Abstract
Increased synchrony within neuroanatomical networks is often observed in neurophysiologic studies of human brain disease. Most often, this phenomenon is ascribed to a compensatory process in the face of injury, though evidence supporting such accounts is limited. Given the known dependence of resting state functional connectivity (rsFC) on underlying structural connectivity (SC), we examine an alternative hypothesis: that topographical changes in SC, specifically particular patterns of disconnection, contribute to increased network rsFC. We obtain measures of rsFC using fMRI and SC using probabilistic tractography in 50 healthy and 28 multiple sclerosis subjects. Using a computational model of neuronal dynamics we simulate BOLD using healthy subject SC to couple regions. We find that altering the model by introducing structural disconnection patterns observed in those multiple sclerosis subjects with high network rsFC generates simulations with high rsFC as well, suggesting that disconnection itself plays a role in producing high network functional connectivity. We then examine SC data in individuals. In multiple sclerosis subjects with high network rsFC we find a preferential disconnection between the relevant network and wider system. We examine the significance of such network isolation by introducing random disconnection into the model. As observed empirically, simulated network rsFC increases with removal of connections bridging a community with the remainder of the brain. We thus show that structural disconnection known to occur in multiple sclerosis contributes to network rsFC changes in multiple sclerosis and further that community isolation is responsible for elevated network functional connectivity.
Keywords: Brain Networks, Resting State Functional Connectivity, Diffusion MRI, Disconnection, Multiple Sclerosis
Introduction
An expanding body of evidence makes clear the importance of interactions between widely distributed neuronal populations in the production of cognition and behavior (Bressler and Menon 2010; Mesulam 1990). Analyses of temporal coherence in activity between distant sites (functional connectivity) reveal neuroanatomical networks corresponding to sets of regions activated by experimental tasks (Fox and Raichle 2007). This covariance structure is altered in neurologic disease and such alterations are associated with behavioral markers (Greicius 2008; Zhang and Raichle 2010). Thus far, this work has been largely descriptive. The mechanism underlying functional connectivity alterations and their behavioral significance remains a central point of inquiry in systems neuroscience (Park and Friston 2013).
Several lines of evidence indicate functional connectivity patterns at multiple spatial scales are constrained by the underlying structural wiring (Honey et al. 2009; Johnston et al. 2008; van den Heuvel et al. 2009). At the same time, functional connectivity measures carry information independent of structural connectivity (Deco et al. 2011; Gonzalez-Castillo et al. 2015; Handwerker et al. 2012; Hutchison et al. 2013; Misic et al. 2016). Given the multitude of factors contributing to network synchronization and the absence of a systematic framework accounting for the complexity of their interactions, interpretation of functional connectivity alterations in clinical populations has been largely speculative. In particular, observations of increased network functional connectivity have been difficult to interpret and are most typically cited as evidence of adaptive neuroplasticity (Jones et al. 2016; Simioni et al. 2016; Venkatesan et al. 2015; Yoo et al. 2007). A more parsimonious explanation, that disease related structural disconnection may itself increase functional connectivity, has not been systematically examined.
Here we investigate the effects of large-scale structural disconnection on resting-state functional connectivity (rsFC) in multiple sclerosis. As early disease pathology is characterized predominantly by focal white matter lesions, multiple sclerosis represents a naturally occurring model of heterogeneous structural disconnection. In initial stages, increased network synchronization is often observed (Roosendaal et al. 2010; Valsasina et al. 2011; Zhou et al. 2014) and is associated with behavioral markers (Faivre et al. 2012; Hawellek et al. 2011). Most commonly these changes are interpreted as evidence of compensatory processes supporting behavioral function in the face of injury. The contribution of underlying structural changes to these observations has not been formally considered.
Biophysical modeling of the dynamics underlying the production of neurophysiologic information may advance investigations of disease beyond descriptive work towards hypothesis-driven analysis (Stephan et al. 2015). In this pilot study we use an established computational model (Goni et al. 2014; Honey et al. 2007; Honey et al. 2009) to show that large-scale structural changes increase network rsFC. We then identify structural features particular to subjects with high rsFC and show, using the model, that rsFC varies with these features. We conclude that simple changes in structural wiring may increase network rsFC.
