ABSTRACT
The reemergence of pertussis or whooping cough in several countries highlights the need for better vaccines. Acellular pertussis vaccines (aPV) contain alum as the adjuvant and elicit Th2-biased immune responses that are less effective in protecting against infection than the reactogenic whole-cell pertussis vaccines (wPV), which elicit primarily a Th1/Th17 response. An important goal for the field is to devise aPV that will induce immune responses similar to those of wPV. We show that Bordetella colonization factor A (BcfA), an outer membrane protein from Bordetella bronchiseptica, has strong adjuvant function and elicits cellular and humoral immune responses to heterologous and Bordetella pertussis antigens. Addition of BcfA to a commercial aPV resulted in greater reduction of B. pertussis numbers from the lungs than that elicited by aPV alone. The more-efficient pathogen clearance was accompanied by increased interleukin-17 (IL-17) and reduced IL-5 and an increased ratio of IgG2/IgG1 antibodies. Thus, our results suggest that BcfA improves aPV-induced responses by modifying the alum-induced Th2-biased aPV response toward Th1/Th17. A redesigned aPV containing BcfA may allow better control of pertussis reemergence by reshaping immune responses to resemble those elicited by wPV immunization.
KEYWORDS: Bordetella pertussis, acellular pertussis vaccines, adjuvant
INTRODUCTION
The Gram-negative bacterium Bordetella pertussis is the etiologic agent of the disease whooping cough, or pertussis. Efficient control of infection and disease and long-lived protective immunity are best provided by whole-cell pertussis vaccines (wPV), which elicit strong Th1/17-skewed cellular and humoral immune responses. However, the reactogenicity of wPV led to their replacement in the United States and other countries with acellular pertussis vaccines (aPV), which induce mixed Th1/2-skewed responses. While current aPV protect immunized individuals against the severe disease, they do not protect efficiently against infection and subsequent interindividual transmission. Adolescents and adults infected with B. pertussis suffer from prolonged cough and can constitute up to 50% of the reported cases (1). These individuals may serve as carriers and transmit the bacteria to infants and children who are either unvaccinated or are too young to be fully vaccinated (2–5). In 2016, greater than 139,000 cases and 89,000 deaths due to pertussis were reported globally by WHO (http://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/passive/pertussis/en/).
Although the mechanisms that lead to reemergence of pertussis and subsequent transmission are multifactorial, lower vaccine efficacy due to less protective Th2-skewed immune responses induced by aPV is one explanation (6–8). Recent studies in murine models showed that experimental aPV with adjuvants that elicit predominantly Th1-skewed responses provide better protection against pertussis infection than alum-adjuvanted vaccines (9–11), suggesting that modification of the current formulations may improve vaccine efficacy. Furthermore, nonhuman primate models of B. pertussis infection demonstrate that immunization with wPV induces protection against infection and subsequent transmission while aPV immunization does not prevent transmission (12). Here we report that BcfA, an outer membrane protein from Bordetella bronchiseptica (13), has strong adjuvant function and activates innate and adaptive immune responses. Immunization of mice with an experimental aPV containing BcfA as the adjuvant induced more-rapid clearance of a B. pertussis infection from mouse lungs than did alum-adjuvanted aPV. Importantly, the addition of BcfA to a commercial aPV (Boostrix) provided immunized mice with better protection against infection than Boostrix alone. Clearance was accompanied by a marked reduction in the Th2 cytokine interleukin-5 (IL-5) and a higher ratio of serum Th1-type antibodies. These results suggest that the addition of BcfA as an adjuvant to approved vaccines may elicit more-protective immune responses than current formulations.
RESULTS
BcfA has adjuvant function.
We previously reported that immunization of mice with BcfA adsorbed to alum protected against a B. bronchiseptica infection (13) and induced a Th1-type immune response. In this report, we tested whether BcfA would enhance humoral and cellular immune responses to heterologous protein antigens. F1 and V are two well-characterized protective antigens of Yersinia pestis (14). Mice (male and female C57BL/6, 8 to 12 weeks old) were immunized and boosted intramuscularly (i.m.) in the forelimb on day 0 and day 28 with 30 μg BcfA plus 10 μg recombinant F1/V fusion protein (BcfA+F1V) (15). BcfA was produced in an Escherichia coli overproduction system, essentially as we described previously (16). Serum was harvested on day 12 postboost and tested by enzyme-linked immunosorbent assay (ELISA). Higher IgG antibody titers against F1, V, and BcfA were detected (Fig. 1A) in mice immunized with BcfA+F1/V than in mice treated with F1/V immunization alone. This result demonstrates that BcfA enhances humoral immune responses to heterologous protein antigens.
FIG 1.
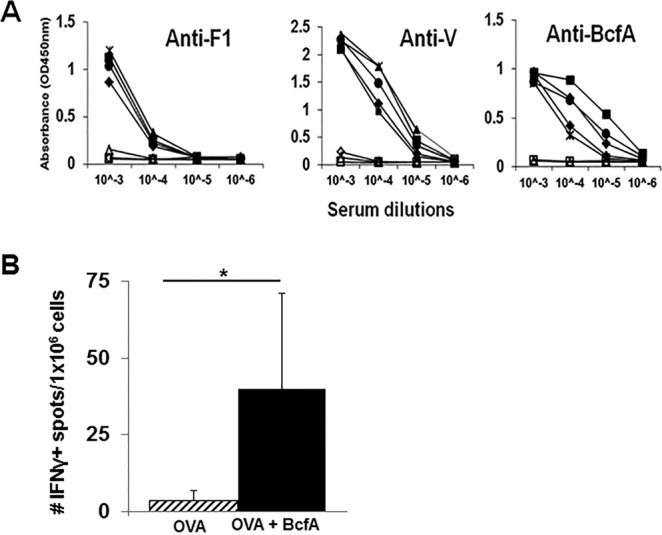
BcfA enhances antibody and CD8+ T cell responses to heterologous protein antigens. (A) C57BL/6 mice (3 to 5 per group) were immunized intramuscularly (i.m.) with 10 μg of F1/V fusion protein either alone (open symbols) or mixed with 30 μg of BcfA protein (closed symbols), on day 0 and day 28. Serum was collected on day 42 (14 days postboost) and tested by ELISA for reactivity against F1, V, and BcfA proteins. One representative experiment of 3 is shown. (B) C57BL/6 mice (3/group) were immunized intraperitoneally on days 0, 14, and 28 with 10 μg of chicken ovalbumin protein either alone or with 30 μg BcfA. Spleens were harvested on day 42, and IFN-γ production in response to stimulation with the H-2Kb-restricted SIINFEKL peptide was tested by ELISPOT. One representative experiment of two is shown. *, P < 0.05.
