Abstract
Background
Fear acquisition and extinction are central constructs in the cognitive-behavioral model of obsessive-compulsive disorder (OCD), which underlies exposure-based cognitive-behavioral therapy (CBT). Youth with OCD may have impairments in fear acquisition and extinction that carry treatment implications.
Methods
Eighty youth [39 OCD, 41 controls (HC)] completed clinical interviews, rating scales, and a differential conditioning task that included habituation, acquisition, and extinction phases. Skin conductance response (SCR) served as the primary dependent measure.
Results
During habituation, participants with OCD exhibited a stronger orienting SCR to initial stimuli relative to HC participants. During acquisition, differential fear conditioning was observed for both groups as evidenced by larger SCRs to the visual conditioned stimulus paired with an aversive unconditioned stimulus (CS+) compared to CS−; OCD participants exhibited a larger SCR to the CS+ relative to HC participants. The absolute magnitude of the unconditioned fear response was significantly larger in participants with OCD, compared to HC participants. During extinction, OCD participants continued to exhibit a differential SCR to the CS+ and CS−, whereas HC participants exhibited diminished SCR to both stimuli.
Conclusions
Participants with OCD exhibit a different pattern of fear extinction relative to HC participants suggestive of greater fear acquisition and impaired inhibitory learning.
Keywords: fear learning, OCD, pediatric, skin conductance, fear acquisition, fear extinction
INTRODUCTION
Obsessive-compulsive disorder (OCD) among children and adolescents has a prevalence of approximately 1-2% (1,2), is a significant public health problem, causes impairment in academic, social and family functioning, and left untreated is frequently unremitting into adulthood (3-5). Currently, two treatment modalities have demonstrated efficacy among pediatric OCD patients, namely pharmacotherapy with serotonin reuptake inhibitors (SRIs) and cognitive-behavioral therapy (CBT) with exposure and response prevention (E/RP; see (6) for a meta-analytic review). Several RCTs examining CBT protocols involving E/RP have provided strong empirical support for treating pediatric OCD (7-9).
In the cognitive-behavioral model, the mechanisms of fear acquisition and extinction play an important conceptual role in explaining symptom development, maintenance and treatment of OCD (10). Conditioned fear occurs when an emotionally neutral stimulus (conditioned stimulus, CS) is paired with an aversive unconditioned stimulus (US), such as the belief that a door handle (CS) is contaminated with germs (US) and contact could cause severe illness/death. Subsequent exposures to the CS produce a conditioned response (CR) such as fear/distress. In extinction, the emotional response to the CS declines through repeated exposure in the absence of the feared outcome (e.g., illness/death) and/or engagement in safety behaviors (e.g., avoidance, compulsive rituals). Extinction does not eradicate the initial CS-US association, but rather forms a new CS-no US association that competes with the existing fear-producing CS-US association (11).
While a fear conditioning model may not account for the entire phenomenology of OCD (e.g., not-just-right experiences, disgust) (12,13), understanding fear conditioning in youth with OCD is clinically relevant. First, as OCD often onsets in childhood (14), examining fear acquisition and extinction processes closer to symptom onset may help to identify whether these processes contribute to OCD phenomenology. Second, a number of youth with OCD exhibit inadequate or incomplete response to CBT in randomized controlled trials (RCTs). In the Pediatric OCD Treatment Study, up to 25% of youth did not respond to CBT and 60% of youth remained symptomatic after treatment (8). Given the central role that fear conditioning concepts are accorded in CBT, a better understanding of these mechanisms may guide treatment selection and thereby improve treatment outcome. For example, it may be that youth with OCD who demonstrate normal fear acquisition and extinction benefit from standard CBT approaches whereas youth with impairments in these processes may require augmentative interventions to ameliorate these deficits to achieve optimal benefit (15, 16). Finally, improved understanding of conditioned fear in OCD may help to inform future research. For example, stronger fear acquisition (“conditionability”) or impaired extinction may be associated with fear circuit abnormalities and/or utilization of different brain regions.
Despite the presumed central role in OCD, inferences about conditioned fear acquisition and extinction have been largely extrapolated from adults with anxiety disorders (17-19). Nanbu and colleagues (20) administered a classical fear conditioning procedure to 39 adults with OCD and 21 community controls. No group difference in skin conductance response (SCR) magnitude during fear acquisition was observed, but there was a trend toward larger SCR during extinction among OCD participants (20). Milad and colleagues (21) used a differential conditioning task to examine differences in SCR magnitude between 21 adults with OCD and 21 community controls. Similar levels of differential fear acquisition were exhibited between groups. No significant group differences or interaction effects were observed during the extinction phase but, OCD subjects showed impaired extinction recall relative to control subjects (23).
However, there are considerable distinctions between adults and youth with OCD that limit generalization without direct study in pediatric populations (22). Age differences in differential fear conditioning have been identified (23-25), such that younger participants are poorer at discriminating conditioned stimuli. Furthermore, age differences in the neurobiology underlying fear acquisition and extinction have been observed; youth recruit more sub-cortical regions (e.g., amygdala and hippocampus), whereas adults use prefrontal cortex regions (25, 26).
