Abstract
Background: Initial Food and Drug Administration-approved artificial pancreas (AP) systems will be hybrid closed-loop systems that require prandial meal announcements and will not eliminate the burden of premeal insulin dosing. Multiple model probabilistic predictive control (MMPPC) is a fully closed-loop system that uses probabilistic estimation of meals to allow for automated meal detection. In this study, we describe the safety and performance of the MMPPC system with announced and unannounced meals in a supervised hotel setting.
Research Design and Methods: The Android phone-based AP system with remote monitoring was tested for 72 h in six adults and four adolescents across three clinical sites with daily exercise and meal challenges involving both three announced (manual bolus by patient) and six unannounced (no bolus by patient) meals. Safety criteria were predefined. Controller aggressiveness was adapted daily based on prior hypoglycemic events.
Results: Mean 24-h continuous glucose monitor (CGM) was 157.4 ± 14.4 mg/dL, with 63.6 ± 9.2% of readings between 70 and 180 mg/dL, 2.9 ± 2.3% of readings <70 mg/dL, and 9.0 ± 3.9% of readings >250 mg/dL. Moderate hyperglycemia was relatively common with 24.6 ± 6.2% of readings between 180 and 250 mg/dL, primarily within 3 h after a meal. Overnight mean CGM was 139.6 ± 27.6 mg/dL, with 77.9 ± 16.4% between 70 and 180 mg/dL, 3.0 ± 4.5% <70 mg/dL, 17.1 ± 14.9% between 180 and 250 mg/dL, and 2.0 ± 4.5%> 250 mg/dL. Postprandial hyperglycemia was more common for unannounced meals compared with announced meals (4-h postmeal CGM 197.8 ± 44.1 vs. 140.6 ± 35.0 mg/dL; P < 0.001). No participants met safety stopping criteria.
Conclusions: MMPPC was safe in a supervised setting despite meal and exercise challenges. Further studies are needed in a less supervised environment.
Keywords: : Fully closed-loop, Artificial pancreas, Type 1 diabetes, Clinical trial
Introduction
Despite rapid advancement in type 1 diabetes (T1D) therapy and technology, current control of glycemia remains suboptimal with <25% of children, adolescents, and young adults meeting American Diabetes Association (ADA) guidelines for glycated hemoglobin (HbA1c) for their age.1,2 This level of glycemic control has occurred despite increased rates of continuous subcutaneous insulin infusion (CSII) pump use of >50% in the United States and rapidly rising rates of continuous glucose monitor (CGM) use.1,3,4 Many have argued that while CSII and CGM have shown benefits in glycemic control in the broader T1D population, optimal benefit will only be seen with automated insulin delivery systems, also known as artificial pancreas (AP) systems, for which insulin delivery is controlled by a dosing algorithm operating in tandem with the CGM and CSII pump.5–7
The Medtronic 670G8 pivotal trial showed overall HbA1c improvement from 7.4% at baseline to 6.9% along with a decreased percent in time ≤70 mg/dL from 6.4% to 3.1% after 3 months of use of the hybrid closed-loop (HCL) system.9,10 Additional work is ongoing for systems being developed around the world by academic and industry groups using single- and/or dual-hormone (e.g., insulin and glucagon) designs.11–22 While improvements seen in glycemic control with these systems are encouraging, users of HCL systems will still experience the burden of carbohydrate counting and will still require premeal insulin bolusing to achieve optimal glycemic control.23 Furthermore, it is unclear what benefit will be seen from HCL in patients with poorer baseline control who often miss or are late in giving meal boluses. For these reasons, development of a fully closed-loop (FCL) AP system, which requires minimal to no user inputs, has been long considered a further step in AP development.6
Model predictive control (MPC) algorithms have emerged as a leading method to achieve FCL AP systems.24,25 A 2017 meta-analysis of published AP studies showed that MPC was the AP algorithm for all FCL systems tested, with the majority using a dual-hormone design.11,12,18,26,27 Additional FCL systems have also been reported elsewhere many of which are dual hormone in design.28–31 Meal detection modules have also been proposed to help achieve FCL AP function.32–38 Other studies have looked at the impact of reduced or missed meal boluses on control in their AP systems.39,40
The multiple model probabilistic predictive control (MMPPC) system is an FCL insulin-only MPC AP that does not require meal announcement and achieves FCL control by two major features not present in most MPC systems: (1) use of uncertainty bands around the prediction of future glucose values that allow insulin-dosing decisions to consider the risk of glucose going below a predefined threshold, and (2) use of population-level assumptions about sleep and meal behavior to enable anticipation and detection of unannounced meals. These features allow meal prediction while attempting to minimize the risk for subsequent hypoglycemia. After demonstrating safety in a hospital and hotel setting using unannounced meals,41 the current studies were conducted to assess safety and performance of the same algorithm with announced and unannounced meals in adults and adolescents in a supervised hotel environment.
Research Design and Methods
Study design
Participants in this hotel study were recruited at three clinical centers (Barbara Davis Center at the University of Colorado Denver, Stanford University, and Icahn School of Medicine at Mount Sinai). The Android phone-based AP system with remote monitoring was tested for 72 h in six adults and four adolescents. Interested patients were eligible to participate in the trial if they had a clinical diagnosis of T1D for at least 12 months requiring insulin therapy, were between 15 and 55 years old, had used an insulin pump for at least 3 months, currently used a Dexcom continuous glucose monitor (G4 or G5), used a total daily dose (TDD) of at least 0.3 U/(kg·d) of insulin, and had an HbA1c between 7.0% and 10.0%. In addition, female participants of childbearing potential needed to use an adequate method of contraception and have a negative pregnancy test. Patients were excluded if they had diabetic ketoacidosis or severe hypoglycemia (including loss of consciousness or seizure) within the previous 6 months, hypoglycemia unawareness, were using a long or intermediate acting insulin or other antidiabetic medications, were participating in another interventional trial, had heart disease or an abnormal electrocardiogram, hypertension, liver or kidney disease, or regularly consumed <100 g of carbohydrates per day.
