Abstract
Background & objectives:
There are reports about the susceptibility of Aedes mosquitoes to ZIKV from various countries, however, no such information is available from Indian sub-continent, although, high level of group cross-reactivity of ZIKV with other flaviviruses has been reported. During outbreak situations, many cases of Dengue (DEN) and Chikungunya (CHIK) are reported. In such scenario, vector mosquitoes are likely to get co-infection/secondary-infection with one or other virus. The present study was carried out to determine the susceptibility of Indian strain of Aedes aegypti to Zika virus (ZIKV) strain (MR-766) and the effect of co-infection/super-infection with either dengue virus (serotype-2) (DENV) or chikungunya virus (CHIKV) on ZIKV replication.
Methods:
Ae. aegypti mosquitoes used in this study were reared for many generations since 1980 at laboratory colony maintained at the ICMR-National Institute of Virology, Pune, India. Transmissibility of ZIKV from infected mosquitoes to suckling mice was also studied. Mosquitoes were experimentally infected with ZIKV and super-infected with either DENV or CHIKV via membrane-feeding route and incubated for 14 days at 28±2°C and humidity of 85±5 per cent. Replication of these viruses in mosquitoes was confirmed using real-time reverse transcription-polymerase chain reaction and immunofluorescence assay. Twenty infected mosquitoes were allowed to feed upon four suckling CD1 mice for about 30 min. Transmission of the ZIKV by infected mosquitoes to suckling mice was confirmed by the appearance of clinical signs and the presence of viral RNA in different organs.
Results:
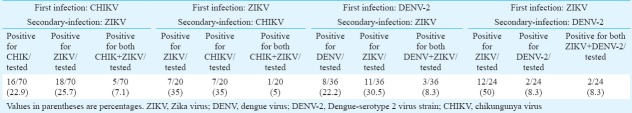
Concomitant infection of mosquitoes with all the three viruses showed simultaneous propagation of all three viruses, confirmed by real time RT-PCR and IFA. Infection of mosquitoes with CHIKV followed by ZIKV showed positivity in individual head squashes (7%) for both viruses using IFA; only 8.3 per cent showed dual positivity with primary infection of ZIKV followed by DENV; 8.3 per cent dual infection positivity was observed when infected with DENV followed by ZIKV; 5 per cent showed dual infection was observed when infected with ZIKV followed by CHIKV. Ae. aegypti was found to be susceptible to ZIKV strain as ZIKV could be detected from the second post-infection day (PID) in infected mosquitoes. Transmission of ZIKV to mice by the bite of infected Ae. aegypti establishes this species as a potential vector.
Interpretation & conclusions:
From super-infection experiments, it was concluded that ZIKV might have a relative advantage in replication dynamics over DENV. Vertical transmission was not observed for ZIKV in experimentally infected mosquitoes (n=920 larvae). Further studies are required to understand the possibility of silently circulating ZIKV in India, which remain non-detected because of lack of surveillance.
Keywords: Aedes aegypti, chikungunya, co-infection, dengue, transmission, Zika
Zika virus (ZIKV) is a mosquito-borne Flavivirus, which was first identified in Uganda in 1947. Later, it was confirmed in humans in 1952 in Uganda and the United Republic of Tanzania1. The first large-scale outbreak of ZIKV infection was reported from the Island of Yap in 20072,3. An association of ZIKV infection with microcephaly and Guillain–Barre syndrome was reported from Brazil4,5. Transmission of this virus occurs primarily through the bite of an infected mosquito, mainly Aedes aegypti, and is considered as the main vector outside Africa6,7. This species also transmits the dengue virus (DENV), chikungunya virus (CHIKV) and yellow fever virus. Determination of vector potential of Ae. aegypti to ZIKV in the presence of DENV and CHIKV can help in predicting its spread and associated risks to newer areas. The highest probability of ZIKV introduction to areas where it has not been reported earlier will be through travel of infected individuals. However, there is a very low probability of introduction of ZIKV by infected mosquitoes, because of the vigilance, quarantine and control measures instituted at international ports and airports, on the backdrop of ZIKV spread and establishment to new areas.
Available reports have shown susceptibility of Ae. aegypti to ZIKV from various countries; however, no such information is available from the Indian subcontinent. During monsoon season, >700,000 cases of dengue and chikungunya are reported annually8. Under such conditions, the entry of two flaviviruses (ZIKV and DENV) in a mosquito by super-infection/secondary infection may affect replication strategy of either of them or both because of the probability of sharing the same receptors and cellular machinery. Considering this fact, this study was conducted to determine the susceptibility of Ae. aegypti to ZIKV and study the dynamics of co-infection and/or secondary-infection with DENV and CHIKV. Attempts were also made to determine the possibility of oral transmission of the ZIKV to infant mice by the bite of infected mosquitoes (infected by oral route) and vertical transmission of ZIKV in experimentally infected mosquitoes.
