Abstract
Two-dimensional Ruddlesden–Popper organic–inorganic hybrid layered perovskites (2D RPs) are solution-grown semiconductors with prospective applications in next-generation optoelectronics. The heat-carrying, low-energy acoustic phonons, which are important for heat management of 2D RP-based devices, have remained unexplored. Here we report on the generation and propagation of coherent longitudinal acoustic phonons along the cross-plane direction of 2D RPs, following separate characterizations of below-bandgap refractive indices. Through experiments on single crystals of systematically varied perovskite layer thickness, we demonstrate significant reduction in both group velocity and propagation length of acoustic phonons in 2D RPs as compared to the three-dimensional methylammonium lead iodide counterpart. As borne out by a minimal coarse-grained model, these vibrational properties arise from a large acoustic impedance mismatch between the alternating layers of perovskite sheets and bulky organic cations. Our results inform on thermal transport in highly impedance-mismatched crystal sub-lattices and provide insights towards design of materials that exhibit highly anisotropic thermal dissipation properties.
Two-dimensional, organic-inorganic hybrid perovskites have sustained research interest due to attractive optoelectronic and excitonic properties. Here, Guo et al. systematically investigate coherent acoustic phonon transport versus layer thickness in these materials with strong acoustic impedance mismatch
Introduction
Hybrid organic–inorganic perovskites such as methylammonium lead halides have emerged as extraordinary light absorbing and emitting materials1,2. Improved understanding of the unique features such as extended carrier lifetime, hot-carrier protection, and dynamic disorder have been advanced by innovative theoretical and experimental approaches3–7. Two-dimensional (2D) Ruddlesden–Popper organic–inorganic hybrid perovskites (2D RPs)8–10, as alternatives to the 3D counterparts, have gained significant attention owing to improved ambient stability11, high luminescence quantum yield12 and strong excitonic effects13,14. The 2D RPs consist of sheets of corner-sharing Pb–X (X= I, Br) octahedra expanding in two dimensions, separated by interdigitating bilayers of organic cations, forming a highly hierarchical architecture. While intensive efforts have revealed their attractive properties such as enhanced optical nonlinearity15, strong electron–phonon interaction16,17, and white-light emission18, no studies so far have addressed phonon transport, a crucial aspect for proper thermal management of 2D RP-based devices. In particular, the interlocked soft organic and hard inorganic sub-lattices raise intriguing questions regarding the existence and properties of low-energy vibrational excitations, and their ability to carry heat along the cross-plane direction of these highly anisotropic materials with natural quantum-well-like electronic structures.
2D RPs additionally present features of superlattices, an attractive material class that can exhibit desirable thermal properties that differ from the bulk limits19 while still preserving the wave nature of phonons20,21. While solution-synthesized 2D RPs can be viewed as superlattices, they qualitatively differ from the engineered all-inorganic versions (e.g., Si–Ge22 and AlAs–GaAs23) due to their atomically abrupt interfaces with minimal misfit dislocations or interfacial atomic mixing, a high degree of long-range order, and, most importantly, the extreme heterogeneity of the atomic masses of the organic and inorganic sub-lattices connected in a serial fashion. Moreover, the van der Waals bonding that holds the structure of 2D RPs together closely resembles the interlayer interactions in 2D van der Waals heterostructures24 and MXenes25. Yet, unlike those analogs, 2D RPs can be grown into single crystals with tens-to-hundreds-of-micron cross-plane dimensions, offering an archetypal system for the study of intrinsic phonon transport characteristics.
Here we investigate coherent longitudinal acoustic phonons (CLAPs) in a series of 2D RPs, (CH3(CH2)3NH3)2(CH3NH3)N−1PbNI3N+1, or (BA)2(MA)N-1PbNI3N+1 (N = 1–6), which are denoted as N = 1–6 for short. Single-crystal transient reflection measurements, aided by the extracted indices of refraction, permit direct access to the cross-plane acoustic phonon group velocities with high fidelity. We find 2D RPs exhibit significantly lower group velocities compared to the 3D counterpart, methylammonium lead iodide (MAPbI3), which corresponds to N = ∞. As captured by a coarse-grained bead-spring model, the reduction in phonon velocity originates from the weak van der Waals bonding between the organic spacers. The unusually large acoustic impedance mismatch between the organic and inorganic layers, and the loose CH3(CH2)m aliphatic tails of the organic spacer layers with partial liquid-like motions lead to substantially stronger suppression of acoustic phonon propagation in 2D RPs as compared to MAPbI3. We expect our findings based on such model material class to be applicable to a broader class of systems, such as nanocrystal arrays26, biological organic–inorganic interfaces27, and self-assembled monolayers on inorganic solids28,29.
Results
Structural characterization of the 2D RPs
The synthesis of 2D RPs reported here followed the previously reported procedures10,30. Figure 1a shows the unit cell of N = 4 as a representative example of the crystal structure. The 2D RPs grow as single-crystal flakes with orthorhombic structures, and have a rectangular parallelepiped crystal habit. We designate [(MA)N−1PbNI3N+1]2− as the inorganic layer (although it contains MA+ cations that are ionically bound to the inorganic lattice), which is comprised of N connected sheets of Pb–I octahedra (Fig. 1a). Each [(BA)2]2+ layer, comprised of two van der Waals-bonded BA+ sheets, is referred to as the organic layer. The characteristic θ–2θ X-ray diffraction peaks (Fig. 1b and Supplementary Table 1) show that the single-crystal flakes have an out-of-plane direction along the b axis, and lateral dimensions coincident with the Pb–I sheets in the a–c plane. Higher-order diffraction peaks (Supplementary Fig. 1) and photoluminescence spectra (Fig. 1c) together reveal that each crystal is composed of a single phase, including the high members (N = 5 and 6) that were thought to be difficult to isolate in pure form31.
Fig. 1.
