ABSTRACT
Embryonic morphogenesis of a complex organism requires proper regulation of patterning and directional growth. Planar cell polarity (PCP) signaling is emerging as a crucial evolutionarily conserved mechanism whereby directional information is conveyed. PCP is thought to be established by global cues, and recent studies have revealed an instructive role of a Wnt signaling gradient in epithelial tissues of both invertebrates and vertebrates. However, it remains unclear whether Wnt/PCP signaling is regulated in a coordinated manner with embryonic patterning during morphogenesis. Here, in mouse developing limbs, we find that apical ectoderm ridge-derived Fgfs required for limb patterning regulate PCP along the proximal-distal axis in a Wnt5a-dependent manner. We demonstrate with genetic evidence that the Wnt5a gradient acts as a global cue that is instructive in establishing PCP in the limb mesenchyme, and that Wnt5a also plays a permissive role to allow Fgf signaling to orient PCP. Our results indicate that limb morphogenesis is regulated by coordination of directional growth and patterning through integration of Wnt5a and Fgf signaling.
KEY WORDS: Fgf, Wnt, Vangl2, Planar cell polarity, PCP, Morphogenesis, Mouse
Summary: During mouse limb morphogenesis, Wnt5a plays both instructive and permissive roles in regulating planar cell polarity by orienting PCP itself, and by allowing apical ectoderm ridge-derived Fgfs to orient PCP.
INTRODUCTION
Planar cell polarity (PCP), a term that originally referred to the coordinated alignment of epithelial cells within a plane perpendicular to their apical-basal axis, is an essential mechanism underlying coordinately polarized cellular and tissue behaviors (Wang and Nathans, 2007; Simons and Mlodzik, 2008; Gray et al., 2011; Yang and Mlodzik, 2015). PCP is required in many symmetry-breaking morphogenetic events in vertebrate development, for instance left-right patterning, sensory hair cell orientation in the inner ear, skin hair orientation and neural tube closure (Montcouquiol et al., 2003; Wang and Nathans, 2007; Devenport and Fuchs, 2008; Song et al., 2010; Hashimoto et al., 2010). Establishment of PCP is first evident molecularly by the uniform asymmetric distribution of the Drosophila core PCP proteins Frizzled, Dishevelled, Van Gogh, Prickle and Flamingo (also known as Starry Night) throughout the polarized tissue (Goodrich and Strutt, 2011; Adler, 2012; Singh and Mlodzik, 2012). Such uniform asymmetric localization is a result of both intracellular and intercellular interactions of the core PCP proteins that amplify and coordinate the initial polarizing signals provided by global cues (Simons and Mlodzik, 2008; Vladar et al., 2009; Goodrich and Strutt, 2011; Wu and Mlodzik, 2009; Yang and Mlodzik, 2015). Several mechanisms have been proposed to regulate PCP establishment by global cues, including cell adhesion gradients, morphogenetic forces and Wnt signaling gradients (Lawrence et al., 1996, 2007; Casal et al., 2002; Aigouy et al., 2010; Matis and Axelrod, 2013; Wu et al., 2013; Chu and Sokol, 2016; Minegishi et al., 2017; Humphries and Mlodzik, 2017). Secreted Wnt molecules have been shown to regulate PCP by binding to the frizzled receptors (Adler et al., 1997; Tomlinson et al., 1997; Lawrence et al., 2004; Dabdoub and Kelley, 2005; Wu and Mlodzik, 2008, 2009; Wu et al., 2013) and Ror2 (Gao et al., 2011; Wang et al., 2011). In vertebrates, Wnt ligands are required to regulate PCP (Rauch et al., 1997; Heisenberg et al., 2000; Kilian et al., 2003; Gros et al., 2009) and Wnt5a genetically interacts with a core PCP protein, Vangl2, in multiple developmental processes (Qian et al., 2007; Wang et al., 2011). Recent studies in Drosophila wing, Xenopus ectoderm and mouse node epithelium also provide evidence for an instructive role of Wnts in establishing PCP (Wu et al., 2013; Chu and Sokol, 2016; Minegishi et al., 2017; Humphries and Mlodzik, 2017).
Embryonic morphogenesis is a complex process that requires proper regulation of both patterning and tissue polarity. Morphogen gradients are well known for their roles in pattern formation and Wnt5a signaling is essential for PCP regulation, but it remains to be elucidated whether there is an intrinsic coordination between tissue patterning and Wnt5a-regulated PCP establishment to ensure proper morphogenesis. The limb is an ideal experimental system for tackling these questions as early limb patterning is controlled by well-defined signaling centers (Zeller et al., 2009) and we have shown previously that Wnt5a signaling is required in mouse for PCP establishment along the proximal-distal (P-D) limb axis in forming chondrocytes (Gao et al., 2011). Wnt5a and fibroblast growth factors (Fgfs) are both required for limb elongation along the P-D axis. Wnt5a is expressed in a P-D gradient in the limb mesoderm and Wnt5a null limbs are truncated with distal digits missing (Parr et al., 1993; Yamaguchi et al., 1999; Fisher et al., 2008). It is well known that Fgfs secreted from the apical ectoderm ridge (AER) play an instructive role in early limb patterning along the P-D axis (Lewandoski et al., 2000; Sun et al., 2002; Mariani et al., 2008). Before chondrogenic mesenchymal condensation occurs, Fgfs induce multiple responses, such as maintaining the progenitor cell pool, regulating mesenchymal differentiation, promoting proliferation, inhibiting apoptosis, acting as chemoattractants or stimulating random cell movements in early limb bud (Niswander et al., 1993; Li and Muneoka, 1999; Sun et al., 2002; Yu and Ornitz, 2008; Bénazéraf et al., 2010; Gros et al., 2010). When Fgf4 and Fgf8 are specifically removed from the AER by Msx2-Cre, the distal limb cartilage is much shortened, but cell death is only restricted to the proximal limb mesenchyme and cell proliferation is largely unchanged (Sun et al., 2002; Mariani et al., 2008), suggesting that Fgf signaling may also regulate elongation of cartilage in the distal limb independently of cell proliferation or survival. We therefore hypothesized that AER-derived Fgf signals may play a role in P-D limb elongation by interacting with Wnt5a/PCP signaling during chondrogenesis.
Vangl2 and Vangl1 are core PCP proteins that show uniform asymmetric localization in a field of planar polarized cells and they have been shown to be required for limb cartilage elongation (Song et al., 2010; Gao et al., 2011; Wang et al., 2011). Vangl2 is present with a random distribution on the membrane of pre-chondrogenic mesenchymal cells before embryonic day (E) 11.5, but quickly becomes asymmetrically localized along the P-D axis in newly differentiated chondrocytes at E12.5. Wnt5a is required for Vangl2 asymmetric localization and it induces Vangl2 phosphorylation in a dose-dependent manner (Gao et al., 2011). As Wnt5a is the only Wnt expressed in a gradient along the P-D axis in the limb mesoderm and is known to be required for cartilage elongation (Parr et al., 1993; Yamaguchi et al., 1999; Fisher et al., 2008; Witte et al., 2009; Gao et al., 2011; Zhu et al., 2012), it is likely that Wnt5a plays an instructive role in the limb mesenchyme to planar polarize the differentiating chondrocytes along the P-D axis.
In this study, we demonstrate a previously unappreciated role of AER-derived Fgf signaling in regulating Wnt5a/PCP signaling during limb morphogenesis, although Wnt5a and Fgf8 did not regulate each other's expression in the developing limb bud. We also show with genetic approaches that Wnt5a signaling plays both instructive and permissive roles in orienting PCP during limb morphogenesis. Our results suggest that PCP and patterning are intrinsically linked during morphogenesis, and that multiple cues regulate PCP in the limb mesoderm.
RESULTS
AER-derived Fgfs are required to regulate PCP, but not Wnt5a expression
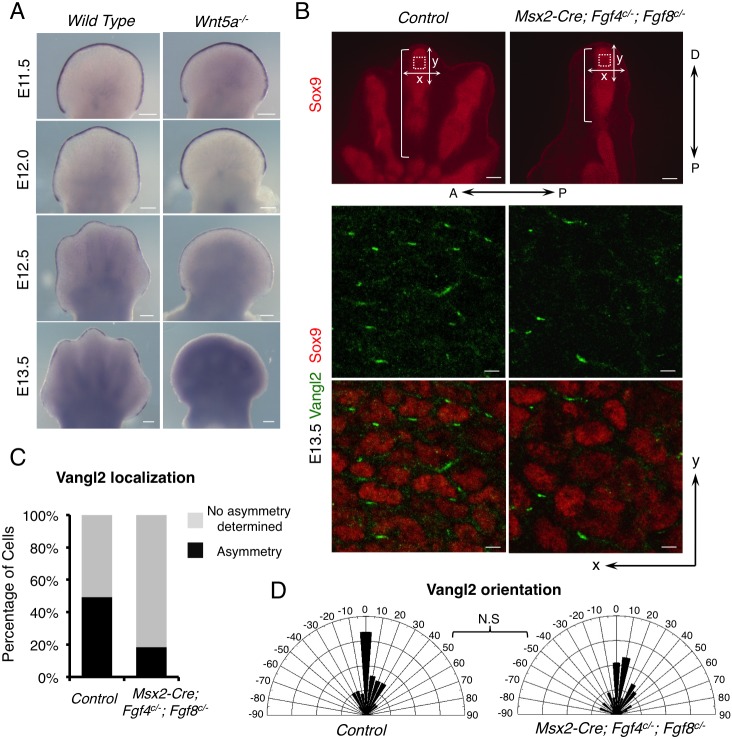
As a first attempt to test the possible interaction between Fgf and Wn5a/PCP signaling, we investigated whether Wnt5a and Fgfs regulated expression of each other. In the wild-type mouse embryo, Fgf8 expression starts in the forming AER at E9.0-E9.5 (Crossley and Martin, 1995; Sun et al., 2002) and is weakened first in the regressing AER overlying the interdigital region at E12.5 after chondrogenesis starts (Pajni-Underwood et al., 2007) (Fig. 1A). In the Wnt5a−/− embryo, Fgf8 expression was indistinguishable from that in the wild-type control until E12.5 (Fig. 1A) (Yamaguchi et al., 1999), at which time the Wnt5a−/− limb phenotype is already apparent (Fig. 1A). Fgf8 expression was weakened after E12.5 and then completely disappeared from the AER at E13.5 when distal chondrogenesis failed in the Wnt5a−/− embryos (Yamaguchi et al., 1999; Topol et al., 2003) (Fig. 1A). But in the wild-type embryo at E13.5, Fgf8 expression was still detected in the AER overlying the elongating digit (Fig. 1A). We therefore concluded that Wnt5a does not directly regulate Fgf8 expression. Earlier cessation of Fgf8 expression in the Wnt5a−/− embryos is more likely to be a result of loss of distal chondrogenesis, not a cause. Loss of distal chondrogenesis in the Wnt5a−/− limb bud likely disrupted the feedback between the phalanx-forming region and Fgf8 expressed in the AER. Moreover, as loss of Fgf8 expression in the Wnt5a−/− limb bud occurred after the normal PCP establishment time, E12.5 (Gao et al., 2011), it is unlikely that loss of PCP is solely due to precocious weakening or loss of Fgf8 expression in the AER. We next investigated whether Fgfs expressed in the AER regulated Wnt5a expression using the Msx2-Cre;Fgf4c/−;Fgf8c/− embryo (Fig. S1A). We found that removal of Fgf4 and Fgf8 from the AER in these mutant embryos did not change the Wnt5a expression pattern, although the limb bud was much smaller, indicating that Fgf4/8 does not regulate Wnt5a expression either.