Materials and Methods
Study Population
Data from 28 subjects was collected from an ongoing longitudinal analysis of advanced imaging markers in relapsing remitting multiple sclerosis (see supplementary appendix). All subjects provided written informed consent under institutional board approval. Multiple sclerosis subjects had a mean age of 40.2, a median Expanded Disability Status Scale (EDSS) of 2.0 and a mean disease duration of 7.2 years. Imaging data from fifty healthy subjects was collected from the MGH/UCLA Consortium Human Connectome Project (HCP, http://www.humanconnectomeproject.org). Demographic and clinical characteristics of each group are presented in the supplementary appendix.
MRI Acquisition and Preprocessing
Multiple sclerosis subject data was acquired on a Siemens 3T Connectom scanner with a 300 mT/m gradient system and 64-channel brain array coil (Keil et al. 2013). The hardware and acquisition protocols were identical to those used to acquire the HCP dataset (http://www.humanconnectomeproject.org/data/documents-and-sops/; see supplemental appendix).
This analysis used a T1-weighted structural scan, a multishell diffusion sequence with acquisition at high b-values, and a resting state blood oxygen level dependent (BOLD) acquisition. Diffusion data was corrected for gradient nonlinearity, eddy current artifact, and motion. For BOLD analyses, slice timing correction, gradient nonlinearity correction, motion correction, smoothing, bandpass filtering, and denoising were performed (see supplemental appendix).
Analysis of Connectivity
Cortical surface reconstruction was performed in Freesurfer version 5.3.0 (Dale et al. 1999) (http://surfer.nmr.mgh.harvard.edu/). The cortical surface was randomly divided into 292 parcels of equal size which were projected onto subject reconstructions (Figure 1, Lower left). Multishell orientation diffusion functions were reconstructed using FSL’s qboot version 5.0.6 (Jenkinson et al. 2012). Probabilistic tractography, using FSL’s probtrackx2, detected the number of samples traversing each region pair (see supplemental appendix). These connection weights were thresholded at 2,500 samples and recorded in a structural connectivity matrix as an index of connection strength. Connection weights followed a power distribution and were resampled to a Gaussian distribution as in prior studies (Honey et al. 2009). Weights were averaged for connections present in at least 50% of controls and included in an aggregate structural connectivity matrix.
Figure 1. Parcellation and Community Detection.
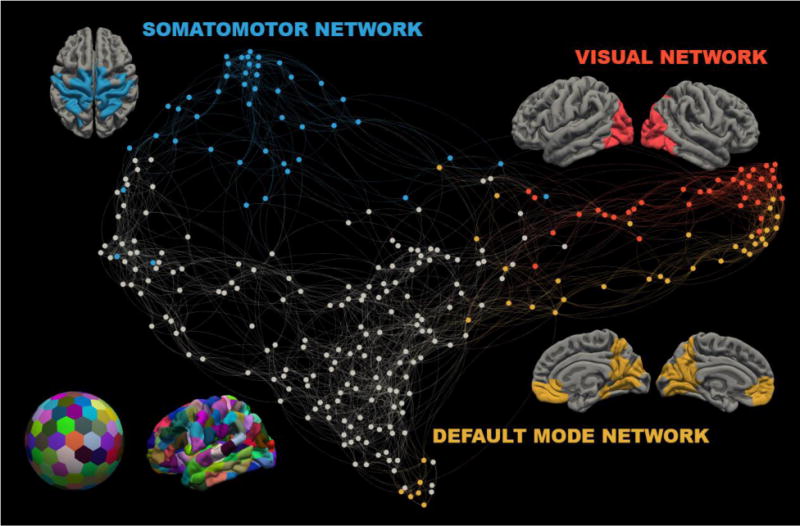
Left bottom: The cortical surface was divided into two hundred ninety-two parcels of equal size and projected onto individual subject surface reconstructions. Center: A spring embedded network diagram. Node color reflects Infomap classification (blue: somatomotor network, yellow: visual network, red: default-mode network, grey nodes are unclassified). For each identified network, its elements are displayed over an average surface.
For every subject, a functional connectivity matrix was constructed from z-scores of pairwise time series correlations between the same parcels. Control subject functional connectivity matrices were averaged to produce an aggregate matrix. We compared aggregate structural and functional connectivity with correlation analysis.
