Abstract
Osteosarcoma, Ewing sarcoma and chondrosarcoma are the three main entities of bone sarcoma which collectively encompass more than 50 heterogeneous entities of rare malignancies. In contrast to osteosarcoma and Ewing sarcoma which mainly affect adolescents and young adults and exhibit a high propensity to metastasise to the lungs, chondrosarcoma is more frequently observed after 40 years of age and is characterised by a high frequency of local recurrence. The combination of chemotherapy, surgical resection and radiotherapy has contributed to an improved outcome for these patients. However, a large number of patients still suffer significant therapy related toxicities or die of refractory and metastatic disease. To better delineate the pathogenesis of bone sarcomas and to identify and test new therapeutic options, major efforts have been invested over the past decades in the development of relevant pre-clinical animal models. Nowadays, in vivo models aspire to mimic all the steps and the clinical features of the human disease as accurately as possible and should ideally be manipulable. Considering these features and given their small size, their conduciveness to experiments, their affordability as well as their human-like bone-microenvironment and immunity, murine pre-clinical models are interesting in the context of these pathologies. This chapter will provide an overview of the murine models of bone sarcomas, paying specific attention for the models induced by inoculation of tumour cells. The genetically-engineered mouse models of bone sarcoma will also be summarized.
Keywords: Bone sarcoma, Osteosarcoma, Ewing sarcoma, Chondrosarcoma, Cell-injection, Murine pre-clinical models, Genetically-engineered mouse models
1. Introduction
The injection of a cell suspension of murine (allograft) or human (xenograft) cancer cells, in orthotopic sites (in close contact to the bone or into the bone medullary cavity) is the most common methods used to induce bone sarcomas in mouse [1], [2]. It has also been possible more recently to utilise the limited material available from patient biopsies (e.g. needle biopsies), and implant such tumour material into immunodeficient [e.g. Patient-Derived Xenografts (PDX)] [3] or immunocompetent animals [4], [5]. The advantage of these PDX bearing mouse models is the possibility of expanding the tumour tissues by retaining the original tumour architecture.
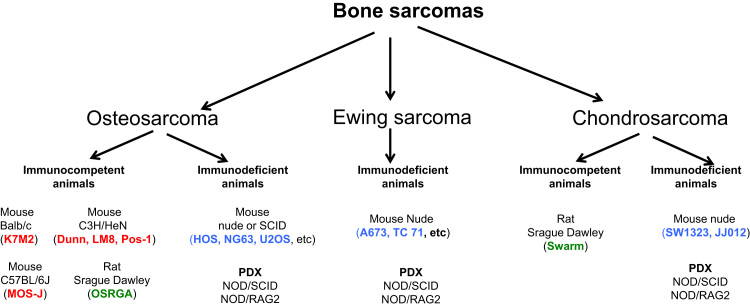
The cell-injection close to the bone is called “paraosseous induction”, in contrast to the “intraosseous model” that consists in cell inoculation into the femur or fibula diaphysis. Immunocompetent (e.g. syngeneic model in C57/BL6 mice or Sprague-Dawley rats) or immunocompromised (xenografts in Nude or SCID mice) models can be used according to the main objective of the studies (Fig. 1). Other heterotopic cell injections are also described in the literature (e.g. subcutaneous, under the renal capsule) however, they do not engage the vicious cycle established between cancer cells and the bone microenvironment and do not mimic all steps of tumour development.
Fig. 1.
Smal animal models available in the literature for the study of primary bone tumours. Cell lines: human (in blue), mouse (in red), rat (in green) orgin. PDX: Patient derived xenograft. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The choice of the model will depend on the goal of the study (e.g. analysis of local tumour growth, imaging of lung metastases). In addition, financial aspects (e.g. relative inexpensive models based on injection of established cell lines versus genetically-engineered models) and availabilities of research tools (e.g. antibodies) are also key parameters that could influence the choice. Independently of their costs, each of these models have several advantages and limitations: i) inoculation of established cell lines may not represent the genetic heterogeneity of the human tumours; ii) genetically engineered models characterised by a spontaneous tumour development can mimic the natural history of the disease with an adapted tumour microenvironment (murine cancer cells in a murine microenvironment); iii) PDX models can maintain the cellular heterogeneity of the initial tumour fragments in a non human microenvironment. The current state of the art concerning the murine strains, the cell lines used, the number of cells injected per animal and some other specific technique-related features will be described in the paragraphs below.
2. Induction of primary bone tumour by cell injections in heterotopic sites
2.1. Induction of bone sarcoma by subcutaneous cell injections
Given the mesenchymal and bone/joint origin of bone sarcomas, their initiation through heterotopic subcutaneous cell injection does not take account of the proper interactions between the tumour cells and their normal bone/muscle/cartilaginous microenvironment. However, this model has the advantage of being technically easy to carry out, a large panel of cancer cell lines and diverse injection sites can be used and the resulting tumours are easily and directly accessible for experiments. Importantly, however, this approach can also address whether the transformed cells have the potential to form tumours in a cell-autonomous way in the absence of their normal environment. In the context of osteosarcoma, the human 143B cell line as well as several c-Fos-transgenic mouse osteosarcoma cells were reported to form tumour masses containing bone after subcutaneous injection [6], [7]. Cancer cells have been also incorporated into acellular Matrigel™ based-matrix to provide an active bio-molecule scaffold from murine origin and facilitate cell engraftment. Utilising such an approach, Duan et al. established osteosarcoma tumours subcutaneously by resuspending KHOS osteosarcoma cells in a 1:1 Matrigel™ volume ratio and injected an amount of 2 × 106 cells per mouse [8]. The use of the Saos-2 human osteosarcoma cells combined with Matrigel™ was also reported. A recent study reports the injection of 3 × 106 cells resuspended in 100 µL of Matrigel™ mix (1:1) in this case [10]. Syngeneic models of osteosarcoma are also available. The Dunn cell line and its derivate LM8 subline are the most frequently used. Dunn cells were originally reported with a low metastatic profile in contrast to its LM8 subline which is highly metastastic. LM8 was initially obtained after 8 successive cycles of in vivo selection [10], [11]. 1–10 × 106 Dunn or LM8 cells resuspended in 200–300 µL of PBS are inoculated subcutaneously into the flank of C3H mice (5- to 8- weeks-old) [12], [13]. The inoculation of LM8 cells results in the development of a primary local tumour and the formation of metastases to the lungs within 4 weeks with an incidence of 100%. Finally, genetically-engineered osteosarcoma cells have also been reported to efficiently grow after subcutaneous injection [13], [14], [15], [16], [17] (Fig. 2). For instance, the low metastatic mouse RF43 osteosarcoma cells and their stable genetically-modified counterparts expressing sFRP2-were injected into nude mice17 Similar studies have also been reported with Ewing Sarcoma cells, with A673 cells being one of the most commonly reported, for drug screening [18]. One to three million A673 cells are sufficient to generate a tumour mass after subcutaneous implantation into the flank or in the inguinal region of nude mice [19], [20]. TC71 and SK-N-MC cell lines were also described to reproduce relevant non-osseous Ewing sarcoma models [21]. Similarly to osteosarcoma, Ewing sarcoma cells (5 × 106 of TC32 cells) suspended in 30% Matrigel™ have been inoculated subcutaneously [22]. Finally, the subcutaneous injection method is also employed to generate chondrosarcomas, as shown by Li et al. [23] and Wang et al. [24], who injected 5 × 106 of SW1353 cells and 106 c-Fos-transformed murine chondrosarcoma cells, respectively, into the hind limbs of nude mice. One million JJ012 human chondrosarcoma cells resuspended in 200 µL of serum-free medium [25] or diluted in 100 µL of medium, concomitantly with 300 µL of Matrigel™ can be also inoculated subcutaneously in the back of nude mice [26].
