Abstract
Sonic Hedgehog (Shh) plays an instrumental role in brain development, fine-tuning processes such as cell proliferation, patterning, and fate specification. Although, mutations in the SHH pathway in humans are associated with various neurodevelopmental disorders, ranging from holoprosencephaly to schizophrenia, its expression pattern in the developing human brain is not well established. We now determined the previously not reported wide expression of SHH in the human fetal cerebral cortex during most of the gestation period (10–40 gestational weeks). This spatiotemporal distribution puts Shh in a position to influence the fundamental processes involved in corticogenesis. SHH expression increased during development, shifting from progenitor cells in the proliferative zones to neurons, both glutamatergic and GABAergic, and astrocytes in upper cortical compartments. Importantly, the expression of its downstream effectors and complementary receptors revealed evolutionary differences in SHH-pathway gene expression between humans and rodents.
Electronic supplementary material
The online version of this article (10.1007/s00429-018-1621-5) contains supplementary material, which is available to authorized users.
Keywords: Cerebral cortex, Human fetal brain, Morphogen, SHH receptors
Introduction
Corticogenesis is a developmental process that requires coordination of the signaling pathways providing mitogenic signals and guiding the regulation of symmetric/asymmetric divisions of neuronal progenitors, positional information, differentiation signals, and a balance of excitation and inhibition in the cortex. Cortical development has been extensively studied in mice, and although it is considered to be generally well conserved in mammals, a number of changes and novelties have emerged during evolution that may underlie the biological basis for the higher cognitive and motor abilities that are specific to humans (Geschwind and Rakic 2013; Silbereis et al. 2016). Sonic Hedgehog (Shh) is a pleiotropic protein that plays a major role in most of the aforementioned processes during corticogenesis, including dorso-ventral patterning, the specification of interneurons and oligodendrocytes, as well as cortical circuitry formation (Fuccillo et al. 2006; Echelard et al. 1993; Ericson et al. 1995; Xu et al. 2005; Tekki-Kessaris et al. 2001; Harwell et al. 2012).
During mouse embryonic development, Shh is highly expressed in the ventral forebrain, where it plays a critical role in patterning during a specific developmental time window (embryonic days 9.5–12.5) (Xu et al. 2005; Fuccillo et al. 2004; Machold et al. 2003). Notably, changes in the concentration of Shh and the duration of Shh exposure influence the specification of different ventral progenitors and their neuronal progeny, which range from hypothalamic and striatal projection neurons to cortical and striatal interneurons (Maroof et al. 2013; Tyson et al. 2015). Despite the sparse expression of Shh in the mouse dorsal telencephalon (Dahmane et al. 2001), conditional inactivation of the Shh pathway leads to the defective proliferation of intermediate progenitor cells and to microcephaly (Komada et al. 2008). These results point to the concentration-dependent functions of this morphogen in both dorsal and ventral forebrain developments.
The significance of SHH in human brain development is illustrated by the dramatic consequences of SHH-pathway gene disruption, which include holoprosencephaly, seizure disorders, language or cognitive impairment, Down syndrome, hyperactivity, and schizophrenia (Heussler et al. 2002; Nanni et al. 1999; Belloni et al. 1996; Odent et al. 1999; Santiago et al. 2006; Currier et al. 2012; Betcheva et al. 2013). Many of these conditions are the result of SHH haploinsufficiency, thus highlighting the importance of SHH gene dosage in humans (Chiang et al. 1996). Although in human embryos (Carnegie stages 12–16), the expression of SHH has been demonstrated ventrally, in the notochord, in the floorplate of the spinal cord, and in the hindbrain (Odent et al. 1999; Hajihosseini et al. 1996), there is a lack of information regarding the sources of SHH in the developing cerebral cortex.
A prerequisite for understanding the physiological role of SHH signaling during cortical development is the determination of its distribution and identification of its cellular sources in the prenatal human cortex. Our initial results, obtained in vitro, showed that at mid-gestation (around 20 gestational weeks, gw), SHH is expressed by radial glia cells (RGCs) and that treatment with exogenous SHH favours the generation of Nkx2.1+ progenitors over calretinin (CalR+) cells, while it has no effect on the generation of pyramidal neurons (Radonjic et al. 2016). In the present study, we used in situ hybridization (ISH) to analyze the distribution of SHH-expressing cells in the prenatal human forebrain. We studied a wide spectrum of gestational ages (8–40 gw), using cryo-sections from throughout the rostro-caudal brain axis. We also combined fluorescence ISH (FISH) with cell-type-specific immunostaining and identified the cell types that express this morphogen. Finally, we analyzed, for the first time, the expression pattern of the known SHH receptors and downstream effectors in comparison with SHH expression in the early and late corticogenesis. These results contribute to the better understanding of cortical development and point to the importance of further studies of SHH signaling in neuropsychiatric disorders.
Materials and methods
Human fetal brain tissue
Human fetal brains (n = 30) from 8 to 40 gw (gestational weeks correspond to postconceptional weeks; full term = 40 gw) (Table S1) were obtained from the Human Fetal Tissue Repository at the Albert Einstein College of Medicine (Bronx), Advanced Bioscience Resources (Alameda, CA), StemEx (Diamond Springs, CA, USA) and the joint MRC/Wellcome Trust-funded Human Developmental Biology Resource (http://www.hdbr.org) after legal abortions with appropriate maternal written consent and approval from the Ethics Committees of the participating institutions. All human materials were handled with special care and following all necessary requirements and regulations set by the Ethics Committee of the University of Connecticut and the Helsinki Declaration. Fetal age was estimated on the basis of weeks after last period, crown-rump length, and anatomical landmarks. In all studied fetuses, ultrasound and gross neuropathological examination were used to exclude those with brain pathology. The dissected tissues were fixed in 4% formaldehyde solution in 0.1 M phosphate buffer, cryoprotected by immersion in 30% sucrose, embedded in Tissue Tek (Sakura), frozen, and preserved in − 80 °C until needed. The tissues used for in situ hybridization (ISH) and immunohistochemistry were cut into 15-µm-thick sections.
In situ hybridization
The human full-coding sequences (CDS) for GLI1, GLI2, GLI3, and PTCH 1 were purchased from Dharmacon. The human SHH CDS was a gift from Cliff Tabin (Marigo et al. 1995) (pBS hShh CT#401 Addgene # 13996). For the SMO, BOC, GAS1, and CDON probes, cDNAs from human fetal brain (18 gw) were used as templates. The riboprobes were generated from a PCR fragment containing the transcription promoter sites T3/T7/SP6 (see Table S2), by in vitro transcription using digoxigenin (DIG)-UTP (Roche) as the label. ISH was performed as previously described (Radonjic et al. 2014). Briefly, cryo-sections (15 µm) were dried at room temperature (RT) for 2 h, fixed for 10 min with 4% paraformaldehyde (PFA), and washed twice in diethyl pyrocarbonate (DEPC)-treated phosphate buffer solution (PBS) before overnight incubation at 70 °C in hybridization buffer containing 1 × DEPC-treated “salts” (200 mM NaCl, 5 mM EDTA, 10 mM Tris, pH 7.5, 5 mM NaH2PO4·2H2O, 5 mM Na2HPO4; Sigma-Aldrich), 50% deionized formamide (Roche), 0.1 mg/mL of RNase-free yeast tRNA (Invitrogen, Carlsbad, CA, USA), 1 × Denhardts (RNase/DNase-free; Invitrogen), 10% dextran sulfate (Sigma-Aldrich) containing 100–500 ngmL of digoxigenin (DIG)-labeled RNA probe. After hybridization, the sections were washed three times in a solution containing 50% formamide 1 × SSC (saline-sodium citrate, Invitrogen) and 0.1% Tween 20 (Sigma-Aldrich) at 65 °C, and twice at RT in 1 × MABT (20 mM maleic acid, 30 mM NaCl, 0.1% Tween 20; Sigma-Aldrich) before incubating in a solution containing 2% blocking reagent (Roche) and 10% heat-inactivated sheep serum in MABT, followed by overnight incubation in alkaline-phosphatase-conjugated anti-DIG antibody (1:1500; Roche). Fast Red (Roche) was used for the fluorescent colorimetric detection of probe (FISH) by incubation in 100 mM Tris, pH 8.2, 400 mM NaCl containing Fast Red for 1–2 h at 37 °C. Alternatively, the TSA (tyramide signal amplification) kit (Perkinelmer) was used for FISH, in combination with anti-DIG-POD antibody (1:500, Roche). The sections were counterstained with bis-benzimide and cover-slipped using Fluoromount G mounting medium. The specificity of the procedure was assessed with a probe corresponding to the sense strand of the respective genes.
Immunohistochemistry after ISH
After ISH, sections were subjected to antigen retrieval in 0.1 M citric acid, pH 9.0, and blocked in 10% normal goat serum (NGS)/PBS containing 0.2% Triton (PBST). Following overnight incubation with primary antibody (see Table S3 for details), the sections were thoroughly washed in PBST and incubated with secondary Alexa 488- or Alexa 555-conjugated antibodies (Life Technologies) for 2 h at RT. Alternatively, an ABC kit was used followed by DAB development. Nuclei were counterstained for 5 min at RT with the nuclear stain bis-benzimide (Sigma).
