Abstract
Objective
Experimental models suggest estrogen has a renoprotective effect, but human studies show variable results. Our objective was to study the association of hormone therapy (HT) and albuminuria in post-menopausal women and to synthesize the results with outcomes from prior studies.
Methods
We analyzed data from post-menopausal women who participated in the second study visit (2000-2004) of the Genetic Epidemiology Network of Arteriopathy (GENOA) study. The exposure was self-reported HT use and the outcome was albuminuria (urine albumin-to-creatinine ratio > 25 mg/g Cr). We also conducted a systematic review and meta-analysis on the association of HT and urine protein in post-menopausal women. Continuous and dichotomous measures of protein excretion were converted to a standardized mean difference (SMD) for each study.
Results
In the GENOA cohort (n = 2217), there were fewer women with albuminuria amongst HT users than non-users (9% vs. 19%, p<0.001). HT use was associated with decreased odds of albuminuria (OR 0.65, 95% CI 0.45-0.95), after adjusting for significant differences in age, race, education, co-morbidities, and the age at and cause of menopause. The SMD of the effect of HT on urine proteinuria/albuminuria in the RCTs (n=3) was 0.02 (95% CI -0.29-0.33) and -0.13(95% CI -0.31-0.05) in the observational studies (n=9). There was significantly less albuminuria among HT users (SMD -0.15, 95% CI -0.27- -0.04) in the 9 studies that only reported albuminuria as an outcome and in the 10 studies with a comparator arm (SMD -0.15 (95% CI -0.26- -0.04)).
Conclusions
HT is associated with decreased odds of albuminuria, but some of the observed benefit may be related to reported outcomes, the presence of a comparator arm and the type of study design.
Keywords: estrogen, hormone therapy, albuminuria, proteinuria, albuminuria
Introduction
Several observational studies have demonstrated a slower decline of kidney function in women with renal disease as compared to men, particularly before the age of menopause.(1-5) Animal studies have shown that sex hormones play an important role in kidney function. In general, estradiol seems to have anti-fibrotic, anti-inflammatory and vasodilatory properties in the kidney.(6, 7) Taken together, this body of evidence has implicated estrogen as a potential renoprotective agent.(8)
Elucidating the role of estrogen in human kidneys is challenging given the complex, multisystemic effects of estrogen and other physiologic changes around menopause. Studies evaluating the association of estrogen-containing hormone therapy (HT) and kidney function, in particular proteinuria and albuminuria, have shown mixed results. Some studies have shown that women using HT have an increased risk of having albuminuria (9, 10), while others have demonstrated a substantially decreased risk.(11, 12) The conflicting results in this area may be due to differing study designs, inclusion and exclusion criteria, and definitions of exposure and outcomes.
In the current study, we used a large, racially and ethnically diverse cohort to study the association of HT and albuminuria. We chose albuminuria as our main outcome of interest as it is an early marker of renal dysfunction and is also associated with cardiovascular disease risk.(13-15) Our objective was to evaluate whether HT use was associated with a decreased risk of albuminuria, after controlling for known risk factors for chronic kidney disease. We also conducted a systematic review and meta-analysis to identify all available studies on HT and proteinuria or albuminuria to see if we could explain the heterogeneity in the existing literature.
Materials and Methods: GENOA
Study Design and Participants
This study included post-menopausal women who participated in the Phase II study visit of the Genetic Epidemiology Network of Arteriopathy (GENOA) study from 12/2000 to 11/2004.(16) GENOA is one of 4 networks in the Family Blood Pressure Program, a multi-center study investigating the genetics of hypertension.(16) GENOA recruited participants of different races and ethnicities from 3 sites: African-Americans from Jackson, MS, Mexican-Americans from Starr County, TX and non-Hispanic whites from Rochester, MN. Sibships with at least 2 hypertensive individuals diagnosed before age 60 were recruited. As part of an effort to reduce confounding during recruitment, the GENOA sibships recruited from Starr County, TX had to have at least 2 siblings with diabetes, given the high incidence of diabetes in this population. All full biologic siblings of recruited siblings were invited to participate in the study. This cross-sectional, post-hoc analysis included post-menopausal participants from GENOA (n=2036 out of 4329 total participants, excluding men and pre-menopausal women). We did not have complete data on albuminuria in the other networks and so they were not included.
Study Visit
All participants gave informed consent and the Institutional Review Board at each clinic site approved all protocols. Questionnaires were administered via personal interviews with trained examiners. They underwent a standard physical exam, blood and urine tests. Body Mass Index (BMI) was calculated as weight (kg)/height (m)2. The diagnosis of hypertension was confirmed if the average of 3 systolic blood pressures (BP) or diastolic BPs were greater than 140 or 90 mm Hg respectively, or if there was a prior diagnosis of hypertension and use of prescription anti-hypertensive medication was documented, including the use of renin-angiotensin-aldosterone (RAAS) blockers. The diagnosis of diabetes was determined by self-report and an ‘ever smoked’ status was defined as having smoked >100 cigarettes at any point in the participant's lifetime. The highest grade of completed education was recorded.
Laboratory Methods
Blood was drawn by venipuncture and urine was collected after an overnight fast of at least 8 hours. Serum and urine creatinine were measured using the Jaffe assay and urine albumin using an immunoturbidity method implemented on an automated chemistry analyzer (Hitachi 911, Roche Diagnostics, Indianapolis, IN). Serum total cholesterol, high-density lipoprotein (HDL) cholesterol and triglyceride concentrations were all measured by standard methodology, also using the Hitachi 911 Chemistry Analyzer. Low-density lipoprotein (LDL) was calculated using the Friedewald equation when the triglyceride concentration was less than 400 mg/dl.
Exposure
The exposure of interest was self-reported HT use in the last month at the time of the study visit, not including topical vaginal estrogen cream. Participants in Rochester, MN and Jackson, MS brought in pill bottles and medications were recorded by trained study personnel, including the use of specific hormone therapy (estrogen vs. estrogen and progesterone). All participants answered a series of questions regarding their menopausal status, including whether they had reached menopause, whether it was natural, surgical, or due to chemotherapy/radiation, the year or age they reached menopause and whether they had taken or used any pills, skin patches or shots for hormone or estrogen therapy in the last month.
Renal Outcomes
Albuminuria was defined as UACR ≥ 25 mg/g Cr on a spot urine sample, consistent with studies on sex-specific thresholds for albuminuria in women.(17) The estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI equation.(18) We defined an abnormal eGFR as < 60 ml/min/1.73 m2.