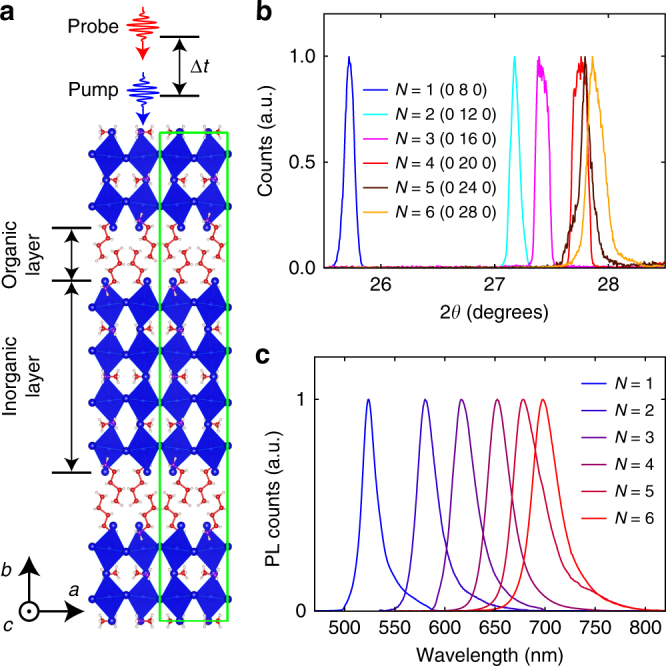
The crystal structure and phase purities of 2D RPs. a The crystal structure of N = 4 with a unit cell enclosed by the green rectangle. The directions of the pump and probe beams with respect to the crystal orientation are illustrated on the top. b Characteristic X-ray diffraction peaks of the single-crystal flakes (N = 1–6) obtained from θ–2θ scans. c Photoluminescence spectra of the 2D RPs
Transient reflection measurements on the 2D RPs
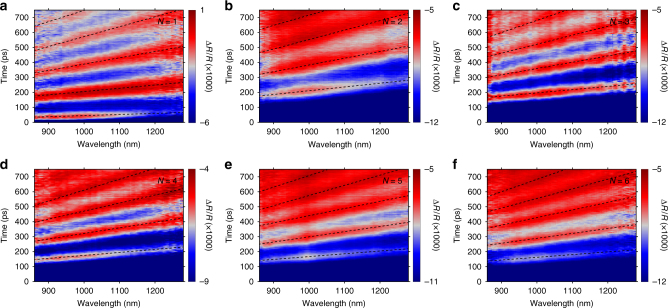
We performed transient reflection measurements using 400-nm (above-bandgap) pumping and near-infrared probing at nearly normal incidence on the a–c plane of the single-crystal flakes (Fig. 1a). The above-bandgap optical pumping excites hot electron–hole pairs, which impulsively heat up the lattice through electron–phonon coupling and launch the CLAPs32,33. The pump-induced heating of the crystal surface produces cross-plane propagating CLAPs (along the b axis) and with it a local, strain-induced refractive index modulation. This propagating lattice wave packet with a locally modulated index reflects the probe light, which interferes with the probe light reflected from the front surface of the crystal, producing time-dependent oscillations in the probe intensity. Figure 2 presents transient ΔR/R spectral maps acquired for N = 1–6 for probe wavelengths between 860 and 1280 nm. The color-coded ΔR/R is the differential change of reflection, defined as [R(t)−R0]/R0 with R0 being the reflection with pump off and R(t) with pump on (at delay time t). We found that probing in the below-bandgap range (where the penetration depth is large) is necessary for the detection of CLAPs, which propagate microns deep into the crystal. The period T of the probe oscillation is related to the CLAP velocity v through , where n′(λ) is the real part of refractive index at wavelength λ. Each period (in time) of the oscillation corresponds to a phonon propagation distance equal to half the wavelength of light in 2D RPs. As a result, the propagation distances of the CLAPs shown in Fig. 2 are on the order of a few microns.
Fig. 2.
Transient reflection measurements of the 2D RP single crystals. a–e ΔR/R spectral maps for N = 1–6, respectively, acquired with 400-nm pump excitation. The scalebar is in unit of ΔR/R (×1000). Fluences used were 0.18, 0.25, 0.25, 0.25, 0.18, and 0.21 mJ cm−2, respectively
Characterization of the refractive indices for the 2D RPs
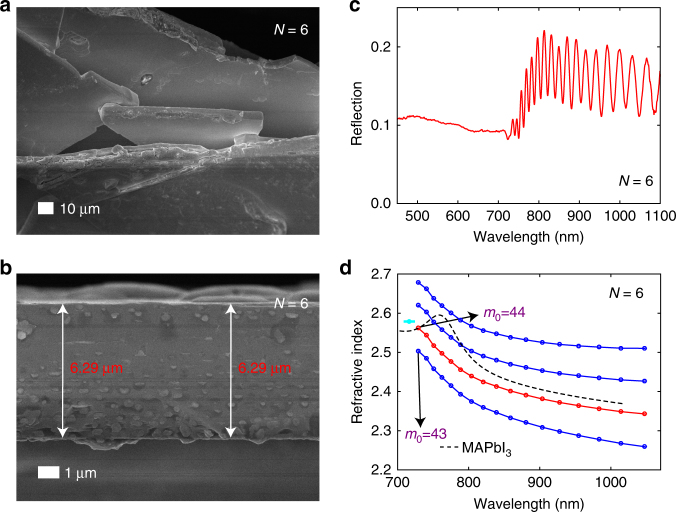
Calculation of v based on T(λ) obtained from Fig. 2 requires knowledge of n′(λ) in the below-bandgap range, which has not been reported for 2D RPs. Here we developed a two-step procedure to determine n′(λ). We first measured the below-bandgap reflection spectra of a-few-µm thick single-crystal flakes. Because near-infrared light fully penetrates the sample, interferences owing to reflection from the top (air–material) and bottom (material–air) interfaces yield a series of maxima and minima in the reflection spectrum, at the respective conditions of and , where l is the sample thickness and m is a positive integer denoted as a mode number (note that each pair of adjacent reflection minimum and maximum has a specific mode number). Figure 3a shows a scanning electron microscopy (SEM) image of an N = 6 flake with a smooth surface. A thickness for this region of 6.29 µm follows from the side-view image (Fig. 3b). The reflection spectrum for this same region (Fig. 3c) exhibits several minima, which were assigned to different mode numbers. With increasing wavelength, n′(λ) decreases monotonically below the bandgap (as dictated by the Kramers–Kronig relation) and with it the mode number . For two neighboring reflection minima, m should differ by one, and assigning a mode number to the bluest minimum (denoted as m0) fixes those for the other minima, which subsequently permits the calculation of n′(λ) over the range where the reflection minima are observed, via the equation . As shown in Fig. 3d, using different trial values for m0 we can produce several n′(λ) curves, only one of which is correct. To resolve this ambiguity, a separate measurement was performed to determine n′(λ) at the absorption edge (where reflection from the bottom interface is suppressed due to light absorption), which is only slightly bluer than the bluest reflection minimum. The detailed procedure appears in Supplementary Note 1 and Supplementary Figs. 2–11. This value of n′(λ), shown as the cyan dot in Fig. 3d, permits the identification of the correct n′(λ) curve, as shown in red. We repeated the same procedure for N = 5–1, with the corresponding results shown in Supplementary Figs. 2–7. Our accurate determination of n′(λ) leverages the strong interference effect, which is attributable to the uniform thickness of the single-crystal flakes, a direct result of the layer-by-layer growth unique to the 2D RPs (Supplementary Fig. 12).
Fig. 3.