In the only examination of conditioned fear acquisition and extinction in youth with OCD to date, McGuire and colleagues (27) administered a differential fear-conditioning task to 41 youth aged 8-17 years (OCD: N=19; mean age 13.3 years; community controls: N=22; mean age 12.6 years). No group differences were found during acquisition, with both groups exhibiting differential fear conditioning but youth with OCD exhibited a unique extinction pattern suggestive of deficits in inhibitory learning. Beyond this preliminary examination, few studies have compared fear conditioning in youth with anxiety disorders and healthy controls (28-34). Findings suggest that fear conditioning produces comparable differential fear learning in anxious and non-anxious youth during acquisition; however, results for extinction are less definitive. Some evidence suggests that anxious youth exhibit resistance to within-session extinction as indicated by a larger SCR to the CS+ than to the CS− (i.e., persistence of differential fear learning) (29, 31, 32, 34). Other studies report no significant difference in extinction between anxious and non-anxious youth (26, 33). Inconsistent findings may be attributed to differences in sample characteristics, conditioning procedures, outcome measures, and unconditioned stimulus.
The present study examined fear conditioning and extinction in 39 youth with OCD and 41 healthy controls (HC) using a novel computer-administered differential conditioning task to assess fear acquisition and extinction (17, 30, 35). Consistent with the available OCD literature, we hypothesized that youth with OCD and HC participants would exhibit comparable fear acquisition. Second, we hypothesized that youth with OCD would exhibit impaired extinction of a fear-conditioned SCR, compared to HC participants.
METHODS
Participants
The sample consisted of 80 youth (49% male) between 8 and 18 years of age that were recruited from three sites (Massachusetts General Hospital [MGH], University of South Florida [USF], and the National Institute of Mental Health [NIMH]). This research was conducted under the approval of each participating institution’s Institutional Review Board, and only after subjects and their legal guardians provided informed consent/assent. Thirty-nine youth had a diagnosis of OCD and were ascertained in a multisite RCT (1R01MH093402, R01MH093381) examining whether d-cycloserine could augment the short-term efficacy of CBT to a greater extent than placebo and CBT in OCD-affected youth (36). An exploratory aim of this multisite RCT examined potential moderators of treatment outcome, including fear extinction learning using a computer-administered paradigm to assess fear acquisition and extinction (17, 30, 35). Participants with OCD were 39 youth who met the following inclusion criteria: a primary or co-primary diagnosis of OCD; between 7 and 17 years of age (inclusive); and a moderate level of obsessive-compulsive symptoms as evidenced by a CY-BOCS total score ≥ 16. Participants with OCD were permitted to be on a psychiatric medication so long as it was stable for at least eight weeks prior to participation. Sixteen (41%) of participants with OCD were on a serotonin reuptake inhibitor (SRI) medication (e.g., clomipramine, fluoxetine, duloxetine, fluvoxamine, sertraline, citalopram, paroxetine).
Healthy control participants were 41 youth recruited in an NIMH-supported study who met the following criteria: between 8 and 17 years of age (inclusive); an IQ ≥ 70; physically healthy; not taking any medication; and without any psychiatric diagnoses (37). All healthy control participants received a comprehensive psychiatric assessment that included the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (37). Healthy control participants were excluded from participation if they met criteria for any current Axis I disorder. Collectively, participants were predominantly non-Hispanic Caucasian (N=31) with a similar representation of males (51%) and females.
Measures
Fear Conditioning Computer Task
A differential fear-conditioning paradigm was used in which participants learned to associate an aversive stimulus (a 95 decibel scream and fear expression) with a paired conditioned stimulus (CS+), but not an unpaired conditioned stimulus (CS−). This paradigm used two female faces with neutral facial expressions to serve as conditioned stimuli (30), and included three phases: habituation (4 presentations of each stimulus face); acquisition (10 presentations of each stimulus face); and extinction (8 presentations of each stimulus face). The CSs were presented for 8 seconds with an inter-stimulus interval ranging from 20-26 seconds for MGH/USF sites and 8-21 seconds at NIMH. During the habituation phase, participants passively viewed the two stimuli (CS+ and CS−) without the presentation of any aversive stimuli. In the acquisition phase, one of the female faces (CS+) was paired with the aversive stimulus for 8 out of 10 presentations, whereas the other female face (CS−) was not paired with the aversive stimulus. The aversive stimulus was presented immediately following CS+ offset and remained on for three seconds (one second in the NIMH trial) along with the fearful expression face, which allowed for the development of the conditioned fear response (CR). The faces were counter balanced at one site (NIMH), but counterbalancing of the faces paired with the aversive stimulus did not occur at the other two sites (MGH and USF). During the extinction phase, the CS+ and CS− were presented repeatedly in the absence of the aversive stimuli. This task has been used in studies with children who have anxiety disorders (25, 28), as well as healthy control samples of youth (23, 28) and adults (28, 38). Skin conductance response served as the primary dependent measure of fear.
At MGH and USF, a computer and Coulbourn Modular Instrument System recorded SCR throughout the task using a Coulbourn Isolated Skin Conductance Coupler. Skin conductance level was recorded through two 9-mm Ag/AgCl electrodes filled with isotonic paste and placed on the hypothenar surface of the participant’s non-dominant hand. Electrodes were separated by 14 mm (the width of the adhesive collar). Participant’s SCR was digitized by a Coulbourn Lablinic Analog to Digital Converter; 10 samples per second were retained for calculating SCR. At NIH, a computer and PsyLab psychophysiological recording system (www.psylab.com) recorded SCR continuously from the medial phalanx of the middle and ring fingers of the left hand using a sampling rate of 1000Hz.
Demographic questionnaire
Parents of participants completed a demographic questionnaire that queried participant’s age, gender, race, ethnicity, and medication status.
