Abstract
Background
An outbreak of Middle East respiratory syndrome coronavirus (MERS-CoV) in Jordan in 2015 involved a variant virus that acquired distinctive deletions in the accessory open reading frames. We conducted a molecular and seroepidemiologic investigation to describe the deletion variant’s transmission patterns and epidemiology.
Methods
We reviewed epidemiologic and medical chart data and analyzed viral genome sequences from respiratory specimens of MERS-CoV cases. In early 2016, sera and standardized interviews were obtained from MERS-CoV cases and their contacts. Sera were evaluated by nucleocapsid and spike protein enzyme immunoassays and microneutralization.
Results
Among 16 cases, 11 (69%) had health care exposure and 5 (31%) were relatives of a known case; 13 (81%) were symptomatic, and 7 (44%) died. Genome sequencing of MERS-CoV from 13 cases revealed 3 transmissible deletions associated with clinical illness during the outbreak. Deletion variant sequences were epidemiologically clustered and linked to a common transmission chain. Interviews and sera were collected from 2 surviving cases, 23 household contacts, and 278 health care contacts; 1 (50%) case, 2 (9%) household contacts, and 3 (1%) health care contacts tested seropositive.
Conclusions
The MERS-CoV deletion variants retained human-to-human transmissibility and caused clinical illness in infected persons despite accumulated mutations. Serology suggested limited transmission beyond that detected during the initial outbreak investigation.
Keywords: coronavirus, emerging infectious disease, genome deletion, Jordan, MERS-CoV, Middle East respiratory syndrome, outbreak investigation, sero-epidemiology
Middle East respiratory syndrome coronavirus (MERS-CoV) causes acute respiratory illness that can progress rapidly to respiratory failure and death in approximately 35%–40% of reported laboratory-confirmed cases [1, 2]. The first known cases of MERS-CoV occurred during an outbreak of severe acute respiratory infections in Zarqa, Jordan, during March–April 2012 [3, 4]. Since then, new cases and clusters of MERS-CoV infections continue to occur within the Arabian Peninsula and have been exported to other countries around the world [5].
During August–October 2015, a MERS-CoV outbreak with 16 laboratory-confirmed cases occurred in hospitals in Amman and Zarqa, Jordan. An outbreak investigation was conducted by the Jordan Ministry of Health (JMoH), followed by a World Health Organization review in September 2015. Sequencing performed by Lamers et al. [6] on samples from 13 outbreak cases found the virus to be associated with a novel recombinant clade that predominated in Saudi Arabia in 2015 [7] and was exported to South Korea and China [8]. Importantly, genetic analysis also revealed 2 unique mutations resulting from substantial deletion events—a 48-nucleotide (nt) in-frame deletion in accessory open reading frame (ORF) 4a present in sequences from 13 outbreak cases and an additional 9-nt in-frame deletion in ORF3 in a subset of case specimens collected later in the outbreak—leading the authors to conclude that all cases were infected following a single introduction of the virus [6]. We conducted an investigation that included additional viral genome and serologic analysis to describe the transmission patterns and epidemiology of MERS-CoV deletion variants during the 2015 Jordan outbreak.
METHODS
The JMoH, US Centers for Disease Control and Prevention (CDC), and Eastern Mediterranean Public Health Network collaboratively conducted a follow-up investigation of the 2015 MERS-CoV outbreak in Jordan during March–April 2016. We reviewed outbreak investigation records, conducted key informant interviews with clinicians and infection control staff at the affected hospitals, and reviewed medical charts of confirmed cases. A confirmed MERS case met the World Health Organization case definition [9] and was laboratory-confirmed at the Jordan Central Public Health Laboratory using MERS-CoV N2 and/or N3 real-time reverse transcription polymerase chain reaction (rRT-PCR) assays [10]. A case was considered health care–associated if they were a patient, visitor, or health care personnel (HCP) at a health care facility during the 14 days before symptom onset (exposure period), and if exposure to a known MERS case occurred exclusively in this setting [11].
To identify previously undetected MERS-CoV infections among contacts of confirmed cases, we interviewed and collected sera from health care and household contacts of confirmed MERS cases from the 2015 outbreak. Additionally, we interviewed and collected sera from available surviving cases. Contacts were identified using contact tracing lists developed by JMoH and the affected hospitals during the initial outbreak investigation. All health care contacts included in the investigation were health care personnel; all household contacts were relatives of confirmed cases, although they did not necessarily live under the same roof. Standardized interview forms were completed, which included questions about types of exposure to confirmed cases, symptoms after exposure, occupation, and testing for MERS-CoV at the time of the outbreak.
Laboratory Methods
Genome Sequencing and Phylogenetic Analysis
MERS-CoV rRT-PCR-positive nasopharyngeal swabs, sputum, tracheal aspirate, or bronchoalveolar lavage samples from 15 of the 16 confirmed cases collected during August–September 2015 were stored at −80°C and shipped to the CDC for molecular analysis. Sample aliquots (200 µL) were extracted on a NucliSens EasyMAG (BioMerieux), and 100 µL of total nucleic acid was recovered. The specimen extracts were retested by MERS-CoV N2 and/or N3 rRT-PCR assays [10], and genome sequencing was performed on confirmed positive samples with sufficient viral load using previously described primer sets and protocol [7].
Nucleotide sequences were aligned using Clustal X, ver. 1.83, implemented in BioEdit, ver. 7.2.5. Phylogenetic analyses were performed using MrBayes, ver. 3.2.6 [12], under a GTR model of nucleotide substitution with 4 categories of γ-distributed rate heterogeneity and a proportion of invariant sites (GTR + 4 + I).
