Abstract
PURPOSE
To evaluate the utility of different optical coherence tomography angiography (OCT A) scan protocols in evaluating retinal changes in non-proliferative diabetic retinopathy.
METHODS
Patients were imaged with the RTVue XR Avanti OCT 3×3 mm and 6×6 mm “Angio Retina” scan protocols. Ability to clearly delineate the foveal avascular zone (FAZ), FAZ remodeling, microaneurysms (MA), capillary non-perfusion, motion and doubling artifacts were evaluated.
RESULTS
46 eyes from 27 patients enrolled. 89% of 3×3 mm versus 59% of 6×6 mm scans clearly delineated the FAZ (p= 0.001). 80% of 3×3 mm versus 43% of 6×6 mm scans demonstrated FAZ remodeling (p= 0.0002). MAs were detected by 57% of 6×6 mm and 35% of 3×3 mm scans (p= 0.003). Capillary non-perfusion was detected in 87% of 3×3 scans versus 89% of 6×6 mm scans (p= 0.99). No significant differences were noted in the incidence of artifacts between the scan sizes (motion artifact p= 0.29, doubling artifact p= 0.29).
CONCLUSIONS
3×3 mm scan delineated FAZ and remodeling better than 6×6 mm scan, likely due to its higher scan density. 6×6 mm scans detected MAs more readily than 3×3 mm, likely due to its larger scan area. There were utility for both 3×3 mm and 6×6 mm scans when evaluating these patients.
Keywords: Non-proliferative diabetic retinopathy, optical coherence tomography angiography, foveal avascular zone visualization, microaneurysm visualization, capillary non-perfusion, image artifacts
Introduction
Diabetic retinopathy is a leading cause of vision loss, as well as the fifth most common cause of preventable blindness worldwide.1,2 While in type 1 diabetics, proliferative diabetic retinopathy remains the most common cause of severe vision loss, diabetic macular edema is the most common cause of vision loss in type 2 diabetics.3 Thus, non-proliferative diabetic retinopathy is an important condition to monitor and manage in diabetic patients.
Traditionally, patients with diabetic retinopathy were monitored with serial fundus ophthalmoscopy in conjunction with ancillary testing, including fluorescein angiography (FA) to assess for areas of neovascularization of the disc or elsewhere, retinal edema and non-perfusion. While FA remains a gold-standard adjunctive test, it requires the injection of dye, and is therefore not suitable for all patients, including those who have poor venous access and allergy to fluorescein (which may include anaphylaxis).4,5 With the advent of optical coherence tomography (OCT), and more recently, OCT angiography (OCT A), it is now possible to assess the anatomy and integrity of the retinal and choroidal vasculature rapidly and non-invasively.4,6–8 It has been used to evaluate a variety of pathologies ranging from neovascular age-related macular degeneration9–11, vein occlusions12,13, to central serous chorioretinopathy14–16. OCT A uses the principle of speckle de-correlation to measure the difference in backscattered OCT signals between sequential B-scans taken at the same location to generate angiographic images.17
Since OCT A is still a relatively new imaging technique, studies are needed to elucidate the different findings in these conditions and to confirm the sensitivity and specificity of the pathologies. Additionally, there is currently no industry standard for the image size of the OCT A enface image and their scan density. For example, the RTVue XR Avanti OCT A (OptoVue Inc., Freemont, CA) has three scan protocols (3×3 mm, a 6×6 mm and 8×8 mm). Each scan protocol consists of 304 × 304 B scans. Thus, the purpose of this study is to compare two of the commercially available OCT A scan protocols— the 3×3 mm and 6×6 mm scans in the detection of various pathologies that may be seen in patients with non-proliferative diabetic retinopathy.
