Abstract
Hot flashes have typically been classified as “symptoms of menopause” that should be tolerated or treated until they resolve. However, mounting evidence points to hot flashes as a manifestation of one or several underlying pathophysiological processes. Associations exist between the presence, timing of onset, severity, and duration of hot flashes and the risk of several neurological (affecting sleep, mood, and cognition) and cardiovascular conditions. In addition, four consistent patterns of vasomotor disturbances have been identified across different countries, making it unlikely that these patterns are solely explained by socioeconomic or cultural factors. The changing hormonal environment of menopause may unmask differences in the autonomic neurovascular control mechanisms that put an individual woman at risk for chronic conditions of aging. These differences may have a genetic basis or may be acquired across the life span and are consistent with the variability of the clinical manifestations of aging observed in women following bilateral oophorectomy. It is time to investigate the pathophysiological mechanisms underlying the four patterns of vasomotor symptoms more closely, and to shift from describing hot flashes as symptoms to be tolerated to manifestations of an underlying autonomic neurovascular dysregulation that need to be addressed.
Keywords: aging, autonomic nervous system, estrogen, hot flashes, menopause, night sweats
“What's in a name? that which we call a rose; by any other name would smell as sweet.”(William Shakespeare, Romeo and Juliet, lines 43-44, Act-II, Scene-II) In this well-known phrase, Juliet stresses that the importance of a person (or a thing) derives from what it is, not from what it is called. However, although names are just labels, they often carry unconscious meaning and bias that can diminish the worth or detract from the attention that the thing may deserve. Take for example, vasomotor symptoms, the “hot flashes” (“hot flushes”) and night sweats that women may experience as they transition to menopause and thereafter. These “flashes” and “sweats” have typically been classified or described as “symptoms of menopause”. But does this description diminish the significance of these manifestations? Labeling hot flashes and night sweats as symptoms of menopause in some ways implies they are merely bothersome symptoms that should be tolerated or treated until they resolve rather than manifestations of one or several underlying pathophysiological processes. Is it time for the scientific and medical community to change the description of hot flashes and night sweats from “menopausal symptoms” to a term that describes the potential underlying pathophysiological phenomenon of autonomic neurovascular dysregulation?
Historically, hot flashes and night sweats have been successfully treated with estrogen in the form of either 17β-estradiol (oral or transdermal preparations) or oral conjugated equine estrogens. Indeed, the practice guidelines from the Endocrine Society, the International Menopause Society, and the North American Menopause Society recommend the use of estrogen to relieve “vasomotor symptoms of menopause.”(1-3) In this line of thinking, vasomotor symptoms represent a chronic condition that is successfully treated with menopausal hormone therapy (MHT). However, the underlying pathophysiological mechanism accounting for and sustaining vasomotor or autonomic neurovascular dysregulation remains incompletely understood.(4)
In studies of women around the world, the prevalence of hot flashes varies across the menopausal transition.(5, 6) In three large, contemporary studies of women who experience hot flashes in the menopausal transition[the Australian Longitudinal Study of Women's Health (ALSWH), the Medical Research Council National Survey of Health and Development (1946 British birth cohort study), and the Study of Women Across the Nation (SWAN, USA)](7-10), four patterns can be identified based on onset, severity, and duration (Figure). In spite of differences in data reporting and terminology used to describe the patterns, their similarity across countries makes it unlikely that the patterns are solely explained by socioeconomic or cultural factors. If these four patterns reflect differences in neuro-regulatory processes or differences in vasomotor instability across women, it may be possible to separate the biological causes from the environmental and socioeconomic causes. In addition, these patterns might differentially associate with other chronic conditions of aging such as sleep disturbances, cognitive and mood disorders, cardiovascular disease, and osteoporosis.(11-13) Thus, we hypothesize that the changing hormonal environment of menopause (natural or surgically induced by oophorectomy) unmasks differences in the autonomic neurovascular control mechanisms that put an individual woman at risk for chronic conditions of aging. These observations can be explained by the unifying hypothesis of eu-estrogenemia proposed by Turner and Kebler.(14) This hypothesis is also supported by the variability in clinical manifestations of aging observed in women following bilateral oophorectomy(15), and by the differences in sleep-related blood pressure variability in menopausal women with insomnia.(16).
