Abstract
Cognitive function declines substantially with age in both humans and animal models. In humans, this decline is associated with decreases in independence and quality of life. Although the methodology for analysis of cognitive function in human models is relatively well established, similar analyses in animal models have many technical issues (e.g., unintended experimenter bias, motivational issues, stress, and testing during the light phase of the light dark cycle) that limit interpretation of the results. These caveats, and others, potentially bias the interpretation of studies in rodents and prevent the application of current tests of learning and memory as part of an overall healthspan assessment in rodent models of aging. The goal of this study was to establish the methodology to assess cognitive function in aging animals that addresses many of these concerns. Here, we use a food reward-based discrimination procedure with minimal stress in C57Bl/6J male mice at 6, 21, and 27 months of age, followed by a reversal task to assess behavioral flexibility. Importantly, the procedures minimize issues related to between-experimenter confounds and are conducted during both the dark and light phases of the light dark cycle in a home-cage setting. During cognitive testing, we were able to assess multiple measures of spontaneous movement and diurnal activity in young and aged mice including, distance moved, velocity, and acceleration over a 90-h period. Both initial discrimination and reversal learning significantly decreased with age and, similar to rats and humans, not all old mice demonstrated impairments in learning with age. These results permitted classification of animals based on their cognitive status. Analysis of movement parameters indicated decreases in distance moved as well as velocity and acceleration with increasing age. Based on these data, we developed preliminary models indicating, as in humans, a close relationship exists between age-related movement parameters and cognitive ability. Our results provide a reliable method for assessing cognitive performance with minimal stress and simultaneously provide key information on movement and diurnal activity. These methods represent a novel approach to developing non-invasive healthspan measures in rodent models that allow standardization across laboratories.
Keywords: Aging, Phenotyper, Behaviour, Automated home-cage, Spatial memory
Introduction
Cognitive decline is a common complication of aging that is manifest, in part, as a decline in processing speed, inductive reasoning, and spatial learning and memory (Hedden and Gabrieli 2004). In humans, impairment of these functions diminishes healthspan and independence. More than half of the otherwise healthy population over age 60 are affected by impairments in spatial learning and memory, which increases with age and with common conditions such as type 2 diabetes, hypertension, and heart disease (Dahle et al. 2009; Okonkwo et al. 2010a, b; Qiu et al. 2005). Although the specific mechanisms of cognitive decline with age have not been established, studies in animal models play a critical role in identifying the molecular and cellular basis for these changes and ultimately developing appropriate interventions.
Murine models have been a particularly important part of translational approaches to study the general mechanisms of aging and specifically have been well suited in assessing the biological basis of interventions that modulate lifespan (Barzilai et al. 2012; Harrison et al. 2014; Zhu et al. 2015, 2016). Nevertheless, lifespan is only one component of aging and it has become apparent that a reliable assessment of healthspan will be essential in order to evaluate interventions that are effective in humans. Unfortunately, the concept of mammalian heathspan is not well defined and, in rodents, may involve highly invasive and/or terminal studies. Measures to date have included assessment of age-related changes in physical function, pathology, metabolism, inflammation, and response to various metabolic and physiological challenges (Ashpole et al. 2017; Giblin et al. 2014; Richardson et al. 2016). These measures and concepts are closely related to resilience (the ability of an organism to respond to physical challenges/stresses and return to homeostasis) and frailty (the decline in function with age) (Bergman et al. 2007; Fried et al. 2001; Schorr et al. 2018; Huffman et al. 2016; Beauchet et al. 2017; Kim et al. 2017). Unfortunately, cognitive function is not routinely addressed or incorporated into these measures and critical questions related to the interaction of “healthspan measures” with cognitive function remain unanswered. In addition, it is not clear whether interventions that are known to influence lifespan have effects on learning and memory. Part of the challenge and controversy in resolving these issues is that there is no rigorous standardization of tests of learning and memory and many of the tests that are routinely used are subject to misinterpretation, confounding, and/or unintended bias. As a result, comparing the results of cognitive tests across laboratories is particularly difficult. Finally, as a result of the interventional nature of the testing and stress associated with the commonly used cognitive assays, they are not appropriate for longitudinal studies (Gulinello et al. 2018; Arakawa and Iguchi 2018; Tanila 2017; Hanell and Marklund 2014).
Part of the difficulty with current testing protocols that are available to assess learning and memory with age is that physical, metabolic, and emotional stress are routinely introduced to motivate the behavior of the animal. Although necessary in many cases, confounding variables are present that have the potential to distort the experimental results, especially when comparing animals of different ages. For example, mild caloric restriction (e.g., 10%) is routinely used to increase motivation for a food reward but results in metabolic stress and decreases insulin levels (Forster and Lal 1999; Soultoukis and Partridge 2016; Weindruch et al. 2001). In addition, tests that require swimming or performance in novel or open field environments increase glucocorticoid levels (Forster and Lal 1999; Soultoukis and Partridge 2016; Weindruch et al. 2001). Finally, the vast majority of analyses are conducted during the light phase of the light/dark cycle when the animal is normally asleep, altering the diurnal rhythm of the animal. Disturbances in sleep have been shown to adversely affect cognitive performance in both human and animal models (Maire et al. 2018; McCoy and Strecker 2011). Thus, there are numerous caveats in analysis of age-related changes in learning and memory that potentially compromise the results of experimental studies and create difficulty in standardization of testing between laboratories. Similar issues have been raised for measures of healthspan (Mandillo et al. 2008). The advancement of our understanding of the etiology and treatment of age-related cognitive dysfunction in general and its relationship to an overall measure of healthspan requires resolution of these issues.
In human studies, the simple measure of walking speed has emerged as one of the most reliable endpoints that is closely associated with physical performance, disability (Ko et al. 2018), and cognitive decline (Suire et al. 2017; Rosso et al. 2017). Despite the importance of walking speed and related measures as a predictor of aging and age-related disease, the physiological basis for this relationship has not been established. Importantly, there are no established measures of spontaneous movement in rodent models. The absence of preclinical information on spontaneous movements and the relationship of these metrics to cognitive function has hindered our ability to develop markers that could (a) form the initial basis of a reliable healthspan measure in rodent models, (b) be used to assess the relationship to cognitive function, and (c) assess the efficacy of interventions that influence lifespan.
In this study, we propose a methodology that allows investigators to measure movement parameters and circadian activity along with cognitive function in mice in a home-cage environment with minimal stress. These tests were developed based on previous literature using observation and analyses of mouse behavior in automated home cages and adopt measures that have been validated in numerous mouse strains with or without neurological deficits (Kramvis et al. 2013; Destici et al. 2013; Remmelink et al. 2016; Remmelink et al. 2015; Dooves et al. 2016). Nevertheless, there have been no studies addressing the ability of this system to monitor deficits in cognition with age. Importantly, the approach used in this study minimizes the physical/emotional stress from investigator handling and stress-based behavioral paradigms that has been an essential part of behavioral testing of rodents. The analyses are conducted in the animal’s home cage, coordinated with the natural circadian rhythm of the animal and use behaviors and motivation that are part of the animal’s normal cognitive domain.