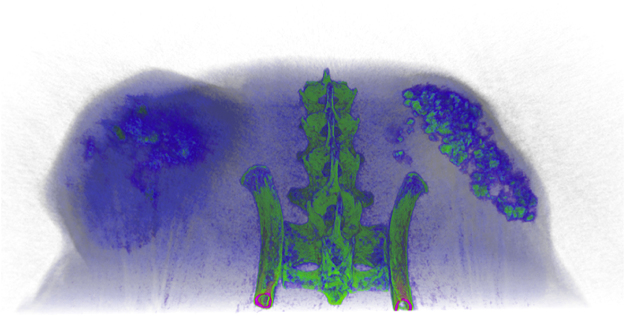
Fig. 2.
Typical view of microCT image of luciferase expressing murine OS cell lines grown on the back flank of Balb/c nu/nu mice. Cells were implanted subcutaneously in matrigel. Mass on the left is control cells (control shRNA) and those on the right is expressing an shRNA directed against Pthr1. Pseudo coloring indicates intensity of the gray scale density of the tumour with green being most dense and blue least dense. (image generated by A. Goradia/M. Russell/C Walkley [13]). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.2. Induction of bone sarcomas after cell injection in a deep non-osseous microenvironment
Intraperitoneal, skeletal muscle and kidney are the main deep heterotopic sites of cancer cell inoculation. Recently, Saos-2 osteosarcoma cells (1 × 106 cells/mouse) were injected intraperitoneally in nude mice, resulting in the induction of osteosarcoma xenograft models [27]. SK-NEP-1 Ewing sarcoma cells were inoculated under the renal capsule of mice, this model displaying the advantage of reproducing the lung metastatic spreading after the inoculation of 1 × 106 cells [28]. Ewing sarcoma tumours were formed after injection of 2 × 106 TC71 Ewing Sarcoma cells into the gastrocnemius muscle [29]. The human JJ012 chondrosarcoma cells (5 × 106 cells resuspended in 100 µL of medium) were directly injected into the lateral tail vein of nude mice to generate a model of disseminated chondrosarcoma [30].
2.3. Induction of bone sarcomas by orthotopic cell injections
The main advantage of the models described hereafter is that they reproduce the original site for the development of the primary bone sarcomas. Nevertheless, despite their location, these models do not allow reproduction of the process of the clonal selection associated both with the tumour growth and the metastatic spreading and may not fully recapitulate the tumour cell-immune interactions occurring in de novo tumours.
2.3.1. Primary-bone tumour induction by para-osseous cell injections
Syngeneic models of osteosarcoma have been generated by injecting 5-week-old male or female C57BL/6 J mice with 1 × 106 MOS-J cells in close proximity to the tibia, whereas the xenogenic models can be induced through the inoculation of 2 × 106 MNNG/HOS cells in Rj;NMRI nude mice with the same method [31], [32] (Fig. 3). As no current syngeneic models of Ewing sarcoma are available, xenografts are conventionally used by injection of 1.5 × 106 TC71 or 1673 Ewing sarcoma cells directly into the nude mice [33]. Of note, this Ewing sarcoma model does not show any metastatic occurrence.
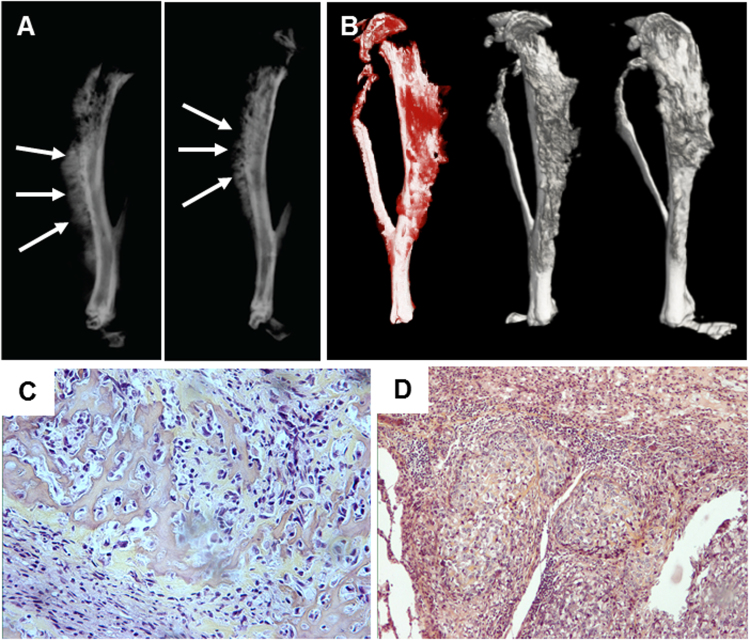
Fig. 3.
