Abstract
Background
Apelin, an endogenous peptide, has recently gained attention due to its positive inotropic effects in heart failure physiopathology. We investigated the relationship between serum apelin levels and the severity of calcific aortic stenosis (AS).
Methods
A total of 68 consecutive patients diagnosed with calcific AS and a control group of 32 subjects were included in the study. The subjects were divided into three group as follows: the control group, the mild-moderate AS group and the severe AS group. Blood samples were obtained from all of the subjects, which were used for biochemical comparisons of apelin 36 and high-sensitive C-reactive protein (hsCRP) levels.
Results
Plasma apelin 36 levels were significantly lower in the patients with severe AS [490 (247-1074) pg/ml] compared to both the mild-moderate AS [209 (97-453) pg/ml] and control [660 (378-1200) pg/ml] groups (p < 0.001). Correlation analysis between the left ventricular mass index and apelin concentrations revealed a significant negative correlation between the two parameters (p < 0.001, r = -0.478).
Conclusions
Our study demonstrated decreased apelin levels and increased hsCRP concentrations in patients with severe calcific AS. Our findings may help to clarify the exact pathophysiologic role of apelin in cardiovascular diseases.
Keywords: Aortic stenosis, Apelin, Apelin 36, Left ventricular hypertrophy
INTRODUCTION
Aortic valve stenosis (AS) is the most common valvular heart disease in developed countries,1 with a 1-year mortality rate up to 14% even if interventional treatment is performed.2 Both the management of the disease and the accompanying structural diseases make it difficult to manage AS.3,4 Although several pathophysiological factors have been proposed regarding the initiation and progression of AS, there is an obvious resemblance between AS and atherosclerosis due to similar histopathological findings.3 In addition, both entities share the same etiologic risk factors including hypertension, smoking and hypercholesterolemia.4 Furthermore, Palta et al. reported that the presence of hypercholesterolemia, smoking, increased serum creatinine, and calcium concentrations accelerate the progression of stenosis similar to atherosclerosis.5 Aortic valve sclerosis is closely related to endothelial dysfunction.6 Kadoglou and coworkers7 reported increased hsCRP levels and decreased apelin levels in patients with both stable and unstable coronary artery disease and acute coronary syndrome compared to control subjects.8 Apelin, first isolated from bovine stomach as a 36 amino acid peptide, is the only known ligand of the human orphan G-protein coupled receptor (APJ).9 Although shorter C-terminal sequences elicit biological activity, apelin-36 and 13 are known to be physiologically active.10 In addition to adipose tissue, brain, lung, and liver, apelin mRNA is richly synthesized in the cardiovascular system, mostly in the right atrium and coronary arteries.11 Data suggest that apelin may have important regulatory effects on cardiac contraction, blood pressure, angioneogenesis, and fluid balance in addition to inhibiting apoptosis. Apelin levels were also found to be decreased in various severe cardiovascular diseases except for obesity and mild compensated heart failure, in which apelin levels were higher compared to controls.12 Furthermore, a recent study reported higher plasma apelin levels and lower apelin/APJ myocardial expression in left ventricular hypertrophy due to chronic pressure overload in patients with AS.13 A direct correlation has also been demonstrated between plasma apelin and left ventricular mass index, a reliable indicator of left ventricular hypertrophy, and its severity.13
To the best of our knowledge, no studies have investigated the relationship between serum apelin 36 levels and the severity of calcific AS. The purpose of this study was to investigate the association between apelin 36 levels and the severity of calcific AS.
MATERIALS AND METHODS
Study population
The present observational study was performed at the Recep Tayyip Erdoğan University Education & Research Hospital between January 2015 and October 2015. A total of 68 consecutive patients diagnosed with calcific AS by 2-D transthoracic echocardiography were included in the study. The patients were categorized into two groups as follows: 34 with mild-moderate AS (18 females, 16 males; mean age: 73.5 ± 9.9 years), and 34 with severe AS (23 females, 11 males; mean age: 76 ± 9.1 years). Thirty-two control subjects with a similar age and gender but without AS (14 females, 16 males; mean age: 73 ± 9.2 years) were also enrolled and gave blood samples which were used for biochemical comparisons of apelin and high-sensitive C-reactive protein (hsCRP) levels. The demographic, clinical and laboratory characteristics of the patients and control subjects were recorded through a systematic review of the patient files on admission. All patients underwent standard transthoracic echocardiography and 12-lead electrocardiography.
