Abstract
Podocyte injury and loss contribute to the progression of glomerular diseases, including diabetic kidney disease. We previously found that the glomerular expression of Sirtuin-1 (SIRT1) is reduced in human diabetic glomeruli and that the podocyte-specific loss of SIRT1 aggravated albuminuria and worsened kidney disease progression in diabetic mice. SIRT1 encodes an NAD-dependent deacetylase that modifies the activity of key transcriptional regulators affected in diabetic kidneys, including NF-κB, STAT3, p53, FOXO4, and PGC1-α. However, whether the increased glomerular SIRT1 activity is sufficient to ameliorate the pathogenesis of diabetic kidney disease has not been explored. We addressed this by inducible podocyte-specific SIRT1 overexpression in diabetic OVE26 mice. The induction of SIRT1 overexpression in podocytes for six weeks in OVE26 mice with established albuminuria attenuated the progression of diabetic glomerulopathy. To further validate the therapeutic potential of increased SIRT1 activity against diabetic kidney disease, we developed a new, potent and selective SIRT1 agonist, BF175. In cultured podocytes BF175 increased SIRT1-mediated activation of PGC1-α and protected against high glucose-mediated mitochondrial injury. In vivo, administration of BF175 for six weeks in OVE26 mice resulted in a marked reduction in albuminuria and in glomerular injury in a manner similar to podocyte-specific SIRT1 overexpression. Both podocyte-specific SIRT1 overexpression and BT175 treatment attenuated diabetes-induced podocyte loss and reduced oxidative stress in glomeruli of OVE26 mice. Thus, increased SIRT1 activity protects against diabetes-induced podocyte injury and effectively mitigates the progression of diabetic kidney disease.
Keywords: SIRT1, diabetic kidney disease, podocytes, PGC1α
INTRODUCTION
Diabetic kidney disease (DKD) is the leading cause of end-stage renal disease in US1. The current therapy for DKD remains limited to the renin-angiotensin system blockade, which only provides partial renoprotection. Thus, many patients on angiotensin converting enzyme inhibitors or angiotensin receptor blockades continue to progress to end stage renal disease2. Therefore there is a large unmet need to develop more potent and safer therapies for patients with DKD.
The sirtuin family of nicotinamide adenine dinucleotide (NAD+)-dependent deacetylases plays an important role in aging 3, metabolism 4, cancer and inflammation 5. Sirtuin-1 (SIRT1) in renal tubular cells has been shown to protect renal tubular cells from cellular stresses associated with aging, cisplatin, and hypoxia 6–8. Our previous studies demonstrated that SIRT1 protein expression is reduced in podocytes and in glomerular cells of human diabetic kidneys, which was consistent with reduced SIRT1 mRNA expression in microdissected glomeruli of diabetic patients 9, 10. We further showed that the global reduction of SIRT1 accelerated DKD progression in db/db mice 10 and that podocyte-specific knockout of Sirt1 similarly accelerated DKD in streptozotocin-induced diabetic mice 10, 11. More recently, renal tubular SIRT1 expression was reported to mitigate diabetic glomerular injury 12. However, whether the increased glomerular SIRT1 expression, particularly in the podocytes, is sufficient to attenuate DKD has not been addressed previously.
On the cellular level, SIRT1 has been shown to regulate autophagy 13, energetic homeostasis 14, mitochondrial biogenesis 15, and apoptosis 16. SIRT1 exerts these biological effects through deacetylation of transcription factors and consequently regulating their activities 17. Among these substrates of SIRT1 are key transcription factors that are implicated in kidney disease progression, such as NF-κB p65 (RelA), STAT3, p53, FOXO, and PGC-1α 18. Systems analysis has revealed that JAK-STAT and NF-κB are key pathways activated in diabetic kidneys 19, 20, and we have recently shown that SIRT1 deacetylates STAT3 and NF-κB p65 to protect kidney from inflammation-induced kidney injury 10. We also demonstrated that the attenuation of proteinuria and podocyte injury in diabetic db/db mice by pyridoxamine treatment was associated with restored SIRT1 expression and reduced NF-κB p65 and STAT3 acetylation and activation 21. A large body of evidence also suggests that p53 mediates apoptosis of podocytes and tubular epithelial cells in DKD 22–24. SIRT1 has been shown to promote cell survival by suppressing p53-dependent apoptosis in response to DNA damage and oxidative stress 16, and recent data suggests that the interplay of SIRT1-p53 pathway controls cellular senescence 25. Furthermore, SIRT1 was also shown to modulate PGC-1α activity and to attenuate aldosterone-induced mitochondrial damage and podocyte injury 26.
Thus, together with the observation that SIRT1 expression is significantly reduced in glomeruli of mouse and human diabetic kidneys and that SIRT1 is a regulator of above-mentioned transcription factors whose roles are implicated in DKD progression, we posited that the increased glomerular expression and/or activity of SIRT1 would confer therapeutic benefit against the disease progression. We have tested this hypothesis in vivo by employing both genetic and pharmacological approaches with the use of the inducible podocyte-specific SIRT1 overexpression mice and a novel SIRT1 agonist, respectively, in OVE26 type 1 diabetic mice.
