Abstract
Aim:
To determine whether interdependence in couples’ sleep (sleep–wake concordance i.e., whether couples are awake or asleep at the same time throughout the night) is associated with two markers of cardiovascular disease (CVD) risk, ambulatory blood pressure (BP) and systemic inflammation.
Methods:
This community-based study is a cross-sectional analysis of 46 adult couples, aged 18–45 years, without known sleep disorders. Percent sleep–wake concordance, the independent variable, was calculated for each individual using actigraphy. Ambulatory BP monitors measured BP across 48 h. Dependent variables included mean sleep systolic BP (SBP) and diastolic BP (DBP), mean wake SBP and DBP, sleep–wake SBP and DBP ratios, and C-reactive protein (CRP). Mixed models were used and were adjusted for age, sex, education, race, and body mass index.
Results:
Higher sleep–wake concordance was associated with lower sleep SBP (b = −.35, SE = .01) and DBP (b = −.22, SE = .10) and lower wake SBP (b = −.26, SE = .12; all p values < .05). Results were moderated by sex; for women, high concordance was associated with lower BP. Men and women with higher sleep–wake concordance also had lower CRP values (b = −.15, SE = .03, p < .05). Sleep–wake concordance was not associated with wake DBP or sleep/wake BP ratios. Significant findings remained after controlling for individual sleep quality, duration, and wake after sleep onset.
Conclusions:
Sleep–wake concordance was associated with sleep BP, and this association was stronger for women. Higher sleep–wake concordance was associated with lower systemic inflammation for men and women. Sleep–wake concordance may be a novel mechanism by which marital relationships are associated with long-term CVD outcomes.
Keywords: Couples’ sleep, cardiovascular disease risk, actigraphy, marital quality, blood pressure, inflammation
Statement of Significance
For many adults, sleep is a shared process between individuals. We found that the degree to which couples are awake or asleep at the same time (concordant) was associated with nighttime blood pressure and systemic inflammation, two putative markers of cardiovascular disease. That is, higher sleep-wake concordance was associated with lower blood pressure and lower inflammation. The link between concordance and CVD risk appears to be independent of individual (ie, each partners’) sleep quality, wake after sleep onset, or sleep duration. Future studies on sleep and cardiovascular disease risk should consider how the shared sleep experience could potentiate CVD risk.
INTRODUCTION
Sleep characteristics are associated with cardiovascular disease (CVD) risk factors in adults,1–5 independent of obstructive sleep apnea.6 For example, self-reported short sleep duration is associated with blood pressure (BP) and risk for hypertension.1–3 Studies on actigraphy-assessed sleep and ambulatory BP indicate that movement during sleep, wake after sleep onset (WASO), and sleep fragmentation are associated with an elevated nighttime/daytime BP ratio,7,8 a strong risk factor for cardiovascular morbidity.9 Insufficient sleep is also linked to systemic inflammation. In particular, both total and partial sleep deprivation are associated with higher C-reactive protein (CRP).10,11
Most of what is known about sleep and CVD risk has been measured in individuals, without accounting for bed partners. However, for roughly two-thirds of US adults,12 sleep is a shared biological and behavioral process that can also be characterized at the level of the dyad. Sleep patterns within couples are interdependent13 because of movement14,15 and sleep timing16 and perhaps because of direct physiological effects of close relationships.17,18 We recently demonstrated that couples’ actigraphy-assessed sleep–wake patterns are concordant, that is, couples were awake or asleep at the same time.19 The degree to which couples’ sleep is concordant also appears to be independent of individually assessed sleep parameters that are associated with CVD risk, such as sleep quality and circadian preference.19
Sleep–wake concordance, defined as the percent of the sleep period when couples are asleep or awake at the same time, can also be viewed as one type of coregulation (i.e., a reciprocally maintained physiological process that promotes the psychological and biological homeostasis of individuals in a relationship).17 Coregulation can be assessed within biological processes,20 such as heart rate and respiration21 and hypothalamic pituitary adrenal axis activity.22 Furthermore, relationship characteristics such as marital satisfaction are associated with coregulation. For example, relationship satisfaction is associated with concordance in cortisol fluctuations throughout the day.22,23 Therefore, sleep–wake concordance characterizes dyadic sleep and may be one way to characterize the degree to which physiological and behavioral coregulation occurs within couples, potentially influencing downstream health outcomes.
Coregulation of physiological processes has been most frequently examined as an outcome of relationship characteristics, or as a physiological process occurring with changes in affect (see reference20 for review). No prior study has examined whether coregulated sleep (i.e., sleep–wake concordance) in couples relates to health outcomes. However, there are several reasons to suspect that sleep–wake concordance is an important predictor of health. First, previous findings on the interdependence of couples’ sleep suggest that sleep–wake concordance may be a unique sleep characteristic. Therefore, it is important to know whether an examination of sleep at the dyadic level adds to existing knowledge on the associations between sleep and health. Second, sleep is a vulnerable physiological process that requires a sense of interpersonal security to downregulate vigilance and arousal,24 interpersonal security at night, in turn, may be associated with hormones, such as oxytocin, which is associated with affiliative behaviors, has stress-attenuating properties, and most recently has been linked to sleep.25,26 The degree to which couples are concordant may relate to other indices of arousal, such as BP, especially during the sleep period. Nighttime BP is strongly predictive of cardiovascular outcomes, independent of daytime measures.27 Finally, couples’ relationships are strongly linked to long-term health outcomes through relationship characteristics (e.g., marital satisfaction) and shared behaviors.17,28–31 In particular, couples’ relationship quality is associated with ambulatory BP32 and systemic inflammation.33 Therefore, couples’ sleep–wake concordance may be a unique, shared behavioral pathway by which both sleep and relationship quality are associated with CVD risk.
In the current study, we followed up on a previous study on couples’ sleep–wake concordance19 to examine the association between couples’ sleep–wake concordance and ambulatory BP during the sleep and wake periods, the sleep/wake BP ratio, and systemic inflammation. BP and inflammation were included as outcomes because they are both risk factors for CVD-related outcomes. We expected that higher concordance would be associated with lower BP levels for both partners. Given previous findings on sleep and systemic inflammation, we also expected that higher concordance would be associated with lower CRP. To determine whether sleep–wake concordance is independent from other individually assessed sleep characteristics, we conducted follow-up analyses controlling for individual sleep quality and total sleep time (TST). Lastly, because coregulation of other physiological systems is linked to marital quality20 and marital quality is associated with CVD risk markers including ambulatory BP (for example see references32,34–36), we examined whether marital adjustment moderated the association between concordance and BP and CRP.
