Abstract
Life expectancy continues to extend, although frailty caused by loss of skeletal muscle mass continues unimpeded. Muscle atrophy caused by withdrawal of motor nerves is a feature of old age, as it is in amyotrophic lateral sclerosis (ALS) in which skeletal muscle denervation results from motoneuron death. In ALS, direct links have been established between motoneuron death and altered nucleocytoplasmic transport, so we ask whether similar defects accompany motoneuron death in normal ageing. We used immunohistochemistry on mouse tissues to explore potential links between neuromuscular junction (NMJ) degeneration, motoneuron death and nucleocytoplasmic transport regulatory proteins. Old age brought neuromuscular degeneration, motoneuron loss and reductions in immunodetectable levels of key nucleocytoplasmic transport proteins in lumbar motoneurons. We then asked whether exercise inhibited these changes and found that active elderly mice experienced less motoneuron death, improved neuromuscular junction morphology and retention of key nucleocytoplasmic transport proteins in lumbar motoneurons. Our results suggest that emergent defects in nucleocytoplasmic transport may contribute to motoneuron death and age-related loss of skeletal muscle mass, and that these defects may be reduced by exercise.
Keywords: Ageing, Sarcopenia, Motoneuron death, Nucleocytoplasmic transport
Introduction
Age-related loss of skeletal muscle mass is a primary feature of sarcopenia, driving morbidity and mortality amongst the elderly, and it is fast becoming a growing health concern in ageing western populations where a demographic shift is seeing increasing numbers of older persons living longer. Muscle mass can be lost progressively in old age by either loss of whole fibres resulting in a decline in fibre number, or by a reduction in fibre cross-sectional area (atrophy). The triggers for these two processes and the cellular mechanisms by which they are enacted may be separate, and this report focuses on increasing our understanding of the cellular events that could lead to muscle fibre atrophy.
Muscle fibre area is a function of the amount of contractile protein retained by the fibre. A change in the amount of contractile protein is triggered by change in usage, with hypertrophy resulting from an increase in usage, and atrophy arising from disuse. Disuse atrophy can arise from changes in activity level, often as a result of lifestyle changes or limb casting, but can also be a consequence of neuromuscular diseases. Disuse atrophy arising from denervation is widely thought to be a major driver of age-related loss of muscle mass (Gillon and Sheard 2015; Rowan et al. 2012). Several studies have described the morphological hallmarks and characteristics of elderly motor nerve terminals, and complete withdrawal of the motor nerve terminal is a feature (Chai et al. 2011; Deschenes 2011; Pannérec et al. 2016). Muscle fibres that lose all connection with a motor nerve terminal remain silent and, unless reinnervated, will experience profound long-term atrophy (Viguie et al. 1997).
Loss of connection between motoneuron and muscle fibre at the neuromuscular junction could be triggered by processes arising in the muscle fibre, which might ultimately result in the death of the motoneuron, or they could be a consequence of other changes affecting the viability of the motoneuron and denervation could be a consequence of those events. Temporal correlation between neuromuscular degeneration and motoneuron death might imply a causal relationship between the two, but the literature on the issue is equivocal. Whilst the existence of age-related neuromuscular deterioration is not in doubt, the existence and magnitude of age-related motoneuron death is less well established, with reports from experiments using old laboratory rodents ranging from 0 to 27% motoneuron loss (Caccia et al. 1979; Chai et al. 2011; Rowan et al. 2012; Valdez et al. 2010).
In addition to its potential role in age-related weakness, neuronal death is a feature of several well-characterised neurodegenerative conditions including Alzheimer’s disease, frontotemporal dementia, Parkinson’s disease and amyotrophic lateral sclerosis (ALS). Each of these diseases has recently been associated with dysregulation of nucleocytoplasmic transport (Freibaum et al. 2015; Jovicic et al. 2015; Zhang et al. 2015). As ALS and sarcopenia share features such as motoneuron loss, muscle denervation and atrophy, and progressive weakness, we wished to investigate whether any age-related loss of neurons features cellular defects in the important regulators of nucleocytoplasmic transport similar to those recently described in the pathogenesis of ALS.
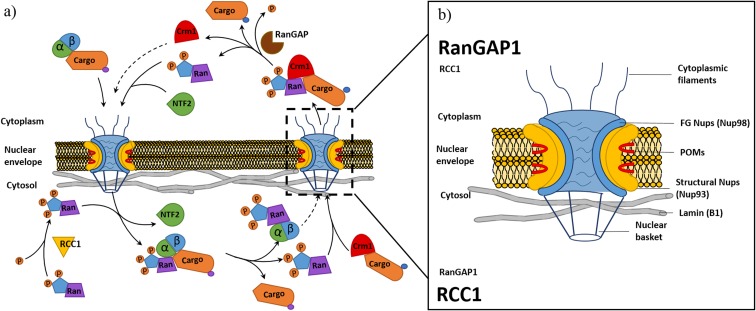
The nuclear envelope (NE) separates gene transcription from protein production (Woulfe 2007), thereby establishing the need for a controlled transport process between cytoplasm and nucleoplasm (nucleocytoplasmic transport) (Fig. 1) (Galy et al. 2003). The NE maintains a selective barrier, with its structural integrity being maintained partly by the presence of the intermediate filament protein Lamin (A/B and C), whilst the selectivity of the transit channel is regulated by the proteins of the nuclear pore complex (NPC). The NPC is a very large multi-protein complex formed of nucleoporins (Nups) arranged into symmetrical rings that traverse the nuclear envelope to form pores (~1000 Å in diameter), which allows for regulated bidirectional transport (Bui et al. 2013; Lin et al. 2016). The Nups that form the NPC are grouped into sub-complexes based on their function: pore membrane proteins (POMs), structural Nups, phenylalanine and glycine repeat (FG) Nups, cytoplasmic filament Nups and nuclear basket Nups (Adams and Wente 2013; Terry and Wente 2009; Vollmer and Antonin 2014) (Fig. 1).
Fig. 1.
Nucleocytoplasmic transport and nuclear pore complex structure: Schematic drawing of classical nuclear import and export processes involved in nucleocytoplasmic transport (a). Nuclear import involves importin-β binding cargo with a nuclear localisation tag (NLS) through the adapter protein importin-α before facilitating import though interactions with nucleoporins (Nups) within the nuclear pore complex (NPC). Upon entry to the nucleus, the Ran GTPase (Ran) bound with guanine triphosphate (RanGTP) binds to the importin complex initiating dissociation of the cargo and return of the import complex to the cytoplasm. The export process involves exportin, chromosome region maintenance 1 (Crm1) binding cargo tagged with a nuclear exiting signal (NES). Crm1 bound to a cargo forms a complex with RanGTP present within the nucleus before export through interaction with Nups within the NPC. Once in the cytoplasm, the cargo is released through hydrolysis of RanGTP to RanGDP by the Ran GTPase activating protein (RanGAP). To maintain the RanGTP/GDP gradient (b), RanGDP is relocated back to the nucleus by nuclear transport factor 2 (NTF2). Once in the nucleus, Ran undergoes a conformational change in response to the regulator of chromosome condensation 1 (RCC1) within the nucleus, stimulating the re-synthesis of RanGTP. The schematic (b) shows the structural arrangement of NPC and nuclear envelope (Lamin), identifying the subcomplexes of the NPC including cytoplasmic filaments, FG Nups, structural Nups, POMs and nuclear basket Nups
The FG Nups are of particular interest, since they feature N terminal phenylalanine and glycine repeats (FG repeats) with hydrophobic tails that form a sieve-like meshwork preventing the unrestricted passage of molecules larger than 40 kDa. A centralised FG Nup, Nup98, has been shown to function as a docking protein for cytosol-mediated import of substrates (D’Angelo and Hetzer 2008; Radu et al. 1995). Dysregulation of the FG Nups may alter the permeability of the NE and disrupt the highly regulated import and export process (Wu et al. 2001). The FG Nups are anchored to the pore complex by a series of structural proteins that span the double-lipid bilayer stabilising the membrane leaflets (structural Nups-Nup93 subcomplex) (D’Angelo et al. 2009; Lin et al. 2016). Structural Nups differ from the other Nups in having a very slow rate of turnover, and many are now thought of as members of a relatively small group of extremely long lived proteins (ELLPs; D’Angelo et al. 2009; Toyama et al. 2013). Structural Nups are believed to remain stable during interphase and disassemble only during metaphase phase of dividing cells; once installed in the NE, they appear to persist for the life of the cell (D’Angelo et al. 2009). This therefore makes these proteins susceptible to accumulation of age-related damage (D’Angelo et al. 2009), potentially contributing to loss of structural integrity and permeability of the NPC especially in postmitotic cells such as neurons.