To test the ability of BcfA to enhance CD8+ T cell responses, we immunized naive C57BL/6 mice intraperitoneally (i.p.) on days 0, 14, and 28 with 100 μg chicken ovalbumin (OVA) protein alone or mixed with 30 μg BcfA. On day 35, spleen cells were stimulated with the SIINFEKL peptide (17) and tested for gamma interferon (IFN-γ) production in an enzyme-linked immunosorbent spot (ELISPOT) assay. This highly immunogenic epitope presented within the OVA protein is presented on the major histocompatibility complex class I (MHC-I) molecule H-2Kb and recognized by CD8+ T cells. It is a widely used model antigen to evaluate CD8+ T cell responses (17). BcfA+OVA immunization increased SIINFEKL-specific CD8+ T cell responses compared with OVA alone, showing that BcfA enhances CD8+ effector responses (Fig. 1B). Together these data show that BcfA has adjuvant activity and can enhance both humoral and cellular responses.
BcfA induces BMDC maturation.
Next, we examined the ability of BcfA to upregulate costimulatory molecule expression and cytokine production by bone marrow-derived dendritic cells (BMDCs) in vitro. BMDCs were generated from C3H/HeJ mice, which are deficient in Toll-like receptor 4 (TLR4). Lipopolysaccharide (LPS) signals through TLR4. Thus, any contribution of the minimal LPS in the BcfA preparation to BMDC activation was avoided by using C3H/HeJ mice (18). Cells were stimulated with BcfA for 24 h and analyzed for the expression of costimulatory molecules by flow cytometry, with supernatant tested for cytokine production by ELISA. Expression of CD40 was highly upregulated, with moderate upregulation of CD80 and CD86 (Fig. 2A). Production of tumor necrosis factor alpha (TNF-α), as well as of the Th1/17-skewing cytokines IL-6 and IL-12/23, was increased upon BcfA stimulation (Fig. 2B). These results suggest that innate immune responses elicited by BcfA support Th1/17-skewed adaptive immune function.
FIG 2.
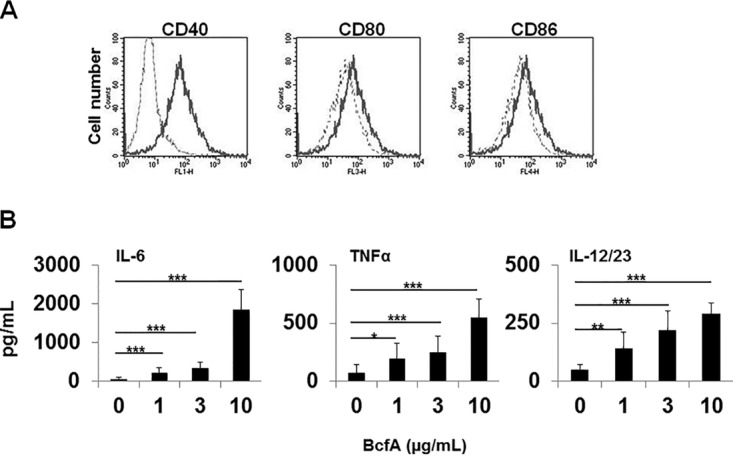
BcfA activates murine bone marrow-derived dendritic cells. Murine BMDCs derived from C3H/HeJ mice were stimulated for 20 to 24 h with various doses of BcfA. The supernatant was collected, and the cells were harvested for analysis of costimulatory molecule expression. (A) Expression of CD40, CD80, and CD86 following BcfA stimulation. Hatched line, medium alone; dark line, BcfA stimulation. One representative well stimulated with 10 μg/ml of BcfA is shown. (B) Supernatant was tested by sandwich ELISA for production of cytokines IL-6, TNF-α, and IL-12/23 common chain. Bars represent the average values for 3 mice tested in triplicate under each condition. One representative experiment of 3 independent experiments is shown. *, P < 0.05; **, P < 0.005; ***, P < 0.001.
BcfA-adjuvanted vaccines elicit protective immune responses against B. pertussis.
