Individuals with familial Alzheimer’s disease (E280A-PSEN1) are impaired in both short- and long-term memory binding, whereas preclinical mutation carriers are impaired only in the former. By combining diffusion tensor MRI with cognitive testing, Parra et al. identify regional cerebral differences in white matter integrity that could account for this dissociation.
Keywords: Alzheimer’s disease, memory, structural MR imaging, biomarkers, psychometry
Individuals with familial Alzheimer’s disease (E280A-PSEN1) are impaired in both short- and long-term memory binding, whereas preclinical mutation carriers are impaired only in the former. By combining diffusion tensor MRI with cognitive testing, Parra et al. identify regional cerebral differences in white matter integrity that could account for this dissociation.
Abstract
Binding information in short-term and long-term memory are functions sensitive to Alzheimer’s disease. They have been found to be affected in patients who meet criteria for familial Alzheimer’s disease due to the mutation E280A of the PSEN1 gene. However, only short-term memory binding has been found to be affected in asymptomatic carriers of this mutation. The neural correlates of this dissociation are poorly understood. The present study used diffusion tensor magnetic resonance imaging to investigate whether the integrity of white matter structures could offer an account. A sample of 19 patients with familial Alzheimer’s disease, 18 asymptomatic carriers and 21 non-carrier controls underwent diffusion tensor magnetic resonance imaging, neuropsychological and memory binding assessment. The short-term memory binding task required participants to detect changes across two consecutive screens displaying arrays of shapes, colours, or shape-colour bindings. The long-term memory binding task was a Paired Associates Learning Test. Performance on these tasks were entered into regression models. Relative to controls, patients with familial Alzheimer’s disease performed poorly on both memory binding tasks. Asymptomatic carriers differed from controls only in the short-term memory binding task. White matter integrity explained poor memory binding performance only in patients with familial Alzheimer’s disease. White matter water diffusion metrics from the frontal lobe accounted for poor performance on both memory binding tasks. Dissociations were found in the genu of corpus callosum which accounted for short-term memory binding impairments and in the hippocampal part of cingulum bundle which accounted for long-term memory binding deficits. The results indicate that white matter structures in the frontal and temporal lobes are vulnerable to the early stages of familial Alzheimer’s disease and their damage is associated with impairments in two memory binding functions known to be markers for Alzheimer’s disease.
Introduction
Although Alzheimer’s disease appears to grossly impair integrative memory functions both in short-term memory (STM) (Parra et al., 2009, 2011; Della Sala et al., 2012) and in long-term memory (LTM) (Buschke et al., 1999; Swainson et al., 2001; O'Connell et al., 2004), these impairments seem to have different origins. STM binding supports the temporary retention of conjunctions of features within object representations, a function needed for the formation of new identity. Associative learning (i.e. LTM binding) enables a flexible representation of the relations between the stimulus’ parts, each holding its own identity and retaining its individual access (Moses and Ryan, 2006; Mayes et al., 2007).
STM binding deficits have been observed in asymptomatic carriers of the mutation E280A of the PSEN1 gene (E280A-PSEN1) who still perform normally on LTM binding tasks, such as the Paired Associates Learning (PAL) test of Wechsler Memory Scale (Wechsler, 1997), which has been found to be sensitive to prodromal and clinical Alzheimer’s disease (Duchek et al., 1991; Elias et al., 2000). In fact, STM and LTM binding deficits in these individuals do not correlate (Parra et al., 2011) reinforcing the notion of different neural substrates. Whereas LTM binding relies on the integrity of cerebral grey matter structures such as the hippocampus, which is known to be targeted by Alzheimer’s disease in its sporadic (Echavarri et al., 2010) and familial variants (Quiroz et al., 2010), a recent functional MRI study indicates that the STM binding function investigated below does not (Parra et al., 2014). Recent behavioural studies have further expanded the evidence in favour of dissociations between these two types of memory representation (Parra et al., 2013).
The STM binding task asks participants to hold together in memory features processed in separate brain regions whereas the LTM binding task (i.e. PAL) asks participants to learn the association between two words. These tasks require effective brain connectivity (O'Reilly et al., 2003; Koenig et al., 2005). It is well recognized that Alzheimer’s disease leads to a disconnection syndrome (Bozzali et al., 2011; Gili et al., 2011), and it is therefore hypothesized that such a syndrome underlies this specific cognitive deficit.
An Alzheimer’s disease disconnection syndrome has been well characterized using EEG-based methods (Dunkin et al., 1994; Cook and Leuchter, 1996) and more recently by resting state functional MRI (Buckner et al., 2005, 2008). Abnormal patterns of brain connectivity in the default mode network appear to characterize the transition from normal ageing to mild cognitive impairment, and from mild cognitive impairment to Alzheimer’s disease (Pihlajamaki and Sperling, 2009; Miao et al., 2011). Furthermore connectivity deficits as assessed by electrophysiological and neuroimaging techniques significantly correlate with cognitive decline both in prodromal (Chua et al., 2008) and clinical Alzheimer’s disease (Duan et al., 2006; Medina and Gaviria, 2008). However, the precise contribution of grey and white matter disruptions to the disconnection syndrome, and its cognitive implications, remains unclear (Johnson et al., 2010; Oishi et al., 2011a).
Abnormalities in white matter integrity can now be more precisely investigated in vivo using diffusion tensor MRI (DT-MRI; Basser, 1995), and have been used to investigate the underpinnings of cognitive deterioration in individuals at increased risk for Alzheimer’s disease such as those with mild cognitive impairment (Chua et al., 2008; Stebbins and Murphy, 2009; Bozzali et al., 2011). These studies are characterized by a great variability in the localization of abnormalities within white matter tracts in mild cognitive impairment patients, e.g. in medial temporal lobe (Fellgiebel et al., 2004; Kantarci et al., 2005), projection fibres including posterior cingulum, thalamic radiations and fornix (Kiuchi et al., 2009; Zhuang et al., 2010), association fibres including superior and inferior longitudinal fasciculi and inferior fronto-occipital fasciculus (Medina et al., 2006; Zhuang et al., 2010), and white matter underlying frontal, temporal, parietal and occipital lobes (Medina et al., 2006; Zhuang et al., 2010; Douaud et al., 2011). Nevertheless, recent evidence suggests that these abnormalities are related to cognitive decline in patients with mild cognitive impairment and seem to develop very early along with still subtle grey matter damage (Bozzali et al., 2011; Gili et al., 2011; Sexton et al., 2011).
Studies of preclinical cases of familial Alzheimer’s disease have also revealed decreased white matter integrity in columns of the fornix and left orbitofrontal lobe in mutation carriers who have gone on to develop familial Alzheimer’s disease, i.e. PSEN1, mutations A431E, L235V, G206A and V717I (Ringman et al., 2007). These patients were completely asymptomatic (Clinical Dementia Rating = 0, cognitively unimpaired) at the time of assessment indicating that reduced white matter integrity may precede the development of clinical symptoms (Ukmar et al., 2008; Wang et al., 2012). Similar disruptions are also observed in other non-Alzheimer’s disease dementias (Borroni et al., 2007; Sweed et al., 2012), suggesting that DT-MRI alone may lack specificity in early identification of Alzheimer’s disease. However, identifying early DT-MRI abnormalities associated with memory binding impairments, which are known to be sensitive to Alzheimer’s disease, would help overcome this limitation. If this hypothesis proves valid, combining assessment of DT-MRI and memory binding performance may unveil brain abnormalities that are more closely related to Alzheimer’s disease pathology. The present study therefore firstly investigated whether differences in white matter integrity detected with DT-MRI are related to STM binding deficits in carriers of the mutation E280A-PSEN1 who were either asymptomatic or had recently met criteria for Alzheimer’s disease. Secondly the study compared regional DT-MRI metrics with performance on both STM binding and LTM binding tasks to investigate further the neural dissociation between these two processes.
Materials and methods
Participants
The participants were members of a large kindred from the Colombian province of Antioquia, South America. They carry the gene mutation E280A of PSEN1, which invariably leads to an autosomal dominant early-onset familial Alzheimer’s disease. This variant of familial Alzheimer’s disease becomes clinically detectable at 47 years of age, on average (Lopera et al., 1997) for a clinical description. Mutation carriers either in the symptomatic or presymptomatic stages of the disease, along with members their family, regularly attend clinical and research appointments at the Health Unit of the Neuroscience Centre of the University of Antioquia. This Health Unit has been monitoring this population for more than 20 years. The participants were approached by the responsible consultants who introduced the study and invited them to take part. All the patients who attended the Unit during the time of the study were given the opportunity to participate. Moreover, patients and relatives who had previously expressed an interest in research and whose contact details were held in the centre’s database were also contacted. Only those expressing an interest were taken forward to the enrolment process which began with the informed consent. The genetic status of these patients is unknown to the centre’s staff and was not revealed to members of the research team until the recruitment process had been completed. This was done using anonymous codes. The study protocol was approved by the Ethics Committee at University of Antioquia, Colombia.
The assessment protocol for all the participants consisted of three phases. First, participants who were not in the centre database (new to the Centre) underwent genetic screening to confirm or exclude the presence of the mutation using the methodology reported by the Alzheimer’s disease Collaborative Group (Clark et al., 1995). Second, all the participants underwent neurological and neuropsychological assessments carried out by expert clinicians and neuropsychologists. Third, all the participants underwent DT-MRI assessment. The first two phases allowed us to allocate participants to three groups: (i) participants with familial Alzheimer’s disease caused by the E280A single PSEN1 mutation; (ii) carriers of the mutation who did not meet Alzheimer’s disease criteria and who were asymptomatic at the time of testing; and (iii) healthy individuals who did not carry the gene mutation, were healthy as confirmed by the clinical interview and were relatives of the members of the other two groups (healthy controls).
A sample of 58 participants entered the study. Data from 32 participants (healthy controls = 6, asymptomatic carriers = 16, familial Alzheimer’s disease = 10) were drawn from previous studies investigating visual STM binding (Parra et al., 2010, 2011). The other new participants were assessed with the same protocol. The first group comprised 19 patients with familial Alzheimer’s disease diagnosed according to the criteria established by the Diagnostic and Statistical Manual of Mental Disorders (fourth edition, text revision), and the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) group (McKhann et al., 1984). Second, the asymptomatic carriers group consisted of 18 participants who met neither Alzheimer’s disease nor mild cognitive impairment criteria at the time of the testing but who were positive for the E280A mutation. Third, the healthy controls group included 21 non-carriers who were relatives of the patients with familial Alzheimer’s disease and asymptomatic carriers. Additional inclusion criteria for the control participants included (i) negative history of neurological or psychiatric disorders; (ii) a Mini-Mental State Examination (MMSE) score ≥ 24; and (iii) no memory complaints as documented by a self-report and family questionnaire.
Asymptomatic carriers and healthy controls were matched according to age, the number of years spent in formal education, and the MMSE scores (Table 1). On average, patients with familial Alzheimer’s disease were older and less educated than the two other groups.
Table 1.