Statistical Analysis
We used Fisher's exact test for categorical variables and Wilcoxon rank sum on continuous variables. We fit linear and logistic regression models for the quantitative and categorical measures of renal function that were adjusted for age, race, education, smoking, diabetes, hypertension, RAAS blockers, history of hypertension in either parent, BMI, HDL, LDL, triglycerides, eGFR < 60 ml/min/1.73 m2, surgical menopause, and age at menopause, all parameters that were significantly different between HT users and non-users. These models were fit with generalized estimating equations to account for sibling relationships in recruitment. Quantitative variables with skewed distributions were log-transformed for all models. We performed a subgroup analysis to evaluate the effect of estrogen vs. estrogen and progesterone therapy in women with medication information.
Materials and Methods: Meta-Analysis
We performed a systematic review and meta-analysis of studies that evaluated the association between HT and albuminuria and/or proteinuria. The review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement.(19) We included observational studies and randomized control trials (RCTs) of post-menopausal women that compared HT users and non-users, though studies comparing women before and after HT use were also included. HT use could be of any duration and could be determined by self-report, pharmacy records or given as an intervention in a trial. Our outcome of interest was any measure of urinary protein excretion (albuminuria or proteinuria). We did not set any limits on length of follow-up, date of publication, study quality, language, or geographic location.
Data Sources and Search Strategy
We conducted a systemic search of several databases to identify relevant articles. The search strategy was developed in consultation with a PhD statistician with expertise in systematic reviews (NM) and was completed on 2/8/2016. We searched 4 databases (with year of inception): Ovid Medline (1946), Embase (1988), Cochrane Central Register of Controlled Trials (1966) and Scopus (1989) for terms related to estrogen, hormone therapy, menopause, kidney, renal or glomerular function, proteinuria and albuminuria (see Supplement for full search strategy). Two independent reviewers (AK and MG) screened all eligible abstracts and full texts. If the reviewers disagreed on inclusion, the abstract was automatically moved to the next stage of full text review. At the full text stage, disagreement was resolved by consensus and if not possible, by a third reviewer. A data extraction form was developed and included details on study design, study population, inclusion/exclusion criteria, the specific exposure or intervention, the length of follow-up and urinary protein and albumin measures, including continuous and dichotomous variables. If the full text was not in English, we identified two independent reviewers that spoke the relevant language to extract data and assess study quality.
Risk of Bias
Risk of bias was reviewed by two independent reviewers using the Newcastle Ottawa Scale for cohort and case-control studies and the Cochrane Risk of Bias Assessment Tool for RCTs.(20, 21) Each study was given a low, moderate and high quality designation based on the criteria deemed to most important by the investigators. The hierarchy of study methodology using the GRADE approach was then combined with the risk of bias assessments to compare the quality of studies across study designs.(21) For example, a low quality RCT was considered comparable to a high quality observational study.
Missing Data and Subgroup Analyses
Certain variables were imputed based on the techniques given in the Cochrane Handbook of Systematic Reviews of Interventions.(21) Subgroup analyses determined a priori included standardized mean difference (SMD) by study type (observational vs. RCTs), the inclusion vs. exclusion of women with diabetes, type of outcome reported (albuminuria vs. proteinuria), estrogen vs. estrogen and progesterone combined HT, and quality. To further explore the reasons for different outcomes, we also evaluated the SMD in observational studies by study type (prospective cohort versus cross-sectional analysis and case-control studies).
Publication Bias
We did not make a funnel plot to evaluate for publication bias, given the small number of studies and our concern that the difference in results between our small and large studies may be the result of study heterogeneity.(22)
Statistical Analysis
The main outcomes of interest were the association of HT use and elevated proteinuria or albuminuria (odds ratio (OR)) or the difference in mean albuminuria or proteinuria associated with HT use. Priority was given to measures adjusted for age and diabetes. Dichotomous and continuous measures of association were converted to SMDs to estimate an effect size for each study. If a study reported both dichotomous and continuous measures, the most appropriately adjusted outcome was preferentially taken. Random effects models were used for to pool the SMDs. Heterogeneity was assessed using the I2 statistic. All analyses were performed with Review Manager, Version 5.
Results: GENOA
Baseline Characteristics
The demographics and medical history of HT users and non-users are shown in Table 1. HT users were significantly younger, more likely to smoke and differed in race/ethnic distribution and level of education. They were less likely to have diabetes, had lower BMI, LDL and triglycerides and higher HDL in serum. The mean (standard deviation (SD)) age at menopause in the cohort was 45.5 (7.2) years. Half of the women reported that onset of menopause was natural (50.9%) and half was surgical (48.9%), with only 4 women reporting menopause due to chemotherapy (n=2), radiation (n=1) or other causes (n=1). In the group of women who reported natural menopause, the mean (SD) age at menopause was 49.1 (5.4) vs. 39.7 (6.9) in those women with surgical menopause. Women who were on HT had an earlier age at menopause and were more likely to have surgical menopause.
Table 1.
Participant Demographics, Medical History and Unadjusted Renal Parameters in Hormone Therapy Users and Non-users.
| Characteristica | HT users (n=574) | HT non-users (n=1462) | p-value |
|---|---|---|---|
| Age, mean (SD) | 60.6 (7.5) | 63.3 (8.8) | <0.001b |
|
| |||
| Race, n(%) | <0.001c | ||
| Non-Hispanic White | 255 (44.4%) | 253 (17.3%) | |
| Hispanic | 80 (13.9%) | 488 (33.4%) | |
| Non-Hispanic Black | 239 (41.6%) | 721 (49.3%) | |
|
| |||
| Education, n(%) | <0.001c | ||
| Less than High School | 66 (11.5%) | 441 (30.1%) | |
| Partial High School | 23 (4.0%) | 63 (4.3%) | |
| High School Graduate/GED | 204 (35.6%) | 396 (27.1%) | |
| Post High School Education | 281 (49.9%) | 562 (38.4%) | |
|
| |||
| Smoking, n(%) | 202 (35.2%) | 417 (28.5%) | 0.004c |
|
| |||
| Hypertension, n(%) | 442 (77.1%) | 1114 (76.3%) | 0.73c |
|
| |||
| Diabetes, n(%) | 117 (20.4%) | 610 (41.7%) | <0.001c |
|
| |||
| BMI | 30.6 (26.4,35.3) | 31.7 (27.7, 36.6) | <0.001d |
|
| |||
| Surgical Menopause, n(%) | 330 (69.1%) | 456 (45.9%) | <0.001c |
|
| |||
| Age at menopause, median (IQR) | 45 (39-50) | 47 (40-51) | <0.001d |
|
| |||
| Total cholesterol (mg/dl) | 202.2 (175.9, 228) | 200.5 (175,229.6) | 0.93d |
|
| |||
| LDL (mg/dl) | 112.8 (90.7, 140.1) | 123.5 (97.0, 149.3) | <0.001d |
|
| |||
| Triglycerides (mg/dl) | 138.5 (95, 193) | 123 (89, 175.6) | <0.001d |
|
| |||
| HDL (md/dl) | 57.8 (49.9, 70.6) | 52.3 (43.5, 62.8) | <0.001d |
|
| |||
| HTN in either parent, n(%) | 445 (77.5%) | 1011 (69.1%) | <0.001c |
|
| |||
| Diabetes in either parent, n(%) | 170 (35.6%) | 347 (35.0%) | 0.86c |
|
| |||
| UACR (mg/g Cr) | 3.3 (1.5, 6.7) | 5.2 (2.2, 16.2) | <0.001d |
|
| |||
| UACR >25 mg/g Cr, n(%) | 53 (9.2%) | 287 (19.0%) | <0.001c |
|
| |||
| Cr (mg/dl) | 0.75 (0.66, 0.86) | 0.75 (0.65, 0.89) | 0.28d |
|
| |||
| eGFR (ml/min/1.73m2) | 91.4 (78.5, 101.7) | 89.7 (74.2, 101.5) | 0.05d |
|
| |||
| eGFR< 60 ml/min/1.73m2, n(%) | 35 (6.1%) | 156 (10.7%) | 0.001c |
Abbreviations: GED = General Education Diploma, HT = hormone therapy, BMI = body-mass index, HDL = high density lipoprotein, LDL = low density lipoprotein, HTN = hypertension, UACR = urinary albumin-to-creatinine ratio, Cr = creatinine, eGFR = estimated glomerular filtration rate, BSA = body-surface area.
