Abstract
The search for and development of radiation countermeasures to treat acute lethal radiation injury has been underway for the past six decades, resulting in the identification of multiple classes of radiation countermeasures. However, to date, only granulocyte colony-stimulating factor (G-CSF, Neupogen) and PEGylated G-CSF (Neulasta) have been approved by the United States Food and Drug Administration (US FDA) for the treatment of hematopoietic acute radiation syndrome (H-ARS). Gamma-tocotrienol (GT3) has demonstrated radioprotective efficacy in murine and nonhuman primate (NHP) models. Currently, this agent is under advanced development as a radioprotector, and we are trying to identify its efficacy biomarkers. In this study, we analyzed global metabolomic changes using ultra-performance liquid chromatography (UPLC) quadrupole time-of-flight mass spectrometry (QTOF-MS). Our pilot study using 16 NHPs (eight NHPs each in GT3- and vehicle-treated groups), with samples obtained from GT3-treated and irradiated NHPs, demonstrates several metabolites that are altered after irradiation, including compounds involved in fatty acid-β oxidation, purine catabolism, and amino acid metabolism. The machine-learning algorithm, Random Forest, separated control, irradiated GT3-treated, and irradiated vehicle-treated NHPs at 12 h and 24 h as evident in a multidimensional scaling plot. Primary metabolites validated included carnitine/acylcarnitines, amino acids, creatine, and xanthine. Overall, GT3-administration reduced high fluctuations in serum metabolite levels, suggesting an overall beneficial effect on animals exposed to radiation. This initial assessment also highlights the utility of metabolomics to determine underlying physiological mechanisms responsible for the radioprotective efficacy of GT3.
Keywords: gamma-radiation, gamma-tocotrienol, metabolites, nonhuman primates, radiation countermeasure, serum
INTRODUCTION
The threat of a nuclear or radiological disaster is a serious concern and a top priority for all US agencies involved in domestic security and public health preparedness as well as for the military. As made evident by the BioShield legislation, the need for new countermeasures that are safe, easily administered, and effective at reducing or eliminating the public health impact of acute high-dose radiation are urgently needed (Singh et al. 2016c). In the event of radiological or nuclear event exposure, medical care would be needed to treat exposure victims developing ARS (DiCarlo et al. 2011; Hall et al. 2012).
Acute radiation injury occurs at whole-body doses above 2 Gy, with symptoms growing in severity as the level of radiation exposure increases (Hall et al. 2012). A dose range of 2 – 6 Gy is characterized by the loss of hematopoietic cell regenerative ability, resulting in H-ARS. In the exposure range of 6 – 10 Gy, hematopoietic symptoms are present in addition to symptoms caused by significant breakdown of the gastrointestinal (GI) system, resulting in GI-ARS. H-ARS and GI-ARS are recognized as the major subsyndromes of ARS which can be treated with radiation countermeasures. The search for radiation countermeasures to lethal radiation injury has been underway for the past several decades, resulting in the identification of multiple classes of radiation countermeasures (Dainiak et al. 2011; Drouet et al. 2014; Herodin et al. 2005; Seed 2005; Singh et al. 2012; Singh et al. 2014; Weiss et al. 2009). However, to date, only two radiomitigators for H-ARS have been approved for use by the US FDA for the mitigation of H-ARS (Farese et al. 2013; Farese et al. 2015; Global Biodefense 2015; Hankey et al. 2015). There is no radioprotector approved by the US FDA that can be used prior to radiation exposure. Further, there is no radioprotector nor radiomitigator approved by US FDA for GI-ARS.
Vitamin E is well known for its antioxidant, anti-inflammatory, and neuroprotective properties (Sen et al. 2006; Singh et al. 2013). Vitamin E represents a family of compounds, acting as antioxidants, that control free-radical production and regulate peroxidation reactions within the body (Singh et al. 2016a). The vitamin E family has eight different isoforms that belong to two groups: four saturated analogues (α, β, γ, and δ) known as tocopherols and four unsaturated analogues called tocotrienols. These eight agents are collectively referred to as tocols. GT3 is a potent inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase. Its antioxidant activity is important reason for evaluating its radioprotective efficacy. During the last 10 years, it has received significant attention and appears to be one of the most encouraging radiation countermeasures among tocols tested to date (Singh et al. 2016a). Further, studies suggest that GT3-stimulated G-CSF is involved in its radioprotective mechanism, at least in the murine model (Kulkarni et al. 2013). Lately, GT3 has been tested as a radioprotector in a pilot study in the NHP model and demonstrated efficacy for improving ionizing radiation-induced cytopenia, neutropenia, and thrombocytopenia in the absence of any supportive care (Singh et al. 2016b). GT3 has also been studied for modulating microRNA in murine and NHP models with an objective to identify its efficacy biomarker (Fendler et al. 2017; Ghosh et al. 2016).