Network Definition
The Infomap algorithm (Rosvall and Bergstrom 2008), a commonly used community detection method, was used to divide the functional connectivity matrix into networks. Clusters visually identified as representing known neuroanatomical networks (Power et al. 2011; Yeo et al. 2011) were selected for further study. rsFC z-scores from all node pairs within a community were averaged to produce a network rsFC index. For each network, this index was collected in all subjects. For each multiple sclerosis subject this index was compared to the control network rsFC distribution. Multiple sclerosis subjects with rsFC index values two standard deviations above the control mean or within one half standard deviation of the control mean were classified as having high or normal network rsFC respectively.
Computational Modeling
Large-scale neuronal dynamics were simulated using an extensively employed neural mass model (Breakspear et al. 2003) in which large populations of densely connected neurons are modeled as chaotic oscillators and weakly coupled on the basis of their structural connectivity (see supplemental appendix). Regional blood volume and deoxyhemoglobin content and the corresponding BOLD signal was estimated using the Balloon-Windkessel hemodynamic model (Friston et al. 2000). Functional connectivity matrices were constructed from simulated time-series as with empirically measured BOLD. Randomized initial conditions produced variability between simulations necessitating multiple simulations for each condition.
We verified that the computational model simulated empirical BOLD by comparing the results of 25 simulations using aggregate control structural data (henceforth referred to as baseline simulations) with empirically measured rsFC. We then examined the effects of disconnection in multiple sclerosis subjects with high and normal network rsFC. For each subject, structural connections absent in that subject were removed from the aggregate control data to produce a modified matrix (Figure 2). 25 simulations were generated using this modified coupling scheme. Simulated subject data was compared to baseline data using a one-way ANOVA with post-hoc one way Dunnett t-tests to account for multiple comparisons.
Figure 2. Modeling Disconnection and Augmented Connections.
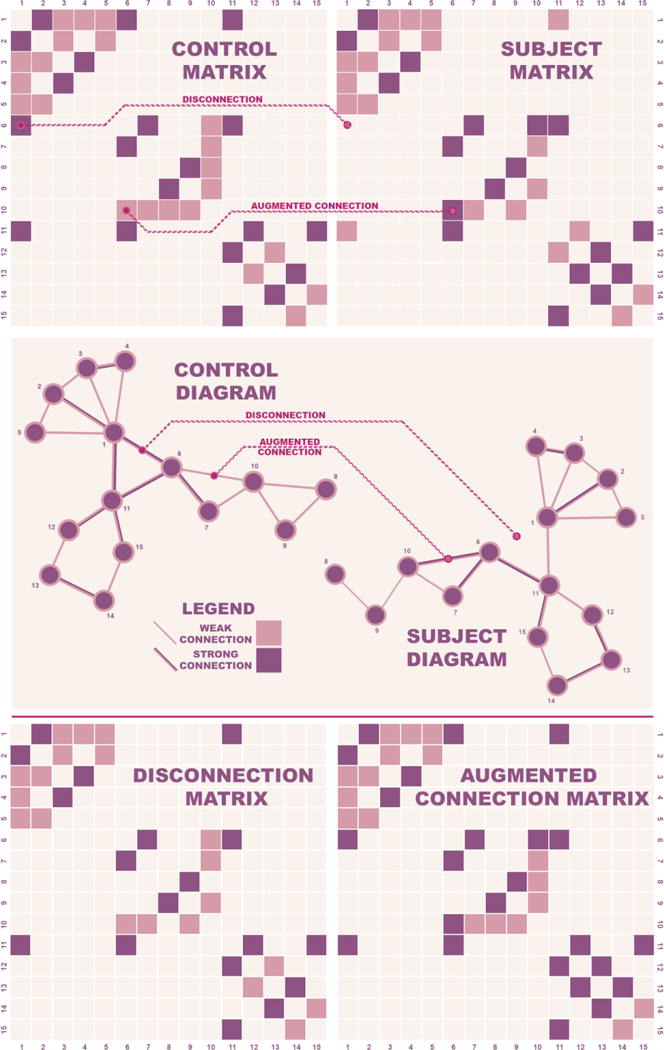
Schematic representations of simple networks illustrate the procedure used to identify salient subject features and investigate their effect.
Top: A sample aggregate control matrix and a sample subject matrix. Examples of disconnections and augmented connections are highlighted.
Middle: Corresponding diagrams for the aggregate control and subject diagrams are displayed. An example each of a disconnection and an augmented connection corresponding to those highlighted in the matrices above are shown.
Bottom: Matrices from control data incorporating either disconnection or augmented connections from the subject. The networks represented differ from the aggregate control matrix only in terms of the relevant features from the individual.
