Abstract
Background and Purpose
Inflammatory cells play a significant role in secondary injury after ischemic stroke. Recent studies have suggested that a lack of autophagy in myeloid cells causes augmented pro-inflammatory cytokine release and prolonged inflammation after tissue injury. In this study, we investigated the roles of myeloid cell autophagy in ischemic brain injury.
Methods
Focal cerebral ischemia was induced via transient middle cerebral artery occlusion in mice with autophagy-deficient myeloid-lineage cells (Atg5flox/flox LysMCre+) and in their littermate controls (Atg5flox/flox). Infarct volume, neurological function, inflammatory cell infiltration, and pro-inflammatory cytokine expression levels were evaluated.
Results
Mice lacking autophagy in myeloid-lineage cells had a lower survival rate over 14 days than control mice (20% vs. 70%, P<0.05). Although there was no difference in infarct volume at 12 hours between the two groups, mice lacking autophagy in myeloid-lineage cells had larger infarct volumes at later time points (3 and 7 days after reperfusion) with worse neurological deficit scores and lower grip test scores. There were a higher number of Iba-1-positive cells and cells expressing M1 marker CD16/32 in mice lacking autophagy in myeloid cells at the later time points. Moreover, these mice had higher expression levels of pro-inflammatory cytokines at later time points; however, there was no difference in Iba-1-positive cells or mRNA levels of pro-inflammatory cytokines at the earlier time point (12 hours after reperfusion).
Conclusions
These data suggest that the lack of myeloid cell autophagy aggravates secondary injury by augmenting and prolonging inflammation after ischemic stroke without affecting the initial injury.
Keywords: Autophagy, Stroke, Inflammation, Secondary injury, Myeloid cell
Introduction
Autophagy is an intracellular degradation process that is critical for cellular homeostasis.1 Engulfment of cytoplasmic materials leads to the formation of the autophagosome, a double-membrane structure inside the cell. The autophagosome fuses with lysosomes, resulting in degradation of the materials within the cell.1 Autophagy is important for sequestering and removing unnecessary cytosol, cytoplasmic organelles, or microbes within cells.2 Autophagy-related protein 5 (ATG5) is essential for autophagosome formation, and deficiency in ATG5 results in failure of autophagy.3, 4 Autophagy was classically recognized as part of a survival mechanism during starvation and a host defense mechanism against infection. However, autophagy was recently found to sequester inflammatory mediators in the cell, and it is emerging as a key process that regulates inflammation. Autophagy can lower basal inflammasome activation and suppress tissue inflammation by continuously removing defunct mitochondria.2
In the brain, autophagy is not only part of basal homeostatic cellular metabolism but is also induced by pathological conditions, including traumatic brain injury and stroke.5–8 By regulating inflammation, autophagy may play significant roles in the initial injury, secondary injury, and recovery after ischemic brain injury. Autophagy in ischemic stroke may be complex, and experimental studies have revealed conflicting findings.8–11 Autophagy may play different roles based on the cell types that undergo autophagy, the timing at which autophagy is modulated, or the severity of brain injury.12, 13
Inflammatory cells have diverse roles in ischemic brain injury, exerting both deleterious and protective effects. Deficiency in the autophagic processes in macrophages can exacerbate atherosclerosis and acute lung injury in mice by promoting a pro-inflammatory milieu14, 15 Autophagy in myeloid-lineage cells, notably macrophages, prevents the excessive release of pro-inflammatory cytokines and suppresses oxidative stress and inflammasome activation.16, 17 Therefore, we hypothesized that autophagy in myeloid-lineage cells, including macrophages, is critical for controlling secondary injury after ischemic brain injury. To investigate the potential roles of myeloid lineage cell autophagy in the pathophysiology of ischemic stroke, especially relating to secondary brain injury and recovery, we utilized mice that have a deficiency in autophagy in myeloid lineage cells.
Materials and Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. Experiments were conducted in accordance with the guidelines and a protocol approved by the University of California, San Francisco, Institutional Animal Care and Use Committee.