Figure.
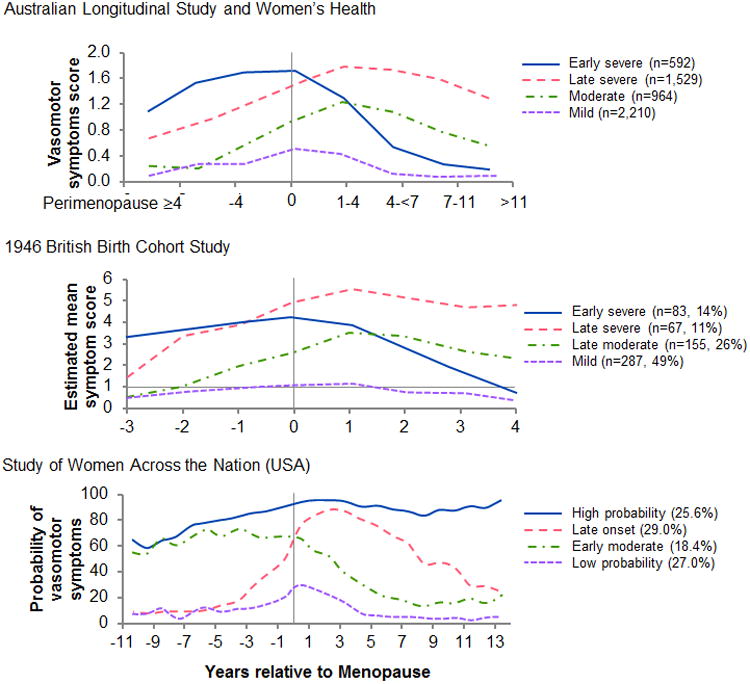
Patterns of vasomotor disturbances defined as a composite including hot flashes and night sweats reported by women transitioning through menopause in three distinct geographical regions: Australia, Great Britain, and the United States. Patterns were derived from data presented in Figure 2b of reference 5 (top panel), Figure 3 of reference 6 (middle panel), and Figure 1 of reference 7 (bottom panel). (Figures are reproduced with permission.)
Studies of the mechanisms underlying the expression of vasomotor instability in women have been hampered by the absence of animal models that recapitulate natural menopause and the various vasomotor patterns in relation to sleep and behavior (mood).(17-19) In addition, progress has been slowed by the lack of convenient, reliable, and standardized tools to objectively monitor vasomotor patterns in women(20), especially in a routine clinical setting, by the lack of standard terminology to describe and report the patterns (see figure), and by the lack of funding for longitudinal studies.
The presence and the severity of hot flashes and night sweats in clinical settings and clinical studies are usually self-reported using a Likert scale classifying the symptoms as none, mild, moderate, or severe. Such reporting may reflect cultural attitudes and care-giver bias in assessing the data and, therefore, does not inform the investigator or the treating physicians of the frequency, pattern, or timing of the events. Nevertheless, even with such an unsophisticated tool, significant associations of “severe” vasomotor patterns with sleep disturbances have been identified. Women with severe vasomotor patterns may need to undergo additional testing for sleep disorders such as obstructive sleep apnea or restless leg syndrome, particularly if the vasomotor symptom but not the sleep disturbances are diminished by MHT.(21-23) In general, the severity of vasomotor symptoms is also associated with an increased risk of cardiovascular disease including endothelial dysfunction, development of carotid intima media thickness, coronary artery calcification, cardiac microvascular disease, and adverse cardiac events.(24-27) However, the risk may vary with both the age of onset of menopause and the severity and type of the vasomotor disturbance (daytime hot flashes, night sweats or both.)(1, 12, 13, 20, 28-30)
Several genetic variants for enzymes involved with estrogen metabolism and for estrogen receptors are associated with onset of menopause and with the presence and severity of vasomotor instability.(11, 31-34) However, it is not known what fraction of the variability in phenotypes, including women who do not experience vasomotor symptoms, is explained by these genetic variants in the estrogen synthesis and signaling pathways, or the contribution of other variants such as those in genes within the noradrenergic or serotonergic signaling pathways. There is a need for additional case-control studies of large numbers of women for whom the absence or the presence, as well as the patterns of vasomotor symptoms, are known. In spite of the limitations of genetic studies, the development of a genotypic algorithm may help health care providers to select the most appropriate dose and formulations of MHT to target specific outcomes.