Integrity of structure and function of the NPC is vital for normal cell functioning and viability. Loss of NPC function is now strongly implicated in the cell loss that is a feature of neurodegenerative disease, but whether the process contributes to cell loss during normal ageing is largely unexplored (Jovicic et al. 2015; Lusk and King 2017; Zhang et al. 2015). Therefore, this study aimed to investigate whether selected nucleocytoplasmic transport proteins are lost from motoneurons during normal ageing, and whether any such event was correlated with either loss of motoneurons or change in structural integrity of the NMJ. Finally, the well-described degenerative changes in elderly muscles and at the elderly NMJ are reported to be reduced in severity or rate of progression by exercise (Valdez et al. 2010; van Praag 2009), so our final aim was to ask whether exercise prevents or reduces the severity of any changes in selected nucleocytoplasmic transport proteins in aged motoneurons.
In this study, we investigated the effect of age and exercise on motoneuron number, levels of key nucleocytoplasmic proteins and neuromuscular junction morphology, with the aim of understanding any changes in relation to sarcopenia.
Material and methods
We sought to determine whether there was any correlation between age-related loss of motoneurons or degenerative change at the neuromuscular junction with change in important regulators of nucleocytoplasmic transport, and to determine whether late-life exercise impacted on change in any age-related parameter. We examined lumbar lateral motor column (LMC) motoneuron number as a function of age by counting motoneurons from Toluidine Blue-stained transverse sections of mouse spinal cord. From the same cohort of animals, we used immunohistochemistry (IHC) on transverse sections of spinal cord to investigate changes in selected nucleocytoplasmic transport proteins, and on sections of skeletal muscle to examine degenerative changes at the NMJ.
Animals
Data were derived from five young (6 months), five elderly (22 months) and five elderly exercised (24 months), female C57Bl/6j mice. All measured parameters in the current study are comparable across the 22–24-month age range in our animals. The C57Bl/6j strain was chosen as it shows several age-related musculoskeletal changes (losses in muscle mass, force-generating capacity and neuromuscular junction integrity) that are similar to those of humans in both degree and relative age of onset (Ballak et al. 2014). The mean life span of the C57Bl/6j strain is 26.7 months (Ballak et al. 2014); young and elderly mice in this study were approximately equivalent to 13 and 76 human years, respectively. Animals were kept under a 12-h light/dark regimen with environment enrichment toys, standard mouse chow and water available ad libitum. Prior consent for all experiments was obtained from the University of Otago Animal Ethics Committee.
Exercised animals
Elderly exercised animals (exercised) were given access to a monitored running wheel for a period of 4 months, starting at 20 months of age. Animals were individually housed in large smooth-walled and topped cages that prevented climbing, allowing any influence of exercise to be associated with running only. Distance covered, time spent running and average running speeds were collected for each animal over the exercise period.
Euthanasia and tissue extraction
Animals were deeply anaesthetised by intraperitoneal injection of sodium pentobarbital and transcardially perfused with heparinized phosphate-buffered saline (PBS), followed by perfusion with 1% paraformaldehyde in warmed phosphate buffer via a peristaltic minipump. Following perfusion, the spinal cord and soleus muscles were carefully removed, cleaned and placed in Tissue-Tek® OCT embedding compound (Sakura, Alphen aan den Rijn, the Netherlands), pre-chilled to 4 °C to optimise snap freezing by partial immersion in L-isopentane chilled to − 170 °C with liquid nitrogen. Specimens were stored at − 80 °C until required for subsequent examination.
Tissue processing
A Leica CM1850 cryostat (Leica, Wetzlar, Germany) was used to cut frozen tissue sections. All sections were mounted on APES (Sigma-Aldrich no. A3648) coated microscope slides and left to dry at room temperature for 30 min.
Muscle
20-μm longitudinal sections of the whole solei were cut and mounted allowing identification and localisation of the motor endplate region within the muscle belly. The use of longitudinal sections allows visualisation of junctions enface throughout the whole thickness of the muscle, whilst whole-mount preparations only allow visualisation of surface junctions.
Spinal cord
16-μm transverse sections of the lumbar enlargement were cut and sections were left to dry at room temperature for 30 min. Serial sections were picked up on numbered microscope slides so they could be subsequently assigned for histological staining for motoneuron counts or for immunohistochemistry using one of five primary antibodies. Every sixth section was allocated to motoneuron counts with the five sections between being allocated to one of the five primary antibodies used to identify nucleocytoplasmic proteins.
Motor cortex
16-μm sections of mouse brains were made in the coronal plane and sections were left to dry at room temperature for 30 min and stored in − 80 °C freezer until use.
Immunohistochemistry
Immunohistochemical protocols were conducted on both transverse sections of brain and spinal cord and longitudinal sections of muscle tissue. All slides were rinsed in Tris-buffered saline (1× TBS) for 30 min prior to processing.
Neuromuscular junction morphology
Slides holding muscle sections were pre-washed in 0.5% sodium dodecyl sulfate (SDS) before washing in glycine (0.1 M phosphate buffer) for 20 min, followed by incubation with anti-synaptophysin primary antibody (SYN-rabbit polyclonal at 1:500, Abcam®, Cambridge, UK, no. AB32594) overnight at 4 °C in the dark. Slides were then washed in several changes of TBS and incubated with the appropriate AlexaFluor488 conjugated anti-species secondary antibody (1:500 dilution; Life Technologies, Carlsbad, CA) in an incubation solution also containing AlexaFluor594-conjugated α-bungarotoxin (α-BTX) (1:500 dilution; Life Technologies, Carlsbad, CA) for 3 h at room temperature. Slides were washed with TBS and coverslipped with glycerol mounting medium.
Spinal cord proteins
Nuclear structural proteins
Slides were incubated in primary antibodies to detect LaminB1 (rabbit polyclonal at 1:500 dilution, Abcam®, Cambridge, UK, AB16048), nucleoporin 93 (Nup93) (rabbit polyclonal at 1:100, Santa Cruz, sc-292099) or nucleoporin 98 (Nup98) (rabbit polyclonal at 1:100, Cell Signalling Technology, C39A3), at room temperature for 4 h.
Nuclear transport proteins
Slides were incubated in primary antibodies to detect Ran GTPase-activating protein 1 (RanGAP1) (rabbit polyclonal at 1:100 dilution; Thermo Fisher, PA1–31482), or Regulator of Chromosome Condensation 1 (RCC1) (rabbit polyclonal at 1:100, Novus-biological, NBP1–85638) across serial transverse sections on separate slides. Slides stained with RCC1 primary antibody underwent a heat-induced epitope retrieval step in citrate-buffered saline (Shi et al. 2001) prior to primary antibody incubation.
All spinal cord sections were washed in several changes of TBS and subsequently incubated with the appropriate AlexaFluor488-conjugated anti-species secondary antibody (1:500 dilution; Life Technologies, Carlsbad, CA) in the dark at 4 °C overnight. Slides were washed with TBS and coverslipped with glycerol mounting medium.
Motor cortex proteins
Identification of the motor cortex region of the brain and the motoneurons within it was made possible by the use of chicken ovalbumin upstream promotor transcription factor-interacting protein 2 (CTIP2) immunohistochemical staining (1:50 dilution; Abcam®, Cambridge UK, AB18465). CTIP2 is a zinc finger transcription factor that has been shown to label the nuclei of cortical spinal projection neurons in layers 5A and 5B of the mature mouse cortex (Tantirigama et al. 2016), allowing us to identify these neurons and measure the levels of Nup98 and Lamin present at the nuclear envelope. Slides stained with CTIP2 primary antibody underwent a heat-induced epitope retrieval step in citrate-buffered saline (Shi et al. 2001) prior to primary antibody incubation at 4 °C overnight, and a secondary incubation for 3 h at room temperature.
Histology
Slides with transverse sections of spinal cord were incubated in Toluidine Blue solution (0.25 g in 250 mL distilled water; Sigma-Aldrich, T3260) for 2 min. then washed in distilled water for 2 min, dehydrated in graded ethanols, cleared in xylene and coverslipped in DPX.