Serology
Serum samples collected during March–April 2016 were shipped to the CDC for MERS-CoV-specific serum antibody testing using MERS-CoV nucleocapsid (N) and spike (S) indirect enzyme-linked immunosorbent assay (ELISA) and microneutralization (MNT) assays [4, 13, 14]. Sera were considered potentially positive when the optical density (OD) values were at or above the screening 0.15 cutoff value for N-ELISA or 0.1 for S-ELISA. Any serum potentially positive by N- or S-ELISA at a screening dilution of 1:400 was subsequently titered with serial 4-fold dilutions (1:100–1:6400) with OD cutoffs of 0.3 for the N and 0.2 for the S-ELISAs to determine end point titer and tested by MNT for confirmation. Neutralizing antibodies to MERS-CoV were detected by MNT assay performed in Biosafety Level 3 (BSL-3) containment, as previously described [4]. The neutralization titer was defined as the reciprocal of the highest serum dilution that completely inhibited the live MERS-CoV-mediated cytopathic effect in Vero cells in at least 1 of the 3 triplicate wells.
N- and S-ELISAs were considered positive with reciprocal end point titers of 400 or greater. MNT were considered positive with reciprocal titers of 20 or greater. MERS-CoV seropositivity was defined as having 2 of 3 positive assays, including N-ELISA, S-ELISA, and MNT, or positive by MNT alone [14]. Sera indeterminate seropositivity was defined as S-ELISA positive, but N-ELISA and MNT negative.
Ethics
This investigation was classified by the CDC as a public health response to an emerging disease outbreak, and Jordanian Institutional Review Board approval was obtained. Verbal consent was obtained in Arabic.
RESULTS
During August–October 2015, 16 laboratory-confirmed MERS-CoV patients were identified in Jordan. These MERS cases received care at 9 hospitals in Jordan, with case clusters associated with 3: hospitals A, B, and C. The median patient age was 55 years (10 months–78 years), 9 (56%) were male, and 13 (81%) were symptomatic (Table 1). Seven (44%) patients died, all of whom had underlying medical conditions. Eleven (69%) cases were health care associated, and 5 (31%) were relatives of a known case. Health care–associated cases included 6 hospital patients, 3 visitors, and 2 HCP.
Table 1.
Demographics, Exposures, and Outcomes Among Confirmed Cases, Jordan, August–October 2015
| Case ID No. | Age, y | Sex | Exposure(s) | Symptoms, Y/N | Admitting Hospital for MERS Illness | Medical Comorbidities | Hospital Outcome |
|---|---|---|---|---|---|---|---|
| 1 | 60 | M | Hospital A, as patient | Y | Hospital E | DM, HTN, CHD/CHF, hypothyroidism | Died |
| 2 | 38 | M | Hospital A, as visitor | Y | Hospital B | Smoker | Survived |
| 3 | 76 | M | Hospital A, as patient | Y | Hospital A | CLL, DM, HTN, CHD | Died |
| 4 | 47 | F | Sister of case 2 | N | NA | None | Survived |
| 5 | 56 | M | Hospital A, as patient | Y | Hospital A | DM, HTN, CHD, MV stenosis, CKD, hepatitis C | Died |
| 6 | 73 | F | Hospital A, as patient | Y | Hospitals F and Ha | DM, HTN, CHD/CHF, atrial fibrillation | Survived |
| 7 | 78 | M | Hospital B, as patient | Y | Hospital B | Lung cancer, DM, COPD | Died |
| 8 | 53 | M | Hospital A, as visitor | Y | Hospital C | DM, HTN, CHD, smoker | Died |
| 9 | 7 | F | Daughter of case 8 | N | Hospital Ha | None | Survived |
| 10 | 0.8 | F | Granddaughter of case 8 | Y | Hospital Ha | None | Survived |
| 11 | 67 | F | Wife of case 3 | Y | Hospital B | DM, HTN | Survived |
| 12 | 28 | M | Hospital C, as HCP | Y | Hospital C | None | Survived |
| 13 | 60 | M | Hospital C, as visitor | Y | Hospital D | DM, HTN | Survived |
| 14 | 69 | F | Hospital C, as patient | Y | Hospital G | Breast cancer, CHD/CHF | Died |
| 15 | 38 | F | Hospital C, as HCP | N | NA | None | Survived |
| 16 | 53 | M | Brother-in-law of case 13 | Y | Hospitals I and D | Smoker | Died |
Abbreviations: CHD, chronic heart disease; CHF, congestive heart failure; CKD, chronic kidney disease; CLL, chronic lymphocytic leukemia; DM, diabetes mellitus; F, female; HCP, health care personnel; HTN, hypertension; M, male; MV, mitral valve.
aHospital H is the referral hospital for patients with MERS in Amman, Jordan.