Methods
Animals
Male C57Bl/6J (N = 33 9–12/group) were obtained from the National Institute on Aging colony at Jackson Laboratories and housed (3–4 per cage) in the Rodent Barrier Facility (RBF) at OUHSC, which is a specific pathogen free facility. Sentinel mice were serologically tested for evidence of exposure to the following at monthly intervals: Sendai virus (SEND), pneumonia virus of mice (PVM), mouse hepatitis virus (MHV), minute virus of mice (MVM), Theiler’s murine encephalomyelitis (TMEV), reovirus (REO), mycoplasma pulmonis (MPUL), epizootic diarrhea of infant mice (EDIM), mouse parvovirus (MPV-1, MPV-2), endo-parasites, ecto-parasites, mycoplasma pulmonis (MPUL), lymphocytic choriomeningitis (LCMV), ectromelia (mousepox) (ECTRO), epizootic diarrhea of infant mice (EDIM), mouse parvovirus (MPV-1, MPV-2), polyoma virus (POLY), mouse adenovirus FL/K87 (MAV 1 & 2), murine cytomegalovirus (MCMV), Encephalitozoon cuniculi (ECUN), cilia-associated respiratory bacillus, (CARB), Helicobacter spp., endo-parasites, ecto-parasites. Animals were maintained in Allentown XJ cages with Anderson’s Enrich-o-cob bedding (Maumee, OH) on a 12-h light/12-h dark cycle at 21 °C and were given access to standard irradiated bacteria-free rodent chow (5053 Pico Lab, Purina Mills, Richmond, IN) and reverse osmosis filtered water ad libitum. All procedures were approved by, and followed the guidelines of, the Institutional Animal Care and Use Committee of OUHSC.
Only males were used in this initial analysis to avoid the potential impact of variations in estrous cycles on behavioral analyses. Two weeks prior to testing, animals were transferred to a specific pathogen-free behavioral testing room where they were housed under identical conditions to the RBF except that the animal room was maintained on a 12:12 L:D cycle (lights on at 6:00 h). Forty-eight (48) hours prior to initiation of testing, animals were singly housed and fed dustless precision rodent pellets (F05684, Bio-Serv, Flemington, NJ) for habituation purposes as well as their regular diet. Water was available ad libitum.
General methods
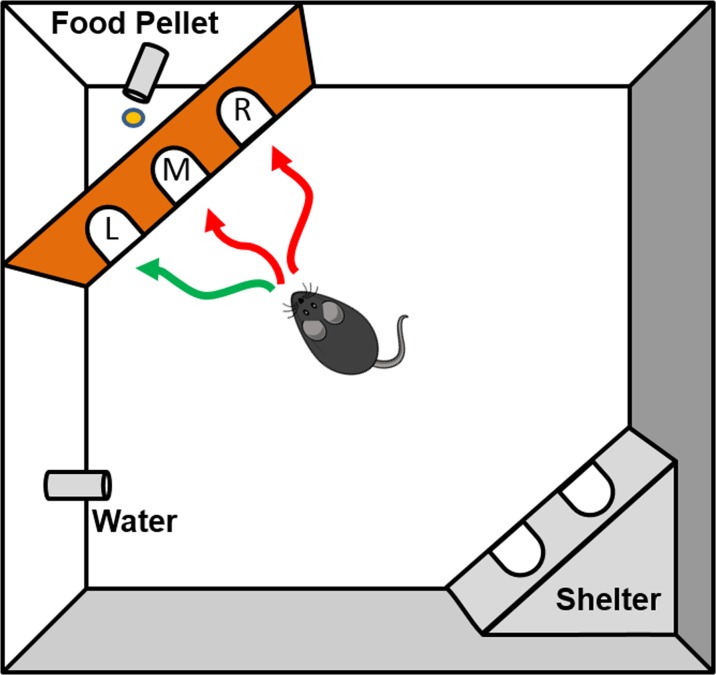
At the beginning of the study, animals were placed individually in Ethovision PhenoTypers (Model 3000, Noldus Information Technology, Netherlands) (Fig. 1) for a 6-h adaptation period. The cages were transparent plastic (L = 30 × W = 30 × H = 35 cm) and bedding was cellulose-free paper (Pure-o’Cel; The Andersons, Maumee, OH). Beginning at 1600 h on day 1, the activity of the animals was continuously recorded for 90 h. An infrared-sensitive video camera above the arena recorded all movements using the X-Y coordinates of the center of gravity of each animal. These data were sampled at a rate of 15 frames per second (fps) and smoothed using EthoVision software (EthoVision XT 11.5, Noldus Information Technology, Wageningen, The Netherlands) as previously described (Maroteaux et al. 2012; Loos et al. 2014). Information on the calculation of these measures is described in detail in previous publications (Maroteaux et al. 2012; Koopmans et al. 2017; Loos et al. 2015). Immediately prior to initiation of video tracking and activation of the task protocol, a food pellet dispenser was connected and a CognitionWall (Noldus Information Technology, Netherlands) was placed into the PhenoTyper, consisting of an insert with three entrances placed in front of the spout of a food dispenser, as described in detail in previous publications (Remmelink et al. 2016; Aarts et al. 2015). Body weights were taken at the beginning and at the end of the study. Four (4) Ethovision cages were available for analysis, requiring nine runs to test all 33 mice. Animals of each age group were randomized to each of the nine runs. The experimental protocol within the PhenoTyper cage as well as data analysis was fully automated and hence blinded to the investigator.
Fig. 1.
Depiction of the Ethovision Phenotyper used for analysis of the effects of aging on spontaneous movement and cognitive function. The cages were transparent plastic (L = 30 × W = 30 × H = 35 cm) and bedding was made of cellulose free paper. Water was available ad libitum and a shelter with nesting material was available for sleeping. The activity of the animals was continuously recorded for 90 h using EthoVision tracking software. An infrared-sensitive video camera above the arena recorded all movements and data were sampled at a rate of 15 fps and smoothed using EthoVision software. Immediately prior to initiation of tracking, a pellet dispenser and a CognitionWall were inserted into the PhenoTyper. The CognitionWall has three entrances (left (L), middle (M), and right (R)) placed in front of a food dispenser. During the initial discrimination phase of the study, the animals were required to enter the CognitionWall through the left entrance and were rewarded with a food pellet using FR5 schedule. The criteria for success were 80% and calculated based on the success rate of the trailing 30 entries. After 48 h, the correct entrance was reversed to the right entrance and the study terminated at 90 h. Data were uploaded to AHCODA-DB (mousedata.sylics.com) for analysis and independently validated using R
Movement parameters
Movement parameters were tracked and the data calculated for each of the 90 h of the study. The results were summarized for the dark and light phases of the L:D cycle. Analysis included distance traveled, maximum velocity, maximum acceleration, and the number and length of moving segments blocked at hourly intervals throughout the light and dark cycles during the experimental period. The calculation of these measures has been described (Remmelink et al. 2016; Maroteaux et al. 2012; Koopmans et al. 2017).