RESULTS
Generation of diabetic mice with inducible podocyte-specific SIRT1 overexpression
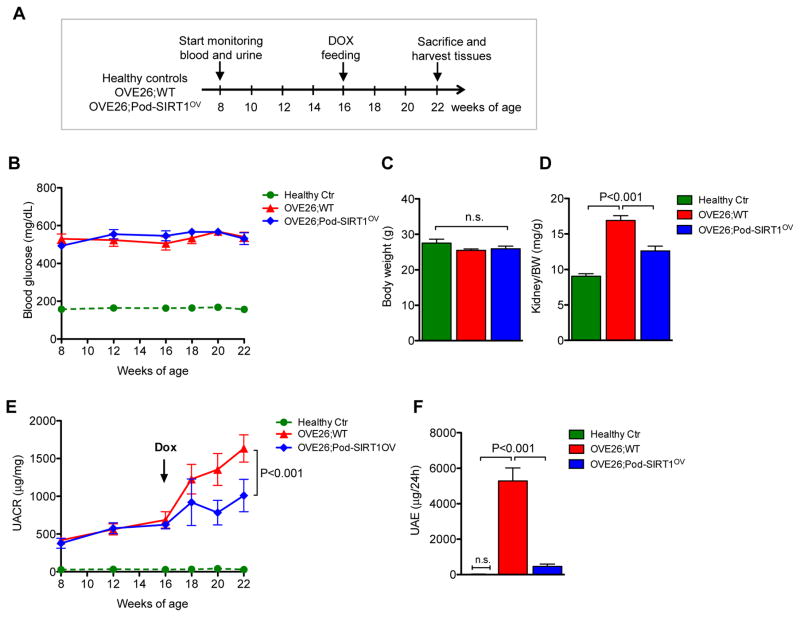
To generate transgenic mice with tetracycline-inducible SIRT1 overexpression, human SIRT1 cDNA (Addgene, Cambridge, MA) was subcloned into pLP-TRE2 vector (Clontech Laboratories), resulting in pLP-TRE2-SIRT1 expression construct as described in the Concise Methods. Doxycycline (Dox)-inducible expression of pLP-TRE2-SIRT1 construct was first confirmed in vitro in U2OS cell line (Supplementary Figure 1A) and used for microinjection to generate the TRE-SIRT1 transgenic mice in the FVB/N background (TRE-SIRT1OV). Inducible SIRT1 overexpression in vivo was first tested in TRE-SIRT1OV mice bred with transgenic mice with the universal expression of reverse tetracycline-controlled transactivator transgene under the cytomegalovirus early enhancer element and chicken beta-actin promoter (CAG-rtTA). Upon Dox supplementation (625mg/kg in chow), there was a robust expression of SIRT1 in kidney cortices of CAGs-rtTA;TRE-SIRT1OV mice (Supplementary Figure 1B). TRE-SIRT1OV mice were subsequently crossed with podocin-rtTA transgenic mice to generate podocin-rtTA;TRE-SIRT1OV (referred hereafter as Pod-SIRT1OV) for podocyte-specific SIRT1 expression. Dox-dependent induction of SIRT1 overexpression in podocytes was confirmed by western blot analysis of primary podocytes isolated from Pod-SIRT1OV mice with or without Dox supplementation (Supplementary Figure 1C). In order to ascertain whether the podocyte-specific overexpression of SIRT1 can mitigate the diabetic kidney injury, Pod-SIRT1OV mice were crossed with type 1 diabetic OVE26 mice 27 to generate OVE26;Pod-SIRT1OV. Their littermates without SIRT1 transgene (OVE26;WT) and age-matched healthy FVB mice were used as controls. Consistent with what was reported previously 27, 28, OVE26 mice exhibited significant albuminuria by age of 16 weeks of age. Thus, we next determined whether the overexpression of SIRT1 in podocytes in OVE26 mice starting at 16 weeks of age can curtail the progress of an established DKD.
Podocyte-specific SIRT1 overexpression attenuates DKD progression in diabetic OVE26 mice
Healthy nondiabetic littermate control, OVE26;WT and OVE26;Pod-SIRT1OV mice were given Dox-supplemented chow starting at 16 weeks of age for 6 weeks as outlined in Figure 1A. Blood glucose and urinary albumin levels were monitored starting at 8 weeks until 22 weeks of age when they were sacrificed. OVE26 mice displayed pronounced hyperglycemia at 8 weeks of age that was sustained throughout the duration of the study (Figure 1B). Dox-induced SIRT1 overexpression in OVE26;Pod-SIRT1OV mice did not affect blood glucose levels or overall body weight in comparison to OVE26;WT mice (Figure 1B–C). However, kidney-to-body weight ratio, which was found to be significantly increased in OVE26;WT mice in comparison to healthy controls, were suppressed in OVE26;Pod-SIRT1OV mice (Figure 1D). Weekly spot collection of urine showed apparent albuminuria in both OVE26;WT and OVE26;Pod-SIRT1OV mice at 8 weeks of age, which had further escalated to by 22 weeks of age in OVE26;WT but was significantly curtailed in OVE26;Pod-SIRT1OV mice (Figure 1E). The reduction in albuminuria by SIRT1 overexpression was further confirmed by 24-hour urine excretion at 22 weeks of age, which showed a marked reduction in OVE26;Pod-SIRT1OV mice as compared to OVE26;WT (Figure 1F).
Figure 1.
Podocyte-specific SIRT1 overexpression attenuates albuminuria in diabetic OVE26 mice. (A) Schema of study design. Blood glucose and urinary albumin of healthy nondiabetic littermate controls, OVE26;WT and OVE26;Pod-SIRT1OV mice were monitored starting at 8 weeks of age. Dox-supplemented chow was administered starting at 16 weeks of age, and all mice were sacrificed at 22 weeks of age. (B) Fasting blood glucose levels of nondiabetic healthy littermate controls (Healthy Ctr), OVE26;WT, and OVE26;Pod-SIRT1 mice. Both diabetic OVE26 mouse groups showed highly elevated blood glucose levels at 8 weeks in comparison to healthy controls, which was sustained throughout the duration of the study. (C) Body weight of mice did not differ between three groups at 22 weeks of age. (D) Kidney-to-body weight (K/BW) was markedly elevated in OVE26;WT compared to healthy control mice, but was significantly reduced in OVE26;Pod-SIRT1OV mice. (E) Urinary albumin-to-creatinine ratio (UACR) of spot urine collected during the duration of the study is shown. (F) Urine albumin excretion (UAE) was measured from the 24-hour urine collected from mice at 22 weeks of age. n=5 to 9 in each group; P-values between groups from one-way or two-way ANOVA analyses are as indicated. n.s., not significant.
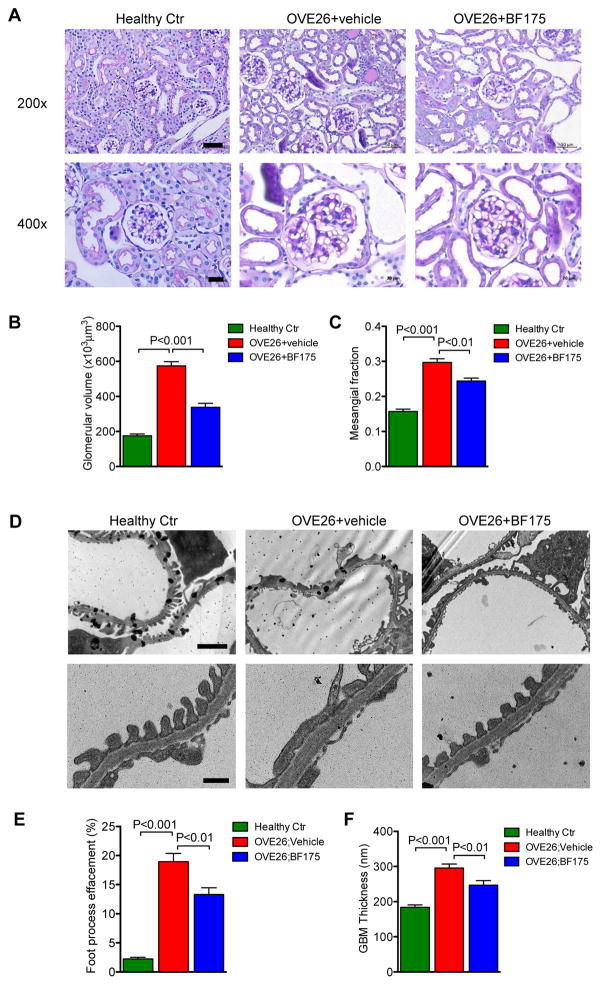
Histologically, although both diabetic groups displayed glomerular hypertrophy and mesangial matrix expansion in comparison to nondiabetic controls, they were both significantly attenuated in OVE26;Pod-SIRT1OV compared to OVE26;WT (Figure 2A–C). Consistently, electron microscopic analysis showed significant reduction in podocyte foot process effacement and glomerular basement membrane (GBM) thickening in OVE26;Pod-SIRT1OV mice in comparison to OVE26;WT mice (Figure 2D). Together, our data suggest that the overexpression of SIRT1 in podocytes in diabetic kidneys mitigates diabetes-induced podocyte injury and effectively blunts the progression of DKD in OVE26 mice.