Quantification of immunolabeled cells
Immunolabeled sections were visualized with an Axioscope microscope (Zeiss) equipped with Axiovision software and photographed using a digital camera. Three samples (22–24 gw) were used for the quantifications presented in Figs. 4 and 5. Nuclear staining allowed the delineation of areas of interest (e.g., CP, SVZ, IZ, and GE). Ten images for each region of interest were observed at 40× magnification and the number of cells was counted using the Photoshop CS6 Count Tool. A descriptive analysis of the data was performed using the Excel Data analysis plug-in. The percentages of double-positive cells expressing SHH are presented together with the standard error of the mean (SEM).
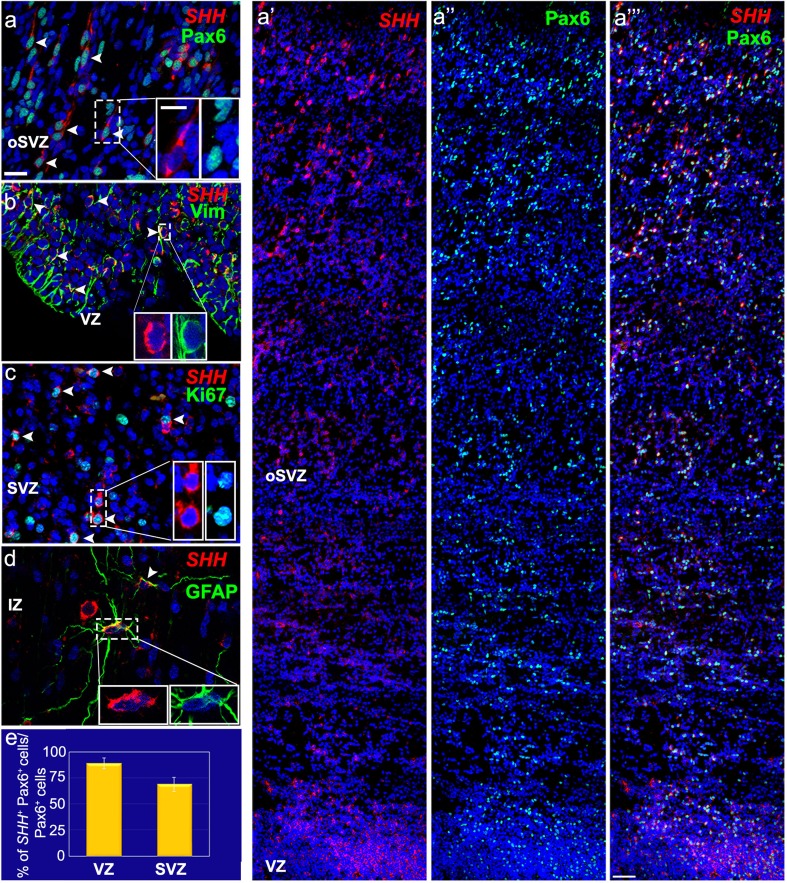
Fig. 4.
Cell-type-specific expression of SHH transcript in the 18–24-gw fetal brain. a′–a‴ Combined ISH for SHH (red) and immunostaining for Pax6 (green) show numerous radial glial cells (RGCs) that co-localize SHH transcript and Pax6 in the proliferative zones (VZ and subventricular zone, SVZ). a-inset Higher magnification of co-labeled cells in the outer SVZ (oSVZ) (arrowheads). b Vimentin staining for RGCs (green) after ISH for SHH (red) confirms that RGCs in the VZ express SHH (arrowheads). c Numerous proliferating SHH + cells, indicated by Ki67 co-expression. The inset shows a higher magnification of the boxed area. d Mature astrocytes (GFAP+) in the IZ/SP express SHH mRNA. e Percentage of total Pax6+ cells that co-express SHH (red) in the VZ (89% ±5.19 SEM) and SVZ (69% ± 6.6 SEM) of 22–24-gw tissues (n = 3). Scale bars: a 25 µm, a-inset 10 µm, a‴ 50 µm
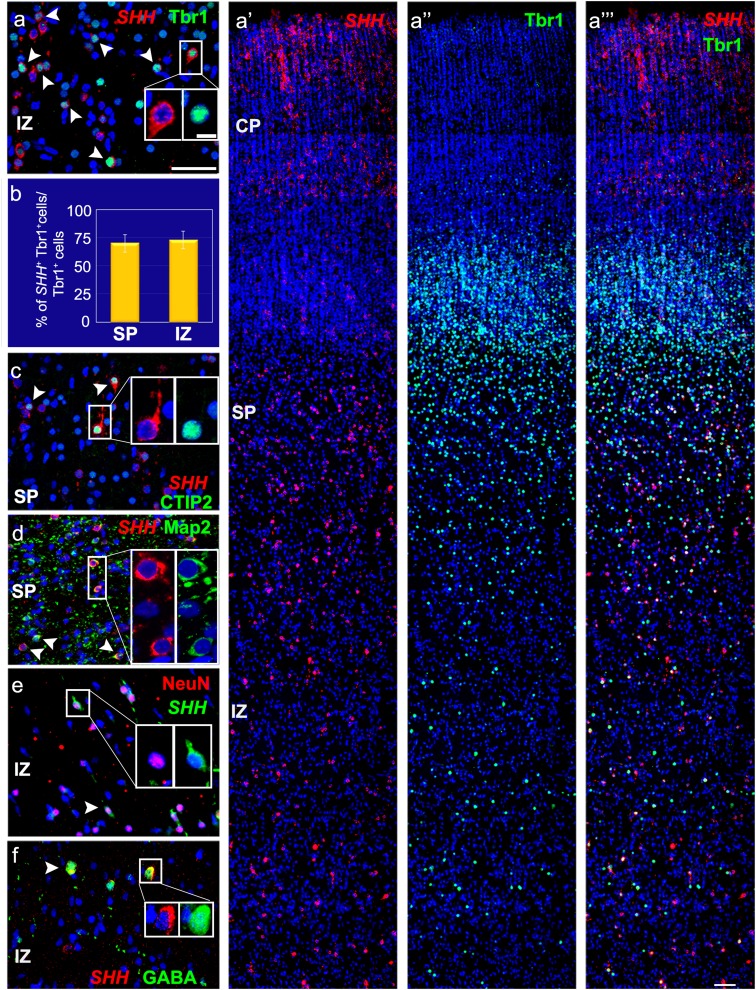
Fig. 5.
Various subtypes of neurons express SHH in the 18–24 gw cortex. a–a‴ Co-labeling for SHH mRNA (red) and Tbr1 (green) reveals many postmitotic projection neurons across the SP and IZ expressing SHH. a′, a″ Single channels. b Percentage of Tbr1+ cells expressing SHH in the SP (70% ±7.6 SEM) and IZ (73% ±7.8 SEM) in 22–24-gw sections (n = 3). c–f SHH is expressed by neurons labeled with CTIP2 (c), MAP2 (d), NeuN (e), and GABA (f). Scale bars: a and a‴ 50 µm, a-inset 10 µm
Results
Distribution of SHH mRNA in the developing human forebrain
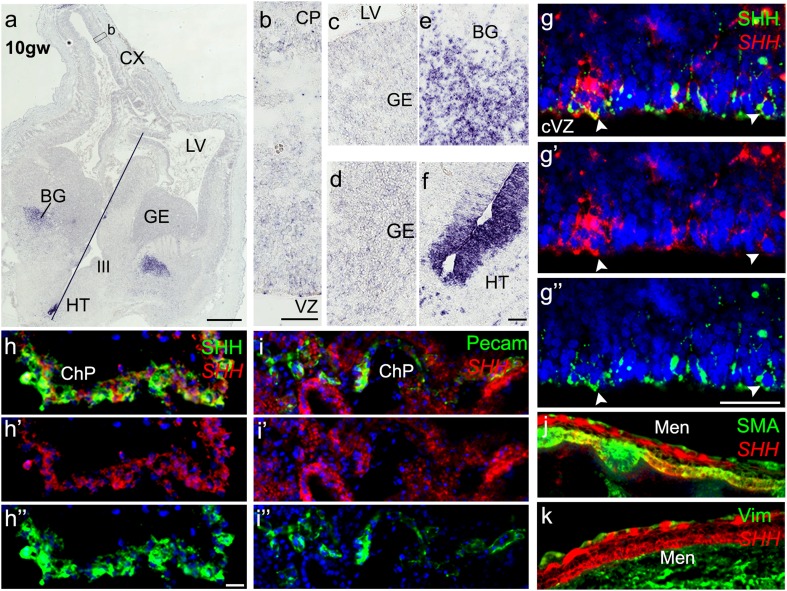
Detailed information on the spatial distribution of SHH in the human developing brain is lacking, in part due to the limited availability of tissue and the failure of most commercially available antibodies to label SHH. We thus used ISH against the human coding sequence of SHH to follow expression during 7 months of the human gestational period (8–40 gw; Table S1). The expression of SHH was highly dynamic, in accordance with the transcriptomics data available from the Allen Brain Institute (Fig. S1a, b). SHH expression in the fetal forebrain increased over the course of development and shifted from the proliferative ventricular (VZ) and subventricular zones (SVZ) (Fig. 1) to the overlying cortical layers (Fig. 2). At the earliest age studied (8–10 gw), Shh mRNA was predominantly restricted to the ventral forebrain, including the midline of the hypothalamus and thalamus, and to the emerging basal ganglia (Fig. 1a, e, f), in a similar pattern detected in the early embryonic mouse brain (Fig. S6a). By contrast, SHH mRNA was only weakly expressed dorsally, in the cortical plate (CP) and cortical VZ (Fig. 1a, b), a result confirmed by TSA-FISH (tyramide signal amplification) and the absence of a signal with the sense probe (Fig. 1g′ and S5). Furthermore, immunostaining with the rabbit monoclonal anti-SHH antibody (Farmer et al. 2016) revealed the definite presence of SHH protein in this area (Fig. 1g, g′′ and Fig. S1c–c′′). The proximal choroid plexus (ChP) was also positive for the SHH transcript and protein (Fig. 1h–h′′). Notably, the levels of SHH were much higher in ChP cells than in the nearby VZ, as observed by the differences in signal intensity at the different exposure times/settings (Fig. S1c–c′′). Thus, it cannot be ruled out that the positive signal for the SHH protein in the cortical VZ reflected the uptake of ChP-secreted SHH by RGCs. However, SHH immunostaining in the cortical VZ appeared in more frontal sections that are much further from the ChP (not present in the same section), suggesting that SHH is produced locally.