Fig. 1.
Fgfs from the AER are required for full PCP in limb development. (A) Whole-mount in situ hybridization of Fgf8 in wild-type and Wnt5a−/− embryonic forelimbs. (B) Compromised Vangl2 asymmetrical localization in distal digits of E13.5 Msx2-Cre; Fgf4c/−; Fgf8c/− forelimbs. The boxed regions were subjected to confocal scanning. Enlarged images of part of the scanned regions are shown in the middle and lower panels. Vangl2 (green) and Sox9 (red) protein are shown by fluorescence immunostaining. The brackets indicate the length of digits. Proximal-distal (P-D) and anterior-posterior (A-P) axes are indicated. x-and y-axes of the images are defined as shown in the upper panel. (C) The percentage of cells with discernible Vangl2 asymmetric localization in control and mutant limbs. (D) Schematics summarizing the quantification of orientation angles of Vangl2. x-axis, angle of orientation (−90° to 90°); y-axis, percentage of cells at angle x. Kolmogorov–Smirnov test, N.S, no significance. (C,D) Number of samples and number of cells analyzed for each genotype: control, N=3, n=325; Msx2-Cre; Fgf4c/−; Fgf8c/−, N=5, n=450. Scale bars: 200 μm (A, upper panel of B); 10 μm (middle and bottom panels of B).
As cell death in the Msx2-Cre;Fgf4c/−;Fgf8c/− embryo is only restricted to the proximal limb mesenchyme and cell proliferation is largely unchanged in both early and late limb bud (Sun et al., 2002; Mariani et al., 2008) (Fig. S1B), we reasoned that besides regulating progenitor cell pool (Sun et al., 2002), AER-Fgfs may also regulate limb elongation through PCP without affecting Wnt5a expression. Loss of or reduced PCP signaling in mouse embryos has been found to cause shortened limb and digits in Vangl1−/−;Vangl2−/− (Fig. S1C) (Song et al., 2010) or Prickle1−/− (Yang et al., 2013) embryos. To test whether Fgfs regulate PCP in the limb mesenchyme, we first examined Vangl2 localization, which is considered to be a functional PCP readout (Wang and Nathans, 2007; Gray et al., 2011; Yang and Mlodzik, 2015), in the newly formed distal digit cartilage where its asymmetric localization is most pronounced (Gao et al., 2011). Two features of Vangl2 localization were measured: the percentage of cells showing asymmetric Vangl2 and the orientation angles of Vangl2 proteins in these cells (see details in Materials and Methods). Changes in one or both of these features alter PCP. We found that the number of cells with discernible asymmetric Vangl2 was significantly reduced in the digits of the Msx2-Cre;Fgf4c/−;Fgf8c/− embryos compared with those of the littermate controls (Fig. 1B,C), but there was no statistically significant change of Vangl2 orientation angles in the Msx2-Cre;Fgf4c/−;Fgf8c/− mutant (Fig. 1D). As PCP signaling induces polarized cellular responses including chondrocyte polarity and arrangement (Li and Dudley, 2009; Wallingford, 2012; Yang and Mlodzik, 2015; Li et al., 2017), we then examined cell shapes and the alignment of distal chondrocytes. The results are shown as length-to-width ratios (LWR) and orientation angles, respectively (see details in Materials and Methods). Indeed, we found that loss of Fgf4 and Fgf8 from the AER altered the cell shape in the limb mesenchyme. In control embryos, distal chondrocytes were elongated with an average LWR of 2 (Fig. S2A). Control chondrocytes were oriented mostly along the x-axis (−20° to +20°) (Fig. S2B). In the mutant, chondrocytes were more round with reduced LWR. The average LWR was close to 1.5. The orientation angles were also increased (Fig. S2). Therefore, a reduction in the proportion of chondrocytes with appropriately biased Vangl2 resulted in altered shape and orientation of chondrocytes, suggesting that Fgf signaling is required for proper PCP signaling in distal limb chondrocytes. Weakened PCP signaling due to absence of Fgf4/8 may lead to weakened intercellular interactions between PCP components such as Vangl2 and Fzd in neighboring cells, which are required to strengthen uniform cell orientation (Yang and Mlodzik, 2015). The shortened digits resulting from loss of AER-Fgf signals could be a combined consequence of reduced progenitor cell pool and directional outgrowth regulated by PCP (Sun et al., 2002; Gao and Yang, 2013).
Fgf signaling plays an instructive role in PCP
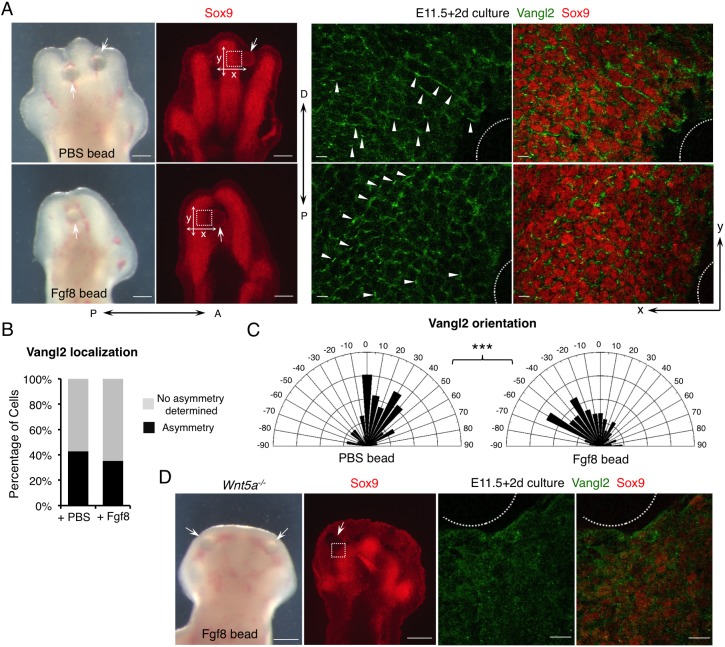
To test directly whether Fgfs play a permissive or an instructive role in regulating PCP in the distal limb, we altered the direction of Fgf signaling gradient in the distal limb. Beads that were soaked in either PBS or Fgf8 protein were inserted into the interdigital mesenchyme at E11.5 when digital condensations were forming and Vangl2 asymmetric localization had not yet been established. We cultured the limb buds with bead implantation for 2 days in a rolling tube under optimized conditions that allow good digit morphogenesis and proper PCP establishment in the wild-type control limb (Fig. 2A, Fig. S3). The cultured limbs implanted with PBS beads showed normal expression of the chondrocyte/chondrogenic progenitor marker Sox9, the chondrocyte marker Col2a1, and Msx1, which marks distal and interdigital limb mesenchyme (Fig. S3B). These data indicate that this ex utero limb culturing system supports normal limb growth and morphogenesis for at least 2 days. In contrast to PBS beads, Fgf8 beads inhibited overall limb chondrogenesis, a known effect of Fgf signaling (Moftah et al., 2002), and, more interestingly, it also caused bending of nearby digits towards the beads (Fig. 2A, Fig. S3A,B). It is unlikely that such bending is due to distorted digit outgrowth as the apoptosis and proliferation patterns in distal limb induced by PBS beads and Fgf8 beads were similar (Fig. S3C). Furthermore, we found that Fgf8 beads did not bend the digit by inducing Wnt5a expression around the bead (Fig. S4A,B). As a control, we confirmed that Fgf8 beads did strongly induce expression of Msx1 and Sprouty4 (Spry4), two transcriptional targets of Fgf signaling (Furthauer et al., 2001) (Fig. S3B, Fig. S4A).
Fig. 2.
Fgf signaling is sufficient to regulate PCP in a Wnt5a-dependent manner. (A) Representative morphological and immunofluorescence images of cultured distal limbs. Beads soaked in PBS or Fgf8 recombinant protein were inserted into E11.5 mouse embryonic forelimbs, which were then cultured for 2 days. The boxed regions lateral to the beads (arrows) were scanned by confocal microscope and representative images are shown in the middle (Vangl2, green) and right-hand panels (Vangl2/Sox9, green/red). The beads are outlined. Arrowheads point to some of the asymmetrically localized Vangl2. P-D and A-P axes are indicated. x- and y-axes of the images are defined as shown in the left-hand panels. (B) The percentage of cells with discernible Vangl2 asymmetric localization in digits implanted with PBS- or Fgf8-soaked beads. (C) Schematics summarizing the quantification of orientation angles of Vangl2. x-axis, angle of orientation (−90° to 90°); y-axis, percentage of cells at angle x. Kolmogorov–Smirnov test, ***P=0.0008. (B,C) PBS beads-implanted limbs N=4, analyzed cells n=711; Fgf8 beads-implanted limbs N=5, analyzed cells n=678. (D) Loss of Vangl2 asymmetric localization in Wnt5a−/− limbs cannot be rescued by Fgf8-soaked beads after 2 days of culture. Boxed region close to the bead (arrow) is shown in the middle (Vangl2, green) and right-hand panels (Vangl2/Sox9, green/red) at higher magnification. The bead is outlined. No Vangl2 asymmetric localization was observed. Cultured Wnt5a−/− limbs: N=4. Scale bars: 200 μm (left-hand panels of A and D); 20 μm (right-hand panels of A and D).
To determine whether the digit-bending effects of Fgf8 beads are caused by altered PCP, we first examined and quantified Vangl2 localization in the chondrocytes around the beads (Fig. 2A-C). First, the percentage of cells with discernible asymmetric Vangl2 were comparable in PBS and Fgf8 bead-implanted limbs (Fig. 2B). Whereas most of the cells around the PBS bead exhibited a Vangl2 localization pattern along the y-axis (orientation angle −45° to +45° with reference to the x-axis), not towards the bead, the number of cells with reoriented Vangl2 localization towards the Fgf8 bead was significantly increased (orientation angle <−45° or >45° with reference to the x-axis) (Fig. 2A,C). In a control experiment, Shh-soaked beads were similarly inserted to the interdigital region, which caused a significant change in digit growth, but PCP was not altered. Most of the cells lateral to the Shh beads were still oriented along the original P-D axis (Fig. S4C), indicating that growth distortion is not sufficient to cause the observed Vangl2 reorientation. Importantly, if Fgf8 caused digit bending independently of PCP, the direction of Vangl2 orientation would be unlikely to be changed (explained in Fig. S5). Interestingly, we observed higher Vangl2 levels and more clearly reoriented Vangl2 localization in cells slightly away from (not adjacent to) the Fgf8 bead (Fig. 2A), probably due to Fgf8-mediated inhibition of chondrogenesis in adjacent cells. Vangl2 asymmetric localization only occurs in chondrocytes with high Sox9 expression (Gao et al., 2011). At the cellular level, we found that although Fgf8 beads did not change the shape of the cells (Fig. S6A), cell orientation was altered significantly (Fig. S6B). More cells around the Fgf8 beads were oriented along the y-axis (<−45° or >45° with reference to the x-axis) instead of the x-axis, indicating that altering the direction of the Fgf signaling gradient reoriented chondrocytes without significantly changing cell shape. These results indicate that Fgf8 can act as an instructive cue to regulate PCP when limb mesenchymal cells are differentiating into chondrocytes. In previous studies, when Fgf8 beads were implanted at a later stage (after PCP establishment), no digit bending was observed (Montero et al., 2001; Hernandez-Martinez et al., 2009).
