Abstract
Background
In the Single Ventricle Reconstruction (SVR) trial, one-year transplant-free survival was better for the Norwood procedure with right ventricle-to-pulmonary artery shunt (RVPAS) compared with a modified Blalock-Taussig shunt (MBTS) in patients with hypoplastic left heart and related syndromes. At 6 years, we compared transplant-free survival and other outcomes between the groups.
Methods
Medical history was collected annually using medical record review, telephone interviews, and the death index. The cohort included 549 patients randomized and treated in the SVR trial.
Results
Transplant-free survival for the RVPAS vs. MBTS groups did not differ at 6 years (64% vs. 59%, P=0.25) or with all available follow-up of 7.1±1.6 years (log-rank P=0.13). The RVPAS vs. MBTS treatment effect had non-proportional hazards (P=0.009); the hazard ratio (HR) for death or transplant favored the RVPAS before Stage II surgery (HR=0.66; 95% CI 0.48–0.92). The effect of shunt type on death or transplant was not statistically significant between Stage II to Fontan surgery (HR 1.36, 95% CI 0.86–2.17, p=0.17) or after the Fontan procedure (HR 0.76, 95% CI 0.33–1.74, p=0.52). By 6 years, RVPAS patients had a higher incidence of catheter interventions (0.38 vs. 0.23/patient-year, P<0.001), primarily due to more interventions between the Stage II and Fontan procedures (HR=1.72, 95% CI 1.00–3.03). Complications did not differ by shunt type; by 6 years, one in five patients had had a thrombotic event and one in six, seizures.
Conclusions
By 6 years, the hazards of death or transplant and catheter interventions were not different between the RVPAS vs. MBTS groups. Children assigned to the RVPAS group had 5% higher transplant-free survival but the difference did not reach statistical significance, and they required more catheter interventions. Both treatment groups have accrued important complications.
Clinical Trial Registration
Registered with ClinicalTrials.gov number, NCT00115934 URL: http://clinicaltrials.gov/show/NCT00115934
Keywords: congenital heart defect, congenital heart disease, cardiac surgery, single ventricle, Norwood procedure
INTRODUCTION
With the development of the Norwood procedure in 1980,1 survival became possible for infants with hypoplastic left heart syndrome (HLHS) and related single right ventricle anomalies. The Norwood procedure uses the native pulmonary artery to provide blood flow to the aorta, leaving the distal pulmonary arteries to be perfused by a systemic-to-pulmonary-artery shunt. In early years, surgeons employed a modified Blalock-Taussig shunt (MBTS) from the subclavian artery to the ipsilateral pulmonary artery. Subsequently, Sano et al.2 described the right ventricular-to-pulmonary artery shunt (RVPAS), which lessens aortic diastolic run off and coronary arterial steal in the early postoperative period, but requires a ventriculotomy in patients with a single right ventricle.
In 2005, we began the Single Ventricle Reconstruction (SVR) Trial, a randomized trial comparing transplant-free survival and morbidities after the Norwood procedure with the RVPAS vs. MBTS in children with HLHS and other single right ventricular disorders.3 We previously reported the outcomes of study patients at ages 1 and 3 years.3, 4 Transplant-free survival proved to be superior in the first year of life among those assigned to the RVPAS. By three years, the shunt groups no longer differed significantly in transplant-free survival, although the hazard ratio for transplant-free survival tended to be better for the MBTS group after one year. Moreover, the RVPAS group had slightly worse right ventricular ejection fraction and a higher rate of unplanned reinterventions.
In this report of the primary endpoint of the SVR Extension Study (SVR II), we sought to compare the shunt groups with respect to transplant–free survival using all available data when the last enrolled patient had reached age six years. We also compared the shunt groups with respect to interventions and morbidities to age 6 years, including echocardiographic right-ventricular ejection fraction (RVEF), catheter interventions, other events and morbidities, and New York Heart Association Class.
METHODS
After trial results are published, the datasets and descriptor files from the SVR II study will be made available to other researchers on the Pediatric Heart Network Public Use Dataset webpage.5
Subjects
Patients were enrolled between May 2005 and July 2008. Eligibility criteria and trial methods for the SVR trial have previously been published.6 Inclusion criteria were a diagnosis of HLHS or a related single, morphologic right ventricular anomaly with a planned Norwood procedure. Among 555 patients originally enrolled, one patient was excluded after withdrawal in week 1, and five did not undergo a Norwood procedure; therefore, the analytic cohort includes 549 patients. The data include at least 6 years of follow-up since the last patient was enrolled in the trial. The Institutional Review Board of each participating center approved this study, and parents/guardians of enrolled patients provided informed consent.