All data presented as n(%) and median (interquartile range) unless otherwise specified.
Student's t-test as variable was normally distributed.
Fisher's Exact Test
Wilcoxon Rank Sum.
Renal Outcomes
The unadjusted renal parameters in HT users and non-users are shown in Table 2. UACR was significantly lower in those on HT versus those who were not (3.3 vs. 5.2 mg/g Cr, p<0.001). The proportion of women with albuminuria (9.2% vs. 19.0%) and eGFR < 60 ml/min/1.73 m2 (6.1% vs. 10.7%) was significantly lower in HT users as compared to non-users.
Table 2.
Logistic Regression Model for urinary albumin-to-creatinine ratio > 25 mg/g Cr in Hormone Therapy users vs. Non-users (n=2036) GENOA all - Non-Hispanic White and Black and Hispanic
| Parameter | Odds Ratio | 95% Confidence Interval | p-value |
|---|---|---|---|
| HT use vs. no usea | 0.65 | 0.45-0.95 | 0.02 |
|
| |||
| Age, per 10 years | 0.92 | 0.78-1.09 | 0.35 |
|
| |||
| Race | |||
| Non-Hispanic White | Ref | -- | -- |
| Non-Hispanic Black | 5.02 | 3.08-8.18 | <0.001 |
| Hispanic | 2.25 | 1.31-3.85 | 0.003 |
|
| |||
| Education | |||
| Post High School Education | Ref | -- | -- |
| High School Graduate or GED or less | 1.61 | 1.15-2.25 | 0.006 |
|
| |||
| Diabetes | 3.89 | 2.86-5.29 | <0.001 |
|
| |||
| Hypertension | 2.67 | 1.74-4.10 | <0.001 |
|
| |||
| Log(BMI) | 1.36 | 0.29-6.38 | 0.70 |
|
| |||
| Smoking | 0.85 | 0.63-1.15 | 0.3 |
|
| |||
| Log(Triglycerides) | 5.34 | 2.55-11.18 | <0.001 |
|
| |||
| Log(HDL) | 3.79 | 1.09-13.12 | 0.04 |
|
| |||
| Log(LDL) | 1.51 | 0.59-3.90 | 0.39 |
|
| |||
| HTN in either parent | 1.04 | 0.78-1.39 | 0.78 |
|
| |||
| RAAS blockers | 0.75 | 0.56-1.01 | 0.06 |
|
| |||
| eGFR< 60 ml/min/1.73 m2 | 3.46 | 2.38-4.98 | <0.001 |
|
| |||
| Surgical menopause | 0.76 | 0.55-1.04 | 0.09 |
|
| |||
| Age at menopause | 0.99 | 0.97-1.01 | 0.16 |
Abbreviations: GED = General Education Diploma, HT = hormone therapy, BMI = body-mass index, HDL = high density lipoprotein, LDL = low density lipoprotein, HTN = hypertension, RAAS = renin-angiotensin-aldosterone system blockers, eGFR =estimated glomerular filtration rate
Adjusted value for age, race, education, smoking, diabetes, hypertension, hypertension in either parent, BMI, HDL, LDL, triglycerides, RAAS blockers, eGFR< 60 ml/min/1.73 m2, surgical menopause (vs. other including natural, chemotherapy and other unspecified causes), and age at menopause.
HT users had a decreased odds of having microalbuminuria after adjusting age alone (OR 0.44, 95% CI 0.32-0.66). After further adjusting for race, education, smoking, diabetes, hypertension, history of hypertension in either parent, BMI, HDL, LDL, triglycerides, use of RAAS blockers, eGFR < 60 ml/min/1.73 m2, surgical menopause and age at menopause, HT users had a decreased odds of having albuminuria (adjusted OR 0.65, 95% CI 0.45-0.95). The odds of having eGFR < 60 ml/min/1.73 m2 was no longer significantly different between the groups after adjustment (adjusted OR 0.74, 95%CI 0.48- 1.14).
In our subgroup analysis of women in GENOA who provided pill bottles (n=1468), 494 women were on HT, of which 362 were on estrogen alone and 102 were on estrogen and progesterone, with the 30 remaining women on other HT combinations (progesterone alone, estrogen and testosterone, etc.). The majority of the 477 women taking estrogen were on conjugated estrogens (n=391, 82.1%) via the oral route (n=412, 86.4%). We found that estrogen alone was significantly associated with a decreased odds of albuminuria (OR 0.63, 95% CI 0.41- 0.96) after adjustment. Estrogen and progesterone therapy was associated with a greater decrease in odds of albuminuria, but this was not significant (OR 0.25, 95% CI: 0.06-1.09).