Figure 2.
Podocyte-specific SIRT1 overexpression attenuates glomerular injury in diabetic OVE26 mice. (A) Representative images of periodic acid-Schiff (PAS)-stained mouse kidneys at 22-weeks of age are shown (top panels: original magnification x200, scale bar: 50μm; bottom panels: original magnification x400, scale bar: 20μm). (B–C) Quantification of glomerular volume (B) and fraction of mesangial area (C) are shown. (D) Electron microscopy was performed to assess ultra-structural changes in podocyte morphology (top panels: original magnification ×12,000, scale bar: 2μm; bottom panels: original magnification ×40,000, scale bar: 500nm). Representative images are shown. (E) Semi-quantification of podocyte effacement (n=5) (F) Measurements of glomerular basement membrane (GBM) thickness (n=5). P-values between groups from one-way ANOVA analyses are as indicated.
BF175 is a new potent agonist of SIRT1
Given that podocyte-specific overexpression of SIRT1 for 6 weeks in OVE26 mice with established DKD was sufficient to mitigate the disease progression, we sought for a pharmacological means of SIRT1 activation as a therapeutic approach against DKD. Although several studies have shown the beneficial effects of SIRT1 agonist resveratrol against DKD injury 18, resveratrol is also associated with renal toxicity 29 and reported to have SIRT1-independent effects 30, 31. Thus, in order to improve the specificity, bioavailability and to lower toxicity, we have synthesized several boron-containing compounds based on the structure of resveratrol using the target-to-hit and structure-activity relationships (SAR) approaches and with computer-aided drug design approach. The introduction of boron atoms into small molecules presents several advantages in that it enhances the interaction with the target molecule and facilitates the molecule’s movement across the cell membrane through their interactions with cell surface glycoproteins 32. We recently employed a similar approach toward a potent inhibitor of homeodomain-interacting protein kinase-2 (HIPK2) that was shown to be highly effective against renal fibrosis 33, as well as toward a novel retinoic acid alpha agonists for CKD treatment 34. Currently existing SIRT1 agonists SRT1720, SRT2183, SRT1460 and SRT2104 are high molecular weight amides containing imidazothiazole based pharmacophore groups 35 (Supplementary Figure 2A). Since amide bonds are prone to proteolytic cleavage, leading to increased toxicity and to off-target effects, we first performed the SAR study based on the structure of resveratrol (Supplementary Figure 2B) and synthesized a small library of compounds. Potential SIRT1 agonists were screened for stimulation of SIRT1 activity, and we found BF175 to be a potent SIRT1 activator (Supplementary Figure 3A). BF175 was a more potent activator of SIRT1 than resveratrol at same concentration of 10μM in cultured human podocytes (Supplementary Figure 3B). Using the drug affinity responsive target stability (DARTS) assay 36,37,33, which is based on the principle that binding of small molecule compound to the target protein changes the protein conformation to increase its stability and confer protection against proteolysis, we also confirmed that BF175 directly binds to SIRT1 (Supplementary Figure 3C).
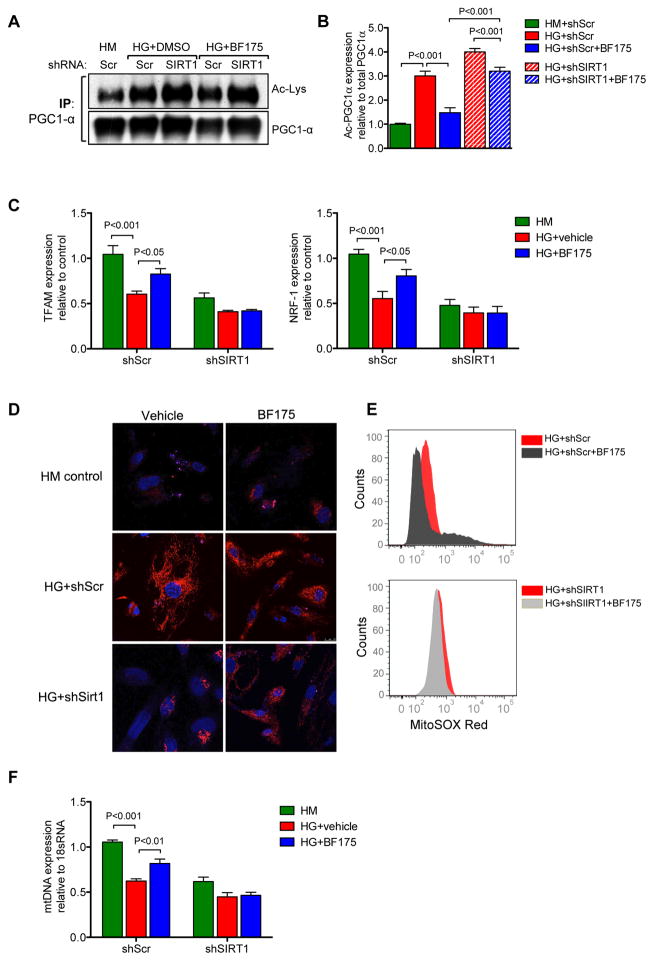
To test the biological effects of BF175 on SIRT1 activity, we next examined the SIRT1-mediated modulation of PGC-1α pathway in cultured human podocytes. Podocytes were transduced with lentivirus expressing either a control scrambled shRNA (shScr) or shRNA against SIRT1 (shSIRT1). As expected, stable transduced shSIRT1 podocytes showed significantly reduced SIRT1 expression in comparison to shScr podocytes (Supplementary Figure 4). Exposure to high glucose media led to elevated levels of acetylated PGC-1α in both shScr and shSIRT1 podocytes in comparison to high mannitol control (Figure 3A–B). Concomitant incubation with BF175 significantly reduced the high glucose-induced PGC-1α in shScr podocytes, but not in shSIRT1 podocytes (Figure 3A–B). Consistently, high glucose treatment led to the reduced expression of PGC-1α-dependent expression of downstream mitochondrial gene expression, NRF-1 and TFAM (Figure 3C). BF175 treatment significantly restored their expression in shScr podocytes in presence of high glucose, but this protective effect was abrogated in shSIRT1 podocytes (Figure 3C). Similarly, BF175 reduced the superoxide production under high glucose conditions in shScr podocytes, but not in shSIRT1 podocytes (Figures 3D–E). Consistent with these observations, the amount of mtDNA that was decreased in high glucose conditions was also significantly restored by BF175 treatment in shScr podocytes, but not in shSIRT1 podocytes (Figure 3F). Together, these results indicate that BF175 is a potent activator of SIRT1 and that it protects the podocytes from high glucose-induced injury by improving the mitochondrial function and homeostasis in a SIRT1-dependent manner. Therefore, we next explored whether BF175 could attenuate DKD progression in OVE26 mice.