Digital photography and image processing
Neuromuscular junction morphology
The neuromuscular endplate was identified in each muscle, and the pre- and post-synaptic apparatuses were photographed to allow the analysis of three parameters: post-synaptic fragmentation, where the post-synaptic apparatus was imaged and the number of stained receptor fragments was counted (Pannérec et al. 2016); synaptic occupancy (overlap between synaptophysin/AChR as a percentage of AChR area); and innervation status (number of innervated post-synaptic endplates as a percentage of total number of endplates identified). Each post-synaptic apparatus was imaged with its pre-synaptic partner (if present) allowing for a measure of both synaptic occupancy and innervation status.
Spinal cord motoneuron counts and measurements
Toluidine Blue-stained sections were scanned using a Leica imperial scanner (Leica, Wetzlar, Germany) at ×40 magnification. Scanned digital images were used to identify the alpha-motoneurons based on size and location criteria. Counted motoneurons had an average orthogonal size of ≥ 20 μm (Woolley et al. 1999) and were located anterior to the central canal and within the lateral margin of the ventral horn. Due to the size of motoneurons and section thickness, split cell double-counting errors could occur, so a split cell correction factor (Abercrombie 1946) was applied. Cell size data were also recorded for analysis.
Nuclear structural and transport proteins
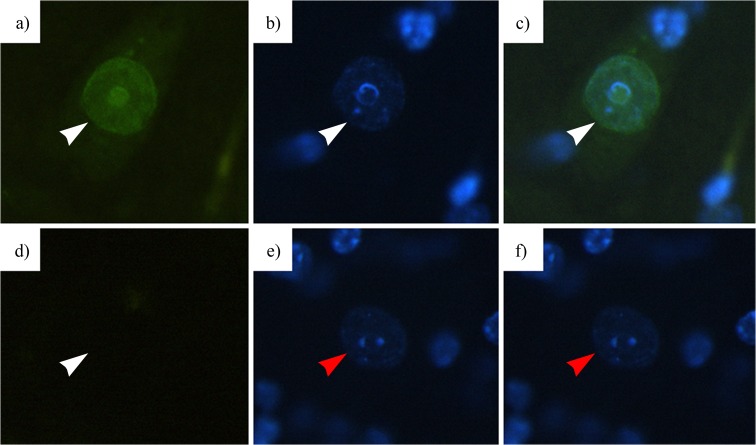
Immunoprocessed spinal cord sections were illuminated using a CoolLED fluorescence illuminator on an Olympus BX-50 (Olympus® Corporation, Tokyo, Japan) compound wide-field fluorescence microscope and digitally photographed with a Spot-RT slider (SPOT Imaging Solutions: Diagnostic Instruments Inc., Sterling Heights, MI) cooled digital microscope camera. Illumination and exposure conditions were carefully controlled to eliminate specimen-to-specimen variation in these parameters and to ensure that variation in grey level values between specimens was not due to variability in imaging conditions. Image region of interest (nucleus) greyscale data was acquired using FoveaPro 4.0 (Reindeer Graphics, Ashville, NC) and ImageJ (National Institutes of Health, Bethesda, MD). Co-staining with 4′,6-diamidino-2-phenylindole (DAPI) was used to verify the presence of motoneuron nuclei during the imaging of each fluorescently labelled protein, whilst negative controls ensured the absence of staining due to non-specific binding of fluorescent probes (Fig. 2).
Fig. 2.
4′,6-Diamidino-2-phenylindole (DAPI) was used to mark nuclei within sections of the spinal cord (white arrow) (b) and was co-localised with nucleocytoplasmic staining (white arrow) (a–c) to verify if the staining measured was present within the envelope of a motoneuron nucleus. Negative control tissues that were incubated without primary antibody were also run and imaged (d, e) to verify that no staining (white arrow) (d) occurred in the presence of DAPI-positive nuclei (red arrow) (e, f), showing that staining was not the result of non-specific secondary antibody binding (scale 20 μm)
We have recently validated the accuracy and reliability of our immunohistochemical measures of relative protein levels on tissue sections (Sheard 2015); these methods are an extension and adaptation of previously validated principles (Matkowskyj et al. 2003). Briefly, we dissolve known amounts of purified protein of interest at a variety of concentrations in agarose gel. We then freeze, section and immunohistochemically process the sections of protein gel on the slides along with the tissue sections. When processed and imaged, the known-concentration protein gel provides an accurate reference enabling reliable relative quantification of protein as detected on identically processed tissue sections.
Statistical analyses
All data was organised and analysed in Microsoft Excel (Microsoft Corporation, Redmond, WA), Prism 7 (GraphPad Software Inc., La Jolla, CA) and R statistical data analysis packages (Davies 2016). Significance was p ≤ 0.05 for all analyses.
Neuromuscular junction morphology
The age and exercise effects on three measured parameters at the neuromuscular junction were compared using an unpaired t test.
Spinal cord motoneuron counts, structural and transport protein levels
Statistical comparison of motoneuron counts between young, elderly and exercised mice were conducted using an unpaired t test. The comparison of structural and transport proteins between young, elderly and exercised mice involved measurement of 50 motoneuron nuclei per animal and five animals per group. The statistical comparison was conducted using a linear mixed model in order to analyse correlation values from multiple mice without violating the assumption of independence (Davies 2016).
Results
Running data
Exercised elderly animals ran between 216 and 810 total kilometres at an average of 4.26 km ± 1.74 per day. These distances are similar to running distances previously recorded in the literature for elderly animals (Lightfoot et al. 2004; McMahon et al. 2014).
The neuromuscular response to age and exercise
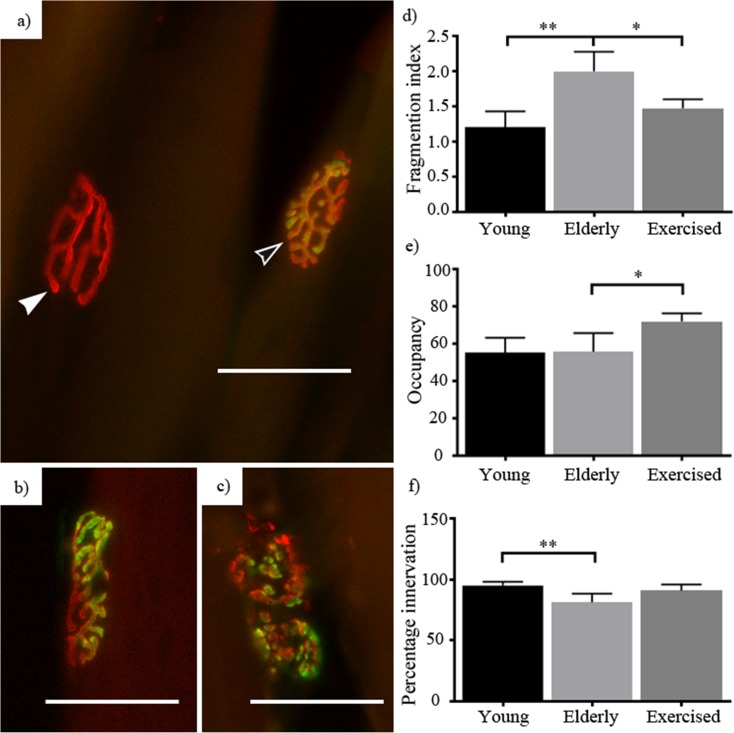
We documented commonly used indices of neuromuscular degradation in our animals (Pannérec et al. 2016; Valdez et al. 2010) (Fig. 3), finding that NMJ fragmentation increased with age (1.2–2 on fragmentation index, p = 0.001) and was reduced with exercise (2–1.5, p = 0.01) (Fig. 3a). Investigation of endplate occupancy revealed no change with age but saw a significant exercise stimulated increase (55–72% occupancy, p = 0.02) (Fig. 3b). Age resulted in a significant reduction in the proportion of innervated fibres present (from 95 to 81%, p = 0.0042), and exercise did not improve that parameter (81–90%) (Fig. 2c). These findings are similar to those reported elsewhere (Deschenes et al. 2013; Pannérec et al. 2016; Valdez et al. 2010).
Fig. 3.