The exclusion criteria were as follows: presence of severe coronary artery disease, peripheral artery disease, congestive heart failure, recent acute coronary syndrome, atrial fibrillation, congenital heart disease, myocarditis, pericarditis, cardiomyopathy, rheumatic or congenital aortic stenosis, gout arthritis, use of diuretics, use of uricosuric or hepatotoxic agents, acute or chronic renal dysfunction, presence of prosthetic heart valves, primary or secondary hyperparathyroidism, malignancies, moderate-severe aortic insufficiency, acute or chronic inflammatory disease, and active infection.
This study was conducted in accordance with the Declaration of Helsinki, and approved by the local ethics committee. Fifty-eight consecutive patients between 40-85 years of age were enrolled after providing written informed consent.
Definitions
Hypertension and diabetes mellitus (DM) were diagnosed according to recent guideline definitions. Hypertension was defined as either a systolic arterial pressure greater than 140 mmHg and/or diastolic arterial pressure of 90 mmHg, or if the patient used antihypertensive drugs.14 DM was confirmed if the patient had a history of DM, or was on a diet, or used antidiabetic drugs, or had a fasting venous blood glucose concentration ≥ 126 mg/dL in previously untreated patients. Hyperlipidemia was defined as fasting total serum cholesterol > 200 mg/dL, low-density lipoprotein (LDL) cholesterol > 130 mg/dL, or serum triglycerides > 180 mg/dL or if the patient used lipid-lowering drugs because of hypercholesterolemia.15 The height and weight of the subjects were recorded, and body mass index (BMI) was calculated as the ratio of weight in kilograms divided by the square of height in meters. Patients smoking 20 or more cigarettes daily at the time of diagnosis were recognized as active smokers.
Transthoracic echocardiography
All of the study participants underwent comprehensive 2-dimensional, M-mode, conventional Doppler and color Doppler echocardiographic examinations to determine the underlying structural heart disease with a Philips ie33 echocardiography system using a 2.5- to 3.5-MHz probe by two experienced cardiologists who were blinded to the patients’ data. A continuity equation was used to calculate aortic valve area. A continuous wave Doppler scan at the level of the aortic valve was used to measure peak aortic velocity, and time velocity integral was used to calculate the mean aortic gradient. AS severity was graded using recent guidelines as follows:16 mild, valve area exceeding 1.5 cm2, transvalvular velocity 2.0 to 2.9 m/s, and mean gradient < 20 mmHg; moderate, valve area of 1.0 to 1.5 cm2, transvalvular velocity 3.0 to 3.9 m/s, and mean gradient 20 to 39 mmHg; and severe, valve area less than 1.0 cm2, transvalvular velocity ≥ 4 m/s, and mean gradient ≥ 40 mmHg.
Biochemical measurements
Fasting venous blood samples were acquired via antecubital fossa in the morning hours. Serum was obtained after centrifugation at 3000 rpm at 4 °C for 15 minutes, and stored at -80 °C until being used for analysis of apelin 36 levels. Fasting blood glucose, postprandial glucose, creatinine, total cholesterol, triglycerides, and high-density lipoprotein cholesterol concentrations were measured routinely using an Abbott Diagnostics C8000i (Abbott, Germany) autoanalyzer in the biochemistry laboratory of our hospital with commercial kits. LDL cholesterol was assayed using Friedwald’s formula for samples with triglycerides ≤ 400 mg/dL.17 Hematological parameters were obtained using a Coulter LH 780 Hematology Analyzer (Beckman Coulter Ireland Inc., Mervue, Galway, Ireland).