Determination of the refractive index for N = 6. a Angular and b side-view of the examined flake taken under SEM. c Reflection spectrum and d several possible n′(λ) curves obtained using different m0 values, with the correct n′(λ) curve shown in red. The cyan dot denotes the refractive index at the absorption onset wavelength determined from a separate step
CLAP velocities of the 2D RPs
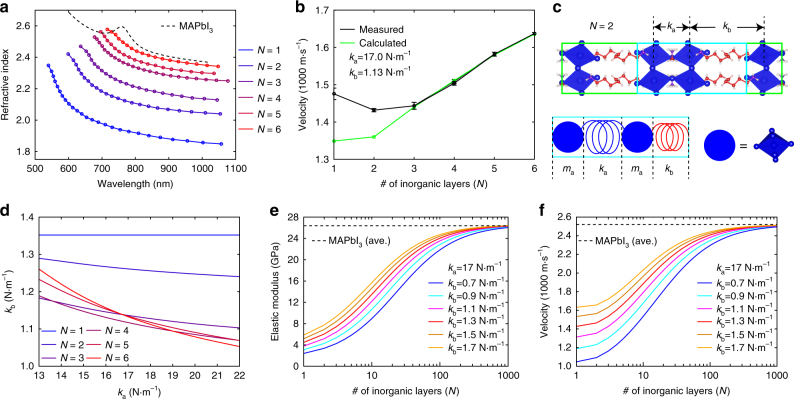
The n′(λ) curves for N = 1–6 together with the reported34 n′(λ) for MAPbI3 appear in Fig. 4a. As expected, n′(λ) decreases with increasing λ (a larger spectral separation from the bandgap, Eg). Furthermore, n′(λ) decreases with a decreasing N, which stems from a larger volumetric ratio of the organic sub-lattice that has lower dielectric constant than the inorganic counterpart. The variation of n′(λ) with Eg is qualitatively consistent with the Moss relation35, written as . With the determined n′(λ), and the CLAP oscillation period T(λ) taken as time difference between the consecutive maxima in the ΔR/R map (averaged over two periods; black dashed lines in Fig. 2), we calculated v and plot it against N in Fig. 4b. Note that from , v can be calculated using T(λ) and n′(λ) at each wavelength, and should be consistent throughout the entire probed window. Figure 4b plots the mean value and standard deviation of v calculated using the ΔR/R map from 860 to 1050 nm, where both T(λ) and n′(λ) are known. The variation in v within the probe wavelength window is as low as 0.7% (averaged for N = 1–6), which confirms the accurate determination of n′(λ). Three interesting observations arise from Fig. 4b. (i) For N=1–6, we find v to be within 1430–1640 m s−1, which is significantly lower than that for N=∞ (between 2340 m s−1 and 2990 m s−1, as discussed later). (ii) Decreasing N from 6 to 2 causes a reduction of v, with the exception of v for N=1 which falls in between that of N=2 and N=3. (iii) Varying N from 1 to 6, which lowers the weight percentage of the organic layer by six times (from 20.75% reduced to 3.89%), does not strongly impact v (which only varies up to 14.4%). The measured values of v for N=1–6 are 1476, 1432, 1444, 1495, 1582 and 1637 m s-1, respectively.
Fig. 4.
The CLAP velocities of 2D RPs. a n′(λ) curves for N = 1–6 in the below-bandgap range, and n′(λ) for N = ∞ taken from literature. b Measured CLAP velocities plotted against N (number of inorganic layers), shown in black. Calculated results from the bead-spring model is shown in green. c Schematic illustration of the coarse-grained bead-spring model. In the upper figure, the green rectangle encloses a single unit cell, half of which, highlighted by a cyan rectangle, was considered in the bead-spring model, as illustrated in the lower figure. d The pairs of the spring constants that give the calculated velocities exactly matching the experimental ones for N = 1–6. Dependence of elastic modulus along the cross-plane direction in e and velocity in f on the number of inorganic layers plotted for various kb values (with ka fixed at 17 N m−1). Result for MAPbI3 (average value of its lower and upper bounds) is shown in black dashed lines
Coarse-grained bead-spring model
The substantial reduction of v upon the periodic inclusion of organic layers in a parent MAPbI3 lattice arises from the distinctively different bond strengths and atomic masses of the two species. The acoustic impedances, defined as ρv, where ρ and v are the mass density and sound velocity of the constituents, are estimated to be 1.11 × 107 kg m−2 s−1 and 9.25 × 105 kg m−2 s−1 for the respective inorganic and organic layers. Such a large impedance mismatch can cause appreciable reduction in CLAP velocity in the composite36. Here ρ and v of liquid n-butylamine (0.74 g cm−3 and 1249.8 m s−1) were assumed for the organic layer37; note that the organic layer in a periodic arrangement can exhibit larger sound velocity than liquid phase of butylamine with random molecular orientations (as true for the smectic-A phase compared to the isotropic phase for liquid crystals38). To gain a deeper understanding, we developed a minimal coarse-grained, one-dimensional (1D) bead-spring model (schematically illustrated in Fig. 4c for the case of N = 2). Such a 1D model is sufficient to capture the essence of the long-wavelength phonon propagation along the cross-plane direction, which is the experimentally measured quantity. Details of the bead-spring model appears in Supplementary Note 2 and Supplementary Fig. 13. First-principles calculations of the phonon band structures were not attempted because of the very large unit cells of the high N members, and the local octahedral distortions and dynamic disorder in lead-halide perovskites, which often result in imaginary modes in the acoustic branches39. In the 1D bead-spring model, we represent each Pb–I octahedral sheet by a blue bead, which has the mass (ma) of one Pb–I octahedron. Two neighboring Pb–I octahedra in direct contact are connected by a blue spring with spring constant ka; two Pb–I octahedra separated by the organic layer are linked by a red spring with spring constant kb. The blue spring has a length of 6.39 Å, which is the average distance between adjacent octahedral sheets in N = 1–6 (which varies within 2.54%). The red spring has a length of 13.24 Å, which is about 7 Å longer than the blue spring, due to the inclusion of the organic layer.
To get a reasonable estimate of ka, we computed the CLAP velocity for a chain of blue beads connected by ka, which we denote as va. Because the octahedral sheets in 2D RPs derive from the (110) planes of the tetragonal MAPbI3 (plane spacing is 6.26 Å), in the sense that the perovskite layers in both cases are stacked in an eclipsed manner (Supplementary Fig. 14), va should be approximately equal to the velocity along the [110] direction of the tetragonal phase, which transforms into the [100] direction of the cubic phase upon the phase transition40. Earlier measurements on MAPbX3 single crystals yielded a velocity of 2340 m s−1 along the [100] direction of the tetragonal MAPbI341, corresponding to the [110] direction for the cubic phase. Hence, the velocity along the [100] direction of the cubic MAPbI3, and with it va, should be greater than 2340 m s−1, since cubic MAPbBr3 is stiffer along the [100] than the [110] direction42, which we assume also holds for MAPbI3. We also measured a velocity of 2990 m s−1 along the [100] direction of the cubic MAPbBr3, which is stiffer than cubic MAPbI3 due to a stronger Pb–Br bond. Therefore, va should lie between 2340 m s−1 and 2990 m s−1, which results in an estimated value for ka within the 13.0–21.0 N m−1 range (Supplementary Fig. 15).