Fig. 1.
Expression of Sonic Hedgehog (SHH) in the human brain at 10 gw. a Coronal sections through both cerebral hemispheres show SHH mRNA signal in the diencephalon (hypothalamus, HT) and basal ganglia (BG). The line indicates the midline. b Low-level SHH expression in the developing cortex, mainly in the ventricular zone (VZ) and cortical plate (CP). c, d Scattered cells weakly expressing Shh in the VZ of the ganglionic eminence (GE) (c) and in other regions of the GE (d). e, f High-level expression in the future basal ganglia (e) and hypothalamic midline (f). g–g″ Both SHH protein (green) and transcript (red) are demonstrated in the cortical VZ in radial glial cells (arrowheads). h Choroid plexus expresses SHH mRNA (red) and protein (green); single channels (h–h″). i Pecam (CD31) immunostaining shows that endothelial cells in the choroid plexus do not express SHH mRNA. j SHH-expressing cells stain with antibody to smooth-muscle actin (green) but not vimentin (green) (k). Scale bars: a 1 mm, b, f and g 50 µm, h 20 µm
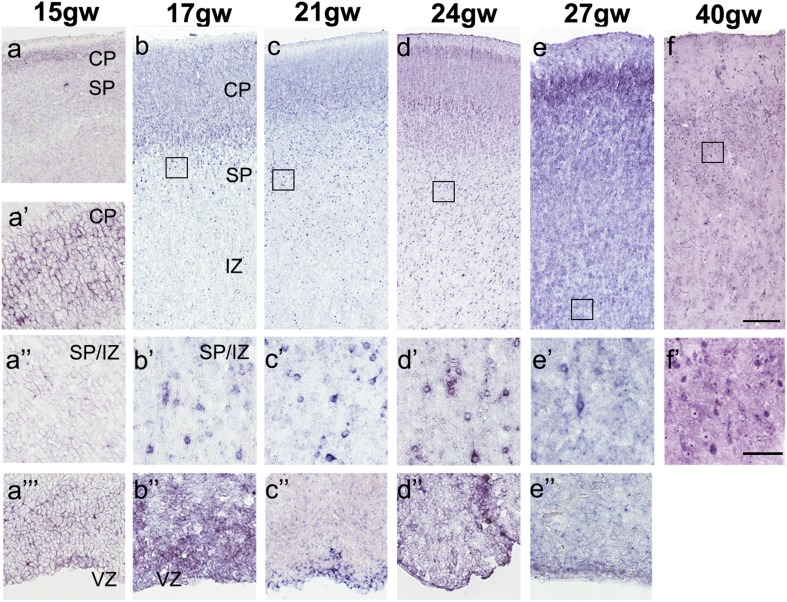
Fig. 2.
Spatiotemporal changes in SHH expression in the human cerebral cortex between 15 and 40 (newborn) gw. a–f Low magnification of the cortical column at the indicated age. a″–f′ High magnification of cells in the subplate (SP)/intermediate zone (IZ) area (indicated boxes). a‴–e″ High magnification of cells in the cortical VZ (except at 40 gw,when there is no VZ). CP cortical plate. Scale bars : f 200 µm, f′ 50 µm
Another source of SHH protein in the developing human brain at this stage is the meninges, which were strongly positive for SHH mRNA and protein. Within the meninges, SHH-expressing cells were those of smooth-muscle lineage, rather than fibroblasts, as demonstrated by co-localization with smooth-muscle actin but not vimentin antibody (Fig. 1j, k). Finally, SHH mRNA was detected in the endothelial cells of blood vessels in the GE stained for the endothelial cell marker PECAM (CD31), indicating that the developing vasculature is an additional source of SHH (Fig. S1d–d′′). Notably, endothelial cells in the ChP did not express SHH (Fig. 1i). Extracortically, SHH mRNA co-localized with SHH protein in the hypothalamic midline and retinal ganglion cells. In addition to validating the specificity of the antibody, this result provided insight into the range of SHH diffusion from its source (Fig. S1e–e′′, f–f′′).
In the subsequent stages of development (15–17 gw), the density and distribution of SHH transcripts increased considerably throughout the forebrain (Fig. 2a, b; Fig. S2a). Strong signal was detected not only in the cortical VZ/SVZ (Fig. 2a′′′, b′′; Fig. S2a), but also in the cortical plate (CP) (Fig. 2a′, b; Fig. S2a), whereas the signal in the intermediate zone (IZ) and subplate (SP) increased dramatically from 15 to 17 gw (Fig. 2a′, b′).
In the next stage of development, at mid-gestation (18–24 gw), SHH expression increased steadily in subpopulations of cells in the expanded outer SVZ (oSVZ) (Figs. 3a, 6f), and in the IZ and SP, the zones through which neurons migrate (Figs. 2, 3a). At the same time, a very strong signal was present in cells located in the ganglionic eminence (GE) and hippocampus (Fig. 6j, S7c and Fig. S3). From 24 gw onwards, the SHH transcript signal in the cVZ decreased progressively, coinciding with a reduction in the size of these structures in late fetal development. This is the first study demonstrating SHH expression in the GE and cVZ| in stages after 14 gw. SHH expression in the upper cortical layers remained high between 24 and 27 gw and persisted in the newborn (40 gw), in which SHH-expressing cells were found in all layers of the cerebral cortex (Fig. 2) and in the hippocampus (Fig. S3). A similar expression pattern was detected in mouse postnatal day 4 (P4) brain (Fig.S7d–d′′).
Fig. 3.
Gradients of SHH mRNA in 23–24 gw brains. a Coronal sections from three tissue blocks (1–3) from frontal to occipital pole show rostro-caudal gradient of SHH mRNA expression. In addition, dorsal areas show higher expression than ventral in each block. b In a single coronal section, the expression of SHH transcripts shows pronounced differences (1–7). Boxed ares show higher magnification along the cerebral cortex. 1 Cingulum; 2–3 dorsal cortex, future somatosensory or motor areas; 4 frontal, preinsular cortex; 5 insula; 6–7 temporal cortex. Scale bars: a 50 µm, b 2 mm, (1) 250 µm and inset 50 µm
Fig. 6.
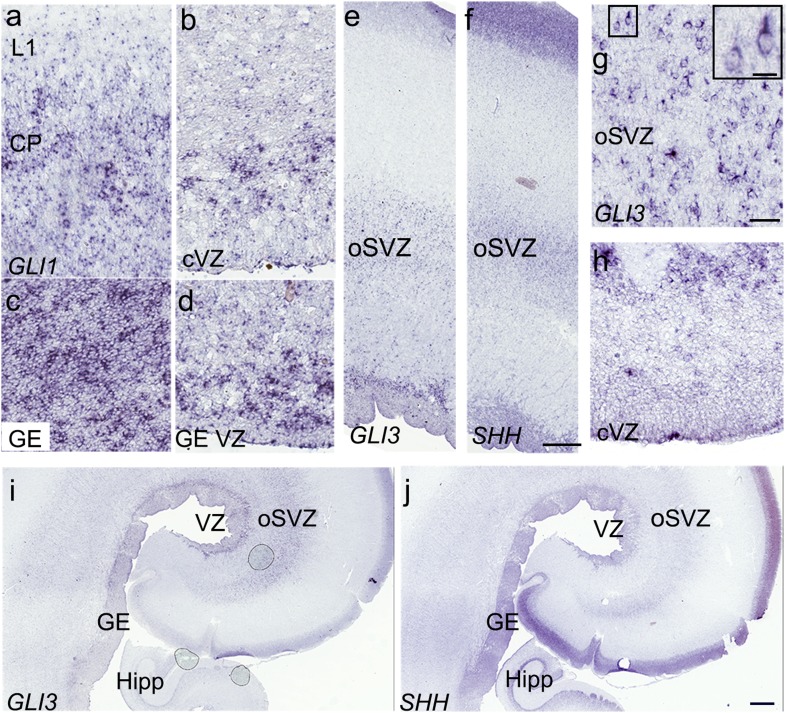
Components of the SHH-signaling pathway are expressed in the human fetal cortex at 22 gw. a, b ISH reveals the cortical expression of GLI1 (CP and VZ), indicative of SHH-signaling activity in these areas. c, d Strong GLI1 expression in the GE suggests higher levels of SHH activity ventrally. e, f GLI3 and SHH transcripts show similar high-density signals in the VZ and oSVZ. g On higher magnification, GLI3+cells in the oSVZ resemble RGCs and are more numerous than in the VZ (h). i, j Comparison of the GLI3 and SHH expression patterns in adjacent coronal sections at the level of the hippocampus (Hipp) of the 22 gw fetal brain. Scale bars: f 500 µm, g 50 µm, g- inset 15 µm, j 1 mm
We then examined whether SHH transcripts were present uniformly along the rostro-caudal and dorso-ventral axis or formed a gradient in these regions of the human fetal cortex. ISH performed in sagittal sections of 15-gw forebrain showed that, although SHH expression appeared slightly higher in the rostral and caudal cortical areas than in the dorsal and ventral areas, the difference over the entire cortex was not significant (Fig. S2).
