Abstract
Tularemia is a severe, zoonotic disease caused by a gram-negative bacterium, Francisella tularensis. We have previously shown that rabbits are a good model of human pneumonic tularemia when exposed to aerosols containing a virulent, type A strain, SCHU S4. We further demonstrated that the live vaccine strain (LVS), an attenuated type B strain, extended time to death when given by scarification. Oral or aerosol vaccination has been previously shown in humans to offer superior protection to parenteral vaccination against respiratory tularemia challenge. Both oral and aerosol vaccination with LVS were well tolerated in the rabbit with only minimal fever and no weight loss after inoculation. Plasma antibody titers against F. tularensis were higher in rabbits that were vaccinated by either oral or aerosol routes compared to scarification. Thirty days after vaccination, all rabbits were challenged with aerosolized SCHU S4. LVS given by scarification extended time to death compared to mock-vaccinated controls. One orally vaccinated rabbit did survive aerosol challenge, however, only aerosol vaccination extended time to death significantly compared to scarification. These results further demonstrate the utility of the rabbit model of pneumonic tularemia in replicating what has been reported in humans and macaques as well as demonstrating the utility of vaccination by oral and respiratory routes against an aerosol tularemia challenge.
Keywords: tularemia, respiratory disease, live attenuated vaccines, mucosal vaccination
The authors describe how altering the route of vaccination improves protection conferred against pneumonic tularemia in rabbits, providing further evidence for the rabbit as a relevant model of human tularemia.
INTRODUCTION
Tularemia (a.k.a. rabbit fever) is a severe zoonotic disease in humans with a mortality rate ≥30% if untreated (Dennis et al.2001; Ellis et al.2002). Tularemia can be spread by arthropod, contact with infected animals or tissues, ingestion or by inhalation. When inhaled, as few as 15 organisms are sufficient to cause disease in humans. In the 1950s, tularemia was the leading cause of laboratory-acquired infection. Both the former Soviet Union and the USA (prior to 1969) developed tularemia as a biological weapon. The causative agent is a gram-negative coccobacillus, Francisella tularensis, a facultative intracellular bacterium. Four subspecies exist, of which subsp. tularensis (a.k.a. type A) is the most virulent and is found (with one exception) only in North America.
Initial vaccines used killed whole-cell bacteria but were not protective against aerosol challenge (Eigelsbach and Downs 1961; Barry, Cole and Santiago 2009). In the 1950s, the Soviet Union passaged a F. tularensis subsp. holarctica (type B) strain in culture and created a live vaccine (Barry, Cole and Santiago 2009). This was given to the USA which passaged the strain further and created what is called the live vaccine strain (LVS). In studies conducted in the 1960s, LVS was found to be generally safe and protected both humans and macaques against low-dose aerosol challenge with a virulent type A strain, SCHU S4 (Saslaw and Carhart 1961; Saslaw et al.1961; Eigelsbach et al.1962). At higher aerosol challenge doses, protection broke down. Subsequent studies showed that oral or aerosol vaccination with LVS provided better protection against aerosol challenge (Hornick and Eigelsbach 1966; Hornick et al.1966; Tulis, Eigelsbach and Hornick 1969). Nevertheless, mucosal LVS vaccination was not pursued further. Subsequent studies in mice and rats have confirmed that oral or intranasal (i.n.) vaccination with LVS protects against SCHU S4 (this is challenge dose and route dependent as well as fairly limited) (Conlan et al.2005; Wu et al.2005; Ray et al.2009; Griffin et al.2015).
We have recently re-established the New Zealand White (NZW) rabbit as a model of pneumonic tularemia (Reed et al.2011). Back in the 1970s, a report had shown that NZW rabbits were susceptible to aerosol challenge with F. tularensis and that LVS vaccination extended the time to death. We found that after inhaling aerosolized SCHU S4, rabbits develop a fever, lose weight and succumb to infection within 4–6 days. Blood drawn post-challenge showed an elevation in erythrocyte sedimentation rate (ESR), lymphopenia and drop in platelets that was associated with the onset of fever. Lymphopenia and more generally leukopenia are common findings in many bacterial infections. ESR is considered a crude measure of the acute phase protein response during an inflammatory response and is generally elevated during an acute infection including human tularemia (Koc et al.2012). The loss of platelets suggested thrombocytopenia. Bacteremia was low or undetectable through the course of the disease in the rabbits. Radiographs showed evidence of severe bronchial pneumonia and bloating in the intestines. At necropsy, gross pathological changes were noted in the lungs, liver and spleen as well as the intestines and kidneys. Bacteria were found in all tissues examined but the levels were quite variable. All of these results were consistent with human pneumonic tularemia. Subsequently, we evaluated derivatives of SCHU S4 as potential vaccines given by scarification in the rabbit model (Reed et al.2014). These derivatives were safe and three of the four derivatives tested protected better than LVS with 27%–40% of rabbits surviving to 28 days post-challenge. In agreement with previously published data, LVS extended time to death but none of the LVS-vaccinated rabbits survived SCHU S4 challenge. To further evaluate the potential of the rabbit as a relevant surrogate for humans in studying protection against pneumonic tularemia, we have subsequently evaluated whether oral or respiratory LVS vaccination could recapitulate what had been reported previously in humans and macaques. Those results are reported here.
MATERIALS AND METHODS
Biosafety and regulatory information
All work with live Francisella tularensis was conducted at biosafety level (BSL)-3 in the University of Pittsburgh, Regional Biocontainment Laboratory (RBL). For respiratory protection, all personnel wore powered air-purifying respirators (3M GVP-1 PAPR with L-series bumpcap) or used a class III biological safety cabinet. Vesphene II se (1:128 dilution, Steris Corporation, Erie, PA, USA) was used to disinfect all liquid wastes and surfaces associated with the agent. All solid wastes, used caging, and animal wastes, were steam sterilized. Animal carcasses were digested via alkaline hydrolysis (Peerless Waste Solutions, Holland, MI, USA). The University of Pittsburgh, RBL is a registered entity with the CDC/USDA for work with F. tularensis.