Peripheral nerve response to age and exercise: young, elderly and exercised neuromuscular junctions were examined for changes associated with age and exercise, on an index of post-synaptic fragmentation (a–c), a percentage of pre-synaptic occupancy of the post-synaptic apparatus and what percentage of the total post-synaptic junctions were innervated. Ageing resulted in both an increase in the index of fragmentation (d) and a decrease in the proportion of innervated junctions (f), without change in the occupancy of innervated junctions (e). Exercised animals showed a significant decline in fragmentation compared to sedentary elderly, without increase in the percentage of innervated junctions compared to sedentary elderly. Exercised animals showed a significant increase in the occupancy of innervated junctions (b). Images d–g show the varying states of the pre- (green) and post-synaptic (red) apparatuses. Including denervated (a—solid white arrow), healthy (a—hollow white arrow), partially innervated (b) and fragmented (c). Data expressed as mean ± SEM *p < 0.05. Young n = 5, elderly n = 5 and exercised n = 4 (scale 50 μm)
Spinal cord response to age and exercise
Alpha-motoneuron counts
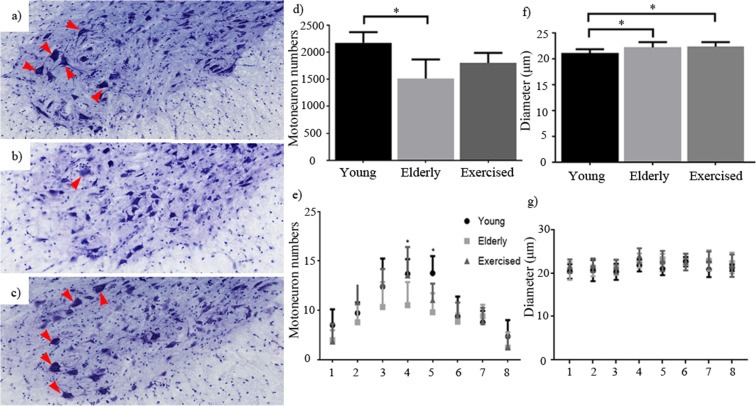
Although previous investigations have looked to document changes in motoneuron number with age, the outcomes have been variable so we looked first to accurately quantify young (Fig. 4c), elderly (Fig. 4d) and exercised (Fig. 4e) motoneuron numbers in the lumbar region of the spinal cord in C57/B6 mice in our colony. On average, young mice had 2172 lumbar alpha-motoneurons (cf. previously published values 2152–3200 (Lance-Jones 1982; McHanwell and Biscoe 1981)). Average counts from elderly (22 month) sedentary animals decreased to approximately 1513, a decline of approximately 30% (p = 0.0159) (Fig. 4a). Although motoneuron number in old exercised animals was higher (mean 1800) than the sedentary elderly group, the difference between the two old groups failed to reach statistical significance. When we looked at motoneuron numbers as a function of position along the rostro-caudal extent of the lumbar LMC (Fig. 4b), we established that motoneurons were not lost evenly along the entire length of the lumbar enlargement. Although the regions (1–8) depicted in Fig. 3 cannot be definitively associated with a known landmark within the lumbar enlargement (L1–L5), use of the motorpool locations within the lateral column and previous literature allow us to indicate the effected region. Using this technique, we see that the greatest losses were confined to the middle third of the lumbar enlargement (Fig. 4b (4 and 5)), where motor pools to the major muscles of the lower leg are located (McHanwell and Biscoe 1981). Interestingly, exercise appeared to attenuate (Fig. 4b (4)) the cell losses at specific locations in the spinal cord, suggesting that some motor pools benefitted from exercise whilst others did not.
Fig. 4.
Motoneuron number and distribution: total lumbar lateral column and alpha motoneuron counts from young (a), elderly (b) and elderly exercised (c) animals (red arrows mark motoneurons counted in each section). Sedentary ageing resulted in significant motoneuron loss, whilst exercise had no significant difference in total motoneuron number compared to both young and elderly animals (d). e Presentation of motoneuron number in a rostral to caudal (1–8, L1 to L5, respectively) position along the lumbar enlargement shows the distribution of each group of animals. Significant declines are evident in elderly (22 months) animals compared to young (6 months) at both positions 4 and 5, with exercised (24 months) animals also showing significantly higher numbers of motoneurons at position 4 compared to elderly animals. f Average motoneuron diameters show that aged animals have slightly larger motoneurons than young; analysis of this distribution across the length of the cord (1–8, L1 to L5, respectively), however, shows no region specific differences between groups (g). Data expressed as mean ± SEM *p < 0.05. Young n = 5, elderly n = 5 and exercised n = 5 (scale 200 μm)
Motoneuron cell somas were measured, and the results indicate that cell bodies continue to increase in size throughout life (Fig. 4f). Comparison of cell size along the length of the LMC (Fig. 4g) revealed no region-specific differences between young, elderly and elderly exercised animals, suggesting that the loss of motoneurons was not specific to any particular size category and therefore is unlikely to represent the specific loss of motoneurons projecting to slow (small-diameter cells) or fast (large-diameter cells) fibre types.
Nuclear membrane and transport proteins
Structural nuclear proteins
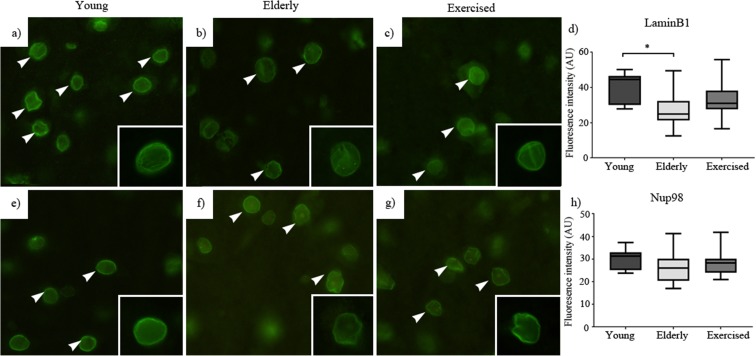
We looked at the relative immunodetectable levels of proteins involved in maintenance of the nuclear envelope (LaminB1 (Fig. 5d)) and in the regulation of the NPC (Nup98 (Fig. 5h) and Nup93 (Fig. 5l)) in young, elderly sedentary and elderly exercised mice. We found that a significant age associated decline in the level of LaminB1 protein in the nucleus (Fig. 5a, b) and that the decline was attenuated by late life exercise (Fig. 5c). Examination of relative levels of the nucleoporins Nup98 and Nup93 showed similar patterns to that of LaminB1, namely a significant age-related decline in immunodetected protein (Fig. 5f, j) that was attenuated by exercise (Fig. 5g, k). It is important to note that the declines in mean population levels of these nucleoporin proteins potentially mask a significant possible outcome, namely that the protein loss was dramatic in a subset of motoneurons whilst other motoneurons retained normal protein levels. This prospect is indicated by the spread of the datasets. Should this event be coupled to motoneuron loss, it is reasonable to think of it being prominent in only that relatively small subset of neurons that had initiated a pathological sequence of events at the time of investigation.
Fig. 5.
Structural and nuclear pore complex proteins: young (a), elderly (b) and exercised (c) animal immunostaining for structural protein LaminB1. Young (e), elderly (f) and exercised (g) animal immunostaining for nuclear pore complex protein, nucleoporin 93. Young (i), elderly (j) and exercised (k) animal immunostaining for nuclear pore complex protein, nucleoporin 98. For each protein and group, ×40 magnification images are in the inset. Sedentary ageing elicited significant decline in immunostaining of LaminB1 (d), nucleoporin 93 (h) and nucleoporin 98 (l) compared to both young and exercised animals. Data expressed as mean ± SEM *p < 0.05. Young n = 5, elderly n = 5 and exercised n = 5; 50 nuclei per animal (scale 20 μm)
Transport proteins
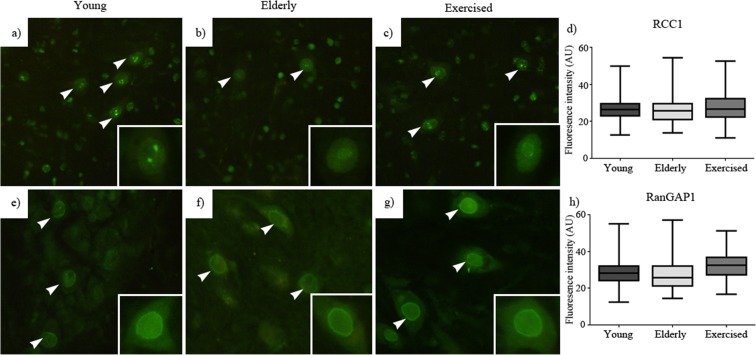
We investigated two proteins with essential roles in the maintenance of the RanGTP/GDP gradient driving nucleocytoplasmic transport, RCC1 and RanGAP1. By contrast with the assays of levels of nucleoporins and LaminB1, neither relative levels nor subcellular locations of these proteins were influenced by age or exercise (Fig. 6f, g).
