Abstract
Objective
To determine if an interpersonal attribution bias associated with self-perception, the externalizing bias, was related to neural activations during mentalization.
Methods
A functional magnetic resonance imaging task involving verbal appraisals measured neural activations when thinking about oneself and others in 59 adults, including healthy women as well as women with and recovered from anorexia nervosa. Whole-brain regressions correlated brain function during mentalization with the externalizing bias measured using the Internal, Personal, and Situational Attributions Questionnaire.
Results
Women with anorexia nervosa had a lower externalizing bias, demonstrating a tendency to self-attribute more negative than positive social interactions, unlike the other groups. The externalizing bias was correlated with activation of the left inferior frontal gyrus and posterior insula, when comparing thinking about others evaluating oneself with direct self-evaluation.
Discussion
Externalizing biases may provide an office-based assay reflecting neurocognitive disturbances in social self-perception that are common during anorexia nervosa.
Keywords: anorexia nervosa, neuroimaging, biological, neuropsychology
INTRODUCTION
Adult anorexia nervosa is a complex psychiatric illness that includes altered perceptions of oneself and others. These include problems both in the physical perception of one’s body and differences in social and emotional perception (Harrison, Tchanturia, Naumann, & Treasure, 2012; McAdams & Smith, 2015; Reville, O'Connor, & Frampton, 2016). Self and other perception engages specific neural regions including temporal, parietal, and frontal regions in healthy people (Pfeifer & Peake, 2012); many of these regions function differently during self-perception in anorexia nervosa (McAdams & Krawczyk, 2014).
Verbal appraisal functional magnetic resonance imaging (fMRI) tasks are widely-utilized to measure social aspects of self and other perception in healthy and diseased populations (Denny, Kober, Wager, & Ochsner, 2012), and rely on comparing differences in the blood oxygen-level dependent (BOLD) signal when completing evaluations about oneself and/or others. Contrasting these states provides insight into the neural circuitry underlying self and other perception. For example, thinking about one’s friend thinking about you versus thinking about oneself directly is a comparison that seeks to isolate the neural substrates involved in mentalization (D'Argembeau et al., 2007). Mentalization is the term ascribed to the process in which a person chooses to think about a situation from another person’s viewpoint. This ability to take another person’s perspective is clinically relevant to many mental illnesses, such that trials of mentalization-based treatment are being examined in many different psychiatric disorders, including eating disorders (Jewell et al., 2016; Kelton-Locke, 2016). Recently, we identified differences in neural activations in women with and recovered from eating disorders during mentalization (McAdams et al., 2016). These data support a hypothesis that neurocognitive changes related to mentalization may be important in recovery, however interpreting neural data in the context of individual patients requires identification of office measures associated with both neural and illness state differences.
Interpersonal attribution biases provide a self-report measure of cognitive biases about oneself and others. Negative externalizing biases, a tendency to blame oneself for negative events more than positive events, are observed in women with eating disorders (Morrison, Waller, & Lawson, 2006; Rotenberg & Flood, 2000; Watkins et al., 2001) and differ when comparing women in sustained weight-recovery following anorexia nervosa to those recently with anorexia nervosa (McAdams, Lohrenz, & Montague, 2015). Cabanis et al. (2013) examined neural activations during verbal interpersonal attributions in healthy subjects, finding that negative self-attributed statements activated the bilateral insula. The insula are associated with social conformity (Wu, Luo, & Feng, 2016), and pathology in anorexia nervosa (Ehrlich et al., 2015; Kerr et al., 2016; Oberndorfer et al., 2013; Shott, Pryor, Yang, & Frank, 2016), leading us to an idea that one’s biases about one’s position in the world might reflect differences in the engagement of the insula during self-relevant mentalization.
Using a verbal appraisal fMRI task, the Social Identity V2 Task, we recently reported on how brain activations during mentalization differed across groups for women with anorexia nervosa, women in recovery following anorexia nervosa, and healthy women (McAdams et al., 2016). The primary aim of that study was to establish how brain function during self-evaluations related to state of disease. The goal of the secondary analysis provided here was to explore whether the externalizing bias, a measure of the valence in one’s self-evaluations, was related to neural activations during mentalization.
METHODS
Participants
Subjects provided written informed consent to participate, approved by the UT Southwestern Institutional Review Board. Fifty-nine women were enrolled, including healthy comparison women (HC, n = 19), women recently with anorexia nervosa (AN-C, DSM-IV criteria for anorexia nervosa in previous 6 months, n = 22), and women in weight-recovery following anorexia nervosa (AN-WR, DSM-IV criteria for anorexia nervosa in lifetime but BMI > 19 for 24 months, n = 18).