Rabbits
Young female NZW rabbits (Robinson Services, Inc., Mocksville, NC and Myrtle's Rabbitry, Thompson Station, TN) were housed in the University of Pittsburgh, RBL at ABSL-3 for the duration of the studies. Prior to vaccination, IPTT-300 temperature/ID chips (BioMedic Data Systems, Seaford, DE, USA) were implanted subcutaneously. Body weight was recorded once in the morning and body temperature was recorded twice daily. Temperature was read using a DAS-7000 reader (BioMedic Data Systems). All studies were approved by the University of Pittsburgh's Institutional Animal Care and Use Committee.
Bacteria
Francisella tularensis LVS or SCHU S4 were originally obtained from Gerald Nau and the Dynport Vaccine Company, respectively, and were stored as single-passage stocks (Reed et al.2011). Francisella tularensis was grown first on cysteine heart agar (CHA) for 2 days prior to growing overnight in Brain Heart Infusion broth using baffled, vented polycarbonate Erlenmeyer flasks (Reed et al.2011). After the exposures were completed, nebulizer and all-glass impinger (AGI) contents were quantified on CHA.
Vaccination
Rabbits were vaccinated by scarification, by oral gavage or by aerosol exposure. For scarification, rabbits were anesthetized by subcutaneous injection of ketamine (80 mg/kg) and xylazine (8 mg/kg); once anesthesia was confirmed, a small area of the dorsal surface was shaved. Approximately 0.1 ml of bacteria at a concentration of 1 × 1010 cfu/ml were placed in a drop on the shaved area and a bifurcated needle (Becton Dickinson) was jabbed through the drop of bacteria into the skin 17 times. The drop was allowed to absorb into the skin, after which the xylazine was reversed by i.m. injection of 0.2–1 mg/kg yohimibine. The scarification site was monitored daily for the first 7 days after vaccination. For oral vaccination, rabbits were anesthetized by subcutaneous injection of ketamine (80 mg/kg) and xylazine (8 mg/kg); once anesthesia was confirmed, a French feeding tube was inserted into the rabbit's stomach and 10 ml of broth containing LVS was delivered into the stomach of the rabbit.
Aerosol exposures
Aerosols of either LVS or SCHU S4 were conducted inside a class III biological safety cabinet (Baker Co., Sanford, ME, USA) located inside the RBL as previously described (Reed et al.2011). Briefly, rabbits were exposed two at a time for 10 min in a nose-only exposure chamber (CH Technologies, Westwood, NJ, USA) using a 3-jet Collison nebulizer while plethysmography data were collected in real time using Buxco XA software (Buxco Research Systems, Wilmington, NC, USA) during the exposure. Aerosol concentration and inhaled (presented) dose were determined as described previously (Roy 2005). The median challenge dose was 1.1 × 103 cfu with a standard deviation of 9.2 × 103.
ELISA
ELISA was performed using standard ELISA procedures. Briefly, dilutions of rabbit sera were incubated for 1 h at 37°C on 96-well plates coated with heat-killed F. tularensis SCHU S4. After washing with PBS-Tween, secondary goat anti-rabbit IgG-HRP (Horseradish Peroxidase) (Fitzgerald Industries, Acton, MA, USA) was added to the plates and incubated for 1 h at 37°C, after which the plates were washed again with PBS-Tween, and BM Chemiluminescence ELISA Substrate (Roche Applied Sciences, Indianapolis, IN) was added to the plates. Plates were then read on an Lmax plate reader (Molecular Devices, Sunnyvale, CA, USA). Median effective concentration (EC50) was determined by four-parameter logistical regression of ELISA data using GraphPad Prism 6.
ESR
Rabbit whole blood collected in EDTA was pipetted using a glass Pasteur pipet into glass Wintrobe tubes. After one h, the degree of sedimentation was recorded in millimeter for each rabbit.
Hematology
Rabbit whole blood collected in EDTA was analyzed on a VetScan HM2 (Abaxis, Union City, CA, USA) to determine the white blood cell (WBC) counts including granulocytes, macrophages and lymphocytes as well as platelets.
Statistical methods
Data were collected and organized using spreadsheets in Microsoft Excel 2007; graphing and statistical analyses were conducted using GraphPad Prism 6.
RESULTS
Impact of route on response to LVS
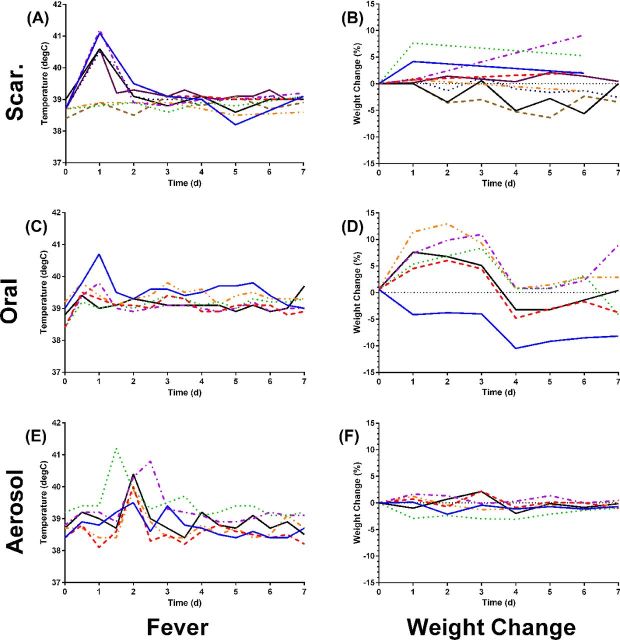
Over the course of five independent experiments, NZW rabbits were vaccinated with 1 × 109 cfu LVS by scarification, oral gavage or inhalation. Six of nine rabbits vaccinated with LVS by scarification developed a fever (40°C –41°C) within 1 day of inoculation, which returned to normal by the second day post-inoculation (Fig. 1A). However, only two of those nine rabbits lost weight in the first week post-inoculation relative to mock-vaccinated controls and only one of those two reached 5% weight loss (Fig. 1B). When LVS was given orally, only one of six rabbits developed a fever post-inoculation and that only on day 1 (Fig. 1C). That same rabbit was the only one to also lose weight after oral LVS inoculation relative to the mock-vaccinated controls (Fig. 1D); by day 14 it had recovered (data not shown). Three of six rabbits vaccinated by inhalation of LVS developed a fever but that fever peaked at different intervals (Fig. 1E). As was seen with the other routes of inoculation, the fever after inhalation of LVS persisted only 1 day. None of the aerosol LVS-vaccinated rabbits lost weight post-inoculation (Fig. 1F).