METHODS
Participants and Procedure
The University of Pittsburgh Institutional Review Board approved this study. This analysis used a previously described sample19 of 48 healthy married couples recruited from the community between the ages of 18 and 45 years. After written informed consent, participants completed an in-person diagnostic evaluation and an in-home overnight screening for sleep-disordered breathing. To be included in the study, couples had to share a bed at least 4 nights per week. Exclusionary criteria included night-shift work, having a current sleep disorder, pregnancy, current psychiatric illness, or substance dependence. Participants who had cardiovascular or renal disease, diabetes, or were taking prescription medications known to affect the autonomic nervous system or sleep were also excluded.
Eligible participants completed a 10-day home assessment and an in-person laboratory assessment. The 10-day home assessment included 10 days of actigraphy (described below) and sleep diaries in which participants logged their individual bed- and wake-times and whether they shared a bed with their spouse the night before. The vast majority of the sample (92.6%) shared a bed for more than 75% of the nights, which is consistent with study eligibility. To avoid undue participant burden, couples were able to choose on what day to begin their 10-day assessment. Study start day was evenly distributed across 6 days (Monday–Saturday). Upon completing their 10-day assessment, participants returned to the research laboratory for a fasting blood draw. Two couples were excluded from all analyses because their 10-day home assessment was completed in a different time frame. Therefore, the final sample included 46 dyads.
Measures
Sleep–Wake Concordance
Each member of the couple wore a wrist actigraph (Actiwatch 64; Philips Respironics) continuously for 10 days. Individuals pressed an event marker on the actigraph when they got into bed and attempted to go to sleep and when they got out of bed and were no longer attempting to sleep. These markers defined the rest interval. If the participant did not press the event marker for a given night (<1% of the time), we used the diary bedtime and wake time to calculate their rest interval.
Sleep–wake state was determined for each 60-s epoch using a previously validated algorithm (Actiware v.5.70.1 Philips Respironics). We used the standard scoring algorithm set at the “medium” sensitivity of 40. Actiwatches for each couple were initialized on the same computer within a few minutes of each other and were programmed to start and stop collecting data at the same time.
The independent variable, percent sleep–wake concordance, has been described in detail in our prior work.19 Briefly, concordance was computed by dividing the number of epochs in which both partners had the same sleep–wake state by the number of total epochs [(No. of concordant epochs / No. of total epochs) * 100]. Concordance was measured based on a dyadic rest interval (i.e., the earliest bedtime and the latest wake time of individuals in the dyad) to capture variation in couples’ sleep throughout the night and timing of sleep (in- and out-of-bed times). A value of 85% means that the couple was concordant for sleep and wake in 85% of epochs within the dyadic rest interval. Percent sleep–wake concordance scores ranged from 54% to 88% (M = 74.8, SD = 7.22). Of the concordant epochs, sleep concordance was 66%; wake concordance was approximately 9%. Therefore, sleep concordance represented the majority of couples’ total sleep–wake concordance.
Our primary analyses were conducted using sleep–wake concordance calculated within the dyadic rest interval. We previously demonstrated that associations between sleep–wake concordance and relationship characteristics were unchanged when restricting sleep–wake concordance to the time interval when both members of the couple were in bed and trying to sleep.19 In the present paper, we again repeated analyses using the restricted time interval. Main effects and interactions described in the Results were in the same direction and still significant at the .05 level. We retained sleep–wake concordance based on the dyadic rest interval because it captures concordance in sleep and sleep timing. Both sleep and sleep timing may help to maintain homeostasis in sleep–wake rhythms, and concordance in sleep timing has been linked to relationship characteristics.37
Blood Pressure
Ambulatory BP monitors (BP; Oscar Suntech) were used to assess BP during the day and night across two consecutive 24-h periods within the 10-day assessment. BP was measured every 30 min across the waking period and every 60 min during the sleep period. BP monitors were programmed using habitual bedtime and wake time. BP during the sleep period was then defined based on actigraphy event markers and individuals’ sleep diary. Systolic and diastolic BPs were averaged across the sleep (sleep SBP and DBP) and wake periods (wake SBP and DBP) over 48 h. The number of usable readings for the wake period ranged from 22 to 46 (M = 32.7, SD = 4.36) and for the sleep period it ranged from 8 to 30 (M = 17.7, SD = 4.21). The sleep/wake SBP and DBP ratios were used as an index of nocturnal BP dipping8,38 and were derived by dividing the average sleep SBP and DBP by the average wake SBP and DBP, respectively.
Systemic Inflammation
Systemic inflammation was assessed with plasma CRP following the 10-day assessment period. Blood was drawn from the antecubital vein in the morning following a 12-h fast. Blood draws were rescheduled for a later date if participants endorsed any signs of acute illness (e.g., fever, congestions, sore throat, or acute infections due to injury). Samples were stored in citrated plasma tubes and frozen at −80°C and assayed in batches. Circulating levels of CRP in plasma were determined by high sensitivity ELISA (R&D Systems) and assayed by the University of Pittsburgh Cancer Institute Behavioral Medicine Core Facility laboratory, which is certified according to the Clinical Laboratory Improvement Amendments (CLIA).
Marital Quality
Marital quality was assessed using the Dyadic Adjustment Scale (DAS).39 The DAS is a widely used, well-validated measure of marital satisfaction, cohesion, consensus, and affection. Each member of the couple completed the measure. The measure includes items such as agreement on important matters in the relationship, demonstration of affection, and on amount of time spent together. Items are rated on a 6-point Likert scale from always agree to always disagree. Raw scores on the DAS range from 0 (low adjustment) to 151 (high adjustment) and ranged from 78 to 143 (M = 121.74) in this sample. The DAS is internally consistent39 and had good internal reliability in our sample (α = .86).
Covariates
Age, sex, education level, race, and body mass index (BMI) were included as covariates. Age was assessed at the consent visit. BMI was calculated based on weight and height measurements taken by a nurse practitioner during a visit to the laboratory. Education level was assessed on a 7-point scale ranging from 1 (less than high school) to 7 (advanced degree). Race was self-identified (Black or African American, American Indian, Asian, or White).
