Abstract
Calorie restriction (CR) triggers benefits for healthspan including decreased risk of cardiometabolic disease (CVD). In an ancillary study to CALERIE 2, a 24-mo 25% CR study, we assessed the cardiometabolic effects of CR in 53 healthy, nonobese (BMI: 22–28 kg/m2) men (n = 17) and women (n = 36). The aim of this study was to investigate whether CR can reduce risk factors for CVD and insulin resistance in nonobese humans and, moreover, to assess whether improvements are exclusive to a period of weight loss or continue during weight maintenance. According to the energy balance method, the 25% CR intervention (n = 34) produced 16.5 ± 1.5% (mean ± SE) and 14.8 ± 1.5% CR after 12 and 24 mo (M12, M24), resulting in significant weight loss (M12 −9 ± 0.5 kg, M24 −9 ± 0.5 kg, P < 0.001). Weight was maintained in the group that continued their habitual diet ad libitum (AL, n = 19). In comparison to AL, 24 mo of CR decreased visceral (−0.5 ± 0.01 kg, P < 0.0001) and subcutaneous abdominal adipose tissue (−1.9 ± 0.2kg, P < 0.001) as well as intramyocellular lipid content (−0.11 ± 0.05%, P = 0.031). Furthermore, CR decreased blood pressure (SBP −8 ± 3 mmHg, P = 0.005; DBP −6 ± 2 mmHg, P < 0.001), total cholesterol (−13.6 ± 5.3 mg/dl, P = 0.001), and LDL-cholesterol (−12.9 ± 4.4 mg/dl, P = 0.005), and the 10-yr risk of CVD-disease was reduced by 30%. Homeostasis model assessment of insulin resistance (HOMA-IR) decreased during weight loss in the CR group (−0.46 ± 0.15, P = 0.003), but this decrease was not maintained during weight maintenance (−0.11 ± 0.15, P = 0.458). In conclusion, sustained CR in healthy, nonobese individuals is beneficial in improving risk factors for cardiovascular and metabolic disease such as visceral adipose tissue mass, ectopic lipid accumulation, blood pressure, and lipid profile, whereas improvements in insulin sensitivity were only transient.
Keywords: caloric restriction, cardiometabolic health, ectopic fat accumulation, physical fitness, visceral adipose tissue
INTRODUCTION
As the average age of the US and worldwide population is increasing, so, too, is the prevalence and incidence of chronic metabolic diseases such as cardiovascular disease (CVD) and type 2 diabetes (47a). Over the past two decades, CVD-related deaths in the US alone increased by more than 30%, which is probably related to an aging population (39). The economic burden of CVD to the society is enormous; one-half of the total health care expenditures in the US ($610 million USD) are devoted to CVD treatment, and current estimates project a threefold increase in these costs by 2030 (15).
With increasing age, physical and mental functionality decline, and the susceptibility to diseases increases (“primary aging”) (18). Vice versa, CVD accelerates the aging process by impairing metabolic health, reducing physical function, and reducing the quality of life (“secondary aging”). The progressive and detrimental interaction between aging and the development of CVD is likely related to a set of factors including increased abdominal adiposity, ectopic lipid accumulation, hypertension, and hyperlipidemia. Treatments to reverse these pathologies may thus attenuate the aging process and the development of CVD.
The largest and longest, controlled studies of calorie restriction (CR) at the National Institute of Aging (n = 121 rhesus monkeys) and at University of Wisconsin at Madison (n = 76 rhesus monkeys) and other studies in nonhuman primates collectively show that CR improves survival and reduces CVD mortality (2, 5, 7, 8, 26). In a review of these studies (25), it was concluded that CR is a promising nutritional intervention to prevent prevalent chronic diseases such as CVD, insulin resistance, and cancer, even in the absence of effects on lifespan. In line with this observation, human retrospective and observational studies have shown that moderate CR has been associated with increased lifespan (50), reduced CVD mortality (17, 43, 50), and improvements in metabolic risk factors (11, 12, 27, 42, 46, 47).
Indeed, numerous randomized clinical trials have supported the potential of CR to improve the cardiometabolic profile of overweight and obese subjects (reviewed in Ref. 14). However, if CR interventions are advocated for lifespan and healthspan extension in all adults, studies are also needed in normal-weight subjects. The most comprehensive assessment of the effects of sustained CR in humans was performed in the initial CALERIE studies followed by a three-site, single protocol across the US in nonobese individuals (9, 16, 33, 37). In an ancillary study to the CALERIE 2 protocol, additional measurements were obtained to evaluate whether CR sustained for 24 mo reduces ectopic fat depots and improves cardiometabolic risk factors (blood pressure and plasma lipid profile). We hypothesized that CR in nonobese subjects significantly improves these outcomes during weight loss (1st year) and that these effects persist during CR-induced weight maintenance throughout the 2-yr intervention.
METHODS
Design
In this multicenter trial (CALERIE 2), participants were randomly allocated to an intervention group aimed to reduce energy intake by 25% (CR) or to a control group with instructions to maintain habitual energy intake on an ad libitum basis (AL) for 24 mo (37). Randomization was in a 2:1 allocation in favor of the CR group and stratified by study site, sex, and BMI dichotomized into normal weight (22.0 ≤ BMI <25.0 kg/m2) or overweight (25.0 ≤ BMI <28.0 kg/m2). The ancillary study is registered at clinicaltrials.gov (NCT02695511) and was monitored by the Institutional Review Board of Pennington Biomedical Research Center. Individuals provided informed consent for the additional visits and procedures.
Subjects
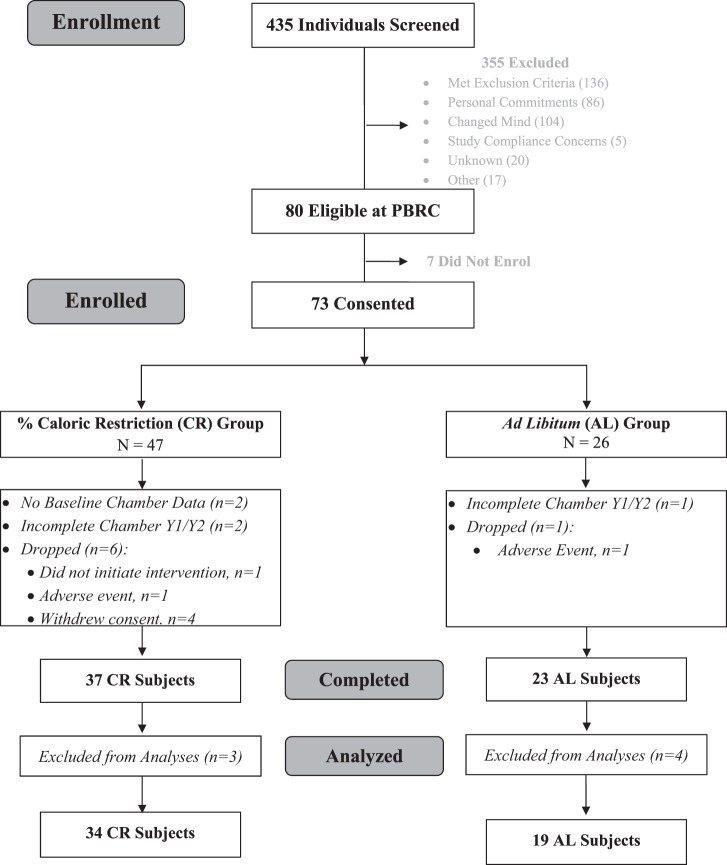
The study was offered to the 80 healthy, nonobese individuals enrolled in CALERIE 2 at Pennington Biomedical Research Center. In addition to the inclusion and exclusion criteria for CALERIE 2 (inclusion: age, 20–50 yr for men, 20–47 yr for women, 22.0 ≤ BMI < 28 kg/m2; exclusion: history or clinical manifestation of CVD and diabetes, abnormal laboratory markers, psychological problems, and regular use of medication except for oral contraceptives), individuals were excluded for contraindications to MRI. To study the true effects of CR, without bias from nonadherent participants, included subjects in the CR group were required to have ≥5% weight loss at month 12 (M12) and/or month 24 (M24), and AL subjects were required to have <5% weight loss. The CONSORT diagram summarizing throughput of individuals in the ancillary study is shown in Fig. 1.
Fig. 1.
CONSORT diagram. Summary of throughput of individuals in the ancillary study.
Study Interventions
From day 1, the CR intervention targeted a sustained 25% restriction of energy intake prescribed according to their energy requirements at baseline. Energy requirements and actual energy intake were determined by the energy-intake balance method, combining measures of daily energy expenditure (doubly labeled water) and changes in body mass, as previously described (31, 34, 36). To facilitate adherence to 25% CR, all meals were provided for the first 27 days of the study, and these included three diets differing in macronutrient composition; standard American (AHA Step 1), Mediterranean, and low fat. Participants also attended regular group and individual meetings with trained interventionists throughout the study (individual meetings weekly in first month, twice monthly with additional biweekly phone contact until M12, and monthly until M24; group meetings monthly from M2 to M24) (36). The intervention was purposely designed to achieve ~15% weight loss during the first 12 mo [commensurate with a 25% CR based on a model of the Phase 1 data (30)] and with sustained CR from baseline in the second year, to promote weight loss maintenance. Participants randomized to the AL group were advised to continue their current diets on a completely ad libitum basis. No specific level of physical activity was required or recommended for either group. All participants received a multivitamin (Nature Made Multi Complete; Pharmavite, Mission Hills, CA) and calcium supplement (1,000 mg/day; Douglas Laboratories, Pittsburgh, PA).