Results: Meta-Analysis
Description of included studies
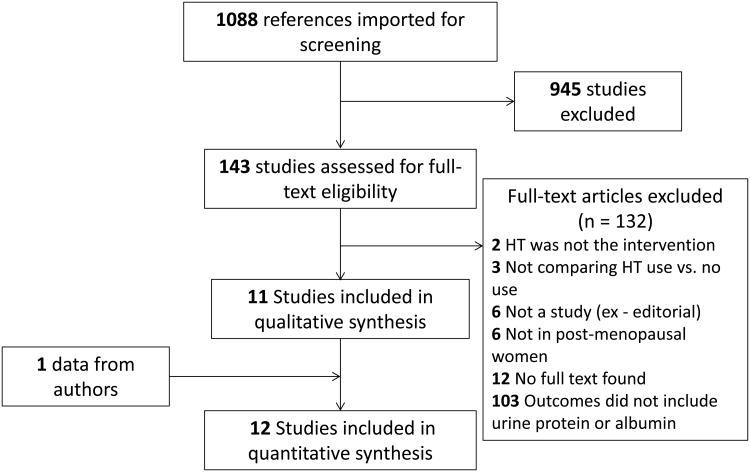
We identified 1088 abstracts for screening and 945 were excluded at the abstract phase (Figure 1). Twelve studies, including our own, were included in the qualitative and quantitative synthesis. The kappa statistic for agreement on inclusion between the reviewers was 0.95 (95% CI 0.86-1.00). Table 3 shows the details of the study designs of included studies. There were 3 RCTs and 9 observational studies. The RCTS were all placebo-controlled and of similar size, with approximately 30 women in each arm. The observational studies included several large cohort studies –including the Nurse's Health Study (NHS) and the Insulin Resistance and Atherosclerosis Study (IRAS).(11, 12) There were two population-based cohort studies – the Rancho Bernando study from a suburban community in Southern California and the Prevention of Renal and End-Stage Disease (PREVEND) cohort from Groningen, Netherlands that was recruited to identify the presence of elevated urine albumin excretion in the population.(9, 10) There were 2 small, uncontrolled, interventional studies where women were given hormone therapy and observed before and after therapy.(23, 24) Overall, there were 8,343 women included in all of the studies. The risk of bias assessments, study quality within each study design category and across study designs are shown in Table 4.
Figure 1.
Flow diagram outlining the identification of studies included in the systematic review and meta-analysis. HT = Hormone Therapy.
Table 3.
Full text articles included in systematic review and meta-analysis.
| Study | Design | Relevant Inclusion/Exclusion Criteria | Intervention or Exposure | Sample Size | Average Age of Women in each group | Length of intervention or exposure |
|---|---|---|---|---|---|---|
| Manning 2002(32) | Randomized Controlled Trial | Type II DM included, but poor control and HbA1C > 10% excluded | Conjugated equine estrogens 0.625 mg/day and medroxyprogesterone acetate 2 mg/day (oral) vs. placebo | HT – 29 Placebo - 32 |
HT – 62 Placebo – 65 |
6 months |
| Machado 2008(30) | Randomized Controlled Trial | Age 40-60, exclude fasting glucose > 100 mg/dl | Estradiol 1 mg/day, oral vs. placebo | HT – 30 Placebo – 30 |
HT – 51.0 Placebo – 51.1 |
6 months |
| Yilmaz 2011(31) | Randomized Controlled Trial | Women after oophorectomy, DM, HTN and renal disease excluded | Estradiol 50 μg/day, transdermal OR conjugated equine estrogens 0.625 mg/day vs. placebo | HT – 30 Placebo – 28 |
Not given | 6 months |
| Szekac 2000(24) | Observational cohort | Included only women with diabetic nephropathy (250-750 mg protein/24 hours) | Estradiol 2 mg/day and norgestrel 0.5 mg/day (oral) | HT – 16 No comparator |
HT – 51.9 | 14 weeks |
| Monster 2000(9) | Observational – case-control | Included only women with microalbuminuria, age 25-75 (PREVEND, population-based cohort) | Current use of HT in last year by pharmacy records | HT – 198 No HT – 1245 |
HT – 60.4 No HT – 58.1 |
Duration of use < and > 5 years reported |
| Fencki 2003(23) | Observational cohort | Included women with DM for 2 years and women with HTN | Estradiol 0.5 mg and norethisterone acetate 0.25 mg/day (transdermal) | HT and DM – 20 HT and HTN - 21 |
HT and DM – 50.9 HT and HTN – 52.6 |
12 weeks |
| Agarwal 2005(11) | Observational – cross-sectional and prospective | Various glucose tolerances (Insulin Resistance Atherosclerosis Study) | Current use of HT | Post-menopausal women at beginning of study – 744 | HT – 57 No HT - 58 |
Average length of HT – 8 years |
| Schopick 2009(12) | Observational cohort | Excluded DM and macroalbuminuria (>355 mg/g Cr) (Nurse's Health Study) | Current or past use of HT | Current HT = 1127 Past Use = 574 No Current HT = 499 |
Current HT – 65.6 No HT – 67.7 |
Duration of use reported up to > 15 years |
| Fung 2011(10) | Observational – cross-sectional and prospective | Excluded residents with unknown menopausal and HT status (Rancho Bernardo Study, population-based cohort) | Current or past use of HT | Post-menopausal women at beginning of study – 1044 | HT – 63.7 No HT – 68.8 |
Past users – 7.9 years and Current users – 16.5 years |
| Kaygusz 2012(41) | Observational cohort | DM and HTN excluded | Estradiol hemihydrate 1.03 mg/day and norethisterone acetate 0.5 mg/day vs. control | HT – 35 No HT – 50 |
HT – 52 No HT – 53.2 |
At least 1 year prior to study |
| Vitolo 2015(42) | Observational, cross-sectional | Normal albumin excretion 6 months prior to the study | Estrogen-containing HT | Post-menopausal women at beginning of study = 374 | HT – 54.4 No HT – 55.6 |
Within the last year |
Abbreviations – HT, hormone therapy, DM, Diabetes Mellitus, HTN, hypertension
Table 4.
Risk of Bias Assessments and Study Quality.