Fig. 6.
Nuclear pore transport proteins: young (a), elderly (b) and exercised (c) animal immunostaining for nuclear transport protein, regulator of chromatin condensation-1 (RCC1). Young (e), elderly (f) and exercised (g) animal immunostaining for nuclear transport protein ran GTPase-activating protein-1 (RanGAP1). In each case, ×40 magnification images are in the insets. Neither sedentary ageing nor exercise resulted in alteration in immunostaining for the nuclear transport protein RanGAP1 (h) or RCC1 (d) as compared to both young. Data expressed as mean ± SEM. *p < 0.05. Young n = 5, elderly n = 5 and exercised n = 5; 50 nuclei per animal (scale 20 μm)
Motor cortex staining
In order to determine whether the pattern of immunostaining we saw in spinal cord was specific to spinal cord or present in neurons generally, we investigated the staining pattern of both LaminB1 and Nup98 within the nucleus of upper motoneurons present within layer V of the motor cortex. Upper motoneurons were identified using immunostaining to CTIP2 (Tantirigama et al. 2016), and their nuclei were localised using DAPI fluorescent staining and the fluorescence intensity of both LaminB1 and Nup98 proteins measured (Fig. 6). The relative immunostaining levels of LaminB1 reduced with age, but the decline was prevented or reversed by exercise whilst Nup98 levels remained unmoved by age or exercise (Fig. 7).
Fig. 7.
Nuclear pore complex transport proteins examined in upper motor neurons of the motor cortex: young (a), elderly (b) and exercised (c) animal immunostaining for LaminB1. Young (e), elderly (f) and exercised (g) animal immunostaining for nucleoporin 98 (Nup98). In each case, ×40 magnification images are located in the insets. Ageing resulted in a significant decline in immunodetected LaminB1 (h) but not Nup98 (d) in these cells. Data expressed as mean ± SEM. *p < 0.05. Young n = 2, elderly n = 5 and exercised n = 3; 50 nuclei per animal (scale 20 μm)
Discussion
We examined age- and exercise-associated changes in total motoneuron number and cellular morphology and specific protein levels at the neuromuscular junction and in cell bodies of alpha-motoneurons, and we present three main findings. First, we replicate previous studies showing that ageing results in increased neuromuscular junction fragmentation and denervation, changes that are attenuated by endurance exercise in elderly animals. Second, we document the age- and location-related loss of lumbar lateral column alpha-motoneurons in elderly animals. Third, we describe age-related reductions in the levels of three key structural nuclear pore and nuclear envelope proteins within alpha-motoneurons and show that those reductions are attenuated by exercise. Overall, findings from this study provide clarification that alpha-motoneurons within the lateral column of the ventral horn are lost with age but in a pattern that appears to be region specific and selectively attenuated by exercise. In addition, key nuclear pore complex and nuclear envelope proteins in alpha-motoneurons also decline with age and their loss is also attenuated by exercise. We believe that this is the first study to show a direct temporal relationship between motoneuron loss, NMJ deterioration and NPC protein decline in elderly mice, and the first to show the preventive influence of endurance exercise on these parameters.
Motoneuron loss
Denervation and the presence of aneural fibres is a widely reported characteristic of elderly muscles, though little about its underlying mechanism is understood (Chai et al. 2011; Deschenes 2004; Gillon and Sheard 2015). The cause of denervation remains unclear, one possibility being that it derives from degeneration of the motoneuron cell body within the spinal cord resulting in withdrawal and degeneration of the intramuscular axons (Aagaard et al. 2010; Rowan et al. 2012). Although one study suggests that age-related neuromuscular degeneration occurs in the absence of neuron loss (Chai et al. 2011), our data show coincidental neuromuscular degeneration (fragmentation and denervation; Fig. 3) and reduction in motoneuron number, though either could be a cause or a consequence of the other.
Evidence for a motoneuron degeneration driving neuromuscular collapse and muscle fibre atrophy within the literature exists in the form of nerve crush, axotomy and whole-limb amputation investigations which point to a prolonged survival of motoneurons within the spinal cord without peripheral input (NMJ) (Johnson and Duberley 1998; Kosmarskaia and Smirnova 1981; Swett et al. 1991; Wu and Kaas 2000). In each case, alpha-motoneurons are seen to persist within the spinal cord for months (3–6 months) before they undergo morphological changes (size and structure) but not always resulting in cell death (Johnson and Duberley 1998; Kosmarskaia and Smirnova 1981). Such prolonged delays between loss of contact with the periphery and motoneuron degeneration suggests that although peripheral synaptic input plays a vital role in modulating motoneuron health (size and morphology), the long life span of peripherally disconnected motoneurons renders their immediate death as a result of neuromuscular collapse unlikely. Instead, we favour the hypothesis that age-associated degeneration of motoneurons drives neuromuscular collapse.
In the current investigation, the age-associated mapping of cell numbers along the length of the lumbar enlargement shows that cell loss varies as a function of rostro-caudal position, with the greatest neuron loss coming from the middle third of the region (L3–L4) (Fig. 4). This region includes the motor pools to the crural muscles of the lower limb (soleus, lateral and medial heads of gastrocnemius and plantaris muscles) (McHanwell and Biscoe 1981). By contrast, no significant neuron loss was detected in the rostral or caudal extremes of the L-LMC, suggesting that the motor pools to the quadriceps, hamstrings and adductor muscles (McHanwell and Biscoe 1981) are either less susceptible or more resistant to age-related changes that result in neuron death. Interestingly, analysis of the motoneuron soma size between young, elderly and exercised animals revealed an age-associated increase in the average diameter of lateral column motoneurons of the lumbar enlargement (Fig. 4f). Although there were no region-specific differences (Fig. 4g), the increase in average motoneuron size may relate either to continued growth of nerve cells or to the existence of motor-unit expansion as a response to motoneuron losses.
Location-dependent variations in susceptibility to age-related degenerative change are also a feature of whole muscle and individual muscle fibres. It appears that weight-bearing muscles of the lower limbs are more prone to atrophy and fibre loss than non-weight bearing muscles (Chai et al. 2011; Holloszy et al. 1991; Sheard and Anderson 2012; Valdez et al. 2010; Valdez et al. 2012). Fast (type II) muscle fibres are thought to be more susceptible to denervation than slow (type I) muscle fibres (Campbell et al. 1973; Cruz-Sanchez et al. 1998; Lexell 1997; McNeil et al. 2005; Tomlinson and Irving 1977). These outcomes are consistent with the suggestion that the effects of ageing may not manifest equally in all motoneurons or muscle fibres and point to the possibility of a susceptibility based on usage pattern, in which phasically active motoneurons and the non-weight-bearing muscles they innervate (quadriceps, adductors and sternomastoid) are more susceptible to loss than tonically active motoneurons and their weight-bearing muscle fibres (soleus, plantaris).
Exercise-stimulated attenuation
The existence of a usage-dependent susceptibility at the neural and muscular level coincides with the therapeutic benefit of exercise seen in both the literature and the current investigation. We found that 4 months of endurance exercise significantly attenuated the loss of NPC proteins in elderly animals whilst also maintaining the integrity of the neuromuscular junction and selectively inhibiting motoneuron loss. Such benefits at both the muscular and motoneuron levels suggest that the health of the cells and the maintenance of the neuromuscular junction are both exercise-related (Bowen et al. 2015; Gomes et al. 2017; Tonnies and Trushina 2017). Previous work shows that the strong anti-oxidant and anti-inflammatory effects of exercise attenuate both the protein degradation present at the muscular level and helps reduce cognitive decline associated with several neurodegenerative diseases (Bowen et al. 2015; Gomes et al. 2017; Tonnies and Trushina 2017).