Molecular Epidemiology
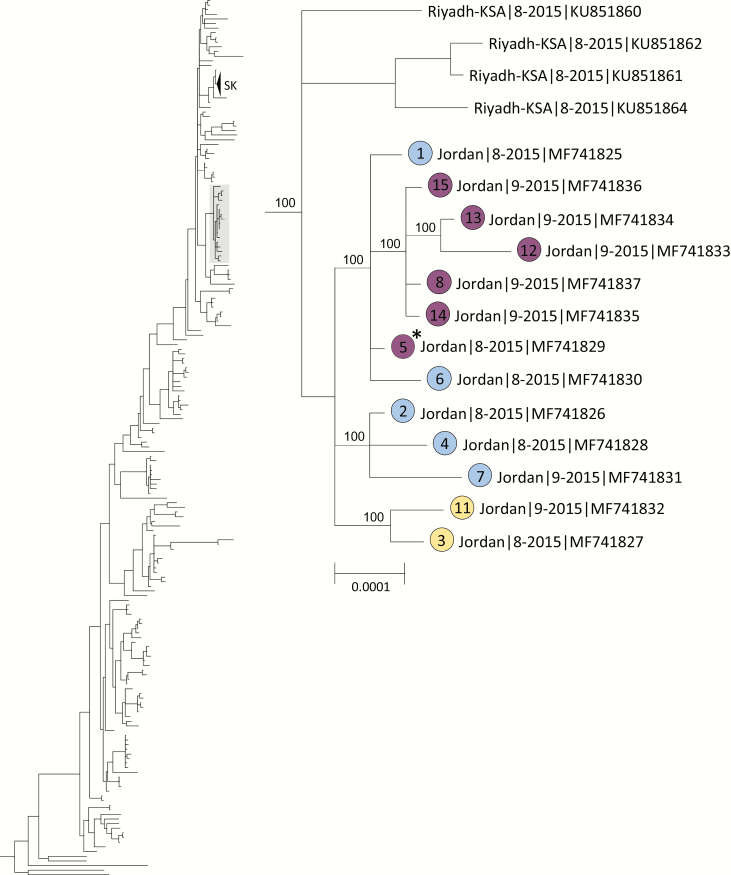
Respiratory specimens from 15 (94%) of the MERS-CoV-positive patients were received by the CDC and confirmed positive by rRT-PCR; 13 specimens representing 11 symptomatic and 2 asymptomatic cases had sufficient viral RNA for genome sequencing. Phylogenetic analysis of the 13 nearly complete genomes (GenBank accession No. MF741825-MF741837) and 240 previously published MERS-CoV genomes showed well-supported clustering of the Jordan outbreak sequences within a previously identified clade defined by a signature recombination (Figure 1) [7, 8]. Jordan outbreak sequences showed the closest similarity with sequences obtained from 4 temporally concurrent but epidemiologically unrelated human MERS cases from Riyadh, Saudi Arabia, in August 2015 (GenBank accession Nos. KU851860–851862 and KU851864), which did not possess any deletion mutations [15].
Figure 1.
Phylogenetic tree constructed from near complete Middle East respiratory syndrome coronavirus (MERS-CoV) genome sequences obtained from the 13 Jordan cases, indicated by solid-colored circles, and 240 previously published genome sequences in GenBank using the program MrBayes v3.2.6 [12] under a general time-reversible (GTR) model of nucleotide (nt) substitution with 4 categories of γ-distributed rate heterogeneity and a proportion of invariant sites (GTR + 4 + I). Clade-credibility values ≥70% are indicated at selected nodes. Circle colors correspond to transmitted deletion mutations: blue indicates 48-nt in-frame deletion in ORF4a; purple indicates 48-nt in-frame deletion in ORF4a and tandem 9-nt in-frame deletion in ORF3; and yellow indicates 48-nt in-frame deletion in ORF4a and tandem 726-nt out-of-frame deletion in ORF4b and 5. Case numbers assigned by the Jordan Ministry of Health are indicated within the circles. The scale bar shows the genetic distance as nt substitutions per site. aFor case 5, 2 sequence variants were detected: (i) a sequence with the ORF4a deletion only and (ii) a sequence with the ORF3 deletion and a tandem deletion in ORF3, 4a and 4b. Only the sequence with the ORF4a deletion is presented.
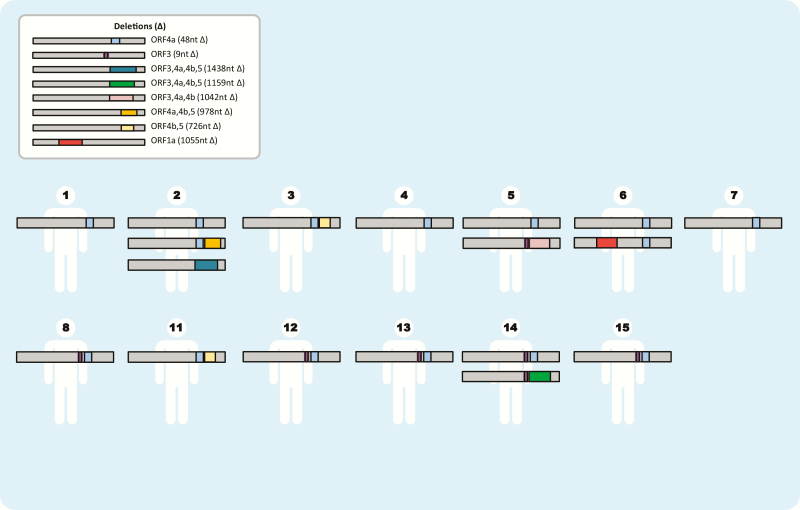
We confirmed the 48-nt (16-aa) in-frame deletion in ORF4a present in all Jordan sequences as well as the tandem 9-nt (3-aa) in-frame deletion in ORF3 in sequences from 5 patients (cases 8, 12, 13, 14, 15) obtained later in the outbreak [6]. Additionally, we identified a third 726-nt out-of-frame deletion in ORF4b and ORF5 (genome position 26339–27064 based on accession number JX869059.2) (Supplementary Table 1) from 2 patients (cases 3, 11). In ORF4a, ORF3, and ORF4b, 5 deletions were all identified in 2 or more epidemiologically linked patients, indicating successful virus transmission. We also identified 5 additional unique deletions ranging in size from 978 to 1438 nt variably involving ORF1a, ORF3, ORF4a, ORF4b, and ORF5 that were only present as mixed variants in individual patients (cases 2, 5, 6, 14) without evidence of transmission (Figure 2).