To determine the independent contribution of sleep–wake concordance, we included individual-level sleep variables as covariates including actigraphy-assessed TST, WASO, sleep quality, bedsharing frequency, and night awakenings due to children. Actigraphy measures were averaged over the 10-day assessment period. TST was defined by the total number of minutes from actigraphy-based sleep onset to sleep offset. WASO was defined as the total number of minutes awake after sleep initiation and before the final rise time. Sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI40).The PSQI measures sleep complaints during the previous month. The scale comprises 18 items and yields a global PSQI score, ranging from 0 to 21, with higher scores indicating poorer sleep quality. The PSQI is a valid assessment of sleep quality, reliably discriminates between good and poor sleepers, and has good internal and test-retest reliability.40,41 Bedsharing frequency was assessed nightly using a daily diary and summarized as a percentage of the 10-day assessment. Higher scores indicated more frequent bedsharing. We included individual reports of bedsharing to account for within-dyad discrepancy of bedsharing, which occurred in approximately 25% of dyads and is common in couples’ research.42,43 Frequency of waking at night due to children was assessed using a 0 to 4 scale, where 0 = does not wake at night because of children, 1 = wake less than once a week, 2 = 1–2 times per week, 3 = 3–4 times per week, and 4 = wake almost every night. Night waking due to children was not normally distributed (i.e., approximately 75% of the sample reported waking less than once a week); therefore, this variable was transformed to a dichotomous (yes/no) variable.
Analytic Plan
All data were checked for normality through visual inspection and statistical diagnostics. One dyad had low sleep–wake concordance (53.45%) that was nearly 3 SDs from the mean of the sample. All models were examined with and without this dyad. In all analyses, there were no changes in the direction of findings; therefore, we retained the dyad to be more inclusive. CRP was positively skewed and was log transformed for all analyses. One dyad was excluded from BP and CRP analyses due to one individual with average systolic blood values over 160 mm Hg during sleep and wake periods (i.e., more than 3 SDs from the sample mean). Four additional dyads are excluded from CRP analyses due to missing CRP values in one member of the dyad.
Sleep–wake concordance was included as a between-dyad predictor variable. Each couple had one concordance value that represented the average sleep–wake concordance over a 10-day period. Mixed modeling was used to account for interdependence in BP and CRP within the dyad. Heterogeneous compound symmetry was chosen as the covariance structure since the couples are distinguishable by sex.44 Age, sex, race, education level, and BMI were included as covariates in all models. We used a full information maximum likelihood estimation method. Within-subject predictors were grand mean centered.45 Effect size was calculated as the difference in variance explained (R2) after sleep concordance was added to a model that already included the above-mentioned covariates. In models that tested interactions between sleep–wake concordance and other between-dyad predictor variables, effect size was calculated by including the interaction term in models that already included all covariates and main effects. For significant associations between concordance and CVD risk markers, we conducted follow-up analyses that additionally controlled for TST, sleep quality, WASO, frequency of bedsharing, or awakenings due to children (entered in separate models). Finally, we tested the Concordance*Marital adjustment interaction to determine whether marital quality influenced the association between significant concordance and CVD risk associations.
RESULTS
Couple and Individual Sample Characteristics
About one-half (45%) of the couples were married 1–4 years; 21% were married < 1 year; 17% were married 5–10 years, 4% were married 11–14 years, and 10% were married > 15 years. Couples were predominantly Caucasian (79.3%). Approximately 35% of the sample woke at night due to children. Other sample characteristics and mean differences between husbands and wives are presented in Table 1. Husbands had higher sleep SBP, wake SBP and DBP, and higher BMI. Wives were also younger and had longer mean actigraphic sleep duration than husbands.
Table 1.
Means, Standard Deviations, and Paired t Tests of Descriptive and Study Variables.
| M (SD) | |||
|---|---|---|---|
| Study variables | Women (n = 46) | Men (n = 46) | t |
| Age (years) | 30.61 (6.15) | 31.43 (5.92) | −2.03* |
| Educationa | 5.37 (1.32) | 5.26 (1.54) | 0.48 |
| BMI (kg/m2) | 24.05 (5.64) | 26.49 (5.77) | 2.55* |
| Sleep SBP (mmHg) | 101.03 (9.9) | 106.10 (13.50) | −2.49* |
| Sleep DBP (mmHg) | 60.52 (6.94) | 61.80 (7.94) | −0.96 |
| Wake SBP (mmHg) | 115.31 (8.56) | 124.28 (10.69) | −7.76* |
| Wake DBP (mmHg) | 73.87 (5.86) | 76.44 (6.30) | −2.42* |
| SBP ratio (sleep SBP/wake SBP; mmHg) | 0.89 (0.05) | 0.86 (0.06) | 1.47 |
| DBP ratio (sleep DBP/wake DBP; mmHg) | 0.81 (0.06) | 0.81 (0.07) | 1.04 |
| CRP levels (mg/L) | 2.20 (2.75) | 1.67 (2.11) | −1.04 |
| Actigraphy-assessed TST (h) | 6.60 (0.76) | 6.30 (0.90) | 2.55* |
| PSQI (total score) | 2.88 (1.7) | 3.13 (1.8) | −0.66 |
| Actigraphy-assessed WASO (min) | 58.34 (27.9) | 60.74 (34.33) | −0.565 |
| Marital adjustment | 121.83 (12.53) | 121.65 (11.06) | 0.083 |
BMI = body mass index; DBP = diastolic blood pressure; PSQI = Pittsburgh Sleep Quality Index; SBP = systolic blood pressure; TST = total sleep time; WASO = wake after sleep onset.
Education is coded on a scale from 1 to 7, with 1 being less than high school and 7 being a postgraduate degree. On average, women and men in this sample had a college degree.
p < .05.
Bivariate Correlations
Zero-order bivariate correlations among predictors and outcomes are presented in Table 2. Husbands’ correlations are shaded and displayed above the diagonal line and wives’ correlations are presented below the diagonal line. The bolded diagonal line represents within-dyad correlations. For wives, sleep–wake concordance was significantly correlated with sleep SBP and DBP, wake DBP, and CRP (all p values < .05) such that higher sleep–wake concordance was associated with lower BP and CRP. Wake DBP and sleep–wake SBP and DBP ratios were in the same direction but not significant. For husbands, higher sleep–wake concordance was significantly correlated with lower CRP. Sleep–wake concordance was not significantly correlated with any of the BP measures for husbands, though correlations were in the same direction as that for wives. Sleep–wake concordance and WASO were negatively correlated for husbands. Marital adjustment was not correlated with sleep–wake concordance for wives or husbands. Within the husband and wife dyad, sleep DBP and wake DBP were significantly and positively correlated. There were no significant correlations between husbands and wives on any other ambulatory BP measures or on CRP. As previously reported, PSQI scores were negatively correlated between wives and husbands.19 TST and WASO between husbands and wives were positively correlated.
Table 2.