We then tested whether the role of Fgf in regulating PCP requires Wnt5a signaling by implanting Fgf8 beads in the distal limb bud of the Wnt5a−/− embryos. Although the implanted Fgf8 beads induced Sprouty4 expression (Fig. S4A), they failed to rescue limb outgrowth or restore Vangl2 asymmetric localization in Sox9+ chondrogenic cells (Fig. 2D). These results indicate that Fgf8 cannot exert its instructive role on PCP in the absence of Wnt5a signaling.
We then investigated the molecular mechanism by which PCP is regulated by Fgf signaling. As we have shown previously that Wnt5a induces Vangl2 phosphorylation, and we and others found that Vangl2 activities depend on its levels of phosphorylation in multiple organisms, including Drosophila, zebrafish, Xenopus and mouse (Gao et al., 2011; Ossipova et al., 2015; Kelly et al., 2016; Yang et al., 2017), we tested whether Fgf could also regulate Vangl2 phosphorylation in limb bud mesenchymal cells. We isolated mouse E10.5-E11.5 limb bud mesenchymal cells and treated them with recombinant Fgf8 and/or Wnt5a proteins. Whereas Wnt5a protein induced Vangl2 phosphorylation as shown by gel mobility shift, Fgf8 protein by itself did not induce obvious gel mobility shift of Vangl2 (Fig. S7A). When applied simultaneously, Fgf8 dose-dependently enhanced Vangl2 phosphorylation induced by a lower Wnt5a dosage (Fig. S7A,B). In addition, at higher doses, Fgf8 also appeared to upregulate Vangl2 protein levels (Fig. S7B). These in vitro data are consistent with the notion that the role of Fgfs in PCP is instructive by promoting Wnt5a-induced Vangl2 phosphorylation and/or protein levels; in this regard, the function of Wnt5a could be permissive, i.e. to allow Fgf signaling to regulate PCP, and an instructive Wnt5a gradient may not be absolutely required for this purpose.
PCP is partially restored by non-graded Wnt5a expression in the limb
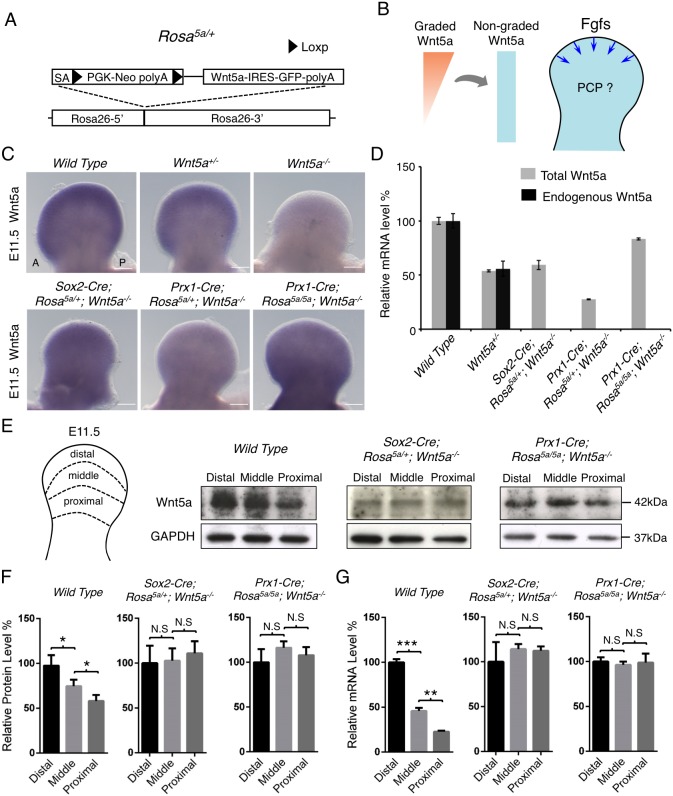
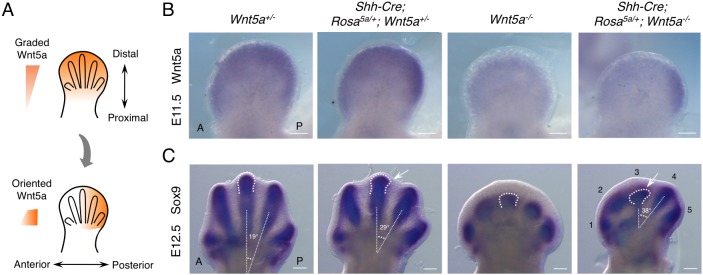
We then investigated whether Wnt5a signaling is indeed instructive in limb PCP as shown in different model systems (Wu et al., 2013; Chu and Sokol, 2016; Minegishi et al., 2017) or only permissive, i.e. allowing other cues, such as Fgf signaling, to orient PCP. Previous studies have also suggested a permissive role of Wnt5a (Heisenberg et al., 2000; Witze et al., 2008). To answer these questions, we replaced the endogenous graded Wnt5a expression with non-graded Wnt5a expression by employing an inducible Wnt5a expression approach (Fig. 3A,B). As the instructive model indicates that graded Wnt5a expression is required for Wnt5a's function in the limb, such replacement should not rescue the PCP defects of Wnt5a null mutant at all. Conversely, if Wnt5a signaling is solely permissive for PCP, the proposed strategy should completely rescue the PCP defects of the Wnt5a mutant. We used the Prx1-Cre and Sox2-Cre mouse lines to activate even Wnt5a expression in the early limb bud mesenchymal cells (Fig. 3C). Prx1-Cre is expressed at E9.5 and Sox2-Cre is expressed in all epiblast cells at E6.5 (Logan et al., 2002; Hayashi et al., 2002). Wnt5a expressions in Prx1-Cre;Rosa5a/+;Wnt5a−/− and Sox2-Cre;Rosa5a/+;Wnt5a−/− embryos were driven by the Rosa26 promoter, which is not graded in developing limbs (Soriano, 1999). We found that the graded Wnt5a expression pattern along the P-D axis of the limb bud was disrupted in Prx1-Cre;Rosa5a/+;Wnt5a−/− and Sox2-Cre;Rosa5a/+; Wnt5a−/− embryos at E11.5, a stage preceding asymmetric Vangl2 localization (Fig. 3C) (Gao et al., 2011). However, Wnt5a expression in Prx1-Cre; Rosa5a/+;Wnt5a−/− embryos was lower compared with that in Wnt5a+/− and Sox2-Cre;Rosa5a/+;Wnt5a−/− embryos (Fig. 3C). This was confirmed by quantitative PCR (qPCR) of Wnt5a expression in the limb buds (Fig. 3D). To increase Wnt5a expression driven by Prx1-Cre, we induced Wnt5a expression from both alleles of the Rosa26 locus by generating Prx1-Cre;Rosa5a/5a;Wnt5a−/− embryos, in which Wnt5a expression was comparable to that of Sox2-Cre;Rosa5a/+;Wnt5a−/− or Wnt5a+/− embryos (Fig. 3C,D). To further test whether this strategy resulted in non-graded Wnt5a expression and protein distribution, we examined both Wnt5a mRNA and Wnt5a protein levels in different regions of the limb buds along the P-D axis by qPCR and western blotting (Fig. 3E-G). The distal-to-proximal gradients of Wnt5a were much diminished in the limb buds of both Prx1-Cre;Rosa5a/5a;Wnt5a−/− and Sox2-Cre;Rosa5a/+;Wnt5a−/− embryos (Fig. 3E-G). These results indicate that, by expressing Wnt5a from the Rosa26 locus in the Wnt5a−/− background, we have created a much flattened Wnt5a pattern along the P-D axis in the developing limb bud.
Fig. 3.
Generation of non-graded Wnt5a expression. (A) Generation of an inducible Wnt5a mouse strain (Rosa5a/+). Mouse Wnt5a cDNA following a PGK-Neo cassette flanked by loxp sequences was knocked into the Rosa26 locus. IRES-GFP sequence was cloned downstream of Wnt5a cDNA. SA, splice acceptor sequence. (B) Schematic of the experimental strategy. The blue arrows represent Fgf signaling from distal limb AER. (C) Wnt5a whole-mount in situ hybridization in E11.5 mouse forelimb buds. A, anterior; P, posterior. Scale bars: 200 μm. (D) Levels of total or endogenous Wnt5a mRNA from E11.5 forelimb buds were quantified by qPCR. Wnt5a mRNA levels were normalized to Gapdh expression. Error bars represent s.d., n=3 repetitions. (E) The E11.5 forelimb buds were dissected into three parts, and each part was subjected to western blot analysis and qPCR. (F,G) Quantification of Wnt5a protein and mRNA levels of the three parts, which were normalized to Gapdh protein and mRNA levels, respectively. Two-tailed t-test, *P<0.05, **P<0.01, ***P<0.001. N.S, no significance. Error bars represent s.d., n=2 repetitions for western blotting, n=3 repetitions for qPCR.
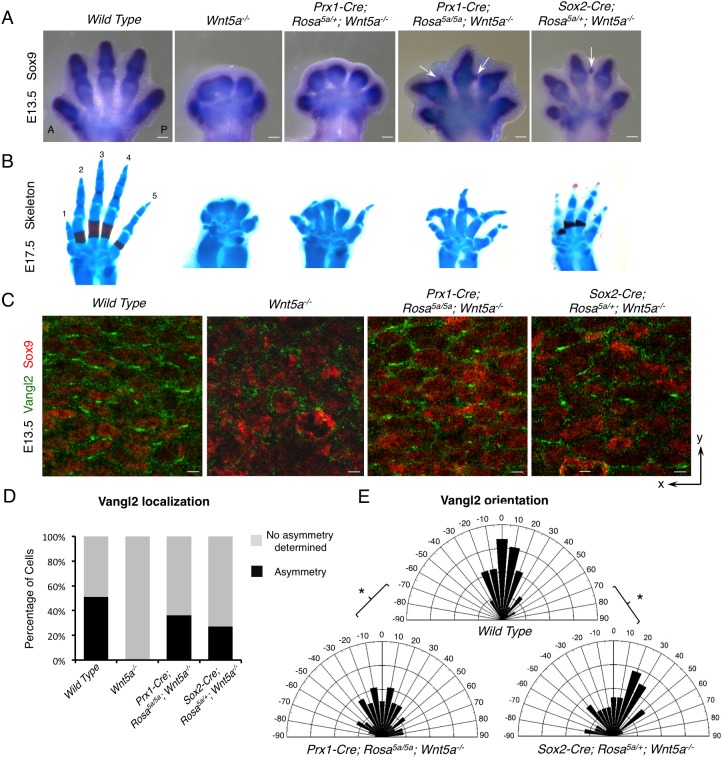
In situ hybridization and cartilage staining of late-stage embryos showed that flattened Wnt5a expression was able to partially rescue digit outgrowth in limbs of both Prx1-Cre;Rosa5a/5a;Wnt5a−/− and Sox2-Cre;Rosa5a/+;Wnt5a−/− embryos (Fig. 4A,B). In support of our previous findings that Wnt5a signaling promotes chondrogenesis (Topol et al., 2003), we found that ectopic Wnt5a expression throughout the limb bud led to rescued digit formation and even ectopic cartilage formation in the interdigital area of the limbs of Prx1-Cre;Rosa5a/5a;Wnt5a−/− and Sox2-Cre;Rosa5a/+;Wnt5a−/− embryos (Fig. 4A, arrows). These results indicate that induced non-graded Wnt5a expression is sufficient to allow normal and ectopic cartilage formation. As the rescued digits were still shorter than the wild-type controls but longer than the Vangl1−/−;Vangl2−/− embryos (Fig. S1C), in which PCP is completely abolished (Song et al., 2010), we reasoned that PCP may also be partially restored in these embryos. Indeed, whereas Vangl2 asymmetric localization was not detected at all in the absence of Wnt5a, it was partially restored in the distal digits of both Prx1-Cre;Rosa5a/5a;Wnt5a−/− and Sox2-Cre; Rosa5a/+; Wnt5a−/− embryos at E13.5 (Fig. 4C). Compared with wild-type control embryos, the number of cells with discernible Vangl2 asymmetric localization was reduced (Fig. 4C,D) and more cells with Vangl2 asymmetric localization deviated from the y-axis in both mutant embryos (Fig. 4E). Consistent with partially restored Vangl2 asymmetry, the shape, and to a lesser extent the orientation, of distal chondrocytes were also partially rescued in both mutant limbs compared with Wnt5a null limb (Fig. S8A,B). The partial rescue of PCP observed both morphologically and molecularly suggests that PCP is still present to a certain extent in the presence of non-graded Wnt5a expression, indicating that other directional cue(s) may have been provided.
