Abstract
The c-Mos proto-oncogene product plays an essential role during meiotic divisions in vertebrate eggs. In Xenopus, it is required for progression of oocyte maturation and meiotic arrest of unfertilized eggs. Its degradation after fertilization is essential to early embryogenesis. In this study we investigated the mechanisms involved in c-Mos degradation. We present in vivo evidence for ubiquitin-dependent degradation of c-Mos in activated eggs. We found that c-Mos degradation is not directly dependent on the anaphase-promoting factor activator Fizzy/cdc20 but requires cyclin degradation. We demonstrate that cyclin B/cdc2 controls in vivo c-Mos phosphorylation and stabilization. Moreover, we show that cyclin B/cdc2 is capable of directly phosphorylating c-Mos in vitro, inducing a similar mobility shift to the one observed in vivo. Tryptic phosphopeptide analysis revealed a practically identical in vivo and in vitro phosphopeptide map and allowed identification of serine-3 as the largely preferential phosphorylation site as previously described (Freeman et al., 1992). Altogether, these results demonstrate that, in vivo, stability of c-Mos is directly regulated by cyclin B/cdc2 kinase activity.
INTRODUCTION
The c-Mos proto-oncogene is a serine-threonine kinase expressed in Xenopus oocytes and plays an important role in germ cell development (Sagata, 1997; for review, see Ferrell, 1999). It is present at very low levels in Xenopus oocytes arrested at prophase of meiosis I. Progesterone secreted by the surrounding follicle cells induces c-Mos protein synthesis and oocyte meiosis resumption. After germinal vesicle breakdown (GVBD) and completion of meiosis I, oocytes enter meiosis II and arrest at metaphase II (Haccard et al., 1993; Colledge et al., 1994; Hashimoto et al., 1994). Subsequent fertilization of eggs leads to an increase in intracellular free Ca2+, and, as a consequence, c-Mos protein is degraded (Watanabe et al., 1991; Lorca et al., 1993; Roy et al., 1996). The biological activity of c-Mos is mediated by the activation of MAP kinase (MAPK) cascade through the phosphorylation of the MAPK-activating kinase, MEK (Nebreda and Hunt, 1993; Posada et al., 1993; Shibuya and Ruderman, 1993). Thus, c-Mos-dependent activation of MAPK plays an important role in inducing GVBD (Sagata, 1997; for a review see Ferrell, 1999) and maintaining a stable metaphase arrest necessary for fertilization (Haccard et al., 1993; Colledge et al., 1994).
Because regulation of c-Mos is essential for entry into both meiotic maturation and embryonic mitosis, the timing of c-Mos activity is tightly regulated at different levels, including control of its abundance and modulation of its kinase activity. In this regard, c-Mos stabilization by phosphorylation of serine residues has been reported to protect the protein from being degraded by the ubiquitination pathway (Nishizawa et al., 1992, 1993). Among the 10 conserved serines found in Xenopus, human, mouse, and chicken c-Mos, only serine-3 (Freeman et al., 1992; Nishizawa et al., 1992), serine-25 (Yang et al., 1996), and serine-16 (Bai et al., 1991; Pham et al., 1999) have been identified as being phosphorylation sites. It has been reported that the phosphorylation of Xenopus c-Mos at serine-3 after GVBD increases c-Mos stability by preventing the recognition of the adjacent residue proline-2, by the ubiquitin-mediated degradation machinery (Nishizawa et al., 1992, 1993). Moreover, recent studies have also demonstrated an additional role of serine-16 phosphorylation in protecting c-Mos from being degraded by the ubiquitination pathway (Pham et al., 1999).
Contrary to c-Mos stabilization, the modulation of c-Mos protein kinase activity by phosphorylation is uncertain. The study of serine-3 phosphorylation with the use of the nonphosphorylable serine-3 to alanine mutant (S3A) led to controversial results. Thus, for example, Freeman et al. (1992) reported that the expression of this mutant can induce oocyte maturation and exhibits cytostatic factor (CSF) activity comparable to the wild-type protein. However, Nishizawa et al. (1993) reported the requirement of serine-3 phosphorylation for CSF activity of c-Mos. Moreover, the results obtained by Chen and Cooper (1995) showed a reduced interaction of S3A mutant with its substrate MEK and the incapacity of this mutant to activate endogenous MAPK when expressed in reticulocyte lysate. Finally, Yang et al. (1998) reported an inhibition of v-Mos activity when the equivalent serine-34 is mutated to alanine. Furthermore, phosphorylation of serine-25 can also modulate c-Mos kinase activity. According to mutagenic studies, serine-25 phosphorylation may be necessary to inhibit activation of c-Mos occurring by phosphorylation at serine-3 (Yang et al., 1998).
Despite the prominent role ascribed to the phosphorylation of c-Mos in the modulation of its stability and activity, little is known about the kinase(s) responsible for c-Mos phosphorylation. Indeed, serine-3 may well be a target of autophosphorylation (Nishizawa et al., 1992), although the phosphorylation of c-Mos on this residue has also been described when the kinase dead (KD) form of this protein was used (Freeman et al., 1992). Moreover, Matten et al. (1996) showed that MAPK could phosphorylate myelin basic protein-Mos fusion protein on serine-3 in vitro. Two different kinases, cyclin B/cdc2 (Bai et al., 1991) and MAPK (Matten et al., 1996), have been described as being capable of phosphorylating serine-16 residue. Moreover, the injection of oocytes with a kinase-minus cdc2 protein blocks c-Mos accumulation but does not affect the rate of c-Mos synthesis (Nebreda et al., 1995). These results suggest the existence of a negative regulation of c-Mos degradation in a cdc2-dependent manner. Finally, Yang et al. (1996) described in vivo and in vitro phosphorylation of serine-25 by protein kinase A.
In this study we investigated the role of cyclin B/cdc2 in monitoring c-Mos stability. Our results provide evidence that the direct phosphorylation of endogenous c-Mos by cyclin B/cdc2 is responsible for its stabilization.
MATERIALS AND METHODS
Recombinant mRNAs and Proteins
The sea urchin cyclin B-glutathione S-transferase (Gst) was obtained as previously described (Abrieu et al., 1996). Cyclin B/cdc2 complex from starfish oocytes and yeast recombinant p13-Suc 1 protein were purified by the use of published protocols (Labbe et al., 1989, 1991). Methyl-ubiquitin was kindly provided by Dr. Olivier Coux. Wild-type and KD c-Mos genes were cloned into the EcoRI site of the Xenopus expression vector pXen1 (MacNicol et al., 1997) to allow production of Gst-Mos mRNAs by SP6 in vitro transcription as described by Fisher et al. (2000). Nontagged wild-type c-Mos mRNA was obtained as previously described by Lorca et al. (1991). S3A c-Mos mutant was generated according to an oligonucleotide-directed in vitro mutagenesis system from Amersham, Little Chalfont, Buckinghamshire, UK. The hyperactive form of Raf mRNA was kindly provided by Dr. Deborah K. Morrison (Cutler and Morrison, 1997).
Xenopus Oocytes, Gst-Pull Downs, and Immunoprecipitations
Isolated stage VI oocytes were obtained as previously described by Faure et al. (1998) and maintained in MMR buffer (5 mM HEPES, pH 7.8, 100 mM NaCl, 2 mM KCl, 0.1 mM EGTA, 1 mM MgCl2, 2 mM CaCl2). When activated oocytes were used, immature oocytes were first incubated overnight in the presence of 1 μM progesterone and subsequently activated by ionophore treatment. Antibodies (50 nl, 1.5 mg/ml α-fizzy), proteins (50 nl, 1 mg/ml cyclin B-Gst, 1.5 mg/ml Suc1, 15 mg/ml methyl-ubiquitin), and mRNAs (50 nl, wild-type c-Mos-Gst mRNA, KD c-Mos-Gst mRNA, wild-type c-Mos mRNA, S3A c-Mos mRNA, hyperactive Raf mRNA, all at 0.5 mg/ml) were microinjected at the indicated times. A mixture of three oocytes per point was homogenized in 30 μl of oocyte buffer (20 mM Tris, pH 7.5, 50 mM NaCl, 50 mM NaF, 10 mM β-glycerophosphate, 5 mM Na4P207, 1 mM EDTA). After extract centrifugation (13,000 rpm for 3 min at 4°C), the clear supernatant was recovered, and the corresponding volume to one oocyte was used for Western blot analysis. When Gst-pull downs were developed, 30 oocytes were homogenized on a total volume of 750 μl of extract buffer. The clear supernatant was then incubated with 30 μl of 50% glutathione-Sepharose beads (Pharmacia, Piscataway, NJ) for 1 h at 4°C, washed twice in RIPA buffer (10 mM NaH2PO4, pH 7.5, 100 mM NaCl, 5 mM EDTA, 1% Triton X-100, 0.5% deoxycholate, 80 mM β-glycerophosphate, 50 nM NaF, 1 mM dithiothreitol), rinsed in 25 mM Tris, pH 7.5, and assayed for cyclin B/cdc2 phosphorylation or immunoblotting. For phosphopeptide mapping, S3A mutant and wild-type c-Mos mRNAs were injected in 30 stage VI oocytes and then incubated overnight with [32P]orthophosphate for in vivo phosphopeptide mapping or 2 h in MMR buffer for the in vitro one. Oocytes were then collected, homogenized, and centrifuged as described above. Three microliters of affinity-purified anti-c-Mos polyclonal antibodies were added to the clear supernatant and incubated for 1 h at 4°C. Subsequently, a total of 20 μl of 50% protein A-Sepharose were added and incubated for an additional 30 min at 4°C. After incubation, immunoprecipitates were extensively washed with RIPA buffer and finally rinsed in 25 mM Tris, pH 7.5.