Demographic variables and cognitive screening
| FAD (n = 19) | AC (n = 18) | HC (n = 21) | ANOVA |
Post-hoc t-tests (P) |
|||
|---|---|---|---|---|---|---|---|
| Mean (SD), (range) | Mean (SD), (range) | Mean (SD), (range) | F (P-value) | FAD versus HC | AC versus HC | FAD versus AC | |
| Age | 47.5 (6.4), (38–66) | 35.1 (5.5), (24–43) | 39.3 (83), (25–54) | 15.46 (<0.001) | 0.001 | 0.137 | <0.001 |
| Education | 7.3 (3.7), (2–14) | 10.2 (3.9), (2–16) | 10.3 (27), (4–13) | 4.50 (<0.005) | 0.024 | 0.993 | 0.038 |
| MMSE | 23.6 (4.3), (17–30) | 29.8 (0.4), (29–30) | 29.6 (07), (28–30) | 39.41 (<0.001) | <0.001 | 0.957 | <0.001 |
AC = asymptomatic carriers; FAD = familial Alzheimer’s disease; HC = healthy controls; MMSE = Mini-Mental State Examination. Significant (P < 0.05) tests highlighted in bold.
Each participant underwent a colour vision assessment using Dvorine pseudo-isochromatic plates (Dvorine, 1963) and a binding perception condition. These assessments were undertaken to rule out the possibility that poor performance on the STM binding task could result from visual or perceptual difficulties. None of the participants recruited for the present study were excluded due to colour vision or perceptual binding problems.
Behavioural assessment
Neuropsychological battery
The neuropsychological battery comprised Spanish translations of the MMSE (Ardila et al., 2000), the PAL Task (Wechsler, 1997), Verbal (Letter-FAS, adapted from Sumerall et al., 1997) and Animal Fluency Tests (from Morris et al., 1989), the Copy and Recall of the Complex Figure of Rey-Osterrieth (Osterrieth, 1944), Part A of the Trail Making Test (Reitan, 1958), the Boston Naming Test (Kaplan et al., 1983), the Wisconsin Card Sorting Test (Berg, 1948), and the Word List Test (Morris et al., 1989).
Visual short-term memory task
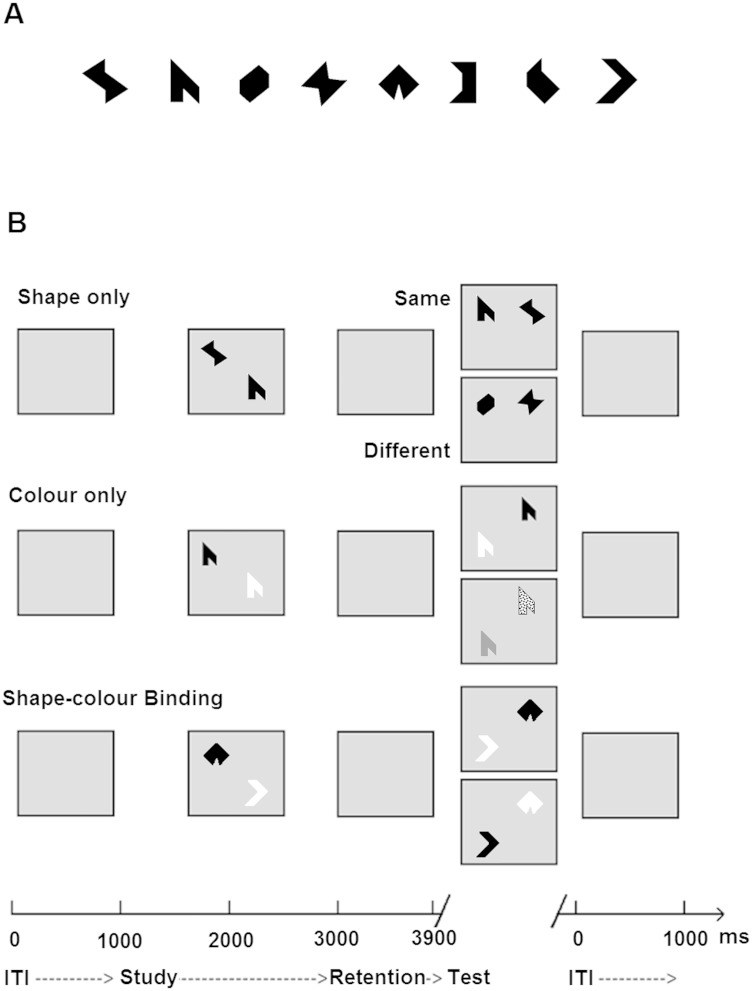
The visual STM task assessed memory for shapes (Fig. 1A), colours, or combinations of the two. Stimuli were randomly selected from a set of eight shapes and eight colours and presented as individual features or as features combined into integrated objects. Each type of stimulus was presented in a separate condition. Three experimental conditions were used (Fig. 1B), each consisting of 15 practice trials followed by 32 test trials leading to a total of 96 test trials per task. Trials were fully randomized across participants and conditions were delivered in a counterbalanced order. In the ‘shape only’ and ‘colour only’ conditions, arrays of shapes or colours were presented in the study display. In the test display for the ‘different’ trials, two new shapes or colours from the study array were replaced with two new shapes or colours. Hence, in these conditions, only visual STM for individual features was required to detect a change. In the ‘shape-colour binding’ condition, combinations of shapes and colours were presented in the study display. In the test display for ‘different’ trials, two shapes swapped the colours in which they had been shown in the study display. Hence, memory for bindings of shape and colour in the study display was required to detect this change. No shape or colour was repeated within a given array. Fifty per cent of the test trials were ‘same’ trials (the study and test displays presented identical items) and 50% were ‘different’ trials.
Figure 1.
Shape-colour binding task. (A) Shapes used as stimuli. (B) The three conditions used in the task.
Trials began with a fixation screen presented for 500 ms. This was followed by an array presented for 2000 ms on a 15” PC screen using a 3 × 3 virtual grid (‘study display’). After a 900 ms retention interval, participants were presented with ‘test display’ and were required to respond orally whether the test stimulus was the ‘same’ or ‘different’ to the one presented in the ‘study display’. The experimenter entered participants’ responses using the keyboard.
Memory load was manipulated to match the general group performance by presenting asymptomatic carriers and healthy controls with arrays of three items and patients with familial Alzheimer’s disease with arrays of two items. Previous studies have shown that manipulating the memory loads allows performance levels in the baseline memory condition to be equated across groups and, thus, any differences between groups in visual STM binding performance cannot be attributed to the baseline differences in memory for single features (Parra et al., 2010).
DT-MRI assessment
Data collection and preprocessing
DT-MRI data were collected using a Siemens Symphony Vision 1.5 T (Siemens Healthcare Sector) clinical scanner, and consisted of one T2-weighted and sets of diffusion-weighted (b = 1000 s/mm2) single-shot, spin-echo, echo-planar volumes acquired with diffusion gradients applied in 12 non-collinear directions. Fifty contiguous slice locations were imaged with a field of view of 220 × 220 mm, an acquisition matrix of 128 × 128 and a slice thickness of 3 mm, giving an acquisition voxel dimension of 1.72 × 1.72 × 3 mm. The repetition and echo times for each echo-planar volume were 7.2 s and 90 ms, respectively.
The DICOM format (http://medical.nema.org) magnitude images were converted into NIfTI-1 format (http://nifti.nimh.nih.gov). Using tools freely available in FSL (FMRIB, Oxford, UK; http://www.fmrib.ox.ac.uk), the DT-MRI data were preprocessed to extract the brain, and bulk patient motion and eddy current induced artefacts removed by registering the diffusion-weighted to the T2-weighted echo-planar volume for each subject (Jenkinson and Smith, 2001). From these MRI data, mean diffusivity and fractional anisotropy volumes were generated for every subject using DTIFIT.
Region of interest placement
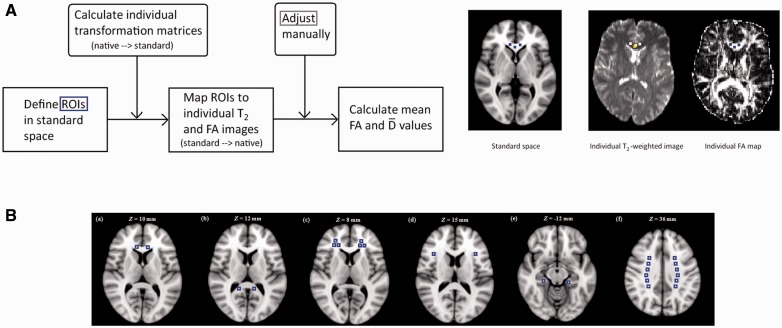
Semi-automated region of interest analysis was performed using ‘in house’ software written in MATLAB (The MathWorks) that allowed multiple small square regions of interest to be placed on the T2-weighted echo-planar volumes and then overlaid on the co-registered mean diffusivity and fractional anisotropy maps automatically using locations defined in Montreal Neurological Institute (MNI; http://www.bic.mni.mcgill.ca) standard space. The software allows the user to interactively move regions of interest if standard to native space registration errors cause white matter regions of interest to be placed over CSF or grey matter structures.
The procedure for obtaining the fractional anisotropy and mean diffusivity values for each region of interest is presented in Fig. 2A. First, MNI coordinates were defined in standard space for each region of interest using the ICBM-DTI-81 white matter atlas (Oishi et al., 2011b) and then selected in FSLview 3.1.8. Either 4, 6 or 12 square regions of interest were defined for each brain structure depending on its size in horizontal view, sizes of which were 3 × 3 × 1 voxels (see Supplementary Table 1 for MNI coordinates of each region of interest and Supplementary Table 2 for parameters used to place regions of interest). Differences in size of the chosen region of interest are explained by anatomical factors (e.g. tract dimension as for the corticospinal tract) and underlying theory (e.g. middle frontal white matter which encompasses tracts found to be impaired in Alzheimer’s disease). An aim of this study was to unveil biomarkers of cognitive impairment in preclinical Alzheimer’s disease. We therefore maximized the likelihood of identifying DTI correlates of behavioural impairments.
Figure 2.
Procedures for DT-MRI assessment. (A) Procedures for obtaining fractional anisotropy (FA) and mean diffusivity values for each region of interest (ROI). (B) Regions of interest (ROIs) meeting the criteria set for our study: (a) genu CC, (b) splenium CC, (c) middle FWM, (d) inferior FWM, (e) CGH, and (f) centrum semiovale. See ‘Materials and methods’ section for a detailed description.
Several square regions of interest were used for each structure to reduce the effects of differences in individual placement. Next, the coordinates were mapped from standard space to each individual’s T2-weighted echo-planar volume using the inverse of the transformation matrix from native to standard space (MNI152_T1_1mm_brain template) determined using affine registration (12 degrees of freedom) provided by FSL’s FLIRT. The placement of the regions of interest in native space was then checked to ensure no overlap with either CSF or grey matter. The T2-weighted echo-planar volumes were used to define the regions of interest to avoid biasing their placement by the underlying fractional anisotropy and mean diffusivity values (Bozzali and Cherubini, 2007). Minor adjustments to region of interest position were performed by an investigator blind to subjects’ genetic or clinical status. It was only to some regions of interest whenever they fell into CSF or grey matter. Finally, the values for fractional anisotropy and mean diffusivity were obtained for each square and then averaged for each region of interest separately.