Bivariate Intercorrelations for Concordance and Relationship and Sleep Measures and CVD Risk Factors (N = 46 dyads).
| Study variables | Sleep–wake concordance | Sleep SBP | Sleep DBP | Wake SBP | Wake DBP | Sleep/wake SBP ratio | Sleep/Wake DBP ratio | CRP | Marital adjustment | Actigraphy- assessed TST | PSQI | Actigraphy- assessed WASO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sleep–wake concordance | × | −.183 | −.150 | −.148 | −.114 | −.129 | −.109 | −.467** | .169 | .025 | −.068 | −.410** |
| Sleep SBP | −.413** | .075 | .802*** | .843*** | .580*** | .708*** | .652*** | 297 | .010 | .235 | .242 | .292* |
| Sleep DBP | −.313* | .821*** | .326 * | .632*** | .794*** | .639*** | .756*** | .449** | −.109 | .166 | .284 | .231 |
| Wake SBP | −.435** | .814*** | .606*** | .069 | .689*** | .220 | .254 | .211 | −.078 | .095 | .341* | .199 |
| Wake DBP | −.287 | .696*** | .749*** | .813*** | .318 * | .163 | .206 | .534*** | −.147 | .131 | .334* | .478** |
| Sleep/wake SBP ratio | −.145 | .650*** | .605*** | .089 | .125 | .084 | .855*** | .246 | .105 | .265 | −.015 | .273 |
| Sleep/Wake DBP ratio | −.162 | .481** | .695*** | .031 | .049 | .780*** | .305 | .150 | −.022 | .128 | .105 | .266 |
| CRP | −.312* | .414** | .280 | .346* | .197 | .256 | .234 | .057 | −.093 | −.008 | ..041 | .067 |
| Marital adjustment | .025 | −.218 | −.347* | −.149 | −.318* | −.170 | −.171 | −.102 | .275 | .331* | −.378* | .026 |
| Actigraphy-assessed TST | .186 | .111 | .029 | .009 | .024 | .185 | .028 | .226 | −.020 | .406 ** | −.145 | .268 |
| PSQI | .178 | .009 | −.073 | .040 | −.014 | −.024 | −.073 | .244 | −.066 | .152 | −.466** | .072 |
| Actigraphy-assessed WASO | −.242 | .053 | −.025 | .033 | .244 | .045 | −.048 | .245 | −.110 | .370* | .260 | .377 * |
DBP = diastolic blood pressure; PSQI = Pittsburgh Sleep Quality Index; SBP = systolic blood pressure; TST = total sleep time; WASO = wake after sleep onset.
Values for husbands are shaded and displayed above the diagonal and values for wives are displayed below the diagonal. The bolded diagonal line represents within-couple correlations on each measure.
p < .05.
p < .01.
p < .001.
Sleep–Wake Concordance on BP and CRP
Main effects and interactions between sleep–wake concordance and BP and CRP using mixed modeling results are presented in Table 3 and summarized below. Unstandardized coefficients (b) and standard errors are reported for each model.
Table 3.
Mixed Model Results of Main Effects of Sleep–Wake Concordance and Sex*Sleep–Wake Concordance Interactions on CVD Risk Factors.
| CVD risk variables | Sex*Concordance interaction | |||||
|---|---|---|---|---|---|---|
| b (SE) | p | R 2 a | b (SE) | p | R 2 b | |
| Wake BP | ||||||
| SBP | −.26 (.12) | .037 | .036 | .53 (.25) | .043 | .053 |
| DBP | −.16 (.09) | .070 | .037 | .18 (.17) | .298 | .011 |
| Sleep BP | ||||||
| SBP | −.35 (.13) | .008 | .041 | .61 (.30) | .050 | .051 |
| DBP | −.22 (.10) | .033 | .043 | .30 (.19) | .126 | .030 |
| Sleep/Wake BP ratio | ||||||
| SBP ratio | −.001 (.001) | .275 | 0.00 | .001 (.001) | .527 | 0.00 |
| DBP ratio | −.001 (.001) | .289 | 0.00 | .002 (.002) | .238 | 0.00 |
| Systemic inflammation | ||||||
| CRP | −.03 (.03) | <.001 | .122 | −.014 (.01) | .375 | .130 |
Covariates included in models: sex, age, race, education, and body mass index.
CRP = C-reactive protein; CVD = cardiovascular disease; DBP = diastolic blood pressure; SBP = systolic blood pressure.
R 2 = amount of variance explained beyond covariates.
R 2 = amount of variance explained beyond covariates and main effect of sleep–wake concordance.
Blood Pressure
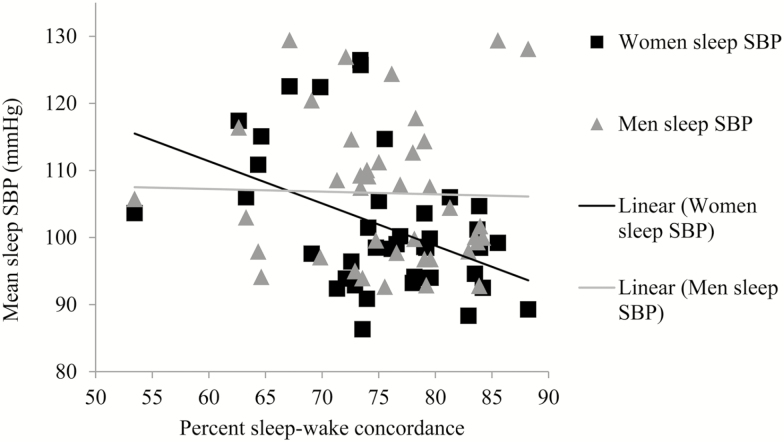
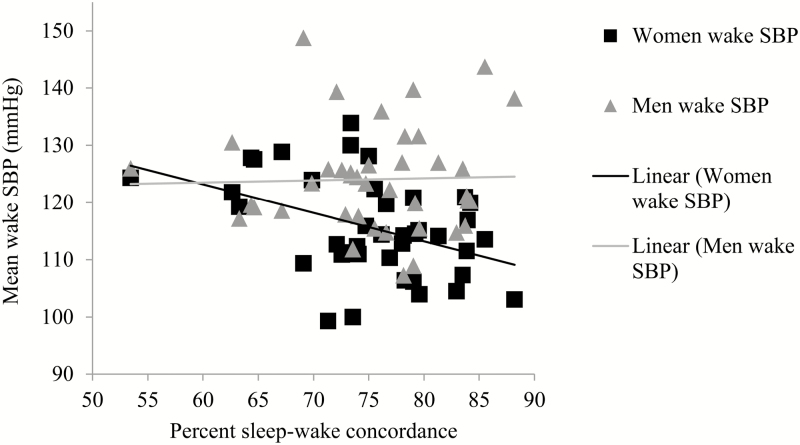
Higher sleep–wake concordance was associated with lower sleep SBP, lower sleep DBP, and lower wake SBP. Sleep–wake concordance was also associated with lower wake DBP, but this effect was only marginal (p = .07). Sleep–wake concordance was not associated with sleep/wake SBP or DBP ratios. The Sex*Sleep–wake concordance interaction was marginally significant for sleep SBP (Figure 1) and significant for wake SBP (Figure 2). In both models, higher sleep–wake concordance was associated with lower SBP in women but not in men. The Sex*Sleep–wake concordance interaction was not significant for any of the other BP variables.