Anthropometrics and Vital Signs
Height was measured at screening using a wall-mounted stadiometer. Weight (Scale Tronix 5200, White Plains, NY) was a metabolic weight measured in the morning after an overnight fast and voiding while wearing a surgical gown, which was subtracted from the total weight. Blood pressure was measured in duplicate while the participant was sitting after a 5-min rest. Mean arterial pressure (MAP) was calculated as follows (2×DBP+SBP)/3.
Body Composition
Body composition [fat mass (FM) and fat-free mass (FFM)] was measured by dual-energy X-ray absorptiometry (DEXA; Hologic QDR 4500A,Bedford, MA). Abdominal adipose tissue (AT) distribution and ectopic lipid accumulation in skeletal muscle and liver were measured by magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS), respectively using a 3.0 T scanner (General Electric, Excite HD System, Milwaukee, WI).
Adipose tissue distribution.
Abdominal tissue (AT) volumes, including total, subcutaneous (SAT), and visceral (VAT) tissues, were quantified between the symphysis pubis and the dome of the diaphragm, using ~8 axial MRI images of 3.4 mm thickness with no interslice gap (38). SliceOmatic 4.2 image analysis software (Tomovision, Montreal, PQ, Canada) was used to analyze images on a PC workstation (Gateway, PIII 500 MHz). All MRI scans were read by the same trained observer.
Muscle and liver lipid content.
Briefly, intramyocellular lipid content (IMCL, soleus muscle) and intrahepatic lipid content (IHL) were measured by the proton magnetic resonance spectroscopy technique (1H-MRS) on a 3-T whole body imaging and spectroscopy system (General Electric, Excite HD System, Milwaukee, WI) using the PRESS box (Point RESolved Spectroscopy) technique as described previously (20, 22).
Clinical Chemistry
Fasting blood samples were obtained, and the following assays were performed at the CALERIE central biochemistry laboratory at University of Vermont: lipids, using a Beckman-Coulter Synchron CX7 (Brea, CA); total cholesterol, by the cholesterol esterase/oxidase/peroxidase method; triacylglycerols, by the GPO-Trinder method; and HDL-cholesterol (HDL-C), by an assay from Trinity Biotech (Jamestown, NY); LDL-cholesterol (LDL-C), calculated using the Friedwald equation (13); glucose, colorimetric reflectance spectrophotometry (Vitros, Ortho Clinical Diagnostics, Rochester, NY); insulin, chemiluminescent immunoassay (Elecsys 2010; Roche Diagnostics, Indianapolis, IN) concentrations, determined from fasting concentrations; and HOMA-IR, calculated as (insulin (µU/ml) × glucose (mg/dl)/405).
Estimates of 10-Year CVD Risk
Ten-year CVD risk was calculated using the sex-specific equations developed by Anderson et al. (1) that include total and HDL-cholesterol (as ratio), systolic blood pressure, age and sex. Smoking, presence of diabetes, and abnormal ECG were set to 0 in the model, since these variables were not evident in the present study.
Maximal Aerobic Capacity and Physical Activity
V̇o2peak was measured using the Cornell incremental treadmill test (44), with the speed and/or grade of the treadmill changing every 2 min, as previously described (32). V̇o2 was measured continuously and calculated at 15-s intervals using a calibrated metabolic cart (TrueOne 2400 or TrueMax 2400; Parvo Medics, Sandy, UT). The single highest 15-s V̇o2 value during the exercise test was considered the V̇o2peak, which was expressed per kilogram of body weight. Physical activity was assessed with the 7-day recall questionnaire (40), in which the amount of time spent sleeping and engaging in moderate, hard, and very hard activity was reported; light activity was calculated by difference.
Statistical Methods
This study was powered by the ability to detect a significant adaptation in energy expenditure (a reduction that is greater than expected, based on the reduction in metabolic mass, the primary outcome) from baseline and to detect differences between the two diet groups (AL vs. CR). A random subject effect was included to account for intraindividual correlations over time. Data are presented as least square means (LSM) ± SE that are derived from the linear mixed model. Contrasts of the LSM were used to compare adjusted mean change between intervention groups and to test for group differences in adjusted mean change at M12 and M24. P values represent the significance of the difference of LSM. All analyses were carried out using SAS/STAT software, version 9.4 of the SAS System for Windows (SAS Institute, Cary, NC). All tests were evaluated using significance level α = 0.05.
RESULTS
At baseline, study groups did not significantly differ in age, weight, BMI, or body fat [CR: n = 34 (24 women), AL: n = 19 (12 women)]. Despite randomization, fasting insulin concentrations and HOMA-IR tended to differ between groups at baseline (Table 1).
Table 1.
Subjects’ characteristics
| AL | CR | P | |
|---|---|---|---|
| N (men, women) | 19 (7, 12) | 34 (10, 24) | |
| Age, yr | 38.7 ± 1.2 | 40.0 ± 1.2 | 0.47 |
| Weight, kg | 71.0 ± 1.9 | 71.9 ± 1.5 | 0.73 |
| Body mass index, kg/m2 | 25.5 ± 0.4 | 25.7 ± 0.3 | 0.70 |
| Normal weight, n | 9 | 14 | |
| Overweight, n | 10 | 20 | |
| Fat-free mass, kg | 48.3 ± 1.9 | 47.8 ± 1.5 | 0.86 |
| Fat mass, kg | 23.4 ± 0.9 | 24.7 ± 0.9 | 0.36 |
| Body fat, % | 32.9 ± 1.3 | 34.2 ± 1.1 | 0.47 |
| Abdominal subcutaneous AT, kg | 5.0 ± 0.3 | 5.1 ± 0.3 | 0.26 |
| Abdominal visceral AT, kg | 0.7 ± 0.2 | 0.7 ± 0.1 | 0.29 |
| Systolic blood pressure, mmHg | 112 ± 2 | 115 ± 2 | 0.95 |
| Diastolic blood pressure, mmHg | 73 ± 2 | 76 ± 1 | 0.83 |
| Glucose, mg/dl | 83 ± 1 | 82 ± 1 | 0.36 |
| Insulin, mU/ml | 6.5 ± 0.5 | 5.1 ± 0.4 | 0.06 |
| HOMA-IR, AU | 1.33 ± 0.11 | 1.05 ± 0.09 | 0.06 |
Data are presented as LSM ± SE. AT, adipose tissue; HOMA-IR, homeostatic model assessment of insulin resistance. P value indicates statistical significance of difference between the calorie restriction (CR) and ad libitum diet (AL) groups.
Body Weight, Body Composition, and Ectopic Fat
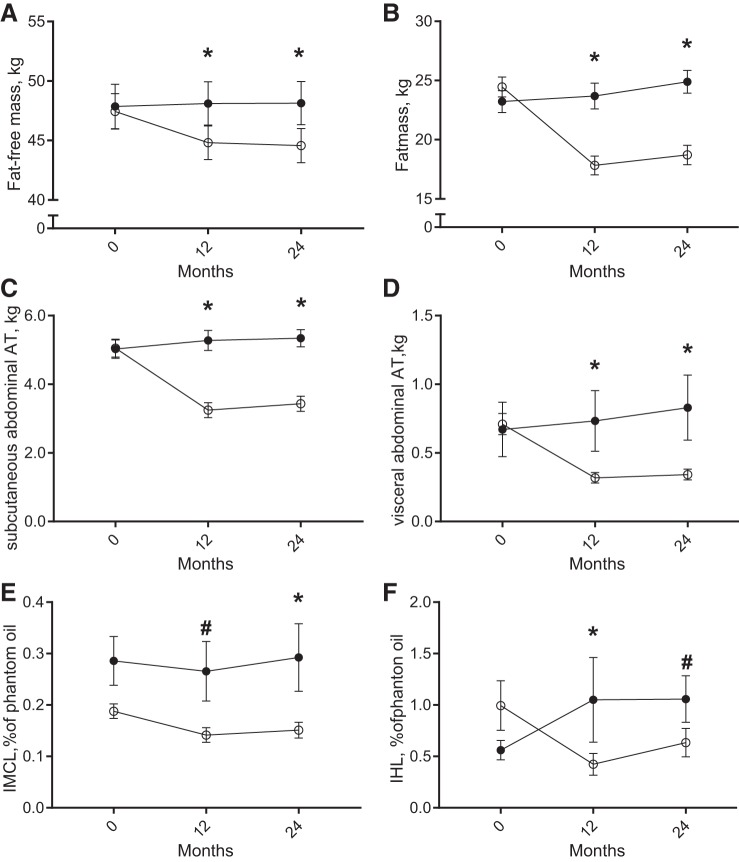
Over the study period of 24 mo, the CR group achieved a mean CR of 14.8 ± 1.5% (16.5 ± 1.5% at M12). Per study design, changes in body weight were different between the groups (P < 0.001). Whereas participants in the AL group slightly increased their body weight over the course of the study (M12: +0.6 ± 0.6 kg, P = 0.30; M24: +1.8 ± 3.0 kg, P = 0.003), CR induced a significant weight loss at M12 (−9.4 ± 0.4 kg, P < 0.001), which was maintained at M24 (CR: −8.7 ± 2.4 kg, P < 0.001). Accordingly, in the CR group, both fat-free mass and fat mass were significantly reduced at both time points (Fig. 2, A and B). Abdominal fat mass in both subcutaneous and visceral depots was significantly decreased by CR at M12 compared with AL and remained decreased at M24 (all, P < 0.001; Fig. 2, C and D). Additionally, ectopic lipid in the liver and skeletal muscle (soleus) was significantly reduced by CR (Fig. 2, E and F). A CR-induced decrease in intrahepatic lipid was significantly different from the change in the AL group at M12, but not at M24 (M12: P = 0.01, M24: P = 0.12; Fig. 2E). Presenting a different pattern, IMCL was reduced at M12, but reached statistically significance only at M24 (M12: P = 0.07, M24: P = 0.03; Fig. 2F).