Atg5flox/flox (Atg5f/f) mice were generous gifts from Dr. Noboru Mizushima (University of Tokyo)3 and LysMCre mice (B6.129P2-Lyz2tm1(cre)Ifo/J)18 were purchased from the Jackson Laboratory (Bar Harbor, ME). Atg5f/f mice were bred with Atg5f/f LysMCre+ mice (mice carrying one allele of LysMCre) to create myeloid lineage cell-specific Atg5 deletion (Atg5f/f LysMCre+ mice carrying one allele of LysMCre) and control littermates (Atg5f/f mice not carrying a LysMCre allele). LysMCre deletes floxed genes in myeloid lineage cells, including macrophages and granulocytes.18–20 LysMCre has a low recombination efficacy in microglia in vivo20 reported as less than 10–25%.20–23
We induced transient focal cerebral ischemia using the left transient middle cerebral artery occlusion (tMCAO) as previously described by others.24, 25 Animals were euthanized at 12 hours, 3 days, and 7 days after reperfusion. We assessed infarct volume, neurological deficits, immune cell infiltration, and inflammatory cytokine mRNA expression as previously described.26–34 Details of the methods are presented in Online Data Supplement.
Statistical Analysis
Two-way ANOVA or two-way ANOVA followed by Bonferroni’s multiple comparisons test when applicable was used for comparison of continuous variables. The ordinal scores (neurological deficit score and grip test score) were analyzed by the Mann–Whitney test. Survival rate was analyzed by Log-rank test. For infarct volume, we calculated a sample size to detect the difference between 40% and 60% in infarct volume assuming alpha of 0.05, power of 0.8, and standard deviation of 10% (G*Power 3.1.9.3). Statistical analyses were performed using Prism 6 software (GraphPad Software, San Diego, CA). P < 0.05 was considered statistically significant. Continuous variables are presented as the mean ± SD, and ordinal scores are presented as the range; median.
Results
Survival and initial injury after ischemic stroke in Atg5flox/flox LysMCre+ mice
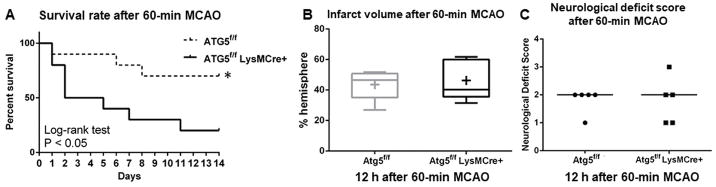
As a first step, we assessed 14-day survival rates after 60-minute MCAO in myeloid lineage-specific ATG5 knockout mice (Atg5f/f LysMCre+) and their control littermates (Atg5f/f). As shown in Figure 1A, myeloid lineage cell-specific ATG5 KO mice (myeloid ATG5 KO) had a significantly lower survival rate than control mice (20 vs. 70%: P < 0.05).
Figure 1.
A lack of autophagy in myeloid cells results in a lower survival rate after 60-minute MCAO without affecting the initial injury. (A) Survival rate over 14 days after 60-minute MCAO (n = 10 each). (B) Infarct volume (n = 5 each). (C) Neurological deficit score (n = 5 each). *: P < 0.05.
To test whether the lower survival rate in myeloid ATG5 KO mice was due to a more severe initial injury after MCAO, we assessed the degree of initial injury by measuring infarct volume and neurological deficit score at 12 hours after 60-minute MCAO. As shown in Figure 1B–C, there were no significant differences in the infarct volume (46 ± 12 vs. 44 ± 9%-hemisphere: mean ± SD: P = 0.73) or neurological deficit score (1–3; 2 vs. 1–2; 2, range; median, P > 0.99) between myeloid ATG5 KO mice and control littermates, indicating no difference in the initial injury.