Cognitive assessment
For the initial discrimination phase of the study, the animal was required to pass through the left entrance of the CognitionWall to obtain a food rewards (Dustless Precision Rodent Pellets, F05684, Bio-Serv, Flemington, NJ), which were dispensed using a Fixed Ratio (FR) 5 schedule. After 48 h of discrimination learning, the task was modified and the correct response was changed to the right entry requiring the animal to extinguish the previous learning and acquire a new response, again rewarded at an FR5 schedule. Success rates for both initial discrimination and reversal learning were calculated post hoc and defined as the percentage of correct entries of the trailing 30 entries through any of the entrances of the CognitionWall. The percent of animals in each age group reaching success rates of 70, 73, 77, 80, 83, 87, and 90% correct were calculated. The following dependent variables to reach a specific success rate were calculated for both initial discrimination and reversal learning: percent of animals reaching criteria, entries to criteria, errors to criteria and time (hours) to criteria.
Statistical analyses
Ethovision export files containing X-Y coordinates and hardware input/output signals were initially uploaded to a password protected area of AHCODA-DB (mousedata.sylics.com, (Koopmans et al. 2017)), independently verified for consistency and quality according to pre-set criteria and processed to generate behavioral parameters using AHCODA software (Synaptologics BV, Amsterdam, The Netherlands). AHCODA incorporates R scripts for generating graphs and statistics, in particular for plotting survival curves of learning performance and assessement of group differences using the Gρ-weighted log-rank test for differences between two or more Kaplan–Meier survival curves. Subsequently, data were downloaded from AHCODA-DB, entered into an Excel spreadsheet, and re-validated before further analysis. To incorporate subject level correlation, a generalized linear mixed model was applied to compare the total distance moved, total time spent moving, number of moving segments, and acceleration among the different age groups. The Kruskal-Wallis test was used to evaluate the differences in time and number of entries for reaching criteria and percent of activity in the dark between different age groups. The differences in the percent of animals achieving the criteria for the initial discrimination and reversal tasks were tested by Fisher’s exact test. Pairwise comparisons were used to compare differences between initial and reversal discrimination in different age groups and were conducted as paired sign tests. All analyses were conducted using the Statistical Analysis Software (SAS) statistical package (SAS, version 9.4, Cary, NC, USA).
Results
Data from 33 mice were collected and uploaded to AHCODA-DB. All files met initial quality criteria and were included in the analyses. The initial discrimination and reversal data from one 21-month-old animal was subsequently eliminated since the animal showed a high preference for one of the entrances and no learning in the reversal. The data presented in this manuscript, as well as additional behavioral data, are available online through the public AHCODA-DB website (public.sylics.com; Logan2018).
Movement data
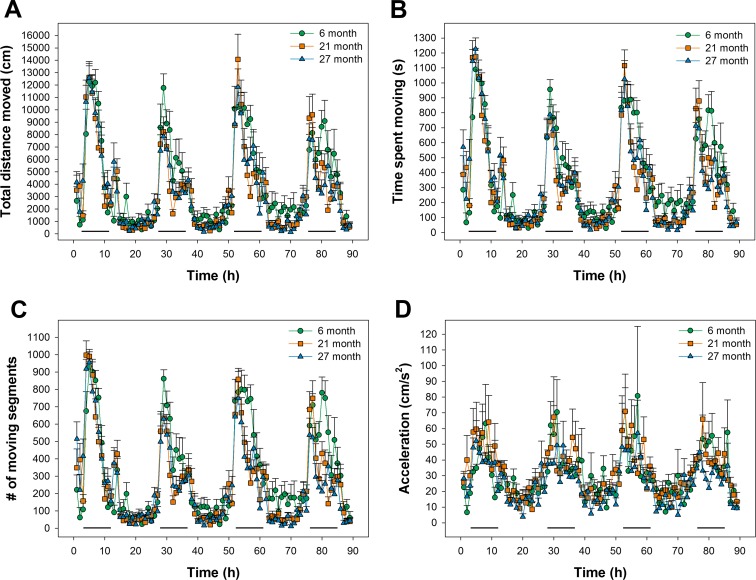
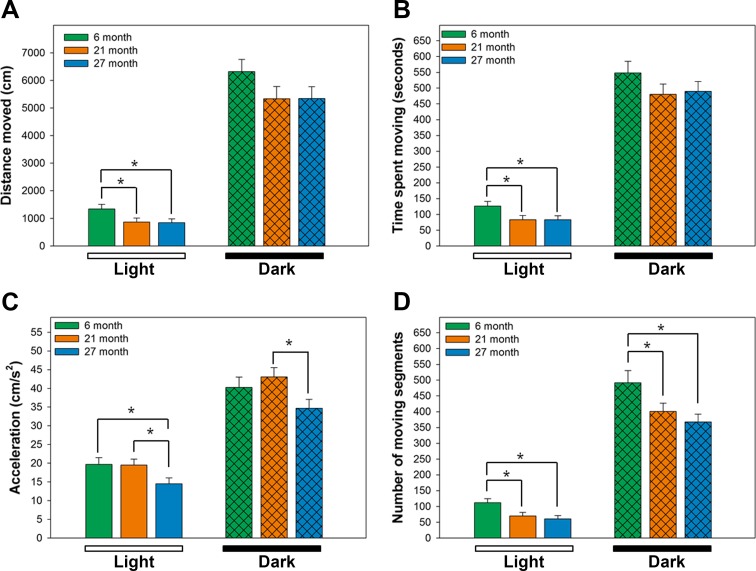
Movement parameters were continuously tracked during the study and a subset of these parameters are detailed in Fig. 2 and summarized in Fig. 3 across the light and dark periods. Clear differences were evident between the light and dark cycles for all groups (p < 0.001). Comparison of the ratio of total distance moved during the dark phase compared to activity during the dark and light phases indicated no differences in diurnal activity between the groups (85.8 ± 2.11, 88.1 ± 1.26 and 87.9 ± 1.17% for 6-, 21-, and 27-month-old animals, respectively). Total distance moved during the light and dark phases of the L:D cycle decreased with age (Fig. 3a). These differences were evident at 21 and 27 months of age compared to 6-month-old animals during the light phase of the cycle (p < 0.05) but did not reach significance during the dark phase. Similar changes were found with time spent moving and number of moving segments (Fig. 3b, c). Segment velocity measures were similar between 6- and 21-month-old animals but decreased at 27 months compared to 21 months of age (p < 0.05, data not shown). Similarly, maximum acceleration (Fig. 3d) was reduced at 27 months of age compared to either the 6- or 21-month groups (p < 0.05).
Fig. 2.
Data derived from tracking animal movements during the 90 h experimental period. Analyses included a total distance moved, b time spent moving, c number of moving segments, and d acceleration. Data are averaged over hourly intervals during both the light and dark phases of the L:D cycle. Dark horizontal lines represent the dark phase of the cycle. Data represent mean + SEM calculated for hourly intervals
Fig. 3.