Fig. 2.
Changes in body composition, abdominal fat, and ectopic fat accumulation during sustained calorie restriction (CR, open symbols, n = 34) or ad libitum diet (AL, filled symbols, n = 19). Data are presented as LSM ± SE. A–B: fat mass (FM, circles) and fat-free mass (FFM, squares). C–D: abdominal subcutaneous (circles) and visceral (squares) adipose tissue (AT, CR: n = 33, AL: n = 18). E: intramyocellular lipid content (IMCL, CR: n = 33, AL: n = 16). F: intrahepatic lipid content (IHL, CR: n = 33, AL: n = 14). *Statistical significance, P < 0.05; #statistical trend, P < 0.10 of the difference of LSM.
Cardiovascular Risk Profile
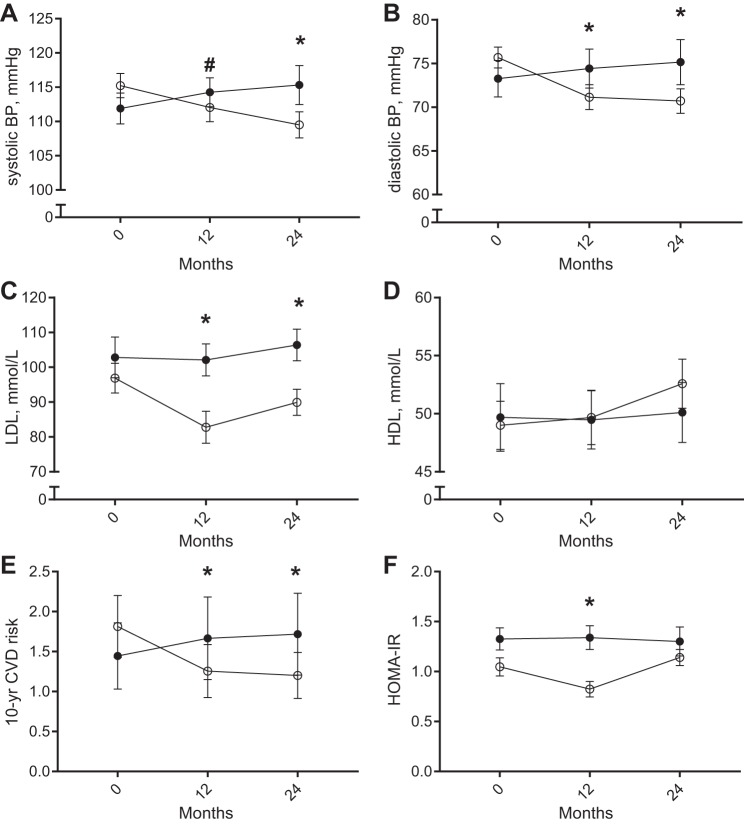
CR induced a decrease in systolic blood pressure over time compared with AL, which reached statistical significance at M24 (Fig. 3A). Diastolic blood pressure was significantly reduced at M12 and remained reduced during the CR-weight maintenance period compared with AL (Fig. 3B). MAP was therefore significantly reduced from baseline at both M12 and M24 in CR subjects (M12: −5.8 ± 2.1, P = 0.007; M24: −7.6 ± 2.0 mmHg, P < 0.001). Plasma LDL-C concentrations significantly decreased in the CR group at M12 and remained lower at M24 compared with the AL group (P = 0.005 and P = 0.006; Fig. 3C), whereas increases of HDL-C were not significant compared with the AL group (P = 0.12 and P = 0.18; Fig. 3D). As a result, the LDL/HDL ratio decreased over time during CR compared with AL (P = 0.001 and P = 0.005) as well as total cholesterol (P = 0.01, P = 0.01) and triglyceride (M12: −17.6 ± 7.9, P = 0.03; M24: −16.4 ± 7.5 mg/dl, P = 0.03) concentrations. By use of total cholesterol, HDL, systolic blood pressure, and age, the 10-yr risk for CVD was significantly lowered by 30% in the CR group at M12 and maintained throughout the 2-yr intervention (P < 0.001 and P = 0.001 at M12 and M24, respectively; Fig. 3E). Finally, insulin resistance assessed by HOMA-IR was significantly decreased in the CR group at M12 (−0.46 ± 0.15, P = 0.003), but the decrease in HOMA-IR was not maintained at M24 (−0.11 ± 0.15, P = 0.46; Fig. 3F).
Fig. 3.
Cardiovascular risk profile changes during CR (open circles, n = 34) and AL (filled circles, n = 19). Data are presented as LSM ± SE. A: systolic blood pressure (SBP). B: diastolic blood pressure (DBP). C: low-density lipoprotein-cholesterol (LDL). D: high-density lipoprotein-cholesterol (HDL), E: 10-yr CVD risk (1), F: HOMA-IR. *Statistical significance, P < 0.05; #statistical trend, P < 0.10 of the difference of LSM.
Physical Performance
V̇o2peak, expressed per body mass, was unchanged in the CR group (M24: +0.46 ± 0.75 ml·kg−1·min−1, P = 0.54), whereas it declined significantly in the AL group (M24: −4.40 ± 0.98 ml·kg−1·min−1, P < 0.001). In line with these findings, no difference between the CR and AL groups was observed for daily physical activity by 7-day recall. The hours spent sleeping or in activities of different intensities did not change differently over time between groups; also daily METs were unaffected by the intervention (M12: −0.16 ± 0.29 kcal·kg body wt−1·day−1, P = 0.57; M24: −0.24 ± 0.31 kcal·kg body wt−1·day−1, P = 0.44). Daily energy expenditure, calculated as function of body mass and physical activity ratio assessed by the 7-day recalls, decreased in the CR compared with the AL group (M12: −182 ± 51 kcal/day, P < 0.001; M24: −135 ± 63 kcal/day, P = 0.04).
DISCUSSION
In this novel study of sustained CR in healthy, nonobese participants, we found beneficial effects of CR on body composition, fat distribution, and cardiometabolic risk factors over a study period of 2 yr. We showed that CR-induced weight loss over the first year of the intervention reduced CVD risk factors including ectopic lipid accumulation, blood pressure, and plasma lipids. Importantly, these improvements were sustained between M12 and M24 of the intervention when energy intake was still below baseline levels, and weight loss from year 1 was maintained. The present study was unique in its duration and in its stringent level of control, which allowed us to describe how true CR affects the cardiometabolic, cardiovascular, and cardiorespiratory profile. In addition, we were able to quantify effects of sustained CR during weight loss maintenance and thus independently of weight loss.
In 2015, the Global Burden of Diseases study (12a) estimated that annually 10.7 million deaths are caused by high blood pressure, 5.2 million by hyperglycemia, 4.3 million by high total cholesterol concentrations, and 4.0 million by a high BMI (>25 kg/m2). Amelioration of these chronic health problems is primarily directed at treatments for individuals that present with clinically increased values for these variables. However, an equally favorable approach would be to tackle disease prevention and thereby delay and/or decelerate the development of chronic disease before manifestation. CR has been proposed as a superior nutritional intervention to attenuate the age-associated decline in chronic diseases with the potential to extend lifespan.