These matrices are then used to couple regions in the computational model to explore the effect of empirically observed structural changes on neuronal dynamics.
We investigated whether structural connections with greater weight in multiple sclerosis subjects compared to the aggregate control, augmented connections, affected rsFC (Figure 2). For each subject with high rsFC we ran 25 simulations in which the aggregate control matrix was modified to incorporate increased connection weights present in that subject (Figure 2). Following these analyses, we examined the topography of disconnection in high and normal rsFC subjects and ran additional simulations with random disconnections introduced on the basis of features discordant between groups.
Results
Structure-Function Relationships
The previously reported concordance between structure and function was replicated in the control population in this analysis. Aggregate structural connection weight was associated with aggregate functional connectivity (Pearson r=.4798; all matrix correlations in this study have associated p-values <10−50; Figure S1). Simulated functional connectivity was highly associated with empirical rsFC across all node pairs (mean Pearson r=.4066; Figure S2).
Network Definition
Three clusters associated with known neuroanatomical networks were identified, specifically the visual, somatomotor, and default mode networks. Four and seven multiple sclerosis subjects had high and normal range empirical rsFCS respectively (subscripts denote network rsFC: somatomotor [rsFCS], visual [rsFCV], default mode [rsFCD]). No multiple sclerosis subjects were found to have high rsFCV or rsFCD as defined in this study. High and normal rsFCS subjects were similar in age (mean 37.8 vs 39.1), disability (median EDSS 2.25 vs 2), and disease duration (mean years 5.5 vs 7.7).
Computational Modeling of Disease
A one-way ANOVA comparing simulated individual subject rsFCS and baseline rsFCS showed significant differences (F(11)=3.430, p=.0002). Post-hoc Dunnett t-tests compared baseline data to data from each simulated subject. Two of four subjects with high rsFC subjects were found to have high simulated rsFCS (p=.0448, p=.0159) after correction for multiple comparisons and a third trended towards significance (p=.0621). None of the seven subjects with normal empirical rsFCS subjects had high simulated connectivity. When structural connection weight was augmented based on weights found in high empirical rsFCS subjects, simulated mean rsFCS was similar to empirical rsFCS for each examined subject.
Topography of Disconnection
The topographical distribution of affected structural connections involving somatomotor nodes was examined in multiple sclerosis subjects with high and normal rsFCS (Figure 3). Though connectivity between groups did not differ in terms of total connections involving network nodes, intra-network connections, and inter-network connections, high rsFCS subjects had a disproportionate ratio of inter-network to total network disconnections (ET-ratio, p=.0216). In other words, though high rsFCS subjects appeared not to have accumulated more pathology than normal rsFCS subjects, connections between the somatomotor nodes and other brain regions were disproportionately affected in these subjects.
Figure 3. Topographical Differences.
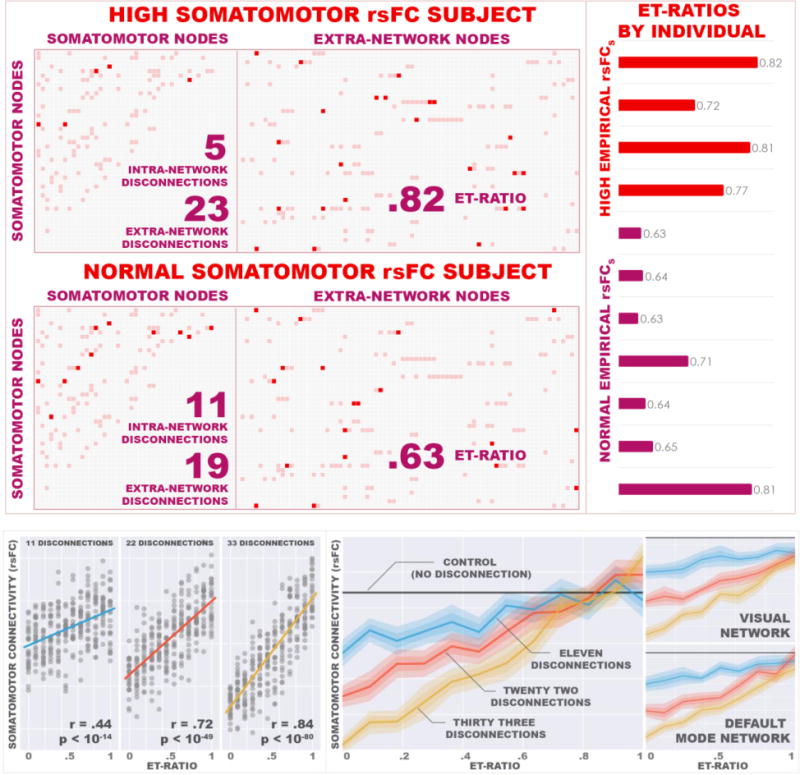
Left: Connection matrices from representative subjects with high rsFCS (left) and normal rsFCS (right) highlight alterations in connection patterns of somatomotor nodes. Rows correspond to somatomotor nodes. The initial forty-six columns of each matrix represent these same nodes while the remainder represent extra-network nodes. Muted red elements indicate connections present in the aggregate control matrix and are identical in both matrices. Bright red elements indicate disconnected edges in the individual. The subjects had similar numbers of total disconnected edges, though the high empirical rsFCS subject has a higher proportion of extra-network disconnections to total disconnections than the normal rsFCS subject. This pattern was seen across the sample and is reflected in the ET-ratio.