Here, we report ionizing radiation-induced metabolic changes in serum samples of GT3 or vehicle-treated NHPs using a global metabolomics approach with an ultra-performance liquid chromatography (UPLC) quadrupole time-of-flight mass spectrometry (QTOF-MS) platform at 12 h and 24 h after 6.5 Gy total body irradiation. We found lower fluctuations of most metabolites in the GT3-treated group, suggesting a beneficial response to the radioprotective efficacy of GT3. Furthermore, this pilot study demonstrates the utility of metabolomics in deciphering the physiological effects of GT3 as a radioprotector.
MATERIALS AND METHODS
Animals and animal care
Sixteen naïve rhesus macaques (Macaca mulatta, Chinese sub strain) (8 males and 8 females) 49 – 68 mo of age, weighing 3.8 – 6.6 kg, were obtained from Primate Products, Inc. (Miami, FL, USA) and quarantined for 6 weeks prior to initiation of the experiment. Animal quarantine, housing, health monitoring, care, and enrichment during the experimental period have been previously described (Singh et al. 2016b). Animals were stratified by gender and body weight increases during the quarantine period and then assigned to GT3-treated and vehicle groups. Due to study-specific reasons, paired housing was not possible during the experiment. The animals were housed individually, but they were able to see and touch conspecifics through the cage divider. This also eliminated the chance of conflict injuries that could have been caused by pair-housing. Animals that are irradiated are more prone to infection as their natural immunity is suppressed. This animal study was conducted in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-International. All procedures involving animals were approved by Institutional Animal Care and Use Committee (IACUC) and Department of Defense Animal Care and Use Review Office (ACURO). This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (National Research Council of the National Academy of Sciences 2011).
Drug Preparation and Administration
Pyrogen-free samples of GT3 and olive oil formulations (50 mg/ml) in 5% Tween-80 in saline, were purchased from Yasoo Health Inc. (Johnson City, TN, USA). The dose of GT3 for each NHP was 37.5 mg/kg, and adjusted precisely to the body weight of individual NHPs. A single administration of the above dose of GT3 without any supportive care was equivalent, in terms of improving hematopoietic recovery, to multiple doses of Neupogen and two doses of Neulasta with full supportive care (including blood products) in the NHP model. Drug or vehicle was administered to 8 animals each (4 males and 4 females) at the dorsal scapular area (between the shoulder blades) 24 h prior to irradiation as described in detail earlier (Singh et al. 2016b).
Radiation exposure
Food was withheld from each animal approximately 12 – 18 h prior to radiation exposure to minimize the occurrence of radiation-induced vomiting. Approximately 30 – 45 min prior to irradiation, NHPs were administered 10 – 15 mg/kg of ketamine hydrochloride intramuscularly for sedation, then placed in custom-made Plexiglas irradiation boxes and secured in a seated position. Two NHPs were placed on the irradiation platform facing away from each other and exposed with a midline dose of 6.5 Gy (LD25-50/60 without supportive care) cobalt-60 γ-radiation at a dose rate of 0.6 Gy/min from both sides (bilateral, simultaneous exposure). To deliver the precise dose, NHPs’ abdominal widths were measured with digital calipers. Animals were observed throughout the irradiation procedure via in-room cameras. Following irradiation, animals were returned to the transport cart and to their cages in the housing area and monitored for recovery from the procedure. Dose rate measurements were based primarily on the alanine/EPR (electron paramagnetic resonance) system as described earlier (Singh et al. 2016b).
Serum sample collection
Blood was collected by venipuncture from the saphenous vein on the caudal aspect of the lower leg, placed in serum separating tubes, allowed to clot for 30 min, and centrifuged (10 min, 400 x g). Serum samples were stored at −70 °C until use.
Chemicals
All reagents were LC-MS grade (Fisher Scientific, Hanover Park, IL) and chemicals (tryptophan, leucine, isoleucine, L-carnitine, creatine, xanthine, methionine, proline, and propionylcarnitine [Sigma-Aldrich, St. Louis, MO]) were of the highest purity available.
Sample preparation and analysis
Serum (5.0 μl) was mixed with 195.0 μl 66% acetonitrile (ACN) and internal standards (2.0 μM debrisoquine [M+H]+ = 176.1188 m/z; 30.0 μM 4-nitrobenzoic acid [M-H]− = 166.0141 m/z), incubated 10 min (on ice), centrifuged for 10 min (13,000 rpm, 4 °C), and injected (2.0 μl). Samples were analyzed by a Waters ACQUITY UPLC coupled to a Xevo® G2 QTOF-MS (Waters, Milford, MA), equipped with a BEH C18 1.7 μm, 2.1 × 50 mm column, and data acquired in both negative and positive electrospray ionization (ESI) modes (Leucine enkephalin (556.2771 [M+H]+ or 554.2615 [M-H]-) was used for Lock-Spray®).