Figure 1.
Unadjusted associations between sleep–wake concordance and sleep SBP values. SBP = systolic blood pressure.
Figure 2.
Unadjusted associations between sleep–wake concordance and wake SBP. SBP = systolic blood pressure.
Systemic Inflammation
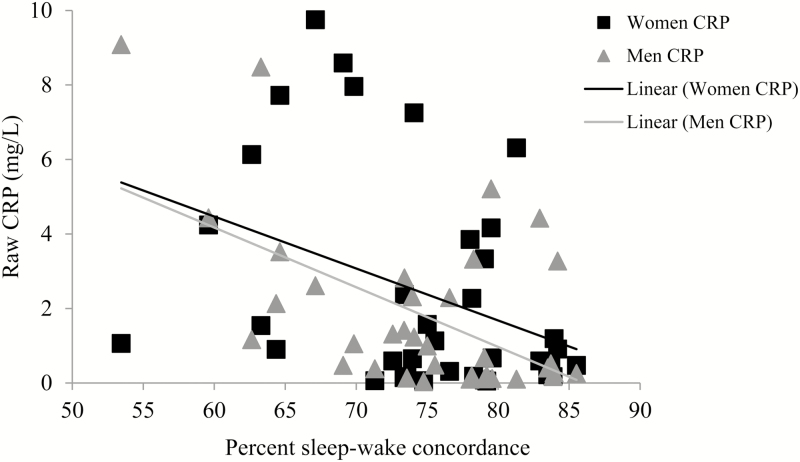
Sleep–wake concordance was significantly associated with CRP (Table 3). As depicted in Figure 3, in both men and women, higher concordance was associated with lower CRP values. The slopes are presented for both men and women in Figure 3; however, the Sex*Sleep–wake concordance interaction was nonsignificant.
Figure 3.
Unadjusted associations between sleep–wake concordance and CRP. CRP = C-reactive protein. Raw values are plotted for interpretability. Five dyads are excluded from analyses and the scatterplot due to missing CRP values in one or both members of the dyad (n = 4) or due to elevated SBP in one member of the dyad (n = 1). Therefore, 10 cases were excluded. Two cases (one dyad) appear to be multivariate outliers; however, we tested this model without the two cases (one dyad) and the findings are in the same direction and significant at the .05 level. Linear trend lines are presented for both men and women; the Sex*Sleep–wake concordance interaction is not significant (b = −.08, SE = .08, p =.282).
Follow-up Analyses
In follow-up analyses, we first tested whether individual variations in sleep quality, TST, and WASO explained some of the observed associations between sleep–wake concordance and sleep SBP, sleep DBP, wake SBP, and CRP. For these significant associations, we additionally controlled for subjective sleep quality (PSQI scores), actigraphy-assessed TST, and WASO in separate models. The main effects of sleep–wake concordance and sleep SBP, sleep DBP, and CRP remained statistically significant and in the same direction after controlling for these individual-level sleep characteristics.
We previously reported that 35% of the sample had children and having children did not influence concordance rates.19 In the current analysis, we additionally covaried for waking at night due to children on significant associations between concordance and CVD risk variables (sleep SBP and DBP, wake DBP, and CRP). The presence of night waking due to children did not have an effect on associations between concordance and CVD risk factors. However, we interpret this finding cautiously because approximately 65% of the sample did not have children.
Finally, we assessed whether variation in bedsharing influenced significant associations between concordance and CVD risk variables (sleep SBP and DBP, wake DBP, and CRP). Bedsharing frequency did not influence the association between concordance and sleep SBP, sleep DBP, or CRP. When bedsharing was included in the association between concordance and wake SBP, the finding was in the same direction, but no longer significant at the .05 level (b = −.221, SE = .12, p = .072).
Does Marital Adjustment Moderate the Association Between Sleep–Wake Concordance and BP and CRP?
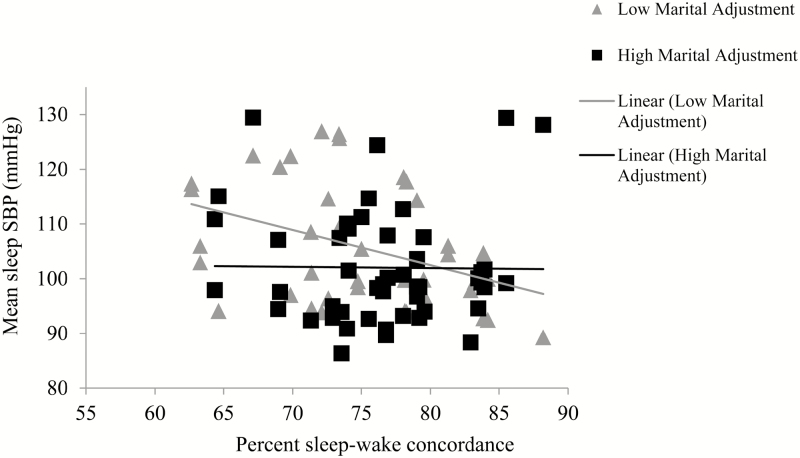
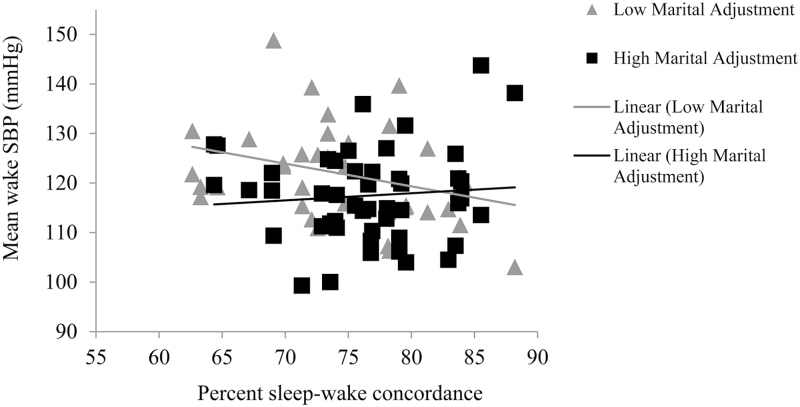
We examined whether marital adjustment moderated significant associations between concordance and CVD risk (wake SBP, sleep SBP and DBP, and CRP). Marital adjustment did not moderate the association between concordance and sleep DBP (b = −.006, SE = .008, p =.439) or CRP (b = −.001, SE = .001, p =.187). The Concordance*Marital adjustment interaction for sleep SBP approached significance (b = .022, SE = .011, p = .062; Figure 4). At higher levels of concordance, there was no effect of marital adjustment on sleep SBP. At lower levels of concordance, however, lower marital adjustment was associated with higher sleep SBP. Marital adjustment also moderated the association between concordance and wake SBP (b = .023, SE = .010, p = .025) and in the same direction as with sleep SBP (see Figure 5).
