Abstract
Objective
Ad libitum high fat diet (HFD) spontaneously increases caloric intake in rodents, which correlates positively with weight gain. However, it remains unclear why rodents overeat HFD. We investigated how changing the proportion of diet that came from HFD might alter daily caloric intake in mice.
Methods
Mice were given 25%, 50%, or 90% of their daily caloric need from HFD, along with ad libitum access to a low-fat rodent chow diet. Food intake was measured daily to determine how these HFD supplements impacted total daily caloric intake. Follow up experiments addressed timing of HFD feeding.
Results
HFD supplements did not alter total caloric intake or body weight. In a follow up experiment, mice consumed ~50% of their daily caloric need from HFD in 30 minutes during the light cycle, a time when mice do not normally consume food.
Conclusions
HFD did not disrupt regulation of total daily caloric intake, even when up to 90% of total calories came from HFD. However, HFD increased daily caloric intake when provided ad libitum, and was readily consumed by mice outside of their normal feeding cycle. Ad libitum HFD appears to induce overconsumption beyond the mechanisms that regulate daily caloric intake.
Keywords: overweight, high-fat diet, obesity, animal models
Introduction
Obesity is a leading public health challenge in the United States [1, 2]. Even after being recognized as a national epidemic in 1999, obesity rates rose steadily throughout the beginning of the 21st century, and currently more than 30% of Americans are obese [3, 4]. Although the causes of the obesity epidemic are complex, increases in food intake appear to be at least partly responsible [5]. Across the globe, increases in food production correlate with increases in obesity rates of different countries, supporting the link between food intake and obesity rate [6, 7]. In addition, high-fat diet (HFD) access causes a spontaneous increase in daily caloric intake in rodents, which correlates positively with weight gain across individuals [8, 9]. Although ad libitum access to a high fat diet increases daily caloric intake and leads to weight gain in rodents [10], the reasons for this are not fully understood. Here, we sought to understand how HFD alters the regulation of daily caloric intake in mice.
There are generally thought to be two systems that govern food intake: homeostatic hunger systems and hedonic reward systems [11]. Homeostatic hunger systems involve endocrine and neural feedback signals of hunger and satiety that work to maintain a body weight “set point” [12]. Dysregulation of this homeostatic control may contribute to obesity [13]. For example, melanocortin 4 receptors (MC4R) are expressed in regions of the brain that control autonomic and endocrine functions [14] and inhibition of MC4R function increases food intake [15]. In addition, leptin is secreted by adipocytes to promote satiety. Mutations that disrupt the production of, or receptors for, leptin result in hyperphagia and obesity in humans [16] and rodents [17]. However, mutations in leptin, MC4R and other genes involved in homeostatic feedback control are rare in humans [14, 18], and do not account for HFD induced obesity in wild-type rodents. Animals maintained on a HFD also do not have deficits in leptin signaling, but instead have high circulating leptin levels [19]. Therefore, deficits in homeostatic hunger signaling do not appear sufficient to explain how HFD alters the regulation of daily caloric intake in rodents.
Instead, we hypothesized that hedonic systems are responsible for HFD induced overconsumption and consequent weight gain. To test this hypothesis, we provided mice with varying proportions of HFD, equal to 25%, 50%, or 90% of their daily caloric intake. They were also given ad libitum access their regular low-fat chow diet. We predicted that: 1) if homeostatic systems were not adequately engaged by HFD, mice would over-consume the low-fat chow and their total daily caloric intake would increase; but 2) if these systems were engaged by HFD, total daily caloric intake would remain stable, regardless of the percentage of their diet that came from HFD. This second possibility was in line with our hypothesis that hedonic systems are responsible for overconsumption of HFD, and was supported by the data: even when mice received up to 90% of their daily calories from HFD, neither total daily caloric intake nor weight increased. We confirmed that these same mice would over-consume HFD, and gain weight, when HFD was provided ad libitum. We concluded that ad libitum HFD leads to overconsumption because of mechanisms outside of the homeostatic regulation of total daily caloric intake. These likely include those that govern hedonic food reward. In a final experiment, we confirmed that mice would eat nearly 50% of their daily caloric need from HFD in a single 30-minute period during the light cycle, a time when mice normally do not eat. Assessing the relative contributions of homeostatic and hedonic regulation of food intake may inform strategies to treat human obesity.