Instruments
Subjects were interviewed using the Structured Clinical Interview for DSM-IV to confirm course and history of anorexia nervosa for AN-C and AN-WR groups, and absence of eating disorders (ED) in the HC group (First, 2002).Clinician-based measures of depression (Quick Inventory of Depression Symptoms, QIDS-CR (Rush et al., 2003)) and anxiety (Structured Interview for Hamilton Anxiety Scale, SIGH-A (Shear et al., 2001)) were obtained.
Self-report assessments were completed by all subjects: Eating Attitudes Test (EAT) (Garner, Olmsted, Bohr, & Garfinkel, 1982), the Body Shape Questionnaire (BSQ) (Rosen, Jones, Ramirez, & Waxman, 1996), the Toronto Alexithymia Scale-20 (TAS) (Bagby, Parker, & Taylor, 1994), and the Self-Liking and Self-Competence Scale (SLSC) (Tafarodi & Swann, 1995). Interpersonal attribution biases (externalizing bias (EB), positive personalizing bias (PPB), and negative personalizing bias (NPB)) were measured using the Internal, Personal, and Situational Attributions Questionnaire (IPSAQ) (Kinderman & Bentall, 1997).
The IPSAQ involves 32 fill-in-the blank answers to a range of random interpersonal events (e.g. receiving a present); subjects are instructed to write down the first thought for why the event happened. Half of the items are positive and half are negative. After writing their response, the subject then selects whether their response attributes causality to themself, their friend, or the situation. The EB is calculated as the difference in the number of self-attributed positive items minus number of self-attributed negative items. Items selected about the friend or the situation are used to calculate positive and negative personalizing biases, a measure of the tendency to attribute good or bad things to other people. The EB has a Cronbach’s alpha of 0.72, and the scale has been validated for healthy subjects and examined in many psychiatric populations (Didehbani et al., 2012; Kinderman & Bentall, 1996, 1997; Mizrahi, Addington, Remington, & Kapur, 2008; Moritz et al., 2011; Pavlickova et al., 2013).
Functional MRI Task and Analysis
Details about administration, data collection, preprocessing, and preliminary data analysis for the Social Identity-V2 task, as well as the group differences in this task were previously published (McAdams et al., 2016). Briefly, the task required participants to agree or disagree with 48 statements about themselves (Self, ex. “I believe I am clumsy”), their friend (Friend, “I believe my friend is timid”), and whilst undergoing mentalization about themselves, (Reflected, “My friend believes I am truthful”). While completing the task, images were acquired using a 3T Philips Achieva MRI scanner, with a 1-shot gradient T2*-weighted echoplanar (EPI) image sequence sensitive to blood oxygen level-dependent (BOLD) contrast with a repetition time (TR) of 2 s, an echo time (TE) was 35 ms, a flip angle was 70°, and volumes consisted of 36 axial slices (4 mm thick, no gap). Preprocessing included spatial realignment to the first volume, normalization to the MNI template, spatial smoothing using a 6 mm 3D Gaussian kernel, and the voxel time-series was high pass filtered (128 s).
The fMRI task data were analyzed using Statistical Parametric Mapping software (SPM8, Wellcome Department of Imaging Neuroscience London, www.fil.ion.ucl.ac.uk/spm) run in Matlab 2012 (http://www.mathworks.com) and viewed in both SPM and xjview (http://www.alivelearn.net/xjview8/). BOLD signal was extracted during the 4-s presentation of each statement to create contrast images for each event. Evoked activation was assessed using multiple regression analysis set as boxcar functions. Each regressor was convolved with a canonical hemodynamic response function (HRF) provided in SPM8 and entered into the modified general linear model (GLM). Parameter estimates (e.g. β values) were extracted from this GLM analysis for each regressor. A first level analysis evaluated task contrasts; only the contrast related to mentalization, Reflected – Self, is considered here. A whole-brain regression of the EB against the neural activations in the Reflected – Self contrast identified regions of interest (ROI). Consistent with the original study and other fMRI tasks involving self-reflection (Ellard, Barlow, Whitfield-Gabrieli, Gabrieli, & Deckersbach, 2017; Yang, Xu, Chen, Shi, & Han, 2016), the imaging threshold was set at a voxel height of 0.005 followed by family-wise-error cluster-correction to P < 0.05.