Summary of spontaneous movement parameters during the light and dark phases. Each movement parameter was averaged over the light and dark phases over the entire 90 h study. a Total distance moved, b total time spent moving (seconds), c number of moving segments, and d acceleration (cm/s2). Data represent mean + SEM for 9–11 animals/age group. *p < 0.05
Cognitive function
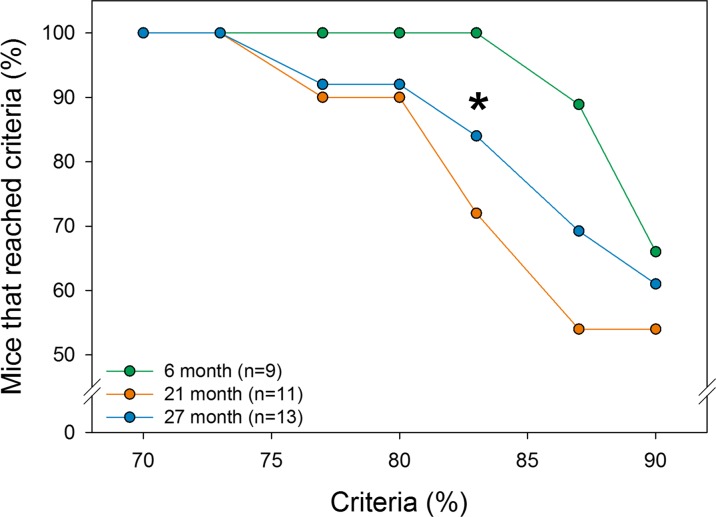
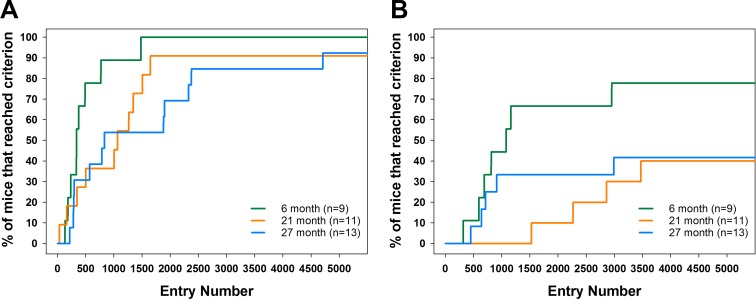
Cognitive function was evaluated using a food reward-based spatial memory task that was divided into two learning phases: (1) initial discrimination learning during the first 45-h block and (2) reversal learning during the second 45 h block. Performance during the initial discrimination learning was evaluated by calculating the number of animals of each age group that were capable of reaching a success rate of 70–90% correct entries based on the trailing 30 entries through any of the entrances of the CognitionWall. All animals were able to reach a 73% success rate (Fig. 4). However, the percentage of 21- and 27-month-old animals reaching higher success rates of 77 and 80% was modestly reduced (p = 0.27, ns). The percentage of 21- and 27-month-old animals reaching success rates above 80% was substantially decreased and was consistently less than the 6-month-old animals. The survival plot for entries required to reach the 80% success rate, a criterion used in previous publications, during the initial discrimination is shown in Fig. 5a.
Fig. 4.
Percentage of mice of each age group that reached criteria during the initial discrimination task. Success rates were calculated as the percentage of correct entries using a moving window based on the last 30 entries into the CognitionWall. Data indicate that 100% of 6-, 21-, and 27-month-old animals reach 73% correct responses but the percent of 21- and 27-month-old animals reaching higher success rates is reduced. At a success rate of 83%, differences were evident between 6 and 21 and 27-month-old animals (*p < 0.05). The 80% success rate was chosen for all subsequent comparisons, as in previous publications
Fig. 5.
a Entries required to reach 80% success rate for each age group during the initial discrimination phase of the study. Each “step” in the curve represents a mouse that reached the learning criterion at the respective number of entries. Log-rank test for differences between two or more Kaplan–Meier survival curves indicates that the cohort of 21- and 27-month-old animals requires more entries to reach the 80% success rate compared with the 6-month-old animals, whereas there was no statistically significant difference between 21- and 27-month animals. b Entries required to reach 80% success rate for each age group during the reversal phase of the study. Data indicate that the cohort of 21- and 27-month-old animals is severely impaired in this task and less than 35% of animals were successful compared to 75% for 6-month-old animals
During the reversal learning phase of the task, 78% of 6-month-old animals were able to complete the task but the success rate for 21- and 27-month-old animals was reduced to 40 and 38%, respectively, (p < 0.01 each). Of those animals that were able to successfully perform the reversal task (Fig. 6), young animals extinguished the previous learning and acquired the new entry position relatively quickly (within 1500 entries). Older animals (21- and 27-month-old animals) required additional entries to each criteria (3000 entries for old and 3500 entries for middle). Of those animals that were able to successfully perform the reversal task (Fig. 5b), young animals extinguished the previous learning and acquired the new entry position relatively quickly (70% within 1500 entries). Older animals (21- and 27-month-old animals) required additional entries to each criteria (> 3000 entries to approximately 40% of the animals in each group).
Fig. 6.
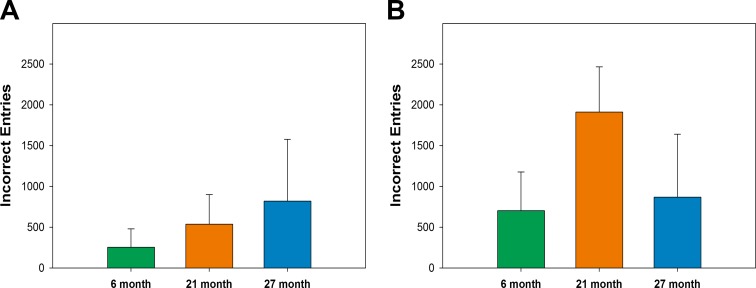
Number of errors (incorrect entries) to reach 80% during the initial discrimination (a) and the reversal (b) phases of the task. Incorrect entries tended to increase with age in the initial discrimination phase. During the reversal, the number of errors increased at 21 months and then declined at 27 months of age. Further analysis indicated that the decline in errors at 27 months of age was due to a reduction in the number of old animals that reached criteria and therefore are not included in the group (refer to Fig. 7)
The number of incorrect entries (errors) to reach criteria is shown in Fig. 6. During initial discrimination, the number of incorrect entries tended to increase with age but these differences failed to reach significance (p < 0.07). During the reversal, the 21-month-old animals that were able to complete the task exhibited an increase in number of errors compared to the 6-month-old group (p < 0.02). Although only 42% of 27-month-old animals were able to complete the reversal task, there was a tendency for a reduced number of errors in those animals. This result was potentially the consequence of excluding animals in this age group that were not able to complete the task and/or differences in the degree of learning (and ease of extinction) that occurred during the initial discrimination.
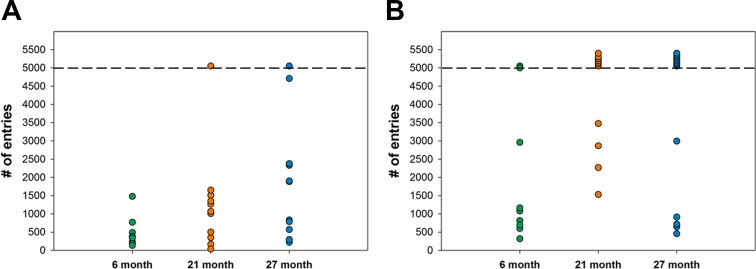
The data for the number of entries to reach 80% criteria are detailed further in Fig. 7. In this figure, the values for individual animals are detailed. At 6 months of age, the data for initial discrimination is largely homogeneous but by 21 months of age, two groups become evident and these differences are magnified by 27 months of age. The 21- and 27-month-old animals with poorer performance in the initial discrimination task were not capable of reaching criteria and therefore in the reversal task and are not shown in this figure. These data indicate that both 21- and 27-month-old cohorts are composed of a heterogeneous group of animals; those that have performance similar to young animals; and those that exhibit an impaired performance compared to young animals. To our knowledge, this is the first cognitive assay that separates mice into subgroups based on their cognitive performance.
