Abstract
Introduction
PTEN is a tumor suppressor gene that inhibits cell proliferation by inhibiting the phosphoinositide 3- kinase (PI3K) signaling pathway. The significance of PTEN mutations resulting in variable PTEN expression and their impact on prognosis of breast cancer is not well established. The objective of our study was to correlate the immunohistochemical expression of PTEN in the four major subtypes of breast carcinoma (Luminal A, Luminal B, HER2 positive, and Triple Negative) in a population of 202 African-American (AA) females with other clinicopathological factors.
Materials and Methods
Tissue microarrays (TMAs) were constructed from FFPE tumor blocks from primary ductal breast carcinomas in 202 African-American females. Five micrometer sections were stained with a mouse monoclonal antibody against PTEN. The sections were evaluated for the intensity of cytoplasmic and nuclear reactivity. Bivariate analysis was done via χ2 analysis and survivability data was calculated via the generation of Kaplan-Meier curves (SPSS v19).
Results
Loss of PTEN expression was associated with ER negative (p=0.021), PR negative (p=0.024) and triple negative (p=0.0024) breast ductal cancers. It was marginally associated with distant metastasis (p=0.074). There was no association between PTEN loss and recurrence-free survival or overall survival.
Conclusion
In our study, a statistically significant association between PTEN loss and the triple negative breast cancers (TNBC) was found in AA women. PTEN inhibits PI3K resulting in decreased activation of downstream effector, mammalian target of rapamycin (mTOR). Loss of PTEN results in cell proliferation through activation of mTOR. Targeted therapy with mTOR inhibitors might be useful in the treatment of TNBC.
Keywords: PTEN, Phosphatidyl inositol 3-kinase (PI3k)/AKT signal transduction pathway, mammalian target of rapamycin (mTOR), triple- negative breast cancer, African American
Introduction
Breast cancer is the leading cause of cancer morbidity and the second most common cause of cancer mortality in women worldwide. Molecular classification of breast cancer by gene expression profiling identified five major subgroups (Luminal A, Luminal B, Her-2 overexpressing, normal breast like and basal phenotype) with clinical implications [1,2,3]. Luminal A and B breast cancers are positive for estrogen and progesterone receptors (ER, PR), and are treatable with currently available targeted therapy [1,2]. Luminal B breast cancers are mitotically active with Ki-67≥14% and may express Her-2. The triple negative breast cancers (TNBC) lack ER, PR and Her2 receptor expression. TNBC are aggressive breast ductal cancers and are associated increased incidence of distant metastasis and decreased overall survival [1,2]. They are also often resistant to conventional chemotherapy. The basaI phenotype lacks ER, PR and Her-2 expression and expresses basal cell markers, including CK5 and high molecular weight cytokeratin. Not all TNBC are basal type and not all basal type breast cancers are triple negative. However, there is significant overlap. No targeted therapy is available for TNBC. The higher incidence of TNBC in African American (AA) women contributes to a higher mortality rate in this group.
Breast cancers in AA women have a higher grade and stage at diagnosis, occur in premenopausal women and are associated with a higher mortality [4,5]. High proliferative activity of TNBC supports the upregulation of growth factor signaling pathway driver genes and downregulation of inhibitors; in the TCGA, there were found to represent potential pathogenetic mechanisms [6]. Recent studies have shown that cell cycle dysregulation plays an important role in TNBC. It may involve loss of critical check points in cell cycle at G1-S phase resulting in increased proliferation. The G1-S phase check point is controlled by p53/Rb gene encoded proteins. They inhibit the transition from G1-S phase of cell cycle. Loss or inhibition of p53/Rb gene might be important in TNBC [6,7].