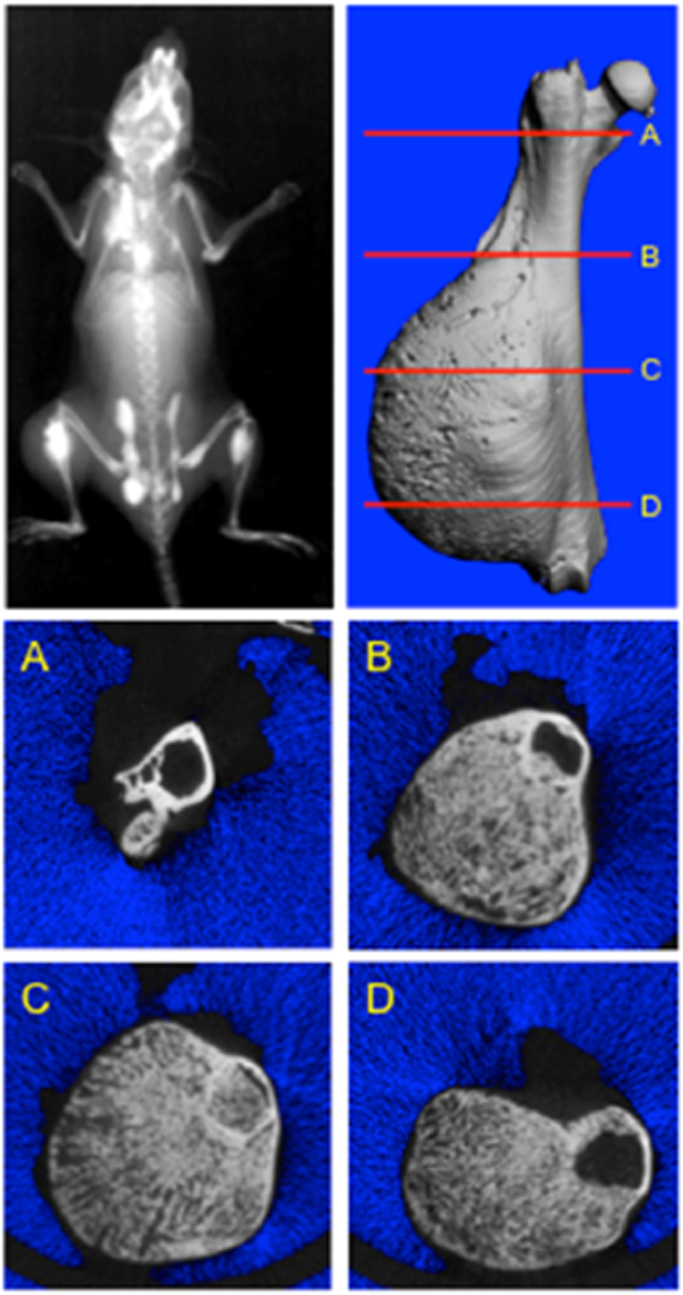
Xenograft model of human osteosarcoma. Bone tumours are induced by para-tibial injection of human HOS-MNNG cells (A, B). Lung metastases can be detected two to three weeks after cell inoculation. Xray (A) and MicroCT (B) analysis of tibial osteosarcoma, three weeks after cell inoculation. leading to spontaneous lung metastases. Tumour are characterised by ectopic bone formation observed by Xray images generated by F. Lamoureux/F. Lézot/D. Heymann) and microCT (images generated by B. Gobin/S. Battaglia/D. Heymann [31]). (C,D) Osteosarcoma developed from intra-tibial injection of OSRGA cells in Sprague Dawley rat. Typical histological feature of an osteoblastic osteosarcoma (primary tumour) (C) leading to the development of lung metastases (D) (Images generated by D. Heymann [4]).
2.3.2. Induction of bone sarcoma by intraosseous injection of cancer cells
Among the currently available methods to generate models of bone sarcomas from cell injections, the intraosseous models are technically the most difficult to achieve and the operator needs to be specifically trained for properly inducing a series of tumour-bearing mice. In a recent study, osteosarcomas were induced using the intratibial injection method using either 1 × 105 human 143B or K7M2L2 osteosarcoma cells resuspended in 10 µL PBS/0.05% EDTA into SCID and BALB/c mice respectively, thus generating highly metastatic pre-clinical models [9]. Similarly, Tome et al. reported 5 × 105 143B-LM4 cells per nude mouse was able to generate tumours in immunodeficient [34]. Another study reports the use of the OS-1 and OS-2 canine osteosarcoma cells (1 × 105 cells resuspended in 10 µL PBS and intra-tibially injected into nude mice), to assess their tumour characteristics and metastatic features [35]. Finally, Shimozaki et al., injected a suspension of 5 × 105 143B cells diluted in Matrigel™, directly into the medullar cavity of the tibia of nude mice [36]. Similarly, isolated osteosarcoma cells isolated from c-Fos transgenic mice maintained on a C57Bl6/J background, were injected intratibially into four week-old female Rag2-/-:IL2Rγ-/- immunocompromised mice (2.5 × 105 per mouse, 5 µL injected with a 29-gauge Hamilton microsyringe). This model results in the formation of lung nodules 14 days after cell inoculation [37] (Fig. 4). In the context of Ewing Sarcoma, the intraosseous injections closely reproduces the human pathology, even if the tumours showed a slowed proliferation rate compared with the soft tissue injected models1 and in osteosarcoma the microenvironment could influence the therapeutic response [38].
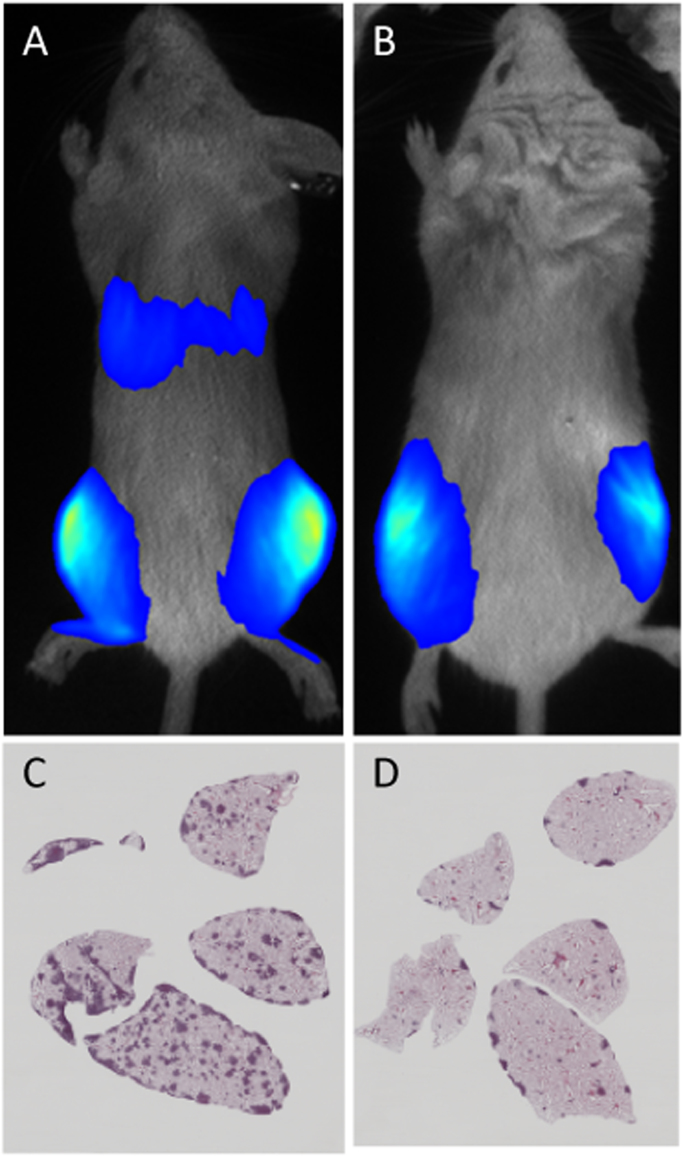
Fig. 4.
Bioluminescence imaging and histology of immunocompromised mice following orthotopic injection of c-Fos transformed murine OS cells showing marked primary tumour formation and lung metastasis. Control OS cells (control shRNA) (A) showing lung metastases are inhibited by expressing an shRNA directed against Fgfr1 (B). H&E stained sections of control (C) and Fgfr1 knockdown (D) lungs show reduced lung nodules. Images generated by C. Zandueta/F. Lecanda (CIMA, Pamplona, Spain) [37].
