Abstract
Objective
This meta-analysis examined treatment efficacy, treatment response, and diagnostic remission effect sizes (ES) and moderators of d-cycloserine (DCS) augmented exposure treatment in randomized controlled trials (RCTs) of individuals with anxiety disorders, obsessive-compulsive disorder (OCD), and posttraumatic stress disorder (PTSD).
Data Sources and Study Selection
Using search terms d-cycloserine AND randomized controlled trial, PubMED (1965-May 2015), PsycInfo, and Scopus were searched for randomized placebo-controlled trials of DCS-augmented exposure therapy for anxiety disorders, OCD, and PTSD.
Data Extraction
Clinical variables and ES were extracted from 20 RCTs (957 participants). A random effects model calculated the ES for treatment efficacy, treatment response, and diagnostic remission using standardized rating scales. Subgroup analyses and meta-regression examined potential moderators.
Results
A small non-significant benefit of DCS augmentation compared to placebo augmentation was identified across treatment efficacy (g=0.15), response (RR=1.08), and remission (RR=1.109), with a moderately significant effect for anxiety disorders specifically (g=0.33, p=.03). At initial follow-up assessments, a small non-significant ES of DCS augmentation compared to placebo was found for treatment efficacy (g=0.21), response (RR=1.06), and remission (RR=1.12). Specific treatment moderators (e.g., comorbidity, medication status, gender, publication year) were found across conditions for both acute treatment and initial follow-up assessments.
Conclusions
DCS does not universally enhance treatment outcomes, but demonstrates promise for anxiety disorders. Distinct treatment moderators may account for discrepant findings across RCTs and disorders. Future trials may be strengthened by accounting for identified moderators in their design, with ongoing research needed on the mechanisms of DCS to tailor treatment protocols and maximize its benefit.
Keywords: treatment outcome, exposure therapy, d-cycloserine, anxiety disorders, obsessive-compulsive disorder, posttraumatic stress disorder
Introduction
Anxiety disorders, obsessive compulsive disorder (OCD), and posttraumatic stress disorder (PTSD) are characterized by clinically significant fear and distress that occur in response to stimuli and/or situational cues.1 These psychiatric conditions collectively affect between 2 and 29% of individuals.2, 3 Psychiatric comorbidity is common for these conditions with co-occurring anxiety and depressive disorders frequently endorsed by individuals.4 Although these disorders possess distinctive diagnostic features and multi-factorial etiologies,1 the mechanisms of fear conditioning and extinction are believed to play a central in symptom acquisition, persistence, and treatment.5
Cognitive-behavioral therapy (CBT) is an efficacious treatment for anxiety disorders, OCD and PTSD, with the core therapeutic component being exposures to feared situations and/or stimuli.6 Although efficacious, several limitations exist with exposure-based CBT for these disorders. These challenges include: inadequate response to exposure-based therapies and/or infrequent diagnostic remission; treatment attrition and/or treatment burden; and limited access and/or availability to trained treatment providers experienced in conducting exposure-based CBT with anxious patients.7, 8 Taken together, there is a clear need to improve therapeutic outcomes and accelerate treatment gains to reduce patient burden and improve therapist availability.
Multiple approaches have been explored to enhance and/or accelerate treatment outcomes for youth and adults with anxiety disorders, OCD, and PTSD. One approach involves the administration of cognitive enhancers to augment exposure-based therapies. Broadly, cognitive enhancers are compounds that influence signaling pathways in brain regions associated with fear learning to enhance neurocircuitry of fear extinction that occurs during exposure-based treatments.9 Although multiple cognitive enhancers have been evaluated in randomized controlled trials (RCTs) among individuals with fear-based psychiatric disorders,8 d-cycloserine (DCS) is the most well-studied cognitive enhancer in both youths and adults. DCS is a partial N-methyl-D-aspartate (NMDA) agonist that has been found to enhance fear extinction in animal studies.10, 11 It is believed that acute DCS administration stimulates NMDA glutamate synapses involved in emotional learning and strengthens extinction learning that takes place in exposure-based treatments,12, 13 which may occur in part through the consolidation of extinction learning.14 Given the parallels between fear extinction in animal studies and human exposure-based therapies, DCS has been evaluated across multiple RCTs with mixed success.15–34
When evaluating the benefit of a treatment approach, it is important to synthesize the empirical evidence to inform clinical decisions.35 Meta-analyses provide a quantitative synthesis of RCTs, and can examine treatment moderators across studies. To date, there have been four meta-analyses that have examined the efficacy of DCS-augmented exposure-based therapies relative to placebo-augmentation across anxiety disorders, OCD, and PTSD.10, 36–38 Norberg and colleagues10 examined DCS across animal and human studies. Although a large effect was found in animal studies (d=1.19), the augmentative effects of DCS were modest among clinical human studies at post-treatment (d=0.60) and follow-up assessments (d=0.47).10 Meanwhile, Bontempo and colleagues36 completed a meta-analysis of 9 RCTs with 273 human participants and found a standardized mean difference (SMD) of 0.46 between DCS-augmented and placebo-augmented treatment at post-treatment. Rodrigues and colleagues37 completed a meta-analysis of 13 RCTs and found a small effect (SMD=0.34) of DCS-augmented treatment relative to placebo-augmentation at post-treatment. Most recently, Ori and colleagues38 completed a meta-analysis of 21 RCTs and found no significant difference in treatment response between DCS augmentation and placebo augmentation treatment [Risk ratio (RR)=1.1, 95% CI=0.89, 1.34). While these meta-analyses initially suggest an overall benefit of DCS in augmenting exposure-based treatments, the additive benefit of DCS appears to have diminished with the inclusion of further RCTs. This diminishing effect may be attributed to a minimal benefit of DCS revealed with further examination, trial design methodologies of later RCTs that utilized full courses of exposure treatments, and/or possible moderators that influence treatment effects.
Notably, across these four meta-analyses, few moderators have been examined. Available moderator analyses have found no association between treatment effects and: dose,10, 36 time of administration,36 number of doses,10, 36 number of treatment sessions,36 diagnostic group,36 methodological quality, 36 and analytic method.36 However, Norberg and colleagues10 found that DCS doses administered closer to therapy sessions exhibited greater effects. Although noteworthy, several limitations exist in these prior examinations that include: small sample size,36 moderator analyses across humans and animal studies,10 and treatment effect extraction from ratings with untested psychometric properties (e.g., subjective fear ratings).10, 36 Additionally, the moderating influence of gender, co-occurring psychiatric conditions, or concurrent medication status were not examined. Furthermore, there has been no evaluation of moderators of treatment response and diagnostic remission.
To address these concerns, this report examined placebo-controlled RCTs of DCS-augmented exposure-based treatment for anxiety disorders, OCD, and PTSD to determine its treatment efficacy and identify the RR of experiencing treatment response or diagnostic remission. We hypothesized that DCS-augmented exposure-based treatment would outperform placebo-augmented exposure-based treatment. Based on prior meta-analyses and the extant literature, several theoretically-derived moderators were examined. First, given the neurobiological differences in fear acquisition and extinction between youth and adults,39 we examined the influence of age. Second, given the variable gender distribution across studies (19%-100%), we examine whether gender influenced treatment effects. Third, as specific disorders (i.e., panic disorder, PTSD, depression) have been related to impediments in fear acquisition and extinction,40 we examined whether co-occurring anxiety or depressive disorders influenced effects. Fourth, as serotonin reuptake inhibitors are common evidence-based treatments for these conditions and have demonstrated mixed evidence on the promotion/impairment of fear extinction,41, 42 we examined the moderating impact of SRIs on treatment effects. Fifth, as some studies utilized full courses of treatment and others employed abbreviated treatment protocols, we examined the influence of therapeutic contact on treatment effects. Finally, following-up on studies that suggest the dose15, 43 and time administration10 may influence DCS outcomes, we investigated wither these factors impacted treatment effects.
Method
Search Strategy
PubMED (1965-May 2015), PsycInfo, and Scopus were searched using the key search terms: “d-cycloserine” and “randomized controlled trial.” Identified abstracts were reviewed independently by two raters for appropriateness (J.F.M and M.S.W). The references of eligible treatment trials and review articles were also searched. Identified abstracts/citations were evaluated for the following inclusion criteria: 1) an RCT, 2) comparison of DCS-augmented exposure-based treatment to placebo-augmented exposure-based treatment; 3) sample that met criteria for an anxiety disorder, OCD, or PTSD; 4) available in English; 5) provision of sufficient data to calculate treatment effects using psychometrically-supported rating scales. When insufficient or incomplete data were present, study investigators were contacted to obtain values.