The equations of motion, considering nearest-neighbor interactions (Supplementary Note 2), result in a set of linear equations, where solving for the low frequency limit yields v. We found that by fixing ka = 17 N m−1 (the average of its lower and upper bound), the experimental results for N = 4–6 are well reproduced with kb = 1.13 N m−1 (Fig. 4b). We note that the effect of Pb–I octahedral distortions on the acoustic phonon velocity along the cross-plane direction is implicitly incorporated into the effective spring constant (ka) that connects the beads. By fixing the value of ka, the differences in octahedral rotations in N = 1–6 are implicitly neglected, because we found41 that octahedral rotations, which occurred in MAPbI3 as temperature is varied from 300 K to 160 K, only results in a minor change of velocity within 10%. Notably, the more than one order of magnitude smaller kb compared to ka possibly arises from (i) the weak van der Waals interactions between the CH3(CH2)m tails of the BA+ cations; (ii) the weak vibrational coupling between the Pb–I octahedron and the amine groups of the BA+ cation. If we fix ka = 17 N m−1 and kb = 1.13 N m−1, the calculated v can match the measured values for N = 4–6, but progressively underestimates v as N further decreases. This can be attributed to an increasing ionic character of the inorganic layer as N decreases from 6 to 1. In particular, because N layers of perovskite, with the chemical formula [(MA)N−1PbNI3N+1]2−, always carry −2 charges to compensate the +2 charges carried by (BA)22+, each perovskite layer on average distributes −2/N charge. As a result, in lower N members, the more negatively charged inorganic layer is more strongly attracted to the positively charged BA+ via Coulomb interactions. Additional calculations were performed in which kb was adjusted to match the velocity for each N, when we swept ka within its lower and upper bounds. As shown in Fig. 4d, for a fixed value of ka within its lower and upper bounds, kb for the lower N members can be 10–30% larger than that for higher N members. For any given member, the small variation of kb (<10%) relative to the variation of ka (13–22 N m−1) stems from the order-of-magnitude difference between kb and ka; such difference dictates that the CLAP velocity is mainly determined by kb, while a relatively large variation of ka does not significantly alter the velocity.
Using the bead-spring model, we further examined the asymptotic behavior of N > 6. The lattice constants along b were extrapolated from those of N = 1–6 (Supplementary Fig. 16). We find each additional inorganic layer on average increases b by 6.203 Å, whereas the average thickness of the organic layer is 7.437 Å. We first calculated the diagonal components of the elastic modulus along the cross-plane direction, denoted as E. Fig. 4e shows the calculated E as a function of N obtained with various kb values, wherein we fix ka at 17 N m−1. The velocity, shown in Fig. 4f, was then evaluated using v = (E/ρ)1/2 where ρ is the mass density. Although v approaches the MAPbI3 limit for large N values, substantial reduction in v can be achieved even beyond N = 6 due to the soft and hard springs connected in series, whereas the optoelectronic properties (e.g., Eg and refractive index) reach the MAPbI3 limit much faster with N. Compared to v, a more dramatic reduction in E is obtained, especially for the low N members, partly due to the smaller ρ. Note that the lowest members (N = 1 and 2) have moduli about five times lower than that of MAPbI3, and are comparable to those of common organic semiconductors (in the few-GPa range), with potential utility in flexible optoelectronic devices43. On the other hand, synthesis of higher 2D RP members (N > 6) may be possible, since pure phases of oxide RP perovskites44 exist up to N = 10.
Changing the organic spacers
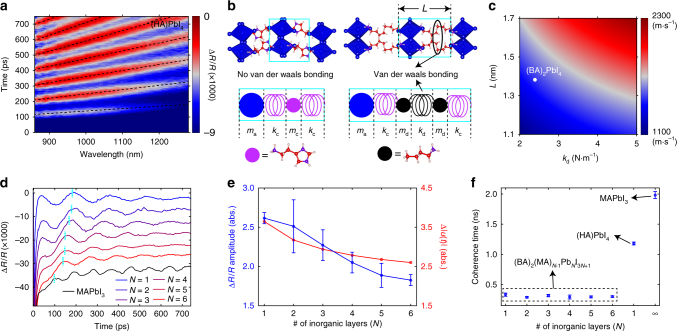
To investigate the origin of the small kb in comparison to ka, we examined another 2D hybrid perovskite, (HA)PbI4, where HA2+ (histammonium, or C3N2H4CH2CH2NH32+) is a dication that separates the individual perovskite layers45. The key distinction between (HA)PbI4 and (BA)2PbI4 (which is denoted as N = 1), is that in (HA)PbI4 two perovskite layers are connected by only one layer of HA2+. As a result, (HA)PbI4 does not have van der Waals bonding that is present in (BA)2PbI4. The transient ΔR/R map (Fig. 5a) together with the out-of-plane n′(λ) for (HA)PbI4 (Supplementary Figs. 17 and 18) obtained with a slightly different method (Supplementary Note 3), permit the determination of its cross-plane acoustic phonon velocity to be 1939 m s−1. This value, notably, is 31% higher than the velocity of N = 1 (1476 m s−1). To understand this significant difference in the velocities between (HA)PbI4 and N = 1, we built another set of coarse-grained models (Fig. 5b), wherein the organic cations are explicitly included (mc and md are the masses of HA2+ and BA+, respectively). The ionic bonding between the amine group and the Pb–I octahedron is represented by a purple spring with spring constant kc, and the van der Waals bonding between the alphatic tails of the BA+ cations is described by a black spring with spring constant kd. Using the velocity for (HA)PbI4, we calculated kc to be 10.3 N m−1, which in turn permitted the estimation of kd to be 2.4 N m−1. The significant difference between kc and kd clearly demonstrates that the slow cross-plane acoustic phonon velocities observed for N = 1 to N = 6 primarily stem from the weak van der Waals interactions between the organic spacer layers, whereas the organic–inorganic interface only plays a secondary role. Based on the model for N = 1, we further evaluated the effect of changing kd and L (the total length of the inorganic and organic layer; see Fig. 5b) on its phonon velocity (Fig. 5c). Although it is expected that increasing kd can significantly increase the velocity, we find that a faster velocity can also be achieved by using a longer organic spacer, through the reduction of the line density of the van der Waals bonds.