Fig. 4.
Non-graded Wnt5a expression partially rescued the PCP defects of the Wnt5a−/− embryo. (A) Sox9 whole-mount in situ hybridization in E13.5 mouse forelimbs. Arrows point to the ectopic cartilage. (B) Alizarin Red and Alcian Blue staining of forelimbs from E17.5 embryos with the genotypes indicated in A. Digits 1-5 are labeled. (C) Representative images of fluorescence immunostaining of Vangl2 (green) and Sox9 (red). x- and y-axes of the images are defined as shown in Fig. S13A. (D) The percentage of cells with discernible Vangl2 asymmetric localization in each genotype. (E) Schematics summarizing the quantification of orientation angles of Vangl2 in each genotype. x-axis, angle of orientation (−90° to 90°); y-axis, percentage of cells at angle x. Kolmogorov–Smirnov test, *P=0.0466 (wild type versus Prx1-Cre; Rosa5a/5a; Wnt5a−/−) and 0.0285 (wild type versus Sox2-Cre; Rosa5a/+; Wnt5a−/−). (D,E) Number of samples and number of cells analyzed for each genotype: wild type, N=2, n=230; Wnt5a−/−, N=2, n=188; Prx1-Cre; Rosa5a/5a; Wnt5a−/−, N=3, n=271; Sox2-Cre; Rosa5a/+; Wnt5a−/−, N=5, n=450. Scale bars: 200 μm (A); 10 μm (C).
As Fgf8 can regulate PCP (Figs 1 and 2) and its expression later in limb development is maintained by elongating digit cartilage in the overlying AER (Fig. 1A) (Gañan et al., 1998; Pizette and Niswander, 1999), we examined Fgf8 expression in Prx1-Cre;Rosa5a/5a;Wnt5a−/− and Sox2-Cre;Rosa5a/+;Wnt5a−/− embryos. Indeed, Fgf8 expression was restored at a level similar to that in wild-type controls in the AER overlying the rescued digit cartilage (Fig. S9A). Therefore, it is possible that the collective effects of non-graded Wnt5a and graded Fgf signaling resulted in a PCP signaling gradient, though less steep than that in the wild-type limb. In support of this, the ectopic cartilage formed in the interdigital region of Prx1-Cre;Rosa5a/5a;Wnt5a−/− embryos showed no Vangl2 asymmetric localization (Fig. 4A, arrows; Fig. S9B), likely due to lack of Wnt5a gradient and weak (or absence of) Fgf signaling in the proximal interdigital region. Taken together, our results suggest that distal digit elongation is regulated by integrated Wnt5a and Fgf signaling in PCP.
Altered Wnt5a gradient re-orients Vangl2 localization
As restored Fgf8 expression is not sufficient to restore PCP completely in the presence of non-graded Wnt5a (Figs 3 and 4), graded Wnt5a expression may still play a major instructive role in directing digit outgrowth by regulating PCP. To test directly the instructive role of Wnt5a in limb mesenchyme, we re-oriented the Wnt5a gradient in the developing limb bud using a Shh-Cre line (Fig. 5A). Shh is expressed in the posterior limb margin in the zone of polarizing activity (ZPA) (Todt and Fallon, 1987; Riddle et al., 1993) and a Shh-expressing cell lineage gives rise to digit 5, digit 4 and part of the posterior half of digit 3 (Harfe et al., 2004) (Fig. S10A). Therefore, we were able to create a posterior-to-anterior Wnt5a gradient by inducing Wnt5a expression from the Rosa26 locus using the Shh-Cre line in the Wnt5a−/− background (Fig. 5A). This new Wnt5a gradient was nearly perpendicular to the endogenous distal-to-proximal one, particularly in digit 3 (Fig. 5A). In the limb of Shh-Cre;Rosa5a/+;Wnt5a−/− embryos, whereas digit 4 and digit 5 are immersed in Wnt5a-expressing cells, digit 3 should experience a steep posterior-to-anterior Wnt5a gradient (Fig. S10A). If the role of Wnt5a is instructive, this operation should lead to reorientation of the asymmetric Vangl2 localization and a corresponding change of digit morphology.
Fig. 5.
Reoriented Wnt5a expression gradient changes digit morphogenesis. (A) Illustration of altering Wnt5a expression gradient from distal-proximal axis to posterior-anterior axis. (B) Wnt5a whole-mount in situ hybridization in mouse E11.5 forelimb buds. (C) Sox9 whole-mount in situ hybridization in mouse E12.5 forelimb buds. Each digit is numbered and the third digit is outlined. The angles between digit 3 and digit 4 were measured using their longitudinal axes. The arrows point to the posteriorly tilted digit 3. A, anterior; P, posterior. Digits 1-5 are labeled. Scale bars: 200 μm.
We first confirmed by in situ hybridization that in the absence of endogenous Wnt5a, Shh-Cre was able to induce a gradient of Wnt5a expression from the Rosa26 locus along the anterior-posterior (A-P) axis of the limb bud at E11.5 (Fig. 5B). At E12.5, elongation of digits 4 and 5 in Shh-Cre;Rosa5a/+;Wnt5a−/− embryos was partially rescued, and digit 4 was oriented towards the posterior limb margin (Fig. 5C). Digit 3, which received much less Wnt5a signal, also grew out towards the posterior side, albeit to a much lesser extent (Fig. 5C). At E13.5, whereas digit 4 outgrowth was still tilted towards the posterior limb, outgrowth of digit 3 stopped (Fig. S10B). This is likely due to the low dose of Wnt5a it received, which was not sufficient to support continuous chondrogenesis. Lethality of the Shh-Cre;Rosa5a/+;Wnt5a+/− mouse precludes the production of Shh-Cre;Rosa5a/5a;Wnt5a−/− embryos to increase the dose. Interestingly, when the endogenous Wnt5a expression with a P-D gradient was progressively reduced and an artificial Wnt5a gradient was induced along the A-P axis in Shh-Cre;Rosa5a/+;Wnt5a+/+, Shh-Cre;Rosa5a/+;Wnt5a+/− and Shh-Cre;Rosa5a/+;Wnt5a−/− mouse embryos, digit 4 bent progressively more to the posterior limb margin (Fig. S10B). Thus, reorientation of the Wnt5a gradient changed the orientation of digit outgrowth.
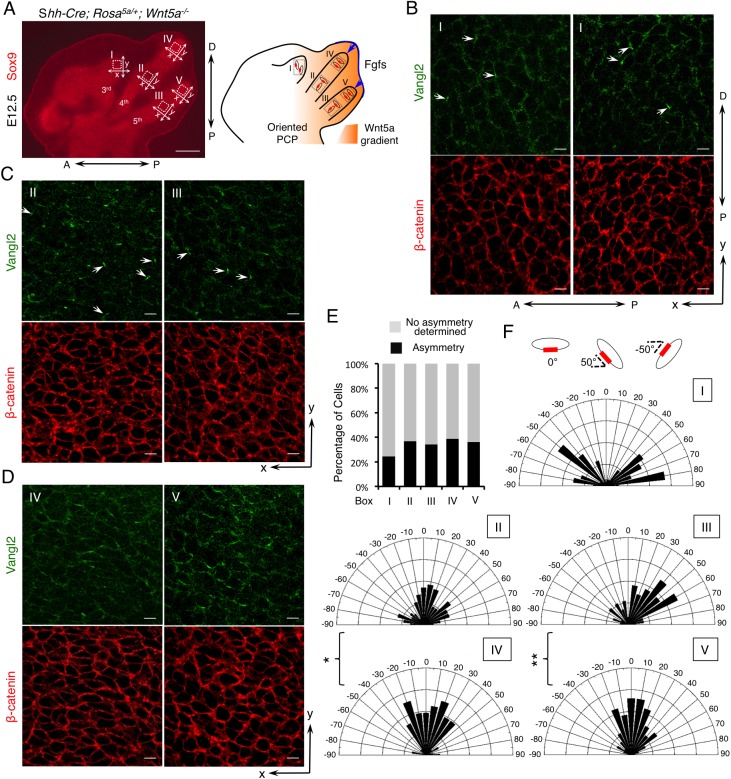
To test whether the observed reorientation of digit outgrowth is associated with altered PCP, we examined Vangl2 localization in Shh-Cre;Rosa5a/+;Wnt5a−/− limbs (Fig. 6). Because of the radial outgrowth of the digits, only the x- and y-axes of digit 3 overlap with the A-P and P-D axes of the whole limb. Although the number of cells with discernible Vangl2 asymmetric localization was comparable in different regions of partially rescued digit 4 and digit 5 (Fig. 6A,E), fewer cells displayed asymmetric Vangl2 in digit 3 likely due to the limited Wnt5a dosage (Fig. 6E). In digit 3, which experienced the steepest Wnt5a gradient (Fig. 6A,B), Vangl2 asymmetric localization was reoriented along the A-P axis (orientation angle <−45° or >45° with reference to the x-axis or A-P axis) in almost all cells with detectable Vangl2 asymmetric localization (Fig. 6B,F). Vangl2 localization in digits 4 and 5, which were completely immersed in Wnt5a-expressing cells, was more complex. In the proximal regions of digits 4 and 5 (Fig. 6A,C), Vangl2 localization in a few cells was reoriented along the x-axis in lieu of the y-axis (orientation angle −45° to +45° with reference to the x-axis) (Fig. 6C,F). But in the distal regions of digits 4 and 5 (Fig. 6A,D), Vangl2 asymmetric localization was largely oriented along the y-axis (Fig. 6D,F). Consistent with the Vangl2 localization pattern, although there was no significant change of chondrocyte shape (Fig. S11A), a large number of cells in digit 3 (region I) had reoriented their major axes along the y-axis (Fig. S11B). In addition, more reorientation was also observed in proximal regions of digits 4 and 5 (regions II and III), compared with distal regions (regions IV and V) (Fig. S11B). These results demonstrate that an altered Wnt5a gradient is able to reorient PCP in forming chondrocytes in digit 3 (region I), does so less well in proximal regions of digits 4 and 5 (regions II and III) and failed to do so in distal regions of digits 4 and 5 (regions IV and V) (Fig. 6A). It is intriguing that, in distal regions of digits 4 and 5, Vangl2 still orients towards the distal end, suggesting another cue was provided. Indeed, Fgf8 expression in the AER was restored in the partially rescued digits 4 and 5 of Shh-Cre;Rosa5a/+;Wnt5a−/− embryos (Fig. 7A). Thus, signal(s) from the distal limb, likely Fgf signaling, may compete with posterior limb-derived Wnt5a signaling to orient chondrocytes and Vangl2 localization, resulting in a more complicated limb phenotype and Vangl2 localization pattern (Fig. 6A).