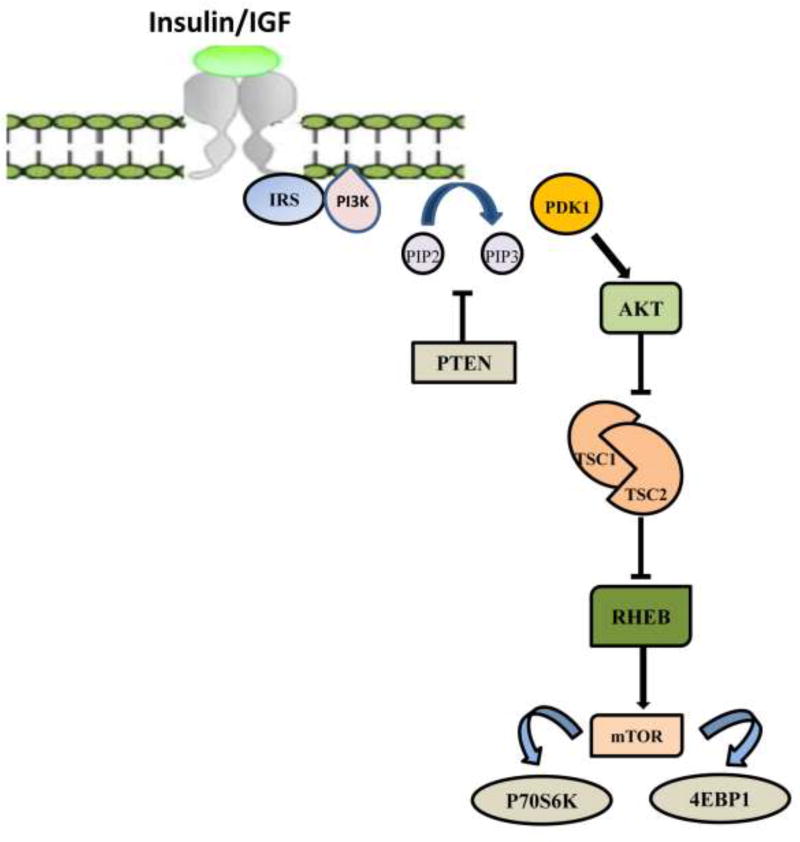
PTEN (phosphatase and tensin homolog) is a tumor suppressor gene, located on chromosome 10, and is a major inhibitor of phosphatidyl inositol 3-kinase (PI3K)/AKT signal transduction pathway. PI3K/AKT signal transduction pathway, on activation by extracellular growth factor ligands (Insulin), promotes cell proliferation [8]. The transmembrane receptor has tyrosine kinase activity. Binding of growth factors to the extracellular domain of the receptor produces activation via autophosphorylation of cytoplasmic tyrosine residues. Activated PI3K phosphorylates cell membrane lipids, leading to the recruitment and activation of AKT, a serine/threonine kinase. Activated AKT in turn phosphorylates several downstream effectors; these are involved in cell cycle proliferation, migration and angiogenesis, which ultimately promote survival and growth of tumor cells. PTEN, a major inhibitor of PI3K/AKT signal transduction pathway, provides physiological counter regulation [Figure 3]. It encodes a phosphatidylinositol-3,4,5-triphosphate 3-phosphatase protein that dephosphorylates phosphatidylinositol-3,4,5-triphosphate (PIP3)to phosphatidylinositol-4,5-biphosphate (PIP2) resulting in the inactivation of AKT and downstream effectors [9]. Loss of PTEN might result in uninhibited activation of PI3K/AKT signal transduction pathway promoting tumorigenesis. An important downstream effector of the PI3K pathway is mammalian target of rapamycin (mTOR) [Figure 3] that can be inhibited by specific drugs such as rapamycin [10,11]. The significance of PTEN loss and activation of proliferating pathways in breast carcinogenesis is poorly understood.
Figure 3.
PI3K/AKT/mTOR signaling pathway
We hypothesize that the lack of PTEN expression in ER negative and triple negative breast cancer could be driving TNBC’s aggressive nature. To begin exploring this hypothesis, we determined the association between PTEN expression and hormone receptor status and triple negative breast cancer status. Specifically, to explore the role of PTEN protein expression, we compared the immunohistochemical expression of PTEN in the four major subtypes of ductal breast cancers (BC) (Luminal A, Luminal B, HER2, and Triple Negative) in a population of 202 African-American (AA) women with other clinicopathological factors including grade, stage, disease-free, and overall survival.
Materials and Methods
Tissue Samples
This study was reviewed and formally exempted by the Howard University Institutional Review Board (IRB-10-MED-24). We analyzed invasive breast ductal carcinomas (IDCs) from 202 AA women diagnosed and treated at the Howard University Hospital between 2000 and 2010. Demographic and clinical information was obtained through the Howard University Cancer Center Tumor Registry.
Tissue Microarrays
A series of tissue microarrays (TMAs) was constructed containing the consecutive primary IDCs (Pantomics, Inc., Richmond, CA). The TMAs consisted of 10 × 16 arrays of 1.0-mm tissue cores from well preserved, morphologically representative tumor cells in archived, formalin-fixed, paraffin-embedded (FFPE) surgical blocks. A precision tissue arrayer (Beecher Instruments, Silver Spring, MD) with two separate core needles for punching the donor and recipient blocks was used. The device also had a micrometer-precise coordinate system for tissue assembly on a multi-tissue block. Two separate tissue cores of IDC represented each surgical case in the TMA. Each tissue core was assigned a unique TMA location number, which was subsequently linked to an Institutional Review Board-approved database containing demographic and clinical data. Using a microtome, 5-µm sections were cut from the TMA blocks and mounted onto Superfrost Plus microscope slides.