Of particular interest to the current investigation is the exercise-induced upregulation of skeletal muscle expression of essential neurotrophic factors including neurotrophin-4 (NT-4) and brain-derived neurotrophic factor (BDNF). Both NT-4 and BDNF act to modulate and maintain the neuromuscular synapse, but are also retrogradely transported to the cell body where they are shown to play essential roles in motoneuron survival (Bartlett et al. 1998; Braun et al. 1996; Funakoshi et al. 1995; Gómez-Pinilla et al. 2001). The removal or addition of either neurotrophic factor is seen to retard or promote neuronal survival and growth, respectively (Braun et al. 1996; Martin et al. 1999; Zhang et al. 2000). Considering the apparent usage-dependent variability in motoneuron loss, and the therapeutic benefits of exercise (increased use), we suggest that if the protection is conferred on the neurons (middle third of lumbar enlargement) that experience a certain type of activity or upregulation of activity, that support is activity dependent and activity regulated and that the loss of motoneurons is likely due to their having fallen below a support threshold due to specific inactivity. We can therefore ask whether exercise attenuates the changes evident in sarcopenia via a usage-dependent mechanism that upregulates key signalling and maintenance proteins essential for maintaining the motoneurons that supply the muscles of the lower limb.
Nuclear lamina
The nuclear lamina consists of intermediate filaments including Lamins (A, B and C), Lamin-associated proteins and membrane-associated proteins which play vital roles in the regulation of both the structural integrity and arrangement of nuclear components (chromatin and nuclear pore proteins) (D’Angelo and Hetzer 2006; Shah et al. 2013). Alteration to components of the nuclear lamina is known to disrupt cellular functioning (Lammerding et al. 2006). Conditions such as Hutchinson-Gilford progeria syndrome are characterised by an early ageing phenotype which has been linked to loss of the lamina protein, LaminA (Lammerding et al. 2006). Altered lamina structure affects nuclear mechanical properties including nuclear stiffness and deformation (Lammerding et al. 2006; Shah et al. 2013) and in some cases has been identified as a marker for senescent cells (Freund et al. 2012). The current investigation identified significant reductions in levels of LaminB1 in old upper and lower motoneuron nuclei, and that these reductions are attenuated in exercised animals (Fig. 5). The dramatic loss of such an integral structural protein within the nuclear lamina may alter the arrangement of key structures within the nucleus (chromatin) and also proteins within the nuclear envelope itself, such as nucleocytoplasmic transport proteins.
Nucleocytoplasmic transport
ALS is described by its caudal to rostral pattern of motoneuron degeneration (Andersen 2006), and a significant subset of cases have recently been linked to defective nucleocytoplasmic transport (NCT) through the C9orf72 mutation (Jovicic et al. 2015; Jovičić et al. 2016; Walker et al. 2017; Zhang et al. 2015). Given that ALS and sarcopenia share several pathophysiological similarities, we asked whether normal ageing also potentially features changes in NCT proteins.
Extremely long-lived proteins are often confined to metabolically quiet cells such as the lens fibres; however, the long life of NPC scaffold proteins including Nup93 renders them potentially susceptible to age-related deterioration by oxidative damage, glycosylation or modification in response to stress (D’Angelo et al. 2009; Li and Kohler 2014; Zachara et al. 2004). Investigations looking specifically at age effects on ELLPs and nucleoporins highlighted increased levels of carbonyl groups (a by-product of oxidation) on a subset of nucleoporins (including Nup93), confirming that these proteins are subject to oxidative stress with age (D’Angelo et al. 2009). Other investigations have highlighted that selected NPC proteins are susceptible to O-linked β-N-acetylglucosamine (O-GlycNac) mediated post-translational modification in response to stress, in which a single sugar (O-GlycNac) is attached to the side chain of some NPC proteins altering their functionality (Li and Kohler 2014; Zachara et al. 2004). In both cases, age-related stress induces a modification to proteins that are not regularly turned over, resulting in degradation or conformational change that potentially alters function and prevents antibody-based detection (Toyama et al. 2013).
The current investigation suggests that age affects the expression or structure of the ELLPs as significant age-related declines were found in immunodetectable levels of Nup93 and Nup98, but not RCC1 or RanGAP1, in alpha-motoneuron nuclei. Also interesting is the non-uniformity of this loss, since only a subset of motoneurons showed the decline and since upper motoneurons of the motor cortex generally showed changes in lamin but not in nuclear pore complex proteins (Fig. 6). The loss of Nup93, a protein linker for the FG-Nups, may result in changes in nuclear permeability due to loss of integrity of the nuclear sieve (Fig. 1). Our investigation of the FG-Nup, Nup98, also demonstrated its significant age-related decline, an observation that aligns with the previously described presence of old “leaky” nuclei due to lost integrity of the nuclear sieve (D’Angelo et al. 2009; Kotwaliwale and Dernburg 2009). The presence of a leaky NPC may result in the inappropriate localisation of nuclear components (proteins and organelles) within the nucleoplasm that can disrupt nuclear homeostasis. Interestingly, the exposure of nucleic acids (DNA) to cytoplasmic components is believed to activate machinery thought to initiate cell death and inflammatory pathways as an autoimmune response (D’Angelo et al. 2009; Kotwaliwale and Dernburg 2009; Lusk and King 2017). Therefore, we propose that the age-related changes to long-lived NPC proteins lead to loss of integrity of the nuclear barrier, leading to altered nuclear permeability and the consequential death of neurons (Woulfe 2007). In regard to skeletal muscle atrophy in old age, each lost motoneuron leaves a number of muscle fibres denervated with the immediate consequence being weakness, and the longer-term consequence being muscle atrophy (Deschenes 2011).
Conclusions
This study examined the effects of age and exercise on neuromuscular junction status, motoneuron number and NPC protein levels in lumbar motoneurons. Normal ageing resulted in reduced immunodetection of the intranuclear intermediate filament protein LaminB1 and the nuclear pore proteins Nup93 and Nup98, loss of lower alpha-motoneurons and degeneration of the neuromuscular junction. We suggest that age-related loss of Nup93 and Nup98 may alter the permeability of the nuclear envelope, thereby contributing to the dysregulation of the nuclear transport process leading to loss of motoneurons, to withdrawal of the motor nerve terminal and to skeletal muscle fibre denervation ultimately leading to weakness and muscle atrophy. Endurance exercise reduced the age-related changes in NPC proteins, protected the NMJ and may have extended the lives of susceptible motoneurons. On the basis of the outcomes of this study, we propose that age-related cellular stressors contribute to the death of lower motoneurons by reducing the effectiveness of the nuclear barrier and the nucleocytoplasmic transport process, and that neuromuscular fragmentation and muscle fibre denervation are symptomatic of dying motoneurons. Endurance exercise delays the onset or slows the progress of these degenerative processes, thereby keeping motoneurons alive and preserving neuromuscular integrity and innervation status.
Acknowledgements
Funding of the study reported in the article was provided by the Department of Physiology as part of a full PhD scholarship. AG was also the grateful recipient of a Universities New Zealand, Henry Kelsey Neurological scholarship, and a Hope Selwyn Foundation, ageing research scholarship.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Contributor Information
Ashley Gillon, Email: ashgillon@gmail.com.
Philip Sheard, Email: phil.sheard@otago.ac.nz.
