Short abstract
Irritable bowel syndrome is a disorder of unknown etiology characterized by widespread, chronic abdominal pain associated with altered bowel movements. Increasing amounts of evidence indicate that stressors presented during gestational periods could have long-term effects on the offspring’s tissue structure and function, which may predispose to gastrointestinal diseases. The aim of the present study is to determine whether prenatal maternal stressis a adverse factor affecting gastrointestinal sensitivity and to investigate possible mechanisms underlying prenatal maternal stress-induced visceral hypersensitivity in adult offspring. Prenatal maternal stress was induced in pregnant Sprague–Dawley rats by exposure to heterotypic intermitent stress from gestational day 7 to delivery. Prenatal maternal stress significantly increased visceromotor response to colorectal distention in adult offspring from the age of 6 weeks to 10 weeks. Prenatal maternal stress also enhanced neuronal excitability including depolarization of resting membrane potentials, reduction in rheobase, and an increase in the number of action potentials evoked by 2× and 3× rheobase current stimultion of colon-specific dorsal root ganglion neurons. Prenatal maternal stress remarkably enhanced expression of cystathionine-β-synthase and Nav1.7 in T13-L2 thoracolumbar dorsal root ganglions both at protein and mRNA levels. Intraperitoneal injection of aminooxyacetic acid, an inhibitor of cystathionine-β-synthase, attenuated prenatal maternal stress-induced visceral hypersensitivity in a dose-dependent manner. A consecutive seven-day administration of aminooxyacetic acid reversed the hyperexcitability of colon-specific dorsal root ganglion neurons and markedly reduced Nav1.7 expression. These results indicate that the presence of multiple psychophysical stressors during pregnancy is associated with visceral hypersensitivity in offspring, which is likely mediated by an upregualtion of cystathionine-β-synthase and Nav1.7 expression. Prenatal maternal stress might be a significant contributor to irritable bowel syndrome, and cystathionine-β-synthase might be a potential target for treatment for chronic visceral hypersensitivity in patients with irritable bowel syndrome.
Keywords: Irritable bowel syndrome, prenatal maternal stress, dorsal root ganglion, visceral hypersensitivity, cystathionine-β-synthase, voltage-gated sodium channel 1.7
Introduction
Irritable bowel syndrome (IBS) is characterized by a chronic recurrent abdominal pain and discomfort associated with an altered bowel habits in the absence of any detectable structural/organic abnormalities.1 However, the pathogenesis of chronic visceral hypersensitivity of IBS remains speculative. There is a growing body of evidence to support the concept that adult health status is likely determined by early life adversities.2 Chronic stress is one of the most common early life events associated with health outcomes which may persist into adulthood.3 According to the “fetal programming hypothesis,” prenatal exposure to suboptimal intrauterine conditions could predispose the individual to chronic disease at adult age.4 Barker suggested that alterations of the intrauterine environment produced individuals who were more prone to develop diseases such as coronary heart disease, type II diabetes mellitus, and hypertension.5 Several animal model-based studies have demonstrated that early psychological stress such as intermittent maternal deprivation impairs the normal development of gastrointestinal track and leads to long-lasting alterations in gastrointestinal functioning.6 However, few studies have investigated effects of stress during pregnancy on visceral sensitivity of the offspring. Psychophysical/social complaints are important to investigate because they are highly prevalent in what are otherwise normal pregnancies.7 Chronic stress causes or exacerbates symptoms such as abdominal pain and cramping in patients with IBS.8 Therefore, it is important and urgent to determine whether and how multiple psychophysical stressors to dam during pregnancy produce gastrointestinal hypersensitivity of rat offspring.
Hydrogen sulfide (H2S), one of the three gaseotransmitters/modulators, has been reported to be involved in many biological functions. It is increasingly recognized as a biologically important signaling molecule in many diseases such as pain and inflammation.9 H2S is synthesized mainly by the endogenous enzymes cystathionine-β-synthase (CBS), cystathionine-γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST).10,11 Previous studies have shown that CBS signaling plays a crucial role in development of inflammatory pain12 and neonatal colonic inflammation-induced visceral hyperalgesia.13 In addition, accumulating evidence suggests specific effects of CBS in fetal programming.14 It is, therefore, interesting to determine whether CBS signaling is involved in visceral hypersensitivity of adult offspring with prenatal maternal stress (PMS). Recently, we reported that CBS-positive colon-specific neurons in dorsal root ganglion (DRG) expressed Nav1.7 channels,15 suggesting an interaction between these two molecules. This was further supported from studies that CBS enhanced the excitability of hind paw-innervating DRG neurons via Nav1.7 channels on extrinsic afferent terminals.16 Nav1.7 channels play an important role in chronic pain,17,18 and its expression is increased in IBS models.19 However, it is unclear whether PMS is a prenatanl factor affecting gastrointestinal sensitivity via activaiton of CBS-Nav1.7 signaling pathway in adult offspring.
Therefore, we hypothesized that the presence of psychosocial/physical stressors to dam during pregnancy is associated with visceral hypersensitivity, which is likely mediated by upregulation of CBS and Nav1.7 in DRGs of the offspring. In the present study, we showed for the first time that gestational physical/psychological stress resulted in colonic visceral hypersensitivity in the offspring. We also demonstrated that PMS significantly enhanced expression of CBS and NaV1.7 in T13-L2 DRGs of offspring. The results of the present study may open up new avenues for preventive strategies with regard to functional gastrointestinal disorders in adult patients.
Materials and methods
Animals
Adult virgin Sprague–Dawley (SD) female rats weighing 200–220 g and their male offspring were housed in plastic cages under controlled conditions: 12 h light–12 h dark (light on 8:00), 20°C–22°C, and food and water available ad libitum. Care and handling of these SD rats were approved by the Institutional Animal Care and Use Committee at Soochow University. Adult female rat was mated with two males in the evening. Vaginal plug was examined daily in the morning (between 9:00 and 10:00). The onset of pregnancy was confirmed by the presence of vaginal plug which was defined as gestational day 1 (GD 1) as described previously.20
Gestational stress procedure
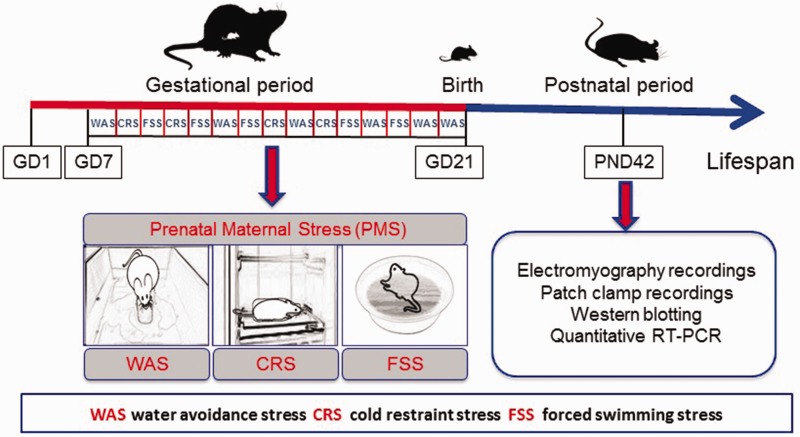
Beginning on the GD 7, the pregnant dams were exposed to a heterotypic intermittent stress until delivery (i.e., GD 21). The stress protocol is shown in Figure 1, as described previously.21 Briefly, three randomly arranged stressors comprise heterotypic stress protocol, of water avoidance stress (WAS) for 60 min, cold restraint stress at 4°C for 45 min, and forced swimming stress for 20 min. Age-matched control pregnant dams were brought to the lab without further handling. Following delivery, pups were left undisturbed in their cages until weaned at the age of three weeks. Most experiments started when these offspring became adult (i.e., at the age of six weeks) unless otherwise described.