| Study | Study Design/Risk of Bias Scale | Main Source of Risk of Bias | Qualitya within study design category | Qualitya across study designs |
|---|---|---|---|---|
| Manning 2002(32) | Randomized Controlled Trial/Cochrane Risk of Bias Tool | Incomplete Outcome Data, selective outcome reporting, significant baseline imbalance | Low | Moderate |
| Machado 2008(30) | Randomized Controlled Trial/Cochrane Risk of Bias Tool | Low risk of bias | High | High |
| Yilmaz 2011(31) | Randomized Controlled Trial/Cochrane Risk of Bias Tool | No description of randomization, allocation concealment or blinding | Moderate | High |
| Szekac 2000(24) | Observational Study/Newcastle-Ottawa Scale, Cohort study | Low risk of bias | High | Moderate |
| Monster 2000(9) | Observational Study/Newcastle-Ottawa Scale, Case-Control study | Low risk of bias | High | Moderate |
| Fencki 2003(23) | Observational Study/Newcastle-Ottawa Scale, Cohort study | Low risk of bias | High | Moderate |
| Agarwal 2005(11) | Observational Study/Newcastle-Ottawa Scale, Cohort study | Low risk of bias | High | Moderate |
| Schopick 2009(12) | Observational Study/Newcastle-Ottawa Scale, Cohort study | Ensuring outcome was not present at beginning | Moderate | Low |
| Fung 2011(10) | Observational Study/Newcastle-Ottawa Scale, Cohort study | Low risk of bias | High | Moderate |
| Kaygusz 2012(41) | Observational Study/Newcastle-Ottawa Scale, Cohort study | Minimal description of cohort, did not ensure outcome was not present at beginning | Low | Very low |
| Vitolo 2015(42) | Observational Study/Newcastle-Ottawa Scale, Cohort study | Minimal description of cohort and exposure | Low | Very low |
| Manning 2002(32) | Observational Study/Newcastle-Ottawa Scale, Cohort study | Ensuring outcome was not present at beginning | Moderate | Low |
Each study was given a very low, low, moderate or high quality designation based on the risk of bias (Newcastle-Ottawa Scale for observational studies and Cochrane Risk of Bias Assessment for Randomized Controlled Trials) and the hierarchy of study methodology
Meta-Analysis
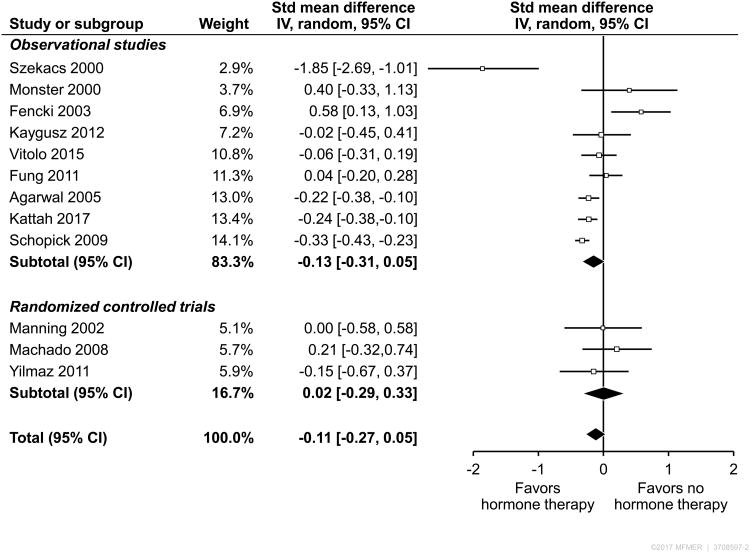
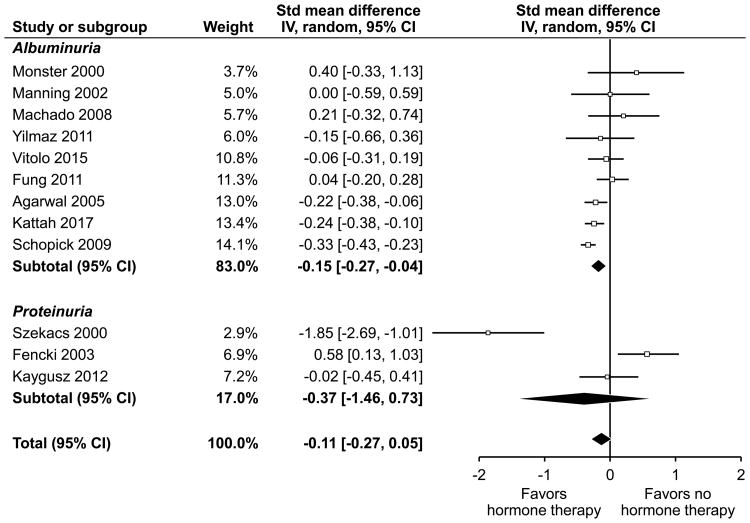
The individual outcomes, units of measure, threshold for an ‘abnormal’ dichotomous variable and adjustments made to the outcomes are shown in Table 5. The forest plot of all included studies is shown in Figure 2. Twelve studies were included in meta-analysis; 9 were observational studies and 3 were RCTS. The SMD of the effect of HT on urine proteinuria/albuminuria in the RCTs was 0.02 (95% CI -0.29–0.33, p = 0.89) and -0.13 (95% CI -0.31-0.05, p=0.15) in the observational studies. Nine studies reported albuminuria, while 3 studies reported proteinuria as an outcome (Figure 3). All three RCT studies reported albuminuria as outcome. The studies that reported albuminuria showed a significant effect in the direction of benefit of HT (-0.15, 95% CI -0.27- -0.04), whereas there was no net effect in the studies that reported proteinuria (-0.37, 95% CI -1.46-0.73). The effect was no longer significant in a sensitivity analysis excluding our own study. The overall pooled estimate of all studies was -0.11 (95% CI -0.27-0.05) consistent with a small, non-significant effect in the direction of benefit of HT. The heterogeneity of the overall effect was high (I2 = 75%).
Table 5.
Outcomes Reported for Individual Studies.