Two sets of outcomes are presented in this analysis. The first are the 2016 outcome measures for AP clinical trials consensus report42 and the consensus CGM metrics presented at the 2017 ADA conference in San Diego, California, which have been proposed for consistency and comparison between AP trials: sensor glucose (SG) percent >250 mg/dL, >180 mg/dL, in target 70–180 mg/dL, <70 mg/dL, <54 mg/dL, mean SG, standard deviation (SD), and coefficient of variation (CV) of SG, and estimated HbA1c (eHbA1c). The second are the metrics proposed a priori and include percentage of subjects with mean CGM <169 mg/dL (equivalent to an eA1c of 7.5%), low blood glucose index,43,44 number of hypoglycemia events for which self-monitoring of blood glucose (SMBG) was <70 mg/dL, <60 mg/dL, and <50 mg/dL separated by at least 30 min, total grams of carbohydrate taken for hypoglycemia, total daily insulin dose, percent time CGM was used, and percent time in closed loop (CL).
Predefined safety criteria were as follows: (1) no more than three SMBG values <50 mg/dL, separated by at least 30 min, for any subject; (2) no more than two episodes with SMBG values remaining >300 mg/dL for >1 h that are unrelated to an infusion set failure; (3) no ketonemia >1.0 mmol/L while the system is functional unless related to an intercurrent illness or infusion set failure; and (4) no seizure or loss of consciousness. The study was approved by the Food and Drug Administration, the institutional review boards at the three clinical sites, and is listed on (www.clinicaltrials.gov, NCT02769884).
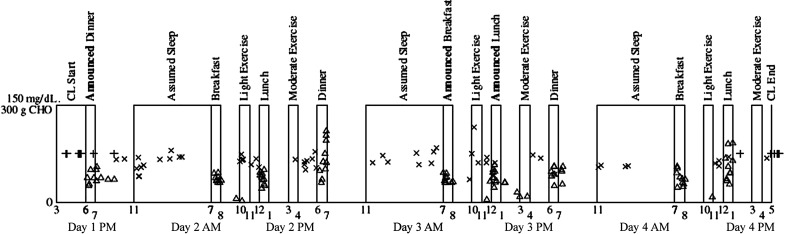
Participants arrived at the study hotel in the afternoon of study day 1 and had a new Dexcom G4 CGM placed to begin the 2-h warm-up with calibration at least 30 min before dinner. CL was initiated by 6 PM. During the 72-h AP phase, participants consumed both announced (premeal bolus administered by the patient based on their insulin to carbohydrate ratio and current glucose level) and unannounced (no meal bolus) meals and exercise (Fig. 1). Meal and exercise times varied by 30–60 min each day in a flexible hotel-based study environment. Each meal time (breakfast, lunch, and dinner) had one announced meal and two unannounced meals. Breakfast consisted of at least 60 g of carbohydrates with low amounts of protein and fat with about 1/3 of carbohydrates consisting of simple carbohydrates (e.g., juice) with the same foods consumed on days 2 (unannounced) and 3 (announced). Foods for lunch and dinner were freely selected by the participants with the only guideline that participants must consume at least 30 g of carbohydrates per meal.
FIG. 1.
Overview of the study protocol with overlaid carbohydrate treatments ( × s represent SMBG values), system activation and deactivation times (+ signs on days 1 and 4), and meals (triangles at times of CHO ingestion). Note that 3 of 10 patients account for 40 of 56 CHO treatments and that some small-dose triangles represent snacks unrelated to risk of hypoglycemia. CHO, carbohydrate; CL, closed loop; SMBG, self-monitoring of blood glucose.
Moderate-intensity exercise of at least 60 min in duration (e.g., running, bicycling, jogging, or playing laser tag) was conducted in the evenings on days 2, 3, and 4. Before moderate-intensity exercise, participants were asked to calibrate the CGM, activate “exercise mode,” and consume 15–30 g of unannounced carbohydrates if their meter glucose value was <140 mg/dL. Light exercise of 30–90-min duration (e.g., walking) was conducted in the mornings on days 2, 3, and 4 without activating exercise mode. Exercise took place daily for all subjects during the specified time windows with no major differences in exercise protocol adherence. On days 2 and 3, following exercise but before dinner, the previous 24-h data were downloaded and reviewed to determine if adjustments should be made to the adaptive lower bound glucose target as detailed hereunder. At 5 PM on day 4, participants were switched back to their home insulin pumps and discharged home.
MMPPC AP system
The MMPPC algorithm was running on the University of Virginia Diabetes Assistant platform using a Roche Accu-Chek Combo insulin pump and Dexcom G4 Platinum CGM with remote web-based monitoring.45,46 The MMPPC algorithm is part of the class of MPC algorithms, which have been previously described and tested in preliminary safety and feasibility trials in hospital and hotel settings.41,47–49 The theory behind the MMPPC system has been described in a previous publication and associated appendix.47 The version of the MMPPC controller being implemented in this trial is essentially the same version implemented in our previous inpatient trial.41 Tuning parameters for the MMPPC system are provided in Supplementary Table S1 (Supplementary Data are available at http://online.liebertpub.com/suppl/doi/10.1089/dia.2017.0424).
In brief, the MMPPC algorithm predicts mean glucose 5 h into the future in a manner similar to other MPC designs and also includes uncertainty bands in the prediction allowing insulin-dosing decisions that directly consider the risk of future hypoglycemia.47–49 The MMPPC algorithm also uses data from the National Health and Nutrition Examination Survey50 and American Time Use Survey51 to provide population-level assumptions about meal and sleep behavior allowing anticipation of future meals to better detect unannounced meals. The model used by the MMPPC algorithm is initialized to the individual patient using the TDD and the basal rate profile. The basal rates are assumed to be countered by the endogenous glucose production. The algorithm initializes the insulin sensitivity using the TDD and the 1800-rule.
The lower bound glucose value, defined as the bound to which the 10% lower bound predictions would be adjusted through insulin delivery, was used by the predictive portion of the algorithm and was adjusted in an adaptive manner in this study. The lower bound value was initially set at 120 mg/dL. If during the previous 24 h there were no hypoglycemic events (defined as meter glucose value <70 mg/dL) unrelated to exercise and the average CGM value was ≥160 mg/dL, then the daytime lower bound glucose target was lowered by 10–20 mg/dL at the investigator's discretion. If there were two hypoglycemic events, then the lower bound target was increased by 10–20 mg/dL. This adaptive assessment was performed in the afternoon of days 2 and 3.