Figure 3.
BF175 is a novel agonist of SIRT1. Immortalized human podocytes were stimulated with high glucose (HG, 30mM) with or without BF175 (10μg/ml) for 24 hours. Treatment with high mannitol (HM, 5mM glucose+25mM mannitol) served as a control. (A) Total cell extracts were subjected to immunoprecipitation (IP) using an anti-PGC-1α antibody and immunoblotting with anti-acetyl lysine (Ac-Lys) or anti-PGC-1α antibodies. Representative image of 3 independent experiments are shown. (B) Densitometric analysis shows the levels of acetylated PGC-1α normalized to total PGC-1α in each group. P-values between groups from one-way ANOVA analyses are indicated. (C) Quantitative PCR analysis of mitochondrial biogenesis genes TFAM (left panel) and NRF-1 (right panel). (D–E) BF175 reduces the oxidative stress under high glucose conditions. Podocytes cultured in high glucose or high mannitol conditions were incubated with MitoSOX red for 15 minutes. Cells were then counterstained with DAPI and imaged (D, scale bar: 25μm) or dissociated and fluorescence intensity of MitoSOX were quantified with fluorescence activated cell sorting (FACS) (E). (F) Quantitative PCR analysis of mitochondrial DNA (mtDNA). P-values between groups from one-way or two-way ANOVA analyses are as indicated. n=3 in each group.
BF175 treatment attenuates DKD progression in diabetic OVE26 mice
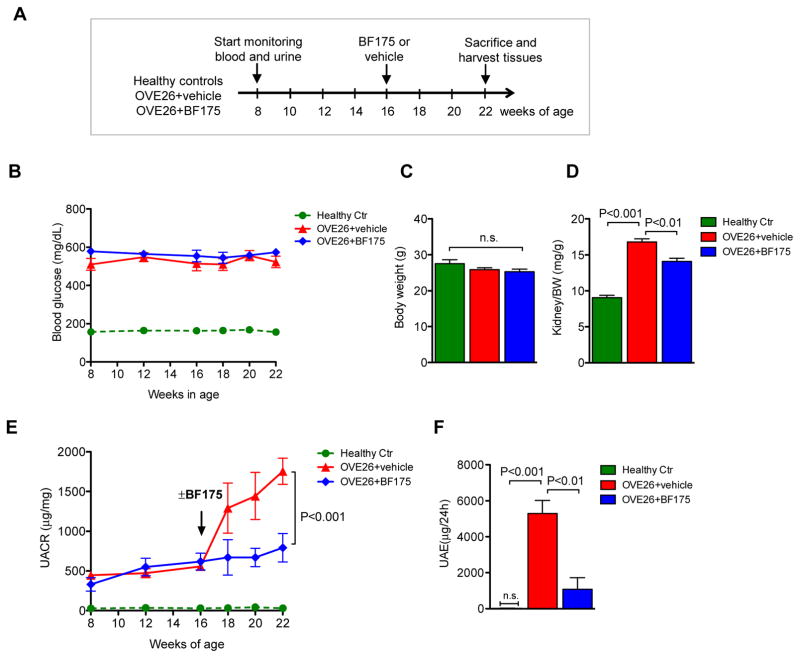
Diabetic OVE26 mice at 16 weeks of age received intraperitoneal injection of either BF175 (0.4mg/kg body weight) or vehicle (5% DMSO in saline) each day for 6 weeks, as outlined in Figure 4A. Similar to what was observed in OVE26;Pod-SIRT1OV mice, BF175 treatment did not affect blood glucose levels or change their body weight for the duration of the treatment (Figure 4B–C). However, BF175 significantly reduced the kidney-to-body weight ratio in OVE26 mice (Figure 4D). Importantly, BF175 markedly reduced albuminuria (Figure 4E–F), glomerular hypertrophy, and mesangial matrix expansion, (Figure 5A–C), similarly to what was observed in OVE26;Pod-SIRT1OV. Consistent with these findings, the extent of podocyte foot process effacement and GBM thickening were markedly diminished with BF175 treatment for 6 weeks (Figure 5D–F). Together, our data indicates that BF175 effectively mitigated diabetic glomerular injury in OVE26 mice.
Figure 4.
BF175 treatment attenuates albuminuria in diabetic OVE26 mice. (A) Schema of study design. Healthy controls and diabetic OVE26 mice were monitored starting at 8 weeks of age. Vehicle or BF175 (0.4mg/kg) was administered intraperitoneally each day starting at 16 weeks of age, and all mice were sacrificed at 22 weeks of age. (B) Fasting blood glucose levels of normal healthy controls (Ctr), vehicle-treated (OVE26+vehicle), and BF175-treated (OVE26+BF175) mice were monitored starting at 8 to 22 weeks of age. Both diabetic OVE26 mouse groups showed highly elevated blood glucose levels at 8 weeks of age in comparison to healthy controls, which was sustained throughout the duration of the study. (C) Body weight of mice did not differ between three groups at 22 weeks of age. (D) Kidney-to-body weight (K/BW) was markedly elevated in OVE26+vehicle compared to healthy control mice, but was significantly reduced in OVE26+BF175 mice. (E) Urinary albumin-to-creatinine ratio (UACR) of spot urine collected during the duration of the study is shown. Black arrow notes the time when BF175 or vehicle treatment commenced. (F) Urine albumin excretion (UAE) was measured from the 24-hour urine collected from mice at 22 weeks of age. n=5 to 9 in each group; P-values between groups from one-way or two-way ANOVA analyses are indicated. n.s., not significant.
Figure 5.
BF175 treatment attenuates glomerular injury in diabetic OVE26 mice. (A) Representative images of PAS-stained mouse kidneys at 22-weeks of age are shown (top panels: original magnification x200, scale bar: 50μm; bottom panels: original magnification x400, scale bar: 20μm). (B–C) Quantification of glomerular volume (B) and fraction of mesangial area (C) are shown. (D) Electron microscopy was performed to assess ultra-structural changes in podocyte morphology (top panels: original magnification ×12,000, scale bar: 2μm; bottom panels: original magnification ×40,000, scale bar: 500nm). Representative images are shown. (E) Semi-quantification of podocyte effacement (n=5) (F) Measurements of glomerular basement membrane (GBM) thickness (n=5). P-values between groups from one-way ANOVA analyses are indicated.
