ABSTRACT
In the adult central nervous system, endothelial and neuronal cells engage in tight cross-talk as key components of the so-called neurovascular unit. Impairment of this important relationship adversely affects tissue homeostasis, as observed in neurodegenerative conditions including Alzheimer's and Parkinson's disease. In development, the influence of neuroprogenitor cells on angiogenesis is poorly understood. Here, we show in mouse that these cells interact intimately with the growing retinal vascular network, and we identify a novel regulatory mechanism of vasculature development mediated by hypoxia-inducible factor 2a (Hif2a). By Cre-lox gene excision, we show that Hif2a in retinal neuroprogenitor cells upregulates the expression of the pro-angiogenic mediators vascular endothelial growth factor and erythropoietin, whereas it locally downregulates the angiogenesis inhibitor endostatin. Importantly, absence of Hif2a in retinal neuroprogenitor cells causes a marked reduction of proliferating endothelial cells at the angiogenic front. This results in delayed retinal vascular development, fewer major retinal vessels and reduced density of the peripheral deep retinal vascular plexus. Our findings demonstrate that retinal neuroprogenitor cells are a crucial component of the developing neurovascular unit.
KEY WORDS: Hif2a, Epas1, Retina, Neuroprogenitor, Endostatin, Angiogenesis, Cre/lox
Summary: Hypoxia-inducible factor 2a expressed in neuroprogenitors promotes angiogenesis in mouse retina by upregulating expression of vascular endothelial growth factor and erythropoietin, and downregulating endostatin.
INTRODUCTION
Oxygen is fundamental for the development, specialization and survival of multicellular organisms (Giaccia et al., 2004). In particular, the central nervous system (CNS) is highly dependent on oxygen homeostasis for normal function and extremely vulnerable to harm from hypoxic injury. Molecular oxygen-sensing mechanisms have evolved to mediate adaptive responses, including the development of new vasculature to meet the oxygen demand of developing tissues (López-Barneo et al., 2001; Giaccia et al., 2004). The main molecular pathway responsible for this is controlled by the basic helix-loop-helix transcription elements known as hypoxia-inducible factors (HIFs). These are heterodimers formed by an oxygen-independent β-subunit and an oxygen-dependent α-subunit, of which three variants exists: Hif1a, Hif2a (also known as EPAS1) and Hif3a (Hu et al., 2003). Hypoxic stabilization of the α-subunit enables dimerization with the β-subunit and binding of the dimer to specific sequences on gene promoters (hypoxia responsive elements, HREs) to regulate transcription and trigger responses to hypoxia including metabolic adaptation, erythropoiesis [via erythropoietin (EPO) synthesis] and angiogenesis through mediators such as vascular endothelial growth factor (VEGF) (Majmundar et al., 2010).
In the CNS, neurons (alongside glia) act as oxygen and nutrient sensors, as well as vascular regulators, by interacting structurally and functionally with blood vessels in the neurovascular unit, impairment of which plays a role in a number of neurological conditions (Hawkins and Davis, 2005). In development, neuronal networking and vascular formation go hand-in-hand, sharing common molecular cues to ensure that developing neuronal networks are supported by an appropriate vascular supply (Segura et al., 2009). The laminar development of the neuroretina and its supporting vasculature offers a valuable example and model system, in which glial and neuroretinal cells mediate a coordinated response to address increasing metabolic demand by sensing local availability of oxygen and nutrients (Fruttiger, 2007). In the mouse, astrocytes direct the formation of the superficial vascular plexus between postnatal day (P) 1 and P8, via both HIF-dependent (Duan et al., 2014) and HIF-independent mechanisms (Scott et al., 2010; Weidemann et al., 2010). The metabolic demand generated in the outer plexiform layer (OPL) triggers the descent of sprouting vessels to form the deep vascular plexus (P7-P12). Vasculature formation reaches completion and maturation by P18 with the development of the intermediate vascular plexus in the inner plexiform layer (IPL) (Fruttiger, 2007). Interestingly, dividing endothelial cells do not respond to hypoxia during the process; in fact, endothelial cell-specific HIF factors are reported to be dispensable for developmental angiogenesis (Duan et al., 2014). Through this highly organized process, retinal vascular networks are structurally optimized to support one of the most metabolically demanding tissues in the body (Yu and Cringle, 2001).
Various mechanisms for the temporal and functional involvement of neuronal cells in vascular development have been proposed. Retinal ganglion cells support the astrocytes in guiding the formation of the superficial vascular plexus (Liu et al., 2013), whereas amacrine and horizontal cells guide deep and intermediate plexus formation and vasculature maturation (Usui et al., 2015) in a VEGF/Hif1a-dependent manner. Neuroprogenitor cells play a role of particular interest, as neurogenesis proceeds in parallel with angiogenesis (Hawkins and Davis, 2005). These multipotent undifferentiated cells respond to the hypoxic conditions of early neural development by self-renewing and populating the tissue (De Filippis and Delia, 2011). In anticipation of the increasing neuronal activity, neuroprogenitor cells promote vascularization and tissue oxygenation, which itself induces and supports neuronal differentiation (De Filippis and Delia, 2011).
The molecular mechanisms of this interaction between the proliferating neuroprogenitors and the developing vasculature are only partially understood. It has been previously established (Caprara et al., 2011) that Hif1a-mediated responses to hypoxia by retinal neuroprogenitor cells are involved in maintaining appropriate VEGF gradients during vascular development (Okabe et al., 2014) and in influencing the formation of the astrocytic network and the deposition of the vascular extracellular matrix (Nakamura-Ishizu et al., 2012). However, the involvement of neuroprogenitor-specific Hif2a has yet to be described, although the importance of Hif2a in vascular development is indicated by the severe retinal vasculature abnormalities observed in Hif2a−/− mice (Ding et al., 2005). Hif2a is structurally similar to Hif1a, but shows distinct localization and often non-redundant responses to hypoxia (Hu et al., 2003; Mowat et al., 2010), especially during development (Scortegagna et al., 2003). Hif2a is the major regulator of EPO, which has powerful neuroprotective and pro-angiogenic effects in both development and disease (Haase, 2013). Additionally, like Hif1a, Hif2a drives VEGF expression (Majmundar et al., 2010).
In the present study, we observed that Hif2a ablation in murine neuroprogenitor cells delayed retinal vascular development, resulting in profound abnormalities persisting into adulthood, including fewer arteries and veins, and reduced density of the deep vascular plexus peripherally. We found reduced expression of VEGF genes and Epo and increased levels of endostatin, which is known for its powerful anti-angiogenic properties in disease (Walia et al., 2015) but has not been previously implicated in vascular development. Overall, our findings contribute to an understanding of the interaction between neuronal and endothelial cells, demonstrating how neuroprogenitors promote normal vascular development through the oxygen-dependent transcription factor Hif2a. These observations may have implications for pathological conditions in which neurovascular cross-talk is impaired.
RESULTS
Hif2a is expressed in RPE, ganglion cells, astrocytes and neuroprogenitors of the postnatal developing retina
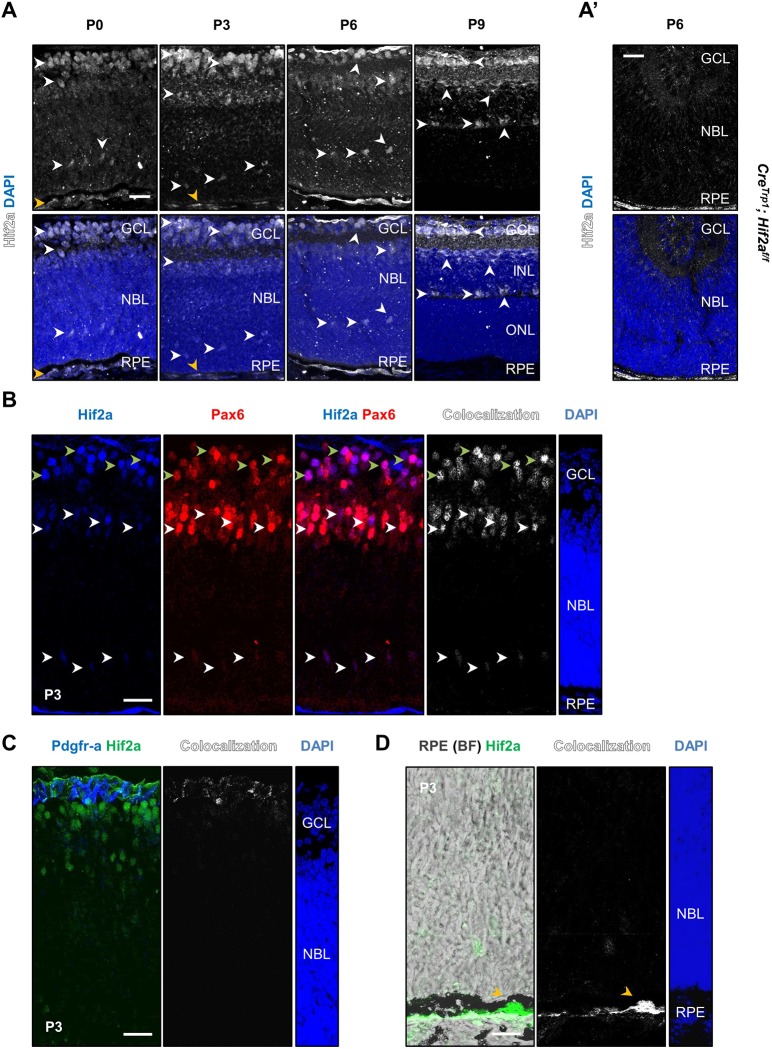
We investigated expression of Hif2a by immunofluorescence in retinal sections at successive postnatal time points (Fig. 1A). Nuclear expression of the protein was detected in the ganglion cell layer (GCL; all ages), the retinal pigment epithelium (RPE; Fig. 1A,D, yellow arrowheads), the neuroblastic layer (NBL; P0 to P6) and the inner nuclear layer (INL; P9). The anatomical location of Hif2a+ cells in the neuroretina suggested their ganglion cell and neuroprogenitor nature (Fig. 1A, white arrowheads), which was confirmed by co-staining with cellular markers on P3 sections: Pax6+ cells in the GCL (Fig. 1B, green arrowheads) were ganglion cells (Hitchcock et al., 1996); Pax6+ cells in the NBL were neuroprogenitors (Marquardt et al., 2001) (Fig. 1B; white arrowheads). Pdgfra+ astrocytes instead showed only limited colocalization with Hif2a (Fig. 1C).
Fig. 1.
Expression of Hif2a in the eye after birth. (A) Hif2a staining was detected in cell nuclei in the GCL at all ages analysed, in the NBL at P0, P3 and P6 and in the INL at P9 (white arrowheads), as well as RPE nuclei (orange arrowheads). (A′) Absence of specific Hif2a signal in P6 CreTrp1; Hif2af/f tissue. (B) Co-labelling of Hif2a and Pax6 in retinal ganglion cells (see green arrowheads) and neuroprogenitors (various locations of the NBL; see white arrowheads). (C) Co-staining with Pdgfra in astrocytes. (D) Hif2a expression in the RPE, visualized under brightfield (BF) through its pigmentation (orange arrowheads). Scale bars: 25 µm.
Cre expression and Cre-mediated recombination affects the postnatal neuroretina in the CreTrp1 line
Because Hif2a appeared to be strongly expressed in neuroprogenitors during postnatal eye development, we sought to determine its role by Cre/lox deletion of Hif2a in these cells.
The CreTrp1 transgenic line expresses Cre recombinase under the tyrosinase-related protein 1 (Tyrp1) promoter and has been historically employed to knock out genes conditionally in pigmented ocular tissues such as the RPE and the iris (Mori et al., 2002). Because concerns regarding cell specificity were previously raised (Thanos et al., 2012), we investigated Cre-mediated recombination by crossing CreTrp1 with the reporter line ROSAmT/mG (Muzumdar et al., 2007). Unexpectedly, at P1 membrane GFP (mGFP) reporter expression was evident not only in the RPE but also throughout the optic nerve and neuroretina (Fig. 2A,B). Although Cre activity appeared strongest in the peripheral retina (Fig. 2A,B), significant activity was also evident in columns of cells in the central retina (Fig. 2C), suggesting clonal expansion of cells resulting from Cre-mediated recombination in a common neuroprogenitor (Reese et al., 1999). Cre recombinase signal was detected by immunohistochemistry in Pax6+ neuroprogenitors of the NBL (Fig. 2D), and active transcription was detected in whole neuroretina (Fig. 2E). Evidence of Cre protein in the outer nuclear layer (ONL) as late as P14 (Fig. 2F, Fig. S1A) indicated that Tyrp1 promoter activity is more sustained than previously described (Mori et al., 2002).
Fig. 2.