| Study | Outcome | Threshold | Main Outcome used in Meta-Analysis | Value of Outcome | Adjustments |
|---|---|---|---|---|---|
| Manning 2002(32) | Albumin-to-creatinine ratio (mg/g Cr) | 30 mg/g Cr | Median (IQR) range of change from baseline to end of intervention | Median (IQR) of changea HT: 2 (-11, 21) Placebo: 2 (-1, 14) |
None |
| Machado 2008(30) | Urine albumin excretion (μg/min) by 12 hour collection | N/A | Mean change in microalbuminuria from baseline to follow-up | Mean (SD) of change HT: -0.16 (2.79) Placebo: -0.86 (3.64) |
None |
| Yilmaz 2011(31) | Microalbumin (mg/L) | N/A | Mean change in microalbuminuria from baseline to follow-up | Mean (SD imputed)b of change HT: -11.5 (24.3) Placebo: -8.2 (18.3) |
None |
| Szekac 2000(24) | 24-hour urine protein (mg/24 hrs) | N/A | Mean change in proteinuria from baseline to follow-up | Mean (SD) of pre- and post-HT Pre-HT: 452(39) Post-HT: 370 (47) |
None |
| Monster 2000(9) | Microalbuminuria | 30-300 mg/g Cr | Odds Ratio – adjusted | OR (95% CI): 2.05 (1.12-3.77) | Age, HTN, DM, obesity, hyperlipidemia, smoking |
| Fencki 2003(23) | 24-hour urine protein (mg/24 hrs) | N/A | Mean change in proteinuria from baseline to follow-up (combined HT and DM) | Mean (SD) of pre- and post-HT Pre-HT: 125.9 (31.9) Post-HT: 142 (21.3) |
None |
| Agarwal 2005(11) | Albumin-to-creatinine ratio (mg/g Cr) | 25 mg/g Cr | Odds Ratio – adjusted | OR (95% CI): Prospective: OR 0.67 (0.43-1.01) Mean (SD) of ln (UACR) HT: 2.16 (0.82) No HT: 2.43 (1.07) |
Age, race, DM, SBP, DBP, triglycerides, HDL, smoking, BMI, GFR |
| Schopick 2009(12) | Top albumin-to-creatinine ratio decile | 9.2 mg/g Cr | Odds Ratio – adjusted | OR (95% CI): 0.55 (0.39-0.77) Median (IQR) of two groups: HT (> 6 years): 2.58 (1.6-4.6)c No HT: 3.5 (2.1-5.8) |
Age, BMI, hypertension and eGFR |
| Fung 2011(10) | Albumin-to-creatinine ratio (mg/g Cr) | 25 mg/g Cr | Odds Ratio - adjusted | Cross-sectional: OR 1.07 (0.73-1.56) Prospective: Mean (SD imputed)b change of ln(UACR) HT: -0.07 (0.8) No HT: 0.31 (0.77) |
Age, weight, DM, smoking, hyperlipidemic, HTN Age |
| Kaygusz 2012(41) | Protein-to-creatinine ratio | N/A | Difference in mean between HT and no HT group | Median (IQR) of two groupsa: HT: 0.08 (0.01-0.97) No HT: 0.09 (0-0.6) |
None |
| Vitolo 2015(42) | Albumin-to-creatinine ratio (mg/g Cr) | N/A | Odds Ratio – unadjusted | OR (95% CI): 0.90 (0.549-1.479) Median (IQR) of two groupsa: HT: 6.45 (2.16-29.1) No HT: 6.15 (2.70-33.9) |
None |
Median taken as mean change and interquartile range converted to standard deviation of change for analysis of continuous outcomes.
Standard deviation of change imputed using the correlation coefficient calculated from Machado et al (correlation coefficient = 0.53).
Combined medians of all groups with > 6 years of hormone therapy and took widest confidence interval for the group.
Abbreviations: Cr = creatinine, IQR = interquartile range, HT = hormone therapy, SD = standard deviation, N/A = not applicable, HTN = hypertension, DM = diabetes, BMI = body-mass index, OR = odds ratio, CI = confidence interval, UACR = urine albumin-to-creatinine ratio
Figure 2.
Forest Plot of the standardized mean difference of the effect of hormone therapy on urine albuminuria/proteinuria in observational studies and randomized controlled trials. Each study is listed according to subgroup, with the corresponding weight, standardized mean difference and 95% Confidence Interval (CI).
Figure 3.
Forest Plot of the standardized mean difference of the effect of hormone therapy on urine albuminuria and proteinuria in all included studies. Each study is listed according to subgroup, with the corresponding weight, standardized mean difference and 95% Confidence Interval (CI).
In a sensitivity analysis, we removed the two studies with no comparator group (23, 24) and the overall pooled estimate was significant at -0.15 (95% CI -0.26- -0.04). No statistically significant difference was observed in any other predefined subgroup analyses (study quality, inclusion/exclusion of diabetics or estrogen vs. estrogen/progesterone). In a sensitivity analysis of observational studies, we pooled only estimates from the 5 studies, including our own (9-12), that were adjusted for important covariates, such as age, diabetes and hypertension (Table 5) and found a significant benefit in the direction of HT (-0.21, 95% CI -0.34 - -0.08). In our analysis of observational study types, we found that cross-sectional and case-control studies (9-11, 25), including our own, had an SMD of -0.06 (95% CI -0.19 – 0.06) for the association of HT use and albuminuria, whereas prospective, cohort studies had a significant SMD of -0.26 (95% CI -0.53 – 0.0). Of note, both Fung and Agarwal et al (10, 11) presented cross-sectional and prospective, cohort analyses and so the results from each portion of these two studies were considered separately in this subgroup analysis and we did not test for interaction as the studies were not independent observations.
Discussion
The association of HT and albuminuria was evaluated in a large, racially and ethnically diverse cohort with well-defined medical co-morbidities. We found that HT use was significantly associated with a 40% decreased odds of albuminuria after accounting for traditional risk factors for chronic kidney disease. We next conducted a systematic review and meta-analysis and identified a heterogeneous body of evidence on the association of HT and urine protein excretion. The overall pooled estimate on the association of HT use and measures of albuminuria or proteinuria was small and not significant, but became significant in the direction of benefit when including only studies that evaluated albuminuria, a more sensitive marker of glomerular permeability, and if the studies with no comparator arms were excluded. We also found a significant benefit in prospective cohort studies, but not in case-control and cross-sectional analyses, nor in randomized controlled trials. After synthesizing our data with the available literature and evaluating the potential causes for heterogeneity, we feel that the association between hormone therapy and reduced albuminuria is truly present and may be due to the biologic effects of estrogen, issues of study design and confounding, or a combination of both.
The rationale behind studying the effect of estrogen on kidney function has come from several different arenas. In experimental methods where one can manipulate the sex hormones separately, estrogen appears to be renoprotective.(8, 26, 27) From a clinical perspective, women have demonstrated slower progression of chronic kidney disease, as was shown in a meta-analysis of 11,345 participantss with non-diabetic renal disease.(28) In the Modification of Diet in Renal Disease study, a post-hoc analysis showed that the decline in eGFR was slower in women than men under the age of 52, but similar after the age of 52, though this effect was lost after multivariate analysis taking into account blood pressure, urine protein measurements and HDL.(2) It is possible that HT use is reducing albuminuria indirectly, such as through effects on blood pressure, as opposed to a direct effect on kidney function. While the Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial did not show any significant effects of conjugated equine estrogen on blood pressure over a 3-year period (29), a study on women undergoing oophorectomy found that there is a significant increase in mean 24-hour blood pressure, night-time blood pressures and forearm vascular resistance immediately after removal of the ovaries that improves after 3 months with transdermal estradiol, suggesting that estrogen is important for blood pressure homeostasis.(30)
Studies on HT use in post-menopausal women have been initiated as a way to understand the effect of estrogen on human kidneys. We identified 9 observational studies, including our own, that have specifically studied this relationship. We found that the effect size of approximately a 40% reduction in the odds of albuminuria was consistent across 3 three observational studies of similar design.(11, 12) The observational study by Fung et al, reported an adjusted odds ratio from the cross-sectional portion of the study which suggested an increased risk of albuminuria, but found that HT use was associated with a decline in albuminuria as a continuous measure in the longitudinal portion of the study.(10) This difference may reflect a survivor bias, whereby the women who survived the prospective portion of the study were younger and healthier. Another outlier in the observational study group is the study by Monster et al.(9) This study differed from the others in that it was a population-based, nested case-control study. The three small RCTs showed overall no effect of HT on albuminuria in a 6-month time frame.(31-33) The consistency of the effect size in a subset of observational studies that differs from other observational studies and RCTs suggest the presence of a systematic bias.