Fig. 5.
CLAP of (HA)PbI4, and the amplitude and coherence time of CLAPs in 2D RPs and MAPbI3. a ΔR/R spectral map for (HA)PbI4 single-crystal flake, excited with 400-nm pump at a fluence of 0.2 mJ cm−2. b Left: crystal structure of (HA)PbI4 (upper figure) and schematic of the corresponding bead-spring model (lower figure). The ionic interaction between the Pb–I octahedron (blue bead) and the HA2+ ion (purple bead) is represented by the purple spring with a spring constant of kc. Right: crystal structure of N = 1 (upper figure) and schematic of the corresponding bead-spring model (lower figure). The van der Waals interaction between the two BA+ ions (black beads) is represented by the black spring with a spring constant of kd. c Dependence of the acoustic phonon velocity of N = 1 on kd and L, with L defined in b. d ΔR/R kinetic traces at 955 nm for N = 1–6 and MAPbI3 (curves are offset for clarify). e Blue: amplitude of the sinusoidal oscillations at the delay times associated with the first ΔR/R peak (marked by cyan dotted lines in d); the plotted amplitudes are normalized by the fluence described in caption of Fig. 2. Red: calculated time-averaged mean displacement of the beads. f Coherence time of the CLAP oscillations. Error bars (i.e., standard deviations) in e and f are based on the fitting of ΔR/R kinetics at ten different wavelengths spanning the probed spectral window
Light-induced lattice dynamics
Figure 5d shows the representative ΔR/R kinetic traces at 955 nm for N = 1–6, and reveals an increasing amplitude of the sinusoidal CLAP oscillation with decreasing N. This is quantitatively illustrated in Fig. 5e, which shows the fitted amplitude of the ΔR/R oscillation at the delay time of the first ΔR/R peak (cyan dashed lines in Fig. 5d), averaged over ten probe wavelengths spanning the monitored spectral window (Supplementary Fig. 19). To gain some understanding of this feature, we calculated , the change of mean bead displacement due to energy injection into the system, using the bead-spring model (Supplementary Note 4 and Supplementary Figs. 20 and 21), which is again plotted in Fig. 5e. A similar dependence of on N is observed, which may contribute to the observed N-dependent oscillation amplitude. Pump wavelength and fluence dependent measurements on N = 2 (strongly quantum confined) and N = 4 (weakly quantum confined), as shown in Supplementary Figs. 22–24, demonstrate that in both cases, the CLAP signatures in the ΔR/R spectral maps can only be obtained via above-bandgap pumping, but not with near-bandgap pumping. Therefore, the thermoelastic effect46, as opposed to deformation potential, is mainly responsible for the CLAP signatures in the transient reflection spectral maps. Fully quantitative assessment of the ΔR/R oscillation amplitude requires knowledge of the photoelastic coefficients (i.e., strain-dependent refractive index change), which is beyond the scope of the present work. Note that the observed variation of oscillation amplitude with N does not mainly arise from a difference in the magnitude of index modulation due to a bandgap change (caused by CLAP-induced strain). Our first-principles calculation of the electronic band structure (Supplementary Fig. 25) shows a reduction in bandgap of 0.01 eV and an increase in bandgap of 0.04 eV for N = 1 and N = 3, respectively, under the application of 1% strain along the b axis. A larger bandgap modulation achieved for the higher members is opposite in trend to the observed variation of the probe oscillation amplitude with N, indicating a negligible contribution from bandgap modifications to the refractive index change. Overall, changes of the band structure are small since the strain is absorbed by the soft organic layer without significant rearrangement of the inorganic sub-lattice; the latter encompasses the optoelectronically relevant conduction and valence bands.
Discussion
The identified CLAP velocities have profound implications on the thermal conductivities (κ) of 2D RPs. In the kinetic theory, κ is approximated as , where C is the heat capacity and Λ is the phonon mean free path. The small v is expected to reduce κ of 2D RPs below that of the MAPbI3 value, which is already low (0.3–0.5 W m−1 K−1 as reported elsewhere47). Additionally, as plotted in Fig. 5f, the probe oscillations of N = 1–6 exhibit about six times shorter coherence times as compared to MAPbI3 obtained from exponential fits (Supplementary Fig. 19), signifying a substantially increased attenuation of the CLAPs. Interestingly, we found that (HA)PbI4 exhibits about four times longer coherence time than N = 1–6. This suggests that that dynamic disorders and large structural fluctuations of the organic spacer molecules at the aliphatic ends, which may undertake liquid motions due to the weak van der Waals interactions, plays an important role in impeding acoustic phonon transport. Strong anharmonicity at the organic–inorganic interfaces stemming from an asymmetric potential landscape may also contribute to phonon scattering (e.g., stronger interfacial reflection and phonon–phonon interactions)48 and suppress cross-plane phonon propagation49,50, which results in the overall short coherence times of the 2D RPs in comparison to 3D MAPbI3. Note that measurements in the below-bandgap range with negligible optical absorption (Supplementary Fig. 11) ensure that optical absorption does not contribute to the observed acoustic phonon attenuation. For N=1–6, a seemingly independence of the coherence time on N may arise from (i) larger defect densities in higher N members which may contribute to additional acoustic phonon scattering14; (ii) a stronger manifestation of the wave-like nature of CLAPs in lower N members with a higher interfacial density20; (iii) lower N member exhibit stronger bonding strength at the organic interface (Fig. 4d), which possibly results in a less pronounced dynamic disorder. More quantitative understanding may be aided by measurements of the phonon mean free paths23,51. Based on the stronger CLAP attenuation, we expect κ of 2D RPs to diminish more strongly than v itself, and heat dissipation of 2D RP-based devices presents an important design consideration. Relatedly, it was shown that superlattices present low thermal conductivities along both the cross-plane and in-plane directions52; in the latter case phonon transport is extremely sensitive to the strengths of interfacial specular and diffuse scattering53. Further exploration of acoustic phonon propagations along the in-plane directions (which have superior charge transport properties) is warranted, and can be enabled by spatially resolved pump-probe techniques54.
In conclusion, we find reduced group velocities and substantially stronger attenuation of acoustic phonons in 2D RPs as compared to MAPbI3, which arises from the large impedance mismatch between the organic and inorganic layers due to the weak van der Waals bonding between the layers. 2D perovskites without van der Waals bonding (e.g., (HA)PbI4) are expected to exhibit higher thermal conductivities for device applications, yet still maintaining strong quantum confinement effects. The demonstrated optical pump-probe technique is particularly powerful for the examination of acoustic phonons in strongly phonon absorptive (or reflective) media at reduced dimensions, where traditional ultrasonic methods38 become problematic. Through a combination of atomic layer deposition and molecular layer deposition, both of which are layer-by-layer growth techniques with digital thickness control55,56, hybrid organic–inorganic superlattices with arbitrary configurations are possible. These layered organic–inorganic hybrids feature an interesting class of materials with tunable acoustic phonon transport characteristics via chemical substitutions57,58, and controllable distortions of the inorganic sub-lattice with corrugated structures59,60.