Methods
Subjects and OCT A Scan Protocols
Patients with history of mild to severe non-proliferative diabetic retinopathy seen at the vitreoretinal service at Shiley Eye Institute, University of California, San Diego between July and December 2015 were eligible. The study was approved by the University of California, San Diego Review Board and was conducted in accordance with the ethical standards stated in the 1964 Declaration of Helsinki. Patients with evidence of significant anterior segment, media opacities, optic nerve or retinal pathologies other than non-proliferative diabetic retinopathy were excluded from the study. The severity of diabetic retinopathy was graded based on the International Clinical Diabetic Retinopathy Severity Scale18. In addition, patients with high myopia or evidence of pathological myopia, including posterior staphyloma or CNV were excluded. Fluorescein angiography were not routinely obtained for patients imaged. However, in instances of severe non-proliferative diabetic retinopathy, fluorescein angiography was obtained to rule out the presence of proliferative disease.
Patients were imaged with the RTVue XR Avanti OCT Angiography 3×3 mm and 6×6 mm “Angio Retina” scan protocols (Figure 1). Two scans from each eye were obtained for each protocol. The scan with the higher signal strength out of the two was selected. When both scans from a protocol had the same signal strength, one scan was selected at random for analysis. All scans had a signal strength of at least 50 out of 100. Both eyes from each patient were imaged, both eyes were included in the study if they both met the inclusion criteria.
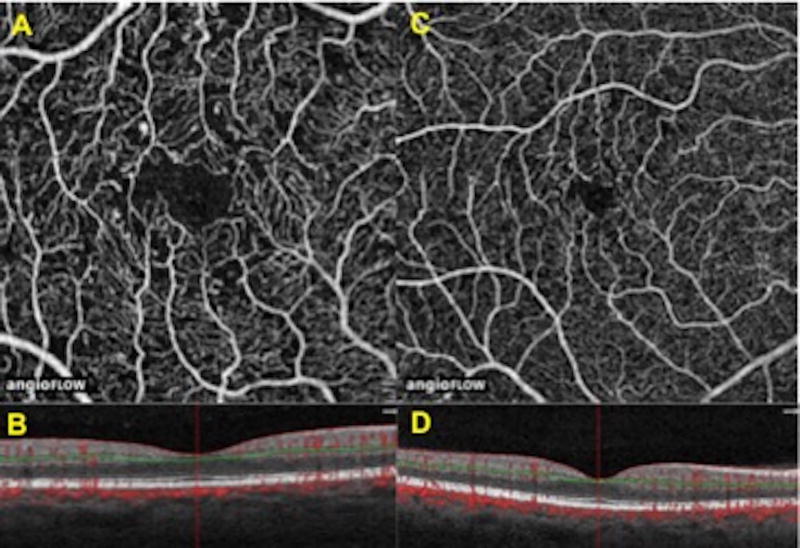
Figure 1. Comparison of 3×3 mm versus 6×6 mm superficial retina OCT angiography scans.
A) Example of 3×3 mm OCT A image.
B) Central horizontal 3 mm OCT B scan. Green line signifies area of segmentation.
C) Example of 6×6 mm OCT A image.
D) Central horizontal 6 mm OCT B scan. Green line signifies area of segmentation.
Image Analysis
Each OCT-A scan was individually analyzed on the Avanti OCT On-screen computer display by a grader (JH). Each individual B scan was analyzed for the presence of macular edema affecting the evaluation of the OCT A image and excluded if present. In addition, scans with edema around the foveal avascular zone were also excluded. The superficial retina scan slab, segmented at the superficial capillary plexus at the ganglion cell layer, was selected for analysis as it could most readily identify the extent of the FAZ. Features analyzed included: clarity of delineation of the foveal avascular zone (FAZ), presence of FAZ remodeling, microaneurysms (MA) and capillary non-perfusion (Figure 2 A and B). Additionally, the prevalence of OCT A scan errors including motion and doubling artifacts were reviewed (Figure 2 C and D):
FAZ delineation: Defined as the ability to completely trace the border of the FAZ 360 degrees around. Any blurriness or disturbance was considered inadequate visualization (Figure 3 C).
FAZ remodeling: Defined as an irregularity in the shape of the FAZ secondary to a deviation in the normal circular appearance of the FAZ. Any non-circular or irregularity was classified as the presence of remodeling.