Procedure
All study procedures were approved by the site’s respective Institutional Review Boards (MGH, USF, and NIMH). Participants with OCD were recruited from two sites (MGH, USF) as part of an ongoing clinical trial, and healthy control participants from an NIMH study. Consent and assent to participate in the fear-conditioning paradigm were obtained from parents and youth, respectively. While parent’s completed demographic questionnaires, youth completed the fear conditioning paradigm. When completing the fear paradigm task, participants at MGH and USF were provided with a brief pause after the habituation phase, but there was no pause at the NIMH site. While a brief pause was also provided after the acquisition phase at one site (NIMH), there was no pause provided at the other two sites (MGH and USF). Aside from these minor distinctions, the paradigm was consistently administered across all three sites.
Data Analysis
Following the method used by Orr et al. 2000 (20), a SCR score for each CS presentation was calculated by subtracting the average SCR during the 2-second interval immediately preceding CS onset from the peak SCR during the 8-second CS interval. A SCR score for each US presentation was calculated by subtracting the average SCR during the last 2 seconds of the CS interval from the peak SCR during the 5-second interval following US onset. At NIMH, SCR was calculated as the difference from peak amplitude within 1-5 seconds following the stimulus onset compared to baseline. In order to address skewness in the SCR distribution, a square-root transformation was applied to the absolute values of all SCRs prior to analysis, with the minus sign replaced if the SCR was negative. Analyses of habituation, acquisition and extinction phases included the 4, 10 and 8 presentations (respectively) of the CS+ and CS−. Trial blocks were created by averaging across every two trials (of the same stimulus type); this step produced for each stimulus (CS+ and CS−), two trial blocks for the habituation phase, five trial blocks for the acquisition phase, and four trial blocks for the extinction phase. For each phase, a repeated-measures analysis of variance (ANOVA) was conducted with SCR as the dependent measure and Diagnostic Group (OCD, HC) as a between-group factor, Stimulus Type (CS+, CS−) as a within-group factor, and Trial Block as the repeated measure. Independent sample t-tests were used to compare the groups’ orienting response (OR), i.e., the SCR to the first trial block of the CS+ and CS−. The unconditioned SCR during the acquisition phase was examined using a repeated-measure ANOVA, with Diagnostic Group as a between-group factor and Trial Block as the repeated measure. For all repeated-measure ANOVAs, significance levels reflect the Greenhouse-Geisser correction for sphericity. Significance was determined using alpha=0.05 and two-tailed tests.
RESULTS
Participants
While there was no significant differences in gender (χ2=0.21, p=0.65) or age [t78=0.54, p=0.59], the OCD group had more Hispanic participants (χ2=9.65, p=0.008) and the HC group had more non-Caucasian participants (χ2=8.03, p=0.005) (see Table 1).
Table 1.
Participant Characteristics For the Entire Sample and by Diagnostic Group
| Whole Sample (N = 80) |
OCD Group (n = 39) |
HC Group (n = 41) |
|
|---|---|---|---|
|
| |||
| N (%) | N (%) | N (%) | |
| Gender | |||
| Male | 39 (49%) | 18 (46%) | 21 (51%) |
| Female | 41 (51%) | 21 (54%) | 20 (49%) |
| Race | |||
| Asian | 1 (1%) | 0 (0%) | 1 (2%) |
| Black | 6 (8%) | 1 (3%) | 5 (12%) |
| White | 69 (86%) | 38 (97%) | 31 (76%) |
| Multi-racial | 3 (4%) | 0 (0%) | 3 (7%) |
| Unknown | 1 (1%) | 0 (0%) | 1 (2%) |
| Ethnicity | |||
| Hispanic | 7 (9%) | 7 (18%) | 0 (0%) |
| Non-Hispanic | 71 (88%) | 32 (82%) | 39 (95%) |
| Unknown | 2 (3%) | 0 (0%) | 2 (5%) |
| SRI Medication Status | 16(20%) | 16(41%) | 0 (0%) |
|
| |||
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Age | 14.04 (2.47) | 14.19 (2.30) | 13.89 (2.65) |
Note: OCD = Obsessive Compulsive Disorder; HC = Healthy Control, SRI = Serotonin Reuptake Inhibitor
Orienting Response (OR)
Given that the ordering of CS+ and CS− presentations was not counterbalanced across sites, the OR was examined using all OCD participants and only those HC participants that received the same stimulus presentation order as the OCD group (39 OCD, 18 HC). The OR was significantly larger in the OCD group (M=0.40, SD=0.23), compared to the HC group (M=0.26, SD=0.24) for the first Trial Block of the to-be CS+, t55=2.07, p=0.04, d=0.60. There was no group difference for the first Trial Block of the to-be CS−, t55=−0.74, p=0.46, d=0.21.
Habituation Phase
As can be seen in Table 2 and Figure 1, there were significant main effects for Group, Stimulus, and Trial Block, as well as a significant Group × Stimulus × Trial Block interaction. The significant three-way interaction suggests that participants showed different patterns of SCRs to the CSs over the two trial blocks, which appears to primarily result from the OCD group’s larger initial responses (OR) to the to-be CS+. Importantly, there was no significant group difference for the second Trial Block of the to-be CS+ (t78=0.86, p=0.39, d=0.19), indicating that the initial group difference was no longer evident prior to beginning the fear acquisition phase.
Table 2.