Parameter estimates were extracted from the ROI and exported to IBM Statistical Package for Social Sciences (SPSS; v.23) for linear regressions and group comparisons. Pearson’s bivariate correlations were computed to assess the relationship between this ROI and the EB, for the entire population and each group. Next, multiple linear regression examined whether any other clinical or cognitive measures were also significantly related to activations within the ROI (p < 0.05), to assess whether the EB was simply a proxy for other clinical factors. Clinical and cognitive measures (QIDS, SIGH-A, EAT, BSQ, TAS, SL, SC, EB, PPB, NPB) and neural activations for the identified ROI were compared for the three groups using one-way ANOVAs followed by Bonferroni-corrected post hoc comparisons.
A supplementary analysis evaluated whether neural responses within Automated Anatomic Labeling (AAL) regions also correlated with the EB focusing on the insula (left, A_LINS; right, A_RINS), as well as the rolandic operculum (left, A_LRO; right, A_RRO); these correlations were Bonferroni-corrected for multiple comparisons as four regions were included (p = 0.0125).
The combination of a whole-brain threshold of 0.005, the use of cluster-correction, and inclusion of standardized anatomical regions for supplemental analysis has been recommended to balance consideration of both Type I error and Type II error in exploratory whole-brain studies utilizing cognitive neuroimaging tasks (Cunningham & Koscik, 2017; Hopfinger, 2017; Lieberman & Cunningham, 2009; Slotnick, 2017).
RESULTS
Amongst all clinical and cognitive measures, the AN-C and AN-WR groups differed only on the EAT (AN-C > AN-WR > HC), in BMI (AN-C < AN-WR & HC) and for the EB of the IPSAQ (AN-C < AN-WR & HC) (Table 1). In addition, the AN-C and the AN-WR groups had more depression, anxiety, alexithymia, and body shape concerns than the HC group, as well as less self-esteem, for both the self-liking and self-competence subscales than the HC group.
Table 1.
Participant Characteristics
| Measure | Participant Group | Statistical Comparisons | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AN-C (n = 22) |
AN-WR (n = 18) |
HC (n = 19) |
|||||||
| Mean(SD) | Range | Mean(SD) | Range | Mean(SD) | Range | F(2,56) | p | Ƞ2 | |
| Age | 27.59(7.65) | 20–45 | 30.22(8.08) | 22–47 | 27.89(5.99) | 21–43 | 0.74 | 0.48 | 0.03 |
| Body Mass Index | 17.64(1.49) | 14.64–20.31 | 22.84(2.73) | 19.20–28.49 | 22.52(2.42) | 19.1–26.6 | 35.24 | <.001^† | 0.56 |
| BSQ | 134.32(37.66) | 59–196 | 114.00(37.91) | 54–189 | 58.16(22.30) | 30–112 | 27.46 | <.001*^ | 0.50 |
| Depression (QIDS-CR) | 6.68(5.28) | 0–16 | 5.11(4.36) | 0–15 | 1.58(2.09) | 0–7 | 7.75 | 0.001*^ | 0.22 |
| Anxiety (SIGH-A) | 10.27(8.72) | 0–29 | 8.33(6.64) | 0–20 | 2.26(2.79) | 0–8 | 7.80 | 0.001*^ | 0.22 |
| Alexithymia (TAS) | 55.50(13.35) | 25–75 | 52.06(11.30) | 30–71 | 38.26(9.69) | 25–58 | 12.11 | <.001*^ | 0.30 |
| Eating Attitudes Test | 39.00(17.94) | 1–66 | 15.72(9.87) | 0–38 | 3.32(3.94) | 0–12 | 43.62 | <.001*^† | 0.61 |
| Self-Liking | 16.36(5.79) | 9–32 | 18.61(6.37) | 8–32 | 31.32(6.15) | 23–40 | 34.48 | <.001*^ | 0.55 |
| Self-Competence | 21.82(5.84) | 8–37 | 24.39(4.47) | 13–31 | 29.11(5.30) | 19–39 | 9.84 | <.001*^ | 0.26 |
| IPSAQ EB | −3.41(5.35) | −14–6 | 1.39(4.73) | −10–9 | 4.58(4.14) | −2–12 | 14.44 | <.001^† | 0.34 |
| IPSAQ PPB | 0.55(0.35) | 0.13–1.00 | 0.48(0.29) | 0.00–1.00 | 0.50(0.25) | 0.00–0.88 | 0.35 | 0.70 | 0.01 |
| IPSAQ NPB | 0.60(0.29) | 0.00–1.00 | 0.70(0.23) | 0.15–1.00 | 0.54(0.24) | 0.25–1.00 | 1.50 | 0.23 | 0.05 |
AN-C = women with anorexia nervosa; AN-WR = women in weight recovery after anorexia nervosa; HC = healthy control women. BSQ = Body Shape Questionnaire; QIDS-CR = Quick Inventory of Depression Symptoms; SIGH-A = Structured Interview for Hamilton Anxiety Scale; TAS= Toronto Alexithymia Scale; IPSAQ = Internal, Personal, and Situational Attribution Questionnaire; EB = externalizing bias; PPB = positive personalizing bias; NPB = negative personalizing bias
AN-WR differs from HC (p < 0.05)
AN-C differs from HC (p < 0.05)
AN-C differs from AN-WR (p < 0.05)
Previously, we reported three main effect of group clusters during the Reflected – Self contrast. These included regions in the bilateral inferior frontal gyri extending into the insula and the dorsal anterior cingulate; the AN-WR group differed from both the AN-C and HC group in all three, the AN-C group differed from the HC group in the left inferior frontal gyrus.