Fig. 7.
Entries required to reach 80% success rate for each age group during the initial discrimination (a) and reversal (b) phases of the study. Each point represents the number of entries required for an individual animal to reach the 80% success rate. Note that 21- and 27-month-old animals separate into two groups—those with performance similar to young animals and those with performance that is impaired compared to young animals. All of the impaired animals identified during the initial discrimination phase of the study at 27 months of age were not able to reach the 80% criteria in the reversal phase of the study. Mice that did not reach criteria are identified in the graph with arbitrary values above 5000 entries (separated by the dashed line)
Food intake and body weight
Immediately prior to the initiation of the study, body weights in 21- and 27-month-old animals were greater than 6-month-old animals (p ≤ 0.05). Both 21- and 27-month-old animals exhibited a modest reduction in body weight compared to 6-month-old animals by the end of the study (p ≤ 0.05, Table 1). Comparison of the number of pellets consumed during the experiment indicated an increased number of food rewards in 21- and 27-month-old animals during the initial discrimination phase of the study followed by a reduction during the reversal phase of the study. The total number of pellets dispensed over the testing period was not different between groups (data not shown). Correlational analyses indicated that there were no significant associations between change in body weight and number or errors or hours to criteria during the initial discrimination or the reversal tasks.
Table 1.
Pre- and post-body weights and pellets dispensed in 6-, 21-, and 27-month-old animals
| Group | Pre-weight (g) |
Post-weight (g) |
Δ weight (%) |
Total pellets dispensed |
|---|---|---|---|---|
| 6 months | 29.6 ± 0.6* | 29.9 ± 0.6 | 0.92 ± 0.37* | 520 ± 56 |
| 21 months | 33.3 ± 0.8 | 31.7 ± 0.9 | − 4.16 ± 1.24 | 519 ± 46 |
| 27 months | 33.3 ± 1.2 | 31.6 ± 0.9 | − 4.45 ± 1.63 | 529 ± 38 |
*Significantly different compared to 21 or 27 months (p ≤ 0.05)
Relationship between movement parameters and cognitive function
To assess the possible relationship between movement parameters and their ability to predict cognitive status, we initially calculated the correlations between movement parameters to assess the relationship between individual movement parameters in the study. Although results indicated significant correlations between a number of these variables (identified in Table 2), the correlation coefficient of some pairwise comparisons indicated that several movement parameters detected differ aspects of movement.
Table 2.
Pairwise correlation matrix for movement variables (Spearman’s rank correlation coefficient: r (p value))
| Variable | Distance moved | Time moving | Average distance/moving segment | Size of moving segment | # of moving segments | Max acceleration | Segment velocity |
|---|---|---|---|---|---|---|---|
| Distance moved | 1 | 0.87 (< 0.01*) |
0.58 (< 0.01*) |
0.30 (0.09) |
0.55 (< 0.01*) |
0.26 (0.15) |
0.61 (< 0.01*) |
| Time moving | – | 1 | 0.23 (0.21) |
0.13 (0.49) |
0.71 (< 0.01*) |
0.23 (0.20) |
0.32 (0.07) |
| Average distance/moving segment | – | – | 1 | 0.79 (< 0.01*) |
− 0.20 (0.28) |
− 0.12 (0.50) |
0.65 (< 0.01*) |
| Size of moving segment | – | – | – | 1 | − 0.44 (< 0.01*) |
− 0.43 (< 0.01*) |
0.15 (0.41) |
| # of moving segments | – | – | – | – | 1 | 0.44 (< 0.01*) |
0.20 (0.27) |
| Max acceleration | – | – | – | – | – | 1 | 0.17 (0.35) |
| Segment velocity | – | – | – | – | – | – | 1 |
To probe the potential relationship between learning and movement parameters, we classified animals by their cognitive status as either “Intact” or “Impaired” based on their performance during the initial discrimination phase of the study. A threshold of 1000 entries to reach 80% criteria was used since 90% of the 6-month-old animals were successful (see Fig. 5a). All animals at or below this threshold were classified as “intact” and animals above this threshold were classified as “Impaired.” We then used univariate logistic regression analysis to assess the relationship between each movement parameter and cognitive status. The results of this analysis are expressed as the “Odds Ratio” and corresponding p values are shown in Table 3. Finally, stepwise model selection using a multivariate logistic model was used to build a model to predict cognitive function from movement parameters (Table 4). Although the model did not reach statistical significance, values for 21- and 27-month-old animals were p = 0.06 and p = 0.08, respectively.
Table 3.
Ability of individual movement parameters to predict cognitive status (univariate regression analysis)
| Variable | Odds ratios (95% CI) | p value |
|---|---|---|
| Distance moved | 1.00 (1.00–1.00) | 0.70 |
| Time moving | 1.00 (1.00–1.00) | 0.46 |
| Average distance/moving segment | 0.80 (0.58–1.09) | 0.16 |
| Size of moving segment | 0.06 (< 0.01–5.90) | 0.23 |
| # of moving segments | 1.01 (0.99–1.03) | 0.32 |
| Max acceleration | 1.08 (0.96–1.20) | 0.20 |
| Segment velocity | 0.89 (0.52–1.51) | 0.65 |
Table 4.
Ability of movement parameters to predict cognitive status (stepwise regression analysis)
| Variable | Odds ratios (95% CI) | p value |
|---|---|---|
| Group | ||
| 6 months | 1.00 | |
| 21 months | 53.75 (0.79–> 999) | 0.06 |
| 27 months | 192.55 (0.56–> 999) | 0.08 |
| Distance moved | 1.00 (1.00–1.00) | 0.70 |
| Time moving | 1.00 (1.00–1.00) | 0.96 |
| Average distance/moving segment | 0.08 (0.001–4.55) | 0.22 |
| Size of moving segment | > 999 (< 0.001–> 999) | 0.21 |
| # of moving segments | 1.05 (0.92–1.20) | 0.48 |
| Max acceleration | 1.08 (0.88–1.32) | 0.48 |
| Segment velocity | 45.77 (0.23–> 999) | 0.16 |
Discussion
In this study, we report a novel method for assessing learning and memory in murine models of aging and assess the relationship of these measures to spontaneous movement parameters. These tests alleviate many of the on-going concerns with behavioral testing and simultaneously provide new endpoints that are relevant to measures in human subjects that are associated with physical performance, disability, and cognitive decline. The analysis of spontaneous movement parameters in our animal models revealed decreases in distance moved, velocity, and acceleration with increasing age. Importantly, these analyses could be separated into the light and dark phases of the L:D cycle permitting analysis of the animal’s circadian activity. Simultaneous assessment of cognitive function, specifically spatial discrimination under an FR5 schedule of reinforcement, demonstrated significant decreases with age in both the initial discrimination and reversal components of the task. Similar to data from human and rat studies (Freeman et al. 2009; Foster et al. 2012), not all aging mice demonstrated impairments in acquisition, which allowed the animals to be classified by their cognitive status. Weight loss was minimal during the procedure, was similar across groups, and was not associated with cognitive performance. Our results indicate that analysis of age-related changes in movement and cognitive function can be conducted simultaneously without the addition of major physical or emotional stresses. The measures detailed here represent a novel and initial approach to developing a comprehensive non-invasive, healthspan assessment in rodent models that includes cognitive function and provide the opportunity to provide a standardized analysis between different laboratories.