ANOVA results for comparisons of SC Responses during the Habituation Phase, Acquisition Phase, and Extinction Phase (N = 80)
| Habituation Phase | F | p | η2p |
|---|---|---|---|
| Group | 4.62 | 0.04 | 0.06 |
| Stimulus | 18.41 | < 0.001 | 0.19 |
| Trial Block | 65.67 | < 0.001 | 0.46 |
| Stimulus × Trial Block | 1.23 | 0.27 | 0.02 |
| Group × Stimulus | 3.22 | 0.08 | 0.04 |
| Group × Trial Block | 0.37 | 0.55 | 0.01 |
| Group × Stimulus × Trial Block | 6.27 | 0.02 | 0.07 |
|
| |||
| Acquisition Phase | F | p | η2p |
|
| |||
| Group | 0.56 | 0.46 | 0.01 |
| Stimulus | 29.82 | < 0.001 | 0.28 |
| Trial Block | 7.22 | < 0.001 | 0.09 |
| Stimulus × Trial Block | 16.28 | < 0.001 | 0.17 |
| Group × Stimulus | 6.49 | 0.01 | 0.08 |
| Group × Trial Block | 1.16 | 0.33 | 0.02 |
| Group × Stimulus × Trial Block | 0.98 | 0.42 | 0.01 |
|
| |||
| Extinction Phase | F | p | η2p |
|
| |||
| Group | 1.50 | 0.23 | 0.02 |
| Stimulus | 5.11 | 0.03 | 0.06 |
| Trial Block | 1.87 | 0.14 | 0.02 |
| Stimulus × Trial Block | 0.39 | 0.75 | 0.01 |
| Group × Stimulus | 4.01 | 0.05 | 0.05 |
| Group × Trial Block | 4.51 | 0.005 | 0.06 |
| Group × Stimulus × Trial Block | 0.94 | 0.42 | 0.01 |
Figure 1.
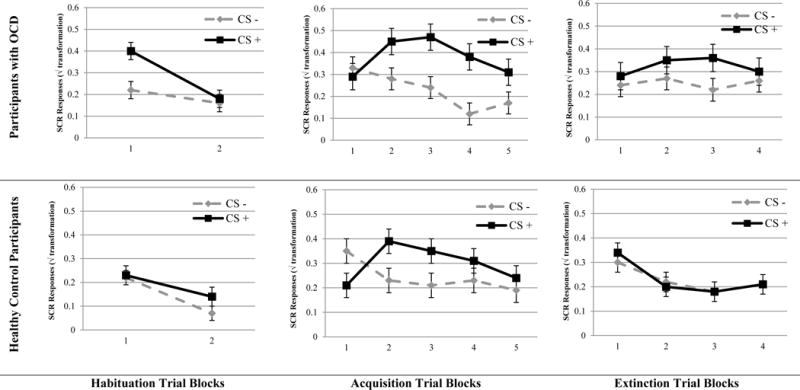
Skin conductance responses across conditioning phases for participants with OCD (n = 39) and healthy control participants (n = 41) with standard error.
Acquisition Phase
As can be seen in Table 2 and Figure 1, there was a significant Group × Stimulus interaction. Participants with OCD exhibited larger differential SCR to the CS+ and CS−, compared to HC participants. Follow-up comparisons for SCRs to the CS+ and CS− revealed larger responses in the OCD (M=0.40, SD=0.24) compared to HC group (M=0.29, SD=0.24) for the CS+ (d=0.45), but not the CS− (OCD, M=0.24, SD=0.18; HC, M=0.24, SD=0.19, d=0.00). These results suggest a stronger differential fear conditioning in the OCD, compared to HC participants. The main effect for Stimulus was also significant, with the CS+ (CS+, M=0.34, SD=0.24) producing larger SCR relative to the CS− (M=0.24, SD=0.18, see Table 2). The main effect for Trial was also significant, with the second trial block (M=0.34, SD=0.23) associated with greater SCR relative to the fourth (M=0.26, SD=0.25) and fifth trial blocks (M=0.23, SD=0.21, Table 2). There was also a difference between the third trial block (M=0.32, SD=0.25) and fifth trial block as well. There was also a significant Stimulus × Trial Block interaction. While participants initially had a greater SCR to the CS− (M=0.34, SD=0.29) compared to the CS+ (M=0.25, SD=0.23, d=0.34) during the first trial block, participants subsequently had a greater SCR to the CS+ compared to the CS− on second (CS+, M=0.42, SD=0.29; CS−, M=0.25, SD=0.25, d=0.63), third (CS+, M=0.41, SD=0.33; CS−, M=0.23, SD=0.27, d=0.60), fourth (CS+, M=0.34, SD=0.30; CS−, M=0.17, SD=0.27, d=0.26), and fifth trial blocks (CS+, M=0.28, SD=0.29; CS−, M=0.18, SD=0.23, d=0.38). This significant Stimulus × Trial interaction supports that differential SCR conditioning occurred across groups and that the differential SCR decreased somewhat across trials, as is commonly observed for SCR in human fear conditioning.
A repeated measure ANOVA was used to examine group differences in the magnitude of SCR to the unconditioned stimulus (95 dB loud scream and fearful facial expression). As can be seen in Figure 2, there were significant main effects for Group (F=45.93, p<0.001, η2p=0.37) and Trial Block (F=59.07, p<0.001, η2p=0.43). Participants with OCD produced larger SCRs to the unconditioned stimuli, compared to HC participants and both groups exhibited decreased SCR across trial blocks. The Group × Trial Block interaction was also significant (F=3.88, p<0.01, η2p=0.05), which suggests that the decrease in SCR over trial blocks differed between groups. Youth with OCD appeared to have a somewhat slower decrease in SCR from the first to second trial block compared to healthy controls (d=0.65), whereas youth with OCD had a greater decrease in SCR from the third to fourth trial block, compared to healthy controls (d=0.38).
Figure 2.