Cre expression is not limited to RPE in the CreTrp1 line. (A) Whole eye section from a P1 CreTrp1 crossed with ROSAmT/mG line. Membrane-localized GFP expression is present throughout the whole neuroretina, particularly in the peripheral region. (B) Retinal and choroid/RPE flatmounts of CreTrp1;ROSAmT/mG P1 eye showing peripheral GFP expression in the retina and RPE. (C) High-magnification images of central and peripheral regions (age: P1); GFP+ cells are organized in columns, reminiscent of developmental clonal expansion. (D) Pax6 and Cre staining of P1 eye section (co-staining highlighted by white arrowheads). (E) RT-qPCR analysis of Cre in P5 whole retinae mRNA extracts. n=5. (F) Cre staining of a P14 eye section (highlighted by white arrowheads). Note the non-specific binding of secondary antibody to blood vessels (red arrowheads; also refer to Fig. S1A). (G) RT-qPCR analysis of Hif1a and Hif2a in whole retina RNA extracts (age: P6). Values are relative percentages of the controls (n=5-8). ANOVA and Tukey's multiple comparison test: *P<0.05, **P<0.01 vs controls; §§P<0.01, §§§P<0.001 vs CreTrp1; ##P<0.01 vs CreTrp1;Hif1af/f; xxP<0.01 vs CreTrp1;Hif2af/f. Scale bars: 250 µm (A); 500 µm (B); 25 µm (C,D,F).
After crossing the CreTrp1 line with Hif1af/f, Hif2af/f and Hif1af/f;Hif2af/f lines (Gruber et al., 2006; Ryan et al., 2000), we investigated the impact of ectopic Cre recombinase expression by assessing the expression of HIF family members in P6 retinae by RT-qPCR (Fig. 2G): Hif1a expression was significantly reduced in CreTrp1;Hif1af/f and CreTrp1;Hif1af/f;Hif2af/f mice, and Hif2a was reduced in CreTrp1;Hif2af/f and CreTrp1;Hif1af/f;Hif2af/f mice.
Overall, the results indicated that Cre-mediated recombination in retinal neuroprogenitors is not limited to ocular pigmented tissues as originally described (Lange et al., 2012; Mori et al., 2002).
As we previously reported a major role for Hif1a regulation in whole eye development by employing the same transgenic line (Lange et al., 2012), we compared eye size and morphology over time and observed no effect (Fig. S1B,C). Adult eyes were macroscopically normal (Fig. S1B) and showed no sign of photoreceptor (PR) degeneration (Fig. S1D,E).
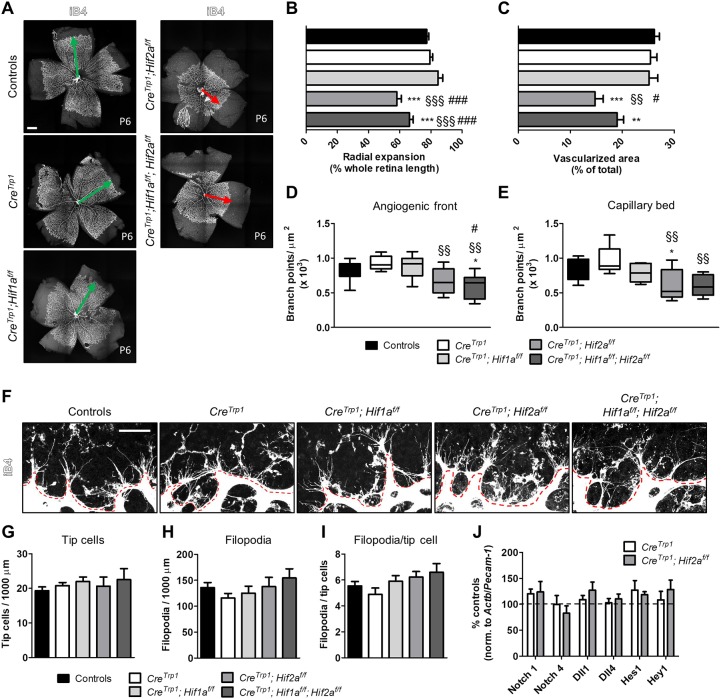
CreTrp1;Hif2af/f and CreTrp1;Hif1af/f;Hif2af/f mice show severely delayed developmental angiogenesis
We examined retinal vascular development at P6 (Fig. 3A, Fig. S2A) and identified a marked delay in primary plexus formation in both CreTrp1;Hif2af/f and CreTrp1;Hif1af/f;Hif2af/f in terms of both radial expansion (Fig. 3B) and vascularized area (Fig. 3C) compared with controls and CreTrp1. In both lines, fewer branch points were present in the advancing angiogenic front (Fig. 3D, Fig. S2A) and mature capillary bed (Fig. 3E, Fig. S2A). Occasionally, the forming vasculature in the Hif2a knockout (KO) retina showed abnormally curved arteries (Fig. S1F), reported to be a sign of vascular pathology (Han, 2012). Hif2a KO lines showed no significant reduction in tip cell differentiation and/or filopodia along the angiogenic front (Fig. 3F-I), nor an increase in vascular regression, identified by empty extracellular matrix sleeves (Phng et al., 2009) (Fig. S2B,C). As sprouting angiogenesis is typically dependent on the Notch signalling pathway in endothelial cells (Hellström et al., 2007), we studied the expression of the major Notch receptors (Notch1 and Notch4), ligands (Dll1 and Dll4) and downstream effectors (Hes1 and Hey1) in the transcriptome of P2 endothelial cells sorted by fluorescence-activated cell sorting (FACS) (Fig. 3J) and, in accordance with the absence of changes at the angiogenic front, we detected no significant differences in cells sorted from Hif2a KO mice compared with cells sorted from CreTrp1 mice.
Fig. 3.
Deletion of Hif2a causes delayed developmental angiogenesis. (A-E) Isolectin B4 (iB4) staining of whole retinal flatmounts (A; age: P6) shows a delayed vascular development in Hif2a KO lines in terms of radial expansion (B), vascularized area (C), and number of branch points in the angiogenic front (D) and in the mature capillary bed (E). Green and red arrows indicate timely and delayed radial vascular expansion, respectively. n=5-39. Also refer to Fig. S2A. In D and E, whiskers are minimum and maximum value; line inside the box is the median. ANOVA and Tukey's multiple comparison test: *P<0.05, **P<0.01, ***P<0.001 vs controls; §§P<0.01, §§§P<0.001 vs CreTrp1; #P<0.05, ###P<0.001 vs CreTrp1;Hif1af/f. (F-I) Images (F) and quantification (G-I) of tip cells and filopodia at the angiogenic front (highlighted by the red dashed line) for all the genotypes analysed. n=6-19 (four fields of view/eye). No significant differences were observed (ANOVA). (J) Notch pathway gene expression assessed by RT-qPCR in FACS-sorted (iB4-FITC labelling) endothelial cells (age: P2). Results are expressed as percentage of controls (normalized to the endothelial marker Pecam1 and the housekeeping gene Actb); no significant differences (ANOVA). Each sample was a pool of 4-8 retinae; n=3-9. Scale bars: 500 µm (A); 25 µm (F).
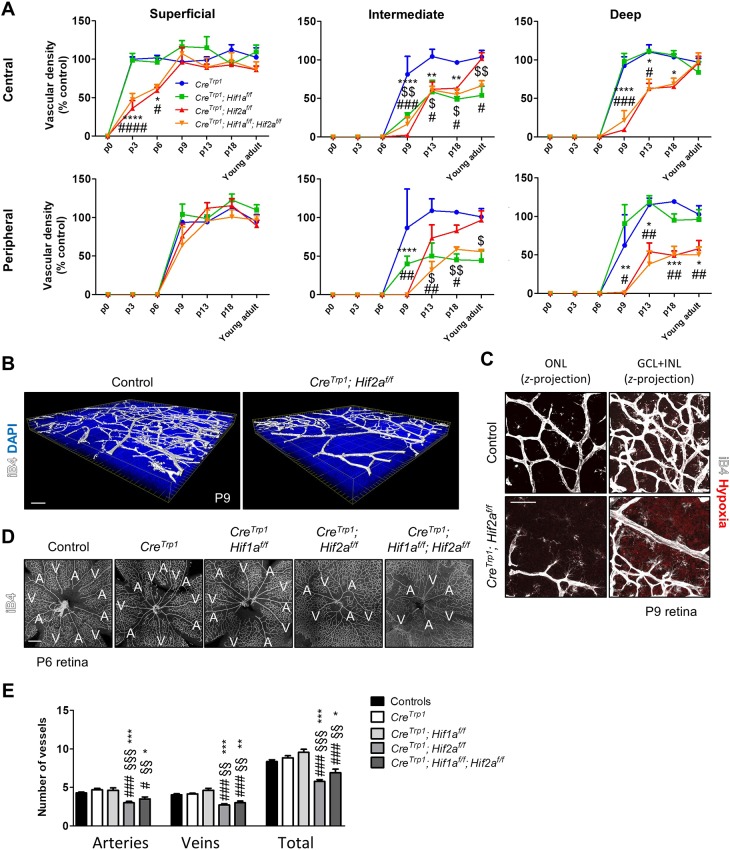
Radial progression of the primary plexus was delayed in Hif2a KO lines compared with CreTrp1 and CreTrp1;Hif1af/f, as measured centrally in terms of vascular density at P3 and P6 (Fig. 4A). Delayed angiogenesis also affected the formation of the deep and intermediate plexi (Fig. 4A,B). Hypoxic tissue between the superficial and the deficient deep plexus was detected at P9 in CreTrp1;Hif2af/f (Fig. 4C).
Fig. 4.
Vascular phenotype in Hif2a KO lines results in delayed plexi formation and fewer arteries and veins. (A) Time-course quantification of deep, intermediate and superficial plexi formation (as a precentage of controls). Refer to Fig. S3 for control plexi images. n=5-12 animals/genotype/time point. ANOVA and Bonferroni multiple comparison test performed versus CreTrp1: $P<0.05, $$P<0.01 CreTrp1;Hif1af/f vs CreTrp1; *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 CreTrp1;Hif2af/f vs CreTrp1; #P<0.05, ##P<0.01, ###P<0.001, ####P<0.0001 CreTrp1;Hif1af/f;Hif2af/f vs CreTrp1. (B) 3D reconstruction of P9 CreTrp1;Hif2af/f vasculature. (C) Persistent hypoxia between the primary and the deep plexi (GCL and INL). (D,E) Images (D) and quantification (E) of IB4 staining revealing reduction in arteries and veins in Hif2a KO lines. A, artery; V, vein. n=8-12. ANOVA and Tukey's multiple comparison test: *P<0.05, **P<0.01, ***P<0.001 vs controls; §§P<0.01, §§§P<0.001 vs CreTrp1; #P<0.05, ###P<0.001 vs CreTrp1;Hif1af/f. Scale bars: 50 µm (B,C); 200 µm (D).
Despite delayed retinal vascular development, the hyaloid vasculature regressed almost normally in Hif2a KO mice: at P13, only a few foetal vessels persisted, and at P18 few remnants were evident in CreTrp1;Hif2af/f and CreTrp1;Hif1af/f;Hif2af/f mice (Fig. S1G).
The pigmented tissue of the iris expresses Cre in the CreTrp1 mouse (Lange et al., 2012; Mori et al., 2002); however, iridal vasculature developed normally in all the lines studied, with no signs of persistent pupillary membrane in adulthood (Fig. S1H).
Vascular abnormalities were still present in young adult Hif2a KO mice at the peripheral deep plexus level (Fig. S3B), but the remaining vasculature eventually developed a normal configuration (Fig. 4A). Hif1a KO lines showed a marked delay in the formation of the intermediate plexus (both centrally and peripherally) (Fig. 4A), consistent with previous observations (Caprara et al., 2011). No changes in terms of vascular permeability were observed (Fig. S1I), indicating development of an intact blood-retinal barrier.
Strikingly, Hif2a KO lines developed fewer arteries and veins (Fig. 4D,E) as confirmed by fluorescein angiography (Fig. S3C), together with an increased number of branch points (Fig. S3D), suggestive of a compensatory mechanism acting to enable fewer major vessels serve a greater portion of the tissue.