Methods
Animals and Diets
All mice (C57/BL6 background) were individually housed under standard conditions (12h light/dark cycle, 23-25C). Mice were weight-matched and randomized to groups before all experiments involving multiple measurements. All procedures were performed in accordance with guidelines from the Animal Care and Use Committee of the National Institute on Diabetes and Digestive and Kidney Diseases.
Diets
Diets included: low-fat chow diet (5001 Rodent Diet; 3.10 kcal/g with 29% energy derived from protein, 13% from fat, and 56% from carbohydrate; LabDiet), 60% HFD (D12492; 5.24 kcal/g with 20% energy derived from protein, 60% from fat, and 20% from carbohydrate; Research Diets), or (for experiments in Figure 2) homemade diets of varying fats percentages of roughly 20%, 30%, 40%, 50%, 60%, or 70% (percentages were determined by kcal). Home-made diets were made by mixing low-fat chow diet with lard (46% of the fat being saturated fat and 50% unsaturated fat) or Crisco vegetable shortening (29% of the fat being saturated fat and 65% unsaturated fat) using a blender.
Figure 2. High fat diet leads to overeating.

Mice were fed diets with varying percentages of fat derived from either lard (A) or vegetable shortening (B) for 3 days. Average daily kcal consumed per mouse per diet is plotted with circles for individual mice. * indicates p<0.05 on Sidak’s multiple comparison test.
Food intake measurements
Food intake measurements were obtained by manually weighing food. Mice were given food in Rodent Cafes (OYC Americas), which were weighed every 24 hours to derive food consumption. All measurements were converted to calories to compare between different diets.
Schedule of diets
For ad libitum high-fat feeding experiments, mice received ad libitum HFD (D12492) for 9 weeks. Intake was measured with Rodent cafes every 2-3 days.
For homemade diet experiments, diets were delivered in a randomized cross-over design where each mouse was tested with 2 homemade diets of the same fat source (lard or vegetable shortening). The repeated test design was employed to limit the number of animals used, and the two measurements were considered independent for the ANOVA.
For high-fat supplement experiments, mice were provided pre-measured HFD supplements of 25%, 50%, or 90% of their estimated normal total daily caloric intake (estimated at 10kcal for all mice). Mice were concurrently provided access to ad libitum chow in a separate Rodent Cafe (OYC Americas) that was weighed daily, 2 hours into the light cycle. Each concentration of HFD supplement was provided for one week and these tests were conducted back-to-back starting with 1 week of the 25% HFD to habituate mice to the diet, followed by 25%, 90%, then 50% HFD supplement phases. HFD supplements were provided daily, 2 hours into the light cycle. Mice were weighed once each week. Following these supplement experiments mice were given ad libitum HFD for 5 weeks. Food intake was measured daily for the first week of this phase.
For intermittent feeding, mice were given a HFD supplement where 50% of their daily caloric needs were given in HFD for 24 days, along with ad libitum access to chow. For the first 16 days, HFD was provided exactly as in Figure 3. The first 8 days was considered habituation while the data from days 9-16 was analyzed. Mice were then provided the same amount of HFD but dispensed intermittently (described below). 800mg HFD, ~8% of their caloric need, was premeasured and dispensed every 4 hours, totaling 50% of their caloric intake (6 dispenses/day). This piece was large enough to visualize in the bedding and we never observed any HFD left over each day. Chow consumption was also measured daily 2 hours into the light cycle after which HFD dispensing started.
Figure 3. Animals fed limited HFD supplements do not overeat.

(A) Schematic of the experiment: mice were given ad libitum access to chow while a subgroup was also given an increasingly higher amount of HFD supplements. (B) Average total kcal consumed per day for each week of varying HFD supplements. (C) Average kcal consumed per condition (bars) and per mouse (circles) for each supplement phase. (D) Weekly average weights per group for the duration of the experiment. * indicates p<0.05 on Sidak’s multiple comparison test.
For experiments measuring food intake in the light cycle, mice were given 1g (3.00 kcal) of chow or 1 g (5.24 kcal) of HFD for 30min, 3 hours after the light cycle. Mice were randomized to receive either chow or HFD. On the following day, mice were given another 30min access to the other diet such that each mouse was tested on both diets.
Open Field Activity
Average speed (cm/s) of mice were assessed using PhenoTyper cages (30 × 30 cm; Noldus IT) and EthoVision video analysis software (Version 11; Noldus IT) following 9 weeks of HFD. Briefly, mice were video-taped from above for 20 minutes, and the position of the mouse was extracted from these videos. Velocity was calculated from position. Minutes 5-20 were used for analysis.