Figure 1.
Schematic representation of gestational stress procedure. Beginning on the day 7 of gestation (GD 7), the pregnant dams were exposed to a heterotypic intermittent stress until delivery (i.e., GD 21). Three randomly arranged stressors comprise heterotypic stress protocol of water avoidance stress (WAS) for 60 min, cold restraint stress (CRS) at 4°C for 45 min, and forced swimming stress (FSS) for 20 min. Following delivery, pups were left undisturbed in their cages until weaned at the age of postnatal day 21 (PND21). Most experiments started when these offspring became adult (i.e., at the age of PND42).
Electromyography recordings
On postnatal week 5, the male offspring rats were anaesthetized with 3.6% chloralic hydras (1 ml/100 g body weight, i.p.), and a pair of noninsulated tips of electrodes (A&E Medical Corp) was stitched in parallel, 10 mm length, 5 mm apart in the abdominal external oblique muscles. The electrodes were threaded subcutaneously to emerge at the back of the neck, where they were secured by medical adhesive tape. After the procedure, rats were housed individually with free access to water and food. Electromyography (EMG) activity from the external oblique muscle was recorded in response to colorectal distention (CRD) at the seventh day after surgery. A flexible latex balloon (8 cm) was inserted into the descending colon and rectum via the anus and held in place by taping the tubing to the tail. Animals with a balloon inserted were placed in small Lucite cubicles for 30 min before CRD. CRD was performed by rapidly inflating the balloon to constant pressures: 20, 40, 60, and 80 mmHg for 20 s followed by 2-min rest. Net value of EMG activities for each distension period was calculated by subtracting the baseline value derived from the average of area under curve for 20 s before and 20 s during the distention period.22,23
Dissociation of DRG neurons and patch-clamp recordings
Colon-specific DRG neurons were labeled by injection of 1, 1′-dioleyl-3, 3, 3′, 3-tetramethylindocarbocyanine methanesulfonate (DiI, Invitrogen) into the colon wall at the age of five weeks.24 One week after DiI injection, rats from PMS and CON group were sacrificed by decapitation. DRG (T13-L2) neurons were bilaterally dissected out and transferred to an ice-cold, oxygenated fresh dissecting solution, containing (in mM) 130 NaCl, 5 KCl, 2 KH2PO4, 1.5 CaCl2, 6 MgSO4, 10 glucose, and 10 HEPES, with pH 7.2 (osmolarity: 305 mOsm). The procedure of DRG neuron dissociation was described previously.25 After a single-cell suspension was obtained, cells were plated onto acid-cleaned glass coverslips. Coverslips containing adherent DRG cells were put in a small recording chamber (0.5 ml volume) and attached to the stage of an inverting microscope (Olympus IX71) fitted for both fluorescence and bright-field microscopy. DiI-labeled neurons were identified by their fluorescence under the fluorescent microscope. For the patch-clamp recording experiments, cells were continuously superfused (1.5 ml/min) at room temperature with normal external solution containing (in mM) 130 NaCl, 5 KCl, 2 KH2PO4, 2.5 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose, with pH adjusted to 7.2 with NaOH (osmolarity: 295–300 mOsm). The detailed whole-cell patch-clamp recording procedure was described previously.26
Western blot
Expressions of CBS and Nav1.7 in T13-L2 DRGs from PMS and CON rats were determined using Western Blot analysis, as previously described in detail.27 The primary antibodies including rabbit anti-CBS (1:1000, Abnova, USA), rabbit anti-Nav1.7 (1:1000, Alomone, USA), or mouse anti-β-actin (1:1000, Sigma, USA) and secondary antibodies including antirabbit peroxidase-conjugated secondary antibody (1:2000; Santa Cruz Biotechnology, CA) and antimouse horseradish peroxidase-conjugated secondary antibody (1:4000, Sigma, USA) were used to probe the target proteins.
Quantitative RT-PCR
Total RNA from DRGs (T13-L2) was extracted using Trizol Reagent (Takaba) according to the manufactures instructions. The cDNA was prepared using M-MLV First Strand kit (Invitrogen) following the supplier’s instructions. The primers used were obtained from Invitrogen, and the sequences of the primers were as follows: CBS primers, forward: CTGCCCGACTCTGTGCGCAA; reverse: CGCAGATGCCACCACCAGGG; Nav1.7 primers, forward: CAGACGGTATCGCCTCAGAC; reverse: GAAGGTGGCGAGATGGTTGA. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers, forward: AAGGTGGTGAAGCAGGCGGC; reverse: GAGCAATGCCAGCCCCAGCA. The Ct value was defined as cycle number at which fluorescence intensity reached a certain threshold where amplification of each target gene was within the linear region of the reaction amplification curves. Relative expression level for each target gene was normalized by Ct value of β-actin using a 2ΔΔCt relative quantification method.28
Application of drugs
AOAA (Sigma) was freshly prepared in normal saline (NS). For behavioral experiment, different doses of AOAA in a volume of 1 mL were intraperitoneally injected 30 min before EMG recordings. For control group, the same volume of NS was injected intraperitoneally. For patch clamp, AOAA was injected intraperitoneally daily for consecutive seven days.
Data analysis
All data are expressed as mean ± SEM Two-way repeated measures analysis of variance (ANOVA) was used to analyze data of EMG recordings for behavioral test. All other data obtained in the present study were analyzed using two-sample t test when it is a normally distributed population or using Mann–Whitney test when it is not. Significant differences were determined using Turkey’s post hoc test. It was considered statistically significant when p value was less than 0.05.