Given the associations between the presence, timing of onset, severity, and duration of hot flashes and the risk of several neurological conditions (sleep, mood, and cognition) and of cardiovascular disease, it is time to investigate the pathophysiological mechanisms underlying the four patterns of vasomotor symptoms more closely in terms of the central pathways that are involved. One such pathway is the hypothalamic-pituitary axis involving the KNDy neurons (kisspeptin, neurokinin B, and dynorphin)projecting to the hypothalamic site of thermoregulation.(35) Indeed, neurokinin B receptor antagonists reduced self-reported hot flashes by 45% over a 90 day period in a small group of women.(36) In the future, it will be important to understand whether these drugs are effective in reducing all patterns of vasomotor symptoms, their efficacy on daytime hot flashes compared to night sweats, and their possible effects on sleep and mood.
Interconnections of the hypothalamus with other areas of the brain related to sleep and mood need to be investigated more closely because vasomotor symptoms can be treated with drugs affecting the serotonin system (i.e., selective serotonin reuptake inhibitors, SSRIs), the noradrenergic system (i.e., alpha2-adrenergic agonists), the cholinergic system, and the gabanergic sytem.(37) However, these treatments have not been explored in relation to patterns of vasomotor symptoms, hormonal status, or risk for chronic diseases of aging (i.e., osteoporosis, cardiovascular disease, and cognitive decline).
In addition to dissecting the interactions between central thermoregulatory pathways and sleep, a greater understanding of potential differences in the autonomic outflow from these centers (sympathetic and parasympathetic activation) and in the neuroeffector tissues is needed. In particular, further research is needed on patterns or changes in peripheral innervation and activation, neurotransmitter synthesis, adrenergic and cholinegic receptor responsiveness of the vascular smooth muscle, and on vascular smooth muscle sensitivity to vasodilatory processes mediated by by the vascular endothelium including those processes requiring activation of guanylate cyclase.(38-40)
Devices to quantitatively monitor physiological parameters associated with hot flashes (skin resistance) are now available so that prospective studies can be designed to examine the relationships between genetic variants affecting hormone metabolism and hormone receptors, and the autonomic neurovascular regulatory processes manifesting as vasomotor symptoms.(41-43) A few key research questions for future inquiry are listed below:
Is there a difference in autonomic function [perhaps measured by peripheral skin or muscle sympathetic nerve activity(44-46)] among women who experience different patterns of autonomic neurovascular dysregulation (none to severe)? How do these patterns relate to the risk of cardiovascular disease, osteoporosis, sleep and mood disorders, or cognitive decline?
Is the difference in autonomic function across women associated with their age of onset of menopause or their genomic profile for estrogen metabolism and signaling? How do these genotypes relate to patterns of autonomic neurovascular dysregulation?
Can more convenient and sensitive monitoring devices be developed to assess patterns of autonomic neurovascular dysregulation (vasomotor symptoms)?
How does the risk for chronic disease change with an individualized MHT dosing regimen?
Considering vasomotor disturbances as manifestations of one or several underlying pathophysiological processes, rather than as a “symptom” of menopause, shifts the focus of scientists and clinicians toward the need to mitigate future chronic disease risk (cardiovascular disease, dementia, and osteoporosis).
Conclusion
Classifying hot flashes and night sweats as symptoms of menopause rather than as manifestations of an underlying autonomic and vascular dysregulation has hampered scientific investigation into the mechanisms controlling these phenomena, and has delayed the understanding of different aging trajectories in women due to estrogen deficiency. “Autonomic neurovascular dysregulation” may not be the only or final term to describe these physiological responses. However, the name describes a physiological phenomenon that can help to focus attention and research on the underlying control mechanisms.
Acknowledgments
Funding: Research of authors contributing to these comments is funded through grants from the National Institutes of Health, P50 AG044170, R01 AG034676, and R01 HL83947.
Footnotes
Conflicts of Interest: Dr. Faubion is a consultant for Mithra Pharmaceuticals and Procter and Gamble.