2.4. Primary bone tumour induction by tumour transplantations
The transplantation of tumour fragments from a donor to a recipient is also another possible strategy to maintain the cellular heterogeneity and the genetic background of the tumour. The engraftment success is variable depending upon the model used and the tumour studied [2], [4], [5]. Such transplantation can be done subcutaneously or directly in close contact to the bone of the animals. A recent study reports the passage of 2 × 2 × 2 mm tumour-fragments from human OHS osteosarcoma cells through subcutaneous transplantation into the rear flank of nude mice as an efficient model to test the targeting ability of a murine monoclonal radio-labeled antibody to the CD146 [39]. Lamoureux et al., described a transplantation method in which the murine POS-1 osteosarcoma cells were first inoculated in the hind footpad of C57BL6 mice until tumour formation was observed [40]. Similarly, 2 × 2 × 2 mm tumour fragments were then excised from these donor mice and transplanted along the tibia in other acceptor mice. A similar approach was used for chondrosarcoma [41], [42] and for genetically engineered mouse models [43], [44].
Several studies indeed report the use of osteosarcoma or Ewing sarcoma PDXs as useful models to perform personalized therapeutic tests. However, these models are still limited by the availability of patient samples, the low rate of engraftment and the cost of immunodeficient animals and the constraining process of mandatory quality control. The most recent work describing osteosarcoma and Ewing sarcoma PDX models have been reported by Stewart et al. [45], [46]. These authors have conducted a comprehensive genetic characterization of both diseases using whole-genome sequencing from tumour fragments or original cell lines isolated from patient biopsies and implanted in NOD/SCID/IL-2Rγ-null mice. They identified recurrent somatic alterations in cancer genomes that may be missed using other methods. Murakami et al., recently performed a subcutaneous implantation of 5 mm fragments from freshly obtained human sarcoma samples, directly onto the flank of nude mice [47]. After three weeks when the tumour diameter reached more than 10 mm, 3 mm3 tumour fragments were then reimplanted in orthotopic sites for reinducing a tumour mass. In the study of Goldstein et al., 3 mm fragments of the DAR PDX (generated from the malignant pleural effusion of a patient suffering from osteosarcoma) and the LR PDX (generated from a pulmonary metastasis of an Osteosarcoma patient) were implanted into either the flank or the pretibial side of mice [48]. The serially passaged tumours were then transplanted in the hindlimb of a single NSG mouse, from which the tumour was grown. This mouse was then sacrificed and 3 mm fragments were washed in Matrigel™ prior to be transferred into the hindlimb of NSG pups. In a study employing PDXs from Ewing sarcomas, NOD/SCID mice were used for the initial tumour engraftment (HSJD-ES-004 and HSJD-ES-006 models, originated from mediastinum-metastasis and from lung-metastasis respectively) and were then passaged into athymic nude mice prior to the assessment of the therapeutic response [3]. Ambati et al., developed PDX models of Ewing sarcoma by passaging initial tumour material two times into NSG mice prior to start the animal treatment [49].
2.5. Genetically-engineered mouse models of bone sarcomas
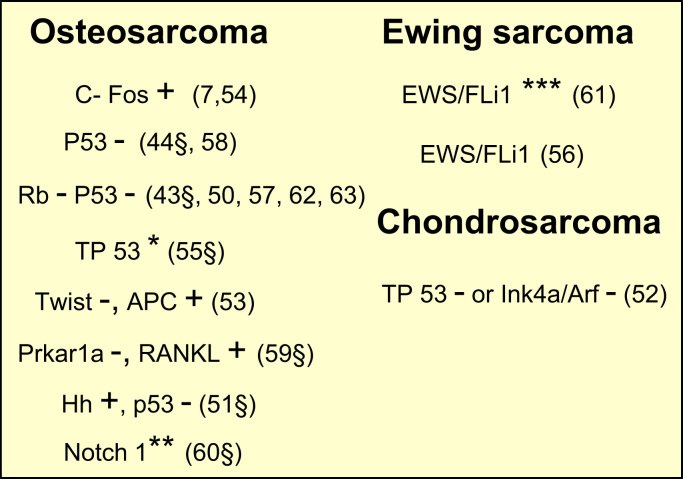
Genetically-engineered mouse models have been characterised to be accurate models in oncology, especially in an attempt to study the tumour onset/development and to delineate the molecular drivers or the genetic initiator events responsible of these pathologies. The main advantage af such models is the formation of spontaneous tumours close to the human context, and can be imaged by conventional approaches (i.e. microCT) (Fig. 5). However, genetically-engineered odel can not summarize all events of human tumours and can not mimick the high molecular heteorgeneity (more specically of osteosarcoma). Ewing sarcoma is an exception in the list of three most frequent bone sarcoma (osteosarcoma, chondrosarcoma, Ewing sarcoma) for which all attempts for developping a genetically-engineered small animal mimicking the human disease has failed (64,65). Despites the technical difficulties, genetically-engineered models in small animals allowed a better understanding of molecular/genetic pathways surrounding bone sarcoma development (Fig. 6, Fig. 7).
Fig. 5.
Overview of the genetically-engineered models in small animals. +; gain; -: deletion; *: mutation; **: restricted expression of the intracellular domain of Notch1 in osteoblast; ***: inducible expression. §: description of metastases.
Fig. 6.
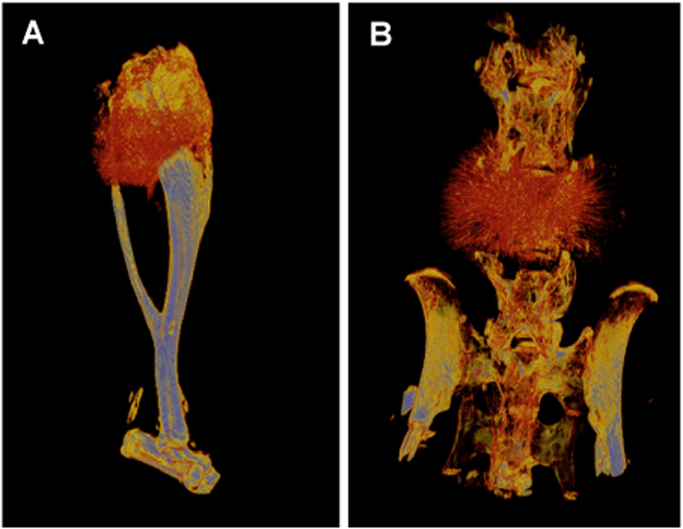
Two different osteosarcomas arising in Osx-Cre p53fl/fl pRbfl/fl animals. Osteosarcoma arising in the tibiae (A) and in the vertebrae (B). Pseudo coloring indicates intensity of the gray scale density of the tumour with blue being most dense and black/crimson least dense. Images generated by A Ng/C Walkley [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [68].
Fig. 7.
Xray of an 8 week-old c-Fos transgenic mouse showing numerous calcified osteosarcomas throughout the skeleton. Tumours are at different stages of development. microCT analysis of a femoral osteosarcoma and serial cross-section images through the length of the femur show both extraosseous and intraosseous tumour growth. MicroCT image generated by L. Suva (Texas A&M University, USA) [7].