Here, in the whole brain regression of the EB against the Reflected – Self contrast for all subjects, one cluster in the left insula was identified as a ROI (Figure 1, LINS, 100 voxels, 4620 mm3, MNI −40, 0, 8, Z = 3.70, cluster pFWE < 0.001). Pearson’s bivariate correlations were computed between the parameter estimates extracted from this cluster for each subject and each subject’s EB for all subjects (Figure 1, LINS-EB, All Subjects, r = 0.59, p < 0.001), as well as for each group separately (AN-C, r = 0.37, p = 0.09; AN-WR, r = 0.55, p = 0.02; HC, r = 0.56, p = 0.01). Using ANOVA, the parameter estimates for this cluster were compared for the three groups, finding significant differences between both clinical groups and the comparison group (LINS, Mean (SD), AN-C, −0.28(0.70), AN-WR, −0.09(0.79), HC 0.70(0.93); F(57) = 13.3, p < 0.001; AN-C < HC, p = 0.001; AN-WR < HC, p = 0.011).
Figure 1.
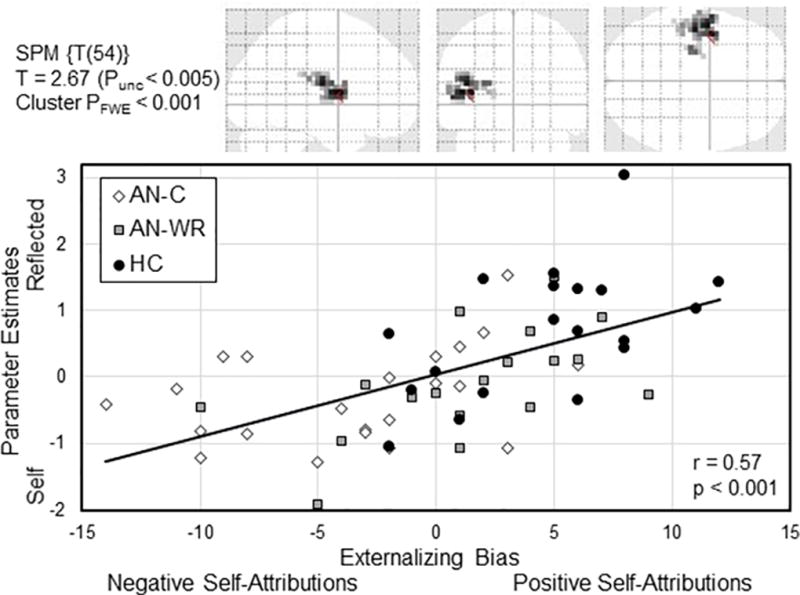
The upper row show the transparent brain representation of the LINS cluster, obtained from the whole-brain regression of the externalizing bias compared to the Reflected – Self contrast of the Social Identity V2 task. Single voxel threshold set to Punc< 0.005, with the voxel extent of 100, cluster PFWE was < 0.001. The scatterplot on the second row shows the extracted parameter estimates within this cluster on the y-axis with the externalizing bias on the x-axis for each subject, with the best-fit regression line for the entire population shown in black (r = 0.57, p < 0.001). Each subject is shown based on their group classification: AN-C, participants currently with anorexia nervosa; AN-WR, participants in weight-recovery with history of anorexia nervosa, and HC, healthy comparison participants.
Next, multiple linear regression considered whether any other factors (Group, Age, BMI, QIDS, SIGH-A, EAT, BSQ, TAS, SL, SC, EB, PPB, NPB) were correlated with neural activations in the LINS cluster. The only significant relationship (F(14,44) = 2.72, p = 0.006, R2 = 0.46) observed remained with the EB (t = 2.25, p = 0.03, R2 = .010).