Fig. 6.
Vangl2 asymmetric localization is partially reoriented by the altered Wnt5a gradient. (A) The forelimb digits of E12.5 Shh-Cre;Rosa5a/+; Wnt5a−/− embryos are shown by Sox9 immunofluorescence staining. The boxed regions I-V are enlarged with Vangl2 (green) and β-catenin (red) double immunofluorescence staining as shown in B-D. P-D and A-P axes are indicated. x- and y-axes of the boxed regions are defined as shown in the left-hand panel. Schematic on the right depicts a summary of the results. The red boxes represent Vangl2 asymmetric localization and the blue arrows represent Fgf signaling cues. (B) Representative 3rd digit (region I). (C) Representative proximal regions of the 4th (region II) and 5th (region III) digits. (D) Representative distal regions of the 4th (region IV) and 5th (region V) digits. Arrows point to cells with asymmetrical Vangl2 localization along the x-axis. (E) The percentage of cells with discernible Vangl2 asymmetric localization shown in B-D. (F) Schematics summarizing the quantification of orientation angles of Vangl2 in each boxed region I-V. x-axis, angle of orientation (−90° to 90°); y-axis, percentage of cells at angle x. Kolmogorov–Smirnov test, **P=0.0128, *P=0.0414. (E,F) Number of samples and number of cells analyzed for each region: N=4, n=232 for region I; N=4, n=294 for region II; N=3, n=196 for region III; N=4, n=327 for region IV; N=2, n=130 for region V. Scale bars: 200 μm (A); 20 μm (B-D).
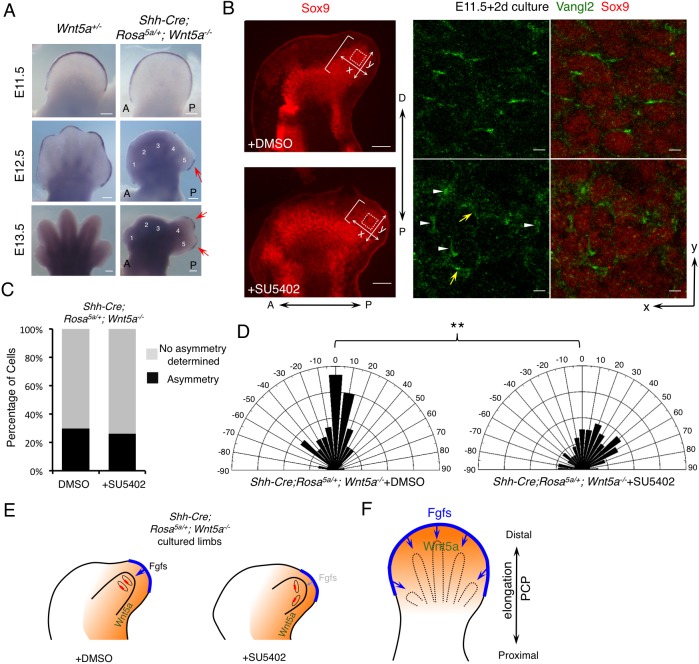
Fig. 7.
Inhibition of distal Fgf signaling renders cells more responsive to oriented Wnt5a signaling. (A) Whole-mount in situ hybridization of Fgf8 in forelimbs of the embryos with the indicated genotypes. Fgf8 expression was restored (red arrows) in the AER overlying the posterior digits of Shh-Cre; Rosa5a/+;Wnt5a−/− embryos. A, anterior; P, posterior. Digits 1-5 are labeled in the mutant. (B) Treatment with Fgf receptor inhibitor (SU5402) changed the direction of Vangl2 asymmetric localization in cultured Shh-Cre;Rosa5a/+;Wnt5a−/− embryonic forelimbs (E11.5+2-day culture). The brackets indicate the length of digit outgrowth. Part of the boxed regions (dotted squares) is shown in the middle (Vangl2, green) and right-hand (Vangl2/Sox9, green/red) panels at higher magnification. White arrowheads point to 90° oriented Vangl2. Yellow arrows point to Vangl2 with other orientations. P-D and A-P axes are indicated. x- and y-axes of the boxed regions are defined as shown in the left-hand panel. (C) The percentage of cells with discernible Vangl2 asymmetric localization in distal chondrocytes of Shh-Cre;Rosa5a/+;Wnt5a−/− limbs after DMSO or SU5402 treatment. (D) Schematics summarizing the quantification of orientation angles of Vangl2 in each group. x-axis, angle of orientation (−90° to 90°); y-axis, percentage of cells at angle x. Kolmogorov–Smirnov test, **P=0.0027. (C,D) DMSO-treated limbs N=6, counted cells n=414; SU5402-treated limbs N=6, counted cells n=406. (E) Schematics summarizing the results shown in B-D. The red boxes represent Vangl2 asymmetric localization and the blue arrows represent Fgf signaling. The orange backgrounds represent Wnt5a signaling. The light blue arrow in the right-hand panel indicates weaker Fgf signaling. (F) Schematic of the proposed model: PCP establishment is coordinately regulated by both Wnt5a and Fgfs signaling. Orange represents graded Wnt5a expression; blue, Fgf signaling from the AER. Scale bars: 200 μm (A and left-hand panels of B); 10 μm (right-hand panels of B).
Inhibition of Fgf signaling enhanced the reorientation response to an altered Wnt5a gradient
To further test whether distal Fgf signaling and posterior limb-derived Wnt5a signaling compete in orienting chondrocytes and Vangl2 asymmetric localization in distal regions of digits 4 and 5 in the Shh-Cre;Rosa5a/+;Wnt5a−/− limb (Fig. 6A), we inhibited Fgf signaling by treating E11.5 limbs with the Fgf receptor inhibitor SU5402 (Mohammadi et al., 1997). In ex utero culture, Shh-Cre; Rosa5a/+;Wnt5a−/− embryos only formed one posterior digit instead of two (Fig. 7B). In this digit, about 20-30% of the distal chondrocytes showed asymmetric Vangl2 (Fig. 7C) and most of them were oriented towards the distal limb margin (orientation angle −45° to +45° with reference to the x-axis) (Fig. 7B,D). Remarkably, in the SU5402-treated limb of Shh-Cre;Rosa5a/+;Wnt5a−/− embryos, there was a slight reduction in the number of cells with asymmetric Vangl2 (Fig. 7C). However, among the cells with asymmetric Vangl2, the percentage that exhibited reorientation induced by posterior Wnt5a signaling (orientation angle <−45° or >+45° with reference to the x-axis) was increased (Fig. 7B,D). Interestingly, comparison of Fig. 7D with Fig. 6F showed that inhibition of Fgf signaling converted the orientation of Vangl2 localization from that observed in the distal digit (Fig. 6F, IV and V), which received strong distal Fgf signaling, to that observed in the proximal digit (Fig. 6F, II and III), which received weak Fgf signaling. These results indicate that inhibition of distal Fgf signaling promoted digit 4/5 distal chondrocyte reorientation, which was hindered by Fgfs expressed in the overlying AER in Shh-Cre;Rosa5a/+;Wnt5a−/− embryos (Fig. 7E). Consistent with the Vangl2 reorientation, whereas there was only slight change in cell shape, cell orientation was altered significantly by SU5402 treatment (Fig. S12A,B). As a result, digit outgrowth was compromised but the width of the digit was increased (Fig. 7B, Fig. S12D).
Taken together, our data show that Wnt5a does play an instructive role in orienting PCP in limb morphogenesis. Because we also found that Fgf signaling could provide an additional instructive cue in the presence of Wnt5a (either graded or non-graded), Wnt5a signaling is also permissive in that it allows Fgf signaling to orient PCP.
DISCUSSION
How directional information is provided and interpreted and how it is coordinated with patterning events to guide morphogenesis are fundamentally important in the development of complex multicellular organisms. Here, we show that Wnt5a plays both instructive and permissive roles in regulating PCP and limb elongation. Our finding that the direction of the Wnt5a gradient determines the orientation of PCP in mouse limb mesenchymal cells is consistent with previous findings that a Wnt signaling gradient provides an instructive cue in controlling PCP in epithelial tissues of Drosophila, Xenopus and mouse (Wu et al., 2013; Chu and Sokol, 2016; Minegishi et al., 2017; Humphries and Mlodzik, 2017). Our data also show that additional instructive cue(s) such as Fgf can orient PCP during limb morphogenesis in a Wnt5a-dependent manner. Wnt5a and Fgf signaling integrate together to establish PCP in early limb chondrocytes (Fig. 7F). As Fgf signaling is required for early limb P-D patterning (Lewandoski et al., 2000; Sun et al., 2002; Mariani et al., 2008), our results further suggest that PCP may be intrinsically linked with patterning events during morphogenesis. Fgf signaling can regulate both pattern formation and PCP, whereas PCP can be regulated by multiple instructive cues.
Among all other morphogen gradients in the developing limb, only Fgf signaling components have been shown to play an instructive role in P-D patterning possibly through regulating cell proliferation/apoptosis, acting as chemoattractants or stimulating random cell movements (Niswander et al., 1993; Li and Muneoka, 1999; Sun et al., 2002; Bénazéraf et al., 2010; Gros et al., 2010). Wnt5a and Fgf8 exert coordinated but distinct effects in mesenchymal cells in the early limb bud before chondrogenesis, with Wnt5a acting as chemoattractant but Fgf8 stimulating random cell movements (Gros et al., 2010). It has been shown that directional cell behaviors, such as directional mesenchymal cell movement and cartilage P-D elongation, rather than graded proliferation, drive preferential elongation of the limb along the P-D axis (Boehm et al., 2010; Hopyan et al., 2011; Gao and Yang, 2013). However, limb P-D elongation occurs mostly at later stages of development via cartilage P-D elongation and chondrocytes do not move. Our genetic and explant culture experiments demonstrated that loss of Fgf4 and Fgf8 led to PCP defects in limb chondrocytes and Fgf8 proteins were able to reorient PCP in the presence of Wnt5a. In this regard, the Wnt5a signal is required to provide a permissive condition for Fgf signaling to orient PCP. Although the exact mechanism remains to be elucidated, one possibility suggested by our data is that Fgf regulates PCP by promoting Wnt5a-induced Vangl2 phosphorylation and/or protein stability to steepen the Wnt/PCP signaling gradient. When the Wnt-induced PCP gradient was flattened or its direction was altered, the remaining Fgf signaling gradient established partial PCP. It is also possible that Wnt5a is only required to promote chondrogenesis, then Fgf8 orients chondrocytes through some other mechanisms. Interestingly, the Fgf-Erk1/2 pathway has been shown to regulate mitotic spindle orientation in the lung epithelium (Tang et al., 2011), and a possible downstream effect of PCP is to control oriented cell division in many contexts (Saburi et al., 2008; Baena-López et al., 2005; Gong et al., 2004; Ciruna et al., 2006). It is unclear whether Fgf controls oriented cell division or cell rearrangement in chondrocytes. But it has been shown that PCP can regulate chondrocyte orientation through daughter cell spreading or a pivoting-like process after oriented cell division. (Li and Dudley, 2009; Romereim et al., 2014; Li et al., 2017).