Results
PMS-induced visceral hypersensitivity in adult male offspring
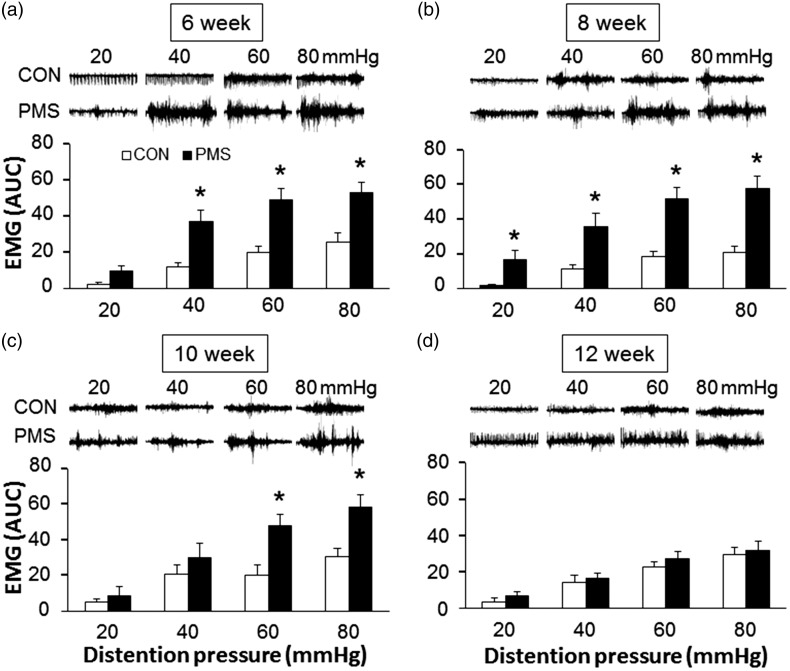
Visceral sensitivity was determined by measuring the EMG in response to CRD at the age of 6–12 weeks in male offspring (Figure 2). PMS significantly enhanced the visceromotor response in male offspring to CRD at pressures of 40, 60, and 80 mmHg at the age of six weeks (Figure 2(a), n = 9 rats for each group, *p < 0.05, compared with age-matched control rats, two-way repeated measures ANOVA) and at the age of eight weeks compared with controls even at pressure of 20 mmHg (Figure 2(b), n = 9 rats for each group, *p < 0.05, two-way repeated measures ANOVA). The increase in visceromotor response persists for four weeks, but there is statistical significance only at pressures of 60 and 80 mmHg at the age of 10 weeks (Figure 2(c), n = 9 rats for each group, *p < 0.05, Mann–Whitney test following Friedman’s ANOVA). No significant increase in the EMG amplitude was observed at the age of 12 weeks (Figure 2(d), n = 9 rats for each group, p > 0.05, Mann–Whitney test following Friedman’s ANOVA). These data indicated that male adult offspring from dams with heterotypical intermittent stress during pregnancy developed visceral hypersensitivity at the age of 6–10 weeks.
Figure 2.
Time course of effects of prenatal maternal stress (PMS) on visceromotor sensitivity to colorectal distention (CRD). Electromyographical (EMG) activities in the external oblique muscle in male offspring rats in response to graded CRD were measured at different time point after PMS. Examples of EMG activities recorded from age-matched control (CON) and PMS rats responding to 20, 40, 60, and 80 mmHg distention pressures were presented at top of each bar graph. The average of EMG amplitude was expressed as area under curve (AUC). (a) PMS significantly increased the magnitude of AUC at 40, 60, and 80 mmHg distention pressures when compared with CON at the age of six weeks (n = 9 for each group, *p < 0.05 vs. CON, two-way repeated measures ANOVA). (b) PMS significantly increased the magnitude of AUC at 20, 40, 60, and 80 mmHg distention pressures when compared with CON at the age of eight weeks (n = 9 for each group, *p < 0.05 vs. CON, two-way repeated measures ANOVA). (c) PMS significantly increased the magnitude of AUC at 60 and 80 mmHg distention pressures when compared with CON at the age of 10 weeks (n = 9 for each group, *p < 0.05 vs CON, Mann-Whitney test following Friedman’s ANOVA). (d) No significant difference between CON and PMS group was observed at the age of 12 weeks (n = 9 for each group, p > 0.05 vs. CON, Mann–Whitney test following Friedman’s ANOVA).
PMS enhanced excitability of colon-specific thoracolumbar DRGs
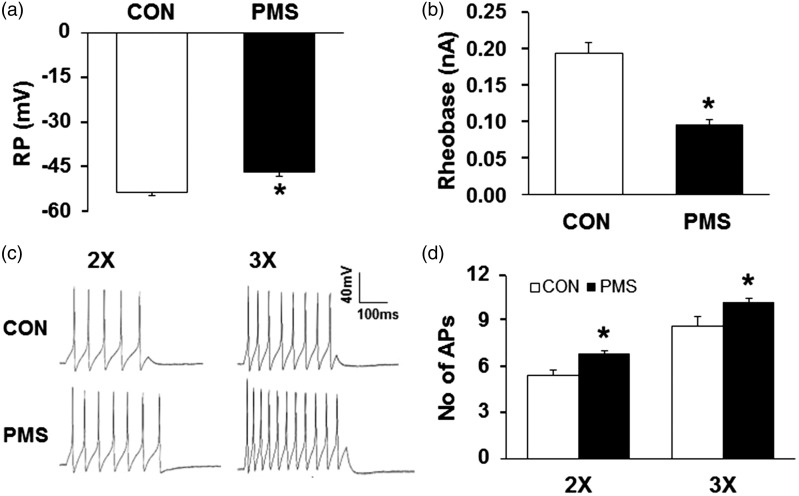
We then examined excitability of colon-specific (T13-L2) DRG neurons in male offspring at the age of six weeks. DRG neurons were acutely dissociated, and the colon innervating neurons were identified by the presence of retrograde labeled DiI. The resting membrane potentials (RP) were −53.57±1.09 mV and −46.52±1.44 mV for age-matched control and PMS rats, respectively. Thus, PMS significantly depolarized RP of colon-specific DRG neurons isolated from PMS male offspring when compared with age-matched controls (Figure 3(a), n = 21 neurons for both groups, *p < 0.05, two-sample t test). PMS also markedly reduced rheobase (Figure 3(b), n = 21 neurons for both groups, *p < 0.05, two-sample t test). Rheobase is the minimal stimulation current, which only evokes an action potential (AP) of recorded neuron. The average rheobase was 0.19±0.01 nA and 0.10±0.01 nA for age-matched control and PMS rats, respectively. In addition, the number of APs evoked at 2× and 3× rheobase current stimulation were greatly increased (Figure 3(c)), compared to neurons from age-matched control rats (Figure 3(d), n = 21 neurons for both groups, *p < 0.05, Mann–Whitney test for 2× rheobase and two-sample t test for 3× rheobase). PMS did not alter the other electrophysiological characteristics such as AP amplitude, duration, and threshold of colon-specific DRG neurons (data not shown).
Figure 3.
