Abstract
Context:
To quantify quadriceps weakness after anterior cruciate ligament reconstruction (ACLR), researchers have often analyzed only peak torque. However, analyzing other characteristics of the waveform, such as the rate of torque development (RTD), time to peak torque (TTP), and central activation ratio (CAR), can lend insight into the underlying neuromuscular factors that regulate torque development.
Objective:
To determine if interlimb neuromuscular asymmetry was present in patients with ACLR at the time of clearance to return to activity.
Design:
Cross-sectional study.
Setting:
Laboratory.
Patients or Other Participants:
A total of 10 individuals serving as controls (6 men, 4 women; age = 23.50 ± 3.44 years, height = 1.73 ± 0.09 m, mass = 71.79 ± 9.91 kg) and 67 patients with ACLR (43 men, 24 women; age = 21.34 ± 5.73 years, height = 1.74 ± 0.11 m, mass = 77.85 ± 16.03 kg, time postsurgery = 7.52 ± 1.36 months) participated.
Main Outcome Measure(s):
Isokinetic (60°/s) and isometric quadriceps strength were measured. Peak torque, TTP, and RTD were calculated across isometric and isokinetic trials, and CAR was calculated from the isometric trials via the superimposed burst. Repeated-measures analyses of variance were used to compare limbs in the ACLR and control groups.
Results:
No between-limbs differences were detected in the control group (P > .05). In the ACLR group, the involved limb demonstrated a longer TTP for isokinetic strength (P = .04; Cohen d effect size [ES] = 0.18; 95% confidence interval [CI] = −0.16, 0.52), lower RTD for isometric (P < .001; Cohen d ES = 0.73; 95% CI = 0.38, 1.08) and isokinetic (P < .001; Cohen d ES = 0.84; 95% CI = 0.49, 1.19) strength, lower CAR (P < .001; Cohen d ES = 0.37; 95% CI = 0.03, 0.71), and lower peak torque for isometric (P < .001; Cohen d ES = 1.28; 95% CI = 0.91, 1.65) and isokinetic (P < .001; Cohen d ES = 1.15; 95% CI = 0.78, 1.52) strength.
Conclusions:
Interlimb asymmetries at return to activity after ACLR appeared to be regulated by several underlying neuromuscular factors. We theorize that interlimb asymmetries in isometric and isokinetic quadriceps strength were associated with changes in muscle architecture. Reduced CAR, TTP, and RTD were also present, indicating a loss of motor-unit recruitment or decrease in firing rate.
Key Words: muscle mechanics, neural activity, limb asymmetry
Key Points
Several underlying neuromuscular factors may regulate interlimb asymmetries at return to activity after anterior cruciate ligament reconstruction.
Changes in muscle volume and cross-sectional area in the anterior cruciate ligament-reconstructed limb may be present concurrently with a shift in muscle-fiber types.
Diminished motoneuron recruitment or a decrease in motor-unit–firing frequency also likely contributed to interlimb differences.
Regaining quadriceps strength is a primary focus of patients pursuing a rehabilitation program after anterior cruciate ligament reconstruction (ACLR). Unfortunately, despite rehabilitation programs aimed at reversing this muscle weakness, authors1−3 of multiple systematic reviews have shown that quadriceps strength deficits persisted for years after ACLR. Lepley and Palmieri-Smith4 directly illustrated this point, reporting that when patients were released to activity, they did not reach a limb symmetry index (quadriceps strength of the injured or reconstructed limb compared with the uninjured limb) of 90%. Recently, Grindem et al5 highlighted the importance of regaining quadriceps symmetry at return to activity, as individuals who returned to sport with a limb symmetry index less than 90% were more likely to sustain another knee injury. Beyond the risk of sustaining another knee injury, the inability to regain quadriceps strength and restore limb symmetry often leads to decreased function4,6,7 and increased risk for developing posttraumatic osteoarthritis.8 To quantify these quadriceps strength asymmetries after ACLR, researchers have often used only discrete points on the quadriceps torque curve (most commonly peak torque) to calculate the limb symmetry index or reductions in normalized quadriceps torque. However, analyzing other characteristics of the waveform may provide investigators with more information about the underlying deficits in quadriceps neuromuscular factors that clinically manifest as a weakness that rehabilitation specialists can target and treat.
Voluntary activation failure (VAF), or an inability to achieve complete activation of a muscle, is often cited as a neurologic mechanism of muscle weakness after ACL injury. It is hypothesized to result from diminished motoneuron recruitment or decreased motor-unit–firing frequency.9 After ACLR, patients have VAF, and in some cases, VAF contributes to quadriceps strength deficits and interlimb asymmetry.4,10,11 Most investigators12−14 have quantified VAF using the central activation ratio (CAR) derived from the superimposed-burst technique, which is achieved by applying exogenous supramaximal stimuli to the muscle while a patient maximally contracts, to determine if the muscle is completely activated or produces additional torque when the stimuli are applied. Whereas not used as often, the time needed to reach peak torque (TTP) and the rate of torque development (RTD) can help to quantify changes in muscle that lead to quadriceps weakness.15 Deficits in the TTP and RTD can be regulated by several underlying factors, including a decline in neural activation,16,17 a shift in fiber types after injury,18 and changes in the contractile elements of muscle fibers or the tendon structure.19−21 To date, a decline in neural activity is thought to be the primary cause of these deficits16; however, given that alterations in neural activity affect muscle-fiber size and type,22,23 it seems plausible that declines in the TTP and RTD are related to alterations in both the neural and morphologic systems.