References
- Aagaard P, Suetta C, Caserotti P, Magnusson SP, Kjær M. Role of the nervous system in sarcopenia and muscle atrophy with aging: strength training as a countermeasure. Scand J Med Sci Sports. 2010;20:49–64. doi: 10.1111/j.1600-0838.2009.01084.x. [DOI] [PubMed] [Google Scholar]
- Abercrombie M (1946) Estimation of nuclear population from microtome sections. Anat Rec 94(2):239–247 [DOI] [PubMed]
- Adams RL, Wente SR. Uncovering nuclear pore complexity with innovation. Cell. 2013;152:1218–1221. doi: 10.1016/j.cell.2013.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen PM. Amyotrophic lateral sclerosis associated with mutations in the CuZn superoxide dismutase gene. Curr Neurol Neurosci Rep. 2006;6:37–46. doi: 10.1007/s11910-996-0008-9. [DOI] [PubMed] [Google Scholar]
- Ballak SB, Degens H, de Haan A, Jaspers RT. Aging related changes in determinants of muscle force generating capacity: a comparison of muscle aging in men and male rodents. Ageing Res Rev. 2014;14:43–55. doi: 10.1016/j.arr.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Bartlett SE, Reynolds AJ, Hendry IA. Retrograde axonal transport of neurotrophins: differences between neuronal populations and implications for motor neuron disease. Immunol Cell Biol. 1998;76:419–423. doi: 10.1046/j.1440-1711.1998.00767.x. [DOI] [PubMed] [Google Scholar]
- Bowen TS, Schuler G, Adams V. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachexia Sarcopenia Muscle. 2015;6:197–207. doi: 10.1002/jcsm.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun S, Croizat B, Lagrange M-C, Warter J-M, Poindron P. Neurotrophins increase motoneurons’ ability to innervate skeletal muscle fibers in rat spinal cord-human muscle cocultures. J Neurol Sci. 1996;136:17–23. doi: 10.1016/0022-510X(95)00315-S. [DOI] [PubMed] [Google Scholar]
- Bui KH, et al. Integrated structural analysis of the human nuclear pore complex scaffold. Cell. 2013;155:1233–1243. doi: 10.1016/j.cell.2013.10.055. [DOI] [PubMed] [Google Scholar]
- Caccia MR, Harris JB, Johnson MA. Morphology and physiology of skeletal muscle in aging rodents. Muscle Nerve. 1979;2:202–212. doi: 10.1002/mus.880020308. [DOI] [PubMed] [Google Scholar]
- Campbell M, McComas A, Petito F. Physiological changes in ageing muscles. J Neurol Neurosurg Psychiatry. 1973;36:174–182. doi: 10.1136/jnnp.36.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai RJ, Vukovic J, Dunlop S, Grounds MD, Shavlakadze T. Striking denervation of neuromuscular junctions without lumbar motoneuron loss in geriatric mouse muscle. PLoS One. 2011;6:e28090. doi: 10.1371/journal.pone.0028090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Sanchez F, Moral A, Tolosa E, De Belleroche J, Rossi M. Evaluation of neuronal loss, astrocytosis and abnormalities of cytoskeletal components of large motor neurons in the human anterior horn in aging. J Neural Transm. 1998;105:689–701. doi: 10.1007/s007020050088. [DOI] [PubMed] [Google Scholar]
- D’Angelo MA, Hetzer MW. The role of the nuclear envelope in cellular organization. Cell Mol Life Sci. 2006;63:316–332. doi: 10.1007/s00018-005-5361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo MA, Hetzer MW. Structure, dynamics and function of nuclear pore complexes. Trends Cell Biol. 2008;18:456–466. doi: 10.1016/j.tcb.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo MA, Raices M, Panowski SH, Hetzer MW. Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell. 2009;136:284–295. doi: 10.1016/j.cell.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies TM (2016) The book of R: a first course in programming and statistics 2016. Network security, vol 9. William Pollock. 10.1016/S1353-4858(16)30084-8
- Deschenes MR. Effects of aging on muscle fibre type and size. Sports Med. 2004;34:809–824. doi: 10.2165/00007256-200434120-00002. [DOI] [PubMed] [Google Scholar]
- Deschenes M. Motor unit and neuromuscular junction remodeling with aging. Curr Aging Sci. 2011;4:209–220. doi: 10.2174/1874609811104030209. [DOI] [PubMed] [Google Scholar]
- Deschenes MR, Hurst TE, Ramser AE, Sherman EG. Pre- to post-synaptic relationships of the neuromuscular junction are held constant across age and muscle fiber type. Dev Neurobiol. 2013;73:744–753. doi: 10.1002/dneu.22095. [DOI] [PubMed] [Google Scholar]
- Freibaum BD, et al. GGGGCC repeat expansion in C9orf72 compromises nucleocytoplasmic transport. Nature. 2015;525(7567):129–133. doi: 10.1038/nature14974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund A, Laberge R-M, Demaria M, Campisi J. LaminB1 loss is a senescence-associated biomarker. Mol Biol Cell. 2012;23:2066–2075. doi: 10.1091/mbc.e11-10-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi H, Belluardo N, Arenas E, Yamamoto Y, Casabona A, Persson H, Ibanez CF. Muscle-derived neurotrophin-4 as an activity-dependent trophic signal for adult motor neurons. Science. 1995;268:1495–1499. doi: 10.1126/science.7770776. [DOI] [PubMed] [Google Scholar]
- Galy V, Mattaj IW, Askjaer P. Caenorhabditis elegans nucleoporins Nup93 and Nup205 determine the limit of nuclear pore complex size exclusion in vivo. Mol Biol Cell. 2003;14:5104–5115. doi: 10.1091/mbc.e03-04-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillon A, Sheard P. Elderly mouse skeletal muscle fibres have a diminished capacity to upregulate NCAM production in response to denervation. Biogerontology. 2015;16:811–823. doi: 10.1007/s10522-015-9608-6. [DOI] [PubMed] [Google Scholar]
- Gomes MJ, et al. Skeletal muscle aging: influence of oxidative stress and physical exercise. Oncotarget. 2017;8:20428–20440. doi: 10.18632/oncotarget.14670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Pinilla F, Ying Z, Opazo P, Roy RR, Edgerton VR. Differential regulation by exercise of BDNF and NT-3 in rat spinal cord and skeletal muscle. Eur J Neurosci. 2001;13:1078–1084. doi: 10.1046/j.0953-816x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Chen M, Cartee GD, Young JC. Skeletal muscle atrophy in old rats: differential changes in the three fiber types. Mech Ageing Dev. 1991;60:199–213. doi: 10.1016/0047-6374(91)90131-I. [DOI] [PubMed] [Google Scholar]
- Johnson IP, Duberley RM. Motoneuron survival and expression of neuropeptides and neurotrophic factor receptors following axotomy in adult and ageing rats. Neurosci. 1998;84:141–150. doi: 10.1016/S0306-4522(97)00500-9. [DOI] [PubMed] [Google Scholar]
- Jovicic A, et al. Modifiers of C9orf72 dipeptide repeat toxicity connect nucleocytoplasmic transport defects to FTD/ALS. Nature Neurosci. 2015;18:1226–1229. doi: 10.1038/nn.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovičić A, Paul JW, Gitler AD. Nuclear transport dysfunction: a common theme in amyotrophic lateral sclerosis and frontotemporal dementia. J Neurochem. 2016;138:134–144. doi: 10.1111/jnc.13642. [DOI] [PubMed] [Google Scholar]
- Kosmarskaia EN, Smirnova NV (1981) Changes in the structure of lamina IX of the lumbar enlargement of the spinal cord in dogs following limb amputation Arkh anat, gistol embriol 81:40–45 [PubMed]
- Kotwaliwale CV, Dernburg AF. Old nuclei spring new leaks. Cell. 2009;136:211–212. doi: 10.1016/j.cell.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Lammerding J, Fong LG, Ji JY, Reue K, Stewart CL, Young SG, Lee RT. Lamins A and C but not Lamin B1 regulate nuclear mechanics. J Biol Chem. 2006;281:25768–25780. doi: 10.1074/jbc.M513511200. [DOI] [PubMed] [Google Scholar]
- Lance-Jones C. Motoneuron cell death in the developing lumbar spinal cord of the mouse. Dev Brain Res. 1982;4:473–479. doi: 10.1016/0165-3806(82)90192-4. [DOI] [PubMed] [Google Scholar]
- Lexell J. Evidence for nervous system degeneration with advancing age. J Nutr. 1997;127:1011S–1013S. doi: 10.1093/jn/127.5.1011S. [DOI] [PubMed] [Google Scholar]
- Li B, Kohler JJ. Glycosylation of the nuclear pore. Traffic. 2014;15:347–361. doi: 10.1111/tra.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightfoot JT, Turner MJ, Daves M, Vordermark A, Kleeberger SR. Genetic influence on daily wheel running activity level. Physiol Genomics. 2004;19:270–276. doi: 10.1152/physiolgenomics.00125.2004. [DOI] [PubMed] [Google Scholar]