References
- 1.Stuenkel CA, Davis SR, Gompel A, Lumsden MA, Murad MH, Pinkerton JV, et al. Treatment of Symptoms of the Menopause: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2015;100(11):3975–4011. doi: 10.1210/jc.2015-2236. [DOI] [PubMed] [Google Scholar]
- 2.de Villiers TJ, Hall JE, Pinkerton JV, Perez SC, Rees M, Yang C, et al. Revised global consensus statement on menopausal hormone therapy. Maturitas. 2016;91:153–5. doi: 10.1016/j.maturitas.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 3.The NHTPSAP. The 2017 hormone therapy position statement of the The North American Menopause Society. Menopause. 2017;24(7):728–53. doi: 10.1097/GME.0000000000000921. [DOI] [PubMed] [Google Scholar]
- 4.Freedman RR. Menopausal hot flashes: mechanisms, endocrinology, treatment. J Steroid Biochem Mol Biol. 2014;142:115–20. doi: 10.1016/j.jsbmb.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman EW, Sherif K. Prevalence of hot flushes and night sweats around the world: a systematic review. Climacteric. 2007;10(3):197–214. doi: 10.1080/13697130601181486. [DOI] [PubMed] [Google Scholar]
- 6.Archer DF, Sturdee DW, Baber R, de Villiers TJ, Pines A, Freedman RR, et al. Menopausal hot flushes and night sweats: where are we now? Climacteric. 2011;14(5):515–28. doi: 10.3109/13697137.2011.608596. [DOI] [PubMed] [Google Scholar]
- 7.Mishra GD, Dobson AJ. Using longitudinal profiles to characterize women's symptoms through midlife: results from a large prospective study. Menopause. 2012;19(5):549–55. doi: 10.1097/gme.0b013e3182358d7c. [DOI] [PubMed] [Google Scholar]
- 8.Mishra GD, Kuh D. Health symptoms during midlife in relation to menopausal transition: British prospective cohort study. BMJ. 2012;344:e402. doi: 10.1136/bmj.e402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tepper PG, Brooks MM, Randolph JF, Jr, Crawford SL, El Khoudary SR, Gold EB, et al. Characterizing the trajectories of vasomotor symptoms across the menopausal transition. Menopause. 2016;23(10):1067–74. doi: 10.1097/GME.0000000000000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avis NE, Crawford SL, Greendale G, Bromberger JT, Everson-Rose SA, Gold EB, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175(4):531–9. doi: 10.1001/jamainternmed.2014.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thurston RC, Joffe H. Vasomotor symptoms and menopause: findings from the Study of Women's Health across the Nation. Obstet Gynecol Clin North Am. 2011;38(3):489–501. doi: 10.1016/j.ogc.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gast GC, Pop VJ, Samsioe GN, Grobbee DE, Nilsson PM, Keyzer JJ, et al. Vasomotor menopausal symptoms are associated with increased risk of coronary heart disease. Menopause. 2011;18(2):146–51. doi: 10.1097/gme.0b013e3181f464fb. [DOI] [PubMed] [Google Scholar]
- 13.Szmuilowicz ED, Manson JE, Rossouw JE, Howard BV, Margolis KL, Greep NC, et al. Vasomotor symptoms and cardiovascular events in postmenopausal women. Menopause. 2011;18(6):603–10. doi: 10.1097/gme.0b013e3182014849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner RJ, Kerber IJ. A theory of eu-estrogenemia: a unifying concept. Menopause. 2017;24(9):1086–97. doi: 10.1097/GME.0000000000000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rocca WA, Gazzuola Rocca L, Smith CY, Grossardt BR, Faubion SS, Shuster LT, et al. Bilateral Oophorectomy and Accelerated Aging: Cause or Effect? J Gerontol A Biol Sci Med Sci. 2017;72:1213–7. doi: 10.1093/gerona/glx026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Zambotti M, Trinder J, Javitz H, Colrain IM, Baker FC. Altered nocturnal blood pressure profiles in women with insomnia disorder in the menopausal transition. Menopause. 2017;24(3):278–87. doi: 10.1097/GME.0000000000000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellino FL. Nonprimate animal models of menopause: workshop report. Menopause. 2000;7(1):14–24. doi: 10.1097/00042192-200007010-00004. [DOI] [PubMed] [Google Scholar]