Assessment of learning and memory is one of the most important aspects of healthspan analysis but the tests that are available have a high degree of variability between laboratories and are subject to unintended bias. It is well recognized that Object recognition and Y maze performance, commonly used as tests of age-related cognitive decline, may be confounded by sensory and motivational differences between the age groups (Gerlai 2001). In addition, these tests may not have the power to detect early age-related differences between groups. Fear conditioning and water-based tests have attempted to standardize motivational components but differences in physical, emotional, and/or metabolic stress remain unknown. The concern is that young and old animals may respond differently to these stresses and therefore the testing paradigm has the potential to confound results of cellular/molecular studies that occur subsequent to the behavioral task. In the present study, the motivation for the discrimination task was an appetitive stimulus and all animals appeared to be motivated to a consistent level as noted by their ability to perform the task during the initial discrimination phase of the study. Notably animals in the different age groups received an equal number of food pellets during the study. Although all behavioral tests introduce some element of confounding (e.g., loss of body weight, stress), the cognitive tests conducted here resulted in no significant differences in mean body weight between groups during the 90 h of testing. Nevertheless, we found that some animals exhibited a decrease in body weight that appeared to be the result of differences in the reversal phase of the study. However, the majority of animals, regardless of age, were able to reach the 80% criteria during the initial discrimination phase of the test and there was not a significant correlation between cognitive performance and weight loss. The results of these studies indicate that learning and memory can be assessed in animals of different ages with minimal disturbance of body weight.
Of particular importance in this study was the observation that 21-month-old animals began to separate into two groups with different levels of performance in the initial discrimination phase of the study. At 27 months of age, the performance was more heterogeneous and animals could clearly be separated based on their performance. Although the present study was designed as a cross-sectional analysis of performance, it will be important to develop the procedures to follow the animals with the poorer performance beginning at 21 months of age using a longitudinal approach and determine whether this was associated with lifespan. Previous studies of lifespan analysis for C57Bl/6 mice in our laboratory indicate that greater than 95% of animals survive at 21 months of age but by 27 months, this is reduced to 76%. Thus, it remains to be determined whether the cognitive tests used here could be part of a more comprehensive healthspan analysis.
In elderly humans, spontaneous walking speed is an important clinical measure closely associated with physical performance, disability, and cognitive decline (Watson et al. 2010; Mielke et al. 2013). Although walking speed is a clinically relevant measure of healthspan in humans, similar measures in preclinical rodent models have not been assessed and those tests that are available for gait analysis (e.g., Treadscan, Cat-walk analysis) assess coordination or, in some cases, maximum running speed (e.g., Treadmill analysis) rather than spontaneous activity. Other tests include balance and coordination (e.g., rotorod), exercise endurance (running during on a treadmill) and muscle function (e.g., grip strength, latency to fall from a wire or inclined plane). In addition to these relatively non-invasive measures of healthspan, several tests include more invasive measures including blood measures (e.g., insulin, glucose) as well as cardiac, kidney and immune function. Each of the latter endpoints are highly invasive and to date there is no healthspan panel in rodents that has been validated to predict disability, disease or lifespan. In addition, tests of cognitive function are an important component of overall healthspan but, as noted previously, their relationship to lifespan has not been assessed. As a result, there are no reliable measures of healthspan that can be used to assess the effectiveness of interventions that modulate lifespan. In the past several years, there have numerous interventions that have been demonstrated to increase lifespan (e.g., rapamycin (Harrison et al. 2014), metformin (Martin-Montalvo et al. 2013), senolytics (Baker et al. 2011)). Nevertheless, the absence of a comprehensive non-invasive healthspan panel for preclinical models that is reliable and reproducible impairs our ability to determine the efficacy of the interventions that module aging.
Despite the fact that none of the movement parameters alone were found to be significantly associated with cognitive status using univariate regression analysis, some measures appeared to be stronger predictors of cognitive status (e.g., average distance/moving segment, max acceleration, size of moving segment) than others. Development of additional measures of spontaneous activity or refinement of these measures may prove to be beneficial. We expected that a combination of these measures may be more effective in predicting cognitive status and stepwise regression using a logistic model approached statistical significance. Based on the number of animals and the variance of the data, our study was underpowered but it does appear that a relationship between movement parameters and cognitive function has the potential to be developed—similar to that in humans.
Although the concept of healthspan in both human subjects and animal models continues to be vague and ill defined, it is likely that none of the measures presented in this study alone are sufficient to develop a comprehensive measure that could evolve into a measure of healthspan. Nevertheless, a combination of cognitive ability, cognitive flexibility and spontaneous movement parameters would appear to be some of the most promising variables that have the potential to support the development of a healthspan index. Importantly, these measures are non-invasive, are amenable to longitudinal analysis and are similar to what has been reported in humans. While we have not assessed the level of stress in these mice, handling mice alone is sufficient to increase corticosterone levels that confounds both metabolic measurements (Ayala et al. 2006, 2010) as well as induce sleep disruptions (Longordo et al. 2011). Based on these previous studies, our methodology utilizes a relatively stress-free home-cage environment with minimal handling. Nevertheless, it will be critical to determine whether these measures can predict lifespan using longitudinal analyses, larger cohorts and multiple strains of animals. The non-invasive and automated approach presented here will likely be invaluable in developing measures to assess the efficacy of interventions that modify both healthspan and lifespan.
We have previously published related cohorts of young and aged mice in the water maze (Logan et al. 2018; VanGuilder Starkey et al. 2013; VanGuilder et al. 2011) as well as other cohorts of aging models (Ashpole et al. 2017). The data presented here represent the initial methodology for the simultaneous assessment of movement, cognitive function and circadian activity in murine models of aging that could form the foundation of a comprehensive healthspan analysis. Importantly, we have shown that we are able to distinguish between cognitively intact and impaired aged animals using the non-invasive methodology in this manuscript as we have in previously published cohorts using the water maze (VanGuilder Starkey et al. 2013; VanGuilder et al. 2011). The analyses that we have conducted to date are not comprehensive and additional measures will need to be added and validated. Importantly, whether these measures predict disease, disability and/or lifespan in normal models of aging and in response to the numerous lifespan-extending interventions requires further validation. In the present study, the number of animals used, although sufficient to detect age-related changes in individual measures, was not sufficient to detect a strong relationship between age-related changes in cognitive status and movement parameters. Nevertheless, there was a clear trend with this analysis. The approach we have initiated here controls several factors that routinely bias healthspan analyses including testing during the light phase of the L:D cycle, differences in handling of animals and differential motivation for performance of the tasks. We expect that the approach presented here may present an opportunity for reliable comparisons between laboratories and potentially evolve into a comprehensive healthspan analysis for mice.