Serum apelin 36 levels were measured using a commercially available kit. After collection of blood for apelin 36 measurements, the serum of all patients was immediately obtained by centrifugation, transferred into cryotubes and stored at -80 °C until assay. Serum apelin 36 was measured using enzyme-linked immunosorbent assay (ELSIA) method (AP36 test kit, Cloud-Clone Corp., Houston, USA) according to the manufacturer’s protocol. Absorbance of each well was determined at 450 nm with a microtiter plate reader (Multiskan GO, Thermo Scientific, Waltham, MA, USA) in the 5th minute. A standard curve was fitted using Titri ELISA software, and was then used to convert sample absorbance readings to apelin 36 concentration. The serum apelin 36 minimum detection range was 7.41 pg/mL. Serum hsCRP levels were measured using the nephelometric method on a UniCel DxC 800 System (Beckman Coulter Inc., USA). The reference range was 0-0.77 mg/dL.
Statistical analysis
Continuous variables were expressed as means ± standard deviations or median (minimum-maximum) values, and categorical variables were presented as percentages. The One-Sample Kolmogorov-Smirnov test was used to evaluate the distribution of continuous variables. Continuous variables were compared between two groups using the Student’s t test or Mann-Whitney U test. The Kruskal-Wallis test was used to compare plasma apelin and hsCRP levels among groups. Categorical variables were compared appropriately with the chi-square or Fisher’s exact tests. Correlations between variables were tested using the Pearson correlation test for normally distributed variables and Spearman correlation test for non-normally distributed variables. All analyses were performed using SPSS software, version 16.0 (SPSS Inc., Chicago, Illinois, USA). A p value < 0.05 was considered to be significant.
RESULTS
The mild to moderate AS group consisted of 34 patients (18 females, 16 males; mean age 72 ± 8.5 years), and the severe AS group also included 34 patients (23 females, 11 males; mean age 73.7 ± 6.8). The control group consisted of 32 patients (17 females, 15 males; mean age 70.7 ± 7.2 years). Baseline demographic, clinical characteristics and echocardiographic parameters of the AS patients by group are presented in Table 1. The groups were similar in terms of age and BMI, and the presence of hypertension, hyperlipidemia, smoking, and DM.
Table 1. Clinical characteristics of the study patients.
| Patients with aortic valve stenosis | Control group (n = 32) | ||
| Mild-moderate AS (n = 34) | Severe AS (n = 34) | ||
| Age, yr | 72 ± 8.5 | 73.7 ± 6.8 | 70.7 ± 7.2 |
| Sex, male/female | 16/18 | 43427 | 17/15 |
| BMI, kg/m2 | 28.5 ± 5.3 | 26.7 ± 2.1 | 30 ± 4.2 |
| Body surface area, m2 | 1.89 ± 0.1 | 1.76 ± 0.1 | 1.8 ± 0.1 |
| Aortic valve area, cm2 | 1.52 ± 0.2# | 0.78 ± 0.1# | |
| Mean pressure gradient, mmHg | 25.9 ± 6# | 49.2 ± 11.9# | |
| Peak pressure gradient, mmHg | 45.9 ± 9.4# | 78.5 ± 17.8# | |
| LV mass index, g/m2 | 121 (69-350)* | 137 (13-185)* | 89 (66-117) |
| LV ejection fraction, % | 61.7 ± 3.7 | 60.8 ± 3.5 | 62.4 ± 3.4 |
| Hypertension, % | 41.2 | 35.3 | 37.5 |
| Diabetes mellitus, % | 23.5 | 20.6 | 12.5 |
| Smoking, % | 11.8 | 14.7 | 9.4 |
| Hyperlipidemia, % | 37.5 | 41.2 | 46.9 |
| Medication | |||
| Beta-blockers, % | 76.5 | 79.4 | 18.8 |
| Statin, % | 32.4 | 50 | 21.9 |
| ACEi-ARB, % | 44.1 | 52.9 | 37.5 |
ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; LV, left ventricular; Significant difference for patients with vs. without heart failure; * p ≤ 0.05; # p < 0.001.