- Lin DH, Stuwe T, Schilbach S, Rundlet EJ, Perriches T, Mobbs G, Fan Y, Thierbach K, Huber FM, Collins LN, Davenport AM, Jeon YE, Hoelz A. Architecture of the symmetric core of the nuclear pore. Science. 2016;352:aaf1015. doi: 10.1126/science.aaf1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusk CP, King MC. The nucleus: keeping it together by keeping it apart. Curr Opin Cell Biol. 2017;44:44–50. doi: 10.1016/j.ceb.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Kaiser A, Price AC (1999) Motor neuron degeneration after sciatic nerve avulsion in adult rat evolves with oxidative stress and is apoptosis. J Neurobiol 40:185–201. https://doi.org/10.1002/(SICI)1097-4695(199908)40:2<185::AID-NEU5>3.0.CO;2-# [PubMed]
- Matkowskyj KA, Cox R, Jensen RT, Benya RV. Quantitative immunohistochemistry by measuring cumulative signal strength accurately measures receptor number. J Histochem Cytochem. 2003;51:205–214. doi: 10.1177/002215540305100209. [DOI] [PubMed] [Google Scholar]
- McHanwell S, Biscoe TJ. The localization of motoneurons supplying the hindlimb muscles of the mouse philosophical transactions of the Royal Society of London B. Biol Sci. 1981;293:477–508. doi: 10.1098/rstb.1981.0082. [DOI] [PubMed] [Google Scholar]
- McMahon CD, et al. Lifelong exercise and locally produced insulin-like growth factor-1 (IGF-1) have a modest influence on reducing age-related muscle wasting in mice. Scand J Med Sci Sports. 2014;24:e423–e435. doi: 10.1111/sms.12200. [DOI] [PubMed] [Google Scholar]
- McNeil CJ, Doherty TJ, Stashuk DW, Rice CL. Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle Nerve. 2005;31:461–467. doi: 10.1002/mus.20276. [DOI] [PubMed] [Google Scholar]
- Pannérec A, Springer M, Migliavacca E, Ireland A, Piasecki M, Karaz S, Jacot G, Métairon S, Danenberg E, Raymond F, Descombes P, McPhee JS, Feige JN. A robust neuromuscular system protects rat and human skeletal muscle from sarcopenia. Aging. 2016;8:712–728. doi: 10.18632/aging.100926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radu A, Moore MS, Blobel G. The peptide repeat domain of nucleoporin Nup98 functions as a docking site in transport across the nuclear pore complex. Cell. 1995;81:215–222. doi: 10.1016/0092-8674(95)90331-3. [DOI] [PubMed] [Google Scholar]
- Rowan SL, Rygiel K, Purves-Smith FM, Solbak NM, Turnbull DM, Hepple RT. Denervation causes fiber atrophy and myosin heavy chain co-expression in senescent skeletal muscle. PLoS One. 2012;7:e29082. doi: 10.1371/journal.pone.0029082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PP, Donahue G, Otte GL, Capell BC, Nelson DM, Cao K, Aggarwala V, Cruickshanks HA, Rai TS, McBryan T, Gregory BD, Adams PD, Berger SL. Lamin B1 depletion in senescent cells triggers large-scale changes in gene expression and the chromatin landscape. Genes Dev. 2013;27:1787–1799. doi: 10.1101/gad.223834.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheard PW, Anderson RD. Age-related loss of muscle fibres is highly variable amongst mouse skeletal muscles. Biogerontology. 2012;13:157–167. doi: 10.1007/s10522-011-9365-0. [DOI] [PubMed] [Google Scholar]
- Sheard P, Brady J. Implementation of internal intensity controls for semi-quantitative immunohistochemistry. Dunedin: New Zealand Microscopy Society; 2015. [Google Scholar]
- Shi S-R, Cote RJ, Taylor CR. Antigen retrieval techniques: current perspectives. J Histochem Cytochem. 2001;49:931–937. doi: 10.1177/002215540104900801. [DOI] [PubMed] [Google Scholar]
- Swett JE, Hong CZ, Miller PG. All peroneal motoneurons of the rat survive crush injury but some fail to reinnervate their original targets. J Comp Neurol. 1991;304:234–252. doi: 10.1002/cne.903040207. [DOI] [PubMed] [Google Scholar]
- Tantirigama MLS, Oswald MJ, Clare AJ, Wicky HE, Day RC, Hughes SM, Empson RM (2016) J Comp Neurol 524(4):829–845 [DOI] [PubMed]
- Terry LJ, Wente SR. Flexible gates: dynamic topologies and functions for FG nucleoporins in nucleocytoplasmic transport. Eukaryot Cell. 2009;8:1814–1827. doi: 10.1128/EC.00225-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson BE, Irving D. The numbers of limb motor neurons in the human lumbosacral cord throughout life. J Neurol Sci. 1977;34:213–219. doi: 10.1016/0022-510X(77)90069-7. [DOI] [PubMed] [Google Scholar]
- Tonnies E, Trushina E. Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzheimers Dis. 2017;57:1105–1121. doi: 10.3233/JAD-161088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama BH, Savas JN, Park SK, Harris MS, Ingolia NT, Yates Iii JR, Hetzer MW. Identification of long-lived proteins reveals exceptional stability of essential cellular structures. Cell. 2013;154:971–982. doi: 10.1016/j.cell.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez G, Tapia JC, Kang H, Clemenson GD, Gage F, Lichtman JW, Sanes JR. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci. 2010;107:14863–14868. doi: 10.1073/pnas.1002220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez G, Tapia JC, Lichtman JW, Fox MA, Sanes JR. Shared resistance to aging and ALS in neuromuscular junctions of specific muscles. PLoS One. 2012;7:e34640. doi: 10.1371/journal.pone.0034640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H. Exercise and the brain: something to chew on. Trends Neurosci. 2009;32:283–290. doi: 10.1016/j.tins.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viguie CA, Lu D-X, Huang S-K, Rengen H, Carlson BM. Quantitative study of the effects of long-term denervation on the extensor digitorum longus muscle of the rat. Anat Rec. 1997;248:346–354. doi: 10.1002/(SICI)1097-0185(199707)248:3<346::AID-AR7>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Vollmer B, Antonin W. The diverse roles of the Nup93/Nic96 complex proteins—structural scaffolds of the nuclear pore complex with additional cellular functions. Biol Chem. 2014;395:515–528. doi: 10.1515/hsz-2013-0285. [DOI] [PubMed] [Google Scholar]
- Walker C et al. (2017) C9orf72 expansion disrupts ATM-mediated chromosomal break repair. Nat Neurosci 20:1225–1235. 10.1038/nn.4604http://www.nature.com/neuro/journal/v20/n9/abs/nn.4604.html#supplementary-information [DOI] [PMC free article] [PubMed]
- Woolley A, Sheard P, Dodds K, Duxson M. Alpha motoneurons are present in normal numbers but with reduced soma size in neurotrophin-3 knockout mice. Neurosci Lett. 1999;272:107–110. doi: 10.1016/S0304-3940(99)00587-X. [DOI] [PubMed] [Google Scholar]
- Woulfe J. Abnormalities of the nucleus and nuclear inclusions in neurodegenerative disease: a work in progress. Neuropathol Appl Neurobiol. 2007;33:2–42. doi: 10.1111/j.1365-2990.2006.00819.x. [DOI] [PubMed] [Google Scholar]
- Wu CWH, Kaas JH. Spinal cord atrophy and reorganization of motoneuron connections following long-standing limb loss in primates. Neuron. 2000;28:967–978. doi: 10.1016/S0896-6273(00)00167-7. [DOI] [PubMed] [Google Scholar]
- Wu X, Kasper LH, Mantcheva RT, Mantchev GT, Springett MJ, van Deursen JMA. Disruption of the FG nucleoporin NUP98 causes selective changes in nuclear pore complex stoichiometry and function. Proc Natl Acad Sci. 2001;98:3191–3196. doi: 10.1073/pnas.051631598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachara NE, O'Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress a survival response of mammalian cells. J Biol Chem. 2004;279:30133–30142. doi: 10.1074/jbc.M403773200. [DOI] [PubMed] [Google Scholar]
- Zhang JY, Luo XG, Xian CJ, Liu ZH, Zhou XF. Endogenous BDNF is required for myelination and regeneration of injured sciatic nerve in rodents. Eur J Neurosci. 2000;12:4171–4180. [PubMed] [Google Scholar]
- Zhang K, Donnelly CJ, Haeusler AR, Grima JC, Machamer JB, Steinwald P, Daley EL, Miller SJ, Cunningham KM, Vidensky S, Gupta S, Thomas MA, Hong I, Chiu SL, Huganir RL, Ostrow LW, Matunis MJ, Wang J, Sattler R, Lloyd TE, Rothstein JD. The C9orf72 repeat expansion disrupts nucleocytoplasmic transport. Nature. 2015;525:56–61. doi: 10.1038/nature14973. [DOI] [PMC free article] [PubMed] [Google Scholar]