- 18.Prelle K, Igl BW, Obendorf M, Girbig D, Lehmann T, Patchev VK. Endpoints of drug discovery for menopausal vasomotor symptoms: interpretation of data from a proxy of disease. Menopause. 2012;19(8):909–15. doi: 10.1097/gme.0b013e318245533f. [DOI] [PubMed] [Google Scholar]
- 19.Koebele SV, Bimonte-Nelson HA. Modeling menopause: The utility of rodents in translational behavioral endocrinology research. Maturitas. 2016;87:5–17. doi: 10.1016/j.maturitas.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thurston RC, Chang Y, Barinas-Mitchell E, Jennings JR, von Kanel R, Landsittel DP, et al. Physiologically assessed hot flashes and endothelial function among midlife women. Menopause. 2017;24(8):886–93. doi: 10.1097/GME.0000000000000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cintron D, Lahr BD, Bailey KR, Santoro N, Lloyd R, Manson JE, et al. Effects of oral versus transdermal menopausal hormone treatments on self-reported sleep domains and their association with vasomotor symptoms in recently menopausal women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS) Menopause. 2017 doi: 10.1097/GME.0000000000000971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cintron D, Lipford M, Larrea-Mantilla L, Spencer-Bonilla G, Lloyd R, Gionfriddo MR, et al. Efficacy of menopausal hormone therapy on sleep quality: systematic review and meta-analysis. Endocrine. 2017;55(3):702–11. doi: 10.1007/s12020-016-1072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao C, Kapoor E, Lipford M, Miller V, Schroeder D, Mara K, et al. Association of vasomotor symptoms and sleep apnea risk in midlife women. 2017;25(4) doi: 10.1097/GME.0000000000001020. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang AJ, Sawaya GF, Vittinghoff E, Lin F, Grady D. Hot flushes, coronary heart disease, and hormone therapy in postmenopausal women. Menopause. 2009;16(4):639–43. doi: 10.1097/gme.0b013e31819c11e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thurston RC, Sutton-Tyrell K, Everson-Rose SA, Hess R, Matthews KA. Hot Flashes and Subclinical Cardiovascular Disease. Findings From the Study of Women's Health Across the Nation Heart Study. Circulation. 2008;118:1234–40. doi: 10.1161/CIRCULATIONAHA.108.776823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muka T, Oliver-Williams C, Colpani V, Kunutsor S, Chowdhury S, Chowdhury R, et al. Association of Vasomotor and Other Menopausal Symptoms with Risk of Cardiovascular Disease: A Systematic Review and Meta-Analysis. PLoS One. 2016;11(6):e0157417. doi: 10.1371/journal.pone.0157417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thurston RC, Johnson BD, Shufelt CL, Braunstein GD, Berga SL, Stanczyk FZ, et al. Menopausal symptoms and cardiovascular disease mortality in the Women's Ischemia Syndrome Evaluation (WISE) Menopause. 2017;24(2):126–32. doi: 10.1097/GME.0000000000000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allison MA, Manson JE, Aragaki A, Langer RD, Rossouw J, Curb D, et al. Vasomotor symptoms and coronary artery calcium in postmenopausal women. Menopause. 2010;17(6):1136–45. doi: 10.1097/gme.0b013e3181e664dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Svartberg J, von Muhlen D, Kritz-Silverstein D, Barrett-Connor E. Vasomotor symptoms and mortality: the Rancho Bernardo Study. Menopause. 2009;16(5):888–91. doi: 10.1097/gme.0b013e3181a4866b. [DOI] [PubMed] [Google Scholar]
- 30.Hitchcock CL, Elliott TG, Norman EG, Stajic V, Teede H, Prior JC. Hot flushes and night sweats differ in associations with cardiovascular markers in healthy early postmenopausal women. Menopause. 2012;19(11):1208–14. doi: 10.1097/gme.0b013e31825541cc. [DOI] [PubMed] [Google Scholar]