Plasma apelin 36 levels were significantly lower in the patients with severe AS compared to those with mild-moderate AS and the control subjects (p < 0.001) (Figure 1). We also found significantly higher hsCRP concentrations in the patients with severe AS compared to the other groups (p < 0.001). Even though serum apelin levels were significantly lower in the mild-moderate AS group compared to the control group [490 (247-1074) vs. 660 (378-1200) pg/ml], the lowest apelin concentrations were observed in the severe AS group [209 (97-453) pg/ml] (Table 2). Spearman’s rank test showed a statistically significant negative correlation between serum apelin 36 levels and mean aortic gradient in the patients with AS (r = -0.637, p < 0.001, Figure 2). There was also a significant negative correlation between the left ventricular mass index and apelin 36 concentrations (p < 0.001, r = -0,478), whereas aortic valve area and apelin 36 had a statistically significant positive association (p < 0.001, r = 0.646).
Figure 1.
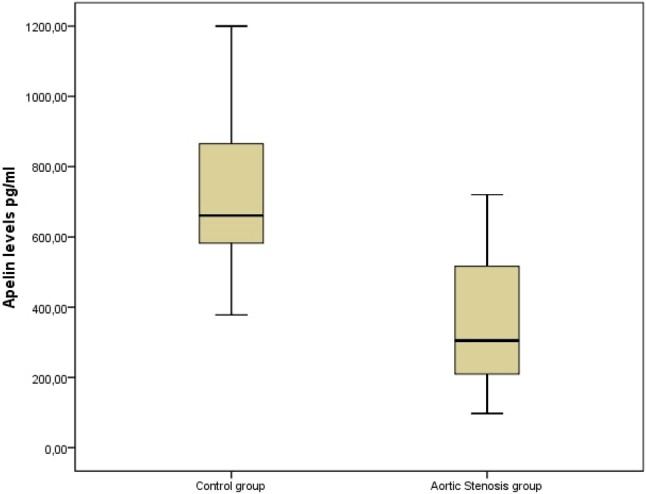
Serum apelin concentrations according to aortic stenosis and control group.
Table 2. Plasma apelin and hsCRP concentrations in patients with AS and the control subjects.
| Patients with AS | Control group (n = 32) | p value | ||
| Mild-moderate AS (n = 34) | Severe AS (n = 34) | |||
| Apelin, ng/ml | 490 (247-1074) | 209 (97-453) | 660 (378-1200) | < 0.001 |
| hs-CRP, mg/L | 0.40 (0.10-1.20) | 0.75 (0.30-2.10) | 0.20 (0.1-0.54) | < 0.001 |
Data are medians with range in parentheses. p values are from Kruskal-Wallis tests across the three groups.
AS, aortic stenosis; hs-CRP, high-sensitive C-reactive protein.
Figure 2.
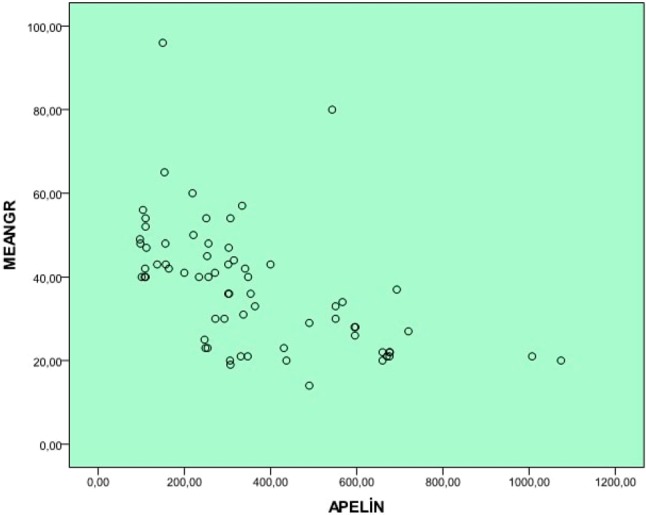
The correlation graph of apelin concentration with mean aortic gradient.