Secondary injury after ischemic stroke in mice lacking autophagy in myeloid lineage cells
As shown above, the mortality of myeloid ATG5 KO mice after 60-minute ischemia was 80%, making it impossible to examine the potential mechanisms by which the lack of autophagy in myeloid lineage cells worsens the outcome after ischemic stroke. Therefore, in the next series of experiments, we induced 30-minute ischemia (i.e., mild injury). We examined infarct volume, neurological functions, and inflammatory cell infiltration at 12 hours, 3 days and 7 days after MCAO. There was no difference in the overall survival between myeloid ATG5 KO mice and control littermates (79% vs. 85%, n = 14 vs. 13, P = 0.66) 7 days after 30-minute ischemia.
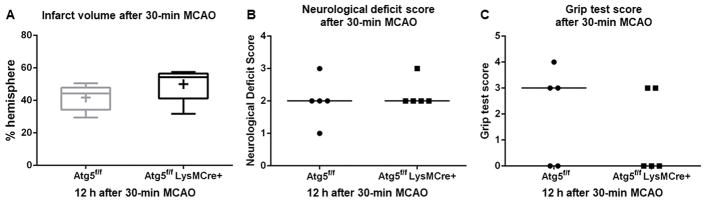
When the initial injury was assessed 12 hours after 30-minute MCAO, similar to the 60-minute occlusion, there was no significant difference in infarct volume (50 ± 9 vs. 42 ± 7%-hemisphere, P = 0.19), neurological deficit score (2–3; 2 vs. 1–3; 2, range; median, P > 0.99), or grip test score (0–3; 3 vs. 0–4; 0, range; median, P = 0.68) (Figure 2) between myeloid ATG5 KO mice and control littermates, again indicating that the lack of myeloid cell autophagy did not affect injury early after stroke.
Figure 2.
The lack of autophagy in myeloid cells did not affect the initial injury after 30-minute MCAO. (n = 5 each). (A) Infarct volume. (B) Neurological deficit score. (C) Grip test score. Day 3.
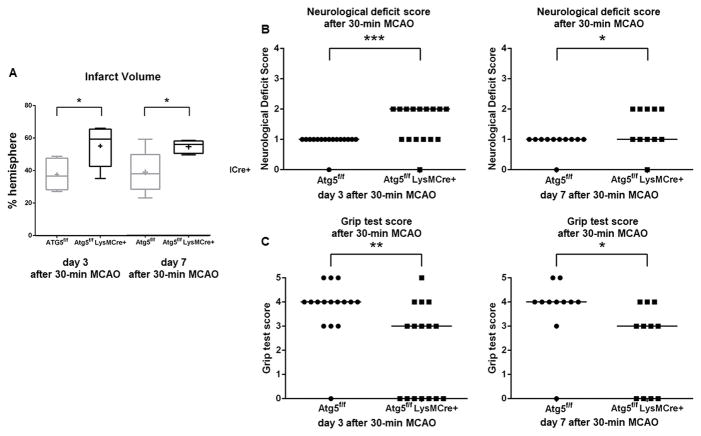
In contrast, as shown in Figure 3, when mice were examined at later time points (days 3 and 7) after 30-minute MCAO, myeloid-specific ATG5 KO mice had significantly worse neurological deficit scores than control mice (day 3: 0–2; 2 vs. 0–1; 1, range; median, P < 0.0005, day 7: 0–2; 1 vs. 0–1; 1, range; median, P < 0.05). Similarly, at 3 and 7 days after 30-minute MCAO, myeloid ATG5 KO mice showed lower grip test scores than control mice (Figure 3) (day 3: 0–5; 3 vs. 0–5; 4, range; median, P < 0.005, day 7: 0–4; 3 vs. 0–5; 4, range; median, P < 0.05).
Figure 3.
Loss of myeloid cell autophagy worsened brain injury and neurological function in the later phase (day 3 and 7) after 30-minute MCAO. (A) Infarct volume (n = 5/group). (B) Neurological deficit score (day 3: n = 16 each; day 7: n = 11 each). (C) Grip test score (day 3: n = 16 each; day 7: n = 11 each). *: P < 0.05; **: P < 0.005; **: P < 0.0005.