However, in later developmental stages (21–24 gw), SHH expression exhibited a high rostral to lower caudal gradient, as illustrated in coronal sections prepared from rostral (frontal), medial, and caudal (occipital) tissue blocks of the 23-gw fetal forebrain (Fig. 3a). Moreover, within each tissue block, SHH signal intensity was consistently stronger in dorsal than in ventral cortical areas (Fig. 3a).
In addition, cortical SHH expression exhibited regional differences, as illustrated in single coronal sections prepared through the level of the thalamus. The signal was always stronger above the insular region of the cortex, in the prospective somatosensory/motor cortices and possibly including Broca’s area (Fig. 3b:1–4), than in the ventrally positioned temporal cortex (Fig. 3b:6–7). This pattern, which first emerged around 19 gw and became prominent at 22–24 gw, confirms the transcriptome data (Fig. S1a, b) that suggest higher expression in the dorsal than ventral cortex for this developmental stage.
These results revealed the complex pattern of SHH expression in the developing cortex and thus a possible role for SHH in cortical arealization during the second and third trimesters of gestation.
Characterization of SHH-expressing cells during mid-gestation
After establishing that SHH mRNA is expressed widely in fetal brain, we asked which cell types express SHH in the developing human cortex. The answer to this question is important, because it might indicate the roles played by SHH during development. We, therefore, subjected the fetal tissue sections to FISH followed by immunohistochemistry with cell-type-specific markers. Specifically, we asked whether during the late second trimester of gestation (20–24 gw). SHH transcripts are expressed by cortical progenitors, that is, RGCs, which are the predominant cell type in the proliferative VZ/SVZ. Using the RGCs markers Pax6, GFAP, and vimentin, we identified cells that co-express SHH mRNA as well as these markers (Fig. 4). Moreover, many of the SHH-expressing cells were proliferating, based on their co-labeling for the proliferation marker Ki67 (Fig. 4c). Quantification of the double-labeled cells showed that 89% (± 5.19 SEM, n = 3) of the Pax6-positive cells in the VZ expressed SHH transcripts, compared to 69% (± 6.6 SEM, n = 3) in the SVZ; thus, at this developmental stage the majority of RGCs in the proliferative zones produced SHH (Fig. 4e).
Since progenitors in the fetal cortex give rise mainly to glutamatergic neurons, SHH-expressing cells in cortical layers were probed with the glutamatergic cell marker Tbr1 (T-box Brain 1) to determine whether they belong to this neuronal population (Fig. 5a–a′′′, b). From the total population of Tbr1+ cells in the subplate and the IZ, around 70% (n = 3) were positive for SHH (Fig. 5b). Thus, in addition to RGCs in the VZ/SVZ, a subpopulation of glutamatergic neurons in cortical layers is source of SHH. This finding was confirmed using another marker for deep cortical layer neurons, CTIP2 (Fig. 5c). Two more general neuronal markers, Map2 and NeuN, were also co-expressed with SHH transcripts in a subpopulation of cortical neurons (Fig. 5d, e).
Co-labeling with GABA (Fig. 5f) or Gad65/67 (Fig. S4a, a′) suggested that at mid-gestation, cortical interneurons are less likely to express SHH transcript than glutamatergic neurons. Since not all SHH mRNA+ cells overlap with neuronal markers in higher cortical layers, we investigated whether non-neuronal populations in the cortex also express SHH transcript. Indeed, GFAP+ astrocytes in the IZ and SP/CP expressed SHH (Fig. 4d), but not oligodendrocytes or microglia labeled with Olig2 or Iba1, respectively (Fig. S4b, c). These data are consistent with a heterogeneous cell population producing Shh during mid-gestation, with the two main sources being RGCs and glutamatergic neurons.
SHH-signaling pathway in the developing human cortex
Despite the demonstration of the widespread expression of SHH in the developing human cortex, whether the classical SHH pathway was activated remained to be tested. Gli1 (GLI zinc finger transcription factor), a transcription factor activated by Shh-signaling, is the most sensitive and reliable read-out for this pathway as, unlike Gli2 and Gli3, it acts only as an activator (Bai et al. 2004). The presence of GLI1 would, therefore, provide proof that the expressed SHH activates the signaling pathway in nearby cells. ISH using the human GLI1 probes on sections from fetuses age 19–24 gw found that the GLI1 transcript was expressed in the cortical VZ and CP/SP as well as in the GE (Fig. 6a–d). The intensity of the GE signal was much stronger, indicating a higher level of SHH expression, and hence SHH-signaling activity, in this area. GLI3, a transcription factor that acts as both a repressor and an activator of the Shh pathway (Ingham and McMahon 2001), was highly expressed in the cortical VZ, in the inner and outer SVZ (Fig. 6e, g–h), and in the hippocampus (Fig. 6i), confirming previously published transcriptomics data showing that GLI3 is expressed by human RGCs (Pollen et al. 2015).
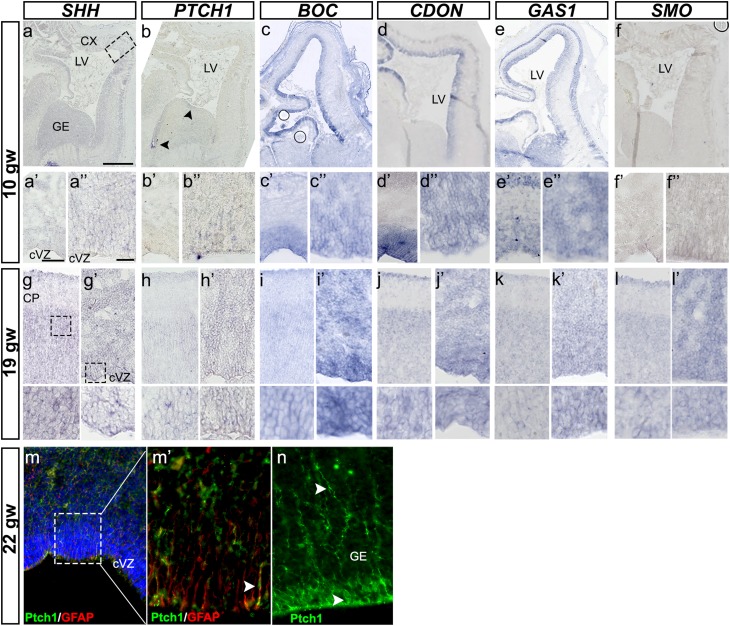
We then asked whether the SHH receptor PATCHED1 (PTCH1) is expressed in the vicinity of SHH-expressing cells. We tested tissues from fetuses of two gestational ages, early (10 gw), when cell proliferation, migration, and neurogenesis/fate specification are predominant, and later, at mid-gestation (19–24 gw), when additional processes such as axonal guidance and synaptogenesis occur. At 10 gw, PTCH1 transcripts were non-detectable in the neocortex (Fig. 7b, b′), while expression was low in the VZ of the GE and in the thalamic and hypothalamic neuroepithelium, paralleling the spatiotemporal pattern of strong SHH expression in these ventral regions. At later stages (19–24 gw), when SHH expression increased in the cortex, PTCH1 was seen in the cortical VZ, although at much lower levels than in ventral areas such as the GE or hypothalamus (Fig. 7h, h′). Immunostaining with PTCH1 antibody verified low- and high-level expression in the cortical VZ and GE, respectively (Fig. 7m, n). Co-labeling with GFAP also demonstrated the expression of this receptor by RGCs in the cortical VZ (Fig. 7m′). A weak PTCH1 mRNA signal was detected in the dentate gyrus of the hippocampus (Fig. S3h).
Fig. 7.