DISCUSSION
In our study we revealed a strong correlation between various parameters denoting the severity of calcific AS and plasma apelin 36 levels. We demonstrated lower apelin 36 levels in patients with severe AS compared to patients with mild-moderate AS and normal control subjects. Additionally, consistent with previous reports, hsCRP levels were correlated with the severity of AS.
Apelin levels have been reported to be lower in patients with various cardiovascular diseases but higher in those with obesity and compensated mild heart failure compared to controls.9 Recent studies have shown lower apelin levels in patients with significant coronary atherosclerosis.7,8 Since there are several shared risk factors for calcific AS and atherosclerosis, lower levels of apelin in both situations may help to reveal the pathophysiologic processes of calcific AS.
Preclinical studies have demonstrated that apelin signaling exerts major effects on both vascular tone and cardiac contractility. Apelin has been identified as an endogenous circulating peptide with potent positive inotropic activity.18 Apelin signaling may also be involved in the regulation of blood pressure, cardiac contractile function, fluid balance, angiogenesis and inhibition of apoptosis. Moreover, apelin has been shown to increase cardiac contractility in vivo and cause a rapid fall in both arterial blood pressure and systemic venous tone with corresponding reductions in left ventricular afterload and preload in rodents.19-23
Japp and coworkers found that apelin exerts direct coronary vasodilation and positive inotropic effects in humans.24 In addition, apelin decreased both peak and end-diastolic left ventricular pressures. The authors also revealed that systemic apelin administration caused a reduction in peripheral vascular resistance accompanied by an increase in cardiac output and heart rate.24
Previous investigations have demonstrated either decreased or unaltered levels of plasma apelin in patients with heart failure compared to control subjects.25-30 In contrast, Chen et al. reported that plasma apelin was increased mostly in patients with mild to moderate heart failure with symptoms and left ventricular dysfunction, whereas apelin levels declined with the transition from moderate to severe heart failure.31 Thus, previous data suggest that a failing cardiac muscle could increase production of this powerful inotropic peptide as a compensatory mechanism to augment inotropic capacity and maintain cardiac output. Our finding of decreased apelin levels in severe AS may be consistent with these observations. However, it must be remembered that our study group included patients with normal ejection fraction, and the patients with mild-moderate AS tended to have lower apelin levels compared to the healthy controls. A recent study demonstrated significantly lower apelin levels in hypertensive patients with left ventricular hypertrophy compared to those without hypertophy.25 In addition, the in vitro part of their study revealed a potent inhibitory effect of apelin to hypertrophic stimuli.25 Similarly, we demonstrated decreased apelin concentrations in both the mild-moderate AS and severe AS groups, however apelin levels were significantly lower in the patients with severe AS compared to those with mild-moderate AS. In contrast to the findings of Chen and coworkers, the decreased apelin levels in our patients with mild-moderate AS and normal ejection fraction may have been due to higher left ventricular mass index (hypertrophy). Aortic valve calcification is an active process which depends on osteoblastic differentiation of aortic valve interstitial cells. Yuan and coworkers demonstrated that apelin attenuates the osteoblastic differentiation of aortic valve interstitial cells.32 Therefore, the inverse correlation between plasma apelin and degree of aortic stenosis in our study group may reflect a low inhibitory effect of apelin culminating in more severe AS, using a different mechanism than heart failure.
A recent study demonstrated reduced circulating apelin levels in patients with essential hypertension, and found that lower plasma apelin was independently associated with an increasing degree of left ventricular systolic and diastolic function impairment.33 In contrast, Helske et al. reported increased serum apelin 12 levels in patients with severe aortic stenosis, and also found different apelin concentrations in the aortic root and coronary sinus of their patients.34 However the control group was considerably younger than the patients in that study. A possible explanation for this contradictory finding either may be due to different concentrations of apelin 36 and 12 levels in the same patient subsets or by differences in ages between the control group and patient group. The demographic characteristics of our patient group and control subjects were similar, which strengthens our results.