Alterations in quadriceps strength can also be quantified through changes in classic muscle-mechanics relationships, such as the torque-length and torque-velocity curves recorded during isometric and isokinetic contractions on a dynamometer.24 Alterations in isometric and isokinetic relationships lend insight into the underlying maladaptations in quadriceps muscle morphology that lead to decreased quadriceps strength after ACLR. For instance, a decrease in the physiological cross-sectional area (CSA; physiological CSA = [muscle volume × cos θ (pennation angle) / fiber length]) of the quadriceps would directly influence the torque-length (isometric) curve by reducing the overall peak torque,25,26 and a change to the torque-velocity curve, derived from isokinetic testing, could result from a shift in the fiber types present within the muscle.27−29
Whereas several researchers have considered neurologic and morphologic factors independently, few have considered these mechanisms together, thereby limiting the information that can be gathered from the data. Furthermore, most investigators examining changes in muscle have used expensive and time-intensive imaging technology (eg, magnetic resonance imaging) although changes in the functional characteristics of muscle could potentially be derived using simpler and more accessible clinical measures. We contend that using noninvasive, clinically accessible techniques to measure changes in the strength profiles of patients with ACLR could provide key insights into the underlying neuromuscular changes associated with quadriceps weakness after ACLR and allow identification of the specific factors that clinicians need to target and treat in rehabilitation. Hence, we hope that, when making return-to-activity decisions, clinicians can use these techniques to inform their clinical practices to optimize recovery. Therefore, the purpose of our study was to determine if interlimb neuromuscular asymmetry was present in patients with ACLR at the time of clearance to return to activity. We hypothesized that, after ACLR, patients would display increased TTP, slower RTD, smaller CAR, and lower peak torque in the involved than the uninvolved limb. Furthermore, we proposed that we would not observe differences between limbs in a group of healthy, uninjured control participants.
METHODS
Participants
A total of 67 patients who had undergone ACLR with patellar tendon grafts and 10 individuals serving as control participants were enrolled in a cross-sectional study design and tested on a single occasion. Participants in the ACLR group were tested after they were cleared to return to full activity by their orthopaedic surgeons at an average of 7.52 ± 1.36 months postsurgery (Table 1). Patients were considered for the ACLR group if they were between the ages of 14 and 30; had sustained an acute ACL tear, which we defined as reporting to a health care professional within 48 hours after injury; had no previous ACL injury or knee surgery; were not pregnant; and did not have a diagnosed heart condition. Data for control participants were collected immediately after they were enrolled in the study (Table 1). Individuals in the control group reported no lower extremity injury in the 6 months before the study, had no history of surgery to their lower extremities or ACL injury, were not pregnant, and had no known heart condition. All participants provided written informed consent, and the study was approved by the Institutional Review Board of the University of Michigan Medical School.
Table 1.
Participant Demographics
| Characteristic |
Group |
|
| Anterior Cruciate Ligament Reconstruction |
Control |
|
| Sex, No. | ||
| Male | 43 | 6 |
| Female | 24 | 4 |
| Age, y (mean ± SD) | 21.34 ± 5.73 | 23.50 ± 3.44 |
| Height, m (mean ± SD) | 1.74 ± 0.11 | 1.73 ± 0.09 |
| Body mass, kg (mean ± SD) | 77.85 ± 16.03 | 71.79 ± 9.91 |
| Graft type | 67 Patellar tendon | NA |
| Time from surgery, mo (mean ± SD) | 7.52 ± 1.36 | NA |
| Isometric quadriceps index (mean ± SD) | 71.97 ± 16.47 | 95.44 ± 6.91 |
| Isokinetic quadriceps index (mean ± SD) | 70.93 ± 22.54 | 100.92 ± 15.86 |
| International Knee Documentation Committee score (mean ± SD) | 81.04 ± 11.02 | 99.19 ± 2.17 |
| Tegner score (mean ± SD)a | 6.38 ± 1.89 | 6.10 ± 2.42 |
| Concomitant injury, No. | 0 Collateral ligament | NA |
| 11 Meniscectomy | ||
| 17 Meniscal repair | ||
Abbreviation: NA, not applicable.
Indicates score at time of testing.
Quadriceps Testing
All participants completed 3 bouts of maximal isometric quadriceps strength testing with a superimposed burst and 3 bouts of maximal isokinetic quadriceps strength testing on each lower extremity. Extremity order and contraction mode were randomized. For all testing, participants were seated upright and fully strapped into an isokinetic dynamometer (model System 3; Biodex Medical Systems, Shirley, NY) with the knee positioned at 90° according to the manufacturer's guidelines and two 7- by 13-cm self-adhesive electrodes (model Dura-Stick II; Chattanooga Group, Hixson, TN) secured over the vastus medialis and lateralis muscles. They practiced the movements on the dynamometer until they were comfortable with the equipment.
Isometric strength and superimposed-burst testing were completed as previously reported.7 Briefly, participants were required to complete maximal voluntary isometric contractions (MVICs) until no improvement in knee-extension torque was visualized. Next, they completed 3 MVICs in which a supramaximal stimulus (model S88 Dual Output Square Pulse Stimulator/SIU8T Stimulus Isolation Unit; Grass Technologies, West Warwick, RI) was delivered to the quadriceps when triggered by a custom-written program (LabVIEW version 8.5; National Instruments, Austin, TX). The stimulus was delivered after the MVIC was reached and subsequently decreased by 1 Nm. The CAR was calculated (CAR = [MVIC / (MVIC + superimposed burst)] × 100), and a value of 100 was used to represent complete quadriceps activation or no VAF.14
Isokinetic strength of the quadriceps was measured at 60°/s in the concentric mode. Patients began each trial at 90° of knee flexion, completely extended the knee, then returned to 90° of flexion. They completed 3 consecutive trials. For all strength testing, we provided visual feedback and oral encouragement.