Statistical analyses
Statistical analyses were performed on all data for the 10 participants analyzed for the CL period (system activation to deactivation) as if the CL system were active. The calculations of glycemic metrics are based on the downloaded CGM values. The overnight period was considered 0:00–06:00, and the daytime period 06:00–0:00. Data are reported as mean ± SD or as a percentage. Glycemic variability was represented as intrasubject SD and CV in CGM values. Statistical significance between announced and unannounced meals was calculated using a paired t-test where an α value of 0.05 was used to determine significance.
Results
Subject characteristics
This study consisted of 10 participants with a mean age of 20.5 ± 5.4 years, 3 female and 7 male, with a mean T1D duration of 9.3 ± 4.6 years (Table 1). Baseline HbA1c was 8.1 ± 0.6% and TDD of insulin was 0.77 ± 0.21 U/(kg·d).
Table 1.
Subject Demographics
| N | 10 |
| Age (years) | 20.5 ± 5.4 |
| Baseline HbA1c (%) | 8.1 ± 0.6 |
| Gender (% female) | 30 |
| Duration of T1D (years) | 9.3 ± 4.6 |
| Weight (kg) | 78.6 ± 11.4 |
| Baseline insulin TDD (U/day) | 61.2 ± 22.0 |
| Baseline insulin TDD (U/(kg·d)) | 0.77 ± 0.21 |
HbA1c, glycated hemoglobin; T1D, type 1 diabetes; TDD, total daily dose.
Glycemic control during hotel period
The mean CGM was 157.4 ± 14.4 mg/dL, and median CGM was 157.8 mg/dL (interquartile range [IQR] 148.3–162.5 mg/dL) with 2.9 ± 2.3% time <70 mg/dL (Table 2). The mean CGM value was <169 mg/dL for 80% of the participants (eHbA1c of <7.5%). Overall participants had 1.0 ± 0.83% <60 mg/dL, 0.31 ± 0.33% <50 mg/dL, and 43.5 ± 9.7% in the narrow target range of 70–140 mg/dL. CL was active for 97.9 ± 1.3% of the hotel stay and CGM was active 98.8 ± 1.3% of the time. Overall percent time in the target range of 70–180 mg/dL was 63.6 ± 9.2%. The overall CV was 38 ± 5% and SD was 59.0 ± 6.9 mg/dL. The time <54 mg/dL was 0.5 ± 0.4%. The mean TDD in CL was 0.73 ± 0.18 U/(kg·d) and was not significantly different from the baseline TDD of 0.77 ± 0.21 U/(kg·d).
Table 2.
Closed-Loop Glycemic Control
| Full day | Overnight (0:00–06:00) | Daytime (06:00–0:00) | |
|---|---|---|---|
| Mean SG (mg/dL) | 157.4 ± 14.4 | 139.6 ± 27.6 | 163.4 ± 15.4 |
| eHbA1c (%) | 7.1 ± 0.5 | 6.5 ± 1.0 | 7.3 ± 0.5 |
| SD (mg/dL) | 59.0 ± 6.9 | 35.8 ± 11.9 | 62.1 ± 6.6 |
| CV (%) | 38 ± 5 | 26 ± 6 | 38 ± 5 |
| LBGI risk score | 7.9 ± 1.7 | 4.8 ± 2.9 | 8.9 ± 1.9 |
| % <54 mg/dL (%) | 0.5 ± 0.4 | 0.6 ± 1.4 | 0.4 ± 0.5 |
| % <70 mg/dL (%) | 2.9 ± 2.3 | 3.0 ± 4.5 | 2.8 ± 2.4 |
| % 70–180 mg/dL (%) | 63.6 ± 9.2 | 77.9 ± 16.4 | 58.8 ± 9.2 |
| % >180 mg/dL (%) | 33.6 ± 10.1 | 19.1 ± 17.8 | 38.4 ± 10.1 |
| % >250 mg/dL (%) | 9.0 ± 3.9 | 2.0 ± 4.5 | 11.4 ± 5.0 |
| TDD (U/day) | 58.8 ± 16.6 | ||
| TDD [U/(kg·d)] | 0.73 ± 0.18 | ||
| % CL active (%) | 97.9 ± 1.3 | ||
| % CGM active (%) | 98.8 ± 1.3 | ||
| Subjects with CGM mean < 169 mg/dL (%) | 80 |
CL, closed loop; CV, coefficient of variation; CGM, continuous glucose monitor; eHbA1c, estimated HbA1c; LBGI, low blood glucose index; SG, sensor glucose; SD, standard deviation.
The mean CGM overnight was 139.6 ± 27.6 mg/dL with 3.0 ± 4.5% of time <70 mg/dL, and 0.6 ± 1.4% time <54 mg/dL. Time in the target range (70–180 mg/dL) was 77.9 ± 16.4%.
During the day, the mean CGM was 163.4 ± 15.4 mg/dL with 2.8 ± 2.4% of time <70 mg/dL. Time in the target range (70–180 mg/dL) during the day was 58.8 ± 9.2%. Hypoglycemia <54 mg/dL was 0.4 ± 0.5% for the daytime period.
Hypoglycemia and safety analysis
No participants experienced severe hypoglycemia with change in mental status or seizure requiring glucagon, intravenous dextrose, or outside intervention. No participants met the study stopping criteria of three SMBG values <50 mg/dL. Participants took 1.9 ± 1.9 (range 0–5.2) carbohydrate treatments of at least 15 g of carbohydrate per day for hypoglycemia (CGM <70 mg/dL) or alerts for predicted hypoglycemia averaging 33 ± 37 g of carbohydrates per day (Table 3). Overall participants had an average of 1.1 ± 1.2 times each day when their SMBG value was <70 mg/dL, 0.4 ± 0.5 events per day <60 mg/dL, and 0.1 ± 0.1 event per day <50 mg/dL. No participants had more than two episodes of SMBG >300 mg/dL or ketonemia >1.0 mmol/L unrelated to infusion set failure.
Table 3.
Hypoglycemic Events
| Events SMBG <50 mg/dL per day | 0.1 ± 0.1 |
| Events SMBG <60 mg/dL per day | 0.4 ± 0.5 |
| Events SMBG <70 mg/dL per day | 1.1 ± 1.2 |
| Hypoglycemic treatments per day | 1.9 ± 1.9 |
| Carbohydrates for hypoglycemia treatment per day (g/day) | 33 ± 37 |
SMBG, self-monitoring of blood glucose.