Japp et al. raised the idea that the loss of apelin may be deleterious in left ventricular pressure overload, and they suggested that the acute administration of apelin may cause peripheral and coronary vasodilatation and increased cardiac output. This finding supports the idea that restoration of cardiac apelin stores could be beneficial in heart failure.24 The combined strong inotropic effect and afterload reduction with vasodilatation and diuresis suggests that apelin could serve as a therapeutic option.24,35-38 The administration of apelin has also been shown to exert a positive inotropic effect in rats with experimental right ventricular failure.33 According to these data, we think that severe calcific AS patients may also benefit from the cardiovascular effects of apelin. This benefit may be achieved in different ways such as slowing disease progression, lessening medication, decreasing hospitalizations for decompensated heart failure, or prolonging the time to valve replacement.
Limitations
Our study has several limitations. The major limitation is the relatively small number of patients. Since the patients with mild-moderate AS had left ventricular hypertrophy and we did not include a control group with left ventricular hypertrophy, we could not define apelin levels in this population independently of left ventricular hypertrophy. We did not perform myocardial strain imaging in order to understand whether or not the decrease in apelin in severe AS was due to subclinical systolic dysfunction. Since our study was cross-sectional and observational, our results cannot implicate causality. Follow-up of patients within a time frame would have given the chance to identify changes in valvular gradients with respect to apelin concentration. Moreover, since we only measured apelin 36 and not the other bioactive forms of apelin, our results only apply to apelin 36.
CONCLUSIONS
Our study demonstrated decreased apelin levels and increased hsCRP concentrations in patients with severe calcific AS. We believe that patients with calcific AS and preserved ejection fraction have lower apelin concentrations. Our findings may help to clarify the exact pathophysiologic role of apelin in cardiovascular diseases.
AUTHOR CONTRIBUTIONS
Duman H, Hamur H, Bahçeci I conceived and designed the study. Duman H, Hamur were responsible for data collection. Hamur H, Duman H, Dilek AR were responsible for data analysis and interpretation. Hamur H, Duman H were responsible for the statistical analyses. Duman H, Demirelli S, Durakoğlugil ME, Erdogan T, Şatıroğlu Ö were responsible for manuscript writing and literature search. All authors approved the final version of the text.
DECLARATION OF INTEREST
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The authors received no financial support for the research, authorship, and/or publication of this article.
REFERENCES
- 1.Tziomalos K, Athyros VG, Karagiannis A, Mikhailidis DP. Established and emerging vascular risk factors and the develop-ment of aortic stenosis: an opportunity for prevention? Expert Opin Ther Targets. 2008;12:809–820. doi: 10.1517/14728222.12.7.809. [DOI] [PubMed] [Google Scholar]
- 2.Chen YH, Chang HH, Chen PL, et al. Procedural characteristics and outcomes of transcatheter aortic valve implantation: a single-center experience of the first 100 inoperable or high surgical risk patients with severe aortic stenosis. Acta Cardiol Sin. 2017;33:339–349. doi: 10.6515/ACS20170620A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sahinarslan A, Vecchio F, MacCarthy P, et al. Dynamics of concomitant functional mitral regurgitation in patients with aortic stenosis undergoing TAVI. Acta Cardiol Sin. 2016;32:477–484. doi: 10.6515/ACS20150629C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yin WH. Transcatheter aortic valve implantation in Taiwan: still evolving! Acta Cardiol Sin. 2017;33:350–352. doi: 10.6515/ACS20170707A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dweck MR, Boon NA, Newby DE. Calcific aortic stenosis: a disease of the valve and the myocardium. J Am Coll Cardiol. 2012;60:1854–1863. doi: 10.1016/j.jacc.2012.02.093. [DOI] [PubMed] [Google Scholar]