We chose regions of interest that met two criteria. First, they comprise tracts relevant to the specific memory functions investigated in this study and second, they have been found to be affected in the preclinical and in the clinical stages of Alzheimer’s disease. The regions of interest targeted by the Alzheimer’s disease pathology (e.g. amyloid plaques) in the preclinical stages were of particular interest (Buckner et al., 2005; Fleisher et al., 2012). The selected regions of interest that met these criteria are shown in Fig. 2B (see also Supplementary Table 1 for the MNI coordinates). They included two regions of the corpus callosum (CC), the genu (central body) corresponding to forceps minor [Fig 2B(a)] and the splenium which includes the forceps major [Fig 2B(b)], both interhemispheric tracts. Two regions were selected from the frontal lobes. One labelled middle frontal white matter (FWM) [Fig 2B(c)], which encompasses the inferior frontal-occipital fasciculus, the anterior thalamic radiations and the lateral projections of the genu which run ipsilaterally. The other was a more inferolateral region of the FWM through which runs the superior longitudinal fasciculus [inferolateral FWM, Fig 2B(d)]. In the medial temporal lobe we selected the hippocampal part of the cingulum bundle [CGH, Fig 2B(e)]. White matter tracts, including cingulum, bilateral superior frontal-occipital fasciculus, and the genu of the CC are known to connect regions of the default mode network (Teipel et al., 2010) which have been consistently found to be affected by Alzheimer’s disease (Sorg et al., 2009; Agosta et al., 2011; Wu et al., 2011). Furthermore, regions of the default mode network, such as the frontal lobes, are associated with working memory performance (Koshino et al., 2014) whereas medial temporal lobe regions are involved in long-term associative memory functions (Ward et al., 2014). These regions have shown a synergistic relationship following brain damage (Maccotta et al., 2007). The final region of interest we considered was the centrum semiovale [Fig 2B(e)] which covers large areas of the corticospinal tract. This was chosen on the assumption of preserved motor functions in the early stages of Alzheimer’s disease (Rose et al., 2000; Huang et al., 2012) and as such served as a comparative control region. One other region found to be relevant in previous DT-MRI studies involving preclinical Alzheimer’s disease is the fornix (Ringman et al., 2007; Molinuevo et al., 2014; Racine et al., 2014). This is a projection tract that connects the medial temporal lobe to other limbic structures and is known to be involved in memory functions (Boespflug et al., 2014). However, technical limitations (i.e. small size and imprecise boundaries) prevented the inclusion of this region of interest in our analysis. Nevertheless, we included the CGH, a white matter tract linking the hippocampus and parahippocampal cortex to the posterior cingulate cortex, known to be relevant for memory function and sensitive to Alzheimer’s disease pathology (Villain et al., 2008; Catheline et al., 2010).
Finally, region of interest selection was independently performed by two investigators of this study. The aim of this procedure was to assess inter-rater reliability of region of interest placement. To this aim, rater 2 (L.P.) randomly selected a subset of participants (n = 5) from the data set initially processed by rater 1 (H.S.). We report on the index of reproducibility and coefficient of variation for the two DT-MRI metrics.
Statistical analyses
Statistical analyses were performed in R version 3.1.1 (R Development Core Team). Group differences in background variables (e.g. age, education, and MMSE score) were examined with ANOVA using Tukey’s test for post hoc comparisons. Hemispheric differences in fractional anisotropy and mean diffusivity values were examined with t-tests.
Group differences in behavioural tasks were tested with linear regression. A model with age, education, and group was created for each task (task ∼ age + education + group) to test for the relationship between these variables and task performance. Post hoc group comparisons were performed with using pairwise least-squares means comparison with the Tukey’s correction for multiple comparisons using lsmeans function from the lsmeans package. Least-squares means were used to extract the group means after controlling for age and education effects.
Group differences in DT-MRI metrics (i.e. fractional anisotropy and mean diffusivity values) for each region of interest were examined with linear regression. A simple model with group as a predictor was created for each region of interest (DT-MRI group) and post hoc group comparisons were performed with pairwise Tukey’s test. This model, and the subsequent models in which ‘Group’ was entered as a predictor, allow the assessment of associations between dependent variables (i.e. DT-MRI measures and behavioural) and group membership. The rationale behind these regression models is that they fit the first intercept and slope for the association between such variables in the first group (in our analysis this was the healthy control group). Then, the models test whether group membership (i.e. asymptomatic carriers or patients with familial Alzheimer’s disease) modifies such associations. The relationship between task performance and DT-MRI metrics was investigated with linear regression by fitting models with DT-MRI variables as predictors for task performance (task ∼ DT-MRI).
Finally, the relationship between task performance, DT-MRI variables, and group membership was examined with correlation and linear regression. Firstly, we examined the correlation between task performance and DT-MRI variables in each region of interest separately in all groups. Second, to visualize the results, we examined the significant correlations revealed in step one more closely with linear regression. The relationship between task performance, group identity and DT-MRI parameters was investigated with linear regression by predicting task performance with group identity, DT-MRI parameter, and their interaction (‘task ∼ group × DT-MRI’). To account for multiple statistical comparisons, all P-values shown were false detection rate (FDR) corrected.
A recent study confirmed that the method reported here to place regions of interest and derive DT-MRI variables provides good reliability (Pettit et al., 2013). However, we performed further inter-rater reliability analysis for the regions of interest chosen for the present study. To this aim, placement of the regions of interest was independently performed by two investigators of this study. Rater 2 (L.P.) randomly selected a subset of participants (n = 5) from the data set initially processed by rater 1 (H.S.). We report on the index of reproducibility and coefficient of variation for the two DT-MRI variables.
Results
Behavioural results
Results from group comparisons of behavioural variables are presented in Table 2. Patients with familial Alzheimer’s disease performed significantly worse than healthy controls on all neuropsychological and visual STM binding tasks except for the Wisconsin Card Sorting Test and Letter fluency task. Patients with familial Alzheimer’s disease performed significantly worse than the asymptomatic carriers on all tasks except for the Copy of the Complex Rey Figure, the Trail Making Test part A, number of attempts to category in the Wisconsin Card Sorting Test, and the shape-colour binding task. Asymptomatic carriers performed significantly worse than healthy controls on the shape-colour binding condition of the visual STM task only.
Table 2.
Neuropsychological performance for the three groups
| Model (task ∼ age + education + group) |
Predictor (beta and P-values) |
Post hoc t-tests (t and P-values) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Task | R2 (P-value) | Age | Education | Group 2 (AC) | Group 3 (FAD) | HC versus AC | HC versus FAD | AC versus FAD | |||
| PAL | 0.47 (<0.001) | −0.2 (0.108) | 0.2 (0.130) | −0.2 (0.438) | −9.6 (<0.001) | 0.8 (0.72) | −4.4 (<0.001) | 3.3 (0.005) | |||
| Complex Rey Figure – copy | 0.30 (<0.001) | −0.2 (0.221) | 0.2 (0.159) | −0.02 (0.939) | −7.0 (0.011) | 0.08 (0.997) | 2.7 (0.03) | 2.3 (0.06) | |||
| Complex Rey Figure – recall | 0.55 (<0.001) | −0.01 (0.953) | 0.2 (0.090) | 0.07 (0.686) | −1.19 (<0.001) | −0.4 (0.913) | 5.6 (<0.001) | 5.3 (<0.001) | |||
| Letter fluency (FAS) | 0.09 (0.068) | − | − | − | − | – | – | – | |||
| Animal fluency | 0.32 (<0.001) | 0.3 (0.059) | 0.3 (0.036) | 0.3 (0.209) | −9.0 (0.001) | −1.3 (0.417) | 3.5 (0.003) | 4.1 (<0.001) | |||
| Boston naming test | 0.35 (<0.001) | 0.2 (0.105) | 0.3 (0.04) | 0.2 (0.475) | −10.0 (<0.001) | −0.7 (0.753) | 3.9 (<0.001) | 4.1 (<0.001) | |||
| Word list – immediate recall | 0.45 (<0.001) | <0.01 (1.000) | 0.02 (0.847) | 0.1 (0.493) | −12.1 (<0.001) | −0.7 (0.771) | 5.2 (<0.001) | 5.2 (<0.001) | |||
| Word list – delayed recall | 0.63 (<0.001) | 0.1 (0.449) | −0.01 (0.913) | −0.04 (0.830) | −15.6 (<0.001) | 0.2 (0.975) | 8.2 (<0.001) | 7.2 (<0.001) | |||
| Word list recognition | 0.54 (<0.001) | −0.1 (0.593) | −0.1 (0.423) | −0.01 (0.960) | −13.5 (<0.001) | 0.05 (0.999) | 6.3 (<0.001) | 5.6 (<0.001) | |||
| Trail Making Test A | 0.38 (<0.001) | 0.3 (0.059) | −0.2 (0.047) | 0.1 (0.827) | 6.39 (0.009) | −0.2 (0.974) | −2.7 (0.025) | −2.1 (0.093) | |||
| WCST number of categories | 0.24 (0.002) | 0.1 (0.320) | 0.2 (0.164) | 0.5 (0.030) | −6.3 (0.035) | −2.2 (0.075) | 2.2 (0.087) | 3.6 (0.002) | |||
| WCST attempt to category | −0.07 (0.967) | − | − | − | − | – | – | – | |||
| Conditions of the VSTM Binding Task | |||||||||||
| Shape only | 0.57 (<0.001) | −0.2 (0.077) | 0.04 (0.697) | −0.3 (0.062) | −13.7 (<0.001) | 1.9 (0.147) | 6.4 (<0.001) | 4.3 (<0.001) | |||
| Colour only | 0.37 (<0.001) | −0.1 (0.333) | 0.07 (0.529) | −0.2 (0.281) | −11.0 (<0.001) | 1.1 (0.525) | 4.3 (<0.001) | 3.0 (0.01) | |||
| Shape-colour binding | 0.42 (<0.001) | −0.1 (0.458) | 0.1 (0.242) | −0.68 (0.001) | −12.6 (<0.001) | 3.5 (0.003) | 5.0 (<0.001) | 2.0 (0.133) | |||
AC = asymptomatic carriers; FAD = familial Alzheimer’s disease; HC = healthy controls; WCST = Wisconsin Card Sorting Test Significant. (P < 0.05) tests highlighted in bold. Beta values shown are standardized P-values for R2 are FDR-corrected.
Education was associated with task performance on the Trail Making Test, Animal Fluency, and Boston Naming Test. Age was not significantly associated with performance in any of the tasks. Importantly, age and education were not associated with visual STM shape-colour binding or PAL tasks, so further analyses with these tasks of interest did not include age or education as a covariate.