Procedures
Given the diversity of measures across studies, a hierarchy of preferred informant ratings for the primary outcome measure was established a priori to limit reporting bias. In order of preference, these informants included clinician ratings, self-report ratings, and parent-report ratings. When multiple ratings by the same informant were available, preference was placed on the most commonly used measure in RCTs. For classification of treatment response, preference was placed on the Clinical Global Impression–Improvement Scale (CGI-Improvement),44 with treatment response classified as a rating of “much improved” or “very much improved”. When the CGI-Improvement was unavailable, a percent reduction or clinical cut-off score based on the primary outcome measure was selected that corresponded with a response on the CGI-Improvement.45–49 For classification of diagnostic remission, preference was placed on the Clinical Global Impression–Severity Scale (CGI-Severity), with a remission rating classified as a rating of “no illness” or “mild illness”.44, 50 When the CGI-Severity was unavailable, a percent reduction or clinical cut-off score based on the primary outcome measure was selected that corresponded with a categorization of remission on the CGI-Severity.45–47
Trials were coded for the following characteristics: sample size, percent male, average participant age, percentage of co-occurring anxiety or depressive disorders, percent on an serotonin reuptake inhibitor (SRI) medication, number of therapy sessions, DCS administration time and number of DCS doses, publication year, and analysis type (intent-to-treat or completer). Study methodology was assessed using a 23-item scale that has been used in other meta-analyses,51, 52 with higher scores corresponding to greater methodological rigor. Trials were coded by two raters to ascertain reliability. Rater disagreement was resolved through discussion and consensus.
Effect Size (ES) Calculation
Given the difference in sample sizes across studies, Hedges’ g was used to calculate treatment efficacy and was calculated in Comprehensive Meta-Analysis (CMA) Version 2.53 Effect sizes were calculated using change scores because it increases the precision of ES estimators by controlling for pretreatment group differences of symptom severity. Pre- and post-treatment means and standard deviations were entered into CMA, and were divided by the pooled post-treatment standard deviation. Effect sizes were standardized so that a positive result indicated that DCS outperformed placebo augmentation in reducing symptom severity. The RR is the ratio of participants exhibiting response or remission with DCS-augmented treatment divided by the probability of patients exhibiting response or remission with placebo-augmented treatment.54 A RR of 1 suggests that response or remission did not differ between conditions, whereas a RR of 3 indicates that the DCS augmentation had a threefold greater probability of exhibiting response or remission. The number of participants experiencing a treatment response and remission were entered into CMA, which calculated the RR for treatment response and diagnostic remission.
Statistical Analyses
Inter-rater agreement of study characteristics and quality ratings were assessed using descriptive statistics and intra-class correlation coefficient (ICC). A random effects model using inverse variance weights examined the ES of DCS-augmented treatment. Separate random effect models examined the RR of DCS-augmented treatment for treatment response and diagnostic remission. A random effects model was chosen because the true ES were expected to vary across trials due to different study characteristics.55 Heterogeneity of ES was assessed using the forest plot, Q statistic, and I2 statistic. Publication bias was assessed by visual inspection of the funnel plot and Egger’s test for bias. When publication bias was present, a trim-and-fill method was applied to account for bias by producing an adjusted summary effect that takes into account possible unpublished studies within the field.55 Finally, moderator variables were analyzed using either method-of-moments meta-regression or an analog to the analysis of variance (ANOVA).
Results
Included Studies and Reliability Ratings
Initial search strategies produced 232 potential abstracts/citations, with 92 abstracts being retrieved for detailed review (see Figure 1). Table 1 displays the 20 RCTs (Anxiety, n=8; OCD, n=7; PTSD, n=5) that met inclusion criteria and produced a final sample size of 957 participants. The average initial follow-up assessment occurred 2.30 months after acute treatment (range: 1-6 months). There was excellent inter-rater agreement between the two raters on categorical and continuous study characteristics (100% agreement), as well as overall study methodological quality (ICC=0.94, 95% CI=0.86, 0.98).
Figure 1.
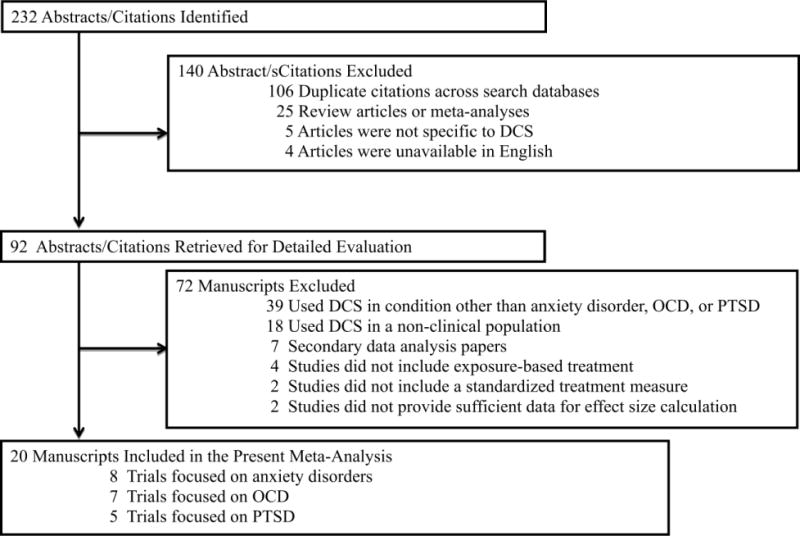
Study selection and Rationale for exclusion
Table 1.
Characteristics of Randomized Controlled Trials of D-Cycloserine Augmented Exposure-Based Cognitive-Behavioral Therapy (CBT)
| Trial | N | Primary Diagnosis | Mean Age | % Male | % Comorbid Depressive Disorders | % ComorbidAnxiety Disorders | % on SRI Med | # of Therapy sessions | DCS Dose Timing in hours | # of DCS Doses | Analysis Type | Method Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ressler et al.15 | 27 | SP | 46 | 41 | 0 | 0 | NR | 2 | −3 | 2 | ITT | 26 |
| Hofmann et al.16 | 32 | SAD | 34 | 67 | 15 | 33 | 33 | 5 | −1 | 4 | COMP | 32 |
| Storch et al.17 | 24 | OCD | 29 | 50 | 38 | 75 | 50 | 12 | −4 | 12 | COMP | 31 |
| Kushner et al.18 | 32 | OCD | 37 | 38 | 25 | 12.5 | 34.4 | 4 | −2 | 4 | ITT | 31 |
| Wilhelm et al.19 | 23 | OCD | 39 | 61 | 26 | 43 | 39 | 10 | −1 | 10 | ITT | 31 |
| Guastella et al.20 | 56 | SAD | 35 | 57 | 16 | 16 | 9 | 5 | −1 | 4 | ITT | 36 |
| Storch et al.21 | 30 | OCD | 12 | 63 | 10 | 27 | 30 | 10 | −1 | 7 | ITT | 36 |
| Otto at al.22 | 28 | PD | 37 | 50 | 17.9 | 28.6 | 54.5 | 5 | −1 | 3 | COMP | 28 |
| Litz et al.23 | 26 | PTSD | 32 | 100 | 30.8 | 11.5 | 7.7 | 6 | −1 | 4 | ITT | 36 |
| de Kleine et al.24 | 67 | PTSD | 38 | 19 | 54 | 42 | 25 | 8 | −1 | 6 | ITT | 36 |
| Nave et al.25 | 20 | SP | 37 | 40 | 5 | 0 | 10 | 1 | −1 | 1 | COMP | 30 |
| Farrell et al.26 | 17 | OCD | 13 | 41 | 12 | 59 | 76 | 9 | −1 | 5 | COMP | 36 |
| Rodebaugh et al.27 | 34 | SAD | 43 | 33 | 47 | 47 | 35 | 1 | 0 | 2 | COMP | 32 |
| Hofmann et al.28 | 169 | SAD | 33 | 57 | 26.6 | 25.4 | 0 | 12 | −1 | 5 | ITT | 38 |
| Tart et al.29 | 29 | SP | 33 | 24 | 34 | 66 | 0 | 2 | 1 | 2 | ITT | 35 |
| Mataix-Coles et al. 30 | 27 | OCD | 15 | 52 | 11 | 48 | 26 | 14 | 1 | 10 | ITT | 34 |
| Scheeringa & Weems31 | 57 | PTSD | 12 | 44 | 61.4 | 59.7 | 10.5 | 12 | −1 | 7 | ITT | 38 |
| Rothbaum et al.32 | 106 | PTSD | 34 | 93 | 77.3 | 12.2 | NR | 6 | −0.5 | 5 | ITT | 29 |
| Difede et al.33 | 25 | PTSD | 46 | 76 | 64 | NR | NR | 12 | −1.5 | 10 | ITT | 35 |
| Andersson et al.34 | 128 | OCD | 35 | 42 | 9 | 25 | 24 | 12 | −1 | 5 | ITT | 39 |
NOTE: NR= Not Reported, SP= Specific Phobia, PD= Panic Disorder, SAD= Social Anxiety Disorder, OCD= Obsessive-Compulsive Disorder, PTSD= Posttraumatic Stress Disorder, COMP=Completer Analysis, ITT=Intent-to-Treat Analysis
DCS-Augmented Outcomes
Acute Efficacy
A random effects meta-analysis found a non-significant effect for DCS- relative to placebo-augmented treatment across all studies (g=0.15, 95% CI: -0.03, 0.32, z=1.65, p=0.10) (see Figure 2). Visual inspection of the forest plot, Q statistic, and I2 statistic identified significant heterogeneity [Q(18)=29.09 p=0.05, I2=38.12%]. Visual inspection of the funnel plot and Egger’s test for bias indicated that publication bias was not significant (t=0.95, p=0.36). When examining diagnostic groups separately, there was a moderately significant effect for anxiety disorders (g=0.33, 95% CI: 0.02, 0.64, z=2.12, p=0.03), and a non-significant effect for both OCD (g=0.01, 95% CI: -0.24, 0.27, z=0.10, p=0.92) and PTSD (g=0.03, 95% CI: -0.30, 0.35, z=0.17, p=0.87). Follow-up comparisons found no significant difference between anxiety disorders and OCD (p=0.12), anxiety disorders and PTSD (p=0.18), or OCD and PTSD (p=0.95).