Data Obtained
Medical history was collected annually using medical record review, telephone interviews with parents or guardians, and the death index. Data obtained included vital status, surgical and catheter-based interventions, medical events, morbidities, and New York Heart Association Class. The primary causes of death between 1 and 6 years were adjudicated by a medical monitor.7 Echocardiograms obtained for clinical indications were interpreted in a core laboratory, and the biplane pyramidal method was used for calculation of RVEF.8
Statistical Analysis
Statistical methods are similar to those used in analysis of data at 3 years.4 The comparison of study outcomes was according to treatment assignment to MBTS or RVPAS (intention-to-treat) unless otherwise specified. We used a Wald test for comparison of 6-year event rates estimated by the Kaplan-Meier method and the log-rank test to determine whether the distributions of time to the earliest occurrence of death or transplantation using all available follow-up differed by assigned shunt. Three patients had their follow-up time censored at the time of biventricular repair. To test the hypothesis that the size of the treatment effect varied by time, we used Cox regression with a time-dependent treatment indicator. The time intervals were as follows: before Stage II surgery, between Stage II surgery until Fontan surgery, and then after Fontan surgery. Cumulative incidence rates for the competing risks of death and transplant were also estimated by shunt group.9
To determine whether the treatment effect differed across patient subgroups, a treatment by subgroup interaction test from Cox regression was used for the following pre-specified subgroups: birth weight: <2500 vs. ≥2500 g; pre-Norwood tricuspid regurgitation with proximal jet width <2.5 vs. ≥2.5 mm; use of deep hypothermic circulatory arrest vs. regional cerebral perfusion during the Norwood procedure; annual surgeon Norwood volume; and annual center single ventricle volume based on the patients screened for the trial. We also examined a subgroup identified post hoc in a previous SVR report,10 defined by the presence or absence of a patent aortic valve and preterm birth (4 subgroups).
Incidence rates of the secondary outcomes related to catheter-based interventions and morbidities occurring by age 6 years were compared by shunt type using Poisson regression. The time to first catheter-based intervention was estimated using the Kaplan-Meier method. We also used Cox regression with a time-dependent treatment indicator by surgical stage to examine differential shunt type effects by time on the catheterization outcomes.
We used all available follow-up and Cox proportional hazards regression to identify pre- and intraoperative risk factors (all variables evaluated are shown in Supplemental Table I) for transplant-free survival. The stepwise selection procedure for the multivariable main effects model included all predictors with a univariate p-value less than 0.2. A test of non-proportionality was performed for each candidate predictor. A two-sample t-test was used to assess whether mean RVEF differed by assigned shunt. For all analyses, including tests of interaction, a p-value of 0.05 was considered significant. All analyses were conducted using SAS version 9.4 (SAS Institute, Inc., Cary, NC) and R version 3.2.1.
RESULTS
At the time of this report, 331 of the original 549 patients were alive without cardiac transplantation with mean follow-up of 7.1±1.6 years. The Fontan procedure had been performed in 328 patients (169 in the RVPAS group) at a mean age of 2.9±0.9 years (IQR, 2.3–3.4 years; range 1.3–6.6 years). Fontan type was extra-cardiac in 55% and lateral tunnel in 45%; 87% were fenestrated. The distributions of these surgical approaches did not differ between the RVPAS vs. MBTS shunt groups. An additional three patients, all in the RVPAS group, had undergone biventricular repair.