Immunological Procedures
The Xenopus anti-c-Mos and anti-active ERK antibodies were obtained from Santa Cruz (SC086, Santa Cruz, CA) and New England Biolabs (9106S, Beverly, MA), respectively. When immunoprecipitations were developed, affinity-purified anti-c-Mos polyclonal antibodies were used. These antibodies were increased in our laboratory by immunizing rabbits with Xenopus c-Mos protein and purified on a pmal-c-Mos column. Affinity-purified antibodies against fizzy and cyclin B2 proteins were obtained as previously described (Abrieu et al., 1996, 1997; Lorca et al., 1998).
Western blots were probed with the primary antibody at 50 ng/ml and the appropriate secondary antibody-horseradish peroxidase conjugate was diluted according to recommendations (Amersham, Arlington Heights, IL). When anti-cyclin B2 was probed in anti-fizzy–microinjected oocytes, protein A-horseradish peroxidase conjugate was used. Immunoblots were revealed by ECL (New England Nuclear, Boston, MA).
Kinase Assays
To analyze c-Mos phosphorylation by purified starfish cyclin B/cdc2 complex, 15 oocytes were microinjected with wild-type or KD c-Mos-Gst mRNAs and incubated for 2 h in MMR buffer. Gst-pull downs were then obtained and assayed for 30 min at room temperature in 35 μl of reaction buffer (25 mM Tris, pH 7.5, 5 mM glutathione, 200 μM ATP, 10 mM MgCl2, 2 μCi of [32P]ATP). Subsequently, a volume of 3 μl of purified starfish cyclin B/cdc2 complex (specific activity: 20 pmol transferred 32P/min) was added.
Phosphopeptide Map Analysis
Wild-type and S3A c-Mos mRNAs microinjected in groups of 30 oocytes were incubated overnight in MMR buffer containing 1 mCi/ml [32P]orthophosphate and recovered by immunoprecipitation. The proteins were then resolved in SDS-PAGE electrophoresis and analyzed by autoradiography. Gel slices containing labeled proteins were then subjected to in-gel digestion with 0.5 μg of porcine trypsin (Promega, Madison, WI ; 5000 U/mg) for 12–14 h. Peptides were separated in the horizontal direction by electrophoresis in 2.5% formic acid/7.8% glacial acetic acid (vol/vol), pH 1.9, for 20 min at 1500 V. Separation in the vertical direction was performed by thin-layer chromatography in a system of 62.5% isobutyric acid/1.9% n-butanol/4.8% pyridine/2.9% glacial acetic acid (vol/vol).
When in vitro phosphopeptide analysis was developed, wild-type and S3A c-Mos mRNAs were microinjected and incubated for 2 h in MMR buffer, and, once immunoprecipitated, the purified proteins were subjected to a cyclin B/cdc2 phosphorylation treatment before the phosphopeptide mapping, as previously described in “Kinase Assays.” The phosphopeptide mapping of the in vivo plus in vitro plus cyclin B/cdc2 mixture was developed by mixing an equal number of counts per minute of both samples.
Phosphatase Assays
For phosphatase assays, oocytes were first homogenized in XB buffer (50 mM sucrose, 100 mM KCl, 0.1 mM CaCl2, 0.1 mM EGTA, 5 mM HEPES, pH 7.7) and subsequently mixed with λ-phosphatase (New England Biolabs) to a final concentration of 8000 U/ml. Reactions were carried out for 30 min at 30°C and the equivalent volume of one oocyte was used in Western blot assays.
c-Mos Ubiquitination Assays
For c-Mos ubiquitination assays, 50 μl of interphasic egg extracts prepared as previously described (Matthews and Colman, 1991) were subjected to RNase A treatment (1 μg/μl) during 20 min. After dithiothreitol (final concentration 1 mM) and placental RNase inhibitor addition (0.5 U/μl), 100 μCi of [35S]methionine alone or a mixture of 100 μCi of [35S]methionine and 50 ng/μl wild-type c-Mos mRNA were added. When indicated, a final concentration of 3 μg/μl methyl-ubiquitin or purified recombinant Gst-ubiquitin was mixed. Extracts were then incubated for an additional period of 3 h at 21°C to allow mRNA translation and c-Mos ubiquitination. Finally, a volume of 5 μl of the reaction products was analyzed by SDS-PAGE and fluorography.
RESULTS
Role of Cyclin B/cdc2-dependent Phosphorylation of c-Mos in c-Mos Stability
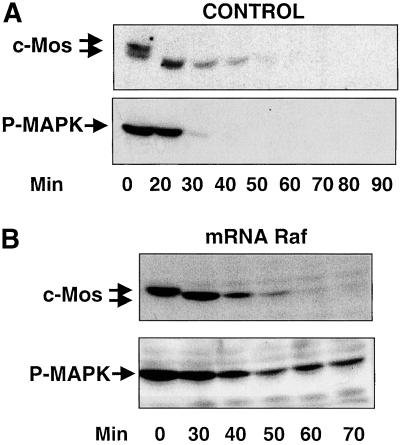
To study in vivo the effect of c-Mos phosphorylation on its stability, mature oocytes were activated with calcium ionophore. Then, endogenous c-Mos protein and activated MAPK (P-MAPK), as an indicator of c-Mos activity, were analyzed by Western blot, at different times postactivation. As shown in Figure 1A, 20 min after ionophore treatment, endogenous c-Mos was dephosphorylated as indicated by its faster electrophoretic mobility (Watanabe et al., 1989). The dephosphorylated form was detected until 40 min and disappeared at ∼50 min after egg activation. In the same experiment, P-MAPK completely disappeared 30 min after ionophore treatment, whereas a substantial level of dephosphorylated c-Mos was still present. Because inactivation of MAPK clearly precedes c-Mos proteolysis, we hypothesized that this inactivation could be necessary to allow c-Mos degradation. To test this possibility, we induced a continuous activation of MAPK by injecting an mRNA encoding a hyperactive form of Raf into immature oocytes. After progesterone treatment, mature oocytes were activated by calcium ionophore. Endogenous c-Mos degradation and MAPK activity were analyzed by immunoblotting. The results of this experiment demonstrate that, although MAPK activity is continuously maintained (Figure 1B, P-MAPK), c-Mos is normally proteolysed (Figure 1B, c-Mos) excluding a possible requirement of MAPK inactivation for c-Mos degradation.
Figure 1.
Role of MAPK in c-Mos stability. (A) Metaphase II-arrested oocytes were activated by ionophore treatment and, at the indicated times, groups of three oocytes per point were homogenized in oocyte buffer. After centrifugation, the supernatants were analyzed by immunoblotting for endogenous c-Mos and P-MAPK. (B) Stage VI oocytes were first microinjected with mRNA encoding a hyperactive form of Raf and incubated overnight with progesterone. The resulting metaphase II-arrested oocytes were activated, homogenized as reported in legend A at the indicated times, and immunoblotted for endogenous c-Mos and P-MAPK proteins.