- 6.Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular health study. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 7.Palta S, Pai AM, Gill KS, Pai RG. New insights into the progression of aortic stenosis: implications for secondary prevention. Circulation. 2000;101:2497–2502. doi: 10.1161/01.cir.101.21.2497. [DOI] [PubMed] [Google Scholar]
- 8.Poggianti E, Venneri L, Chubuchny V, et al. Aortic valve sclerosis is associated with systemic endothelial dysfunction. J Am Coll Cardiol. 2003;41:136–141. doi: 10.1016/s0735-1097(02)02622-0. [DOI] [PubMed] [Google Scholar]
- 9.Kadoglou NP, Lampropoulos S, Kapelouzou A, et al. Serum levels of apelin and ghrelin in patients with acute coronary syndromes and established coronary artery disease--Kozani Study. Transl Res. 2010;155:238–246. doi: 10.1016/j.trsl.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Zeituon MH. Correlation of serum apelin level with coronary calcium score in patients with suspected coronary artery disease. The Egyptian Heart Journal. 2014;66:1–35. [Google Scholar]
- 11.Tatemoto K, Hosoya M, Habata Y, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–476. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 12.Maguire JJ, Kleinz MJ, Pitkin SL, Davenport AP. [Pyr1]apelin-13 identified as the predominant apelin isoform in the human heart: vasoactive mechanisms and inotropic action in disease. Hypertension. 2009;54:598–604. doi: 10.1161/HYPERTENSIONAHA.109.134619. [DOI] [PubMed] [Google Scholar]
- 13.Kleinz MJ, Davenport AP. Immunocytochemical localization of the endogenous vasoactive peptide apelin to human vascular and endocardial endothelial cells. Regul Pept. 2004;118:119–125. doi: 10.1016/j.regpep.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Charles CJ. Update on apelin peptides as putative targets for cardiovascular drug discovery. Expert Opin Drug Discov. 2011;6:633–644. doi: 10.1517/17460441.2011.571251. [DOI] [PubMed] [Google Scholar]
- 15.Falcão-Pires I, Gonçalves N, Gavina C, et al. Correlation between plasma levels of apelin and myocardial hypertrophy in rats and humans: possible target for treatment? Expert Opin Ther Targets. 2010;14:231–241. doi: 10.1517/14728220903485685. [DOI] [PubMed] [Google Scholar]
- 16.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 17.Reiner Z, Catapano AL, De Backer G, et al. ESC/EAS guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32:1769–1818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 18.Nishimura RA, et al. AHA/ACC valvular heart disease guideline. Circulation. 2014;129:000–000. [Google Scholar]
- 19.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 20.Szokodi I, Tavi P, Foldes G, et al. Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility. Circ Res. 2002;91:434–440. doi: 10.1161/01.res.0000033522.37861.69. [DOI] [PubMed] [Google Scholar]
- 21.Ashley EA, Powers J, Chen M, et al. The endogenous peptide apelin potently improves cardiac contractility and reduces cardiac loading in vivo. Cardiovasc Res. 2005;65:73–82. doi: 10.1016/j.cardiores.2004.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atluri P, Morine KJ, Liao GP, et al. Ischemic heart failure enhances endogenous myocardial apelin and APJ receptor expression. Cell Mol Biol Lett. 2007;12:127–138. doi: 10.2478/s11658-006-0058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tatemoto K, Takayama K, Zou MX, et al. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul Pept. 2001;99:87–92. doi: 10.1016/s0167-0115(01)00236-1. [DOI] [PubMed] [Google Scholar]
- 24.Cheng X, Cheng XS, Pang CC. Venous dilator effect of apelin, an endogenous peptide ligand for the orphan APJ receptor, in conscious rats. Eur J Pharmacol. 2003;470:171–175. doi: 10.1016/s0014-2999(03)01821-1. [DOI] [PubMed] [Google Scholar]