Body Composition and Energy Expenditure Calculations
Energy expenditure was determined using an energy balance calculation [20, 21]:
Body composition (fat and fat free mass) was measured using 1H-NMR spectroscopy (EchoMRI-100H; Echo Medical Systems) while metabolized energy intake was calculated from food intake measurements.
Intermittent HFD dispensing
We made a home-cage compatible device to drop premeasured HFD every two hours. 12 wells to hold HFD were 3D printed and mounted onto a 24-hour clock movement (3D file available at: https://kravitzlab.github.io/SnackClock/). The clock movement rotated the dividers and pushed food into the cage at 4-hour intervals.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism (Version 6.07; GraphPad Software). Two-tailed Student’s t test, one-way repeated-measured ANOVA, or two-way repeated-measured ANOVA were used when appropriate and as stated. Sidak’s multiple comparison test was used for post hoc comparisons. Results were considered significant at an alpha of p< 0.05.
Results
We provided mice (n=16) ad libitum access to HFD for 9 weeks and measured food intake, weight, and body composition, from which we calculated energy expenditure (Fig 1) [20, 21]. Weight and total HFD intake over 9 weeks were positively correlated (Fig 1A, R2 = 0.73, p<0.0001), indicating that weight gain was associated with caloric intake [9, 22]. Weight was also positively correlated with energy expenditure (Fig 1B, R2 = 0.33, p<0.05), while movement in an open field arena did not correlate with weight (Fig 1C, R2 = 0.04, p=0.44). Food intake and energy expenditure were also positively correlated (Fig 1D, R2 = 0.77, p<0.0001). These results are consistent with the conclusion that ad libitum HFD increases both total calorie intake and energy expenditure [22–24].
Figure 1. Food intake, weights and energy expenditure all positively correlate.
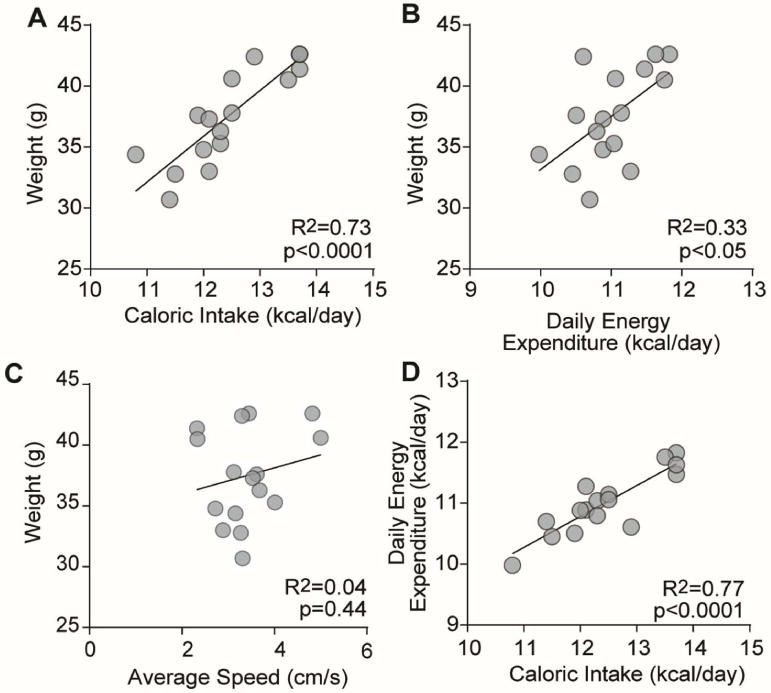
(A) weights plotted with food intake, (B) weights plotted with daily energy expenditure, (C) weights plotted with average speed in an open field test, and (D) daily energy expenditure plotted with food intake per mouse.
We next asked whether the fat content of the diet was sufficient to drive over consumption. Mice were fed homemade diets containing a range of fat (14%, 20%, 30%, 40%, 50%, 60%, or 70%) from either lard or vegetable shortening (Fig 2). Each diet exposure lasted 3 days, and included a control group that was only tested on chow (n=8). Experimental animals (n=48) were tested in a randomized design such that each mouse was tested on two homemade diets. By two-way ANOVA, we detected a significant effect of fat % (F (6, 98) = 17.21, P<0.0001) and fat source (F (1, 98) = 7.391, P=0.0078), but no significant interaction between them (F (6, 98) = 0.764, P=0.5999). Post-hoc tests (Sidak’s multiple comparison test) revealed that all diets with added fat were associated with higher total caloric intake than chow (all p<0.05, Figure 2). Despite the significant effect of fat source by ANOVA, post-hoc tests did not reveal any significant difference between fat source in any individual diet composition (all p>0.08).