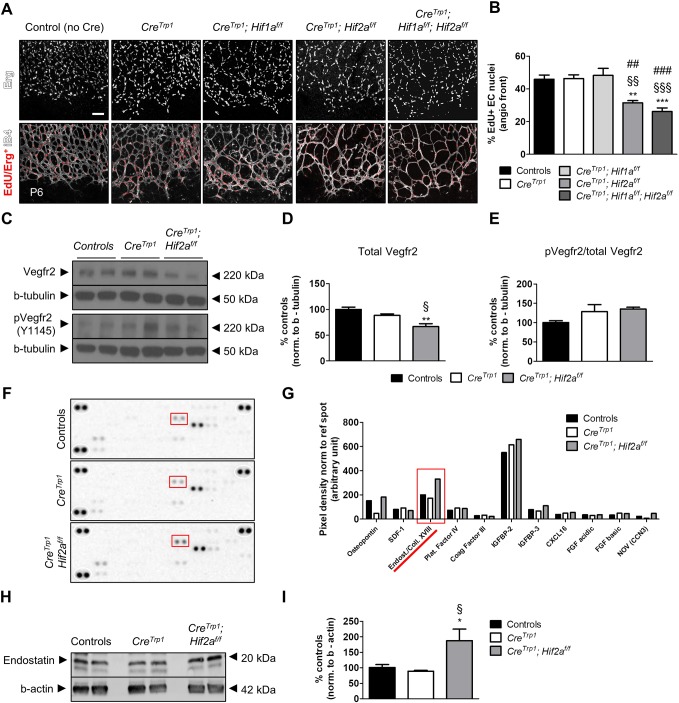
CreTrp1; Hif2af/f and CreTrp1;Hif1af/f;Hif2af/f retinae show decreased endothelial proliferation, normal VEGF/Vegfr2 signalling and increased endostatin levels
At P6, the proportion of actively dividing endothelial cells at the angiogenic front [visualized by 5-ethynyl-2′-deoxyuridine (EdU) incorporation] was significantly reduced in CreTrp1;Hif2af/f and CreTrp1;Hif1af/f;Hif2af/f mice (Fig. 5A,B), as was the total number of endothelial cells (Fig. S4).
Fig. 5.
Hif2a KO lines show reduced endothelial cells division, normal Vegfr2 activation and increased endostatin levels. (A) EdU inclusion in endothelial cell nuclei (stained with Erg antibody) at the angiogenic front (age: P6). (B) Quantification of the data shown in A. n=5; 4 fields/animal quantified. ANOVA and Tukey's multiple comparison test: **P<0.01, ***P<0.001 vs controls; §§P<0.01, §§§P<0.001 vs CreTrp1; ##P<0.01, ###P<0.001 vs CreTrp1;Hif1af/f. (C) Representative western blot of whole retina lysates (age: P5) probed for total Vegfr2, phosphorylated Vegfr2 (Y1145) and β-tubulin. (D,E) Densitometry of quantification of total Vegfr2 (D) and the ratio of pVegfr2 over total Vegfr2 (E). Values are expressed as percentage relative to controls; n=4-6. ANOVA and Tukey's multiple comparison test: **P<0.01 vs controls; §P<0.05 vs CreTrp1. (F) Angiogenesis proteome profiler array of P6 whole retina lysates (pooled n=10 retinae). (G) Densitometry of spot pixels for different analytes detected in F. Red boxes in F and G highlight endostatin/collagen XVIII spots and pixel densities, respectively. (H) Representative western blot of whole retina lysates (age: P6) probed for endostatin and β-actin. (I) Densitometry of endostatin bands in H, normalized to β-actin. Values are expressed as percentage relative to controls; n=4-6 animals. ANOVA and Tukey's multiple comparison test: *P<0.05 vs. controls; §P<0.05 vs CreTrp1. Scale bar: 10 µm.
Endothelial cell migration and proliferation during development are mainly driven by VEGF-A through interaction with VEGFR2 on tip and stalk cells (Gerhardt et al., 2003) and decoy regulation of VEGF-A content by neural progenitors (Okabe et al., 2014). Retinae of P5 CreTrp1;Hif2af/f presented slightly reduced levels of Vegfr2 (Fig. 5C,D), possibly as a consequence of the reduced endothelial cell number (Fig. S4), but the ratio of active (phosphorylated) receptor over total Vegfr2 was unaffected (Fig. 5C-E). Although limited in its cell specificity (Stenzel et al., 2011), this whole tissue analysis suggested that intracellular VEGF signalling was not significantly impaired in this genotype and that factors other than VEGF-A may have been involved in driving the phenotype.
To investigate signalling molecules that might account for the delayed angiogenesis in Hif2a KO lines, we performed angiogenesis-specific proteome profiling of whole P6 retinal protein extracts. The most prominent change was detected for endostatin/collagen XVIII (Fig. 5F,G, red). Quantitative analysis of the 20 kDa collagen XVIII-derived anti-angiogenic fragment endostatin (Walia et al., 2015) by western blotting demonstrated significantly higher levels in CreTrp1;Hif2af/f mice (Fig. 5H,I).
Cre-mediated recombination occurs in neuroprogenitors in the CreTrp1 line
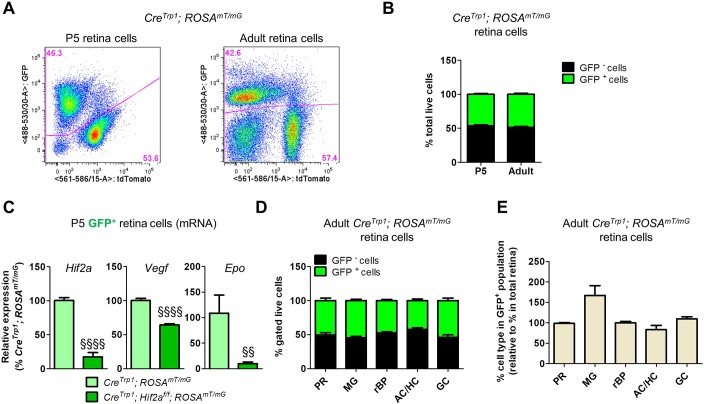
To determine the extent of Cre-mediated recombination in the CreTrp1 line, we performed flow cytometry on CreTrp1;ROSAmT/mG dissociated retinae (Fig. 6A). We found that almost 50% of live retinal cells from neuroretinae of both young and adult mice were affected (Fig. 6B) (46.08±1.18% at P5; 48.57±1.40% in adults). qPCR analysis on mRNA from FACS-sorted mGFP+ cells from CreTrp1;ROSAmT/mG and CreTrp1;Hif2af/f;ROSAmT/mG showed substantial reductions in Hif2a and its main transcriptionally regulated targets, Vegf and Epo (Fig. 6C). Interestingly, few GFP+ cells could be detected in CreTrp1;ROSAmT/mG dissociated brain tissue, whereas no GFP+ cells could be detected in spleen (Fig. S5C).
Fig. 6.
Characterization of neuroretina cells expressing Cre recombinase in the CreTrp1 line. (A) Representative flow cytometry (P5 and young adult) of CreTrp1;ROSAmT/mG. (B) Quantification of total live GFP+ and GFP− cells in CreTrp1;ROSAmT/mG retinae. n=24-52 animals/age. (C) RT-qPCR analysis of Hif2a, Vegf and Epo expression in GFP+ cells FACS-sorted retina cells from P5 CreTrp1;ROSAmT/mG. n=6-7. ANOVA and Tukey's multiple comparison test: §§P<0.01, §§§§P<0.0001 vs CreTrp1;ROSAmT/mG. (D) Quantification of GFP+ and GFP− cells for each subpopulation analysed by flow cytometry on adult dissociated CreTrp1;ROSAmT/mG retinae. Please refer to Tables S1 and S2 for markers and reference percentages. (E) Histogram comparing the composition of the GFP+ subpopulation with the composition of the whole retina of adult CreTrp1;ROSAmT/mG mice (values expressed as percentage of the total retina composition). n=5-9. AC, amacrine cells; GC, ganglion cells; HC, horizontal cells; MG, Müller glia; PR, photoreceptors; rBP, rod bipolar cells.
In the mouse retina, neurogenesis is characterized by two waves of cellular differentiation. The first (E11-E18) is characterized by the differentiation of early neuroprogenitors into ganglion cells, horizontal cells, cone photoreceptors, approximately half of the rod photoreceptors and a subset of amacrine cells. During the second wave (P0 to P8), remaining late neuroprogenitor cells divide asymmetrically to generate the remaining rod photoreceptors and amacrine cells, all bipolar cells and Müller glia (Cepko, 2014). We immuno-phenotyped GFP+ cells from adult CreTrp1;ROSAmT/mG retinae by flow cytometry (refer to Tables S1, S2 and S3 and Fig. S5 for strategy and markers used). All major neuroretinal cell types showed highly similar proportions of GFP positivity at approximately 50% of the total live subpopulation (Fig. 6D). Each major retinal cell type was represented within the mGFP+ subpopulation similarly to whole dissociated retinae, with the exception that Müller glia cells were over-represented (Fig. 6E). This evidence suggested that Cre-mediated recombination occurred in around 50% of an early embryonic retinal neuroprogenitor, from which late retinal neuroprogenitors and every major cell type of the neuroretina originated.
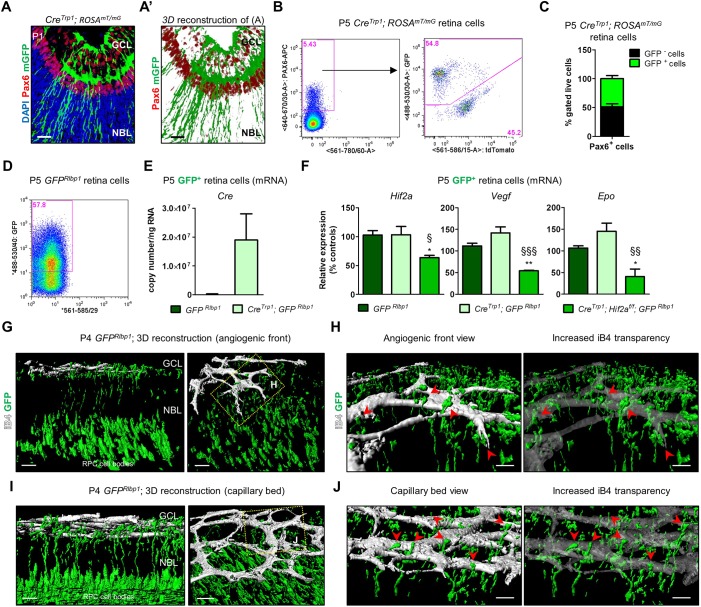
Murine retinal angiogenesis starts at P0 and late neuroprogenitor cells are known to be involved in this process (Caprara et al., 2011; Nakamura-Ishizu et al., 2012; Okabe et al., 2014). We sought to confirm whether late neuroprogenitors were affected by Cre-mediated recombination. We first assessed Pax6, a marker of neuroprogenitors (Marquardt et al., 2001) as well as ganglion cells and a fraction of amacrine cells (Hitchcock et al., 1996), and its colocalization with mGFP in P1 retina of CreTrp1;ROSAmT/mG mice (Fig. 7A,A′). Numerous mGFP+ cells appeared to ensheath Pax6+ nuclei, particularly those present in the NBL, most of which at this stage of retinal development are dividing/differentiating late neuroprogenitors (Marquardt et al., 2001; Nakamura-Ishizu et al., 2012). Quantification by flow cytometry showed that 50% of Pax6+ cells stained positive for GFP in P5 CreTrp1;ROSAmT/mG retinae (Fig. 7B,C).
Fig. 7.
Cre expression affects late neuroprogenitors in the CreTrp1 line. (A) P1 section from the CreTrp1 line showing colocalization of mGFP+ cells and the ganglion cell/neuroprogenitor marker Pax6 (false-coloured in red for better visualization). mTomato signal not shown. (A′) 3D reconstruction of the image shown in A. (B) Representative flow cytometry of P5 Pax6+/mGFP+ cells in P5 CreTrp1;ROSAmT/mG retina. (C) Quantification of GFP+ and GFP− Pax6+ cells. n=7 animals/age. (D) Representative flow cytometry of GFP+ cells in P5 CreTrp1;GFPRlbp1 retina. (E) RT-qPCR analysis of Cre copy number in GFP+ cells FACS-sorted from indicated genotypes (age: P5). n=5-14. (F) RT-qPCR analysis of Hif2a and downstream targets Vegf and Epo on FACS-sorted GFP+ cells (age: P5). n=4-5 samples. Values are normalized to GFP+ cells isolated from GFPRlbp1. ANOVA and Tukey's multiple comparison test: *P<0.05, **P<0.01 vs controls; §P<0.05, §§P<0.01, §§§P<0.001 vs CreTrp1. (G) 3D reconstruction of P4 GFPRlbp1 (two orientations of same confocal z-stack) shows points of contact between GFP+ retinal neuroprogenitors' projections and endothelial cells. (H) Magnification of the boxed area in G. Image with increased transparency aids appreciation of the interactions (see red arrowheads). (I,J) Similarly, late neuroprogenitors interact with the mature capillary bed. Scale bars: 25 µm (A,A′); 20 µm (G,I); 10 µm (H,J).