In addition, myeloid ATG5 KO mice had significantly larger infarct volumes than control mice at day 3 (55 ± 12 vs. 38 ± 9%-hemisphere, P < 0.05) and at day 7 (55 ± 4 vs. 39 ± 12%-hemisphere, P < 0.05) whether analyzed pairwise or analyzing all four groups together (Figure 3A). There was no difference in infarct volume between day 3 and day 7 in each group. These data indicate that the lack of autophagy in myeloid cells worsens the secondary injury after ischemic stroke.
Inflammatory cell infiltration
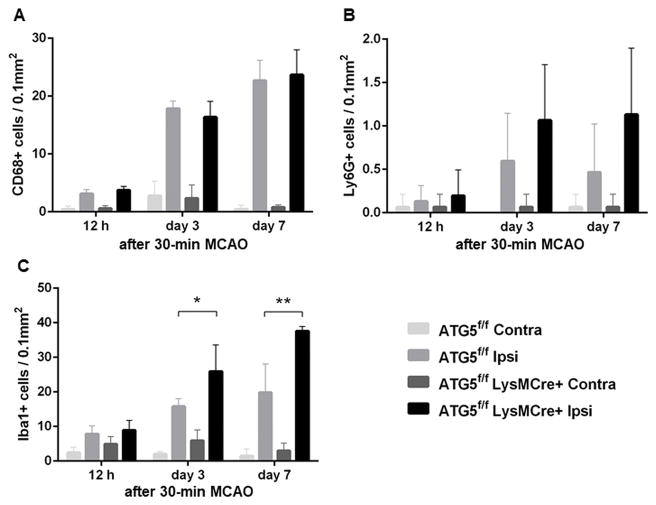
To investigate the underlying mechanism by which the lack of autophagy in myeloid lineage cells worsens secondary injury after ischemic stroke, we first assessed Lys6G-positive cells, CD68-positive cells, and Iba1-positive cells in the peri-infarct area at 12 hours, 3 days, and 7 days after 30-minute MCAO. Thirty-minute MCAO caused a significant increase in the number of both CD68-positive cells and Lys6G-positive cells in the peri-infarct area in both myeloid ATG5 KO mice and control mice at all three time points (Figure 4). However, there was no difference in the number of CD68-positive cells or Lys6G-positive infiltrating cells in the peri-infarct area between myeloid ATG5 KO mice and control mice at any time point. (Figure 4A–B).
Figure 4.
Inflammatory cell dynamics after 30-minute MCAO.
(A) CD68-positive cell infiltration. (B) Ly6G-positive cell infiltration. (C) Iba1-positive cell infiltration. (n = 5/group/time point). *: P < 0.05; **: P < 0.005.
Although there was no difference in the number of Iba1-positive cells at 12 hours, there was a significantly higher number of Iba1-positive cells in myeloid ATG5 KO mice than in control mice at the later time points (day 3: 25.9 ± 6.9 vs. 15.8 ± 2.0 cells / 0.1 mm2, P < 0.05; day 7: 37.6 ± 1.2 vs. 19.8 ± 7.4 cells / 0.1 mm2, P < 0.005) (Figure 4C).
We also assessed M1 and M2 polarization as previously described.35–39 There was no difference in M1 or M2 marker positive cells between myeloid ATG5 KO mice and control mice at 12 hours after MCAO (Online Data Supplement). At later time points, numbers of both M1 and M2 marker positive cells increased. However, the increase was more pronounced in M1 marker positive cells than in M2 marker positive cells. Seven days after MCAO, myeloid ATG5 KO mice had a significantly higher number of M1 marker positive cells than control mice (Online Data Supplement). In contrast, there was no significant difference in the number of M2 marker positive cells between myeloid ATG5 KO mice and control mice at any time point.