Figure 4.
Marital adjustment moderates the association between sleep–wake concordance and sleep SBP in men and women (unadjusted association presented). SBP = systolic blood pressure. Mean marital adjustment is 122; low marital adjustment line represents cases that are below the group mean and high marital adjustment line represents cases that are at or above the group mean.
Figure 5.
Marital adjustment moderates the association between sleep–wake concordance and wake SBP in men and women (unadjusted association presented). SBP = systolic blood pressure. Mean marital adjustment is 122; low marital adjustment line represents cases that are below the group mean and high marital adjustment line represents cases that are at or above the group mean.
DISCUSSION
Higher sleep–wake concordance in couples was associated with lower sleep SBP and DBP and lower wake SBP. These effects were stronger in women than in men. Sleep–wake concordance was not associated with wake DBP or the sleep/wake SBP and DBP ratios. Higher concordance was associated with lower CRP, and there was no evidence of gender differences in this effect. Marital adjustment moderated the association between concordance and wake SBP, such that lower marital adjustment was associated with higher wake SBP. The marital adjustment by concordance interaction for sleep SBP was in the same direction, but did not reach significance at the .05 level. Marital adjustment did not moderate the association between concordance and CRP or concordance and sleep DBP.
The finding that sleep–wake concordance is associated with lower sleep BP adds a dyadic sleep association to previous findings in which individually assessed sleep parameters, such as sleep quality and TST, are associated with BP.2,3,7 In addition, sleep–wake concordance was associated with CRP, which adds to a limited number of studies on individuals’ sleep and inflammation.46 We previously demonstrated that sleep–wake concordance was not correlated with circadian preference and sleep quality.19 In the current study, findings between concordance and BP and CRP remained significant even after controlling for TST, sleep quality, WASO, and awakenings due to children. Therefore, current findings provide further support for sleep–wake concordance as a novel sleep measure that takes into account dyadic sleep patterns.
Concordance was not associated with sleep/wake BP ratio, an index of nocturnal BP dipping. Given that this was a normotensive population, it is likely that sleep BP drove the sleep/wake ratio since most individuals in our sample had lower BP at night. However, nighttime BP itself is a strong predictor of CVD risk27 and CVD mortality.47 Moreover, concordance was strongly linked to CRP, which is a risk marker for later development of heart disease in healthy adults.48 Concordance was not associated with wake DBP, and the magnitude of the association between concordance and wake SBP was less robust than the associations between concordance and sleep BP. It is possible that associations between concordance and BP are stronger when couples are in closer proximity. For example, in an examination of spousal presence on cardiovascular responses, spousal presence is associated with reduced cardiovascular reactivity.49 Overall, the associations between concordance and sleep BP and CRP suggest that dyadic sleep patterns may be one mechanism by which couples’ relationships are linked to CVD risk.
A secondary aim of the current study was to test whether marital adjustment, a type of overall marital quality, moderated the association between concordance and CVD risk. We previously demonstrated that marital satisfaction moderated associations between concordance and attachment style.19 Given that marital quality is associated with better couple-level outcomes,28 we expected that the association between concordance and CVD risk factors would vary based on individual scores on marital adjustment. The associations between concordance and sleep SBP and wake SBP were each moderated by marital adjustment. For both findings, at higher levels of concordance, there was no effect of marital adjustment on SBP. At lower levels of concordance, lower marital adjustment was associated with higher sleep SBP. This suggests that higher concordance may buffer lower levels of marital adjustment. Alternatively, low marital adjustment may potentiate the association between low concordance and higher CRP. However, many of the couples in the current study were satisfied in their marriage; therefore, it will be important to obtain greater diversity in marital quality in future studies. That marital adjustment moderated the association between concordance and BP is partially consistent with previous findings on marital satisfaction and ambulatory BP. Holt-Lunstad and colleagues found that higher marital satisfaction was associated with lower wake SBP; however, they did not find associations between marital satisfaction and nocturnal SBP or DBP.32
In addition to novel findings on sleep and health, the current findings add to our general understanding and knowledge about coregulation and health. To our knowledge, this is the first study to demonstrate an association between one type of physiological coregulation (sleep) and another biological process (BP and CRP). In general, very few studies have examined physiological coregulation in the context of couples’ relationships and few studies have examined sleep in a dyadic context.13–15,37 Our findings suggest that coregulation of sleep may be one way that couples, especially women, downregulate cardiovascular arousal. One function of coregulation is to regulate biology within optimal bounds.50 Biological regulation is also thought to be most effective when in proximity to trusted others.51 Coregulated sleep, therefore, may be one way to maintain physiological balance in other biological systems, such as sympathetic activity, immune functioning, and hormones associated with both affiliation and attenuated stress response, such as oxytocin.17,52,53 In the current study, sex interactions indicated stronger relationships between concordance and SBP for women than for men. As with previous studies on relationship factors and physiological outcomes in couples,28 women may be more sensitive to potential benefits of sleep–wake concordance. However, for both men and women, greater sleep–wake concordance was associated with lower CRP, which suggests that concordance is also associated with beneficial outcomes for men. Despite these intriguing findings, research on coregulation of biology and research on sleep in a dyadic context are still in the early stages.18 We speculate that sleep–wake concordance may be a type of attachment behavior that fosters regulation of the stress response and immune systems. Future research on coregulation will help determine whether coregulation in other biological systems is also associated with downregulation of physiological arousal and immune functioning, or whether this is unique to sleep–wake concordance.