Increase in neuronal excitability of colon-specific DRG neurons of PMS male offspring. (a) PMS significantly depolarized the resting membrane potential (RP) in colon-specific DRG neurons (n = 21 for each group, *p < 0.05 vs. CON, two-sample t test). (b) PMS resulted in a marked reduction in rheobase of colon-specific DRG neurons (n = 21 for each group, *p < 0.05 vs. CON, two-sample t test). (c) Representative traces of action potentials (APs) evoked by 300-ms depolarizing current pulses injected through the patch pipette in DRG neurons from CON and PMS rats under current-clamp conditions. PMS resulted in a significant increase in the number of APs induced by a 2× and 3× rheobase current injection in colon-specific DRG neurons. (d) Bar graph shows a significant increase in average of the number of APs in colon-specific DRG neurons from PMS rats (n = 21 for each group, *p < 0.05 vs. CON, Mann-Whitney test for 2x rheobase and two-sample t test for 3× rheobase).
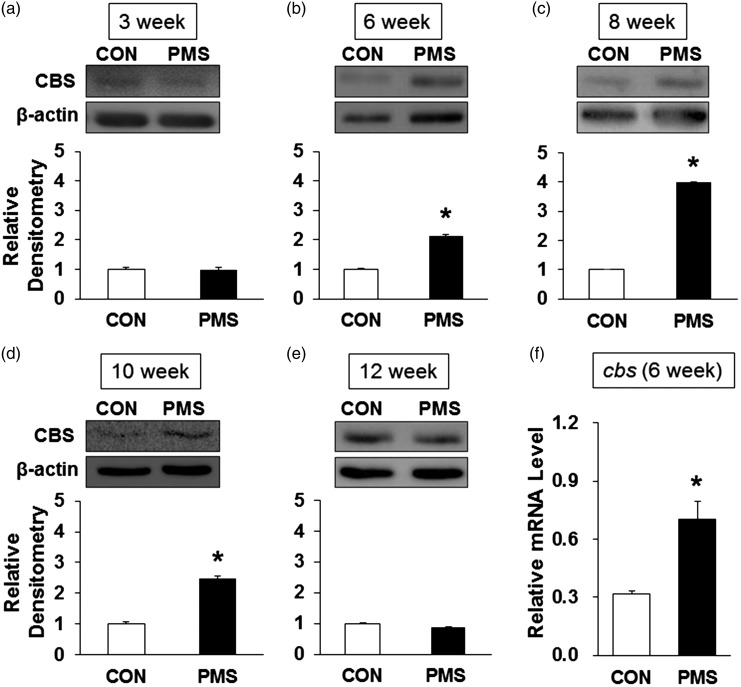
PMS upregulated Nav1.7 expression both at protein and mRNA levels
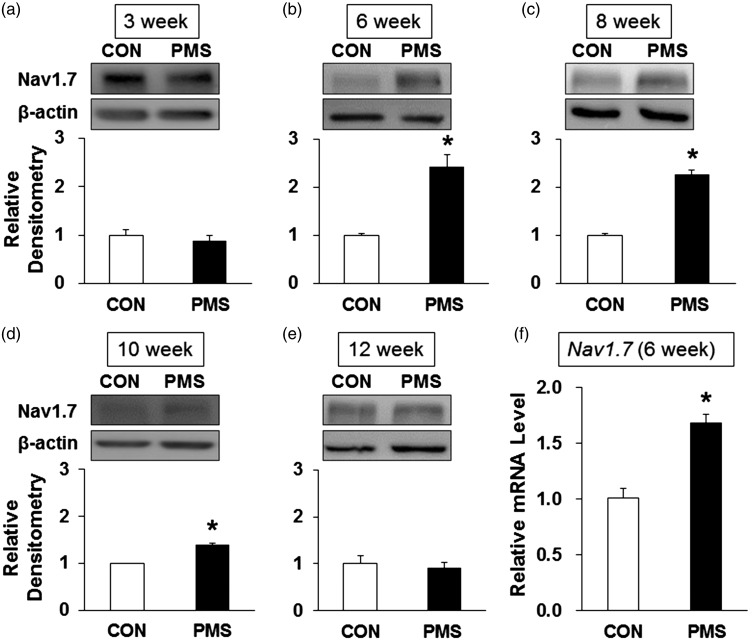
In our previous study, we have confirmed that Nav1.7 was expressed in colon thoracolumbar DRGs.15 We then determined whether PMS altered the Nav1.7 expression by Western blotting and qPCR analysis. Total protein and mRNA were extracted from T13–L2 DRGs of PMS and control rats. As shown in Figure 4, expression of Nav1.7 protein was obviously increased at the age of 6, 8, and 10 weeks in PMS offspring when compared with age-matched controls (Figure 4(b) to (d), n = 4 for each group, *p < 0.05, two-sample t test). However, no change was detected for Nav1.7 protein expression at the age of 3 and 12 weeks (Figure 4(a) and (e), n = 4 for each group, p > 0.05, two-sample t test). Besides, Nav1.7 mRNA level was also increased at the age of six weeks compared to controls (Figure 4(f), n = 4 for each group, *p < 0.05, two-sample t test).
Figure 4.
PMS upregulated the Nav1.7 expression. (a) There was no change in Nav1.7 expression at the age of three weeks (n = 4 rats in each group, p > 0.05 vs. CON, two-sample t test). (b) PMS significantly increased Nav1.7 expression in T13-L2 DRGs at the age of six weeks compared with CON (n = 4 rats in each group, *p < 0.05 vs. CON, two-sample t test). (c) PMS significantly increased Nav1.7 expression in T13-L2 DRGs at the age of eight weeks compared with CON (n = 4 rats in each group, *p < 0.05 vs. CON, two-sample t test). (d) PMS significantly increased Nav1.7 expression in colon DRGs at the age of 10 weeks compared with CON (n = 4 rats in each group, *p < 0.05 vs. CON, two-sample t test). (e) There was no change in Nav1.7 expression at the age of 12 weeks (n = 4 rats in each group, p > 0.05 vs. CON, two-sample t test). (f) PMS treatment significantly enhanced mRNA level of Nav1.7 at the age of six weeks compared with CON (n = 4 rats for each group, *p < 0.05 vs. CON, two-sample t test).
PMS upregulated CBS expression both at protein and mRNA levels
We then examined whether PMS altered CBS expression in male offspring. Total protein and mRNA were extracted from T13–L2 DRGs from male offspring and control rats. Western blotting was performed for CBS expression at protein levels. As shown in Figure 5(a), there is no change in CBS protein expression at the age of three weeks in PMS offspring (n = 4 for each group, p > 0 .05, two-sample t test). However, CBS expression was markedly increased at the age of six weeks when compared with age-matched controls (Figure 5(b), n = 4 for each group, *p < 0.05, two-sample t test). Expression of CBS protein maintained at a higher level from 8 to 10 weeks in PMS offspring than age-matched controls (Figure 5(c) and (d), n = 4, *p < 0.05, two-sample t test) and returned to baseline normal level at the age of 12 weeks (Figure 5(e), n = 4 for each group, p > 0 .05, two-sample t test). These results were parallelly correlated with Nav1.7 expression and with behavior data as well. To further determine expression of CBS gene at transcriptional level, CBS mRNA was measured from male offspring and control rats at the age of six weeks by qPCR analysis. PMS significantly enhanced the mRNA level of CBS (Figure 5(f), *p < 0.05, n = 4 for each group, two-sample t test).