Inflammatory cytokines in the infarcted hemisphere
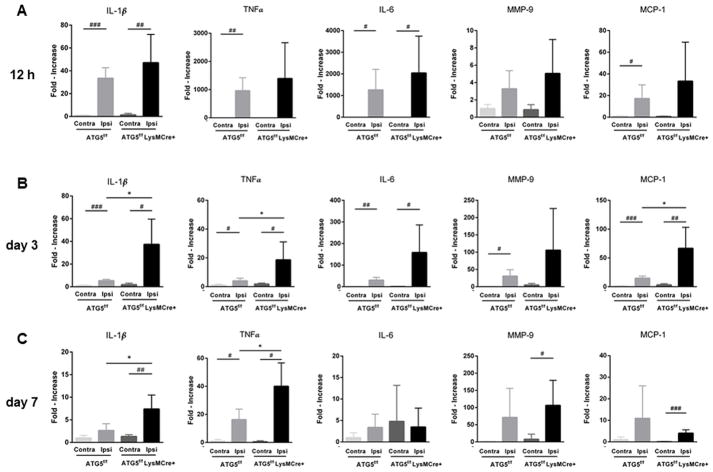
The mRNA expression levels of inflammatory cytokines in the infarcted hemisphere (ipsilateral) and the contralateral hemisphere were assessed by real-time PCR 12 hours, 3 days, and 7 days after 30-minute MCAO. At all three time points, the infarcted hemisphere had significantly higher levels of IL-1β, TNF-α, IL-6, MMP-9, and MCP-1 than the contralateral hemisphere (Figure 5).
Figure 5.
Myeloid lineage-specific ATG5 KO mice had higher pro-inflammatory cytokine expression levels in the later phase (day 3 and 7) after 30 min MCAO. (n = 5/group/time point) when assessed by real-time RT-PCR. *: P < 0.05 (vs. ATG5 KO ipsilateral side); # : P < 0.05 (vs. contralateral side); ##: P < 0.005 (vs. contralateral side); ### : P < 0.0005 (vs. contralateral side).
When the mRNA levels of the infarcted hemisphere were compared between myeloid ATG5 KO and control mice, there was no difference in any of these cytokines at the early time point (12 hours). However, at the later time points (3 and 7 days), myeloid ATG5 KO mice showed higher expression of IL-1β (3 days: 37.4 ± 22.3 vs. 5.4 ± 1.2, P < 0.05; 7 days: 7.4 ± 3.1 vs. 2.6 ± 1.5, P < 0.05), TNF-α (3 days: 18.6 ± 12.5 vs. 4.0 ± 1.9, P < 0.05; 7 days: 40 ± 16.7 vs. 16.3 ± 7.5, P < 0.05), and MCP-1 (3 days: 66.7 ± 36.5 vs. 14.8 ± 3.7, P < 0.05), further supporting a role for myeloid cell autophagy in secondary injury, but not in initial injury, after ischemic stroke.
Discussion
In this study, we found that a lack of autophagy in myeloid lineage cells, including macrophages and neutrophils, did not affect the initial infarct volume or neurological score measured at 12 hours; however, it worsened long-term survival after 60-minute ischemia. Similarly, when the 30-minute ischemia model was used, there was no difference in the infarct volume or neurological outcomes at 12 hours after 30-minute ischemia between myeloid lineage cell-specific ATG5 KO mice and control mice; however, myeloid lineage cell-specific ATG5 KO mice had larger infarct volumes and worse neurological outcomes at later time points, specifically 3 days and 7 days. These observations indicate that autophagy in myeloid lineage cells plays an important role in controlling secondary injury and potentially promoting recovery after ischemic brain injury.
Autophagy in myeloid lineage cells may play several roles after ischemic brain injury. Shortly after brain ischemia, myeloid lineage cells, including macrophages and neutrophils, can be attracted to the infarcted area and the surrounding penumbra and release pro-inflammatory cytokines; the resulting inflammation promotes further injury. It was not surprising that we found no effect of myeloid-lineage cell autophagy deficiency on the initial infarct volume, as the initial damage may be primarily a function of the primary brain ischemia caused by the lack of cerebral blood flow. Moreover, it is possible that the lack of autophagy does not affect the ability of macrophages and neutrophils to home and infiltrate into the injury site as a part of the initial inflammatory response.