Our results indicated that Wnt/PCP signaling can receive inputs from other signaling pathways such as those mediated by Fgfs that regulate patterning, which forms a molecular basis for coordination of different developmental events that occur at the same time as morphogenesis. Notably, in addition to its limb AER expression, Fgf8 is also expressed in the tail bud and the frontonasal process where Wnt5a is expressed (Heikinheimo et al., 1994; Yamaguchi et al., 1999; Dubrulle and Pourquié, 2004). As PCP mutant mice exhibited severe directional outgrowth defects in those places (Song et al., 2010; Gao, 2012), the mechanism of coordinating patterning and PCP by Wnt5a and Fgf signaling may be fundamentally important in morphogenesis of other tissues and organs. In tissues or organs bigger than the limb bud, signaling gradients across a longer distance is required to orient PCP in a larger group of cells. In the tail bud, both Wnt5a and Fgf8 are expressed in a gradient across a larger spatial scale (Yamaguchi et al., 1999; Dubrulle and Pourquié, 2004). A steeper gradient may also provide a more robust directional cue by making a bigger signaling difference within the same spatial scale. PCP regulation by a Wnt5a signaling gradient could be enhanced in responding cells by Fgf signaling or modified by other signaling molecules. The effects of other signaling cues become more apparent when the Wnt5a signaling gradient is diminished or altered in its direction. Indeed, we uncovered the effects of Fgf signaling on PCP when the Wnt5a gradient was flattened in this study. This may explain why permissive Wnt11 can rescue the zebrafish slb mutant phenotype (Heisenberg et al., 2000) and is in line with a previous report that in cultured melanoma cells Wnt5a also acts permissively to allow directional migration in response to other positional cues such as a CXCL12 chemokine gradient (Witze et al., 2008). In addition, the Wnt antagonist secreted frizzled-related protein (Sfrp) family members Sfrp1, -2 and -5 can regulate PCP during early trunk formation (Satoh et al., 2008). A recent study also provided genetic evidence that opposing gradients of Wnt5a/b and their Sfrp inhibitors polarize node cells along the A-P body axis (Minegishi et al., 2017). Interestingly, Sfrp2, Sfrp3 and Wif1 are expressed in the limb mesenchyme (Witte et al., 2009). It is possible that Sfrps and Wif1 also shape the Wnt signaling gradient during limb morphogenesis. Other Wnts may contribute to limb PCP. However, as PCP is completely lost in the Wnt5a null mutant limb, the activities of other Wnts in regulating PCP are likely to be much weaker. In addition, as a feedback mechanism, the Wnt5a gradient may also be shaped by Wnt-binding PCP signaling components such as frizzled receptors and Ror2, membrane availability of which can be regulated by Wnts (van Amerongen, 2012). These observations highlight the complexity of Wnt-regulated PCP and future studies are required to understand how global cues are formed and accurately deciphered by responding cells.
MATERIALS AND METHODS
Mouse lines and breeding
Prx1-Cre, Sox2-Cre and Shh-Cre:EGFP mice were purchased from the Jackson Laboratories. Wnt5a+/−, Msx2-Cre, Fgf4c/c and Fgf8c/c mouse strains have been described previously (Yamaguchi et al., 1999; Logan et al., 2002; Hayashi et al., 2002; Harfe et al., 2004; Sun et al., 2002; Lewandoski et al., 2000). The inducible Wnt5a line (Rosa5a/+) was generated by knocking the mouse Wnt5a cDNA into the Rosa26 locus in embryonic stem cells. A PGK-Neo cassette flanked by loxp sites was inserted before the Wnt5a cDNA. The details of generation of Rosa5a/+ have been described previously (Cha et al., 2014). This study was approved by the ethics committees of the National Human Genome Research Institute, National Institutes of Health, USA, and the University of Hong Kong, China.
Antibodies and proteins
Sox9 (1:150, H-90, Santa Cruz), Vangl2 (1:100, N-13, Santa Cruz), β-catenin (1:500, #610154, BD Transduction Laboratories), cleaved caspase 3 (1:100, #9661, Cell Signaling) and p-Histone H3 (1:500, #9701, Cell Signaling) antibodies were used for immunofluorescence staining. Wnt5a (1:100, BAF645, R&D Systems), Vangl2 (1:200, gift of M. Montcouquiol, Neurocentre Magendie, France), GAPDH (1:10,000, G8795, Sigma), alpha-tubulin (1:10,000, ab7291, Abcam), Erk1/2 (1:1000, #9102, Cell Signaling) and p-Erk1/2 (1:1000, #9106, Cell Signaling) antibodies were used for western blotting. Recombinant proteins were purchased from R&D Systems: Wnt5a (645-WN-010), FGF-8b (423-F8-025), Shh (461-SH-025).
Immunofluorescence and confocal microscopy
Embryos were fixed in 4% paraformaldehyde (PFA) in PBS for 30 min at 4°C and then processed for cryosectioning. The tissue sections were permeabilized for 5 min in 0.5% Triton X-100 in PBS, blocked for 1 h in 5% bovine serum albumin and 0.1% Triton X-100 in PBS and incubated with primary antibodies overnight at 4°C. Secondary antibodies coupled to Alexa Fluor 488 or 594 (Invitrogen) were diluted 1:500 and applied to the sections for 1 h at room temperature. The longitudinal axis of the target digit was defined as the vertical y-axis (an example is shown in Fig. S13A). Confocal images were acquired using a Zeiss LSM 510 NLO Meta system (63× oil objective). Projected z-stack images were acquired at 0.3 μm intervals for ∼6 μm and projected by Zeiss LSM 510 software.
Quantification of Vangl2 localization pattern
First, projected z-stack images were used to manually count the number of cells with polarized Vangl2. Cells were classified into two categories: ‘Asymmetry’ and ‘No asymmetry determined’. A cell with polarized Vangl2 (any orientation direction) was classed as ‘Asymmetry’, but a cell with no polarized Vangl2 was classed as ‘No asymmetry determined’. An example is illustrated in Fig. S13B. Next, the orientation angle of asymmetric Vangl2 localization was measured and quantified using CellProfiler 2.1.1 (http://cellprofiler.org/). CellProfiler automatically identifies objects in terms of the custom-designed pipeline and the preset parameters (e.g. the range of the object size, the intensity threshold). The orientation of the Vangl2 staining is determined by the angle of the identified object between its major axis and the x-axis of the image (in degrees ranging from −90° to 90°). An example is illustrated in Fig. S13C. In mesenchymal cells, the rich extracellular matrix allows us to determine where Vangl2 localizes between neighboring cells (see Fig. S14 for examples). Notably, within the 6 μm that was scanned, only a fraction of the digit cells showed discernible Vangl2 asymmetric localization.
Quantification of chondrocytes orientation and shape
Immunofluorescence images of chondrocytes with DAPI or Sox9 (nucleus) and β-catenin (cell membrane) staining were processed using CellProfiler 2.1.1, a software application used to measure cell phenotypes quantitatively. The orientation of the cell is determined by the angle of the identified cell object between its major axis and the x-axis of the image (in degrees ranging from −90° to 90°) with the y-axis defined as the longitudinal axis of a particular digit. An example is illustrated in Fig. S15. The shape of the cell is reflected by the LWR of the cell. The cell length (the longest dimension of the cell) was measured as ‘Major-Axis-Length’ and the cell width (the shortest dimension of the cell) was measured as ‘Minor-Axis-Length’ automatically by CellProfiler. Data were analyzed using GraphPad Prism 6 (two-tailed t-test for LWR and Kolmogorov–Smirnov test for orientation angle distribution). The orientation schematics were plotted using Origin 9 software.
Limb culturing
The E11.5 embryonic forelimbs were dissected in pre-warmed DMEM containing 10% fetal bovine serum (FBS), penicillin/streptomycin (pen/strep, Thermo Fisher Scientific) and 20 mM HEPES and cultured in pre-warmed 1:1 mixed DMEM (10% FBS, pen/strep) and freshly prepared rat serum on a rotator at 37°C with 5% CO2 for 2 days. The culturing medium was changed every 24 h. This culturing method allows digit morphogenesis comparable to in vivo digit development at E12.5-E13.5, a time point when PCP has been already established. Fgf receptor inhibitor (10 μM, SU5402, Sigma) was directly added into the culturing medium and changed every 24 h.
Bead implantation
Bead implantation experiments were performed according to procedures described previously (Niswander et al., 1993). Heparin acrylic beads (H5263, Sigma) were washed in PBS four to six times and then soaked in 2-3 μl 1 mg/ml FGF-8b recombinant protein (423-F8-025, R&D Systems) for at least 1 h at room temperature. Control beads were soaked in PBS. The beads were inserted into the interdigital mesenchyme of the distal wild-type limb when digital condensations were just visible (∼E11.5) or distal limb mesenchyme distal to digit formation in the Wnt5a−/− limb bud.
Limb mesenchymal cell culturing
E10.5-E11.5 limb buds were isolated in calcium- and magnesium-free PBS buffer containing 0.1% glucose and pen/strep. The limb buds were then digested in calcium- and magnesium-free PBS buffer containing 0.01% trypsin, 0.002% EDTA, 0.1% glucose and pen/strep for 20-30 min at 37°C. The mesenchymal cells were completely disassociated by pipetting up and down and then they were transferred to an equal volume of culture medium (10% FBS in DMEM). The cells were filtered through a Nitex filter (20 μm mesh, 03-20/14, Sefar America) to eliminate fibrous materials and tissue debris and were plated. The cells were confluent 24 h later. Confluent cells were treated with recombinant proteins (Fgf8, 250 ng/ml and Wnt5a 100 ng/ml or 500 ng/ml) for 2 h after 6 h of serum starvation (0.2% FBS in DMEM).
Skeletal preparation
E17.5 fetuses were eviscerated, fixed overnight in 100% ethanol, and then transferred into 100% acetone, followed by staining with Alizarin Red S (bone) and Alcian Blue (cartilage). Embryos were cleared using 1% KOH and stored and photographed in 80% glycerol.
In situ hybridization
Whole-mount in situ hybridizations of mouse embryos using digoxigenin-labeled antisense RNA were performed as described previously (Wilkinson and Nieto, 1993). Riboprobes used in this study have been described previously: Sox9 (Bi et al., 1999), Fgf8 (Crossley and Martin, 1995) and Sprouty4 (Minowada et al., 1999). The antisense riboprobe of Wnt5a, which does not detect Wnt5a transcripts from the Wnt5a null locus, was synthesized based on a 190 bp PCR product using the primers 5′-GGAATATTAAGCCCGGGAGTG-3′ and 5′-AGAGAGGCTGTGCACCTATGAT-3′.
Quantitative PCR
Total RNA was extracted using Trizol reagent (Invitrogen) and reverse transcribed using the SuperScript First Strand Synthesis System. RT-PCR (Invitrogen) was performed according to the manufacturer's instructions. Quantitative PCR using SYBR Select Master Mix (Applied Biosystems) was performed on 7500 Fast Real-Time PCR system (Applied Biosystems). The following primers were used: endogenous mouse Wnt5a, 5′-ATTGTCCCCCAAGGCTTAAC-3′ and 5′-CTTCTATAACAACCTGGGCGAAG-3′; total mouse Wnt5a, 5′-CCATGTCTTCCAAGTTCTTCCTA-3′ and 5′-CCAGACACTCCATGACACTTACA-3′; mouse Sprouty4, 5′-GCAGCGTCCCTGTGAATCC-3′ and 5′-TCTGGTCAATGGGTAAGATGGT-3′. Gapdh mRNA level was used as internal control.