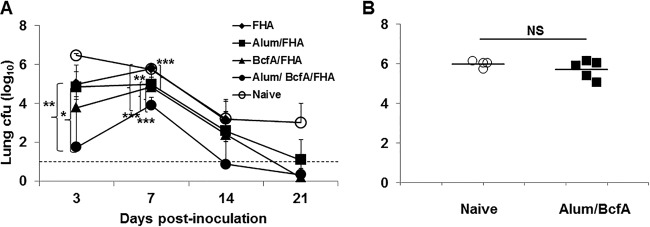
Based on the above-described results that demonstrated BcfA's adjuvant function, we tested whether an experimental vaccine containing BcfA as the adjuvant would elicit protective immune responses against a B. pertussis challenge. C57BL/6 mice were immunized with filamentous hemagglutinin (FHA; an antigen in many current aPV) alone, with alum and FHA (alum/FHA), or with Bcf and FHA (BcfA/FHA). The bacterial load of B. pertussis strain Bp536 in the lungs was determined from day 3 to day 21 postinoculation (Fig. 3A). BcfA/FHA immunization resulted in a lower bacterial burden in the lungs than did FHA alone (P = 0.007) and was similar to the protection provided by alum/FHA immunization (P = 0.0053 versus alum/FHA; P = 0.0037 versus BcfA/FHA). Alum/BcfA/FHA immunization elicited the most-significant reduction in lung bacterial numbers (P < 0.0001) across all time points, demonstrating an additive effect of combining alum and BcfA as adjuvants. These data suggest that the combination of BcfA and alum may provide better protection than either adjuvant alone.
FIG 3.
The combination of adjuvants BcfA and alum elicits better clearance of a B. pertussis infection from the lungs than either adjuvant alone. (A) Mice were immunized with the indicated vaccines and challenged intranasally with Bp536. CFU in the lung were enumerated on days 3 to 21 postinoculation. Each point is the average for 4 or 5 mice per group. One representative experiment of two experiments is shown. *, P ≤ 0.05; **, P ≤ 0.005; ***, P ≤ 0.001. (B) Mice were immunized i.m. on day 0 and boosted on day 28 with 30 μg BcfA/50 μg alum and challenged on day 14 postboost. Lungs were harvested on day 4 postchallenge. NS, not significant.
BcfA does not function as a protective antigen for B. pertussis.
BcfA is a pseudogene in B. pertussis (19; our unpublished analyses) and thus was not expected to be a protective antigen. To confirm that mice immunized with BcfA alone are not protected from colonization with B. pertussis, mice were immunized and boosted with 30 μg of BcfA adsorbed to alum (alum/BcfA) as described above and challenged with 5 × 105 CFU of Bp536 on day 14 postboost. On day 4 postinoculation, lung colonization was similar in naive and alum/BcfA-immunized mice (Fig. 3B) showing that BcfA is not a protective antigen for B. pertussis.
BcfA-containing vaccines downregulate Th2 cytokine production.
To evaluate the immune response elicited by BcfA-containing vaccines, spleen cells from mice immunized with the above-described vaccines were harvested on days 3 to 4 postinoculation and stimulated with FHA. Seven days poststimulation, the supernatant was tested for the presence of IFN-γ, IL-17, and IL-5. Similar levels of IFN-γ (Fig. 4A) and IL-17 (Fig. 4B) were produced by spleen cells from all vaccinations. Notably, there was a marked reduction in IL-5 production from spleen cells of mice immunized with a BcfA-containing vaccine (Fig. 4C). This result suggests that BcfA attenuates production of Th2 cytokines and highlights the ability of BcfA to modify alum-induced immune responses.
FIG 4.
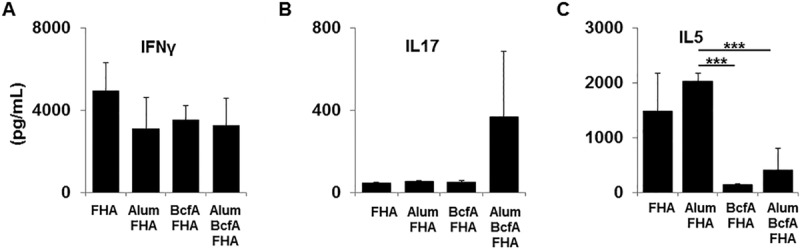
Reduced Th2 cytokine production from spleen cells of mice immunized with BcfA-adjuvanted vaccines. Spleens from immunized mice harvested at day 3 postinoculation were stimulated for 7 days with FHA. Supernatant was tested by sandwich ELISA for the presence of IFN-γ (A), IL-17 (B), and IL-5 (C). One representative experiment of two, with 4 or 5 mice per group, is shown. *, P < 0.05; **, P < 0.005; ***, P < 0.001.
BcfA enhances B. pertussis clearance from mouse lungs mediated by an approved aPV.
We next determined whether the addition of BcfA would enhance the protection mediated by a currently approved commercial vaccine, Boostrix, which contains FHA, Prn (pertactin), PT (pertussis toxin), and aluminum hydroxide as the adjuvant, as well as diphtheria and tetanus toxoid. C57BL/6 mice were immunized i.m. on days 0 and 28 with 1/5 the human dose of Boostrix alone or mixed with 30 μg BcfA and then were challenged intranasally with Bp536 on days 12 to 14 postboost. We selected this dose of aPV since it has been used by others to test vaccine-induced immune responses in mice (20–23). Bacterial colonies in the lungs were enumerated on days 1, 4, 7, and 14 postchallenge. Mice immunized with aPV+BcfA had significantly lower numbers of CFU in the lungs on days 1 and 4 postchallenge than did mice immunized with aPV alone (P = 0.005) (Fig. 5). By day 14, both vaccines had cleared the infection from the lungs. These results show that inclusion of BcfA in the vaccination enhanced the protection provided by an approved aPV.
FIG 5.
