow-up available. 18 of them were found intracranial progress of BM. At a median follow-up of 11 months, there was only one new lesion in area of hippocampus (<5 mm).
Discussion
In our radiological study, we tried to report the incidence of HI not only limited to 5 mm, but also extends to wider margins, which would be more practical in radiotherapeutic strategy. Our results showed that in patients with initial diagnosis of BM from solid tumour, mainly with lung cancer and breast cancer, the frequency of HI was 3.1, 5.7 and 8.4%, respectively, using border from hippocampus extending 5, 10 and 20 mm to the peripheral intracranial structure. The frequency of HI increases with the number of BM, which increases significantly in patients with 5 or more BMs (OR: 3.84 and 3.60 in 10 and 20 mm), more significantly with 6 or more BMs (OR: 5.20, 5.38 and 3.84 in 5,10 and 20 mm). These data might be helpful in better defining the oncological feasibility and technical reference in applying HA-WBRT technique. In patients with 1 to 4 BMs, the HA zones could be reached in 10 mm from hippocampus. While in those with more than 5 BMs, the HA-WBRT should be more cautious.
In the published data, the frequency of HI in patients with initial diagnosis of BM is low, which provides the scientific hypothesis of HA-WBRT. In Gondi’s study,15 the incidence of PH involvement (hippocampus plus 5 mm margin) was 8.6% per patient, with 3.0% of direct involvement. Similar results were reported in Ghia’s study.16 In their study, the incidence of HI is higher than in our study. Possible explanation is the difference between the studied populations, e.g. the percentage of primary small cell lung cancer in our study. In Gondi’s study, patients with primary small cell lung cancer and melanoma had higher incidence of melanoma patients. Similar to our results were reported by Guo’s and coll14. Wan’s study17 found that the rate of metastases was 3.4 and 4.3% in hippocampus and subventricular zone.Wu’s study18 found that 4.1% of patients had direct HI and 5.5% had PH involvement. Although the overall incidence of HI is low, there still exists some discrepancy from different series indicating different clinical and histological factors might influence the risk of HI.
With available risk factors, we found that the risk of HI increases significantly and continuously with number of BMs. Patients with 5 or more BMs had higher risk of HI, more significantly when number increases to 6 or more, which is consistent with Guo’s study.14 Sun et al also reported that the HI was significantly more frequent in patients with BMs ≥10 than those with 1–3 (OR 9.919, 95% CI 2.388–41.179, p = 0.002).19 Age and BM status (synchronous or metachronous) were also reported to influence the risk of HI in other studies, although they were negative in our series. Therefore, we suggest that HA-WBRT should be cautious in patients with number of BM of 5 or above.
After brain irradiation from the described, brain necrosis can conduct to dementia or decline of memory.20 Study from Chang E et al21 showed that patients with WBRT could present early neuro-cognitive defect within the first 4 months after the treatment. The RTOG 0214 Phase III study, comparing prophylactic cranial irradiation with observation in patients with locally advanced (Stage IIIa or IIIb) non-small cell lung cancer22 showed that the higher incidence of the memory decline and delayed recall was found in the period of 6 to 12 months.23 The neurocognitive sequel after cranial irradiation is subsequent to the radiation-induced vascular injury, which will result in ischemia and hypoxia in the hippocampal area but also to associated systemic treatments and the direct tumour infiltration.
The injury of hippocampus is related to the radiation dose and volume ratio.24,25 In animal study, Michelle Monje et al in rat models described radiation dose-dependent effect on neurotoxicity, with a single fraction of 10 Gy inducing 62% reduction in neural stem cell proliferation and a 97% reduction in hippocampal neurogenesis.10,26 Another preclinical study in mice models, the whole-body irradiation with 0, 0.5, 2 or 4 Gy of γ-rays reported the behavioral dysfunction in the group at a dose rate of 2 Gy.27 Therefore, margin definition in PH region is an important factor to decrease the toxicity. A closer margin in PH contouring is safer in terms of oncological safety, while wider margin requires less technical challenge. A reasonable comprise is, therefore, important to achieve the balance between intracranial tumour control and technical feasibility.