The current study has several limitations. Individuals in this study were heterosexual, healthy, and generally happily married couples, which likely limited the range of findings on marital quality and CVD risk factors. Studies of sleep–wake concordance with culturally diverse couples who have greater health diversity are warranted. Higher sleep–wake concordance in this study was associated with more advantageous markers of health. However, it is important to consider how this could vary depending on the sleep health of the individuals in the relationships. A considerable strength of the study was that couples were free from sleep disorders, including insomnia and obstructive sleep apnea (determined by in-home objective apnea screening). However, we did not specifically assess snoring, which has been shown to be associated with poor sleep quality in the bedpartner,54 and thus, may have introduced some heterogeneity in our results. Among couples with sleep disorders, greater sleep–wake concordance may actually serve to potentiate health risk, as sleep disorders in one or both members may exacerbate sleep disturbances.55 Moreover, it is possible that couples with lower sleep–wake concordance have other relative relationship strengths. For example, in one cross-sectional study, well-adjusted couples who had different bed-timing preferences (owls versus larks) demonstrated flexible problem-solving skills.56 The current findings are also cross-sectional, which limits the ability to draw conclusions on the direction of the findings. To provide a stronger test of the directionality of the association, a useful next step would be to examine night-to-night variation in sleep–wake concordance in conjunction with day-to-day relationship factors and health outcomes. For example, Hasler and Troxel found that lower sleep onset concordance predicted more negative interactions the next day,37 but they did not examine whether nightly concordance was associated with daily fluctuations in any physiological outcome. In addition, in future couples’ sleep research, it will be useful to consider how social jetlag influences the association between concordance and CVD risk given recent research on the importance of shifts in sleep timing.57 Finally, we defined coregulation as concordance in sleep and wake patterns; however, other types of coregulation are certainly possible50 in dyadic sleep. For example, it will be useful to explore time-lagged effects (e.g., partner A influences partner B) and coregulation in staging (i.e., architecture) using polysomnography.
In conclusion, the current study is the first to demonstrate that sleep–wake concordance is linked to lower BP, particularly for women, and to lower systemic inflammation for men and women in healthy, well-adjusted couples. These results, along with previous findings regarding marital satisfaction and concordance, suggest that couples’ sleep–wake concordance relates to couples’ psychological and physiological health. This suggests that sleep–wake concordance may be a novel mechanism by which couples’ relationships are associated with long-term health outcomes, such as CVD. Taken more broadly, our findings also provide more support for consideration of close relationships when evaluating individual sleep in research and clinical settings.
FUNDING
Support for this study was provided by the National Heart, Lung, and Blood Institute (NHLBI) K23HL093220 and HL112646 (PI: WMT). Support for HEG and MRC was provided by NHLBI T32 HL082610 (PI: DJB). Support for CEK was provided by NHLBI K23 HL118318 (PI: CE). Additional support for HEG was provided by the Sleep Research Society Foundation 013-JP-16 (PI: HEG).
DISCLOSURE STATEMENT
DJB has served as a paid consultant on scientific advisory boards for the following companies: Merck, CME Outfitters, and Medscape. Remaining authors have no conflicts to disclose.
REFERENCES
- 1. Cappuccio FP, Stranges S, Kandala NB, et al. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007; 50(4): 693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006; 29(8): 1009–1014. [DOI] [PubMed] [Google Scholar]
- 3. Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006; 47: 833–839. [DOI] [PubMed] [Google Scholar]
- 4. Quan SF. Sleep disturbances and their relationship to cardiovascular disease. Am J Lifestyle Med. 2009; 3(1 Suppl): 55S–59S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003; 163(2): 205–209. [DOI] [PubMed] [Google Scholar]
- 6. Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010; 122(4): 352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Loredo JS, Nelesen R, Ancoli-Israel S, Dimsdale JE. Sleep quality and blood pressure dipping in normal adults. Sleep. 2004; 27(6): 1097–1103. [DOI] [PubMed] [Google Scholar]
- 8. Matthews KA, Kamarck TW, H Hall M, et al. Blood pressure dipping and sleep disturbance in African-American and Caucasian men and women. Am J Hypertens. 2008; 21(7): 826–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verdecchia P, Angeli F, Borgioni C, Gattobigio R, Reboldi G. Ambulatory blood pressure and cardiovascular outcome in relation to perceived sleep deprivation. Hypertension. 2007; 49(4): 777–783. [DOI] [PubMed] [Google Scholar]
- 10. Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004; 43(4): 678–683. [DOI] [PubMed] [Google Scholar]
- 11. van Leeuwen WM, Lehto M, Karisola P, et al. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS One. 2009; 4:e4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. National Sleep Foundation. 2005 Sleep in America Poll. Washington, DC: National Sleep Foundation; 2005: 21–30. [Google Scholar]
- 13. Meadows R, Arber S, Venn S, Hislop J, Stanley N. Exploring the interdependence of couples’ rest-wake cycles: an actigraphic study. Chronobiol Int. 2009; 26(1): 80–92. [DOI] [PubMed] [Google Scholar]
- 14. Pankhurst FP, Horne JA. The influence of bed partners on movement during sleep. Sleep. 1994; 17(4): 308–315. [DOI] [PubMed] [Google Scholar]
- 15. Meadows R, Venn S, Hislop J, Stanley N, Arber S. Investigating couples’ sleep: an evaluation of actigraphic analysis techniques. J Sleep Res. 2005; 14(4): 377–386. [DOI] [PubMed] [Google Scholar]
- 16. Dé Waterman AL, Kerkhof G. Sleep-wake patterns of partners. Percept Mot Skills. 1998; 86: 1141–1142. [DOI] [PubMed] [Google Scholar]
- 17. Sbarra DA, Hazan C. Coregulation, dysregulation, self-regulation: an integrative analysis and empirical agenda for understanding adult attachment, separation, loss, and recovery. Pers Soc Psychol Rev. 2008; 12(2): 141–167. [DOI] [PubMed] [Google Scholar]
- 18. Troxel WM, Robles TF, Hall M, Buysse DJ. Marital quality and the marital bed: examining the covariation between relationship quality and sleep. Sleep Med Rev. 2007; 11(5): 389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gunn HE, Buysse DJ, Hasler BP, Begley A, Troxel WM. Sleep concordance in couples is associated with relationship characteristics. Sleep. 2015; 38(6): 933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Timmons AC, Margolin G, Saxbe DE. Physiological linkage in couples and its implications for individual and interpersonal functioning: a literature review. J Fam Psychol. 2015; 29(5): 720–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferrer E, Helm JL. Dynamical systems modeling of physiological coregulation in dyadic interactions. Int J Psychophysiol. 2013; 88(3): 296–308. [DOI] [PubMed] [Google Scholar]