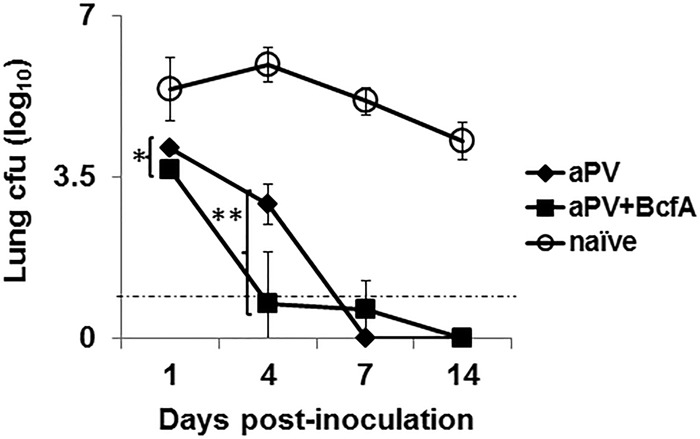
Addition of BcfA to an approved aPV enhances bacterial clearance from the lungs of immunized mice. C57BL/6 mice were immunized i.m. on day 0 and day 28 with 1/5 the human dose of aPV (Boostrix) or 1/5 the human dose of aPV plus 30 μg BcfA (aPV+BcfA) and challenged on days 12 to 14 postboost. Lung colonies were enumerated on the indicated days. Each point is the average for 5 mice per group with the experiment repeated once. Open circles, naive (unimmunized) mice challenged with bacteria; closed diamonds, aPV (Boostrix) alone; closed squares, aPV+BcfA. *, P < 0.05; **, P < 0.01.
BcfA remodels aPV-induced cytokine responses by attenuating Th2 cytokine production.
We determined whether addition of BcfA to the aPV would modify the cytokines produced by immune spleen cells. Spleens of mice immunized with aPV or aPV+BcfA were stimulated with antigens FHA, Prn, or PT. High IFN-γ production was detected and was unchanged by the addition of BcfA to the vaccine (Fig. 6A). There was a moderate increase in FHA-specific IL-17 in aPV+BcfA-immunized mice (Fig. 6B). As observed with the experimental vaccine containing FHA alone, there was a marked decline in IL-5 secretion from spleens of aPV+BcfA-immunized mice (Fig. 6C), demonstrating the ability of BcfA to modulate cytokine responses in a complex vaccine formulation.
FIG 6.
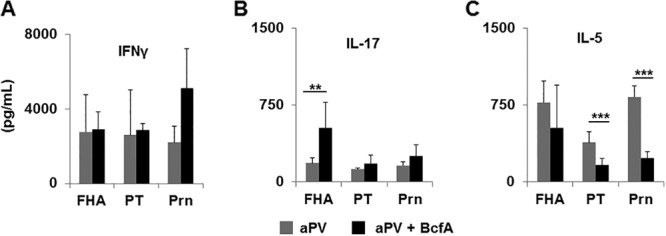
Addition of BcfA to aPV increases IL-17 and decreases IL-5 production from immune spleen cells. Spleens from aPV- and aPV/BcfA-immunized mice were dissociated and stimulated in vitro for 7 days with FHA, Prn, or PT. Supernatant was tested by ELISA for IFN-γ (A), IL-17 (B), and IL-5 (C). **, P < 0.01; ***, P < 0.005.
Th1-skewed antibodies are more prevalent in the serum of aPV+BcfA-immunized mice.
Alum-adjuvanted vaccines elicit primarily IgG1 antibodies, while wPV elicit IgG1 and IgG2 antibodies. We tested whether the addition of BcfA would affect the quantity or composition of serum antibodies elicited by immunization. Total serum IgG levels were not significantly different in mice immunized with aPV alone and in those immunized with aPV+BcfA (data not shown). We determined the ratios of the Th1 isotypes IgG2b and IgG2c to the Th2 isotype IgG1 and found increased ratios of FHA- and PT-specific IgG2b and FHA-specific IgG2c antibodies (Fig. 7), suggesting that the predominantly Th2-biased antibody response elicited by alum was altered to the more-protective Th1-skewed isotypes by the addition of BcfA to the aPV.
FIG 7.
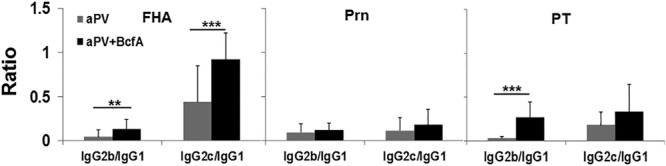
The ratio of Th1-type antibodies is increased in the serum of aPV/BcfA-immunized mice. Serum collected on day 7 postinoculation was tested by multiplex Luminex assay for the presence of FHA-, PRN-, and PT-specific antibodies. The total IgG levels and isotypes were determined, and the ratio of IgG2b/c to IgG1 was calculated. **, P < 0.05; ***, P < 0.005.
DISCUSSION
An active area of investigation to control pertussis resurgence is the development and validation of novel adjuvants and/or antigens that elicit protective responses similar to those elicited by wPV and extend the duration of vaccine-mediated protection. Studies in mice and baboons (12) suggest that wPV provide better protection against infection because they elicit Th1/17 T cell and antibody responses. In contrast, aPV elicit mixed Th1/2 responses and are less protective than wPV. The failure of current vaccines is attributed to this dichotomy of immune responses between wPV and aPV (24). Next-generation aPV may be more easily developed by modifying currently approved aPV than by developing and validating an entirely new formulation.
We report here the adjuvant and immune-modulatory properties of BcfA, an outer membrane protein from B. bronchiseptica. BcfA enhanced antibody responses to F1/V, a fusion protein from Y. pestis, and increased CD8+ T cell responses to the model antigen OVA, demonstrating its ability to augment humoral and cellular immune responses.
BcfA was produced in an E. coli overproduction system, with careful removal of endotoxin. The amount of LPS in each injection (∼4 endotoxin units [EU]) was well below the endotoxin levels present in current aPV (<100 EU/dose) (25, 26). This quantity of LPS was insufficient to trigger BMDCs from C57BL/6 mice (data not shown). Furthermore, BcfA stimulation upregulated costimulatory molecule expression and elicited cytokine production from BMDCs of TLR4 knockout mice, demonstrating that activation of innate immune cell function is not mediated by the minimal LPS in the BcfA preparation.