There are multiple reasons why mice might overeat a HFD. It is possible that the diet does not adequately engage the homeostatic mechanisms regulating total daily caloric intake. Alternatively, it is possible that mice over-consume the diet in spite of these mechanisms. We examined this in a new group of mice (n=24) that were given 25%, 50%, and 90% of their daily caloric need (estimated at 10kcal) from HFD supplements for one week each (Fig 3A). These mice also had ad libitum access their regular chow diet for the duration of the study. Relative to control mice (n=8) that were maintained on chow across the entire experiment, mice receiving HFD supplements did not increase their total daily caloric intake. This supports the contention that calories from HFD engage the regulatory mechanisms that govern daily total caloric intake (Fig 3B–C). A repeated-measures one-way ANOVA revealed a significant interaction between experimental group and preload amount (F (3, 90) = 7.569, p=0.0001). Interestingly, this interaction was driven by the 25% and 50% (but not 90%) preload groups eating slightly less than the control group (Figure 3B–C, Sidak’s multiple comparison test p<0.05). In addition, mice receiving HFD supplements did not gain weight during this experiment, supporting the conclusion that their caloric intake remained stable (Figure 3D, 2-tailed t-test, p=0.73). Next, we provided these same mice with ad libitum access to HFD and measured their total daily caloric intake along with their weights for 5 weeks. As expected, animals over-ate and gained weight when the HFD was provided ad libitum (Figure 4A–C, p<0.0001 for both comparisons).
Figure 4. Animals fed ad libitum HFD overeat.
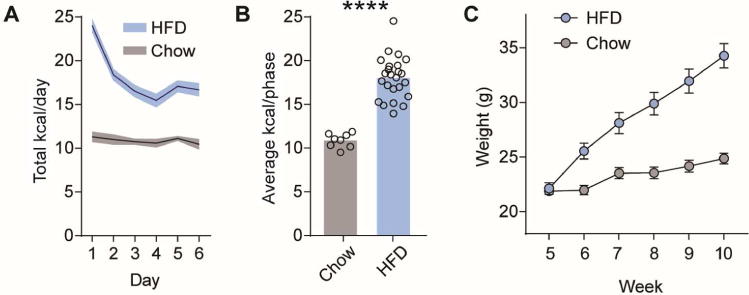
(A) Average total kcal consumed per day for a week of mice fed ad libitum HFD or chow. (B) Average kcal consumed per condition (bars) and per mouse (circles). (C) Weekly average weights per group. **** for p<0.0001.
One unique aspect of this experimental design was that the supplements were administered only once per day, whereas mice normally consume ad libitum diets throughout the day and night. To test if the timing of dispensing affected our results, we designed a device that would drop pre-measured HFD pieces into the cage at 4-hour intervals (Fig 5A–C). We measured total food intake in a new group of mice (n=8) that received a single dispense of HFD containing 50% of their daily need, once each day for 8 days, along with ad libitum chow (this feeding paradigm was identical to the 50% supplement group in Fig 3). For the following 8 days, mice continued to receive the same total amount of HFD, but the HFD was divided into 6 portions that dropped once every 4 hours. Mice ate slightly more when the HFD was dispensed gradually (Figure 5D–E, average difference: 0.5kCal/day, p<0.05), but not approaching ad libitum levels (Fig 4A). While we may have identified a mechanism by which food timing affects total caloric intake, it is important to note the effect size was small, accounting for ~5% of their daily caloric need. On ad libitum HFD, mice over-eat each day by ~50-100% in the first week. We conclude that timing of the dispensing did not account for the lack of increase in total caloric intake following limited exposure to HFD.
Figure 5. Animals fed HFD in multiple dispenses do not overeat, but do binge on HFD.