DT-MRI metrics
The inter-rater reliability analysis indicated excellent reproducibility of region of interest measurements with the standard deviation of the difference between repeated measures of mean diffusivity and fractional anisotropy being 37 × 10−6 mm2/s (mean of measurements 739 × 10−6 mm2/s) and 0.034 (mean 0.349), respectively. This yielded coefficients of variation of 5.0% for mean diffusivity (range 0.0 for centrum semiovale to 8.51% for CGH) and 9.8% for fractional anisotropy (range 0.0 for centrum semiovale to 12.3% for CGH), which compares well with values for other studies using region of interest analysis (Shenkin et al., 2005).
Initial comparisons between fractional anisotropy and mean diffusivity values from corresponding regions of interest in left and right hemispheres revealed hemispheric differences in all regions either in fractional anisotropy, mean diffusivity, or both. Therefore, all the reported analyses were conducted for hemispheres separately (Table 3). Group comparisons between asymptomatic carriers and controls revealed no significant differences in either fractional anisotropy or mean diffusivity values. However, group comparisons between patients with familial Alzheimer’s disease and controls showed that patients with familial Alzheimer’s disease had higher mean diffusivity in CGH and genu CC bilaterally, left inferolateral FWM, and left splenium CC. Also, group comparisons between patients with familial Alzheimer’s disease and asymptomatic carriers showed that patients with familial Alzheimer’s disease had higher mean diffusivity in genu CC bilaterally, left inferolateral FWM, right CGH, and left splenium CC.
Table 3.
Significant group differences in DT-MRI measures in regions of interests
| Model (DT-MRI ∼ group) |
Predictor (beta and P-values) |
Post-hoc t-tests (t and P-values) |
||||
|---|---|---|---|---|---|---|
| DT-MRI | R2 (P-value) | Group 2 (AC) | Group 3 (FAD) | HC versus AC | HC versus FAD | AC versus FAD |
| FA left mFWM | 0.04 (0.224) | − | − | − | − | − |
| FA right mFWM | 0.007 (0.417) | − | − | − | − | − |
| FA left iFWM | 0.03 (0.224) | − | − | − | − | − |
| FA right iFWM | −0.005 (0.495) | − | − | − | − | − |
| FA left gCC | 0.06 (0.133) | − | − | − | − | − |
| FA right gCC | −0.01 (0.568) | − | − | − | − | − |
| FA left sCC | −0.03 (0.850) | − | − | − | − | − |
| FA right sCC | −0.01 (0.527) | − | − | − | − | − |
| FA left CGH | 0.10 (0.069) | − | − | − | − | − |
| FA right CGH | 0.03 (0.224) | − | − | − | − | − |
| FA left CS | −0.003 (0.481) | − | − | − | − | − |
| FA right CS | 0.06 (0.146) | − | − | − | − | − |
| 〈D〉 left mFWM | 0.07 (0.133) | − | − | − | − | − |
| 〈D〉 right mFWM | 0.08 (0.096) | − | − | − | − | − |
| 〈D〉 left iFWM | 0.15 (0.024) | 0.04 (0.861) | 0.76 (0.003) | −0.2 (0.983) | −3.1 (0.008) | −2.9 (0.016) |
| 〈D〉 right iFWM | 0.03 (0.234) | − | − | − | − | − |
| 〈D〉 left gCC | 0.14 (0.024) | 0.07 (0.781) | 0.8 (0.003) | −0.3 (0.958) | −3.1 (0.008) | −2.8 (0.020) |
| 〈D〉 right gCC | 0.13 (0.036) | −0.01 (0.969) | 0.70 (0.007) | 0.04 (0.999) | −2.8 (0.018) | −2.8 (0.020) |
| 〈D〉 left sCC | 0.15 (0.024) | −0.2 (0.409) | 0.63 (0.012) | 0.8 (0.685) | −2.6 (0.032) | −3.3 (0.004) |
| 〈D〉 right sCC | 0.005 (0.417) | − | − | − | − | − |
| 〈D〉 left CGH | 0.16 (0.024) | 0.3 (0.287) | 0.85 (0.001) | −1.1 (0.533) | −3.5 (0.003) | −2.4 (0.054) |
| 〈D〉 right CGH | 0.24 (0.005) | −0.1 (0.693) | 0.86 (<0.001) | 0.4 (0.917) | −3.7 (0.001) | −4.0 (<0.001) |
| 〈D〉 left CS | 0.11 (0.055) | − | − | − | − | − |
| 〈D〉 right CS | 0.005 (0.417) | − | − | − | − | − |
〈D〉 = mean diffusivity; AC = asymptomatic carriers; CGH = hippocampal part of cingulum bundle; CS = centrum semiovale; FA = fractional anisotropy; FAD = familial Alzheimer’s disease; gCC = genu of corpus callosum; HC = healthy controls; iFWM = inferior frontal white matter; mFWM = middle frontal white matter; sCC = splenium of corpus callosum. Significant (P < 0.05) tests highlighted in bold. P-values for R2 are FDR-corrected.
Relationship between DT-MRI metrics and behavioural tasks
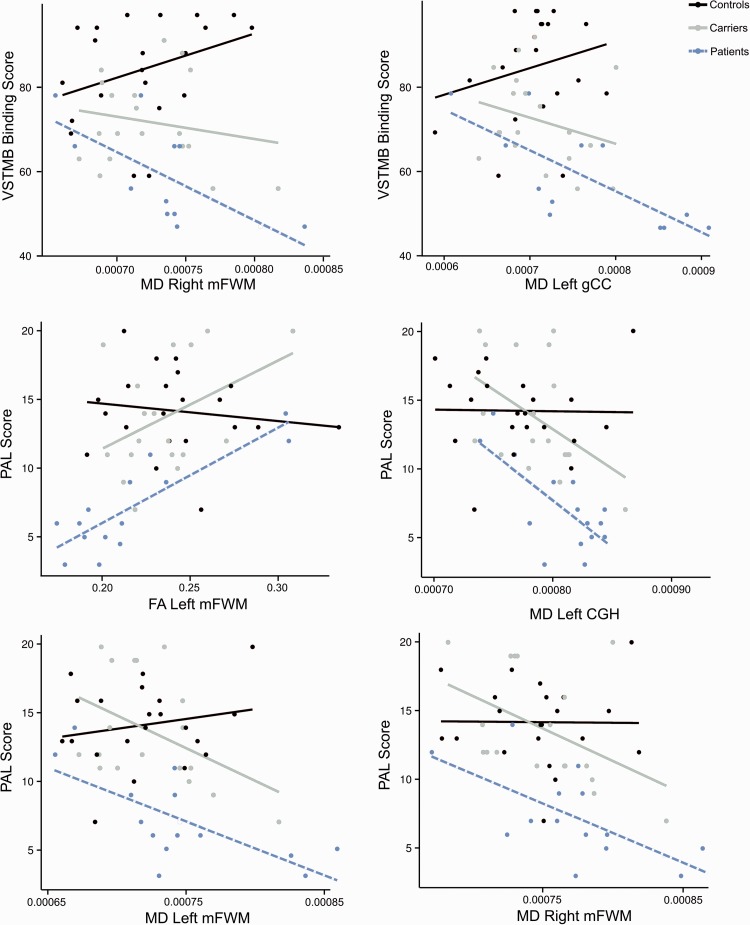
To identify the regions of interest that might be related to deficits in STM binding, the shape-colour binding condition was chosen for further analysis as this was the only condition of the STM binding task that differentiated between the three study groups. Performance on the PAL task was also included for comparison due to the reported sensitivity of this memory function to the early stages of Alzheimer’s disease (Swainson et al., 2001; Fowler et al., 2002). Although in the current study asymptomatic carriers were not significantly impaired on the PAL, in previous studies this task accounted for a large proportion of variance between carriers and controls who were taken from the same population (Parra et al., 2010). Our data show a significant group effect in the shape-colour binding task (model P < 0.001, adjusted R2 = 0.42) and PAL task (model P < 0.001, adjusted R2 = 0.43).
In the shape-colour binding task, better performance was significantly predicted by mean diffusivity values in bilateral genu CC, left inferolateral FWM, and left CGH (Table 4). Fractional anisotropy values did not significantly predict task performance. When examining the groups separately, we found that task performance correlated significantly with mean diffusivity values in right middle FWM (r = −0.80, P = 0.036) and left genu CC (r = −0.75, P = 0.04) only in the familial Alzheimer’s disease group (Fig. 3). In the other groups the correlations were not significant (Supplementary Table 3). The ‘task ∼ group × DT-MRI’ model with variables showing significant correlations revealed that the slope of the familial Alzheimer’s disease group significantly differed from that of controls for both middle FWM and bilateral genu CC (all P < 0.05).
Table 4.
Variance explained by DT-MRI parameters in task performance in the STM binding task and the PAL task
| Model (task ∼ DT-MRI) | VSTM Shape-Colour Binding |
PAL |
||
|---|---|---|---|---|
| FA | 〈D〉 | FA | 〈D〉 | |
| R2 (P-value), beta | R2 (P-value), beta | R2 (P-value), beta | R2 (P-value), beta | |
| Left mFWM | 0.04 (0.157), 0.23 | 0.01 (0.276), −0.18 | 0.18 (0.007), 0.44 | 0.14 (0.012), −0.40 |
| Right mFWM | 0.05 (0.102), 0.27 | 0.02 (0.257), 0.19 | 0.02 (0.243), 0.20 | 0.20 (0.007), −0.46 |
| Left iFWM | −0.02 (0.951), 0.009 | 0.10 (0.040), −0.34 | 0.00 (0.371), 0.15 | 0.17 (0.007), −0.43 |
| Right iFWM | −0.00 (0.437), −0.13 | 0.08 (0.064), −0.31 | 0.01 (0.325), 0.16 | 0.06 (0.096), −0.27 |
| Left gCC | −0.00 (0.414), 0.14 | 0.15 (0.009), −0.41 | 0.05 (0.115), 0.25 | 0.22 (0.007), −0.48 |
| Right gCC | −0.01 (0.501), 0.11 | 0.16 (0.009), −0.42 | −0.01 (0.501), 0.11 | 0.17 (0.007), −0.43 |
| Left sCC | −0.02 (0.728), 0.06 | 0.03 (0.182), −0.22 | 0.06 (0.094), 0.27 | 0.18 (0.024), −0.45 |
| Right sCC | −0.01 (0.501), 0.07 | −0.02 (0.734), −0.06 | 0.10 (0.032), 0.35 | 0.09 (0.040), −0.33 |
| Left CGH | −0.00 (0.414), 0.14 | 0.15 (0.009), −0.41 | 0.13 (0.015), 0.39 | 0.20 (0.007), −0.46 |
| Right CGH | 0.05 (0.112), 0.26 | 0.08 (0.066), −0.31 | 0.05 (0.107), 0.26 | 0.18 (0.007), −0.44 |
| Left CS | −0.02 (0.782), −0.05 | 0.06 (0.094), −0.28 | −0.02 (0.857), 0.03 | 0.15 (0.009), −0.41 |
| Right CS | 0.01 (0.325), 0.17 | 0.01 (0.299), −0.18 | 0.07 (0.071), 0.29 | 0.07 (0.007), −0.30 |
〈D〉 = mean diffusivity; CGH = hippocampal part of cingulum bundle; CS = centrum semiovale; FA = Fractional Anisotropy; gCC = genu of corpus callosum; iFWM = inferior frontal white matter; mFWM = middle frontal white matter; sCC = splenium of corpus callosum. Significant (P < 0.05) tests highlighted in grey. P-values are FDR-corrected.