Announced versus unannounced meal analysis
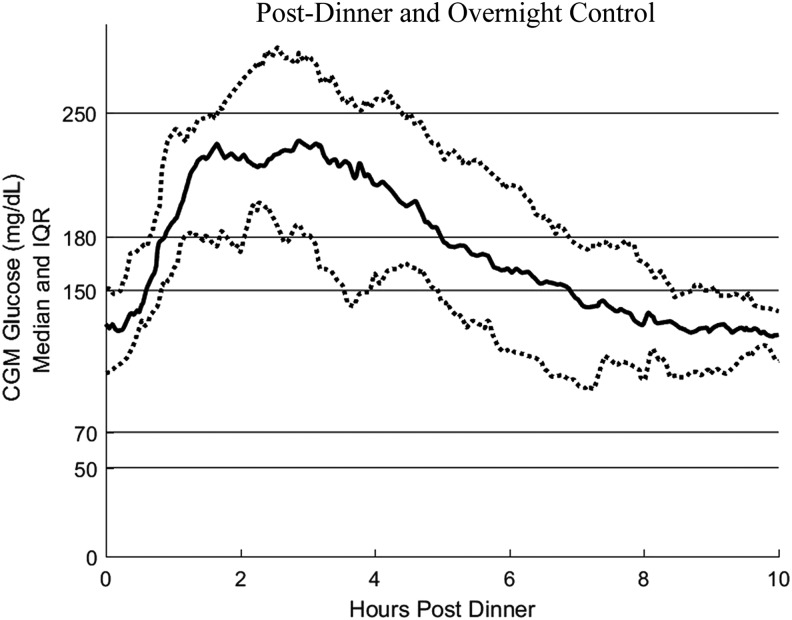
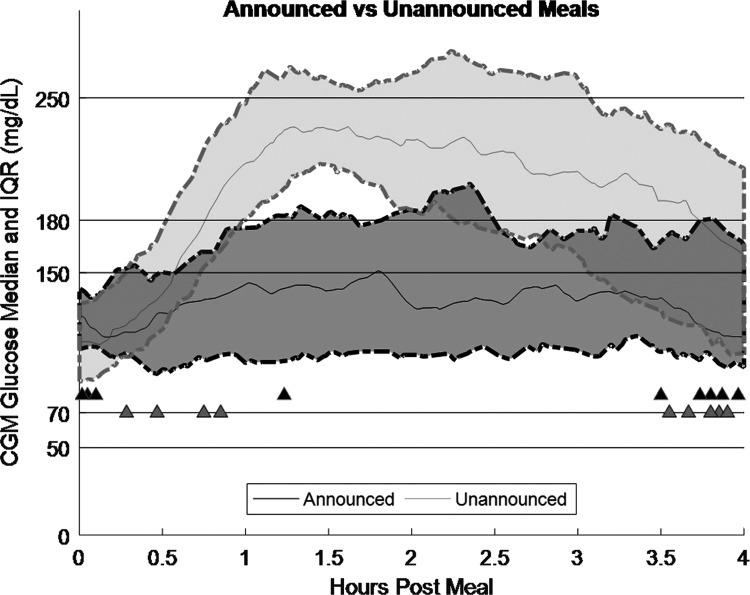
Each participant consumed breakfast, lunch, and dinner meals with one being preannounced and two being unannounced (Fig. 1). Overall meal sizes did not differ significantly between announced and unannounced meals (75 ± 18 vs. 91 ± 40 g of carbohydrate/meal; P = 0.28). Each meal was analyzed for the 4 h after the start of the meal with the postdinner and overnight period providing a longer window into the fasting postmeal period (Fig. 2). Across all meals, the CGM average was significantly lower for announced than for unannounced meals (140.6 ± 35.0 vs. 197.8 ± 44.1 mg/dL; P < 0.001) (Table 4; Fig. 3). Announced meals also produced significantly better glycemia based on CGM SD (30.1 ± 14.7 vs. 50.8 ± 14.2 mg/dL; P < 0.001) and CV (21.3 ± 9.3 vs. 26.8 ± 9.7; P < 0.001), CGM percent >250 mg/dL (2.2 ± 6.1 vs. 22.8 ± 24.0%; P < 0.001), CGM percent >180 mg/dL (20.6 ± 25.7 vs. 60.9 ± 23.3%; P < 0.001), and maximum CGM value (194.6 ± 46.6 vs. 268.2 ± 44.4 mg/dL; P < 0.001).
FIG. 2.
Postdinner and overnight glycemic control. Solid center line represents the average, and dotted lines represent the 25th and 75th percentiles. CGM, continuous glucose monitor; IQR, interquartile range.
Table 4.