Data Analysis
A custom MATLAB (The MathWorks, Inc, Natick, MA) code was used to analyze torque data. Peak isometric and isokinetic strength were normalized (Nm/kg), and trials were averaged. The first isokinetic trial was not included in any of our analyses because participants started from a stationary position and did not move at a consistent velocity. Onset of muscle contraction was defined at 7.5 Nm15 to ensure that baseline movement had been exceeded; this was verified by visual inspection to guarantee continuous torque generation. The RTD was calculated from the onset of torque to peak torque every 10 milliseconds and averaged across the trial (RTD = [(Δtorque / Δtime) / mass]).20,30 The TTP was calculated (TTP = time of peak torque – time of onset of torque) for both isometric and isokinetic data.15 A representation of how the isometric and isokinetic data were analyzed is shown in Figures 1 and 2, respectively.
Figure 1.
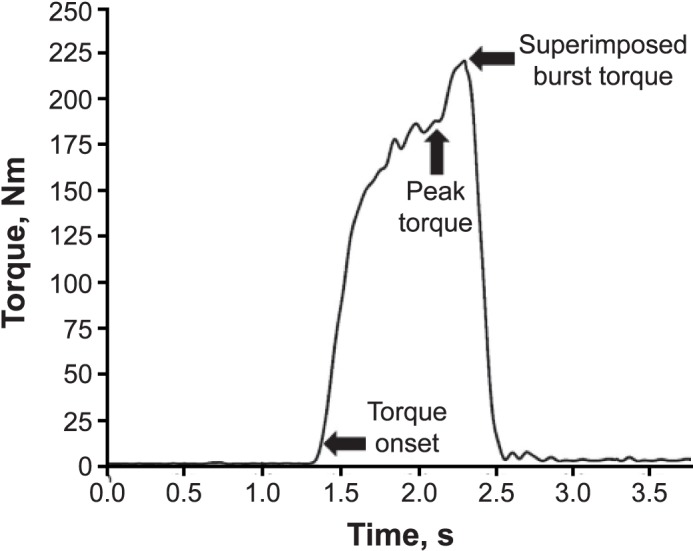
A single trial of isometric knee-extension torque data that includes the superimposed-burst procedure. Arrows represent torque onset, peak torque, and superimposed burst torque. Rate of torque development and time to peak torque were calculated on the curve bounded by torque onset and peak torque. Succeeding the maximal torque value, indicated by the second arrow, was the superimposed-burst torque indicating involuntary maximal torque.
Figure 2.
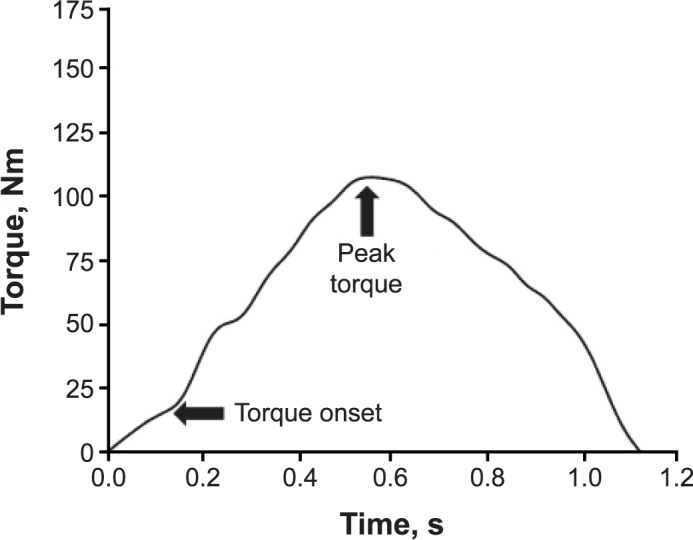
A single trial of isokinetic knee-extension torque data from participant X. Arrows indicate torque onset and peak torque. Rate of torque development and time to peak torque were calculated on the curve bounded by arrows.
Statistical Analysis
Descriptive statistics were computed for patient demographic information. Multiple 1 × 2 repeated-measures analysis-of-variance models were computed to compare limbs (involved, uninvolved) for the ACLR group. We completed the same analyses for the control group, with limb (left versus right) comparisons to ensure no interlimb asymmetries existed in this group. The dependent variables examined in the analyses were isometric and isokinetic peak torque, isometric and isokinetic RTD, isometric and isokinetic TTP, and CAR. For descriptive purposes, the percentage differences/symmetry values ([involved limb / uninvolved limb] × 100) for the dependent measures were also calculated.7,15 To determine if interlimb asymmetries were clinically meaningful, we conducted standardized effect sizes (ESs) and 95% confidence intervals (CIs) and interpreted ESs as weak (<0.5), moderate (0.5–0.79), or strong (>0.8).31 The α level was set a priori at P ≤ .05. All statistical analyses were performed in SPSS (version 22; IBM Corp, Armonk, NY).
RESULTS
Demographic information for all participants is reported in Table 1. As expected in the control group, we found no differences between limbs for any isometric or isokinetic variables (P > .05), with weak ESs and wide CIs (Cohen d range = 0.04−0.28; 95% CI = −1.12, 1.16; Table 2). These results indicated that, in a healthy population, irrelevant differences in isometric or isokinetic strength variables existed between limbs (Table 2).
Table 2.