MAs: Seen as out-pouching of the retinal vasculature. It could be seen as a focal area of hyper-reflectivity abutting a retinal blood vessel. On B scan OCT, it could be seen as a hyperreflective spot in the inner retina (Figure 3 A and B).
Capillary non-perfusion: Described as discrete areas on the OCT A scan where there was clear absence of the normal retinal vessels.
Motion artifact: Seen as linear distortions seen on the OCT A image, either as a waviness or abrupt discontinuity and shifting of the retinal vessels.
Doubling artifact: The presence of two or more identical OCT A images that were clearly non-overlapping.
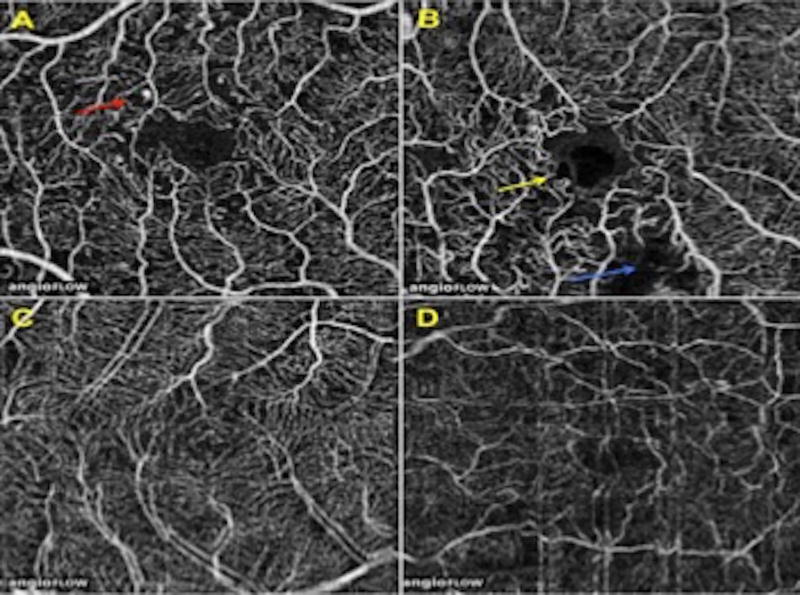
Figure 2. Examples of non-proliferative diabetic retinopathy features and artifacts.
A) Microaneurysms indicated by red arrow.
B) Foveal avascular zone remodeling (yellow arrow) and capillary non-perfusion (blue arrow).
C) Doubling artifact.
D) Motion artifact.
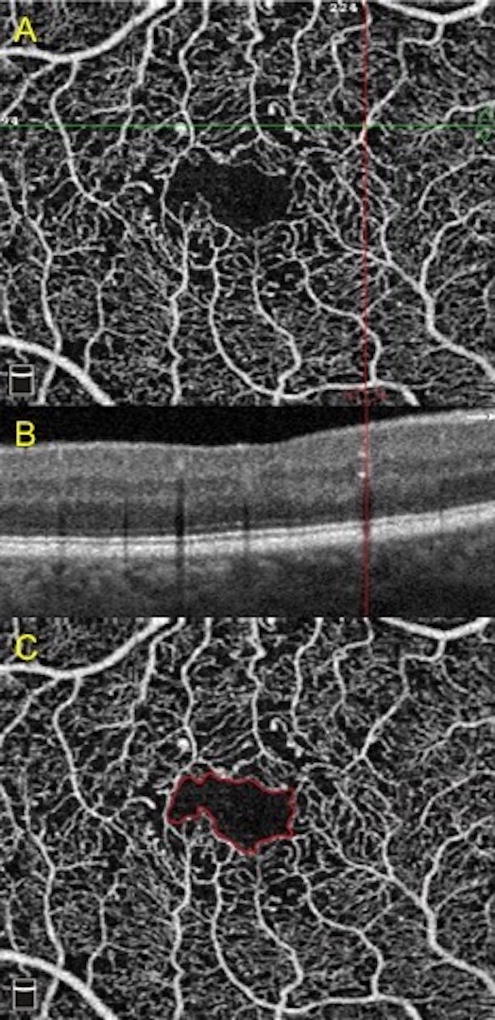
Figure 3. Examples of changes to foveal avascular zone and presence of microaneurysms.