To validate these findings, we crossed CreTrp1 with the GFPRlbp1 mouse, in which postnatal GFP expression labels late neuroprogenitors (Vázquez-Chona et al., 2009). FACS-sorted GFP+ cells from P5 CreTrp1;GFPRlbp1 retinae showed active Cre recombinase expression (Fig. 7E), whereas P5 GFP+ retinal neuroprogenitors isolated from CreTrp1;Hif2af/f;GFPRlbp1 presented significantly reduced expression of Hif2a, Vegf and Epo (Fig. 7F). These findings demonstrate the extent of Cre expression and Cre-mediated excision of floxed genes in late neuroprogenitors of CreTrp1 lines.
To understand in more detail how late neuroprogenitors are structurally related to the forming vasculature, we performed isolectin B4 (iB4) staining on whole-mounted retinae of P4 wild-type GFPRlbp1 mice, followed by three-dimensional rendering. Long cellular processes originated from GFP+ late neuroprogenitors were visible between the NBL and the GCL, intercalating with the forming vasculature (Fig. 7G). Neuroprogenitor processes engaged in a close association with endothelial cells at the angiogenic front (Fig. 7H, red arrowheads) as well as with post-mitotic endothelial cells in the capillary bed (Fig. 7I,J, red arrowheads).
Retinal ganglion cells also stain positive for Pax6 (Hitchcock et al., 1996) and are key regulators of astrocytes and endothelial cells during developmental angiogenesis, through mechanisms that appear to be independent of HIF (Sapieha et al., 2008). We observed colocalization of the ganglion cell marker neurofilament with GFP in CreTrp1;ROSAmT/mG mice at P5 (Fig. S6A-B′). As the arrangement of axonal extensions was similar, both centrally and peripherally (Fig. S6C), a pronounced effect on ganglion cells viability by Cre-mediated recombination and/or downregulation of HIF factors appears unlikely.
Other perivascular cells are affected by Cre-mediated excision but do not show strong phenotypic alterations
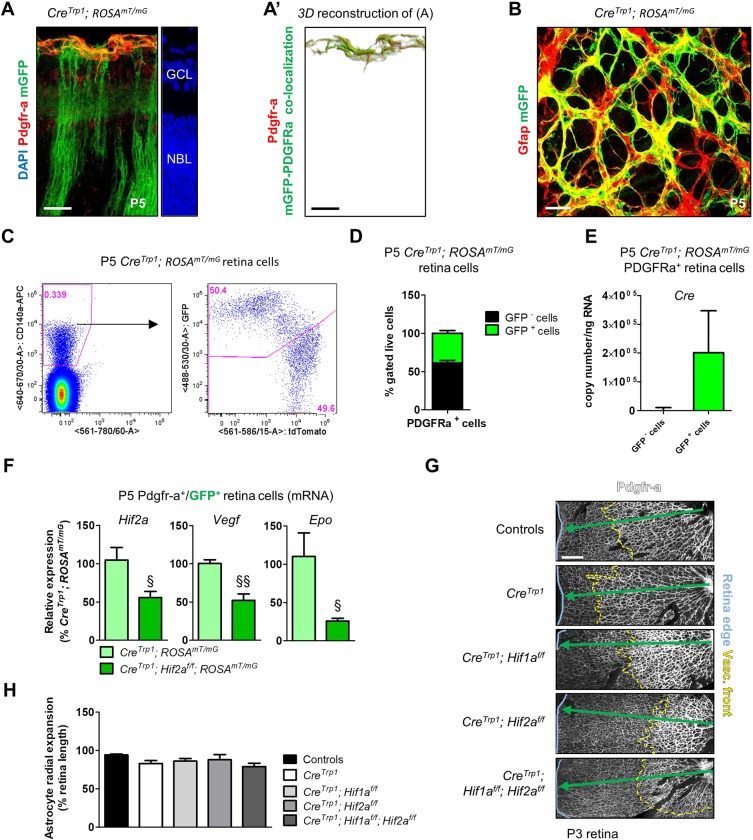
Retinal astrocytes play a key role in retina postnatal vascular development (Fruttiger, 2007). Unexpectedly, we found clear mGFP colocalization with the immature astrocyte marker Pdgfra (Fig. 8A,A′) and the mature astrocyte marker Gfap (Fig. 8B) in P5 CreTrp1;ROSAmT/mG eyes. By flow cytometry, we confirmed GFP and Pdgfra colocalization in around 40% of astrocytes (Fig. 8C,D), detected active Cre expression in GFP+ astrocytes from P5 CreTrp1;ROSAmT/mG mouse (Fig. 8E) and found a marked reduction in Hif2a, Vegf and Epo expression in GFP+ astrocytes derived from Hif2a KO mouse (Fig. 8F).
Fig. 8.
Although affected by Cre-mediated excision, astrocytes invade the developing retina in a timely manner. (A) P5 section from CreTrp1;ROSAmT/mG line showing colocalization between GFP+ cells and the astrocyte marker Pdgfra (false-coloured in red for better visualization). mTomato signal not shown. (A′) 3D reconstruction of the image shown in A. (B) z-stack projection of a P5 flat-mounted retina from a CreTrp1;ROSAmT/mG mouse showing the astrocyte marker Gfap (false-coloured in red for better visualization; tdTomato not shown) and mGFP colocalization. (C) Representative flow cytometry of Pdgfra+/GFP+ cells in P5 CreTrp1;ROSAmT/mG dissociated retina. (D) Quantification of GFP+ and GFP− in Pdgfra+ cell population. n=13 animals. (E) RT-qPCR analysis of Cre copy number in GFP+ and GFP− FACS-sorted Pdgfra+ cells from P5 CreTrp1;ROSAmT/mG. Values are expressed as copy number/ng of retro-transcribed RNA (n=8). (F) RT-qPCR analysis of Hif2a and its targets Vegf and Epo on Pdgfra+/GFP+ cells FACS-sorted from P5 CreTrp1;ROSAmT/mG and CreTrp1;Hif2af/f;ROSAmT/mG lines. Values are relative percentage of CreTrp1;ROSAmT/mG (n=4-5). ANOVA and Tukey's multiple comparison test: §P<0.05, §§P<0.01 vs CreTrp1;ROSAmT/mG. (G) Retinal wholemounts (age: P3) stained with Pdgfra antibody. Tissue edges are highlighted with a light blue line, angiogenic front with a yellow dashed line. Green arrow indicates normal astrocyte radial expansion. (H) Quantification of astrocyte radial expansion. n=4-6; 4 fields of view analysed/sample. No significant differences were observed (ANOVA). Scale bars: 25 µm (A-B); 200 µm (G).
Although previous reports (Scott et al., 2010; Weidemann et al., 2010) found no role for astrocyte-derived HIFs or VEGF in developmental retina angiogenesis, a more recent study (Duan et al., 2014) identified the importance of astrocyte-specific Hif2a in regulating astrocyte proliferation and network formation, with consequent alterations on vascular formation. We identified in CreTrp1;Hif2af/f mice no clear morphological differences in the astrocytic network at P3 (Fig. 8G,H), consistent with a possible difference in the penetrance or efficiency of Cre recombinase in astrocytic lineages.
Hif1a in retinal neuroprogenitors can influence the deposition of extracellular fibronectin by retinal astrocytes (Nakamura-Ishizu et al., 2012); we identified similar defects in fibronectin deposition in Hif1a KO lines but no substantial difference in Hif2a KO mice (Fig. S7).
Pericytes participate in developmental angiogenesis and contribute to vessel stability and maturation (Bergers and Song, 2005). We detected around 20% of Ng2 (Cspg4)+ pericytes to be GFP+ in P5 CreTrp1;ROSAmT/mG (Fig. S8A,B), but this did not impact on the positioning of pericytes around forming vasculature (Fig. S8C,D).
Resident myeloid cells (microglia) are required to promote branching patterning and tip cell filopodia formation (Fantin et al., 2010). The CreTrp1;ROSAmT/mG mouse showed approximately 30% of CD45 (Ptprc)+/CD11b (Itgam)+ cells positive for GFP (Fig. S8E,F) but we identified no difference (Fig. S8G) at the angiogenic front of Hif2a KO lines.
Viral vector delivery of Cre recombinase to a subset of Hif2af/f late retinal neuroprogenitors recapitulates the delayed vascular development of Hif2a KO mice
To investigate the role of late neuroprogenitor-specific Hif2a in directing normal retinal vascular development, we used a recombinant adeno-associated virus (AAV) serotype 7m8 (Dalkara et al., 2013) to deliver the reporter gene RFP or Cre recombinase under the control of the neuroprogenitor-specific promoter nestin (Tronche et al., 1999).
To target late neuroprogenitors efficiently, we injected AAV-7m8 intravitreally (Fig. S9A) soon after birth (P0.5-P1) and collected tissue at P6 (Fig. S9B). Other collection time points (P4, P18) were chosen to assess viral transduction and tropism. First, we observed nestin-driven expression of RFP 3 days post-injection (P4) in cells at various levels of the NBL (Fig. 9A) with elongated morphology consistent with that of neuroprogenitors (Surzenko et al., 2013). To confirm viral tropism, GFPRlbp1 mice were injected with AAV-Nestin.RFP and P4 dissociated retinae were analysed by flow cytometry. Such an approach is sufficiently sensitive to assess close-to-total colocalization of transgene (RFP) expression in GFP-expressing cells (Fig. 9B,C), supporting the high progenitor-specific tropism of this vector configuration. Nonetheless, this investigative design is likely to underestimate AAV-Nestin tropism and efficiency of transduction owing to unavoidable experimental limitations: the relatively short time interval between AAV injection and analysis does not allow identification of all cells transduced, some of which express as yet undetectable levels of fluorescent protein; the ongoing fast division of neuroprogenitors causes dilution of the episomal AAV vector genome in daughter cells, and the nestin promoter is inactive in neuroprogenitors differentiating into neurons or Müller glia.
Fig. 9.
Viral delivery of Cre recombinase to late neuroprogenitors in the Hif2af/f mice recapitulates the vascular phenotype observed in CreTrp1;Hif2af/f mice. (A) Representative image of a wild-type eye injected with AAV-Nestin.RFP. (B,C) Representative flow cytometry of RFP virus in GFPRlbp1 mice (B) and quantification of RFP/GFP co-labelling (C). n=6. (D) Quantification of cell types by flow cytometry of AAV-Nestin.Cre-injected ROSAmT/mG retinae, harvested and dissociated at P18. MG, Müller glia; PR, photoreceptors; rBP, rod bipolar cells; undet, undetermined. (E) iB4-stained retinal flatmounts injected with AAV-Nestin.Cre in one eye and AAV-Null in the contralateral eye (genotypes indicated). Green and red arrows indicate timely and delayed radial vascular expansion, respectively; blue line marks the edge of the flat-mounted tissue. (F) Quantification of vascular development. n=7-20. (G) Quantification of branch points in the angiogenic front and in the mature capillary bed. Orange (angiogenic front) and yellow (capillary bed) boxes in E represent examples of fields used for quantification. Values are expressed as box plots (n=7-15) as in Fig. 2D,E. ANOVA and Bonferroni multiple comparison test (F,G): *P<0.05, **P<0.01 vs Hif2af/f;ROSAmT/mG/AAV-Null; #P<0.05, ###P<0.001 vs ROSAmT/mG/AAV-Null; §P<0.05, §§P<0.01, §§§P<0.001 vs ROSAmT/mG/AAV-Nestin.Cre. (H) Representative images of arteries (A) and veins (V). (I) Large vessel quantification. n=7-20. Unpaired t-test: *P<0.05 vs ROSAmT/mG/AAV-Null. (J) Representative western blot of whole retinae (age: P6) for endostatin and β-actin. AAV-Null-treated eye and AAV-Nestin.Cre-treated eye loaded side-by-side. (K) Densitometry of endostatin bands in J, normalized to β-actin. Values are expressed as percentage relative to controls; n=11. ANOVA and Tukey's multiple comparison test: *P<0.05 vs AAV-Null treated eyes. Scale bars: 25 µm (A); 250 µm (E,H).
The efficacy of the AAV-Nestin.Cre vector was validated by intravitreal administration to ROSAmT/mG eyes, where it produced columns of GFP+ cells in the ONL (Fig. S9C), reminiscent of those seen in the CreTrp1 mouse (Fig. 5C). Sorted GFP+ cells from virally injected Hif2af/f;GFPRlbp1 mice showed limited reduction in Hif2a expression (Fig. S9C), in accordance with the limited number of GFP+ cells infected.