Increased SIRT1 function attenuates podocyte loss in diabetic OVE26 mice
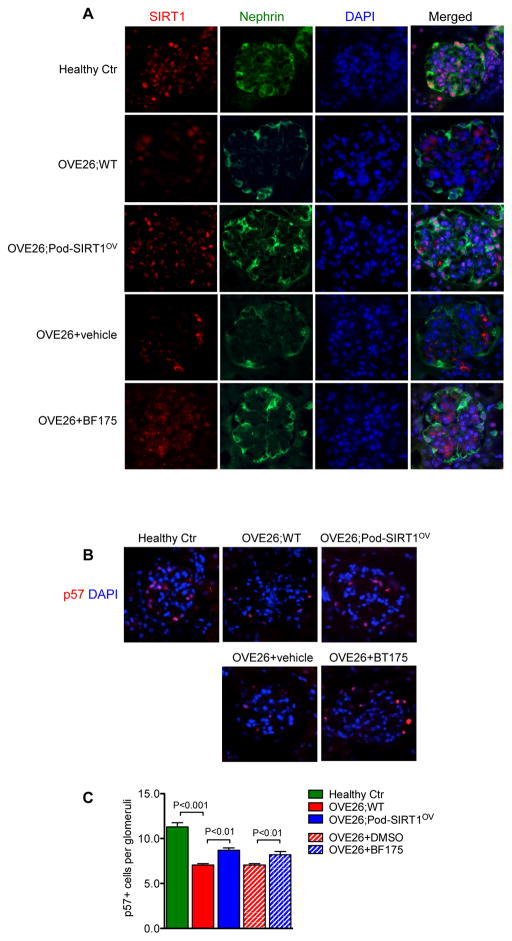
We further observed that the podocyte-specific induction of SIRT1 overexpression or BF175 administration in OVE26 mice significantly restored the expression of podocyte marker nephrin in OVE26 mice (Figure 6A). Quantification of podocytes by p57 immunostaining showed a marked decrease in podocyte number in OVE26 mice compared to healthy controls (Figure 6B–C). Both OVE26;Pod-SIRT1OV and mice treated with BF175 showed increased podocyte number compared to control OVE26, indicating that increased SIRT1 function protected against podocyte loss in OVE26 glomeruli.
Figure 6.
Podocyte-specific SIRT1 overexpression or BF175 treatment increases podocyte marker expression and podocyte number in diabetic OVE26 mice. (A) Representative images of glomerular expression of SIRT1 (red) and podocin (green) are shown of healthy control, OVE26 with or without podocyte SIRT1 overexpression, and OVE26 with or without BF175 treatment. Scale bar: 20μm. (B–C) Representative images of glomerular p57 expression (B) and their quantification (C) are shown. Scale bar: 20μm. P-values between groups from one-way ANOVA analyses are as indicated. n=5 in each group.
Increased SIRT1 function reduces oxidative stress in glomeruli of diabetic OVE26 mice
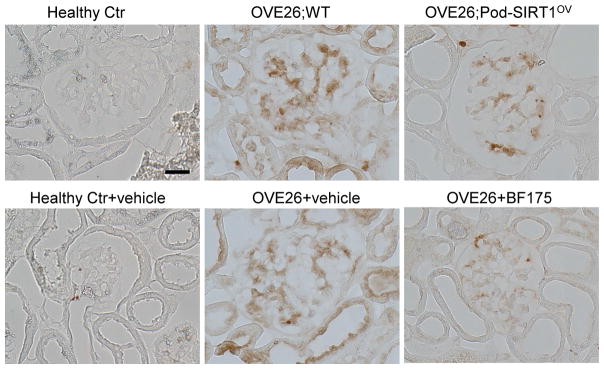
Given that BF175 significantly improved mitochondrial function and reduced the superoxide production in cultured podocytes exposed to high glucose, we next examined the effects of increased podocyte SIRT1 expression or BF175 on the extent of oxidative stress in the diabetic glomeruli. Immunohistochemical staining showed a strong upregulation of nitro-tyrosine expression in the glomeruli of OVE26;WT and in vehicle-treated OVE26 mice, which was markedly suppressed in glomeruli of both OVE26;Pod-SIRT1OV and in BF175-treated OVE26 mice (Figure 7). These results suggests that the renoprotection observed by increased SIRT1 function is mediated in part through the improved mitochondrial function and reduced oxidative stress in diabetic glomeruli of OVE26 mice. In sum, our data demonstrates that increased podocyte-specific expression of SIRT1 effectively thwarts the DKD progression and that BF175 is a novel SIRT1 agonist that can be further developed as a potential therapy against DKD.
Figure 7.
Podocyte-specific SIRT1 overexpression or BF175 treatment reduces oxidative stress in glomeruli of diabetic OVE26 mice. Representative images of nitro-tyrosine immunostaining in paraffin-embedded kidney sections. Scale bar: 20μm.
DISCUSSION
Many studies have shown that the loss of SIRT1 expression and/or activity worsens kidney disease, including DKD 12, 18. Our previous work support a important role of SIRT1 in protection of podocytes against diabetic injury, as loss of SIRT1 led to aggravated disease progression in murine models of DKD 10, 11. We now show that the induction of SIRT1 expression in podocytes is sufficient to attenuate DKD progression in OVE26 mice, further corroborating an essential role of SIRT1 against diabetes-induced podocyte injury and glomerulopathy. In addition, our findings also provide a strong basis for SIRT1 as a potential drug target for treatment of DKD. Toward this end, we also report in vitro and in vivo findings of a novel SIRT1 activator BF175. We found that BF175 led to improved mitochondrial function and reduced oxidative stress in podocytes under high glucose conditions, and 6 weeks of BF175 treatment in vivo significantly hindered the progression of DKD in OVE26 mice.
Although resveratrol is a well-known SIRT1 activator, the use of resveratrol has been associated with renal toxicity in rodents 29, and recent reports questions its specificity of SIRT1 activation 30,31. Therefore, we sought to develop new selective SIRT1 agonist that would be associated with less toxicity. From the potential candidates that developed using the SAR approach based on the structure of resveratrol, we have identified BF175 as a potent SIRT1 agonist, assessed both directly through SIRT1 activity measurements and indirectly through downstream transcription factor PGC-1α activation and mitochondrial function. DARTS assay confirmed that BF175 bound directly to SIRT1, and by using SIRT1-knockdown podocytes, we showed that the protective effects of BF175 were SIRT1-dependent. In vivo, BF175 significantly attenuated proteinuria and kidney injury in diabetic OVE26 mice in a manner similar to what was observed in Pod-SIRT1OV mice. As BF175 is administered systemically, in addition to protecting podocytes from diabetic injury, it is likely to confer renoprotection through activation of SIRT1 in other cell types. For instance, it was shown recently that the function of proximal tubular SIRT1 attenuates diabetic albuminuria in db/db mice 12. Therefore, it is possible that BF175 activates proximal tubular SIRT1 to enhance renoprotection in diabetic kidneys. More in vivo studies using cell-specific SIRT1 knockout mice in conjunction with BF175 treatments are required to assess the cell type-specific contribution of renoprotection conferred by BF175 in the setting of DKD. We also did not find any obvious signs of toxicity of BF175 at the dosage used (0.4 mg/kg) after 6 weeks of treatment in diabetic mice. The BF175-treated mice were indistinguishable in behavior and physical activity, and maintained normal body weight as compared to vehicle-treated mice. However, more studies are required in the future to determine the pharmacokinetics, pharmacodynamics and toxicity of BF175 in greater detail.