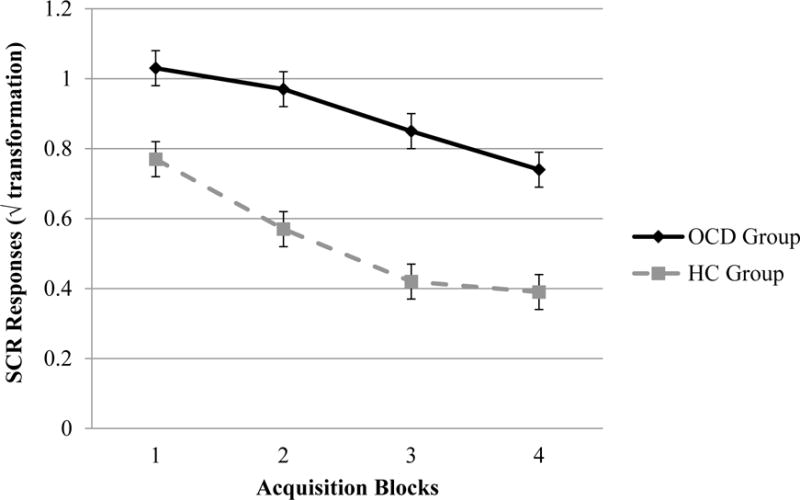
Comparisons of unconditioned skin conductance responses to the loud scream during acquisition phase CS+ trials between participants with OCD (n = 39) and healthy control participants (n = 41) with standard error.
Extinction Phase
As can be seen in Table 2 and Figure 1, there was a significant Group × Stimulus interaction during extinction, indicating that larger conditioned differential SCRs observed during acquisition in the OCD, compared to HC, group persisted into the extinction phase. When separately comparing responses to the CS+ and CS−, participants with OCD had larger SCRs to the CS+ (OCD, M=0.32, SD=0.23; HC, M=0.23, SD=0.23, d=0.40), but not the CS - compared to HC participants (OCD, M=0.25, SD=0.21; HC, M=0.23, SD=0.21, d=0.10). There was also a significant Group × Trial Block interaction, indicating that the pattern of SCRs across extinction trial blocks differed between OCD and HC groups. Youth with OCD exhibited overall larger SCRs, compared to controls, in the second (OCD, M=0.31, SD=0.24; HC, M=0.21, SD=0.24, d=0.42) and third (OCD, M=0.29, SD=0.25; HC, M=0.18, SD=0.25, d=0.44) trial blocks, while there was no notable group difference in SCR magnitude for the first (OCD, M=0.26, SD=0.24; HC, M=0.32, SD=0.24, d=−0.25) and last trial blocks (OCD, M=0.28, SD=0.26; HC, M=0.21, SD=0.26, d=0.27).
DISCUSSION
To our knowledge, this study represents the largest examination of conditioned fear acquisition and extinction in youth with OCD. In summary, youth with OCD exhibited a larger orienting response, larger unconditioned and conditioned fear response to the CS+ during acquisition, and impaired extinction to the CS+. These results suggest that youth with OCD had greater orienting response to novel stimuli, greater fear acquisition, and slowed extinction compared to healthy controls. Taken together, these results suggest that youth with OCD may be more responsive to acquiring conditioned fear and exhibit impaired inhibitory learning that results in diminished fear extinction.
During habituation in a cohort of youth with OCD and healthy controls, participants with OCD produced a significantly larger SC OR, compared to HC participants. This finding suggests that youth with OCD are more reactive to novel stimuli presentations, relative to HC participants and is consistent with extant literature suggesting that individuals with OCD have early attention biases to threat (39). In addition, we found that during fear acquisition, the youth with OCD produced larger SCRs to the aversive stimulus that served as the unconditioned stimulus. This suggests that youth with OCD have a heightened sensitivity to aversive stimuli, which could, in part, explain their heightened attention bias to threat.
During the acquisition phase, both OCD and HC participants exhibited robust differential fear conditioning. However, contrary to our hypothesis, OCD participants exhibited stronger differential fear acquisition, compared to HC participants. The stronger conditioned fear observed in the OCD group was evident in their increased SCR magnitude to the CS+, i.e., the fear cue, while they produced SCRs to the CS−, i.e., safety cue, that were comparable to those of the HC group. This finding is similar to our preliminary examination of fear extinction in youth with OCD (27), but had a greater magnitude and statistical significance in our larger sample. Notably, these findings are contrary to adult OCD studies of fear conditioning (where greater prefrontal involvement is hypothesized to occur) that found no difference in fear acquisition (20, 21, 25, 26), However, these findings are similar to several examinations of fear extinction in child anxiety disorders (28, 29, 34), which suggest that the youth with these conditions acquire fear to a greater degree relative to HC participants.
During extinction, the OCD and HC groups differed in their pattern of responses to the conditioned stimuli. While HC participants extinguished fear to the CS+ and CS−, participants with OCD continued to exhibit a differential SCR to the stimuli. Moreover, magnitude of the SCR to the CS+ was larger for OCD participants compared to HC participants. However, this difference was not observed in the CS−. Taken together, the persistence of conditioned fear and greater magnitude of conditioned fear to the CS+ suggests slower learning of safety signals after fear conditioning, which may represent a deficit in inhibitory learning. While initial CBT models emphasize within-and-between session habituation as the central mechanism for CBT (40), research suggests that inhibitory learning may be a key therapeutic component (41, 42). Indeed, within-and-between session habituation in CBT has not been found to predict treatment outcome for youth with OCD (43, 44). A deficit in inhibitory learning may explain disparate exposure-based CBT outcomes among youth with OCD and offers preliminary support for consideration of CBT protocols that optimize inhibitory learning (42).