(A) Schematic of the experiment: mice were first given a HFD supplement with 50% of their daily caloric intake provided at a single time daily for 8 days or mice were then given the same total amount of HFD, but dispensed gradually every 4 hours for a total of 6 dispenses. (B) 3D illustration of the HFD dispenser and (C) position of dispenser in a home cage. (D) Average total kcal consumed per day for a week with 50% of the mice’s caloric need provided by a HFD supplement given at one time (day 1-8) then dispensed gradually every 4 hours (day 9-16). (E) Average kcal consumed per HFD dispensing count. (F) Total amount (kcal) of HFD or chow consumed by mice in 30 minutes during the light cycle. * for p<0.05 and **** for p< 0.0001.
Finally, we predicted that mice would over-consume HFD, but not low-fat chow, at a time when they would not normally be hungry. We gave mice access to ad libitum HFD or chow for 30 min at the beginning of their light cycle (new mice, n=32, randomized cross-over design). As predicted, mice consumed ~50% of their daily caloric need from high fat diet in this period, but only 9% of chow (Figure 5E, 2-tailed paired t-test, p<0.0001).
Discussion
The obesity epidemic observed in industrialized nations has been attributed in part to the wide availability of highly palatable, calorically dense foods [5, 7]. Foods that are high in fat are often over-consumed and contribute to weight gain in both animals and humans, but it is unclear how these foods impact the homeostatic regulation of daily caloric intake. Here, we used mice to address how HFD impacts short term hyperphagia. We demonstrated that mice do not overeat or gain weight, even when up to 90% of their normal daily caloric need came from HFD. Yet, the same mice over-ate and gained weight when given ad libitum HFD access. This suggests that calories from HFD adequately engage the mechanisms regulating total daily caloric intake, leading us to speculate that hedonic hunger may be the primary contributor to overconsumption of HFD in mice.
We first asked whether weight gained on a HFD was associated with increases in energy consumption or decreases in energy expenditure. After 9 weeks of ad libitum HFD, body weight was positively correlated with food intake, but also positively correlated with energy expenditure. This increase in energy expenditure in the heaviest mice may be due to HFD-induced thermogenesis [25, 26]. Human studies have also shown that people with obesity have higher absolute levels of energy expenditure than lean individuals [24, 27, 28], but their energy expenditure tends to be lower when adjusted for body weight or fat-free mass [27, 28]. Many of these studies measured energy expenditure involved cross-sectional comparisons of lean and obese individuals who were presumably in energy balance. Yet the mice that gained weight in our study had not reached energy balance and may therefore have increased energy expenditure to compensate for their positive energy balance. Regardless of this distinction, we conclude that high fat diet induced weight gain was associated with increases in energy intake, but not decreases in energy expenditure.
Next, we tested whether the source of fat affected total daily caloric intake. Although the effect of specific fats on health are controversial [29], unsaturated fats are generally more beneficial for health than saturated fats [30, 31]. To determine if fat source effected daily caloric intake, we provided mice with ad libitum diets with added fat from lard (46% of the fat being saturated fat and 50% unsaturated fat) or vegetable shortening (29% of the fat being saturated fat and 65% unsaturated fat). We found no significant effect of the fat source on total daily caloric intake, but mice over-ate both diets with higher total fat. This suggests that HFD overeating may be due to similar mechanisms in different fat types, although our examination of this subject here was limited to two sources of fat. In addition, we only measured daily calorie intake, and cannot draw conclusions about other health related outcomes from these fats. Compatible with our results, a review of 13 studies [32] demonstrated that prolonged HFD feeding increased body weight with similar effects observed in animal and plant-based fats (diets ranged between 40-60% fat from lard, milk fat, coconut fat, olive oil, or safflower oil for 20-300 days in rats and mice). In contrast, a study [33] showed that caloric intake, weight, and fat gains were greater with 14 weeks of lard-based HFD (60% fat from lard), relative to vegetable-based HFD (60% fat from vegetable shortening) in rats. Similarly, another study [34] showed that female rats who were given ad libitum access to a diet high in saturated fats (67% fat from butter or lard) for 50 days failed to adjust their intake based on energy density, while rats with a diet high in unsaturated fat (67% fat from canola oil) did. As humans consume a wide variety of fats, further investigation of the effect of specific fat sources on energy intake and expenditure is needed.