Control-Group Data
| Quadriceps Strength |
Limb (Mean ± SD) |
Cohen d Effect Size (95% Confidence Interval) |
|
| Dominant |
Nondominant |
||
| Isometric | |||
| Peak torque, Nm/kg | 3.14 ± 0.93 | 3.26 ± 0.84 | 0.13 (−1.01, 0.75) |
| Time to peak torque, s | 1.37 ± 0.83 | 1.44 ± 0.93 | 0.08 (−0.95, 0.80) |
| Rate of torque development, Nm·s−1·kg−1 | 1.55 ± 0.76 | 1.58 ± 0.80 | 0.04 (−0.91, 0.84) |
| Central activation ratio, % | 95.71 ± 3.54 | 96.56 ± 3.16 | 0.24 (−1.12, 0.64) |
| Isokinetic | |||
| Peak torque, Nm/kg | 2.28 ± 0.55 | 2.24 ± 0.33 | 0.08 (−0.79, 0.96) |
| Time to peak torque, s | 0.53 ± 0.13 | 0.49 ± 0.14 | 0.28 (−0.60, 1.16) |
| Rate of torque development, Nm·s−1·kg−1 | 4.47 ± 1.68 | 5.01 ± 2.57 | 0.24 (−1.12, 0.64) |
In contrast, the ACLR group exhibited interlimb asymmetries (Table 3). Specifically, lower and strong clinically meaningful reductions in isometric peak torque (−28.5%, P < .001; Cohen d = 1.28; 95% CI = 0.91, 1.65) and isokinetic peak torque (−31.5%; P < .001; Cohen d = 1.15; 95% CI = 0.78, 1.52) were found in the involved compared with the uninvolved limb (Table 3). Similarly, we noted interlimb asymmetries for the CAR (−4%; P < .001; Cohen d = 0.37; 95% CI = 0.03, 0.71) and the RTD (isometric: −30%; P < .001; Cohen d = 0.73; 95% CI = 0.38, 1.08; isokinetic = 36%; P < .001; Cohen d = 0.84; 95% CI = 0.49, 1.19) in the involved compared with the uninvolved limb, with weak to moderate ESs. Isokinetic TTP was also 8% longer in the involved than in the uninvolved limb, but the difference was not clinically meaningful (P = .04; Cohen d = 0.18; 95% CI = −0.16, 0.52). The isometric TTP was not different between limbs (P = .98).
Table 3.
Anterior Cruciate Ligament Reconstruction Group Data
| Quadriceps Strength |
Limb (Mean ± SD) |
Cohen d Effect Size (95% Confidence Interval) |
|
| Involved |
Uninvolved |
||
| Isometric | |||
| Peak torque, Nm/kg | 2.34 ± 0.68a | 3.24 ± 0.72 | 1.28 (0.91, 1.65) |
| Time to peak torque, s | 1.94 ± 0.82 | 1.94 ± 0.73 | 0.00 (−0.34, 0.34) |
| Rate of torque development, Nm·s−1·kg−1 | 0.70 ± 0.34a | 0.98 ± 0.42 | 0.73 (0.38, 1.08) |
| Central activation ratio, % | 88.9 ± 9.40a | 92.01 ± 7.06 | 0.37 (0.03, 0.71) |
| Isokinetic | |||
| Peak torque, Nm/kg | 1.43 ± 0.50a | 2.05 ± 0.57 | 1.15 (0.78, 1.52) |
| Time to peak torque, s | 0.54 ± 0.17b | 0.51 ± 0.16 | 0.18 (−0.16, 0.52) |
| Rate of torque development, Nm·s−1·kg−1 | 2.89 ± 1.63a | 4.36 ± 1.84 | 0.84 (0.49, 1.19) |
Indicates difference from the uninvolved limb (P ≤ .001).
Indicates difference from the uninvolved limb (P = .04).
DISCUSSION
Like so many others, we showed that quadriceps weakness was common and interlimb asymmetries were present at return to activity after ACLR. Yet for the first time, by analyzing other characteristics of the waveform and using classic muscle-mechanics relationships (ie, force-length [isometric] and force-velocity [isokinetic] relationships), we were able to provide insight into the underlying neuromuscular factors that may contribute to these limb-strength asymmetries. We observed that persistent deficits in neuromuscular function were present at return to activity. The biggest regulator of these interlimb deficits appeared to be deficits in isometric and isokinetic peak torque production, which are functional mechanical properties of muscle that are highly related to changes in muscle architecture.25,27,29,32,33 Specifically, we found large interlimb deficits with strong ESs and narrow CIs during isometric testing; this muscle-mechanic alteration is related to changes in muscle volume and physiological CSA25,26 and can also be regulated by a change in pennation angle.27,34 Additionally, interlimb differences in quadriceps strength were identified during isokinetic testing,27–29 a functional mechanical change in muscle that is highly related to muscle phenotyping. Importantly, altered measures of neural activity were also present during both contraction types (isometric and isokinetic), suggesting that neural factors were also contributing to interlimb asymmetries at return to activity.
Isometric Interlimb Asymmetries
Isometric peak torque was 28.5% lower in the involved than in the uninvolved limb. With the greatest ES and narrowest 95% CI (Table 3), this clinically meaningful and statistically different decrease in peak torque indicated a deficit in quadriceps function that, in part, may be explained by a mechanical change in muscle due to a loss of sarcomeres in parallel or a change in the pennation of the muscle.27,32,33,35−37 Whereas we did not directly analyze these possibilities, several authors using imaging technologies found that patients with ACLR experienced reduced total muscle volume38,39 and anatomic CSA.40 Recently, Kuenze et al41 used magnetic resonance imaging and custom-written software to quantify individual variations in quadriceps muscle volume and reported that reductions in the vastus intermedius muscle belly were most strongly related to a decline in isometric quadriceps force production. We examined this characteristic of muscle using classic muscle-mechanics relationships to gain insight into the underlying morphologic changes, which is a cost-effective technique for clinicians to employ. Although we did not directly assess muscle morphology, our simplified clinical measures of quadriceps isometric torque production agree with previously reported38−41 changes in anatomic variants of muscle that likely led to a lack of quadriceps force production.