Finally, in the supplemental analysis using the AAL-defined regions, a similar relationship was observed between the EB and the left rolandic operculum (A_LRO, r = 0.50, p < 0.001 for all subjects), but not for the other regions (A_LINS, r = 0.25, p = 0.06; A_RINS, r = 0.17, p = 0.18; A_RRO, r = 0.29, p = 0.03).
DISCUSSION
The externalizing bias was related to activation of a cluster within the left insula and the left rolandic operculum during mentalization in the Social Identity-V2 task, and activations within this region differed based on clinical group. These data support further research examining how the insula’s role in social perspective-taking and self-attribution relates to anorexia nervosa. The reported relationship suggests that individuals who tend to think well of themselves in interpersonal scenarios use the left operculum more during mentalization, but individuals who think poorly of themselves use this area less during mentalization. Thus, the externalizing bias may reflect differences in neural processing related to pre-existing biases about one’s social status during self-evaluations.
Although the insula are involved in interoception (Cauda et al., 2012), they are also critical for the cognitive processing of self and others (Hu et al., 2016; Sperduti, Delaveau, Fossati, & Nadel, 2011), and transdiagnostically implicated in the psychopathology of mood, addictive, and anxiety disorders (Downar, Blumberger, & Daskalakis, 2016; Goodkind et al., 2015; Price & Drevets, 2012). Different subregions of the insula have been related to different modalities, including sensorimotor, cognitive, olfacto-gustatory, social-emotional, and an anterior-dorsal area that integrates across them (Kurth, Zilles, Fox, Laird, & Eickhoff, 2010). The left operculum, is linked to sensorimotor experiences, including pain (Garcia-Larrea, 2012). Of relevance, both social and physical pain modulate the insula (Eisenberger, 2012). Murphy et al. (Murphy, Brewer, Catmur, & Bird, 2017) recently proposed a neurodevelopmental view of how interoceptive disturbances may contribute to psychopathology, proposing that interoceptive deficiencies contribute to risky behaviors in adolescents but social emotional impairments in adults.
In anorexia nervosa, the insula have been proposed as neural regions that may be related to the clinical symptom of feeling fat (Frank, 2015) whereas in depression the insula have been postulated to be responsible for negative self-relevant ruminations (Hamilton, Farmer, Fogelman, & Gotlib, 2015; Hao et al., 2015). Importantly, both depressive and eating disorder symptoms can include a negative perception of oneself relative to others (I feel ashamed; I feel fat). In a recent meta-analysis examining functional MRI studies of social conformity, Wu et al. (2016) found that the insula become more active during behavior that deviates from social norms. Consistent with that role, we demonstrate here that modulation of the left insula during mentalization depends upon the valence of one’s own biases towards oneself. The left insula may then contribute to multiple symptom dimensions observed in anorexia nervosa, including physical dissatisfaction about one’s body and differences in one’s social perception of self.
There are limitations to this study. Previously published group differences related to the main effects of this task included a cluster of the left inferior gyrus extending into the insula in which the AN-WR group showed different modulation relative to both the AN-C and HC groups during mentalization (McAdams et al., 2016). That cluster, although smaller, anterior, and lateral, partially overlapped with the left insula cluster identified here with whole-brain regression. However, the multiple regression showed that only the externalizing bias, and not clinical group or any other measure, was related to this cluster. Another limitation is the use of only women, and a mixed population composed of AN-C, AN-WR, and HC. Causality and directionality cannot be determined in a correlation– does the externalizing bias change the left insula activity during mentalization or does this neural difference underlie the altered externalizing biases? Future studies will be necessary to determine whether changes in clinical symptoms are associated with neural changes to the insula and/or changes in the externalizing bias.
In conclusion, in concert with the two-year clinical outcomes data collected from many of these participants (See Harper et al.), the externalizing bias appears to be relevant to both brain function and illness state in anorexia nervosa. Externalizing biases may be related to several aspects of anorexia nervosa including body perception, restriction, and social perceptions; this measure should be evaluated in the context of both clinical interventions and neuroimaging studies in anorexia nervosa to clarify these relationships. In addition, assessment of mentalization as well as mentalization-based therapies are just beginning to be explored in anorexia nervosa, and these studies will further our understanding of the role of mentalization in this disease (Balestrieri et al., 2015; Jewell et al., 2016; Kuipers, van Loenhout, van der Ark, & Bekker, 2016).
Acknowledgments
Funding: Dr. McAdams receives salary support from NIMH K23 MH093684.
Footnotes
CONFLICTS
All authors report no biomedical financial interests or potential conflicts of interest.