One potential source of bias could be the healthy user bias, which is well described in the literature on HT and cardiovascular disease.(34) The NHS and a large systematic review of observational studies both demonstrated a reduced risk of coronary heart disease in HT users,(35, 36) while two large RCTs, the Heart and Estrogen-progestin Replacement Study and the Women's Health Initiative (WHI), demonstrated an increased risk of cardiovascular disease in HT users.(37, 38) The healthy user bias suggests that women who take HT are fundamentally different than women who do not and that those differences are strongly associated with decreased cardiovascular risk, or in our case, a decreased risk of albuminuria. HT use in our cohort was not only associated with a more favorable metabolic profile, but also race and level of education. Despite adjustment for these confounders, there is still a risk for residual confounding, particularly for socioeconomic factors, which play a significant role in disease.(39)
An important factor we could not address in our study is when in relation to menopause HT was started, which has been shown to be an important consideration.(40) In the NHS, the women on HT for the longest (> 15 years), underwent menopause at the youngest age and had the largest decrease in risk of albuminuria.(12) A study by Ahmed et al demonstrated a larger decline in eGFR in HT users as compared to non-users in an elderly population, which could reflect the importance of woman's age on the effects of HT.(41)
Our study has several limitations. We had no information on how long women were taking HT and did not have longitudinal measurements of renal function. The study visit in GENOA relied heavily on self-report by survey, however, we were able to confirm HT use in a subset of women that had their pill bottles reviewed (n=1468) and found that self-report of hormone use was accurate in 96.6% of cases. Blood pressure and lipid measurements were taken directly as part of the study, as well. As the majority of women were on oral conjugated estrogens, these results may not be applicable to women on other formulations, such as transdermal bioidentical hormones. The participants in the GENOA study were recruited on the basis of hypertension and/or diabetes and so this may limit the generalizability of the results. We tried to address these limitations by putting our study in the context of a systematic review. We had to impute several values for the meta-analysis, but we used conservative estimates and sensitivity analyses to ensure that our assumptions were not affecting our results. The overall quality of the evidence is low to moderate. While we were able to find a statistically significant association between HT use and albuminuria, the clinical significance of this finding is unclear.
Conclusion
While the biologic basis for estrogen having a potentially beneficial effect on renal function is strong, the results in human studies are mixed. We found that HT was associated with a reduced risk of albuminuria, consistent with the results of other observational studies of a similar design. In our meta-analysis, we found that studies evaluating specifically albuminuria, as opposed to total proteinuria, showed a net benefit of HT. Additional physiologic studies in humans are needed to further elucidate the effects of sex hormones on renal function.
Supplementary Material
Supplemental Digital Content: SDC 1 – Word document with search strategy for meta-analysis
Acknowledgments
Patricia Erwin for her help designing the search strategy.
Funding: This publication was supported by Grant Number UL1 TR000 135 from the National Center for Advancing Translational Sciences (NCATS), as well as Grant Numbers HL054464, HL054457, HL054481, HL087660, and HL119443 from the National Heart, Lung, and Blood Institute (NHLBI) Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Disclosures: None. The results of this study have not been published previously in whole or part, except in abstract form.
References
- 1.Berg UB. Differences in decline in GFR with age between males and females. Reference data on clearances of inulin and PAH in potential kidney donors. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2006;21(9):2577–82. doi: 10.1093/ndt/gfl227. [DOI] [PubMed] [Google Scholar]
- 2.Coggins CH, Breyer Lewis J, Caggiula AW, Castaldo LS, Klahr S, Wang SR. Differences between women and men with chronic renal disease. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1998;13(6):1430–7. doi: 10.1093/ndt/13.6.1430. [DOI] [PubMed] [Google Scholar]
- 3.Neugarten J, Acharya A, Lei J, Silbiger S. Selective estrogen receptor modulators suppress mesangial cell collagen synthesis. Am J Physiol Renal Physiol. 2000;279(2):F309–18. doi: 10.1152/ajprenal.2000.279.2.F309. [DOI] [PubMed] [Google Scholar]
- 4.Xu R, Zhang LX, Zhang PH, Wang F, Zuo L, Wang HY. Gender differences in age-related decline in glomerular filtration rates in healthy people and chronic kidney disease patients. BMC Nephrol. 2010;11:20. doi: 10.1186/1471-2369-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kummer S, von Gersdorff G, Kemper MJ, Oh J. The influence of gender and sexual hormones on incidence and outcome of chronic kidney disease. Pediatr Nephrol. 2012;27(8):1213–9. doi: 10.1007/s00467-011-1963-1. [DOI] [PubMed] [Google Scholar]
- 6.Lane PH. Estrogen receptors in the kidney: lessons from genetically altered mice. Gender medicine. 2008;5 Suppl A:S11–8. doi: 10.1016/j.genm.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Gross ML, Adamczak M, Rabe T, Harbi NA, Krtil J, Koch A, et al. Beneficial Effects of Estrogens on Indices of Renal Damage in Uninephrectomized SHRsp Rats. Journal of the American Society of Nephrology : JASN. 2004;15(2):348–58. doi: 10.1097/01.asn.0000105993.63023.d8. [DOI] [PubMed] [Google Scholar]
- 8.Neugarten J. Gender and the progression of renal disease. Journal of the American Society of Nephrology : JASN. 2002;13(11):2807–9. doi: 10.1097/01.asn.0000035846.89753.d4. [DOI] [PubMed] [Google Scholar]
- 9.Monster TB, Janssen WM, de Jong PE, de Jong-van den Berg LT Prevention of R, Vascular End Stage Disease Study G. Oral contraceptive use and hormone replacement therapy are associated with microalbuminuria. Arch Intern Med. 2001;161(16):2000–5. doi: 10.1001/archinte.161.16.2000. [DOI] [PubMed] [Google Scholar]
- 10.Fung MM, Poddar S, Bettencourt R, Jassal SK, Barrett-Connor E. A cross-sectional and 10-year prospective study of postmenopausal estrogen therapy and blood pressure, renal function, and albuminuria: the Rancho Bernardo Study. Menopause. 2011;18(6):629–37. doi: 10.1097/gme.0b013e3181fca9c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwal M, Selvan V, Freedman BI, Liu Y, Wagenknecht LE. The relationship between albuminuria and hormone therapy in postmenopausal women. Am J Kidney Dis. 2005;45(6):1019–25. doi: 10.1053/j.ajkd.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 12.Schopick EL, Fisher ND, Lin J, Forman JP, Curhan GC. Post-menopausal hormone use and albuminuria. Nephrol Dial Transplant. 2009;24(12):3739–44. doi: 10.1093/ndt/gfp321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouchi R, Babazono T, Yoshida N, Nyumura I, Toya K, Hayashi T, et al. Association of albuminuria and reduced estimated glomerular filtration rate with incident stroke and coronary artery disease in patients with type 2 diabetes. Hypertens Res. 2010;33(12):1298–304. doi: 10.1038/hr.2010.170. [DOI] [PubMed] [Google Scholar]
- 14.Gansevoort RT, Nauta FL, Bakker SJ. Albuminuria: all you need to predict outcomes in chronic kidney disease? Curr Opin Nephrol Hypertens. 2010;19(6):513–8. doi: 10.1097/MNH.0b013e32833e4ce1. [DOI] [PubMed] [Google Scholar]
- 15.Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106(14):1777–82. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 16.Multi-center genetic study of hypertension: The Family Blood Pressure Program (FBPP) Hypertension. 2002;39(1):3–9. doi: 10.1161/hy1201.100415. [DOI] [PubMed] [Google Scholar]
- 17.Mattix HJ, Hsu CY, Shaykevich S, Curhan G. Use of the albumin/creatinine ratio to detect microalbuminuria: implications of sex and race. Journal of the American Society of Nephrology : JASN. 2002;13(4):1034–9. doi: 10.1681/ASN.V1341034. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009;3(3):e123–30. [PMC free article] [PubMed] [Google Scholar]
- 20.GA Wells BS, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2014 Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 21.Collaboration TC. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. 2011 Available from: www.cochrane-handbook.org.