Immunohistochemistry
Immunohistochemistry (IHC) was performed on TMA sections of FFPE tumor tissue. The polymer-HRP system was utilized for immunostaining. Following deparaffinization and rehydration of the tissue sections, heat-induced epitope retrieval at pH 9.0 was performed. Five micrometer sections were stained with mouse monoclonal antibodies against against PTEN (6H2.1, Dako). Primary antibody detection was carried out using a polymer-based detection system with staining development achieved by incubation with 3,3´-diaminobenzadine (DAB) and DAB Enhancer (Envision Plus, DAKO, Carpinteria, CA). IHC staining was performed at Quest Diagnostics (Chantilly, VA).
Immunohistochemically stained sections were scored by two independent observers (TN and FK) blinded to the clinical outcome. The cytoplasmic and/or nuclear immunoreaction was scored based on intensity whereby a score of 2 = positive (equal in intensity to normal epithelial cells), a score of 1 = weak (reduced intensity as compared with normal epithelial cells), and a score of 0 = negative (no immunoreaction).
Breast subtypes were defined using immunohistochemical expression of estrogen receptor (ER), progesterone receptor (PR), and HER2 based on criteria established in the literature. Luminal A was characterized by strong expression of ER and PR (H-score ≥200), HER2 negativity, and Ki-67 proliferation <14%. Luminal B was characterized by Ki-67 ≥ 14% and HER2-negative or HER2 positive. The HER2 subtype was hormone receptor-negative with only HER2 positivity. The triple-negative subtype lacked expression of ER, PR, and HER2.
Statistical Analysis
To determine the correlation between PTEN expression and clinicopathological variables,(e.g. ER, PR, HER2, subtype, grade, stage, age, overall survival, and recurrence), bivariate analysis was done using the chi square test or Fisher’s exact test, when appropriate. The association between PTEN expression and breast molecular subtypes and categorical variables was analyzed using logistic regression, where odds ratios (OR) and 95% confidence intervals (CI) were calculated. To examine the correlation between variables, overall survival, and disease-free survival, Kaplan-Meier survival analyses were carried out. Estimates were considered statistically significant for two-tailed values of P< 0.05. All analyses were carried out using the SPSS 12.0 statistical program (SPSS Inc., Chicago, IL).
Results
Characteristics of the Study Population
Clinical and pathological characteristics of the study population are summarized in Table 1. There were 202 patients diagnosed with infiltrating ductal carcinomas, with adequate FFPE tumor tissue for analysis, at our institution from 2000 to 2010. The majority of the tumor blocks (67.8%) came from women over the age of 50 (mean=57.65, SD=13.03), most of whom (76.2%) had no cancer recurrence. In this population, 43%, 47.7%, and 13.9% of tumors were ER−, PR−, and HER2-positive, respectively. The most prevalent breast cancer subtype was luminal A (ER+ or PR+, HER2−; 43.5%), followed by triple-negative (ER−, PR−, HER2−; 33.7%). Greater than 70% of all tumors were stage I and II; however, they tended to be higher grade, with Grade 3 tumors comprising 67.4% of the tumors in the study population.
Table 1.
Clinical and pathological characteristics of the study population
| Number | Percentage % | ||
|---|---|---|---|
| n = 202 | |||
| Age | |||
| <50 | 62 | 32.1 | |
| >50 | 131 | 67.8 | |
| Estrogen Receptor | |||
| Positive | 110 | 57.0 | |
| Negative | 83 | 43.0 | |
| Progesterone Receptor | |||
| Positive | 92 | 47.7 | |
| Negative | 101 | 52.3 | |
| HER2 Status | |||
| Positive | 27 | 14.0 | |
| Negative | 166 | 86.0 | |
| Subtype* | |||
| Luminal A | 84 | 43.5 | |
| Luminal B | 27 | 14.0 | |
| Her2 | 17 | 8.8 | |
| Triple Negative | 65 | 33.7 | |
| Pathologic Stage Group | |||
| 1 | 60 | 31.1 | |
| 2 | 80 | 41.5 | |
| 3 | 40 | 20.7 | |
| 4 | 12 | 6.2 | |
| Unknown | 1 | 0.5 | |
| Grade | |||
| I | 9 | 4.6 | |
| II | 54 | 28.0 | |
| III | 130 | 67.4 | |
| Recurrence | |||
| None | 147 | 76.2 | |
| Loco-regional | 8 | 4.1 | |
| Distant | 21 | 10.9 | |
| Never Disease free | 8 | 4.1 | |
| Unknown | 9 | 4.7 |
ER, estrogen receptor; PR, progesterone receptor.