The LysM Cre driver is highly efficient in recombining genes in macrophages and monocytes,40–42 and has been shown to cause gene recombination in granulocytes.43–45 Although LysM Cre appears reliable in recombining target genes in cultured microglia, recombination by LysM Cre driver in microglia in vivo not efficient,20–23 reported as less than 10–25%.21–23 Therefore, it is more likely that the worse outcomes after ischemic stroke observed in this study in Atg5f/f LysMCre+ mice are due to the lack of autophagy in macrophages, monocytes, and possibly granulocytes. However, we cannot completely exclude a possible contribution of the lack of ATG5 in a small subset of microglia to the worse outcomes.
There are likely several mechanisms by which autophagy in myeloid lineage cells affects secondary injury and recovery after ischemic brain injury. By effectively removing endogenous inflammasome activators, including reactive oxygen species and defunct mitochondria DNA,2, 46 autophagy may suppress the overall inflammasome activity and reduce the secondary injury that is partly mediated by sustained and aberrant inflammation in the penumbra. In addition, efferocytosis —the removal of dead or abnormal cells—during the resolution of inflammation may be defective in macrophages lacking autophagy,14, 47 resulting in excessive inflammation during the sub-acute and chronic phases after ischemic brain injury. Alternatively, a lack of autophagic death of macrophages and neutrophils may cause persistent inflammation by effectively slowing the resolution of the initial inflammation.
Our findings on M1 and M2 polarization indicate that the lack of autophagy in myeloid lineage cells promotes M1 polarization. Increased M1 polarization during recovery from stroke may lead to sustained inflammation, likely contributing to the worse long-term neurological outcomes and mortality in myeloid lineage-specific ATG5 KO mice. Although the precise mechanism by which autophagy deficiency in myeloid lineage cells leads to worse long-term survival and neurological outcomes in this model is unknown, the increased M1 polarization further supports more severe inflammation in myeloid lineage cell-specific ATG5 KO mice than in control mice after ischemic brain injury.
Promotion or enhancement of autophagy in myeloid lineage cells after brain ischemia may have therapeutic potential for improving stroke outcomes. However, autophagy in other cell types may have different roles. There are conflicting reports regarding the effects of indiscriminate modulation of autophagy in stroke models,13, 48–50 probably reflecting differential roles of autophagy in different cell types. The roles of autophagy after stroke are likely dependent on the cell type, severity of injury, and timing.16
In this study, we assessed infarct volumes at 12 hours, 3 days, and 7 days after ischemic stroke. It has been reported that the core infarct rapidly expands and becomes complete by 6–12 hours after 30 minutes of MCA occlusion in mice.51–53 Further expansion of the infarct after 12 hours is thought to reflect expanding ischemic damage in the penumbra.51, 53 Therefore, the time point at 12 hours is appropriate to assess the potential role of autophagy in the initial and early injury in this model. While some of the sub-acute or delayed damage may occur 1 or 2 days after ischemia, previous studies indicated that there were no significant changes in infarct volume between 24 hours and 3 days after 30 minutes of MCA occlusion in mice.53, 54 Therefore, our data at 3 days is sufficient in assessing possible sub-acute or delayed damages. Nevertheless, assessment of infarct volumes at more frequent time points may be useful in further characterizing dynamic changes in the absence of myeloid lineage autophagy in ischemic stroke.
While some of the past studies have used Iba1 and CD68 as a microglial and macrophage maker, respectively, some fractions of microglia appear to express CD68 and infiltrating monocytes/macrophages in the brain may express Iba1.55–57 Therefore, these markers may not be able to distinguish infiltrating macrophages from microglias.58 Recent reports suggest that transmembrane protein 119 (Tmem119) may act as a specific marker for microglia and that an antibody against Tmem 119 does cross-react with infiltrating monocytes/macrophages.59 For future studies, generation of chimeric mice or the adoptive transfer of monocyte/macrophage populations may be needed to precisely distinguish these two cell types.
While serial imaging using MRI or CT could be useful in longitudinally assessing evolution of infarct volume,60, 61 here we assessed infarct volume at multiple time points using Cresyl violet staining. Infarct volume measured by histological methods closely correlates with that determined by MRI imaging,60, 61 and Cresyl violet staining is established as a highly reliable method to measure infarct volume, especially studies assessing long-term outcomes.62–64 Further characterization of roles of myeloid cell autophagy may be aided by the use of serial imaging using MRI or CT.