Figure 2.
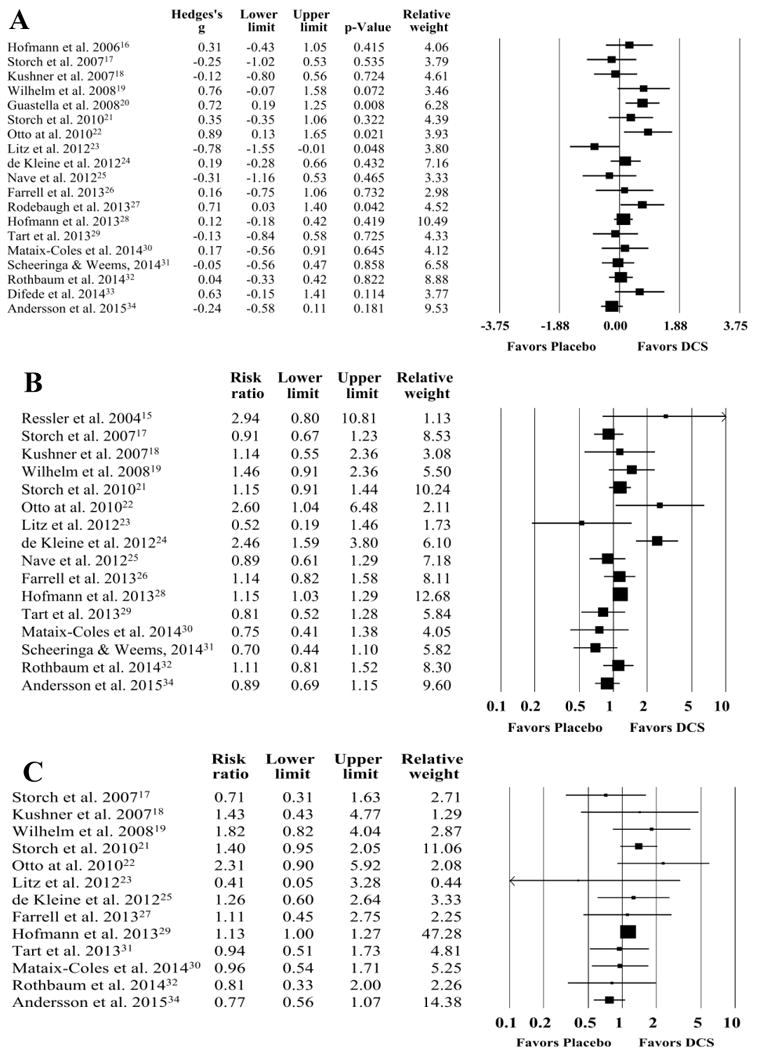
Forest Plots for Acute Treatment Efficacy (A), Treatment Response (B), and Diagnostic Remission (C) in D-Cycloserine or Placebo Augmentation Trials of Exposure-Based Treatment. Upper and Lower Limits in Forest plots represent 95% confidence intervals.
Acute Treatment Response
A random effects meta-analysis found a non-significant effect for DCS- relative to placebo-augmented treatment (RR=1.08, 95% CI: 0.94, 1.25, z=1.08, p=0.28) with significant heterogeneity [Q(15)=35.45, p=0.002, I2=57.68%] (Figure 2). Visual inspection of the funnel plot and Egger’s test for bias indicated that publication bias was not present (t=0.03, p=0.97). When examining diagnostic groups separately, there was a non-significant effect for anxiety disorders (RR=1.12, 95% CI: 0.83, 1.50, z=0.75, p=0.45), OCD (RR=1.04, 95% CI: 0.91, 1.18, z=0.51, p=0.61), and PTSD (RR=1.07, 95% CI: 0.59, 2.96, z=0.23, p=0.82). Follow-up comparisons found no significant difference between anxiety disorders and OCD (p=0.63), anxiety disorders and PTSD (p=0.90), or OCD and PTSD (p=0.91).
Acute Treatment Remission
A random effects meta-analysis found a non-significant effect for DCS relative to placebo augmented treatment (RR=1.09, 95% CI: 0.95, 1.25, z=1.16, p=0.25) with minimal heterogeneity present [Q(12)=13.33, p=0.35, I2=9.97%] (Figure 2). Visual inspection of the funnel plot and Egger’s test for bias indicated that publication bias was not significant (t=0.18, p=0.86). When examining diagnostic groups separately, there was a non-significant effect for anxiety disorders (RR=1.15, 95% CI: 0.89, 1.50, z=1.06, p=0.29), OCD (RR=1.09, 95% CI: 0.80, 1.38, z=0.38, p=0.71), and PTSD (RR=0.99, 95% CI: 0.57, 1.71, z=-0.05, p=0.96). Follow-up comparisons found no significant difference between anxiety disorders and OCD (p=0.64), anxiety disorders and PTSD (p=0.62), or OCD and PTSD (p=0.84).
Follow-up Efficacy
A random effects meta-analysis found a non-significant effect for DCS- relative to placebo-augmented treatment (g=0.21, 95% CI: -0.05, 0.46, z=1.60, p=0.11) (see Figure 3). Visual inspection of the forest plot, Q statistic, and I2 statistic identified significant heterogeneity [Q(15)=44.94, p<0.001, I2=66.62%]. Visual inspection of the funnel plot and Egger’s test for bias indicated that publication bias was not significant (t=1.24, p=0.23). When examining diagnostic groups separately, there was a non-significant effect for anxiety disorders (g=0.54, 95% CI: -0.15, 1.24, z=1.53, p=0.13), OCD (g=0.10, 95% CI: -0.22, 0.42, z=0.62, p=0.53) and PTSD (g=-0.05, 95% CI: -0.28, 0.18, z=-0.42, p=0.68). Follow-up comparisons found no significant difference between anxiety disorders and OCD (p=0.26), anxiety disorders and PTSD (p=0.11), or OCD and PTSD (p=0.45).
Figure 3.