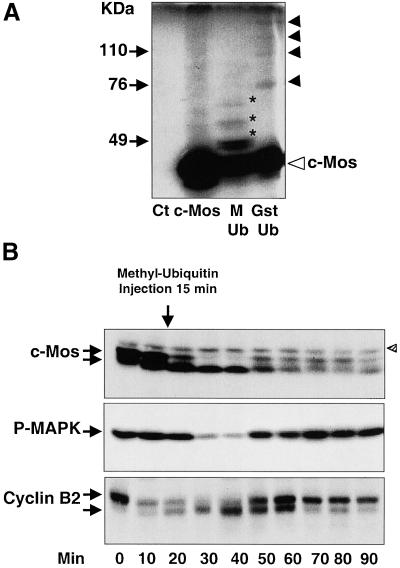
In the early stage of oocyte maturation, instability of c-Mos has been correlated with the presence of c-Mos-ubiquitin conjugates (Nishizawa et al., 1992). It is known that methyl-ubiquitin blocks the elongation of ubiquitin chains (Ziegenhagen et al., 1990; Hershko et al., 1991). In Figure 2A, wild-type c-Mos mRNA was added to interphase egg extracts in the presence of [35S]methionine (lane c-Mos). Subsequently, methylated-ubiquitin (lane M-Ub) or purified recombinant Gst-ubiquitin (Gst-Ub) was added. As analyzed by SDS-PAGE, the translation products contained the bulk c-Mos polypeptide (38 kDa, open triangle) and a slowly migrating smeared background (lane c-Mos). We attribute these slowly migrating bands to a modified form of the c-Mos protein because they were not present when no c-Mos mRNA was added to the [35S]methionine-containing interphase extracts (lane Ct). These forms correspond to ubiquitinated c-Mos because they moved to higher molecular weights when recombinant Gst-ubiquitin was added to the reaction mixture (lane Gst-Ub, arrowheads; note that the molecular weight of the first band of modified c-Mos, 76 kDa, coincides with the expected molecular weight of the mono-Gst–ubiquitinated form of this protein). Moreover, as expected, the ubiquitin chain elongation of c-Mos was severely reduced by the addition of methyl-ubiquitin to the reaction mixture (lane M-Ub, asterisks denote c-Mos bands that agree with the predicted molecular weights of one to three ubiquitin-containing c-Mos conjugates).
Figure 2.
c-Mos ubiquitination and degradation in activated oocytes. (A) Fresh interphase egg extracts were mixed with [35S]methionine alone after RNase A treatment (see MATERIALS AND METHODS) (Ct); [35S]methionine and wild-type c-Mos mRNA (c-Mos); [35S]methionine, wild-type c-Mos mRNA, and methyl-ubiquitin (M-Ub); or [35S]methionine, wild-type c-Mos mRNA, and Gst-ubiquitin (Gst-Ub), and incubated during 3 h at 21°C to allow mRNA translation and c-Mos ubiquitination. Proteins were resolved by SDS-PAGE, subjected to fluorography, and revealed by autoradiography. Bands corresponding to methyl-ubiquitin-c-Mos and Gst-ubiquitin-c-Mos conjugates are indicated by asterisks and arrowheads, respectively. Nonubiquitinated c-Mos is indicated by an open triangle. (B) Mature oocytes were activated by ionophore treatment and, 15 min later (indicated by the arrow), microinjected with methyl-ubiquitin. Oocytes were homogenized at the indicated times and subjected to Western blot for endogenous c-Mos, P-MAPK, and cyclin B2 proteins. Shaded triangle indicates the presence of an unspecific band occasionally recognized by anti-c-Mos antibodies. The equivalent of one oocyte was loaded per lane.
Because ubiquitin chain elongation is believed to be required to allow proteosome-dependent protein degradation, we investigated, in vivo, the effect of methyl-ubiquitin on c-Mos degradation by injecting it into activated oocytes. We chose microinjection 15 min after ionophore treatment, just before the initiation of c-Mos degradation, to prevent possible indirect effects induced by the inhibition of the ubiquitination of other proteins such as cyclin B. As shown in Figure 2B (c-Mos) the microinjection of methyl-ubiquitin efficiently induced the stabilization of endogenous c-Mos protein up to 90 min postactivation without preventing c-Mos dephosphorylation (Figure 2B, c-Mos, 30 and 40 min). Surprisingly, we found that the P-MAPK signal dropped considerably, between 30 and 40 min, at a time when the bulk of c-Mos was still present (Figure 2B, P-MAPK). The P-MAPK signal increased again at 50 min concomitantly with a rephosphorylation of c-Mos. This increase of P-MAPK signal, as well as rephosphorylation of c-Mos, faithfully correlated with the activation of cyclin B/cdc2 kinase (note timing of cyclin B2 phosphorylation-dependent shift as an indication of cyclin B/cdc2 activation, Figure 2B, Cyclin B2).
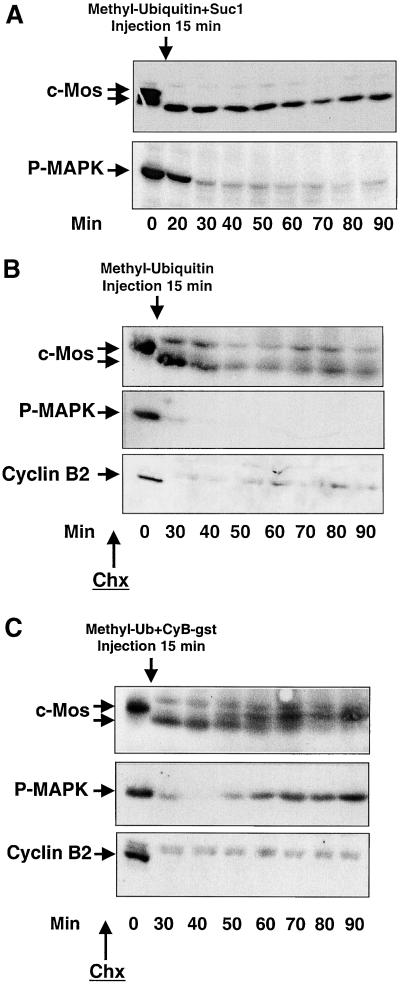
To test whether cyclin B/cdc2 plays a role in the rephosphorylation of c-Mos and P-MAPK, we inhibited cyclin B/cdc2 reactivation by simultaneously microinjecting the cyclin B/cdc2 partner, Suc1 and methyl-ubiquitin. As shown in Figure 3A, concomitant microinjection of methyl-ubiquitin and Suc1 15 min postactivation induced a definitive dephosphorylation of endogenous c-Mos at 20 min (c-Mos). Likewise, MAPK was inactivated at 30 min, and no reactivation was subsequently observed (P-MAPK).
Figure 3.
Inhibition of cyclin B/cdc2 kinase activity prevents the reactivation of P-MAPK in methyl-ubiquitin–microinjected oocytes. (A) Oocytes were simultaneously injected with a mixture of methyl-ubiquitin and the cyclin B/cdc2 partner, Suc1, 15 min postionophore treatment. At different times they were homogenized and subjected to SDS-PAGE and immunoblot to anti-c-Mos or anti-P-MAPK antibodies. (B) Oocytes were activated as in A and injected with methyl-ubiquitin 15 min postactivation. Before ionophore treatment (30 min) and throughout the experiment, oocytes were incubated with 100 μg/ml Chx to inhibit protein synthesis. (C) Similar to B except for a concomitant microinjection of methyl-ubiquitin and the recombinant protein cyclin B-Gst (arrow). The equivalent of one oocyte was loaded per lane.
Although previous studies have demonstrated that microinjection of Suc1 blocks cdc2 tyrosine dephosphorylation of cyclin B/cdc2 complexes and kinase activation (Dunphy and Newport, 1989), we wanted to confirm independently of Suc1 the possible dependency of c-Mos phosphorylation on cyclin B/cdc2 kinase. For this, we injected methyl-ubiquitin into activated oocytes preincubated with the protein synthesis inhibitor cycloheximide (Chx), which prevents cyclin B synthesis and thus cdc2 reactivation (Figure 3B, Cyclin B2). The results of this experiment show a stabilization of endogenous c-Mos in its unphosphorylated form for as long as 90 min without any further rephosphorylation (Figure 3B, c-Mos). MAPK was definitively inactivated at 30 min (Figure 3B, P-MAPK), although a great part of c-Mos was still present. We next repeated this experience except for the simultaneous microinjection of methyl-ubiquitin and recombinant cyclin B-Gst 15 min postionophore treatment. At variance with results shown in Figure 2B, in which ectopic cyclin B was not microinjected, all unphosphorylated c-Mos was rephosphorylated 50 min postactivation (Figure 3C, c-Mos). Similarly, MAPK was reactivated at the same time (Figure 3C, P-MAPK)
Inhibition of Cyclin B Proteolysis after Egg Activation Prevents c-Mos Dephosphorylation and Degradation
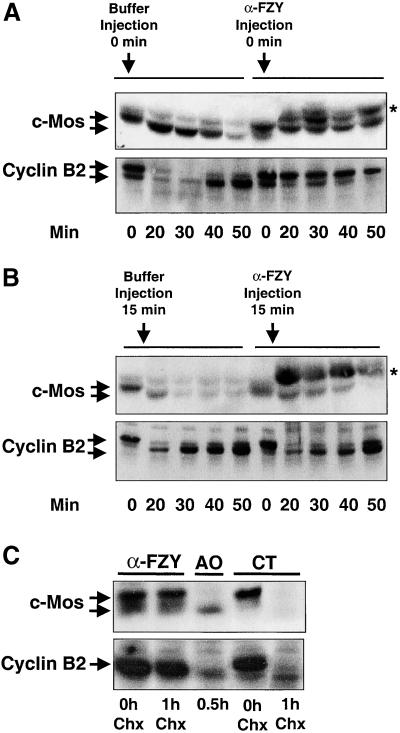
We previously showed that anti-fizzy antibodies (α-FZY) block the APCfizzy-dependent proteolysis of cyclin A and B (Lorca et al., 1998). We microinjected metaphase II-arrested oocytes with α-FZY or with the same volume of buffer in controls. Because pricking by itself is sufficient to trigger a Ca2+ transient in Xenopus eggs, activation occurred in both cases. As shown in Figure 4A, endogenous c-Mos in buffer-injected oocytes presented a faster mobility form 20 min after activation as compared with inactivated eggs or activated oocytes injected with α-FZY. Moreover, it disappeared completely at 50 min in controls, whereas it was stable throughout the whole experiment in α-FZY–injected oocytes. In the same assay cyclin B2 was entirely degraded after 20 min in buffer-injected oocytes but remained stable in the α-FZY–injected oocytes (Figure 4A, Cyclin B2). Thus, the injection of α-FZY blocks c-Mos dephosphorylation and degradation after egg activation. These results are consistent with a potential role of cyclin B/cdc2 kinase in c-Mos phosphorylation, although a direct effect of α-FZY in the c-Mos degradation pathway was not excluded. To eliminate this possibility, buffer or α-FZY were injected 15 min after activation, by the time when cyclin B is already degraded. No suppression of endogenous c-Mos degradation was observed (Figure 4B, c-Mos) when α-FZY were injected once cyclin B2 was proteolysed (Figure 4B, Cyclin B2). These results indicate that the effect of α-FZY on c-Mos degradation is not direct and depends, presumably, on their effect on cyclin B degradation. Thus, inhibition of cyclin B degradation by α-FZY maintains cyclin B/cdc2 kinase activity that may phosphorylate c-Mos and, as a consequence, prevents c-Mos degradation.