Transplant-free survival
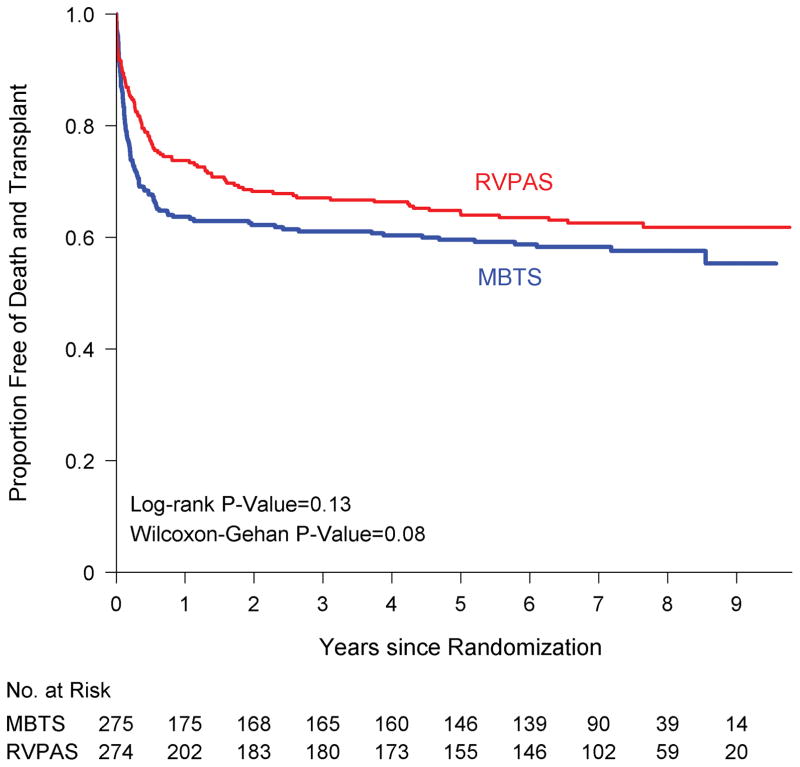
The assigned shunt groups did not differ in transplant-free survival (log-rank P=0.13, Figure 1). At 6 years, transplant-free survival rates for the RVPAS vs. MBTS groups were 59% vs. 64% (P=0.25; Table 1), and death or transplant had occurred in 99 RVPAS patients (87 deaths, 12 transplants) and 113 MBTS patients (103 deaths, 10 transplants). Altogether, in the RVPAS vs. MBTS groups respectively, death or transplant occurred in 72 vs. 100 patients by one year post randomization, in 18 vs. 7 patients between 1 and 3 years, and in 9 vs. 6 patients after three years up to 6 years of age. The shunt groups did not differ significantly in rates of all-cause mortality or cardiac transplantation (Supplemental Figure I). Although our primary analysis was performed according to intent to treat, study inferences were similar when examining the shunt in place at the end of the Norwood procedure. An additional 6 death or transplant events occurred after 6 years, 3 each in the RVPAS and MBTS groups. Timing of deaths and cardiac transplantation in the combined shunt groups according to surgical stage are summarized in Supplemental Table II.
Figure 1.
Comparison of the assigned shunt types in their freedom from the composite end point of death or cardiac transplantation (ie, transplantation-free survival). RVPAS indicates right ventricle–to–pulmonary artery shunt; and MBTS, modified Blalock-Taussig shunt.
Table I.
Clinical Events from Norwood to Age 6 Years by Shunt Type
| Outcome | All (n=549) | RVPAS* (n=274) | MBTS† (n=275) | P-value‡ |
|---|---|---|---|---|
| No. of Patients | ||||
| Death or cardiac transplant | 212 | 99 | 113 | 0.25 |
| Death (prior to transplant) | 190 | 87 | 103 | |
| Cardiac transplant | 22 | 12 | 10 | |
| Death/transplant ≤1 year | 172 | 72 | 100 | |
| Death/transplant >1 to ≤3 years | 25 | 18 | 7 | |
| Death/transplant >3 to ≤6 years | 15 | 9 | 6 | |
| Incidence per 100 patient-years | ||||
| Cardiac surgeries | 100.6 | 100.8 | 100.4 | 0.93 |
| Catheter procedures | 30.7 | 37.8 | 23.0 | <0.001 |
| Interventional catheterizations | 21.5 | 25.3 | 17.4 | <0.001 |
| Complications | 176.3 | 175.7 | 177.0 | 0.83 |
| No. of Patients (%) | ||||
| Pacemaker placed | 14 (2.6%) | 8 (2.9%) | 6 (2.2%) | 0.60 |
| Thrombotic event | 86 (15.7%) | 42 (15.3%) | 44 (16.0%) | 0.91 |
| Stroke | 38 (6.9%) | 23 (8.4%) | 15 (5.5%) | 0.18 |
| Seizure | 71 (12.9%) | 40 (14.6%) | 31 (11.3%) | 0.26 |
| PLE§ | 17 (3.1%) | 12 (4.4%) | 5 (1.8%) | 0.09 |
| Plastic bronchitis | 3 (0.5%) | 1 (0.4%) | 2 (0.7%) | 1.00 |
RVPAS = right-ventricular-to-pulmonary-artery shunt MBTS = modified Blalock-Taussig shunt
MBTS = modified Blalock-Taussig shunt
P-values for incidence rate and proportion comparisons are based on Poisson regression and Fisher exact test, respectively. P-value for comparison of 6-year death/transplant is based on Wald test of the pointwise Kaplan-Meier event rate estimates at 6 years.
PLE = protein-losing enteropathy
The magnitude of the treatment effect on transplant-free survival varied over the study period (Proportional Hazards [PH] P=0.009). Follow-up was divided into three periods based on the time of Stage II surgery and Fontan procedure. The RVPAS group, compared with the MBTS group, had a lower hazard of death or transplant prior to the Stage II procedure (P=0.003): hazard ratio (HR), HR=0.66 (95% Confidence Interval [CI] 0.48–0.92). The shunt effect on death or transplant was not significant after the Stage II procedure, but the estimates for the two later periods were in opposite directions: Stage II to Fontan, RVPAS vs. MBTS HR 1.36 (95% CI 0.86–2.17, p=0.17); and post-Fontan, RVPAS vs. MBTS HR 0.76 (95% CI 0.33–1.74, p=0.52).