There are a myriad of studies in obese subjects showing the benefits of diet-induced weight loss on improvements in cardiometabolic risks (14). We have also previously demonstrated in shorter-term CR studies (6 mo), that overweight individuals experience that weight-loss induced changes ectopic fat, insulin sensitivity, and reduced 10-yr risk for CVD (9, 16, 33, 34). In the current 2-yr study in normal weight and slightly overweight (up to BMI 27.9 kg/m2), the CR intervention significantly reduced the mean BMI (−3.7 ± 0.3 kg/m2) and therefore reduced the prevalence of overweight in the CR group (prevalence of BMI >25; M0: n = 20, M12: n = 0, M24: n = 3 in the CR group; M0: n = 10, M12: n = 11, M24: n = 11 in the AL group). Of the lost body weight, importantly the loss of fat mass was larger than the loss of fat-free mass (70% FM vs. 30% FFM). Neither BMI at enrollment nor sex had a significant effect on weight change or changes in body composition outcomes (10), including the improvements in abdominal fat distribution, ectopic lipid, insulin resistance, and the 10-yr risk for CVD. Because adipose tissue depots differ in morphology, metabolism (24), and association with CVD (3), we quantified abdominal VAT and SAT depots using MRI. VAT, the depot most strongly associated with CVD incidence, decreased by more than 50% in the CR group after 12 mo, and SAT by more than 30%, which is in line with the results of CR imposed for six months (19, 35). The extent of the reductions of VAT and SAT in the present study are, to our knowledge, unprecedented in nonobese individuals and remarkable given the fairly low tissue masses of these depots at baseline (<1 kg and ~5 kg, respectively).
Next, we studied whether CR would induce changes in the plasma profile of cardiometabolic risk factors. We (23) reported previously a reduced CVD risk following 6 mo of CR, and now in this longer study, we confirmed this reduction after 12 and 24 mo (CR: −30%, AL: +15%) but with similar changes in weight. Interestingly, individual factors contributing to the reduced risk for CVD in the 6-mo study, including plasma lipids and blood pressure, were not significantly improved with 6 mo of CR. This finding emphasizes the unique importance of sustained CR, beyond weight loss for benefits to factors related to healthspan.
A central factor in the development of obesity, CVD, and aging is insulin resistance. To investigate possible mechanisms that may relate to reported changes in insulin sensitivity after 6 and 12 mo of CR (16), we measured ectopic lipid accumulation in skeletal muscle (IMCL) and the liver (IHL) using MRS. In contrast to the transient improvement of systemic insulin resistance, there was not a CR-induced improvement in IMCL content after 6 [CALERIE 1 (19)] or 12 mo, but after 24 mo. However the 15% reduction in IMCL that we observed is similar to the effect size observed in obese individuals with comparable weight loss after 4 mo (45), so, although not significant in our study, the reduction in IMCL may be implying some health benefits to the CR subjects. Interestingly, as for insulin resistance, the observed decrease in IHL content after 12 mo disappeared during the weight maintenance period, although percent body fat and abdominal VAT remained reduced. The parallel patterns of hepatic lipid content and systemic insulin resistance support the close relationship between hepatic and systemic metabolism and confirm earlier reports of improved hepatic metabolism following CR (reviewed in Ref. 41). We are not able to fully explain why sustained CR and weight loss was insufficient to maintain an improvement in insulin sensitivity and ectopic lipid accumulation. One might argue that this finding is driven by a reduced compliance with the CR intervention; however, the level of CR achieved from baseline throughout year 2 was estimated at 13.2% CR, only a 2% change from year 1. Alternatively, we acknowledge that the effect size may have been limited by studying a healthy, insulin-sensitive population. Therefore the individuals likely did not have substantially elevated liver fat or insulin resistance (HOMA-IR: 1.05 ± 0.09, liver fat: 1.0 ± 0.2%) as opposed to obese or nonalcoholic fatty liver disease patients who are the subject of most reported studies. Last, HOMA-IR is a crude measure of systemic insulin resistance. Characterization of the CR-induced insulin sensitivity effects on adipose tissue, liver, and skeletal muscle by means of a hyperinsulinemic-euglycemic clamp would provide a more sensitive measurement and thereby allow more definitive conclusions on the effects of CR on insulin sensitivity and its role in reducing cardiometabolic risks.
Finally, we assessed whether the reduction of caloric intake and the loss of body mass affected physical fitness. We found no significant change in maximal oxygen consumption per body weight (V̇o2peak and V̇o2max) in the CR-group after 12 and 24 mo. Our findings are in line with previous reports that showed no improvement in aerobic capacity if body (fat) mass is lost through diet, as opposed to weight loss through exercise (21, 45, 48, 49). We conclude that CR-induced improvements on cardiometabolic health appear to be independent of physical fitness.
To our knowledge, this is the first study designed to assess effects of controlled CR during and after weight loss in the same study. In the latter part of the study, CR was maintained from baseline, and the beneficial effects of CR on cardiometabolic risk factors were preserved, but not enhanced. On the basis of this observation, we conclude that reduced and sustained calorie intake is the main driver of the improvements in the cardiometabolic profile. The benefit of long-term CR has been supported by data collected on individuals who self-administer CR. These individuals, who were mostly normal weight at CR onset, have followed a regimen of self-imposed CR for an average of 15 yr and present a remarkably low metabolic risk profile (reviewed in Ref. 29). For example, it has been reported that no member of the CRON society has developed any chronic disease so far, and no use of medication is reported either (4).
In conclusion, we show that the classical CVD risk factors were all improved by sustained CR in nonobese participants. The improved CVD risk profile was associated with a significant reduction in overall adiposity as well as reduced abdominal fat and ectopic lipids, which are highly predictive for the development of chronic metabolic diseases with human aging. We hypothesize that sustained CR would not only prolong average life expectancy by reducing CVD/mortality (secondary aging) but more importantly would improve healthspan, even in individuals who are already “healthy” and not obese. Because sustained CR reduced lipid accumulation in all measured adipose tissue depots did not improve aerobic fitness, we further hypothesize that improvement of cardiometabolic health with CR is mediated through improved body composition and not physical fitness. Arguably the most striking finding of the present study is that the subjects were not obese or at high risk for the development of CVD but were still responsive to the effects of CR on measures of the cardiometabolic profile. Now, it has to be investigated whether such improvements can prevent or delay the onset of metabolic complications in later life of nonobese subjects in a comparable manner, as it has been established for the obese and suggested by data of the CR society members.
GRANTS
This work was supported by the National Institute of Aging [Grants R01 AG-029914, R01 AG-030226, R01 AG-031797, and U01 AG-020478]
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.M., L.A.G., H.H., and L.M.R. analyzed data; J.M., L.A.G., E.R., and L.M.R. interpreted results of experiments; J.M. prepared figures; J.M. drafted manuscript; J.M., L.A.G., S.R.S., E.R., and L.M.R. edited and revised manuscript; J.M., L.A.G., S.R.S., H.H., E.R., and L.M.R. approved final version of manuscript; S.R.S., E.R., and L.M.R. conceived and designed research; S.R.S., E.R., and L.M.R. performed experiments.
ACKNOWLEDGMENTS
We are indebted to the commitment of the study participants who invested over two years of their lives to participate in this clinical trial. The efforts of the CALERIE data coordinating center (James Rochon, William Krauss, and Manjushri Bhapkah) are also acknowledged and greatly appreciated.
Current address of S. R. Smith: Translational Research Institute for Metabolism and Diabetes, Florida Hospital and Sanford-Burnham Medical Research Institute, 301 E. Princeton St., Orlando, FL 32804.