An experimental vaccine containing BcfA as the adjuvant (BcfA/FHA) displayed protective efficacy similar to that of a vaccine containing alum (alum/FHA), while the vaccine containing the combination of these adjuvants (BcfA/alum/FHA) was much more effective than either adjuvant alone. Importantly, improved protective efficacy was also observed when BcfA was added to Boostrix, an approved aPV, further strengthening the observation that BcfA enhances the protection mediated by alum-adjuvanted vaccines. Immunization with BcfA alone adsorbed to alum did not reduce lung colony numbers, demonstrating that BcfA does not serve as an additional antigenic target.
The most notable observation with respect to development of an improved vaccine for pertussis was the finding that BcfA modified alum-induced immune responses. Alum/FHA or aPV immunization induced high levels of the Th2 cytokine IL-5, while spleens of mice immunized with a BcfA-containing vaccine produced markedly lower IL-5. The levels of IFN-γ and IL-17 elicited by alum, BcfA, or the combination of the two were similar, suggesting that BcfA skews cytokine responses toward Th1/17 by attenuating the Th2 response. Low levels of IL-4 were detected but were not different between aPV and aPV+BcfA groups (data not shown). The observation that murine BMDCs stimulated with BcfA in vitro produced the Th1/17-skewing innate cytokines IL-6 and IL-12/23 but not the Th2-skewing cytokines IL-4 or IL-10 (data not shown) suggests that this skewing may occur at the level of dendritic cell maturation and initiation of the adaptive immune response. The ability of BcfA to skew immune responses toward Th1/17 by attenuating Th2 cytokine production is a distinct advantage, since the Th1/2-skewed responses elicited by the current adjuvant, alum, are less protective than the Th1/17 responses elicited by the wPV (9–11). The role of CD4+ T cells in protective immunity against B. pertussis is well established (7, 11, 27–30). Thus, the FHA- and Prn-specific responses detected here suggest that the adjuvant activity of BcfA augments CD4+ T cell responses as well.
Whole-cell pertussis vaccines elicit Th1-type antibodies that persist longer in children than the Th2-skewed responses elicited by aPV (31). Experimental adjuvants that elicit a higher proportion of Th1-type antibodies provide better protection against a B. pertussis infection in mice than do alum-adjuvanted vaccines (10, 32). Although total serum IgG levels were similar, a higher ratio of IgG2 to IgG1 was observed in aPV+BcfA-immunized animals, suggesting that BcfA modifies the alum-induced antibody response.
Current aPV may be improved by incorporation of additional antigens that broaden the immunological targets (33) or novel adjuvants that amplify or modify the response. One such novel target, a lipoprotein identified recently in B. pertussis, has dual function as an antigen and an adjuvant (10). However, BcfA is not a protective antigen for B. pertussis, showing that the addition of an adjuvant to experimental and approved alum-adjuvanted aPV is powerful enough to modify aPV-induced immune responses without the need for additional immune targets.
The development of allelic variations in B. pertussis specifically in antigens included in the pertussis vaccines has been proposed as an explanation for the current resurgence in pertussis. While this hypothesis is debatable, it is striking that since the introduction of aPV, genetic changes have been observed in genes encoding FHA, PRN, PT, and Fim2,3. Additionally, many circulating strains either are deficient in certain vaccine antigens or produce larger amounts of the remaining vaccine antigens (34, 35). In this context, the addition of an adjuvant that does not function as a protective antigen would avoid vaccine-mediated selection pressure and the development of BcfA-negative escape variants. However, selection against other current aPV antigens may still occur, and inclusion of additional antigenic targets may be necessary to elicit an optimal response to aPV antigen-deficient circulating strains.
Thus, a modified vaccine that includes BcfA may remodel immune responses in adolescents and adults with waning immunity to the more protective Th1/17-skewed phenotype and thereby increase the longevity of protection against a B. pertussis infection.
MATERIALS AND METHODS
Animals.
All experiments were reviewed and approved by the WFUSM Institutional Animal Care and Use Committee. C57BL/6 mice (male and female, 8 to 12 weeks old) were bred in-house. C3H/HeJ mice were purchased from Jackson Laboratories.
Reagents.
Purified FHA for immunizations was purchased from Kaketsuken (Japan) and was free of detectable endotoxin as tested using the Limulus amebocyte lysate (LAL) test (Lonza). Purified proteins FHA, Prn, and PT for ELISAs were from List Biologicals. Boostrix (GlaxoSmithKline) was purchased from the Wake Forest School of Medicine (WFSM) pharmacy. BcfA was overproduced in E. coli and purified essentially as described previously (16), with size exclusion chromatography as an additional step. Residual LPS was removed using MustangQ filters or polymyxin B agarose. Endotoxin was quantified and was ≤150 pg/μg protein (∼4 EU/30-μg injection). Alhydrogel, polymyxin B agarose, and fetal bovine serum (FBS) were from Sigma-Aldrich (St. Louis, MO). ELISA kits were from eBioscience, and antibodies for flow cytometry were from eBioscience (Thermo Fisher), BD Biosciences (San Jose, CA), or Tonbo Biosciences (San Diego, CA). RPMI was from Lonza (Walkersville, MD). Biotinylated secondary antibodies were from BioLegend (San Diego, CA). F1/V fusion protein was provided by Steven Mizel, Wake Forest University Health Sciences (WFUHS). Ovalbumin protein, SIINFEKL peptide, and anti-TLR2 antibody were from InvivoGen (San Diego, CA). MagPlex C magnetic microspheres were from Luminex Corporation.