Multiple mechanisms have been proposed as to the renoprotective mechanism of SIRT1 in DKD 18, 38. SIRT1 is also a key regulator of cellular senescence, autophagy, and metabolism, all of which are dysregulated in DKD. We previously showed that SIRT1 improves DKD by inhibition of NF-κB and STAT3-mediated inflammatory response 10. Consistent with the known function of SIRT1 to maintain normal mitochondrial function by deacetylation of PGC-1α and activation of PPARγ 26, we now show that increased SIRT1 activity by BF175 induces deacetylation of PGC-1α and activation of PPARγ-targeted gene expression in podocytes in vitro and reduces oxidative stress in glomeruli of diabetic kidneys of OVE26 mice in vivo. Therefore, the renoprotection afforded by increased SIRT1 activity is mediated through the interaction of multiple downstream cellular pathways. Future studies are required to confirm which of these SIRT1-mediated pathways are essential for its renoprotective function against diabetic kidney injury. In summary, we have demonstrated that the induction of SIRT1 expression in podocytes or the activation of SIRT1 by a new agonist BF175 attenuates albuminuria and podocyte injury in diabetic mice. Our study strongly supports a therapeutic role of SIRT1 in DKD.
METHODS
Mouse models
To generate inducible SIRT1 overexpression transgenic mice, human SIRT1 cDNA (purchased from Addgene, #1791) was subcloned into pLP-TRE2 vector (Clontech Laboratories), resulting in pLP-TRE2-SIRT1 expression construct. The sequence and orientation of SIRT1 insert were confirmed by restriction endonuclease digestion and DNA sequencing. Doxycycline-inducible expression of SIRT1 was first confirmed in the Tet-ON U2OS cell line (Clontech Laboratories). pLP-TRE2-SIRT1 was then used for microinjection to generate the TRE-SIRT1 transgenic mice in FVB/N background (TRE-SIRT1OV). Founder mice with germ-line transmission of the transgene were crossed with podocin-rtTA mice (Jackson Laboratory, FVB/N-Tg(NPHS2-rtTA2*M2)1Jbk/J) to generate inducible podocyte-specific SIRT1 overexpression mice (Pod-SIRT1OV). Pod-SIRT1OV was further crossed with diabetic OVE26 mice (Jackson Laboratory, FVB(Cg)-Tg(Cryaa-Tag,Ins2-CALM1)26Ove/PneJ) to generate the OVE26;Pod-SIRT1OV mice. OVE26 littermates without TRE-SIRT1 transgene were used as wildtype controls (OVE26;WT). Age-matched normal FVB/N mice were used as healthy controls. Genotyping primer sets used were as follows: SIRT1 (forward: 5′-AATAGGCGTATCACGAGGCCCTTTCG-3′; reverse: 5′-GGAGCCGCCGGGCTGAAG-3′), rtTA (forward: 5′-GAACAACGCCAAGTCATTCCG-3′, reverse: 5′-TACGCAGTGTAAAGTGG-3′); INS2 (forward: 5′-ACTCCAAGTGGAGGCTGA GA-3′; reverse: 5′-TCCTTCCACAAACCCATAGC-3′). SIRT1 expression was induced starting at 16 weeks of age by feeding Dox-supplemented chow (625mg/kg chow, Envigo Inc.). For in vivo BF175 treatments, mice received intraperitoneal injection of 0.4mg/kg of BF175 or vehicle (5% DMSO in saline) per day starting at 16 weeks of age until 22 weeks of age (n=5–9 in each group). After the mice were killed, blood, urine, and kidney tissue were collected. Animal studies were performed in accordance with the guidelines of and approved by the Institutional Animal Care and Use Committee at the Icahn School of Medicine at Mount Sinai.
Measurement of blood glucose and urine albumin
Blood glucose was measured using Accu-Check Aviva glucometer from tail-vein blood sample each week. Urine albumin was determined using a commercial ELISA kit (Bethyl Laboratory Inc., Houston, Texas). Urine creatinine levels were measured in the same samples using the Creatinine Colorimetric Assay Kit (Cayman Chemical, MI) according to the manufacturer’s instructions. 24-hour urine collections in the metabolic cages were also used for determination of urinary albumin excretion.
Kidney histology
Kidney samples were fixed in 10% formalin, embedded in paraffin and sectioned to 4-μm thickness. Sections were stained with periodic acid–Schiff (PAS) for analysis of glomerular area and mesangial matrix expansion. Images were taken at ×400 magnification using Zeiss AX10 microscope (Carl Zeiss Jena, Toronto, ON, Canada). Relative mesangial area was expressed as percentage of mesangial to glomerular surface area. Quantification of mesangial expansion was based on a minimum of 10 glomeruli per section in a blinded fashion, under x400 magnification. Mouse glomerular volume (GV) was calculated from the cross-sectional area with the formula VG = β/k (AG)3/2, where β = 1.38 is the shape coefficient for a sphere, and k = 1.1 is the size distribution coefficient. For transmission electron microscopy (TEM), kidney cortex samples fixed in 2.5% glutaraldehyde were divided into sections, mounted on a copper grid, and images were acquired using the Hitachi H7650 microscope (Hitachi, Tokyo, Japan) as previously described 39. Quantification of foot process (FP) effacement and GBM width was performed using ImageJ software on digitized TEM images, as previously described 40. For quantification of GBM thickness, a mean of 55 measurements (>600μm GBM lengths) was taken per mouse (from podocyte to endothelial cell membranes) at random sites where the GBM was displayed in best cross-section. The same glomeruli were scored for degree of podocyte effacement, defined as the percentage of total glomerular capillary surface area over which the podocyte foot processes were effaced.
Immunofluorescence and immunohistochemical staining
Immunofluorescence staining was conducted on frozen or paraffin kidney sections using standard procedures. Briefly, kidney sections were incubated with primary antibodies at 4°C overnight. Primary antibodies used were: anti-p57 (#8298, Cell signaling, Danvers, MA), anti-SIRT1 (#110304, Abcam, Cambridge, MA), anti-nephrin (ab58968, Abcam, Cambridge, MA). After washing with PBS, sections were incubated with Alexa Fluor-488 or Alexa Fluor-568-labeled second antibodies at room temperature for 1 hour. After nuclei were counterstained with 6-diamidino-2-phenylindole, slides were mounted with Aqua PolyMount (Polysciences, Inc., Warrington, PA). Images were acquired using an AxioVision II Microscope with a digital camera (Carl Zeiss, Dublin, CA). For immunohistochemical staining of nitro-tyrosine, deparaffinized kidney sections were incubated with anti-nitrotysine antibody (SC-32757, Santa Cruz Biotechnology Inc., Dallas, TX). After washing, sections were incubated with an anti-rabbit biotinylated secondary antibody at room temperature, and then with the avidin–biotin–peroxidase complex (Vectastain Elite ABC Kit, Vector Laboratories). The reaction products were developed using the 3, 3′-diaminobenzidine substrate and mounted.