Figure 5.
PMS upregulated the CBS expression. (a) There was no change in CBS expression at the age of three weeks (n = 4 rats in each group, p > 0.05 vs. CON, two-sample t test). (b) PMS significantly increased CBS expression in T13-L2 DRGs at the age of six weeks compared with CON (n = 4 rats in each group, *p < 0.05 vs. CON, two-sample t test). (c) PMS significantly increased CBS expression in T13-L2 DRGs at the age of eight weeks compared with CON (n = 4 rats in each group, *p < 0.05 vs. CON, two-sample t test). (d) PMS significantly increased CBS expression in colon DRGs at the age of 10 weeks compared with CON (n = 4 rats in each group, *p < 0.05 vs. CON, two-sample t test). (e) There was no change in CBS expression at the age of 12 weeks (n = 4 rats in each group, p > 0.05 vs. CON, two-sample t test). (f) PMS treatment significantly enhanced mRNA level of CBS at the age of six weeks compared with CON (n = 4 rats for each group, *p < 0.05 vs. CON, two-sample t test).
CBS inhibitor reversed the upregulation of Nav1.7 expression in PMS offspring
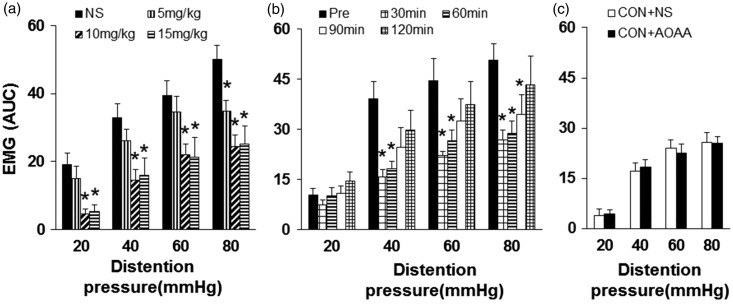
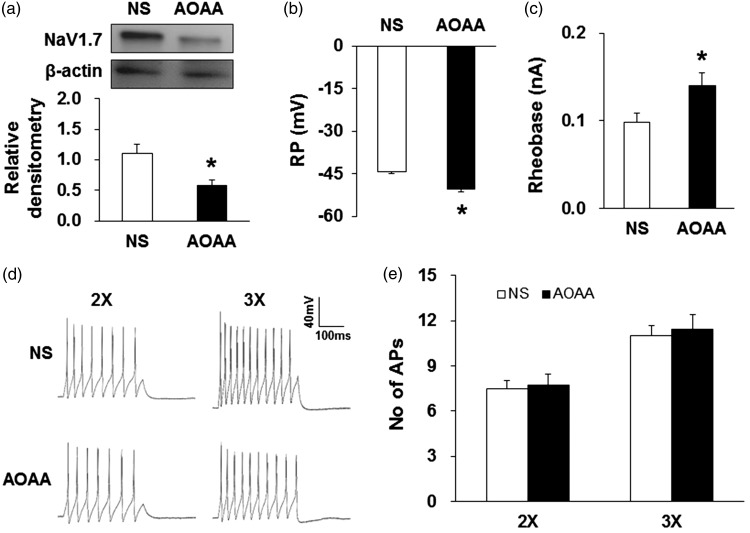
Because Nav1.7 and CBS co-expressed in colon-specific thoracolumbar DRGs,15 we further investigated whether aminooxyacetic acid (AOAA), an inhibitor of CBS, administration reversed the upregulation of Nav1.7 expression in PMS male offspring. AOAA was dissolved in NS. Since 10 mg/kg AOAA produced the optimal analgesic effect according to dose-dependent experiments (Figure 7(a)), male offspring received an injection of 10 mg/kg AOAA daily for consecutive seven days at the age of five weeks. Thirty minutes after last injection of AOAA, T13–L2 DRGs were harvested. Western blotting results showed that expression of Nav1.7 protein in T13–L2 DRGs was significantly decreased after AOAA administration when compared with NS-treated group (Figure 6(a), n = 4 for each group, *p < 0.05, two-sample t test).
Figure 7.
CBS inhibitor attenuated the EMG amplitude. (a) Effects of administration of three doses of AOAA (5, 10 and 15 mg/kg, n = 8 for each dose) in PMS rats. EMG activities were recorded 30 min after intraperitoneal injection of AOAA. The doses of 10 and 15 mg/kg AOAA completely abrogated the PMS-induced hypersensitivity compared with NS-treated group (n = 8 for each group, *p < 0.05 vs. NS, two-way repeated measures ANOVA). (b) Time course of prolonged analgesic effect of AOAA treatment (10 mg/kg, i.p.). AOAA effect lasted for ∼90 min (n = 8 for each group, *p < 0.05 vs. Pre, two-way repeated measures ANOVA). (c) The intraperitoneal injection of AOAA at 10 mg/kg did not produce significant effects on CON rats (n = 8 for each group, p > 0.05 vs. NS, two-way repeated measures ANOVA).
Figure 6.
CBS inhibitor reversed upregulation of Nav1.7 expression and neuronal excitability. AOAA at 10 mg/kg was intraperitoneally injected for consecutive seven days. (a) Nav1.7 expression was reduced in T13-L2 DRGs when compared with normal saline (NS) group (n = 4 for each group, *p < 0.05 vs. NS, two-sample t test). (b) AOAA treatment hyperpolarized RP of colon-specific DRG neurons (n = 22 for each group, *p < 0.05 vs. NS, two-sample t test). (c) AOAA treatment significantly enhanced rheobase (n = 22 for each group, *p < 0.05 vs. NS, two-sample t test). (d) Examples of APs induced by 300-ms depolarizing current pulses injected through the patch pipette in colon-specific DRG neurons from NS- and AOAA-treated PMS rats. (e) AP numbers were not changed after injection of 10 mg/kg AOAA for seven days (n = 22 for each group, p > 0.05 vs. NS, two-sample t test).
CBS inhibitor reversed the enhanced excitability of colon-specific DRG neurons
Next, we investigated whether AOAA decreased the enhanced excitability of colon-specific DRG neurons of PMS offspring. Thirty minutes after last injection of AOAA, T13-L2 DRGs were dissected out immediately. The passive and active membrane properties of colon-specific DRG neurons were defined. RP of colon-specific DRG neurons from AOAA-treated rats was significantly more hyperpolarized when compared with NS-treated rats (Figure 6(b), n = 22 for each group, *p < 0.05, two-sample t test). In neurons from AOAA-treated rats, the rheobases were significantly increased (Figure 6(c), n = 22 for each group, *p < 0.05, two-sample t test). However, the number of APs evoked by 2× and 3× rheobase current stimulation was not altered (Figure 6(d) and (e), n = 22 for each group, p > 0.05, two-sample t test). These data suggested that AOAA treatment reversed the enhanced neuronal excitability of colon-specific DRG neurons of PMS male offspring.