A) Microaneurysm at intersection of horizontal and vertical lines.
B) Corresponding microaneurysm as hyperreflective spot in the inner retina marked by the vertical line.
C) Delineation of the foveal avascular zone in solid line.
The presence or absence of each of the features or artifacts was compared using the McNemar chi-square test. All statistical analysis were done using Microsoft Excel (Microsoft Corp, Redmond, WA).
Results
A total of 46 eyes from 27 patients were enrolled in the study. Average age of the patients was 63 (standard deviation 11)— 13 of whom were females and 14 were males. 38 eyes had mild, 4 eyes had moderate and 4 with severe non-proliferative diabetic retinopathy. 5 eyes demonstrated non-foveal avascular zone diabetic macular edema on the OCT B-scans.
Of the 27 patients in this study, 22 had a history of hypertension, 16 had hyperlipidemia, 2 patients were current smokers and 10 were former smokers. 5 had concurrent renal dysfunction. Hemoglobin A1C data was available for 23 patients, the average was 6.8%.
Analysis of OCT A Features
89% (41/46) of the 3×3 mm scans clearly demonstrated the extent of the FAZ, compared to 59% (27/46) of the 6×6 mm scans (p= 0.001; Table 1). FAZ remodeling was seen in 80% (37/46) of the 3×3 mm images, compared to 43% (20/46) of the 6×6 mm scans (p= 0.0002). MAs were detected by 57% (26/46) of the 6×6 mm and 35% (16/46) of the 3×3 mm scans (p= 0.003). The presence of capillary non-perfusion was similar between the two groups: 87% (40/46) of the 3×3 scans versus 89% (41/46) of the 6×6 mm scans (p= 0.99).
Table 1.
Comparison of Various OCT Angiography Scan Sizes.
| Scan | Foveal Avascular Zone Clarity |
Foveal Avascular Zone Remodel |
Microaneurysms | Capillary Non- perfusion |
Motion Artifact |
Doubling Artifact |
|---|---|---|---|---|---|---|
| 3×3 mm | 89% (41) | 80% (37) | 35% (16) | 87% (40) | 13% (6) | 24% (10) |
| 6×6 mm | 60% (28) | 46% (21) | 57% (26) | 89% (41) | 20% (9) | 13% (6) |
| P-value | 0.001 | 0.0002 | 0.003 | 0.99 | 0.29 | 0.29 |
Numbers in parenthesis refer to number of eyes.
Error Analysis
In terms of the incidence of motion artifacts, it was seen in 13% (6/46) of 3×3 mm scans and 20% (9/46) of 6×6 mm scans. The difference was not statistically significant (p= 0.29). 22% (10/46) of the 3×3 mm scans and 13% (6/46) of the 6×6 mm scans showed doubling artifacts. There were no significant differences noted in the incidence of this error (p= 0.29).
Discussion
This study demonstrated that there were significant differences noted in the prevalence of the various pathologies seen in patients with non-proliferative diabetic retinopathy between the two OCT A scan sizes. The 3×3 mm scans more clearly delineated the extent of the FAZ as well as remodeling of the FAZ compared to the 6×6 mm scans. The 6×6 mm scans more clearly demonstrated the presence of MAs compared to the 3×3 mm scans. Both 3×3 mm as well as the 6×6 mm scans showed similar incidence of capillary non-perfusion. Additionally, both scan protocols showed similar incidences of motion and doubling artifacts.
The RTVue XR Avanti has a scan rate of 70,000 A scans per second with a 5 micrometer axial and 15 micrometer transverse resolution. It acquires scans consisting of 304 × 304 B scans (304 A scans per line) with 2 repeats, which are then averaged. It has the options of 3×3 mm, 6×6 mm and 8×8 mm scan sizes (with each protocol taking about 3 seconds). Since all scan protocols, regardless of the scan volume, consists of 304 × 304 B scans, the scan density for the 3×3 mm is higher than that for the 6×6 mm scans. Thus, given the higher image resolution of the 3×3 mm compared to the 6×6 mm scans, it is intuitive that the 3×3 mm protocol more clearly delineates findings like the clarity of the FAZ extent as well as remodeling of the FAZ.