Neurologic asymmetries in isometric muscle function were also present in the ACLR group. Specifically, a 4% reduction in VAF and a 30% decrease in isometric RTD were found in the involved versus the uninvolved limb. Compared with the muscle mechanical regulators of isometric torque production that we described, these neurologic asymmetries produced weaker ESs and wider CIs, indicating that neurologic deficits in quadriceps function were likely a secondary contributor to quadriceps isometric asymmetries (Table 3). Nevertheless, reduced quadriceps CAR was present in the involved limb, indicating that diminished motoneuron recruitment or decreased motor-unit–firing frequency was likely contributing to reduced isometric quadriceps strength and interlimb asymmetries.7,9,42 This VAF finding is supported by the decreased isometric RTD in the involved limb, which was a factor in quadriceps torque production that was also regulated by altered neural drive.43−46
Isokinetic Interlimb Asymmetries
Interlimb asymmetries in quadriceps isokinetic force production were also present in the involved limb at return to activity. Specifically, peak isokinetic torque was 31.5% lower in the involved than in the uninvolved limb (Table 3). This clinically meaningful and significant reduction in the torque-velocity relationship in the involved limb can be explained, in part, by classic muscle mechanics, as a shift in the torque-velocity relationship is known to be highly influenced by a change in the distribution of muscle-fiber types.27−29,35 A limitation of our work is that we collected isokinetic torque at 60°/s, which is a speed known to be influenced by both fast-twitch and slow-twitch fiber types.28 However, given that we used maximum isokinetic torque to compute asymmetries between limbs, it seems highly plausible that interlimb morphologic differences in isokinetic torque were being regulated by a shift in muscle phenotype from fast-twitch to slow-twitch fibers in the involved limb.28,29 Notably, this shift in muscle phenotype has been shown to have a debilitating effect on athletes' performance,28,47 and alterations in the torque-velocity relationship were present before ACLR and at 3 and 6 months postsurgery.24 Our observations suggest that alterations in muscle phenotype may be an important underlying morphologic factor that likely contributed to interlimb asymmetry in patients with ACLR. This concept of a shift in muscle phenotype has been investigated by others48,49 who analyzed muscle biopsies after ACLR and found a reduction in type II muscle fibers. Researchers50 have also used isokinetic dynamometry after ACLR and theorized that selective type II fiber atrophy may be present, as the involved quadriceps muscle appeared to be more resistant to fatigue than the contralateral limb.
Similar to the isometric results, patients with ACLR in our study demonstrated a 36% slower isokinetic RTD in the involved than in the uninvolved limb. This decrease in isokinetic RTD supports previous theories of researchers15,51 who suggested that a lower RTD may be determined by a lack of afferent feedback from the reconstructed ACL, ultimately inhibiting the recruitment of high-threshold motor units. A lower RTD may also indicate a longer period is needed for higher-threshold motor units to activate.16 Again, the weak to moderate ESs and wider CIs stemming from the neurologic data suggested that alterations in muscle-mechanics relationships may play a bigger role in the primary causes of isokinetic interlimb asymmetries (Table 3). Interestingly, whereas VAF was present immediately after ACLR and ACL rehabilitation, our data support the concept that VAF may have a minimal effect on interlimb asymmetries at return to activity.4 However, it seems plausible that the large VAF observed immediately after ACL injury and surgery may have an important indirect effect on long-term quadriceps function, as alterations in neural activity can change muscle phenotypes. Using animal models, researchers22,23,52 have confirmed that neurologic deficits can directly influence muscle-fiber type. Hence, the immediate decreases in neural activity that are commonly seen after ACL injury and surgery provide a compelling mechanism for the prolonged shifts in muscle-fiber type that, in turn, contribute to interlimb asymmetries at return to activity. Given the noninvasive nature of our study and the lack of longitudinal measures, we cannot directly conclude that alterations in neural activity are causing these prolonged deficits in muscle mechanics; however, the theory is interesting and well supported by animal models that can be tested in future studies. Research including direct measures of muscle morphology and VAF is needed to determine if changes in quadriceps muscle strength are more strongly influenced by morphologic factors than by VAF.
Clinical Importance
Despite the best efforts of clinicians, at the time of return to activity, patients with ACLR often have interlimb asymmetries. Our isometric and isokinetic data support the idea that limb asymmetries after ACLR are related to a decline in muscle CSA or pennation angle in the involved limb in concert with potential shifting in muscle-fiber types. Underlying deficits in quadriceps VAF also appear to contribute to these interlimb differences. Given that neurologic deficits occur in the population with ACLR, it is possible that they may be a secondary mechanism of prolonged quadriceps weakness at return to activity. Taken together, our data suggest that simply measuring only discrete points on the quadriceps torque curve, with the most common being peak torque at return to activity, may not provide the patient or clinician with enough data points to make a fully informed return-to-activity decision. The addition of other objective criteria that can provide insight into protracted interlimb asymmetries is fundamental to understanding the underlying neuromuscular factors that could inhibit performance. Readiness for sport is more than being able to produce a high level of force, and we encourage clinicians to consider using some of the outcome measures described in this paper as part of a battery of standard tests to aid in decision making.