Figure 3.
Regressions with region of interest, memory binding and group. Fitted regression lines for the shape-colour binding (top) and the PAL Task (middle and bottom) and DT-MRI variables for each group separately in the regions where significant correlations between DT-MRI metrics and memory performance were found. Dots show the observed data in raw values and lines represent fitted regression lines. CGH = hippocampal part of cingulum bundle; FA = fractional anisotropy; mFWM = middle FWM; MD = mean diffusivity; gCC = genu CC.
In the PAL task, better performance was significantly predicted by fractional anisotropy values in left middle FWM, right splenium CC, left CGH, and by mean diffusivity values in all regions of interest except the right inferolateral FWM and right centrum semiovale (Table 4). When examining the groups separately, we found that task performance correlated significantly with fractional anisotropy values in left middle FWM (r = 0.85, P = 0.002) and with mean diffusivity values in left middle FWM (r = −0.69, P = 0.036), right middle FWM (r = −0.66, P = 0.048), left inferolateral FWM (r = 0.70, P = 0.036), and left CGH (r = −0.72, P = 0.036) in the familial Alzheimer’s disease group (Fig. 3). Although the correlations in the asymptomatic carriers group were all non-significant, the effect sizes were of middle or large magnitude as compared with small in the healthy control group (Supplementary Table 3). The ‘task ∼ group × DT-MRI’ model with variables showing significant correlations revealed that the slope of the familial Alzheimer’s disease group significantly differed from that of controls for fractional anisotropy values in left middle FWM, mean diffusivity in left middle FWM, left inferolateral FWM, and left CGH (all P < 0.05).
Discussion
The present study was designed to investigate whether white matter integrity detected with DT-MRI was associated with deficits in memory binding functions known to be sensitive to the early stages of Alzheimer’s disease. This hypothesis was assessed in a unique population of carriers of the mutation E280A-PSEN1 who were either in the asymptomatic stages or had recently met criteria for Alzheimer’s disease. The main findings of this study were: (i) white matter integrity in frontal regions (middle FWM) and in the anterior part of corpus callosum (genu CC) accounted for a significant proportion of variance of STM binding performance; (ii) white matter integrity in frontal regions (middle FWM and inferolateral FWM) and in the hippocampal part of cingulum bundle (CGH) accounted for a significant proportion of variance in performance on the PAL task; and (iii) these associations proved significant in the clinical but not in the preclinical stages of familial Alzheimer’s disease. Before we discuss the implications of these findings for our current understanding of memory decline in Alzheimer’s disease, we briefly address the distinction between these two memory systems.
STM binding is an integrative memory function known to support the conjunction of features necessary to create objects’ identity (Staresina and Davachi, 2010). Such a function relies on regions along the visual ventral stream but is independent of the hippocampus (Parra et al., 2014). Associative memory is an integrative memory function responsible for linking aspects of complex experiences, each with their own identity, into relational representations. Such a memory function cannot be carried out without an intact hippocampus (Moses and Ryan, 2006; Mayes et al., 2007). Conjunctive (STM binding) and relational (associative memory) binding functions have been found to dissociate also in STM (Parra et al., 2013). A recent hypothesis paper has suggested that context-free memory (e.g. STM binding) declines in the subhippocampal phase of Alzheimer’s disease, which seems to occur very early in the disease process (Braak stages I–II). However, context-rich memory (e.g. associative memory) is impacted during the hippocampal phase of Alzheimer’s disease which appears to correspond to more advanced diseases stages (Braak stages III–VI) (Didic et al., 2011). This ongoing debate is relevant to our current study as our data revealed different patterns of dissociation for behavioural and DT-MRI variables.
Unlike previous studies which have consistently reported differences in white matter integrity in the presymptomatic stages of familial Alzheimer’s disease (Ringman et al., 2007; Ryan et al., 2013), in the present study we failed to find significant differences in DT-MRI metrics between asymptomatic carriers and controls. There are some key differences between the present study and those reported earlier which may explain this lack of replication. First, Ringman et al. (2007) investigated DT-MRI metrics in a heterogeneous group of carriers of different mutations either in the PSEN1 (A431E, n = 11; L235V, n = 7; G206A, n = 1) or APP gene (V717I, n = 4). This raises the question of whether such diverse genotypes may yield phenotypic expressions which contributed differently to the reported outcomes. In our study we assessed a sample taken from a population that carries a single mutation of PSEN1 (i.e. E280A). Second, the study by Ryan et al. (2013) investigated an even more genetically heterogeneous sample of carriers of PSEN1 mutation who were also older than the carriers investigated in the present study (mean age = 37.8 years, SD = 4.7). Age is an important factor in these dominantly inherited forms of Alzheimer’s disease as it unequivocally indicates time to clinical expression. It has been recently observed that the PSEN1 mutation affecting the individuals from the Colombian kindred (i.e. E280A) leads to accumulation of amyloid deposits from 28 through to 37 years of age (Fleisher et al., 2012). The asymptomatic carriers investigated here had a mean age of 35. The contributions of the amyloid pathology to white matter disruptions in Alzheimer’s disease are well known (Racine et al., 2014). These earlier findings together with our current results suggest that our sample of E280A-PSEN1 mutation carriers was in a stage of preclinical familial Alzheimer’s disease where structural damage to white matter tracts is not yet evident.
Consistent with previous studies (Parra et al., 2010, 2011), mutation carriers who did and did not meet criteria for familial Alzheimer’s disease presented with significant STM binding impairments. However, we observed that lower white matter integrity values were associated with STM binding impairments only in symptomatic carriers of the E280A-PSEN1 mutation. These differences were driven by increased mean diffusivity in frontal white matter and genu CC. One other study investigating white matter integrity in familial Alzheimer’s disease of which we are aware (Ringman et al., 2007), showed disruption in left frontal white matter (region of interest specified as two voxels in inferior FWM). Although Ringman et al. (2007) implemented a definition of frontal white matter different from our own, our results are complementary and suggest that FWM disturbance is an early anatomical signature of Alzheimer’s disease (see also Oishi et al., 2011a). Furthermore the present study indicates that these white matter differences are associated with a decline of memory functions known to be affected in the preclinical stages of the disease, namely STM binding.
The role of frontal lobes (and connecting structures such as genu CC) in working memory (Owen, 2000) and in binding functions in particular (Prabhakaran et al., 2000; Sala and Courtney, 2007) has been recognized. Neuroimaging studies have documented the involvement of frontal regions, e.g. Brodmann area 10 and dorso-lateral prefrontal cortex, during feature binding in working memory (Prabhakaran et al., 2000; Mitchell et al., 2006) and have suggested that changes in the activity of these regions are related to reduced binding abilities in older adults (Mitchell et al., 2006). Here this association is further strengthened with the finding of prefrontal tract abnormalities related to STM binding deficits in individuals who have recently met criteria for familial Alzheimer’s disease. Neuronal degeneration in Alzheimer’s disease seems to begin in the neuronal periphery rather than in the cell body (Pigino et al., 2003; Stokin et al., 2005), and these early abnormalities appear to be associated with amyloid pathology (Gunawardena and Goldstein, 2001; Racine et al., 2014). Factors such as altered myelin and oligodendrocytes, axonal degeneration, and vascular pathologies, are some proposed mechanisms (Englund and Brun, 1990; Sjobeck et al., 2005; Bartzokis et al., 2007).
Severity of FWM damage in Alzheimer’s disease is closely related to parenchymal Abeta load (Chalmers et al., 2005). Recent studies in E280A-PSEN1 mutation carriers taken from the same population studied here show that amyloid-β deposits in frontal regions begin at the age of 27 years (range: 26.2–28.9) and reach a plateau at the age of 36.2 years (range: 35.1–39.3) (Fleisher et al., 2012). This is more than 10 years before the average age of onset of this form of familial Alzheimer’s disease. Of note, this is precisely the age at which we first see STM binding deficits in asymptomatic carriers of this mutation (Parra et al., 2010, 2011), supporting the notion that these events, i.e. amyloid-β load, white matter degeneration and STM binding deficits, may be associated. However, contrary to our predictions, white matter integrity in the investigated region of interest did not correlate with STM binding performance in the preclinical stages of familial Alzheimer’s disease. This raises the question of what disease mechanism could trigger such an early memory decline. A potential account could be offered by the Neuroplasticity Hypothesis of Alzheimer’s disease (Teter and Ashford, 2002). Early amyloidosis in the course of Alzheimer’s disease may disrupt synaptic transmission leading to neuronal connectivity impairments (Spires-Jones and Hyman, 2014). White matter synaptic disruption precedes both white matter tract anomalies and neurodegeneration (Alix and Domingues, 2011). Therefore, different memory binding functions may be affected by different white matter events which would range from early synaptic dysfunction (conjunctive binding functions) to large-scale network disruptions (relational binding functions). Such a hypothesis will benefit from future animal and human research.
Previous studies have reported that reduced white matter integrity in Alzheimer’s disease leads to a disruption of the topological organization of large-scale structural networks (Lo et al., 2010). We found that damage in middle FWM and genu CC were both related to poor performance on the STM binding task. The genu CC is a major white matter structure hosting tracts (e.g. forceps minor) which connect the dorsolateral prefrontal cortex across hemispheres (Barbas and Pandya, 1984). The strength of such connectivity seems to reflect changes in response to task demands (Tang et al., 2010) or training (Takeuchi et al., 2010). In the context of the present study, the association between poor performance on the binding condition of the STM task and increased mean diffusivity both in regional and trans-hemispheric white matter tracks may well reflect the demands for top-down attentional control. A recent functional MRI study which used the same STM binding task as the current investigation reported binding-specific activation in posterior parietal regions (Parra et al., 2014) which are also known to be part of the network supporting top-down attentional control (Gazzaley and Nobre, 2012). Therefore, the hypothesis that STM binding deficits may be explained, at least in part, by impaired structural connectivity early in the course of familial Alzheimer’s disease seems to be one supported by these data.
This study also sheds light on the neural substrate of PAL deficits in Alzheimer’s disease and demonstrates that lower white matter integrity in frontal regions (middle FWM) and in the hippocampal part of cingulum bundle (CGH) accounts for a significant proportion of variance of performance on the PAL task in symptomatic carriers of the mutation. In line with previous studies, the patients with familial Alzheimer’s disease assessed in the present study presented with associative learning deficits (Lowndes and Savage, 2007; Didic et al., 2011). Associative memory, also known as relational binding (Moses and Ryan, 2006; Mayes et al., 2007), seems to rely on the integrity of grey matter located in frontal regions and the hippocampus (Cer and O'Reilly, 2006) as well as on the effective connectivity between these regions (Fellgiebel and Yakushev, 2011; Yassa, 2011). There is evidence that associative learning declines in the prodromal stages of late-onset sporadic Alzheimer’s disease (Swainson et al., 2001; Fowler et al., 2002). Previous neuroimaging studies in this population have demonstrated functional reorganization of medial temporal lobe structures, including the hippocampus, when asymptomatic carriers with an average age of 33.7 years completed a face-name association task (Quiroz et al., 2010). The authors suggested that functional changes within the hippocampal memory system occur years before cognitive decline in familial Alzheimer’s disease. In fact, Parra et al. (2010, 2011) showed that STM binding and PAL exhibit a gradual and continued decline in groups of carriers whose age approached the average age of onset of this familial Alzheimer’s disease variant. This decline stood out from the neuropsychological background and was found to be earlier and much steeper in the former function. The results presented here suggest that these very early PAL impairments in the early stages of familial Alzheimer’s disease are not solely due to the impact of neurodegeneration on grey matter structures (Quiroz et al., 2013), but also to lower white matter integrity of those tracts connecting them.
