Abstract
Insulin-like growth factor (IGF)-I and ephrin ligand (efn)–receptor (Eph) signaling are both crucial for bone cell function and skeletal development and maintenance. IGF-I signaling is the major mediator of growth hormone-induced bone growth, but a host of different signals and factors regulate IGF-I signaling at the systemic and local levels. Disruption of the Igf1 gene results in reduced peak bone mass in both experimental animal models and humans. Additionally, efn–Eph signaling is a complex system which, particularly through cell–cell interactions, contributes to the development and differentiation of many bone cell types. Recent evidence has demonstrated several ways in which the IGF-I and efn–Eph signaling pathways interact with and depend upon each other to regulate bone cell function. While much remains to be elucidated, the interaction between these two signaling pathways opens a vast array of new opportunities for investigation into the mechanisms of and potential therapies for skeletal conditions such as osteoporosis and fracture repair.
Keywords: IGF-I, Efn, Eph, Bone
1. Introduction
Osteoporosis is a debilitating disease affecting millions worldwide. In the United States alone, more than 10 million people have osteoporosis, and that number is predicted to rise, increasing the burden of suffering as well as healthcare costs on individuals as well as society at large. By the year 2025, the annual rate of osteoporosis-related fractures in the United States has been projected to be greater than 3 million, leading to an economic burden of more than $25 billion per year (Burge et al. 2007). Osteoporosis, a disease characterized by low bone mass and compromised skeletal microarchitecture, occurs when elevated rates of bone resorption are not sufficiently compensated for by increased bone formation. Frequently, osteoporosis occurs in the context of accelerated bone resorption due to sex hormone deficiency and aging in postmenopausal women. While several current therapeutic strategies have achieved some success at slowing the rate of bone loss, devastating osteoporotic fractures still occur at an alarming rate, emphasizing both the importance of achieving a high peak bone mass during development and the necessity of investigating the mechanisms regulating bone growth and maintenance in order to develop new therapies to treat and prevent osteoporosis. Much work has gone into understanding of the origin and functions of cartilage-forming chondrocytes, bone matrix-producing osteoblasts and osteocytes, and bone-resorbing osteoclasts, and these studies have revealed that the network of signals regulating these cells and their functions is complex. Indeed, there are many growth factors which contribute to the regulation of skeletal development. Two growth factor signaling pathways which exert many effects on skeletal development are the IGF-I and efn–Eph pathways. The focus of this review is to explore the extent to which each of these two signaling pathways has been studied with respect to its contribution to skeletal development and to review what is currently known about the ways in which the interactions of these two pathways may enhance our understanding of skeletal regulation and lead to opportunities to discover novel therapies for osteoporosis and bone repair.
2. IGF Signaling in Bone
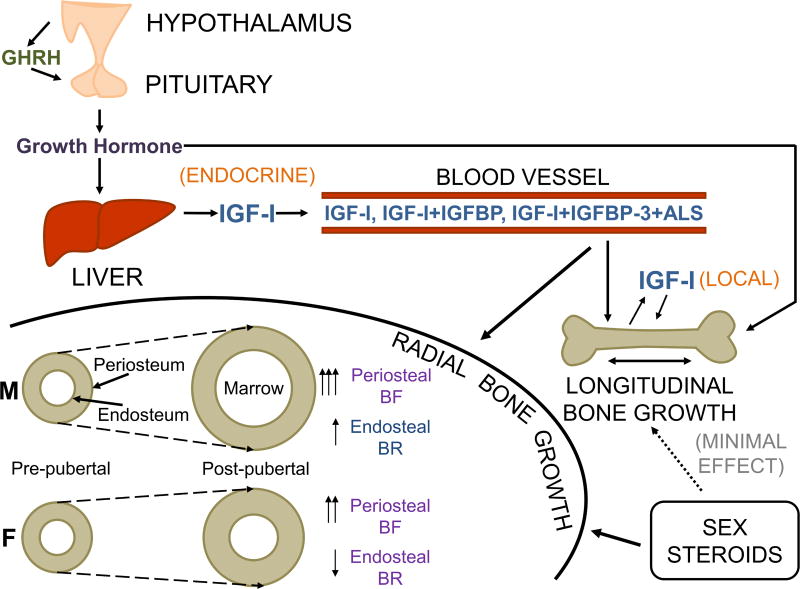
Insulin-like growth factors (IGFs) are the most abundant growth factors in bone matrix and are critical regulators of bone growth and maintenance. IGF-I and -II are small peptide hormones which are structurally similar to insulin, and they act by binding the type I IGF receptor (IGF-IR), a receptor tyrosine kinase which is also structurally similar to the insulin receptor (Centrella et al. 1990; Slootweg et al. 1990). During intrauterine development, IGF-II is responsible for growth, while IGF-I predominantly regulates skeletal growth and maintenance during postnatal life. As a member of the growth hormone (GH)/IGF axis, IGF-I is responsible for mediating a significant proportion of GH’s effects on bone (Fig. 1).
Figure 1. A model of GH/IGF-I and sex hormone signaling interacting to regulate bone growth.
GH effects on the skeletal system is mediated via both endocrine and local mechanisms through hepatic IGF-I and local modulation of IGF-I expression in bone cells, respectively. Sex steroids contribute relatively little to longitudinal bone growth, but they interact with IGF-I signaling to regulate radial bone growth. The interaction of IGF-I and sex steroid signaling results in skeletal sexual dimorphism in which, during pubertal growth, male (M) periosteal bone formation (BF) and endosteal bone resorption (BR) are both increased, producing bones with a larger diameter as well as a larger marrow cavity, while female (F) periosteal BF is increased but endosteal BR is decreased, leading to smaller bones with more preservation of cortical bone (Adapted with permission from Lindsey and Mohan, 2015).
a. GH regulation of IGF-I
The systemic, endocrine actions of IGF-I are largely due to GH-induced hepatic IGF-I secretion into the circulation. In fact, conditional hepatic deletion of the Igf1 gene (Sjögren et al. 1999; Yakar et al. 1999) or GH receptor (Ghr) gene (Fan et al. 2009) in mice resulted in up to 90% reductions in systemic circulating IGF-I levels. Furthermore, up to 75% of circulating IGF-I is bound up in a ternary complex with IGF binding protein 3 (IGFBP3) and an acid-labile subunit (ALS) which is also produced in the liver in response to GH signaling. Growth hormone-induced hepatic secretion of circulating, endocrine-acting IGF-I may be responsible for up to 30% of body weight (Stratikopoulos et al. 2008). However, IGF-I also acts locally in an autocrine/paracrine manner. An additional 39% of body weight may be due to local IGF-I production, of which 4% may be accounted for by GH-induced IGF-I production in peripheral tissues (Stratikopoulos et al. 2008).
Since IGF-I is known to interact with efn–Eph signaling and IGF-I mediates many of GH’s effects on bone, this review focuses primarily on the contribution of IGF signaling to skeletal development. However, the entire GH/IGF axis is crucial for skeletal development, and other recent reviews have examined the GH/IGF axis as a whole (Locatelli & Bianchi 2014; Lindsey & Mohan 2015; Liu et al. 2016). Furthermore, some evidence suggests that the GHR and downstream effectors of GH signaling Janus kinase 2 (JAK2) and signal transducer and activator of transcription 5B (STAT5B) may complex with EphA4 to enhance Igf1 expression, suggesting a more complex relationship between the GH/IGF axis and efn–Eph signaling which deserves further investigation (Jing et al. 2012; see below).
b. Systemic regulation and effects of IGF-I
Many systemic signals in addition to GH are known to regulate IGF-I. For example, circulating parathyroid hormone (PTH) is known to enhance osteoblast proliferation, differentiation, and survival by increasing local production of IGF-I (Linkhart & Mohan 1989; McCarthy et al. 1989; Miyakoshi et al. 2001a; Bikle et al. 2002; Yamaguchi et al. 2005). Additionally, sex steroids cause an increase in IGF-I expression during puberty (Christoforidis et al. 2005; Veldhuis et al. 2005). In fact, crosstalk between the sex steroid and GH/IGF-I axes is crucial for linear bone growth and acquisition, and GH/IGF-I may contribute to the development of skeletal sexual dimorphism (Liu et al. 2016). Furthermore, thyroid hormone (TH) increases both hepatic and skeletal IGF-I expression during a critical prepubertal growth period in mice in which TH is a more critical regulator of skeletal growth than GH; thus, IGF-I is thought to mediate many TH effects on the skeleton (Xing et al. 2012; Cheng et al. 2016). Conversely, glucocorticoids and 1,25-dihidroxyvitamin D3 [1,25-(OH)2D3] have been shown to downregulate IGF-I expression (Chen et al. 1991; Scharla et al. 1991; Canalis 2005). Thus, there is ample evidence in the literature to suggest that many of the systemic effects of calcium regulating hormones on bone are mediated in part via regulation of IGF actions.
c. Local regulation and effects of IGF-I
At the local level, many growth factors and other signals in addition to GH are involved in regulating IGF expression. For example, fibroblast growth factor (FGF) 2, transforming growth factor (TGF)-β1, bone morphogenetic protein (BMP) 7, and interleukin-1 are known to influence expression of IGF-I in bone cells (Tremollieres et al. 1991; Knutsen et al. 1995; McCarthy & Centrella 2001; Zhang et al. 2002a). These studies have shown that the actions of many of the local growth factors on IGF-I expression vary depending on both cell type and stage of differentiation.
IGF activity, however, is dependent upon more than just the regulation of IGF expression levels. IGF-I interacts with several IGF binding proteins (IGFBP-1 through -6) which serve to regulate IGF-I activity at both the systemic and local levels. Up to 75% of the IGF-I in plasma is bound up in a ternary complex with IGFBP-3 and an acid-labile subunit (ALS), and in this way IGFBP-3 regulates the concentration of active, free IGF-I available in circulation for signaling (Bagi et al. 1994; Rosen et al. 1994; Jones & Clemmons 1995; Rajaram et al. 1997). Not all IGFBPs affect IGF-I signaling in the same way; generally, IGFBP-3 and -5 enhance IGF-I’s effect on osteoblasts by prolonging its half-life in circulation while IGFBP-1, -2, -4, and -6 inhibit IGF-I’s effect on osteoblasts by preventing its interaction with the type I IGF receptor (IGF-IR) (Govoni et al. 2005). Moreover, IGFBPs themselves are regulated at the expression level by systemic and local factors including PTH, 1,25-(OH)2D3, glucocorticoids, estrogen, retinoic acid, IGFs, TGF-β, BMPs, PDGF, and interleukins (Knutsen et al. 1995; Chevalley et al. 1996; Gabbitas & Canalis 1996; Honda et al. 1996; Hayden et al. 1997; Malpe et al. 1997; Kveiborg et al. 2001; Denger et al. 2008; DeMambro et al. 2015). IGFBP activity is also regulated by IGFBP proteases including pregnancy-associated plasma protein (PAPP)-A, PAPP-A2 (Kanzaki et al. 1994; Conover 1995; Qin et al. 2006; Tanner et al. 2008; Christians et al. 2013), and ADAM-9 (Mohan et al. 2002). Regulation of IGF activity by IGFBPs and, by extension, regulation of the IGFBPs themselves are therefore further crucial determinants of IGF signaling in bone.
d. IGF-I and Mechanotransduction
Considerable experimental evidence indicates that mechanical strain is a key physiological regulator of bone formation. IGF-I plays an important role in mechanotransduction and the skeletal response to mechanical strain (Tian et al. 2017). Mechanical loading leads to a rapid increase in IGF-I expression in bones (Lean et al. 1995; Xing et al. 2005; Reijnders et al. 2007), an effect which is mediated via an integrin-dependent phosphorylation of the IGF-I receptor (IGF-IR) (Kapur et al. 2005; Lau et al. 2006). In vivo overexpression of Igf1 in mouse osteoblasts led to increased periosteal bone formation in response to low-magnitude loading (Gross et al. 2002), and osteoblasts exposed to dynamic strain demonstrate increased activation of PI3K/Akt and β-catenin, downstream effectors of IGF-I signaling (Sunters et al. 2010). Furthermore, conditional knockout of Igf1 in osteoblasts expressing type I collagen (Col-I, Kesavan et al. 2011) or osteocytes (Lau et al. 2013) prevented an osteogenic response to mechanical loading. In osteocytes, lack of Igf1 disrupted mechanical strain-induced changes in expression of sclerostin and Wnt10b (Lau et al. 2013). In addition to its contributions to the response of osteoblasts and osteocytes to mechanical strain, IGF-I signaling may even play a role in mediating the ability of mechanical forces to regulate the proliferation, survival, and osteogenic differentiation of bone marrow derived mesenchymal stem cells (Sakata et al. 2003, 2004; Chen et al. 2017; Wang et al. 2017). Thus, IGF-I signaling is essential for the skeletal response to mechanical stimuli.