To determine which stage(s) of late neuroprogenitors is transduced by AAV-Nestin vectors, we collected AAV-Nestin.Cre-injected ROSAmT/mG eyes after completion of retinal development (P18) and characterized the widespread (Fig. 9E) GFP+ population by flow cytometry and immunohistochemistry, which identified CD73 (Nt5e)+ photoreceptors (43%), Müller glia (38%) and Pkc-α+ rod-bipolar cells (5%; Fig. 9D, Fig. S9F,G). The absence of reliable GFP/cone arrestin colocalization (Fig. S9H), typical morphology of rod photoreceptor cell bodies and outer segments (Fig. S9I, yellow and white arrowheads, respectively) and absent colocalization of the latter with PNA-stained cone outer segments (Fig. S9J,K) confirmed rod identity of GFP+ photoreceptors. We concluded that AAV-Nestin recombinant vectors injected at P0.5-P1 almost exclusively target the latest subclass of retinal neuroprogenitors, which generates both Müller glia and a proportion of rods (Cepko, 2014). With this in mind, we investigated the effect of virally mediated Hif2a ablation in these cells on retinal angiogenesis (Fig. 9E). Retinae from Hif2af/f;ROSAmT/mG mice injected with AAV-Nestin.Cre showed a significant reduction in radial expansion, vascularized area and branch points both at the angiogenic front and in the capillary bed (Fig. 9F,G). In addition, the number of major arteries and veins was also reduced (Fig. 9H,I). Similarly to CreTrp1;Hif2af/f mice (Fig. 4H,I), AAV-Nestin.Cre-injected Hif2af/f retinae showed increased levels of the anti-angiogenic peptide endostatin compared with AAV-Null-treated eyes (Fig. 9J,K).
Overall, these results closely replicate the phenotype observed in CreTrp1;Hif2af/f and strongly support the hypothesis that late neuroprogenitor-specific Hif2a is a crucial regulator of retinal angiogenesis/vascular development.
As the CreTrp1 line was originally designed to target the RPE specifically and this tissue presented strong postnatal Hif2a expression, we investigated the role of RPE-specific Hif2a on retinal angiogenesis using a similar viral vector approach. To target the RPE, we chose to use a recombinant HIV1-based lentiviral vector and a ubiquitous promoter (spleen focus-forming virus promoter, SFFV) (Balaggan and Ali, 2012). A single vector administration was performed subretinally into ROSAmT/mG and Hif2af/f;ROSAmT/mG mice (Fig. S10A,B). Between 20% and 40% of the RPE monolayer was transduced (Fig. S10C) and no other cell types appeared to be GFP+ by P12 (Fig. S10D). At P6, we detected no differences in terms of radial expansion and vascularized area (Fig. S10E-G), nor of numbers of arteries and veins (Fig. S10H,I). Therefore, we concluded that RPE-specific Hif2a is unlikely to influence retinal vascular development.
Viral vector delivery of Hif2a to Hif2a KO late neuroprogenitor cells partially rescues the vascular phenotype
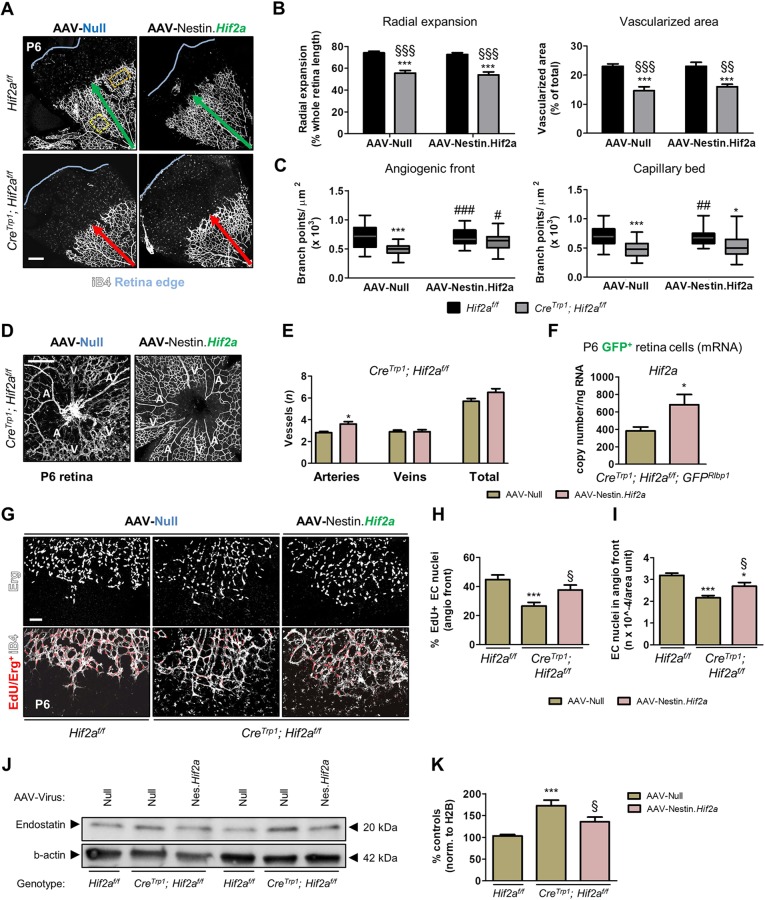
To rescue the vascular phenotype of Hif2a KO mice, we cloned the Hif2a coding sequence into the AAV-Nestin vector and delivered it intravitreally to CreTrp1;Hif2af/f mice following the same experimental paradigm (Fig. S9A,B). Hif2a expression was significantly increased in GFP+ cells sorted from P6 Hif2a-injected CreTrp1;Hif2af/f;GFPRlbp1 eyes compared with AAV-Null-treated eyes (Fig. 10F). No change in radial expansion and vascularized area (Fig. 10A,B) was detected in CreTrp1;Hif2af/f mice treated with AAV-Nestin.Hif2a, which might be a result of limitations in this experimental set-up compared with the previously described AAV-Nestin.Cre experiment, including: insufficient levels of Hif2a expression per cell, transient transgene expression due to cell differentiation or cell division, short treatment window. Nonetheless, CreTrp1;Hif2af/f mice treated with AAV-Nestin.Hif2a presented more branch points at the angiogenic front (Fig. 10C), as well as more arteries (Fig. 10D,E), and these changes were accompanied by an increase in the percentage of dividing endothelial cell nuclei (Fig. 10G,H) as well as an increase in the number of total endothelial nuclei at the angiogenic front (Fig. 10G,I). In CreTrp1;Hif2af/f mice, retinal endostatin was also significantly attenuated by AAV-Nestin.Hif2a administration to levels closer to those detected in Hif2af/f mice (Fig. 10J,K). These findings strongly support the hypothesis that neuroprogenitor-specific Hif2a promotes endothelial cell division and vascular bed formation during normal development.
Fig. 10.
Delivery of Hif2a cDNA to CreTrp1;Hif2af/f neuroprogenitors partially rescues the vascular phenotype. (A) Images of iB4-stained retinal flatmounts injected with AAV-Nestin.Hif2a and AAV-Null controlaterally (genotypes: Hif2af/f and CreTrp1;Hif2af/f). Green and red arrows indicate timely and delayed radial vascular expansion, respectively; blue line marks the edge of the flat-mounted tissue. (B) Quantification of vascular development in terms of radial expansion and vascularized area. n=10-25. (C) Quantification of branch points in the angiogenic front and in the mature capillary bed. Orange (angiogenic front) and yellow (capillary bed) boxes in A represent examples of fields used for quantification. Values are expressed as box plots (n=14-25) as in Fig. 2D,E. Two-way ANOVA and Bonferroni multiple comparison test (B,C): *P<0.05, ***P<0.001 vs Hif2af/f/AAV-Null; §§P<0.01, §§§P<0.001 vs Hif2af/f/AAV-Nestin.Hif2a; #P<0.05, ##P<0.01, ###P<0.001 vs CreTrp1; Hif2af/f/AAV-Null. (D) Arteries (A) and veins (V) in CreTrp1;Hif2af/f AAV-injected eyes. (E) Large vessel quantification. n=20-26. Unpaired t-test: *P<0.05 vs AAV-Null. (F) RT-qPCR analysis of Hif2a copy number in GFP+ FACS-sorted cells from P6 CreTrp1;Hif2af/f;GFPRlbp1 virally injected retinae. Values are expressed as copy number/ng of retro-transcribed RNA (n=6). Unpaired t-test: *P<0.05 vs Null-injected eyes. (G) EdU inclusion in endothelial nuclei (stained with Erg antibody) at the angiogenic front (age: P6). (H,I) Quantification of EdU+/Erg+ nuclei at the angiogenic front in G; n=9-13; 4 fields/animal quantified. ANOVA and Tukey's multiple comparison test: *P<0.05, ***P<0.001 vs Hif2af/f/AAV-Null; §P<0.05 vs CreTrp1;Hif2af/f/AAV-Null. (J) Representative western blot of whole retina lysates (age: P6) probed for endostatin and β-actin. Hif2af/f and CreTrp1;Hif2af/f loaded side-by-side are littermates. AAV-Null-treated eye and AAV-Nestin.Hif2a-treated eye loaded side-by-side. (K) Densitometry of endostatin bands in J, normalized to β-actin. Values are expressed as percentage of controls (n=8). ANOVA and Tukey's multiple comparison test: ***P<0.001 vs Hif2af/f/AAV-Null eyes; §P<0.05 vs CreTrp1;Hif2af/f/AAV-Null. Scale bars: 250 µm (A,D); 100 µm (G).
DISCUSSION
Owing to its accessible laminar anatomy and its uniquely high metabolic demand, the murine retina has been used extensively to study the consequences of neuronal hypoxia (Caprara et al., 2011; Usui et al., 2015). Historically, astrocytes have been considered the primary oxygen sentinels of the retina; however, their importance is questioned by evidence of the dispensable role of the HIF pathway in these cells (Scott et al., 2010; Weidemann et al., 2010). Recent studies have elucidated the role played by neuronal cells as oxygen and nutrient sensors, highlighting their ability to drive and regulate physiological and pathological angiogenesis (Nakamura-Ishizu et al., 2012; Usui et al., 2015). In the current study, we present evidence for a novel mechanism, centred on Hif2a and neuroprogenitors of the retina, which dynamically interact with the forming vasculature at the angiogenic front, promoting vascular development in an astrocyte-independent manner. Interestingly, the iridal vasculature, which is also dependent on appropriate oxygen management (Lange et al., 2012), developed normally (Fig. S1), making our findings strictly specific to retinal angiogenesis.
Initial evidence was obtained from experiments performed on the CreTrp1 line, designed as a tool to generate efficient RPE-specific knockouts (Mori et al., 2002). However, the promoter driving Cre [tyrosinase-related protein 1 (Tyrp1), an enzyme involved in pigment formation] is not RPE specific: we have shown (Fig. 2) diffuse postnatal Cre expression and Cre-mediated recombination in the neuroretina, in a peripheral-to-central pattern. This is reminiscent of the CrePax6 line, which has been extensively used to target neuroprogenitors and neurons in similar studies (Nakamura-Ishizu et al., 2012; Okabe et al., 2014). One possible explanation for the ectopic Cre recombinase expression in retinal neuroprogenitors is the involvement of the ciliary body (CB). This region hosts a pool of multipotent stem cells specialized to become precursor cells for retinal neurogenesis and anterior eye development (Frøen et al., 2013). Because this region of the eye shares a common developmental origin with pigmented tissues (such as the RPE and the iris pigmented epithelium), early Tyrp1-mediated Cre expression may be induced in an upstream common multipotent neuroprogenitor in the CreTrp1 mouse. As we detected occasional cells affected by Cre-mediated excision in the brain (Fig. S5C), which also contains distinctive pigmented cells (i.e. the neurons in the substantia nigra pars compacta), this might indicate Cre expression as a result of the early activation of the pigment synthesis pathway. However, this does not explain why non-neuronal cells such as astrocytes, pericytes and microglia are also affected by Cre-mediated excision in this line (Fig. 8, Fig. S8). The distinctive phagocytic abilities of these cells, their close proximity and functional interaction with developing neurons and their putative ability to undergo cell fusion/material transfer (Ogle et al., 2005) offers a plausible explanation.
Off-targeting effects in the Cre/lox system are well recognized, making data interpretation often difficult (Harno et al., 2013). We included the Cre-expressing group as a control, and confirmed our modified, neuroprogenitor-centred hypothesis through a more reliable cell-specific viral vector-based approach. The severity of the angiogenic delay observed in Hif2a KO lines, compared with related ones (Nakamura-Ishizu et al., 2012), was particularly pronounced (Figs 3 and 4), with persistent differences in the deep vascular plexus evident to adulthood. In the wild-type neuroretina, Hif2a stabilization was detected until (at least) P9 in areas rich in neuroprogenitors/differentiating neurons/glia (Fig. 1), even though the ongoing vascularization should have already improved the deep retinal hypoxia typical of the first week of life. This observation is consistent with the hypothesis that Hif2a is more susceptible to chronic (Lin et al., 2011) and subtle (Appelhoffl et al., 2004) levels of hypoxia compared with Hif1a and, therefore, more dynamically modulated.