3. Conclusion
In this chapter we have outlined the currently available murine models for bone sarcomas induced both by injection of established tumour cell line and from transplantation of primary patient-derived tumour material, as well as providing an overview of the genetically-induced mouse models. In addition, the entire experimental procedure of the para- and intra-osseous injections are outlined here. The murine models are valuable tools in the field of oncology research, however each one has both advantages and limitations and the choice of a particular model should be careful considered depending upon the goals of the study. Furthermore, osteosarcomas naturally occur in large breed dogs (pet dogs/companion animals) and have been characterised to display clinical features comparable with human OS. In this context, it is conceivable that the spontaneous canine models will be further employed, in an attempt to better understand the normal biology of the bone as well as to find novel biomarkers and innovative therapeutic approaches [65], [66], [67], [68].
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgement
This work was supported by the Bone Cancer Research Trust (UK, research project number 144681).
Contributor Information
Carl R. Walkley, Email: cwalkley@svi.edu.au.
Agamemnon E. Grigoriadis, Email: agi.grigoriadis@kcl.ac.uk.
Dominique Heymann, Email: dominique.heymann@univ-nantes.fr.
References
- 1.Odri G.A., Dumoucel S., Picarda G., Battaglia S., Lamoureux F., Corradini N., Rousseau J., Tirode F., Laud K., Delattre O., Gouin F., Heymann D., Redini F. Zoledronic acid as a new adjuvant therapeutic strategy for Ewing's sarcoma patients. Cancer Res. 2010;70:7610–7619. doi: 10.1158/0008-5472.CAN-09-4272. [DOI] [PubMed] [Google Scholar]
- 2.Cherrier B., Gouin F., Heymann M.F., Thiéry J.P., Rédini F., Heymann D., Duteille F. A new experimental rat model of osteosarcoma established by intrafemoral tumor cell inoculation, useful for biology and therapy investigations. Tumour Biol. 2005;26:121–130. doi: 10.1159/000086483. [DOI] [PubMed] [Google Scholar]
- 3.Ordóñez J.L., Amaral A.T., Carcaboso A.M., Herrero-Martín D., del Carmen García-Macías M., Sevillano V., Alonso D., Pascual-Pasto G., San-Segundo L., Vila-Ubach M., Rodrigues T., Fraile S., Teodosio C., Mayo-Iscar A., Aracil M., Galmarini C.M., Tirado O.M., Mora J., de Álava E. The PARP inhibitor olaparib enhances the sensitivity of Ewing sarcoma to trabectedin. Oncotarget. 2015;8:26027–26040. doi: 10.18632/oncotarget.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heymann D., Ory B., Blanchard F., Heymann M.F., Coipeau P., Charrier C., Couillaud S., Thiery J.P., Gouin F., Redini F. Enhanced tumor regression and tissue repair when zoledronic acid is combined with ifosfamide in rat osteosarcoma. Bone. 2005;37:74–86. doi: 10.1016/j.bone.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 5.Grimaud E., Damiens C., Rousselle A.V., Passuti N., Heymann D., Gouin F. Bone remodelling and tumour grade modifications induced by interactions between bone and swarm rat chondrosarcoma. Histol. Histopathol. 2002;17:1103–1111. doi: 10.14670/HH-17.1103. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Z.F., Sun T.W., Chen F., Zuo D.Q., Wang H.S., Hua Y.Q., Cai Z.D., Tan J.Z. Calcium phosphate-phosphorylated adenosine hybrid microspheres for anti-osteosarcoma drug delivery and osteogenic differentiation. Biomaterials. 2017;121:1–14. doi: 10.1016/j.biomaterials.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 7.Grigoriadis A.E., Schellander K., Wang Z.Q., Wagner E.F. Osteoblasts are target cells for transformation in c-fos transgenic mice. J. Cell Biol. 1993;122:685–701. doi: 10.1083/jcb.122.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duan Z., Gao Y., Shen J., Choy E., Cote G., Harmon D., Bernstein K., Lozano-Calderon S., Mankin H., Hornicek F.J. miR-15b modulates multidrug resistance in human osteosarcoma in vitro and in vivo. Mol. Oncol. 2017;11:151–166. doi: 10.1002/1878-0261.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meier D., Botter S.M., Campanile C., Robl B., Gräfe S., Pellegrini G., Born W., Fuchs B. Foscan and Foslip based photodynamic therapy in osteosarcoma in vitro and in intratibial mouse models. Int. J. Cancer. 2017;140:1680–1692. doi: 10.1002/ijc.30572. [DOI] [PubMed] [Google Scholar]
- 10.Fidler I.J., Kripke M.L. Metastasis results from preexisting variant cells within a malignant tumor. Science. 1977;197:893–895. doi: 10.1126/science.887927. [DOI] [PubMed] [Google Scholar]
- 11.Asai T., Ueda T., Itoh K., Yoshioka K., Aoki Y., Mori S., Yoshikawa H., Asai T. Establishment and characterization of a murine osteosarcoma cell line (LM8) with high metastatic potential to the lung. Int. J. Cancer. 1998;76:418–422. doi: 10.1002/(sici)1097-0215(19980504)76:3<418::aid-ijc21>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Arlt M.J., Banke I.J., Walters D.K., Puskas G.J., Steinmann P., Muff R., Born W., Fuchs B. LacZ transgene expression in the subcutaneous Dunn/LM8 osteosarcoma mouse model allows for the identification of micrometastasis. J. Orthop. Res. 2011;29:938–946. doi: 10.1002/jor.21304. [DOI] [PubMed] [Google Scholar]
- 13.Ho P.W., Godadia A., Russell M.R., Chalk A.M., Milley K.M., Baker E.K., Danks J.A., Slavin J.L., Walia M., Dickins R.A., Martin T.J., Walkley C.R. Knockdown of PTHR1 in osteosarcoma cells decreases invasion and growth and increases tumor differentiation in vivo. Oncogene. 2015;34:2922–2933. doi: 10.1038/onc.2014.217. [DOI] [PubMed] [Google Scholar]
- 14.Baker E.K., Taylor S., Gupte A., Sharp P.P., Walia M., Walsh N.C., Zannettino A.C., Chalk A.M., Burns C.J., Walkley C.R. BET inhibitors induce apoptosis through a MYC independent mechanism and synergise with CDK inhibitors to kill osteosarcoma cells. Sci. Rep. 2015;5 doi: 10.1038/srep10120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhattacharya S., Chalk A.M., Ng A.L., Martin T.J., Zannettino A.C., Purton L.E., Lu J., Baker E.K., Walkley C.R. Increased miR-155-5p and reduced miR-148a-3p contribute to the suppression of osteosarcoma cell death. Oncogene. 2016;35:5282–5294. doi: 10.1038/onc.2016.68. [DOI] [PubMed] [Google Scholar]