Right: ET-ratios. Subjects with high empirical rsFCS have higher ET-ratios than subjects with normal rsFCS indicating that in high rsFCS subjects, edges bridging the network to the wider system are preferentially implicated.
Using the computational model, we then systematically examined whether this property was relevant to observed high rsFCS. Random disconnections involving somatomotor nodes were introduced into the control structural connectivity matrix. Holding the total number of disconnections constant, we ran simulations at varying ET-ratios. For every ratio examined, we ran 25 simulations each with a random set of disconnections. We then repeated the entirety of this process twice varying the total number of disconnections (Figure 4, Left). We found a positive relationship between the ratio and rsFCS (11 total disconnections r=.4398, p=1.2782×10−15; 22 disconnections r=.7234, p=7.3371×10−50; 33 disconnections r=.8391, p=8.7871×10−81). At high ET-ratios, rsFCS was increased above that of baseline simulations. This exercise was repeated for the default mode and visual networks with similar results (Figure 4, Right).
Figure 4. The Effects of Extra-Network Disconnection.
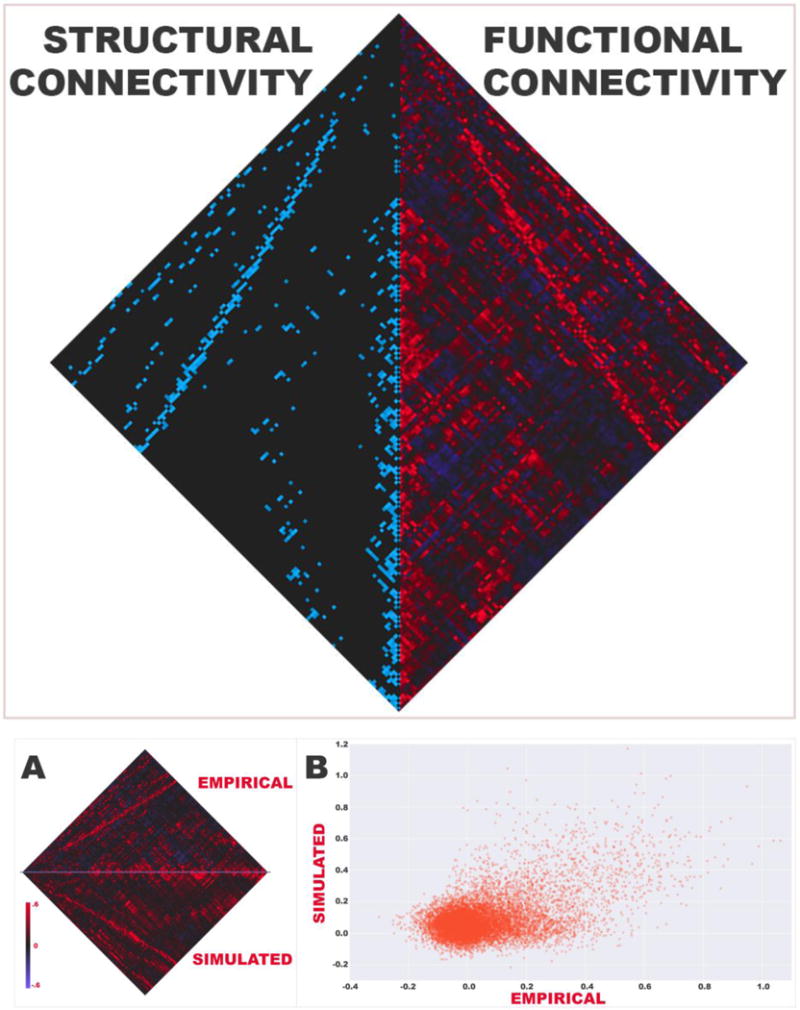
Left: rsFCS measurements from simulations incorporating random disconnections are plotted against ET-ratio. An ET-ratio of zero represents the condition in which all disconnections are between network nodes; when disconnections are distributed exclusively amongst extra-network edges, the ET-ratio is 1. Panels show varying absolute disconnection numbers (11 - left, 22 - middle, 33 - right) with regression lines superimposed.