Data processing, statistical analysis, and marker validation
Deconvolution and peak alignment on the total ion chromatogram (TIC) was performed using Progenesis QI (Nonlinear Dynamics, Newcastle, UK). Pre-processed data was initially analyzed with the in-house statistical package MetaboLyzer (Mak et al. 2014) for univariate analysis as previously described (Pannkuk et al. 2016b). Putative ion pathways were determined by comparing to Kyoto encyclopedia of genes and genomes (KEGG) (Kanehisa et al. 2000). Complete presence ions (≥70%) were analyzed with a standard singular value decomposition based principal component analysis (PCA) using in house software and a multi-dimensional scaling (MDS) plot and heatmap was generated with Random Forest (RF) using R v 2.15.2. A subset of statistically significant ions were identified (identification: human metabolome database (HMDB); significance: Welch’s t-test [p < 0.05]) and were chosen for validation against pure standards (diluted in 1:1 ACN:H2O) using tandem MS and the METLIN tandem MS database. Validated and normalized ions were checked for normality with a Shapiro-Wilk test, homogenous variances with a Levene’s test, sphericity using Geisser-Greenhouse epsilon value, significance (p < 0.05) with a repeated measures ANOVA, and graphed using GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA; SAS v 9.4, Cary, NC).
RESULTS
Biomarker identification and validation
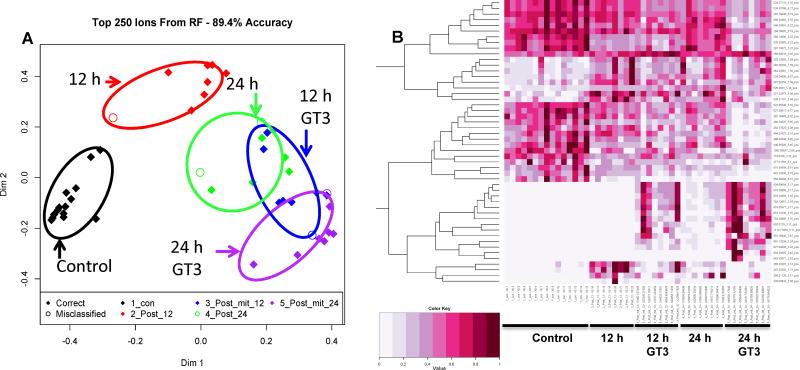
The 12 and 24 h post-irradiation time points clearly separated from the untreated control samples for both vehicle and GT3-treated groups when compared with an unsupervised PCA (Supplementary figure 1). The top 250 ions in ESI+ mode had an accuracy of 89.4%, and the top 137 ions in ESI− mode had an accuracy of 66% (figure 1). Multivariate data analysis of the proximity matrix from the machine learning RF algorithm indicated all NHP groups exposed to 60Co γ-radiation separated from the unirradiated control group, with the GT3-treated group separating the most on dimension 1 and the vehicle-treated group separating more on dimension 2 (figure 1). Manual inspection of the heatmap generated by RF indicated that some variation may be due to putative steroids present in the 12 h vehicle group and putative sphingomyelin, phosphatidylserine, and gangliosides in the GT3 group.
Figure 1.
A) MDS plot and B) heatmap generated by Random Forest analysis comparing metabolomic signatures (ESI+) of serum biomarkers from NHPs exposed to 6.5 Gy γ-radiation after 12 and 24 h. One group was administered GT3 as a radioprotector 24 h prior to irradiation. Higher separation was seen between GT3 and control groups along dimension 1 while the non-GT3 group separated the greatest from the control along dimension 2 at 12 h.
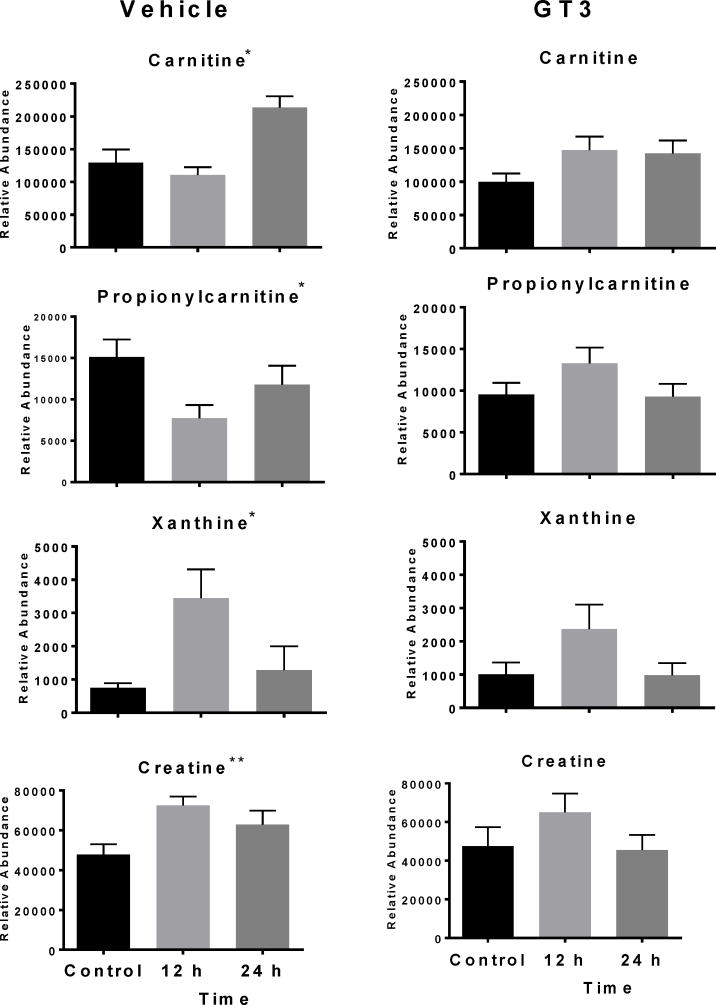
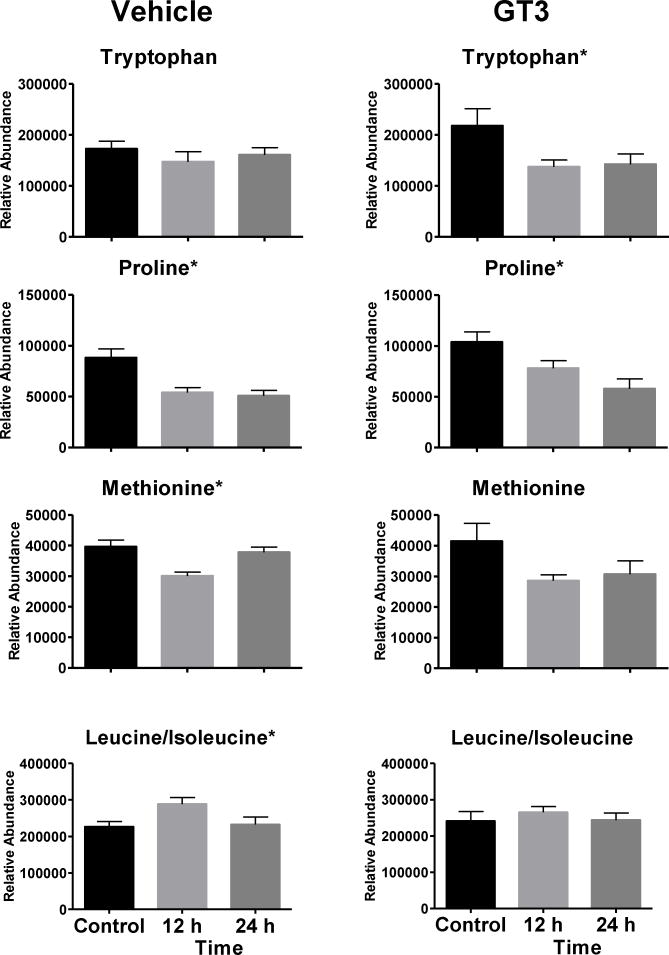