REFERENCES
- 1.Anderson KM, Wilson PW, Odell PM, Kannel WB. An updated coronary risk profile. A statement for health professionals. Circulation 83: 356–362, 1991. doi: 10.1161/01.CIR.83.1.356. [DOI] [PubMed] [Google Scholar]
- 2.Bodkin NL, Alexander TM, Ortmeyer HK, Johnson E, Hansen BC. Mortality and morbidity in laboratory-maintained Rhesus monkeys and effects of long-term dietary restriction. J Gerontol A Biol Sci Med Sci 58: B212–B219, 2003. doi: 10.1093/gerona/58.3.B212. [DOI] [PubMed] [Google Scholar]
- 3.Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol 62: 921–925, 2013. doi: 10.1016/j.jacc.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cava E, Fontana L. Will calorie restriction work in humans? Aging (Albany NY) 5: 507–514, 2013. doi: 10.18632/aging.100581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cefalu WT, Wang ZQ, Bell-Farrow AD, Collins J, Morgan T, Wagner JD. Caloric restriction and cardiovascular aging in cynomolgus monkeys (Macaca fascicularis): metabolic, physiologic, and atherosclerotic measures from a 4-year intervention trial. J Gerontol A Biol Sci Med Sci 59: B1007–B1014, 2004. doi: 10.1093/gerona/59.10.B1007. [DOI] [PubMed] [Google Scholar]
- 7.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325: 201–204, 2009. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun 5: 3557, 2014. doi: 10.1038/ncomms4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, Cheatham RA, Tyler S, Tsay M, McCrory MA, Lichtenstein AH, Dallal GE, Dutta C, Bhapkar MV, Delany JP, Saltzman E, Roberts SB. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr 85: 1023–1030, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Das SK, Roberts SB, Bhapkar MV, Villareal DT, Fontana L, Martin CK, Racette SB, Fuss PJ, Kraus WE, Wong WW, Saltzman E, Pieper CF, Fielding RA, Schwartz AV, Ravussin E, Redman LM; CALERIE-2 Study Group . Body-composition changes in the Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy (CALERIE)-2 study: a 2-y randomized controlled trial of calorie restriction in nonobese humans. Am J Clin Nutr 105: 913–927, 2017. doi: 10.3945/ajcn.116.137232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci USA 101: 6659–6663, 2004. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontana L, Villareal DT, Weiss EP, Racette SB, Steger-May K, Klein S, Holloszy JO; Washington University School of Medicine CALERIE Group . Calorie restriction or exercise: effects on coronary heart disease risk factors. A randomized, controlled trial. Am J Physiol Endocrinol Metab 293: E197–E202, 2007. doi: 10.1152/ajpendo.00102.2007. [DOI] [PubMed] [Google Scholar]
- 12a.Forouzanfar MH, Afshin A, Alexander LT, Anderson HR, Bhutta ZA, Biryukov S, Brauer M, Burnett R, Cercy K, Charlson FJ, Cohen AJ, Dandona L, Estep K, Ferrari AJ, Frostad JJ, Fullman N, Gething PW, Godwin WW, Griswold M, Hay SI, Kinfu Y, Kyu HH, Larson HJ, Liang X, Lim SS, Liu PY, Lopez AD, Lozano R, Marczak L, Mensah GA, Mokdad AH, Moradi-Lakeh M, Naghavi M, Neal B, Reitsma MB, Roth GA, Salomon JA, Sur PJ, Vos T, Wagner JA, Wang H, Zhao Y, Zhou M, Aasvang GM, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, Abdulle AM, Abera SF, Abraham B, Abu-Raddad LJ, Abyu GY, Adebiyi AO, Adedeji IA, Ademi Z, Adou AK, Adsuar JC, Agardh EE, Agarwal A, Agrawal A, Kiadaliri AA, Ajala ON, Akinyemiju TF, Al-Aly Z, Alam K, Alam NKM, Aldhahri SF, Aldridge RW, Alemu ZA, Ali R, Alkerwi A, Alla F, Allebeck P, Alsharif U, Altirkawi KA, Martin EA, Alvis-Guzman N, Amare AT, Amberbir A, Amegah AK, Amini H, Ammar W, Amrock SM, Andersen HH, Anderson BO, Antonio CAT, Anwari P, Ärnlöv J, Artaman A, Asayesh H, Asghar RJ, Assadi R, Atique S, Avokpaho EFGA, Awasthi A, Quintanilla BPA, Azzopardi P, Bacha U, Badawi A, Bahit MC, Balakrishnan K, Barac A, Barber RM, Barker-Collo SL, Bärnighausen T, Barquera S, Barregard L, Barrero LH, Basu S, Batis C, Bazargan-Hejazi S, Beardsley J, Bedi N, Beghi E, Bell B, Bell ML, Bello AK, Bennett DA, Bensenor IM, Berhane A, Bernabé E, Betsu BD, Beyene AS, Bhala N, Bhansali A, Bhatt S, Biadgilign S, Bikbov B, Bisanzio D, Bjertness E, Blore JD, Borschmann R, Boufous S, Bourne RRA, Brainin M, Brazinova A, Breitborde NJK, Brenner H, Broday DM, Brugha TS, Brunekreef B, Butt ZA, Cahill LE, Calabria B, Campos-Nonato IR, Cárdenas R, Carpenter DO, Carrero JJ, Casey DC, Castañeda-Orjuela CA, Rivas JC, Castro RE, Catalá-López F, Chang J-C, Chiang PP-C, Chibalabala M, Chimed-Ochir O, Chisumpa VH, Chitheer AA, Choi J-YJ, Christensen H, Christopher DJ, Ciobanu LG, Coates MM, Colquhoun SM, Manzano AGC, Cooper LT, Cooperrider K, Cornaby L, Cortinovis M, Crump JA, Cuevas-Nasu L, Damasceno A, Dandona R, Darby SC, Dargan PI, das Neves J, Davis AC, Davletov K, de Castro EF, De la Cruz-Góngora V, De Leo D, Degenhardt L, Del Gobbo LC, del Pozo-Cruz B, Dellavalle RP, Deribew A, Jarlais DCD, Dharmaratne SD, Dhillon PK, Diaz-Torné C, Dicker D, Ding EL, Dorsey ER, Doyle KE, Driscoll TR, Duan L, Dubey M, Duncan BB, Elyazar I, Endries AY, Ermakov SP, Erskine HE, Eshrati B, Esteghamati A, Fahimi S, Faraon EJA, Farid TA, Farinha CSS, Faro A, Farvid MS, Farzadfar F, Feigin VL, Fereshtehnejad S-M, Fernandes JG, Fischer F, Fitchett JRA, Fleming T, Foigt N, Foreman K, Fowkes FGR, Franklin RC, Fürst T, Futran ND, Gakidou E, Garcia-Basteiro AL, Gebrehiwot TT, Gebremedhin AT, Geleijnse JM, Gessner BD, Giref AZ, Giroud M, Gishu MD, Giussani G, Goenka S, Gomez-Cabrera MC, Gomez-Dantes H, Gona P, Goodridge A, Gopalani SV, Gotay CC, Goto A, Gouda HN, Gugnani HC, Guillemin F, Guo Y, Gupta R, Gupta R, Gutiérrez RA, Haagsma JA, Hafezi-Nejad N, Haile D, Hailu GB, Halasa YA, Hamadeh RR, Hamidi S, Handal AJ, Hankey GJ, Hao Y, Harb HL, Harikrishnan S, Haro JM, Hassanvand MS, Hassen TA, Havmoeller R, Heredia-Pi IB, Hernández-Llanes NF, Heydarpour P, Hoek HW, Hoffman HJ, Horino M, Horita N, Hosgood HD, Hoy DG, Hsairi M, Htet AS, Hu G, Huang JJ, Husseini A, Hutchings SJ, Huybrechts I, Iburg KM, Idrisov BT, Ileanu BV, Inoue M, Jacobs TA, Jacobsen KH, Jahanmehr N, Jakovljevic MB, Jansen HAFM, Jassal SK, Javanbakht M, Jayaraman SP, Jayatilleke AU, Jee SH, Jeemon P, Jha V, Jiang Y, Jibat T, Jin Y, Johnson CO, Jonas JB, Kabir Z, Kalkonde Y, Kamal R, Kan H, Karch A, Karema CK, Karimkhani C, Kasaeian A, Kaul A, Kawakami N, Kazi DS, Keiyoro PN, Kemmer L, Kemp AH, Kengne AP, Keren A, Kesavachandran CN, Khader YS, Khan AR, Khan EA, Khan G, Khang Y-H, Khatibzadeh S, Khera S, Khoja TAM, Khubchandani J, Kieling C, Kim C, Kim D, Kimokoti RW, Kissoon N, Kivipelto M, Knibbs LD, Kokubo Y, Kopec JA, Koul PA, Koyanagi A, Kravchenko M, Kromhout H, Krueger H, Ku T, Defo BK, Kuchenbecker RS, Bicer BK, Kuipers EJ, Kumar GA, Kwan GF, Lal DK, Lalloo R, Lallukka T, Lan Q, Larsson A, Latif AA, Lawrynowicz AEB, Leasher JL, Leigh J, Leung J, Levi M, Li X, Li Y, Liang J, Liu S, Lloyd BK, Logroscino G, Lotufo PA, Lunevicius R, MacIntyre M, Mahdavi M, Majdan M, Majeed A, Malekzadeh R, Malta DC, Manamo WAA, Mapoma CC, Marcenes W, Martin RV, Martinez-Raga J, Masiye F, Matsushita K, Matzopoulos R, Mayosi BM, McGrath JJ, McKee M, Meaney PA, Medina C, Mehari A, Mejia-Rodriguez F, Mekonnen AB, Melaku YA, Memish ZA, Mendoza W, Mensink GBM, Meretoja A, Meretoja TJ, Mesfin YM, Mhimbira FA, Millear A, Miller TR, Mills EJ, Mirarefin M, Misganaw A, Mock CN, Mohammadi