e. Nutrition and IGF-I
Diet and nutritional status are additional important regulators of IGF-I (Bonjour 2016). Under conditions of restricted dietary intake, IGF-I levels are reduced via several mechanisms: hepatic GH receptors are reduced in number, decreasing IGF-I production, and rates of IGF-I degradation and clearance are increased (Thissen et al. 2004). Furthermore, osteoblasts appear to be resistant to IGF-I action in the context of protein restriction (Bourrin et al. 2000). This IGF-I response to alternations in dietary protein intake specifically has been confirmed in both in vivo animal studies (Ammann et al. 2000; Bourrin et al. 2000) and patients after hip fracture (Schürch et al. 1998). The interaction of dietary protein and IGF-I may thus influence skeletal health on a number of levels including attainment of peak bone mass (Bonjour et al. 2007), skeletal response to physical activity (Chevalley et al. 2008), and bone loss in the context of calorie-poor conditions such as intensive exercise and anorexia nervosa (Russell et al. 1994; Grinspoon et al. 2000; Gremion et al. 2001; Warren et al. 2002; Misra et al. 2008). Additionally, epidemiological studies indicate that higher protein intake may be protective against bone loss, musculoskeletal deterioration, and decreased IGF-I levels, particularly in the elderly (Dawson-Hughes et al. 2004; Gaffney-Stomberg et al. 2009; Bonjour 2016). Adequate nutrition and protein intake is thus crucial for skeletal development and maintenance.
f. Transgenic mouse models of IGF-I action
The question of the relative contributions of endocrine versus local IGF-I action to skeletal growth and maintenance has also been extensively studied. Several transgenic mouse models indicate that systemic IGF-I has a much larger effect on cortical bone size and expansion than on longitudinal bone growth. Mice with conditional disruption of hepatic Igf1 (Sjögren et al. 1999; Yakar et al. 1999) or GH receptor (Ghr, Fan et al. 2009) had little to no reduction in linear bone growth despite up to 90% reductions in circulating IGF-I levels. Furthermore, mice with conditional disruption of hepatic IGF-I along with total ALS or total ALS and total IGFBP-3 had up to 97.5% decreases in circulating IGF-I levels; these mice had relatively small changes in body length, but the triple negative mice had a 50% reduction in cortical bone (Sjögren et al. 1999; Yakar et al. 1999, 2002, 2009), a similar reduction to that seen in mice lacking total IGF-I (Mohan et al. 2003). However, restoration of hepatic IGF-I production in total Igf1 knockout mice rescued body size by approximately 30% (Stratikopoulos et al. 2008), indicating that endocrine IGF-I action may play a role in skeletal growth (Nordstrom et al. 2011; List et al. 2014).
Local expression of IGF-I is also critical for bone function. Overexpression of IGF-I in mouse osteoblasts in vivo has led to increased osteoblast activity, bone formation, and bone remodeling (Zhao et al. 2000; Jiang et al. 2006), and overexpression of regulators of IGF bioavailability including IGFBPs and their proteases suggests that local availability of IGF-I is an important determinant of bone formation (Miyakoshi et al. 1999; Richman et al. 1999; Zhao et al. 2000; Miyakoshi et al. 2001b; Devlin et al. 2002; Qin et al. 2006). Conditional disruption of Igf1 and Igf1r specifically in osteoblasts confirmed that, while serum levels of IGF-I were unchanged, a lack of local IGF-I significantly impaired bone mineral density (BMD), bone size, and bone formation measures (Zhang et al. 2002b; Govoni et al. 2007a). Furthermore, local IGF-I expression in chondrocytes is also necessary for skeletal development; chondrocyte-specific Igf1 disruption in mice decreased bone length and BMD and impaired growth plate organization and function (Govoni et al. 2007b; Wang et al. 2011, 2015). Thus, both systemic and local IGF-I contribute significantly to skeletal growth and maintenance.
g. IGF-I and osteoporosis
It has been well established that growth hormone (GH) secretion decreases with age (Corpas et al. 1993; Müller et al. 1999). Accordingly, levels of IGF-I in the serum and bone decrease with age as well (Benbassat et al. 1997; Seck et al. 1999). However, in addition to decreased levels of IGF-I with age, osteoprogenitor cells from aged rats do not respond as effectively to IGF-I (Tanaka & Liang 1996), indicating that the skeleton is particularly susceptible to deterioration with age. In fact, age is a major risk factor for osteoporosis, and IGF-I may play an important mechanistic role in explaining age-related bone loss.
Epidemiological evidence suggests that IGF-I levels are well correlated with bone mineral density in men and postmenopausal women (Muñoz-Torres et al. 2001; Gillberg et al. 2002), and a cross-sectional study indicated that elderly women who had sustained femoral neck fractures had significantly decreased levels of IGF-I, IGF-II, IGFBP-3, and IGFBP-5 (Boonen et al. 1999). Moreover, several recent studies have also observed relationships between deficiencies in components of the IGF system and increased rates of fracture in both women and men (Ohlsson et al. 2011; Paccou et al. 2012; van Varsseveld et al. 2015; Lundin et al. 2016). Recombinant human IGF-I has even undergone clinical trials as an osteoporosis treatment, with mainly positive results (Locatelli & Bianchi 2014). However, additional studies have reported little to no effect of IGF-I levels on fracture risk (Kassem et al. 1994; Seck et al. 1999; Gillberg et al. 2001; Martini et al. 2001; Zofková et al. 2001). Thus, while IGF-I may play a role in age-related bone loss and osteoporosis, further studies are needed to elucidate its precise contribution. Taken together, both animal studies and human clinical studies dealing with different aspects of bone formation have established IGF-I as a critical mediator of bone growth and homeostasis.
3. Ephrin/Eph Signaling in Bone
The ephrins are large families of membrane-bound ligands (efn: ephrin family receptor interacting proteins) and receptor tyrosine kinase receptors (Eph: erythropoietin-producing human hepatocellular receptors) that mediate cell–cell communication within and between tissues in a wide variety of biological functions. Efn–Eph mediate cell proliferation and migration, and they are critical in the regulation of adhesion, repulsion, and tension which segregates cells to establish and maintain tissue boundaries during development (Cayuso et al. 2015). The efns and Ephs are broadly expressed during tissue development and repair. These functions have also implicated the efns and Ephs in the dysregulated adhesion and motility that promotes tumor metastasis.
There are two families of ephrin ligands. In mammals, they comprise the five-member ephrin A (efnA) family of glycosylphosphatidylinositol (GPI)-linked ligands and the three-member ephrin B (efnB) family of transmembrane ligands. Efn-binding receptors are all receptor tyrosine kinase (RTK) receptors, but they belong to either the nine-member A receptor (EphA) family or the five-member B receptor (EphB) family. Both EphA and EphB have an extracellular efn-binding domain attached to two fibronectin-III repeats and the intracellular tyrosine kinase, sterile alpha motif (SAM) and postsynaptic density protein 95/disks large/zonula occludens-1 (PDZ)-binding domains. Ligand–receptor interactions can be promiscuous within the ligand–receptor family, but, with few exceptions, signaling is restricted between ligands and receptors within either the A or B family.
a. Forward and reverse signaling
EfnB and EphB regulation of tissue development and homeostasis is noteworthy because this family can signal in the forward direction, from ligand through receptor, as well as in the reverse direction, from receptor through ligand, through a PDZ binding motif connected to the intracellular cytoplasmic domain. Reverse signaling from EphA through efnA is not well characterized, but this pathway might utilize regulatory proteins recruited to efnA clusters (Davy et al. 1999).
Eph receptor and binding diversity permits a wide array of forward and reverse signaling options to mediate their functions. EphA regulates the JAK/STAT pathway, while EphB regulates PI3 kinase-mediated proliferation. Activation of the Rho GTPases by EphA or EphB mediates actin effects on cell shape and movement. Activation of the Ras GTPase pathways generally leads to negative regulation of the MAP kinase pathway, resulting in reduced proliferation and migration by EphA as well as reduced adhesion by EphB (Edwards & Mundy 2008). Reverse signaling from EphB2 through efnB1 promotes stromal cell differentiation to osteoblasts. In this case, PDZ domains from efnB1 and the PDZ-containing protein Na/H exchange regulatory factor 1 (NHERF1) form a complex with protein tyrosine phosphatase (PTPN13) and the PDZ domain-binding transcriptional coactivator (TAZ) to promote Osx transcription and osteoblast differentiation (Xing et al. 2010). Other studies have demonstrated the interaction of efns with various adaptor proteins, further increasing the potential of efn–Eph signaling to interact with diverse signaling partners. For example, the SH2/SH3 adaptor Grb4 binds the cytoplasmic tail of enfB1. The SH3 domains of these adaptor proteins can in turn recruit a number of SH3-binding partners (e.g., Axin, Pak1, FAK, and Paxillin) to the efnB1 signaling complex and participate in propagating the reverse signal in a complex manner (Cowan & Henkemeyer 2001). The diversity of efn–Eph signaling functions is thus the product of the ability of efn–Eph to signal in both a forward and reverse manner and the presence of cross-talk between the several efn–Eph pathways, non-efn–Eph signaling pathways (e.g., the fibroblast growth factor pathway; Sawada et al. 2010), and different adaptor proteins. Thus, dissecting intracellular signaling pathway cross-talk among different cell types presents a challenge in characterizing efn–Eph effects.
Additionally, ligand and receptor functions can be diversified and adjusted by higher order ligand and receptor associations resulting from clustering. Clustering can involve the extracellular receptor domain as well as the intracellular RTK and SAM domains. Homo-oligomerization can follow interactions between Ephs that follow the formation of the initial ligand–receptor tetramer. Additionally, interfamily hetero-oligomers have been observed to cluster and produce different activation or inhibition states according the relative levels of expression within the cluster (Janes et al. 2011; Jurek et al. 2016). Conformational changes following Eph binding, while limited when compared to other RTKs, might explain some of the interfamily binding between ligands and receptors (Janes et al. 2012). The role of proteolytic cleavage in further regulating efn–Eph signaling has been reviewed elsewhere (Atapattu et al. 2014). Additionally, splice variants in EphA7 have been observed; kinase domain deficient variants bias receptor function from repulsion toward adhesion (Holmberg et al. 2000). Considering the wide array of receptors, the ability to signal in forward and reverse directions, and the extracellular and intracellular variations, modulation of the efn–Eph system of cell signaling is indeed complex.
b. Mechanisms of Efn–Eph signaling in skeletal development
The efns and Ephs have been best characterized during the development of various murine tissues. Global knockout mouse studies have demonstrated that efnA2 and EphA7 mediate neural development by inhibiting proliferation. EfnB2 patterns the somites and regulates neural cell development (Davy & Soriano 2007). Neurogenesis, axonal guidance, and vasculogenesis are regulated by different efns and Ephs through both forward and reverse signaling (Zhang & Hughes 2006; Wilkinson 2014). Given their established functions in cell motility and adherence, it is not surprising that members of both the A and B families of ligands and receptors have been associated with neo-angiogenesis and invasiveness of several types of tumors (Brantley-Sieders & Chen 2004; Kumar et al. 2006), with deleterious effects attributed to their expression levels in the affected tissues (Campbell & Robbins 2008).
A variety of efns and Ephs are expressed in various tissues during the normal inflammatory response to injury and in chronic inflammation, where they mediate diverse processes such as vascular permeability and cell motility (Coulthard et al. 2012). EfnB1 and EphB2 expression is upregulated during skin wound repair and drives the re-epithelialization necessary for healing through the down-regulation of tight junctions, the release of adherens, and the reduction in actinomysin tension (Nunan et al. 2015). Efns and Ephs mediate growth factor-regulated angiogenesis during development, and they would also be expected to mediate the repair of skeletal tissues in a similar fashion.