Given the multifaceted nature of these interlimb asymmetries and the lack of current rehabilitation strategies that can return patients with ACLR to activity with 90% limb symmetry,2,3 it is clear that alternative rehabilitation strategies capable of targeting these underlying neuromuscular deficits are also needed. To treat alterations in the involved limb, emerging evidence indicates that eccentric exercise after ACLR is particularly beneficial to promoting muscle volume,53 selective recruitment of type II fibers, and volitional muscle activity.10 In animal models, high-intensity neuromuscular electrical stimulation reduced fibrotic tissue formation54 and atrogene levels of muscle atrophy.55 Promising data are also available that support whole-body–vibration training to promote improved neuromuscular control56 and blood-flow–restriction therapy to increase fiber-type recruitment,57 satellite cell proliferation,58 muscle activation,59 and muscle volume.58,60
LIMITATIONS
Our study had limitations. Notably, our isokinetic data were not collected at the previously reported optimal velocities for the correlation to changes in muscle-fiber types. Whereas peak power output can predict fiber-type distribution, MacIntosh et al28 determined that the strongest relationship between the percentage of fast-twitch fibers and power output occurred when isokinetic strength was collected at angular velocities of 180°/s or greater. We collected our data at 60°/s, as this is a common mode of isokinetic testing used at return to activity in our primary orthopaedic clinic. Although this speed has a moderate correlation with shifts in fiber types,28 the observation of multiple isokinetic velocities would be crucial for drawing further conclusions about shifts in fiber types and the torque velocity curve. Our data were also limited because we did not simultaneously collect electromyography data during isometric and isokinetic testing. Having continuous electromyography data during testing would have allowed us to evaluate how changes in neural activity were continuously contributing to maximal torque-production efforts. In addition, we did not directly collect any muscle-morphology data. Whereas dynamometer testing is well suited for examining fiber-type distribution,29 future work would benefit from confirming these results using muscle biopsies and imaging.
CONCLUSIONS
Using classic muscle-mechanics relationships and noninvasive measures, we found that interlimb asymmetries at return to activity after ACLR appeared to be regulated by several underlying neuromuscular factors. Our data supported the idea that a change in muscle CSA or pennation angle in the involved limb may be present concurrently with a shift in muscle-fiber types. Underlying deficits in quadriceps VAF also appeared to contribute to interlimb differences, but these neurologic deficits seemed to be a secondary mechanism of prolonged quadriceps weakness.
ACKNOWLEDGMENTS
This study was supported by Grant No. K08 AR05315201A2 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (Dr Palmieri-Smith) and by Grant Nos. UL1TR000433 and UL1TR002240 from the National Center for Advancing Translational Sciences of the National Institutes of Health (University of Michigan). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1. Failla MJ., Arundale AJ., Logerstedt DS., Snyder-Mackler L. Controversies in knee rehabilitation: anterior cruciate ligament injury. . 2015; 34 2: 301– 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lepley LK. Deficits in quadriceps strength and patient-oriented outcomes at return to activity after ACL reconstruction: a review of the current literature. . 2015; 7 3: 231– 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Palmieri-Smith RM., Thomas AC., Wojtys EM. Maximizing quadriceps strength after ACL reconstruction. . 2008; 27 3: 405– 424. [DOI] [PubMed] [Google Scholar]
- 4. Lepley LK., Palmieri-Smith RM. Quadriceps strength, muscle activation failure, and patient-reported function at the time of return to activity in patients following anterior cruciate ligament reconstruction: a cross-sectional study. . 2015; 45 12: 1017– 1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grindem H., Snyder-Mackler L., Moksnes H., Engebretsen L., Risberg MA. Simple decision rules can reduce reinjury risk by 84% after ACL reconstruction: the Delaware-Oslo ACL cohort study. . 2016; 50 13: 804– 808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ageberg E., Thomeé R., Neeter C., Silbernagel KG., Roos EM. Muscle strength and functional performance in patients with anterior cruciate ligament injury treated with training and surgical reconstruction or training only: a two to five-year followup. . 2008; 59 12: 1773– 1779. [DOI] [PubMed] [Google Scholar]
- 7. Palmieri-Smith RM., Lepley LK. Quadriceps strength asymmetry after anterior cruciate ligament reconstruction alters knee joint biomechanics and functional performance at time of return to activity. . 2015; 43 7: 1662– 1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tourville TW., Jarrell KM., Naud S., Slauterbeck JR., Johnson RJ., Beynnon BD. Relationship between isokinetic strength and tibiofemoral joint space width changes after anterior cruciate ligament reconstruction. . 2014; 42 2: 302– 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ingersoll CD., Grindstaff TL., Pietrosimone BG., Hart JM. Neuromuscular consequences of anterior cruciate ligament injury. . 2008; 27 3: 383– 404, vii. [DOI] [PubMed] [Google Scholar]