Cell Culture
Conditionally immortalized human podocytes were obtained from Dr. Moin Saleem (University of Bristol, UK) 41. Cells were cultured in RPMI 1640 medium (Gibco, Gaithersburg, MD) containing 10% FBS, 1% insulin-transferrin-selenium-A supplement, 0.5% penicillin and streptomycin at 5% CO2 humidified environment in 33°C under growth-permiss ive (GP) conditions or in 37°C growth-restrictive (GR) conditions. Podocytes were differentiated in GR condition for 5 to 7 days before treatments. For high glucose treatment, cells were serum-starved for 4 hours, followed by incubation with the medium containing 5mM glucose supplemented with either 25mM mannitol or additional 25mM glucose for 48 hours. SIRT1 agonist BF175 (10μg/ml) or vehicle (DMSO) was added at the time of high glucose or mannitol incubation.
Stable knockdown of SIRT1 in human podocytes
Lentiviral vector expressing either scrambled shRNA or SIRT1 shRNA (MISSION shRNA, Bacterial Glycerol Stock NM_012238) was purchased from Sigma-Aldrich (St. Louis, MO). Lentiviral particles were produced by co-transfecting HEK293T cells with, pCD/NL-BH*ΔΔΔ packaging plasmid (Addgene #17531) and VSV-G-encoding pLTR-G plasmid (Addgene #17577). For transduction of podocytes, cells were incubated with viral supernatants supplemented with 8 μg/ml polybrene were incubated for 24 hours, followed by puromycin selection for additional 72 hours prior to use in all studies.
Mitochondrial ROS production assay
Human podocytes were seeded in 35mm glass bottom dishes (MatTek Corporation, MA) and allowed to differentiate at 37°C. Following the 48-hour exp osure of high mannitol of high glucose conditions as described above, 5μM MitoSOX (Thermo Fisher Scientific, Waltham, MA) was added and incubated further for 15 min at 37°C. Subsequently, cells we re washed and used for confocal microscopy imaging with excitation at 510nm. A portion of the cells was trypsinized, and the intensity of MitoSOX was determined by FACS.
mtDNA Quantification
Total DNA from cultured human podocytes was extracted using the DNeasy Blood kit (Qiagen, Valencia, CA) for detection of mitochondrial DNA (mtDNA) content by real-time PCR. Sequences of primers used are as follows: mtDNA (Forward: 5′-CGATTCTTTACCTTTCACTTCATCTT-3′; Reverse: 5′-GAGGGCGTCTTTGATTGTGT -3′); 18s rRNA (Forward: 5′-GCGGTTCTATTTTGTTGGTTTT -3′; Reverse: 5′-ACCTCCGACTTTCGTTCTTG-3′). 18S rRNA served as controls for mtDNA for reaction efficiency. The results were analyzed using the comparative cycle threshold (2−ΔΔCt) method.
RNA Extraction and real time PCR
Total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA). 500 ng total RNA was reverse transcribed to cDNA using the SuperScriptIII First Strand Synthesis System (Invitrogen, Carlsbad, CA). Real-Time PCR was performed using the SYBR Green Master Mix with 7600 Real-Time PCR System (Applied Biosystems, Waltham, MA, USA). Gene level was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and 2−ΔΔCt method was used for the analysis of relative gene expression. The primer sets used are as follows: hTFAM (forward: 5′-CCGAGGTGGTTTTCATCTGT-3′; reverse: 5′-GCATCTGGGTTCTGAGCTTT-3′), hNRF-1 (forward: 5′-GCTGATGAAGACTCGCCTTCT-3′; reverse: 5′-TACATGAGGCCGTTTCCGTTT-3′), and hGAPDH (forward: 5′-AATTGAGCCCGCAGCCTCCC-3′; reverse: 5′-CCAGGCGCCCAATACGACCA-3′).
Immunoprecipitation and western blotting
Cells were lysed with RIPA buffer containing 50mM Tris, pH 8.0, 150mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, supplemented with protease and phosphatase inhibitors and with 5mM nicotinamide and 1μM Trichostatin A to inhibit deacetylase activity. Lysates were incubated with anti-PGC-1α antibody (Cell Signaling, Danvers, MA) overnight at 4°C and the precipitated materials were used for western blot analysis using anti-acetylated lysine antibody (Cell Signaling, Danvers, MA) or with anti-PGC-1α antibody.
SIRT1 activity assay
SIRT1 deacetylase activity was measured using a direct fluorometric assay kit (#10010401, Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s protocol with addition of vehicle, resveratrol, or BF175 with their concentrations as indicated in the text.
Statistical Analyses
Data are reported as mean±SEM. One-way or two-way ANOVA followed by Bonferroni correction was used for comparison between groups. GraphPad Prism software was used for statistical analyses. All experiments were repeated at least three times, and representative experiments are shown. Data were considered statistically significant when p<0.05.
Supplementary Material
Acknowledgments
QH is supported by the China Scholarship Council (No.201503170082) and National Natural Science Foundation of China (No.81470949); JCH is supported by NIH 1R01DK078897, 1R01DK088541, 1R01DK109683, P01DK56492 and VA Merit Award IBX000345C; PYC and KL are supported by NIH 5R01DK098126.
Footnotes
AUTHOR CONTRIBUTIONS
JCH, KL, PYC, GC and XC conceived and designed the experiments; QH, LZ, ZL, and BL performed experiments; BD contributed reagents and materials; JCH, KL, PYC, QH, and LZ analyzed the data; JCH, KL, QH and ZL wrote and edited the manuscript.