Expression of SHH receptors in the fetal human brain at 10 and 19 gw. a–a″ ISH for SHH on a coronal section from a 10-gw brain, as a reference for all SHH receptors in sections from the same fetus. Higher magnification of the boxed areas in (a): a′ cortex, a″ cVZ. Weak signal is detected in the cortical VZ and GE. b–b″ ISH for PTCH1 receptor reveals very low expression in the VZ (b″). BOC expression is much more prominent along the cortical VZ and GE VZ (c–c″), whereas CDON expression is restricted to the cortical VZ (d–d″). GAS1 is the only receptor expressed in both the VZ and CP, in addition to the GE (e–e″). SMOOTHENED (SMO) is hardly detected in the VZ at this stage (f–f″). g–l′ SHH and all its receptors in contiguous sections of 19-gw brain. Two areas are presented for each gene: CP and VZ/SVZ. m Co-staining of 22-gw brain with Patched1 and GFAP antibodies shows that RGCs in the cortical VZ express this receptor. m′ Higher magnification of the boxed area in (m). n Ptch1 immunoreaction in the same section reveals a strong expression in the GE. a 1 mm, a′ 150 µm, a″ 25 µm
In addition to Ptch1, three other membrane-associated proteins, the structurally related Boc (biregional Cdon-binding) and Cdon (cell-adhesion-molecule-related/down-regulated by oncogenes) proteins and the vertebrate-only Gas1 (growth-arrest-specific 1) protein, are thought to function as positive modulators of Shh signaling by enhancing the presentation of Shh to its receptor, Ptch1 (Tenzen et al. 2006; Allen et al. 2007; Martinelli and Fan 2007). Both Boc and Cdon promote Shh-dependent cell-fate specification and axon guidance (Tenzen et al. 2006; Okada et al. 2006), while Gas1 regulates the ventral specification of neural tube progenitors (Allen et al. 2007; Martinelli and Fan 2007) and CGNP (cerebellar granule neural progenitors) proliferation (Liu et al. 2001). Boc, Gas1, and Cdon are required for successful Shh signaling (Izzi et al. 2011). We found that BOC and GAS1 were strongly expressed at 10 gw in cortical VZ and GE, whereas CDON was present only in the cortical VZ (Fig. 7c–e). GAS1 was also expressed in the CP at this stage. The ChP cells, however, express only BOC at 10 gw (Fig. S8b). At later stages (19–24 gw), the expression of BOC and CDON was similar to that in earlier stages: high in the cortical VZ and SVZ and low in the CP/SP and GE (Fig. 7i–i′, j–j′). GAS1 expression in the CP was lower than at 10 gw, but remained high in the cortical VZ, oSVZ, and GE (Fig. 7k–k′). Finally, expression of the SHH signal transducer, SMO (smoothened) was weak in the 10-gw brain (Fig. 7f, f′′), but increased in the 19-gw cortical VZ and in the CP (Fig. 7l–l′). SMO expression was also detected in the 10-gw ChP (Fig. S8a). Thus, in summary, all three receptors (BOC, CDON, and GAS1) were expressed in the VZ during early fetal development, pointing to their role in cell proliferation. CDON and GAS1 were expressed at higher levels than BOC in the CP, suggesting their additional roles in cortical development.
Discussion
SHH is crucial for human brain development and changes in its signaling lead to distinct neuropathologies (Heussler et al. 2002; Nanni et al. 1999; Belloni et al. 1996; Odent et al. 1999; Santiago et al. 2006; Currier et al. 2012). Despite its importance, information on SHH expression profile in the developing human brain is still fragmentary. Here, we provided evidence of the expression of SHH and the transcription factors, and receptors necessary for its signaling, in specific cortical layers and cell types. Our study spans the course of most of the gestational period. The spatiotemporal distribution suggests the involvement of SHH in diverse developmental processes in the human telencephalon, including cell proliferation and cell-fate specification in the VZ/SVZ, the subsequent migration of newly generated neurons, synaptogenesis, and circuit formation.
Our results agree with those of the previous studies (Odent et al. 1999), in which SHH mRNA was mostly detected in the ventral structures of the human brain during early stages (8–10 gw), with very low signal in the developing cerebral cortex. Given that a high concentration of SHH is known to induce patterning and cell-fate specification, whereas low levels regulate proliferation (Komada 2012; Komada et al. 2008; Wang et al. 2016a, b) this pattern of signal localization suggests that SHH regulates patterning and cell-fate specification in the human ventral forebrain and proliferation in the dorsal forebrain. SHH transcripts were clearly detected in the cortical VZ/SVZ at 15 gw, which is consistent with our previous in vitro results showing that this morphogen is secreted by dorsal RGCs and induces their proliferation (Radonjic et al. 2016). The function of Shh in proliferation and regulation of symmetric/asymmetric divisions of intermediate progenitors in mice (Dave et al. 2011) points to a role as a mitogen in the human cortical proliferative zone. This role is probably maintained even past mid-gestation, since neurogenesis in humans continues up to 27 gw, later than previously known (Malik et al. 2013). Between 15 and 27 gw, SHH was also expressed in the IZ, SP, and CP, where the intensity of the signal increased over time. Furthermore, we observed robust inter-regional and areal differences in the expression of SHH during mid-to-late gestation in fetal neocortex. Although it is difficult to interpret this spatiotemporal expression pattern, these differences most likely have biological origin and they are not due to tissue quality or other variation as observed in all cases studied. The SHH-positive cells in developing cortical layers were mostly neurons expressing Map2 or NeuN, with a subpopulation expressing glutamatergic markers (Tbr1 or CTIP2), as previously reported for the early postnatal mouse brain (Harwell et al. 2012). The SHH transcript was also identified in a subpopulation of GABAergic cells, in accordance with the results of a single study in mouse (Komada et al. 2008). Given that Shh in mice plays a role in neuronal migration, axonal guidance (Fuccillo et al. 2006; Baudoin et al. 2012; Bourikas et al. 2005; Charron et al. 2003; Yam et al. 2009), and synaptic connectivity (Harwell et al. 2012), it is likely that it has similar functions in the developing human fetal neocortex. Moreover, the finding that GFAP-labeled astrocytes in the upper IZ, SP, and CP were positive for SHH suggests a role in numerous processes in which astrocytes are involved, such as synapse formation and synaptic plasticity (Farmer et al. 2016; Eroglu and Barres 2010) as well as the formation and maintenance of the blood–brain barrier (BBB). Indeed, recent work demonstrated that Hedgehog signaling promoted the formation and integrity of the BBB as well as the immune quiescence of the central nervous system (Alvarez et al. 2011). Microglia and oligodendrocytes were negative for SHH, but Olig2+ cells were found in close proximity to SHH-expressing cells, which correlates well with our previous in vitro study showing that SHH promotes the generation and maintenance of forebrain Olig2 progenitors (Ortega et al. 2013).
Our finding of GLI1 in the fetal cortical VZ and CP suggests active SHH signaling in the developing human cortex. This result is in line with our previous demonstration of functional SHH signaling in cortical RGCs in vitro, as indicated by the increased levels of GLI1 and PTCH1 after SHH treatment (Radonjic et al. 2016). In the present study, we provided evidence of PTCH1 (protein) as well as BOC, GAS1, and CDON expression in the human RGCs of the cortical VZ, which suggest an autocrine function for SHH in these progenitors. The role of these receptors has not been studied in cortical development, but they are known as positive modulators of Shh signaling in mice, in both cell proliferation in the cerebellum (Liu et al. 2001; Izzi et al. 2011) and cell-fate specification in neural tube progenitors (Allen et al. 2007; Martinelli and Fan 2007). A recent study has shown that Boc, Cdon, and Gas1 are necessary components of the Shh receptor complex and essential in Shh signal transduction in vertebrates (Izzi et al. 2011). Their strong expression in the human cortical VZ in association with the low-level expression of SHH and PTCH1 during early stages (10 gw) suggests that they could act as enhancers of SHH signaling. Impaired function of BOC, CDON, and GAS1 appears to underlie holoprosencephaly, both in humans (Clement et al. 2007; Ribeiro et al. 2010; Pineda-Alvarez et al. 2012; Bae et al. 2011) and in mice (Seppala et al. 2007; Zhang et al. 2006; McLellan et al. 2008). Interestingly, we found that BOC is strongly expressed in the human, but not in the mouse cortical VZ (Fig. S6, brainatlas.org), suggesting a species-specific difference. Indeed, comparative epigenetic profiling of human, monkey, and mouse brain tissue identified epigenetic gains (promoters and enhancers with gained activity) in genes involved in human corticogenesis, including BOC, SHH, NKX2.1, PTCH1, and GLI3 (Reilly et al. 2015).
Recent studies have suggested that the transcriptional programs associated with interneuron development in human are very similar in the GE and cortical VZ (Miller et al. 2014), which points to a role for SHH in interneuron fate specification, in addition to cell proliferation, in the cortical VZ. Indeed, our previous in vitro results show that SHH affects the commitment of some cortical RGCs to interneuronal fate (Radonjic et al. 2016). This points to the need for a better understanding of the origin and development of human cortical interneurons (Radonjic et al. 2014; Alzu’bi et al. 2017; Clowry et al. 2010). Such studies are likely to provide important insights into the pathogenesis of human neuropsychiatric disorders such as schizophrenia, in which dysfunction of GABAergic interneurons has been implicated (Benes and Berretta 2001; Guidotti et al. 2005; Lewis et al. 2005; Selemon and Zecevic 2015).