- 22. Saxbe D, Repetti RL. For better or worse? Coregulation of couples’ cortisol levels and mood states. J Pers Soc Psychol. 2010; 98(1): 92–103. [DOI] [PubMed] [Google Scholar]
- 23. Liu S, Rovine MJ, Klein LC, Almeida DM. Synchrony of diurnal cortisol pattern in couples. J Fam Psychol. 2013; 27(4): 579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dahl RE, El-Sheikh M. Considering sleep in a family context: introduction to the special issue. J Fam Psychol. 2007; 21(1): 1–3. [DOI] [PubMed] [Google Scholar]
- 25. Troxel WM. It’s More than sex: exploring the dyadic nature of sleep and implications for health. Psychosom Med. 2010; 72(6): 578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blagrove M, Fouquet NC, Baird AL, et al. Association of salivary-assessed oxytocin and cortisol levels with time of night and sleep stage. J Neural Transm (Vienna). 2012; 119(10): 1223–1232. [DOI] [PubMed] [Google Scholar]
- 27. Tsioufis C, Andrikou I, Thomopoulos C, Syrseloudis D, Stergiou G, Stefanadis C. Increased nighttime blood pressure or nondipping profile for prediction of cardiovascular outcomes. J Hum Hypertens. 2011; 25(5): 281–293. [DOI] [PubMed] [Google Scholar]
- 28. Kiecolt-Glaser JK, Newton TL. Marriage and health: his and hers. Psychol Bull. 2001; 127(4): 472–503. [DOI] [PubMed] [Google Scholar]
- 29. Pietromonaco PR, Uchino BNDSC Close relationship processes and health: implications for attachment theory for health and disease. Health Psychol. 2014; 32(5): 499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meyler D, Stimpson JP, Peek MK. Health concordance within couples: a systematic review. Soc Sci Med. 2007; 64(11): 2297–2310. [DOI] [PubMed] [Google Scholar]
- 31. Robles TF, Slatcher RB, Trombello JM, McGinn MM. Marital quality and health: a meta-analytic review. Psychol Bull. 2014; 140(1): 140–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Holt-Lunstad J, Birmingham W, Jones BQ. Is there something unique about marriage? The relative impact of marital status, relationship quality, and network social support on ambulatory blood pressure and mental health. Ann Behav Med. 2008; 35: 239–244. [DOI] [PubMed] [Google Scholar]
- 33. Sbarra DA. Marriage protects men from clinically meaningful elevations in C-reactive protein: results from the National Social Life, Health, and Aging Project (NSHAP). Psychosom Med. 2009; 71(8): 828–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Troxel WM, Matthews KA, Gallo LC, Kuller LH. Marital quality and occurrence of the metabolic syndrome in women. Arch Intern Med. 2005; 165(9): 1022–1027. [DOI] [PubMed] [Google Scholar]
- 35. Smith TW, Uchino BN, MacKenzie J, et al. Effects of couple interactions and relationship quality on plasma oxytocin and cardiovascular reactivity: empirical findings and methodological considerations. Int J Psychophysiol. 2013; 88: 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith TW, Uchino BN, Berg CA, Florsheim P. Marital discord and coronary artery disease: a comparison of behaviorally defined discrete groups. J Consult Clin Psychol. 2012; 80(1): 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hasler BP, Troxel WM. Couples’ nighttime sleep efficiency and concordance: evidence for bidirectional associations with daytime relationship functioning. Psychosom Med. 2010; 72(8): 794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fagard RH, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA. Night-day blood pressure ratio and dipping pattern as predictors of death and cardiovascular events in hypertension. J Hum Hypertens. 2009; 23: 645–653. [DOI] [PubMed] [Google Scholar]
- 39. Spanier G. Measuring dyadic adjustment: new scales for assessing the quality of marriage and similar dyads. J Marriage Fam. 1976; 38: 15–28. [Google Scholar]
- 40. Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989; 28(2): 193–213. [DOI] [PubMed] [Google Scholar]
- 41. Morin CM, Espie CA. Insomnia: A Clinical Guide to Assessment and Treatment. New York, NY: Kluwer Academic/Plenum Publishers; 2003. [Google Scholar]
- 42. Jacobson NS, Moore D. Spouses as observers of the events in their relationship. J Consult Clin Psychol. 1981; 49(2): 269–277. [DOI] [PubMed] [Google Scholar]
- 43. Christensen A, Nies DC. The spouse observation checklist: empirical analysis and critique. Am J Fam Ther. 1980; 8(2): 69–79. [Google Scholar]
- 44. Kenny DAKDA&CWL. Dyadic Data Analysis. New York, NY: The Guilford Press; 2006. [Google Scholar]
- 45. Enders CK, Tofighi D. Centering predictor variables in cross-sectional multilevel models: a new look at an old issue. Psychol Methods. 2007; 12(2): 121–138. [DOI] [PubMed] [Google Scholar]
- 46. Miller MA, Kandala NB, Kivimaki M, et al. Gender differences in the cross-sectional relationships between sleep duration and markers of inflammation: Whitehall II study. Sleep. 2009; 32: 857–864. [PMC free article] [PubMed] [Google Scholar]
- 47. Fagard RH, Celis H, Thijs L, et al. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension. 2008; 51: 55–61. [DOI] [PubMed] [Google Scholar]
- 48. Koenig W, Sund M, Fröhlich M, et al. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999; 99: 237–242. [DOI] [PubMed] [Google Scholar]
- 49. Phillips AC, Carroll D, Hunt K, Der G. The effects of the spontaneous presence of a spouse/partner and others on cardiovascular reactions to an acute psychological challenge. Psychophysiology. 2006; 43: 633–640. [DOI] [PubMed] [Google Scholar]
- 50. Butler EA, Randall AK. Emotional coregulation in close relationships. Emot Rev. 2013; 5(2): 202–210. [Google Scholar]
- 51. Beckes L, Coan JA. Social baseline theory: the role of social proximity in emotion and economy of action. Soc Personal Psychol Compass. 2011; 5: 976–988. [Google Scholar]
- 52. Holt-Lunstad J, Birmingham WA, Light KC. Influence of a “warm touch” support enhancement intervention among married couples on ambulatory blood pressure, oxytocin, alpha amylase, and cortisol. Psychosom Med. 2008; 70: 976–985. [DOI] [PubMed] [Google Scholar]
- 53. Diamond LM. Contributions of psychophysiology to research on adult attachment: review and recommendations. Personal Soc Psychol Rev. 2001; 5(4): 276–295. [Google Scholar]
- 54. Beninati W, Harris CD, Herold DL, Shepard JW., Jr The effect of snoring and obstructive sleep apnea on the sleep quality of bed partners. Mayo Clin Proc. 1999; 74(10): 955–958. [DOI] [PubMed] [Google Scholar]
- 55. Ulfberg J, Carter N, Talbäck M, Edling C. Adverse health effects among women living with heavy snorers. Health Care Women Int. 2000; 21: 81–90. [DOI] [PubMed] [Google Scholar]
- 56. Larson JH, Crane D, Smith CW. Morning and night couples: the effect of wake and sleep patterns on marital adjustment. J Marital Fam Ther. 1991; 17: 53–65. [Google Scholar]
- 57. Parsons MJ, Moffitt TE, Gregory AM, et al. Social jetlag, obesity and metabolic disorder: investigation in a cohort study. Int J Obes (Lond). 2015; 39(5): 842–848. [DOI] [PMC free article] [PubMed] [Google Scholar]