Metabolomics data analysis requires validation of putative metabolites identified by matching the retention time and MS/MS fragmentation of compounds in the samples to a pure standard using the same analytical platform (putative ions listed in Supplementary Table 1). Univariate data analysis by repeated measures ANOVA was used to compare validated ions at 12 and 24 h for NHPs receiving either a vehicle or GT3 as a radioprotector 24 h prior to irradiation. In animals not administered GT3, xanthine (p = 0.034) and creatine (p = 0.053) increased at 12 h; xanthine returned closer to pre-irradiation levels at 24 h, and creatine levels were slightly lower than levels at 12 h (figure 2). Levels did not change significantly in animals receiving GT3 (xanthine [p = 0.178] and creatine [p = 0.368]), although a similar trend was observed. Carnitine and propionylcarnitine showed slight decreases at 12 h in the vehicle group (carnitine [p < 0.001], propionylcarnitine [p = 0.008]), while levels in the GT3-treated group did not significantly change (carnitine [p = 0.174], propionylcarnitine [p = 0.127]). Minor changes were observed in levels of one non-essential amino acid and three essential amino acids (figure 3). Methionine significantly changed in the vehicle group (p = 0.001, F = 11.720) but not the GT3-treated group (p = 0.068, F = 3.269); however, with the difference in within-group variation, a higher sample size may be needed for this metabolite. Proline (a non-essential amino acid) decreased significantly in both groups (vehicle [p = 0.004], GT3 [p = 0.007]) and tryptophan decreased significantly only in the GT3 group (p = 0.026) and not the vehicle group (p = 0.589). Leucine/isoleucine (p = 0.048) increased at 12 h while returning closer to pre-irradiation level but not to the GT3-treated group level (p = 0.562). Changes in tryptophan have been linked to the host microbiota rather than a direct product from radiation exposure (Kurland et al. 2015; Ó Broin et al. 2015).
Figure 2.
Dose response of serum biomarkers from NHPs exposed to 6.5 Gy γ-radiation after 12 and 24 h (Mean ± SEM). Graphs in the left column represent vehicle group, and graphs in the right column represent the GT3 group. The GT3 group had a lower fold change of xanthine (fold change 2.35 vs. 4.57) and creatine (fold change 1.37 vs. 1.52) 12 h post-irradiation than the vehicle group. The GT3 group had lower increases in carnitine (* indicates significant value p < 0.05, **p = 0.053).
Figure 3.
Dose response of serum biomarkers from NHPs exposed to 6.5 Gy γ-radiation after 12 and 24 h (Mean ± SEM). Graphs in the left column represent vehicle group and graphs in the right column represent the GT3 group. Minor differences in amino acid concentration are observed between the two groups (* indicates significant value p < 0.05).