Immunizations.
C57BL/6 mice were lightly anesthetized with 2.5% isoflurane–O2 and immunized intramuscularly (i.m.) in the forelimb on day 0 and boosted on day 28 with 100 μl of vaccine containing 1.5 μg FHA alone, FHA adsorbed to 50 μg Alhydrogel (aluminum hydroxide), 30 μg BcfA adsorbed to 50 μg Alhydrogel, or FHA plus 30 μg BcfA mixed and then adsorbed to 50 μg Alhydrogel (30 min at room temperature). For aPV immunization, C57BL/6 mice were immunized i.m. on day 0 and day 28 with 100 μl (1/5 the human dose) of Boostrix alone or Boostrix mixed with 30 μg BcfA.
Bacterial challenge.
The laboratory strain of B. pertussis, Bp536, was grown to log phase in Stainer-Scholte medium in room air at 37°C overnight and diluted in phosphate-buffered saline (PBS) to 5 × 105 CFU/50 μl PBS. On days 12 to 14 postboost, mice were lightly anesthetized with 2.5% isoflurane–O2, and the 50-μl inoculum was delivered to both nares.
Colony enumeration.
Mice were euthanized at various days postchallenge, and the lungs were harvested in PBS–2% casein. Tissues were dissociated by Dounce homogenization, and dilutions were plated on Bordet-Gengou agar plates containing 10% sheep's blood, streptomycin (100 μg/ml), and nalidixic acid (20 μg/ml). Antibiotics were stored at 4°C prior to addition to growth medium. Colonies were counted after 4 days of incubation at 37°C. Centrifuged supernatants were stored for cytokine analysis.
ELISA analysis.
Spleens were dissociated, and red blood cells (RBCs) were lysed, plated at 2.5 × 106 cells/ml of complete T cell medium (RPMI, 10% FBS, Gentamicin, 5 × 10−5 M 2-mercaptoethanol [2-ME]) in 48 wells, and stimulated with 0.5 to 1 μg/ml FHA or medium alone. Supernatant was collected on day 7 poststimulation. Production of IFN-γ, IL-5, and IL-17 was quantified using sandwich ELISAs (eBioscience) according to the manufacturer's instructions.
Bone marrow dendritic cell (BMDC) generation and stimulation.
Bone marrow was isolated from the arms and legs of C3H/HeJ mice and plated in 10-cm petri dishes. BMDCs were generated essentially as described previously (36) and stimulated with 40 ng/ml of granulocyte-macrophage colony-stimulating factor (GM-CSF) in T cell medium. One-half of the medium was removed every 2 days and replaced with fresh T cell medium containing GM-CSF. On day 7 of culture, BMDCs were plated at 2.5 × 106 cells/well in 48-well plates and stimulated with medium alone or BcfA at 1, 3, or 10 μg/ml. Supernatant was collected at 20 to 24 h poststimulation and analyzed by ELISA for production of IL-6, TNF-α, and IL-12/23. The stimulated BMDCs were harvested and stained for expression of CD11c, CD40, CD80, and CD86. Marker expression was determined on a BD FACSCanto or FACSCalibur and analyzed using FACSDiva or Cellquest software, respectively.
Multiplex antibody analysis.
Purified antigens were coupled through an amine linkage to MagPlex C magnetic microspheres (Luminex Corporation), each with a unique fluorescent bead region address, and combined to form a 5-plex microarray. Mouse serum or lung homogenates were diluted in assay buffer, PBS–0.1% Brij-35–1% bovine serum albumin (BSA), pH 7.2, and incubated with the beads for 2 h at room temperature (r.t.) in the dark while shaking at 800 rpm. After washing, appropriate biotinylated detection antibody was added, i.e., goat anti-mouse total IgG, rat anti-IgG1, rat anti-IgG2a, rat anti-IgG2b, or goat anti-IgG2c, at a 1:250 dilution in assay buffer for 1 h at r.t. After washing, streptavidin-phycoerythrin (SA-PE) at 1:250 in assay buffer was added for 1 h with shaking. Unbound SA-PE was removed by washing, and the beads were resuspended in 100 μl PBS prior to reading on a Luminex 200 flow cytometer. Antibody isotype and subclass values are reported in arbitrary fluorescent intensity units.
Statistical analysis.
Bacterial colonies in the lung were evaluated using a 2-way analysis of variance (ANOVA) model that included terms for time, group, and a time-by-group interaction. If the interaction was not significant, then the ANOVA model was refit to compare groups adjusting for time. Additional ANOVA models were fit at each time point separately to examine which specific time points had statistically significant differences among the groups. In these analyses, multiple-comparison adjustments were made using the Student Newman-Keuls multiple-range test approach. Student's t test was used to determine differences in ELISAs, with P values of ≤0.05 considered significant.
ACKNOWLEDGMENTS
We thank Kacy Yount, Marisela Velasquez de Kamm, and Amy Medford for technical assistance, Steven Mizel for providing F1/V fusion protein, and Stephanie Seveau and Amy Lovett-Racke for critical review of the manuscript. We acknowledge the Flow Cytometry Shared Resource of the Comprehensive Cancer Center at WFUHS (supported in part by NIH/NCI P30 CA121291-37).
This work was supported by NIAID/RPRC subcontract HHSN272201200005C (to R. Deora), NIH/NIAID 1R01AI125560-01 (to P. Dubey and R. Deora), pilot HHSN272201200005C/AI/NIAID NIH HHS/United States (to S. Quataert) and Wake Forest Innovations.