- 10. Lepley LK., Wojtys EM., Palmieri-Smith RM. Combination of eccentric exercise and neuromuscular electrical stimulation to improve quadriceps function post-ACL reconstruction. . 2015; 22 3: 270– 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pietrosimone BG., Lepley AS., Ericksen HM., Clements A., Sohn DH., Gribble PA. Neural excitability alterations after anterior cruciate ligament reconstruction. . 2015; 50 6: 665– 674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rutherford O., Jones D., Newham D. Clinical and experimental application of the percutaneous twitch superimposition technique for the study of human muscle activation. . 1986; 49 11: 1288– 1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Snyder-Mackler L., Delitto A., Stralka SW., Bailey SL. Use of electrical stimulation to enhance recovery of quadriceps femoris muscle force production in patients following anterior cruciate ligament reconstruction. . 1994; 74 10: 901– 907. [DOI] [PubMed] [Google Scholar]
- 14. Stackhouse SK., Dean JC., Lee SC., Binder-MacLeod SA. Measurement of central activation failure of the quadriceps femoris in healthy adults. . 2000; 23 11: 1706– 1712. [DOI] [PubMed] [Google Scholar]
- 15. Hsieh CJ., Indelicato PA., Moser MW., Vandenborne K., Chmielewski TL. Speed, not magnitude, of knee extensor torque production is associated with self-reported knee function early after anterior cruciate ligament reconstruction. . 2015; 23 11: 3214– 3220. [DOI] [PubMed] [Google Scholar]
- 16. de Ruiter CJ., Kooistra RD., Paalman MI., de Haan A. Initial phase of maximal voluntary and electrically stimulated knee extension torque development at different knee angles. . 2004; 97 5: 1693– 1701. [DOI] [PubMed] [Google Scholar]
- 17. Van Cutsem M., Duchateau J., Hainaut K. Changes in single motor unit behaviour contribute to the increase in contraction speed after dynamic training in humans. . 1998; 513 pt 1: 295– 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burnham R., Martin T., Stein R., Bell G., MacLean I., Steadward R. Skeletal muscle fibre type transformation following spinal cord injury. . 1997; 35 2: 86– 91. [DOI] [PubMed] [Google Scholar]
- 19. Bojsen-Moller J., Magnusson SP., Rasmussen LR., Kjaer M., Aagaard P. Muscle performance during maximal isometric and dynamic contractions is influenced by the stiffness of the tendinous structures. . 2005; 99 3: 986– 994. [DOI] [PubMed] [Google Scholar]
- 20. Callahan DM., Tourville TW., Slauterbeck JR., et al. Reduced rate of knee extensor torque development in older adults with knee osteoarthritis is associated with intrinsic muscle contractile deficits. . 2015; 72: 16– 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reeves ND., Narici MV., Maganaris CN. Strength training alters the viscoelastic properties of tendons in elderly humans. . 2003; 28 1: 74– 81. [DOI] [PubMed] [Google Scholar]
- 22. Lu DX., Huang SK., Carlson BM. Electron microscopic study of long-term denervated rat skeletal muscle. . 1997; 248 3: 355– 365. [DOI] [PubMed] [Google Scholar]
- 23. Sunderland S., Ray L. Denervation changes in mammalian striated muscle. . 1950; 13 3: 159– 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hsiao SF., Chou PH., Hsu HC., Lue YJ. Changes of muscle mechanics associated with anterior cruciate ligament deficiency and reconstruction. . 2014; 28 2: 390– 400. [DOI] [PubMed] [Google Scholar]
- 25. Blazevich AJ., Coleman DR., Horne S., Cannavan D. Anatomical predictors of maximum isometric and concentric knee extensor moment. . 2009; 105 6: 869– 878. [DOI] [PubMed] [Google Scholar]
- 26. Narici MV., Roi GS., Landoni L., Minetti AE., Cerretelli P. Changes in force, cross-sectional area and neural activation during strength training and detraining of the human quadriceps. . 1989; 59 4: 310– 319. [DOI] [PubMed] [Google Scholar]
- 27. Epstein M., Herzog W. . New York, NY: Wiley; 1998: 22– 43. [Google Scholar]
- 28. MacIntosh BR., Herzog W., Suter E., Wiley JP., Sokolosky J. Human skeletal muscle fibre types and force: velocity properties. . 1993; 67 6: 499– 506. [DOI] [PubMed] [Google Scholar]
- 29. Suter E., Herzog W., Sokolosky J., Wiley JP., Macintosh BR. Muscle fiber type distribution as estimated by Cybex testing and by muscle biopsy. . 1993; 25 3: 363– 370. [PubMed] [Google Scholar]
- 30. Kline PW., Morgan KD., Johnson DL., Ireland ML., Noehren B. Impaired quadriceps rate of torque development and knee mechanics after anterior cruciate ligament reconstruction with patellar tendon autograft. . 2015; 43 10: 2553– 2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Revised ed. New York, NY: Academic Press; 1977: 145– 178. [Google Scholar]
- 32. Herzog W., Guimaraes A., Anton M., Carter-Erdman K. Moment-length relations of rectus femoris muscles of speed skaters/cyclists and runners. . 1991; 23 11: 1289– 1296. [PubMed] [Google Scholar]
- 33. Lieber RL., Loren GJ., Friden J. In vivo measurement of human wrist extensor muscle sarcomere length changes. . 1994; 71 3: 874– 881. [DOI] [PubMed] [Google Scholar]
- 34. Kawakami Y., Abe T., Kuno SY., Fukunaga T. Training-induced changes in muscle architecture and specific tension. . 1995; 72 1−2: 37– 43. [DOI] [PubMed] [Google Scholar]
- 35. Wisdom KM., Delp SL., Kuhl E. Use it or lose it: multiscale skeletal muscle adaptation to mechanical stimuli. . 2015; 14 2: 195– 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tabary J., Tardieu C., Tardieu G., Tabary C., Gagnard L. Functional adaptation of sarcomere number of normal cat muscle. . 1976; 72 3: 277– 291. [PubMed] [Google Scholar]