COMPETING INTEREST STATEMENT
The authors declare that they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.USRDS. Annual Data Report: Atlas of End-Stage-Renal-Disease in the United States. 2011 [Google Scholar]
- 2.de Zeeuw D. Unmet need in renal protection--do we need a more comprehensive approach? Contrib Nephrol. 2011;171:157–160. doi: 10.1159/000327337. [DOI] [PubMed] [Google Scholar]
- 3.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 4.Moynihan KA, Grimm AA, Plueger MM, et al. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105–117. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Yang H, Bi Y, Xue L, et al. Multifaceted Modulation of SIRT1 in Cancer and Inflammation. Crit Rev Oncog. 2015;20:49–64. doi: 10.1615/critrevoncog.2014012374. [DOI] [PubMed] [Google Scholar]
- 6.He W, Wang Y, Zhang MZ, et al. Sirt1 activation protects the mouse renal medulla from oxidative injury. J Clin Invest. 2010;120:1056–1068. doi: 10.1172/JCI41563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasegawa K, Wakino S, Yoshioka K, et al. Kidney-specific overexpression of Sirt1 protects against acute kidney injury by retaining peroxisome function. J Biol Chem. 2010;285:13045–13056. doi: 10.1074/jbc.M109.067728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kume S, Uzu T, Horiike K, et al. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest. 2010;120:1043–1055. doi: 10.1172/JCI41376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chuang PY, Yu Q, Fang W, et al. Advanced glycation endproducts induce podocyte apoptosis by activation of the FOXO4 transcription factor. Kidney Int. 2007;72:965–976. doi: 10.1038/sj.ki.5002456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu R, Zhong Y, Li X, et al. Role of transcription factor acetylation in diabetic kidney disease. Diabetes. 2014;63:2440–2453. doi: 10.2337/db13-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chuang PY, Xu J, Dai Y, et al. In vivo RNA interference models of inducible and reversible Sirt1 knockdown in kidney cells. Am J Pathol. 2014;184:1940–1956. doi: 10.1016/j.ajpath.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasegawa K, Wakino S, Simic P, et al. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat Med. 2013;19:1496–1504. doi: 10.1038/nm.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salminen A, Kaarniranta K. SIRT1: regulation of longevity via autophagy. Cell Signal. 2009;21:1356–1360. doi: 10.1016/j.cellsig.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Rodgers JT, Lerin C, Haas W, et al. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 15.Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta. 2011;1813:1269–1278. doi: 10.1016/j.bbamcr.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo J, Nikolaev AY, Imai S, et al. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 17.Nakagawa T, Guarente L. Sirtuins at a glance. J Cell Sci. 2011;124:833–838. doi: 10.1242/jcs.081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yacoub R, Lee K, He JC. The Role of SIRT1 in Diabetic Kidney Disease. Front Endocrinol (Lausanne) 2014;5:166. doi: 10.3389/fendo.2014.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berthier CC, Zhang H, Schin M, et al. Enhanced expression of Janus kinase-signal transducer and activator of transcription pathway members in human diabetic nephropathy. Diabetes. 2009;58:469–477. doi: 10.2337/db08-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmid H, Boucherot A, Yasuda Y, et al. Modular activation of nuclear factor-kappaB transcriptional programs in human diabetic nephropathy. Diabetes. 2006;55:2993–3003. doi: 10.2337/db06-0477. [DOI] [PubMed] [Google Scholar]
- 21.Chuang PY, Dai Y, Liu R, et al. Alteration of forkhead box O (foxo4) acetylation mediates apoptosis of podocytes in diabetes mellitus. PLoS One. 2011;6:e23566. doi: 10.1371/journal.pone.0023566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niranjan T, Bielesz B, Gruenwald A, et al. The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat Med. 2008;14:290–298. doi: 10.1038/nm1731. [DOI] [PubMed] [Google Scholar]
- 23.Tikoo K, Tripathi DN, Kabra DG, et al. Intermittent fasting prevents the progression of type I diabetic nephropathy in rats and changes the expression of Sir2 and p53. FEBS Lett. 2007;581:1071–1078. doi: 10.1016/j.febslet.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Brezniceanu ML, Liu F, Wei CC, et al. Catalase overexpression attenuates angiotensinogen expression and apoptosis in diabetic mice. Kidney Int. 2007;71:912–923. doi: 10.1038/sj.ki.5002188. [DOI] [PubMed] [Google Scholar]
- 25.Tran D, Bergholz J, Zhang H, et al. Insulin-like growth factor-1 regulates the SIRT1-p53 pathway in cellular senescence. Aging Cell. 2014;13:669–678. doi: 10.1111/acel.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan Y, Huang S, Wang W, et al. Activation of peroxisome proliferator-activated receptor-gamma coactivator 1alpha ameliorates mitochondrial dysfunction and protects podocytes from aldosterone-induced injury. Kidney Int. 2012;82:771–789. doi: 10.1038/ki.2012.188. [DOI] [PubMed] [Google Scholar]
- 27.Zheng S, Noonan WT, Metreveli NS, et al. Development of late-stage diabetic nephropathy in OVE26 diabetic mice. Diabetes. 2004;53:3248–3257. doi: 10.2337/diabetes.53.12.3248. [DOI] [PubMed] [Google Scholar]
- 28.Xu J, Huang Y, Li F, et al. FVB mouse genotype confers susceptibility to OVE26 diabetic albuminuria. Am J Physiol Renal Physiol. 2010;299:F487–494. doi: 10.1152/ajprenal.00018.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crowell JA, Korytko PJ, Morrissey RL, et al. Resveratrol-associated renal toxicity. Toxicol Sci. 2004;82:614–619. doi: 10.1093/toxsci/kfh263. [DOI] [PubMed] [Google Scholar]
- 30.Beher D, Wu J, Cumine S, et al. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem Biol Drug Des. 2009;74:619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- 31.Pacholec M, Bleasdale JE, Chrunyk B, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Das BC, Thapa P, Karki R, et al. Boron chemicals in diagnosis and therapeutics. Future Med Chem. 2013;5:653–676. doi: 10.4155/fmc.13.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu R, Das B, Xiao W, et al. A Novel Inhibitor of Homeodomain Interacting Protein Kinase 2 Mitigates Kidney Fibrosis through Inhibition of the TGF-beta1/Smad3 Pathway. J Am Soc Nephrol. 2017 doi: 10.1681/ASN.2016080841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhong Y, Wu Y, Liu R, et al. Novel retinoic acid receptor alpha agonists for treatment of kidney disease. PLoS One. 2011;6:e27945. doi: 10.1371/journal.pone.0027945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hubbard BP, Sinclair DA. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends in pharmacological sciences. 2014;35:146–154. doi: 10.1016/j.tips.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lomenick B, Hao R, Jonai N, et al. Target identification using drug affinity responsive target stability (DARTS) Proc Natl Acad Sci U S A. 2009;106:21984–21989. doi: 10.1073/pnas.0910040106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pai MY, Lomenick B, Hwang H, et al. Drug affinity responsive target stability (DARTS) for small-molecule target identification. Methods Mol Biol. 2015;1263:287–298. doi: 10.1007/978-1-4939-2269-7_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wakino S, Hasegawa K, Itoh H. Sirtuin and metabolic kidney disease. Kidney Int. 2015;88:691–698. doi: 10.1038/ki.2015.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mallipattu SK, Liu R, Zhong Y, et al. Expression of HIV transgene aggravates kidney injury in diabetic mice. Kidney Int. 2013;83:626–634. doi: 10.1038/ki.2012.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reiniger N, Lau K, McCalla D, et al. Deletion of the receptor for advanced glycation end products reduces glomerulosclerosis and preserves renal function in the diabetic OVE26 mouse. Diabetes. 2010;59:2043–2054. doi: 10.2337/db09-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saleem MA, O’Hare MJ, Reiser J, et al. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol. 2002;13:630–638. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.