Figure 2.
Middle East respiratory syndrome coronavirus (MERS-CoV) deletion variants detected in individual cases, Jordan 2015. MERS-CoV deletions not drawn to scale. Deletion (∆): superscript numbers indicate genome assignment.
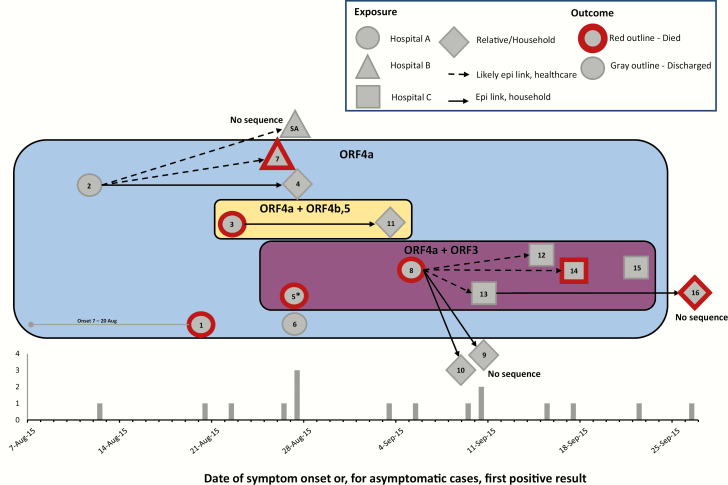
The viral transmission patterns deduced independently through the epidemiologic investigation corresponded to the grouping of cases by deletions observed in their viral genomes (Figure 3). Three hospital clusters were identified; the earliest cases occurred at hospital A, and index cases for the hospital B and C clusters both had epidemiologic links to hospital A. The 2 cases with the earliest dates of illness onset (cases 1 and 2) were both present at the same coronary care unit (CCU) in hospital A on a single day (case 1 as a patient and case 2 as a visitor); however, no direct contact was documented between them. Before the 2015 outbreak involving the MERS-CoV deletion variants, the last identified MERS case in Jordan had been 8 months earlier, in December 2014. In the 2015 outbreak, cases 1 and 2 had both recently traveled to Jordan from locations on the Arabian Peninsula (Jeddah, Saudi Arabia, and Kuwait City, Kuwait, respectively). Interestingly, viruses genetically similar to the viruses detected in cases 1 and 2 in Jordan, but without deletions, were concurrently circulating in Riyadh, Saudi Arabia, during the period corresponding to the earliest cases in Jordan (August 2015) [15]. Neither case 1 nor case 2 reported contact with a known MERS-CoV patient, camels, or a health care setting outside of Jordan during their exposure period. A review of previous CCU admissions did not reveal other likely index case candidates. Genome sequence analysis could not definitively identify the antecedent virus. Despite an extensive investigation, epidemiologic evidence did not definitively determine which of these 2 cases may have been the index case. Subsequently, 3 patients (cases 3, 5, and 6) and 1 visitor (case 8) at hospital A were identified as cases. Cases 5 and 6 had been admitted in the CCU at hospital A MERS before symptom onset. No cases were identified among HCP at hospital A, including from the CCU. The wife of case 3 tested positive (case 11) shortly after case 3 died; a 726-nt deletion in the ORF4b and ORF5 genes was shared between this couple.
Figure 3.
Proposed transmission pathways and epidemiologic curve and for confirmed Middle East respiratory syndrome (MERS) cases, Jordan, 2015. Transmitted deletion variants are indicated by superimposed colored boxes. Box colors correspond to those used for case markers in Figure 1. The epidemiologic curve shows the date of illness onset or, for asymptomatic cases, the date of first positive MERS-CoV test. Case patients are indicated by the gray elements; element shape corresponds to the likely site of exposure. Case numbers are indicated within the elements, and “SA” indicates a patient detected in Saudi Arabia. A red outline indicates that the patient died. Arrows indicate likely epidemiologic links (dashed lines for likely healthcare links and solid lines for household links). For cases which may have overlapped in space or time but for which we have insufficient data to confidently propose direction of direct or indirect transmission links, no lines are displayed. *For case 5, 2 sequence variants were detected: (i) a sequence with the ORF4a deletion only and (ii) a sequence with the ORF3 deletion and a tandem deletion in ORF3, 4a and 4b.
The hospital B cluster index case was case 2, who visited a relative at hospital A and subsequently became ill with respiratory symptoms within 2 days of his last exposure to hospital A. Case 2 was admitted to hospital B with severe respiratory illness and tested positive for MERS-CoV. His sister, case 4, was not symptomatic and was tested as a contact. Case 7 was a patient at hospital B during the period that case 2 was admitted. Additionally, 1 patient admitted at hospital B during the outbreak period became symptomatic with acute respiratory symptoms 10 days after discharge and was diagnosed with MERS-CoV after returning home to Saudi Arabia. She was not counted among the 16 Jordanian cases. The patient had returned to Jeddah, Saudi Arabia, just before onset of respiratory symptoms and was subsequently hospitalized there, transmitting MERS-CoV to an HCP in Jeddah. This case was detected during an investigation of otherwise unexplained hospital-associated MERS-CoV in Saudi Arabia [16] and could represent export of the deletion variant virus from Jordan to Saudi Arabia; however, sequences were not available for confirmation.