The 6-year composite event rates conditional on transplant-free survival to one year (a pre-specified landmark analysis) were 13.8% for RVPAS and 7.7% for MBTS (overall log-rank test P=0.056). The shunt groups did not differ with respect to primary causes of death after 1 year (Supplemental Table III).
In pre-specified subgroup analyses (Supplemental Table IV), interaction of shunt by subgroup was statistically significant only for annual Norwood surgeon volume (P=0.046), with MBTS having superior transplant-free survival (P=0.04) in the highest volume stratum, which only included 2 surgeons; in the lower volume strata, the RVPAS was beneficial or no shunt difference could be detected.
We performed multivariable Cox regression to determine predictors of worse transplant-free survival, including only covariates measured prior to and during the Norwood procedure (Table 2). An interaction of shunt type by time periods defined by surgical stage was fixed in the model. The MBTS was a risk factor only prior to the Stage II procedure. The RVPAS group had a higher event rate between the Stage II and Fontan procedures, but the association was not significant (HR=1.50, p=0.09). Other independent predictors of adverse outcome included presence of total anomalous pulmonary venous return, presence of a genetic syndrome or unknown genetic status (compared with absence of a genetic syndrome), moderate-to-severe tricuspid regurgitation prior to the Norwood procedure, use of extracorporeal membrane oxygenation during the Norwood procedure, and lower surgeon annual Norwood volume.
Table II.
Multivariable Cox Regression Model for Transplant-Free Survival (N=534; 209 death/transplant events)
| Characteristic | Hazard Ratio (95% CI) | P |
|---|---|---|
| Shunt x Time interaction | 0.03 | |
| RVPAS* vs. MBTS†, prior to Stage II procedure | 0.71 (0.51,1.0) | 0.03 |
| RVPAS vs MBTS, Stage II to Fontan procedure | 1.50 (0.93,2.40) | 0.09 |
| RVPAS vs MBTS, post-Fontan procedure | 0.84 (0.36, 1.93) | 0.68 |
| Total anomalous pulmonary venous return | 3.25 (1.39,7.60) | 0.007 |
| Genetic syndrome | <.001 | |
| Yes | 2.78 (1.56,4.95) | |
| Unknown (no genetic evaluation) | 3.27 (2.45,4.36) | |
| No | Ref | |
| Pre-Norwood moderate/severe tricuspid regurgitation | 1.77 (1.23,2.56) | 0.002 |
| ECMO‡ used in Operating Room | 4.68 (3.06,7.15) | <.001 |
| Surgeon Annual Norwood volume, per 1 unit increase | 0.95 (0.93,0.98) | <0.001 |
RVPAS = right ventricular-to-pulmonary artery shunt;
MBTS = modified Blalock-Taussig shunt,
ECMO = Extracorporeal membrane oxygenation.
Cardiac catheterization
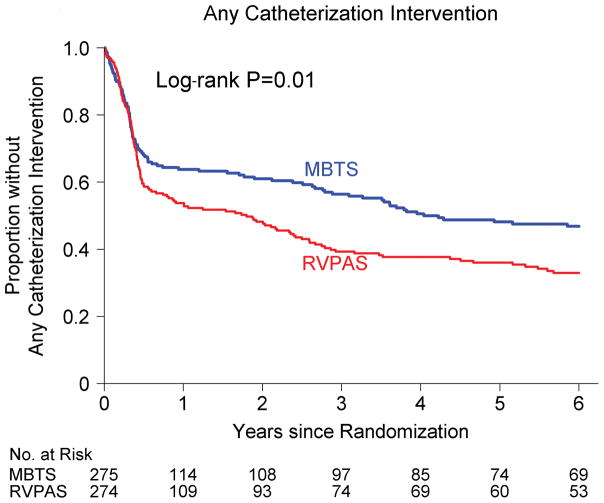
In the period from the Norwood procedure to age 6 years, the incidence of catheter interventions was higher in patients in the RVPAS vs. MBTS group (P<0.001; Figure 2). Compared with the MBTS group, RVPAS patients underwent their first catheter intervention of any type at an earlier time (P=0.01; 67% vs. 53% by 6 years). They also underwent earlier coil occlusion of collaterals (P=0.001; 36% vs. 23% by 6 years); this difference for the RVPAS vs. MBTS groups was primarily due to coiling of aortopulmonary collaterals (P<0.001; 31% vs. 17% by 6 years) and to a lesser degree due to coiling of veno-venous collaterals (P=0.10; 12% vs. 7% by 6 years). The two shunt groups had a similar time to first balloon angioplasty (P=0.54; 38% vs. 33% by 6 years; P=0.55 after adjusting for center) and stenting (P=0.054; 22% vs. 14% by 6 years; P=0.67 after adjusting for center).
Figure 2.
Comparison of the assigned shunt types in their freedom from any catheter interventions. RVPAS indicates right ventricle–to–pulmonary artery shunt; and MBTS, modified Blalock-Taussig shunt.