Announced Versus Unannounced Meal Analysis
| Announced | Unannounced | Difference | P | |
|---|---|---|---|---|
| All meals | ||||
| CGM average (mg/dL) | 140.6 ± 35.0 | 197.8 ± 44.1 | 57.2 ± 16.0 | <0.001 |
| CGM SD (mg/dL) | 30.1 ± 14.7 | 50.8 ± 14.2 | 20.7 ± 5.6 | <0.001 |
| CGM CV (%) | 21.3 ± 9.3 | 26.8 ± 9.7 | 5.5 ± 3.7 | <0.001 |
| % >250 mg/dL (%) | 2.2 ± 6.1 | 22.8 ± 24.0 | 20.6 ± 7.7 | <0.001 |
| % >180 mg/dL (%) | 20.6 ± 25.7 | 60.9 ± 23.3 | 40.3 ± 9.3 | <0.001 |
| CGM maximum (mg/dL) | 194.6 ± 46.6 | 268.2 ± 44.4 | 73.7 ± 17.5 | <0.001 |
| Meal size (carbohydrate g) | 75 ± 18 | 91 ± 40 | 16 ± 13.3 | 0.28 |
| Manual meal bolus (U) | 9.6 ± 3.7 | 0 ± 0 | 9.6 ± 0.8 | <0.001 |
| Total insulin −1 to +4 h from meal (U) | 15.0 ± 4.8 | 14.5 ± 5.8 | 0.5 ± 2.1 | 0.84 |
| Breakfast | ||||
| CGM average (mg/dL) | 133.9 ± 27.5 | 174.4 ± 38.1 | 40.5 ± 13.5 | 0.0088 |
| CGM SD (mg/dL) | 28.3 ± 12.5 | 52.0 ± 11.6 | 23.7 ± 4.6 | <0.001 |
| CGM CV (%) | 20.5 ± 6.4 | 31.5 ± 11.1 | 11.0 ± 3.8 | <0.001 |
| % >250 mg/dL (%) | 2.3 ± 7.2 | 11.7 ± 15.2 | 9.5 ± 5.1 | 0.1794 |
| % >180 mg/dL (%) | 10.6 ± 16.2 | 49.6 ± 24.5 | 39.0 ± 8.5 | <0.001 |
| CGM maximum (mg/dL) | 185.9 ± 39.4 | 256.9 ± 37.3 | 71.0 ± 14.7 | <0.001 |
| Lunch | ||||
| CGM average (mg/dL) | 140.3 ± 37.3 | 199.4 ± 50.7 | 59.1 ± 18.1 | 0.003 |
| CGM SD (mg/dL) | 33.2 ± 14.1 | 49.1 ± 15.3 | 15.9 ± 5.8 | 0.021 |
| CGM CV (%) | 24.4 ± 11.8 | 25.5 ± 8.9 | 1.1 ± 3.9 | <0.001 |
| % >250 mg/dL (%) | 1.6 ± 3.8 | 22.2 ± 26.3 | 20.6 ± 8.4 | 0.046 |
| % >180 mg/dL (%) | 22.6 ± 28.3 | 61.6 ± 23.3 | 39.0 ± 9.7 | 0.009 |
| CGM maximum (mg/dL) | 201.5 ± 48.0 | 262.6 ± 52.5 | 61.1 ± 19.8 | 0.046 |
| Dinner | ||||
| CGM average (mg/dL) | 147.5 ± 41.1 | 219.5 ± 30.6 | 72.0 ± 13.3 | <0.001 |
| CGM SD (mg/dL) | 28.7 ± 17.9 | 51.3 ± 16.1 | 22.5 ± 6.5 | <0.001 |
| CGM CV (%) | 19.0 ± 9.1 | 23.3 ± 7.1 | 4.3 ± 3.0 | <0.001 |
| % >250 mg/dL (%) | 2.7 ± 7.3 | 34.5 ± 24.6 | 31.7 ± 7.9 | <0.001 |
| % >180 mg/dL (%) | 28.7 ± 29.5 | 71.5 ± 17.0 | 42.8 ± 8.5 | <0.001 |
| CGM maximum (mg/dL) | 196.4 ± 54.9 | 285.2 ± 38.7 | 88.9 ± 17.3 | <0.001 |
Analyzed as the time from the start of the meal until 4 h after unless otherwise noted.
FIG. 3.
Comparison of postprandial CGM response for 30 announced and 60 unannounced meals. Unannounced meals <35 g CHO were omitted from this analysis. Dark gray represents announced meals, and light gray represents unannounced meals. Solid line is median, and dashed lines are the 25th and 75th percentiles. Triangles represent hypoglycemia treatments, with the dark fill color occurring with announced meals and the light gray fill color corresponding to unannounced meals.
Insulin delivered per meal between announced and unannounced meals was analyzed as 1 h before the meal to 4 h after the meal as IOB (insulin on board) before the meal may have impacted MMPPC unannounced meal performance. Both announced and unannounced meals required relatively similar average per meal insulin doses (15.0 ± 4.8 vs. 14.5 ± 5.8 U/meal; P = 0.84). During the 4 h after a meal, announced meals had an average of 1.8% CGM time <70 mg/dL and unannounced meals had 2.3% CGM time <70 mg/dL (nonsignificant difference). Additional analysis was conducted to look at the frequency at which participants crossed from >70 mg/dL to <70 mg/dL after a meal. For announced meals, there were 0.27 downward crossings of 70 mg/dL per meal, while for unannounced meals there were 0.17 (nonsignificant difference).
When broken down by individual meals, similar patterns are seen for CGM average, SD, CV, percent >180 mg/dL, and CGM maximum value across all meals. Comparison of breakfast CGM percent >250 mg/dL was not significantly different between announced and unannounced meals.
Discussion
This outpatient trial of the FCL MMPPC AP system demonstrates the safety of this emerging system based on the predefined safety criteria. The MMPPC system successfully maintained glycemic control with an average CGM of <169 mg/dL for 80% of the participants, an eHbA1c of <7.5%, which was one of the predefined outcomes of the study. The overall CGM average was 157.4 ± 14.4 mg/dL and only 1.0 ± 0.8% of values <60 mg/dL. In a supervised setting, participants were able to keep the AP active for 97.9 ± 1.3% of the time. The MMPPC system was safe with no participants experiencing more than one SMBG value <50 mg/dL and no severe hypoglycemic or ketotic events, which were the predefined safety criteria for this trial. Participants did, however, require 1.9 ± 1.9 carbohydrate interventions per day to prevent or correct hypoglycemia (33 ± 37 g of carbohydrate per day), which is higher than desired.
A previous inpatient trial of the MMPPC algorithm with unannounced meals was assessed with four subjects, and the algorithm was then revised to improve performance, and assessed with six additional subjects. CGM averages were 167 and 140 mg/dL with percent time in target range of 70–180 mg/dL of 62% and 78%, respectively.48 For the second group, the 3-h postmeal average CGM was 156 mg/dL. The number of hypoglycemia treatments was 0.52 per patient-day. Use of the MMPPC system with unannounced meals was also tested in a 10-patient inpatient setting and 15-patient hotel setting and these studies showed average CGM values of 152 and 158 mg/dL with percent time in range of 70–180 mg/dL of 70% and 68%, respectively.41 For the inpatient setting, there were 1.68 hypoglycemia treatments per patient-day and for the hotel cohort there were 0.47 treatments per patient-day. In the hotel cohort of the previous trial, several participants had missed meals, which did result in hypoglycemia.
The current study tested the safety of the MMPPC controller when meals were both announced and unannounced with slightly varying times (30–60 min), although with no missed meals. The results are similar with the previous studies with an overall CGM average in the 150–160 mg/dL range, percent <70 mg/dL in the 2% to 3% range, percent <54 mg/dL of <0.5%, and hypoglycemic interventions per patient-day of 1.9. In the current trial involving announced and unannounced meals, the CGM average was similar at 157.4 ± 14.4 mg/dL although the number of hypoglycemia interventions was higher at 1.9 ± 1.9 per day (range 0–5.2 treatments per day). The high variability in treatment number between subjects could suggest differences in model-fit, possibly related to reliance of this system on the participant's home settings.