Carpineto et al utilized the 3×3 mm scan protocol and showed that FAZ measurements were both reproducible and repeatable in normal eyes.19 Takase et al also demonstrated that the 3×3 mm OCT A scans were able to delineate the extent of the FAZ and showed enlargement in eyes with diabetic retinopathy.20 These studies both selected the 3×3 mm scans over the other protocols, likely to take advantage of their higher scan density.
Ishibazawa et al demonstrated that MAs could be clearly delineated with a 3×3 mm protocol in a pilot study utilizing the RTVue XR Avanti.21 This study showed that the 6×6 mm scans were more likely to pick up MAs. This was likely because a scan protocol with a wider area of coverage like the 6×6 mm were more likely to show the presence of more diffuse or scattered pathologies like MAs.
Spaide et al discussed various imaging artifacts which may occur with OCT A.22 The authors discussed that motion artifacts may be the result of ocular saccades, and thus one way to decrease this incidence was to acquire dual OCT B scans, one horizontally and another vertically. Then the software corrected for motion artifacts by estimating and translating the eye motion for each A scan and subsequently comparing the volumes. The utilization of software correction could introduce other imaging artifacts such as doubling artifacts. The incidence of motion and doubling artifacts were clinically assessed in this study. We found that they were not significantly different between the protocols, as the scan acquisition time was similar for both scans. The presence of both artifacts was relatively high. This illustrates that further research will be necessary to increase scan speeds in order to decrease the incidence of motion artifacts, and improvements to the software will also be needed to decrease the prevalence of doubling artifacts.
In the present study, only retinal changes in the superficial capillary plexus were evaluated. While retinopathy may affect both superficial as well as the deep capillary plexus in diabetic patients, we focused on the superficial plexus in this study, as we could more readily identify the pathologies. Ishibazawa et al evaluated OCT A images in patients with diabetic retinopathy in both the superficial as well as the deep capillary plexus utilizing 3×3 mm OCT A scans21. They observed that areas of retinal non-perfusion were greater in the superficial than the deep capillary plexus and suggested that the deep capillaries could be relatively more spared compared to capillaries in the superficial plexus secondary to anatomical differences. A potential future study could be to do a comparative study looking at 3×3 mm vs 6×6 mm scan sizes to evaluate MAs, FAZs and capillary non-perfusion status in the deep capillary plexus. We suspect the results would be similar to the current study as FAZ remodeling would still benefit from the higher scan resolution of a 3×3 mm scan and MA identification would benefit from the wider field of view of a 6×6 mm scan.
Limitations of the study include the absence of FA to compare to OCT A images. De Carlo et al compared the incidence of MAs on OCT A versus FA and found that while OCT A was able to visualize most of the MAs seen on FA, some MAs were only noted on OCT A and not on FA, and vice versa.2,17 Thus future studies may be necessary to compare the sensitivity and specificity of OCT A versus FA findings. Since this study only analyzed data obtained from RTVue XR Avanti, another possible future direction would be a comparison of the various OCT angiography devices, like the Zeiss Cirrus HD-OCT Model 5000 with Angioplex OCT A capability (which uses a spectral domain OCT with a scan rate of 68,000 A scans/second with scans consisting of 350×350 A scans).23 Since this study only evaluated the presence versus absence of different retinal findings, another potential future study could be to quantitatively assess pathologies such as actual number or MAs or volume of capillary non-perfusion measured using the different OCT A scan protocols. Another limitation of the study is the presence of macular edema in 5 eyes, which may interfere with the detection of features such as MAs and FAZ measurements. However, we have correlated the OCT B scans to the OCT A scans and the presence of edema in our patients did not appear to have affected the quality of the images. This study only evaluated OCT A scans at the level of the superficial capillary plexus and thus this may miss potential retinal changes that may occur at other locations, such as in the deep capillary plexus. In this study, the grader was not blinded as to the relative size of the image being graded (3×3 mm or 6×6 mm) because the purpose of the study was to evaluate the scans under real-world, standard clinical conditions, and as such masking would be difficult logistically as the scan size could be recognized by the reader. However, this is another potential limitation to the study, especially when evaluating central findings like FAZ remodeling or delineation. Another limitation was the inclusion of two eyes in some patients and one eye in other patients. Lastly, only one observer was used to evaluate the OCT scans, thus this may also be a potential source of bias.