It is worth noting that the analysis of DT-MRI metrics during PAL task performance revealed that this function relies on a more extended network than visual STM binding (Table 4). In previous studies we have found that STM binding was specifically affected by Alzheimer’s disease relative to other non-Alzheimer’s disease dementias (Della Sala et al., 2012). We have suggested that a potential cause for this high specificity may lie at a neuroanatomical level. We have recently demonstrated that normal performance on the STM binding task presented here does not require an intact hippocampus (Parra et al., 2013, 2014). In fact, we previously showed that performance on the STM binding and PAL tasks did not correlate in carriers of the mutations E280A-PSEN1 (Parra et al., 2011). However, associative learning does decline in other non-Alzheimer’s disease dementias (Taylor et al., 1990; Dimitrov et al., 1999; Clague et al., 2005) and also in healthy ageing (Naveh-Benjamin et al., 2007; Old and Naveh-Benjamin, 2008) rendering this task less specific both for the early detection of Alzheimer’s disease and its differential diagnosis. In the present study we found that these functions previously dissociated at behavioural and anatomical level, also dissociate when the integrity of white matter structure is considered, reinforcing the notion that memory binding functions in STM and in LTM have different neural correlates. The fact that PAL relies on a widespread network whereas the STM binding relies on more restricted network may well explain the different pattern of sensitivity and specificity shown by these two memory binding functions.
A question that may arise from this study is whether the associations between lower white matter integrity and specific memory impairments reported here are typical of Alzheimer’s disease or a phenotypic expression of this specific mutation (i.e. E280A-PSEN1). There is no straightforward answer to this question as the links between genotype and phenotype in Alzheimer’s disease are poorly understood (Holmes, 2002). However, a recent study suggests that when it comes to STM binding, sporadic and familial variants of Alzheimer’s disease share a common phenotype (Parra et al., 2011). Moreover, the findings of DT-MRI studies of both sporadic and familial variants have been complementary suggesting that although triggered by different mechanisms, the clinical expression of white matter damage in these forms of Alzheimer’s disease may also share phenotypic features (Gold et al., 2012).
We acknowledge some limitations of this study. First, we used region of interest analyses which introduce some subjective components to the placement of region of interest structures of interest. However, we took great care in both the selection of regions of interest and their placement, while checks were performed to ensure they were placed solely in white matter structures. Furthermore, we assessed the inter-rater reliability as reported in a previous study (Pettit et al., 2013) and this analysis confirmed a high reliability of this methods. We therefore consider it unlikely that issues related to the placement of regions of interest may have had an influence on the results reported here. Second, the lack of associations between memory binding performance and DT-MRI metrics in asymptomatic carriers may reflect limited power because of the relative small sample assessed in this study. This is supported by the finding of middle to large effect sizes for the correlational analyses between PAL performance and DTI-metrics in this group. Finally, it is worth mentioning some technical difficulties we encountered in identifying regions of interest such as the fornix, which is proving relevant as a biomarker for Alzheimer’s disease (Oishi and Lyketsos, 2014). Although we failed to find significant associations between white matter integrity in the selected theory-driven region of interest and STM binding performance in the preclinical stages of familial Alzheimer’s disease, there may still be white matter structures which are relevant to this cognitive function. Nevertheless, we have provided reliable evidence of dissociation between the integrity of white matter structures and the two memory functions investigated here, namely STM binding and associative learning.
In sum, the present study showed that reduced white matter integrity, in frontal lobes, corpus callosum and medial temporal lobes, can account for memory binding impairments in familial Alzheimer’s disease. In the early stages of familial Alzheimer’s disease, white matter integrity explained deficits in memory binding functions which rely on large-scale networks such as PAL. However, deficits in memory binding functions which need more selective networks do not seem to be accounted for by white matter disruption in asymptomatic individuals who will unequivocally develop familial Alzheimer’s disease. Future studies should investigate what particular disease mechanisms underpin such an early memory decline.
Funding
M.A.P. is supported by Alzheimer’s Society, Grant # AS-R42303. The study is also sponsored by Colciencias, Grants 1115‐408‐20512 and 1115‐343‐19127 awarded to the Neuroscience Group, University of Antioquia, Colombia in collaboration with M.A.P. and S.D.S. The project was also partially supported by ALFA Eurocaribean Neurosciences Network, contract AML/B7‐311/97/0666/II-0322-FA-FCD-FI-FC, in which S.D.S. and F.L. were partners. This work was conducted within the context of The University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology, part of the cross council Lifelong Health and Wellbeing Initiative (MR/K026992/1).
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviations
- CC
corpus callosum
- CGH
hippocampal part of the cingulum bundle
- DT-MRI
diffusion tensor MRI
- FWM
frontal white matter
- LTM
long term memory
- PAL
Paired Associates Learning
- STM
short-term memory
References
- Agosta F, Pievani M, Geroldi C, Copetti M, Frisoni GB, Filippi M. Resting state fMRI in Alzheimer's disease: beyond the default mode network. Neurobiol Aging. 2011;33:1564–78. doi: 10.1016/j.neurobiolaging.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Alix JJ, Domingues AM. White matter synapses: form, function, and dysfunction. Neurology. 2011;76:397–404. doi: 10.1212/WNL.0b013e3182088273. [DOI] [PubMed] [Google Scholar]
- Ardila A, Lopera F, Rosselli M, Moreno S, Madrigal L, Arango-Lasprilla JC, et al. Neuropsychological profile of a large kindred with familial Alzheimer's disease caused by the E280A single presenilin-1 mutation. Arch Clin Neuropsychol. 2000;15:515–28. [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Topography of commissural fibers of the prefrontal cortex in the rhesus monkey. Exp Brain Res. 1984;55:187–91. doi: 10.1007/BF00240516. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Mintz J. Human brain myelination and amyloid beta deposition in Alzheimer's disease. Alzheimers Dement. 2007;3:122–5. doi: 10.1016/j.jalz.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8:333–44. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Berg EA. A simple objective test for measuring flexibility in thinking. J Gen Psychol. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- Boespflug EL, Eliassen J, Welge J, Krikorian R. Associative learning and regional white matter deficits in mild cognitive impairment. J Alzheimers Dis. 2014;41:421–30. doi: 10.3233/JAD-131682. [DOI] [PubMed] [Google Scholar]
- Borroni B, Brambati SM, Agosti C, Gipponi S, Bellelli G, Gasparotti R, et al. Evidence of white matter changes on diffusion tensor imaging in frontotemporal dementia. Arch Neurol. 2007;64:246–51. doi: 10.1001/archneur.64.2.246. [DOI] [PubMed] [Google Scholar]
- Bozzali M, Cherubini A. Diffusion tensor MRI to investigate dementias: a brief review. Magn Reson Imaging. 2007;25:969–77. doi: 10.1016/j.mri.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Bozzali M, Padovani A, Caltagirone C, Borroni B. Regional grey matter loss and brain disconnection across Alzheimer disease evolution. Curr Med Chem. 2011;18:2452–8. doi: 10.2174/092986711795843263. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–17. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschke H, Kuslansky G, Katz M, Stewart WF, Sliwinski MJ, Eckholdt HM, et al. Screening for dementia with the memory impairment screen. Neurology. 1999;52:231–8. doi: 10.1212/wnl.52.2.231. [DOI] [PubMed] [Google Scholar]
- Catheline G, Periot O, Amirault M, Braun M, Dartigues JF, Auriacombe S, et al. Distinctive alterations of the cingulum bundle during aging and Alzheimer's disease. Neurobiol Aging. 2010;31:1582–92. doi: 10.1016/j.neurobiolaging.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Cer DM, O'Reilly RC. Neural mechanisms of binding in the hippocampus and neocortex: insights from computational models. In: Zimmer HD, Mecklinger A, Lindenberger U, editors. Handbook of binding and memory, perspective from cognitive neuroscience. New York: Oxford University Press; 2006. pp. 193–220. [Google Scholar]
- Chalmers K, Wilcock G, Love S. Contributors to white matter damage in the frontal lobe in Alzheimer's disease. Neuropathol Appl Neurobiol. 2005;31:623–31. doi: 10.1111/j.1365-2990.2005.00678.x. [DOI] [PubMed] [Google Scholar]
- Chua TC, Wen W, Slavin MJ, Sachdev PS. Diffusion tensor imaging in mild cognitive impairment and Alzheimer's disease: a review. Curr Opin Neurol. 2008;21:83–92. doi: 10.1097/WCO.0b013e3282f4594b. [DOI] [PubMed] [Google Scholar]
- Clague F, Dudas RB, Thompson SA, Graham KS, Hodges JR. Multidimensional measures of person knowledge and spatial associative learning: can these be applied to the differentiation of Alzheimer's disease from frontotemporal and vascular dementia? Neuropsychologia. 2005;43:1338–50. doi: 10.1016/j.neuropsychologia.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Clark RF, Hutton M, Fuldner M, Froelich S, Karran E, Talbot C, et al. The structure of the presenilin 1 (S182) gene and identification of six novel mutations in early onset AD families. Nat Genet. 1995;11:219–22. doi: 10.1038/ng1095-219. [DOI] [PubMed] [Google Scholar]
- Cook IA, Leuchter AF. Synaptic dysfunction in Alzheimer's disease: clinical assessment using quantitative EEG. Behav Brain Res. 1996;78:15–23. doi: 10.1016/0166-4328(95)00214-6. [DOI] [PubMed] [Google Scholar]