Methods
Chemicals
All chemicals were purchased from Sigma-Aldrich and used as received. Methylammonium iodide was synthesized by neutralizing equimolar amounts of 57% w/w aqueous hydriodic acid (HI) and 40% w/w aqueous methylamine (CH3NH2). The white precipitate was harvested by evaporating the solvent using a rotary evaporator at 60 °C under reduced pressure.
Materials synthesis
The 2D RPs were synthesized following the reported literature procedure10,45, in a 20 mmol basis derived from the total Pb content. In a typical procedure, PbO powder (4464 mg, 20 mmol) was dissolved in a mixture of 57% w/w aqueous HI solution (25.0 ml, 190 mmol) and 50% aqueous H3PO2 (3.4 ml, 15.5 mmol), by heating to boiling under constant magnetic stirring for about 5 min, forming a bright yellow solution. Subsequent addition of solid CH3NH3Cl, of 20 × (N−1)/N mmol, to the hot yellow solution initially caused the precipitation of a black powder which rapidly re-dissolved under stirring to yield a clear bright yellow solution. In a separate beaker, n-CH3(CH2)3NH2, of 20-(20 × (N−1)/N) mmol, was neutralized with aqueous HI solution (57% w/w, 5 ml, 38 mmol) in an ice bath resulting in a clear, pale yellow solution. Addition of the n-CH3(CH2)3NH3I solution to the PbI2 solution initially produced a black precipitate which was subsequently dissolved on heating the combined solution to boiling. The stirring was then discontinued, and the solution was left to cool to room temperature during which time rectangular-shaped crystals started to crystallize. The precipitation was deemed to be complete after about 24 h. The crystals were isolated by suction filtration and thoroughly dried under reduced pressure. The purity of the materials was confirmed by powder diffraction, and absorption and photoluminescence spectroscopy.
Static characterization
Microscopic reflection measurements were performed with a Filmetrics F40 thin-film analyser. Transmission spectra were collected using a customized microscope system. SEM experiments were taken with a JEOL 7500 scanning electron microscope. The scalebar of our SEM tool was calibrated using a calibration standard specimen. Single-crystal θ–2θ X-ray diffraction was performed with an X-ray diffractometer (Bruker D8 Discover).
Transient reflection measurements
Femtosecond transient absorption measurements were performed using a 35 fs titanium:sapphire laser operating at 800 nm at a 2 kHz repetition rate. 400-nm pump pulses were generated via frequency-doubling of the 800-nm amplifier output using a BBO crystal. Pump pulses of other wavelengths were generated from an optical parametric amplifier. The pump pulses were reduced in repetition rate down to 1 kHz with a mechanical chopper. Near-infrared probe pulses were produced by focusing a portion of the amplifier output to a 12-mm-thick YAG crystal.
Band structure calculation
The electronic band structures were computed using density functional theory as implemented in the Quantum Espresso package61 with LDA norm-conserving pseudopotentials. A plane wave kinetic energy cut-off of 80 Ry and a k-point grid of 4 × 1 × 4 are used in the calculations. All atomic positions are relaxed to forces less than 0.001 Ry Bohr−1. The change in bandgap due to a tensile strain of 1% along the b axis is computed for N = 1 and 3.
Data availability
All relevant data used in the article are available from the authors.
Electronic supplementary material
Acknowledgements
The work was performed at the Center for Nanoscale Materials, a U.S. Department of Energy Office of Science User Facility, and supported by the U.S. Department of Energy, Office of Science, under Contract No. DE-AC02-06CH11357. This material is based upon work supported by Laboratory Directed Research and Development (LDRD) funding from Argonne National Laboratory, provided by the Director, Office of Science, of the U.S. Department of Energy under contract DE-AC02-06CH11357. Work at Northwestern University was supported by grant SC0012541 from the US Department of Energy, Office of Science (sample synthesis, processing and structural characterization).
Author contributions
P.G. and R.D.S. designed the research. P.G. performed all measurements and coarse-grained modeling. C.C.S. and L.M. synthesized the single crystals. S.S. and P.D. carried out the DFT calculations. J.B.K. contributed to the analysis of results. P.G. wrote the manuscript with input from all authors. R.D.S. supervised the project.
Competing interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-04429-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang W, Eperon GE, Snaith HJ. Metal halide perovskites for energy applications. Nat. Energy. 2016;1:16048. doi: 10.1038/nenergy.2016.48. [DOI] [Google Scholar]
- 2.Tan ZK, et al. Bright light-emitting diodes based on organometal halide perovskite. Nat. Nanotechnol. 2014;9:687–692. doi: 10.1038/nnano.2014.149. [DOI] [PubMed] [Google Scholar]
- 3.Hutter EM, et al. Direct-indirect character of the bandgap in methylammonium lead iodide perovskite. Nat. Mater. 2017;16:115–120. doi: 10.1038/nmat4765. [DOI] [PubMed] [Google Scholar]
- 4.Guo Z, et al. Long-range hot-carrier transport in hybrid perovskites visualized by ultrafast microscopy. Science. 2017;356:59–62. doi: 10.1126/science.aam7744. [DOI] [PubMed] [Google Scholar]
- 5.Yaffe O, et al. Local polar fluctuations in lead halide perovskite crystals. Phys. Rev. Lett. 2017;118:136001. doi: 10.1103/PhysRevLett.118.136001. [DOI] [PubMed] [Google Scholar]
- 6.Zhu H, et al. Screening in crystalline liquids protects energetic carriers in hybrid perovskites. Science. 2016;353:1409–1413. doi: 10.1126/science.aaf9570. [DOI] [PubMed] [Google Scholar]