This study demonstrated that there was clinical utility for both the higher scan resolution obtainable with a smaller (3×3 mm) scan, as well as the larger field of view obtained with a larger (6×6 mm) OCT A scan protocols in assessing patients with non-proliferative diabetic retinopathy. Given the relatively quick scan acquisition time (about 3 seconds), it would be both clinically feasible and helpful to obtain and analyze both scans in the care of these patients.
Summary statement.
Two different optical coherence tomography angiography scan protocols were compared to evaluate their utilities in imaging various non-proliferative diabetic retinopathy pathologies. While smaller field of view scans more clearly delineated the anatomy of the foveal avascular zone, the larger scan protocol better demonstrated microaneurysms and capillary non-perfusion.
Acknowledgments
Financial Support
Supported in part by a core grant from the National Eye Institute P30 EY022589, an unrestricted grant from Research to Prevent Blindness, NY (WRF). The funding organizations had no role in the design or conduct of this research.
Footnotes
Authors with financial/conflicting interests are listed after references.
The data in this study was previously presented at the American Society of Retina Specialists Meeting 2016 in San Francisco, CA.
Financial Disclosures
Joseph Ho, none.
Kunny Dans, none.
Qisheng You, none.
Eric N. Nudleman, none.
William R. Freeman, none.
References
- 1.Bourne RR, Stevens GA, White RA, et al. Causes of vision loss worldwide, 1990–2010: a systematic analysis. Lancet Glob Health. 2013;1:e339–49. doi: 10.1016/S2214-109X(13)70113-X. [DOI] [PubMed] [Google Scholar]
- 2.Salz DA, de Carlo TE, Adhi M, et al. Select Features of Diabetic Retinopathy on Swept-Source Optical Coherence Tomographic Angiography Compared With Fluorescein Angiography and Normal Eyes. JAMA ophthalmology. 2016;134:644–50. doi: 10.1001/jamaophthalmol.2016.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lightman S, Towler HM. Diabetic retinopathy. Clin Cornerstone. 2003;5:12–21. doi: 10.1016/s1098-3597(03)90015-9. [DOI] [PubMed] [Google Scholar]
- 4.Novais EA, Roisman L, de Oliveira PR, et al. Optical Coherence Tomography Angiography of Chorioretinal Diseases. Ophthalmic Surg Lasers Imaging Retina. 2016;47:848–61. doi: 10.3928/23258160-20160901-09. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Saez MP, Ordoqui E, Tornero P, et al. Fluorescein-induced allergic reaction. Ann Allergy Asthma Immunol. 1998;81:428–30. doi: 10.1016/S1081-1206(10)63140-7. [DOI] [PubMed] [Google Scholar]
- 6.Spaide RF, Klancnik JM, Jr, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA ophthalmology. 2015;133:45–50. doi: 10.1001/jamaophthalmol.2014.3616. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz DM, Fingler J, Kim DY, et al. Phase-variance optical coherence tomography: a technique for noninvasive angiography. Ophthalmology. 2014;121:180–7. doi: 10.1016/j.ophtha.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi W, Mohler KJ, Potsaid B, et al. Choriocapillaris and choroidal microvasculature imaging with ultrahigh speed OCT angiography. PLoS One. 2013;8:e81499. doi: 10.1371/journal.pone.0081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang D, Jia Y, Rispoli M, Tan O, Lumbroso B. Optical Coherence Tomography Angiography of Time Course of Choroidal Neovascularization in Response to Anti-Angiogenic Treatment. Retina. 