CBS inhibitor attenuated visceral hypersensitivity
To determine whether CBS involves in the PMS-induced visceral hypersensitivity, we then investigated effect of AOAA on the enhanced visceromotor response to CRD in PMS rats. Different doses (5, 10, and 15 mg/kg) were given intraperitoneally 30 min before EMG recordings. The maximal effect observed in this experiment was at the dose of 10 mg/kg body weight. NS injection had no effect on EMGs in PMS rats (Figure 7(a), n = 8 for each group, *p < 0.05, two-way repeated measures ANOVA). To examine the time course of AOAA effect, AOAA at 10 mg/kg was intraperitoneally injected. The analgesic effect produced by 10 mg/kg AOAA persisted for ∼60 min. The analgesic effect on EMG induced by 80 mmHg distention only observed 90 min after AOAA injection (Figure 7(b), n = 8 for each group, *p < 0.05, two-way repeated measures ANOVA). On the contrary, AOAA at 10 mg/kg did not produce any effect on EMG in control rats (Figure 7(c), n = 8 for each group, p > 0.05, two-way repeated measures ANOVA).
Discussion
The data reported here, for the first time, that the prenatal presence of multiple psychophysical stressors was associated with visceral hypersensitivity in the offspring at the age of 6–10 weeks. The timing of stress assessment in pregnancy may be a key to study the potential fetal programming effects. In the rat, exposure to glucocorticoids in the final week of gestation causes adult hypertension in the offspring,29 whereas the window of sensitivity in sheep is earlier in gestation.30 The assessment of stress in the current study took place in the second week of gestation, thus assessing experienced stress in the preceding first trimester weeks. The first trimester is often considered the trimester with the highest fetal vulnerability because of the development of critical, basal systems, including the formation of gastrointestinal tract and nervous system.31 Although future experiments would be helpful to determine how the stage of pregnancy plays a role in the association between maternal stress and fetal programming and to identify the exact windows of sensitivity, the present findings suggested that this model would be a good animal model to investigate the pathophysiologic mechanisms of visceral hypersensitivity in patients with functional gastrointestinal disorders such as IBS.
Several mechanisms underlying the association between psychosocial stress during pregnancy and fetal programming have been proposed although none of them has been extensively investigated.7 One mechanism involves gestational and maternal inflammation.32. Gestational psychosocial factors lead to increased inflammation, which influences the development of adult gastrointestinal disease. Maternal psychosocial factors contribute to higher circulating levels of inflammation markers, such as H2S33 and the proinflammatory cytokines interleukin (IL)-1β, IL-6, tumor necrosis factor-α, and TRL4,34 which are involved in many pain condition, such as bone cancer pain35,36 and neuropathic pain.37 As reviewed by Entringer et al., H2S had a direct programming effects of maternal inflammation.38 In the present study, we showed that expression of the endogenous H2S enzyme CBS was greatly enhanced in the male offspring after PMS, indicating that CBS-H2S signaling might be an important player in the development of visceral hypersensitivity of PMS offspring. Roles of other inflammation mediators need to be further investigated. Another potential pathway is the influence of psychosocial stress on the maternal hypothalamic–pituitary–adrenal (HPA) axis. Psychosocial stress leads to hypersecretion of the glucocorticoid cortisol, which may influence the development of the fetal HPA axis and immune function as well.39 Hyperactivity of the offspring’s HPA axis is in turn associated with risk factors for gastrointestinal diseases such as IBS and functional dyspepsia.40,41 Intrauterine growth retardation could be another mechanism by which maternal stress affects offspring gastrointestinal sensitivity. The association between maternal stress and fetal growth restriction has been well-documented,42 as well as the association between low birth weight and an increased risk of high functional gastrointestinal disorders in later life. Also preterm birth has been linked to altered HPA function in children.43 Additionally, maternal (preexisting) visceral hypersensitivity may be a factor explaining the association between prenatal stress and the offspring’s visceral hypersensitivity because literature suggests that stress is associated with alterations in blood pressure and glucose concentration.5 Unfortunately, we do not have data from assessments in the pregnant dams with or without PMS. The reason why we did not perform any measurements during stress protocol and the time period of maternal care is simply because we attempted to minimize the unexpected effect from these procedures. Future studies are definitely needed to determine whether dams with PMS display an enhanced visceral sensitivity during maternal period and the role in mediating visceral hypersensitivity in offspring rats. Nevertheless, PMS-induced visceral hypersensitivity in the offspring may be multifactual. Increase in CBS expression and its signaling might be one of the significant contributing factors.
The PMS offspring showed a significant increase in neuronal excitability. This conclusion is based on the following findings. First, colon-specific DRG neurons from animals with PMS displayed more depolarized RP than the age-matched controls, indicating that these neurons are spontaneously active. Second, these neurons exhibited lower current thresholds for initiating an AP compared with age-matched controls. Finally, these neurons had enhanced firing frequencies in response to a standardized stimulation compared with controls. These data suggest that peripheral sensitization might contribute to the visceral hypersensitivity in the offspring rats from dams with PMS. However, the precise mechanisms underlying the neuronal hyperexcitability remain to be further investigated under PMS condition. Neuronal excitability is controlled by many ion channels such as voltage-gated sodium and potassium channels.44,45 In the present study, expression of Nav1.7 was enhanced at the age of 6, 8, and 10 weeks in T13-L2 DRGs, which correlated with the exacerbation of visceral hypersensitivity, suggesting a role of NaV1.7 in the neuronal hyperexcitability. The elevated expression of Nav1.7 was in line with CBS at the same time point in T13-L2 DRGs. AOAA, an inhibitor of CBS, decreased the expression of Nav1.7. Furthermore, AOAA administration significantly attenuated visceral hypersensitivity. This indicates that upregulation of Nav1.7 expression is mediated by CBS signaling underlying the visceral hypersensitivity of PMS adult rat offspring. The detailed mechanisms warrant further investigation. Although contributions from other types of ion channels remain to be elucidated, it is interesting to study the molecular mechanism by which the expression of Nav1.7 was upregulated.