- 7.Herz LM. Charge-carrier dynamics in organic-inorganic metal halide perovskites. Ann. Rev. Phys. Chem. 2016;67:65–89. doi: 10.1146/annurev-physchem-040215-112222. [DOI] [PubMed] [Google Scholar]
- 8.Kagan CR, Mitzi DB, Dimitrakopoulos CD. Organic-inorganic hybrid materials as semiconducting channels in thin-film field-effect transistors. Science. 1999;286:945–947. doi: 10.1126/science.286.5441.945. [DOI] [PubMed] [Google Scholar]
- 9.Dou L, et al. Atomically thin two-dimensional organic-inorganic hybrid perovskites. Science. 2015;349:1518–1521. doi: 10.1126/science.aac7660. [DOI] [PubMed] [Google Scholar]
- 10.Stoumpos CC, et al. Ruddlesden–Popper hybrid lead iodide perovskite 2D homologous semiconductors. Chem. Mater. 2016;28:2852–2867. doi: 10.1021/acs.chemmater.6b00847. [DOI] [Google Scholar]
- 11.Tsai H, et al. High-efficiency two-dimensional Ruddlesden–Popper perovskite solar cells. Nature. 2016;536:312–316. doi: 10.1038/nature18306. [DOI] [PubMed] [Google Scholar]
- 12.Quan LN, et al. Tailoring the energy landscape in quasi-2D halide perovskites enables efficient green-light emission. Nano Lett. 2017;17:3701–3709. doi: 10.1021/acs.nanolett.7b00976. [DOI] [PubMed] [Google Scholar]
- 13.Hong X, Ishihara T, Nurmikko AV. Dielectric confinement effect on excitons in PbI4-based layered semiconductors. Phys. Rev. B. 1992;45:6961–6964. doi: 10.1103/PhysRevB.45.6961. [DOI] [PubMed] [Google Scholar]
- 14.Blancon JC, et al. Extremely efficient internal exciton dissociation through edge states in layered 2D perovskites. Science. 2017;355:1288–1292. doi: 10.1126/science.aal4211. [DOI] [PubMed] [Google Scholar]
- 15.Saouma FO, Stoumpos CC, Wong J, Kanatzidis MG, Jang JI. Selective enhancement of optical nonlinearity in two-dimensional organic-inorganic lead iodide perovskites. Nat. Commun. 2017;8:742. doi: 10.1038/s41467-017-00788-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Straus DB, et al. Direct observation of electron–phonon coupling and slow vibrational relaxation in organic–inorganic hybrid perovskites. J. Am. Chem. Soc. 2016;138:13798–13801. doi: 10.1021/jacs.6b08175. [DOI] [PubMed] [Google Scholar]
- 17.Guo Z, Wu X, Zhu T, Zhu X, Huang L. Electron–phonon scattering in atomically thin 2D perovskites. ACS Nano. 2016;10:9992–9998. doi: 10.1021/acsnano.6b04265. [DOI] [PubMed] [Google Scholar]
- 18.Hu T, et al. Mechanism for broadband white-light emission from two-dimensional (110) hybrid perovskites. J. Phys. Chem. Lett. 2016;7:2258–2263. doi: 10.1021/acs.jpclett.6b00793. [DOI] [PubMed] [Google Scholar]
- 19.Chiritescu C, et al. Ultralow thermal conductivity in disordered, layered WSe2 crystals. Science. 2007;315:351–353. doi: 10.1126/science.1136494. [DOI] [PubMed] [Google Scholar]
- 20.Ravichandran J, et al. Crossover from incoherent to coherent phonon scattering in epitaxial oxide superlattices. Nat. Mater. 2014;13:168–172. doi: 10.1038/nmat3826. [DOI] [PubMed] [Google Scholar]
- 21.Simkin MV, Mahan GD. Minimum thermal conductivity of superlattices. Phys. Rev. Lett. 2000;84:927–930. doi: 10.1103/PhysRevLett.84.927. [DOI] [PubMed] [Google Scholar]
- 22.Lee SM, Cahill DG, Venkatasubramanian R. Thermal conductivity of Si–Ge superlattices. Appl. Phys. Lett. 1997;70:2957–2959. doi: 10.1063/1.118755. [DOI] [Google Scholar]
- 23.Luckyanova MN, et al. Coherent phonon heat conduction in superlattices. Science. 2012;338:936–939. doi: 10.1126/science.1225549. [DOI] [PubMed] [Google Scholar]
- 24.Geim AK, Grigorieva IV. Van der Waals heterostructures. Nature. 2013;499:419–425. doi: 10.1038/nature12385. [DOI] [PubMed] [Google Scholar]
- 25.Naguib M, Mochalin VN, Barsoum MW, Gogotsi Y. 25th anniversary article: MXenes: a new family of two-dimensional materials. Adv. Mater. 2014;26:992–1005. doi: 10.1002/adma.201304138. [DOI] [PubMed] [Google Scholar]
- 26.Ong WL, Rupich SM, Talapin DV, McGaughey AJH, Malen JA. Surface chemistry mediates thermal transport in three-dimensional nanocrystal arrays. Nat. Mater. 2013;12:410. doi: 10.1038/nmat3596. [DOI] [PubMed] [Google Scholar]
- 27.Loynachan CN, et al. Targeted magnetic nanoparticles for remote magnetothermal disruption of amyloid-β aggregates. Adv. Healthc. Mater. 2015;4:2100–2109. doi: 10.1002/adhm.201500487. [DOI] [PubMed] [Google Scholar]
- 28.Love JC, Estroff LA, Kriebel JK, Nuzzo RG, Whitesides GM. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005;105:1103–1170. doi: 10.1021/cr0300789. [DOI] [PubMed] [Google Scholar]
- 29.Losego MD, Grady ME, Sottos NR, Cahill DG, Braun PV. Effects of chemical bonding on heat transport across interfaces. Nat. Mater. 2012;11:502–506. doi: 10.1038/nmat3303. [DOI] [PubMed] [Google Scholar]
- 30.Stoumpos CC, et al. High members of the 2D Ruddlesden-Popper halide perovskites: synthesis, optical properties, and solar cells of (CH3(CH2)3NH3)2(CH3NH3)4Pb5I16. Chemistry. 2017;2:427–440. doi: 10.1016/j.chempr.2017.02.004. [DOI] [Google Scholar]
- 31.Papavassiliou GC. Three- and low-dimensional inorganic semiconductors. Prog. Solid State Chem. 1997;25:125–270. doi: 10.1016/S0079-6786(97)80886-2. [DOI] [Google Scholar]
- 32.Thomsen C, Grahn HT, Maris HJ, Tauc J. Surface generation and detection of phonons by picosecond light pulses. Phys. Rev. B. 1986;34:4129–4138. doi: 10.1103/PhysRevB.34.4129. [DOI] [PubMed] [Google Scholar]
- 33.Mante PA, Stoumpos CC, Kanatzidis MG, Yartsev A. Electron–acoustic phonon coupling in single crystal CH3NH3PbI3 perovskites revealed by coherent acoustic phonons. Nat. Commun. 2017;8:14398. doi: 10.1038/ncomms14398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leguy AMA, et al. Experimental and theoretical optical properties of methylammonium lead halide perovskites. Nanoscale. 2016;8:6317–6327. doi: 10.1039/C5NR05435D. [DOI] [PubMed] [Google Scholar]