Right: The left panel demonstrates rsFCS means with 68% and 95% confidence intervals of simulation data represented in (B). The dark grey line represents rsFCS from control simulations (essentially the no disconnection condition). Note that at ET-ratios of one, rsFCS does not vary substantially with number of disconnections suggesting that a small number of disconnections is sufficient to produce the maximum effect. The analogous data for simulations involving the visual (top right), and default mode (bottom right) networks show a similar relationship.
Discussion
We examined the effects of large-scale structural changes on network functional connectivity in MS. Structural disconnection patterns in subjects with high empirical network rsFC simulated high rsFC in the computational model, demonstrating that structural disconnection contributes to the observed increase in functional connectivity. Further reinforcing this point, the model did not simulate high rsFC from disconnection patterns seen in normal empirical rsFC subjects or from augmentation patterns in high rsFC subjects.
Despite considerable interest in functional connectivity alterations in disease, a comprehensive account of the salient factors influencing neuronal dynamics has yet to emerge. A sophisticated interpretation of the mechanism underlying such observations is critical in discerning their relevance and advancing the technology towards clinical application. Observations of increased network rsFC in disease are commonly suspected to reflect adaptive neuroplasticity (Hawellek et al. 2011; Schoonheim et al. 2010; Zhou et al. 2014) though evidence substantiating these interpretations is limited. While future work may validate these accounts, our results suggest simple alterations in wiring account for some of the variance in network rsFC. A more cautious approach to interpretation is clearly needed.
Structure-Function Relationships
Our results demonstrate that the organization of the wider system in which a network is embedded affects its internal dynamics. A particular topographical alteration, an uncoupling of network nodes from outside nodes, increases network functional connectivity. High empirical rsFCS occurred when the network was disproportionately isolated (Figure 3). Further, when random disconnections perturbed the system, a strong relationship emerged between inter-network disconnection and rsFC (Figure 4).
Structural changes promoting community isolation have been shown to increase synchronization in other contexts. This is typical of systems of coupled phase oscillators operating in modular networks; local synchrony in these contexts varies inversely with inter-network coupling (Skardal and Restrepo 2012). Neuroimaging work anticipates this finding as well. A recent graph theoretical analysis showed node-to-node rsFC associates inversely with nodal degree along elements of their shortest path (Goni et al. 2014). As nodes along the shortest path are stripped of external connection, rsFC between endpoints increases. In these contexts, external signal contaminates synchronization between the nodes of interest; removing outside influence increases internal coherence. The present analysis shows that this same principle affects brain network dynamics.
Disconnection in Multiple Sclerosis
Current work characterizing rsFC in multiple sclerosis suggests early increased network rsFC (Faivre et al. 2012; Hawellek et al. 2011; Roosendaal et al. 2010; Zhou et al. 2014) with subsequent pseudonormalization and eventual reduction (Rocca et al. 2010; Rocca et al. 2012). Considering our findings, this progression may reflect the accumulation of subcortical pathology. Specifically, an early predilection for inter-network connection may produce increased network rsFC with subsequent reduction resulting from accrual of further lesion burden. Graph theoretical work on structural network characteristics in early multiple sclerosis show increased modularity, local efficiency and clustering (Fleischer et al. 2016; Muthuraman et al. 2016), findings consistent with selective culling of inter-network connection. Interestingly, our results suggest that the effect of network isolation on rsFC reaches a ceiling with little disconnection burden. At all but the highest ET-ratios, increased burden lowered rsFC (Figure 4). In other words, a small amount of inter-network disconnection is necessary to produce maximum network rsFC attributable to network isolation with additional burden resulting in reduction. This effect may contribute to the initial rise and subsequent fall in rsFC.