The efns and Ephs are widely expressed in skeletal tissues, as they are in most tissues. The efna2, Epha2, Epha4, efnb1, and efnb2 genes are expressed in osteoclasts induced to differentiate in vitro (Irie et al. 2009); several ligand and receptor genes of both the A and B families are expressed in differentiating osteoblasts in vitro (Zhao et al. 2006; Matsuo & Otaki 2012). In vivo, efnb2 gene and efnB2 protein expression in osteoblasts and osteoclasts was promoted by PTH and parathyroid hormone-related peptide (PTHrP). The authors suggest that the anabolic effect of PTH and PTHrP could be mediated by efnB2 action on osteoblasts that express EphB (Allan et al. 2008). These characteristics of efn–Eph expression have important implications for the maintenance of homeostasis between osteoblasts and osteoclasts and the balance between bone formation and bone resorption.
Bone lineage motility and differentiation are regulated by efn–Eph binding. EfnB2 and EphB4 interactions mediate the mobilization from the bone marrow to the blood of efnB2-expressing bone marrow hematopoietic precursors through the EphB4-expressing sinusoidal cells. Antibody-dependent inhibition of this interaction and dominant-negative EphB4 receptor studies established that the mechanism was dependent on forward cell–cell signaling (Kwak et al. 2016). In vitro spreading and migration assays on human mesenchymal stem cells (MSCs) using soluble efnB and EphB established that forward signaling through EphB2 promoted MSC spreading, but reverse signaling through efnB inhibited MSC attachment and spreading. Moreover, differentiation of MSCs to the chondrogenic or osteogenic lineages was promoted by reverse signaling from EphB2 through efnB1 and efnB2 (Arthur et al. 2011). EfnB family regulation of bone marrow MSCs is important for several aspects of bone formation and possibly bone repair.
It was the observation that spontaneous efn and Eph mutations resulted in abnormalities in embryonic skeletal development that first implicated efn–Eph as important regulators in bone. Additional studies in mice with targeted deletions of different efn and Eph genes have further established the importance of efn–Eph signaling in skeletal development and characterized its effects in the regulation of bone cell differentiation as described below.
The developmental functions of efn–Eph in the skeleton have been best described in craniofacial patterning abnormalities. EphA4 has been demonstrated in Twist1 and Epha4 mutant mice to mediate Twist1 regulation of osteogenic cell migration to the coronal sutures and to then exclude these cells from the sutures. A failure of EphA4 signaling produced craniosynostosis in these models (Ting et al. 2009). In mice with efnb1 mutations, it is believed that X-linked mosaicism of efnB1 impaired cell sorting. Using signaling-deficient mutations, forward and reverse signaling between efnB1 and EphB, which mediate adhesion and repulsion in mesenchymal cell condensations, was demonstrated to regulate this aspect of craniofacial development (Davy et al. 2004). The calvarial defect phenotype in efnb1 mutants was similar to that observed in neural crest cell sorting and was the result of impaired gap junction communication involving efnB1 in early osteogenic precursor development (Davy et al. 2006). Similar craniofacial abnormalities are produced by efnb1 signaling mutations in human pathology (Compagni et al. 2003). However, other studies have implicated unidirectional forward signaling in this phenotype. EfnB1 patterning of palate development is accomplished through forward signaling interactions with EphB2 and EphB3 receptors, as demonstrated by combination Ephb2 forward-signaling deficient mutants/Ephb3 knockout mice, which developed the cleft palate. The defect resided in reduced proliferation (Risley et al. 2009) of the palatal mesenchymal cells mediated by these EphBs. Recent studies have postulated that the separation of the sutures results from efnB1 forward signaling mediated by Rho-associated protein kinase (ROCK). ROCK signaling modifies intracellular actin distributions and the resulting cortical tension of efnB1-expressing cells, maintaining the suture separating them from non-efnB1-expressing cells (O’Neill et al. 2016).
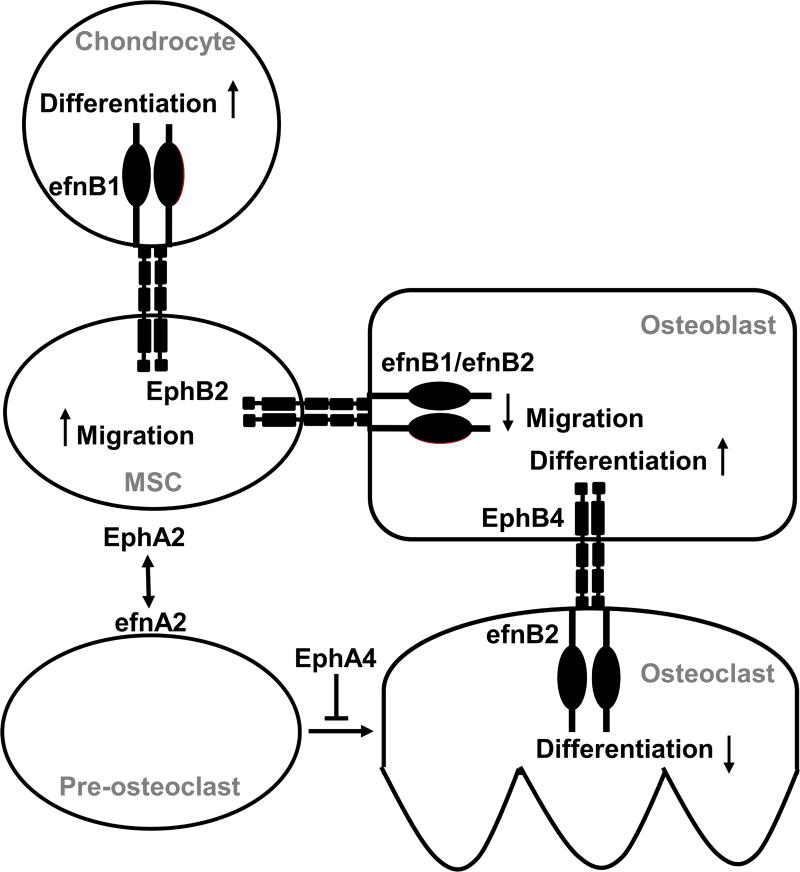
The efnA and EphA families also mediate communication between osteoblasts and osteoclasts. Interactions between efnA2 and EphA4 enhance osteoclast differentiation but inhibit osteoblast differentiation, as osteoblast differentiation was enhanced in Epha4-deficient osteoblasts in response to efna2 overexpression (Irie et al. 2009). This effect must bias the initiation of remodeling away from bone formation, although multiple efn–Eph interactions may mediate this communication (Fig. 2). EfnA2 also interacts with EphA2 in promoting osteoclastogenesis to promote osteoporotic resorption; ovariectomy in rats increased efnA2–EphA2 signaling, osteoclast development, and trabecular bone deterioration, effects that were reduced in vitro by the inhibition of efnA2 and EphA2 expression and in vivo by estradiol treatment (Liu et al. 2017). These observations suggest efnA–EphA modulation as an alternative to hormone related therapy for osteoporosis.
Figure 2. A model for efn–Eph regulation of bone cell interaction in bone development and homeostasis.
Several efns and Ephs mediate growth plate development during bone growth. Interactions between osteoblasts (OB), osteoclasts (OC), mesenchymal stem cells (MSC), and chondrocytes are mediated by different ligand–receptor combinations, coordinating bone cell development and activity through forward and reverse signaling. Osteoblast development is mediated by forward signaling through EphB4 from efnB2 (Zhao et al. 2006) and reverse signaling through efnB1/efnB2 from EphB2 (Arthur et al. 2011). Osteoclast development is mediated by efnA2 and EphA4 (Irie et al. 2009; Liu et al. 2017) as well as through reverse signaling from EphB4 through efnB2 (Zhao et al. 2006).
Studies in mice with targeted disruption of the Epha4 gene in osteoclasts have shown that EphA4 functions are diverse. EphA4 expression inhibited the activity but not the development of osteoclasts through the altered phosphorylation of stimulatory and inhibitory mediators of osteoclast activity (Stiffel et al. 2014). EphA5 has been determined to be an inhibitor of osteogenic development of bone marrow stromal cells in culture (Yamada et al. 2013). EphA5 inhibition is overcome by the exogenous addition of dexamethasone (Yamada et al. 2016), suggesting potential interaction between a systemic hormone and a local growth factor in regulating osteogenesis, although the mechanism remains to be elucidated. Developmental studies in Hoxa13 knockout mice implicate EphA7 expression in mediating Hoxa13-dependent mesenchymal cell adherence and chondrocyte condensations in the knockout autopods, which display digit fusions (Stadler et al. 2001). The EphAs therefore participate in different aspects of skeletal development and homeostasis.
Studies in efnb1 knockout mice established that efnB1 can promote different aspects of bone homeostasis. An osteoblast (Osx)-specific knockout of efnb1 resulted in altered long bone development, with impaired trabecular and cortical bone formation. A decrease in osteoblasts and increase in osteoclast formation suggests that efnB1 can mediate bone formation and resorption (Nguyen et al. 2016). Other investigations have determined the mechanism of intracellular signaling that mediate this effect. Transgenic overexpression of efnb1 in murine osteoblasts increased osteoblast differentiation and mineralization in vitro, increased Osx expression and trabecular bone formation in vivo, and reduced osteoclastogenesis through EphB2 in response to mechanical loading of the long bones (Cheng et al. 2013). Further studies also examined the role of efnB1 in the osteoclast lineage and established that deletion of efnb1 from myeloid cells increased osteoclastogenesis. Trabecular bone was reduced in the knockout mice without changes in bone formation. In vitro studies implicated reverse signaling of efnB1 through EphB2 as a mechanism for this effect and a role for efnB1 as a negative regulator of osteoclast differentiation (Cheng et al. 2012).
Deletion of efnb2 in osteoblasts resulted in a significant reduction in bone formation in knockout mice in vivo and reduced mineralization and expression of late stage osteoblast differentiation markers in vitro. Efnb2 deletion has been observed to increase apoptosis in the osteoblast lineage, suggesting yet another mode for this efn in promoting bone formation (Tonna et al. 2014).
c. Role of Efn–Eph in cell–cell communication
Multiple efn–Eph interactions mediate several aspects of bone development and homeostasis, but the efnB2–EphB4 ligand–receptor signaling pair is especially well-studied in the mediation of osteoblast–osteoclast communication that maintains a homeostasis between bone formation and bone remodeling in the skeleton (Edwards & Mundy 2008). In vitro and in vivo studies established that the efnB2–EphB4 ligand–receptor pair is especially important for bone homeostasis, as forward signaling from efnB2 through EphB4 promoted bone formation from osteoblasts, while reverse signaling from EphB4 through efnB2 inhibited osteoclastogenesis by suppressing NFATc1 (Zhao et al. 2006). In this case, an in vivo reduction in osteoblast development and bone formation would be expected in osteoblast marker-specific Ephb4 knockout mice, while an increase in osteoclast development and bone resorption functions would be expected in osteoclast marker-specific efnb2 knockout mice. However, the osteoclast-specific efnb2 knockout mice in this same study exhibited only a slight increase in osteoclast number without a change in bone volume, osteoblast surface, or bone mineral density (Zhao et al. 2006). In contrast, a myeloid lineage efnb1 knockout mouse also driven by Lys2-cre expression did present a phenotype with increased resorption (Cheng et al. 2012), which suggests that efnB1 is a negative regulator of osteoclast function and that there is a redundancy in the efnBs, and possibly the Ephs, in osteoblast–osteoclast coupling. In either case, a functional demonstration of forward or reverse signaling in osteoblast–osteoclast coupling, respectively, would require that the phenotype be accompanied by a reduction in signaling from an EphB receptor in an osteoclast-specific efnb knockout or efnB-specific signaling to a specific receptor in an osteoblast-specific Ephb knockout.