Material & Methods
All the experiments in this study were approved by the Institutional Animal Ethics Committee (Project ID: MCL1701; CPCSEA Registration No. 43/GO/C/1999/CPCSEA) and the Institutional Biosafety Committee. All mosquitoes used in the experiments were sacrificed by freezing at the end of the study. Considering the biosafety concerns, manipulations of ZIKV was carried out in the maximum containment laboratory. The study period was 2016.
In vitro propagation of Zika virus (ZIKV), dengue virus (DENV) and chikungunya virus (CHIKV): The ZIKV strain (MR-766, African strain of ZIKV, country of origin Uganda, GenBank ID DQ859059, Date of isolation – 20/04/1947) was obtained from the European Union and European Virus Archive goes Global project. Virus stock used in the present study was prepared using Vero cells (CCL81; Source: ATCC CCL-81TM CRL-1586TM) infected at a multiplicity of infection of 0.01. ZIKV was titrated in Vero cells (CCL-81) using the standard protocol as described by Reed and Muench9. TCID50(50% tissue culture infective dose) calculation was done as per the Reed and Muench formula. This experiment was repeated three times.
The Dengue-serotype 2 virus (DENV-2) strain (American genotype cluster; strain No. 803347) was isolated from Kolkata, India, in 1980. The CHIKV strain (African genotype; strain No. 061573) was isolated in 2006 from Andhra Pradesh, India. These two viruses were obtained from the virus repository of the ICMR-National Institute of Virology, Pune. These were also grown in Vero cells (CCL-81) and used further for all the experiments in the present study.
Viral infection in mosquitoes
Mosquito inoculation by intrathoracic inoculation (ITI) route: Approximately 4-5 days post-emergence, female mosquitoes were inoculated via intrathoracic inoculation (ITI) route with either ZIKV or DENV or CHIKV in separate groups, respectively, from the frozen virus stocks (0.2 μl/mosquito)10,11. The inoculated mosquitoes were maintained at temperature 28°C±2°C and humidity 85±5 per cent. During the incubation period of 7-14 days, they were maintained at 10 per cent glucose solution soaked in cotton wool pledgets.
Mosquito's infection by oral route: Female mosquitoes aged 4-5 days (n=200) were experimentally infected with viral stock by allowing them to feed on an infectious blood meal containing 0.5 ml of the either ZIKV or DENV or CHIKV (in separate batches of mosquitoes) and 1.5 ml of the defibrinated chicken (white leghorn) blood through an artificial membrane (Parafilm, American National Can, USA)12. The membrane-fed mosquitoes were incubated at 28°C±2°C with 85±5 per cent humidity, in individual jars separately, each holding mosquitoes infected with either of the above three viruses. During the incubation period, they were maintained at 10 per cent glucose solution soaked in cotton. Mosquitoes were held for seven days after the first (primary) infective feeding. The gravid and the semi-gravid female individuals laid the eggs by 5th-7th post-infection days (PIDs). Once the egg laying process was complete, the female mosquitos were re-fed on the second virus infective blood meal (in four different super-infection combinations) to attempt the super-infection.
Detection of DENV, CHIKV and ZIKV using qualitative Trioplex real-time reverse transcription polymerase chain reaction (RT-PCR): RNA was extracted from infected cell culture supernatant/mosquito pool/specimens of mice using the QIAamp Viral RNA Mini Kit (Qiagen, Germany) as per the manufacturer's protocol. The Trioplex real-time reverse transcription-polymerase chain reaction (RT-PCR) kits were provided by Centers for Disease Control and Prevention (CDC), USA, and the reaction was performed using the standard protocol provided with the kit (dyes used for probes, DENV: FAM; CHIKV: VIC; ZIKV: Texas Red). PCR measurement was performed using BioRad CFX96 Real-Time PCR detection system (Bio-Rad Laboratories, Inc., USA) under following thermal parameters: 50°C for 30 min, 95°C for 2 min, followed by 45 cycles of 95°C for 15 sec and 60°C for 1 min. This assay was used to detect DENV, CHIKV and ZIKV simultaneously. The primer and probes mix for DENV was specific for the detection of 5'UTR region of all four DENV serotypes, i.e. DENV1, DENV2, DENV3 and DENV4; primer and probe mix for CHIKV and ZIKV were specific for nSP1 region and the envelope region, respectively13,14,15.