Figure 1.
Fever and weight changes after LVS inoculation by different routes. Rabbits were inoculated with LVS by scarification (A, B), orally (C, D) or inhalation of a small-particle aerosol (E, F) and monitored for 7 days to record changes in body temperature and weight. Each graph shows values for individual rabbits in each group; temperature was recorded twice daily while weight was recorded once daily.
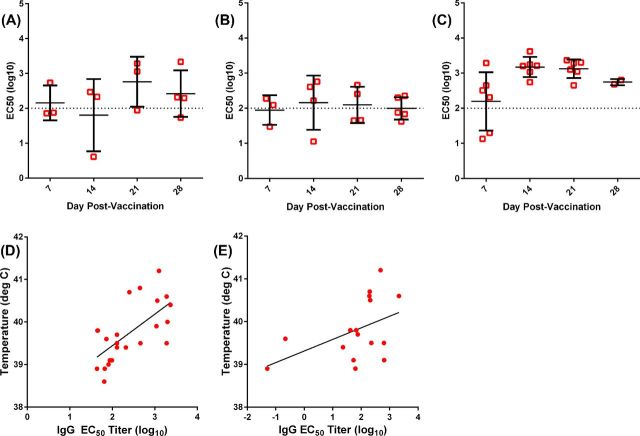
In addition to monitoring the physiological response to vaccination, the rabbits were bled weekly to assess the plasma antibody response to vaccination. The results are shown in Fig. 2. Rabbits vaccinated with LVS by scarification had low plasma IgG titers against heat-killed SCHU S4, peaking at day 21 post-vaccination and dropping slightly at day 28 although the differences were not significant (Fig. 2A). Similarly, orally vaccinated rabbits had low plasma IgG responses, although a few rabbits had higher titers at days 14 and 21 (Fig. 2B). In contrast, aerosol-vaccinated rabbits had significantly higher levels of plasma of IgG at days 14 and 21, relative to day 7 (P = 0.041 and 0.026, respectively by Mann–Whitney test) (Fig. 2C). These differences were also significant compared to scarification at day 14 (P = 0.027) and oral vaccination at day 21 (P = 0.021). Plasma IgG titers on day 21 roughly correlated with the febrile response to LVS vaccination (Fig. 2D; r2 = 0.434) while day 28 IgG titers did not (Fig. 2E, r2 = 0.215), and plasma IgG titers on day 7 and 14 even less so (data not shown).
Figure 2.
Plasma IgG response to LVS inoculation. Rabbits were inoculated with LVS by scarification (A), orally (B) or inhalation of a small-particle aerosol (C). Rabbits were bled on days 7, 14, 21 and 28 post-vaccination, and antibody titers were assessed by ELISA using heat-killed SCHU S4 as the antigen. Antibody titer is shown as the median effective concentration determined by four-parameter logistical regression analysis. Graphs show individual rabbits (open symbols) with the mean and standard deviation for each group/time point (bars). Linear regression analysis is shown for day 21 (D) and day 28 (E) post-vaccination plasma IgG titers and the maximum body temperature recorded post-vaccination across all rabbits including mock-vaccinated controls. Graphs show individual rabbits (symbols) and the linear trend (black line). Plasma IgG response to LVS inoculation. Rabbits were inoculated with LVS by scarification (A), orally (B) or inhalation of a small-particle aerosol (C). Rabbits were bled on days 7, 14, 21 and 28 post-vaccination and antibody titers were assessed by ELISA using heat-killed SCHU S4 as the antigen. Antibody titer is shown as the median effective concentration determined by four-parameter logistical regression analysis. Graphs show individual rabbits (open symbols) with the mean and standard deviation for each group/time point (bars). Linear regression analysis is shown for day 21 (D) and day 28 (E) post-vaccination plasma IgG titers and the maximum body temperature recorded post-vaccination across all rabbits including mock-vaccinated controls. Graphs show individual rabbits (symbols) and the linear trend (black line).
Impact of vaccination route on protection against SCHU S4 aerosol challenge
Thirty days after vaccinations, rabbits were challenged by aerosol exposure to virulent SCHU S4. Inhaled doses ranged from 1000 to10 000 cfu (43–430 LD50), but there was no significant difference between mock-vaccinated and LVS-vaccinated groups as far as mean dose (data not shown). Mock-vaccinated rabbits succumbed within 7 days of challenge, with a mean of 4.8 days and standard deviation of 0.8 days as shown in Fig. 3. As we have seen previously, scarification with LVS extended the time to death to a maximum of 10 days with a mean of 7 days and a standard deviation of 1.3 days. Oral vaccination with LVS extended time to death with a mean of 6.6 days and a standard deviation of 2.2 days with one rabbit surviving challenge. No prior published results have reported an LVS-vaccinated rabbit surviving SCHU S4 challenge. Aerosol vaccination with LVS extended the time to death the farthest with a mean of 10 days and a standard deviation of 4.5 days. When compared using a Mantel–Cox test, all three routes were better than mock-vaccinated rabbits (P < 0.0001) but only aerosol vaccination was significantly better than scarification (P = 0.5509 for oral versus scarification, P = 0.0428 for aerosol versus scarification). There was no significant difference, however, between oral and aerosol vaccination (P = 0.7757).
Figure 3.