Figure 4.
Inhibition of cyclin B proteolysis prevents c-Mos dephosphorylation and degradation. (A) Mature oocytes were microinjected with affinity-purified α-FZY or the same volume of MMR buffer. Microinjection by itself triggered Ca2+ transient and oocyte activation. Once activated, oocytes were homogenized at the indicated times and subjected to immunoblot analysis for the endogenous c-Mos and cyclin B2 proteins. (B) Similar to A but oocytes were first activated with ionophore treatment and, 15 min later, microinjected with α-FZY or MMR buffer. At the indicated times endogenous c-Mos and cyclin B2 amounts were analyzed by Western blot. Asterisks in A and B denote the presence in the Western blots of an unspecific band due to the recognition of microinjected α-FZY by the secondary antibody. (C) Stage VI oocytes were microinjected with affinity-purified α-FZY or the same volume of MMR buffer (CT) and stimulated to undergo oocyte maturation with progesterone. Post-GVBD protein synthesis (30 min) was blocked by the addition of a final concentration of 100 μg/ml Chx. Oocytes were homogenized before (0 h Chx) or 1 h after Chx incubation (1 h Chx) and analyzed for endogenous c-Mos and cyclin B2 levels by immunoblotting. One activated oocyte homogenized 0.5-h postionophore treatment (AO, 0.5 h) was also included in the Western blot to compare phosphorylated and dephosphorylated forms of endogenous c-Mos. The equivalent of one oocyte was loaded per lane.
The mechanism of c-Mos stabilization by phosphorylation has not only been described in metaphase II but also in maturing oocytes (Watanabe et al., 1989; Nishizawa et al., 1992). To test whether cyclin B/cdc2 kinase could also be involved in the stabilization of this protein during oocyte maturation, we blocked possible cyclin B degradation in stage VI oocytes by injecting α-FZY before subjecting them to progesterone. Thirty minutes post-GVBD, protein synthesis was blocked by the addition of Chx during 1 h. Then oocytes were homogenized and used for immunoblot analysis with anti-c-Mos and anti-cyclin B2 antibodies. As shown in Figure 4C, both c-Mos and cyclin B2 proteins completely disappeared after 1 h of Chx treatment in the control. In contrast, no drop in the amount of the two proteins was observed in α-FZY–injected oocytes when protein synthesis was suppressed for 1 h (compare α-FZY, lane 0 h Chx, with lane 1 h Chx). These results are consistent with the view that, not only in parthenogenetically activated oocytes but also in maturing oocytes, inhibition of cyclin B induces stabilization of c-Mos protein by maintenance of its phosphorylated state.
Injection of Recombinant Cyclin B after Egg Activation Prevents c-Mos Degradation by Inducing Its Rephosphorylation
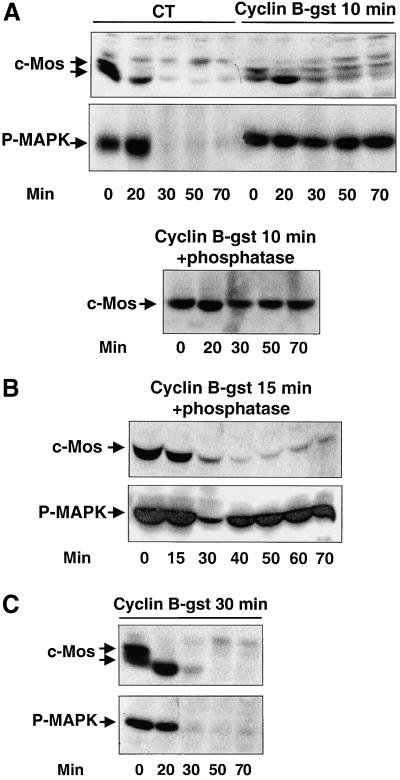
To confirm the role of cyclin B/cdc2 in c-Mos stabilization, recombinant cyclin B-Gst was injected into activated oocytes at different times (10, 15, and 30 min) postionophore treatment. The injection of cyclin B-Gst at 10 min, when endogenous cyclin B had undergo complete degradation (data not shown), did not prevent c-Mos dephosphorylation. However, at 30 min, endogenous c-Mos was rephosphorylated and stabilized (compare Figure 5A, c-Mos, CT, and Cyclin B-gst 10 min). The time elapsed between cyclin B-Gst injection and c-Mos rephosphorylation reflects the time necessary for microinjected cyclin B-Gst and newly synthesized cyclin B to form active complexes with cdc2, as judged by the phosphorylated state of the cyclin B/cdc2 substrate Cdc25 (data not shown). It is worth noting that different signal intensities of c-Mos were observed when comparing the unphosphorylated and phosphorylated forms. We attribute these differences to a reduced capacity of c-Mos antibodies to recognize the shifted c-Mos phosphorylated form. Indeed, no differences in c-Mos amount were observed throughout the experiment, when the same samples were first dephosphorylated by pretreatment with λ-phosphatase before analysis by SDS-PAGE (Figure 5A, c-Mos, Cyclin B-gst 10 min + phosphatase). Once again, activated MAPK was correlated with the concomitant presence of c-Mos and active cyclin B/cdc2 kinase, except at 20 min when only c-Mos was present (Figure 5A, P-MAPK, Cyclin B-Gst 10 min).
Figure 5.
Injection of recombinant cyclin B after egg activation induces rephosphorylation of c-Mos. (A) Mature oocytes were activated by ionophore treatment and 10 min postactivation were injected with either 50 nl of recombinant cyclin B-Gst (Cyclin B-gst 10 min) or the same volume of MMR buffer (CT). At the indicated times after ionophore treatment, oocytes were homogenized and subjected to immunoblot for endogenous c-Mos and P-MAPK proteins. In the lower panel samples from A were pretreated with λ-phosphatase as indicated in MATERIALS AND METHODS, subjected to Western blot, and probed with anti-c-Mos antibodies (Cyclin B-gst 10 min + phosphatase). (B) The same experiment as in A but cyclin B-Gst was injected 15 min postactivation. Samples were pretreated with λ-phosphatase before Western blot analysis (Cyclin B-gst 15 min + phosphatase). (C) Similar to A) except for the injection of cyclin B-Gst 30 min postactivation (Cyclin B-gst 30 min). The equivalent of one oocyte was loaded per lane.
When cyclin B-Gst was injected 15 min postactivation, endogenous c-Mos degradation had already started and only one-tenth of the initial amount of this protein was left. The remaining c-Mos protein was detected by Western blot until 30 min postactivation, i.e., before cyclin B/cdc2 activation (data not shown). The subsequent presence of this protein was detected only when the samples were pretreated with λ-phosphatase, suggesting that residual c-Mos was, in fact, rephosphorylated (Figure 5B, c-Mos). Moreover, MAPK was reactivated again to attain the original levels 40 min postionophore treatment. Finally, the injection of cyclin B-Gst 30 min after egg activation, when c-Mos was completely degraded, did not trigger MAPK reactivation (Figure 5C, c-Mos, P-MAPK). This observation confirms that the effect of cyclin B/cdc2 on MAPK activation requires the presence of c-Mos. Moreover, these results demonstrate that, in the presence of active cyclin B/cdc2, maximal phosphorylation of MAPK can be obtained by as little as approximately one-tenth of the amount of c-Mos observed in mature oocytes (compare Figure 5B, P-MAPK and c-Mos).