- 31.Moyer AM, de Andrade M, Weinshilboum RM, Miller VM. Influence of SULT1A1 genetic variation on age at menopause, estrogen levels, and response to hormone therapy in recently postmenopausal white women. Menopause. 2016;23(8):863–9. doi: 10.1097/GME.0000000000000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moyer AM, Miller VM, Faubion SS. Could personalized management of menopause based on genomics become a reality? Pharmacogenomics. 2016;17(7):659–62. doi: 10.2217/pgs.16.17. [DOI] [PubMed] [Google Scholar]
- 33.Crandall CJ, Crawford SL, Gold EB. Vasomotor symptom prevalence is associated with polymorphisms in sex steroid-metabolizing enzymes and receptors. Am J Med. 2006;119(9 Suppl 1):S52–60. doi: 10.1016/j.amjmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Johansson H, Gray KP, Pagani O, Regan MM, Viale G, Aristarco V, et al. Impact of CYP19A1 and ESR1 variants on early-onset side effects during combined endocrine therapy in the TEXT trial. Breast Cancer Res. 2016;18(1):110. doi: 10.1186/s13058-016-0771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34(3):211–27. doi: 10.1016/j.yfrne.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prague JK, Roberts RE, Comninos AN, Clarke S, Jayasena CN, Nash Z, et al. Neurokinin 3 receptor antagonism as a novel treatment for menopausal hot flushes: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389(10081):1809–20. doi: 10.1016/S0140-6736(17)30823-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rapkin AJ. Vasomotor symptoms in menopause: physiologic condition and central nervous system approaches to treatment. Am J Obstet Gynecol. 2007;196(2):97–106. doi: 10.1016/j.ajog.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 38.Tuomikoski P, Ebert P, Groop PH, Haapalahti P, Hautamaki H, Ronnback M, et al. Effect of hot flushes on vascular function: a randomized controlled trial. Obstet Gynecol. 2009;114(4):777–85. doi: 10.1097/AOG.0b013e3181b6f268. [DOI] [PubMed] [Google Scholar]
- 39.Tuomikoski P, Ebert P, Groop PH, Haapalahti P, Hautamaki H, Ronnback M, et al. Evidence for a role of hot flushes in vascular function in recently postmenopausal women. Obstet Gynecol. 2009;113(4):902–8. doi: 10.1097/AOG.0b013e31819cac04. [DOI] [PubMed] [Google Scholar]
- 40.Sassarini J, Fox H, Ferrell W, Sattar N, Lumsden MA. Vascular function and cardiovascular risk factors in women with severe flushing. Clin Endocrinol (Oxf) 2011;74(1):97–103. doi: 10.1111/j.1365-2265.2010.03921.x. [DOI] [PubMed] [Google Scholar]
- 41.Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension. 2005;45(4):522–5. doi: 10.1161/01.HYP.0000160318.46725.46. [DOI] [PubMed] [Google Scholar]
- 42.Liu CC, Kuo TB, Yang CC. Effects of estrogen on gender-related autonomic differences in humans. Am J Physiol (Heart Circ Physiol 42) 2003;285(5):H2188–93. doi: 10.1152/ajpheart.00256.2003. [DOI] [PubMed] [Google Scholar]
- 43.Mercuro G, Podda A, Pitzalis L, Zoncu S, Mascia M, Melis GB, et al. Evidence of a role of endogenous estrogen in the modulation of autonomic nervous system. Am J Cardiol. 2000;85(6):787–9. A9. doi: 10.1016/s0002-9149(99)00865-6. [DOI] [PubMed] [Google Scholar]
- 44.Charkoudian N, Hart ECJ, Barnes JN, Joyner MJ. Autonomic control of body temperature and blood pressure: influences of female sex hormones. Clin Auton Res. 2017;27(3):149–55. doi: 10.1007/s10286-017-0420-z. [DOI] [PubMed] [Google Scholar]
- 45.Hart EC, Head GA, Carter JR, Wallin BG, May CN, Hamza SM, et al. Recording sympathetic nerve activity in conscious humans and other mammals: guidelines and the road to standardization. Am J Physiol (Heart Circ Physiol 42) 2017;312(5):H1031–H51. doi: 10.1152/ajpheart.00703.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Low DA, Hubing KA, Del Coso J, Crandall CG. Mechanisms of cutaneous vasodilation during the postmenopausal hot flash. Menopause. 2011;18(4):359–65. doi: 10.1097/gme.0b013e3181f7a17a. [DOI] [PMC free article] [PubMed] [Google Scholar]