A, Mohammed S, Mola GLD, Monasta L, Hernandez JCM, Montico M, Morawska L, Mori R, Mozaffarian D, Mueller UO, Mullany E, Mumford JE, Murthy GVS, Nachega JB, Naheed A, Nangia V, Nassiri N, Newton JN, Ng M, Nguyen QL, Nisar MI, Pete PMN, Norheim OF, Norman RE, Norrving B, Nyakarahuka L, Obermeyer CM, Ogbo FA, Oh I-H, Oladimeji O, Olivares PR, Olsen H, Olusanya BO, Olusanya JO, Opio JN, Oren E, Orozco R, Ortiz A, Ota E, Pa M, Pana A, Park E-K, Parry CD, Parsaeian M, Patel T, Caicedo AJP, Patil ST, Patten SB, Patton GC, Pearce N, Pereira DM, Perico N, Pesudovs K, Petzold M, Phillips MR, Piel FB, Pillay JD, Plass D, Polinder S, Pond CD, Pope CA, Pope D, Popova S, Poulton RG, Pourmalek F, Prasad NM, Qorbani M, Rabiee RHS, Radfar A, Rafay A, Rahimi-Movaghar V, Rahman M, Rahman MHU, Rahman SU, Rai RK, Rajsic S, Raju M, Ram U, Rana SM, Ranganathan K, Rao P, García CAR, Refaat AH, Rehm CD, Rehm J, Reinig N, Remuzzi G, Resnikoff S, Ribeiro AL, Rivera JA, Roba HS, Rodriguez A, Rodriguez-Ramirez S, Rojas-Rueda D, Roman Y, Ronfani L, Roshandel G, Rothenbacher D, Roy A, Saleh MM, Sanabria JR, Sanchez-Riera L, Sanchez-Niño MD, Sánchez-Pimienta TG, Sandar L, Santomauro DF, Santos IS, Sarmiento-Suarez R, Sartorius B, Satpathy M, Savic M, Sawhney M, Schmidhuber J, Schmidt MI, Schneider IJC, Schöttker B, Schutte AE, Schwebel DC, Scott JG, Seedat S, Sepanlou SG, Servan-Mori EE, Shaddick G, Shaheen A, Shahraz S, Shaikh MA, Levy TS, Sharma R, She J, Sheikhbahaei S, Shen J, Sheth KN, Shi P, Shibuya K, Shigematsu M, Shin M-J, Shiri R, Shishani K, Shiue I, Shrime MG, Sigfusdottir ID, Silva DAS, Silveira DGA, Silverberg JI, Simard EP, Sindi S, Singh A, Singh JA, Singh PK, Slepak EL, Soljak M, Soneji S, Sorensen RJD, Sposato LA, Sreeramareddy CT, Stathopoulou V, Steckling N, Steel N, Stein DJ, Stein MB, Stöckl H, Stranges S, Stroumpoulis K, Sunguya BF, Swaminathan S, Sykes BL, Szoeke CEI, Tabarés-Seisdedos R, Takahashi K, Talongwa RT, Tandon N, Tanne D, Tavakkoli M, Taye BW, Taylor HR, Tedla BA, Tefera WM, Tegegne TK, Tekle DY, Terkawi AS, Thakur JS, Thomas BA, Thomas ML, Thomson AJ, Thorne-Lyman AL, Thrift AG, Thurston GD, Tillmann T, Tobe-Gai R, Tobollik M, Topor-Madry R, Topouzis F, Towbin JA, Tran BX, Dimbuene ZT, Tsilimparis N, Tura AK, Tuzcu EM, Tyrovolas S, Ukwaja KN, Undurraga EA, Uneke CJ, Uthman OA, van Donkelaar A, van Os J, Varakin YY, Vasankari T, Veerman JL, Venketasubramanian N, Violante FS, Vollset SE, Wagner GR, Waller SG, Wang JL, Wang L, Wang Y, Weichenthal S, Weiderpass E, Weintraub RG, Werdecker A, Westerman R, Whiteford HA, Wijeratne T, Wiysonge CS, Wolfe CDA, Won S, Woolf AD, Wubshet M, Xavier D, Xu G, Yadav AK, Yakob B, Yalew AZ, Yano Y, Yaseri M, Ye P, Yip P, Yonemoto N, Yoon S-J, Younis MZ, Yu C, Zaidi Z, Zaki MES, Zhu J, Zipkin B, Zodpey S, Zuhlke LJ, Murray CJL. GBD 2015 Risk Factors Collaborators . Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388: 1659–1724, 2016. doi: 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499–502, 1972. [PubMed] [Google Scholar]
- 14.García-Prieto CF, Fernández-Alfonso MS. Caloric restriction as a strategy to improve vascular dysfunction in metabolic disorders. Nutrients 8: 370, 2016. doi: 10.3390/nu8060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ; American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research . Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 123: 933–944, 2011. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 16.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E; Pennington CALERIE Team . Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA 295: 1539–1548, 2006. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hindhede M. The effect of food restriction during war on mortality in Copenhagen. J Am Med Assoc 74: 381–382, 1920. doi: 10.1001/jama.1920.02620060015005. [DOI] [Google Scholar]
- 18.Holloszy JO. The biology of aging. Mayo Clin Proc 75, Suppl: S3–S8, 2000. [PubMed] [Google Scholar]
- 19.Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, Smith SR, Alfonso A, Ravussin E. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care 29: 1337–1344, 2006. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larson-Meyer DE, Newcomer BR, Heilbronn LK, Volaufova J, Smith SR, Alfonso AJ, Lefevre M, Rood JC, Williamson DA, Ravussin E; Pennington CALERIE Team . Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function. Obesity (Silver Spring) 16: 1355–1362, 2008. doi: 10.1038/oby.2008.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larson-Meyer DE, Redman L, Heilbronn LK, Martin CK, Ravussin E. Caloric restriction with or without exercise: the fitness versus fatness debate. Med Sci Sports Exerc 42: 152–159, 2010. doi: 10.1249/MSS.0b013e3181ad7f17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larson-Meyer DE, Smith SR, Heilbronn LK, Kelley DE, Ravussin E, Newcomer BR; Look AHEAD Adipose Research Group . Muscle-associated triglyceride measured by computed tomography and magnetic resonance spectroscopy. Obesity (Silver Spring) 14: 73–87, 2006. doi: 10.1038/oby.2006.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lefevre M, Redman LM, Heilbronn LK, Smith JV, Martin CK, Rood JC, Greenway FL, Williamson DA, Smith SR, Ravussin E; Pennington CALERIE Team . Caloric restriction alone and with exercise improves CVD risk in healthy nonobese individuals. Atherosclerosis 203: 206–213, 2009. doi: 10.1016/j.atherosclerosis.2008.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marinou K, Hodson L, Vasan SK, Fielding BA, Banerjee R, Brismar K, Koutsilieris M, Clark A, Neville MJ, Karpe F. Structural and functional properties of deep abdominal subcutaneous adipose tissue explain its association with insulin resistance and cardiovascular risk in men. Diabetes Care 37: 821–829, 2014. doi: 10.2337/dc13-1353. [DOI] [PubMed] [Google Scholar]
- 25.Mattison JA, Colman RJ, Beasley TM, Allison DB, Kemnitz JW, Roth GS, Ingram DK, Weindruch R, de Cabo R, Anderson RM. Caloric restriction improves health and survival of rhesus monkeys. Nat Commun 8: 14063, 2017. doi: 10.1038/ncomms14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 489: 318–321, 2012. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer TE, Kovács SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol 47: 398–402, 2006. doi: 10.1016/j.jacc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- 29.Most J, Tosti V, Redman LM, Fontana L. Calorie restriction in humans: an update. Ageing Res Rev 39: 36–45, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pieper C, Redman L, Racette S, Roberts S, Bhapkar M, Rochon J, Martin C, Kraus W, Das S, Williamson D, Ravussin E. Development of adherence metrics for caloric restriction interventions. Clin Trials 8: 155–164, 2011. doi: 10.1177/1740774511398369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Racette SB, Das SK, Bhapkar M, Hadley EC, Roberts SB, Ravussin E, Pieper C, DeLany JP, Kraus WE, Rochon J, Redman LM; CALERIE Study Group . Approaches for quantifying energy intake and %calorie restriction during calorie restriction interventions in humans: the multicenter CALERIE study. Am J Physiol Endocrinol Metab 302: E441–E448, 2012. doi: 10.1152/ajpendo.00290.