Luminal A: ER+ or PR+, HER2−; luminal B: ER+ or PR+, HER2+ ; triple-negative: ER−, PR−, HER2−; Her2+: ER−, PR−, HER2+
Immunohistochemical Detection of PTEN and Analysis
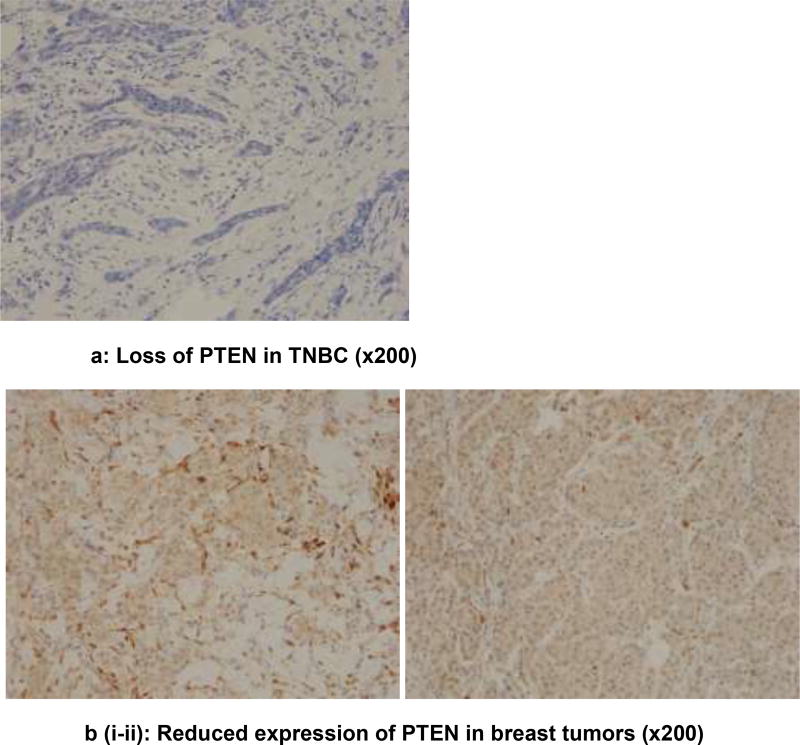
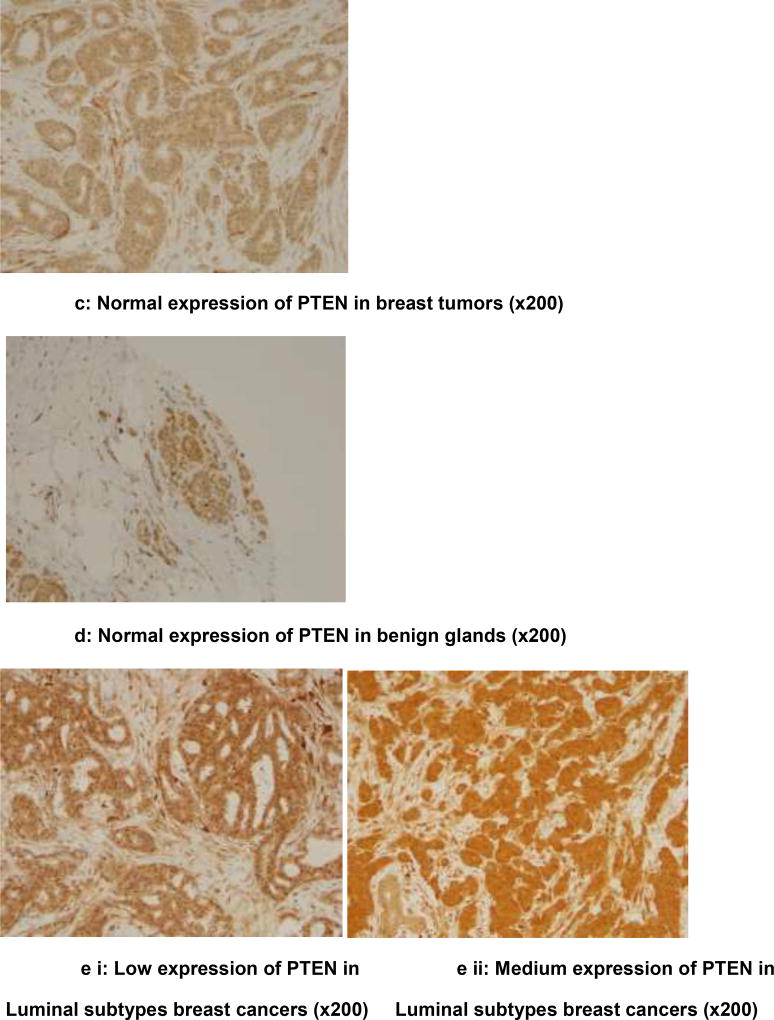
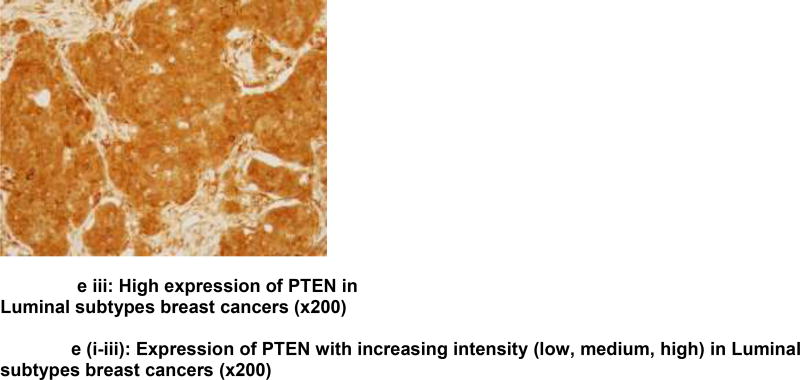
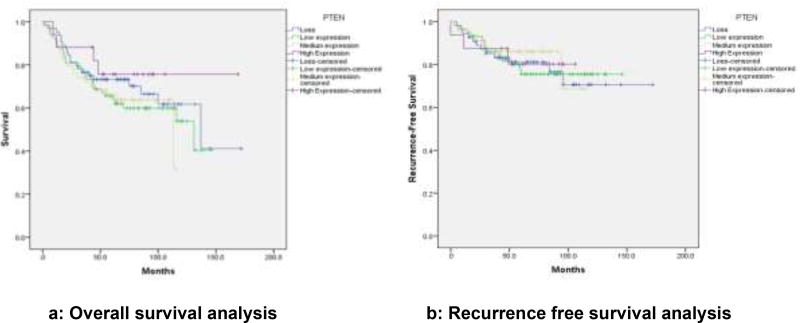
Analysis is based on 193 cases due to loss of some cores in the TMAs. Chi square analysis revealed an association between PTEN loss and ER (ER−: 41.38% vs. ER+: 23.93%; p=0.044); PR (PR−: 41.12% vs. PR+: 20.62%; p=0.014), non-luminal breast cancers (non-luminal: 41.86% vs. Luminal 23.73%; p=0 04), TNBC (TNBC: 44.12% vs. non-TNBC: 25.19%; p=0.036), Immunohistochemical detection of PTEN expression in the benign breast glands, normal and reduced expression in breast tumor, loss of expression in triple negative subtype with basal phenotype and increased intensity of expression (low, medium, high) in luminal subtypes is shown in figures 1a–e. Triple negative subtype shows no expression of PTEN; and luminal A subtype shows strong and diffuse expression of PTEN. Loss of PTEN was not associated with overall or recurrence-free survival [Figure 2].
Figure 1.
a) PTEN protein expression (×200) in triple negative breast cancers showing loss of PTEN expression
b) Reduced expression of PTEN in breast tumors (×200)
c) Normal expression of PTEN in breast tumors (×200)
d) Normal expression of PTEN in benign glands (×200)
e) Increased expression of PTEN (low, medium, high) in Luminal subtypes breast cancers (×200)
Figure 2.
a) Overall survival associated with PTEN expression
b) Recurrence-free survival analysis associated with PTEN expression
Discussion