Review of the literature shows several other FCL systems under different stages of development.11,12,28–31,38,41 The Doyle/Dassau group at Harvard/Sansum has also tested an FCL MPC single-hormone design with results reported by Harvey et al.30 They reported results for 12 subjects during a 24-h admission during which subjects consumed two unannounced meals of 50 and 40 g of carbohydrates along with two optional snacks of 16 g of carbohydrates. Average SG was 153 ± 16.4 mg/dL with 80% time 70–180 mg/dL overall and with 69% and 61% in target after each unannounced meal. The results for this single-hormone FCL system showed similar mean SG to MMPPC with better percent time in target range.
The Damiano/Russell bionic pancreas system was tested in a randomized crossover trial of 43 adults with optional meal announcement.12 Participants bolused 5.6 times per day during the control period and announced meals 2.6 times per day while on the AP system. In post hoc analysis, they found “no correlation between number of meal announcements during the bionic pancreas period and the treatment effect for either of the coprimary outcomes [mean CGM and % time <60 mg/dL].” They report a mean CGM value of 140 ± 11 mg/dL and percent time <60 mg/dL of 0.6 ± 0.6% for the bionic pancreas group during the trial. These results appear similar to that achieved with the MMPPC system for announced meals (mean CGM 140.6 ± 35 mg/dL) and significantly better than that achieved for unannounced meals (mean CGM 197.8 ± 44.1 mg/dL).
The PCDIAB consortium in the Netherlands is developing a bihormonal FCL system. An initial feasibility study on this system reported results for 11 patients in a monitored home setting comparing 2 days of CL control (without meal or exercise announcement) with 2 days of open-loop control.29 The authors report CL median (IQR) of 133 (40) mg/dL for day 1 and 139 (41) mg/dL for day 2. Time in the target range of 70–180 mg/dL was 79.2 (16.9)% for day 1 and 76.5 (23.9)% for day 2 with time <70 mg/dL as 2.1 (7.61)% for day 1 and 2.8 (9.8)% for day 2. Despite administration of glucagon, oral carbohydrates were required on average 1.4 times per day per patient and no differences were seen in oral carbohydrate administration between the two study arms. A follow-up study for this group looked at 10 patients using the bihormonal system at home for 3 days.28 This study showed median (IQR) glucose control of 131 (126–137) mg/dL with 84.7 (82.2–87.8)% time in the target range of 70–180 mg/dL and 1.3 (0.2–3.2)% <70 mg/dL. The overall results for this bihormonal system show average glycemic control and percent time in target range, somewhat better than for our system with similar rates of hypoglycemia. It should be noted that this improvement in control of about 18–26 mg/dL comes with added cost, complexity, and inconvenience of using a second infusion set site with the addition of glucagon.
Comparison of announced versus unannounced meals in this study shows that overall, announced meals performed better than unannounced meals by 40.5–72.0 mg/dL for the average CGM value in the 4 h after the meal. This finding supports the long-held doctrine that bolusing before eating is superior to bolusing after eating as the MMPPC AP system delivers insulin in response to the meal glycemic rise. The previous iteration of the MMPPC system did not allow for premeal bolusing and this feature was added as an optional module in response to feedback from the patients participating in the earlier trials. While outpatient glycemic control for unannounced meals may be somewhat suboptimal given the current limits on insulin pharmacodynamics, CGM accuracy, and insulin delivery speed, these findings need to be taken in the context of current diabetes burden and average glycemic control. The frequency and impact of missed meal boluses have also been studied, with a series of studies by Olinder et al. showing that almost 40% of adolescents on CSII had missed >15% of their meal doses and that elevated HbA1c values could be explained by the frequency of missed meal boluses.52 In addition, they found that adolescents frequently “lose focus” around meal times and simply forget to bolus resulting in significantly worsened glycemic outcomes.53 Implementation of an FCL system in adolescents and similar populations holds the potential to reduce average CGM value, decrease hypoglycemic exposure, and decrease the burden of carbohydrate counting and meal announcement concurrently.
In the afternoons of days 2 and 3, the MMPPC algorithm was adapted by adjusting the lower bound for the hypoglycemia threshold. The MMPPC algorithm tries to inject insulin so that the future glucose level will fall below a threshold (threshold) a set percentage of the time (lower bound). For the initial six participants, we adapted the threshold. For the last four participants, we adapted the lower bound percentage. Participant adjustments are outlined in Supplementary Table S2. The threshold led to more hyperglycemia than was desirable. Adaption of the algorithm was an exploratory aim to provide information for further algorithm refinement in this project. There was thus insignificant power to more robustly explore the impact of algorithm adaption on glycemic control.
This study has several notable limitations. The design was based on safety and feasibility assessment and as such there was no control group against which to assess efficacy. The study period of 72 h was brief in comparison to recent HCL trials, although similar to other FCL trials. Participants were in a hotel setting and were closely monitored by research staff. The comparison of announced versus unannounced meals is limited by the fact that bolus status was not randomized either between or within patients. Participants also required more carbohydrate treatments per day to prevent hypoglycemia than would be desired for a commercial system. A strength of this study is that announced and unannounced meals were both performed in the same environment during the same study with patients serving as their own relative controls. The study was conducted in an outpatient environment providing more generalizability than would be provided by a hospital-based trial. This trial included both adults and adolescents enabling safety justification in multiple age cohorts. Use of an FCL AP is novel and highly desired by patients and providers alike.
Overall, this MMPPC FCL AP system was shown to be safe in a monitored hotel setting in adults and adolescents. Both announced and unannounced meals were safely performed with the same base algorithm with the announced meals showing superior glycemic control to unannounced meals. Further work on this project includes incorporation of additional accelerometry detection elements as well as continued refinement of the CL algorithm. Future studies should also test emerging ultrarapid insulin, which, along with improved sensor accuracy, may help to enable more generalizable outpatient studies.