- Della Sala S, Parra MA, Fabi K, Luzzi S, Abrahams S. Short-term memory binding is impaired in AD but not in non-AD dementias. Neuropsychologia. 2012;50:833–40. doi: 10.1016/j.neuropsychologia.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Didic M, Barbeau EJ, Felician O, Tramoni E, Guedj E, Poncet M, et al. Which memory system is impaired first in Alzheimer's disease? J Alzheimers Dis. 2011;27:11–22. doi: 10.3233/JAD-2011-110557. [DOI] [PubMed] [Google Scholar]
- Dimitrov M, Granetz J, Peterson M, Hollnagel C, Alexander G, Grafman J. Associative learning impairments in patients with frontal lobe damage. Brain Cogn. 1999;41:213–30. doi: 10.1006/brcg.1999.1121. [DOI] [PubMed] [Google Scholar]
- Douaud G, Jbabdi S, Behrens TE, Menke RA, Gass A, Monsch AU, et al. DTI measures in crossing-fibre areas: increased diffusion anisotropy reveals early white matter alteration in MCI and mild Alzheimer's disease. Neuroimage. 2011;55:880–90. doi: 10.1016/j.neuroimage.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan JH, Wang HQ, Xu J, Lin X, Chen SQ, Kang Z, et al. White matter damage of patients with Alzheimer's disease correlated with the decreased cognitive function. Surg Radiol Anat. 2006;28:150–6. doi: 10.1007/s00276-006-0111-2. [DOI] [PubMed] [Google Scholar]
- Duchek JM, Cheney M, Ferraro FR, Storandt M. Paired associate learning in senile dementia of the Alzheimer type. Arch Neurol. 1991;48:1038–40. doi: 10.1001/archneur.1991.00530220054019. [DOI] [PubMed] [Google Scholar]
- Dunkin JJ, Leuchter AF, Newton TF, Cook IA. Reduced EEG coherence in dementia: state or trait marker? Biol Psychiatry. 1994;35:870–9. doi: 10.1016/0006-3223(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Dvorine I. Quantitative classification of color blind. J Gen Psychol. 1963;68:255–65. [Google Scholar]
- Echavarri C, Aalten P, Uylings HB, Jacobs HI, Visser PJ, Gronenschild EH, et al. Atrophy in the parahippocampal gyrus as an early biomarker of Alzheimer's disease. Brain Struct Funct. 2010;215:265–71. doi: 10.1007/s00429-010-0283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias MF, Beiser A, Wolf PA, Au R, White RF, D'Agostino RB. The preclinical phase of alzheimer disease: A 22-year prospective study of the Framingham Cohort. Arch Neurol. 2000;57:808–13. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- Englund E, Brun A. White matter changes in dementia of Alzheimer's type: the difference in vulnerability between cell compartments. Histopathology. 1990;16:433–9. doi: 10.1111/j.1365-2559.1990.tb01542.x. [DOI] [PubMed] [Google Scholar]
- Fellgiebel A, Wille P, Muller MJ, Winterer G, Scheurich A, Vucurevic G, et al. Ultrastructural hippocampal and white matter alterations in mild cognitive impairment: a diffusion tensor imaging study. Dement Geriatr Cogn Disord. 2004;18:101–8. doi: 10.1159/000077817. [DOI] [PubMed] [Google Scholar]
- Fellgiebel A, Yakushev I. Diffusion tensor imaging of the hippocampus in MCI and early Alzheimer's disease. J Alzheimers Dis. 2011;26:257–62. doi: 10.3233/JAD-2011-0001. [DOI] [PubMed] [Google Scholar]
- Fleisher AS, Chen K, Quiroz YT, Jakimovich LJ, Gomez MG, Langois CM, et al. Florbetapir PET analysis of amyloid-beta deposition in the presenilin 1 E280A autosomal dominant Alzheimer's disease kindred: a cross-sectional study. Lancet Neurol. 2012;11:1057–65. doi: 10.1016/S1474-4422(12)70227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler KS, Saling MM, Conway EL, Semple JM, Louis WJ. Paired associate performance in the early detection of DAT. J Int Neuropsychol Soc. 2002;8:58–71. [PubMed] [Google Scholar]
- Gazzaley A, Nobre AC. Top-down modulation: bridging selective attention and working memory. Trends Cogn Sci. 2012;16:129–35. doi: 10.1016/j.tics.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gili T, Cercignani M, Serra L, Perri R, Giove F, Maraviglia B, et al. Regional brain atrophy and functional disconnection across Alzheimer's disease evolution. J Neurol Neurosurg Psychiatry. 2011;82:58–66. doi: 10.1136/jnnp.2009.199935. [DOI] [PubMed] [Google Scholar]
- Gold BT, Johnson NF, Powell DK, Smith CD. White matter integrity and vulnerability to Alzheimer's disease: preliminary findings and future directions. Biochim Biophys Acta. 2012;1822:416–22. doi: 10.1016/j.bbadis.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardena S, Goldstein LS. Disruption of axonal transport and neuronal viability by amyloid precursor protein mutations in Drosophila. Neuron. 2001;32:389–401. doi: 10.1016/s0896-6273(01)00496-2. [DOI] [PubMed] [Google Scholar]
- Holmes C. Genotype and phenotype in Alzheimer's disease. Br J Psychiatry. 2002;180:131–4. doi: 10.1192/bjp.180.2.131. [DOI] [PubMed] [Google Scholar]
- Huang H, Fan X, Weiner M, Martin-Cook K, Xiao G, Davis J, et al. Distinctive disruption patterns of white matter tracts in Alzheimer's disease with full diffusion tensor characterization. Neurobiol Aging. 2012;33:2029–45. doi: 10.1016/j.neurobiolaging.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Johnson DK, Barrow W, Anderson R, Harsha A, Honea R, Brooks WM, et al. Diagnostic utility of cerebral white matter integrity in early Alzheimer's disease. Int J Neurosci. 2010;120:544–50. doi: 10.3109/00207454.2010.494788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Petersen RC, Boeve BF, Knopman DS, Weigand SD, O'Brien PC, et al. DWI predicts future progression to Alzheimer disease in amnestic mild cognitive impairment. Neurology. 2005;64:902–4. doi: 10.1212/01.WNL.0000153076.46126.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Gooidglass H, Weintraub S. Boston naming test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- Kiuchi K, Morikawa M, Taoka T, Nagashima T, Yamauchi T, Makinodan M, et al. Abnormalities of the uncinate fasciculus and posterior cingulate fasciculus in mild cognitive impairment and early Alzheimer's disease: a diffusion tensor tractography study. Brain Res. 2009;1287:184–91. doi: 10.1016/j.brainres.2009.06.052. [DOI] [PubMed] [Google Scholar]
- Koenig T, Studer D, Hubl D, Melie L, Strik WK. Brain connectivity at different time-scales measured with EEG. Philos Trans R Soc Lond B Biol Sci. 2005;360:1015–23. doi: 10.1098/rstb.2005.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshino H, Minamoto T, Yaoi K, Osaka M, Osaka N. Coactivation of the default mode network regions and working memory network regions during task preparation. Sci Rep. 2014;4:5954. doi: 10.1038/srep05954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CY, Wang PN, Chou KH, Wang J, He Y, Lin CP. Diffusion tensor tractography reveals abnormal topological organization in structural cortical networks in Alzheimer's disease. J Neurosci. 2010;30:16876–85. doi: 10.1523/JNEUROSCI.4136-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopera F, Ardilla A, Martinez A, Madrigal L, rango-Viana JC, Lemere CA, et al. Clinical features of early-onset Alzheimer disease in a large kindred with an E280A presenilin-1 mutation. JAMA. 1997;277:793–9. [PubMed] [Google Scholar]
- Lowndes G, Savage G. Early detection of memory impairment in Alzheimer's disease: a neurocognitive perspective on assessment. Neuropsychol Rev. 2007;17:193–202. doi: 10.1007/s11065-007-9032-z. [DOI] [PubMed] [Google Scholar]
- Maccotta L, Buckner RL, Gilliam FG, Ojemann JG. Changing frontal contributions to memory before and after medial temporal lobectomy. Cereb Cortex. 2007;17:443–56. doi: 10.1093/cercor/bhj161. [DOI] [PubMed] [Google Scholar]
- Mayes A, Montaldi D, Migo E. Associative memory and the medial temporal lobes. Trends Cogn Sci. 2007;11:126–35. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Medina D, Detoledo-Morrell L, Urresta F, Gabrieli JD, Moseley M, Fleischman D, et al. White matter changes in mild cognitive impairment and AD: a diffusion tensor imaging study. Neurobiol Aging. 2006;27:663–72. doi: 10.1016/j.neurobiolaging.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Medina DA, Gaviria M. Diffusion tensor imaging investigations in Alzheimer's disease: the resurgence of white matter compromise in the cortical dysfunction of the aging brain. Neuropsychiatr Dis Treat. 2008;4:737–42. doi: 10.2147/ndt.s3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao X, Wu X, Li R, Chen K, Yao L. Altered connectivity pattern of hubs in default-mode network with Alzheimer's disease: an Granger causality modeling approach. PLoS One. 2011;6:e25546. doi: 10.1371/journal.pone.0025546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ, Raye CL, Johnson MK, Greene EJ. An fMRI investigation of short-term source memory in young and older adults. Neuroimage. 2006;30:627–33. doi: 10.1016/j.neuroimage.2005.09.039. [DOI] [PubMed] [Google Scholar]
- Molinuevo JL, Ripolles P, Simo M, Llado A, Olives J, Balasa M, et al. White matter changes in preclinical Alzheimer's disease: a magnetic resonance imaging-diffusion tensor imaging study on cognitively normal older people with positive amyloid beta protein 42 levels. Neurobiol Aging. 2014;35:2671–80. doi: 10.1016/j.neurobiolaging.2014.05.027. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van BG, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–65. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Moses SN, Ryan JD. A comparison and evaluation of the predictions of relational and conjunctive accounts of hippocampal function. Hippocampus. 2006;16:43–65. doi: 10.1002/hipo.20131. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Brav TK, Levy O. The associative memory deficit of older adults: the role of strategy utilization. Psychol Aging. 2007;22:202–8. doi: 10.1037/0882-7974.22.1.202. [DOI] [PubMed] [Google Scholar]
- O'Connell H, Coen R, Kidd N, Warsi M, Chin AV, Lawlor BA. Early detection of Alzheimer's disease (AD) using the CANTAB paired Associates Learning Test. Int J Geriatr Psychiatry. 2004;19:1207–8. doi: 10.1002/gps.1180. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC, Busby RS, Soto R. Three forms of binding and their neural substrates: alternatives to temporal synchrony. In: Cleeremans A, editor. The unity of consciousness: binding, integration, and dissociation. Oxford: Oxford University Press; 2003. pp. 168–92. [Google Scholar]
- Oishi K, Mielke MM, Albert M, Lyketsos CG, Mori S. DTI analyses and clinical applications in Alzheimer's disease. J Alzheimers Dis. 2011a;26:287–96. doi: 10.3233/JAD-2011-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Faria AV, Zijl P, Mori S. MRI atlas of human white matter. New York: Academic Press; 2011b. [Google Scholar]
- Oishi K, Lyketsos CG. Alzheimer's disease and the fornix. Front Aging Neurosci. 2014;6:241. doi: 10.3389/fnagi.2014.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old SR, Naveh-Benjamin M. Differential effects of age on item and associative measures of memory: a meta-analysis. Psychol Aging. 2008;23:104–18. doi: 10.1037/0882-7974.23.1.104. [DOI] [PubMed] [Google Scholar]