In vitro, efnB2 expression and efnB2–EphB4 interaction in response to PTH or PTHrP treatment has been associated with the differentiation of stromal cells to mineralizing osteoblasts (Allan et al. 2008). EphB4 inhibition by soluble EphB4 inhibited efnB2–EphB4 signaling and the expression of late-stage osteoblast differentiation markers and promoted osteoclast formation, even preventing the anabolic response to PTH. This effect required osteoblasts in vitro, indicating that signaling between efnB2 and EphB4 within the osteoblast lineage is required for osteoblast differentiation and osteoclast formation (Takyar et al. 2013). These results suggest that efnB2–EphB4 regulation of bone formation and resorption might be complicated by the expression and function of multiple efns and Ephs during the development of the bone cell lineages. Indeed, this interaction extends to skeletal pathology in the adult; elevated EphB4 expression in osteoarthritis chondrocytes was reduced by an in vitro application of efnB2, which increased Col-II gene expression, a marker of chondrocyte development, and decreased markers of bone resorption (Kwan Tat et al. 2009).
In addition to its interactions with EphB4 in bone homeostasis, efnB2 is important in calvarial bone formation (Benson et al. 2012). Embryonic expression of efnB2 is found in the calvarial sutures and periosteum but expression is confined to the sutures of the adult murine calvariae. It promotes the expression of bone formation markers in vitro and calvarial bone formation in organ culture, possibly through EphB1 and EphB2 signaling. In vivo, efnB2 might mediate bone formation through Ephs outside of the efnB2–EphB4 signaling circuit, as it promotes bone formation in the calvariae, where EphB4, its preferred receptor, is not expressed.
Other efnB ligand–receptor signaling combinations could regulate osteoblast functions through osteoclasts. The anti-resorptive alendronate has been demonstrated to increase the expression of efnB1, EphB1, and EphB3 in mice and decrease bone sialoprotein and osteonectin, markers of bone formation that were dependent on pre-osteoclasts. Because the expression of efnB1 and these Ephs was present on osteoclasts and osteoblasts, respectively, the authors conclude that efnB1 expressed in osteoclasts inhibits osteoblast differentiation (Shimizu et al. 2012). This signaling appears similar to that of efnB2 and EphB4 in maintaining bone homeostasis (Zhao et al. 2006) and suggests a degree of functional redundancy among the efns and Ephs.
Efn–Eph functions have also been associated with bone repair. In addition to its role in calvarial bone formation during development, efnB2 expression is elevated at sites of calvarial bone injury, suggesting regulation of the bone formation phase of bone repair (Benson et al. 2012). The Col-I-mediated overexpression of transgenic Ephb4 in osteoblasts in a murine model of femur fracture healing increased osteogenic progenitors and decreased osteoclasts, which resulted in increased bone formation and reduced bone remodeling within the fracture callus (Arthur et al. 2013). These results might involve efnB2–EphB4 signaling, as an increase in EphB4 would be expected to decrease osteoclastogenesis predicted by reverse signaling through efnB2 and thereby increase bony callus formation (Zhao et al. 2006). Alternatively, EphB4 might function by forward signaling from different efnBs. Thus, interaction between efns in one cell type and their receptors in different cell type can lead to enrichment of various cell types that contribute to key cellular processes involved in fracture repair.
4. Interactions between IGF and Efn–Eph Signaling
There is now substantial evidence in the literature to suggest that interactions between chondrocytes, osteoblasts, and osteoclasts are important in the regulation of bone metabolism. In terms of molecular signals that contribute to the interactions between the various bone cell types, IGF-I and efn–Eph signaling have received considerable attention since studies using various knockout mouse models and in vitro cell culture systems demonstrate that these signaling pathways are critical in bone and are required for stimulation of bone formation by a number of key anabolic agents including PTH and mechanical strain. Therefore, it is likely that IGF-I and efn–Eph signaling pathways interact to regulate bone metabolism during normal and disease states. Accordingly, recent studies provide evidence that some of the actions of IGF-I in the skeleton are mediated via regulation of efn–Eph signaling as described below.
Recent studies support a role for IGF-I interactions with efn–Eph signaling in postnatal skeletal growth. Global Epha4 knockout mice displayed a dramatic general reduction in body size that was gene dose-dependent in heterozygotes. Bone growth was affected; there was a reduction in the length of the epiphyseal growth plates of the long bones consistent with growth reduction and not achondroplasia. This phenotype was accompanied by low plasma IGF-I levels and markedly reduced Igf1 mRNA in the liver and other tissues. Since GH and GHR levels were unchanged, the reduction in IGF-I production was not due to decreased GH signal. Rather, EphA4 forms a complex with the GHR and downstream GH effectors JAK2 and STAT5B to enhance IGF-I production in response to GH. This effect was largely JAK2-dependent, although some direct effect on STAT5B was observed. Thus, in the absence of EphA4, decreased production of IGF-I resulted in a small body size (Jing et al. 2012).
Further molecular studies characterized the binding of JAK2 and EphA4 to the GHR, the phosphorylation status of EphA4, GHR, JAK2, and STAT5B resulting from their mutual interactions, and the subsequent nuclear translocation of STAT5B. While GH canonically activates STAT5B via the GHR and JAK2, efn–EphA4 signaling was found to induce STAT5B activation independent of JAK2. The GHR was required for this JAK2-independent activation of STAT5B, although its precise role remains to be elucidated (Sawada et al. 2017). These studies reveal a global role for EphA4 functions in the body, in contrast to tissue-specific knockout models that demonstrate its regulation of osteoclast functions.
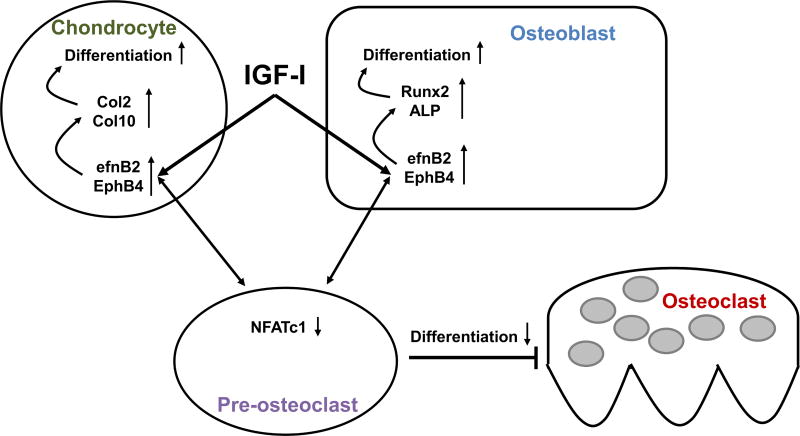
IGF-I is an important regulator of endochondral bone formation, and its functions have been demonstrated to be mediated by efnB2–EphB4 signaling (Wang et al. 2014). In vivo, the normal expression was reduced in Igf1 knockout mice and in the growth plates of Col2- or Osx-specific Igf1r knockout mice (Wang et al. 2015). In vitro, inhibition of efnB2 by deletion or EphB4 expression with a specific receptor inhibitor reduced the expression of osteogenic markers in response to IGF-I. In vitro blocking of EphB2 and EphB4 interaction through specific inhibition of efnB2 or EphB4 also reduced the development of osteoclasts and osteoblasts, respectively, in response to IGF-I (Fig. 3). With respect to chondrocytes, in vitro inhibition of the efnB2–EphB4 interaction reduced the expression of markers of chondrocyte and osteoclast development in response to IGF-I. These results provide strong evidence for efnB2–EphB4 functions in cell–cell contact for IGF-I-induced bone development and for intermittent PTH-induced bone development, as PTH regulates IGF-I functions.
Figure 3. A model of the interaction of IGF-I and efn–Eph signaling in skeletal metabolism.
IGF-I can act directly on chondrocytes and osteoblasts, increasing efnB2–EphB4 signaling and leading to differentiation of each cell type by increasing expression of key transcription factors. Through reverse signaling, efnB2–EphB4 interaction can decrease NFATc1 expression in osteoclast precursors and inhibit formation of differentiated, mature multinucleated osteoclasts (Zhao et al. 2006; Kwan Tat et al. 2009; Wang et al. 2015).
There is also now evidence in the literature that IGF-I can interact with efn–Eph signaling to regulate cellular processes. For example, IGF-I has been shown to regulate efnB1 functions following tooth injury. Tooth injury in mice with dentin matrix protein (dmp)-1-driven deletion of Igf1r demonstrate reduced efnb1 expression and impaired tooth repair. In response to the dental pulp capping procedure, Igf1 expression was increased. However, specific inhibition of IGF-IR signaling pathways revealed that efnb1 and Ephb2 expression was mediated by different pathways. EfnB1 and EphB2 signaling therefore mediate IGF-I functions during tooth repair (Matsumura et al. 2017).
Skeletal myogenesis requires the activation of the PI3K/Akt cascade but is inhibited by the ERK1/2 cascade. IGF-I induces myogenesis; however, IGF-I is known to activate both the PI3K/Akt and ERK1/2 cascades. EfnA–EphA signaling downregulates the Ras/ERK1/2 pathway. In vitro activation of efnA1 enhanced the ability of IGF-I to increase myogenic differentiation, an effect which was dependent upon the ability of EphA to decrease ERK1/2 signaling. Furthermore, the ability of IGF-I to induce myogenesis was inhibited by downregulation of efnA–EphA, an effect which was rescued by inactivation of ERK1/2. EfnA–EphA-mediated suppression of ERK1/2 is thus required for IGF-I to induce myogenesis (Minami et al. 2011).
Studies of protein associations also have benefited from the development of databases that predict interactions between proteins. Significant changes in gene expression obtained from microarray data can be merged with protein–protein interaction (PPI) databases to identify proteins that might interact to regulate the condition. By merging miRNA microarray data and PPI predictions, IGF-I and EphA4 proteins also were identified within two separate subnetworks of interacting proteins in OA (Wang et al. 2013). Although a functional connection between IGF-I and EphA4 was not investigated, their presence in the OA PPI network suggests an interaction between them is possible and that such a database approach might be valuable in identifying candidate proteins that could interact with large and widely expressed families of genes, such as the efns and the Ephs.
Recent investigations have only begun to characterize the interactions of IGF-I, efns, and Ephs in the skeleton. Given the importance of IGF-I as a growth factor in several tissues and the widespread expression and importance of the efns and Ephs in tissue development, it is probable the efn and Eph interactions with IGF-I, as well as other growth factors, are extensive and remain to be elucidated.
5. Summary
The IGF-I and efn–Eph signaling pathways are both critical regulators of bone cell function and skeletal development and maintenance. Many different signals and factors regulate IGF-I signaling at the systemic and local levels, making it a point of integration for several different pathways which induce bone growth and maintenance. Efn–Eph signaling is a complex system which, particularly through cell–cell interactions, contributes to the development and differentiation of many bone cell types. Thus, the fact that recent studies have established the interaction between these two signaling pathways opens a vast array of new opportunities for investigation into the mechanisms of and potential therapies for skeletal conditions such as osteoporosis and fracture repair.
6. Future Research Directions
While the role of IGF-I signaling in bone has been studied in great detail, the role of IGF-II in human bone metabolism remains to be elucidated. In rodents, IGF-II is primarily a fetal growth factor, and its expression declines during postnatal growth while IGF-I expression increases. In contrast, IGF-II is expressed at higher levels in adult human tissues including bone. The question of whether IGF-II participates significantly in adult bone metabolism and osteoporosis pathogenesis thus remains to be established. Furthermore, while recent evidence suggests a role for IGF-I in age-related bone loss and osteoporosis, the extent of its contribution and usefulness as an anabolic therapy for osteoporosis deserve further consideration and study. Additionally, work remains to be done in elucidating the particular roles of the various efns and Ephs in each of the bone cell types and their contributions to the regulation of skeletal growth. The precise roles of efn–Eph and IGF-I signaling in fracture repair also remain to be uncovered. Finally, the mechanisms by which IGF-I and efn–Eph signaling interact to regulate skeletal metabolism and growth must be determined with an emphasis towards developing novel strategies for treatment of osteoporosis and bone repair.
Acknowledgments
Funding
SM is a recipient of a Senior Research Career Scientist Award from the US Department of Veterans Affairs. This work was supported by funding from a National Institute of Arthritis and Musculoskeletal and Skin Diseases R01 grant (AR048139) to SM, a Veterans Administration BLR&D merit review grant (101-BX-002519) to CHR, and the National Institutes of Health IMSD grant to the LLU Center for Health Disparities and Molecular Medicine (2 R25 GM060507).