Survival of LVS-vaccinated rabbits after aerosol challenge with SCHU S4. Thirty days after vaccination, rabbits were exposed to aerosolized SCHU S4. Graph shows percent survival in each group using a Kaplan-Meier plot over 28 days post-challenge.
In addition to recording survival, rabbits were monitored for changes in body temperature and weight (Fig. 4). Similar to what we have published previously, rabbits vaccinated with LVS by scarification develop a fever around 2–3 days after challenge, in the same time frame as mock-vaccinated controls. That fever response is maintained until the rabbits succumbed to the infection. They also lose weight in the same time frame. While some of the LVS-scarification rabbits lost up to 15%–20% of their body weight, others only lost 5%–10% but still succumbed to the infection. With the LVS-oral vaccination group, fevers also began and peaked in the same range as mock-vaccinated controls but unlike the mock-vaccinated or LVS-scarification groups, the fever response was more variable over the course of the post-challenge period. One set of LVS-oral rabbits lost weight fairly quickly and succumbed within 5 days while three others lost weight more slowly. The lone LVS-oral survivor did develop a fever but that fever gradually reduced over the 15-day monitoring period while it only lost a modest amount of weight, suggesting that the immune response was able to control and eventually eliminate the infection. LVS-aerosol rabbits had the most variable fever response of all the vaccinated groups and weight loss was generally slower across the entire group although all the rabbits in that group did eventually succumb to the disease. We evaluated whether pre-challenge plasma IgG anti-SCHU S4 titer predicted time to death or fever in the rabbits. Across all rabbits, linear regression analysis of day 14 and day 21 plasma IgG titer indicated a correlation with body temperature on day 4 (r2 = 0.44 and 0.56, respectively) but not with time to death (r2 = 0.18 for both). Plasma IgG titer from other time points did not correlate well with either febrile response or survival and the lone surviving rabbit in the oral LVS vaccination group did not have the highest titer seen at any time point pre-challenge (data not shown).
Figure 4.
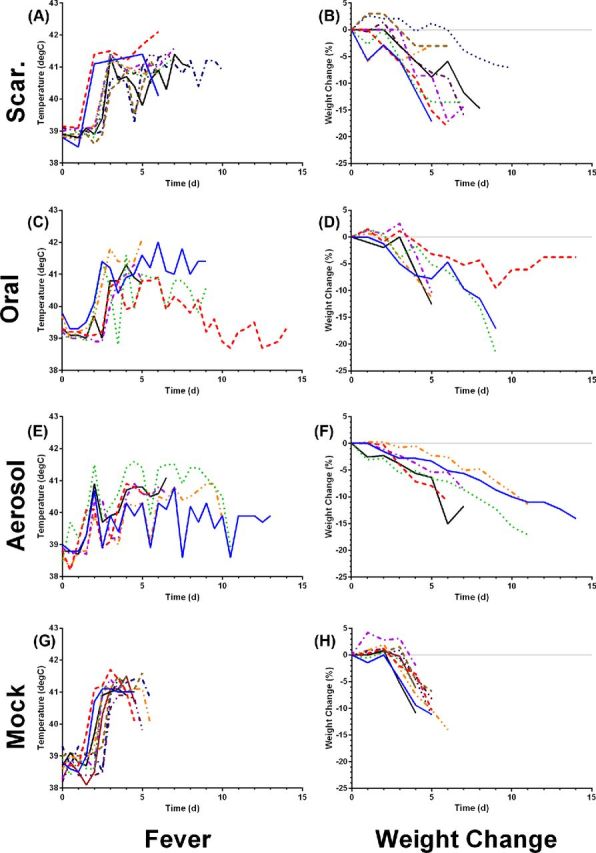
Fever and weight changes after aerosol challenge with SCHU S4. Rabbits inoculated with LVS by scarification (A, B), orally (C, D), inhalation of a small-particle aerosol (E, F) or mock vaccinated (G, H) were monitored for 14 days after SCHU S4 challenge to record changes in body temperature and weight. Each graph shows values for individual rabbits in each group; temperature was recorded twice daily while weight was recorded once daily.
Blood samples were collected post-challenge and evaluated for changes in leukocytes by CBC analysis and for changes in ESR. In mock-vaccinated rabbits, total WBC counts drop from baseline on days 4 and 5 post-challenge with a slight recovery on day 6 (Fig. 5A); this appears to be due to a loss of lymphocytes (Fig. 5B) as granulocyte counts and monocyte counts are largely unchanged (Fig. 5C and D, respectively). This is consistent with what we have reported previously in naive rabbits exposed to aerosolized Francisella tularensis SCHU S4 (Reed et al.2011). WBC counts remained largely unchanged in LVS-scarification or LVS-aerosol rabbits but did decline in LVS-oral rabbits. As with the mock-vaccinated rabbits, the decline in oral LVS WBC was largely due to lymphopenia. There was an increase in granulocytes in the LVS-aerosol and LVS-oral groups on day 8; this increase was not significantly different from the granulocyte counts seen in LVS-scarification rabbits, although in part this was due to the relatively small number of rabbits being sampled at that time (n = 3/group for LVS oral and LVS aerosol, n = 1 for LVS scarification). In all four groups, mean platelet levels dropped from baseline on day 4. In some rabbits, no platelets were counted on day 4, suggesting thrombocytopenia. Using a Mann–Whitney test, the decline in platelet counts was highly significant for the mock-vaccinated rabbits (P = 0.0003), significant in the LVS-aerosol rabbits (P = 0.04) and not significant in the LVS-scarification or LVS-oral groups (P = 0.3333 and 0.6741, respectively).
Figure 5.