Several limitations to the reported work should be considered. First, the conclusion that youth with OCD exhibit greater conditioning than healthy controls must be tempered by the fact that the magnitude of the unconditioned response to the 95 decibel scream during acquisition was significantly larger in our OCD sample, compared to the HC participants. This finding suggests that the unconditioned stimulus was perceived as more aversive by the youth with OCD. Given a larger unconditioned response, one might expect to see a stronger conditioned response and slower extinction. It is possible that, had an unconditioned stimulus that was determined to be comparably aversive for the OCD and HC groups been used, acquisition and extinction of the conditioned fear response would have been comparable. Future studies should consider possible methods for establishing levels of an unconditioned stimulus that are comparably aversive across groups. Second, the cases and controls were recruited in two separate, albeit parallel, studies that used similar but not identical equipment. The paradigm was similar but not exactly the same between sites. Third, although the largest study to date, this study had a modest sample size. Although larger than adult OCD samples (21), results that trended towards statistical significance may emerge more robustly in a larger sample. Fourth, the significance value was set at α=0.05 for all analyses and no corrections for multiple comparisons were conducted. Although this may have impacted significance, it would not have impacted the magnitude of effects. Fifth, the order of presentation of the two faces that served as CS+ and CS− was varied at one site (NIMH), but not at the other two sites (MGH and USF). The finding that the to-be CS+ face elicited a stronger SCR in the OCD participants during habituation compared to the HC participants could indicate that the faces were perceived differently regardless of the paired US. However when using only HC participants that experienced the same ordering of stimulus presentations to control for this potential bias, results were unchanged. Sixth, there was a slight difference in paradigms used. The break between acquisition and extinction in healthy control sample may have allowed for greater habituation to occur, whereas youth with OCD did not have this break. Finally, the magnitude of conditioning can be influenced by study specific methodology (e.g., task, UCS, sample characteristics) (26). Thus, findings from the present study may be limited to our conditioning procedure and sample characteristics.
These initial findings highlight several possible directions for future OCD research. First, given variable findings during extinction in fear conditioning studies of youth with anxiety disorders (33), replication and extension of these findings is warranted. Second, given the evidence of greater differential fear conditioning among youth with OCD, it would be interesting to examine associations with specific fear circuit abnormalities and/or utilization of different brain regions. Indeed, as youth and adults exhibit neurobiological differences in fear extinction circuitry (25), this greater differential fear conditioning may be associated with a specific clinical presentation, symptom course, and/or treatment profile. Third, there is a growing body of evidence that highlights the importance of inhibitory learning in exposure therapy (41). Although predominantly focused on adults with anxiety disorders (41), the incorporation of inhibitory-learning based CBT may prove beneficial in strengthening observed inhibitory deficits and maximize the therapeutic benefit for youth with OCD (42).
CONCLUSIONS
Participants with OCD exhibit a different pattern of fear extinction relative to HC participants suggestive of greater fear acquisition and impaired inhibitory learning. A deficit in inhibitory learning may explain these findings as well as disparate exposure-based CBT outcomes among youth with OCD. This study offers preliminary support for consideration of CBT protocols that optimize inhibitory learning in the treatment of youth with OCD.
Acknowledgments
The authors acknowledge Chelsea Ale, Ph.D., Noah Berman, Ph.D., Jennifer Britton, Ph.D., David Greenblatt, M.D., Marni Jacob, Ph.D., Nicole McBride, B.S., Scott Orr, Ph.D., Jennifer Park, Ph.D., Julia McQuade, Ph.D., David Pauls, Ph.D., Christine Cooper-Vince, Ph.D., Kathleen Carey, CNS, Anne Chosak Ph.D., Allison Cooperman B.A, Angelina Gómez, B.A., Ashley Brown, B.A., Kesley Ramsey, B.A., Robert Selles, M.A., Abigail Stark, B.A., Alyssa Faro, B.A., and Monica S. Wu, M.A.
Support for this article comes in part from the National Institute of Mental Health (NIMH) of the National Institutes of Health (NIH) under awards 1R01MH093402 and R01MH093381 to the first and last authors. Dr. Geller has also received funding from Eli Lilly, Otsuka, Glaxo Smith Kline, Forest Pharmaceuticals (lifetime) and Neurocrine Bioscience (last 3 years). Dr. Pine is supported by the NIMH Intramural Research Program. Dr. Murphy received funding in the last 3 years from NIH/NIMH, CDC, MGH, PANDAS Network, Otsuka Pharmaceuticals, Shire Pharmaceuticals, Pfizer, Inc., Auspex, Neurocrine and Astra Zeneca. Travel support received from the Tourette Syndrome Association and for various CME presentations.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH or NIH. Dr. Britton is supported by R00MH091183.
Footnotes
AUTHOR DISCLOSURES
Dr. McGuire, Dr. Orr, Dr. Small and Dr. Wilhelm report no biomedical financial interests or potential conflicts of interest.