- Osterrieth PA. Le test de copie d'une figure complex: contribution a l'etude de la perception et de la memoire. Arch Psychol. 1944;30:206–356. [Google Scholar]
- Owen AM. The role of the lateral frontal cortex in mnemonic processing: the contribution of functional neuroimaging. [Review]. Exp Brain Res. 2000;133:33–43. doi: 10.1007/s002210000398. [DOI] [PubMed] [Google Scholar]
- Parra MA, Della Sala S, Logie RH, Morcom AM. Neural correlates of shape-color binding in visual working memory. Neuropsychologia. 2014;52:27–36. doi: 10.1016/j.neuropsychologia.2013.09.036. [DOI] [PubMed] [Google Scholar]
- Parra MA, Fabi K, Luzzi S, Cubelli R, Hernandez VM, Della Sala S. Relational and conjunctive binding functions dissociate in short-term memory. Neurocase. 2013;21:56–66. doi: 10.1080/13554794.2013.860177. [DOI] [PubMed] [Google Scholar]
- Parra MA, Abrahams S, Fabi K, Logie R, Luzzi S, Della Sala S. Short-term memory binding deficits in Alzheimer's disease. Brain. 2009;132:1057–66. doi: 10.1093/brain/awp036. [DOI] [PubMed] [Google Scholar]
- Parra MA, Abrahams S, Logie RH, Mendez LG, Lopera F, Della Sala S. Visual short-term memory binding deficits in familial Alzheimer's disease. Brain. 2010;133:2702–13. doi: 10.1093/brain/awq148. [DOI] [PubMed] [Google Scholar]
- Parra MA, Sala SD, Abrahams S, Logie RH, Mendez LG, Lopera F. Specific deficit of colour-colour short-term memory binding in sporadic and familial Alzheimer's disease. Neuropsychologia. 2011;49:1943–52. doi: 10.1016/j.neuropsychologia.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Pettit LD, Bastin ME, Smith C, Bak TH, Gillingwater TH, Abrahams S. Executive deficits, not processing speed relates to abnormalities in distinct prefrontal tracts in amyotrophic lateral sclerosis. Brain. 2013;136:3290–304. doi: 10.1093/brain/awt243. [DOI] [PubMed] [Google Scholar]
- Pigino G, Morfini G, Pelsman A, Mattson MP, Brady ST, Busciglio J. Alzheimer's presenilin 1 mutations impair kinesin-based axonal transport. J Neurosci. 2003;23:4499–508. doi: 10.1523/JNEUROSCI.23-11-04499.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajamaki M, Sperling RA. Functional MRI assessment of task-induced deactivation of the default mode network in Alzheimer's disease and at-risk older individuals. Behav Neurol. 2009;21:77–91. doi: 10.3233/BEN-2009-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran V, Narayanan K, Zhao Z, Gabrieli JD. Integration of diverse information in working memory within the frontal lobe. Nat Neurosci. 2000;3:85–90. doi: 10.1038/71156. [DOI] [PubMed] [Google Scholar]
- Quiroz YT, Stern CE, Reiman EM, Brickhouse M, Ruiz A, Sperling RA, et al. Cortical atrophy in presymptomatic Alzheimer's disease presenilin 1 mutation carriers. J Neurol Neurosurg Psychiatry. 2013;84:556–61. doi: 10.1136/jnnp-2012-303299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz YT, Budson AE, Celone K, Ruiz A, Newmark R, Castrillón G, et al. Hippocampal hyperactivation in presymptomatic familial Alzheimer's disease. Ann Neurol. 2010;68:865–75. doi: 10.1002/ana.22105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine AM, Adluru N, Alexander AL, Christian BT, Okonkwo OC, Oh J, et al. Associations between white matter microstructure and amyloid burden in preclinical Alzheimer's disease: a multimodal imaging investigation. Neuroimage Clin. 2014;4:604–14. doi: 10.1016/j.nicl.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM. Validity of the Trail Making test as an indicator of organic brain damage. Percept Motor Skills. 1958;8:271–6. [Google Scholar]
- Ringman JM, O'Neill J, Geschwind D, Medina L, Apostolova LG, Rodriguez Y, et al. Diffusion tensor imaging in preclinical and presymptomatic carriers of familial Alzheimer's disease mutations. Brain. 2007;130:1767–76. doi: 10.1093/brain/awm102. [DOI] [PubMed] [Google Scholar]
- Rose SE, Chen F, Chalk JB, Zelaya FO, Strugnell WE, Benson M, et al. Loss of connectivity in Alzheimer's disease: an evaluation of white matter tract integrity with colour coded MR diffusion tensor imaging. J Neurol Neurosurg Psychiatry. 2000;69:528–30. doi: 10.1136/jnnp.69.4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan NS, Keihaninejad S, Shakespeare TJ, Lehmann M, Crutch SJ, Malone IB, et al. Magnetic resonance imaging evidence for presymptomatic change in thalamus and caudate in familial Alzheimer's disease. Brain. 2013;136:1399–414. doi: 10.1093/brain/awt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala JB, Courtney SM. Binding of what and where during working memory maintenance. Cortex. 2007;43:5–21. doi: 10.1016/s0010-9452(08)70442-8. [DOI] [PubMed] [Google Scholar]
- Sexton CE, Kalu UG, Filippini N, Mackay CE, Ebmeier KP. A meta-analysis of diffusion tensor imaging in mild cognitive impairment and Alzheimer's disease. Neurobiol Aging. 2011;32:2322. doi: 10.1016/j.neurobiolaging.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Shenkin SD, Bastin ME, Macgillivray TJ, Deary IJ, Starr JM, Rivers CS, et al. Cognitive correlates of cerebral white matter lesions and water diffusion tensor parameters in community-dwelling older people. Cerebrovasc Dis. 2005;20:310–18. doi: 10.1159/000087930. [DOI] [PubMed] [Google Scholar]
- Sjobeck M, Haglund M, Englund E. Decreasing myelin density reflected increasing white matter pathology in Alzheimer's disease—a neuropathological study. Int J Geriatr Psychiatry. 2005;20:919–26. doi: 10.1002/gps.1384. [DOI] [PubMed] [Google Scholar]
- Sorg C, Riedl V, Perneczky R, Kurz A, Wohlschlager AM. Impact of Alzheimer's disease on the functional connectivity of spontaneous brain activity. Curr Alzheimer Res. 2009;6:541–53. doi: 10.2174/156720509790147106. [DOI] [PubMed] [Google Scholar]
- Spires-Jones TL, Hyman BT. The intersection of amyloid beta and tau at synapses in Alzheimer's disease. Neuron. 2014;82:756–71. doi: 10.1016/j.neuron.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Object unitization and associative memory formation are supported by distinct brain regions. J Neurosci. 2010;30:9890–7. doi: 10.1523/JNEUROSCI.0826-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins GT, Murphy CM. Diffusion tensor imaging in Alzheimer's disease and mild cognitive impairment. Behav Neurol. 2009;21:39–49. doi: 10.3233/BEN-2009-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokin GB, Lillo C, Falzone TL, Brusch RG, Rockenstein E, Mount SL, et al. Axonopathy and transport deficits early in the pathogenesis of Alzheimer's disease. Science. 2005;307:1282–8. doi: 10.1126/science.1105681. [DOI] [PubMed] [Google Scholar]
- Sumerall SW, Timmons PL, James AL, Ewing MJ, Oehlert ME. Expanded norms for the Controlled Oral Word Association Test. J Clin Psychol. 1997;53:517–21. doi: 10.1002/(sici)1097-4679(199708)53:5<517::aid-jclp14>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Swainson R, Hodges JR, Galton CJ, Semple J, Michael A, Dunn BD, et al. Early detection and differential diagnosis of Alzheimer's disease and depression with neuropsychological tasks. Dement Geriatr Cogn Disord. 2001;12:265–80. doi: 10.1159/000051269. [DOI] [PubMed] [Google Scholar]
- Sweed HS, Abdul-Rahman SA, Abdel-Aal WM, Abdul-Rahman LA. Clock drawing testing and diffusion tensor imaging among vascular dementia versus Alzheimer's disease. European Geriatric Medicine. 2012;3:349–55. [Google Scholar]
- Takeuchi H, Sekiguchi A, Taki Y, Yokoyama S, Yomogida Y, Komuro N, et al. Training of working memory impacts structural connectivity. J Neurosci. 2010;30:3297–303. doi: 10.1523/JNEUROSCI.4611-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang CY, Eaves EL, Ng JC, Carpenter DM, Mai X, Schroeder DH, et al. Brain networks for working memory and factors of intelligence assessed in males and females with fMRI and DTI. Intelligence. 2010;38:293–303. [Google Scholar]
- Taylor AE, Saint-Cyr JA, Lang AE. Memory and learning in early Parkinson's disease: evidence for a "frontal lobe syndrome". Brain Cogn. 1990;13:211–32. doi: 10.1016/0278-2626(90)90051-o. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Bokde AL, Meindl T, Amaro E, Jr, Soldner J, Reiser MF, et al. White matter microstructure underlying default mode network connectivity in the human brain. Neuroimage. 2010;49:2021–32. doi: 10.1016/j.neuroimage.2009.10.067. [DOI] [PubMed] [Google Scholar]
- Teter B, Ashford JW. Neuroplasticity in Alzheimer's disease. J Neurosci Res. 2002;70:402–37. doi: 10.1002/jnr.10441. [DOI] [PubMed] [Google Scholar]
- Ukmar M, Makuc E, Onor ML, Garbin G, Trevisiol M, Cova MA. Evaluation of white matter damage in patients with Alzheimer's disease and in patients with mild cognitive impairment by using diffusion tensor imaging. Radiol Med. 2008;113:915–22. doi: 10.1007/s11547-008-0286-1. [DOI] [PubMed] [Google Scholar]
- Villain N, Desgranges B, Viader F, De lS V, Mezenge F, Landeau B, et al. Relationships between hippocampal atrophy, white matter disruption, and gray matter hypometabolism in Alzheimer's disease. J Neurosci. 2008;28:6174–81. doi: 10.1523/JNEUROSCI.1392-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zuo X, Dai Z, Xia M, Zhao Z, Zhao X, et al. Disrupted Functional Brain Connectome in Individuals at Risk for Alzheimer's Disease. Biol Psychiatry. 2012;73:472–81. doi: 10.1016/j.biopsych.2012.03.026. [DOI] [PubMed] [Google Scholar]
- Ward AM, Schultz AP, Huijbers W, van Dijk KR, Hedden T, Sperling RA. The parahippocampal gyrus links the default-mode cortical network with the medial temporal lobe memory system. Hum Brain Mapp. 2014;35:1061–73. doi: 10.1002/hbm.22234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. The Wechsler memory scale-III UK manual. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- Wu X, Li R, Fleisher AS, Reiman EM, Guan X, Zhang Y, et al. Altered default mode network connectivity in Alzheimer's disease–a resting functional MRI and Bayesian network study. Hum Brain Mapp. 2011;32:1868–81. doi: 10.1002/hbm.21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA. Searching for novel biomarkers using high resolution diffusion tensor imaging. J Alzheimers Dis. 2011;26 3:297–305. doi: 10.3233/JAD-2011-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang L, Wen W, Zhu W, Trollor J, Kochan N, Crawford J, et al. White matter integrity in mild cognitive impairment: a tract-based spatial statistics study. Neuroimage. 2010;53:16–25. doi: 10.1016/j.neuroimage.2010.05.068. [DOI] [PubMed] [Google Scholar]