Supplementary Material
Acknowledgments
The authors thank all the patients who participated in this clinical trial, as well as their families and support teams. This work was funded by a grant from the National Institutes of Health (Grant No. 1R01DK102188-01). The authors acknowledge the work done by the diabetes technology teams at Stanford University, the Barbara Davis Center, and Mt. Sinai, who contributed many overnight and weekend hours to this project.
Author Disclosure Statement
G.P.F. conducts research supported by Medtronic, Tandem, Insulet, and Dexcom and has been a consultant for Abbott and a paid speaker and advisory board member for Dexcom. T.T.L. has received research funding from Medtronic and Tandem, and currently is employed by Insulet. S.D.P. works for Type Zero technologies. C.L. receives research support from Roche, Dexcom, and Senseonics, and is an advisory board member for Novo Nordisk. R.P.W. reports research support from Bigfoot Biomedical, MannKind Corporation, Novo Nordisk, Xeris Pharmaceuticals, and Dexcom and has been a consultant for Eli Lilly and Co and Novo Nordisk. D.M.M. is on the advisory board for Insulet, is a consultant for Abbott Diabetes Care, and receives research funding from Medtronic, Roche, and Dexcom. B.W.B. has served as a consultant for Becton, Dickinson and Company. B.A.B. has received research support from Medtronic, Dexcom, Insulet, Roche, Tandem, and Bigfoot Biomedical and is on advisory boards for Sanofi and Novo Nordisk, and was a consultant for Dexcom. F.M.C., D.L., D.P.H., N.B., G.K., L.M., P.C., and C.L. report no conflicts of interest.
References
- 1.Miller KM, Foster NC, Beck RW, et al. : Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D exchange clinic registry. Diabetes Care 2015;38:971–978 [DOI] [PubMed] [Google Scholar]
- 2.Ly TT, Buckingham BA, DeSalvo DJ, et al. : Day-and-night closed-loop control using the unified safety system in adolescents with type 1 diabetes at camp. Diabetes Care 2016;39:e106–e107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sherr JL, Hermann JM, Campbell F, et al. : Use of insulin pump therapy in children and adolescents with type 1 diabetes and its impact on metabolic control: comparison of results from three large, transatlantic paediatric registries. Diabetologia 2016;59:87–91 [DOI] [PubMed] [Google Scholar]
- 4.Forlenza GP, Pyle LL, Maahs DM, Dunn TC: Ambulatory glucose profile analysis of the juvenile diabetes research foundation continuous glucose monitoring dataset-Applications to the pediatric diabetes population. Pediatr Diabetes 2016;18:622–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kowalski AJ: Can we really close the loop and how soon? Accelerating the availability of an artificial pancreas: a roadmap to better diabetes outcomes. Diabetes Technol Ther 2009;11(Suppl 1):S113–S119 [DOI] [PubMed] [Google Scholar]
- 6.Kowalski A: Pathway to artificial pancreas systems revisited: moving downstream. Diabetes Care 2015;38:1036–1043 [DOI] [PubMed] [Google Scholar]
- 7.Forlenza GP, Buckingham B, Maahs DM: Progress in diabetes technology: developments in insulin pumps, continuous glucose monitors, and progress towards the artificial pancreas. J Pediatr 2016;169:13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Food and Drug Administration. FDA approves first automated insulin delivery device for type 1 diabetes. September 28, 2016. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522974.htm (accessed September28, 2016)
- 9.Bergenstal RM, Garg S, Weinzimer SA, et al. : Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA 2016;316:1407–1408 [DOI] [PubMed] [Google Scholar]
- 10.Garg SK, Weinzimer SA, Tamborlane WV, et al. : Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2017;19:155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weisman A, Bai JW, Cardinez M, et al. : Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol 2017;5:501–512 [DOI] [PubMed] [Google Scholar]
- 12.El-Khatib FH, Balliro C, Hillard MA, et al. : Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomised crossover trial. Lancet 2017;389:369–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forlenza GP, Deshpande S, Ly TT, et al. : Application of zone model predictive control artificial pancreas during extended use of infusion set and sensor: a randomized crossover-controlled home-use trial. Diabetes Care 2017;40:1096–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forlenza GP, Raghinaru D, Cameron F, et al. : Predictive hyperglycemia and hypoglycemia minimization: In-home double-blind randomized controlled evaluation in children and young adolescents. Pediatr Diabetes 2017;1–9. https://doi.org/10.1111/pedi.12603 [DOI] [PMC free article] [PubMed]
- 15.Huyett LM, Ly TT, Forlenza GP, et al. : Outpatient closed-loop control with unannounced moderate exercise in adolescents using zone model predictive control. Diabetes Technol Ther 2017;19:331–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nimri R, Bratina N, Kordonouri O, et al. : MD-logic overnight type 1 diabetes control in home settings: a multicentre, multinational, single blind randomized trial. Diabetes Obes Metab 2017;19:553–561 [DOI] [PubMed] [Google Scholar]
- 17.Taleb N, Emami A, Suppere C, et al. : Efficacy of single-hormone and dual-hormone artificial pancreas during continuous and interval exercise in adult patients with type 1 diabetes: randomised controlled crossover trial. Diabetologia 2016;59:2561–2571 [DOI] [PubMed] [Google Scholar]
- 18.Russell SJ, Hillard MA, Balliro C, et al. : Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. Lancet Diabetes Endocrinol 2016;4:233–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breton MD, Chernavvsky DR, Forlenza GP, et al. : Closed-loop control during intense prolonged outdoor exercise in adolescents with type 1 diabetes: the artificial pancreas ski study. Diabetes Care 2017;40:1644–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kovatchev B, Cheng P, Anderson SM, et al. : Feasibility of long-term closed-loop control: a multicenter 6-month trial of 24/7 automated insulin delivery. Diabetes Technol Ther 2017;19:18–24 [DOI] [PubMed] [Google Scholar]
- 21.Anderson SM, Raghinaru D, Pinsker JE, et al. : Multinational home use of closed-loop control is safe and effective. Diabetes Care 2016;39:1143–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobs PG, El Youssef J, Reddy R, et al. : Randomized trial of a dual-hormone artificial pancreas with dosing adjustment during exercise compared with no adjustment and sensor-augmented pump therapy. Diabetes Obes Metab 2016;18:1110–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Messer LH, Forlenza GP, Wadwa RP, et al. : The dawn of automated insulin delivery: a new clinical framework to conceptualize insulin administration. Pediatr Diabetes 2018;19:14–17 [DOI] [PubMed] [Google Scholar]