The mechanism for over-eating of HFDs and its subsequent weight gain likely involves a combination of multiple feedback mechanisms, including homeostatic, hedonic (palatability), and cognitive feedback [35]. Here, administration of HFD supplements consisting of up to 90% of daily caloric requirements did not lead to increased total daily caloric intake. Additionally, the timing of HFD delivery (single or multiple dispenses) did not alter this finding. Importantly, these same mice overate and gained weight when given ad libitum access to a HFD. We conclude that HFD appropriately engaged the mechanisms underlying homeostatic regulation of total daily caloric intake, and overeating on ad libitum HFD occurs despite such mechanisms. In a direct test of this conclusion, we found that mice ate approximately 50% of their daily caloric needs when given access to HFD for 30 min during their light phase, a time when they normally do not consume large amounts of food. In contrast, they consumed only a minimal quantity of chow (9% of their caloric needs) under the same conditions. Although rodents can alter their circadian rhythm in anticipation of palatable foods [36], we do not think this impacted our results because we were not assessing food intake at specific time points. Additionally, the mice in this experiment could not have been expecting HFD since we used a new cohort of mice with limited exposure to HFD. Our findings relate to those reported by Hurley and colleagues who used a 2 meal paradigm to decipher the homeostatic and hedonic drives of food intake [37]. In these studies, fasted rats were given access to standard chow for 2 hours, followed by 15 minutes of chow or Western Diet (17% protein, 43% carbohydrate, 41% fat; 4.7 kcal/g). Rats doubled their caloric intake of WD over 2 weeks, but did not increase intake of chow.
Our findings may support weight management strategies that limit access to high-fat foods at specific times of the day. Temporally restricting access to a 45% and 60% HFD (fat from lard) for 8 hours a day protected mice against obesity over 4 and 12 weeks [38, 39], and even led to weight loss in obese mice [39]. Similarly, 12 hour restriction of HFD inhibited the development of obesity in mice for at least 17 weeks [40]. A study of overweight humans also showed that reducing eating duration (from >14 hours to 10-11 hours per day) reduced body weight and increased self-reported energy levels in overweight individuals [41]. However, more empirical tests are needed to understand the extent of time-restricted weight loss interventions.
One reason animals overeat HFD may be because of its palatability; HFDs are more palatable than low-fat diets and are spontaneously overconsumed by animals [42]. Palatable foods activate brain reward circuits including the ventral tegmental area, nucleus accumbens, amygdalar complex, and prefrontal cortex [43, 44]. Activation of these regions may drive consumption of food beyond homeostatic needs, termed “hedonic hunger.” Hedonic hunger has been increasingly recognized for its contribution to positive energy balance and development of obesity [45]. However, assessing the relative contribution of hedonic pathways to overconsumption of a HFD is difficult, as the behavioral and neurobiological alterations depend on dietary fat content, type of fat, inclusion of other dietary components [46]. Furthermore, hedonic and homeostatic mechanisms are not exclusive of each other; eating activates areas of the brain known to regulate food intake, as well as areas regulating reward and motivation processing [44]. However, hedonic mechanisms can override regulatory mechanisms and cause overconsumption, as when one eats a high calorie dessert after a satiating meal.
We conclude that HFD engages mechanisms that regulate total daily caloric intake, yet if provided ad libitum, animals over-consume HFD despite these mechanisms. This supports the case for HFD induced overeating and weight gain being driven by hedonic, and not homeostatic, hunger in mice. However, feedback control mechanisms related to energy balance are complex and interactive, and developing interventions to prevent excess energy intake or achieve weight loss in humans will require further understanding of these mechanisms.
What is already known about this subject?
Mice over-eat ad libitum high fat diet, which leads to weight gain
High fat diets activate brain reward systems
Hedonic and homeostatic mechanisms interact to regulate daily caloric intake
What does this study add?
Ad libitum high fat diet intake correlates positively with both weight gain and energy expenditure
Limited high fat diet exposure did not disrupt regulation of total daily caloric intake
Mice over-eat high fat diet at times when they are normally not hungry
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). We thank the members of the Kravitz lab for comments and Dr. Gina Battaglia of ScienceDocs.com for editing support.
Footnotes
Disclosure: The authors declared no conflict of interest.
Author Contributions: KPN, WCF, MAA, and AVK designed the experiments. JAL, KPN, WCF, CS, and MAA carried out the experiments. All authors were involved in the data analysis and interpretation. KPN made the food distribution device. JAL and AVK generated the figures and wrote the manuscript. All authors discussed results and commented on the manuscript.