Our comparison between the two main hypoxia responsive α-subunits supports a more prominent role of Hif2a in the developing neuroretina, consistent with the findings of previous studies (Rattner et al., 2014). Although aware of the well-described developmental role played by neuroprogenitor-specific Hif1a (Caprara et al., 2011), the dramatic consequences of Hif2a ablation were unexpected, not only in terms of temporal delay of angiogenesis and reduction in vascular complexity (Figs 3 and 4), but also in terms of irreversible abnormalities such as the reduction in the number of major retinal vessels and vascular density of the intermediate plexus (Fig. 4, Fig. S3). Similar phenotypes have been linked in previous studies to changes in vascular regression/pruning, tip cell behaviour, chemotaxis and Vegf signalling; remarkably, such parameters remained unchanged (Fig. 3, Fig. S2B,C), as were Vegfr2 activation (Fig. 5C-E) and the expression of Notch/Dll components in endothelial cells (Fig. 3J). Instead, late retinal neuroprogenitors of the CreTrp1;Hif2af/f mouse showed transcriptional reduction in Vegf and Epo, well known to be angio-modulatory factors. As VEGF isoforms are the major drivers of sprouting angiogenesis (Gerhardt et al., 2003), vascular outgrowth, capillary bed complexity (Ruhrberg et al., 2002) and artery/vein patterning (Dela Paz and D'Amore, 2009), this indicates a likely mechanism of Hif2a/progenitor-dependent regulation of developmental angiogenesis. Moreover, our own findings are in close accordance with the impact of CreNestin-mediated Vegfa ablation in brain neuroprogenitors (Fantin et al., 2010), resulting in reduced vascular branching despite similar tip cell numbers. Persistent hypoxia in the neuroretina (Fig. 4) may also contribute to the defects observed in artery/vein patterning, as described in an earlier report (Claxton and Fruttiger, 2005). Epo is well recognized for both its neuroprotective and pro-angiogenic properties (Haase, 2013); although its mechanism of action has yet to be elucidated, the results of this study provide further evidence of its participation in developmental angiogenesis as previously proposed (Caprara et al., 2011).
Our experimental evidence (Figs 5, 9 and 10) suggested endostatin as another key player. This small protein fragment is derived from the proteolytic cleavage of the extracellular matrix component collagen XVIII operated by an array of proteases including metalloproteases (MMPs) and cathepsins (Walia et al., 2015), some of which (MMP3 and MMP9) are released during neurogenesis by neuroprogenitors to promote matrix remodelling and neural migration (Barkho et al., 2009). Endostatin is known for its paracrine anti-angiogenic properties, mainly exerted by blocking endothelial cells migration and proliferation via cell-cycle arrest and blockade of VEGF/VEGFR2 interaction and signalling (Walia et al., 2015). Although its role in vascular disease has been well described, evidence for its involvement in developmental angiogenesis is limited to one in vitro study (Schmidt et al., 2004). We speculate that hypoxic neuroprogenitors in the avascular peripheral retina and avascular deeper cellular layers, while engaging in dynamic interactions with the forming vasculature through their cellular processes, suppress endostatin release in the vascular microenvironment to enable rapid tissue vascularization; in addition, they contribute to the formation of a pro-angiogenic molecular gradient ahead of the vascular front, as previously described (Gerhardt et al., 2003; Okabe et al., 2014). Once neuroprogenitors are reached by the forming vasculature and start sensing the physiological oxygen level, suppression is relieved and basal levels of endostatin help stabilize and maintain mature vasculature. This hypothesis is consistent with the endogenous anti-angiogenic nature of endostatin and with the observation that mutations in the collagen XVIII gene Col18a1 that downregulate endostatin generation and/or activity predispose to the development of certain tumours with a prominent vascular component (Iughetti et al., 2001).
Overall, our study describes new levels of structural and molecular association between neuronal and endothelial cells, indicating the importance of retinal neuroprogenitor/Hif2a-centred mechanisms in the cross-talk between neurogenesis and angiogenesis. More broadly, these findings further our understanding of neuro-vascular interactions during normal development, and model the pathological consequences of miscommunication at the neurovascular unit.
MATERIALS AND METHODS
Animals
All in vivo procedures were conducted following ethical approval of University College London and under the regulation of the UK Home Office Animals (Scientific Procedures) Act 1986. This study complied with the ARVO Statement for the Use of Animals in Ophthalmology and Vision Research. All genetic combinations were maintained on a C57BL/6J background and kept on a standard 12/12 h light/dark cycle with food ad libitum. Genotyping was performed as previously described (Lange et al., 2012). The following lines were used (all under C57BL/6J background): transgenic mice expressing Cre recombinase under the control of the RPE cell-specific tyrosinase-related protein 1 promoter Tyrp1, referred to as CreTrp1 (Lange et al., 2012; Mori et al., 2002), Hif1af/f (Ryan et al., 2000), Hif2af/f (Gruber et al., 2006) and combinations as reported in the Results section. Some of these combinations were crossed with ROSA26mT/mG (Muzumdar et al., 2007) or with GFPRlbp1 (Vázquez-Chona et al., 2009). The group ‘controls’ includes all the Cre-negative genotypes (wild-type C57BL/6J and HIF floxed lines), as preliminary analysis revealed no differences in basal HIF factor expression and comparable vasculature development.
To collect pups in a consistent manner at precise postnatal developmental stages, the morning of discovery of a new litter was designated P0. Adult mice were 4-6 weeks old.
Fluorescein and indocyanine green angiography
To analyse retinal and corneal vascular phenotype, we performed fluorescein (FA) and indocyanine green (ICG) angiography, respectively. Mice were anaesthetized by intraperitoneal injections of Dormitor (1 mg/ml; Pfizer Pharmaceuticals) and ketamine (100 mg/ml; Fort Dodge Animal Health, UK) mixed with sterile water in 5:3:42 ratio. Mice were further injected intraperitoneally with 0.2 ml fluorescein (20 mg/ml) or ICG (5 mg/ml) in PBS. Pupils were dilated using 1% (v/v) tropicamide and images were captured on a HRA2 scanning laser ophthalmoscope (Heidelberg Engineering) as previously described (Luhmann et al., 2009).
Intravitreal injections
Pups aged between P0.5 and P1 were anaesthetized by brief ice exposure (Kim et al., 2014). Treatments were randomly assigned to left or right eyes before each litter was injected. For vasculature development experiments, one eye was injected with either AAV-7m8.Nestin.Cre or AAV-7m8.Nestin.Hif2a, whereas the controlateral with AAV-7m8.Null eye lids were incised with a 30G needle and eyes were prolapsed. Injections were performed with the aid of an operating microscope. The tip (1 mm) of a 10 mm 34-gauge needle, mounted on a 5 ml Hamilton syringe was inserted slowly through the sclera, below the corneal limbus, until the tip of the needle was visible (bevel down) underneath the lens. Vector suspension (0.4 µl) was injected carefully, monitoring eye swelling. After 10 s, the needle was slowly withdrawn and possible reflux noted down. Pups were quickly moved to a heated mat to help post-injection recovery and then transferred back to the mother.
Evans Blue permeability assay
Evans Blue (Sigma-Aldrich) was prepared (30 mg/ml) in normal saline solution [0.9% (w/v) NaCl; Sigma-Aldrich] and sonicated for 5 min. The solution was then filtered through a 5 µm filter (Millipore). Mice were injected intraperitoneally at 4 ml/kg of body weight (Manaenko et al., 2011) and the dye was left circulating for 16 h before overdosing the animal with 0.2 ml pentobarbital (intraperitoneally), collecting a sample of blood by cardiac puncture and transcardially perfusing with ice-cold PBS to remove unbound dye. Eyes were enucleated, retinae dissected and wet weight calculated. Blood-retinal barrier properties were assessed by extracting and measuring bound dye from serum and retinae as reported (Xu et al., 2001).
Preparation of flat-mount samples, cryosections and immunofluorescence
Ocular size was assessed on freshly enucleated eyes at different time points as reported (Lange et al., 2012). Eyes were then fixed in 4% (w/v) paraformaldehyde (PFA) on ice for 2 h and either dissected for RPE/choroid or retina flat-mounts as previously described (Fruttiger et al., 1996) or prepared for cryosectioning as described (Mowat et al., 2010).
Wholemounts were immunostained as described (Pitulescu et al., 2010), whereas eye sections were immunostained as reported previously (Mowat et al., 2010). In both cases, primary antibody was incubated overnight at 4°C and secondary antibody was incubated for at least 2 h at room temperature (RT); each step was followed by extensive washes. Nuclei were stained by incubation (15 min; RT) with Hoechst 33342 (10 μM) in PBS before mounting. Nuclei rows were counted in four randomly selected sections/animal, both centrally and peripherally.
Vasculature and resident myeloid cells were visualized by overnight incubation at 4°C with biotin-conjugated Bandeiraea simplicifolia isolectin B4 (iB4; Sigma-Aldrich) at 1:200, followed by Alexa 633-conjugated streptavidin incubation for 2 h at RT. Cone outer segments were visualized by overnight incubation at 4°C with biotin-conjugated peanut agglutinin (PNA; Vector Laboratories) at 1:500, followed by Alexa 633-conjugated streptavidin incubation for 2 h at RT. Hypoxiprobe staining was performed as reported (Gardner et al., 2017). For three-dimensional reconstructions, immunostained flatmounts were cleared before image acquisition following a published protocol (Hama et al., 2011) with modifications. Details of the antibodies used for immunohistochemistry can be found in Table S3.
Imaging and quantification
Retinal sections and flatmounts were imaged on a confocal microscope (Leica TCS SPE, Leica Microsystems). Mosaic stack images were generated to visualize the entire retinal vasculature at 512×512 pixel resolution with a 10× dry objective, whereas higher resolution 1 µm-thick z-stacks were taken at 1024×1024 pixel resolution with a 40× oil-immersion objective. Stacks were z-projected using the confocal microscope's proprietary software (Leica LAS AF). Image quantification was performed using ImageJ software (NIH, US); the counter was masked to the genotype and treatment received. For vascular quantification, 12 measurements of the forming vascular front (normalized to the whole retina extension) were performed and averaged for each retina sample. For characterization of the angiogenic front and capillary bed, four regions per retina were quantified and the mean calculated. When required, z-stacks were three-dimensionally reconstructed and surface rendered using Imaris software (Bitplane).
EdU administration and visualization
Proliferating endothelial cells at the angiogenic front were visualized by EdU incorporation, following recommended guidelines from a commercially available kit (Click-iT EdU kit, Life Technologies).
Pups were injected intraperitoneally with 10 µl/g body weight 20 mM EdU solution and returned to the mother. Four hours later pups were culled by cervical dislocation, eyes collected and fixed in 4% (w/v) PFA for 1 h at RT. After performing standard immunofluorescence for other vascular markers, dissociated retinae were incubated in EdU reaction cocktail as per protocol for 30 min at RT, protecting samples from light. After extensive washes in 0.3% (v/v) Triton X-100 in PBS, samples were flat-mounted and visualized by confocal microscopy.
Production of recombinant adeno-associated viral (AAV) and lentiviral particles
Cre recombinase-coding sequence was cloned into an AAV-ready pD10 backbone, into which the nestin promoter (Lothian and Lendahl, 1997) was added or into a Lenti-ready pLenti.SFFV promoter backbone (Tschernutter et al., 2005). The red fluorescent protein (RFP) and the murine Hif2a coding sequences were obtained from a plasmid available in-house and by PCR amplification on murine retinal cDNA, respectively, and cloned into the pD10.Nestin promoter backbone. Empty pD10 backbone served to produce non-expressing (Null) viral particles. The resulting constructs were used to generate AAV-7m8 recombinant particles (Dalkara et al., 2013) and VSV-G lentiviral particles as previously described (Smith et al., 2003; Tschernutter et al., 2005). AAV viral particles were purified through an AVB Sepharose column and concentrated to a final volume of 150-200 μl sterile PBS using Vivaspin 4 (10 kDa) concentrators (Sartorius). Lentiviral particles were concentrated by ultra-centrifugation (50,000 g; 2.5 h, 4°C) to a final volume of 200 μl sterile PBS.
Tissue dissociation, immunostaining, flow cytometry sample acquisition and cell sorting
Dissected mouse retinae were dissociated into a single-cell suspension using a Papain neurosphere dissociation kit (Miltenyi Biotec), according to the manufacturer's instructions, for subsequent cell staining. Spleen and brain samples were homogenized using a 2.5 ml syringe plunger and a 70 µm filter.