We found significant non-proportional hazards for catheter intervention of any type (PH P=0.02) and for coil insertion (PH P=0.003), particularly coil placement for aortopulmonary collaterals (PH P=0.001). The hazard ratio for catheter intervention of any type in the RVPAS vs. MBTS groups varied over time (P=0.014; Supplemental Table V): prior to Stage II HR=1.34 (95% CI, 0.97–1.84); Stage II to Fontan HR=1.74 (95% CI 1.00–3.00), and post-Fontan HR=1.29 (95% CI 0.67–2.48). Conversely, the effect of RVPAS vs. MBTS shunt type on coil placement for any indication diminished over time (P=0.001): < prior to Stage II, HR=9.55 (30 vs. 3 events); Stage II to Fontan, HR=1.45 (33 vs. 24 events), and post-Fontan, HR=0.91 (10 vs. 12 events). A similar pattern over time was observed for coil placement for aortopulmonary collaterals. We found no significant interactions of shunt type and subgroup factors in analyses of catheter procedures.
Right ventricular function and clinical events
RVEF could be accurately measured in only a small subset of patients. At 6 years, the RVPAS vs. MBTS groups did not differ in mean RVEF (46±6 [n=61] vs. 45±6 [n=55]; P=0.60), even after adjustment for factors that were predictive of RVEF (P=0.40, adjusted for gender, socioeconomic status score,10 and at least moderate tricuspid regurgitation).
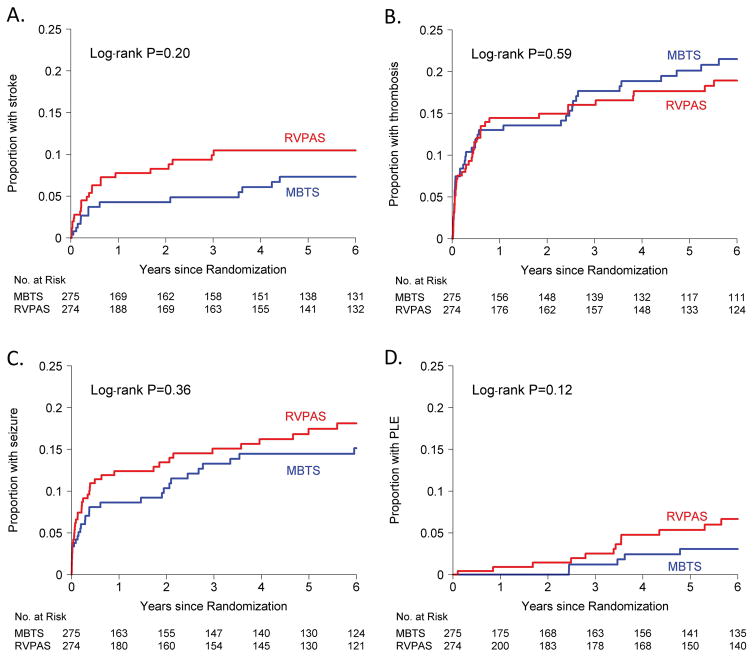
The shunt groups did not differ significantly with respect to clinical events and morbidities (Table 1, Figure 3); the overall incidence of seizures in the first 6 years was 3.4 events per 100 patient-years; stroke, 1.8 events per 100 patient-years; protein losing enteropathy, 0.82 events per 100 patient-years; and plastic bronchitis was 0.14 events per 100 patient-years. By age six years, approximately one in five patients had had a thrombotic event and approximately one in six had had seizures. NYHA class was similar for the two shunt groups at 6 years, with 71% in Class I, 21% in Class II, 4% in Class III, and 5% in Class IV in the overall cohort.
Figure 3.
Kaplan-Meier estimates of the proportion of patients with stroke (Panel A), 6-year incidence for RVPAS vs. MBTS, 10% vs. 7%; Thrombotic events (Panel B), 6-year incidence RVPAS vs. MBTS, 19% vs. 21%; Seizures (Panel C), 6-year incidence for RVPAS vs. MBTS, 18% vs. 15%; and PLE = protein losing enteropathy (Panel D), 6-year incidence for RVPAS vs. MBTS, 7% vs. 3%. RVPAS indicates right ventricle–to–pulmonary artery shunt; and MBTS, modified Blalock-Taussig shunt.
DISCUSSION
Advances in surgical and catheter techniques, as well as postoperative care, have improved the survival of patients with even the most critical forms of congenital heart disease. Among congenital heart defects, HLHS serves as an exemplar of the extraordinary pharmacologic, technical, and procedural innovations that have transformed the outlook of children with previously fatal defects. The SVR trial is the first multicenter randomized, prospective trial to compare outcomes of two congenital heart operations, highlighting the evolution of the field of congenital heart surgery from earlier landmark achievements to evidence-based surgical practice that focuses not only on survival but also on longer-term complications of life-saving therapies11.