X-gal staining
Embryos were fixed in fixative (0.5% formaldehyde, 0.1% glutaraldehyde, 2 mM MgCl2, 5 mM EGTA, 0.02% NP-40) for 15 min. Cryosectioned tissue was stained with X-gal buffer [5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 2 mM MgCl2, 0.01% sodium deoxycholate, 0.02% NP-40] for 2-4 h at 37°C and post-fixed in 4% PFA.
Supplementary Material
Acknowledgements
We thank C. Rivas and E. Escobar for their assistance in the mouse work; M. Montcouquiol (Neurocentre Magendie, France) for the rabbit Vangl2 antibodies; and S. Mackem (NCI, NIH) for sharing the Shh-Cre:EGFP mice.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: B.G., Y.Y.; Methodology: B.G., R.A., H.S., M.B.L., T.P.Y., Y.Y.; Formal analysis: B.G., W.Y., M.J.A.; Investigation: B.G., R.A., W.Y., C.L., M.J.A., R.R.L.; Resources: B.G., R.A., H.S., M.J.A., M.B.L., T.P.Y., Y.Y.; Writing - original draft: B.G., Y.Y.; Writing - review & editing: B.G., M.B.L., T.P.Y., Y.Y.; Supervision: B.G., M.B.L., T.P.Y., Y.Y.; Project administration: B.G., Y.Y.; Funding acquisition: B.G., M.B.L., T.P.Y., Y.Y.
Funding
The work in the Yang laboratory was supported by the intramural research program of the National Human Genome Research Institute at the National Institutes of Health (NIH) and an NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases grant (R01AR070877). The work in the Gao laboratory was supported by University of Hong Kong start-up funds and the Research Grants Council, University Grants Committee (27115317). The work in the Yamaguchi and Lewandoski laboratories was supported by the intramural research program of the National Cancer Institute, Center for Cancer Research. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.163824.supplemental
References
- Adler P. N. (2012). The frizzled/stan pathway and planar cell polarity in the Drosophila wing. 101, 1-31. 10.1016/B978-0-12-394592-1.00001-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler P. N., Krasnow R. E. and Liu J. (1997). Tissue polarity points from cells that have higher Frizzled levels towards cells that have lower Frizzled levels. 7, 940-949. 10.1016/S0960-9822(06)00413-1 [DOI] [PubMed] [Google Scholar]
- Aigouy B., Farhadifar R., Staple D. B., Sagner A., Röper J.-C., Jülicher F. and Eaton S. (2010). Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. 142, 773-786. 10.1016/j.cell.2010.07.042 [DOI] [PubMed] [Google Scholar]
- Baena-López L. A., Baonza A. and García-Bellido A. (2005). The orientation of cell divisions determines the shape of Drosophila organs. 15, 1640-1644. 10.1016/j.cub.2005.07.062 [DOI] [PubMed] [Google Scholar]
- Bénazéraf B., Francois P., Baker R. E., Denans N., Little C. D. and Pourquié O. (2010). A random cell motility gradient downstream of FGF controls elongation of an amniote embryo. 466, 248-252. 10.1038/nature09151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi W., Deng J. M., Zhang Z., Behringer R. R. and de Crombrugghe B. (1999). Sox9 is required for cartilage formation. 22, 85-89. 10.1038/8792 [DOI] [PubMed] [Google Scholar]
- Boehm B., Westerberg H., Lesnicar-Pucko G., Raja S., Rautschka M., Cotterell J., Swoger J. and Sharpe J. (2010). The role of spatially controlled cell proliferation in limb bud morphogenesis. 8, e1000420 10.1371/journal.pbio.1000420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal J., Struhl G. and Lawrence P. A. (2002). Developmental compartments and planar polarity in Drosophila. 12, 1189-1198. 10.1016/S0960-9822(02)00974-0 [DOI] [PubMed] [Google Scholar]
- Cha J., Bartos A., Park C., Sun X., Li Y., Cha S.-W., Ajima R., Ho H.-Y. H., Yamaguchi T. P. and Dey S. K. (2014). Appropriate crypt formation in the uterus for embryo homing and implantation requires Wnt5a-ROR signaling. 8, 382-392. 10.1016/j.celrep.2014.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C.-W. and Sokol S. Y. (2016). Wnt proteins can direct planar cell polarity in vertebrate ectoderm. 5, e16463 10.7554/eLife.16463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruna B., Jenny A., Lee D., Mlodzik M. and Schier A. F. (2006). Planar cell polarity signalling couples cell division and morphogenesis during neurulation. 439, 220-224. 10.1038/nature04375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley P. H. and Martin G. R. (1995). The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. 121, 439-451. [DOI] [PubMed] [Google Scholar]
- Dabdoub A. and Kelley M. W. (2005). Planar cell polarity and a potential role for a Wnt morphogen gradient in stereociliary bundle orientation in the mammalian inner ear. 64, 446-457. 10.1002/neu.20171 [DOI] [PubMed] [Google Scholar]
- Devenport D. and Fuchs E. (2008). Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. 10, 1257-1268. 10.1038/ncb1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrulle J. and Pourquié O. (2004). fgf8 mRNA decay establishes a gradient that couples axial elongation to patterning in the vertebrate embryo. 427, 419-422. 10.1038/nature02216 [DOI] [PubMed] [Google Scholar]
- Fisher M. E., Clelland A. K., Bain A., Baldock R. A., Murphy P., Downie H., Tickle C., Davidson D. R. and Buckland R. A. (2008). Integrating technologies for comparing 3D gene expression domains in the developing chick limb. 317, 13-23. 10.1016/j.ydbio.2008.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furthauer M., Reifers F., Brand M., Thisse B. and Thisse C. (2001). sprouty4 acts in vivo as a feedback-induced antagonist of FGF signaling in zebrafish. 128, 2175-2186. [DOI] [PubMed] [Google Scholar]
- Gañan Y., Macias D., Basco R. D., Merino R. and Hurle J. M. (1998). Morphological diversity of the avian foot is related with the pattern of msx gene expression in the developing autopod. 196, 33-41. 10.1006/dbio.1997.8843 [DOI] [PubMed] [Google Scholar]
- Gao B. (2012). Wnt regulation of planar cell polarity (PCP). 101, 263-295. 10.1016/B978-0-12-394592-1.00008-9 [DOI] [PubMed] [Google Scholar]
- Gao B. and Yang Y. (2013). Planar cell polarity in vertebrate limb morphogenesis. 23, 438-444. 10.1016/j.gde.2013.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B., Song H., Bishop K., Elliot G., Garrett L., English M. A., Andre P., Robinson J., Sood R., Minami Y. et al. (2011). Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. 20, 163-176. 10.1016/j.devcel.2011.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y., Mo C. and Fraser S. E. (2004). Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. 430, 689-693. 10.1038/nature02796 [DOI] [PubMed] [Google Scholar]
- Goodrich L. V. and Strutt D. (2011). Principles of planar polarity in animal development. 138, 1877-1892. 10.1242/dev.054080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R. S., Roszko I. and Solnica-Krezel L. (2011). Planar cell polarity: coordinating morphogenetic cell behaviors with embryonic polarity. 21, 120-133. 10.1016/j.devcel.2011.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros J., Serralbo O. and Marcelle C. (2009). WNT11 acts as a directional cue to organize the elongation of early muscle fibres. 457, 589-593. 10.1038/nature07564 [DOI] [PubMed] [Google Scholar]
- Gros J., Hu J. K.-H., Vinegoni C., Feruglio P. F., Weissleder R. and Tabin C. J. (2010). WNT5A/JNK and FGF/MAPK pathways regulate the cellular events shaping the vertebrate limb bud. 20, 1993-2002. 10.1016/j.cub.2010.09.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harfe B. D., Scherz P. J., Nissim S., Tian H., Mcmahon A. P. and Tabin C. J. (2004). Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. 118, 517-528. 10.1016/j.cell.2004.07.024 [DOI] [PubMed] [Google Scholar]
- Hashimoto M., Shinohara K., Wang J., Ikeuchi S., Yoshiba S., Meno C., Nonaka S., Takada S., Hatta K., Wynshaw-Boris A. et al. (2010). Planar polarization of node cells determines the rotational axis of node cilia. 12, 170-176. 10.1038/ncb2020 [DOI] [PubMed] [Google Scholar]
- Hayashi S., Lewis P., Pevny L. and Mcmahon A. P. (2002). Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. 119 Suppl. 1, S97-S101. 10.1016/S0925-4773(03)00099-6 [DOI] [PubMed] [Google Scholar]
- Heikinheimo M., Lawshé A., Shackleford G. M., Wilson D. B. and Macarthur C. A. (1994). Fgf-8 expression in the post-gastrulation mouse suggests roles in the development of the face, limbs and central nervous system. 48, 129-138. 10.1016/0925-4773(94)90022-1 [DOI] [PubMed] [Google Scholar]
- Heisenberg C.-P., Tada M., Rauch G.-J., Saúde L., Concha M. L., Geisler R., Stemple D. L., Smith J. C. and Wilson S. W. (2000). Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. 405, 76-81. 10.1038/35011068 [DOI] [PubMed] [Google Scholar]
- Hernandez-Martinez R., Castro-Obregon S. and Covarrubias L. (2009). Progressive interdigital cell death: regulation by the antagonistic interaction between fibroblast growth factor 8 and retinoic acid. 136, 3669-3678. 10.1242/dev.041954 [DOI] [PubMed] [Google Scholar]
- Hopyan S., Sharpe J. and Yang Y. (2011). Budding behaviors: growth of the limb as a model of morphogenesis. 240, 1054-1062. 10.1002/dvdy.22601 [DOI] [PubMed] [Google Scholar]
- Humphries A. C. and Mlodzik M. (2017). From instruction to output: Wnt/PCP signaling in development and cancer. 51, 110-116. 10.1016/j.ceb.2017.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly L. K., Wu J., Yanfeng W. A. and Mlodzik M. (2016). Frizzled-induced Van Gogh phosphorylation by CK1epsilon promotes asymmetric localization of core PCP factors in Drosophila. 16, 344-356. 10.1016/j.celrep.2016.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian B., Mansukoski H., Barbosa F. C., Ulrich F., Tada M. and Heisenberg C.-P. (2003). The role of Ppt/Wnt5 in regulating cell shape and movement during zebrafish gastrulation. 120, 467-476. 10.1016/S0925-4773(03)00004-2 [DOI] [PubMed] [Google Scholar]
- Lawrence P. A., Sanson B. and Vincent J. P. (1996). Compartments, wingless and engrailed: patterning the ventral epidermis of Drosophila embryos. 122, 4095-4103. [DOI] [PubMed] [Google Scholar]
- Lawrence P. A., Casal J. and Struhl G. (2004). Cell interactions and planar polarity in the abdominal epidermis of Drosophila. 131, 4651-4664. 10.1242/dev.01351 [DOI] [PubMed] [Google Scholar]
- Lawrence P. A., Struhl G. and Casal J. (2007). Planar cell polarity: one or two pathways? 8, 555-563. 10.1038/nrg2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandoski M., Sun X. and Martin G. R. (2000). Fgf8 signalling from the AER is essential for normal limb development. 26, 460-463. 10.1038/82609 [DOI] [PubMed] [Google Scholar]
- Li Y. and Dudley A. T. (2009). Noncanonical frizzled signaling regulates cell polarity of growth plate chondrocytes. 136, 1083-1092. 10.1242/dev.023820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. and Muneoka K. (1999). Cell migration and chick limb development: chemotactic action of FGF-4 and the AER. 211, 335-347. 10.1006/dbio.1999.9317 [DOI] [PubMed] [Google Scholar]