References
- 1.Douglass HM, Moffitt TE, Dar R, McGee R, Silva P. Obsessive-compulsive disorder in a birth cohort of 18-year-olds. Prevalence and predictors. J Am Acad Child Adolesc Psychiatry. 1995;34:1424–1431. doi: 10.1097/00004583-199511000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Zohar AH. The epidemiology of obsessive-compulsive disorder in children and adolescents. Child Adolesc Psychiatr Clin N Am. 1999;8:445–460. [PubMed] [Google Scholar]
- 3.Leonard HL, Swedo SE, Lenane MC, Rettew DC, Hamburger SD, Bartko JJ, Rapoport JL. A 2- to 7-year follow-up study of 54 obsessive-compulsive children and adolescents. Arch Gen Psychiatry. 1993;50:429–439. doi: 10.1001/archpsyc.1993.01820180023003. [DOI] [PubMed] [Google Scholar]
- 4.Piacentini J, Bergman RL, Keller M, McCracken J. Functional impairment in children and adolescents with obsessive-compulsive disorder. J Child Adolesc Psychopharmacol. 2003;13(Suppl 1):S61–S69. doi: 10.1089/104454603322126359. [DOI] [PubMed] [Google Scholar]
- 5.Swedo SE, Rapoport JL, Leonard H, Lenane M, Cheslow D. Obsessive-compulsive disorder in children and adolescents. Clinical phenomenology of 70 consecutive cases. Arch Gen Psychiatiatry. 1989;46:335–341. doi: 10.1001/archpsyc.1989.01810040041007. [DOI] [PubMed] [Google Scholar]
- 6.McGuire JF, Piacentini J, Lewin AB, Brennan EA, Murphy TK, Storch EA. A meta-analysis of cognitive behavior therapy and medication for child obsessive-compulsive disorder: Moderators of treatment efficacy, response, and remission. Depress Anxiety. 2015;32:580–593. doi: 10.1002/da.22389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman J, Sapyta J, Garcia A, Compton S, Khanna M, Flessner C, FitzGerald D, Mauro C, Dingfelder R, Benito K, Harrison J, Curry J, Foa E, March J, Moore P, Franklin M. Family-based treatment of early childhood obsessive-compulsive disorder: the pediatric obsessive-compulsive disorder treatment study for young children (pots jr)—a randomized clinical trial. JAMA Psychiatry. 2014;71:689–698. doi: 10.1001/jamapsychiatry.2014.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.POTS. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: The pediatric ocd treatment study (pots) randomized controlled trial. JAMA. 2004;292:1969–1976. doi: 10.1001/jama.292.16.1969. [DOI] [PubMed] [Google Scholar]
- 9.Storch EA, Geffken GR, Merlo LJ, Mann G, Duke D, Munson M, Adkins J, Grabill KM, Murphy TK, Goodman WK. Family-based cognitive-behavioral therapy for pediatric obsessive-compulsive disorder: Comparison of intensive and weekly approaches. J Am Acad Child Adolesc Psychiatry. 2007;46:469–478. doi: 10.1097/chi.0b013e31803062e7. [DOI] [PubMed] [Google Scholar]
- 10.Abramowitz JS, Taylor S, McKay D. Obsessive-compulsive disorder. Lancet. 2009;374:491–499. doi: 10.1016/S0140-6736(09)60240-3. [DOI] [PubMed] [Google Scholar]
- 11.Myers KMDM. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 12.Coles ME, Heimberg RG, Frost RO, Steketee G. Not just right experiences and obsessive-compulsive features: Experimental and self-monitoring perspectives. Behav Res Ther. 2005;43:153–167. doi: 10.1016/j.brat.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Olatunji BO, Tart CD, Ciesielski BG, McGrath PB, Smits JAJ. Specificity of disgust vulnerability in the distinction and treatment of ocd. J Psychiatr Res. 2011;45:1236–1242. doi: 10.1016/j.jpsychires.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nestadt G, Samuels J, Riddle M, Bienvenu J, Liang K-Y, LaBuda M, Walkup J, Grados M, Hoehn-Saric R. A family study of obsessive-compulsive disorder. Arch Gen Psychiatry. 2000;57:358–363. doi: 10.1001/archpsyc.57.4.358. [DOI] [PubMed] [Google Scholar]
- 15.Riemann BC, Kuckertz JM, Rozenman M, Weersing VR, Amir N. Augmentation of youth cognitive behavioral and pharmacological interventions with attention modification: A preliminary investigation. Depress Anxiety. 2013;30:822–828. doi: 10.1002/da.22127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shechner T, Rimon-Chakir A, Britton JC, Lotan D, Apter A, Bliese PD, Pine DS, Bar-Haim Y. Attention bias modification treatment augmenting effects on cognitive behavioral therapy in children with anxiety: Randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2014;53:61–71. doi: 10.1016/j.jaac.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, Pine DS. Classical fear conditioning in the anxiety disorders: A meta-analysis. Behav Res Ther. 2005;43:1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Michael T, Blechert J, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in panic disorder: Enhanced resistance to extinction. J Abnorm Psychol. 2007;116:612–617. doi: 10.1037/0021-843X.116.3.612. [DOI] [PubMed] [Google Scholar]
- 19.Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol. 2000;109:290–298. [PubMed] [Google Scholar]
- 20.Nanbu M, Kurayama T, Nakazawa K, Matsuzawa D, Komiya Z, Haraguchi T, Ogura H, Hashimoto T, Yoshida S, Iyo M, Shimizu E. Impaired p50 suppression in fear extinction in obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:317–322. doi: 10.1016/j.pnpbp.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Milad MR, Furtak SC, Greenberg JL, Keshaviah A, Im JJ, Falkenstein MJ, Jenike M, Rauch SL, Wilhelm S. Deficits in conditioned fear extinction in obsessive-compulsive disorder and neurobiological changes in the fear circuit. JAMA Psychiatry. 2013;70:608–618. doi: 10.1001/jamapsychiatry.2013.914. [DOI] [PubMed] [Google Scholar]