Our study evaluated PTEN expression in breast ductal cancers from AA women. Our results demonstrate a significant association of PTEN loss with ER negative (p=0.044), PR negative (p=0.014) and triple negative (p=0.036) breast ductal cancers. These findings further support the results of previous studies, which have linked PTEN loss in breast cancers with negative-hormone-receptor and basal-like phenotypes [12,13]. There was also marginal association with distant metastasis. Our study did not show any association with PTEN loss and recurrence free survival or overall survival. These findings are in contrast to some of the previously published studies that showed significant association between PTEN loss and disease free survival specifically in AA women [12]. Some other studies have shown significant association between PTEN loss and disease free and overall survival. These studies have suggested that PTEN loss might predict aggressive behavior and poor clinical outcome in patients with breast cancer [13]. Loss of PTEN results in uncontrolled activation of PI3K/AKT signal transduction pathway that may underlie increased proliferative activity in TNBC. The use of targeted therapy against upstream and downstream effectors of PI3K/AKT pathway in combination with chemotherapy might be useful for aggressive TNBC.
In the United States, the incidence of breast cancer has been increasing in AA women since 2008. The overall incidence has been equal in AA and Caucasian women since 2012 [14]. The breast cancer mortality is higher in AA women as reflected by a lower five year survival rate (77%) when compared to Caucasian women (90%) [15].TNBC is more frequent in premenopausal AA women (39%) compared to either postmenopausal AA Women (14%) or in non-African Americans of any age (16%) [16]. The higher incidence of TNBC may account for more rapid progression and decreased survival in AA women with breast cancer. Approximately 70% of TNBC are basal phenotype with high proliferative activity and increased incidence of lymph node and distant metastasis. In our study, 67% of TNBC expressed CK5, a basal marker.