References
- Bagby RM, Parker JD, Taylor GJ. The twenty-item Toronto Alexithymia Scale--I. Item selection and cross-validation of the factor structure. Journal of Psychosomatic Research. 1994;38(1):23–32. doi: 10.1016/0022-3999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Balestrieri M, Zuanon S, Pellizzari J, Zappoli-Thyrion E, Ciano R, Res TM. Mentalization in eating disorders: A preliminary trial comparing mentalization-based treatment (MBT) with a psychodynamic-oriented treatment. Eating and Weight Disorders. 2015;20(4):525–528. doi: 10.1007/s40519-015-0204-1. [DOI] [PubMed] [Google Scholar]
- Cabanis M, Pyka M, Mehl S, Muller BW, Loos-Jankowiak S, Winterer G, Wölwer W, Musso F, Klingberg S, Rapp AM, Langohr K, Wiedemann G, Herrlich J, Walter H, Wagner M, Schnell K, Vogeley K, Kockler H, Shah NJ, Stöcker T, Thienel R, Pauly K, Krug A, Kircher T. The precuneus and the insula in self-attributional processes. Cognitive, Affective, and Behavioral Neuroscience. 2013;13(2):330–345. doi: 10.3758/s13415-012-0143-5. [DOI] [PubMed] [Google Scholar]
- Cauda F, Costa T, Torta DM, Sacco K, D'Agata F, Duca S, Geminiani G, Vercelli A. Meta-analytic clustering of the insular cortex: Characterizing the meta-analytic connectivity of the insula when involved in active tasks. Neuroimage. 2012;62(1):343–355. doi: 10.1016/j.neuroimage.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham WA, Koscik TR. Balancing Type I and Type II error concerns in fMRI through compartmentalized analysis. Cognitive Neuroscience. 2017:1–3. doi: 10.1080/17588928.2017.1299122. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A, Maquet P, Salmon E. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. Journal of Cognitive Neuroscience. 2007;19(6):935–944. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of Cognitive Neuroscience. 2012;24(8):1742–1752. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didehbani N, Shad MU, Kandalaft MR, Allen TT, Tamminga CA, Krawczyk DC, Chapman SB. Brief report: Insight in to illness and social attributional style in Asperger's syndrome. Journal of Autism and Developmental Disorders. 2012;42(12):2754–2760. doi: 10.1007/s10803-012-1532-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J, Blumberger DM, Daskalakis ZJ. The neural crossroads of psychiatric illness: an emerging target for brain stimulation. Trends in Cognitive Sciences. 2016;20(2):107–120. doi: 10.1016/j.tics.2015.10.007. [DOI] [PubMed] [Google Scholar]
- Ehrlich S, Lord AR, Geisler D, Borchardt V, Boehm I, Seidel M, Ritschel F, Schulze A, King JA, Weidner K, Roessner V, Walter M. Reduced functional connectivity in the thalamo-insular subnetwork in patients with acute anorexia nervosa. Human Brain Mapping. 2015;36(5):1772–1781. doi: 10.1002/hbm.22736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI. The neural bases of social pain: Evidence for shared representations with physical pain. Psychosomatic Medicine. 2012;74(2):126–135. doi: 10.1097/PSY.0b013e3182464dd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellard KK, Barlow DH, Whitfield-Gabrieli S, Gabrieli JD, Deckersbach T. Neural correlates of emotion acceptance versus worry or suppression in generalized anxiety disorder. Social Cognitive and Affective Neuroscience. 2017 doi: 10.1093/scan/nsx025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Frank GK. Advances from neuroimaging studies in eating disorders. CNS Spectrums. 2015;20(4):391–400. doi: 10.1017/S1092852915000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Larrea L. The posterior insular-opercular region and the search of a primary cortex for pain. Neurophysiologie Clinique. 2012;42(5):299–313. doi: 10.1016/j.neucli.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Garner DM, Olmsted MP, Bohr Y, Garfinkel PE. The eating attitudes test: Psychometric features and clinical correlates. Psychological Medicine. 1982;12(4):871–878. doi: 10.1017/s0033291700049163. [DOI] [PubMed] [Google Scholar]
- Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, Ortega BN, Zaiko YV, Roach EL, Korgaonkar MS, Grieve SM, Galatzer-Levy I, Fox PT, Etkin A. Identification of a common neurobiological substrate for mental illness. Journal of the American Medical Association Psychiatry. 2015;72(4):305–315. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Farmer M, Fogelman P, Gotlib IH. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biological Psychiatry. 2015;78(4):224–230. doi: 10.1016/j.biopsych.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L, Yang J, Wang Y, Zhang S, Xie P, Luo Q, Ren G, Qiu J. Neural correlates of causal attribution in negative events of depressed patients: Evidence from an fMRI study. Clinical Neurophysiology. 2015;126(7):1331–1337. doi: 10.1016/j.clinph.2014.10.146. [DOI] [PubMed] [Google Scholar]