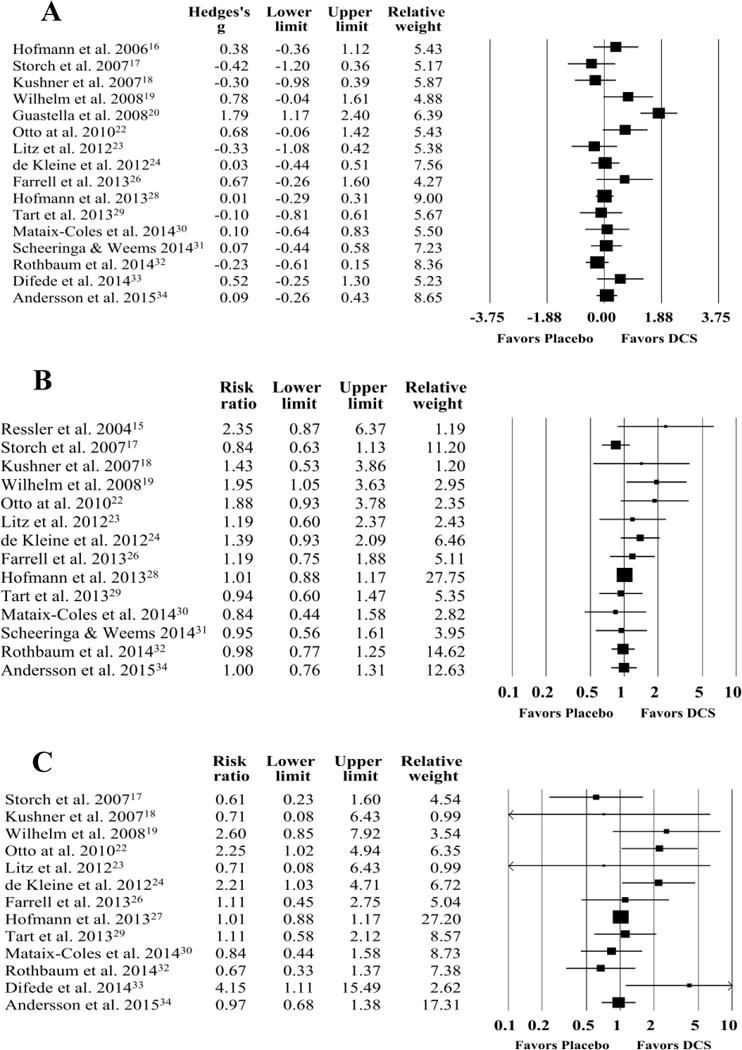
Forest Plots for Follow-Up Treatment Efficacy (A), Treatment Response (B), and Diagnostic Remission (C) in D-Cycloserine or Placebo Augmentation Trials of Exposure-Based Treatment Upper and Lower Limits in Forest plots represent 95% confidence intervals.
Follow-up Treatment Response
A random effects meta-analysis found a non-significant effect for DCS relative to placebo augmented treatment (RR=1.06, 95% CI: 0.95, 1.18, z=0.96, p=0.34) with some heterogeneity present [Q(13)=15.33, p=0.23, I2=15.21%] (Figure 3). Visual inspection of the funnel plot and Egger’s test for bias suggested that publication bias existed (t=2.25, p=0.04). A trim-and-fill method was applied and three studies were trimmed, which produced a revised summary effect that was also non-significant (RR=1.02, 95% CI: 0.87, 1.16). When examining diagnostic groups separately, there was a non-significant effect for anxiety disorders (RR=1.17, 95% CI: 0.85, 1.61, z=0.97, p=0.33), OCD (RR=1.04, 95% CI: 0.84, 1.30, z=0.39, p=0.69), and PTSD (RR=1.07, 95% CI: 0.88, 1.28, z=0.66, p=0.51). Follow-up comparisons found no significant difference between anxiety disorders and OCD (p=0.56), anxiety disorders and PTSD (p=0.62), or OCD and PTSD (p=0.89).
Follow-up Diagnostic Remission
A random effects meta-analysis found a non-significant effect for DCS relative to placebo augmented treatment (RR=1.12, 95% CI: 0.90, 1.40, z=1.01 p=0.32) with moderate heterogeneity [Q(12)=17.96, p=0.12, I2=33.20%] (Figure 3). Visual inspection of the funnel plot and Egger’s test for bias suggested that publication bias was not significant (t=1.07, p=0.31). When examining diagnostic groups separately, there was a non-significant effect for anxiety disorders (RR=1.20, 95% CI: 0.81, 1.77, z=0.89, p=0.38), OCD (RR=0.97, 95% CI: 0.74, 1.27, z=-0.21, p=0.84), and PTSD (RR=1.49, 95% CI: 0.61, 3.65, z=0.87, p=0.38). Follow-up comparisons found no significant difference between anxiety disorders and OCD (p=0.40), anxiety disorders and PTSD (p=0.66), or OCD and PTSD (p=0.37).
Moderators of DCS-Augmented Treatment
Table 2 presents treatment moderators examined across DCS-augmentation trials for both acute and follow-up treatment efficacy, treatment response, and symptom remission.
Table 2.
Regression Analyses and Analog to ANOVA Examining Moderators of Treatment Efficacy, Treatment Response, and Remission for DCS-Augmented CBT Trials
| Study Characteristics | Treatment Efficacy (n=19) | Treatment Response (n=16) | Diagnostic Remission (n=13) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | z | p | B | SE | z | p | B | SE | z | p | |
| Sample Size | < −0.01 | < 0.01 | −0.83 | 0.41 | < 0.01 | < 0.01 | 0.13 | 0.90 | < −0.01 | < 0.01 | −0.73 | 0.46 |
| Percent Male | < −0.01 | < 0.01 | −0.29 | 0.77 | < −0.01 | < 0.01 | −0.80 | 0.42 | < 0.01 | 0.01 | 0.48 | 0.63 |
| Mean Participant Age | 0.01 | 0.01 | 0.87 | 0.38 | 0.01 | 0.01 | 1.49 | 0.14 | −0.01 | 0.01 | −0.60 | 0.55 |
| Percent Comorbid Depressive Disorders | < 0.01 | < 0.01 | 0.03 | 0.97 | < 0.01 | < 0.01 | 0.27 | 0.79 | < − 0.01 | < 0.01 | −0.14 | 0.89 |
| Percent Comorbid Anxiety Disorders | < 0.01 | < 0.01 | 0.29 | 0.77 | < −0.01 | < 0.01 | −0.67 | 0.50 | < − 0.01 | < 0.01 | −0.40 | 0.69 |
| Percent on SRI Medication | 0.01 | 0.01 | 1.20 | 0.23 | < 0.01 | < 0.01 | 1.11 | 0.27 | < 0.01 | < 0.01 | 0.58 | 0.56 |
| Number of Therapy Sessions | −0.01 | 0.02 | −0.57 | 0.57 | −0.01 | 0.02 | −0.57 | 0.57 | −0.02 | 0.03 | −0.66 | 0.51 |
| Time of DCS Administration | 0.06 | 0.10 | 0.59 | 0.55 | −0.03 | 0.07 | −0.52 | 0.61 | < 0.01 | 0.09 | 0.02 | 0.98 |
| Number of DCS Doses | 0.01 | 0.04 | 0.26 | 0.79 | < −0.01 | 0.03 | −0.19 | 0.85 | < 0.01 | 0.04 | 0.07 | 0.95 |
| Publication Year | −0.04 | 0.03 | −1.39 | 0.17 | −0.04 | 0.03 | −1.45 | 0.15 | −0.07 | 0.03 | −2.11 | 0.04 |
| Methodological Quality Rating | −0.03 | 0.03 | −1.06 | 0.29 | −0.02 | 0.02 | −1.00 | 0.32 | −0.03 | 0.03 | −1.10 | 0.27 |
| Q | (df) | p | Q | (df) | p | Q | (df) | p | ||||
| Primary Diagnostic Classification | 2.80 | 2 | 0.25 | 0.24 | 2 | 0.89 | 0.36 | 2 | 0.84 | |||
| Child versus Adult | 0.03 | 1 | 0.86 | 0.83 | 1 | 0.36 | 0.81 | 1 | 0.37 | |||
| Analysis Type (ITT/COMP) | 0.62 | 1 | 0.43 | 0.05 | 1 | 0.82 | 0.07 | 1 | 0.79 | |||
|
| ||||||||||||
| Follow-Up Treatment Efficacy (n=16) | Follow-Up Treatment Response (n=14) | Follow-Up Diagnostic Remission (n=13) | ||||||||||
| Study Characteristics | B | SE | z | p | B | SE | z | p | B | SE | z | p |
| Sample Size | < −0.01 | < 0.01 | −0.74 | 0.46 | < −0.01 | < 0.01 | −0.80 | 0.42 | < −0.01 | < 0.01 | −1.07 | 0.29 |
| Percent Male | < −0.01 | 0.01 | −0.11 | 0.91 | < −0.01 | < 0.01 | −0.59 | 0.55 | −0.01 | 0.01 | −0.96 | 0.34 |
| Mean Participant Age | 0.01 | 0.02 | 0.42 | 0.67 | 0.01 | 0.01 | 1.32 | 0.19 | 0.03 | 0.01 | 1.70 | 0.09 |
| Percent Comorbid Depressive Disorders | −0.01 | 0.01 | −1.45 | 0.15 | < −0.01 | < 0.01 | −0.66 | 0.51 | < 0.01 | 0.01 | 0.32 | 0.75 |
| Percent Comorbid Anxiety Disorders | < −0.01 | 0.01 | −0.47 | 0.64 | < −0.01 | < 0.01 | −0.96 | 0.34 | < 0.01 | 0.01 | 0.26 | 0.80 |
| Percent on SRI Medication | < 0.01 | 0.01 | 0.42 | 0.67 | < 0.01 | < 0.01 | 0.70 | 0.48 | < 0.01 | < 0.01 | 0.82 | 0.41 |
| Number of Therapy Visits | −0.02 | 0.04 | −0.48 | 0.63 | −0.02 | 0.02 | −1.33 | 0.18 | −0.02 | 0.03 | −0.73 | 0.47 |
| Time of DCS Administration | 0.05 | 0.13 | 0.40 | 0.69 | 0.01 | 0.05 | 0.26 | 0.79 | < −0.01 | 0.11 | −0.01 | 0.99 |
| Number of DCS Doses | −0.02 | 0.05 | −0.33 | 0.74 | −0.02 | 0.02 | −1.02 | 0.31 | < −0.01 | 0.05 | −0.10 | 0.92 |
| Publication Year | −0.05 | 0.04 | −1.21 | 0.23 | −0.02 | 0.02 | −1.02 | 0.31 | −0.05 | 0.05 | −0.88 | 0.38 |
| Methodological Quality Rating | 0.01 | 0.04 | 0.23 | 0.82 | −0.01 | 0.02 | −0.72 | 0.47 | −0.01 | 0.03 | −0.43 | 0.67 |
| Follow-Up Duration | −0.13 | 0.10 | −1.31 | 0.19 | < −0.01 | 0.07 | −0.05 | 0.96 | 0.08 | 0.11 | 0.71 | 0.48 |
| Q | (df) | p | Q | (df) | p | Q | (df) | P | ||||
| Primary Diagnostic Classification | 2.73 | 2 | 0.26 | 0.36 | 2 | 0.84 | 1.31 | 2 | 0.52 | |||
| Child Versus Adult | 0.01 | 1 | 0.92 | 0.13 | 1 | 0.72 | 0.68 | 1 | 0.41 | |||