Another limitation of this study is that we used relatively young male mice to study the roles of autophagy of myeloid lineage cells in ischemic stroke. Our intention was to explore the potential role of myeloid cell lineage autophagy in ischemic stroke, thus using animals of both sexes and of different ages is beyond the scope of the present study. Ultimately, these additional important biological variables will need to be tested.
Summary
The lack of autophagy in myeloid linage cells decreased survival and aggravated secondary injury after ischemic stroke, with higher levels of pro-inflammatory cytokines. Our findings indicate that autophagy in myeloid cells plays a protective role in ischemic injury and may be a potential therapeutic target.
Supplementary Material
Acknowledgments
None.
Sources of Funding
This project was supported by R01NS055876 (TH), R01NS082280 (TH), RO1NS084396 (RGG) and RO1NS080177 (RGG) from the National Institute of Neurological Disorders and Stroke (NIH/NINDS), and Fight Like Frank Chair of Research Grant and Team Cindy Escape from Alcatraz Chair of Research Grant from Brain Aneurysm Foundation (P0525796) (CR).
Abbreviations
- ATG5
Autophagy-related 5 protein
- MCAO
middle cerebral artery occlusion
- IL-1β
interleukin-1β
- TNF-α
tumor necrosis factor α
- IL-6
interleukin-6
- MMP-9
matrix metalloproteinase-9
- MCP-1
monocyte chemotactic protein-1
Footnotes
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
References
- 1.Mizushima N, Komatsu M. Autophagy: Renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 2.Deretic V. Autophagy: An emerging immunological paradigm. J Immunol. 2012;189:15–20. doi: 10.4049/jimmunol.1102108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 4.Mizushima N, Ohsumi Y, Yoshimori T. Autophagosome formation in mammalian cells. Cell Struct Funct. 2002;27:421–429. doi: 10.1247/csf.27.421. [DOI] [PubMed] [Google Scholar]
- 5.Lai Y, Hickey RW, Chen Y, Bayir H, Sullivan ML, Chu CT, et al. Autophagy is increased after traumatic brain injury in mice and is partially inhibited by the antioxidant gamma-glutamylcysteinyl ethyl ester. J Cereb Blood Flow Metab. 2008;28:540–550. doi: 10.1038/sj.jcbfm.9600551. [DOI] [PubMed] [Google Scholar]
- 6.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 7.Ginet V, Puyal J, Clarke PG, Truttmann AC. Enhancement of autophagic flux after neonatal cerebral hypoxia-ischemia and its region-specific relationship to apoptotic mechanisms. Am J Pathol. 2009;175:1962–1974. doi: 10.2353/ajpath.2009.090463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baek SH, Noh AR, Kim KA, Akram M, Shin YJ, Kim ES, et al. Modulation of mitochondrial function and autophagy mediates carnosine neuroprotection against ischemic brain damage. Stroke. 2014;45:2438–2443. doi: 10.1161/STROKEAHA.114.005183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang P, Xu TY, Wei K, Guan YF, Wang X, Xu H, et al. Arrb1/beta-arrestin-1 mediates neuroprotection through coordination of becn1-dependent autophagy in cerebral ischemia. Autophagy. 2014;10:1535–1548. doi: 10.4161/auto.29203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang P, Liang J, Li Y, Li J, Yang X, Zhang X, et al. Down-regulation of mirna-30a alleviates cerebral ischemic injury through enhancing beclin 1-mediated autophagy. Neurochem Res. 2014;39:1279–1291. doi: 10.1007/s11064-014-1310-6. [DOI] [PubMed] [Google Scholar]
- 11.Bu Q, Liu X, Zhu Y, Liu Y, Wang Y. W007b protects brain against ischemia-reperfusion injury in rats through inhibiting inflammation, apoptosis and autophagy. Brain research. 2014;1558:100–108. doi: 10.1016/j.brainres.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 12.Puyal J, Clarke PG. Targeting autophagy to prevent neonatal stroke damage. Autophagy. 2009;5:1060–1061. doi: 10.4161/auto.5.7.9728. [DOI] [PubMed] [Google Scholar]