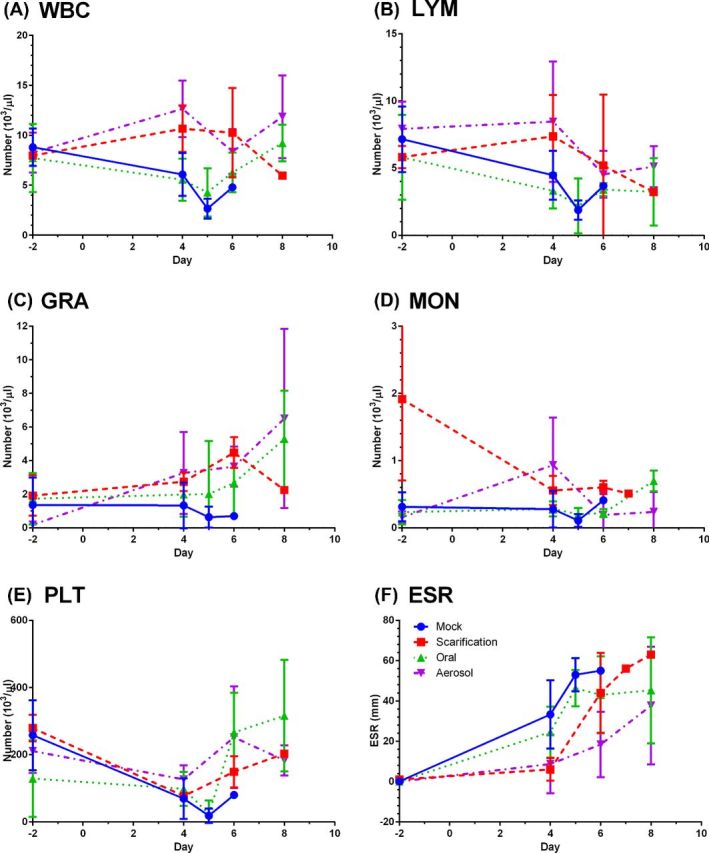
Lymphopenia, thrombocytopenia and elevated ESR after aerosol challenge with SCHU S4. Blood samples collected pre- and post-exposure were analyzed for changes in WBC (A), lymphocytes (B), granulocytes (C), monocytes (D), platelets (E) and ESR (F). Graphs show means at each time point with error bars showing the standard deviation for mock (solid lines, circles) as well as rabbits vaccinated with LVS by scarification (dashed lines, squares), oral inoculation (dotted lines, triangles) or by aerosol (dot-dash lines, inverted triangles).
As we have seen previously, ESR increased dramatically in mock-vaccinated rabbits with a mean of 33.3 mm on day 4 post-challenge and continued to increase through days 5 and 6, reaching >60 mm in some rabbits before they succumb. In contrast, ESR levels were lower in the vaccinated rabbits after challenge compared to the mock-vaccinated rabbits. On day 4, LVS-oral rabbits averaged 24.5 mm while LVS-scarification and LVS-aerosol rabbits averaged 6.0 and 8.6, respectively. By one-way ANOVA, day 4 ESRs were significantly different from baseline in mock-vaccinated rabbits (P < 0.0001) but not in any of the LVS-vaccinated groups. After day 4, ESR increased in all of the vaccinated groups, averaging between 40 and 60 on day 8. This is curious because vaccinated rabbits did develop a fever response to infection, albeit not as high as naive or mock-vaccinated controls, suggesting there are differences in the inflammatory response.
Rabbits were euthanized when moribund (or at day 28 post-challenge for the one survivor) and necropsied to collect organs for determination of bacterial load (Fig. 6). Historical controls were used in the mock-vaccinated group. Bacterial load in spleen, lung and liver was highly variable in the mock-vaccinated group. Similar to what we had previously reported in naive rabbits, after adjusting for weight, the bacterial load was higher in the spleen than in the lung or liver in all groups. The geometric mean SCHU S4 load in LVS-scarification and LVS-oral groups was overall slightly lower than the mock-vaccinated, while in the aerosol LVS-vaccinated rabbits, the difference from the mock-vaccinated rabbits was more prominent. These differences, however, were not significant by one-way ANOVA. The lone surviving rabbit from the oral LVS group had no detectable bacteria in the spleen, liver or lung (data not shown).
Figure 6.
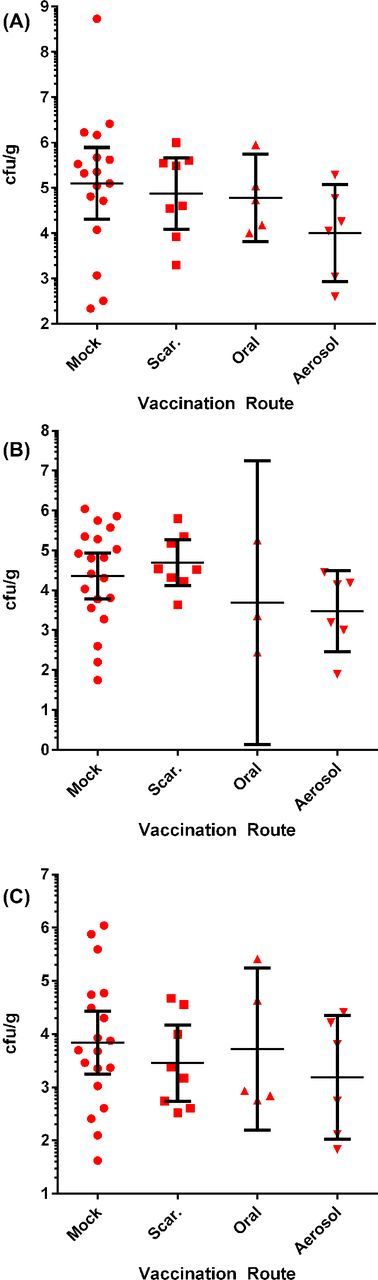
Organ bacterial load in mock-vaccinated or LVS-vaccinated rabbits that succumbed to pneumonic tularemia. Rabbits were euthanized when moribund and organs were collected and frozen for later analysis to determine bacterial load. Graphs show individual rabbits (symbols) in each group with the mean and standard deviation for each group/time point (bars) for samples taken from (A) spleen, (B) lung and (C) liver.