Detection of viral antigen by indirect immunofluorescence assay (IFA): On the 12th PID, head squashes of ZIKV-infected mosquitoes via ITI route and uninfected controls were prepared. The head squashes were screened for fluorescence under a compound fluorescent microscope (Olympus, BX61, USA)16. Dengue immune serum raised in rabbit was adsorbed with mosquito tissue and mouse liver powder. This adsorbed immune serum was found to be reactive to both DENV and ZIKV by indirect immunofluorescence assay (IFA) detection in mosquitoes, respectively. However, since dual infection studies were conducted, Zika immune serum raised in mice and dengue immune serum raised in rabbit were used for the respective virus detection. Using similar procedures, laboratory raised chikungunya virus immune serum in rabbit was adsorbed with mosquito tissue and mouse liver powder and was used in super-infection IFA experiments in the study. ZIKV hyperimmune serum raised in the mice at 1:50 dilution and 1:150 dilution of fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Sigma, USA) were used for the detection of ZIKV in infected mosquito head squashes by IFA. Dilution of 1:150 of the DENV hyperimmune serum and 1:100 dilution of CHIKV hyperimmune serum, both raised in rabbit in combination with 1:150 dilution and 1:250 dilution of goat anti-rabbit IgG FITC conjugate, were used for the detection of DENV and CHIKV, respectively, in the infected mosquito head squashes by IFA in all the experiments.
Replication kinetics of ZIKV in mosquitoes: Mosquitoes were infected with ZIKV via ITI route (n=100) and by oral feeding method (membrane fed route) (n=200) in two separate groups. Five mosquitoes were harvested on alternate days from 0 to 12th PID from both the ITI-infected and membrane-fed groups in each experiment. Mosquitoes were frozen for some time to make them immobilized. These were homogenized in 1 ml of 1× PBS solution using the Tissue Lyser II Machine (Qiagen) for five minutes at frequency of 30 oscillations /second, and RNA was extracted by QiAamp Mini Viral RNA Mini Kit using the manufacturer's protocol. RNA was extracted and the ZIKV titre in the post-fed blood meal mixture was determined by Trioplex qualitative real-time RT-PCR.
Replication of ZIKV in the salivary glands of mosquitoes: Groups of mosquitoes (n=75 mosquitoes/group) were infected with either DENV or CHIKV or ZIKV, respectively, in three individual groups via ITI route. On seventh PID, pools of infected mosquitoes (n=5 whole mosquitoes/pool) and of infected mosquito salivary glands (n=10 salivary glands/pool), which were dissected and washed twice in normal buffer saline containing 0.3 per cent Triton-X 100, were tested qualitatively by Trioplex real-time RT-PCR17.
Per cent positivity of mosquitoes with DENV/CHIKV and ZIKV by IFA method on individual mosquito head squashes: Per cent positivity of Ae. aegypti to these viruses was studied by infecting the mosquitoes with either DENV or ZIKV or CHIKV in three individual batches by oral route (membrane-feeding route). After 14th PID, all live individual-infected mosquito head squashes were tested by IFA11,12,13,14,15,16.
Susceptibility of mosquitoes to ZIKV, CHIKV and DENV in different combinations of infections and secondary/super-infections: Mixing virus and blood in the ratio 1:4 was carried out on four different experiments for oral infections with ZIKV. Following combinations of infections and secondary-infections were attempted 14 days after the first feeding cycle: (i) first infection with CHIKV and secondary infection with ZIKV; (ii) first infection with ZIKV and secondary infection with CHIKV; (iii) first infection with DENV and secondary infection with ZIKV; and (iv) first infection with ZIKV and secondary infection with DENV.
In each experiment, female mosquitoes aged 4-5 days (n=200) were provided with the first infectious meal containing blood (white leghorn fowl) plus respective virus (DENV or CHIKV or ZIKV). Further, the fed mosquitoes were segregated out and incubated. Mosquitoes were held for seven days after the first (primary) infective feeding. The gravid and the semi-gravid female mosquitoes laid the eggs by 5-7 PIDs. Once the egg laying process was complete (i.e. seven days after the primary infective feeding), the female mosquitoes were re-fed on the second virus infective blood meal to attempt the super-infection. The combination of primary infection and secondary/super-infection with another virus, both via membrane-feeding route, was completed.
On 14th PID (post-primary infection), head squashes of the infected mosquitoes were tested by preparing sandwich smears of heads of individual mosquitoes on two separate slides (mirror impressions) for the examination of two different viruses. Detection of the respective virus in the head squash was done by IFA using the respective adsorbed hyperimmune serum stock. The mosquito bodies were pooled and tested by Trioplex real-time RT-PCR to detect the presence of the respective viruses qualitatively and thereby determine the dissemination rate. Minimum two replicates of all the above experiments were performed.