The case introducing the deletion variant to hospital C by evidence of onset time (case 8) had visited his daughter at hospital A during the period when MERS-CoV patients were admitted at hospital A. He became ill approximately 5–6 days after visiting hospital A and was admitted at hospital C for care. Subsequently, 1 patient (case 14), 1 visitor (case 13), and 2 health care personnel (cases 12 and 15) at hospital C were identified with MERS-CoV infection. The 9-nt deletion in the ORF3 gene was detected among all identified cases linked to the hospital C cluster. The visit of case 8 to hospital A corresponded temporally with the hospitalization of case 5 at hospital A, whose specimen contained 2 genome sequence variants, 1 with the ORF4a deletion only and a second with the 9-nt ORF3 deletion noted above and a downstream 1042-nt out-of-frame deletion involving ORF3, 4a, and 4b.
Seroepidemiologic Investigation
Interviews and sera were obtained from 301 contacts of the 16 confirmed MERS-CoV cases, including 278 health care and 23 household contacts, as well as 2 surviving confirmed cases (Supplementary Table 2). Of the 278 health care contacts, 3 (1%) were seropositive and 4 (1%) were serologically indeterminate. Seropositive HCP included a nurse, a respiratory therapist, and a radiology clerk. Serologically indeterminate HCP included 2 respiratory therapists, a nurse, and a housekeeper (Table 2). The seropositive respiratory therapist reported fever and cough within 14 days of exposure to a MERS-CoV patient; all others did not report symptoms. The seropositive radiology clerk and symptomatic respiratory therapist tested negative for MERS-CoV by rRT-PCR during the initial outbreak investigation.
Table 2.
Characteristics and Exposures of Seropositive and Serologically Indeterminate Health Care and Household Contacts of Confirmed MERS-CoV Cases, Jordan, March–April 2016
| ID | Serology Results (Reciprocal Titers) N-ELISA S-ELISA MNT |
Serology Interpretation | Case Contact | Occupation or Relationship to Case | Location of Exposure(s) | Age, y | Sex | Symptomsa | Medical Comorbidities |
MERS-CoV RT-PCR Test During Outbreak Investigationb | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Health care contacts | ||||||||||||
| HC-1 | 1600 | 1600 | 40 | Seropositive | 2 | Nurse | Hospital B | 24 | M | None | Smoker | No |
| HC-2 | 1600 | 1600 | <20 | Seropositive | 8, 14 | Respiratory therapist | Hospital C | 49 | M | Fever, nonproductive cough | Smoker | Yes; negative |
| HC-3 | 1600 | 1600 | <20 | Seropositive | 12 | Radiology clerk | Hospital C | 30 | F | None | Smoker | Yes; negative |
| HC-4 | <100 | 400 | <20 | Indeterminate | 8, 12, 14 | Respiratory therapist | Hospital C | 25 | M | None | Smoker | No |
| HC-5 | <100 | 400 | <20 | Indeterminate | 13, 16 | Nurse | Hospital D | 31 | M | None | Smoker | No |
| HC-6 | <100 | 400 | <20 | Indeterminate | 13, 16 | Respiratory therapist | Hospital D | 47 | M | None | DM, smoker | No |
| HC-7 | <100 | 400 | <20 | Indeterminate | 2, 11 | Housekeeper | Hospital B | 33 | F | None | Pregnant | No |
| Household contacts | ||||||||||||
| HH-1 | 100 | 400 | 40 | Seropositive | 1 | Brother | Visited in hospital | 63 | M | None | DM, HTN, smoker | Yes; negative |
| HH-2 | 1600 | 1600 | 40 | Seropositive | 2, 4 | Sister | Visited in hospital | 43 | F | Fever, rhinorrhea, sore throat, SOB | None | Yes; negative |
| HH-3 | 100 | 400 | <20 | Indeterminate | 16 | Son | Visited in hospital, cared for at home | 30 | M | None | Smoker | Yes; negative |
| HH-4 | <100 | 400 | <20 | Indeterminate | 12 | Niece | Lived in same household | 4 | F | None | None | Yes; negative |
Abbreviations: DM, diabetes mellitus; HC, health care; HH, household; HTN, hypertension; MERS-CoV, Middle East respiratory syndrome coronavirus; MNT, microneutralization assay; N-ELISA, nucleocapsid protein enzyme-linked immunosorbent assay; RT-PCR, reverse transcriptase polymerase chain reaction; S-ELISA, spike protein enzyme-linked immunosorbent assay; SOB, shortness of breath.
aReported symptoms within 14 days of exposure to a confirmed case.
bSelf-report; positive responses were confirmed using records from the Jordan Ministry of Health; timing of testing in relation to last exposure to confirmed cases unknown.
Seropositive and serologically indeterminate HCP were detected from 3 hospitals: B, C, and D (Table 2). Of the 58 hospital A HCP tested, none were seropositive or serologically indeterminate. At hospitals B and C, health care–associated transmission was identified at the time of the outbreak; at hospital D, where 2 serologically indeterminate HCP were detected, health care–associated transmission had not been documented. At hospital C, the investigation revealed a seropositive respiratory therapist who cared for both case 8 hospital C (index case) and case 14, who later developed symptoms after hospital discharge. The 2 cases briefly overlapped on the same floor, and case 8 immediately preceded case 14 in a particular radiology suite; however, no direct contact between the patients was reported. Case 15, a nurse at hospital C, also cared for both case 8 and case 14, but her suspected onset followed the exposure periods of these cases.