HA-WBRT requires special technique because of the unique anatomy and small volume of hippocampus. Intensity modulated radiation therapy, including helical tomotherapy and LINAC-based (linear accelerator-based) intensity modulated radiation therapy28 is capable of delivering highly conformal dose distribution.25 In RTOG 0933 study showed better tolerance.11
Intracranial disease-progression per se is other important factor to neurocognitive function decline. Mehta et al have shown a relationship between tumour volume shrinkage subsequent to WBRT with improved survival and neurocognitive function preservation.29 Despite the emerging trends of SRS or fractionated stereotactic radiotherapy, the place of WBRT remains associated with better intracranial tumour control in patients with multiple lesions. Aoyama et al3 studied 132 patients with 1–4 BMs with maximum diameter less than 3 cm who were randomized to receive WBRT plus SRS or SRS alone. Although WBRT plus SRS did not improve overall survival and the local recurrence rate was significantly higher in the group receiving SRS alone. Another recent study showed that time to intracranial recurrence was significantly shorter in the SRS alone group compared with SRS plus WBRT. Intracranial tumour progression at 3, 6 and 12 months were 6.3, 11.6 and 15.0% with SRS plus WBRT vs 24.7, 35.3 and 49.5% with SRS alone (p < 0.001).5 Systemic therapy in association with effective radiotherapy can significantly improve the survival after BM from solid tumours, especially targeted therapy in patients with BM from primary epidermal growth factor receptor-mutant non-small cell lung cancer or HER2 over-expression breast cancer.30–33 Neurocognitive function preservation could offer a much higher priority following the improved prognosis of BM.34–36
The major limitation of our study is a single centre radiological retrospective review. Further study is needed to confirm the subset of patients who might most benefit from HA-WBRT based on radiological and oncological follow-up with evaluation of the patients’ prognosis.
In conclusion, our study has shown that the initial situation of metastatic lesions in the region of hippocampus is low in patients with BM from different primary malignancies. Hippocampus avoidance is feasible in most patients with BM based on professional radiological diagnosis and careful delineation in every patient as well as adapted radiotherapy technique.
Footnotes
This study was supported in part by the National Natural Science Foundation of China (grant 81172504, grant 81673102, grant 81602791).
Contributor Information
Yi-Min Han, Email: uofox9@hotmail.com.
Gang Cai, Email: caigang11855@rjh.com.cn.
Wei-Min Chai, Email: uofox9@hotmail.com.
Cheng Xu, Email: xucheng60@126.com.
Lu Cao, Email: caolu_163@ymail.com.
Dan Ou, Email: olga_oudan@163.com.
Jia-Yi Chen, Email: chenjiayi0188@aliyun.com.
Youlia M. Kirova, Email: youlia.kirova@curie.fr.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–32.https://doi.org/10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 2.Eichler AF, Loeffler JS. Multidisciplinary management of brain metastases. Oncologist 2007; 12: 884–98.https://doi.org/10.1634/theoncologist.12-7-884 [DOI] [PubMed] [Google Scholar]
- 3.Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 2006; 295: 2483–91.https://doi.org/10.1001/jama.295.21.2483 [DOI] [PubMed] [Google Scholar]