| Analysis Type (ITT/COMP) | 0.22 | 1 | 0.64 | 0.10 | 1 | 0.75 | 0.06 | 1 | 0.81 | |||
Acute Outcomes
Across studies, trials published earlier were associated with larger RR for diagnostic remission (see Table 2). There were no other significant moderators across the full sample of trials for acute treatment efficacy, response, or remission.
When examining diagnostic groups separately, larger DCS effects on treatment efficacy were associated with a greater percentage of participants taking SRI medications in anxiety disorder trials (n=7) (B=0.01, SE=0.01, z=2.02, p=0.04), and a greater percentage of male participants in OCD trials (n=7) (B=0.03, SE=0.01, z=2.08, p=0.04). No moderators of acute treatment efficacy were found for PTSD.
In terms of acute treatment response, larger DCS effects were associated with a greater percentage of participants on SRI medications in PTSD trials (n=4) (B=0.09, SE=0.02, z=4.29, p<0.001), while no significant moderators emerged for anxiety disorders or OCD.
Although no moderators of acute diagnostic remission were identified for anxiety or PTSD trials, multiple moderators were identified for OCD trials (n=7). Larger DCS treatment effects were associated with a greater percentage of male participants (B=0.03, SE=0.01, z=2.32, p=0.02), while smaller DCS effects were associated with larger sample sizes (B≤-0.01, SE=<0.01, z=-2.14, p=0.03) and more recent publications (B=-0.07, SE=0.04, z=-2.02, p=0.04).
Follow-Up Outcomes
No significant moderators were identified across trials for treatment efficacy, response, or remission at initial follow-up (see Table 2).
When examining diagnostic groups separately, larger DCS effects on treatment efficacy were associated with greater participant mean age for anxiety disorders (n=5) (B=0.56, SE=0.21, z=2.71, p=0.007). No moderators of treatment efficacy at follow-up were found for OCD or PTSD.
In terms of follow-up treatment response, larger DCS effects were associated with greater participant mean age for anxiety disorders (n=5)(B=0.07, SE=0.04, z=1.96, p=0.05). However, smaller DCS effects were associated with greater co-occurring depressive disorders (B=-0.03, SE=0.02, z=-2.10, p=0.04), greater methodological quality (B=-0.06, SE=0.03, z=2.10, p=0.04), and more recently published studies (B=-0.11, SE=0.05, z=-2.19, p=0.03) for anxiety disorders. No moderators of treatment response were found for OCD or PTSD.
Although no moderators of diagnostic remission at follow-up were identified for OCD trials, multiple moderators were identified for both anxiety disorders (n=3) and PTSD (n=4). Larger DCS effects were associated with greater mean participant age (B=0.33, SE=0.17, z=1.96, p=0.05) and percentage on an SRI medication (B=0.01, SE=0.01, z=1.95, p=0.05), whereas smaller DCS effects were associated with greater methodological quality (B=-0.07, SE=0.04, z=-1.92, p=0.05) and more recently published studies (B=-0.26, SE=0.14, z=-1.95, p=0.05) in anxiety disorder trials. Additionally, intent-to-treat studies (RR=1.02) had smaller DCS effects relative to completer analyses among anxiety disorder trials (RR=2.25), Q(1)=3.80, p=0.05. For PTSD trials, greater DCS treatment effects were associated with a greater percentage of co-occurring anxiety disorders (B=0.04, SE=0.02, z=2.28, p=0.02), more therapy sessions (B=0.33, SE=0.12, z=2.66, p=0.007), earlier DCS dosing (B=-1.91, SE=0.70, z=-2.73, p=0.006), more DCS doses (B=0.33, SE=0.16, z=2.04, p=0.04), and greater methodological quality (B=0.17, SE=0.09, z=1.93, p=0.05).
Discussion
This study evaluated treatment efficacy, response, and diagnostic remission of DCS-augmented treatment for anxiety disorders, OCD, and PTSD during acute treatment and at follow-up assessments using validated ratings. While prior meta-analyses have provided initial support for DCS, this meta-analysis addressed several limitations of prior investigations such as small sample size,36 use of psychometrically validated rating scales to calculate effects,10, 36 and limited examination of moderators37, 38 across three clinically relevant measures of therapeutic outcome. Moreover, this study completed an extensive examination of theoretically-derived treatment moderators across outcomes. Findings suggest minimal benefit of DCS relative to placebo augmentation across all three outcomes, with the most robust effect observed among anxiety disorders during acute treatment (g=0.33). This finding may be understood in several ways. First, the minimal effect of DCS across trials may be attributed to the robust effect sizes of existing exposure-based treatments in which there is limited room for improvement. Second, as DCS can influence memory reconsolidation,56 conditions that have more fear-based psychopathology and well-defined exposure targets (e.g., public speaking, specific phobias) may enable better fear memory reconsolidation in treatment. Conversely, conditions that have greater heterogeneity such as OCD may be influenced by sample-dependent characteristics of symptom presentation (e.g., fear-based symptoms versus not-just-right symptoms). Third, given the potential importance of between- and within-session habituation,32, 57 anxiety disorders may allow for better habituation within the session due to contained triggers. Meanwhile from an inhibitory learning model perspective,58 it may be that within session habituation serves as a possible marker that the prior fear association is adequately inhibited and fear extinction associations are strengthened. Given the contained nature of anxiety triggers predominantly studied in DCS trials (e.g., SAD, specific phobias), this may be more easily achieved for anxiety disorders relative to conditions that have more expansive triggers like OCD and PTSD. Fourth, as OCD and PTSD often have greater psychiatric comorbidities,2, 3 it may be that specific co-occurring conditions impede extinction learning targeted in treatment. While DCS augmentation can provide some enhancement of outcomes beyond placebo, further research is needed to clarify mechanisms and disentangle its effect across disorders.