We found that, by 6 years after assignment to an RVPAS vs. MBTS during the Norwood procedure, children with HLHS and other single RV anomalies no longer had a statistically significant difference in their transplant-free survival. Specifically, survival among children assigned to the RVPAS was better before the second stage of palliation was performed, but they had a similar hazard of death or transplant after this time. There was an interaction of shunt type with annual Norwood volume, with the highest volume and most senior surgeons having better outcomes using the MBTS. These data support the importance of tailoring the choice of shunt to the experience level of the surgeon, and also highlight the case-volume relationship with Norwood procedure outcomes. Children in the RVPAS group had a higher incidence of catheter interventions before undergoing the Fontan procedure. After the Fontan procedure, the hazard ratios were similar in the two shunt groups. Finally, right ventricular ejection fraction, clinical events and morbidities, and New York Heart Association Class were similar among survivors in the two shunt groups.
Few studies have prospectively followed children with HLHS from birth through early school years. Reports based on smaller cohorts have shown that the Fontan circulation is characterized by elevated filling pressure and low cardiac index, with a cascade of end-organ morbidities including hepatic fibrosis and cirrhosis, lymphatic disorders including protein losing enteropathy and plastic bronchitis, late renal insufficiency, electrophysiological abnormalities, and thromboembolic events.12–21 The SVR trial, which has prospectively gathered extensive data beginning before the Norwood procedure, provides a unique window into the early evolution of morbidities known to be associated with staged palliation to the Fontan procedure. The SVR trial was conceived with the hypothesis that an early survival advantage expected from the RVPAS strategy might be balanced by adverse late outcomes of ventricular dysfunction and arrhythmia.12 However, to date, we have found little sustained advantage of the initial systemic to pulmonary artery shunt on morbidities among survivors following the Fontan procedure. By 6 years, the rates of development of Fontan morbidities were similar in the two groups. However, cumulative Fontan morbidities in the combined shunt groups were already considerable: one in five children had had an event of thrombotic nature noted in routine clinical care; approximately 15% had had at least one seizure; and 7.5% had had a stroke. These data highlight that morbidity begins early in life for children with single right ventricle, and has a steady increase even in the early years after Fontan.
Our study should be considered in light of some limitations. We did not standardize the materials or techniques used for construction of the RVPAS, nor did we collect information in the dataset on the surgical techniques used to create or repair the right ventriculotomy in RVPAS patients. As is typical for congenital heart surgery, surgical techniques for the RVPAS vary among surgeons between and even within institutions, and RVPAS methods have continued to evolve since study enrollment.22–24 The most important potential long-term disadvantage of the RVPAS is diminution of right ventricular function related to the right ventriculotomy. Because of limitations in echocardiographic techniques for evaluation of right ventricular ejection fraction after the RVPAS, RVEF could not be calculated for many patients, nor could we assess regional right ventricular wall motion or impact of focal scarring and dyskinesis. As this cohort continues to be observed longitudinally, cardiac MRI is supplanting echocardiography as a primary cardiac outcome variable.
Additional limitations include that some effects of the two shunt strategies, such as effects on right ventricular function or pulmonary artery architecture, may only become manifest in later years after the Fontan procedure. The RVPAS group underwent more interventions before the Fontan procedure and, although not statistically significant, had a somewhat higher incidence of most complications. One potential reason for these findings is that the RVPAS causes more pulmonary artery distortion. If so, alterations in pulmonary artery architecture related to the RVPAS could adversely affect the long-term functional capacity of those with an initial RVPAS. Exercise capacity is currently being tested at ages 10–12 years in children who were enrolled in the SVR trial. Finally, whereas the difference in transplant-free survival of children in the RVPAS vs. MBTS treatment groups (64% vs. 59% at age 6 years) was not statistically significant, the magnitude of this difference could be clinically important.
In summary, at 6 years in the SVR trial, the difference in transplant-free survival for those assigned to the RVPAS vs. MBTS is not statistically significant. The 6-year incidence of catheter interventions is higher in patients in the RVPAS vs. MBTS group, although the increased hazard was present only prior to the Fontan procedure. Both treatment groups have accrued important morbidities. Participants in the SVR trial are undergoing continued surveillance to characterize their longer-term outcomes and risk factors for adverse events.
Supplementary Material
CLINICAL PERSPECTIVE.
What Is New?
We compared transplant-free survival and other outcomes at 6 years after the Norwood procedure with right ventricle–to–pulmonary artery shunt (RVPAS), compared with a modified Blalock-Taussig shunt (MBTS) in children enrolled in the SVR Trial.
The RVPAS group had similar transplant-free survival at 6 years but required more catheter interventions before the Fontan procedure.
Right ventricular ejection fraction, New York Heart Association class, and complications did not differ by shunt type.