Cyclin B/cdc2 Directly Phosphorylates c-Mos Protein
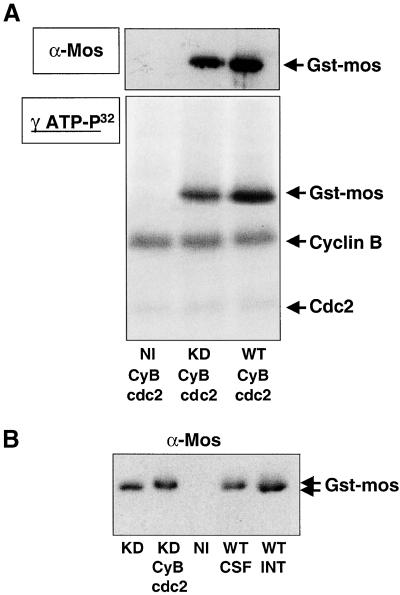
To determine whether the phosphorylation of c-Mos could be directly performed by cyclin B/cdc2, we microinjected Gst-tagged wild-type or KD c-Mos mRNA into stage VI oocytes. Two hours later, oocytes were homogenized; ectopic wild-type and KD c-Mos-Gst were purified by Gst-pull down and used as the in vitro substrate for affinity-purified starfish cyclin B/cdc2 (Labbe et al., 1991) in the presence of γ-ATP- 32P. One-tenth of the final reaction mixture was resolved by SDS-PAGE and immunoblotted with anti-c-Mos antibodies to quantify ectopic c-Mos (Figure 6A, α-Mos). The remaining sample was also resolved by SDS-PAGE but subjected to autoradiography to detect phosphorylated proteins (Figure 6A, γ-ATP-P32). As shown in Figure 6A, both, KD and wild-type c-Mos-Gst were phosphorylated by purified starfish cyclin B/cdc2 complex (γ-ATP-P32, lanes KD CyB/cdc2 and WT CyB/cdc2). This phosphorylation seemed very similar, if not identical, in both cases, because the different intensity signals observed in the autoradiography were correlated with differences in the amount of the recovered protein (compare Figure 6A, γ-ATP-P32 and α-Mos). No band corresponding to c-Mos-Gst was observed when in vitro phosphorylation was performed with a Gst-pull down obtained from noninjected oocytes (lane NI CyB/cdc2).
Figure 6.
Cyclin B/cdc2 directly phosphorylates c-Mos protein. (A) Stage VI oocytes were injected with Gst-tagged wild-type or KD c-Mos mRNA and incubated for 2 h in MMR buffer. Subsequently, Gst-pull downs from these oocytes (lane KD CyB/cdc2 and lane WT CyB/cdc2) as well as from uninjected oocytes (lane NI CyB/cdc2) were isolated and subjected to phosphorylation with purified starfish cyclin B/cdc2 complex as described in MATERIALS AND METHODS. One-tenth of the final reaction mixture was resolved by SDS-PAGE and immunoblotted with anti-c-Mos antibodies (α-Mos). The remaining sample was subjected to electrophoresis and autoradiography to detect phosphorylated proteins (γ-ATP-P32). The presence of phosphorylated bands corresponding to KD or wild-type c-Mos-Gst, cyclin B, and Cdc2 proteins are indicated. (B) Western blot of Gst-pull downs of c-Mos obtained from uninjected oocytes (NI) and microinjected oocytes with wild-type Gst-tagged c-Mos mRNA (WT) or KD Gst-tagged c-Mos mRNA (KD). Stage VI oocytes were injected with the corresponding mRNA and incubated overnight with MMR buffer. After MMR incubation, oocytes microinjected with wild-type c-Mos-Gst mRNA were divided in two groups. One of these groups was subjected to ionophore activation to obtain interphasic oocytes (lane WT-INT) where c-Mos-Gst was dephosphorylated. Subsequently wild-type c-Mos-Gst protein was recovered by Gst-pull down from both groups, activated (lane WT-INT) and nonactivated oocytes (lane WT-CSF) and subjected to SDS-PAGE and immunoblot for endogenous c-Mos protein. Nonmatured oocytes microinjected with KD c-Mos-Gst mRNA were also divided in two groups and KD c-Mos-Gst protein of both groups was recovered by Gst-pull down. One of these groups was subjected to a subsequent in vitro phosphorylation with purified starfish cyclin B/cdc2 complex to induce c-Mos phosphorylation (lane KD CyB/cdc2). A Gst-pull down from uninjected oocytes (lane NI) was used as a control.
The Western blot presented in Figure 6B shows that direct phosphorylation of KD c-Mos-Gst by purified starfish cyclin B/cdc2 reduces its electrophoretic mobility (lanes KD and KD-cyB/cdc2) in the same way observed in ovo for ectopically expressed c-Mos-Gst after treatment with ionophore of the microinjected CSF-arrested oocytes (compare lane WT CSF to lane WT INT). These results show that the resulting phosphorylation stoichiometry is at least one phosphate per molecule of c-Mos-Gst, because all c-Mos-Gst was shifted. Thus, because cyclin B/cdc2 is capable of phosphorylating KD and wild-type c-Mos-Gst to the same extent and induces in vitro a shift in KD c-Mos-Gst similar to the one obtained in wild-type c-Mos-Gst in vivo, we can conclude that the phosphorylation of c-Mos by cyclin B/cdc2 is direct and that c-Mos autophosphorylation is not required in this process.
Analysis of the Phosphorylation Pattern of c-Mos Induced by Cyclin B/cdc2
To test whether the in vivo phosphorylation pattern of c-Mos corresponds to the one induced in vitro by purified starfish cyclin B/cdc2, we compared the two-dimensional tryptic phosphopeptide maps of c-Mos untagged protein phosphorylated in either of these conditions. Because serine-3 has been reported to be the major phosphorylation site of Xenopus c-Mos (Freeman et al., 1992), we identified the phosphopeptide containing this amino acid by comparing the phosphopeptide maps of wild-type c-Mos and serine-3 to alanine mutant (S3A).
To obtain in vivo phosphorylation, mRNAs encoding either the S3A mutant or the wild-type c-Mos protein were microinjected into stage VI oocytes and labeled overnight with [32P]orthophosphate. The corresponding c-Mos protein was purified by immunoprecipitation and digested with trypsin. In these experiments overexpression of the S3A mutant induced GVBD with an efficiency similar to the wild-type c-Mos protein.
For in vitro phosphopeptide mapping, the same mRNAs were microinjected in stage VI oocytes and incubated for 2 h in MMR buffer. Subsequently, the S3A mutant and the wild-type c-Mos proteins were purified by immunoprecipitation, subjected to in vitro phosphorylation with purified starfish cyclin B/cdc2 complex, and digested with trypsin.
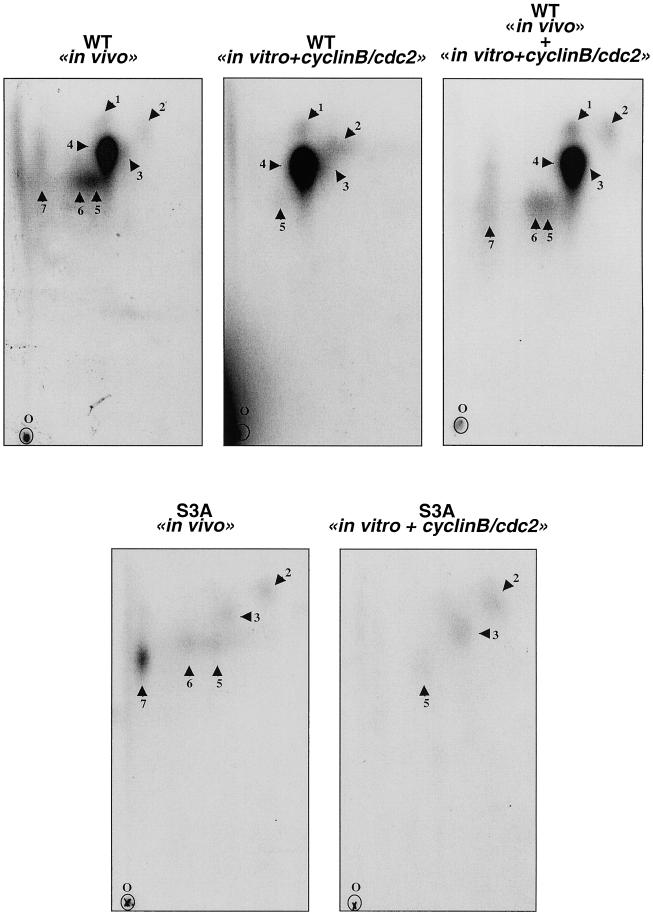
As shown in Figure 7 (WT in vivo), seven tryptic phosphopeptides were obtained when in vivo phospholabeling was performed with the wild-type c-Mos protein. However, the S3A mutant exhibited a different in vivo phosphorylation pattern (Figure 7, S3A in vivo). The peptide map of the S3A c-Mos mutant lacked tryptic peptides 1 and 4. Because the mutant and wild-type proteins differ by only a single residue, we reasoned that peptides 1 and 4 were likely to be overlapping by containing serine-3 and that, in fact, peptide 1 could represent a partial digestion of peptide 4. In agreement with what was previously described by Freeman et al. (1992), serine-3–containing peptides 1 and 4 represent the major phosphopeptides of the in vivo phosphorylated wild-type c-Mos.