Footnotes
Declaration of Interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Allan EH, Häusler KD, Wei T, Gooi JH, Quinn JMW, Crimeen-Irwin B, Pompolo S, Sims NA, Gillespie MT, Onyia JE, et al. EphrinB2 regulation by PTH and PTHrP revealed by molecular profiling in differentiating osteoblasts. Journal of Bone and Mineral Research. 2008;23:1170–1181. doi: 10.1359/jbmr.080324. [DOI] [PubMed] [Google Scholar]
- Ammann P, Bourrin S, Bonjour JP, Meyer JM, Rizzoli R. Protein undernutrition-induced bone loss is associated with decreased IGF-I levels and estrogen deficiency. Journal of Bone and Mineral Research. 2000;15:683–690. doi: 10.1359/jbmr.2000.15.4.683. [DOI] [PubMed] [Google Scholar]
- Arthur A, Zannettino A, Panagopoulos R, Koblar SA, Sims NA, Stylianou C, Matsuo K, Gronthos S. EphB/ephrin-B interactions mediate human MSC attachment, migration and osteochondral differentiation. Bone. 2011;48:533–542. doi: 10.1016/j.bone.2010.10.180. [DOI] [PubMed] [Google Scholar]
- Arthur A, Panagopoulos RA, Cooper L, Menicanin D, Parkinson IH, Codrington JD, Vandyke K, Zannettino ACW, Koblar SA, Sims NA, et al. EphB4 enhances the process of endochondral ossification and inhibits remodeling during bone fracture repair. Journal of Bone and Mineral Research. 2013;28:926–935. doi: 10.1002/jbmr.1821. [DOI] [PubMed] [Google Scholar]
- Atapattu L, Lackmann M, Janes PW. The role of proteases in regulating Eph/ephrin signaling. Cell Adhesion & Migration. 2014;8:294–307. doi: 10.4161/19336918.2014.970026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagi CM, Brommage R, Deleon L, Adams S, Rosen D, Sommer A. Benefit of systemically administered rhIGF-I and rhIGF-I/IGFBP-3 on cancellous bone in ovariectomized rats. Journal of Bone and Mineral Research. 1994;9:1301–1312. doi: 10.1002/jbmr.5650090820. [DOI] [PubMed] [Google Scholar]
- Benbassat CA, Maki KC, Unterman TG. Circulating levels of insulin-like growth factor (IGF) binding protein-1 and-3 in aging men: relationships to insulin, glucose, IGF, and dehydroepiandrosterone sulfate levels and anthropometric measures. The Journal of Clinical Endocrinology and Metabolism. 1997;82:1484–1491. doi: 10.1210/jcem.82.5.3930. [DOI] [PubMed] [Google Scholar]
- Benson MD, Opperman LA, Westerlund J, Fernandez CR, San Miguel S, Henkemeyer M, Chenaux G. Ephrin-B stimulation of calvarial bone formation. Developmental Dynamics. 2012;241:1901–1910. doi: 10.1002/dvdy.23874. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Sakata T, Leary C, Elalieh H, Ginzinger D, Rosen CJ, Beamer W, Majumdar S, Halloran BP. Insulin-like growth factor I is required for the anabolic actions of parathyroid hormone on mouse bone. Journal of Bone and Mineral Research. 2002;17:1570–1578. doi: 10.1359/jbmr.2002.17.9.1570. [DOI] [PubMed] [Google Scholar]
- Bonjour J-P. The dietary protein, IGF-I, skeletal health axis. Hormone Molecular Biology and Clinical Investigation. 2016;28:39–53. doi: 10.1515/hmbci-2016-0003. [DOI] [PubMed] [Google Scholar]
- Bonjour J-P, Chevalley T, Rizzoli R, Ferrari S. Gene-environment interactions in the skeletal response to nutrition and exercise during growth. Medicine and Sport Science. 2007;51:64–80. doi: 10.1159/000103005. [DOI] [PubMed] [Google Scholar]
- Boonen S, Mohan S, Dequeker J, Aerssens J, Vanderschueren D, Verbeke G, Broos P, Bouillon R, Baylink DJ. Down-regulation of the serum stimulatory components of the insulin-like growth factor (IGF) system (IGF-I, IGF-II, IGF binding protein [BP]-3, and IGFBP-5) in age-related (type II) femoral neck osteoporosis. Journal of Bone and Mineral Research. 1999;14:2150–2158. doi: 10.1359/jbmr.1999.14.12.2150. [DOI] [PubMed] [Google Scholar]
- Bourrin S, Ammann P, Bonjour JP, Rizzoli R. Dietary protein restriction lowers plasma insulin-like growth factor I (IGF-I), impairs cortical bone formation, and induces osteoblastic resistance to IGF-I in adult female rats. Endocrinology. 2000;141:3149–3155. doi: 10.1210/endo.141.9.7633. [DOI] [PubMed] [Google Scholar]
- Brantley-Sieders DM, Chen J. Eph receptor tyrosine kinases in angiogenesis: from development to disease. Angiogenesis. 2004;7:17–28. doi: 10.1023/B:AGEN.0000037340.33788.87. [DOI] [PubMed] [Google Scholar]
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. Journal of Bone and Mineral Research. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- Campbell TN, Robbins SM. The Eph receptor/ephrin system: an emerging player in the invasion game. Current Issues in Molecular Biology. 2008;10:61–66. [PubMed] [Google Scholar]
- Canalis E. Mechanisms of glucocorticoid action in bone. Current Osteoporosis Reports. 2005;3:98–102. doi: 10.1007/s11914-005-0017-7. [DOI] [PubMed] [Google Scholar]
- Cayuso J, Xu Q, Wilkinson DG. Mechanisms of boundary formation by Eph receptor and ephrin signaling. Developmental Biology. 2015;401:122–131. doi: 10.1016/j.ydbio.2014.11.013. [DOI] [PubMed] [Google Scholar]
- Centrella M, McCarthy TL, Canalis E. Receptors for insulin-like growth factors-I and -II in osteoblast-enriched cultures from fetal rat bone. Endocrinology. 1990;126:39–44. doi: 10.1210/endo-126-1-39. [DOI] [PubMed] [Google Scholar]
- Chen TL, Chang LY, Bates RL, Perlman AJ. Dexamethasone and 1,25-dihydroxyvitamin D3 modulation of insulin-like growth factor-binding proteins in rat osteoblast-like cell cultures. Endocrinology. 1991;128:73–80. doi: 10.1210/endo-128-1-73. [DOI] [PubMed] [Google Scholar]
- Chen C-Y, Tseng K-Y, Lai Y-L, Chen Y-S, Lin F-H, Lin S. Overexpression of Insulin-Like Growth Factor 1 Enhanced the Osteogenic Capability of Aging Bone Marrow Mesenchymal Stem Cells. Theranostics. 2017;7:1598–1611. doi: 10.7150/thno.16637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Zhao SL, Nelson B, Kesavan C, Qin X, Wergedal J, Mohan S, Xing W. Targeted disruption of ephrin B1 in cells of myeloid lineage increases osteoclast differentiation and bone resorption in mice. PloS One. 2012;7:e32887. doi: 10.1371/journal.pone.0032887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Kesavan C, Mohan S, Qin X, Alarcon CM, Wergedal J, Xing W. Transgenic overexpression of ephrin b1 in bone cells promotes bone formation and an anabolic response to mechanical loading in mice. PloS One. 2013;8:e69051. doi: 10.1371/journal.pone.0069051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Xing W, Pourteymoor S, Mohan S. Effects of Thyroxine (T4), 3,5,3’-triiodo-L-thyronine (T3) and their Metabolites on Osteoblast Differentiation. Calcified Tissue International. 2016;99:435–442. doi: 10.1007/s00223-016-0159-x. [DOI] [PubMed] [Google Scholar]
- Chevalley T, Strong DD, Mohan S, Baylink D, Linkhart TA. Evidence for a role for insulin-like growth factor binding proteins in glucocorticoid inhibition of normal human osteoblast-like cell proliferation. European Journal of Endocrinology. 1996;134:591–601. doi: 10.1530/eje.0.1340591. [DOI] [PubMed] [Google Scholar]
- Chevalley T, Bonjour J-P, Ferrari S, Rizzoli R. High-protein intake enhances the positive impact of physical activity on BMC in prepubertal boys. Journal of Bone and Mineral Research. 2008;23:131–142. doi: 10.1359/jbmr.070907. [DOI] [PubMed] [Google Scholar]
- Christians JK, de Zwaan DR, Fung SHY. Pregnancy associated plasma protein A2 (PAPP-A2) affects bone size and shape and contributes to natural variation in postnatal growth in mice. PloS One. 2013;8:e56260. doi: 10.1371/journal.pone.0056260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoforidis A, Maniadaki I, Stanhope R. Growth hormone / insulin-like growth factor-1 axis during puberty. Pediatric Endocrinology Reviews. 2005;3:5–10. [PubMed] [Google Scholar]
- Compagni A, Logan M, Klein R, Adams RH. Control of skeletal patterning by ephrinB1-EphB interactions. Developmental Cell. 2003;5:217–230. doi: 10.1016/s1534-5807(03)00198-9. [DOI] [PubMed] [Google Scholar]
- Conover CA. Insulin-like growth factor binding protein proteolysis in bone cell models. Progress in Growth Factor Research. 1995;6:301–309. doi: 10.1016/0955-2235(95)00032-1. [DOI] [PubMed] [Google Scholar]
- Corpas E, Harman SM, Blackman MR. Human growth hormone and human aging. Endocrine Reviews. 1993;14:20–39. doi: 10.1210/edrv-14-1-20. [DOI] [PubMed] [Google Scholar]
- Coulthard MG, Morgan M, Woodruff TM, Arumugam TV, Taylor SM, Carpenter TC, Lackmann M, Boyd AW. Eph/Ephrin signaling in injury and inflammation. The American Journal of Pathology. 2012;181:1493–1503. doi: 10.1016/j.ajpath.2012.06.043. [DOI] [PubMed] [Google Scholar]
- Cowan CA, Henkemeyer M. The SH2/SH3 adaptor Grb4 transduces B-ephrin reverse signals. Nature. 2001;413:174–179. doi: 10.1038/35093123. [DOI] [PubMed] [Google Scholar]
- Davy A, Soriano P. Ephrin-B2 forward signaling regulates somite patterning and neural crest cell development. Developmental Biology. 2007;304:182–193. doi: 10.1016/j.ydbio.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy A, Gale NW, Murray EW, Klinghoffer RA, Soriano P, Feuerstein C, Robbins SM. Compartmentalized signaling by GPI-anchored ephrin-A5 requires the Fyn tyrosine kinase to regulate cellular adhesion. Genes & Development. 1999;13:3125–3135. doi: 10.1101/gad.13.23.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy A, Aubin J, Soriano P. Ephrin-B1 forward and reverse signaling are required during mouse development. Genes & Development. 2004;18:572–583. doi: 10.1101/gad.1171704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy A, Bush JO, Soriano P. Inhibition of gap junction communication at ectopic Eph/ephrin boundaries underlies craniofrontonasal syndrome. PLoS Biology. 2006;4:e315. doi: 10.1371/journal.pbio.0040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson-Hughes B, Harris SS, Rasmussen H, Song L, Dallal GE. Effect of dietary protein supplements on calcium excretion in healthy older men and women. The Journal of Clinical Endocrinology and Metabolism. 2004;89:1169–1173. doi: 10.1210/jc.2003-031466. [DOI] [PubMed] [Google Scholar]
- DeMambro VE, Le PT, Guntur AR, Maridas DE, Canalis E, Nagano K, Baron R, Clemmons DR, Rosen CJ. Igfbp2 deletion in ovariectomized mice enhances energy expenditure but accelerates bone loss. Endocrinology. 2015 doi: 10.1210/en.2014-1452. en20141452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denger S, Bähr-Ivacevic T, Brand H, Reid G, Blake J, Seifert M, Lin C-Y, May K, Benes V, Liu ET, et al. Transcriptome profiling of estrogen-regulated genes in human primary osteoblasts reveals an osteoblast-specific regulation of the insulin-like growth factor binding protein 4 gene. Molecular Endocrinology. 2008;22:361–379. doi: 10.1210/me.2007-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin RD, Du Z, Buccilli V, Jorgetti V, Canalis E. Transgenic mice overexpressing insulin-like growth factor binding protein-5 display transiently decreased osteoblastic function and osteopenia. Endocrinology. 2002;143:3955–3962. doi: 10.1210/en.2002-220129. [DOI] [PubMed] [Google Scholar]