- 16.Walia M.K., Ho P.M., Taylor S., Ng A.J., Gupte A., Chalk A.M., Zannettino A.C., Martin T.J., Walkley C.R. Activation of PTHrP-cAMP-CREB1 signaling following p53 loss is essential for osteosarcoma initiation and maintenance. Elife. 2016;5 doi: 10.7554/eLife.13446. (pii: e13446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Techavichit P., Gao Y., Kurenbekova L., Shuck R., Donehower L.A., Yustein J.T. Secreted Frizzled-related protein 2 (sFRP2) promotes osteosarcoma invasion and metastatic potential. BMC Cancer. 2016;16:869. doi: 10.1186/s12885-016-2909-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teicher B.A., Bagley R.G., Rouleau C., Kruger A., Ren Y., Kurtzberg L. Characteristics of human Ewing/PNET sarcoma models. Ann. Saudi Med. 2011;31:174–182. doi: 10.4103/0256-4947.78206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rouleau C., Gianolio D.A., Smale R., Roth S.D., Krumbholz R., Harper J., Munroe K.J., Green T.L., Horten B.C., Schmid S.M., Teicher B.A. Anti-Endosialin antibody-drug conjugate: potential in sarcoma and other malignancies. Mol. Cancer Ther. 2015;14:2081–2089. doi: 10.1158/1535-7163.MCT-15-0312. [DOI] [PubMed] [Google Scholar]
- 20.Ren C., Ren T., Yang K., Wang S., Bao X., Zhang F., Guo W. Inhibition of SOX2 induces cell apoptosis and G1/S arrest in Ewing's sarcoma through the PI3K/Akt pathway. J. Exp. Clin. Cancer Res. 2016;35:44. doi: 10.1186/s13046-016-0321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hensel T., Giorgi C., Schmidt O., Calzada-Wack J., Neff F., Buch T., Niggli F.K., Schäfer B.W., Burdach S., Richter G.H. Targeting the EWS-ETS transcriptional program by BET bromodomain inhibition in Ewing sarcoma. Oncotarget. 2016;7:1451–1463. doi: 10.18632/oncotarget.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kennedy A.L., Vallurupalli M., Chen L., Crompton B., Cowley G., Vazquez F., Weir B.A., Tsherniak A., Parasuraman S., Kim S., Alexe G., Stegmaier K. Functional, chemical genomic, and super-enhancer screening identify sensitivity to cyclin D1/CDK4 pathway inhibition in Ewing sarcoma. Oncotarget. 2015;6:30178–30193. doi: 10.18632/oncotarget.4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J., Wang L., Liu Z., Zu C., Xing F., Yang P., Yang Y., Dang X., Wang K. MicroRNA-494 inhibits cell proliferation and invasion of chondrosarcoma cells in vivo and in vitro by directly targeting SOX9. Oncotarget. 2015;6:26216–26229. doi: 10.18632/oncotarget.4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z.Q., Grigoriadis A.E., Möhle-Steinlein U., Wagner E.F. A novel target cell for c-fos-induced oncogenesis: development of chondrogenic tumours in embryonic stem cell chimeras. EMBO J. 1991;110:2437–2450. doi: 10.1002/j.1460-2075.1991.tb07783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu G.T., Huang Y.L., Tzeng H.E., Tsai C.H., Wang S.W., Tang C.H. CCL5 promotes vascular endothelial growth factor expression and induces angiogenesis by down-regulating miR-199a in human chondrosarcoma cells. Cancer Lett. 2015;357:476–487. doi: 10.1016/j.canlet.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 26.Sun X., Charbonneau C., Wei L., Chen Q., Terek R.M. miR-181a targets RGS16 to promote chondrosarcoma growth, angiogenesis, and metastasis. Mol. Cancer Res. 2015;13:1347–1357. doi: 10.1158/1541-7786.MCR-14-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen C.H., Li C.J., Tai I.C., Lin X.H., Hsu H.K., Ho M.L. The fractionated toona sinensis leaf extract induces apoptosis of human osteosarcoma cells and inhibits tumour growth in a murine xenograft model. Integr. Cancer Ther. 2017;16:397–405. doi: 10.1177/1534735416675951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hesketh A.J., Maloney C., Behr C.A., Edelman M.C., Glick R.D., Al-Abed Y., Symons M., Soffer S.Z., Steinberg B.M. The macrophage inhibitor CNI-1493 blocks metastasis in a mouse model of Ewing sarcoma through inhibition of extravasation. PLoS ONE. 2015;10:e0145197. doi: 10.1371/journal.pone.0145197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osgood C.L., Maloney N., Kidd C.G., Kitchen-Goosen S., Segars L., Gebregiorgis M., Woldemichael G.M., He M., Sankar S., Lessnick S.L., Kang M., Smith M., Turner L., Madaj Z.B., Winn M.E., Núñez L.E., González-Sabín J., Helman L.J., Morís F., Grohar P.J. Identification of mithramycin analogues with improved targeting of the EWS-FLI1 transcription factor. Clin. Cancer Res. 2016;22:4105–4118. doi: 10.1158/1078-0432.CCR-15-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J.C., Lin C.Y., Fong Y.C., Hsu C.J., Tsai C.H., Su J.L., Tang C.H. Amphiregulin enhances alpha6beta1 integrin expression and cell motility in human chondrosarcoma cells through Ras/Raf/MEK/ERK/AP-1 pathway. Oncotarget. 2015;6:11434–11446. doi: 10.18632/oncotarget.3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gobin B., Baud'huin M., Lamoureux F., Ory B., Charrier C., Lanel R., Battaglia S., Redini F., Lezot F., Blanchard F., Heymann D. BYL719, a new alpha-specific PI3K inhibitor: single administration and in combination with conventional chemotherapy for the treatment of osteosarcoma. Int. J. Cancer. 2015;136:784–796. doi: 10.1002/ijc.29040. [DOI] [PubMed] [Google Scholar]
- 32.Lamoureux F., Baud'huin M., Rodriguez Calleja L., Jacques C., Berreur M., Rédini F., Lecanda F., Bradner J.E., Heymann D., Ory B. Selective inhibition of BET bromodomain epigenetic signalling interferes with the bone-associated tumour vicious cycle. Nat. Commun. 2014;5:511. doi: 10.1038/ncomms4511. [DOI] [PubMed] [Google Scholar]