- 35.Moss TS. Relations between the refractive index and energy gap of semiconductors. Phys. Status Solidi B. 1985;131:415–427. doi: 10.1002/pssb.2221310202. [DOI] [Google Scholar]
- 36.Tamura S, Hurley DC, Wolfe JP. Acoustic-phonon propagation in superlattices. Phys. Rev. B. 1988;38:1427–1449. doi: 10.1103/PhysRevB.38.1427. [DOI] [PubMed] [Google Scholar]
- 37.Góralski P, Wasiak M, Bald A. Heat capacities, speeds of sound, and isothermal compressibilities of some n-Amines and tri-n-amines at 298.15 K. J. Chem. Eng. Data. 2002;47:83–86. doi: 10.1021/je010206v. [DOI] [Google Scholar]
- 38.Miyano K, Ketterson JB. Ultrasonic study of liquid crystals. Phys. Rev. A. 1975;12:615–635. doi: 10.1103/PhysRevA.12.615. [DOI] [Google Scholar]
- 39.Yang J, et al. Acoustic-optical phonon up-conversion and hot-phonon bottleneck in lead-halide perovskites. Nat. Commun. 2017;8:14120. doi: 10.1038/ncomms14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitfield PS, et al. Structures, phase transitions and tricritical behavior of the hybrid perovskite methyl ammonium lead iodide. Sci. Rep. 2016;6:35685. doi: 10.1038/srep35685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo P, et al. Polar fluctuations in metal halide perovskites uncovered by acoustic phonon anomalies. ACS Energy Lett. 2017;2:2463–2469. doi: 10.1021/acsenergylett.7b00790. [DOI] [Google Scholar]
- 42.Létoublon A, et al. Elastic constants, optical phonons, and molecular relaxations in the high temperature plastic phase of the CH3NH3PbBr3 hybrid perovskite. J. Phys. Chem. Lett. 2016;7:3776–3784. doi: 10.1021/acs.jpclett.6b01709. [DOI] [PubMed] [Google Scholar]
- 43.Tahk D, Lee HH, Khang DY. Elastic moduli of organic electronic materials by the buckling method. Macromolecules. 2009;42:7079–7083. doi: 10.1021/ma900137k. [DOI] [Google Scholar]
- 44.Lee CH, et al. Effect of reduced dimensionality on the optical band gap of SrTiO3. Appl. Phys. Lett. 2013;102:122901. doi: 10.1063/1.4798241. [DOI] [Google Scholar]
- 45.Mao L, et al. Role of organic counterion in lead- and tin-based two-dimensional semiconducting iodide perovskites and application in planar solar cells. Chem. Mater. 2016;28:7781–7792. doi: 10.1021/acs.chemmater.6b03054. [DOI] [Google Scholar]
- 46.Ruello P, Gusev VE. Physical mechanisms of coherent acoustic phonons generation by ultrafast laser action. Ultrasonics. 2015;56:21–35. doi: 10.1016/j.ultras.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Pisoni A, et al. Ultra-low thermal conductivity in organic–inorganic hybrid perovskite CH3NH3PbI3. J. Phys. Chem. Lett. 2014;5:2488–2492. doi: 10.1021/jz5012109. [DOI] [PubMed] [Google Scholar]
- 48.Maldovan M. Phonon wave interference and thermal bandgap materials. Nat. Mater. 2015;14:667–674. doi: 10.1038/nmat4308. [DOI] [PubMed] [Google Scholar]
- 49.Li D, McGaughey AJH. Phonon dynamics at surfaces and interfaces and its implications in energy transport in nanostructured materials—an opinion paper. Nanoscale Microsc. Thermophys. Eng. 2015;19:166–182. doi: 10.1080/15567265.2015.1035199. [DOI] [Google Scholar]
- 50.Ong WL, et al. Orientational order controls crystalline and amorphous thermal transport in superatomic crystals. Nat. Mater. 2017;16:83–88. doi: 10.1038/nmat4739. [DOI] [PubMed] [Google Scholar]
- 51.Elbaz GA, et al. Phonon speed, not scattering, differentiates thermal transport in lead halide perovskites. Nano Lett. 2017;17:5734–5739. doi: 10.1021/acs.nanolett.7b02696. [DOI] [PubMed] [Google Scholar]
- 52.Yao T. Thermal properties of AlAs/GaAs superlattices. Appl. Phys. Lett. 1987;51:1798–1800. doi: 10.1063/1.98526. [DOI] [Google Scholar]
- 53.Chen G. Size and interface effects on thermal conductivity of superlattices and periodic thin-film structures. J. Heat Transf. 1997;119:220–229. doi: 10.1115/1.2824212. [DOI] [Google Scholar]
- 54.Lomonosov AM, et al. Exceptional elastic anisotropy of hybrid organic–inorganic perovskite CH3NH3PbBr3 measured by laser ultrasonic technique. Phys. Status Solidi RRL. 2016;10:606–612. doi: 10.1002/pssr.201600156. [DOI] [Google Scholar]
- 55.Van Bui H, Grillo F, van Ommen JR. Atomic and molecular layer deposition: off the beaten track. Chem. Commun. 2017;53:45–71. doi: 10.1039/C7CC02835K. [DOI] [PubMed] [Google Scholar]
- 56.Meng X. An overview of molecular layer deposition for organic and organic-inorganic hybrid materials: mechanisms, growth characteristics, and promising applications. J. Mater. Chem. A. 2017;5:18326–18378. doi: 10.1039/C7TA04449F. [DOI] [Google Scholar]
- 57.Peng W, et al. Ultralow self-doping in two-dimensional hybrid perovskite single crystals. Nano Lett. 2017;17:4759–4767. doi: 10.1021/acs.nanolett.7b01475. [DOI] [PubMed] [Google Scholar]
- 58.Li W, et al. Chemically diverse and multifunctional hybrid organic–inorganic perovskites. Nat. Rev. Mater. 2017;2:16099. doi: 10.1038/natrevmats.2016.99. [DOI] [Google Scholar]
- 59.Mao L, Wu Y, Stoumpos CC, Wasielewski MR, Kanatzidis MG. White-light emission and structural distortion in new corrugated two-dimensional lead bromide perovskites. J. Am. Chem. Soc. 2017;139:5210–5215. doi: 10.1021/jacs.7b01312. [DOI] [PubMed] [Google Scholar]
- 60.Saidaminov MI, Mohammed OF, Bakr OM. Low-dimensional-networked metal halide perovskites: the next big thing. ACS Energy Lett. 2017;2:889–896. doi: 10.1021/acsenergylett.6b00705. [DOI] [Google Scholar]
- 61.Giannozzi P, et al. QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter. 2009;21:395502. doi: 10.1088/0953-8984/21/39/395502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data used in the article are available from the authors.