Author contributions: J. Jennings-Gee conducted experiments and analyzed data, S. Quataert conducted experiments and analyzed data, T. Ganguly conducted experiments, R. D'Agostino, Jr., conducted statistical analysis, and P. Dubey and R. Deora designed and conducted experiments, analyzed data, and wrote the paper.
REFERENCES
- 1.McNabb SJ, Jajosky RA, Hall-Baker PA, Adams DA, Sharp P, Anderson WJ, Javier AJ, Jones GJ, Nitschke DA, Worshams CA, Richard RA Jr. 2007. Summary of notifiable diseases—United States, 2005. MMWR Morb Mortal Wkly Rep 54:1–92. [PubMed] [Google Scholar]
- 2.Yeh SH, Mink CM. 2006. Shift in the epidemiology of pertussis infection: an indication for pertussis vaccine boosters for adults? Drugs 66:731–741. doi: 10.2165/00003495-200666060-00001. [DOI] [PubMed] [Google Scholar]
- 3.Gilberg S, Njamkepo E, Du Chatelet IP, Partouche H, Gueirard P, Ghasarossian C, Schlumberger M, Guiso N. 2002. Evidence of Bordetella pertussis infection in adults presenting with persistent cough in a french area with very high whole-cell vaccine coverage. J Infect Dis 186:415–418. doi: 10.1086/341511. [DOI] [PubMed] [Google Scholar]
- 4.de Greeff SC, Mooi FR, Westerhof A, Verbakel JM, Peeters MF, Heuvelman CJ, Notermans DW, Elvers LH, Schellekens JF, de Melker HE. 2010. Pertussis disease burden in the household: how to protect young infants. Clin Infect Dis 50:1339–1345. doi: 10.1086/652281. [DOI] [PubMed] [Google Scholar]
- 5.Wendelboe AM, Njamkepo E, Bourillon A, Floret DD, Gaudelus J, Gerber M, Grimprel E, Greenberg D, Halperin S, Liese J, Munoz-Rivas F, Teyssou R, Guiso N, Van Rie A. 2007. Transmission of Bordetella pertussis to young infants. Pediatr Infect Dis J 26:293–299. doi: 10.1097/01.inf.0000258699.64164.6d. [DOI] [PubMed] [Google Scholar]
- 6.Guiso N. 2009. Bordetella pertussis and pertussis vaccines. Clin Infect Dis 49:1565–1569. doi: 10.1086/644733. [DOI] [PubMed] [Google Scholar]
- 7.Higgs R, Higgins SC, Ross PJ, Mills KH. 2012. Immunity to the respiratory pathogen Bordetella pertussis. Mucosal Immunol 5:485–500. doi: 10.1038/mi.2012.54. [DOI] [PubMed] [Google Scholar]
- 8.Mooi FR. 2010. Bordetella pertussis and vaccination: the persistence of a genetically monomorphic pathogen. Infect Genet Evol 10:36–49. doi: 10.1016/j.meegid.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Asokanathan C, Corbel M, Xing D. 2013. A CpG-containing oligodeoxynucleotide adjuvant for acellular pertussis vaccine improves the protective response against Bordetella pertussis. Hum Vaccin Immunother 9:325–331. doi: 10.4161/hv.22755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunne A, Mielke LA, Allen AC, Sutton CE, Higgs R, Cunningham CC, Higgins SC, Mills KH. 2015. A novel TLR2 agonist from Bordetella pertussis is a potent adjuvant that promotes protective immunity with an acellular pertussis vaccine. Mucosal Immunol 8:607–617. doi: 10.1038/mi.2014.93. [DOI] [PubMed] [Google Scholar]
- 11.Ross PJ, Sutton CE, Higgins S, Allen AC, Walsh K, Misiak A, Lavelle EC, McLoughlin RM, Mills KH. 2013. Relative contribution of Th1 and Th17 cells in adaptive immunity to Bordetella pertussis: towards the rational design of an improved acellular pertussis vaccine. PLoS Pathog 9:e1003264. doi: 10.1371/journal.ppat.1003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warfel JM, Merkel TJ. 2014. The baboon model of pertussis: effective use and lessons for pertussis vaccines. Expert Rev Vaccines 13:1241–1252. doi: 10.1586/14760584.2014.946016. [DOI] [PubMed] [Google Scholar]
- 13.Sukumar N, Love CF, Conover MS, Kock ND, Dubey P, Deora R. 2009. Active and passive immunizations with Bordetella colonization factor A protect mice against respiratory challenge with Bordetella bronchiseptica. Infect Immun 77:885–895. doi: 10.1128/IAI.01076-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honko AN, Sriranganathan N, Lees CJ, Mizel SB. 2006. Flagellin is an effective adjuvant for immunization against lethal respiratory challenge with Yersinia pestis. Infect Immun 74:1113–1120. doi: 10.1128/IAI.74.2.1113-1120.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mizel SB, Graff AH, Sriranganathan N, Ervin S, Lees CJ, Lively MO, Hantgan RR, Thomas MJ, Wood J, Bell B. 2009. Flagellin-F1-V fusion protein is an effective plague vaccine in mice and two species of nonhuman primates. Clin Vaccine Immunol 16:21–28. doi: 10.1128/CVI.00333-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sukumar N, Mishra M, Sloan GP, Ogi T, Deora R. 2007. Differential Bvg phase-dependent regulation and combinatorial role in pathogenesis of two Bordetella paralogs, BipA and BcfA. J Bacteriol 189:3695–3704. doi: 10.1128/JB.00009-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rotzschke O, Falk K, Stevanovic S, Jung G, Walden P, Rammensee HG. 1991. Exact prediction of a natural T cell epitope. Eur J Immunol 21:2891–2894. doi: 10.1002/eji.1830211136. [DOI] [PubMed] [Google Scholar]
- 18.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol 162:3749–3752. [PubMed] [Google Scholar]