When examining treatment moderators, a greater percentage of participants taking SRI medications was associated with greater DCS effects during acute treatment for anxiety disorders and PTSD. This finding is consistent with animal research suggesting synergy between antidepressant drugs and fear extinction.41 Although different than the finding of an attenuated response with SRI medication in adults with OCD,34 SRI medications can reduce negative biases in information processing hypothesized to play a central role in fear-based psychopathology.59, 60 Thus, SRIs may indirectly strengthen the appropriate formation of fear extinction learning that occurs treatment. Conversely, other animal model studies (e.g., Burghardt et al.42) suggest that the chronic use of antidepressants may impede fear extinction. Thus, it may be that SRI medications may be interacting with brain-derived neurotrophin factor (BDNF) to enhance synaptic plasticity of fear extinction targeted by DCS in exposure-based treatments.61 Among OCD trials, a greater percentage of male participants was associated with larger DCS effects in acute treatment. Although this suggests a possible gender difference in DCS response, it may also be attributed to tic-related OCD that has a greater male preponderance.62 Indeed, tic-related OCD serves a DSM specifier and has been suggested to be phenomenologically distinct from non-tic OCD with a differential treatment response profile.1, 62 Additionally, a larger sample size and more recently published studies were associated with smaller DCS effects in OCD trials, suggesting that DCS’s broad benefit diminishes in larger studies. As OCD has a heterogeneous presentation, subtype analyses may prove useful in clarifying whether specific OCD symptom dimensions respond better to DCS augmentation (e.g., fear-based symptoms versus not-just-right symptoms).
When examining moderators at initial follow-up assessments, a greater average participant age and percentage of participants on SRI medications was associated with larger DCS benefit for anxiety disorders. Meanwhile, greater depressive disorder, methodological quality, and recently published studies were associated with reduced DCS effects. While reduced effects for methodological quality and publication year may be anticipated, greater co-occurring depressive disorders may interfere with extinction learning targeted in treatment.63 It would be interesting to examine whether co-occurring depression moderated DCS effects among anxiety disorder trials with patient-level data. Among PTSD trials, a greater percentage of anxiety disorders, treatment sessions, and DCS doses were associated with greater DCS effects. As greater co-occurring anxiety disorders may be associated with greater fear-based psychopathology, this may provide further evidence for DCS’s benefit in extinction augmentation of fear-based psychopathology. Notably, extinction enhancement may not necessarily yield treatment enhancement, especially for symptoms not directly tied to fear. While the positive association between DCS effects and more treatment sessions makes clinical sense, the findings regarding a positive association with more DCS doses is somewhat counter-intuitive based on the literature.10 This particular effect may be attributed to the robust results in diagnostic remission that occurred at follow-up in a trial that had the highest DCS doses.33
Several limitations should be considered when interpreting these findings. First, there was inconsistent reporting of variables needed to calculate treatment efficacy, response, and diagnostic remission. Although study investigators were contacted to obtain these data, this resulted in different studies being included for different outcomes. Second, while this evaluation emphasized the importance of standardized rating scales, it is important to note that these studies focused on acute outcomes (often in truncated treatment protocols) and were not designed with the goal of diagnostic remission. Third, this evaluation examined three outcomes across multiple study characteristic across and within psychiatric conditions. This raises some concerns related to family-wise error. While theoretically-derived moderators were selected for analysis, caution in interpreting these findings as definitive is warranted. Fourth, this study was well powered to detect treatment moderators across trials, but had modest power to detect treatment moderators within disorders.55 As such, non-significant moderators should not be interpreted as a conclusive lack of association within conditions. Future research should examine identified treatment moderators in patient-level data to confirm these findings.64 Finally, there were limited characteristics available for extraction across RCTs. Thus, there may be unexamined factors such as within- and between-session habituation that could influence DCS effects.32, 57
In summary, these meta-analytic findings suggest that DCS augmentation yields a small-to-moderate albeit non-significant benefit across disorders, with treatment moderators varying across conditions. These findings highlight three directions for future research. First, the heterogeneous findings across RCTs highlight the importance of preclinical research on cognitive enhancers. As human RCTs are complicated by the interaction of pharmacological agents, human behavior, co-occurring conditions, and treatment protocols, preclinical research in animals can prove useful to elucidate possible biomarkers of DCS response (e.g., BDNF levels, SRI medications). This line of research may be useful to clarifying whether SRI medications potentiate or impede DCS treatment response, and better understand individualized responses to cognitive enhancers. Second, further research is needed to understand DCS’s mechanism of action in humans. For instance, recent research suggests that DCS enhances fear extinction in specific phobias via mechanisms of generalization and context learning.65 Additionally, an ongoing trial aims to further evaluate the dose timing aspect of DCS and will also explore a biological marker of fear conditioning in adults with anxiety disorders.66 While these two studies are noteworthy, considerably more well-designed studies are needed to evaluate its mechanisms in humans; such studies should include idiographic patient factors, therapy-specific reactions, and phenotypes rather than broad demographic stratifications.67 Additionally, when considering study designs, it may be useful to account for identified treatment moderators in the design process (e.g., stratifying randomization by SRI medication status). Finally, the present findings regarding disorder-specific moderation support the importance of treatment moderators as a source of important information to individualize and/or maximize the augmentative benefit of DCS in fear-based psychopathology. Future work employing patient-level analyses within large RCTs and/or aggregating data from similarly designed RCTs within specific disorders may be helpful in furthering our understanding in this area.
Clinical Points.
Given the mixed findings among randomized controlled trials of d-cycloserine augmentation of exposure-based treatments, evaluation of possible moderators was examined.
In contrast to previous meta-analyses, a minimal benefit was observed for d-cycloserine for augmenting exposure-based treatments for anxiety disorders, with no significant benefit for obsessive compulsive disorder (OCD) and posttraumatic stress disorder (PTSD).
Moderator analyses identified several significant moderators within and across disorders that explain discrepant findings and warrant further examination.
Acknowledgments
The authors would like to express their appreciation to the study investigators for their responsiveness and/or assistance in obtaining data not published in their original article.
Funding Source: The authors report no conflicts of interest. Funding for this article comes in part from the National Institutes of Mental Health (NIMH) through T32MH073517 that supports Dr. McGuire. The views expressed within this article represent those of the authors, were not influenced by this funding source, and are not intended to represent the position of NIMH. Dr. McGuire also receives support from the Tourette Association of American. Ms. Wu reports no relevant financial disclosures. Dr. Piacentini has received support from his work from National Institute of Mental Health (NIMH), Tourette Syndrome Association (TSA), Pfizer and the Pettit Family Foundation; book royalties from Oxford University Press and Guilford Publications, and speaking honoraria from the Tourette Syndrome Association, International OCD Foundation (IOCDF), and Trichotillomania Learning Center. Dr. McCraken has received grant or research support from NIH, Seaside Therapeutics, Roche, and Otsuka. He has served as a consultant to BioMarin and PharmaNet. Dr. Storch has received support from the NIH, CDC, Agency for Healthcare Research and Quality, IOCDF, and Ortho-McNeil Scientific Affairs. He receives textbook honorarium from Springer publishers, American Psychological Association, Lawrence Erlbaum and Wiley-Blackwell. He is a consultant for Prophase, Inc. and Rogers Memorial Hospital, and is on the Speaker’s Bureau and Scientific Advisory Board for the IOCDF. He receives research support from the All Children’s Hospital Guild Endowed Chair.