DISCUSSION
The findings reported here expand on our previous study with attenuated live vaccines for Francisella tularensis and demonstrate that vaccination of rabbits with LVS by mucosal/respiratory routes can extend the time to death even farther than has been reported for parenteral inoculation (Baskerville and Hambleton 1976; Reed et al.2014). While we believe the live-attenuated SCHU S4 derivatives are a better choice for a licensed human vaccine, we felt that the data from these mucosal LVS vaccination studies are important as they argue for the relevance of the rabbit model for tularemia vaccine studies to satisfy the FDA's Animal Rule (FDA 2002; FDA 2014). The results reported here demonstrate that the response to LVS in rabbits resembles what was reported in macaques and humans in seminal studies done in the 1960s (Eigelsbach et al.1961, 1962; Hornick and Eigelsbach 1966; Tulis, Eigelsbach and Hornick 1969) demonstrating the superior protection afforded by LVS vaccination delivered by oral or aerosol inoculation against aerosol challenge with SCHU S4.
Other studies have reported similar findings in mice, demonstrating that oral or i.n. inoculation with LVS provides better protection (against i.n. LVS challenge) or extend time to death (against SCHU S4) relative to parenteral vaccination with LVS (Conlan et al.2005; Wu et al.2005; Ray et al.2009; Griffin et al.2015). However, mice are extremely susceptible to F. tularensis including strains that are fully attenuated (such as LVS) in other mammals (Lyons and Wu 2007). This suggests that mechanisms that protect mice against virulent F. tularensis may not be the same as those that protect other species that are not as susceptible. For this reason, mice are not considered to be a model that would be suitable for ‘pivotal’ efficacy studies to support licensure of a tularemia vaccine under the FDA Animal Rule. Although the FDA is careful to say that it may be possible to use one animal model to license a drug or biologic under the Animal Rule, they state that in most cases, multiple relevant animal models will be necessary for the pivotal efficacy studies (FDA 2014).
Rats are also an alternative model of tularemia that other groups are pursuing. First described as a potential model in the late 1970s, rats are more inherently resistant to F. tularensis than other lab animals that are used in tularemia studies (Kostiala, McGregor and Logie 1975; Jemski 1981; Lyons and Wu 2007; Raymond and Conlan 2009). It has been suggested that this inherent resistance makes a case for the rat as a relevant model as it more closely resembles human susceptibility. However, parenteral inoculation with LVS confers 100% protection against respiratory challenge with SCHU S4 and not all negative controls succumb to challenge (Jemski 1981; Wu et al.2009; Chu et al.2014). LVS did not confer 100% protection in humans (McCrumb 1961). Second, it is not possible to demonstrate protection better than 100%, so it would be difficult to prove in the rat model that a new vaccine/route is better than parenteral inoculation with LVS (although possibly higher challenge doses might show breakthrough). For example, it would not have been possible to use rats in the current study to assess whether mucosal or respiratory inoculation with LVS would confer better protection than parenteral inoculation. Lastly, challenge studies with rats have almost exclusively used intratracheal inoculation with virulent F. tularensis (Wu et al.2009; Ray et al.2010; Chu et al.2014). We would argue that ‘both’ the rat and rabbit models are essential and relevant for evaluating potential tularemia vaccines and determining immunological mechanisms and correlates of protection.
Because the nature of the attenuating mutations is uncertain, the potential for reversion unclear and the failure to protect humans or macaques at high aerosol challenge doses, LVS is not considered a suitable candidate for a licensed vaccine against pneumonic tularemia (Marohn and Barry 2013). We have previously shown that attenuated mutants of SCHU S4 offer superior protection against i.n. challenge in mice and aerosol challenge in the rabbit model (Reed et al.2014; Santiago et al.2015). However, LVS is more than suitable as a tool for understanding immunological mechanisms important in protective vaccine-induced adaptive immune responses or in defining correlates of protection. As demonstrated in the data reported here, we have successfully used LVS as a tool to explore the protection conferred by alternate routes of vaccination.
One surprising finding in these studies was that oral and aerosol LVS vaccination resulted in higher plasma IgG levels against F. tularensis than LVS given by scarification. There is an abundance of evidence from other studies that LVS rapidly disseminates from the site of inoculation to other tissues and organs, can persist for up to 21 days in mice, and that persistence is important for protection against later type A challenge (De Pascalis et al.2012, 2014; Griffin et al.2015). The fever response to vaccination reported here would suggest that dissemination occurred in these rabbits, although it is notable that the fever response in the aerosol group was delayed compared to the scarification and oral groups. The fever response after challenge was also lower in the aerosol-vaccinated group, suggesting better control of infection in the lungs. In agreement with this, bacterial loads in all three organs examined were lower in the aerosol-vaccinated group although the differences were not significant. This data suggest that the LVS persists in the lungs longer after aerosol vaccination than inoculation by oral or scarification and it is this persistence that generates the higher plasma IgG levels and results in the extended time to death after aerosol challenge. We would also note the difference in the ELISA assay used here (using heat-killed SCHU S4) as opposed to our prior vaccine studies that used LVS endotoxin as the antigen (Reed et al.2014). Not surprisingly, responses were stronger to whole heat-killed organism but what was intriguing was the relationship between the febrile response to vaccination, the level of plasma IgG achieved and the response to infection. The data suggest that antibody may serve as a correlate of protection against challenge. Other studies have suggested that antibody may play a role in protection against tularemia and passive immunization has been used as therapy against human tularemia (Foshay 1940; Saslaw and Carhart 1961; Drabick et al.1994; Mara-Koosham et al.2011). There is some controversy in this regard and others have proposed cellular mechanisms and shown that these responses can correlate with protection (Elkins, Cowley and Bosio 2007; Kirimanjeswara et al.2008; Rawool et al.2008; De Pascalis et al.2012, 2014). This will be expanded upon in future studies (D.S. Reed, manuscript in preparation).