Studies on vertical transmission of ZIKV in mosquitoes: On the 4th PID, mosquitoes infected with ZIKV via ITI method (n=100) were subjected to artificial membrane feeding on normal chicken blood to obtain eggs12,18. Three to four days after feeding, eggs from the first gonotrophic cycle (G-1) were collected and larvae were pooled at fourth instar stage. In the case of membrane-fed mosquitoes, 3-4 days after the ZIKV infectious blood meal, the eggs from G-1 were collected and larvae were pooled at fourth instar stage (50 larvae/ pool). A total number of 920 fourth instar larvae of the G-1 from ITI route (n=420) and membrane-feeding route (n=500) ZIKV-infected mosquitoes were tested by Trioplex real-time RT-PCR.
ZIKV transmission to infant mice by infected mosquitoes: Female mosquitoes (n=100 mosquitoes per group) were infected with ZIKV by membrane-feeding route and ITI infection method in two separate batches, respectively. On the seventh PID, 20 infected mosquitoes from each of the two individual batch of mosquitoes were allowed to feed on two day old infant suckling CD1 mice (n=4 mice/batch of infected mosquito groups.), for a period of 30 min11. The mice were monitored for the appearance of sickness. Various organs from four sick mice were harvested. These organs as well as serum of mice were tested for the presence of ZIKV by Trioplex real-time RT-PCR.
Statistical analysis: The mean log copy number of the virus between ITI and oral-fed mosquitoes on the post-incubation period was compared by paired t test. The per cent positivity of mosquitoes for each of the three viruses, viz. CHIKV, ZIKV and DENV, was compared by 2×2 Chi-square test. The per cent positivity of mosquitoes to ZIKV, CHIKV and DENV in different combinations of infections and secondary/super-infections was compared by Mid-P exact test. Mid-P exact test was also used to compare dissemination rate of ZIKV between different combinations of infections and super-infections with CHIKV and DENV.
Results
In vitro propagation of ZIKV, DENV and CHIKV: The TCID50 titre of the laboratory-propagated ZIKV (MR-766 strain), DENV and CHIKV frozen stocks used in all the experiments for the study was found to be 105.5 TCID50/ml for ZIKV and 106 TCID50/ml for both DENV and CHIKV, respectively.
Detection of DENV, CHIKV and ZIKV using qualitative Trioplex real-time RT-PCR: Results of the Trioplex real-time RT-PCR for each of the ZIKV/CHIKV-specific and DENV-specific primer and probe sets were interpreted as positive when reactions generated a fluorescence curve that crossed the threshold within <38 cycles.
Replication kinetics of ZIKV in mosquitoes:Mosquitoes were infected with ZIKV by two different routes and subjected to qualitative Trioplex real-time RT-PCR. ZIKV RNA could be detected from the second PID onwards in the infected mosquitoes by both the routes. A logarithmic growth curve showed that the viral load increased with the incubation period (Fig. 1). It was observed that the mean log copy number for ITI-infected mosquitoes (10.13) was significantly higher than that for membrane fed (9.03) batch (P= 0.03).
Fig. 1.
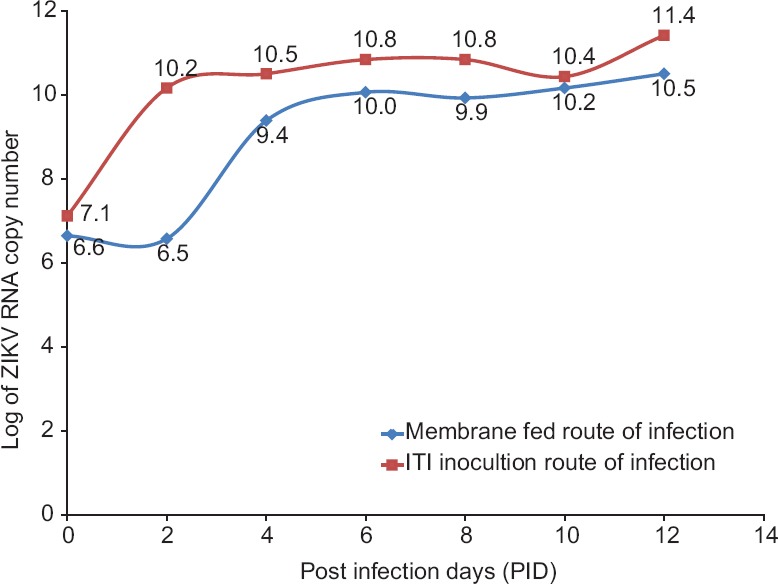
Replication kinetics of Zika virus in Indian strain of Aedes aegypti infected via intrathoracic inoculation (ITI) and membrane-feeding routes.