DISCUSSION
GT3 is a promising radiation countermeasure being developed as a radioprotector which can be administered prior to radiation exposure for the benefit of first responders and military personnel (Singh et al. 2016a). It has demonstrated significant radioprotective efficacy in mouse and NHP models against ionizing radiation, and its radioprotective efficacy has been shown to be mediated through G-CSF in the murine model (Kulkarni et al. 2013). Currently, GT3 is under advanced development with the support from US Department of Defense funding with an objective to submit an investigational new drug (IND) application to US FDA. Its efficacy biomarker identification is gaining momentum to push this agent for FDA approval following Animal Rule (U.S. Food and Drug Administration Guidance for Industry: Product Developoment Under the Animal Rule 2015). Recently, GT3 has also been studied for modulating microRNA (miRNA) in murine and NHP models with an objective to identify its efficacy biomarker (Fendler et al. 2017; Ghosh et al. 2016). We have shown that three microRNAs (miR-30a, miR-126, and miR-375) correlate with the radioprotective efficacy of GT3 in NHPs; these miRNAs in GT3-treated 60Co total body γ-irradiated animals resembled the unirradiated animals (Fendler et al. 2017). Exposure to ionizing radiation stimulates a set of complex biological responses including gene expression and protein synthesis that ultimately leads to dysregulation of metabolic processes. Recently, there are several reports showing metabolites as radiation biomarkers in NHP urine and serum (Pannkuk et al. 2017a; Pannkuk et al. 2017b). In the present pilot study, we used a global metabolomics approach to determine metabolomic serum changes in NHPs administered radioprotective GT3 or vehicle and exposed to 6.5 Gy total body radiation.
Xanthine is a precursor of uric acid and is a compound involved in purine catabolism, indicating DNA damage and increased oxidation, and has been identified as a radiation exposure marker in urine in earlier studies (Johnson et al. 2012; Laiakis et al. 2014; Manna et al. 2013; Tyburski et al. 2009), but opposite trends between genders has been observed (Pannkuk et al. 2015). Since only four NHPs of each gender were used in each group, gender based analysis was not performed in this study. While previous studies have indicated significantly a higher xanthine concentration in urine, with the greatest fold change at 24 h, the present serum signature indicates higher fold change at 12 h with lower levels at 24 h (Johnson et al. 2012; Tyburski et al. 2009). It is possible that higher xanthine levels are observed in serum at 12 h as a direct product of DNA damage and is being eliminated from the body at 24 h when its concentration is higher in urine. Similarly, a slight increase in serum creatine levels was observed at 12 h for both GT3 and vehicle-treated groups; however, levels returned closer to basal levels in the GT3 group. Creatine is a well-known marker of radiation exposure in NHP urine (Johnson et al. 2012; Pannkuk et al. 2015) and may indicate decreased muscle activity. As GT3 lowered xanthine and creatine concentration at both 12 and 24 h post-irradiation compared to the vehicle-treated NHPs, this suggests beneficial effects in animals exposed to ionizing radiation.
Carnitines and acylcarnitines play important roles in fatty acid-β oxidation. Perturbations to biofluid carnitine/acylcarnitine concentrations has been implicated in many diverse diseases (Flanagan et al. 2010) and as consequences of radiation exposure from both internal and external sources (Goudarzi et al. 2014; Laiakis et al. 2012; Pannkuk et al. 2015). Generally, fold changes of carnitine are much greater in urine than serum (Pannkuk et al. 2017b). An estimated 95% of carnitine may be reabsorbed in the kidneys. However, exposure to radiation causes renal failure, limiting the amount reabsorbed and demonstrating higher concentration in urine. Our present study demonstrates lower levels of serum carnitine and propionylcarnitine at 12 h in the vehicle group, with higher concentrations at 24 h. This may be due to decreased reabsorption efficiency at 12 h due to renal failure in the vehicle group compared to the GT3 group. Increases at 24 h in the vehicle-treated animals may represent increased cellular leakage of these metabolites, indicating longer lasting deficiencies to fatty acid-β oxidation. Carnitine and propionylcarnitine returned to basal levels in the GT3 group at 24 h. The reduced fluctuations observed in carnitine and propionylcarnitine may indicate positive effects on fatty acid metabolism by GT3 administration prior to irradiation.
Changes in NHP serum amino acid concentrations have been observed 7 d post-irradiation (Pannkuk et al. 2017b; Pannkuk et al. 2016a; Pannkuk et al. 2016b). Of the four amino acids validated, proline (the one non-essential amino acid detected) significantly decreased in both groups at 12 h and 24 h. Two essential amino acids (methionine and leucine/isoleucine) significantly fluctuated in the vehicle group but not the GT3 group. Tryptophan significantly decreased in the GT3 group but did not change in the vehicle group. Tryptophan may be a microbial metabolite and is required for serotonin production, involved in GI inflammation, and important in tricarboxylic acid (TCA) cycle function and energy metabolism (Goudarzi et al. 2016; Lamas et al. 2016; Ó Broin et al. 2015). Serotonin is an important GI regulatory factor, and the host microbiota can increase biosynthesis (Yano et al. 2015). Given the effects of irradiation on GI function, the interactions of GT3 on host microbiota, serotonin synthesis from tryptophan, and GI inflammation may be an interesting area for further research.