- 4.Kocher M, Soffietti R, Abacioglu U, Villà S, Fauchon F, Baumert BG, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 2011; 29: 134–41.https://doi.org/10.1200/JCO.2010.30.1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA 2016; 316: 401–9.https://doi.org/10.1001/jama.2016.9839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greene-Schloesser D, Moore E, Robbins ME. Molecular pathways: radiation-induced cognitive impairment. Clin Cancer Res 2013; 19: 2294–300.https://doi.org/10.1158/1078-0432.CCR-11-2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McTyre E, Scott J, Chinnaiyan P. Whole brain radiotherapy for brain metastasis. Surg Neurol Int 2013; 4(Suppl. 4): S236–44.https://doi.org/10.4103/2152-7806.111301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Attia A, Rapp SR, Case LD, D’Agostino R, Lesser G, Naughton M, et al. Phase II study of Ginkgo biloba in irradiated brain tumor patients: effect on cognitive function, quality of life, and mood. J Neurooncol 2012; 109: 357–63.https://doi.org/10.1007/s11060-012-0901-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redmond KJ, Mahone EM, Horska A. Association between radiation dose to neuronal progenitor cell niches and temporal lobes and performance on neuropsychological testing in children: a prospective study. Neuro Oncol 2013; 15: 1455: 1455https://doi.org/10.1093/neuonc/not213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res 2003; 63: 4021–7. [PubMed] [Google Scholar]
- 11.Gondi V, Pugh SL, Tome WA, Caine C, Corn B, Kanner A, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol 2014; 32: 3810–6.https://doi.org/10.1200/JCO.2014.57.2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gondi V, Cui Y, Mehta MP, Manfredi D, Xiao Y, Galvin JM. Real-time pretreatment review limits unacceptable deviations on a cooperative group radiation therapy technique trial: quality assurance results of RTOG 0933. Int J Rad Oncol Biol Phys 2015; 91: 564–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo T, Fa-Jin L, Shi J, Xin-You LI, Chen Y, Yan XG, et al. Optimization of MR sequences for brain metastases. Chin J Intervent Imaging & Therapy 2014; 11: 279. [Google Scholar]
- 14.Guo WL, He ZY, Chen Y, Zhou D, Tang K, Wang P, et al. Clinical Features of Brain Metastases in Small Cell Lung Cancer: an Implication for Hippocampal Sparing Whole Brain Radiation Therapy. Transl Oncol 2017; 10: 54–8.https://doi.org/10.1016/j.tranon.2016.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gondi V, Tome WA, Marsh J, Struck A, Ghia A, Turian JV, et al. Estimated risk of perihippocampal disease progression after hippocampal avoidance during whole-brain radiotherapy: safety profile for RTOG 0933. Radiother Oncol 2010; 95: 327–31.https://doi.org/10.1016/j.radonc.2010.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghia A, Tome WA, Thomas S, Cannon G, Khuntia D, Kuo JS. Distribution of brain metastases in relation to the hippocampus: implications for neurocognitive functional preservation. Int J Rad Oncol Biol Phys 2007; 68: 971–7. [DOI] [PubMed] [Google Scholar]
- 17.Wan JF, Zhang SJ, Wang L, Zhao KL. Implications for preserving neural stem cells in whole brain radiotherapy and prophylactic cranial irradiation: a review of 2270 metastases in 488 patients. J Radiat Res 2013; 54: 285–91.https://doi.org/10.1093/jrr/rrs085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu SG, Rao MY, Zhou J, Lin Q, Wang ZJ, Chen YX, et al. Distribution of metastatic disease in the brain in relation to the hippocampus: a retrospective single-center analysis of 6064 metastases in 632 patients. Oncotarget 2015; 6: 44030–6.https://doi.org/10.18632/oncotarget.5828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu SG, Sun JY, Tong Q, Li FY, He ZY, Sg W. Clinical features of brain metastases in breast cancer: an implication for hippocampal-sparing whole-brain radiation therapy. Ther Clin Risk Manag 2016; 12: 1849–53.https://doi.org/10.2147/TCRM.S124212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology 1989; 39: 789–96.https://doi.org/10.1212/WNL.39.6.789 [DOI] [PubMed] [Google Scholar]
- 21.Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 2009; 10: 1037–44.https://doi.org/10.1016/S1470-2045(09)70263-3 [DOI] [PubMed] [Google Scholar]
- 22.Gore E. RTOG 0214: a phase III comparison of prophylactic cranial irradiation versus observation in patients with locally advanced non-small cell lung cancer. Clin Adv Hematol Oncol 2005; 3: 625–6. [PubMed] [Google Scholar]