Of the 23 household contacts, 2 (9%) were seropositive and an additional 2 (9%) were serologically indeterminate. Reported household contact included living in the same household as a confirmed MERS-CoV case, caring for the case at home, and visiting the case in the hospital (Table 2). One seropositive household contact had fever and respiratory symptoms within 14 days of contact with a confirmed case. All household contacts tested negative for MERS-CoV by rRT-PCR during the initial investigation.
Of the 2 surviving confirmed MERS-CoV patients tested, 1 (50%) was seropositive by MNT (case 12) and 1 was seronegative (case 15). Case 12 was a nurse with no comorbidities who had severe illness requiring hospitalization. Case 15 was a nurse with no comorbidities who was documented as asymptomatic.
DISCUSSION
The MERS-CoV ORF 4a deletion variant and additional mutations observed over the course of this outbreak, including deletions in ORFs 3, 4b, and 5, were epidemiologically clustered in time and space, suggesting a single transmission chain and demonstrating human-to-human transmissibility of accumulated deletions during the outbreak. Although all outbreak specimens exhibited the ORF4a deletion, an additional deletion in ORF3, which was observed later in the outbreak first reported by Lamers et al. [6], was predominantly associated with a cluster at a single hospital. Additionally, we observed transmission of a unique 726nt deletion spanning ORF4b and ORF5 between a husband and wife as well as detection of multiple other deletion variants within the same hosts. The overall epidemiology of this outbreak, including prominent transmission among health care contacts, a less pronounced role of household transmission, a high case fatality rate (44%), presence of comorbidities among fatal cases, and retrospective evidence of asymptomatic infections, is all consistent with previous descriptions of hospital outbreaks, despite the distinctive genomic variation we observed [17, 18]. The genomic variability of this MERS-CoV deletion virus and the accumulation of genetic mutations observed as the outbreak continued, as well as information from other recent reports [19, 20], highlight the critical importance of ongoing contemporaneous molecular and epidemiologic surveillance for the persistent emergence of MERS-CoV.
This outbreak was unique in having many deletions detected from multiple patients, the accumulation of deletion mutations over time, and that these deletion variants retained transmissibility and virulence. The observed deletion mutations allowed us to link all outbreak patients to a single originating virus (ORF4a) and revealed some degree of transmission directionality in the outbreak. We observed multiple deletion mutations of varying size mostly distributed among accessory ORFs of the Jordan outbreak viruses. Deletion variants of MERS-CoV present as mixed infections with wild-type virus involving the ORF5, and E protein genes [20] and spike protein gene [19] have been previously reported from patients.
MERS-CoV has 5 group-specific ORFs (3, 4a, 4b, 5, and 8b) that encode accessory proteins whose functions are not fully characterized [21]. Although certain ORFs have been shown to be nonessential for virus replication in vitro [22], evidence exists that they may play a role in modulation of the host innate immune response [23–25]. For example, ORF 4a, ORF 4b, and ORF 5 proteins have been shown to be potent interferon antagonists [23]. Using an ORF3-5 deleted MERS-CoV infectious clone, Baric et al. observed increased stimulation of the interferon response in vitro and reduced viral replication and pathogenesis in a mouse model, compared with a wild-type virus [26]. Although deletions in accessory ORFs hypothetically could modulate the host response during infection, no apparent changes in pathogenicity were observed during our investigation; variant viruses with deletions in ORF 4a, ORFs 3, and 4a, and ORFs 4a, 4b, 5 each resulted in symptomatic illness and death in some cases. The ORF 4a deletion we observed is in-frame and located within the dsRNA-binding domain. The impact on the functionality of the protein with the deletion is unknown and warrants future study. Notably, deletions in accessory ORFs were also observed in SARS-CoV after its introduction to human hosts, and viruses containing a specific ORF 8 deletion were predominant later in the SARS epidemic [25, 27]. Viral genomic changes may have arisen due to selective pressures exerted by the human host, or due to the lack of such pressures on genes that are required specifically for sustained replication in camels, as suggested by Lamers et al. [6].
The combination of serologic and epidemiologic data improved our understanding of viral transmission patterns, including a seropositive HCP that may have been the link between 2 patient cases. We found that the majority of seropositive or indeterminate HCP were either respiratory therapists or nurses, demonstrating risks for these occupations in outbreak settings [28]. We also identified a nonclinical HCP (radiology clerk) who was seropositive. Infection among nonclinical HCP has been less commonly reported; however, these findings highlight the importance of including nonclinical HCP in contact tracing and monitoring efforts and the need to further evaluate specific health care activities associated with risk of MERS-CoV transmission.