We report here data supporting the relevance of the rabbit model for evaluating vaccines to protect against pneumonic tularemia as the outcome of our studies resembles what has been reported in macaques and humans. In agreement with past reports, oral or respiratory inoculation of LVS improved the protection conferred against aerosol challenge with SCHU S4 (Eigelsbach et al.1961, 1962; Hornick and Eigelsbach 1966; Hornick et al.1966; Tulis, Eigelsbach and Hornick 1969). Considering that vaccination at the site of pathogen entry has long been known to offer superior protection against disease (oral poliovirus vaccination, respiratory vaccination for plague, measles and influenza) (Lebedinskii et al.1979; Sabin 1985; Cutts, Clements and Bennett 1997; Jackson et al.1999), these routes should be considered in design and optimizing vaccination against pathogens that would enter via the respiratory tract. This is particularly relevant to biodefense as inhalation is considered the most likely route of exposure in the event of a bioterror or biowarfare attack.
Acknowledgments
We thank the Division of Laboratory Animal Resource's veterinary technicians who work in the University of Pittsburgh and assisted with these studies.
FUNDING
The research described herein was sponsored by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health, grants U01 AI077909-01 and R01 A102966-01A1.
Conflict of interest. None declared.
REFERENCES
- Barry EM, Cole LE, Santiago AE. Vaccines against tularemia. Hum vaccines. 2009;5:832–8. doi: 10.4161/hv.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskerville A, Hambleton P. Pathogenesis and pathology of respiratory tularaemia in the rabbit. Brit J Exp Pathol. 1976;57:339–47. [PMC free article] [PubMed] [Google Scholar]
- Chu P, Cunningham AL, Yu JJ, et al. Live attenuated Francisella novicida vaccine protects against Francisella tularensis pulmonary challenge in rats and non-human primates. PLoS Pathog. 2014;10:e1004439. doi: 10.1371/journal.ppat.1004439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlan JW, Shen H, KuoLee R, et al. Aerosol-, but not intradermal-immunization with the live vaccine strain of Francisella tularensis protects mice against subsequent aerosol challenge with a highly virulent type A strain of the pathogen by an alpha beta T cell- and interferon gamma-dependent mechanism. Vaccine. 2005;23:2477–85. doi: 10.1016/j.vaccine.2004.10.034. [DOI] [PubMed] [Google Scholar]
- Cutts FT, Clements CJ, Bennett JV. Alternative routes of measles immunization: a review. Biologicals. 1997;25:323–38. doi: 10.1006/biol.1997.0103. [DOI] [PubMed] [Google Scholar]
- De Pascalis R, Chou AY, Bosio CM, et al. Development of functional and molecular correlates of vaccine-induced protection for a model intracellular pathogen, F. tularensis LVS. PLoS Pathog. 2012;8:e1002494. doi: 10.1371/journal.ppat.1002494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pascalis R, Chou AY, Ryden P, et al. Models derived from in vitro analyses of spleen, liver, and lung leukocyte functions predict vaccine efficacy against the Francisella tularensis live vaccine strain (LVS) mBio. 2014;5:e00936. doi: 10.1128/mBio.00936-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis DT, Inglesby TV, Henderson DA, et al. Tularemia as a biological weapon: medical and public health management. J Am Med Assoc. 2001;285:2763–73. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- Drabick JJ, Narayanan RB, Williams JC, et al. Passive protection of mice against lethal Francisella tularensis (live tularemia vaccine strain) infection by the sera of human recipients of the live tularemia vaccine. Am J Med Sci. 1994;308:83–7. doi: 10.1097/00000441-199408000-00003. [DOI] [PubMed] [Google Scholar]
- Eigelsbach HT, Downs CM. Prophylactic effectiveness of live and killed tularemia vaccines. I. Production of vaccine and evaluation in the white mouse and guinea pig. J Immunol. 1961;87:415–25. [PubMed] [Google Scholar]
- Eigelsbach HT, Tulis JJ, McGavran MH, et al. LIVE TULAREMIA VACCINE I.: Host-parasite relationship in monkeys vaccinated intracutaneously or aerogenically. J Bacteriol. 1962;84:1020–7. doi: 10.1128/jb.84.5.1020-1027.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigelsbach HT, Tulis JJ, Overholt EL, et al. Aerogenic immunization of the monkey and guinea pig with live tularemia vaccine. P Soc Exp Biol Med. 1961;108:732–4. doi: 10.3181/00379727-108-27049. [DOI] [PubMed] [Google Scholar]
- Elkins KL, Cowley SC, Bosio CM. Innate and adaptive immunity to Francisella. Ann NY Acad Sci. 2007;1105:284–324. doi: 10.1196/annals.1409.014. [DOI] [PubMed] [Google Scholar]
- Ellis J, Oyston PC, Green M, et al. Tularemia. Clin Microbiol Rev. 2002;15:631–46. doi: 10.1128/CMR.15.4.631-646.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA. New drug and biological drug products; evidence needed to demonstrate effectiveness of new drugs when human efficacy studies are not ethical or feasible. Final rule. Fed Regist. 2002;67:37988–98. [PubMed] [Google Scholar]
- FDA. Guidance for industry: animal models - essential elements to address efficacy under the animal rule. 2014. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm399217.pdf.