2015;35:2260–4. doi: 10.1097/IAE.0000000000000846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palejwala NV, Jia Y, Gao SS, et al. Detection of Nonexudative Choroidal Neovascularization in Age-Related Macular Degeneration with Optical Coherence Tomography Angiography. Retina. 2015;35:2204–11. doi: 10.1097/IAE.0000000000000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang MC, de Carlo TE, Baumal CR, et al. Correlation of Spectral Domain Optical Coherence Tomography Angiography and Clinical Activity in Neovascular Age-Related Macular Degeneration. Retina. 2016;36:2265–73. doi: 10.1097/IAE.0000000000001102. [DOI] [PubMed] [Google Scholar]
- 12.Kang JW, Yoo R, Jo YH, Kim HC. Correlation of Microvascular Structures on Optical Coherence Tomography Angiography with Visual Acuity in Retinal Vein Occlusion. Retina. 2016 doi: 10.1097/IAE.0000000000001403. [DOI] [PubMed] [Google Scholar]
- 13.Spaide RF, Lee JK, Klancnik JK, Jr, Gross NE. Optical coherence tomography of branch retinal vein occlusion. Retina. 2003;23:343–7. doi: 10.1097/00006982-200306000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Chan SY, Wang Q, Wei WB, Jonas JB. Optical Coherence Tomographic Angiography in Central Serous Chorioretinopathy. Retina. 2016;36:2051–8. doi: 10.1097/IAE.0000000000001064. [DOI] [PubMed] [Google Scholar]
- 15.McClintic SM, Jia Y, Huang D, Bailey ST. Optical coherence tomographic angiography of choroidal neovascularization associated with central serous chorioretinopathy. JAMA ophthalmology. 2015;133:1212–4. doi: 10.1001/jamaophthalmol.2015.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quaranta-El Maftouhi M, El Maftouhi A, Eandi CM. Chronic central serous chorioretinopathy imaged by optical coherence tomographic angiography. Am J Ophthalmol. 2015;160:581–7. e1. doi: 10.1016/j.ajo.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 17.de Carlo TE, Romano A, Waheed NK, Duker JS. A review of optical coherence tomography angiography (OCTA) Int J Retina Vitreous. 2015;1:5. doi: 10.1186/s40942-015-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkinson CP, Ferris FL, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–82. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 19.Carpineto P, Mastropasqua R, Marchini G, Toto L, Di Nicola M, Di Antonio L. Reproducibility and repeatability of foveal avascular zone measurements in healthy subjects by optical coherence tomography angiography. Br J Ophthalmol. 2016;100:671–6. doi: 10.1136/bjophthalmol-2015-307330. [DOI] [PubMed] [Google Scholar]
- 20.Takase N, Nozaki M, Kato A, Ozeki H, Yoshida M, Ogura Y. Enlargement of Foveal Avascular Zone in Diabetic Eyes Evaluated by En Face Optical Coherence Tomography Angiography. Retina. 2015;35:2377–83. doi: 10.1097/IAE.0000000000000849. [DOI] [PubMed] [Google Scholar]
- 21.Ishibazawa A, Nagaoka T, Takahashi A, et al. Optical Coherence Tomography Angiography in Diabetic Retinopathy: A Prospective Pilot Study. Am J Ophthalmol. 2015;160:35–44. e1. doi: 10.1016/j.ajo.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 22.Spaide RF, Fujimoto JG, Waheed NK. Image Artifacts in Optical Coherence Tomography Angiography. Retina. 2015;35:2163–80. doi: 10.1097/IAE.0000000000000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenfeld PJ, Durbin MK, Roisman L, et al. ZEISS Angioplex Spectral Domain Optical Coherence Tomography Angiography: Technical Aspects. Dev Ophthalmol. 2016;56:18–29. doi: 10.1159/000442773. [DOI] [PubMed] [Google Scholar]