Indian strain of Aedes aegyptiwas highly susceptible to ZIKV (strain MR-766) infection: On seventh PID, viral RNA was measured in the salivary glands of mosquitoes infected with either DENV or CHIKV or ZIKV via ITI route in three individual groups. Qualitative analysis of Trioplex real-time RT-PCR revealed that highest viral RNA was detected in infected pools of salivary glands (Log viral load=12.3) as well as whole-infected mosquitoes (Log viral load=11.1) of ZIKV, as compared to DENV-infected salivary gland pools (Log viral load=11.1) and whole-infected mosquitoes (Log viral load=10.6). CHIKV-infected pools of the salivary glands (Log viral load=7.9) as well as whole-infected mosquitoes (Log viral load=7.1) also showed positive results (Fig. 2). Data revealed that between the two flaviviruses, ZIKV multiplication occurs at a faster rate in Indian strain of Ae. aegypti in comparison to DENV.
Figure 2.
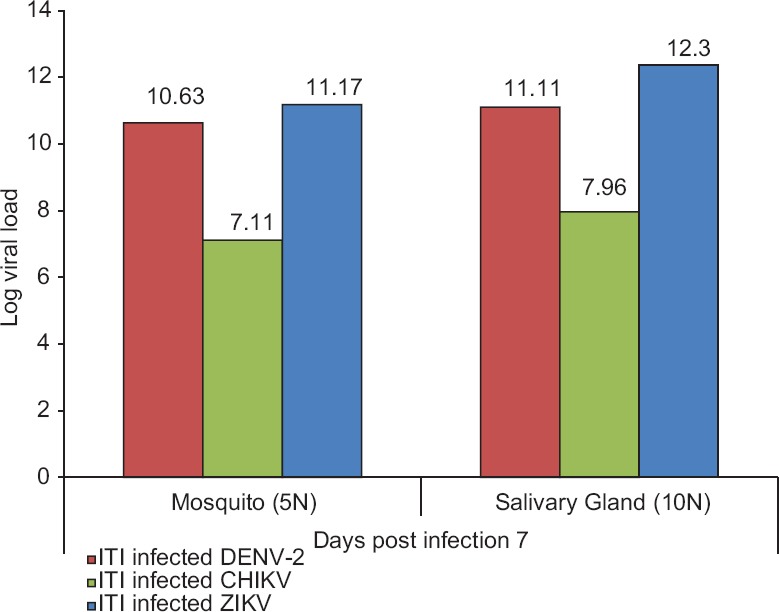
Detection of viral RNA in pools of infected salivary glands (n=10) and whole Aedes aegypti mosquitoes (n=5) on seventh post-infection day after virus infected via intrathoracic inoculation (ITI) route.
Per cent positivity of mosquitoes with DENV/ CHIKV and ZIKV by IFA method on individual mosquito head squashes: Per cent positivity of mosquitoes infected with DENV, CHIKV and ZIKV was determined by individual screening of the head squashes of infected mosquito (membrane-feeding route) using IFA method. Data showed that when mosquitoes were infected separately with ZIKV, CHIKV and DENV, per cent positives were 43.3, 40 and 26.6 per cent, respectively. It was found that the per cent positivity of Ae. aegypti was higher in ZIKV and CHIKV than DENV.
Susceptibility of mosquitoes to ZIKV, DENV and CHIKV in different combinations of infections and secondary/super-infections:First infection with CHIKV and secondary infection with ZIKV revealed that by immunostaining, only seven percent head squashes were positive for both the viruses, while 25.7 and 22.9 per cent were positive only for ZIKV and CHIKV, respectively (Table). Qualitative detection of viral RNA positivity by Trioplex real-time RT-PCR on pooled mosquitoes showed positivity for CHIKV RNA (Ct value=30) and ZIKV RNA (Ct value=26) in super-infected mosquito bodies. The difference between the dissemination rate of these two viruses was not significant.
Table.
Percentage positivity of super-infected mosquitoes screened by dual immunofluorescence assay (IFA) on individual mosquito head squashes on 14th PID.
First infection with ZIKV and secondary infection with CHIKV revealed that by immunostaining, only five per cent head squashes were positive for both the viruses and 35 per cent mosquito head squashes were positive only for ZIKV and 35 per cent mosquito head squashes were positive for only CHIKV virus subsequently (Table). Qualitative detection of viral RNA positivity by Trioplex real-time RT-PCR of the super-infected mosquito bodies showed positivity for ZIKV viral RNA (Ct value=37) and CHIKV viral RNA (Ct value=32) The difference between the dissemination rate of these two viruses was not statistically significant.
First infection with DENV and secondary infection with ZIKV revealed that by immunostaining, only 8.3 per cent head squashes were positive for both the viruses, while 30.5 and 22.2 per cent were positive only for ZIKV and DENV, respectively (Table). Qualitative detection of viral RNA positivity by Trioplex real-time RT-PCR of the super-infected mosquito bodies showed positivity for DENV RNA (Ct value=21) and ZIKV RNA (Ct value=19). The difference between the dissemination rate of these two viruses was not significant.