- Li Y., Li A., Junge J. and Bronner M. (2017). Planar cell polarity signaling coordinates oriented cell division and cell rearrangement in clonally expanding growth plate cartilage. 6, e23279 10.7554/eLife.23279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan M., Martin J. F., Nagy A., Lobe C., Olson E. N. and Tabin C. J. (2002). Expression of Cre recombinase in the developing mouse limb bud driven by a Prxl enhancer. 33, 77-80. 10.1002/gene.10092 [DOI] [PubMed] [Google Scholar]
- Mariani F. V., Ahn C. P. and Martin G. R. (2008). Genetic evidence that FGFs have an instructive role in limb proximal-distal patterning. 453, 401-405. 10.1038/nature06876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matis M. and Axelrod J. D. (2013). Regulation of PCP by the Fat signaling pathway. 27, 2207-2220. 10.1101/gad.228098.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi K., Hashimoto M., Ajima R., Takaoka K., Shinohara K., Ikawa Y., Nishimura H., Mcmahon A. P., Willert K., Okada Y. et al. (2017). A Wnt5 activity asymmetry and intercellular signaling via PCP proteins polarize node cells for left-right symmetry breaking. 40, 439-452.e4. 10.1016/j.devcel.2017.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minowada G., Jarvis L. A., Chi C. L., Neubuser A., Sun X., Hacohen N., Krasnow M. A. and Martin G. R. (1999). Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. 126, 4465-4475. [DOI] [PubMed] [Google Scholar]
- Moftah M. Z., Downie S. A., Bronstein N. B., Mezentseva N., Pu J., Maher P. A. and Newman S. A. (2002). Ectodermal FGFs induce perinodular inhibition of limb chondrogenesis in vitro and in vivo via FGF receptor 2. 249, 270-282. 10.1006/dbio.2002.0766 [DOI] [PubMed] [Google Scholar]
- Mohammadi M., Mcmahon G., Sun L., Tang C., Hirth P., Yeh B. K., Hubbard S. R. and Schlessinger J. (1997). Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. 276, 955-960. 10.1126/science.276.5314.955 [DOI] [PubMed] [Google Scholar]
- Montcouquiol M., Rachel R. A., Lanford P. J., Copeland N. G., Jenkins N. A. and Kelley M. W. (2003). Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. 423, 173-177. 10.1038/nature01618 [DOI] [PubMed] [Google Scholar]
- Montero J. A., Ganan Y., Macias D., Rodriguez-Leon J., Sanz-Ezquerro J. J., Merino R., Chimal-Monroy J., Nieto M. A. and Hurle J. M. (2001). Role of FGFs in the control of programmed cell death during limb development. 128, 2075-2084. [DOI] [PubMed] [Google Scholar]
- Niswander L., Tickle C., Vogel A., Booth I. and Martin G. R. (1993). FGF-4 replaces the apical ectodermal ridge and directs outgrowth and patterning of the limb. 75, 579-587. 10.1016/0092-8674(93)90391-3 [DOI] [PubMed] [Google Scholar]
- Ossipova O., Kim K. and Sokol S. Y. (2015). Planar polarization of Vangl2 in the vertebrate neural plate is controlled by Wnt and Myosin II signaling. 4, 722-730. 10.1242/bio.201511676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajni-Underwood S., Wilson C. P., Elder C., Mishina Y. and Lewandoski M. (2007). BMP signals control limb bud interdigital programmed cell death by regulating FGF signaling. 134, 2359-2368. 10.1242/dev.001677 [DOI] [PubMed] [Google Scholar]
- Parr B. A., Shea M. J., Vassileva G. and Mcmahon A. P. (1993). Mouse Wnt genes exhibit discrete domains of expression in the early embryonic CNS and limb buds. 119, 247-261. [DOI] [PubMed] [Google Scholar]
- Pizette S. and Niswander L. (1999). BMPs negatively regulate structure and function of the limb apical ectodermal ridge. 126, 883-894. [DOI] [PubMed] [Google Scholar]
- Qian D., Jones C., Rzadzinska A., Mark S., Zhang X., Steel K. P., Dai X. and Chen P. (2007). Wnt5a functions in planar cell polarity regulation in mice. 306, 121-133. 10.1016/j.ydbio.2007.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch G. J., Hammerschmidt M., Blader P., Schauerte H. E., Strahle U., Ingham P. W., Mcmahon A. P. and Haffter P. (1997). Wnt5 is required for tail formation in the zebrafish embryo. 62, 227-234. 10.1101/SQB.1997.062.01.028 [DOI] [PubMed] [Google Scholar]
- Riddle R. D., Johnson R. L., Laufer E. and Tabin C. (1993). Sonic hedgehog mediates the polarizing activity of the ZPA. 75, 1401-1416. 10.1016/0092-8674(93)90626-2 [DOI] [PubMed] [Google Scholar]
- Romereim S. M., Conoan N. H., Chen B. and Dudley A. T. (2014). A dynamic cell adhesion surface regulates tissue architecture in growth plate cartilage. 141, 2085-2095. 10.1242/dev.105452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saburi S., Hester I., Fischer E., Pontoglio M., Eremina V., Gessler M., Quaggin S. E., Harrison R., Mount R. and Mcneill H. (2008). Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. 40, 1010-1015. 10.1038/ng.179 [DOI] [PubMed] [Google Scholar]
- Satoh W., Matsuyama M., Takemura H., Aizawa S. and Shimono A. (2008). Sfrp1, Sfrp2, and Sfrp5 regulate the Wnt/beta-catenin and the planar cell polarity pathways during early trunk formation in mouse. 46, 92-103. 10.1002/dvg.20369 [DOI] [PubMed] [Google Scholar]
- Simons M. and Mlodzik M. (2008). Planar cell polarity signaling: from fly development to human disease. 42, 517-540. 10.1146/annurev.genet.42.110807.091432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J. and Mlodzik M. (2012). Planar cell polarity signaling: coordination of cellular orientation across tissues. 1, 479-499. 10.1002/wdev.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H., Hu J., Chen W., Elliott G., Andre P., Gao B. and Yang Y. (2010). Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. 466, 378-382. 10.1038/nature09129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. 21, 70-71. 10.1038/5007 [DOI] [PubMed] [Google Scholar]
- Sun X., Mariani F. V. and Martin G. R. (2002). Functions of FGF signalling from the apical ectodermal ridge in limb development. 418, 501-508. 10.1038/nature00902 [DOI] [PubMed] [Google Scholar]
- Tang N., Marshall W. F., Mcmahon M., Metzger R. J. and Martin G. R. (2011). Control of mitotic spindle angle by the RAS-regulated ERK1/2 pathway determines lung tube shape. 333, 342-345. 10.1126/science.1204831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todt W. L. and Fallon J. F. (1987). Posterior apical ectodermal ridge removal in the chick wing bud triggers a series of events resulting in defective anterior pattern formation. 101, 501-515. [DOI] [PubMed] [Google Scholar]
- Tomlinson A., Strapps W. R. and Heemskerk J. (1997). Linking Frizzled and Wnt signaling in Drosophila development. 124, 4515-4521. [DOI] [PubMed] [Google Scholar]
- Topol L., Jiang X., Choi H., Garrett-Beal L., Carolan P. J. and Yang Y. (2003). Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. 162, 899-908. 10.1083/jcb.200303158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Amerongen R. (2012). Alternative Wnt pathways and receptors. 4, a007914 10.1101/cshperspect.a007914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladar E. K., Antic D. and Axelrod J. D. (2009). Planar cell polarity signaling: the developing cell's compass. 1, a002964 10.1101/cshperspect.a002964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford J. B. (2012). Planar cell polarity and the developmental control of cell behavior in vertebrate embryos. 28, 627-653. 10.1146/annurev-cellbio-092910-154208 [DOI] [PubMed] [Google Scholar]
- Wang Y. and Nathans J. (2007). Tissue/planar cell polarity in vertebrates: new insights and new questions. 134, 647-658. 10.1242/dev.02772 [DOI] [PubMed] [Google Scholar]
- Wang B., Sinha T., Jiao K., Serra R. and Wang J. (2011). Disruption of PCP signaling causes limb morphogenesis and skeletal defects and may underlie Robinow syndrome and brachydactyly type B. 20, 271-285. 10.1093/hmg/ddq462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D. G. and Nieto M. A. (1993). Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. 225, 361-373. 10.1016/0076-6879(93)25025-W [DOI] [PubMed] [Google Scholar]
- Witte F., Dokas J., Neuendorf F., Mundlos S. and Stricker S. (2009). Comprehensive expression analysis of all Wnt genes and their major secreted antagonists during mouse limb development and cartilage differentiation. 9, 215-223. 10.1016/j.gep.2008.12.009 [DOI] [PubMed] [Google Scholar]
- Witze E. S., Litman E. S., Argast G. M., Moon R. T. and Ahn N. G. (2008). Wnt5a control of cell polarity and directional movement by polarized redistribution of adhesion receptors. 320, 365-369. 10.1126/science.1151250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. and Mlodzik M. (2008). The frizzled extracellular domain is a ligand for Van Gogh/Stbm during nonautonomous planar cell polarity signaling. 15, 462-469. 10.1016/j.devcel.2008.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. and Mlodzik M. (2009). A quest for the mechanism regulating global planar cell polarity of tissues. 19, 295-305. 10.1016/j.tcb.2009.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Roman A.-C., Carvajal-Gonzalez J. M. and Mlodzik M. (2013). Wg and Wnt4 provide long-range directional input to planar cell polarity orientation in Drosophila. 15, 1045-1055. 10.1038/ncb2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T. P., Bradley A., Mcmahon A. P. and Jones S. (1999). A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. 126, 1211-1223. [DOI] [PubMed] [Google Scholar]
- Yang Y. and Mlodzik M. (2015). Wnt-frizzled/planar cell polarity signaling: cellular orientation by facing the wind (Wnt). 31, 623-646. 10.1146/annurev-cellbio-100814-125315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T., Bassuk A. G. and Fritzsch B. (2013). Prickle1 stunts limb growth through alteration of cell polarity and gene expression. 242, 1293-1306. 10.1002/dvdy.24025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Garrett L., Feng D., Elliott G., Liu X., Wang N., Wong Y. M., Choi N. T., Yang Y. and Gao B. (2017). Wnt-induced Vangl2 phosphorylation is dose-dependently required for planar cell polarity in mammalian development. 27, 1466-1484. 10.1038/cr.2017.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K. and Ornitz D. M. (2008). FGF signaling regulates mesenchymal differentiation and skeletal patterning along the limb bud proximodistal axis. 135, 483-491. 10.1242/dev.013268 [DOI] [PubMed] [Google Scholar]
- Zeller R., López-Ríos J. and Zuniga A. (2009). Vertebrate limb bud development: moving towards integrative analysis of organogenesis. 10, 845-858. 10.1038/nrg2681 [DOI] [PubMed] [Google Scholar]
- Zhu X., Zhu H., Zhang L., Huang S., Cao J., Ma G., Feng G., He L., Yang Y. and Guo X. (2012). Wls-mediated Wnts differentially regulate distal limb patterning and tissue morphogenesis. 365, 328-338. 10.1016/j.ydbio.2012.02.019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.