In conclusion, the present study fills the gap in our knowledge about the presence of SHH in the developing human cortex which may enhance our understanding of human corticogenesis and the pathologies associated with defective SHH signaling.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1 a RNA-seq data from http://www.brainspan.org/rnaseq/ show the dynamic expression of Sonic Hedgehog (SHH) during brain development in utero. The different cortical areas were grouped in “dorsal” and “ventral” groups to show that during 18–22 gw SHH expression is higher in dorsal than in ventral regions. b The same data, grouped in the different cortical areas, show the changes in SHH expression per area along the gestational timeline. c–c″ Three different exposure times highlight the difference in SHH expression levels between choroid plexus (ChP) cells and the RGCs in the proximal ventricular zone (VZ; arrows). d–d″ SHH transcript is expressed by blood vessel endothelial cells labelled with the endothelial marker PECAM in the ganglionic eminence (GE) at 10 gw. e–e″ Co-labeling for SHH mRNA (red) and protein (green) in the hypothalamic midline reveals the specificity of the antibody and the range of diffusion of the secreted protein.f–f″ Expression of both SHH protein (green) and transcript (red) in the human fetal retina confirms the specificity of the antibody in humans, as previously shown in the mouse (TIF 14429 KB)
Fig. S2 Gradients of SHH mRNA in 15-gw brain. a A sagittal section of a 15-gw forebrain shows a slight rostro-caudal gradient, best seen on higher magnification of the boxed areas presented on the right (TIF 7220 KB)
Fig. S3 Expression of SHH in the human fetal hippocampus. a–c Distribution of SHH transcripts in the hippocampus of 17-, 22-, and 40-gw tissue. b′–b″″ Higher magnification of the boxed areas illustrated in (b) shows SHH expression in the different areas of the 22-gw hippocampus. d, e Fluorescence ISH for SHH and Pax6 staining reveal that only some of Pax6+ cells in dentate gyrus (DG) and CA1 co-express SHH in the 22-gw hippocampus. f SHH is expressed by Tbr1+ cells in the DG of the 22-gw hippocampus. f′ Higher magnification of the double-positive cells in (f). g–l Expression of SHH receptors and downstream molecules in the 19-gw hippocampus shown in contiguous sections. Scale bars a, b 1mm, b′ 100µm, f′ 50µm (TIF 7928 KB)
Fig. S4 a Coronal medial section of the 21-gw fetal brain stained for SHH mRNA (blue) and Gad67 protein (brown) reveals co-labeled cells. a′ Higher magnification of the boxed area in (a). b Double-positive cells are not seen in a tissue section from a 23-gw brain treated for SHH (red) followed by Olig2 staining (green). b′, b″ Higher magnification of the interventricular zone (IZ) and subplate (SP) areas. c Microglial cells (Iba1, light blue) and SHH mRNA (red) staining do not co-label cells in the 10-gw human cortex. Scale bars: a 150µm, a′ 100µm, b′ 50µm (TIF 3754 KB)
Fig. S5 Sense/control in situ for SHH in 10- and 19-gw tissue. Scale bars: 2mm (TIF 9398 KB)
Fig. S6 Expression pattern of Shh-signaling genes in the embryonic mouse brain. Data obtained from the Allen Brain Atlas (TIF 11830 KB)
Fig. S7 In situ hybridizations on mouse brain tissue with the human SHH antisense (AS) and sense (S) probe. Only (b) was probed with the sense probe. Scale bars: a 400µm, c 500µm, c’ 100µm, d 1mm, d″ 150µm (TIF 5166 KB)
Fig. S8 Expression of SMO and SHH receptors in the 10 gw Choroid Plexus. a SMO expression, b BOC, c GAS1 and d CDON. Scale bar: 50µm (TIF 5016 KB)
Acknowledgements
This work was supported by NIH Grants 2R01NSO41489 and Subcontract 5R01DA023999-07(NZ). Human fetal tissue was procured from Advanced Bioscience Resources (ABR, Alameda, CA), and the Joint Medical Research Council/Welcome Trust (Grant No. 099175/Z/12/Z Human Developmental Biology Resource http://hdbr.org), Newcastle upon Tyne, England. We are grateful to Dr Suzie Scales (Genetech) for the Shh antibody, Dr Cliff Tabin (Harvard) for the SHH plasmid and Dr Bill Andrews (UCL) for critical reading of the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Tissue was obtained from the Human Fetal Tissue Repository at the Albert Einstein College of Medicine (Bronx), Advanced Bioscience Resources (Alameda, CA), StemEx (Diamond Springs, CA, USA) and the joint MRC/Wellcome Trust-funded Human Developmental Biology Resource (http://www.hdbr.org) after legal abortions with informed maternal written consent and approval from the Ethics Committees of the participating institutions. All human material was handled with special care and in accordance with the ethical standards set by the Ethics Committee of the University of Connecticut and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This work was funded by NIH Grants 2R01NSO41489 and Subcontract 5R01DA023999-07(NZ).
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s00429-018-1621-5) contains supplementary material, which is available to authorized users.
Contributor Information
Fani Memi, Email: fani.memi@ucl.ac.uk.
Nevena Radonjić, Email: radonjic@uchc.edu.
References
- Allen BL, Tenzen T, McMahon AP. The Hedgehog-binding proteins Gas1 and Cdo cooperate to positively regulate Shh signaling during mouse development. Genes Dev. 2007;21(10):1244–1257. doi: 10.1101/gad.1543607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bourbonniere L, Bernard M, van Horssen J, de Vries HE, Charron F, Prat A. The Hedgehog pathway promotes blood–brain barrier integrity and CNS immune quiescence. Science. 2011;334(6063):1727–1731. doi: 10.1126/science.1206936. [DOI] [PubMed] [Google Scholar]
- Alzu'bi A, Lindsay S, Kerwin J, Looi SJ, Khalil F, Clowry CJ. Distinct cortical and sub-cortical neurogenic domains for GABAergic interneuron precursor transcription factors NKX2.1, OLIG2 and COUP-TFII in early fetal human telencephalon. Brain struct funct. 2017;222(5):2309–2328. doi: 10.1007/s00429-016-1343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae GU, Domene S, Roessler E, Schachter K, Kang JS, Muenke M, Krauss RS. Mutations in CDON, encoding a hedgehog receptor, result in holoprosencephaly and defective interactions with other hedgehog receptors. Am J Hum Genet. 2011;89(2):231–240. doi: 10.1016/j.ajhg.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai CB, Stephen D, Joyner AL. All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3. Dev cell. 2004;6(1):103–115. doi: 10.1016/S1534-5807(03)00394-0. [DOI] [PubMed] [Google Scholar]
- Baudoin JP, Viou L, Launay PS, Luccardini C, Espeso Gil S, Kiyasova V, Irinopoulou T, Alvarez C, Rio JP, Boudier T, Lechaire JP, Kessaris N, Spassky N, Metin C. Tangentially migrating neurons assemble a primary cilium that promotes their reorientation to the cortical plate. Neuron. 2012;76(6):1108–1122. doi: 10.1016/j.neuron.2012.10.027. [DOI] [PubMed] [Google Scholar]
- Belloni E, Muenke M, Roessler E, Traverso G, Siegel-Bartelt J, Frumkin A, Mitchell HF, Donis-Keller H, Helms C, Hing AV, Heng HH, Koop B, Martindale D, Rommens JM, Tsui LC, Scherer SW. Identification of sonic hedgehog as a candidate gene responsible for holoprosencephaly. Nat Genet. 1996;14(3):353–356. doi: 10.1038/ng1196-353. [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25(1):1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Betcheva ET, Yosifova AG, Mushiroda T, Kubo M, Takahashi A, Karachanak SK, Zaharieva IT, Hadjidekova SP, Dimova II, Vazharova RV, Stoyanov DS, Milanova VK, Tolev T, Kirov G, Kamatani N, Toncheva DI, Nakamura Y. Whole-genome-wide association study in the Bulgarian population reveals HHAT as schizophrenia susceptibility gene. Psychiatr Genet. 2013;23(1):11–19. doi: 10.1097/YPG.0b013e3283586343. [DOI] [PubMed] [Google Scholar]
- Bourikas D, Pekarik V, Baeriswyl T, Grunditz A, Sadhu R, Nardo M, Stoeckli ET. Sonic hedgehog guides commissural axons along the longitudinal axis of the spinal cord. Nat Neurosci. 2005;8(3):297–304. doi: 10.1038/nn1396. [DOI] [PubMed] [Google Scholar]
- Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113(1):11–23. doi: 10.1016/S0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking sonic hedgehog gene function. Nature. 1996;383(6599):407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17(2):165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowry G, Molnar Z, Rakic P. Renewed focus on the developing human neocortex. J Anat. 2010;27(4):276–288. doi: 10.1111/j.1469-7580.2010.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier DG, Polk RC, Reeves RH. A sonic hedgehog (Shh) response deficit in trisomic cells may be a common denominator for multiple features of Down syndrome. Prog Brain Res. 2012;197:223–236. doi: 10.1016/B978-0-444-54299-1.00011-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmane N, Sanchez P, Gitton Y, Palma V, Sun T, Beyna M, Weiner H, Ruiz i Altaba A. The sonic hedgehog–Gli pathway regulates dorsal brain growth and tumorigenesis. Development. 2001;128(24):5201–5212. doi: 10.1242/dev.128.24.5201. [DOI] [PubMed] [Google Scholar]
- Dave RK, Ellis T, Toumpas MC, Robson JP, Julian E, Adolphe C, Bartlett PF, Cooper HM, Reynolds BA, Wainwright BJ. Sonic hedgehog and notch signaling can cooperate to regulate neurogenic divisions of neocortical progenitors. PLoS One. 2011;6(2):e14680. doi: 10.1371/journal.pone.0014680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75(7):1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- Ericson J, Muhr J, Placzek M, Lints T, Jessell TM, Edlund T. Sonic hedgehog induces the differentiation of ventral forebrain neurons: a common signal for ventral patterning within the neural tube. Cell. 1995;81(5):747–756. doi: 10.1016/0092-8674(95)90536-7. [DOI] [PubMed] [Google Scholar]
- Eroglu C, Barres BA. Regulation of synaptic connectivity by glia. Nature. 2010;468(7321):223–231. doi: 10.1038/nature09612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer WT, Abrahamsson T, Chierzi S, Lui C, Zaelzer C, Jones EV, Bally BP, Chen GG, Theroux JF, Peng J, Bourque CW, Charron F, Ernst C, Sjostrom PJ, Murai KK. Neurons diversify astrocytes in the adult brain through sonic hedgehog signaling. Science. 2016;351(6275):849–854. doi: 10.1126/science.aab3103. [DOI] [PubMed] [Google Scholar]
- Fuccillo M, Rallu M, McMahon AP, Fishell G. Temporal requirement for hedgehog signaling in ventral telencephalic patterning. Development. 2004;131(20):5031–5040. doi: 10.1242/dev.01349. [DOI] [PubMed] [Google Scholar]
- Fuccillo M, Joyner AL, Fishell G. Morphogen to mitogen: the multiple roles of hedgehog signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7(10):772–783. doi: 10.1038/nrn1990. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Rakic P. Cortical evolution: judge the brain by its cover. Neuron. 2013;80(3):633–647. doi: 10.1016/j.neuron.2013.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Dong E, Grayson DR, Veldic M, Zhang X, Costa E. GABAergic dysfunction in schizophrenia: new treatment strategies on the horizon. Psychopharmacology. 2005;180(2):191–205. doi: 10.1007/s00213-005-2212-8. [DOI] [PubMed] [Google Scholar]
- Hajihosseini M, Tham TN, Dubois-Dalcq M. Origin of oligodendrocytes within the human spinal cord. J Neurosci. 1996;16(24):7981–7994. doi: 10.1523/JNEUROSCI.16-24-07981.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwell CC, Parker PR, Gee SM, Okada A, McConnell SK, Kreitzer AC, Kriegstein AR. Sonic hedgehog expression in corticofugal projection neurons directs cortical microcircuit formation. Neuron. 2012;73(6):1116–1126. doi: 10.1016/j.neuron.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heussler HS, Suri M, Young ID, Muenke M. Extreme variability of expression of a sonic hedgehog mutation: attention difficulties and holoprosencephaly. Arch Dis Child. 2002;86(4):293–296. doi: 10.1136/adc.86.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15(23):3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- Izzi L, Levesque M, Morin S, Laniel D, Wilkes BC, Mille F, Krauss RS, McMahon AP, Allen BL, Charron F. Boc and Gas1 each form distinct Shh receptor complexes with Ptch1 and are required for Shh-mediated cell proliferation. Dev Cell. 2011;20(6):788–801. doi: 10.1016/j.devcel.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komada M. Sonic hedgehog signaling coordinates the proliferation and differentiation of neural stem/progenitor cells by regulating cell cycle kinetics during development of the neocortex. Congenit Anom (Kyoto) 2012;52(2):72–77. doi: 10.1111/j.1741-4520.2012.00368.x. [DOI] [PubMed] [Google Scholar]
- Komada M, Saitsu H, Kinboshi M, Miura T, Shiota K, Ishibashi M. Hedgehog signaling is involved in development of the neocortex. Development. 2008;135(16):2717–2727. doi: 10.1242/dev.015891. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6(4):312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Liu Y, May NR, Fan CM. Growth arrest specific gene 1 is a positive growth regulator for the cerebellum. Dev Biol. 2001;236(1):30–45. doi: 10.1006/dbio.2000.0146. [DOI] [PubMed] [Google Scholar]
- Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, Gritli-Linde A, Dellovade T, Porter JA, Rubin LL, Dudek H, McMahon AP, Fishell G. Sonic hedgehog Is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39(6):937–950. doi: 10.1016/S0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- Malik S, Vinukonda G, Vose LR, Diamond D, Bhimavarapu BB, Hu F, Zia MT, Hevner R, Zecevic N, Ballabh P. Neurogenesis continues in the third trimester of pregnancy and is suppressed by premature birth. J Neurosci. 2013;33(2):411–423. doi: 10.1523/JNEUROSCI.4445-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marigo V, Roberts DJ, Lee SM, Tsukurov O, Levi T, Gastier JM, Epstein DJ, Gilbert DJ, Copeland NG, Seidman CE, et al. Cloning, expression, and chromosomal location of SHH and IHH: two human homologues of the Drosophila segment polarity gene hedgehog. Genomics. 1995;28(1):44–51. doi: 10.1006/geno.1995.1104. [DOI] [PubMed] [Google Scholar]
- Maroof AM, Keros S, Tyson JA, Ying SW, Ganat YM, Merkle FT, Liu B, Goulburn A, Stanley EG, Elefanty AG, Widmer HR, Eggan K, Goldstein PA, Anderson SA, Studer L. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell. 2013;12(5):559–572. doi: 10.1016/j.stem.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli DC, Fan CM. Gas1 extends the range of Hedgehog action by facilitating its signaling. Genes Dev. 2007;21(10):1231–1243. doi: 10.1101/gad.1546307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan JS, Zheng X, Hauk G, Ghirlando R, Beachy PA, Leahy DJ. The mode of Hedgehog binding to Ihog homologues is not conserved across different phyla. Nature. 2008;455(7215):979–983. doi: 10.1038/nature07358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JA, Ding SL, Sunkin SM, Smith KA, Ng L, Szafer A, Ebbert A, Riley ZL, Royall JJ, Aiona K, Arnold JM, Bennet C, Bertagnolli D, Brouner K, Butler S, Caldejon S, Carey A, Cuhaciyan C, Dalley RA, Dee N, Dolbeare TA, Facer BA, Feng D, Fliss TP, Gee G, Goldy J, Gourley L, Gregor BW, Gu G, Howard RE, Jochim JM, Kuan CL, Lau C, Lee CK, Lee F, Lemon TA, Lesnar P, McMurray B, Mastan N, Mosqueda N, Naluai-Cecchini T, Ngo NK, Nyhus J, Oldre A, Olson E, Parente J, Parker PD, Parry SE, Stevens A, Pletikos M, Reding M, Roll K, Sandman D, Sarreal M, Shapouri S, Shapovalova NV, Shen EH, Sjoquist N, Slaughterbeck CR, Smith M, Sodt AJ, Williams D, Zollei L, Fischl B, Gerstein MB, Geschwind DH, Glass IA, Hawrylycz MJ, Hevner RF, Huang H, Jones AR, Knowles JA, Levitt P, Phillips JW, Sestan N, Wohnoutka P, Dang C, Bernard A, Hohmann JG, Lein ES. Transcriptional landscape of the prenatal human brain. Nature. 2014;508(7495):199–206. doi: 10.1038/nature13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanni L, Ming JE, Bocian M, Steinhaus K, Bianchi DW, Die-Smulders C, Giannotti A, Imaizumi K, Jones KL, Campo MD, Martin RA, Meinecke P, Pierpont ME, Robin NH, Young ID, Roessler E, Muenke M. The mutational spectrum of the sonic hedgehog gene in holoprosencephaly: SHH mutations cause a significant proportion of autosomal dominant holoprosencephaly. Hum Mol Genet. 1999;8(13):2479–2488. doi: 10.1093/hmg/8.13.2479. [DOI] [PubMed] [Google Scholar]
- Odent S, Atti-Bitach T, Blayau M, Mathieu M, Aug J, Delezo de AL, Gall JY, Le Marec B, Munnich A, David V, Vekemans M. Expression of the sonic hedgehog (SHH) gene during early human development and phenotypic expression of new mutations causing holoprosencephaly. Hum Mol Genet. 1999;8(9):1683–1689. doi: 10.1093/hmg/8.9.1683. [DOI] [PubMed] [Google Scholar]
- Okada A, Charron F, Morin S, Shin DS, Wong K, Fabre PJ, Tessier-Lavigne M, McConnell SK. Boc is a receptor for sonic hedgehog in the guidance of commissural axons. Nature. 2006;444(7117):369–373. doi: 10.1038/nature05246. [DOI] [PubMed] [Google Scholar]