CBS, CSE, and 3-MST are important enzymes for generation of endogenous H2S in mammals.11 They have been found in many types of mammalian cells including the central nervous system and peripheral tissues as well.46 In this study, our data provide additional evidence to confirm the ideal that CBS plays an important role in stress-induced visceral hypersensitivity. AOAA, an inhibitor of CBS, significantly mitigates visceral hyperalgesia in PMS offspring in a dose- and time-dependent manner. However, AOAA did not produce any significant effect in healthy control rats, suggesting a specific analgesic effect of AOAA. Furthermore, AOAA treatment remarkably decreased excitability of colon-specific DRG neurons. This conclusion was evidenced by the findings shown in Figure 6. AOAA treatment not only significantly hyperpolarized RP of colon-specific DRG neurons but also enhanced the rheobase. However, the firing frequencies in response to a 2× and 3× rheobase current stimulation were not altered in AOAA-treated group compared with NS-treated groups. These data suggest that AOAA treatment desensitized the colon-specific DRG neurons, thus leading to the reduction in EMG amplitudes. In line with the previous studies that CBS rather than CSE was upregulated in colon DRGs under a rat model of neonatal colonic inflammation,13 the present studies further demonstrated that CBS was upregulated both at protein and mRNA levels in a rat model of visceral hyperalgesia induced by PMS, indicating that CBS might be a major enzyme responsible for the endogenous production of H2S in these cells under chronic stressed conditions. Further experiments are needed to determine whether CSE or 3-MST was involved in the enhanced visceral responses in this model.
In summary, our findings suggest that the presence of multiple psychosocial stressors during pregnancy is associated with visceral hyperalgesia in the adult offspring. Prenatal exposure to stress could be indicated as a factor underlying the pathogenesis of functional gastrointestinal diseases. Although the reported association with offspring visceral hyperalgesia requires further investigation, upregulation of Nav1.7 expression mediated by CBS signaling would be a mechanism underlying the visceral hypersensitivity in the offspring. Prevention of maternal stress in the child’s early stages of life may be valuable to improve gastrointestinal health later in life.
Author Contributions
HJW researched, analyzed data, and wrote the manuscript. XX researched and analyzed data. RHX researched and analyzed data. YYR researched and analyzed data. PAZ analyzed data. XJZ analyzed data. GYX designed and supervised the experiments and edited the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from National Natural Science Foundation of China (81471137, 31730040, and 81230024) and from the Priority Academic Program Development of Jiangsu Higher Education Institutions of China.
References
- 1.Schoenfeld PS. Advances in IBS 2016: A Review of Current and Emerging Data. Gastroenterol Hepatol (N Y) 2016; 12: 1–11. [PMC free article] [PubMed] [Google Scholar]
- 2.Liu RT. Childhood adversities and depression in adulthood: current findings and future directions. Clin Psychol (New York) 2017; 24: 140–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodgson DM, Nakamura T, Walker AK. Prophylactic role for complementary and alternative medicine in perinatal programming of adult health. Complement Med Res 2007; 14: 92–101. [DOI] [PubMed] [Google Scholar]
- 4.Gluckma PD andHanson MA. Predictive adaptive responses and human disease. In: The fetal matrix. Evolution, development and disease Cambridge, UK: Cambridge Universiy Press, 2005, pp. 78–102.
- 5.Barker DJ. The Wellcome Foundation lecture, 1994. The fetal origins of adult disease. Proc Biol Sci 1995; 262: 37–43. [DOI] [PubMed] [Google Scholar]
- 6.Tjong YW. Neonatal maternal separation elevates thalamic corticotrophin releasing factor type 1 receptor expression response to colonic distension in rat. Neuro Endocrinol Lett 2010; 31: 215–220. [PubMed] [Google Scholar]
- 7.Woods SM Melville JL Guo Y Fan M-Y, andGavin A.. Psychosocial stress during pregnancy. Am J Obstet Gynecol 2010; 202: 61 e1–e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vicario M Alonso C Guilarte M Serra J Martínez C González-Castro AM Lobo B Antolín M Andreu AL García-Arumí E Casellas M Saperas E Malagelada JR Azpiroz F, andSantos J.. Chronic psychosocial stress induces reversible mitochondrial damage and corticotropin-releasing factor receptor type-1 upregulation in the rat intestine and IBS-like gut dysfunction. Psychoneuroendocrinology 2012; 37: 65–77. [DOI] [PubMed] [Google Scholar]
- 9.Zanardo RCO Brancaleone V Distrutti E Fiorucci S Cirino G, andWallace JL.. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. Faseb J 2006; 20: 2118–2120. [DOI] [PubMed] [Google Scholar]
- 10.Eto K andKimura H.. A novel enhancing mechanism for hydrogen sulfide-producing activity of cystathionine beta-synthase. J Biol Chem 2002; 277: 42680–42685. [DOI] [PubMed] [Google Scholar]
- 11.Kabil O, Vitvitsky V, Xie P, Banerjee R. The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxid Redox Signal 2011; 15: 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qi F Zhou Y Xiao Y Tao J Gu J Jiang X, andXu G-Y.. Promoter demethylation of cystathionine-beta-synthetase gene contributes to inflammatory pain in rats. Pain 2013; 154: 34–45. [DOI] [PubMed] [Google Scholar]
- 13.Xu G-Y Winston JH Shenoy M Zhou S Chen JDZ, andPasricha PJ.. The endogenous hydrogen sulfide producing enzyme cystathionine-beta synthase contributes to visceral hypersensitivity in a rat model of irritable bowel syndrome. Mol Pain 2009; 5: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorman DC Brenneman KA Struve MF Miller KL James RA Marshall MW, andFoster PMD.. Fertility and developmental neurotoxicity effects of inhaled hydrogen sulfide in Sprague-Dawley rats. Neurotoxicol Teratol 2000; 22: 71–84. [DOI] [PubMed] [Google Scholar]
- 15.Qu R Tao J Wang Y Zhou Y Wu G Xiao Y Hu C-Y Jiang X, andXu G-Y.. Neonatal colonic inflammation sensitizes voltage-gated Na(+) channels via upregulation of cystathionine beta-synthetase expression in rat primary sensory neurons. Am J Physiol Gastrointest Liver Physiol 2013; 304: G763–G772. [DOI] [PubMed] [Google Scholar]
- 16.Yan J, Hu S, Zou K, Xu M, Wang Q, Miao X, Yu SP, Xu G-Y. Inhibition of cystathionine beta-synthetase suppresses sodium channel activities of dorsal root ganglion neurons of rats with lumbar disc herniation. Sci Rep 2016; 6: 38188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berta T Qadri Y Tan P-H, andJi R-R.. Targeting dorsal root ganglia and primary sensory neurons for the treatment of chronic pain. Expert Opin Ther Targets 2017; 21: 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y. NaV1.7 as a Pharmacogenomic Target for Pain: Moving Toward Precision Medicine. Trends Pharmacol Sci 2018; 39: 258–275. [DOI] [PubMed] [Google Scholar]