- 25.Lee DK, Saldivia VR, Nguyen T, et al. Modification of the terminal residue of apelin-13 antagonizes its hypotensive action. Endocrinology. 2005;146:231–236. doi: 10.1210/en.2004-0359. [DOI] [PubMed] [Google Scholar]
- 26.Japp AG, Cruden NL, Barnes G, et al. Acute cardiovascular effects of apelin in humans: potential role in patients with chronic heart failure. Circulation. 2010;121:1818–1827. doi: 10.1161/CIRCULATIONAHA.109.911339. [DOI] [PubMed] [Google Scholar]
- 27.Ye L, Ding F, Zhang L, et al. Serum apelin is associated with left ventricular hypertrophy in untreated hypertension patients. J Transl Med. 2015;13:290. doi: 10.1186/s12967-015-0635-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chong KS, Gardner RS, Morton JJ, et al. Plasma concentrations of the novel peptide apelin are decreased in patients with chronic heart failure. Eur J Heart Fail. 2006;8:355–360. doi: 10.1016/j.ejheart.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Foldes G, Horkay F, Szokodi I, et al. Circulating and cardiac levels of apelin, the novel ligand of the orphan receptor APJ, in patients with heart failure. Biochem Biophys Res Commun. 2003;308:480–485. doi: 10.1016/s0006-291x(03)01424-4. [DOI] [PubMed] [Google Scholar]
- 30.Francia P, Salvati A, Balla C, et al. Cardiac resynchronization therapy increases plasma levels of the endogenous inotrope apelin. Eur J Heart Fail. 2007;9:306–309. doi: 10.1016/j.ejheart.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Miettinen KH, Magga J, Vuolteenaho O, et al. Utility of plasma apelin and other indices of cardiac dysfunction in the clinical assessment of patients with dilated cardiomyopathy. Regul Pept. 2007;140:178–184. doi: 10.1016/j.regpep.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Van Kimmenade RR, Januzzi JL, Jr., Ellinor PT, et al. Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. J Am Coll Cardiol. 2006;48:1217–1224. doi: 10.1016/j.jacc.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 33.Chen MM, Ashley EA, Deng DX, et al. Novel role for the potent endogenous inotrope apelin in human cardiac dysfunction. Circulation. 2003;108:1432–1439. doi: 10.1161/01.CIR.0000091235.94914.75. [DOI] [PubMed] [Google Scholar]
- 34.Yuan ZS, Liao X, et al. Apelin attenuates the osteoblastic differentiation of aortic valve interstitial cells via the ERK and PI3-K/Akt pathways. Amino Acids. 2015;47:2475–2482. doi: 10.1007/s00726-015-2020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Przewlocka-Kosmala M, Kotwica T, Mysiak A, Kosmala W. Reduced circulating apelin in essential hypertension and its association with cardiac dysfunction. J Hypertens. 2011;29:971–979. doi: 10.1097/HJH.0b013e328344da76. [DOI] [PubMed] [Google Scholar]
- 36.Helske S, Kovanen PT, Lommi J, et al. Transcardiac gradients of circulating apelin: extraction by normal hearts vs. release by hearts failing due to pressure overload. J Appl Physiol. 2010;109:1744–1748. doi: 10.1152/japplphysiol.00474.2010. [DOI] [PubMed] [Google Scholar]
- 37.Berry MF, Pirolli TJ, Jayasankar V, et al. Apelin has in vivo inotropic effects on normal and failing hearts. Circulation. 2004;110(Suppl 1):II187–II193. doi: 10.1161/01.CIR.0000138382.57325.5c. [DOI] [PubMed] [Google Scholar]
- 38.Dai T, Ramirez-Correa G, Gao WD. Apelin increases contractility in failing cardiac muscle. Eur J Pharmacol. 2006;553:222–228. doi: 10.1016/j.ejphar.2006.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jia YX, Pan CS, Zhang J, et al. Apelin protects myocardial injury induced by isoproterenol in rats. Regul Pept. 2006;133:147–154. doi: 10.1016/j.regpep.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 40.Lee DK, George SR, O’Dowd BF. Unravelling the roles of the apelin system: prospective therapeutic applications in heart failure and obesity. Trends Pharmacol Sci. 2006;27:190–194. doi: 10.1016/j.tips.2006.02.006. [DOI] [PubMed] [Google Scholar]