- 33.Jacques C., Lamoureux F., Baud'huin M., Rodriguez Calleja L., Quillard T., Amiaud J., Tirode F., Rédini F., Bradner J.E., Heymann D., Ory B. Targeting the epigenetic readers in Ewing sarcoma inhibits the oncogenic transcription factor EWS/Fli1. Oncotarget. 2016;7:24125–24140. doi: 10.18632/oncotarget.8214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tome Y., Kimura H., Sugimoto N., Tsuchiya H., Kanaya F., Bouvet M., Hoffman R.M. The disintegrin echistatin in combination with doxorubicin targets high-metastatic human osteosarcoma overexpressing alphanubeta3 integrin in chick embryo and nude mouse models. Oncotarget. 2016;7:87031–87036. doi: 10.18632/oncotarget.13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scott M.C., Tomiyasu H., Garbe J.R., Cornax I., Amaya C., O'Sullivan M.G., Subramanian S., Bryan B., Modiano J.F. Heterotypic mouse models of canine osteosarcoma recapitulate tumour heterogeneity and biological behavior. Dis. Model Mech. 2016;9:1435–1444. doi: 10.1242/dmm.026849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimozaki S., Yamamoto N., Domoto T., Nishida H., Hayashi K., Kimura H., Takeuchi A., Miwa S., Igarashi K., Kato T., Aoki Y., Higuchi T., Hirose M., Hoffman R.M., Minamoto T., Tsuchiya H. Efficacy of glycogen synthase kinase-3beta. targeting against osteosarcoma via activation of beta-catenin. Oncotarget. 2016;7:77038–77051. doi: 10.18632/oncotarget.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kashima D.G., Zandueta C., Perurena N., Thomas D.P., Sunters A., Vuillier C., Bozec A., El-Emir E., Miletich I., Patiño-Garcia A., Lecanda F., Grigoriadis A.E. Regulation of osteosarcoma cell lung metastasis by the c-Fos/AP-1 target FGFR1. Oncogene. 2016;35:2852–2861. doi: 10.1038/onc.2015.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crenn V., Biteau K., Maiaud J., Dumars C., Guiho R., Vidal L., Le Nail L.R., Heymann D., Moreau A., Gouin F., Redini F. Bone microenvironment has an influence on the histological response of osteosarcoma to chemotherapy: retrospective analysis and preclinical modeling. Am. J. Cancer Res. 2017;7:2333–2349. [PMC free article] [PubMed] [Google Scholar]
- 39.Westrom S., Bønsdorff T.B., Abbas N., Bruland Ø.S., Jonasdottir T.J., Mælandsmo G.M., Larsen R.H. Evaluation of CD146 as target for radioimmunotherapy against osteosarcoma. PLoS ONE. 2016;11:e0165382. doi: 10.1371/journal.pone.0165382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamoureux F., Richard P., Wittrant Y., Battaglia S., Pilet P., Trichet V., Blanchard F., Gouin F., Pitard B., Heymann D., Redini F. Therapeutic relevance of osteoprotegerin gene therapy in osteosarcoma: blockade of the vicious cycle between tumour cell proliferation and bone resorption. Cancer Res. 2007;67:7308–7318. doi: 10.1158/0008-5472.CAN-06-4130. [DOI] [PubMed] [Google Scholar]
- 41.Monderer D., Luseau A., Bellec A., David E., Ponsolle S., Saiagh S., Bercegeay S., Piloquet P., Denis M.G., Lodé L., Rédini F., Biger M., Heymann D.M.F., Le Bot R., Gouin F., Blanchard F. New chondrosarcoma cell lines and mouse models to study the link between chondrogenesis and chemoresistance. Lab. Investig. 2013;93:100–114. doi: 10.1038/labinvest.2013.101. [DOI] [PubMed] [Google Scholar]
- 42.Gouin F., Ory B., Rédini F., Heymann D. Zoledronic acid slows down rat primary chondrosarcoma development, recurrent tumor progression after intralesional curretage and increases overall survival. Int. J. Cancer. 2006;119:980–984. doi: 10.1002/ijc.21951. [DOI] [PubMed] [Google Scholar]
- 43.Walkley C.R., Qudsi R., Sankaran V.G., Perry J.A., Gostissa M., Roth S.I., Rodda S.J., Snay E., Dunning P., Fahey F.H., Alt F.W., McMahon A.P., Orkin S.H. Conditional mouse osteosarcoma, dependent on p53 loss and potentiated by loss of Rb, mimics the human disease. Genes Dev. 2008;22:1662–1676. doi: 10.1101/gad.1656808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mutsaers A.J., Ng A.J., Baker E.K., Russell M.R., Chalk A.M., Wall M., Liddicoat B.J., Ho P.W., Slavin J.L., Goradia A., Martin T.J., Purton L.E., Dickins R.A., Walkley C.R. Modeling distinct osteosarcoma subtypes in vivo using Cre:lox and lineage-restricted transgenic shRNA. Bone. 2013;55 doi: 10.1016/j.bone.2013.02.016. (166-1quist 78) [DOI] [PubMed] [Google Scholar]
- 45.Stewart E., Goshorn R., Bradley C., Griffiths L.M., Benavente C., Twarog N.R., Miller G.M., Caufield W., Freeman B.B., 3rd, Bahrami A., Pappo A., Wu J., Loh A., Karlström Å., Calabrese C., Gordon B., Tsurkan L., Hatfield M.J.L., Potter P.M., Snyder S.E., Thiagarajan S., Shirinifard A., Sablauer A., Shelat A.A., Dyer M.A. Targeting the DNA repair pathway in Ewing sarcoma. Cell Rep. 2014;9:829–841. doi: 10.1016/j.celrep.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stewart E., Federico S., Karlstrom A., Shelat A., Sablauer A., Pappo A., Dyer M.A. The childhood solid tumor network: a new resource for the developmental biology and oncology research communities. Dev. Biol. 2016;411:287–293. doi: 10.1016/j.ydbio.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murakami T., Igarashi K., Kawaguchi K., Kiyuna T., Zhang Y., Zhao M., Hiroshima Y., Nelson S.D., Dry S.M., Li Y., Yanagawa J., Federman T.N., Singh A., Elliott I., Matsuyama R., Chishima T., Tanaka K., Endo I., Eilber F.C., Hoffman R.M. Tumour-targeting Salmonella typhimurium A1-R regresses an osteosarcoma in a patient-derived xenograft model resistant to a molecular-targeting drug. Oncotarget. 2016;7:12783–12790. doi: 10.18632/oncotarget.14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldstein S.D., Trucco M., Bautista Guzman W., Hayashi M., Loeb D.M. A monoclonal antibody against the Wnt signaling inhibitor dickkopf-1 inhibits osteosarcoma metastasis in a preclinical model. Oncotarget. 2016;7:21114–21123. doi: 10.18632/oncotarget.8522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ambati S.R., Shieh J.H., Pera B., Lopes E.C., Chaudhry A., Wong E.W., Saxena A., Su T.L., Moore M.A. BO-1055, a novel DNA cross-linking agent with remarkable low myelotoxicity shows potent activity in sarcoma models. Oncotarget. 2016;7:43062–43075. doi: 10.18632/oncotarget.9657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berman S.D., Calo E., Landman A.S., Danielian P.S., Miller E.S., West J.C., Fonhoue B.D., Caron A., Bronson R., Bouxsein M.L., Mukherjee S., Lees J.A. Metastatic osteosarcoma induced by inactivation of Rb and p53 in the osteoblast lineage. Proc. Natl. Acad. Sci. USA. 2008;105:11851–11856. doi: 10.1073/pnas.0805462105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chan L.H., Wang W., Yeung W., Deng Y., Yuan P., Mak K.K. Hedgehog signaling induces osteosarcoma development through Yap1 and H19 overexpression. Oncogene. 2014;33:4857–4866. doi: 10.1038/onc.2013.433. [DOI] [PubMed] [Google Scholar]