First infection with ZIKV and secondary infection with DENV revealed that by immunostaining, only 8.3 per cent were positive for both the viruses while 50 and 8.3 per cent were positive only for ZIKV and DENV, respectively Table. Qualitative detection of viral RNA positivity by Trioplex real time RT-PCR of the super-infected mosquito bodies showed positivity for ZIKV RNA (Ct value=17.7) while DENV RNA was not detected. The dissemination rate of ZIKV was significantly higher as indicated by immunostaining than that of DENV (P< 0.001).
Vertical transmission: All of the 920 G-1 fourth instar larvae tested were found negative for ZIKV. Vertical transmission did not occur with ZIKV in this small group of mosquito population. Based on these small data, it was difficult to conclude on vertical transmission in the present study.
Effective transmission of ZIKV to infant mice by infected mosquitoes: Twenty infected mosquitoes were allowed to feed upon infant CD1 mice. On testing, all four infected (two sick mice/group of infected mosquitoes) mice showed sickness after 4th-5th PID. Sickness signs included neurological manifestations such as trembling and paralysis, no suckling of milk, lethargic condition, lay-down posture and group leaving habit. The mice became moribund in the terminal stage leading to the death. High levels of viral RNA detected in necropsy-collected organs and blood samples suggested that the infected mosquito bite transmitted virus to the mice (Fig. 3).
Figure 3.
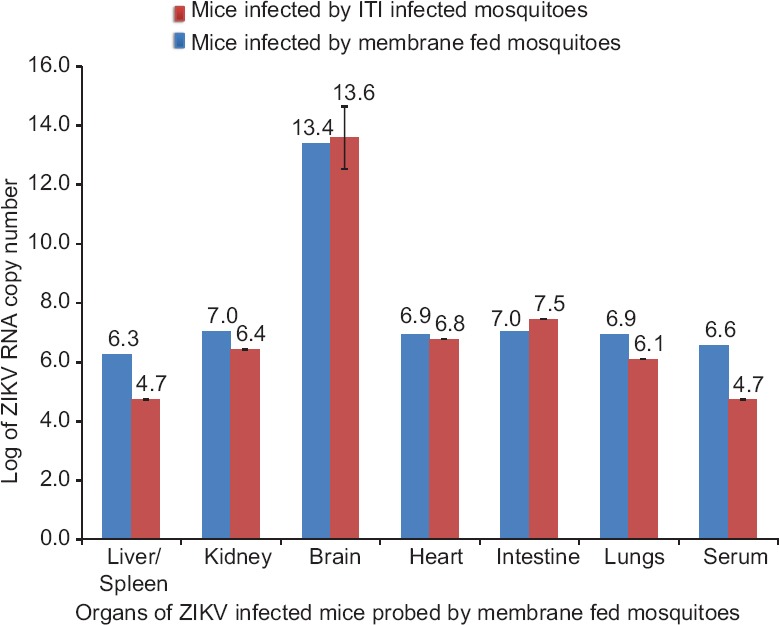
Detection of Zika virus (ZIKV) in various organs of infant CD1 mice bitten by membrane-fed Zika virus-infected mosquitoes. ITI, intrathoracic inoculation.
Discussion
In a recent declaration made by WHO, the Ministry of Health and Family Welfare, Government of India reported three laboratory-confirmed cases of ZIKV disease from Ahmedabad, Gujarat, India19. This puts India in the Risk Group 2 Category for ZIKV transmission and suggests low-level transmission of ZIKV and possibility of new cases in the future. DENV and CHIKV diseases are already endemic in India8. In the present study, attempts were made to get an in-depth perspective of the potential of ZIKV infectivity to already infected Ae. Aegypti with DENV and CHIKV. ZIKV multiplied in the Indian strain of Ae. aegypti after ingesting a viraemic blood meal and could transmit the virus to infant mice. Results showed that ZIKV multiplied effectively in mosquitoes; despite having prior infection with Flavivirus (DENV) and/or Alphavirus (CHIKV). This mosquito species is a daytime biter, and during this period, human hosts are also active. Thus, it is likely that mosquitoes tend to get disturbed feeding and cannot complete blood meal to full satiation in a single prick20. It has been observed that due to their multiple hosts feeding pattern, the hungry (host-seeking) Ae. aegypti tends to bite more than one individual for obtaining a complete blood meal. In this process, there is a potential chance of a single infective mosquito spreading the infection to multiple persons with multiple gonotrophic cycles by this long-lived vector. Similarly, in such a scenario, single mosquito may pick up more than two virus infections. The multiplication of the ZIKV in primary infection and/or secondary infection status and its transmission potential has an important epidemiological dimension in densely populated countries like India.