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Racette SB, Rochon J, Uhrich ML, Villareal DT, Das SK, Fontana L, Bhapkar M, Martin CK, Redman LM, Fuss PJ, Roberts SB, Kraus WE. Effects of 2 years of calorie restriction on aerobic capacity and muscle strength. Med Sci Sports Exerc 49: 2240–2249, 2017. doi: 10.1249/MSS.0000000000001353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Racette SB, Weiss EP, Villareal DT, Arif H, Steger-May K, Schechtman KB, Fontana L, Klein S, Holloszy JO; Washington University School of Medicine CALERIE Group . One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci Med Sci 61: 943–950, 2006. doi: 10.1093/gerona/61.9.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ravussin E, Redman LM, Rochon J, Das SK, Fontana L, Kraus WE, Romashkan S, Williamson DA, Meydani SN, Villareal DT, Smith SR, Stein RI, Scott TM, Stewart TM, Saltzman E, Klein S, Bhapkar M, Martin CK, Gilhooly CH, Holloszy JO, Hadley EC, Roberts SB; CALERIE Study Team . A 2-year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. J Gerontol A Biol Sci Med Sci 70: 1097–1104, 2015. doi: 10.1093/gerona/glv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Redman LM, Heilbronn LK, Martin CK, Alfonso A, Smith SR, Ravussin E; Pennington CALERIE Team . Effect of calorie restriction with or without exercise on body composition and fat distribution. J Clin Endocrinol Metab 92: 865–872, 2007. doi: 10.1210/jc.2006-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rickman AD, Williamson DA, Martin CK, Gilhooly CH, Stein RI, Bales CW, Roberts S, Das SK. The CALERIE Study: design and methods of an innovative 25% caloric restriction intervention. Contemp Clin Trials 32: 874–881, 2011. doi: 10.1016/j.cct.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rochon J, Bales CW, Ravussin E, Redman LM, Holloszy JO, Racette SB, Roberts SB, Das SK, Romashkan S, Galan KM, Hadley EC, Kraus WE; CALERIE Study Team . Design and conduct of the CALERIE study: comprehensive assessment of the long-term effects of reducing intake of energy. J Gerontol A Biol Sci Med Sci 66A: 97–108, 2011. doi: 10.1093/gerona/glq168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ross R. Magnetic resonance imaging provides new insights into the characterization of adipose and lean tissue distribution. Can J Physiol Pharmacol 74: 778–785, 1996. doi: 10.1139/y96-072. [DOI] [PubMed] [Google Scholar]
- 39.Roth GA, Huffman MD, Moran AE, Feigin V, Mensah GA, Naghavi M, Murray CJ. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation 132: 1667–1678, 2015. doi: 10.1161/CIRCULATIONAHA.114.008720. [DOI] [PubMed] [Google Scholar]
- 40.Sallis JF, Haskell WL, Wood PD, Fortmann SP, Rogers T, Blair SN, Paffenbarger RS Jr. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol 121: 91–106, 1985. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 41.is Samuel VT, Shulman GI. Nonalcoholic fatty liver disease as a nexus of metabolic and hepatic diseases. Cell Metab 27: 22–41, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stein PK, Soare A, Meyer TE, Cangemi R, Holloszy JO, Fontana L. Caloric restriction may reverse age-related autonomic decline in humans. Aging Cell 11: 644–650, 2012. doi: 10.1111/j.1474-9726.2012.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strom A, Jensen RA. Mortality from circulatory diseases in Norway 1940–1945. Lancet 257: 126–129, 1951. doi: 10.1016/S0140-6736(51)91210-X. [DOI] [PubMed] [Google Scholar]
- 44.Tamesis B, Stelken A, Byers S, Shaw L, Younis L, Miller DD, Chaitman BR. Comparison of the Asymptomatic Cardiac Ischemia Pilot and modified Asymptomatic Cardiac Ischemia Pilot versus Bruce and Cornell exercise protocols. Am J Cardiol 72: 715–720, 1993. doi: 10.1016/0002-9149(93)90891-F. [DOI] [PubMed] [Google Scholar]
- 45.Toledo FG, Menshikova EV, Azuma K, Radiková Z, Kelley CA, Ritov VB, Kelley DE. Mitochondrial capacity in skeletal muscle is not stimulated by weight loss despite increases in insulin action and decreases in intramyocellular lipid content. Diabetes 57: 987–994, 2008. doi: 10.2337/db07-1429. [DOI] [PubMed] [Google Scholar]
- 46.Walford RL, Harris SB, Gunion MW. The calorically restricted low-fat nutrient-dense diet in Biosphere 2 significantly lowers blood glucose, total leukocyte count, cholesterol, and blood pressure in humans. Proc Natl Acad Sci USA 89: 11533–11537, 1992. doi: 10.1073/pnas.89.23.11533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walford RL, Mock D, Verdery R, MacCallum T. Calorie restriction in biosphere 2: alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-year period. J Gerontol A Biol Sci Med Sci 57: B211–B224, 2002. doi: 10.1093/gerona/57.6.B211. [DOI] [PubMed] [Google Scholar]
- 47a.Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, Casey DC, Charlson FJ, Chen AZ, Coates MM, Coggeshall M, Dandona L, Dicker DJ, Erskine HE, Ferrari AJ, Fitzmaurice C, Foreman K, Forouzanfar MH, Fraser MS, Fullman N, Gething PW, Goldberg EM, Graetz N, Haagsma JA, Hay SI, Huynh C, Johnson CO, Kassebaum NJ, Kinfu Y, Kulikoff XR, Kutz M, Kyu HH, Larson HJ, Leung J, Liang X, Lim SS, Lind M, Lozano R, Marquez N, Mensah GA, Mikesell J, Mokdad AH, Mooney MD, Nguyen G, Nsoesie E, Pigott DM, Pinho C, Roth GA, Salomon JA, Sandar L, Silpakit N, Sligar A, Sorensen RJD, Stanaway J, Steiner C, Teeple S, Thomas BA, Troeger C, VanderZanden A, Vollset SE, Wanga V, Whiteford HA, Wolock T, Zoeckler L, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, Abera SF, Abreu DMX, Abu-Raddad LJ, Abyu GY, Achoki T, Adelekan AL, Ademi Z, Adou AK, Adsuar JC, Afanvi KA, Afshin A, Agardh EE, Agarwal A, Agrawal A, Kiadaliri AA, Ajala ON, Akanda AS, Akinyemi RO, Akinyemiju TF, Akseer N, Lami FHA, Alabed S, Al-Aly Z, Alam K, Alam NKM, Alasfoor D, Aldhahri SF, Aldridge RW, Alegretti MA, Aleman AV, Alemu ZA, Alexander LT, Alhabib S, Ali R, Alkerwi A, Alla F, Allebeck P, Al-Raddadi R, Alsharif U, Altirkawi KA, Martin EA, Alvis-Guzman N, Amare AT, Amegah AK, Ameh EA, Amini H, Ammar W, Amrock SM, Andersen HH, Anderson BO, Anderson GM, Antonio CAT, Aregay AF, Ärnlöv J, Arsenijevic VSA, Artaman A, Asayesh H, Asghar RJ, Atique S, Avokpaho EFGA, Awasthi A, Azzopardi P, Bacha U, Badawi A, Bahit MC, Balakrishnan K, Banerjee A, Barac A, Barker-Collo SL, Bärnighausen T, Barregard L, Barrero LH, Basu A, Basu S, Bayou YT, Bazargan-Hejazi S, Beardsley J, Bedi N, Beghi E, Belay HA, Bell B, Bell ML, Bello AK, Bennett DA, Bensenor IM, Berhane A, Bernabé E, Betsu BD, Beyene AS, Bhala N, Bhalla A, Biadgilign S, Bikbov B, Abdulhak AAB, Biroscak BJ, Biryukov S, Bjertness E, Blore JD, Blosser CD, Bohensky MA, Borschmann R, Bose D, Bourne RRA, Brainin M, Brayne CEG, Brazinova A, Breitborde NJK, Brenner H, Brewer JD, Brown A, Brown J, Brugha TS, Buckle GC, Butt ZA, Calabria B, Campos-Nonato IR, Campuzano JC, Carapetis JR, Cárdenas R, Carpenter DO, Carrero JJ, Castañeda-Orjuela CA, Rivas JC, Catalá-López F, Cavalleri F, Cercy K, Cerda J, Chen W, Chew A, Chiang PP-C, Chibalabala M, Chibueze CE, Chimed-Ochir O, Chisumpa VH, Choi J-YJ, Chowdhury R, Christensen H, Christopher DJ, Ciobanu LG, Cirillo M, Cohen AJ, Colistro V, Colomar M, Colquhoun SM, Cooper C, Cooper LT, Cortinovis M, Cowie BC, Crump JA, Damsere-Derry J, Danawi H, Dandona R, Daoud F, Darby SC, Dargan PI, das Neves J, Davey G, Davis AC, Davitoiu DV, de Castro EF, de Jager P, Leo DD, Degenhardt L, Dellavalle RP, Deribe K, Deribew