Footnotes
Previous Presentation: None
Contributor Information
Joseph F. McGuire, Semel Institute for Neuroscience and Human Behavior, University of California Los Angeles, Los Angeles, CA
Monica S. Wu, Department of Psychology, University of South Florida, Tampa, FL, Department of Pediatrics, University of South Florida, Tampa, FL
John Piacentini, Semel Institute for Neuroscience and Human Behavior, University of California Los Angeles, Los Angeles, CA, Department of Pediatrics, University of South Florida, Tampa, FL
James T. McCracken, Semel Institute for Neuroscience and Human Behavior, University of California Los Angeles, Los Angeles, CA
Eric A. Storch, Department of Psychology, University of South Florida, Tampa, FL, Department of Pediatrics, University of South Florida, Tampa, FL, Departments of Psychiatry and Behavioral Neuroscience, University of South Florida, Tampa, FL, Rogers Behavioral Health – Tampa Bay, Tampa, FL, All Children's Hospital, Johns Hopkins Medicine, St. Petersburg, FL, Department of Health Policy and Management, University of South Florida, Tampa, FL
References
- 1.American Psychiatric Association. Diagnostic and Statistic Manual of Mental Disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 2.Ruscio A, Stein D, Chiu W, Kessler R. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15(1):53–63. doi: 10.1038/mp.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005 Jun;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 5.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12(2):120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 6.Olatunji BO, Cisler JM, Deacon BJ. Efficacy of cognitive behavioral therapy for anxiety disorders: a review of meta-analytic findings. Psychiatr Clin North Am. 2010;33(3):557–577. doi: 10.1016/j.psc.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Deacon BJ, Farrell NR, Kemp JJ, et al. Assessing therapist reservations about exposure therapy for anxiety disorders: The Therapist Beliefs about Exposure Scale. J Anxiety Disord. 2013;27(8):772–780. doi: 10.1016/j.janxdis.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 8.McGuire JF, Lewin AB, Storch EA. Enhancing exposure therapy for anxiety disorders, obsessive-compulsive disorder and post-traumatic stress disorder. Expert Rev Neurother. 2014;14(8):893–910. doi: 10.1586/14737175.2014.934677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singewald N, Schmuckermair C, Whittle N, Holmes A, Ressler K. Pharmacology of cognitive enhancers for exposure-based therapy of fear, anxiety and trauma-related disorders. Pharmacol Ther. 2015;149:150–190. doi: 10.1016/j.pharmthera.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63(12):1118–1126. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald PJ, Seemann JR, Maren S. Can fear extinction be enhanced? A review of pharmacological and behavioral findings. Brain Res Bull. 2013 doi: 10.1016/j.brainresbull.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ledgerwood L, Richardson R, Cranney J. D-cycloserine and the facilitation of extinction of conditioned fear: consequences for reinstatement. Behav Neurosci. 2004;118(3):505–513. doi: 10.1037/0735-7044.118.3.505. [DOI] [PubMed] [Google Scholar]
- 13.Rothbaum BO. Critical parameters for D-cycloserine enhancement of cognitive-behaviorial therapy for obsessive-compulsive disorder. Am J Psychiatry. 2008;165(3):293–296. doi: 10.1176/appi.ajp.2007.07121871. [DOI] [PubMed] [Google Scholar]
- 14.Kalisch R, Holt B, Petrovic P, et al. The NMDA agonist D-cycloserine facilitates fear memory consolidation in humans. Cereb Cortex. 2009;19(1):187–196. doi: 10.1093/cercor/bhn076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ressler KJ, Rothbaum BO, Tannenbaum L, et al. Cognitive Enhancers as Adjuncts to Psychotherapy: Use of D-Cycloserine in Phobic Individuals to Facilitate Extinctionof Fear. Arch Gen Psychiatry. 2004;61(11):1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 16.Hofmann SG, Meuret AE, Smits JA, et al. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch Gen Psychiatry. 2006;63(3):298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- 17.Storch EA, Merlo LJ, Bengtson M, et al. D-cycloserine does not enhance exposure–response prevention therapy in obsessive–compulsive disorder. Int Clin Psychopharmacol. 2007;22(4):230–237. doi: 10.1097/YIC.0b013e32819f8480. [DOI] [PubMed] [Google Scholar]
- 18.Kushner MG, Kim SW, Donahue C, et al. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol Psychiatry. 2007;62(8):835–838. doi: 10.1016/j.biopsych.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 19.Wilhelm S, Buhlmann U, Tolin D, et al. Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. Am J Psychiatry. 2008;165(3):335–341. doi: 10.1176/appi.ajp.2007.07050776. [DOI] [PubMed] [Google Scholar]
- 20.Guastella AJ, Richardson R, Lovibond PF, et al. A randomized controlled trial of D-cycloserine enhancement of exposure therapy for social anxiety disorder. Biol Psychiatry. 2008;63(6):544–549. doi: 10.1016/j.biopsych.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Storch EA, Murphy TK, Goodman WK, et al. A preliminary study of D-cycloserine augmentation of cognitive-behavioral therapy in pediatric obsessive-compulsive disorder. Biol Psychiatry. 2010;68(11):1073–1076. doi: 10.1016/j.biopsych.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otto MW, Tolin DF, Simon NM, et al. Efficacy of d-cycloserine for enhancing response to cognitive-behavior therapy for panic disorder. Biol Psychiatry. 2010;67(4):365–370. doi: 10.1016/j.biopsych.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 23.Litz BT, Salters-Pedneault K, Steenkamp MM, et al. A randomized placebo-controlled trial of D-cycloserine and exposure therapy for posttraumatic stress disorder. J Psychiatr Res. 2012;46(9):1184–1190. doi: 10.1016/j.jpsychires.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 24.de Kleine RA, Hendriks G-J, Kusters WJ, Broekman TG, van Minnen A. A randomized placebo-controlled trial of D-cycloserine to enhance exposure therapy for posttraumatic stress disorder. Biol Psychiatry. 2012;71(11):962–968. doi: 10.1016/j.biopsych.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 25.Nave AM, Tolin DF, Stevens MC. Exposure therapy, D-Cycloserine, and functional magnetic resonance imaging in patients with snake phobia: a randomized pilot study. J Clin Psychiatry. 2012;73(9):1179–1186. doi: 10.4088/JCP.11m07564. [DOI] [PubMed] [Google Scholar]
- 26.Farrell LJ, Waters AM, Boschen MJ, et al. Difficult-To-Treat Pediatric Obsessive-Compulsive Disorder: Feasibility and Preliminary Results of a Randomized Pilot Trial of D-Cycloserine-Augmented Behavior Therapy. Depress Anxiety. 2013;30(8):723–731. doi: 10.1002/da.22132. [DOI] [PubMed] [Google Scholar]
- 27.Rodebaugh TL, Levinson CA, Lenze EJ. A high-throughput clinical assay for testing drug facilitation of exposure therapy. Depress Anxiety. 2013 Jul;30(7):631–637. doi: 10.1002/da.22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofmann SG, Smits JA, Rosenfield D, et al. D-cycloserine as an augmentation strategy with cognitive-behavioral therapy for social anxiety disorder. Am J Psychiatry. 2013;170(7):751–758. doi: 10.1176/appi.ajp.2013.12070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tart CD, Handelsman PR, DeBoer LB, et al. Augmentation of exposure therapy with post-session administration of D-cycloserine. J Psychiatr Res. 2013;47(2):168–174. doi: 10.1016/j.jpsychires.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mataix-Cols D, Turner C, Monzani B, et al. Cognitive-behavioural therapy with post-session D-cycloserine augmentation for paediatric obsessive-compulsive disorder: pilot randomised controlled trial. Br J Psychiatry. 2014;204(1):77–78. doi: 10.1192/bjp.bp.113.126284. [DOI] [PubMed] [Google Scholar]