- Edwards CM, Mundy GR. Eph receptors and ephrin signaling pathways: a role in bone homeostasis. International Journal of Medical Sciences. 2008;5:263–272. doi: 10.7150/ijms.5.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Menon RK, Cohen P, Hwang D, Clemens T, DiGirolamo DJ, Kopchick JJ, Le Roith D, Trucco M, Sperling MA. Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. The Journal of Biological Chemistry. 2009;284:19937–19944. doi: 10.1074/jbc.M109.014308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbitas B, Canalis E. Retinoic acid stimulates the transcription of insulin-like growth factor binding protein-6 in skeletal cells. Journal of Cellular Physiology. 1996;169:15–22. doi: 10.1002/(SICI)1097-4652(199610)169:1<15::AID-JCP2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Gaffney-Stomberg E, Insogna KL, Rodriguez NR, Kerstetter JE. Increasing dietary protein requirements in elderly people for optimal muscle and bone health. Journal of the American Geriatrics Society. 2009;57:1073–1079. doi: 10.1111/j.1532-5415.2009.02285.x. [DOI] [PubMed] [Google Scholar]
- Gillberg P, Johansson AG, Blum WF, Groth T, Ljunghall S. Growth hormone secretion and sensitivity in men with idiopathic osteoporosis. Calcified Tissue International. 2001;68:67–73. doi: 10.1007/BF02678143. [DOI] [PubMed] [Google Scholar]
- Gillberg P, Olofsson H, Mallmin H, Blum WF, Ljunghall S, Nilsson AG. Bone mineral density in femoral neck is positively correlated to circulating insulin-like growth factor (IGF)-I and IGF-binding protein (IGFBP)-3 in Swedish men. Calcified Tissue International. 2002;70:22–29. doi: 10.1007/s002230020048. [DOI] [PubMed] [Google Scholar]
- Govoni KE, Baylink DJ, Mohan S. The multi-functional role of insulin-like growth factor binding proteins in bone. Pediatr Nephrol. 2005;20:261–268. doi: 10.1007/s00467-004-1658-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govoni KE, Wergedal JE, Florin L, Angel P, Baylink DJ, Mohan S. Conditional deletion of insulin-like growth factor-I in collagen type 1alpha2-expressing cells results in postnatal lethality and a dramatic reduction in bone accretion. Endocrinology. 2007a;148:5706–5715. doi: 10.1210/en.2007-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govoni KE, Lee SK, Chung YS, Behringer RR, Wergedal JE, Baylink DJ, Mohan S. Disruption of insulin-like growth factor-I expression in type IIalphaI collagen-expressing cells reduces bone length and width in mice. Physiol Genomics. 2007b;30:354–362. doi: 10.1152/physiolgenomics.00022.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremion G, Rizzoli R, Slosman D, Theintz G, Bonjour JP. Oligo-amenorrheic long-distance runners may lose more bone in spine than in femur. Medicine and Science in Sports and Exercise. 2001;33:15–21. doi: 10.1097/00005768-200101000-00004. [DOI] [PubMed] [Google Scholar]
- Grinspoon S, Thomas E, Pitts S, Gross E, Mickley D, Miller K, Herzog D, Klibanski A. Prevalence and predictive factors for regional osteopenia in women with anorexia nervosa. Annals of Internal Medicine. 2000;133:790–794. doi: 10.7326/0003-4819-133-10-200011210-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross TS, Srinivasan S, Liu CC, Clemens TL, Bain SD. Noninvasive loading of the murine tibia: an in vivo model for the study of mechanotransduction. Journal of Bone and Mineral Research. 2002;17:493–501. doi: 10.1359/jbmr.2002.17.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden JM, Strong DD, Baylink DJ, Powell DR, Sampath TK, Mohan S. Osteogenic protein-1 stimulates production of insulin-like growth factor binding protein-3 nuclear transcripts in human osteosarcoma cells. Endocrinology. 1997;138:4240–4247. doi: 10.1210/endo.138.10.5457. [DOI] [PubMed] [Google Scholar]
- Holmberg J, Clarke DL, Frisén J. Regulation of repulsion versus adhesion by different splice forms of an Eph receptor. Nature. 2000;408:203–206. doi: 10.1038/35041577. [DOI] [PubMed] [Google Scholar]
- Honda Y, Landale EC, Strong DD, Baylink DJ, Mohan S. Recombinant synthesis of insulin-like growth factor-binding protein-4 (IGFBP-4): Development, validation, and application of a radioimmunoassay for IGFBP-4 in human serum and other biological fluids. The Journal of Clinical Endocrinology and Metabolism. 1996;81:1389–1396. doi: 10.1210/jcem.81.4.8636339. [DOI] [PubMed] [Google Scholar]
- Irie N, Takada Y, Watanabe Y, Matsuzaki Y, Naruse C, Asano M, Iwakura Y, Suda T, Matsuo K. Bidirectional signaling through ephrinA2-EphA2 enhances osteoclastogenesis and suppresses osteoblastogenesis. The Journal of Biological Chemistry. 2009;284:14637–14644. doi: 10.1074/jbc.M807598200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes PW, Griesshaber B, Atapattu L, Nievergall E, Hii LL, Mensinga A, Chheang C, Day BW, Boyd AW, Bastiaens PI, et al. Eph receptor function is modulated by heterooligomerization of A and B type Eph receptors. The Journal of Cell Biology. 2011;195:1033–1045. doi: 10.1083/jcb.201104037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes PW, Nievergall E, Lackmann M. Concepts and consequences of Eph receptor clustering. Seminars in Cell & Developmental Biology. 2012;23:43–50. doi: 10.1016/j.semcdb.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Jiang J, Lichtler AC, Gronowicz GA, Adams DJ, Clark SH, Rosen CJ, Kream BE. Transgenic mice with osteoblast-targeted insulin-like growth factor-I show increased bone remodeling. Bone. 2006;39:494–504. doi: 10.1016/j.bone.2006.02.068. [DOI] [PubMed] [Google Scholar]
- Jing X, Miyajima M, Sawada T, Chen Q, Iida K, Furushima K, Arai D, Chihara K, Sakaguchi K. Crosstalk of humoral and cell-cell contact-mediated signals in postnatal body growth. Cell Reports. 2012;2:652–665. doi: 10.1016/j.celrep.2012.08.021. [DOI] [PubMed] [Google Scholar]
- Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocrine Reviews. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- Jurek A, Genander M, Kundu P, Catchpole T, He X, Strååt K, Sabelström H, Xu N-J, Pettersson S, Henkemeyer M, et al. Eph receptor interclass cooperation is required for the regulation of cell proliferation. Experimental Cell Research. 2016;348:10–22. doi: 10.1016/j.yexcr.2016.08.017. [DOI] [PubMed] [Google Scholar]
- Kanzaki S, Hilliker S, Baylink DJ, Mohan S. Evidence that human bone cells in culture produce insulin-like growth factor-binding protein-4 and-5 proteases. Endocrinology. 1994;134:383–392. doi: 10.1210/endo.134.1.7506211. [DOI] [PubMed] [Google Scholar]
- Kapur S, Mohan S, Baylink DJ, Lau K-HW. Fluid shear stress synergizes with insulin-like growth factor-I (IGF-I) on osteoblast proliferation through integrin-dependent activation of IGF-I mitogenic signaling pathway. The Journal of Biological Chemistry. 2005;280:20163–20170. doi: 10.1074/jbc.M501460200. [DOI] [PubMed] [Google Scholar]
- Kassem M, Brixen K, Blum W, Mosekilde L, Eriksen EF. No evidence for reduced spontaneous or growth-hormone-stimulated serum levels of insulin-like growth factor (IGF)-I, IGF-II or IGF binding protein 3 in women with spinal osteoporosis. European Journal of Endocrinology. 1994;131:150–155. doi: 10.1530/eje.0.1310150. [DOI] [PubMed] [Google Scholar]
- Kesavan C, Wergedal JE, Lau K-HW, Mohan S. Conditional disruption of IGF-I gene in type 1α collagen-expressing cells shows an essential role of IGF-I in skeletal anabolic response to loading. American Journal of Physiology. Endocrinology and Metabolism. 2011;301:E1191–1197. doi: 10.1152/ajpendo.00440.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutsen R, Honda Y, Strong DD, Sampath TK, Baylink DJ, Mohan S. Regulation of insulin-like growth factor system components by osteogenic protein-1 in human bone cells. Endocrinology. 1995;136:857–865. doi: 10.1210/endo.136.3.7532581. [DOI] [PubMed] [Google Scholar]
- Kumar SR, Singh J, Xia G, Krasnoperov V, Hassanieh L, Ley EJ, Scehnet J, Kumar NG, Hawes D, Press MF, et al. Receptor tyrosine kinase EphB4 is a survival factor in breast cancer. The American Journal of Pathology. 2006;169:279–293. doi: 10.2353/ajpath.2006.050889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kveiborg M, Flyvbjerg A, Eriksen EF, Kassem M. 1,25-Dihydroxyvitamin D3 stimulates the production of insulin-like growth factor-binding proteins-2, -3 and -4 in human bone marrow stromal cells. European Journal of Endocrinology. 2001;144:549–557. doi: 10.1530/eje.0.1440549. [DOI] [PubMed] [Google Scholar]
- Kwak H, Salvucci O, Weigert R, Martinez-Torrecuadrada JL, Henkemeyer M, Poulos MG, Butler JM, Tosato G. Sinusoidal ephrin receptor EPHB4 controls hematopoietic progenitor cell mobilization from bone marrow. The Journal of Clinical Investigation. 2016;126:4554–4568. doi: 10.1172/JCI87848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan Tat S, Pelletier J-P, Amiable N, Boileau C, Lavigne M, Martel-Pelletier J. Treatment with ephrin B2 positively impacts the abnormal metabolism of human osteoarthritic chondrocytes. Arthritis Research & Therapy. 2009;11:R119. doi: 10.1186/ar2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau K-HW, Kapur S, Kesavan C, Baylink DJ. Up-regulation of the Wnt, estrogen receptor, insulin-like growth factor-I, and bone morphogenetic protein pathways in C57BL/6J osteoblasts as opposed to C3H/HeJ osteoblasts in part contributes to the differential anabolic response to fluid shear. The Journal of Biological Chemistry. 2006;281:9576–9588. doi: 10.1074/jbc.M509205200. [DOI] [PubMed] [Google Scholar]
- Lau K-HW, Baylink DJ, Zhou X-D, Rodriguez D, Bonewald LF, Li Z, Ruffoni D, Müller R, Kesavan C, Sheng MH-C. Osteocyte-derived insulin-like growth factor I is essential for determining bone mechanosensitivity. American Journal of Physiology. Endocrinology and Metabolism. 2013;305:E271–281. doi: 10.1152/ajpendo.00092.2013. [DOI] [PubMed] [Google Scholar]
- Lean JM, Jagger CJ, Chambers TJ, Chow JW. Increased insulin-like growth factor I mRNA expression in rat osteocytes in response to mechanical stimulation. The American Journal of Physiology. 1995;268:E318–327. doi: 10.1152/ajpendo.1995.268.2.E318. [DOI] [PubMed] [Google Scholar]
- Lindsey RC, Mohan S. Skeletal Effects of Growth Hormone and Insulin-like Growth Factor-I Therapy. Molecular and Cellular Endocrinology. 2015 doi: 10.1016/j.mce.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkhart TA, Mohan S. Parathyroid hormone stimulates release of insulin-like growth factor-I (IGF-I) and IGF-II from neonatal mouse calvaria in organ culture. Endocrinology. 1989;125:1484–1491. doi: 10.1210/endo-125-3-1484. [DOI] [PubMed] [Google Scholar]