References
- 1.National Center for Health, S., Health, United States. Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. National Center for Health Statistics (US); Hyattsville (MD): 2016. [PubMed] [Google Scholar]
- 2.McGinnis JM, Foege WH. Actual causes of death in the United States. Jama. 1993;270(18):2207–12. [PubMed] [Google Scholar]
- 3.Mokdad AH, et al. The spread of the obesity epidemic in the United States, 1991-1998. Jama. 1999;282(16):1519–22. doi: 10.1001/jama.282.16.1519. [DOI] [PubMed] [Google Scholar]
- 4.Dietz WH. The response of the US Centers for Disease Control and Prevention to the obesity epidemic. Annu Rev Public Health. 2015;36:575–96. doi: 10.1146/annurev-publhealth-031914-122415. [DOI] [PubMed] [Google Scholar]
- 5.Swinburn B, Sacks G, Ravussin E. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. Am J Clin Nutr. 2009;90(6):1453–6. doi: 10.3945/ajcn.2009.28595. [DOI] [PubMed] [Google Scholar]
- 6.Vandevijvere S, et al. Increased food energy supply as a major driver of the obesity epidemic: a global analysis. Bull World Health Organ. 2015;93(7):446–56. doi: 10.2471/BLT.14.150565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zobel EH, et al. Global Changes in Food Supply and the Obesity Epidemic. Curr Obes Rep. 2016;5(4):449–455. doi: 10.1007/s13679-016-0233-8. [DOI] [PubMed] [Google Scholar]
- 8.Friend DM, et al. Basal Ganglia Dysfunction Contributes to Physical Inactivity in Obesity. Cell Metab. 2017;25(2):312–321. doi: 10.1016/j.cmet.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y, et al. Variations in body weight, food intake and body composition after long-term high-fat diet feeding in C57BL/6J Mice. Obesity (Silver Spring) 2014;22(10):2147–55. doi: 10.1002/oby.20811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mickelsen O, Takahashi S, Craig C. Experimental obesity. I. Production of obesity in rats by feeding high-fat diets. J Nutr. 1955;57(4):541–54. doi: 10.1093/jn/57.4.541. [DOI] [PubMed] [Google Scholar]
- 11.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36(2):199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 12.Elmquist JK, et al. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol. 2005;493(1):63–71. doi: 10.1002/cne.20786. [DOI] [PubMed] [Google Scholar]
- 13.Austin J, Marks D. Hormonal regulators of appetite. Int J Pediatr Endocrinol. 2009;2009:141753. doi: 10.1155/2009/141753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farooqi IS, et al. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Invest. 2000;106(2):271–9. doi: 10.1172/JCI9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adan RAH, et al. The MC4 receptor and control of appetite. Br J Pharmacol. 2006;149(7):815–27. doi: 10.1038/sj.bjp.0706929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farooqi IS, O’Rahilly S. Leptin: a pivotal regulator of human energy homeostasis. Am J Clin Nutr. 2009;89(3):980S–984S. doi: 10.3945/ajcn.2008.26788C. [DOI] [PubMed] [Google Scholar]
- 17.Friedman JM. Obesity: Causes and control of excess body fat. Nature. 2009;459(7245):340–2. doi: 10.1038/459340a. [DOI] [PubMed] [Google Scholar]
- 18.Xia Q, Grant SFA. The genetics of human obesity. Ann N Y Acad Sci. 2013;1281(1):178–90. doi: 10.1111/nyas.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frederich RC, et al. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1(12):1311–4. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 20.Guo J, Hall KD. Estimating the continuous-time dynamics of energy and fat metabolism in mice. PLoS Comput Biol. 2009;5(9):e1000511. doi: 10.1371/journal.pcbi.1000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravussin Y, et al. Estimating energy expenditure in mice using an energy balance technique. Int J Obes (Lond) 2013;37(3):399–403. doi: 10.1038/ijo.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friend DM, et al. Basal Ganglia Dysfunction Contributes to Physical Inactivity in Obesity. Cell Metabolism. 2017;25(2):312–321. doi: 10.1016/j.cmet.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chong PK, et al. Energy expenditure in lean and obese diabetic patients using the doubly labelled water method. Diabet Med. 1993;10(8):729–35. doi: 10.1111/j.1464-5491.1993.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 24.Das SK, et al. Energy expenditure is very high in extremely obese women. The Journal of nutrition. 2004;134(6):1412–1416. doi: 10.1093/jn/134.6.1412. [DOI] [PubMed] [Google Scholar]