- 22.Farrell L, Barrett P, Piacentini J. Obsessive-compulsive disorder across the developmental trajectory: Clinical correlates in children, adolescents and adults. Behaviour Change. 2006;23:103–120. [Google Scholar]
- 23.Glenn CR, Klein DN, Lissek S, Britton JC, Pine DS, Hajcak G. The development of fear learning and generalization in 8-13 year-olds. Dev Psychobiol. 2012;54:675–684. doi: 10.1002/dev.20616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jovanovic T, Nylocks KM, Gamwell KL, Smith A, Davis TA, Norrholm SD, Bradley B. Development of fear acquisition and extinction in children: Effects of age and anxiety. Neurobiol Learn Mem. 2014;113:135–142. doi: 10.1016/j.nlm.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lau JY, Britton JC, Nelson EE, Angold A, Ernst M, Goldwin M, Grillon C, Leibenluft E, Lissek S, Norcross M, Shiffrin N, Pine DS. Distinct neural signatures of threat learning in adolescents and adults. Proc Natl Acad Sci USA. 2011;108:4500–4505. doi: 10.1073/pnas.1005494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shechner T, Hong M, Britton JC, Pine DS, Fox NA. Fear conditioning and extinction across development: Evidence from human studies and animal models. Biol Psychol. 2014;100:1–12. doi: 10.1016/j.biopsycho.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGuire JF, Orr SP, Wu MS, Lewin AB, Small BJ, Phares V, Murphy TK, Wilhelm S, Pine DS, Geller DA, Storch EA. Fear conditioning and extinction in youth with obsessive-compulsive disorder. Manuscript in Submission. 2015 doi: 10.1002/da.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Britton JC, Grillon C, Lissek S, Norcross MA, Szuhany KL, Chen G, Ernst M, Nelson EE, Leibenluft E, Shechner T, Pine DS. Response to learned threat: An fmri study in adolescent and adult anxiety. Am J Psychiatry. 2013;170:1195–1204. doi: 10.1176/appi.ajp.2013.12050651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Craske MG, Waters AM, Bergman RL, Naliboff B, Lipp OV, Negoro H, Ornitz EM. Is aversive learning a marker of risk for anxiety disorders in children? Behav Res Ther. 2008;46:954–967. doi: 10.1016/j.brat.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lau JYF, Lissek S, Nelson EE, Lee Y, Roberson-Nay R, Poeth K, Jenness J, Ernst M, Grillon C, Pine DS. Fear conditioning in adolescents with anxiety disorders: Results from a novel experimental paradigm. J Am Acad Child Adolesc Psychiatry. 2008;47:94–102. doi: 10.1097/chi.0b01e31815a5f01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liberman LC, Lipp OV, Spence SH, March S. Evidence for retarded extinction of aversive learning in anxious children. Behav Res Ther. 2006;44:1491–1502. doi: 10.1016/j.brat.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Pliszka SR, Hatch JP, Borcherding SH, Rogeness GA. Classical conditioning in children with attention deficit hyperactivity disorder (adhd) and anxiety disorders: A test of quay’s model. J Abnorm Child Psychol. 1993;21:411–423. doi: 10.1007/BF01261601. [DOI] [PubMed] [Google Scholar]
- 33.Shechner T, Britton JC, Ronkin EG, Jarcho JM, Mash JA, Michalska KJ, Leibenluft E, Pine DS. Fear conditioning and extinction in anxious and nonanxious youth and adults: Examining a novel developmentally appropriate fear-conditioning task. Depress Anxiety. 2015;32:277–288. doi: 10.1002/da.22318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waters AM, Henry J, Neumann DL. Aversive pavlovian conditioning in childhood anxiety disorders: Impaired response inhibition and resistance to extinction. J Abnorm Psychol. 2009;118:311–321. doi: 10.1037/a0015635. [DOI] [PubMed] [Google Scholar]
- 35.Bouton ME. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- 36.McGuire JF, Lewin AB, Geller DA, Brown A, Ramsey K, Mutch J, Mittelman A, Micco J, Jordan C, Wilhelm S, Murphy TK, Small BJ, Storch EA. Advances in the treatment of pediatric ocd: Rationale and design for the evaluation of d-cycloserine with exposure and response prevention. Neuropsychiatry (London) 2012;2:1–14. doi: 10.2217/npy.12.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (k-sads-pl): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 38.Haddad AD, Xu M, Raeder S, Lau JY. Measuring the role of conditioning and stimulus generalisation in common fears and worries. Cognition Emotion. 2013;27:914–22. doi: 10.1080/02699931.2012.747428. [DOI] [PubMed] [Google Scholar]
- 39.Thomas SJ, Gonsalvez CJ, Johnstone SJ. Neural time course of threat-related attentional bias and interference in panic and obsessive–compulsive disorders. Biol Psychol. 2013;94:116–129. doi: 10.1016/j.biopsycho.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 40.Foa EB, Kozak MJ. Emotional processing of fear: Exposure to corrective information. Psychological Bull. 1986;99:20–35. [PubMed] [Google Scholar]
- 41.Craske MG, Liao B, Brown L, Vervliet B. Role of inhibition in exposure therapy. J Exp Psychopathology. 2012;3:322–345. [Google Scholar]
- 42.Craske MG, Treanor M, Conway CC, Zbozinek T, Vervliet B. Maximizing exposure therapy: An inhibitory learning approach. Behav Res Ther. 2014;58:10–23. doi: 10.1016/j.brat.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kircanski K, Peris TS. Exposure and response prevention process predicts treatment outcome in youth with ocd. J Abnorm Child Psychol. 2014;43:543–552. doi: 10.1007/s10802-014-9917-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kircanski K, Wu M, Piacentini J. Reduction of subjective distress in cbt for childhood ocd: Nature of change, predictors, and relation to treatment outcome. J Anxiety Disord. 2014;28:125–132. doi: 10.1016/j.janxdis.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