Our study demonstrates selective loss of PTEN in TNBC as compared to luminal A and B tumors in AA women, supporting the important role of unregulated activation of PI3K/AKT signal transduction in the pathogenesis of these cancers. TNBC are highly aggressive tumors with increased incidence of distant metastasis. They are resistant to currently available targeted therapy because they lack hormone and HER2 receptors. One of the downstream proteins in PI3K/AKT pathway, mammalian target of Rapamycin (mTOR), can be inhibited by currently available drugs, such as rapamycin. Targeting the TNBC with rapamycin in combination with chemotherapy may be a useful therapeutic option for the highly aggressive tumors, which demonstrate PTEN loss. The mTOR inhibitors are being used in combination with chemotherapy in phase II/III randomized clinical trials [17,18]. Everolimus, an mTOR kinase inhibitor, was associated with a statistically marginally (0.075) higher TNBC clinical response rate when used in conjunction with other chemotherapeutic drugs [19] and could represent a promising drug for TNBC. Recent studies have shown promising results with combination of mTOR inhibitors and chemotherapy [31].
Some studies have evaluated the role of upstream inhibitors of PI3K/AKT pathway in combination with chemotherapy [20]. MK-2206, an AKT inhibitor, was associated with increased toxicity at different doses [21]. One of the major limiting factors is development of drug toxicity when several therapeutic agents are used in combination because they inhibit proliferation pathways at multiple points [31].
HER2 overexpressing breast ductal cancers are high grade aggressive breast ductal cancers that lack ER and PR receptors but can be treated with trastuzumab. Some studies have shown association between PTEN loss and trastuzumab resistant HER2 positive breast ductal cancers [22,23]. The activation of downstream signaling pathway secondary to loss of PTEN results in trastuzumab resistance. It would be interesting to evaluate the role of mTOR inhibitors such as rapamycin in trastuzumab resistant advanced HER2 positive breast ductal cancers.
Loss of PTEN has been reported in the development of other cancers such as colorectal carcinoma [24]. Germline mutations in PTEN predisposes to multiorgan system carcinogenesis (Breast, Endometrial, Thyroid) and multiple hamartomatous polyps in the gastrointestinal tract known as Cowden syndrome [25,26]. The somatic mutations of PTEN are involved in the pathogenesis of endometrial hyperplasia, endometrial carcinoma [27,28] and glioblastoma, an aggressive brain tumor (WHO Grade, IV) [29]. A few studies have shown an association between PTEN loss and aggressive prostate cancers with high Gleason score and advanced stage [30].
Conclusion
Our study shows significant association between PTEN loss and TNBC, a subtype which correlates with progression and decreased survival in AA women. Breast cancers have heterogeneous carcinogenesis at the molecular level. Identification and understanding of molecular pathogenesis that underlies TNBC is critical to develop therapeutic targets. Signal transduction pathways are expressed by epithelial cells and are required for cell proliferation. Unregulated activation of these pathways suggests potential pathogenetic mechanism for carcinogenesis. Loss of PTEN and uncontrolled activation of PI3K/AKT signal transduction pathway may play a role in the pathogenesis of TNBC. mTOR might be responsible for propagation and aggressiveness of TNBC; and, thus, mTOR inhibitors may represent potential molecular targets for therapy in TNBC, occurring with increased incidence in minority populations. Further analysis of larger cohorts of minority populations with TNBC that might benefit from mTOR targeted inhibition is needed.
Table 2.
Association between ER, PR, HER2, Luminal subtypes, TNBC, distant metastasis and PTEN expression
| Estrogen Receptor Expression | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| PTEN | Negative | % | Positive | % | Chi- Square |
p- value |
| Loss | 36 | 41.38% | 28 | 23.93% | 8.095 | 0.044 |
| Low expression | 28 | 32.18% | 41 | 35.04% | ||
| Medium expression | 18 | 20.69% | 36 | 30.77% | ||
| High Expression | 5 | 5.75% | 12 | 10.26% | ||
| Progesterone Receptor Expression | ||||||
|
| ||||||
| PTEN | Negative | % | Positive | % | ||
|
| ||||||
| Loss | 44 | 41.12% | 20 | 20.62% | 10.576 | 0.014 |
| Low expression | 33 | 30.84% | 36 | 37.11% | ||
| Medium expression | 22 | 20.56% | 32 | 32.99% | ||
| High Expression | 8 | 7.48% | 9 | 9.28% | ||
| HER2 Expression | ||||||
|
| ||||||
| PTEN | Negative | % | Positive | % | ||
|
| ||||||
| Loss | 56 | 32.00% | 8 | 27.59% | 3.808 | 0.283 |
| Low expression | 57 | 32.57% | 12 | 41.38% | ||
| Medium expression | 45 | 25.71% | 9 | 31.03% | ||
| High Expression | 17 | 9.71% | 0 | 0.00% | ||
| Breast Cancer Luminal Subtype | ||||||
|
| ||||||
| PTEN | Non-Luminal | % | Luminal | % | ||
|
| ||||||
| Loss | 36 | 41.86% | 28 | 23.73% | 8.329 | 0.04 |
| Low expression | 27 | 31.40% | 42 | 35.59% | ||
| Medium expression | 18 | 20.93% | 36 | 30.51% | ||
| High Expression | 5 | 5.81% | 12 | 10.17% | ||
| TNBC Subtype | ||||||
|
| ||||||
| PTEN | Non-TNBC | % | TNBC | % | ||
|
| ||||||
| Loss | 34 | 25.19% | 30 | 44.12% | 8.559 | 0.036 |
| Low expression | 47 | 34.81% | 21 | 30.88% | ||
| Medium expression | 42 | 31.11% | 12 | 17.65% | ||
| High Expression | 12 | 8.89% | 5 | 7.35% | ||
| Metastasis | ||||||
|
| ||||||
| PTEN | No Mets | % | Mets | % | ||
|
| ||||||
| Negative | 105 | 62.13% | 24 | 77.42% | 2.674 | 0.102 |
| Positive | 64 | 37.87% | 7 | 22.58% | ||
Acknowledgments
We would like to acknowledge Pantomics, Inc. for constructing the TMAs and Quest Diagnostics for performing the immunohistochemical staining of the TMAs.