- 24.Bequette BW: Algorithms for a closed-loop artificial pancreas: the case for model predictive control. J Diabetes Sci Technol 2013;7:1632–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doyle FJ, III, Huyett LM, Lee JB, et al. : Closed-loop artificial pancreas systems: engineering the algorithms. Diabetes Care 2014;37:1191–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russell SJ, El-Khatib FH, Sinha M, et al. : Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med 2014;371:313–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haidar A, Legault L, Matteau-Pelletier L, et al. : Outpatient overnight glucose control with dual-hormone artificial pancreas, single-hormone artificial pancreas, or conventional insulin pump therapy in children and adolescents with type 1 diabetes: an open-label, randomised controlled trial. Lancet Diabetes Endocrinol 2015;3:595–604 [DOI] [PubMed] [Google Scholar]
- 28.Blauw H, van Bon AC, Koops R, DeVries JH: Performance and safety of an integrated bihormonal artificial pancreas for fully automated glucose control at home. Diabetes Obes Metab 2016;18:671–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Bon AC, Luijf YM, Koebrugge R, et al. : feasibility of a portable bihormonal closed-loop system to control glucose excursions at home under free-living conditions for 48 hours. Diabetes Technol Ther 2014;16:131–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harvey RA, Dassau E, Bevier WC, et al. : Clinical evaluation of an automated artificial pancreas using zone-model predictive control and health monitoring system. Diabetes Technol Ther 2014;16:348–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gingras V, Rabasa-Lhoret R, Messier V, et al. : Efficacy of dual-hormone artificial pancreas to alleviate the carbohydrate-counting burden of type 1 diabetes: a randomized crossover trial. Diabetes Metab 2016;42:47–54 [DOI] [PubMed] [Google Scholar]
- 32.Cinar A: Multivariable adaptive artificial pancreas system in type 1 diabetes. Curr Diab Rep 2017;17:88. [DOI] [PubMed] [Google Scholar]
- 33.Turksoy K, Samadi S, Feng J, et al. : Meal detection in patients with type 1 diabetes: a new module for the multivariable adaptive artificial pancreas control system. IEEE J Biomed Health Inform 2016;20:47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samadi S, Turksoy K, Hajizadeh I, et al. : Meal detection and carbohydrate estimation using continuous glucose sensor data. IEEE J Biomed Health Inform 2017;21:619–627 [DOI] [PubMed] [Google Scholar]
- 35.Harvey RA, Dassau E, Zisser H, et al. : Design of the glucose rate increase detector: a meal detection module for the health monitoring system. J Diabetes Sci Technol 2014;8:307–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weimer J, Chen S, Peleckis A, et al. : Physiology-invariant meal detection for type 1 diabetes. Diabetes Technol Ther 2016;18:616–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cameron F, Niemeyer G, Buckingham BA: Probabilistic evolving meal detection and estimation of meal total glucose appearance. J Diabetes Sci Technol 2009;3:1022–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turksoy K, Bayrak ES, Quinn L, et al. : Multivariable adaptive closed-loop control of an artificial pancreas without meal and activity announcement. Diabetes Technol Ther 2013;15:386–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elleri D, Maltoni G, Allen JM, et al. : Safety of closed-loop therapy during reduction or omission of meal boluses in adolescents with type 1 diabetes: a randomized clinical trial. Diabetes Obes Metab 2014;16:1174–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chernavvsky DR, DeBoer MD, Keith-Hynes P, et al. : Use of an artificial pancreas among adolescents for a missed snack bolus and an underestimated meal bolus. Pediatr Diabetes 2016;17:28–35 [DOI] [PubMed] [Google Scholar]
- 41.Cameron FM, Ly TT, Buckingham BA, et al. : Closed-loop control without meal announcement in type 1 diabetes. Diabetes Technol Ther 2017;19:527–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maahs DM, Buckingham BA, Castle JR, et al. : Outcome measures for artificial pancreas clinical trials: a consensus report. Diabetes Care 2016;39:1175–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kovatchev BP, Cox DJ, Gonder-Frederick LA, Clarke W: Symmetrization of the blood glucose measurement scale and its applications. Diabetes Care 1997;20:1655–1658 [DOI] [PubMed] [Google Scholar]
- 44.Kovatchev BP, Cox DJ, Gonder-Frederick LA, et al. : Assessment of risk for severe hypoglycemia among adults with IDDM: validation of the low blood glucose index. Diabetes Care 1998;21:1870–1875 [DOI] [PubMed] [Google Scholar]
- 45.Keith-Hynes P, Guerlain S, Mize B, et al. : DiAs user interface: a patient-centric interface for mobile artificial pancreas systems. J Diabetes Sci Technol 2013;7:1416–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Place J, Robert A, Ben Brahim N, et al. : DiAs web monitoring: a real-time remote monitoring system designed for artificial pancreas outpatient trials. J Diabetes Sci Technol 2013;7:1427–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cameron F, Niemeyer G, Bequette BW: Extended multiple model prediction with application to blood glucose regulation. J Process Control 2012;22:1422–1432 [Google Scholar]
- 48.Cameron F, Niemeyer G, Wilson DM, et al. : Inpatient trial of an artificial pancreas based on multiple model probabilistic predictive control with repeated large unannounced meals. Diabetes Technol Ther 2014;16:728–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cameron F, Bequette BW, Wilson DM, et al. : A closed-loop artificial pancreas based on risk management. J Diabetes Sci Technol 2011;5:368–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.CDC, NCHS: National Health and Nutrition Examination Survey Data. Hyattsville, MD: Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS), 2004 [Google Scholar]
- 51.Kopecky P, Mraz M, Blaha J, et al. : The use of continuous glucose monitoring combined with computer-based eMPC algorithm for tight glucose control in cardiosurgical ICU. Biomed Res Int 2013;2013:186439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olinder AL, Kernell A, Smide B: Missed bolus doses: devastating for metabolic control in CSII-treated adolescents with type 1 diabetes. Pediatr Diabetes 2009;10:142–148 [DOI] [PubMed] [Google Scholar]
- 53.Olinder AL, Nyhlin KT, Smide B: Reasons for missed meal-time insulin boluses from the perspective of adolescents using insulin pumps: “lost focus.” Pediatr Diabetes 2011;12:402–409 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.