- 22.Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. Bmj. 2006;333(7568):597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fenkci S, Fenkci V, Yilmazer M, Serteser M, Koken T. Effects of short-term transdermal hormone replacement therapy on glycaemic control, lipid metabolism, C-reactive protein and proteinuria in postmenopausal women with type 2 diabetes or hypertension. Hum Reprod. 2003;18(4):866–70. doi: 10.1093/humrep/deg146. [DOI] [PubMed] [Google Scholar]
- 24.Szekacs B, Vajo Z, Varbiro S, Kakucs R, Vaslaki L, Acs N, et al. Postmenopausal hormone replacement improves proteinuria and impaired creatinine clearance in type 2 diabetes mellitus and hypertension. Bjog. 2000;107(8):1017–21. doi: 10.1111/j.1471-0528.2000.tb10406.x. [DOI] [PubMed] [Google Scholar]
- 25.Vitolo E, Comassi M, Caputo MT, Solini A. Hormone replacement therapy, renal function and heart ultrasonographic parameters in postmenopausal women: An observational study. International Journal of Clinical Practice. 2015;69(6):632–7. doi: 10.1111/ijcp.12597. [DOI] [PubMed] [Google Scholar]
- 26.Tofovic SP, Dubey RK, Jackson EK. 2-Hydroxyestradiol attenuates the development of obesity, the metabolic syndrome, and vascular and renal dysfunction in obese ZSF1 rats. J Pharmacol Exp Ther. 2001;299(3):973–7. [PubMed] [Google Scholar]
- 27.Silbiger S, Lei J, Ziyadeh FN, Neugarten J. Estradiol reverses TGF-beta1-stimulated type IV collagen gene transcription in murine mesangial cells. Am J Physiol. 1998;274(6 Pt 2):F1113–8. doi: 10.1152/ajprenal.1998.274.6.F1113. [DOI] [PubMed] [Google Scholar]
- 28.Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. Journal of the American Society of Nephrology : JASN. 2000;11(2):319–29. doi: 10.1681/ASN.V112319. [DOI] [PubMed] [Google Scholar]
- 29.Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. Jama. 1995;273(3):199–208. [PubMed] [Google Scholar]
- 30.Mercuro G, Zoncu S, Saiu F, Mascia M, Melis GB, Rosano GM. Menopause induced by oophorectomy reveals a role of ovarian estrogen on the maintenance of pressure homeostasis. Maturitas. 2004;47(2):131–8. doi: 10.1016/s0378-5122(03)00252-4. [DOI] [PubMed] [Google Scholar]
- 31.Machado RB, Careta MF, Balducci GP, Araújo TS, Bernardes CR. Effects of estrogen therapy on microalbuminuria in healthy post-menopausal women. 2008;24(12):681–5. doi: 10.1080/09513590802444159. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=med5&AN=19172536. [DOI] [PubMed] [Google Scholar]
- 32.Yilmaz SA. Effects of estrogen replacement therapy to the renal function in postmenopausal women. Turk Jinekoloji ve Obstetrik Dernegi Dergisi. 2011;8(4):249–58. [Google Scholar]
- 33.Manning PJ, Sutherland WH, Allum AR, de Jong SA, Jones SD. HRT does not improve urinary albumin excretion in postmenopausal diabetic women. Diabetes Res Clin Pract. 2003;60(1):33–9. doi: 10.1016/s0168-8227(02)00279-6. [DOI] [PubMed] [Google Scholar]
- 34.Shrank WH, Patrick AR, Brookhart MA. Healthy user and related biases in observational studies of preventive interventions: A primer for physicians. Journal of General Internal Medicine. 2011;26(5):546–50. doi: 10.1007/s11606-010-1609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stampfer MJ, Colditz GA, Willett WC, Manson JE, Rosner B, Speizer FE, et al. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses' health study. The New England journal of medicine. 1991;325(11):756–62. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- 36.Grady D, Rubin SM, Petitti DB, Fox CS, Black D, Ettinger B, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med. 1992;117(12):1016–37. doi: 10.7326/0003-4819-117-12-1016. [DOI] [PubMed] [Google Scholar]
- 37.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. Jama. 1998;280(7):605–13. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 38.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. Jama. 2002;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 39.von Elm E, Egger M. The scandal of poor epidemiological research. Bmj. 2004;329(7471):868–9. doi: 10.1136/bmj.329.7471.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. Jama. 2007;297(13):1465–77. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 41.Ahmed SB, Culleton BF, Tonelli M, Klarenbach SW, Macrae JM, Zhang J, et al. Oral estrogen therapy in postmenopausal women is associated with loss of kidney function. Kidney Int. 2008;74(3):370–6. doi: 10.1038/ki.2008.205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content: SDC 1 – Word document with search strategy for meta-analysis