Financial Support: This project has been funded in whole or in part with Federal funds (UL1RR031975) from the National Center for Research Resources (NCRR), National Institutes of Health, through the Clinical and Translational Science Awards Program (CTSA), a trademark of DHHS, part of the Roadmap Initiative, “Re-Engineering the Clinical Research Enterprise,” (G12 RR003048) from the RCMI Program at Howard University, Division of Research Infrastructure, National Center for Research Resources, NIH and (U54 CA091431) the Howard University Cancer Center/ Johns Hopkins Cancer Center Partnership, National Cancer Institute, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors declare no conflicts of interest.
References
- 1.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Lonning PE, Borresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001 Sep 11;98(19):10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lonning PE, Brown PO, Borresen-Dale AL, Botstein D. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003 Jul 8;100(14):8418–23. doi: 10.1073/pnas.0932692100. Epub 2003 Jun 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charafe-Jauffret E, Ginestier C, Monville F, Fekairi S, Jacquemier J, Birnbaum D, Bertucci F. How to best classify breast cancer: conventional and novel classifications (review) Int. J. Oncol. 2005;27(5):1307–1313. [PubMed] [Google Scholar]
- 4.American Cancer Society. Cancer Facts and Figures for African Americans 2009–2010. Atlanta: American Cancer Society; 2009. [Google Scholar]
- 5.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 6.Horiuchi D, Kusdra L, Huskey NE, Chandriani S, Lenburg ME, Gonzalez-Angulo AM, Creasman KJ, Bazarov AV, Smyth JW, Davis SE, Yaswen P, Mills GB, Esserman LJ, Goga A. MYC pathway activation in triple-negative breast cancer is synthetic lethal with CDK inhibition. doi: 10.1084/jem.20111512. Published March 19, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slingerland J, Pagano M. Regulation of the cdk inhibitor p27 and its deregulation in cancer. J. Cell. Physiol. 183:10–17. doi: 10.1002/(SICI)1097-4652(200004)183:1<10::AID-JCP2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 8.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002 May 31;296(5573):1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 9.Cantley LC, Neel BG. New insights into tumor suppression: Pten suppresses tumor formation by restraining the phosphoinostide 3-kinase/Akt pathway. Proc Natl Acad Sci U S A. 1999 Apr 13;96(8):4240–5. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meric Berstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009;27:2278–87. doi: 10.1200/JCO.2008.20.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Podsypanina K, Lee RT, Politis C, et al. An inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase activity in Pten+/− mice. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(18):10320–10325. doi: 10.1073/pnas.171060098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y, Sarkissyan M, Elshimali Y, Vadgama JV. Triple negative breast tumors in African-American and Hispanic/Latina women are high in CD44+, low in CD24+, and have loss of PTEN. PLoS One. 2013 Oct 22;8(10):e78259. doi: 10.1371/journal.pone.0078259. eCollection 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Shuting, Shen Yanwei, Wang Mengying, Yang Jiao, Lv Meng, Li Pan, Chen Zheling, Yang Jin. Loss of PTEN expression in breast cancer: association with clinicopathological characteristics and prognosis. Oncotarget. 2017 May 9;8(19):32043–32054. doi: 10.18632/oncotarget.16761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeSantis CE, Fedewa SA, Sauer AG, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. doi: 10.3322/caac.21320. http://onlinelibrary.wiley.com/doi/10.3322/caac.21320/epdf. [DOI] [PubMed]
- 15.American Cancer Society. Cancer Facts and Figures for African Americans 2009–2010. Atlanta: American Cancer Society; 2009. [Google Scholar]