Cumulative incidence of morbidities by 6 years included 20% with a thrombotic event, 15% with a seizure, and 7.5% with stroke.
What Are the Clinical Implications?
The RVPAS strategy carries a survival advantage prior to Stage II surgery, but a greater hazard of catheter interventions until the Fontan procedure is performed.
After the Fontan procedure, there is no sustained advantage of the initial systemic to pulmonary artery shunt on transplant-free survival or catheter intervention.
Morbidity begins early in life and steadily increases for children in both shunt groups.
These data emphasize the importance of continued follow-up of this cohort, and the need to find new strategies to improve the long-term outlook for those with single ventricle anomalies.
Acknowledgments
SOURCES OF FUNDING
This work was supported by grants (HL068269, HL068270, HL068279, HL068281, HL068285, HL068288, HL068290, HL068292, and HL085057) from the National Heart, Lung, and Blood Institute (NHLBI). This work is solely the responsibility of the authors and do not necessarily represent the official views of NHLBI or NIH.
The authors thank Chenwei Hu for assistance in statistical analyses.
Footnotes
DISCLOSURES
No relevant conflicts to disclose.
References
- 1.Norwood WI, Kirklin JK, Sanders SP. Hypoplastic left heart syndrome: experience with palliative surgery. Am J Cardiol. 1980;45:87–91. doi: 10.1016/0002-9149(80)90224-6. [DOI] [PubMed] [Google Scholar]
- 2.Sano S, Ishino K, Kawada M, Arai S, Kasahara S, Asai T, Masuda Z, Takeuchi M, Ohtsuki S. Right ventricle-pulmonary artery shunt in first-stage palliation of hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2003;126:504–509. doi: 10.1016/s0022-5223(02)73575-7. [DOI] [PubMed] [Google Scholar]
- 3.Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Lu M, Goldberg CS, Tabbutt S, Frommelt PC, Ghanayem NS, Laussen PC, Rhodes JF, Lewis AB, Mital S, Ravishankar C, Williams IA, Dunbar-Masterson C, Atz AM, Colan S, Minich LL, Pizarro C, Kanter KR, Jaggers J, Jacobs JP, Krawczeski CD, Pike N, McCrindle BW, Virzi L, Gaynor JW. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362:1980–1992. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newburger JW, Sleeper LA, Frommelt PC, Pearson GD, Mahle WT, Chen S, Dunbar-Masterson C, Mital S, Williams IA, Ghanayem NS, Goldberg CS, Jacobs JP, Krawczeski CD, Lewis AB, Pasquali SK, Pizarro C, Gruber PJ, Atz AM, Khaikin S, Gaynor JW, Ohye RG Pediatric Heart Network I. Transplantation-free survival and interventions at 3 years in the single ventricle reconstruction trial. Circulation. 2014;129:2013–2020. doi: 10.1161/CIRCULATIONAHA.113.006191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.www.pediatricheartnetwork.org/ForResearchers/PHNPublicUseDatasets.PHN Public Use Datasets website.
- 6.Ohye RG, Gaynor JW, Ghanayem NS, Goldberg CS, Laussen PC, Frommelt PC, Newburger JW, Pearson GD, Tabbutt S, Wernovsky G, Wruck LM, Atz AM, Colan SD, Jaggers J, McCrindle BW, Prakash A, Puchalski MD, Sleeper LA, Stylianou MP, Mahony L. Design and rationale of a randomized trial comparing the Blalock-Taussig and right ventricle-pulmonary artery shunts in the Norwood procedure. J Thorac Cardiovasc Surg. 2008;136:968–975. doi: 10.1016/j.jtcvs.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohye RG, Schonbeck JV, Eghtesady P, Laussen PC, Pizarro C, Shrader P, Frank DU, Graham EM, Hill KD, Jacobs JP, Kanter KR, Kirsh JA, Lambert LM, Lewis AB, Ravishankar C, Tweddell JS, Williams IA, Pearson GD. Cause, timing, and location of death in the Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012;144:907–914. doi: 10.1016/j.jtcvs.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atallah-Yunes NH, Kavey RE, Bove EL, Smith FC, Kveselis DA, Byrum CJ, Gaum WE. Postoperative assessment of a modified surgical approach to repair of tetralogy of Fallot. Long-term follow-up. Circulation. 1996;94:II22–II26. [PubMed] [Google Scholar]