- Ortega JA, Radonjic NV, Zecevic N. Sonic hedgehog promotes generation and maintenance of human forebrain Olig2 progenitors. Front Cell Neurosci. 2013;7:254. doi: 10.3389/fncel.2013.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda-Alvarez DE, Roessler E, Hu P, Srivastava K, Solomon BD, Siple CE, Fan CM, Muenke M. Missense substitutions in the GAS1 protein present in holoprosencephaly patients reduce the affinity for its ligand, SHH. Hum Genet. 2012;131(2):301–310. doi: 10.1007/s00439-011-1078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollen AA, Nowakowski Tomasz J, Chen J, Retallack H, Sandoval-Espinosa C, Nicholas Cory R, Shuga J, Liu Siyuan J, Oldham Michael C, Diaz A, Lim Daniel A, Leyrat Anne A, West Jay A, Kriegstein Arnold R. Molecular Identity of human outer radial glia during cortical development. Cell. 2015;163(1):55–67. doi: 10.1016/j.cell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radonjic NV, Ayoub AE, Memi F, Yu X, Maroof A, Jakovcevski I, Anderson SA, Rakic P, Zecevic N. Diversity of cortical interneurons in primates: the role of the dorsal proliferative niche. Cell Rep. 2014;9(6):2139–2151. doi: 10.1016/j.celrep.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radonjic NV, Memi F, Ortega JA, Glidden N, Zhan H, Zecevic N. The role of sonic hedgehog in the specification of human cortical progenitors in vitro. Cereb cortex. 2016;26(1):131–143. doi: 10.1093/cercor/bhu183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly SK, Yin J, Ayoub AE, Emera D, Leng J, Cotney J, Sarro R, Rakic P, Noonan JP. Evolutionary genomics. Evolutionary changes in promoter and enhancer activity during human corticogenesis. Science. 2015;347(6226):1155–1159. doi: 10.1126/science.1260943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro LA, Quiezi RG, Nascimento A, Bertolacini CP, Richieri-Costa A. Holoprosencephaly and holoprosencephaly-like phenotype and GAS1 DNA sequence changes: report of four Brazilian patients. Am J Med Genet A. 2010;152a(7):1688–1694. doi: 10.1002/ajmg.a.33466. [DOI] [PubMed] [Google Scholar]
- Santiago G, Abramides DV, De-Vitto LP, Ribeiro LA, Meira SG, Jr, Richieri-Costa A. Language skills and neuropsychological performance in patients with SHH mutations and a holoprosencephaly-like phenotype. Am J Med Genet A. 2006;140(19):2085–2090. doi: 10.1002/ajmg.a.31311. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Zecevic N. Schizophrenia: a tale of two critical periods for prefrontal cortical development. Transl Psychiatry. 2015;5:e623. doi: 10.1038/tp.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppala M, Depew MJ, Martinelli DC, Fan CM, Sharpe PT, Cobourne MT. Gas1 is a modifier for holoprosencephaly and genetically interacts with sonic hedgehog. J Clin Invest. 2007;117(6):1575–1584. doi: 10.1172/JCI32032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbereis JC, Pochareddy S, Zhu Y, Li M, Sestan N. The cellular and molecular landscapes of the developing human central nervous system. Neuron. 2016;89(2):248–268. doi: 10.1016/j.neuron.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekki-Kessaris N, Woodruff R, Hall AC, Gaffield W, Kimura S, Stiles CD, Rowitch DH, Richardson WD. Hedgehog-dependent oligodendrocyte lineage specification in the telencephalon. Development. 2001;128(13):2545–2554. doi: 10.1242/dev.128.13.2545. [DOI] [PubMed] [Google Scholar]
- Tenzen T, Allen BL, Cole F, Kang JS, Krauss RS, McMahon AP. The cell surface membrane proteins Cdo and Boc are components and targets of the Hedgehog signaling pathway and feedback network in mice. Dev Cell. 2006;10(5):647–656. doi: 10.1016/j.devcel.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Tyson JA, Goldberg EM, Maroof AM, Xu Q, Petros TJ, Anderson SA. Duration of culture and sonic hedgehog signaling differentially specify PV versus SST cortical interneuron fates from embryonic stem cells. Development. 2015;142(7):1267–1278. doi: 10.1242/dev.111526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Hou S, Han YG. Hedgehog signaling promotes basal progenitor expansion and the growth and folding of the neocortex. Nat Neurosci. 2016;19(7):888–896. doi: 10.1038/nn.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wu Q, Yang P, Wang C, Liu J, Ding W, Liu W, Bai Y, Yang Y, Wang H, Gao S, Wang X. LSD1 co-repressor Rcor2 orchestrates neurogenesis in the developing mouse brain. Nat Commun. 2016;7:10481. doi: 10.1038/ncomms10481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Wonders CP, Anderson SA. Sonic hedgehog maintains the identity of cortical interneuron progenitors in the ventral telencephalon. Development. 2005;132(22):4987–4998. doi: 10.1242/dev.02090. [DOI] [PubMed] [Google Scholar]
- Yam PT, Langlois SD, Morin S, Charron F. Sonic hedgehog guides axons through a noncanonical, Src-family-kinase-dependent signaling pathway. Neuron. 2009;62(3):349–362. doi: 10.1016/j.neuron.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Zhang W, Kang JS, Cole F, Yi MJ, Krauss RS. Cdo functions at multiple points in the sonic hedgehog pathway, and Cdo-deficient mice accurately model human holoprosencephaly. Dev cell. 2006;10(5):657–665. doi: 10.1016/j.devcel.2006.04.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 a RNA-seq data from http://www.brainspan.org/rnaseq/ show the dynamic expression of Sonic Hedgehog (SHH) during brain development in utero. The different cortical areas were grouped in “dorsal” and “ventral” groups to show that during 18–22 gw SHH expression is higher in dorsal than in ventral regions. b The same data, grouped in the different cortical areas, show the changes in SHH expression per area along the gestational timeline. c–c″ Three different exposure times highlight the difference in SHH expression levels between choroid plexus (ChP) cells and the RGCs in the proximal ventricular zone (VZ; arrows). d–d″ SHH transcript is expressed by blood vessel endothelial cells labelled with the endothelial marker PECAM in the ganglionic eminence (GE) at 10 gw. e–e″ Co-labeling for SHH mRNA (red) and protein (green) in the hypothalamic midline reveals the specificity of the antibody and the range of diffusion of the secreted protein.f–f″ Expression of both SHH protein (green) and transcript (red) in the human fetal retina confirms the specificity of the antibody in humans, as previously shown in the mouse (TIF 14429 KB)
Fig. S2 Gradients of SHH mRNA in 15-gw brain. a A sagittal section of a 15-gw forebrain shows a slight rostro-caudal gradient, best seen on higher magnification of the boxed areas presented on the right (TIF 7220 KB)
Fig. S3 Expression of SHH in the human fetal hippocampus. a–c Distribution of SHH transcripts in the hippocampus of 17-, 22-, and 40-gw tissue. b′–b″″ Higher magnification of the boxed areas illustrated in (b) shows SHH expression in the different areas of the 22-gw hippocampus. d, e Fluorescence ISH for SHH and Pax6 staining reveal that only some of Pax6+ cells in dentate gyrus (DG) and CA1 co-express SHH in the 22-gw hippocampus. f SHH is expressed by Tbr1+ cells in the DG of the 22-gw hippocampus. f′ Higher magnification of the double-positive cells in (f). g–l Expression of SHH receptors and downstream molecules in the 19-gw hippocampus shown in contiguous sections. Scale bars a, b 1mm, b′ 100µm, f′ 50µm (TIF 7928 KB)
Fig. S4 a Coronal medial section of the 21-gw fetal brain stained for SHH mRNA (blue) and Gad67 protein (brown) reveals co-labeled cells. a′ Higher magnification of the boxed area in (a). b Double-positive cells are not seen in a tissue section from a 23-gw brain treated for SHH (red) followed by Olig2 staining (green). b′, b″ Higher magnification of the interventricular zone (IZ) and subplate (SP) areas. c Microglial cells (Iba1, light blue) and SHH mRNA (red) staining do not co-label cells in the 10-gw human cortex. Scale bars: a 150µm, a′ 100µm, b′ 50µm (TIF 3754 KB)
Fig. S5 Sense/control in situ for SHH in 10- and 19-gw tissue. Scale bars: 2mm (TIF 9398 KB)
Fig. S6 Expression pattern of Shh-signaling genes in the embryonic mouse brain. Data obtained from the Allen Brain Atlas (TIF 11830 KB)
Fig. S7 In situ hybridizations on mouse brain tissue with the human SHH antisense (AS) and sense (S) probe. Only (b) was probed with the sense probe. Scale bars: a 400µm, c 500µm, c’ 100µm, d 1mm, d″ 150µm (TIF 5166 KB)
Fig. S8 Expression of SMO and SHH receptors in the 10 gw Choroid Plexus. a SMO expression, b BOC, c GAS1 and d CDON. Scale bar: 50µm (TIF 5016 KB)