- 31.Scheeringa MS, Weems CF. Randomized placebo-controlled D-Cycloserine with cognitive behavior therapy for pediatric posttraumatic stress. J Child Adolesc Psychopharmacol. 2014;24(2):69–77. doi: 10.1089/cap.2013.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rothbaum BO, Price M, Jovanovic T, et al. A randomized, double-blind evaluation of D-cycloserine or alprazolam combined with virtual reality exposure therapy for posttraumatic stress disorder in Iraq and Afghanistan War veterans. Am J Psychiatry. 2014;171(6):640–648. doi: 10.1176/appi.ajp.2014.13121625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Difede J, Cukor J, Wyka K, et al. D-cycloserine augmentation of exposure therapy for post-traumatic stress disorder: a pilot randomized clinical trial. Neuropsychopharmacology. 2014;39(5):1052–1058. doi: 10.1038/npp.2013.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersson E, Hedman E, Enander J, et al. d-Cycloserine vs Placebo as Adjunct to Cognitive Behavioral Therapy for Obsessive-Compulsive Disorder and Interaction With Antidepressants: A Randomized Clinical Trial. JAMA Psychiatry. 2015;72(7):659–667. doi: 10.1001/jamapsychiatry.2015.0546. [DOI] [PubMed] [Google Scholar]
- 35.Murad MH, Montori VM. Synthesizing evidence: shifting the focus from individual studies to the body of evidence. JAMA. 2013;309(21):2217–2218. doi: 10.1001/jama.2013.5616. [DOI] [PubMed] [Google Scholar]
- 36.Bontempo MA, Panza MKE, Bloch MH. Meta-Analysis: D-cycloserine Augmentation of Behavioral Therapy for the Treatment of Anxiety Disorders. J Clin Psychiatry. 2012;73(4):533. doi: 10.4088/JCP.11r07356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodrigues H, Figueira I, Lopes A, et al. Does d-cycloserine enhance exposure therapy for anxiety disorders in humans? A meta-analysis. PloS one. 2014;9(7):e93519. doi: 10.1371/journal.pone.0093519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ori R, Amos T, Bergman H, Soares-Weiser K, Ipser J, Stein D. Augmentation of cognitive and behavioural therapies (CBT) with d-cycloserine for anxiety and related disorders. Cochrane Database Syst Rev. 2015;5:CD007803–CD007803. doi: 10.1002/14651858.CD007803.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Britton JC, Grillon C, Lissek S, et al. Response to Learned Threat: An fMRI study in adolescent and adult anxiety. Am J Psychiatry. 2013;170(10):1195–1204. doi: 10.1176/appi.ajp.2013.12050651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Otto MW, Moshier SJ, Kinner DG, Simon NM, Pollack MH, Orr SP. De novo fear conditioning across diagnostic groups in the affective disorders: evidence for learning impairments. Behav Ther. 2014;45(5):619–629. doi: 10.1016/j.beth.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karpova NN, Pickenhagen A, Lindholm J, et al. Fear erasure in mice requires synergy between antidepressant drugs and extinction training. Science. 2011;334(6063):1731–1734. doi: 10.1126/science.1214592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burghardt NS, Sigurdsson T, Gorman JM, McEwen BS, LeDoux JE. Chronic antidepressant treatment impairs the acquisition of fear extinction. Biol Psychiatry. 2013;73(11):1078–1086. doi: 10.1016/j.biopsych.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ledgerwood L, Richardson R, Cranney J. Effects of D-cycloserine on extinction of conditioned freezing. Behav Neurosci. 2003;117(2):341. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- 44.Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: National Institute for Mental Health; 1976. Clinical Global Impressions; pp. 218–222. [Google Scholar]
- 45.Bandelow B, Baldwin DS, Dolberg OT, Andersen HF, Stein DJ. What is the threshold for symptomatic response and remission for major depressive disorder, panic disorder, social anxiety disorder, and generalized anxiety disorder? J Clin Psychiatry. 2006;67(9):1428–1434. doi: 10.4088/jcp.v67n0914. [DOI] [PubMed] [Google Scholar]
- 46.Storch EA, Lewin AB, De Nadai AS, Murphy TK. Defining treatment response and remission in obsessive-compulsive disorder: a signal detection analysis of the children's yale-brown obsessive compulsive scale. J Am Acad Child Adolesc Psychiatry. 2010;49(7):708–717. doi: 10.1016/j.jaac.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 47.Lewin AB, De Nadai AS, Park J, Goodman WK, Murphy TK, Storch EA. Refining clinical judgment of treatment outcome in obsessive–compulsive disorder. Psychiatry Res. 2011;185(3):394–401. doi: 10.1016/j.psychres.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 48.Schnurr PP, Friedman MJ, Engel CC, et al. Cognitive behavioral therapy for posttraumatic stress disorder in women: a randomized controlled trial. JAMA. 2007;297(8):820–830. doi: 10.1001/jama.297.8.820. [DOI] [PubMed] [Google Scholar]
- 49.Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety. 2001;13(3):132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- 50.Tolin DF, Abramowitz JS, Diefenbach GJ. Defining response in clinical trials for obsessive-compulsive disorder: a signal detection analysis of the Yale-Brown obsessive compulsive scale. J Clin Psychiatry. 2005;66(12):1549–1557. doi: 10.4088/jcp.v66n1209. [DOI] [PubMed] [Google Scholar]
- 51.Moncrieff J, Churchill R, Drummond DC, McGuire H. Development of a quality assessment instrument for trials of treatments for depression and neurosis. Int J Meth Psych Res. 2001;10(3):126–133. [Google Scholar]
- 52.McGuire JF, Piacentini J, Lewin AB, Brennan EA, Murphy TK, Storch EA. A Meta-Analysis of Cognitive Behavior Therapy and Medication for Child Obsessive Compulsive Disorder: Moderators of Treatment Efficacy, Response, and Remission. Depress Anxiety. 2015;32(8):580–593. doi: 10.1002/da.22389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta-analysis. 2. Englewood, NJ: Biostat; 2005. [Google Scholar]
- 54.McGough JJ, Faraone SV. Estimating the size of treatment effects: moving beyond p values. Psychiatry (Edgmont) 2009;6(10):21. [PMC free article] [PubMed] [Google Scholar]
- 55.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. Wiley; 2009. [Google Scholar]
- 56.Lee JL, Gardner RJ, Butler VJ, Everitt BJ. D-cycloserine potentiates the reconsolidation of cocaine-associated memories. Learn Mem. 2009;16(1):82–85. doi: 10.1101/lm.1186609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smits JA, Rosenfield D, Otto MW, et al. D-Cycloserine enhancement of fear extinction is specific to successful exposure sessions: evidence from the treatment of height phobia. Biol Psychiatry. 2013;73(11):1054–1058. doi: 10.1016/j.biopsych.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Craske MG, Treanor M, Conway CC, Zbozinek T, Vervliet B. Maximizing exposure therapy: an inhibitory learning approach. Behav Res Ther. 2014;58:10–23. doi: 10.1016/j.brat.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harmer CJ, Shelley NC, Cowen PJ, Goodwin GM. Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. Am J Psychiatry. 2004;161:1256–1263. doi: 10.1176/appi.ajp.161.7.1256. [DOI] [PubMed] [Google Scholar]
- 60.Cisler JM, Koster EH. Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clin Psychol Rev. 2010;30(2):203–216. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andero R, Ressler KJ. Fear extinction and BDNF: translating animal models of PTSD to the clinic. Genes Brain Behav. 2012;11(5):503–512. doi: 10.1111/j.1601-183X.2012.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leckman JF, Denys D, Simpson HB, et al. Obsessive–compulsive disorder: a review of the diagnostic criteria and possible subtypes and dimensional specifiers for DSM‐V. Depress Anxiety. 2010;27(6):507–527. doi: 10.1002/da.20669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abramowitz JS, Franklin ME, Street GP, Kozak MJ, Foa EB. Effects of comorbid depression on response to treatment for obsessive-compulsive disorder. Behav Ther. 2000;31(3):517–528. [Google Scholar]
- 64.Bloch MH. Meta-analysis and moderator analysis: can the field develop further? J Am Acad Child Adolesc Psychiatry. 2014;53(2):135–137. doi: 10.1016/j.jaac.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Byrne SP, Rapee RM, Richardson R, Malhi GS, Jones M, Hudson JL. D-cycloserine enhances generalization of fear extinction in children. Depress Anxiety. 2015;32(6):408–414. doi: 10.1002/da.22356. [DOI] [PubMed] [Google Scholar]
- 66.Hofmann SG, Carpenter JK, Otto MW, Rosenfield D, Smits JA, Pollack MH. Dose Timing of D-cycloserine to Augment Cognitive Behavioral Therapy for Social Anxiety: Study Design and Rationale. Contemp Clin Trials. 2015;43:223–230. doi: 10.1016/j.cct.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tuerk PW. Starting From Something: Augmenting Exposure Therapy and Methods of Inquiry. Am J Psychiatry. 2014;171(10):1034–1037. doi: 10.1176/appi.ajp.2014.14070880. [DOI] [PubMed] [Google Scholar]