- 23.Abayomi OK. Pathogenesis of irradiation-induced cognitive dysfunction. Acta Oncol 1996; 35: 659–63.https://doi.org/10.3109/02841869609083995 [DOI] [PubMed] [Google Scholar]
- 24.Jalali R, Mallick I, Dutta D, Goswami S, Gupta T, Munshi A. Factors influencing neurocognitive outcomes in young patients with benign and low-grade brain tumors treated with stereotactic conformal radiotherapy. Int J Rad Oncol Biol Phys 2010; 77: 974–9. [DOI] [PubMed] [Google Scholar]
- 25.Gondi V, Tolakanahalli R, Mehta MP, Tewatia D, Rowley H, Kuo JS, et al. Hippocampal-sparing whole-brain radiotherapy: a "how-to" technique using helical tomotherapy and linear accelerator-based intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2010; 78: 1244–52.https://doi.org/10.1016/j.ijrobp.2010.01.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med 2002; 8: 955–62.https://doi.org/10.1038/nm749 [DOI] [PubMed] [Google Scholar]
- 27.Kim JS, Lee HJ, Kim JC, Kang SS, Bae CS, Shin T, et al. Transient impairment of hippocampus-dependent learning and memory in relatively low-dose of acute radiation syndrome is associated with inhibition of hippocampal neurogenesis. J Radiat Res 2008; 49: 517: 517–26.https://doi.org/10.1269/jrr.08020 [DOI] [PubMed] [Google Scholar]
- 28.Rong Y, Evans J, Xu-Welliver M, Pickett C, Jia G, Chen Q, et al. Dosimetric evaluation of intensity-modulated radiotherapy, volumetric modulated arc therapy, and helical tomotherapy for hippocampal-avoidance whole brain radiotherapy. PLoS One 2015; 10: e0126222https://doi.org/10.1371/journal.pone.0126222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Bentzen SM, Renschler M, Mehta MP. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol 2007; 25: 1260–6.https://doi.org/10.1200/JCO.2006.09.2536 [DOI] [PubMed] [Google Scholar]
- 30.Lin NU, Diéras V, Paul D, Lossignol D, Christodoulou C, Stemmler HJ, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res 2009; 15: 1452–9.https://doi.org/10.1158/1078-0432.CCR-08-1080 [DOI] [PubMed] [Google Scholar]
- 31.Chen J, Zhang Q, Yu X, Zhang Z, Guo X. Outcome of brain metastases from HER 2–positive breast cancer: difference in survival benefit from anti-HER 2 treatment after WBRT with regard to prior targeted therapy. Int J Radiat Oncol Biol Phys 2014; 90: S247https://doi.org/10.1016/j.ijrobp.2014.05.870 [Google Scholar]
- 32.Yang TJ, Wu AJ. Cranial irradiation in patients with EGFR-mutant non-small cell lung cancer brain metastases. Transl Lung Cancer Res 2016; 5: 134–7.https://doi.org/10.3978/j.issn.2218-6751.2016.01.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Welsh JW, Komaki R, Amini A, Munsell MF, Unger W, Allen PK, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol 2013; 31: 895–902.https://doi.org/10.1200/JCO.2011.40.1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magnuson WJ, Lester-Coll NH, Wu AJ, Yang TJ, Lockney NA, Gerber NK, et al. Management of brain metastases in tyrosine kinase inhibitor–naïve epidermal growth factor receptor–mutant non–small-cell lung cancer: a retrospective multi-institutional analysis. J Clin Oncol 2017; 35: 1070–7.https://doi.org/10.1200/JCO.2016.69.7144 [DOI] [PubMed] [Google Scholar]
- 35.Zhang Q, Chen J, Yu X, Cai G, Yang Z, Cao L, et al. Survival benefit of anti-HER2 therapy after whole-brain radiotherapy in HER2-positive breast cancer patients with brain metastasis. Breast Cancer 2016; 23: 732–9.https://doi.org/10.1007/s12282-015-0631-x [DOI] [PubMed] [Google Scholar]
- 36.Hayashi N, Niikura N, Masuda N, Takashima S, Nakamura R, Watanabe K, et al. Prognostic factors of HER2-positive breast cancer patients who develop brain metastasis: a multicenter retrospective analysis. Breast Cancer Res Treat 2015; 149: 277–84.https://doi.org/10.1007/s10549-014-3237-7 [DOI] [PubMed] [Google Scholar]