- 19.Campaniello MA Harrington AM Martin CM Ashley Blackshaw L Brierley SM, andHughes PA.. Activation of colo-rectal high-threshold afferent nerves by interleukin-2 is tetrodotoxin-sensitive and upregulated in a mouse model of chronic visceral hypersensitivity. Neurogastroenterol Motil 2016; 28: 54–63. [DOI] [PubMed] [Google Scholar]
- 20.Sayem ASM Giribabu N Muniandy S, andSalleh N.. Effects of thyroxine on expression of proteins related to thyroid hormone functions (TR-alpha, TR-beta, RXR and ERK1/2) in uterus during peri-implantation period. Biomed Pharmacother 2017; 96: 1016–1021. [DOI] [PubMed] [Google Scholar]
- 21.Winston JH, Xu GY, Sarna SK. Adrenergic stimulation mediates visceral hypersensitivity to colorectal distension following heterotypic chronic stress. Gastroenterology 2010; 138: 294–304.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsson M Arvidsson S Ekman C, andBayati A.. A model for chronic quantitative studies of colorectal sensitivity using balloon distension in conscious mice – effects of opioid receptor agonists. Neurogastroenterol Motil 2003; 15: 371–381. [DOI] [PubMed] [Google Scholar]
- 23.Qi D-B Zhang S-H Zhang Y-H Wu S-Q, andLi W-M.. A rat model for studying electroacupuncture analgesia on acute visceral hyperalgesia. Exp Anim 2018; 67: 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu G-Y Shenoy M Winston JH Mittal S, andPasricha PJ.. P2X receptor-mediated visceral hyperalgesia in a rat model of chronic visceral hypersensitivity. Gut 2008; 57: 1230–1237. [DOI] [PubMed] [Google Scholar]
- 25.Xu GY, Huang LY. Peripheral inflammation sensitizes P2X receptor-mediated responses in rat dorsal root ganglion neurons. J Neurosci 2002; 22: 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang H-H Hu J Zhou Y-L Hu S Wang Y-M Chen W Xiao Y Huang L-YM Jiang X, andXu G-Y.. Promoted interaction of nuclear factor-kappaB with demethylated cystathionine-beta-synthetase gene contributes to gastric hypersensitivity in diabetic rats. J Neurosci 2013; 33: 9028–9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H-H Hu J Zhou Y-L Hu S Wang Y-M Chen W Xiao Y Huang L-YM Jiang X, andXu G-Y.. Promoted interaction of nuclear factor-kappa B with demethylated purinergic P2X3 receptor gene contributes to neuropathic pain in rats with diabetes. Diabetes 2015; 64: 4272–4284. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y-L Jiang G-Q Wei J Zhang H-H Chen W Zhu H Hu S Jiang X, andXu G-Y.. Enhanced binding capability of nuclear factor-kappaB with demethylated P2X3 receptor gene contributes to cancer pain in rats. Pain 2015; 156: 1892–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roghair RD Wemmie JA Volk KA Scholz TD Lamb FS, andSegar JL.. Maternal antioxidant blocks programmed cardiovascular and behavioural stress responses in adult mice. Clin Sci (Sci) 2011; 121: 427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dodic M May CN Wintour EM, andCoghlan JP.. An early prenatal exposure to excess glucocorticoid leads to hypertensive offspring in sheep. Clin Sci (Sci) 1998; 94: 149–155. [DOI] [PubMed] [Google Scholar]
- 31.Lazinski MJ, Shea AK, Steiner M. Effects of maternal prenatal stress on offspring development: a commentary. Arch Womens Ment Health 2008; 11: 363–375. [DOI] [PubMed] [Google Scholar]
- 32.Leff-Gelman P Mancilla-Herrera I Flores-Ramos M Cruz-Fuentes C Reyes-Grajeda JP García-Cuétara MDel P Bugnot-Pérez MD, andPulido-Ascencio DE.. The immune system and the role of inflammation in perinatal depression. Neurosci Bull 2016; 32: 398–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whiteman M Le Trionnaire S Chopra M Fox B, andWhatmore J.. Emerging role of hydrogen sulfide in health and disease: critical appraisal of biomarkers and pharmacological tools. Clin Sci (Sci) 2011; 121: 459–488. [DOI] [PubMed] [Google Scholar]
- 34.Coussons-Read ME, Okun ML, Nettles CD. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain Behav Immun 2007; 21: 343–350. [DOI] [PubMed] [Google Scholar]
- 35.Kong X Wei J Wang D Zhu X Zhou Y Wang S Xu GY, andJiang GQ.. Upregulation of spinal voltage-dependent anion channel 1 contributes to bone cancer pain hypersensitivity in rats. Neurosci Bull 2017; 33: 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei J Li M Wang D Zhu H Kong X Wang S Zhou YL Ju Z Xu GY, andJiang GQ.. Overexpression of suppressor of cytokine signaling 3 in dorsal root ganglion attenuates cancer-induced pain in rats. Mol Pain 2017; 13: 1744806916688901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Y Li RJ Li M Liu X Zhu HY Ju Z Miao X, andXu GY.. Overexpression of GRK6 attenuates neuropathic pain via suppression of CXCR2 in rat dorsal root ganglion. Mol Pain 2016; 12: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Entringer S, Buss C, Wadhwa PD. Prenatal stress and developmental programming of human health and disease risk: concepts and integration of empirical findings. Curr Opin Endocrinol Diabetes Obes 2010; 17: 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bellinger DL, Lubahn C, Lorton C. Maternal Early life stress effects on immune function: relevance to immunotoxicology. J Immunotoxicol 2008; 5: 419–444. [DOI] [PubMed] [Google Scholar]
- 40.Glover V O'Connor TG, andO'Donnell K.. Prenatal stress and the programming of the HPA axis. Neurosci Biobehav Rev 2010; 35: 17–22. [DOI] [PubMed] [Google Scholar]
- 41.O'Donnell K, O'Connor TG, Glover V. Prenatal stress and neurodevelopment of the child: focus on the HPA axis and role of the placenta. Dev Neurosci 2009; 31: 285–292. [DOI] [PubMed] [Google Scholar]
- 42.Lobel M. Conceptualizations, measurement, and effects of prenatal maternal stress on birth outcomes. J Behav Med 1994; 17: 225–272. [DOI] [PubMed] [Google Scholar]
- 43.Holsti L Weinberg J Whitfield MF, andGrunau RE.. Relationships between adrenocorticotropic hormone and cortisol are altered during clustered nursing care in preterm infants born at extremely low gestational age. Early Hum Dev 2007; 83: 341–348. [DOI] [PubMed] [Google Scholar]
- 44.Zhu HY Liu X Miao X Li D Wang S, andXu GY.. Up-regulation of CXCR4 expression contributes to persistent abdominal pain in rats with chronic pancreatitis. Mol Pain 2017; 13: 1744806917697979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pan HL Liu BL Lin W, andZhang YQ.. Modulation of Nav1.8 by Lysophosphatidic Acid in the Induction of Bone Cancer Pain. Neurosci Bull 2016; 32: 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao Q Zhang L Yang G Xu C, andWang R.. Butyrate-stimulated H2S production in colon cancer cells. Antioxid Redox Signal 2010; 12: 1101–1109. [DOI] [PubMed] [Google Scholar]