Earlier studies showed that ZIKV RNA concentrations in positive acute serum samples ranged between 101 and 105 copies/ml, whereas it ranged from 103 to 108 RNA copies/ml in DENV and CHIKV, respectively21,22. The viraemic phase extends over 5-8 days for these viruses. Interestingly, the overall level of viraemia of ZIKV in humans is reported to be relatively low and persists for up to a week23.
Once ZIKV crosses the gut barrier, it gets transmitted and replicates rapidly in salivary glands. Based on results of the study, the rate of replication between the two flaviviruses - Zika and dengue, ZIKV replicated faster than dengue and Zika viral RNA could be detected in mosquito pools from the second PID onwards, unlike dengue, which took a minimum of seven days to be detected. However, the testing of collected saliva of infected mosquitoes might be beneficial in making direct comparisons of infectivity, which could be listed as a drawback of the study. During infection and super-infection, ZIKV and DENV might share the same receptors and cellular replication machinery in mosquitoes, and once high replicating ZIKV gets entry into the mosquito body, it is likely that the second flaviviruses replication may get affected significantly. However, in the present study, it was observed that this difference was not significant in the case of primary infection with ZIKA and super-infection with DEN virus. It was evident that ZIKV multiplied simultaneously with DENV, but the rate of replication of ZIKV was higher even in the presence of DENV. Data suggested that ZIKV replicated in the presence of DENV and there was no obvious effect of the presence of other flaviviruses in the mosquitoes.
Vertical transmission helps in the persistence of arboviruses in nature, but in our experiments, based on the limited number of larvae pool of G-1, we could not observe the vertical transmission. This might provide a potential mechanism for the virus to survive during adverse conditions. However, unless filial infection rates are higher, vertical transmission has no epidemiological significance. Therefore, for conclusive understanding, there is a need to determine this phenomenon in nature by detecting the virus in field-collected larval stages11.
In the ZIKV transmission studies in mice, highest viral load was recorded in the brain, which might have been the cause of death of mice. However, transmission experiments with CHIKV and DENV would have helped to clarify the comparative analysis of the transmission dynamics between the super-infected mosquitoes and could be listed as one of the drawbacks of the present study. The presence of Aedes mosquitoes in densely populated areas is one of the major concerns in most tropical and subtropical countries24,25. In such a scenario, the entry of an acute phase ZIKV-infected person might lead to localized focus and subsequent spread and transmission of the disease by the vector in the resident community.
In conclusion, the results of our study indicated that the Indian strain of Ae. aegypti exhibited susceptibility and transmissibility to the ZIKV strain. Ae. aegypti is abundantly found in India and is the competent vector for the ZIKV. Therefore, this study warrants the need to perform vector surveillance and control in the country to know the possibility of silently circulating ZIKV strains in India.
Acknowledgment
Authors acknowledge the strong support and encouragement received from Dr Soumya Swaminathan, the then Secretary to Government of India, Department of Health Research, Ministry of Health and Family Welfare, and Director-General, Indian Council of Medical Research. Authors thank the European Union and European Virus Archive goes Global project for supplying the ZIKV strain, and Centres for Disease Control and Prevention, USA, for supplying us Trioplex real-time reverse transcription polymerase chain reaction kit. Technical support provided by Servshri Sanjay Gopale, Manoj Kadam and Madhav Acharya, and Ms Divya Zawar Bhattad, Ms Savita Patil, is acknowledged. Authors thank Shri A. Walimbe for statistical analysis of the data.
Footnotes
Financial support & sponsorship: We received a grant from the Indian Council of Medical Research, New Delhi, India for conducting this study. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Conflicts of Interest: None.