- Harrison A, Tchanturia K, Naumann U, Treasure J. Social emotional functioning and cognitive styles in eating disorders. The British Journal of Clinical Psychology. 2012;51(3):261–279. doi: 10.1111/j.2044-8260.2011.02026.x. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB. Replication and innovation versus a perfect '.05'. Cognitive Neuroscience. 2017:1–2. doi: 10.1080/17588928.2017.1297296. [DOI] [PubMed] [Google Scholar]
- Hu C, Di X, Eickhoff SB, Zhang M, Peng K, Guo H, Sui J. Distinct and common aspects of physical and psychological self-representation in the brain: A meta-analysis of self-bias in facial and self-referential judgements. Neuroscience and Biobehavioral Reviews. 2016;61:197–207. doi: 10.1016/j.neubiorev.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Jewell T, Collyer H, Gardner T, Tchanturia K, Simic M, Fonagy P, Eisler I. Attachment and mentalization and their association with child and adolescent eating pathology: A systematic review. The International Journal of Eating Disorders. 2016;49(4):354–373. doi: 10.1002/eat.22473. [DOI] [PubMed] [Google Scholar]
- Kelton-Locke S. Eating disorders, impaired mentalization, and attachment: Implications for child and adolescent family treatment. Journal of Infant, Child, and Adolescent Psychotherapy. 2016;15(4):337–356. doi: 10.1080/15289168.2016.1257239. [DOI] [Google Scholar]
- Kerr KL, Moseman SE, Avery JA, Bodurka J, Zucker NL, Simmons WK. Altered insula activity during visceral interoception in weight-restored patients with anorexia nervosa. Neuropsychopharmacology. 2016;41(2):521–528. doi: 10.1038/npp.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinderman P, Bentall RP. A new measure of causal locus: The internal, personal and situational attributions questionnaire. Personality and Individual Differences. 1996;20(2):261–264. doi: 10.1016/0191-8869(95)00186-7. [DOI] [Google Scholar]
- Kinderman P, Bentall RP. Causal attributions in paranoia and depression: internal, personal, and situational attributions for negative events. Journal of Abnormal Psychology. 1997;106(2):341–345. doi: 10.1037//0021-843x.106.2.341. [DOI] [PubMed] [Google Scholar]
- Kuipers GS, van Loenhout Z, van der Ark LA, Bekker MH. Attachment insecurity, mentalization and their relation to symptoms in eating disorder patients. Attachment and Human Development. 2016;18(3):250–272. doi: 10.1080/14616734.2015.1136660. [DOI] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: Functional differentiation and integration within the human insula revealed by meta-analysis. Brain Structure and Function. 2010;214(5–6):519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4(4):423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams CJ, Jeon-Slaughter H, Evans S, Lohrenz T, Montague PR, Krawczyk DC. Neural differences in self-perception during illness and after weight-recovery in anorexia nervosa. Social Cognitive and Affective Neuroscience. 2016;11(11):1823–1831. doi: 10.1093/scan/nsw092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams CJ, Krawczyk DC. Who am I? How do I look? Neural differences in self-identity in anorexia nervosa. Social Cognitive and Affective Neuroscience. 2014;9(1):12–21. doi: 10.1093/scan/nss093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams CJ, Lohrenz T, Montague PR. Neural responses to kindness and malevolence differ in illness and recovery in women with anorexia nervosa. Human Brain Mapping. 2015;36(12):5207–5219. doi: 10.1002/hbm.23005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams CJ, Smith W. Neural correlates of eating disorders: translational potential. Neuroscience and neuroeconomics. 2015;4:35–49. doi: 10.2147/NAN.S76699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi R, Addington J, Remington G, Kapur S. Attribution style as a factor in psychosis and symptom resolution. Schizophrenia Research. 2008;104(1–3):220–227. doi: 10.1016/j.schres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Moritz S, Schilling L, Wingenfeld K, Kother U, Wittekind C, Terfehr K, Spitzer C. Psychotic-like cognitive biases in borderline personality disorder. Journal of Behavior Therapy and Experimental Psychiatry. 2011;42(3):349–354. doi: 10.1016/j.jbtep.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Morrison T, Waller G, Lawson R. Attributional style in the eating disorders. The Journal of Nervous and Mental Disease. 2006;194(4):303–305. doi: 10.1097/01.nmd.0000208114.79179.7e. [DOI] [PubMed] [Google Scholar]