- 19.Parkhill J, Sebaihia M, Preston A, Murphy LD, Thomson N, Harris DE, Holden MT, Churcher CM, Bentley SD, Mungall KL, Cerdeno-Tarraga AM, Temple L, James K, Harris B, Quail MA, Achtman M, Atkin R, Baker S, Basham D, Bason N, Cherevach I, Chillingworth T, Collins M, Cronin A, Davis P, Doggett J, Feltwell T, Goble A, Hamlin N, Hauser H, Holroyd S, Jagels K, Leather S, Moule S, Norberczak H, O'Neil S, Ormond D, Price C, Rabbinowitsch E, Rutter S, Sanders M, Saunders D, Seeger K, Sharp S, Simmonds M, Skelton J, Squares R, Squares S, Stevens K, Unwin L, Whitehead S, Barrell BG, Maskell DJ. 2003. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat Genet 35:32–40. doi: 10.1038/ng1227. [DOI] [PubMed] [Google Scholar]
- 20.Kwon HJ, Han SB, Kim BR, Kang KR, Huh DH, Choi GS, Ahn DH, Kang JH. 2017. Assessment of safety and efficacy against Bordetella pertussis of a new tetanus-reduced dose diphtheria-acellular pertussis vaccine in a murine model. BMC Infect Dis 17:247. doi: 10.1186/s12879-017-2369-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long GH, Karanikas AT, Harvill ET, Read AF, Hudson PJ. 2010. Acellular pertussis vaccination facilitates Bordetella parapertussis infection in a rodent model of bordetellosis. Proc Biol Sci 277:2017–2025. doi: 10.1098/rspb.2010.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mielcarek N, Debrie AS, Mahieux S, Locht C. 2010. Dose response of attenuated Bordetella pertussis BPZE1-induced protection in mice. Clin Vaccine Immunol 17:317–324. doi: 10.1128/CVI.00322-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Misiak A, Leuzzi R, Allen AC, Galletti B, Baudner BC, D'Oro U, O'Hagan DT, Pizza M, Seubert A, Mills KHG. 2017. Addition of a TLR7 agonist to an acellular pertussis vaccine enhances Th1 and Th17 responses and protective immunity in a mouse model. Vaccine 35:5256–5263. doi: 10.1016/j.vaccine.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Brummelman J, Wilk MM, Han WG, van Els CA, Mills KH. 2015. Roads to the development of improved pertussis vaccines paved by immunology. Pathog Dis 73:ftv067. doi: 10.1093/femspd/ftv067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brito LA, Singh M. 2011. Acceptable levels of endotoxin in vaccine formulations during preclinical research. J Pharm Sci 100:34–37. doi: 10.1002/jps.22267. [DOI] [PubMed] [Google Scholar]
- 26.Geier DA, Geier MR. 2002. Clinical implications of endotoxin concentrations in vaccines. Ann Pharmacother 36:776–780. doi: 10.1345/aph.1A410. [DOI] [PubMed] [Google Scholar]
- 27.Barbic J, Leef MF, Burns DL, Shahin RD. 1997. Role of gamma interferon in natural clearance of Bordetella pertussis infection. Infect Immun 65:4904–4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahon BP, Sheahan BJ, Griffin F, Murphy G, Mills KH. 1997. Atypical disease after Bordetella pertussis respiratory infection of mice with targeted disruptions of interferon-gamma receptor or immunoglobulin mu chain genes. J Exp Med 186:1843–1851. doi: 10.1084/jem.186.11.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mills KH, Barnard A, Watkins J, Redhead K. 1993. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect Immun 61:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryan M, Murphy G, Gothefors L, Nilsson L, Storsaeter J, Mills KH. 1997. Bordetella pertussis respiratory infection in children is associated with preferential activation of type 1 T helper cells. J Infect Dis 175:1246–1250. doi: 10.1086/593682. [DOI] [PubMed] [Google Scholar]
- 31.Bancroft T, Dillon MB, da Silva Antunes R, Paul S, Peters B, Crotty S, Lindestam Arlehamn CS, Sette A. 2016. Th1 versus Th2 T cell polarization by whole-cell and acellular childhood pertussis vaccines persists upon re-immunization in adolescence and adulthood. Cell Immunol 304-305:35–43. doi: 10.1016/j.cellimm.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brummelman J, Helm K, Hamstra HJ, van der Ley P, Boog CJ, Han WG, van Els CA. 2015. Modulation of the CD4(+) T cell response after acellular pertussis vaccination in the presence of TLR4 ligation. Vaccine 33:1483–1491. doi: 10.1016/j.vaccine.2015.01.063. [DOI] [PubMed] [Google Scholar]
- 33.Vaughan K, Seymour E, Peters B, Sette A. 2014. Substantial gaps in knowledge of Bordetella pertussis antibody and T cell epitopes relevant for natural immunity and vaccine efficacy. Hum Immunol 75:440–451. doi: 10.1016/j.humimm.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Gouw D, Hermans PW, Bootsma HJ, Zomer A, Heuvelman K, Diavatopoulos DA, Mooi FR. 2014. Differentially expressed genes in Bordetella pertussis strains belonging to a lineage which recently spread globally. PLoS One 9:e84523. doi: 10.1371/journal.pone.0084523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mooi FR, van Loo IH, van Gent M, He Q, Bart MJ, Heuvelman KJ, de Greeff SC, Diavatopoulos D, Teunis P, Nagelkerke N, Mertsola J. 2009. Bordetella pertussis strains with increased toxin production associated with pertussis resurgence. Emerg Infect Dis 15:1206–1213. doi: 10.3201/eid1508.081511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med 176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]