Previous studies on MERS-CoV antibody kinetics suggest that serologic investigations might underestimate mild and asymptomatic infections [29]. Individuals with mild or asymptomatic infection appear to less reliably develop a detectable antibody response after infection, although antibodies have been observed to persist for nearly 3 years after infection in even young persons without significant comorbidities [13, 29–31]. In our seroepidemiologic investigation, we found that case 15, a mildly ill, rRT-PCR- and sequence-confirmed MERS case at the time of the outbreak, tested seronegative approximately 7 months later. Conversely, we identified individuals who reported no symptoms and tested MERS-CoV negative during outbreak contact tracing but were subsequently seropositive. Several exposed individuals had detectable anti-MERS-CoV S-serum antibodies by ELISA, including 1 who exhibited acute respiratory symptoms but did not have detectable neutralizing serum antibodies (serologic result classified as indeterminate). These individuals may have developed anti-S antibodies to non-neutralizing epitopes, neutralizing antibodies may have decayed to below detectable levels at the time we collected the sera (~8 months after infection), or they may have never mounted robust antibody responses during the initial acute infection. The natural history of the immune response to MERS-CoV infection as related to disease severity needs further investigation. The role of mildly ill or asymptomatic individuals in transmission of MERS-CoV remains a particularly critical question in health care settings where patients with underlying conditions might be at increased risk for infection.
Direct epidemiologic links between some health care–associated cases could not be established. In addition to potentially unrecognized human cases, indirect contact or fomite transmission in this outbreak also remains a possibility. Although contamination of environmental surfaces is a suspected mode of transmission for some respiratory viruses in certain settings [32], this has not been confirmed as a route of transmission for MERS-CoV. Key informant interviews with hospital infection control officers and clinicians working during the outbreak, as well as hygienic observations during the investigation, suggest that patients admitted before MERS-CoV was recognized could have contaminated shared hospital equipment with transmissible virus (specifically, an x-ray table and portable echocardiogram machine). Viable MERS-CoV has been recovered from surfaces after 48 hours at 20°C and 40% relative humidity [33], suggesting plausibility that MERS-CoV survives on indoor surfaces in a climate-controlled setting. Viral contamination of commonly handled surfaces in a hospital setting was reported during the 2015 South Korea outbreak [34, 35]. The importance of fomite transmission compared with other transmission routes in nosocomial outbreaks of MERS-CoV remains uncertain but could have important implications for infection prevention and control guidance.
Our investigation had several limitations. The seroepidemiologic study was conducted 6–8 months after the outbreak, and seropositive individuals having antibody titers wane below detectable levels might have been missed. Additionally, we were conservative in the interpretation of indeterminate serology results as this result could represent cross-reactivity or a partial or waning immune response. We did not capture all health care and household contacts in our seroepidemiologic investigation, and we are unable to assess whether those captured were similar to those not captured. An overall limitation of the MERS-CoV outbreak investigation is that RT-PCR testing of contacts might miss cases depending on the timing of nasopharyngeal swab swab collection. The Sanger sequencing method used in our study may not have detected sequence variants (small deletions or insertions or single base polymorphisms) present at low levels in the samples and therefore likely underestimates the complexity of the virus populations present.
In conclusion, we found that the epidemiology of a MERS-CoV deletion variant did not appear to deviate substantially from previous MERS-CoV outbreaks that did not exhibit these mutations. Our investigation revealed that the MERS cases and clusters detected in multiple hospitals were all linked to a common transmission chain with a single introduction event. The juxtaposition of molecular, serologic, and epidemiologic data revealed that the ORF4a genetic deletion and additional deletions observed over the course of the outbreak were epidemiologically clustered, yielding an important approach for using molecular information to support epidemiological findings and strengthen our understanding of MERS-CoV transmission. Close monitoring of MERS-CoV genomic variation [19] and the epidemiology of infection remains critical amidst continued reporting of sporadic cases and outbreaks of MERS-CoV on the Arabian Peninsula.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
We would like to acknowledge the efforts of Dr. Noha Farag (CDC) in providing translational assistance, performing data entry, and serving as a liaison to Jordan. We would like to acknowledge Ms. Joana Yu, Ms. Christina Chommanard, and Ms. Jessica Rudd (all CDC) for performing data entry. We would like to acknowledge the important contributions in technical support, collaboration, and guidance during this investigation from the Eastern Mediterranean Public Health Network. We also acknowledge and deeply appreciate the support given to this investigation by staff and infection control officers at each of the involved hospitals.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Financial support. M.M.L. was supported by the Erasmus Graduate Program Infection & Immunity (NWO grant No. 022.005.032).