- 9.Fine JG, RJ A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 10.Tweddell JS, Sleeper LA, Ohye RG, Williams IA, Mahony L, Pizarro C, Pemberton VL, Frommelt PC, Bradley SM, Cnota JF, Hirsch J, Kirshbom PM, Li JS, Pike N, Puchalski M, Ravishankar C, Jacobs JP, Laussen PC, McCrindle BW. Intermediate-term mortality and cardiac transplantation in infants with single-ventricle lesions: risk factors and their interaction with shunt type. J Thorac Cardiovasc Surg. 2012;144:152–159. doi: 10.1016/j.jtcvs.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Triedman JK, Newburger JW. Trends in Congenital Heart Disease: The Next Decade. Circulation. 2016;133:2716–2733. doi: 10.1161/CIRCULATIONAHA.116.023544. [DOI] [PubMed] [Google Scholar]
- 12.Opotowsky AR, Baraona FR, Mc Causland FR, Loukas B, Landzberg E, Landzberg MJ, Sabbisetti V, Waikar SS. Estimated glomerular filtration rate and urine biomarkers in patients with single-ventricle Fontan circulation. Heart. 2016;103:434–442. doi: 10.1136/heartjnl-2016-309729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rychik J. The Relentless Effects of the Fontan Paradox. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2016;19:37–43. doi: 10.1053/j.pcsu.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Rogers LS, Glatz AC, Ravishankar C, Spray TL, Nicolson SC, Rychik J, Rush CH, Gaynor JW, Goldberg DJ. 18 years of the Fontan operation at a single institution: results from 771 consecutive patients. J Am Coll Cardiol. 2012;60:1018–1025. doi: 10.1016/j.jacc.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Rychik J, Goldberg DJ. Late consequences of the Fontan operation. Circulation. 2014;130:1525–1528. doi: 10.1161/CIRCULATIONAHA.114.005341. [DOI] [PubMed] [Google Scholar]
- 16.Stephenson EA, Lu M, Berul CI, Etheridge SP, Idriss SF, Margossian R, Reed JH, Prakash A, Sleeper LA, Vetter VL, Blaufox AD Pediatric Heart Network I. Arrhythmias in a contemporary fontan cohort: prevalence and clinical associations in a multicenter cross-sectional study. J Am Coll Cardiol. 2010;56:890–896. doi: 10.1016/j.jacc.2010.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carins TA, Shi WY, Iyengar AJ, Nisbet A, Forsdick V, Zannino D, Gentles T, Radford DJ, Justo R, Celermajer DS, Bullock A, Winlaw D, Wheaton G, Grigg L, d’Udekem Y. Long-term outcomes after first-onset arrhythmia in Fontan physiology. J Thorac Cardiovasc Surg. 2016;152:1355–1363e1. doi: 10.1016/j.jtcvs.2016.07.073. [DOI] [PubMed] [Google Scholar]
- 18.Pundi KN, Pundi KN, Johnson JN, Dearani JA, Li Z, Driscoll DJ, Wackel PL, McLeod CJ, Cetta F, Cannon BC. Sudden cardiac death and late arrhythmias after the Fontan operation. Congenit Heart Dis. 2016;12:17–23. doi: 10.1111/chd.12401. [DOI] [PubMed] [Google Scholar]
- 19.Alexander ME. Pushing back the cliff (but there probably still is a cliff): Arrhythmia, late outcome, and uncertainty in Fontan patients. J Thorac Cardiovasc Surg. 2016;152:1364–1365. doi: 10.1016/j.jtcvs.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 20.Balling G. Fontan Anticoagulation: A Never-Ending Debate? J Am Coll Cardiol. 2016;68:1320–1322. doi: 10.1016/j.jacc.2016.06.050. [DOI] [PubMed] [Google Scholar]
- 21.Egbe AC, Connolly HM, McLeod CJ, Ammash NM, Niaz T, Yogeswaran V, Poterucha JT, Qureshi MY, Driscoll DJ. Thrombotic and Embolic Complications Associated With Atrial Arrhythmia After Fontan Operation: Role of Prophylactic Therapy. J Am Coll Cardiol. 2016;68:1312–1319. doi: 10.1016/j.jacc.2016.06.056. [DOI] [PubMed] [Google Scholar]
- 22.Bentham JR, Baird CW, Porras DP, Rathod RH, Marshall AC. A reinforced right-ventricle-to-pulmonary-artery conduit for the stage-1 Norwood procedure improves pulmonary artery growth. J Thorac Cardiovasc Surg. 2015;149:1502–1508e1. doi: 10.1016/j.jtcvs.2015.02.046. [DOI] [PubMed] [Google Scholar]
- 23.Baird CW, Myers PO, Borisuk M, Pigula FA, Emani SM. Ring-reinforced Sano conduit at Norwood stage I reduces proximal conduit obstruction. Ann Thorac Surg. 2015;99:171–179. doi: 10.1016/j.athoracsur.2014.08.078. [DOI] [PubMed] [Google Scholar]
- 24.Tweddell JS, Mitchell ME, Woods RK, Spray TL, Quintessenza JA. Construction of the right ventricle-to-pulmonary artery conduit in the Norwood: The “Dunk” technique. Oper Tech Thorac Cardiovasc Surg. 2012;17:81–98. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.