Acknowledgements
This work was supported by the funding sources: T32AG052363; NIH R01AG038747; R01NS056218; R01AG057424 to WES.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- Aarts E, Maroteaux G, Loos M, Koopmans B, Kovačević J, Smit AB, Verhage M, Sluis Sv, Neuro-BSIK Mouse Phenomics Consortium The light spot test: measuring anxiety in mice in an automated home-cage environment. Behav Brain Res. 2015;294:123–130. doi: 10.1016/j.bbr.2015.06.011. [DOI] [PubMed] [Google Scholar]
- Arakawa H, Iguchi Y (2018) Ethological and multi-behavioral analysis of learning and memory performance in laboratory rodent models. Neurosci Res S0168-0102(17):30714–9. 10.1016/j.neures.2018.02.001 [DOI] [PubMed]
- Ashpole NM, Logan S, Yabluchanskiy A, Mitschelen MC, Yan H, Farley JA, Hodges EL, Ungvari Z, Csiszar A, Chen S, Georgescu C, Hubbard GB, Ikeno Y, Sonntag WE. IGF-1 has sexually dimorphic, pleiotropic, and time-dependent effects on healthspan, pathology, and lifespan. Geroscience. 2017;39(2):129–145. doi: 10.1007/s11357-017-9971-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes. 2006;55(2):390–397. doi: 10.2337/diabetes.55.02.06.db05-0686. [DOI] [PubMed] [Google Scholar]
- Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI, Wasserman DH, McGuinness OP, for the NIH Mouse Metabolic Phenotyping Center Consortium Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech. 2010;3(9–10):525–534. doi: 10.1242/dmm.006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61(6):1315–1322. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchet O, Launay CP, Sekhon H, Barthelemy JC, Roche F, Chabot J, Levinoff EJ, Allali G. Association of increased gait variability while dual tasking and cognitive decline: results from a prospective longitudinal cohort pilot study. Geroscience. 2017;39:439–445. doi: 10.1007/s11357-017-9992-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, Karunananthan S, Wolfson C (2007) Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A Biol Sci Med Sci 62(7):731–737 [DOI] [PMC free article] [PubMed]
- Dahle CL, Jacobs BS, Raz N. Aging, vascular risk, and cognition: blood glucose, pulse pressure, and cognitive performance in healthy adults. Psychol Aging. 2009;24(1):154–162. doi: 10.1037/a0014283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destici E, Jacobs EH, Tamanini F, Loos M, van der Horst GTJ, Oklejewicz M. Altered phase-relationship between peripheral oscillators and environmental time in Cry1 or Cry2 deficient mouse models for early and late chronotypes. PLoS One. 2013;8(12):e83602. doi: 10.1371/journal.pone.0083602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooves S, Bugiani M, Postma NL, Polder E, Land N, Horan ST, van Deijk ALF, van de Kreeke A, Jacobs G, Vuong C, Klooster J, Kamermans M, Wortel J, Loos M, Wisse LE, Scheper GC, Abbink TEM, Heine VM, van der Knaap MS. Astrocytes are central in the pathomechanisms of vanishing white matter. J Clin Invest. 2016;126(4):1512–1524. doi: 10.1172/JCI83908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster MJ, Lal H. Estimating age-related changes in psychomotor function: influence of practice and of level of caloric intake in different genotypes. Neurobiol Aging. 1999;20(2):167–176. doi: 10.1016/S0197-4580(99)00041-X. [DOI] [PubMed] [Google Scholar]
- Foster TC, Defazio RA, Bizon JL. Characterizing cognitive aging of spatial and contextual memory in animal models. Front Aging Neurosci. 2012;4:12. doi: 10.3389/fnagi.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman WM, VanGuilder HD, Bennett C, Sonntag WE. Cognitive performance and age-related changes in the hippocampal proteome. Neuroscience. 2009;159(1):183–195. doi: 10.1016/j.neuroscience.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- Gerlai R. Behavioral tests of hippocampal function: simple paradigms complex problems. Behav Brain Res. 2001;125(1–2):269–277. doi: 10.1016/S0166-4328(01)00296-0. [DOI] [PubMed] [Google Scholar]
- Giblin W, Skinner ME, Lombard DB. Sirtuins: guardians of mammalian healthspan. Trends Genet. 2014;30(7):271–286. doi: 10.1016/j.tig.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulinello M, Mitchell HA, Chang Q, Timothy O'Brien W, Zhou Z, Abel T, et al (2018) Rigor and reproducibility in rodent behavioral research. Neurobiol Learn Mem S1074-7427(18):30001–7. 10.1016/j.nlm.2018.01.001 [DOI] [PMC free article] [PubMed]
- Hanell A, Marklund N (2014) Structured evaluation of rodent behavioral tests used in drug discovery research. Front Behav Neurosci 8:252 [DOI] [PMC free article] [PubMed]
- Harrison DE, Strong R, Allison DB, Ames BN, Astle CM, Atamna H, Fernandez E, Flurkey K, Javors MA, Nadon NL, Nelson JF, Pletcher S, Simpkins JW, Smith D, Wilkinson JE, Miller RA. Acarbose, 17-alpha-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13(2):273–282. doi: 10.1111/acel.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5(2):87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Huffman DM, Schafer MJ, LeBrasseur NK. Energetic interventions for healthspan and resiliency with aging. Exp Gerontol. 2016;86:73–83. doi: 10.1016/j.exger.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Myers L, Wyckoff J, Cherry KE, Jazwinski SM. The frailty index outperforms DNA methylation age and its derivatives as an indicator of biological age. Geroscience. 2017;39(1):83–92. doi: 10.1007/s11357-017-9960-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko SU, Jerome GJ, Simonsick EM, Studenski S, Ferrucci L (2018) Differential gait patterns by falls history and knee pain status in healthy older adults: results from the Baltimore longitudinal study of aging. J Aging Phys Act:1–18 [DOI] [PMC free article] [PubMed]
- Koopmans B, Smit AB, Verhage M, Loos M. AHCODA-DB: a data repository with web-based mining tools for the analysis of automated high-content mouse phenomics data. BMC Bioinformatics. 2017;18(1):200. doi: 10.1186/s12859-017-1612-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramvis I, et al. Hyperactivity, perseveration and increased responding during attentional rule acquisition in the fragile X mouse model. Front Behav Neurosci. 2013;7:172. doi: 10.3389/fnbeh.2013.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan S, Pharaoh GA, Marlin MC, Masser DR, Matsuzaki S, Wronowski B, Yeganeh A, Parks EE, Premkumar P, Farley JA, Owen DB, Humphries KM, Kinter M, Freeman WM, Szweda LI, van Remmen H, Sonntag WE. Insulin-like growth factor receptor signaling regulates working memory, mitochondrial metabolism, and amyloid-beta uptake in astrocytes. Mol Metab. 2018;9:141–155. doi: 10.1016/j.molmet.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longordo F, Fan J, Steimer T, Kopp C, Lüthi A. Do mice habituate to “gentle handling?” A comparison of resting behavior, corticosterone levels and synaptic function in handled and undisturbed C57BL/6J mice. Sleep. 2011;34(5):679–681. doi: 10.1093/sleep/34.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos M, Koopmans B, Aarts E, Maroteaux G, van der Sluis S, Neuro-BSIK Mouse Phenomics Consortium. Verhage M, Smit AB. Sheltering behavior and locomotor activity in 11 genetically diverse common inbred mouse strains using home-cage monitoring. PLoS One. 2014;9(9):e108563. doi: 10.1371/journal.pone.0108563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos M, et al. Within-strain variation in behavior differs consistently between common inbred strains of mice. Mamm Genome. 2015;26(7–8):348–354. doi: 10.1007/s00335-015-9578-7. [DOI] [PubMed] [Google Scholar]