Limitations and Conclusions
This pilot analysis paves the way for work in larger samples. Limited study size is related to sampling from a cross-sectional cohort with varying disease durations. Replication in an early multiple sclerosis population, the stage at which high network rsFC is typically reported, may raise confidence in our findings.
This analysis shows that structural disconnection patterns in disease produce increased network functional connectivity, a significant departure from prevailing accounts. Our results contribute to a growing literature on brain structure-function relationships and interpretation of neurophysiologic markers. Finally, this work adds to an emerging literature leveraging computational modeling towards hypothesis-driven analysis, an important step in advancing connectivity imaging towards clinical application.
Supplementary Material
Acknowledgments
Data collection and sharing for this project was provided by the MGH-USC Human Connectome Project (Principal Investigators: Bruce Rosen, M.D., Ph.D., Arthur W. Toga, Ph.D., Van J. Weeden, MD). HCP funding was provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute of Mental Health (NIMH), and the National Institute of Neurological Disorders and Stroke (NINDS). HCP data are disseminated by the Laboratory of Neuro Imaging at the University of California, Los Angeles.
Funding: The authors disclosed receipt of the following financial support for the research and/or authorship of this article: This work was supported by grants from the National Multiple Sclerosis Society PP1853 (ECK), and FG 20131-A-1 (KRP) and the National Institutes of Health K23NS078044-04 (ECK).
Eric Klawiter has received research grants from Atlas5D, Biogen, EMD Serono and Roche; and consulting fees from Acorda, Atlas5D, Biogen, EMD Serono, Genentech and Shire.
Footnotes
Compliance with Ethical Standards
Conflict of Interest: The remaining authors declare they have no conflicts of interest.
Research involving human participants: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All subjects provided written informed consent under institutional board approval. This article does not contain any studies with animals performed by any of the authors.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- Breakspear M, Terry JR, Friston KJ. Modulation of excitatory synaptic coupling facilitates synchronization and complex dynamics in a biophysical model of neuronal dynamics. Network. 2003;14:703–732. [PubMed] [Google Scholar]
- Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends in cognitive sciences. 2010;14:277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Deco G, Jirsa VK, McIntosh AR. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nature reviews Neuroscience. 2011;12:43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- Faivre A, et al. Assessing brain connectivity at rest is clinically relevant in early multiple sclerosis. Multiple sclerosis. 2012;18:1251–1258. doi: 10.1177/1352458511435930. [DOI] [PubMed] [Google Scholar]
- Fleischer V, et al. Increased structural white and grey matter network connectivity compensates for functional decline in early multiple sclerosis. Multiple sclerosis. 2016 doi: 10.1177/1352458516651503. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature reviews Neuroscience. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Mechelli A, Turner R, Price CJ. Nonlinear responses in fMRI: the Balloon model, Volterra kernels, and other hemodynamics. NeuroImage. 2000;12:466–477. doi: 10.1006/nimg.2000.0630. [DOI] [PubMed] [Google Scholar]
- Goni J, et al. Resting-brain functional connectivity predicted by analytic measures of network communication. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:833–838. doi: 10.1073/pnas.1315529111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Castillo J, Hoy CW, Handwerker DA, Robinson ME, Buchanan LC, Saad ZS, Bandettini PA. Tracking ongoing cognition in individuals using brief, whole-brain functional connectivity patterns. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:8762–8767. doi: 10.1073/pnas.1501242112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Current opinion in neurology. 2008;21:424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Handwerker DA, Roopchansingh V, Gonzalez-Castillo J, Bandettini PA. Periodic changes in fMRI connectivity. NeuroImage. 2012;63:1712–1719. doi: 10.1016/j.neuroimage.2012.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawellek DJ, Hipp JF, Lewis CM, Corbetta M, Engel AK. Increased functional connectivity indicates the severity of cognitive impairment in multiple sclerosis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:19066–19071. doi: 10.1073/pnas.1110024108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Kotter R, Breakspear M, Sporns O. Network structure of cerebral cortex shapes functional connectivity on multiple time scales. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10240–10245. doi: 10.1073/pnas.0701519104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, et al. Dynamic functional connectivity: promise, issues, and interpretations. NeuroImage. 2013;80:360–378. doi: 10.1016/j.neuroimage.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. NeuroImage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Johnston JM, et al. Loss of resting interhemispheric functional connectivity after complete section of the corpus callosum. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:6453–6458. doi: 10.1523/JNEUROSCI.0573-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, et al. Cascading network failure across the Alzheimer’s disease spectrum. Brain : a journal of neurology. 2016;139:547–562. doi: 10.1093/brain/awv338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil B, et al. A 64-channel 3T array coil for accelerated brain MRI. Magnetic resonance in medicine. 2013;70:248–258. doi: 10.1002/mrm.24427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Annals of neurology. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Misic B, Betzel RF, de Reus MA, van den Heuvel MP, Berman MG, McIntosh AR, Sporns O. Network-Level Structure-Function Relationships in Human Neocortex. Cereb Cortex. 2016;26:3285–3296. doi: 10.1093/cercor/bhw089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuraman M, Fleischer V, Kolber P, Luessi F, Zipp F, Groppa S. Structural Brain Network Characteristics Can Differentiate CIS from Early RRMS. Front Neurosci. 2016;10:14. doi: 10.3389/fnins.2016.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Friston K. Structural and functional brain networks: from connections to cognition. Science. 2013;342:1238411. doi: 10.1126/science.1238411. [DOI] [PubMed] [Google Scholar]
- Power JD, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca MA, et al. Default-mode network dysfunction and cognitive impairment in progressive MS. Neurology. 2010;74:1252–1259. doi: 10.1212/WNL.0b013e3181d9ed91. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Valsasina P, Martinelli V, Misci P, Falini A, Comi G, Filippi M. Large-scale neuronal network dysfunction in relapsing-remitting multiple sclerosis. Neurology. 2012;79:1449–1457. doi: 10.1212/WNL.0b013e31826d5f10. [DOI] [PubMed] [Google Scholar]
- Roosendaal SD, Schoonheim MM, Hulst HE, Sanz-Arigita EJ, Smith SM, Geurts JJ, Barkhof F. Resting state networks change in clinically isolated syndrome. Brain : a journal of neurology. 2010;133:1612–1621. doi: 10.1093/brain/awq058. [DOI] [PubMed] [Google Scholar]
- Rosvall M, Bergstrom CT. Maps of random walks on complex networks reveal community structure. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1118–1123. doi: 10.1073/pnas.0706851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonheim MM, Geurts JJ, Barkhof F. The limits of functional reorganization in multiple sclerosis. Neurology. 2010;74:1246–1247. doi: 10.1212/WNL.0b013e3181db9957. [DOI] [PubMed] [Google Scholar]
- Simioni AC, Dagher A, Fellows LK. Compensatory striatal-cerebellar connectivity in mild-moderate Parkinson’s disease. NeuroImage Clinical. 2016;10:54–62. doi: 10.1016/j.nicl.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skardal PS, Restrepo JG. Hierarchical synchrony of phase oscillators in modular networks. Physical review E, Statistical, nonlinear, and soft matter physics. 2012;85:016208. doi: 10.1103/PhysRevE.85.016208. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Iglesias S, Heinzle J, Diaconescu AO. Translational Perspectives for Computational Neuroimaging. Neuron. 2015;87:716–732. doi: 10.1016/j.neuron.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Valsasina P, et al. A multicentre study of motor functional connectivity changes in patients with multiple sclerosis. The European journal of neuroscience. 2011;33:1256–1263. doi: 10.1111/j.1460-9568.2011.07623.x. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RC, Kahn RS, Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Human brain mapping. 2009;30:3127–3141. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan UM, Dennis NA, Hillary FG. Chronology and chronicity of altered resting-state functional connectivity after traumatic brain injury. Journal of neurotrauma. 2015;32:252–264. doi: 10.1089/neu.2013.3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SS, Hu PT, Gujar N, Jolesz FA, Walker MP. A deficit in the ability to form new human memories without sleep. Nature neuroscience. 2007;10:385–392. doi: 10.1038/nn1851. [DOI] [PubMed] [Google Scholar]
- Zhang D, Raichle ME. Disease and the brain’s dark energy. Nature reviews Neurology. 2010;6:15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- Zhou F, et al. Altered inter-subregion connectivity of the default mode network in relapsing remitting multiple sclerosis: a functional and structural connectivity study. PloS one. 2014;9:e101198. doi: 10.1371/journal.pone.0101198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