- Murphy J, Brewer R, Catmur C, Bird G. Interoception and psychopathology: A developmental neuroscience perspective. Developmental Cognitive Neuroscience. 2017;23:45–56. doi: 10.1016/j.dcn.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberndorfer T, Simmons A, McCurdy D, Strigo I, Matthews S, Yang T, Irvine Z, Kaye W. Greater anterior insula activation during anticipation of food images in women recovered from anorexia nervosa versus controls. Psychiatry Research. 2013;214(2):132–141. doi: 10.1016/j.pscychresns.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlickova H, Varese F, Turnbull O, Scott J, Morriss R, Kinderman P, Paykel E, Bentall RP. Symptom-specific self-referential cognitive processes in bipolar disorder: a longitudinal analysis. Psychological Medicine. 2013;43(9):1895–1907. doi: 10.1017/S0033291712002711. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Peake SJ. Self-development: Integrating cognitive, socioemotional, and neuroimaging perspectives. Developmental Cognitive Neuroscience. 2012;2(1):55–69. doi: 10.1016/j.dcn.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends in Cognitive Sciences. 2012;16(1):61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Reville MC, O'Connor L, Frampton I. Literature review of cognitive neuroscience and anorexia nervosa. Current Psychiatry Reports. 2016;18(2):18. doi: 10.1007/s11920-015-0651-4. [DOI] [PubMed] [Google Scholar]
- Rosen JC, Jones A, Ramirez E, Waxman S. Body Shape Questionnaire: Studies of validity and reliability. The International Journal of Eating Disorders. 1996;20(3):315–319. doi: 10.1002/(SICI)1098-108X(199611)20:3<315::AID-EAT11>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Rotenberg KJ, Flood D. Dietary restraint, attributional styles for eating, and preloading effects. Eating Behaviors. 2000;1(1):63–78. doi: 10.1016/s1471-0153(00)00005-2. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstien S, Manber R, Thase ME, Kocsis JH, Keller MB. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biological Psychiatry. 2003;54(5):573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- Shear MK, Vander Bilt J, Rucci P, Endicott J, Lydiard B, Otto MW, Pollack MH, Chandler L, Williams J, Ali A, Frank DM. Reliability and validity of a structured interview guide for the Hamilton Anxiety Rating Scale (SIGH-A) Depression and Anxiety. 2001;13(4):166–178. doi:10.1002/da.1033 [pii] [PubMed] [Google Scholar]
- Shott ME, Pryor TL, Yang TT, Frank GK. Greater insula white matter fiber connectivity in women recovered from anorexia nervosa. Neuropsychopharmacology. 2016;41(2):498–507. doi: 10.1038/npp.2015.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick SD. Cluster success: fMRI inferences for spatial extent have acceptable false-positive rates. Cognitive Neuroscience. 2017:1–6. doi: 10.1080/17588928.2017.1319350. [DOI] [PubMed] [Google Scholar]
- Sperduti M, Delaveau P, Fossati P, Nadel J. Different brain structures related to self- and external-agency attribution: A brief review and meta-analysis. Brain Stucture and Function. 2011;216(2):151–157. doi: 10.1007/s00429-010-0298-1. [DOI] [PubMed] [Google Scholar]
- Tafarodi RW, Swann WB., Jr Self-linking and self-competence as dimensions of global self-esteem: Initial validation of a measure. Journal of Personality Assessment. 1995;65(2):322–342. doi: 10.1207/s15327752jpa6502_8. [DOI] [PubMed] [Google Scholar]
- Watkins JA, Sargent RG, Miller PM, Ureda JR, Drane WJ, Richler DL. A study of the attribution style, self-efficacy, and dietary restraint in female binge and non-binge eaters. Eating and Weight Disorders. 2001;6(4):188–196. doi: 10.1007/BF03339742. [DOI] [PubMed] [Google Scholar]
- Wu H, Luo Y, Feng C. Neural signatures of social conformity: A coordinate-based activation likelihood estimation meta-analysis of functional brain imaging studies. Neuroscience and Biobehavioral Reviews. 2016;71:101–111. doi: 10.1016/j.neubiorev.2016.08.038. [DOI] [PubMed] [Google Scholar]
- Yang J, Xu X, Chen Y, Shi Z, Han S. Trait self-esteem and neural activities related to self-evaluation and social feedback. Scientific Reports. 2016;6:20274. doi: 10.1038/srep20274. [DOI] [PMC free article] [PubMed] [Google Scholar]