Once dissociated, samples for extracellular staining (see Table S3) were spun down at 320 g for 5 min and stained in the dark in DMEM+ medium (supplemented with 2% foetal calf serum and 10 mM HEPES) for 30 min on ice. Non-directly conjugated anti-NG2 antibody was followed by 30 min incubation with goat anti-rabbit AF633 secondary antibody (Thermo Fisher Scientific). Cells were then washed and re-suspended in fresh DMEM+, filtered and stained with either SYTOX Blue (Thermo Fisher Scientific) at a final concentration of 0.3 mM or DRAQ7 (Biostatus) at a final concentration of 0.1 mM just before sample acquisition and/or cell sorting.
Instead, samples for intracellular staining (see Table S3) were spun down at 320 g for 5 min and stained in the dark with the LIVE/DEAD Fixable Violet Cell Stain (Thermo Fisher Scientific) diluted in DMEM+ media for 30 min on ice. Cells were then washed with DMEM+ media and fixed in 2% PFA for 30 min on ice in the dark. Cells were washed once with PBS and then re-suspended in True Nuclear Transcription Factor permeabilization buffer (BioLegend) for 30 min on ice, in the dark. Cells were then washed and re-suspended in neat donkey serum for 10 min. All the primary antibodies were diluted in True Nuclear permeabilization buffer and added directly to the appropriate samples containing the donkey serum for an additional 30 min on ice, in the dark. With the exception of directly conjugated antibodies, samples were then washed with PBS and re-suspended in neat donkey serum for 10 min just prior to the addition of either goat anti-mouse-AF633 or goat anti-rabbit-AF633 secondary antibody (Thermo Fisher Scientific) for 30 min on ice, in the dark. Samples were then washed and re-suspended in PBS. Please refer to Tables S1 and S3 for more detailed antibody information.
The samples were acquired using a 5-laser BD LSR Fortessa X-20 Analyser or acquired and sorted on a 5-laser BD Influx cell sorter, both equipped with 355 nm (UV), 405 nm (violet), 488 nm (blue), 561 nm (yellow) and 640 nm (red) lasers. Prior to cell acquisition, samples were filtered through a 35 µm cell strainer to prevent cellular aggregation.
Real-time PCR
Whole retinae were homogenized and RNA was extracted using RNeasy Mini Kit (Qiagen). RNA from cells sorted into TRIzol plus (Thermo Fisher Scientific) was extracted using Direct-zol microprep RNA kit (Zymo Research). cDNA preparation was performed using QuantiTect Reverse Transcription Kit (Qiagen) following manufacturer's instructions. Absolute and relative quantification of genes of interest was quantified by real-time probe-based PCR against endogenous Actb (and Pecam1 for sorted endothelial cells) expression levels. Real-time PCR mastermix was used as per recommended guidelines (Quantabio). Primers were first assessed for optimal efficiency of amplification on known amounts of cDNA. Assays for Hif1a and Hif2a were designed so that the reverse primer bound within the second exon, which is flanked by loxP sites in Hif1af/f and Hif2af/f lines. This allowed detection of the gene knocked out in Cre/loxP lines. Primers for VEGF genes were designed to detect all VEGF isoforms. For absolute quantification (Hif2a; Cre), in-house plasmids bearing the coding sequence of the target genes were used. Primer sequences (Thermo Fisher Scientific) and probes (Roche Diagnostics) are reported in Table S4.
Western blotting
Total and phosphorylated (Y1145) VEGFR2 and endostatin levels were determined on whole retinae, snap-frozen in liquid nitrogen after dissection. Tissue was lysed by repeated pipetting and following incubation for 30 min on ice with RIPA buffer (Thermo Fisher Scientific) with phosphatase (Thermo Fisher Scientific) and protease inhibitor (Sigma-Aldrich). Equal amounts of protein were run on reducing 4-12% gradient sodium SDS-PAGE gels (Thermo Fisher Scientific). Housekeeping proteins β-actin or β-tubulin were probed as loading controls. Separated proteins were electrotransferred to PVDF membranes (Millipore), blocked for 1 h at RT in 5% (w/v) bovine serum albumin, 0.05% (v/v) Tween-20 in PBS and then incubated overnight at 4°C with primary antibodies (see Table S3). After extensive washes in 0.05% (v/v) Tween-20 in PBS, membranes were incubated in secondary antibodies for 2 h at RT. Chemiluminescence detection was performed using a Fujifilm LAS-1000 Luminescence Image analyser after incubation with enhanced luminescence reagent (ECL plus; GE Healthcare). Band intensities were quantified using ImageJ software and normalized to the housekeeping protein. For genotype comparisons, control and KO samples were obtained from the same litter to control for intragroup variability, whereas CreTrp1 was obtained from litters of the most similar age possible. For treatment comparison, eyes were paired and compared with the Null-treated contralateral eye.
Mouse angiogenesis proteome profiler array
Retinal protein extracts (processed exactly as for western blot analysis) were analysed for angiogenesis-related molecules on a proteome profiler array (R&D Systems) and developed according to the manufacturer's guidelines. Images were acquired on a Nikon LAS4000 CCD imager and densitometry was performed with ImageJ. Membranes were normalized to each other using positive control spots and background reading (Chu et al., 2016).
Statistical analysis
Data are presented as mean±s.e.m. and sample sizes are reported in each figure legend. Each experiment on animals was performed on at least two independent litters of a given genotype. Data were plotted and analysed for statistical significance using Graphpad Prism software. First, normal distribution was assessed for each group by the Kolmogorov–Smirnov test. Parametric statistical tests (two-tailed t-test and one- or two-way ANOVA) were used to compare the averages of two or more groups, with post-hoc Tukey's or Bonferroni's multiple comparison tests. P-values less than 0.05 were considered statistically significant.
Supplementary Material
Acknowledgements
We would like to thank Laura A Hervas for technical assistance; Prof. John Greenwood (UCL) for Imaris software access; Prof. D. Antonetti for discussion on the manuscript; Dr C. Lin for technical assistance with the ocular permeability experiments; the staff at the Biological Resources Unit at the UCL Institute of Ophthalmology for help with mouse husbandry; and members of the Gene and Cell Therapy Group (UCL Institute of Ophthalmology) for constructive discussion.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: E.C., S.E.L., G.D., M.R., U.F.L., A.J.S., J.W.B.B.; Methodology: E.C., S.E.L., R.D.S., A.K., G.D., M.R., R.N.M., Y.D., T.M.; Validation: E.C.; Formal analysis: E.C.; Investigation: E.C., S.E.L., R.D.S., A.K., G.D., J.H., J.R., Y.D.; Resources: M.R., R.N.M., T.M., N.D.A.; Data curation: E.C., R.D.S.; Writing - original draft: E.C., A.J.S., J.W.B.B.; Writing - review & editing: S.E.L., G.D., A.J.S., R.R.A., J.W.B.B.; Visualization: E.C., R.D.S., A.K., G.D., M.R.; Supervision: R.R.A., J.W.B.B.; Project administration: A.J.S., J.W.B.B.; Funding acquisition: R.R.A., J.W.B.B.
Funding
E.C., R.R.A. and J.W.B.B. are supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital and UCL Institute of Ophthalmology. J.W.B.B. was supported by a NIHR Research Professorship. S.E.L. was supported by a Medical Research Council/Fight for Sight Clinical Research Training Fellowship (2012-1333). Deposited in PMC for release after 6 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.157511.supplemental
References
- Appelhoffl R. J., Tian Y.-M., Raval R. R., Turley H., Harris A. L., Pugh C. W., Ratcliffe P. J. and Gleadle J. M. (2004). Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. 279, 38458-38465. 10.1074/jbc.M406026200 [DOI] [PubMed] [Google Scholar]
- Balaggan K. S. and Ali R. R. (2012). Ocular gene delivery using lentiviral vectors. 19, 145-153. 10.1038/gt.2011.153 [DOI] [PubMed] [Google Scholar]
- Barkho B. Z., Munoz A., Li X., Li L., Cunningham A. and Zhao X. (2009). Endogenous matrix metalloproteinase (MMP)-3 and MMP-9 promote the differentiation and migration of adult neural progenitor cells in response to chemokines. 26, 3139-3149. 10.1634/stemcells.2008-0519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G. and Song S. (2005). The role of pericytes in blood-vessel formation and maintenance. 7, 452-464. 10.1215/S1152851705000232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprara C., Thiersch M., Lange C., Joly S., Samardzija M. and Grimm C. (2011). HIF1A is essential for the development of the intermediate plexus of the retinal vasculature. 52, 2109-2117. 10.1167/iovs.10-6222 [DOI] [PubMed] [Google Scholar]
- Cepko C. (2014). Intrinsically different retinal progenitor cells produce specific types of progeny. 15, 615-627. 10.1038/nrn3767 [DOI] [PubMed] [Google Scholar]
- Chu C. J., Gardner P. J., Copland D. A., Liyanage S. E., Gonzalez-Cordero A., Kleine Holthaus S.-M., Luhmann U. F. O., Smith A. J., Ali R. R. and Dick A. D. (2016). Multimodal analysis of ocular inflammation using the endotoxin- induced uveitis mouse model. 9, 473-481. 10.1242/dmm.022475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claxton S. and Fruttiger M. (2005). Oxygen modifies artery differentiation and network morphogenesis in the retinal vasculature. 233, 822-828. 10.1002/dvdy.20407 [DOI] [PubMed] [Google Scholar]
- Dalkara D., Byrne L. C., Klimczak R. R., Visel M., Yin L., Merigan W. H., Flannery J. G. and Schaffer D. V. (2013). In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. 5, 189ra76 10.1126/scitranslmed.3005708 [DOI] [PubMed] [Google Scholar]
- De Filippis L. and Delia D. (2011). Hypoxia in the regulation of neural stem cells. 68, 2831-2844. 10.1007/s00018-011-0723-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dela Paz N. G. and D'Amore P. A. (2009). Arterial versus venous endothelial cells. 335, 5-16. 10.1007/s00441-008-0706-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding K., Scortegagna M., Seaman R., Birch D. G. and Garcia J. A. (2005). Retinal disease in mice lacking hypoxia-inducible transcription factor-2α. 46, 1010-1016. 10.1167/iovs.04-0788 [DOI] [PubMed] [Google Scholar]
- Duan L. J., Takeda K. and Fong G. H. (2014). Hypoxia inducible factor-2a regulates the development of retinal astrocytic network by maintaining adequate supply of astrocyte progenitors. 9, 1-12. 10.1371/journal.pone.0084736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantin A., Vieira J. M., Gestri G., Denti L., Schwarz Q., Prykhozhij S., Peri F., Wilson S. W. and Ruhrberg C. (2010). Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. 116, 829-840. 10.1182/blood-2009-12-257832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøen R., Johnsen E. O., Nicolaissen B., Facskó A., Petrovski G. and Moe M. C. (2013). Does the adult human ciliary body epithelium contain “true” retinal stem cells? 2013, 1-7. 10.1155/2013/531579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruttiger M. (2007). Development of the retinal vasculature. 10, 77-88. 10.1007/s10456-007-9065-1 [DOI] [PubMed] [Google Scholar]
- Fruttiger M., Calver A. R., Kru W. H., Mudhar H. S., Michalovich D., Takakura N., Nishikawa S. I. and Richardson W. D. (1996). PDGF mediates a neuron–astrocyte interaction in the developing retina. 17, 1117-1131. 10.1016/S0896-6273(00)80244-5 [DOI] [PubMed] [Google Scholar]
- Gardner P. J., Liyanage S. E., Cristante E., Sampson R. D., Dick A. D., Ali R. R. and Bainbridge J. W. (2017). Hypoxia inducible factors are dispensable for myeloid cell migration into the inflamed mouse eye. 7, 40830 10.1038/srep40830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt H., Golding M., Fruttiger M., Ruhrberg C., Lundkvist A., Abramsson A., Jeltsch M., Mitchell C., Alitalo K., Shima D. et al. (2003). VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. 161, 1163-1177. 10.1083/jcb.200302047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaccia A. J., Simon M. C. and Johnson R. (2004). The biology of hypoxia: the role of oxygen sensing in development, normal function, and disease. 18, 2183-2194. 10.1101/gad.1243304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber M., Hu C.-J., Johnson R. S., Brown E. J., Keith B. and Simon M. C. (2006). Acute postnatal ablation of Hif-2a results in anemia. 104, 2301-2306. 10.1073/pnas.0608382104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase V. H. (2013). Regulation of erythropoiesis by hypoxia-inducible factors. 27, 41-53. 10.1016/j.blre.2012.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama H., Kurokawa H., Kawano H., Ando R., Shimogori T., Noda H., Fukami K., Sakaue-sawano A. and Miyawaki A. (2011). Sca l e : a chemical approach for fluorescence imaging and reconstruction of transparent mouse brain. 14, 1481-1488. 10.1038/nn.2928 [DOI] [PubMed] [Google Scholar]