CONCLUSION
There is growing interest in the physiological effects of tocotrienols, not only as a radioprotector but also in several disease types (Torquato et al. 2016). Here, we present an initial assessment of the utility of metabolomics to determine mechanisms of GT3 radioprotective efficacy. Overall, GT3-administration reduced fluctuations of validated metabolites that may indicate positive physiological effects on DNA damage, increased muscle function, fatty acid synthesis, renal function, and amino acid metabolism. Tryptophan levels decreased in the GT3 administered group, possibly representing an important interplay between GI damage from radiation, GT3 effects on the host microbiota, and tryptophan metabolism. Future work may incorporate more metabolomic analytical platforms and transcriptomics with current metabolomic and miRNA studies to explore the efficacy of GT3 as a radiation countermeasure.
Supplementary Material
Table 1.
Radiation biomarkers of NHP serum with and without γ-tocotrienol
| Metabolite name | Adduct | Formula | RTmin | Experimental m/z | P-valueGT3 group | P-valueVehicle group |
|---|---|---|---|---|---|---|
| Methionine | H+ | C5H11NO2S | 0.38 | 150.0584 | 0.068 | 0.001* |
| L-Carnitine | H+ | C7H15NO3 | 0.33 | 162.1125 | 0.174 | <0.001* |
| Creatine | H+ | C4H9N3O2 | 0.33 | 132.0765 | 0.040* | 0.053 |
| Propionylcarnitine | H+ | C10H19NO4 | 0.38 | 218.1386 | 0.127 | 0.008* |
| Tryptophan | H+ | C11H12N2O2 | 1.63 | 205.0969 | 0.026* | 0.588 |
| Proline | H+ | C5H9NO2 | 0.35 | 116.0706 | 0.007* | 0.004* |
| Leucine/Isoleucine | H+ | C6H13NO2 | 0.57 | 132.1019 | 0.562 | 0.048* |
| Xanthine | H- | C5H4N4O2 | 0.37 | 151.0254 | 0.178 | 0.034* |
Notes. P value from repeated measures ANOVA;
indicates significant value P<0.05
Acknowledgments
Source of Funding: This work was funded by the National Institutes of Health (National Institute of Allergy and Infectious Diseases) grant 1R01AI101798 (P.I. Albert J. Fornace, Jr.), partial support from the National Cancer Institute grant P30CA051008 (P.I. Louis Weiner), and a grant from the Defense Threat Reduction Agency grant CBM.RAD.01.10.AR.005 (P.I. Vijay K. Singh). Dr. Pannkuk was supported by a PHS Grant # 5 T32 CA 9686-20.
The opinions or assertions expressed herein are those of the authors and are not endorsements from Uniformed Services University of the Health Sciences, or the US Department of Defense. The authors would like to thank the Lombardi Comprehensive Cancer Metabolomics Shared Resource (MSR) for data acquisition. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Conflicts of Interest: No conflict declared.
References
- Dainiak N, Gent RN, Carr Z, Schneider R, Bader J, Buglova E, Chao N, Coleman CN, Ganser A, Gorin C, Hauer-Jensen M, Huff LA, Lillis-Hearne P, Maekawa K, Nemhauser J, Powles R, Schunemann H, Shapiro A, Stenke L, Valverde N, Weinstock D, White D, Albanese J, Meineke V. First global consensus for evidence-based management of the hematopoietic syndrome resulting from exposure to ionizing radiation. Disaster Med Public Health Prep. 2011;5(3):202–212. doi: 10.1001/dmp.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCarlo AL, Maher C, Hick JL, Hanfling D, Dainiak N, Chao N, Bader JL, Coleman CN, Weinstock DM. Radiation injury after a nuclear detonation: Medical consequences and the need for scarce resources allocation. Disaster Med Public Health Prep. 2011;5(Suppl 1):S32–44. doi: 10.1001/dmp.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouet M, Herodin F. Mitigating radiation-induced toxicity: an overview of new approaches developed at the French Military Biomedical Research Institute. Health Phys. 2014;106(6):682–688. doi: 10.1097/HP.0000000000000039. [DOI] [PubMed] [Google Scholar]
- Farese AM, Cohen MV, Katz BP, Smith CP, Gibbs A, Cohen DM, MacVittie TJ. Filgrastim improves survival in lethally irradiated nonhuman primates. Radiat Res. 2013;179(1):89–100. doi: 10.1667/RR3049.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese AM, MacVittie TJ. Filgrastim for the treatment of hematopoietic acute radiation syndrome. Drugs Today (Barc) 2015;51(9):537–548. doi: 10.1358/dot.2015.51.9.2386730. [DOI] [PubMed] [Google Scholar]