- 16.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 17.Mayer IA, Jovanovic B, Abramson VG, Mayer EL, Sanders ME, Bardia A, Dillon PM, Kuba MG, Carpenter JT, Chang JC, Lehmann BD, Meszoely IM, Grau A, Shyr Y, Arteaga CL, Chen X, et al. Abstract PD1-6: A randomized phase II neoadjuvant study of cisplatin, paclitaxel with or without everolimus (an mTOR inhibitor) in patients with stage II/III triple-negative breast cancer (TNBC) Cancer Research. 2014;73(24 Supplement) PD1-6-PD1-6. [Google Scholar]
- 18.Jerusalem G, Rorive A, Collignon J. Use of mTOR inhibitors in the treatment of breast cancer: an evaluation of factors that influence patient outcomes. Breast Cancer (Dove Med Press) 2014;6:43–57. doi: 10.2147/BCTT.S38679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fouque A, Jean M, van de Weghe P, Legembre P. Review of PI3K/mTOR inhibitors entering clinical trials to treat triple negative breast cancers. Recent Pat Anticancer Drug Discov. 2016;11(3):283–96. doi: 10.2174/1574892811666160519113731. [DOI] [PubMed] [Google Scholar]
- 21.Kalinsky K, Sparano JA, Andreopoulou E, Taback B, Wlechmann LS, Feldman SM, Ananthakrishnan P, Hibshoosh H, Manavaian J, Crew KD, Maurer MA, Hershman DL. Presurgical evaluation of the AKT inhibitor MK-2206 in patients with operable invasive breast cancer. Journal of Clinical Oncology. 2014;32(15) doi: 10.1007/s12094-018-1888-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, Klos KS, Li P, Monia BP, Nguyen NT, Hortobagyi GN, Hung MC, Yu D. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004 Aug;6(2):117–27. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 23.Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 24.Zhou XP, Loukola A, Salovaara R, Nystrom-Lahti M, Peltomäki P, de la Chapelle A, et al. PTEN mutational spectra, expression levels, and subcellular localization in microsatellite stable and unstable colorectal cancers. Am J Pathol. 2002;161:439–47. doi: 10.1016/S0002-9440(10)64200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liaw D, Marsh DJ, Li J, Dahia PLM, Wang SI, Zheng Z, Bose S, Call KM, Tsou HC, Peacocke M, Eng C, Parsons R. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 1997;16:64–67. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- 26.Eng C, Peacocke M. PTEN and inherited hamartoma-cancer syndromes. Nat Genet. 1998;19:223. doi: 10.1038/897. [DOI] [PubMed] [Google Scholar]
- 27.Levine RL, Cargile CB, Blazes MS, van Rees B, Kurman RJ, Ellenson LH. PTEN mutations and microsatellite instability in complex atypical hyperplasia, a precursor lesion to uterine endometrioid carcinoma. Cancer Res. 1998;58:3254–3258. [PubMed] [Google Scholar]
- 28.Tashiro H, Blazes MS, Wu R, Cho KR, Bose S, Wang SI, Li J, Parsons R, Ellenson LH. Mutations in PTEN are frequent in endometrial carcinoma but rare in other common gynecological malignancies. Cancer Res. 1997;57:3935–3940. [PubMed] [Google Scholar]
- 29.Wang SI, Puc J, Li J, Bruce JN, Cairns P, Sidransky D, Parsons R. Somatic mutations of PTEN in glioblastoma multiforme. Cancer Res. 1997;57:4183–4186. [PubMed] [Google Scholar]
- 30.McMenamin ME, Soung P, Perera S, et al. Loss of PTEN expression in paraffin-embedded primary prostate cancer correlates with high Gleason score and advanced stage. Cancer Res. 1999;59:4291–4296. [PubMed] [Google Scholar]
- 31.Massihnia D, Galvano A, Fanale D, Perez A, Castiglia M, Incorvaia L, Listì A, Rizzo S, Cicero G, Bazan V, Castorina S, Russo A. Triple negative breast cancer: shedding light onto the role of pi3k/akt/mtor pathway. Oncotarget. 2016 Sep;137(37):60712–60722. doi: 10.18632/oncotarget.10858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yunokawa M, Koizumi F, Kitamura Y, Katanasaka Y, Okamoto N, Kodaira M, Yonemori K, Shimizu C, Ando M, Masutomi K, Yoshida T, Fujiwara Y, Tamura K. Efficacy of everolimus, a novel mTOR inhibitor, against basal-like triple-negative breast cancer cells. Cancer Sci. 2012;103(9):1665–1671. doi: 10.1111/j.1349-7006.2012.02359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Depowski PL, Rosenthal SI, Ross JS. Loss of expression of the PTEN gene protein product is associated with poor outcome in breast cancer. Mod Pathol. 2001;14:672–676. doi: 10.1038/modpathol.3880371. [DOI] [PubMed] [Google Scholar]