A, Dharmaratne SD, Dhillon PK, Diaz-Torné C, Ding EL, dos Santos KPB, Dossou E, Driscoll TR, Duan L, Dubey M, Duncan BB, Ellenbogen RG, Ellingsen CL, Elyazar I, Endries AY, Ermakov SP, Eshrati B, Esteghamati A, Estep K, Faghmous IDA, Fahimi S, Faraon EJA, Farid TA, Farinha CSS, Faro A, Farvid MS, Farzadfar F, Feigin VL, Fereshtehnejad S-M, Fernandes JG, Fernandes JC, Fischer F, Fitchett JRA, Flaxman A, Foigt N, Fowkes FGR, Franca EB, Franklin RC, Friedman J, Frostad J, Fürst T, Futran ND, Gall SL, Gambashidze K, Gamkrelidze A, Ganguly P, Gankpé FG, Gebre T, Gebrehiwot TT, Gebremedhin AT, Gebru AA, Geleijnse JM, Gessner BD, Ghoshal AG, Gibney KB, Gillum RF, Gilmour S, Giref AZ, Giroud M, Gishu MD, Giussani G, Glaser E, Godwin WW, Gomez-Dantes H, Gona P, Goodridge A, Gopalani SV, Gosselin RA, Gotay CC, Goto A, Gouda HN, Greaves F, Gugnani HC, Gupta R, Gupta R, Gupta V, Gutiérrez RA, Hafezi-Nejad N, Haile D, Hailu AD, Hailu GB, Halasa YA, Hamadeh RR, Hamidi S, Hancock J, Handal AJ, Hankey GJ, Hao Y, Harb HL, Harikrishnan S, Haro JM, Havmoeller R, Heckbert SR, Heredia-Pi IB, Heydarpour P, Hilderink HBM, Hoek HW, Hogg RS, Horino M, Horita N, Hosgood HD, Hotez PJ, Hoy DG, Hsairi M, Htet AS, Htike MMT, Hu G, Huang C, Huang H, Huiart L, Husseini A, Huybrechts I, Huynh G, Iburg KM, Innos K, Inoue M, Iyer VJ, Jacobs TA, Jacobsen KH, Jahanmehr N, Jakovljevic MB, James P, Javanbakht M, Jayaraman SP, Jayatilleke AU, Jeemon P, Jensen PN, Jha V, Jiang G, Jiang Y, Jibat T, Jimenez-Corona A, Jonas JB, Joshi TK, Kabir Z, Kamal R, Kan H, Kant S, Karch A, Karema CK, Karimkhani C, Karletsos D, Karthikeyan G, Kasaeian A, Katibeh M, Kaul A, Kawakami N, Kayibanda JF, Keiyoro PN, Kemmer L, Kemp AH, Kengne AP, Keren A, Kereselidze M, Kesavachandran CN, Khader YS, Khalil IA, Khan AR, Khan EA, Khang Y-H, Khera S, Khoja TAM, Kieling C, Kim D, Kim YJ, Kissela BM, Kissoon N, Knibbs LD, Knudsen AK, Kokubo Y, Kolte D, Kopec JA, Kosen S, Koul PA, Koyanagi A, Krog NH, Defo BK, Bicer BK, Kudom AA, Kuipers EJ, Kulkarni VS, Kumar GA, Kwan GF, Lal A, Lal DK, Lalloo R, Lallukka T, Lam H, Lam JO, Langan SM, Lansingh VC, Larsson A, Laryea DO, Latif AA, Lawrynowicz AEB, Leigh J, Levi M, Li Y, Lindsay MP, Lipshultz SE, Liu PY, Liu S, Liu Y, Lo L-T, Logroscino G, Lotufo PA, Lucas RM, Lunevicius R, Lyons RA, Ma S, Machado VMP, Mackay MT, MacLachlan JH, Razek HMAE, Magdy M, Razek AE, Majdan M, Majeed A, Malekzadeh R, Manamo WAA, Mandisarisa J, Mangalam S, Mapoma CC, Marcenes W, Margolis DJ, Martin GR, Martinez-Raga J, Marzan MB, Masiye F, Mason-Jones AJ, Massano J, Matzopoulos R, Mayosi BM, McGarvey ST, McGrath JJ, McKee M, McMahon BJ, Meaney PA, Mehari A, Mehndiratta MM, Mejia-Rodriguez F, Mekonnen AB, Melaku YA, Memiah P, Memish ZA, Mendoza W, Meretoja A, Meretoja TJ, Mhimbira FA, Micha R, Millear A, Miller TR, Mirarefin M, Misganaw A, Mock CN, Mohammad KA, Mohammadi A, Mohammed S, Mohan V, Mola GLD, Monasta L, Hernandez JCM, Montero P, Montico M, Montine TJ, Moradi-Lakeh M, Morawska L, Morgan K, Mori R, Mozaffarian D, Mueller UO, Murthy GVS, Murthy S, Musa KI, Nachega JB, Nagel G, Naidoo KS, Naik N, Naldi L, Nangia V, Nash D, Nejjari C, Neupane S, Newton CR, Newton JN, Ng M, Ngalesoni FN, de Dieu Ngirabega J, Nguyen QL, Nisar MI, Pete PMN, Nomura M, Norheim OF, Norman PE, Norrving B, Nyakarahuka L, Ogbo FA, Ohkubo T, Ojelabi FA, Olivares PR, Olusanya BO, Olusanya JO, Opio JN, Oren E, Ortiz A, Osman M, Ota E, Ozdemir R, Pa M, Pain A, Pandian JD, Pant PR, Papachristou C, Park E-K, Park J-H, Parry CD, Parsaeian M, Caicedo AJP, Patten SB, Patton GC, Paul VK, Pearce N, Pedro JM, Stokic LP, Pereira DM, Perico N, Pesudovs K, Petzold M, Phillips MR, Piel FB, Pillay JD, Plass D, Platts-Mills JA, Polinder S, Pope CA, Popova S, Poulton RG, Pourmalek F, Prabhakaran D, Qorbani M, Quame-Amaglo J, Quistberg DA, Rafay A, Rahimi K, Rahimi-Movaghar V, Rahman M, Rahman MHU, Rahman SU, Rai RK, Rajavi Z, Rajsic S, Raju M, Rakovac I, Rana SM, Ranabhat CL, Rangaswamy T, Rao P, Rao SR, Refaat AH, Rehm J, Reitsma MB, Remuzzi G, Resnikoff S, Ribeiro AL, Ricci S, Blancas MJR, Roberts B, Roca A, Rojas-Rueda D, Ronfani L, Roshandel G, Rothenbacher D, Roy A, Roy NK, Ruhago GM, Sagar R, Saha S, Sahathevan R, Saleh MM, Sanabria JR, Sanchez-Niño MD, Sanchez-Riera L, Santos IS, Sarmiento-Suarez R, Sartorius B, Satpathy M, Savic M, Sawhney M, Schaub MP, Schmidt MI, Schneider IJC, Schöttker B, Schutte AE, Schwebel DC, Seedat S, Sepanlou SG, Servan-Mori EE, Shackelford KA, Shaddick G, Shaheen A, Shahraz S, Shaikh MA, Shakh-Nazarova M, Sharma R, She J, Sheikhbahaei S, Shen J, Shen Z, Shepard DS, Sheth KN, Shetty BP, Shi P, Shibuya K, Shin M-J, Shiri R, Shiue I, Shrime MG, Sigfusdottir ID, Silberberg DH, Silva DAS, Silveira DGA, Silverberg JI, Simard EP, Singh A, Singh GM, Singh JA, Singh OP, Singh PK, Singh V, Soneji S, Søreide K, Soriano JB, Sposato LA, Sreeramareddy CT, Stathopoulou V, Stein DJ, Stein MB, Stranges S, Stroumpoulis K, Sunguya BF, Sur P, Swaminathan S, Sykes BL, Szoeke CEI, Tabarés-Seisdedos R, Tabb KM, Takahashi K, Takala JS, Talongwa RT, Tandon N, Tavakkoli M, Taye B, Taylor HR, Ao BJT, Tedla BA, Tefera WM, Have MT, Terkawi AS, Tesfay FH, Tessema GA, Thomson AJ, Thorne-Lyman AL, Thrift AG, Thurston GD, Tillmann T, Tirschwell DL, Tonelli M, Topor-Madry R, Topouzis F, Towbin JA, Traebert J, Tran BX, Truelsen T, Trujillo U, Tura AK, Tuzcu EM, Uchendu US, Ukwaja KN, Undurraga EA, Uthman OA, Dingenen RV, van Donkelaar A, Vasankari T, Vasconcelos AMN, Venketasubramanian N, Vidavalur R, Vijayakumar L, Villalpando S, Violante FS, Vlassov VV, Wagner JA, Wagner GR, Wallin MT, Wang L, Watkins DA, Weichenthal S, Weiderpass E, Weintraub RG, Werdecker A, Westerman R, White RA, Wijeratne T, Wilkinson JD, Williams HC, Wiysonge CS, Woldeyohannes SM, Wolfe CDA, Won S, Wong JQ, Woolf AD, Xavier D, Xiao Q, Xu G, Yakob B, Yalew AZ, Yan LL, Yano Y, Yaseri M, Ye P, Yebyo HG, Yip P, Yirsaw BD, Yonemoto N, Yonga G, Younis MZ, Yu S, Zaidi Z, Zaki MES, Zannad F, Zavala DE, Zeeb H, Zeleke BM, Zhang H, Zodpey S, Zonies D, Zuhlke LJ, Vos T, Lopez AD, Murray CJL. GBD 2015 Mortality and Causes of Death Collaborators . Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388: 1459–1544, 2016. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiss EP, Albert SG, Reeds DN, Kress KS, McDaniel JL, Klein S, Villareal DT. Effects of matched weight loss from calorie restriction, exercise, or both on cardiovascular disease risk factors: a randomized intervention trial. Am J Clin Nutr 104: 576–586, 2016. doi: 10.3945/ajcn.116.131391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Ehsani AA, Holloszy JO; Washington University School of Medicine CALERIE Group . Lower extremity muscle size and strength and aerobic capacity decrease with caloric restriction but not with exercise-induced weight loss. J Appl Physiol (1985) 102: 634–640, 2007. doi: 10.1152/japplphysiol.00853.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willcox BJ, Willcox DC, Todoriki H, Fujiyoshi A, Yano K, He Q, Curb JD, Suzuki M. Caloric restriction, the traditional Okinawan diet, and healthy aging: the diet of the world’s longest-lived people and its potential impact on morbidity and life span. Ann N Y Acad Sci 1114: 434–455, 2007. doi: 10.1196/annals.1396.037. [DOI] [PubMed] [Google Scholar]