- Fendler W, Malachowska B, Meghani K, Konstantinopoulos PA, Guha C, Singh VK, Chowdhury D. Evolutionarily conserved serum microRNAs predict radiation-induced fatality in nonhuman primates. Sci Transl Med. 2017;9(379):eaal2408. doi: 10.1126/scitranslmed.aal2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan JL, Simmons PA, Vehige J, Willcox MD, Garrett Q. Role of carnitine in disease. Nutr Metab (Lond) 2010;7:30. doi: 10.1186/1743-7075-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh SP, Pathak R, Kumar P, Biswas S, Bhattacharyya S, Kumar VP, Hauer-Jensen M, Biswas R. Gamma-tocotrienol modulates radiation-induced microRNA expression in mouse spleen. Radiat Res. 2016;185(5):485–495. doi: 10.1667/RR14248.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Biodefense. [Last accessed 05 July 2016];Neulasta approved for treatment of acute radiation syndrome. 2015 Available at: http://globalbiodefense.com/2015/12/03/pegfilgrastim-neulasta-acute-radiation-syndrome/
- Goudarzi M, Mak TD, Jacobs JP, Moon BH, Strawn SJ, Braun J, Brenner DJ, Fornace AJ, Jr, Li HH. An Integrated multi-omic approach to assess radiation injury on the host-microbiome axis. Radiat Res. 2016;186(3):219–234. doi: 10.1667/RR14306.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudarzi M, Weber W, Mak TD, Chung J, Doyle-Eisele M, Melo D, Brenner DJ, Guilmette RA, Fornace AJ. Development of urinary biomarkers for internal exposure by cesium-137 using a metabolomics approach in mice. Radiat Res. 2014;181(1):54–64. doi: 10.1667/RR13479.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall EJ, Giaccia AJ. Radiobiology for the Radiobiologist. Philadelphia, PA: Lippincott Williams and Wilkins; 2012. [Google Scholar]
- Hankey KG, Farese AM, Blaauw EC, Gibbs AM, Smith CP, Katz BP, Tong Y, Prado KL, MacVittie TJ. Pegfilgrastim improves survival of lethally irradiated nonhuman primates. Radiat Res. 2015;183(6):643–655. doi: 10.1667/RR13940.1. [DOI] [PubMed] [Google Scholar]
- Herodin F, Drouet M. Cytokine-based treatment of accidentally irradiated victims and new approaches. Exp Hematol. 2005;33(10):1071–1080. doi: 10.1016/j.exphem.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Johnson CH, Patterson AD, Krausz KW, Kalinich JF, Tyburski JB, Kang DW, Luecke H, Gonzalez FJ, Blakely WF, Idle JR. Radiation metabolomics. 5. Identification of urinary biomarkers of ionizing radiation exposure in nonhuman primates by mass spectrometry-based metabolomics. Radiat Res. 2012;178(4):328–340. doi: 10.1667/rr2950.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni S, Singh PK, Ghosh SP, Posarac A, Singh VK. Granulocyte colony-stimulating factor antibody abrogates radioprotective efficacy of gamma-tocotrienol, a promising radiation countermeasure. Cytokine. 2013;62(2):278–285. doi: 10.1016/j.cyto.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Kurland IJ, Broin PO, Golden A, Su G, Meng F, Liu L, Mohney R, Kulkarni S, Guha C. Integrative metabolic signatures for hepatic radiation injury. PLoS One. 2015;10(6):e0124795. doi: 10.1371/journal.pone.0124795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiakis EC, Hyduke DR, Fornace AJ. Comparison of mouse urinary metabolic profiles after exposure to the inflammatory stressors gamma radiation and lipopolysaccharide. Radiat Res. 2012;177(2):187–199. doi: 10.1667/rr2771.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiakis EC, Mak TD, Anizan S, Amundson SA, Barker CA, Wolden SL, Brenner DJ, Fornace AJ., Jr Development of a metabolomic radiation signature in urine from patients undergoing total body irradiation. Radiat Res. 2014;181(4):350–361. doi: 10.1667/RR13567.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas B, Richard ML, Leducq V, Pham HP, Michel ML, Da Costa G, Bridonneau C, Jegou S, Hoffmann TW, Natividad JM, Brot L, Taleb S, Couturier-Maillard A, Nion-Larmurier I, Merabtene F, Seksik P, Bourrier A, Cosnes J, Ryffel B, Beaugerie L, Launay JM, Langella P, Xavier RJ, Sokol H. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 2016;22(6):598–605. doi: 10.1038/nm.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak TD, Laiakis EC, Goudarzi M, Fornace AJ., Jr MetaboLyzer: a novel statistical workflow for analyzing Postprocessed LC-MS metabolomics data. Anal Chem. 2014;86(1):506–513. doi: 10.1021/ac402477z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna SK, Krausz KW, Bonzo JA, Idle JR, Gonzalez FJ. Metabolomics reveals aging-associated attenuation of noninvasive radiation biomarkers in mice: potential role of polyamine catabolism and incoherent DNA damage-repair. J Proteome Res. 2013;12(5):2269–2281. doi: 10.1021/pr400161k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council of the National Academy of Sciences. Guide for the care and use of laboratory animals. Washington, DC: National Academies Press; 2011. [Google Scholar]