Figure 7.
Tryptic phosphopeptide maps of in vivo and in vitro phosphorylated serine-3 to alanine mutant (S3A) and wild-type c-Mos (WT). For in vivo phosphopeptide mapping, oocytes microinjected with the WT and S3A mRNAs were incubated in MMR containing [32P]orthophosphate overnight. The phosphorylated c-Mos proteins were purified by immunoprecipitation with affinity-purified anti-c-Mos antibodies and subjected to 10% SDS-PAGE. When phosphotryptic analysis was developed in S3A and WT c-Mos proteins phosphorylated in vitro by cyclin B/cdc2, oocytes were microinjected with the corresponding mRNAs and incubated for 2 h in MMR buffer. c-Mos proteins were subsequently purified by immunoprecipitation with affinity-purified anti-c-Mos antibodies, subjected to in vitro phosphorylation with purified starfish cyclin B/cdc2 complex, and loaded onto 10% SDS-PAGE (see MATERIALS AND METHODS). The proteins from both in vivo and in vitro phosphorylation were then in-gel digested with trypsin. The resultant phosphopeptides were separated on a thin-layer chromatography plates by electrophoresis in the horizontal direction and by chromatography in the vertical direction, as indicated. O indicates the origin. Phosphopeptides were detected by autoradiography and numbered from 1 to 7.
When wild-type c-Mos protein was phosphorylated in vitro with purified starfish cyclin B/cdc2 complex (Figure 7, WT in vitro + cyclin B/cdc2), we observed the presence of five of the seven peptides obtained in vivo, including the major serine-3–containing phosphopeptides 1 and 4 (Figure 7, WT in vivo + in vitro + cyclin B/cdc2). This indicates that cyclin B/cdc2 is capable of inducing a very similar phosphopeptide map in vitro (except for peptides 6 and 7) with the same intensity pattern as the one obtained in vivo. Moreover, as with the in vivo results, in vitro phosphorylation of c-Mos by cyclin B/cdc2 yielded serine-3 as the major phosphorylation site (peptides 1 and 4).
As expected, in vitro phosphopeptide mapping of S3A mutant presented only three of the seven peptides (peptides 2, 3, and 5; Figure 7, S3A in vitro + cyclin B/cdc2), because peptides corresponding to phosphorylation in serine-3, peptides 1 and 4, were obviously not present, and as in the wild-type c-Mos protein, peptides 6 and 7 were not phosphorylated by cyclin B/cdc2 in vitro.
Thus, from these results, we can conclude that in vitro phosphorylation of c-Mos by cyclin B/cdc2 yields a pattern of Ser-3–containing phosphopeptide identical to the one obtained in vivo. Thus, cyclin B/cdc2 is very likely to be the kinase that phosphorylates serine-3, the major in vivo phosphorylation site of Xenopus c-Mos.
DISCUSSION
Throughout oocyte maturation and subsequently during the first mitotic cell cycle, the c-Mos–dependent MAPK cascade and cyclin B/cdc2 kinase are associated with the control of cell cycle progression. Interplay between these two kinases affects the major events of meiotic maturation, including the suppression of DNA replication, the segregation of meiotic chromosomes, and the prevention of parthenogenetic activation (reviewed by Abrieu et al., 2001). Inactivation of c-Mos is essential for eggs to enter the first embryonic cell cycle: microinjection of recombinant c-Mos into early embryos either prevents cyclin B/cdc2 kinase reactivation, thereby inducing G2 arrest (Abrieu et al., 1997; Walter et al., 1997; Fisher et al., 1998; Murakami and Van Woude, 1998), or induces metaphase arrest (Sagata et al., 1989; Haccard et al., 1993).
The main aim of this article was to analyze the mechanism responsible for inactivation of c-Mos at the end of meiotic maturation in Xenopus oocytes.
By preventing inactivation of cyclin B/cdc2 kinase or by inducing its precocious activation during the first embryonic cell cycle, we provide evidence for a tight correlation among M-phase promoting factor activity, c-Mos phosphorylation, and stabilization in Xenopus oocytes. Moreover, we demonstrate that cyclin B/cdc2 directly phosphorylates wild-type and KD mutant forms of c-Mos in vitro, with a stoichiometry capable of inducing a total mobility shift of this protein similar to the one observed in vivo. Phosphopeptide maps of in vitro and in vivo phosphorylated c-Mos reveal a very similar phosphopeptide pattern including, in both cases, serine-3 as the major phosphorylation site, as previously described (Freeman et al., 1992). Taken together, these observations strongly support, if not prove, the idea that cyclin B/cdc2 is directly responsible for the in vivo phosphorylation of c-Mos on serine-3 and for its stabilization during meiotic maturation in Xenopus oocytes.
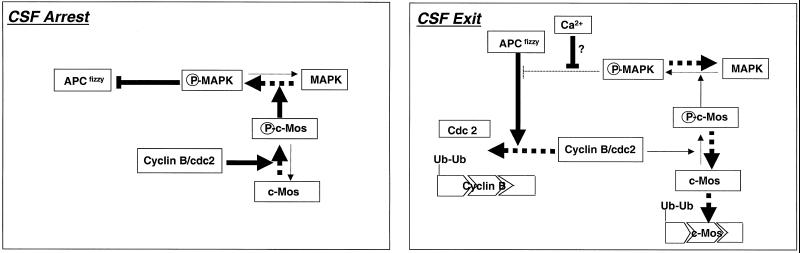
We also present in vivo evidence that c-Mos is degraded by an ubiquitination pathway, because, on one hand, injection of methyl-ubiquitin in activated eggs prevents dephosphorylated c-Mos from being degraded and, on the other hand, this protein efficiently inhibits elongation of ubiquitin chains of c-Mos conjugates in interphase extracts. Moreover, after egg activation, we show that, although APCfizzy function is required for the c-Mos dephosphorylation step by allowing cyclin degradation and cdc2 kinase inactivation, its function is no longer required once c-Mos is dephosphorylated. Most likely, as schematically shown in Figure 8, increase of the intracellular free Ca2+ level resulting from ionophore treatment induces activation of APCfizzy-dependent degradation of cyclin B, without affecting the ubiquitin-dependent degradation pathway of c-Mos, which may depend exclusively on its phosphorylation state. Our results strongly suggest that c-Mos degradation is the exclusive consequence of its dephosphorylation due to the loss of cyclin B/cdc2 activity after cyclin B degradation. Moreover, in metaphase II-arrested oocytes, the c-Mos/MAPK pathway negatively regulates APC-dependent proteolysis of cyclin B (Abrieu et al., 1997; Bhatt and Ferrell, 1999; Gross et al., 2000). In other words, a tight reciprocal regulation between cyclin B/cdc2 and c-Mos ensures the CSF arrest. Cyclin B/cdc2 activity represses c-Mos degradation by maintaining c-Mos phosphorylation and c-Mos inhibits cyclin B degradation by suppressing activation of the APCfizzy-dependent degradation pathway. To exit this static state, an external event, fertilization, is required. Egg fertilization, through the Ca2+-calmodulin pathway, induces the breaking off of this closed network by allowing cyclin proteolysis without the need of an MAPK pathway inactivation.
Figure 8.
A model to explain the mechanisms by which the tight reciprocal regulation between c-Mos and cyclin B/cdc2 assures CSF arrest and CSF exit. Discontinuous arrows denote different states of the same protein. Continuous arrows represent the direct or indirect effect of one protein on another. Thick arrows signal direction of the pathway in each situation. Mechanisms by which intracellular Ca2+ releases the MAPK-dependent inhibition of APC are unknown. Ub, ubiquitin.
We also found that, although unphosphorylated c-Mos could be stabilized by methyl-ubiquitin in Chx-treated and -activated eggs, MAPK is normally inactivated. Moreover, ectopic activation of cyclin B/cdc2 kinase by the injection of recombinant cyclin B-Gst during the first mitotic cell cycle either prevents MAPK inactivation or induces its full reactivation but only if some c-Mos protein is still present. These findings can be explained by two different hypotheses. According to the first one, c-Mos and cyclin B/cdc2 act synergistically to activate the MAPK pathway. According to the second hypothesis, cyclin B/cdc2 kinase modulates, through c-Mos phosphorylation, not only its stabilization but also the activity of this protein and, as a consequence, MAPK activation.