- Foshay L. Tularaemia: a summary of certain aspects of disease including methods for early detection and the results of serum treatment in 600 patients. Medicine. 1940;19:1–81. [Google Scholar]
- Griffin AJ, Crane DD, Wehrly TD, et al. Successful protection against tularemia in C57BL/6 mice is correlated with expansion of Francisella tularensis-specific effector T cells. Clin Vaccine Immunol. 2015;22:119–28. doi: 10.1128/CVI.00648-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornick RB, Dawkins AT, Eigelsbach HT, et al. Oral tularemia vaccine in man. Antimicrob Agents Ch. 1966;6:11–4. doi: 10.1128/AAC.6.1.11. [DOI] [PubMed] [Google Scholar]
- Hornick RB, Eigelsbach HT. Aerogenic immunization of man with live tularemia vaccine. Bacteriol Rev. 1966;30:532–8. doi: 10.1128/br.30.3.532-538.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LA, Holmes SJ, Mendelman PM, et al. Safety of a trivalent live attenuated intranasal influenza vaccine, FluMist, administered in addition to parenteral trivalent inactivated influenza vaccine to seniors with chronic medical conditions. Vaccine. 1999;17:1905–9. doi: 10.1016/s0264-410x(98)00471-x. [DOI] [PubMed] [Google Scholar]
- Jemski JV. Respiratory tularemia: comparison of selected routes of vaccination in Fischer 344 rats. Infect Immun. 1981;34:766–72. doi: 10.1128/iai.34.3.766-772.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirimanjeswara GS, Olmos S, Bakshi CS, et al. Humoral and cell-mediated immunity to the intracellular pathogen Francisella tularensis. Immunol Rev. 2008;225:244–55. doi: 10.1111/j.1600-065X.2008.00689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koc S, Duygu F, Sogut E, et al. Clinical and laboratory findings of tularemia: a retrospective analysis. Kulak Burun Bogaz Ihtis Derg. 2012;22:26–31. doi: 10.5606/kbbihtisas.2012.005. [DOI] [PubMed] [Google Scholar]
- Kostiala AA, McGregor DD, Logie PS. Tularaemia in the rat. I. The cellular basis on host resistance to infection. Immunology. 1975;28:855–69. [PMC free article] [PubMed] [Google Scholar]
- Lebedinskii VA, Chicherin Iu V, Evstigneev VI, et al. Assessment of the effectiveness of different methods of immunization with live plague vaccine EB in aerosol infections. Zh Mikrob Epid Immun. 1979;9:11–4. [PubMed] [Google Scholar]
- Lyons CR, Wu TH. Animal models of Francisella tularensis infection. Ann NY Acad Sci. 2007;1105:238–65. doi: 10.1196/annals.1409.003. [DOI] [PubMed] [Google Scholar]
- Mara-Koosham G, Hutt JA, Lyons CR, et al. Antibodies contribute to effective vaccination against respiratory infection by type A Francisella tularensis strains. Infect Immun. 2011;79:1770–8. doi: 10.1128/IAI.00605-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marohn ME, Barry EM. Live attenuated tularemia vaccines: recent developments and future goals. Vaccine. 2013;31:3485–91. doi: 10.1016/j.vaccine.2013.05.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrumb FR. Aerosol infection of man with Pasteurella Tularensis. Bacteriol Rev. 1961;25:262–7. doi: 10.1128/br.25.3.262-267.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawool DB, Bitsaktsis C, Li Y, et al. Utilization of Fc receptors as a mucosal vaccine strategy against an intracellular bacterium, Francisella tularensis. J Immunol. 2008;180:5548–57. doi: 10.4049/jimmunol.180.8.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray HJ, Chu P, Wu TH, et al. The Fischer 344 rat reflects human susceptibility to Francisella pulmonary challenge and provides a new platform for virulence and protection studies. PLoS One. 2010;5:e9952. doi: 10.1371/journal.pone.0009952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray HJ, Cong Y, Murthy AK, et al. Oral live vaccine strain-induced protective immunity against pulmonary Francisella tularensis challenge is mediated by CD4+ T cells and antibodies, including immunoglobulin A. Clin Vaccine Immunol. 2009;16:444–52. doi: 10.1128/CVI.00405-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CR, Conlan JW. Differential susceptibility of Sprague-Dawley and Fischer 344 rats to infection by Francisella tularensis. Microb Pathogenesis. 2009;46:231–4. doi: 10.1016/j.micpath.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Reed DS, Smith L, Dunsmore T, et al. Pneumonic tularemia in rabbits resembles the human disease as illustrated by radiographic and hematological changes after infection. PLoS One. 2011;6:e24654. doi: 10.1371/journal.pone.0024654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed DS, Smith LP, Cole KS, et al. Live attenuated mutants of Francisella tularensis protect rabbits against aerosol challenge with a virulent type A strain. Infect Immun. 2014;82:2098–105. doi: 10.1128/IAI.01498-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy CJ, Pitt LM. Infectious disease aerobiology: aerosol challenge methods. In: Swearengen JR, editor. Biodefense: Research Methodology and Animal Models. Boca Raton, FL: CRC Press; 2005. pp. 61–76. [Google Scholar]
- Sabin AB. Oral poliovirus vaccine: history of its development and use and current challenge to eliminate poliomyelitis from the world. J Infect Dis. 1985;151:420–36. doi: 10.1093/infdis/151.3.420. [DOI] [PubMed] [Google Scholar]
- Santiago AE, Mann BJ, Qin A, et al. Characterization of Francisella tularensis Schu S4 defined mutants as live-attenuated vaccine candidates. Pathog dis. 2015;73 doi: 10.1093/femspd/ftw036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saslaw S, Carhart S. Studies with tularemia vaccines in volunteers. III. Serologic aspects following intracutaneous or respiratory challenge in both vaccinated and nonvaccinated volunteers. Am J Med Sci. 1961;241:689–99. [PubMed] [Google Scholar]
- Saslaw S, Eigelsbach HT, Prior JA, et al. Tularemia vaccine study. II. Respiratory challenge. Arch Intern Med. 1961;107:702–14. doi: 10.1001/archinte.1961.03620050068007. [DOI] [PubMed] [Google Scholar]
- Tulis JJ, Eigelsbach HT, Hornick RB. Oral vaccination against tularemia in the monkeys. P Soc Exp Biol Med. 1969;132:893–7. doi: 10.3181/00379727-132-34331. [DOI] [PubMed] [Google Scholar]
- Wu TH, Hutt JA, Garrison KA, et al. Intranasal vaccination induces protective immunity against intranasal infection with virulent Francisella tularensis biovar A. Infect Immun. 2005;73:2644–54. doi: 10.1128/IAI.73.5.2644-2654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TH, Zsemlye JL, Statom GL, et al. Vaccination of Fischer 344 rats against pulmonary infections by Francisella tularensis type A strains. Vaccine. 2009;27:4684–93. doi: 10.1016/j.vaccine.2009.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]