- 25.Rothwell NJ, Stock MJ. Energy expenditure of ‘cafeteria’-fed rats determined from measurements of energy balance and indirect calorimetry. J Physiol. 1982;328:371–7. doi: 10.1113/jphysiol.1982.sp014270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothwell N, Stock M, Warwick B. The effect of high fat and high carbohydrate cafeteria diets on diet-induced thermogenesis in the rat. International journal of obesity. 1983;7(3):263–270. [PubMed] [Google Scholar]
- 27.DeLany JP, et al. High energy expenditure masks low physical activity in obesity. International journal of obesity (2005) 2013;37(7):1006–11. doi: 10.1038/ijo.2012.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elbelt U, et al. Differences of energy expenditure and physical activity patterns in subjects with various degrees of obesity. Clinical Nutrition. 2010;29(6):766–772. doi: 10.1016/j.clnu.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Svendsen K. Saturated fat –a never ending story? 2017;61(1) doi: 10.1080/16546628.2017.1377572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammad S, Pu S, Jones PJ. Current Evidence Supporting the Link Between Dietary Fatty Acids and Cardiovascular Disease. Lipids. 2016;51(5):507–517. doi: 10.1007/s11745-015-4113-x. [DOI] [PubMed] [Google Scholar]
- 31.Willett WC. Dietary fats and coronary heart disease. J Intern Med. 2012;272(1):13–24. doi: 10.1111/j.1365-2796.2012.02553.x. [DOI] [PubMed] [Google Scholar]
- 32.Buettner R, Schölmerich J, Bollheimer LC. High-fat Diets: Modeling the Metabolic Disorders of Human Obesity in Rodents*. Obesity. 2007;15(4):798–808. doi: 10.1038/oby.2007.608. [DOI] [PubMed] [Google Scholar]
- 33.Kubant R, et al. A comparison of effects of lard and hydrogenated vegetable shortening on the development of high-fat diet-induced obesity in rats. Nutrition & Diabetes. 2015;5(12):e188–e188. doi: 10.1038/nutd.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hariri N, Gougeon R, Thibault L. A highly saturated fat-rich diet is more obesogenic than diets with lower saturated fat content. Nutrition Research. 2010;30(9):632–643. doi: 10.1016/j.nutres.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Hall KD, Hammond RA, Rahmandad H. Dynamic interplay among homeostatic, hedonic, and cognitive feedback circuits regulating body weight. American Journal of Public Health. 2014;104(7):1169–1175. doi: 10.2105/AJPH.2014.301931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsu CT, et al. Palatable Meal Anticipation in Mice. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hurley MM, et al. Pituitary Adenylate-Cyclase Activating Polypeptide Regulates Hunger- and Palatability-Induced Binge Eating. Frontiers in Neuroscience. 2016;10(383) doi: 10.3389/fnins.2016.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haraguchi A, et al. Controlling access time to a high-fat diet during the inactive period protects against obesity in mice. Chronobiology International. 2014;31(8):935–944. doi: 10.3109/07420528.2014.931413. [DOI] [PubMed] [Google Scholar]
- 39.Chaix A, et al. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metabolism. 2014;20(6):991–1005. doi: 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hatori M, et al. Time-Restricted Feeding without Reducing Caloric Intake Prevents Metabolic Diseases in Mice Fed a High-Fat Diet. Cell Metabolism. 2012;15(6):848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gill S, Panda S. A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metabolism. 2015;22(5):789–798. doi: 10.1016/j.cmet.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van de Giessen E, et al. Free-Choice and No-Choice High-Fat Diets Affect Striatal Dopamine D2/3 Receptor Availability, Caloric Intake, and Adiposity. Obesity. 2012;20(8):1738–1740. doi: 10.1038/oby.2012.17. [DOI] [PubMed] [Google Scholar]
- 43.Kenny Paul J. Reward Mechanisms in Obesity: New Insights and Future Directions. Neuron. 2011;69(4):664–679. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 2011;15(1):37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lowe MR, Butryn ML. Hedonic hunger: A new dimension of appetite? Physiology and Behavior. 2007;91(4):432–439. doi: 10.1016/j.physbeh.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 46.Pandit R, et al. Dietary factors affect food reward and motivation to eat. Obes Facts. 2012;5(2):221–42. doi: 10.1159/000338073. [DOI] [PubMed] [Google Scholar]