- Broin ÓP, Vaitheesvaran B, Saha S, Hartil K, Chen EI, Goldman D, Fleming WH, Kurland IJ, Guha C, Golden A. Intestinal microbiota-derived metabolomic blood plasma markers for prior radiation injury. Int J Radiat Oncol Biol Phys. 2015;91(2):360–367. doi: 10.1016/j.ijrobp.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannkuk EL, Fornace AJ, Jr, Laiakis EC. Metabolomic applications in radiation biodosimetry: exploring radiation effects through small molecules. Int J Radiat Biol. 2017 Oct;93(10):1151–1176. doi: 10.1080/09553002.2016.1269218. Epub 2017 Jan 12. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannkuk EL, Laiakis EC, Authier S, Wong K, Fornace AJ., Jr Gas chromatography/mass spectrometry metabolomics of urine and serum from nonhuman primates exposed to ionizing radiation: Impacts on the tricarboxylic acid cycle and protein metabolism. J Proteome Res. 2017b;16(5):2091–2100. doi: 10.1021/acs.jproteome.7b00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannkuk EL, Laiakis EC, Authier S, Wong K, Fornace AJ., Jr Global metabolomic identification of long-term dose-dependent urinary biomarkers in nonhuman primates exposed to ionizing radiation. Radiat Res. 2015;184(2):121–133. doi: 10.1667/rr14091.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannkuk EL, Laiakis EC, Authier S, Wong K, Fornace AJ., Jr Targeted metabolomics of nonhuman primate serum after exposure to ionizing radiation: Potential tools for high-throughput biodosimetry. RSC Adv. 2016a;6(56):51192–51202. doi: 10.1039/C6RA07757A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannkuk EL, Laiakis EC, Mak TD, Astarita G, Authier S, Wong K, Fornace AJ., Jr A lipidomic and metabolomic serum signature from nonhuman primates exposed to ionizing radiation. Metabolomics. 2016b;12(5) doi: 10.1007/s11306-016-1010-0. pii: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed TM. Radiation protectants: current status and future prospects. Health Phys. 2005;89(5):531–545. doi: 10.1097/01.hp.0000175153.19745.25. [DOI] [PubMed] [Google Scholar]
- Sen CK, Khanna S, Roy S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006;78(18):2088–2098. doi: 10.1016/j.lfs.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VK, Beattie LA, Seed TM. Vitamin E: Tocopherols and tocotrienols as potential radiation countermeasures. J Radiat Res. 2013;54(6):973–988. doi: 10.1093/jrr/rrt048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VK, Ducey EJ, Brown DS, Whitnall MH. A review of radiation countermeasure work ongoing at the Armed Forces Radiobiology Research Institute. Int J Radiat Biol. 2012;88(4):296–310. doi: 10.3109/09553002.2012.652726. [DOI] [PubMed] [Google Scholar]
- Singh VK, Hauer-Jensen M. Gamma-tocotrienol as a promising countermeasure for acute radiation syndrome: Current status. Int J Mol Sci. 2016a;17(5):e663. doi: 10.3390/ijms17050663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VK, Kulkarni S, Fatanmi OO, Wise SY, Newman VL, Romaine PL, Hendrickson H, Gulani J, Ghosh SP, Kumar KS, Hauer-Jensen M. Radioprotective efficacy of gamma-tocotrienol in nonhuman primates. Radiat Res. 2016b;185(3):285–298. doi: 10.1667/RR14127.1. [DOI] [PubMed] [Google Scholar]
- Singh VK, Newman VL, Romaine PL, Wise SY, Seed TM. Radiation countermeasure agents: An update (2011 – 2014) Expert Opin Ther Pat. 2014;24(11):1229–1255. doi: 10.1517/13543776.2014.964684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VK, Romaine PL, Newman VL, Seed TM. Medical countermeasures for unwanted CBRN exposures: part II radiological and nuclear threats with review of recent countermeasure patents. Expert Opin Ther Pat. 2016c;26(12):1399–1408. doi: 10.1080/13543776.2016.1231805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torquato P, Ripa O, Giusepponi D, Galarini R, Bartolini D, Wallert M, Pellegrino R, Cruciani G, Lorkowski S, Birringer M, Mazzini F, Galli F. Analytical strategies to assess the functional metabolome of vitamin E. J Pharm Biomed Anal. 2016;124:399–412. doi: 10.1016/j.jpba.2016.01.056. [DOI] [PubMed] [Google Scholar]
- Tyburski JB, Patterson AD, Krausz KW, Slavik J, Fornace AJ, Jr, Gonzalez FJ, Idle JR. Radiation metabolomics. 2. Dose- and time-dependent urinary excretion of deaminated purines and pyrimidines after sublethal gamma-radiation exposure in mice. Radiat Res. 2009;172(1):42–57. doi: 10.1667/RR1703.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. [Last accessed 18 July 2016];Guidance for industry: Product developoment under the Animal Rule. 2015 Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM399217.pdf.
- Weiss JF, Landauer MR. History and development of radiation-protective agents. Int J Radiat Biol. 2009;85(7):539–573. doi: 10.1080/09553000902985144. [DOI] [PubMed] [Google Scholar]
- Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.