- List EO, Berryman DE, Funk K, Jara A, Kelder B, Wang F, Stout MB, Zhi X, Sun L, White TA, et al. Liver-specific GH receptor gene-disrupted (LiGHRKO) mice have decreased endocrine IGF-I, increased local IGF-I, and altered body size, body composition, and adipokine profiles. Endocrinology. 2014;155:1793–1805. doi: 10.1210/en.2013-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Mohan S, Yakar S. Does the GH/IGF-1 axis contribute to skeletal sexual dimorphism? Evidence from mouse studies. Growth Hormone & IGF Research. 2016;27:7–17. doi: 10.1016/j.ghir.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhou L, Yang X, Liu Q, Yang L, Zheng C, Zhao Y, Zhang Z, Luo X. 17β-estradiol attenuates ovariectomy-induced bone deterioration through the suppression of the ephA2/ephrinA2 signaling pathway. Molecular Medicine Reports. 2017 doi: 10.3892/mmr.2017.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locatelli V, Bianchi VE. Effect of GH/IGF-1 on Bone Metabolism and Osteoporsosis. International Journal of Endocrinology. 2014;2014 doi: 10.1155/2014/235060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin H, Sääf M, Strender L-E, Nyren S, Johansson S-E, Salminen H. High Serum Insulin-Like Growth Factor-Binding Protein 1 (IGFBP-1) is Associated with High Fracture Risk Independent of Insulin-Like Growth Factor 1 (IGF-I) Calcified Tissue International. 2016;99:333–339. doi: 10.1007/s00223-016-0152-4. [DOI] [PubMed] [Google Scholar]
- Malpe R, Baylink DJ, Linkhart TA, Wergedal JE, Mohan S. Insulin-like growth factor (IGF)-I, -II, IGF binding proteins (IGFBP)-3, -4, and -5 levels in the conditioned media of normal human bone cells are skeletal site-dependent. Journal of Bone and Mineral Research. 1997;12:423–430. doi: 10.1359/jbmr.1997.12.3.423. [DOI] [PubMed] [Google Scholar]
- Martini G, Valenti R, Giovani S, Franci B, Campagna S, Nuti R. Influence of insulin-like growth factor-1 and leptin on bone mass in healthy postmenopausal women. Bone. 2001;28:113–117. doi: 10.1016/s8756-3282(00)00408-7. [DOI] [PubMed] [Google Scholar]
- Matsumura S, Quispe-Salcedo A, Schiller CM, Shin JS, Locke BM, Yakar S, Shimizu E. IGF-1 Mediates EphrinB1 Activation in Regulating Tertiary Dentin Formation. Journal of Dental Research. 2017;96:1153–1161. doi: 10.1177/0022034517708572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K, Otaki N. Bone cell interactions through Eph/ephrin: bone modeling, remodeling and associated diseases. Cell Adhesion & Migration. 2012;6:148–156. doi: 10.4161/cam.20888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy TL, Centrella M. Local IGF-I expression and bone formation. Growth Hormone & IGF Research. 2001;11:213–219. doi: 10.1054/ghir.2001.0236. [DOI] [PubMed] [Google Scholar]
- McCarthy TL, Centrella M, Canalis E. Parathyroid hormone enhances the transcript and polypeptide levels of insulin-like growth factor I in osteoblast-enriched cultures from fetal rat bone. Endocrinology. 1989;124:1247–1253. doi: 10.1210/endo-124-3-1247. [DOI] [PubMed] [Google Scholar]
- Minami M, Koyama T, Wakayama Y, Fukuhara S, Mochizuki N. EphrinA/EphA signal facilitates insulin-like growth factor-I-induced myogenic differentiation through suppression of the Ras/extracellular signal-regulated kinase 1/2 cascade in myoblast cell lines. Molecular Biology of the Cell. 2011;22:3508–3519. doi: 10.1091/mbc.E11-03-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra M, Prabhakaran R, Miller KK, Goldstein MA, Mickley D, Clauss L, Lockhart P, Cord J, Herzog DB, Katzman DK, et al. Prognostic indicators of changes in bone density measures in adolescent girls with anorexia nervosa-II. The Journal of Clinical Endocrinology and Metabolism. 2008;93:1292–1297. doi: 10.1210/jc.2007-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakoshi N, Richman C, Qin X, Baylink DJ, Mohan S. Effects of recombinant insulin-like growth factor-binding protein-4 on bone formation parameters in mice. Endocrinology. 1999;140:5719–5728. doi: 10.1210/endo.140.12.7175. [DOI] [PubMed] [Google Scholar]
- Miyakoshi N, Kasukawa Y, Linkhart TA, Baylink DJ, Mohan S. Evidence that anabolic effects of PTH on bone require IGF-I in growing mice. Endocrinology. 2001a;142:4349–4356. doi: 10.1210/endo.142.10.8436. [DOI] [PubMed] [Google Scholar]
- Miyakoshi N, Richman C, Kasukawa Y, Linkhart TA, Baylink DJ, Mohan S. Evidence that IGF-binding protein-5 functions as a growth factor. J Clin Invest. 2001b;107:73–81. doi: 10.1172/jci10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan S, Thompson GR, Amaar YG, Hathaway G, Tschesche H, Baylink DJ. ADAM-9 is an insulin-like growth factor binding protein-5 protease produced and secreted by human osteoblasts. Biochemistry. 2002;41:15394–15403. doi: 10.1021/bi026458q. [DOI] [PubMed] [Google Scholar]
- Mohan S, Richman C, Guo R, Amaar Y, Donahue LR, Wergedal J, Baylink DJ. Insulin-like growth factor regulates peak bone mineral density in mice by both growth hormone-dependent and -independent mechanisms. Endocrinology. 2003;144:929–936. doi: 10.1210/en.2002-220948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller EE, Locatelli V, Cocchi D. Neuroendocrine control of growth hormone secretion. Physiological Reviews. 1999;79:511–607. doi: 10.1152/physrev.1999.79.2.511. [DOI] [PubMed] [Google Scholar]
- Muñoz-Torres M, Mezquita-Raya P, Lopez-Rodriguez F, Torres-Vela E, de Dios Luna J, Escobar-Jimenez F. The contribution of IGF-I to skeletal integrity in postmenopausal women. Clinical Endocrinology. 2001;55:759–766. doi: 10.1046/j.1365-2265.2001.01390.x. [DOI] [PubMed] [Google Scholar]
- Nguyen TM, Arthur A, Paton S, Hemming S, Panagopoulos R, Codrington J, Walkley CR, Zannettino ACW, Gronthos S. Loss of ephrinB1 in osteogenic progenitor cells impedes endochondral ossification and compromises bone strength integrity during skeletal development. Bone. 2016;93:12–21. doi: 10.1016/j.bone.2016.09.009. [DOI] [PubMed] [Google Scholar]
- Nordstrom SM, Tran JL, Sos BC, Wagner K-U, Weiss EJ. Liver-derived IGF-I contributes to GH-dependent increases in lean mass and bone mineral density in mice with comparable levels of circulating GH. Molecular Endocrinology. 2011;25:1223–1230. doi: 10.1210/me.2011-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunan R, Campbell J, Mori R, Pitulescu ME, Jiang WG, Harding KG, Adams RH, Nobes CD, Martin P. Ephrin-Bs Drive Junctional Downregulation and Actin Stress Fiber Disassembly to Enable Wound Re-epithelialization. Cell Reports. 2015;13:1380–1395. doi: 10.1016/j.celrep.2015.09.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson C, Mellström D, Carlzon D, Orwoll E, Ljunggren O, Karlsson MK, Vandenput L. Older men with low serum IGF-1 have an increased risk of incident fractures: the MrOS Sweden study. Journal of Bone and Mineral Research. 2011;26:865–872. doi: 10.1002/jbmr.281. [DOI] [PubMed] [Google Scholar]
- O’Neill AK, Kindberg AA, Niethamer TK, Larson AR, Ho H-YH, Greenberg ME, Bush JO. Unidirectional Eph/ephrin signaling creates a cortical actomyosin differential to drive cell segregation. The Journal of Cell Biology. 2016;215:217–229. doi: 10.1083/jcb.201604097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paccou J, Dewailly J, Cortet B. Reduced levels of serum IGF-1 is related to the presence of osteoporotic fractures in male idiopathic osteoporosis. Joint, Bone, Spine: Revue Du Rhumatisme. 2012;79:78–82. doi: 10.1016/j.jbspin.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Qin X, Wergedal JE, Rehage M, Tran K, Newton J, Lam P, Baylink DJ, Mohan S. Pregnancy-associated plasma protein-A increases osteoblast proliferation in vitro and bone formation in vivo. Endocrinology. 2006;147:5653–5661. doi: 10.1210/en.2006-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaram S, Baylink DJ, Mohan S. Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocrine Reviews. 1997;18:801–831. doi: 10.1210/edrv.18.6.0321. [DOI] [PubMed] [Google Scholar]
- Reijnders CMA, Bravenboer N, Tromp AM, Blankenstein MA, Lips P. Effect of mechanical loading on insulin-like growth factor-I gene expression in rat tibia. The Journal of Endocrinology. 2007;192:131–140. doi: 10.1677/joe.1.06880. [DOI] [PubMed] [Google Scholar]
- Richman C, Baylink DJ, Lang K, Dony C, Mohan S. Recombinant human insulin-like growth factor-binding protein-5 stimulates bone formation parameters in vitro and in vivo. Endocrinology. 1999;140:4699–4705. doi: 10.1210/endo.140.10.7081. [DOI] [PubMed] [Google Scholar]
- Risley M, Garrod D, Henkemeyer M, McLean W. EphB2 and EphB3 forward signalling are required for palate development. Mechanisms of Development. 2009;126:230–239. doi: 10.1016/j.mod.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Rosen CJ, Donahue LR, Hunter SJ. Insulin-like growth factors and bone: the osteoporosis connection. Proceedings of the Society for Experimental Biology and Medicine. 1994;206:83–102. doi: 10.3181/00379727-206-43726. [DOI] [PubMed] [Google Scholar]
- Russell JD, Mira M, Allen BJ, Stewart PM, Vizzard J, Arthur B, Beumont PJ. Protein repletion and treatment in anorexia nervosa. The American Journal of Clinical Nutrition. 1994;59:98–102. doi: 10.1093/ajcn/59.1.98. [DOI] [PubMed] [Google Scholar]
- Sakata T, Halloran BP, Elalieh HZ, Munson SJ, Rudner L, Venton L, Ginzinger D, Rosen CJ, Bikle DD. Skeletal unloading induces resistance to insulin-like growth factor I on bone formation. Bone. 2003;32:669–680. doi: 10.1016/s8756-3282(03)00088-7. [DOI] [PubMed] [Google Scholar]
- Sakata T, Wang Y, Halloran BP, Elalieh HZ, Cao J, Bikle DD. Skeletal unloading induces resistance to insulin-like growth factor-I (IGF-I) by inhibiting activation of the IGF-I signaling pathways. Journal of Bone and Mineral Research. 2004;19:436–446. doi: 10.1359/JBMR.0301241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada T, Jing X, Zhang Y, Shimada E, Yokote H, Miyajima M, Sakaguchi K. Ternary complex formation of EphA4, FGFR and FRS2α plays an important role in the proliferation of embryonic neural stem/progenitor cells. Genes to Cells. 2010;15:297–311. doi: 10.1111/j.1365-2443.2010.01391.x. [DOI] [PubMed] [Google Scholar]
- Sawada T, Arai D, Jing X, Miyajima M, Frank SJ, Sakaguchi K. Molecular interactions of EphA4, growth hormone receptor, Janus kinase 2, and signal transducer and activator of transcription 5B. PloS One. 2017;12:e0180785. doi: 10.1371/journal.pone.0180785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharla SH, Strong DD, Mohan S, Baylink DJ, Linkhart TA. 1,25-Dihydroxyvitamin D3 differentially regulates the production of insulin-like growth factor I (IGF-I) and IGF-binding protein-4 in mouse osteoblasts. Endocrinology. 1991;129:3139–3146. doi: 10.1210/endo-129-6-3139. [DOI] [PubMed] [Google Scholar]
- Schürch MA, Rizzoli R, Slosman D, Vadas L, Vergnaud P, Bonjour JP. Protein supplements increase serum insulin-like growth factor-I levels and attenuate proximal femur bone loss in patients with recent hip fracture. A randomized, double-blind, placebo-controlled trial. Annals of Internal Medicine. 1998;128:801–809. doi: 10.7326/0003-4819-128-10-199805150-00002. [DOI] [PubMed] [Google Scholar]
- Seck T, Bretz A, Krempien R, Krempien B, Ziegler R, Pfeilschifter J. Age-related changes in insulin-like growth factor I and II in human femoral cortical bone: lack of correlation with bone mass. Bone. 1999;24:387–393. doi: 10.1016/s8756-3282(98)00186-0. [DOI] [PubMed] [Google Scholar]