We have demonstrated a tight regulatory relationship between MAPK and MPF, not only in arrest and exit from metaphase II but also during metaphase I-metaphase II transition. During this period, cyclin B/cdc2 is continuously ensuring the stability of c-Mos by inducing its phosphorylation. However, no decrease of c-Mos level is observed throughout metaphase I-metaphase II transition; meanwhile, MPF activity oscillates, reaching a minimum level at 80 min post-GBVD (Ohsumi et al., 1994). How could cyclin B/cdc2 stabilize c-Mos during this period? It is well established that, although cyclin B is proteolysed in the metaphase I-II transition, a residual MPF activity, as well as c-Mos/MAPK pathway function, is always present and, in fact, necessary to prevent chromosome decondensation, pronucleus formation, and rereplication (Daar et al., 1991; Kanki and Donoghue, 1991; Furuno et al., 1994; Picard et al. 1996; Gross et al., 2000; Iwabuchi et al., 2000). This residual level of cyclin B/cdc2 is most likely sufficient to phosphorylate and stabilize c-Mos, thereby inducing full MAPK activation. In this regard, it has been previously reported that, although the maximum levels of c-Mos were obtained at metaphase II, total activation of MAPK was already achieved at metaphase I, when threefold less of c-Mos levels were observed (Roy et al., 1996). Another possibility could be that the delay between cyclin B degradation and c-Mos dephosphorylation is sufficient to cover the period of low cyclin B/cdc2 activity. Contrary to what has been previously described (Roy et al., 1996), our results suggest that there is no turnover of c-Mos during metaphase I- metaphase II transition because, as soon as c-Mos is synthesized, it is phosphorylated and thus stabilized during this period. Indeed, when the cyclin B degradation pathway was blocked in maturing oocytes, only a minor accumulation of c-Mos was observed as compared with huge accumulations of cyclin B (Castro, Lorca, and Labbé, unpublished results). In fact, previous works describing this turnover were based on the disappearance of c-Mos when protein synthesis was inhibited by Chx. This discrepancy can now be clearly explained by the simultaneous disappearance of cyclin B and c-Mos induced by Chx. The resulting whole inactivation of cdc2 kinase activity, which normally does not occur during metaphase I-metaphase II transition (Ohsumi et al., 1994; Iwabuchi et al., 2000), leads to c-Mos dephosphorylation and degradation, although physiologically this protein seems almost completely stabilized during this period.
In conclusion, cyclin B/cdc2 stabilizes c-Mos by direct phosphorylation and, conversely, stabilized c-Mos maintains cyclin B/cdc2 kinase at a level sufficient to allow progression in the absence of DNA replication from metaphase I to metaphase II and arrest at metaphase II. In this way, the establishment of the MPF/MAPK-positive feedback assures the correct progression of meiosis and CSF arrest in vertebrate oocytes.
ACKNOWLEDGMENTS
We thank Dr. O. Coux and Dr. D.K. Morrison for providing methyl-ubiquitin and the hyperactive form of Raf mRNA, respectively. Grateful acknowledgment is due to Dr. M. Dorée for critical reading of the manuscript and to J.P. Capony for his technical assistance with phosphomapping analysis. This work was supported by the Association pour la Recherche sur le Cancer (J.C.L.) and by the Ligue Nationale Contre le Cancer (T.L. and S.G.). L.M.J. was a recipient of the Ligue Nationale Contre le Cancer.
REFERENCES
- Abrieu A, Doree M, Fisher D. The interplay between cyclin B-cdc2 kinase (MPF), and MAP kinase during maturation of oocytes. J Cell Sci. 2001;114:257–267. doi: 10.1242/jcs.114.2.257. [DOI] [PubMed] [Google Scholar]
- Abrieu A, Fisher D, Simon MN, Doree M, Picard A. MAPK inactivation is required for the G2 to M-phase transition of the first mitotic cell cycle. EMBO J. 1997;16:6407–6413. doi: 10.1093/emboj/16.21.6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrieu A, Lorca T, Labbe JC, Morin N, Keyse S, Doree M. MAP kinase does not inactivate, but rather prevents the cyclin degradation pathway from being turned on in Xenopus egg extracts. J Cell Sci. 1996;109:239–246. doi: 10.1242/jcs.109.1.239. [DOI] [PubMed] [Google Scholar]
- Bai W, Singh B, Karshin WL, Shonk RA, Arlinghaus RB. Phosphorylation of v-mos Ser 47 by the mitotic form of p34cdc2. Oncogene. 1991;6:1715–1723. [PubMed] [Google Scholar]
- Bhatt RR, Ferrell JE., Jr The protein kinase p90 rsk as an essential mediator of cytostatic factor activity [see comments] Science. 1999;286:1362–1365. doi: 10.1126/science.286.5443.1362. [DOI] [PubMed] [Google Scholar]
- Chen M, Cooper JA. Ser-3 is important for regulating Mos interaction with and stimulation of mitogen-activated protein kinase kinase. Mol Cell Biol. 1995;15:4727–4734. doi: 10.1128/mcb.15.9.4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colledge WH, Carlton MB, Udy GB, Evans MJ. Disruption of c-mos causes parthenogenetic development of unfertilized mouse eggs. Nature. 1994;370:65–68. doi: 10.1038/370065a0. [DOI] [PubMed] [Google Scholar]
- Cutler RE, Jr, Morrison DK. Mammalian Raf-1 is activated by mutations that restore Raf signaling in Drosophila. EMBO J. 1997;16:1953–1960. doi: 10.1093/emboj/16.8.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daar I, Paules RS, Vande Woude GF. A characterization of cytostatic factor activity from Xenopus eggs and c-mos-transformed cells. J Cell Biol. 1991;114:329–335. doi: 10.1083/jcb.114.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy WG, Newport JW. Fission yeast p13 blocks mitotic activation and tyrosine dephosphorylation of the Xenopus cdc2 protein kinase. Cell. 1989;58:181–191. doi: 10.1016/0092-8674(89)90414-5. [DOI] [PubMed] [Google Scholar]
- Faure S, Morin N, Doree M. Inactivation of protein kinase A is not required for c-mos translation during meiotic maturation of Xenopus oocytes. Oncogene. 1998;17:1215–1221. doi: 10.1038/sj.onc.1202056. [DOI] [PubMed] [Google Scholar]
- Ferrell JE., Jr Xenopus oocyte maturation: new lessons from a good egg. Bioessays. 1999;21:833–842. doi: 10.1002/(SICI)1521-1878(199910)21:10<833::AID-BIES5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Fisher D, Abrieu A, Simon MN, Keyse S, Verge V, Doree M, Picard A. MAPK inactivation is required only for G2-M phase transition in early embryogenesis cell cycles of the starfishes Marthasterias glacialis and Astropecten aranciacus. Dev Biol. 1998;202:1–13. doi: 10.1006/dbio.1998.8981. [DOI] [PubMed] [Google Scholar]
- Fisher DL, Mandart E, Doree M. Hsp90 is required for c-Mos activation and biphasic MAP kinase activation in Xenopus oocytes. EMBO J. 2000;19:1516–1524. doi: 10.1093/emboj/19.7.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman RS, Meyer AN, Li J, Donoghue DJ. Phosphorylation of conserved serine residues does not regulate the ability of mosxe protein kinase to induce oocyte maturation or function as cytostatic factor. J Cell Biol. 1992;116:725–735. doi: 10.1083/jcb.116.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuno N, Nishizawa M, Okazaki K, Tanaka H, Iwashita J, Nakajo N, Ogawa Y, Sagata N. Suppression of DNA replication via Mos function during meiotic divisions in Xenopus oocytes. EMBO J. 1994;13:2399–2410. doi: 10.1002/j.1460-2075.1994.tb06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross SD, Schwab MS, Taieb FE, Lewellyn AL, Qian YW, Maller JL. The critical role of the MAP kinase pathway in meiosis II in Xenopus oocytes is mediated by p90(Rsk) Curr Biol, 2000;10:430–438. doi: 10.1016/s0960-9822(00)00425-5. [DOI] [PubMed] [Google Scholar]