- 37. Fridén J., Seger J., Sjöström M., Ekblom B. Adaptive response in human skeletal muscle subjected to prolonged eccentric training. . 1983; 4 3: 177– 183. [DOI] [PubMed] [Google Scholar]
- 38. Konishi Y., Ikeda K., Nishino A., Sunaga M., Aihara Y., Fukubayashi T. Relationship between quadriceps femoris muscle volume and muscle torque after anterior cruciate ligament repair. . 2007; 17 6: 656– 661. [DOI] [PubMed] [Google Scholar]
- 39. Konishi Y., Oda T., Tsukazaki S., Kinugasa R., Fukubayashi T. Relationship between quadriceps femoris muscle volume and muscle torque at least 18 months after anterior cruciate ligament reconstruction. . 2012; 22 6: 791– 796. [DOI] [PubMed] [Google Scholar]
- 40. Thomas AC., Wojtys EM., Brandon C., Palmieri-Smith RM. Muscle atrophy contributes to quadriceps weakness after anterior cruciate ligament reconstruction. . 2016; 19 1: 7– 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kuenze CM., Blemker SS., Hart JM. Quadriceps function relates to muscle size following ACL reconstruction. . 2016; 34 9: 1656– 1662. [DOI] [PubMed] [Google Scholar]
- 42. Palmieri-Smith RM., Thomas AC. A neuromuscular mechanism of posttraumatic osteoarthritis associated with ACL injury. . 2009; 37 3: 147– 153. [DOI] [PubMed] [Google Scholar]
- 43. Aagaard P., Simonsen EB., Andersen JL., Magnusson P., Dyhre-Poulsen P. Increased rate of force development and neural drive of human skeletal muscle following resistance training. . 2002; 93 4: 1318– 1326. [DOI] [PubMed] [Google Scholar]
- 44. Andersen LL., Aagaard P. Influence of maximal muscle strength and intrinsic muscle contractile properties on contractile rate of force development. . 2006; 96 1: 46– 52. [DOI] [PubMed] [Google Scholar]
- 45. Angelozzi M., Madama M., Corsica C., et al. Rate of force development as an adjunctive outcome measure for return-to-sport decisions after anterior cruciate ligament reconstruction. . 2012; 42 9: 772– 780. [DOI] [PubMed] [Google Scholar]
- 46. Hsieh CJ., Indelicato PA., Moser MW., Vandenborne K., Chmielewski TL. Speed, not magnitude, of knee extensor torque production is associated with self-reported knee function early after anterior cruciate ligament reconstruction. . 2015; 23 11: 3214– 3220. [DOI] [PubMed] [Google Scholar]
- 47. Wilson JM., Loenneke JP., Jo E., Wilson GJ., Zourdos MC., Kim JS. The effects of endurance, strength, and power training on muscle fiber type shifting. . 2012; 26 6: 1724– 1729. [DOI] [PubMed] [Google Scholar]
- 48. Lopresti C., Kirkendall DT., Street GM., Dudley AW. Quadriceps insufficiency following repair of the anterior cruciate ligament. . 1988; 9 7: 245– 249. [DOI] [PubMed] [Google Scholar]
- 49. Noehren B., Andersen A., Hardy P., et al. Cellular and morphological alterations in the vastus lateralis muscle as the result of ACL injury and reconstruction. . 2016; 98 18: 1541– 1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Snyder-Mackler L., Binder-Macleod SA., Williams PR. Fatigability of human quadriceps femoris muscle following anterior cruciate ligament reconstruction. . 1993; 25 7: 783– 789. [DOI] [PubMed] [Google Scholar]
- 51. Konishi Y., Fukubayashi T., Takeshita D. Mechanism of quadriceps femoris muscle weakness in patients with anterior cruciate ligament reconstruction. . 2002; 12 6: 371– 375. [DOI] [PubMed] [Google Scholar]
- 52. Bassel-Duby R., Olson EN. Signaling pathways in skeletal muscle remodeling. . 2006; 75: 19– 37. [DOI] [PubMed] [Google Scholar]
- 53. Gerber JP., Marcus RL., Dibble LE., Greis PE., Burks RT., LaStayo PC. Effects of early progressive eccentric exercise on muscle size and function after anterior cruciate ligament reconstruction: a 1-year follow-up study of a randomized clinical trial. . 2009; 89 1: 51– 59. [DOI] [PubMed] [Google Scholar]
- 54. Durigan JL., Peviani SM., Delfino GB., de Souza Jose RJ., Parra T., Salvini TF. Neuromuscular electrical stimulation induces beneficial adaptations in the extracellular matrix of quadriceps muscle after anterior cruciate ligament transection of rats. . 2014; 93 11: 948– 961. [DOI] [PubMed] [Google Scholar]
- 55. Durigan JL., Delfino GB., Peviani SM., et al. Neuromuscular electrical stimulation alters gene expression and delays quadriceps muscle atrophy of rats after anterior cruciate ligament transection. . 2014; 49 1: 120– 128. [DOI] [PubMed] [Google Scholar]
- 56. Pamukoff DN., Pietrosimone B., Lewek MD., et al. Whole-body and local muscle vibration immediately improve quadriceps function in individuals with anterior cruciate ligament reconstruction. . 2016; 97 7: 1121– 1129. [DOI] [PubMed] [Google Scholar]
- 57. Yasuda T., Abe T., Brechue WF., et al. Venous blood gas and metabolite response to low-intensity muscle contractions with external limb compression. . 2010; 59 10: 1510– 1519. [DOI] [PubMed] [Google Scholar]
- 58. Nielsen JL., Aagaard P., Bech RD., et al. Proliferation of myogenic stem cells in human skeletal muscle in response to low-load resistance training with blood flow restriction. . 2012; 590 17: 4351– 4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Loenneke JP., Kim D., Fahs CA., et al. Effects of exercise with and without different degrees of blood flow restriction on torque and muscle activation. . 2015; 51 5: 713– 721. [DOI] [PubMed] [Google Scholar]
- 60. Loenneke JP., Kim D., Fahs CA., et al. The influence of exercise load with and without different levels of blood flow restriction on acute changes in muscle thickness and lactate. . 2017; 37 6: 734– 740. [DOI] [PubMed] [Google Scholar]