- Shimizu E, Tamasi J, Partridge NC. Alendronate affects osteoblast functions by crosstalk through EphrinB1-EphB. Journal of Dental Research. 2012;91:268–274. doi: 10.1177/0022034511432170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, LeRoith D, Törnell J, Isaksson OG, Jansson JO, et al. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7088–7092. doi: 10.1073/pnas.96.12.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slootweg MC, Hoogerbrugge CM, de Poorter TL, Duursma SA, van Buul-Offers SC. The presence of classical insulin-like growth factor (IGF) type-I and -II receptors on mouse osteoblasts: autocrine/paracrine growth effect of IGFs? The Journal of Endocrinology. 1990;125:271–277. doi: 10.1677/joe.0.1250271. [DOI] [PubMed] [Google Scholar]
- Stadler HS, Higgins KM, Capecchi MR. Loss of Eph-receptor expression correlates with loss of cell adhesion and chondrogenic capacity in Hoxa13 mutant limbs. Development. 2001;128:4177–4188. doi: 10.1242/dev.128.21.4177. [DOI] [PubMed] [Google Scholar]
- Stiffel V, Amoui M, Sheng MH-C, Mohan S, Lau K-HW. EphA4 receptor is a novel negative regulator of osteoclast activity. Journal of Bone and Mineral Research. 2014;29:804–819. doi: 10.1002/jbmr.2084. [DOI] [PubMed] [Google Scholar]
- Stratikopoulos E, Szabolcs M, Dragatsis I, Klinakis A, Efstratiadis A. The hormonal action of IGF1 in postnatal mouse growth. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19378–19383. doi: 10.1073/pnas.0809223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunters A, Armstrong VJ, Zaman G, Kypta RM, Kawano Y, Lanyon LE, Price JS. Mechano-transduction in osteoblastic cells involves strain-regulated estrogen receptor alpha-mediated control of insulin-like growth factor (IGF) I receptor sensitivity to Ambient IGF, leading to phosphatidylinositol 3-kinase/AKT-dependent Wnt/LRP5 receptor-independent activation of beta-catenin signaling. The Journal of Biological Chemistry. 2010;285:8743–8758. doi: 10.1074/jbc.M109.027086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takyar FM, Tonna S, Ho PWM, Crimeen-Irwin B, Baker EK, Martin TJ, Sims NA. EphrinB2/EphB4 inhibition in the osteoblast lineage modifies the anabolic response to parathyroid hormone. Journal of Bone and Mineral Research. 2013;28:912–925. doi: 10.1002/jbmr.1820. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Liang CT. Mitogenic activity but not phenotype expression of rat osteoprogenitor cells in response to IGF-I is impaired in aged rats. Mechanisms of Ageing and Development. 1996;92:1–10. doi: 10.1016/s0047-6374(96)01793-9. [DOI] [PubMed] [Google Scholar]
- Tanner SJ, Hefferan TE, Rosen CJ, Conover CA. Impact of pregnancy-associated plasma protein-a deletion on the adult murine skeleton. Journal of Bone and Mineral Research. 2008;23:655–662. doi: 10.1359/jbmr.071210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thissen J-P, Beauloye V, Ketelslegers J-M, Underwood LE. Regulation of insulin-like growth factor-I by nutrition. In: Houston MS, Holly JMP, Feldman EL, editors. IGF and Nutrition in Health and Disease. Totowa, NJ: Humana Press; 2004. pp. 25–52. [Google Scholar]
- Tian F, Wang Y, Bikle DD. IGF-1 signaling mediated cell-specific skeletal mechano-transduction. Journal of Orthopaedic Research. 2017 doi: 10.1002/jor.23767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting M-C, Wu NL, Roybal PG, Sun J, Liu L, Yen Y, Maxson RE. EphA4 as an effector of Twist1 in the guidance of osteogenic precursor cells during calvarial bone growth and in craniosynostosis. Development. 2009;136:855–864. doi: 10.1242/dev.028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonna S, Takyar FM, Vrahnas C, Crimeen-Irwin B, Ho PWM, Poulton IJ, Brennan HJ, McGregor NE, Allan EH, Nguyen H, et al. EphrinB2 signaling in osteoblasts promotes bone mineralization by preventing apoptosis. FASEB Journal. 2014;28:4482–4496. doi: 10.1096/fj.14-254300. [DOI] [PubMed] [Google Scholar]
- Tremollieres FA, Strong DD, Baylink DJ, Mohan S. Insulin-like growth factor II and transforming growth factor beta 1 regulate insulin-like growth factor I secretion in mouse bone cells. Acta Endocrinologica. 1991;125:538–546. doi: 10.1530/acta.0.1250538. [DOI] [PubMed] [Google Scholar]
- van Varsseveld NC, Sohl E, Drent ML, Lips P. Gender-Specific Associations of Serum Insulin-Like Growth Factor-1 With Bone Health and Fractures in Older Persons. The Journal of Clinical Endocrinology and Metabolism. 2015;100:4272–4281. doi: 10.1210/jc.2015-2549. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Frystyk J, Iranmanesh A, Ørskov H. Testosterone and estradiol regulate free insulin-like growth factor I (IGF-I), IGF binding protein 1 (IGFBP-1), and dimeric IGF-I/IGFBP-1 concentrations. The Journal of Clinical Endocrinology and Metabolism. 2005;90:2941–2947. doi: 10.1210/jc.2004-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cheng Z, Elalieh HZ, Nakamura E, Nguyen M-T, Mackem S, Clemens TL, Bikle DD, Chang W. IGF-1R signaling in chondrocytes modulates growth plate development by interacting with the PTHrP/Ihh pathway. Journal of Bone and Mineral Research. 2011;26:1437–1446. doi: 10.1002/jbmr.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Liu C, Zhang Y, Hao Y, Zhang X, Zhang YM. Protein interaction and microRNA network analysis in osteoarthritis meniscal cells. Genetics and Molecular Research. 2013;12:738–746. doi: 10.4238/2013.March.13.2. [DOI] [PubMed] [Google Scholar]
- Wang Y, Menendez A, Fong C, ElAlieh HZ, Chang W, Bikle DD. Ephrin B2/EphB4 mediates the actions of IGF-I signaling in regulating endochondral bone formation. Journal of Bone and Mineral Research. 2014;29:1900–1913. doi: 10.1002/jbmr.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Menendez A, Fong C, ElAlieh HZ, Kubota T, Long R, Bikle DD. IGF-I Signaling in Osterix-Expressing Cells Regulates Secondary Ossification Center Formation, Growth Plate Maturation, and Metaphyseal Formation during Postnatal Bone Development. Journal of Bone and Mineral Research. 2015 doi: 10.1002/jbmr.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Shan S, Wang C, Wang J, Li J, Hu G, Dai K, Li Q, Zhang X. Mechanical stimulation promote the osteogenic differentiation of bone marrow stromal cells through epigenetic regulation of Sonic Hedgehog. Experimental Cell Research. 2017;352:346–356. doi: 10.1016/j.yexcr.2017.02.021. [DOI] [PubMed] [Google Scholar]
- Warren MP, Brooks-Gunn J, Fox RP, Holderness CC, Hyle EP, Hamilton WG. Osteopenia in exercise-associated amenorrhea using ballet dancers as a model: a longitudinal study. The Journal of Clinical Endocrinology and Metabolism. 2002;87:3162–3168. doi: 10.1210/jcem.87.7.8637. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG. Regulation of cell differentiation by Eph receptor and ephrin signaling. Cell Adhesion & Migration. 2014;8:339–348. doi: 10.4161/19336918.2014.970007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing W, Baylink D, Kesavan C, Hu Y, Kapoor S, Chadwick RB, Mohan S. Global gene expression analysis in the bones reveals involvement of several novel genes and pathways in mediating an anabolic response of mechanical loading in mice. Journal of Cellular Biochemistry. 2005;96:1049–1060. doi: 10.1002/jcb.20606. [DOI] [PubMed] [Google Scholar]
- Xing W, Kim J, Wergedal J, Chen S-T, Mohan S. Ephrin B1 regulates bone marrow stromal cell differentiation and bone formation by influencing TAZ transactivation via complex formation with NHERF1. Molecular and Cellular Biology. 2010;30:711–721. doi: 10.1128/MCB.00610-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing W, Govoni KE, Donahue LR, Kesavan C, Wergedal J, Long C, Bassett JHD, Gogakos A, Wojcicka A, Williams GR, et al. Genetic evidence that thyroid hormone is indispensable for prepubertal insulin-like growth factor-I expression and bone acquisition in mice. Journal of Bone and Mineral Research. 2012;27:1067–1079. doi: 10.1002/jbmr.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu J-L, Ooi GT, Setser J, Frystyk J, Boisclair YR, et al. Circulating levels of IGF-1 directly regulate bone growth and density. The Journal of Clinical Investigation. 2002;110:771–781. doi: 10.1172/JCI15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S, Rosen CJ, Bouxsein ML, Sun H, Mejia W, Kawashima Y, Wu Y, Emerton K, Williams V, Jepsen K, et al. Serum complexes of insulin-like growth factor-1 modulate skeletal integrity and carbohydrate metabolism. FASEB Journal. 2009;23:709–719. doi: 10.1096/fj.08-118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Yuasa M, Masaoka T, Taniyama T, Maehara H, Torigoe I, Yoshii T, Shinomiya K, Okawa A, Sotome S. After repeated division, bone marrow stromal cells express inhibitory factors with osteogenic capabilities, and EphA5 is a primary candidate. Bone. 2013;57:343–354. doi: 10.1016/j.bone.2013.08.028. [DOI] [PubMed] [Google Scholar]
- Yamada T, Yoshii T, Yasuda H, Okawa A, Sotome S. Dexamethasone Regulates EphA5, a Potential Inhibitory Factor with Osteogenic Capability of Human Bone Marrow Stromal Cells. Stem Cells International. 2016;2016:1301608. doi: 10.1155/2016/1301608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Ogata N, Shinoda Y, Akune T, Kamekura S, Terauchi Y, Kadowaki T, Hoshi K, Chung U-I, Nakamura K, et al. Insulin receptor substrate-1 is required for bone anabolic function of parathyroid hormone in mice. Endocrinology. 2005;146:2620–2628. doi: 10.1210/en.2004-1511. [DOI] [PubMed] [Google Scholar]
- Zhang J, Hughes S. Role of the ephrin and Eph receptor tyrosine kinase families in angiogenesis and development of the cardiovascular system. The Journal of Pathology. 2006;208:453–461. doi: 10.1002/path.1937. [DOI] [PubMed] [Google Scholar]
- Zhang X, Sobue T, Hurley MM. FGF-2 increases colony formation, PTH receptor, and IGF-1 mRNA in mouse marrow stromal cells. Biochemical and Biophysical Research Communications. 2002a;290:526–531. doi: 10.1006/bbrc.2001.6217. [DOI] [PubMed] [Google Scholar]
- Zhang M, Xuan S, Bouxsein ML, von Stechow D, Akeno N, Faugere MC, Malluche H, Zhao G, Rosen CJ, Efstratiadis A, et al. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. The Journal of Biological Chemistry. 2002b;277:44005–44012. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- Zhao G, Monier-Faugere MC, Langub MC, Geng Z, Nakayama T, Pike JW, Chernausek SD, Rosen CJ, Donahue LR, Malluche HH, et al. Targeted overexpression of insulin-like growth factor I to osteoblasts of transgenic mice: increased trabecular bone volume without increased osteoblast proliferation. Endocrinology. 2000;141:2674–2682. doi: 10.1210/endo.141.7.7585. [DOI] [PubMed] [Google Scholar]
- Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, Suda T, Matsuo K. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metabolism. 2006;4:111–121. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Zofková I, Bahbouh R, Bendlová B. Systemic insulin-like growth factor-I, insulin and vitamin D status in relation to age-associated bone loss in women. Experimental and Clinical Endocrinology & Diabetes. 2001;109:267–272. doi: 10.1055/s-2001-16346. [DOI] [PubMed] [Google Scholar]