- Haccard O, Sarcevic B, Lewellyn A, Hartley R, Roy L, Izumi T, Erikson E, Maller JL. Induction of metaphase arrest in cleaving Xenopus embryos by MAP kinase. Science. 1993;262:1262–1265. doi: 10.1126/science.8235656. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Watanabe N, Furuta Y, Tamemoto H, Sagata N, Yokoyama M, Okazaki K, Nagayoshi M, Takeda N, Ikawa Y, et al. Parthenogenetic activation of oocytes in c-mos-deficient. Nature. 1994;370:68–71. doi: 10.1038/370068a0. [DOI] [PubMed] [Google Scholar]
- Hershko A, Ganoth D, Pehrson J, Palazzo RE, Cohen LH. Methylated ubiquitin inhibits cyclin degradation in clam embryo extracts. J Biol Chem. 1991;266:16376–16379. [PubMed] [Google Scholar]
- Iwabuchi M, Ohsumi K, Yamamoto TM, Sawada W, Kishimoto T. Residual cdc2 activity remaining at meiosis I exit is essential for meiotic M-M transition in Xenopus oocyte extracts. EMBO J. 2000;19:4513–4523. doi: 10.1093/emboj/19.17.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanki JP, Donoghue DJ. Progression from meiosis I to meiosis II in Xenopus oocytes requires de novo translation of the mosxe protooncogene. Proc Natl Acad Sci USA, 1991;88:5794–5798. doi: 10.1073/pnas.88.13.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbe JC, Capony JP, Caput D, Cavadore JC, Derancourt J, Kaghad M, Lelias JM, Picard A, Doree M. MPF from starfish oocytes at first meiotic metaphase is a heterodimer containing one molecule of cdc2 and one molecule of cyclin B. EMBO J. 1989;10:3053–3058. doi: 10.1002/j.1460-2075.1989.tb08456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbe JC, Cavadore JC, Doree M. M phase-specific cdc2 kinase: preparation from starfish oocytes and properties. Methods Enzymol. 1991;200:291–301. doi: 10.1016/0076-6879(91)00147-o. [DOI] [PubMed] [Google Scholar]
- Lorca T, Castro A, Martinez AM, Vigneron S, Morin N, Sigrist S, Lehner C, Doree M, Labbe JC. Fizzy is required for activation of the APC/cyclosome in Xenopus egg extracts. EMBO J. 1998;17:3565–3575. doi: 10.1093/emboj/17.13.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorca T, Cruzalegui FH, Fesquet D, Cavadore JC, Mery J, Means A, Doree M. Calmodulin-dependent protein kinase II mediates inactivation of MPF and CSF upon fertilization of Xenopus eggs. Nature. 1993;366:270–273. doi: 10.1038/366270a0. [DOI] [PubMed] [Google Scholar]
- Lorca T, Galas S, Fesquet D, Devault A, Cavadore JC, Doree M. Degradation of the proto-oncogene product p39mos is not necessary for cyclin proteolysis and exit from meiotic metaphase: requirement for a Ca(2+)-calmodulin dependent event. EMBO J. 1991;10:2087–2093. doi: 10.1002/j.1460-2075.1991.tb07741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNicol MC, Pot D, MacNicol A. pXen, a utility vector for expression of GST-fusion proteins in Xenopus laevis oocytes and embryos. Gene. 1997;196:25–29. doi: 10.1016/s0378-1119(97)00171-6. [DOI] [PubMed] [Google Scholar]
- Matten WT, Copeland TD, Ahn NG, Vande Woude GF. Positive feedback between MAP kinase and Mos during Xenopus oocyte maturation. Dev Biol. 1996;179:485–492. doi: 10.1006/dbio.1996.0277. [DOI] [PubMed] [Google Scholar]
- Matthews G, Colman A. A highly efficient, cell-free translation/translocation system prepared from Xenopus eggs. Nucleic Acids Res. 1991;19:6405–6412. doi: 10.1093/nar/19.23.6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami MS, Van Woude GF. Analysis of the early embryonic cell cycles of Xenopus: regulation of cell cycle length by Xe-Wee1 and Mos. Development. 1998;125:237–248. doi: 10.1242/dev.125.2.237. [DOI] [PubMed] [Google Scholar]
- Nebreda AR, Gannon JV, Hunt T. Newly synthesized protein(s) must associate with p34cdc2 to activate MAP kinase and MPF during progesterone-induced maturation of Xenopus oocytes. EMBO J. 1995;14:5597–5607. doi: 10.1002/j.1460-2075.1995.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebreda AR, Hunt T. The c-mos proto-oncogene protein kinase turns on and maintains the activity of MAP kinase, but not MPF, in cell-free extracts of Xenopus oocytes and eggs. EMBO J. 1993;12:1979–1986. doi: 10.1002/j.1460-2075.1993.tb05847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa M, Furuno N, Okazaki K, Tanaka H, Ogawa Y, Sagata N. Degradation of Mos by the N-terminal proline (Pro2)-dependent ubiquitin pathway on fertilization of Xenopus eggs: possible significance of natural selection for Pro2 in Mos. EMBO J. 1993;12:4021–4027. doi: 10.1002/j.1460-2075.1993.tb06080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa M, Okazaki K, Furuno N, Watanabe N, Sagata N. The ‘second-codon rule’ and autophosphorylation govern the stability and activity of Mos during the meiotic cell cycle in Xenopus oocytes. EMBO J. 1992;11:2433–2446. doi: 10.1002/j.1460-2075.1992.tb05308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsumi K, Sawada W, Kishimoto T. Meiosis-specific cell cycle regulation in maturing Xenopus oocytes. J Cell Sci. 1994;107:3005–3013. doi: 10.1242/jcs.107.11.3005. [DOI] [PubMed] [Google Scholar]
- Pham CD, Vuyyuru VB, Yang Y, Bai W, Singh B. Evidence for an important role of serine 16 and its phosphorylation in the stabilization of c-Mos. Oncogene. 1999;18:4287–4294. doi: 10.1038/sj.onc.1202804. [DOI] [PubMed] [Google Scholar]
- Picard A, Galas S, Peaucellier G, Doree M. Newly assembled cyclin B-cdc2 kinase is required to suppress DNA replication between meiosis I and meiosis II in starfish oocytes. EMBO J. 1996;15:3590–3598. [PMC free article] [PubMed] [Google Scholar]
- Posada J, Yew N, Ahn NG, Vande Woude GF, Cooper JA. Mos stimulates MAP kinase in Xenopus oocytes and activates a MAP kinase kinase in vitro. Mol Cell Biol. 1993;13:2546–2553. doi: 10.1128/mcb.13.4.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy LM, Haccard O, Izumi T, Lattes BG, Lewellyn AL, Maller JL. Mos proto-oncogene function during oocyte maturation in Xenopus. Oncogene. 1996;12:2203–2211. [PubMed] [Google Scholar]
- Sagata N. What does Mos do in oocytes and somatic cells? Bioessays. 1997;19:13–21. doi: 10.1002/bies.950190105. [DOI] [PubMed] [Google Scholar]
- Sagata N, Daar I, Oskarsson M, Showalter SD, Vande Woude GF. The product of the mos proto-oncogene as a candidate for oocyte maturation. Science. 1989;245:643–646. doi: 10.1126/science.2474853. [DOI] [PubMed] [Google Scholar]
- Shibuya EK, Ruderman JV. Mos induces the in vitro activation of mitogen-activated protein kinases in lysates of frog oocytes and mammalian somatic cells. Mol Biol Cell. 1993;4:781–790. doi: 10.1091/mbc.4.8.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter SA, Guadagno TM, Ferrell JE., Jr Induction of a G2 phase arrest in Xenopus egg extracts by activation of p42 MAP kinase. Mol Biol Cell. 1997;8:2157–2169. doi: 10.1091/mbc.8.11.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Hunt T, Ikawa Y, Sagata N. Independent inactivation of MPF and cytostatic factor (Mos) upon fertilization of Xenopus eggs. Nature. 1991;352:247–248. doi: 10.1038/352247a0. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Vande Woude GF, Ikawa Y, Sagata N. Specific proteolysis of the c-mos proto-oncogene product by calpain on fertilization of Xenopus eggs. Nature. 1989;342:505–511. doi: 10.1038/342505a0. [DOI] [PubMed] [Google Scholar]
- Yang Y, Herrmann CH, Arlinghaus RB, Singh B. Inhibition of v-Mos kinase activity by protein kinase A. Mol Cell Biol. 1996;16:800–809. doi: 10.1128/mcb.16.3.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Pham CD, Vuyyuru VB, Liu H, Arlinghaus RB, Singh B. Evidence of a functional interaction between serine 3 and serine 25 Mos phosphorylation sites: a dominant inhibitory role of serine 25 phosphorylation on Mos protein kinase. J Biol Chem, 1998;273:15946–15953. doi: 10.1074/jbc.273.26.15946. [DOI] [PubMed] [Google Scholar]
- Ziegenhagen R, Goldberg M, Rakutt WD, Jennissen HP. Multiple ubiquitination of calmodulin results in one polyubiquitin chain linked to calmodulin. FEBS Lett. 1990;271:71–75. doi: 10.1016/0014-5793(90)80374-r. [DOI] [PubMed] [Google Scholar]