References
- 1.Dick GW, Kitchen SF, Haddow AJ. Zika virus I Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–20. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 2.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14:1232–9. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grard G, Caron M, Mombo IM, Nkoghe D, Mboui Ondo S, Jiolle D, et al. Zika virus in Gabon (Central Africa)-2007: A new threat from Aedes albopictus? PLoS Negl Trop Dis. 2014;8:e2681. doi: 10.1371/journal.pntd.0002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campos GS, Bandeira AC, Sardi SI. Zika virus outbreak, Bahia, Brazil. Emerg Infect Dis. 2015;21:1885–6. doi: 10.3201/eid2110.150847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teixeira MG, Costa Mda C, de Oliveira WK, Nunes ML, Rodrigues LC. The epidemic of Zika virus-related microcephaly in Brazil: Detection, control, etiology, and future scenarios. Am J Public Health. 2016;106:601–5. doi: 10.2105/AJPH.2016.303113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreira-de-Brito A, Ribeiro IP, Miranda RM, Fernandes RS, Campos SS, Silva KA, et al. First detection of natural infection of Aedes aegypti with Zika virus in Brazil and throughout South America. Mem Inst Oswaldo Cruz. 2016;111:655–8. doi: 10.1590/0074-02760160332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musso D, Gubler DJ. Zika virus. Clin Microbiol Rev. 2016;29:487–524. doi: 10.1128/CMR.00072-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cecilia D. Current status of dengue and Chikungunya in India. WHO South East Asia J Public Health. 2014;3:22–6. doi: 10.4103/2224-3151.206879. [DOI] [PubMed] [Google Scholar]
- 9.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–7. [Google Scholar]
- 10.Mourya DT, Gokhale MD, Kumar R. Xenodiagnosis: Use of mosquitoes for the diagnosis of arboviral infections. J Vector Borne Dis. 2007;44:233–40. [PubMed] [Google Scholar]
- 11.Mourya DT. Absence of transovarial transmission of Chikungunya virus in Aedes aegypti & Ae. albopictus mosquitoes. Indian J Med Res. 1987;85:593–5. [PubMed] [Google Scholar]
- 12.Mourya DT, Gokhale MD, Barde PV, Padbidri VS. A simple artificial membrane-feeding method for mosquitoes. Trans R Soc Trop Med Hyg. 2000;94:460. doi: 10.1016/s0035-9203(00)90141-x. [DOI] [PubMed] [Google Scholar]
- 13.Waggoner JJ, Gresh L, Mohamed-Hadley A, Ballesteros G, Davila MJ, Tellez Y, et al. Single-reaction multiplex reverse transcription PCR for detection of Zika, Chikungunya, and dengue viruses. Emerg Infect Dis. 2016;22:1295–7. doi: 10.3201/eid2207.160326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson BW, Russell BJ, Lanciotti RS. Serotype-specific detection of dengue viruses in a fourplex real-time reverse transcriptase PCR assay. J Clin Microbiol. 2005;43:4977–83. doi: 10.1128/JCM.43.10.4977-4983.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laue T, Emmerich P, Schmitz H. Detection of dengue virus RNA in patients after primary or secondary dengue infection by using the TaqMan automated amplification system. J Clin Microbiol. 1999;37:2543–7. doi: 10.1128/jcm.37.8.2543-2547.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mourya DT, Thakare JR, Gokhale MD, Powers AM, Hundekar SL, Jayakumar PC, et al. Isolation of Chikungunya virus from Aedes aegypti mosquitoes collected in the town of Yawat, Pune district, Maharashtra state, India. Acta Virol. 2001;45:305–9. [PubMed] [Google Scholar]
- 17.Mourya DT. A simple method for dissecting the salivary glands of mosquitoes. Indian J Malariol. 2001;38:43–4. [PubMed] [Google Scholar]
- 18.Thangamani S, Huang J, Hart CE, Guzman H, Tesh RB. Vertical transmission of Zika virus in Aedes aegypti mosquitoes. Am J Trop Med Hyg. 2016;95:1169–73. doi: 10.4269/ajtmh.16-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. Zika virus Infection-India. Disease Outbreak News. [accessed on May 29, 2017]. Available from: http://www.who.int/csr/don/26-may-2017-zika-ind/en/
- 20.Platt KB, Linthicum KJ, Myint KS, Innis BL, Lerdthusnee K, Vaughn DW, et al. Impact of dengue virus infection on feeding behavior of Aedes aegypti. Am J Trop Med Hyg. 1997;57:119–25. doi: 10.4269/ajtmh.1997.57.119. [DOI] [PubMed] [Google Scholar]
- 21.Boorman JP, Porterfield JS. A simple technique for infection of mosquitoes with viruses; transmission of Zika virus. Trans R Soc Trop Med Hyg. 1956;50:238–42. doi: 10.1016/0035-9203(56)90029-3. [DOI] [PubMed] [Google Scholar]
- 22.Ayres CFJ. Identification of Zika virus vectors and implications for control. Lancet Infect Dis. 2016;16:278–9. doi: 10.1016/S1473-3099(16)00073-6. [DOI] [PubMed] [Google Scholar]
- 23.Waggoner JJ, Gresh L, Vargas MJ, Ballesteros G, Tellez Y, Soda KJ, et al. Viremia and clinical presentation in Nicaraguan patients infected with Zika virus, Chikungunya virus, and Dengue virus. Clin Infect Dis. 2016;63:1584–90. doi: 10.1093/cid/ciw589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li MI, Wong PSJ, Ng LC, Tan CH. Oral susceptibility of Singapore Aedes (Stegomyia) Aegypti (Linnaeus) to Zika virus. PLoS Negl Trop Dis. 2012;6:e1792. doi: 10.1371/journal.pntd.0001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanciotti RS, Kosoy OL, Laven JJ, Panella AJ, Velez JO, Lambert AJ, et al. Chikungunya virus in US travelers returning from India, 2006. Emerg Infect Dis. 2007;13:764–7. doi: 10.3201/eid1305.070015. [DOI] [PMC free article] [PubMed] [Google Scholar]