- Han H. (2012). Twisted blood vessels: symptoms, etiology and biomechanical mechanisms. 49, 185-197. 10.1159/000335123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harno E., Cottrell E. C. and White A. (2013). Metabolic pitfalls of CNS cre-based technology. 18, 21-28. 10.1016/j.cmet.2013.05.019 [DOI] [PubMed] [Google Scholar]
- Hawkins B. T. and Davis T. P. (2005). The blood-brain barrier/neurovascular unit in health and disease. 57, 173-185. 10.1124/pr.57.2.4 [DOI] [PubMed] [Google Scholar]
- Hellström M., Phng L.-K., Hofmann J. J., Wallgard E., Coultas L., Lindblom P., Alva J., Nilsson A.-K., Karlsson L., Gaiano N. et al. (2007). Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. 445, 776-780. 10.1038/nature05571 [DOI] [PubMed] [Google Scholar]
- Hitchcock P., Macdonald R., VanDeRyt J. and Wilson S. (1996). Antibodies against Pax6 immunostain amacrine and ganglion cells and neuronal progenitors, but not rod precursors, in the normal and regenerating retina of the goldfish. 29, 399-413. [DOI] [PubMed] [Google Scholar]
- Hu C.-J., Wang L.-Y., Chodosh L. A., Keith B. and Simon M. C. (2003). Differential roles of hypoxia-inducible factor 1 alpha (HIF-1 alpha) and HIF-2 alpha in hypoxic gene regulation. 23, 9361-9374. 10.1128/MCB.23.24.9361-9374.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iughetti P., Suzuki O., Godoi P. H. C., Alves V. A. F., Sertié A. L., Zorick T., Soares F., Camargo A., Moreira E. S., Di Loreto C. et al. (2001). A polymorphism in endostatin, an angiogenesis inhibitor, predisposes for the development of prostatic adenocarcinoma. 61, 7375-7378. [PubMed] [Google Scholar]
- Kim J.-Y., Grunke S. D., Levites Y., Golde T. E. and Jankowsky J. L. (2014). Intracerebroventricular viral injection of the neonatal mouse brain for persistent and widespread neuronal transduction. 91, 51863-51873. 10.3791/51863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C. A. K., Luhmann U. F. O., Mowat F. M., Georgiadis A., West E. L., Abrahams S., Sayed H., Powner M. B., Fruttiger M., Smith A. J. et al. (2012). Von Hippel-Lindau protein in the RPE is essential for normal ocular growth and vascular development. 139, 2340-2350. 10.1242/dev.070813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q., Cong X. and Yun Z. (2011). Differential hypoxic regulation of hypoxia-inducible factors 1 and 2. 9, 757-765. 10.1158/1541-7786.MCR-11-0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Kim S.-Y., Fu Y., Wu X., Ng L., Swaroop A. and Forrest D. (2013). An isoform of retinoid-related orphan receptor β directs differentiation of retinal amacrine and horizontal interneurons. 4, 1813 10.1038/ncomms2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Barneo J., Pardal R. and Ortega-Sáenz P. (2001). Cellular mechanism of oxygen sensing. 63, 259-287. 10.1146/annurev.physiol.63.1.259 [DOI] [PubMed] [Google Scholar]
- Lothian C. and Lendahl U. (1997). An evolutionarily conserved region in the second lntron of the human nestin gene directs gene exmession to CNS progenitor cells and to early neural crest cells. 9, 452-462. 10.1111/j.1460-9568.1997.tb01622.x [DOI] [PubMed] [Google Scholar]
- Luhmann U. F. O., Robbie S., Munro P. M. G., Barker S. E., Duran Y., Luong V., Fitzke F. W., Bainbridge J. W. B., Ali R. R. and Maclaren R. E. (2009). The drusenlike phenotype in aging Ccl2-knockout mice is caused by an accelerated accumulation of swollen autofluorescent subretinal macrophages. 50, 5934-5943. 10.1167/iovs.09-3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majmundar A. J., Wong W. J. and Simon M. C. (2010). Hypoxia-inducible factors and the response to hypoxic stress. 40, 294-309. 10.1016/j.molcel.2010.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaenko A., Chen H., Kammer J., Zhang J. H. and Tang J. (2011). Comparison Evans Blue injection routes: Intravenous versus intraperitoneal, for measurement of blood-brain barrier in a mice hemorrhage model. 195, 206-210. 10.1016/j.jneumeth.2010.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt T., Ashery-padan R., Andrejewski N., Scardigli R., Guillemot F. and Gruss P. (2001). Pax6 is required for the multipotent state of retinal progenitor cells. 105, 43-55. 10.1016/S0092-8674(01)00295-1 [DOI] [PubMed] [Google Scholar]
- Mori M., Metzger D., Garnier J., Chambon P. and Mark M. (2002). Site-specific somatic mutagenesis in the retinal pigment epithelium. 43, 1384-1388. [PubMed] [Google Scholar]
- Mowat F. M., Luhmann U. F. O., Smith A. J., Lange C., Duran Y., Harten S., Shukla D., Maxwell P. H., Ali R. R. and Bainbridge J. W. B. (2010). HIF-1alpha and HIF-2alpha are differentially activated in distinct cell populations in retinal ischaemia. 5, 1-9. 10.1371/journal.pone.0011103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar M., Tasic B., Miyamichi K., Li L. and Luo L. (2007). A global double-fluorescent Cre reporter mouse. 45, 593-605. 10.1002/dvg.20335 [DOI] [PubMed] [Google Scholar]
- Nakamura-Ishizu A., Kurihara T., Okuno Y., Ozawa Y., Kishi K., Goda N., Tsubota K., Okano H., Suda T. and Kubota Y. (2012). The formation of an angiogenic astrocyte template is regulated by the neuroretina in a HIF-1-dependent manner. 363, 106-114. 10.1016/j.ydbio.2011.12.027 [DOI] [PubMed] [Google Scholar]
- Ogle B. M., Cascalho M. and Platt J. L. (2005). Biological implications of cell fusion. 6, 567-575. 10.1038/nrm1678 [DOI] [PubMed] [Google Scholar]
- Okabe K., Kobayashi S., Yamada T., Kurihara T., Tai-Nagara I., Miyamoto T., Mukouyama Y. S., Sato T. N., Suda T., Ema M. et al. (2014). Neurons limit angiogenesis by titrating VEGF in retina. 159, 584-596. 10.1016/j.cell.2014.09.025 [DOI] [PubMed] [Google Scholar]
- Phng L.-K., Potente M., Leslie J. D., Babbage J., Nyqvist D., Lobov I., Ondr J. K., Rao S., Lang R. A., Thurston G. et al. (2009). Nrarp coordinates endothelial notch and Wnt signaling to control vessel density in angiogenesis. 16, 70-82. 10.1016/j.devcel.2008.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitulescu M. E., Schmidt I., Benedito R. and Adams R. H. (2010). Inducible gene targeting in the neonatal vasculature and analysis of retinal angiogenesis in mice. 5, 1518-1534. 10.1038/nprot.2010.113 [DOI] [PubMed] [Google Scholar]
- Rattner A., Wang Y., Zhou Y., Williams J. and Nathans J. (2014). The role of the hypoxia response in shaping retinal vascular development in the absence of norrin/frizzled4 signaling. 55, 8614-8625. 10.1167/iovs.14-15693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese B. E., Necessary B. D., Tam P. P. L., Faulkner-Jones B. and Tan S. S. (1999). Clonal expansion and cell dispersion in the developing mouse retina. 11, 2965-2978. 10.1046/j.1460-9568.1999.00712.x [DOI] [PubMed] [Google Scholar]
- Ruhrberg C., Gerhardt H., Golding M., Watson R., Ioannidou S., Fujisawa H., Betsholtz C. and Shima D. T. (2002). Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. 16, 2684-2698. 10.1101/gad.242002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan H. E., Poloni M., Mcnulty W., Elson D., Gassmann M., Arbeit J. M. and Johnson R. S. (2000). Advances in brief hypoxia-inducible factor-1 α is a positive factor in solid tumor growth. 60, 4010-4015. [PubMed] [Google Scholar]
- Sapieha P., Sirinyan M., Hamel D., Zaniolo K., Joyal J.-S., Cho J.-H., Honoré J.-C., Kermorvant-Duchemin E., Varma D. R., Tremblay S. et al. (2008). The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. 14, 1067-1076. 10.1038/nm.1873 [DOI] [PubMed] [Google Scholar]
- Schmidt A., Wenzel D., Ferring I., Kazemi S., Sasaki T., Hescheler J., Timpl R., Addicks K., Fleischmann B. K. and Bloch W. (2004). Influence of endostatin on embryonic vasculo- and angiogenesis. 230, 468-480. 10.1002/dvdy.20072 [DOI] [PubMed] [Google Scholar]
- Scortegagna M., Ding K., Oktay Y., Gaur A., Thurmond F., Yan L.-J., Marck B. T., Matsumoto A. M., Shelton J. M., Richardson J. A. et al. (2003). Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. 35, 331-340. 10.1038/ng1266 [DOI] [PubMed] [Google Scholar]
- Scott A., Powner M. B., Gandhi P., Clarkin C., Gutmann D. H., Johnson R. S., Ferrara N. and Fruttiger M. (2010). Astrocyte-derived vascular endothelial growth factor stabilizes vessels in the developing retinal vasculature. 5, e11863 10.1371/journal.pone.0011863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura I., De Smet F., Hohensinner P. J., Ruiz de Almodovar C. and Carmeliet P. (2009). The neurovascular link in health and disease: an update. 15, 439-451. 10.1016/j.molmed.2009.08.005 [DOI] [PubMed] [Google Scholar]
- Smith A. J., Schlichtenbrede F. C., Tschernutter M., Bainbridge J. W., Thrasher A. J. and Ali R. R. (2003). AAV-mediated gene transfer slows photoreceptor loss in the RCS rat model of retinitis pigmentosa. 8, 188-195. 10.1016/S1525-0016(03)00144-8 [DOI] [PubMed] [Google Scholar]
- Stenzel D., Lundkvist A., Sauvaget D., Busse M., Graupera M., van der Flier A., Wijelath E. S., Murray J., Sobel M., Costell M. et al. (2011). Integrin-dependent and -independent functions of astrocytic fibronectin in retinal angiogenesis. 138, 4451-4463. 10.1242/dev.071381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surzenko N., Crowl T., Bachleda A., Langer L. and Pevny L. (2013). SOX2 maintains the quiescent progenitor cell state of postnatal retinal Muller glia. 140, 1445-1456. 10.1242/dev.071878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos A., Morizane Y., Murakami Y., Giani A., Mantopoulos D., Kayama M., Roh M. I., Michaud N., Pawlyk B., Sandberg M. et al. (2012). Evidence for baseline retinal pigment epithelium pathology in the Trp1-Cre mouse. 180, 1917-1927. 10.1016/j.ajpath.2012.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronche F., Kellendonk C., Kretz O., Gass P., Anlag K., Orban P. C., Bock R., Klein R. and Schütz G. (1999). Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. 23, 99-103. 10.1038/12703 [DOI] [PubMed] [Google Scholar]
- Tschernutter M., Schlichtenbrede F. C., Howe S., Balaggan K. S., Munro P. M., Bainbridge J. W. B., Thrasher A. J., Smith A. J. and Ali R. R. (2005). Long-term preservation of retinal function in the RCS rat model of retinitis pigmentosa following lentivirus-mediated gene therapy. 12, 694-701. 10.1038/sj.gt.3302460 [DOI] [PubMed] [Google Scholar]
- Usui Y., Westenskow P. D., Kurihara T., Aguilar E., Sakimoto S., Paris L. P., Wittgrove C., Feitelberg D., Friedlander M. S. H., Moreno S. K. et al. (2015). Neurovascular crosstalk between interneurons and capillaries is required for vision. 125, 2335-2346. 10.1172/JCI80297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Chona F., Clark A. M. and Levine E. M. (2009). Rlbp1 promoter drives robust Muller glial GFP expression in transgenic mice. 50, 3996-4003. 10.1167/iovs.08-3189 [DOI] [PubMed] [Google Scholar]
- Walia A., Yang J., Huang Y., Rosenblatt M., Chang J. and Azar D. (2015). Endostatin's emerging roles in angiogenesis, lymphangiogenesis, disease, and clinical applications. 1850, 2422-2438. 10.1016/j.bbagen.2015.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann A., Krohne T. U., Aguilar E., Kurihara T., Dorrell M. I., Simon M. C., Haase V. H., Friedlander M. and Johnson R. S. (2010). Astrocyte hypoxic response is essential for pathological but not developmental angiogenesis of the retina. 58, 1177-1185. 10.1002/glia.20997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q., Qaum T. and Adamis A. P. (2001). Sensitive blood – retinal barrier breakdown quantitation using evans blue. 42, 789-794. [PubMed] [Google Scholar]
- Yu D.-Y. and Cringle S. J. (2001). Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. 20, 175-208. 10.1016/S1350-9462(00)00027-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.