- 52.de Andrea C.E., Zhu J.F., Jin H., Bovee J.V., Jones K.B. Cell cycle deregulation and mosaic loss of Ext1 drive peripheral chondrosarcomagenesis in the mouse and reveal an intrinsic cilia deficiency. J. Pathol. 2015;236:210–218. doi: 10.1002/path.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Entz-Werle N., Choquet P., Neuville A., Kuchler-Bopp S., Clauss F., Danse J.M., Simo-Noumbissie P., Guérin E., Gaub M.P., Freund J.N., Boehm N., Constantinesco A., Lutz P., Guenot D., Perrin-Schmitt F. Targeted apc;twist double-mutant mice: a new model of spontaneous osteosarcoma that mimics the human disease. Transl. Oncol. 2010;3:344–353. doi: 10.1593/tlo.10169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grigoriadis A.E., Schellander K., Wang Z.Q., Wagner E.F. Osteoblasts are target cells for transformation in c-fos transgenic mice. J. Cell Biol. 1993;122:685–701. doi: 10.1083/jcb.122.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hansen S.A., Hart M.L., Busi S., Parker A T., Goerndt A., Jones K., Amos-Landgraf J.M., Bryda E.C. Fischer-344 Tp53-knockout rats exhibit a high rate of bone and brain neoplasia with frequent metastasis. Dis. Model Mech. 2016;9:1139–1146. doi: 10.1242/dmm.025767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin P.P., Pandey M.K., Jin F., Xiong S., Deavers M., Parant J.M., Lozano G. EWS-FLI1 induces developmental abnormalities and accelerates sarcoma formation in a transgenic mouse model. Cancer Res. 2008;68:8968–8975. doi: 10.1158/0008-5472.CAN-08-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu Y., Gitelis S., Lei G., Ding M., Maki C., Mira R.R., Zheng Q. Research findings working with the p53 and Rb1 targeted osteosarcoma mouse model. Am. J. Cancer Res. 2014;4:34–244. [PMC free article] [PubMed] [Google Scholar]
- 58.Mutsaers A.J., Ng A.J., Baker E.K., Russell M.R., Chalk A.M., Wall M., Liddicoat B.J., Ho P.W., Slavin J.L., Goradia A., Martin T.J., Purton L.E., Dickins R.A., Walkley C.R. Modeling distinct osteosarcoma subtypes in vivo using Cre:lox and lineage-restricted transgenic shRNA. Bone. 2013;55:166–178. doi: 10.1016/j.bone.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 59.Molyneux S.D., Grappa M.A.Di., Beristain A.G., McKee T.D., Wai D.H, Paderova J., Kashyap M., Hu P., Maiuri T., Narala S.R., Stambolic V., Squire J., Penninger J., Sanchez O., Triche T.J., Wood G.A., Kirschner L.S., Khokha R. Prkar1a is an osteosarcoma tumor suppressor that defines a molecular subclass in mice. J. Clin. Investig. 2010;120:3310–3325. doi: 10.1172/JCI42391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tao T.J., Jiang M.M., Jiang L., Salvo J.S., Zeng H.C., Dawson B., Bertin T.K., Rao P.H., Chen R., Donehower L.A., Gannon F., Lee B.H. Notch activation as a driver of osteogenic sarcoma. Cancer Cell. 2014;26:390–401. doi: 10.1016/j.ccr.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Torchia E.C., Boyd K., Rehg J.E., Qu C., Baker S.J. EWS/FLI−1 induces rapid onset of myeloid/erythroid leukemia in mice. Mol. Cell Biol. 2007;27:7918–7934. doi: 10.1128/MCB.00099-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walkley C.R., Qudsi R., Sankaran V.G., Perry J.A., Gostissa M., Roth S.I., Rodda S.J., Snay E., Dunning P., Fahey F.H., Alt F.W., McMahon A.P., Orkin S.H. Conditional mouse osteosarcoma, dependent on p53 loss and potentiated by loss of Rb, mimics the human disease. Genes Dev. 2008;22:1662–1676. doi: 10.1101/gad.1656808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Quist T., Jin H., Zhu J.F., Smith-Fry K., Capecchi M.R., Jones K.B. The impact of osteoblastic differentiation on osteosarcomagenesis in the mouse. Oncogene. 2015;34:4278–4284. doi: 10.1038/onc.2014.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Minas T.Z., Surdez D., Javaheri T., Tanaka M., Howarth M., Kang H.J., Han J., Han Z.Y., Sax B., Kream B.E., Hong S.H., Çelik H., Tirode F., Tuckermann J., Toretsky J.A., Kenner L., Kovar H., Lee S., Sweet-Cordero E.A., Nakamura T., Moriggl R., Delattre O., Üren A. Combined experience of six independent laboratories attempting to create an Ewing sarcoma mouse model. Oncotarget. 2017;8:34141–34163. doi: 10.18632/oncotarget.9388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fenger J.M., Roberts R.D., Iwenofu O.H., Bear M.D., Zhang X., Couto J.I., Modiano J.F., Kisseberth W.C., London C.A. miR-9 is overexpressed in spontaneous canine osteosarcoma and promotes a metastatic phenotype including invasion and migration in osteoblasts and osteosarcoma cell lines. BMC Cancer. 2016;16:784. doi: 10.1186/s12885-016-2837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mason N.J., Gnanandarajah J.S., Engiles J.B., Gray F., Laughlin D., Gaurnier-Hausser A., Wallecha A., Huebner M., Paterson Y. Immunotherapy with a HER2-targeting listeria induces HER2-specific immunity and demonstrates potential therapeutic effects in a phase I trial in canine osteosarcoma. Clin. Cancer Res. 2016;22:4380–4390. doi: 10.1158/1078-0432.CCR-16-0088. [DOI] [PubMed] [Google Scholar]
- 67.Khanna C., Fan T.M., Gorlick R., Helman L.J., Kleinerman E.S., Adamson P.C., Houghtonn P.J., Tap W.D., Welch D.R., Steeg P.S., Merlino G., Sorensen P.H., Meltzer P., Kirsch D.G., Janeway K.A., Weigel B., Randall L., Withrow S.J., Paoloni M., Kaplan R., Teicher B.A., Seibel N.L., Smith M., Uren A., Patel S.R., Trent J., Savage S.A., Mirabello L., Reinke D., Barkaukas D.A., Krailo M., Bernstein M. Toward a drug development path that targets metastatic progression in osteosarcoma. Clin. Cancer Res. 2014;20:4200–4209. doi: 10.1158/1078-0432.CCR-13-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ng A.V.J., Walia K.M.K., Smeets M.F., Mutsaers A.J., Sims N.A., Purton L.E., Walsh N.C., Martin T.J., Walkley C.R. The DNA helicase recql4 is required for normal osteoblast expansion and osteosarcoma formation. PLoS Genet. 2015;11:e1005160. doi: 10.1371/journal.pgen.1005160. [DOI] [PMC free article] [PubMed] [Google Scholar]