- Maire M, Reichert CF, Gabel V, Viola AU, Phillips C, Berthomier C, Borgwardt S, Cajochen C, Schmidt C. Human brain patterns underlying vigilant attention: impact of sleep debt, circadian phase and attentional engagement. Sci Rep. 2018;8(1):970. doi: 10.1038/s41598-017-17022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandillo S, Tucci V, Hölter SM, Meziane H, Banchaabouchi MA, Kallnik M, Lad HV, Nolan PM, Ouagazzal AM, Coghill EL, Gale K, Golini E, Jacquot S, Krezel W, Parker A, Riet F, Schneider I, Marazziti D, Auwerx J, Brown SDM, Chambon P, Rosenthal N, Tocchini-Valentini G, Wurst W. Reliability, robustness, and reproducibility in mouse behavioral phenotyping: a cross-laboratory study. Physiol Genomics. 2008;34(3):243–255. doi: 10.1152/physiolgenomics.90207.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroteaux G, Loos M, van der Sluis S, Koopmans B, Aarts E, van Gassen K, Geurts A, The NeuroBSIK Mouse Phenomics Consortium. Largaespada DA, Spruijt BM, Stiedl O, Smit AB, Verhage M. High-throughput phenotyping of avoidance learning in mice discriminates different genotypes and identifies a novel gene. Genes Brain Behav. 2012;11(7):772–784. doi: 10.1111/j.1601-183X.2012.00820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Montalvo A, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy JG, Strecker RE. The cognitive cost of sleep lost. Neurobiol Learn Mem. 2011;96(4):564–582. doi: 10.1016/j.nlm.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Roberts RO, Savica R, Cha R, Drubach DI, Christianson T, Pankratz VS, Geda YE, Machulda MM, Ivnik RJ, Knopman DS, Boeve BF, Rocca WA, Petersen RC. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci. 2013;68(8):929–937. doi: 10.1093/gerona/gls256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonkwo OC, Alosco ML, Griffith HR, Mielke MM, Shaw LM, Trojanowski JQ, Tremont G, Alzheimer's Disease Neuroimaging Initiative Cerebrospinal fluid abnormalities and rate of decline in everyday function across the dementia spectrum: normal aging, mild cognitive impairment, and Alzheimer disease. Arch Neurol. 2010;67(6):688–696. doi: 10.1001/archneurol.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonkwo OC, Cohen RA, Gunstad J, Tremont G, Alosco ML, Poppas A. Longitudinal trajectories of cognitive decline among older adults with cardiovascular disease. Cerebrovasc Dis. 2010;30(4):362–373. doi: 10.1159/000319564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4(8):487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- Remmelink E, Loos M, Koopmans B, Aarts E, van der Sluis S, Smit AB, Verhage M, Neuro-BSIK Mouse Phenomics Consortium A 1-night operant learning task without food-restriction differentiates among mouse strains in an automated home-cage environment. Behav Brain Res. 2015;283:53–60. doi: 10.1016/j.bbr.2015.01.020. [DOI] [PubMed] [Google Scholar]
- Remmelink E, Aartsma-Rus A, Smit AB, Verhage M, Loos M, van Putten M. Cognitive flexibility deficits in a mouse model for the absence of full-length dystrophin. Genes Brain Behav. 2016;15(6):558–567. doi: 10.1111/gbb.12301. [DOI] [PubMed] [Google Scholar]
- Richardson A, Fischer KE, Speakman JR, de Cabo R, Mitchell SJ, Peterson CA, Rabinovitch P, Chiao YA, Taffet G, Miller RA, Rentería RC, Bower J, Ingram DK, Ladiges WC, Ikeno Y, Sierra F, Austad SN. Measures of healthspan as indices of aging in mice—a recommendation. J Gerontol A Biol Sci Med Sci. 2016;71(4):427–430. doi: 10.1093/gerona/glv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso AL, Verghese J, Metti AL, Boudreau RM, Aizenstein HJ, Kritchevsky S, Harris T, Yaffe K, Satterfield S, Studenski S, Rosano C. Slowing gait and risk for cognitive impairment: the hippocampus as a shared neural substrate. Neurology. 2017;89(4):336–342. doi: 10.1212/WNL.0000000000004153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorr A, Carter C, Ladiges W. The potential use of physical resilience to predict healthy aging. Pathobiol Aging Age Relat Dis. 2018;8(1):1403844. doi: 10.1080/20010001.2017.1403844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soultoukis GA, Partridge L. Dietary protein, metabolism, and aging. Annu Rev Biochem. 2016;85:5–34. doi: 10.1146/annurev-biochem-060815-014422. [DOI] [PubMed] [Google Scholar]
- Suire CN, Eitan E, Shaffer NC, Tian Q, Studenski S, Mattson MP, Kapogiannis D. Walking speed decline in older adults is associated with elevated pro-BDNF in plasma extracellular vesicles. Exp Gerontol. 2017;98:209–216. doi: 10.1016/j.exger.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanila H (2017) Testing cognitive functions in rodent disease models: Present pitfalls and future perspectives. Behav Brain Res S0166-4328(17)30634–4. 10.1016/j.bbr.2017.05.040 [DOI] [PubMed]
- VanGuilder Starkey HD, Sonntag WE, Freeman WM. Increased hippocampal NgR1 signaling machinery in aged rats with deficits of spatial cognition. Eur J Neurosci. 2013;37(10):1643–1658. doi: 10.1111/ejn.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanGuilder HD, Farley JA, Yan H, van Kirk CA, Mitschelen M, Sonntag WE, Freeman WM. Hippocampal dysregulation of synaptic plasticity-associated proteins with age-related cognitive decline. Neurobiol Dis. 2011;43(1):201–212. doi: 10.1016/j.nbd.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson NL, et al. Executive function, memory, and gait speed decline in well-functioning older adults. J Gerontol A Biol Sci Med Sci. 2010;65(10):1093–1100. doi: 10.1093/gerona/glq111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, et al. Caloric restriction mimetics: metabolic interventions. J Gerontol A Biol Sci Med Sci. 2001;56 Spec No 1:20–33. doi: 10.1093/gerona/56.suppl_1.20. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O'Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ, Kirkland JL. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14(4):644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, Pirtskhalava T, Giorgadze N, Johnson KO, Giles CB, Wren JD, Niedernhofer LJ, Robbins PD, Kirkland JL. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell. 2016;15(3):428–435. doi: 10.1111/acel.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]