Potential conflicts of interest. B.L.H. has applied for patents on MERS-CoV. No other conflicts are reported. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med 2014; 160:389–97. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV). Available at: http://www.who.int/emergencies/mers-cov/en/. Accessed 30 May 2017. [Google Scholar]
- 3. Hijawi B, Abdallat M, Sayaydeh A, et al. Novel coronavirus infections in Jordan, April 2012: epidemiological findings from a retrospective investigation. East Mediterr Health J 2013; 19(Suppl 1):S12–8. [PubMed] [Google Scholar]
- 4. Al-Abdallat MM, Payne DC, Alqasrawi S, et al. ; Jordan MERS-CoV Investigation Team Hospital-associated outbreak of Middle East respiratory syndrome coronavirus: a serologic, epidemiologic, and clinical description. Clin Infect Dis 2014; 59:1225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization. Global Alert and Response (GAR): Middle East respiratory syndrome coronavirus. Available at: http://www.who.int/csr/disease/coronavirus_infections/en. Accessed 16 May 2018. [Google Scholar]
- 6. Lamers MM, Raj VS, Shafei M, et al. Deletion variants of Middle East respiratory syndrome coronavirus from humans, Jordan, 2015. Emerg Infect Dis 2016; 22:716–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Assiri AM, Midgley CM, Abedi GR, et al. Epidemiology of a novel recombinant MERS-CoV in humans in Saudi Arabia. J Infect Dis 2016; 22:2020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Y, Liu D, Shi W, et al. Origin and possible genetic recombination of the Middle East respiratory syndrome coronavirus from the first imported case in China: phylogenetics and coalescence analysis. MBio 2015; 6:e01280–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization. Laboratory testing for Middle East respiratory syndrome coronavirus (MERS-CoV), interim guidance. Updated January 2018. Available at: http://www.who.int/csr/disease/coronavirus_infections/mers-laboratory-testing/en. Accessed 16 May 2018. [Google Scholar]
- 10. Lu X, Whitaker B, Sakthivel SK, et al. Real-time reverse transcription-PCR assay panel for Middle East respiratory syndrome coronavirus. J Clin Microbiol 2014; 52:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hunter JC, Nguyen D, Aden B, et al. Transmission of Middle East respiratory syndrome coronavirus infections in healthcare settings, Abu Dhabi. Emerg Infect Dis 2016; 22:647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001; 17:754–5. [DOI] [PubMed] [Google Scholar]
- 13. Payne DC, Iblan I, Rha B, et al. Persistence of antibodies against Middle East respiratory syndrome coronavirus. Emerg Infect Dis 2016; 22:1824–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Trivedi S, Miao C, Al-Abdallat MM, et al. Inclusion of MERS-spike protein ELISA in algorithm to determine serologic evidence of MERS-CoV infection. J Med Virol 2018; 90:367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Assiri AM, Biggs HM, Abedi GR, et al. Increase in Middle East respiratory syndrome-coronavirus cases in Saudi Arabia linked to hospital outbreak with continued circulation of recombinant virus, July 1–August 31, 2015. Open Forum Infect Dis 2016; 3:ofw165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shalhoub S, Abdraboh S, Palma R, et al. MERS-CoV in a healthcare worker in Jeddah, Saudi Arabia: an index case investigation. J Hosp Infect 2016; 93:309–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Drosten C, Meyer B, Müller MA, et al. Transmission of MERS-coronavirus in household contacts. N Engl J Med 2014; 371:828–35. [DOI] [PubMed] [Google Scholar]
- 18. Oboho IK, Tomczyk SM, Al-Asmari AM, et al. 2014 MERS-CoV outbreak in Jeddah—a link to health care facilities. N Engl J Med 2015; 372:846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lu X, Rowe LA, Frace M, et al. Spike gene deletion quasispecies in serum of patient with acute MERS-CoV infection. J Med Virol 2017; 89:542–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xie Q, Cao Y, Su J, et al. Two deletion variants of Middle East respiratory syndrome coronavirus found in a patient with characteristic symptoms. Arch Virol 2017; 162:2445–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Boheemen S, de Graaf M, Lauber C, et al. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. MBio 2012; 3(6):e00473–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scobey T, Yount BL, Sims AC, et al. Reverse genetics with a full-length infectious cDNA of the Middle East respiratory syndrome coronavirus. Proc Natl Acad Sci U S A 2013; 110:16157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang Y, Zhang L, Geng H, et al. The structural and accessory proteins M, ORF 4a, ORF 4b, and ORF 5 of Middle East respiratory syndrome coronavirus (MERS-CoV) are potent interferon antagonists. Protein Cell 2013; 4:951–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thornbrough JM, Jha BK, Yount B, et al. Middle East respiratory syndrome coronavirus NS4b protein inhibits host RNase L activation. MBio 2016; 7:e00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rabouw HH, Langereis MA, Knaap RC, et al. Middle East respiratory coronavirus accessory protein 4a inhibits PKR-mediated antiviral stress responses. PLoS Pathog 2016; 12:e1005982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Menachery VD, Mitchell HD, Cockrell AS, et al. MERS-CoV accessory ORFs play key role for infection and pathogenesis. MBio 2017; 8(4):e00665–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oostra M, de Haan CA, Rottier PJ. The 29-nucleotide deletion present in human but not in animal severe acute respiratory syndrome coronaviruses disrupts the functional expression of open reading frame 8. J Virol 2007; 81:13876–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alraddadi BM, Al-Salmi HS, Jacobs-Slifka K, et al. Risk factors for Middle East respiratory syndrome coronavirus infection among healthcare personnel. Emerg Infect Dis 2016; 22:1915–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pyoeng Gyun C, Perera RAPM, Wan Beom P, et al. MERS-CoV antibody responses 1 year after symptom onset, South Korea, 2015. Emerg Infect Dis 2017; 23:1079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alshukairi AN, Khalid I, Ahmed WA, et al. Antibody response and disease severity in healthcare worker MERS survivors. Emerg Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Park WB, Perera RA, Choe PG, et al. Kinetics of serologic responses to MERS coronavirus infection in humans, South Korea. Emerg Infect Dis 2015; 21: 2186–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boone SA, Gerba CP. Significance of fomites in the spread of respiratory and enteric viral disease. Appl Environ Microbiol 2007; 73:1687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van Doremalen N, Bushmaker T, Munster VJ. Stability of Middle East respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Euro Surveill 2013; 18:pii: 20590. [DOI] [PubMed] [Google Scholar]
- 34. Bin SY, Heo JY, Song MS, et al. Environmental contamination and viral shedding in MERS patients during MERS-CoV outbreak in South Korea. Clin Infect Dis 2016; 62:755–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim SH, Chang SY, Sung M, et al. Extensive viable Middle East respiratory syndrome (MERS) coronavirus contamination in air and surrounding environment in MERS isolation wards. Clin Infect Dis 2016; 63:363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.