- 13.Wang W, Kang J, Li H, Su J, Wu J, Xu Y, et al. Regulation of endoplasmic reticulum stress in rat cortex by p62/zip through the keap1-nrf2-are signalling pathway after transient focal cerebral ischaemia. Brain Inj. 2013;27:924–933. doi: 10.3109/02699052.2013.793397. [DOI] [PubMed] [Google Scholar]
- 14.Razani B, Feng C, Coleman T, Emanuel R, Wen H, Hwang S, et al. Autophagy links inflammasomes to atherosclerotic progression. Cell Metab. 2012;15:534–544. doi: 10.1016/j.cmet.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao X, Sluimer JC, Wang Y, Subramanian M, Brown K, Pattison JS, et al. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab. 2012;15:545–553. doi: 10.1016/j.cmet.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Han D, Sun M, Feng J. A combination of remote ischemic perconditioning and cerebral ischemic postconditioning inhibits autophagy to attenuate plasma hmgb1 and induce neuroprotection against stroke in rat. J Mol Neurosci. 2016;58:424–431. doi: 10.1007/s12031-016-0724-9. [DOI] [PubMed] [Google Scholar]
- 17.Hansson GK. How to chew up cells: Lessons for the atherosclerotic plaque. Circ Res. 2012;111:669–671. doi: 10.1161/CIRCRESAHA.112.268151. [DOI] [PubMed] [Google Scholar]
- 18.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using lysmcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 19.Abram CL, Roberge GL, Hu Y, Lowell CA. Comparative analysis of the efficiency and specificity of myeloid-cre deleting strains using rosa-eyfp reporter mice. J Immunol Methods. 2014;408:89–100. doi: 10.1016/j.jim.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldmann T, Wieghofer P, Muller PF, Wolf Y, Varol D, Yona S, et al. A new type of microglia gene targeting shows tak1 to be pivotal in cns autoimmune inflammation. Nat Neurosci. 2013;16:1618–1626. doi: 10.1038/nn.3531. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Wegener JE, Huang TW, Sripathy S, De Jesus-Cortes H, Xu P, et al. Wild-type microglia do not reverse pathology in mouse models of rett syndrome. Nature. 2015;521:E1–4. doi: 10.1038/nature14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao H, Danzi MC, Choi CS, Taherian M, Dalby-Hansen C, Ellman DG, et al. Opposing functions of microglial and macrophagic tnfr2 in the pathogenesis of experimental autoimmune encephalomyelitis. Cell Rep. 2017;18:198–212. doi: 10.1016/j.celrep.2016.11.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hucke S, Flossdorf J, Grutzke B, Dunay IR, Frenzel K, Jungverdorben J, et al. Licensing of myeloid cells promotes central nervous system autoimmunity and is controlled by peroxisome proliferator-activated receptor gamma. Brain. 2012;135:1586–1605. doi: 10.1093/brain/aws058. [DOI] [PubMed] [Google Scholar]
- 24.Yang G, Chan PH, Chen J, Carlson E, Chen SF, Weinstein P, et al. Human copper-zinc superoxide dismutase transgenic mice are highly resistant to reperfusion injury after focal cerebral ischemia. Stroke. 1994;25:165–170. doi: 10.1161/01.str.25.1.165. [DOI] [PubMed] [Google Scholar]
- 25.Xiong X, Barreto GE, Xu L, Ouyang YB, Xie X, Giffard RG. Increased brain injury and worsened neurological outcome in interleukin-4 knockout mice after transient focal cerebral ischemia. Stroke. 2011;42:2026–2032. doi: 10.1161/STROKEAHA.110.593772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraft P, Gob E, Schuhmann MK, Gobel K, Deppermann C, Thielmann I, et al. Fty720 ameliorates acute ischemic stroke in mice by reducing thrombo-inflammation but not by direct neuroprotection. Stroke. 2013;44:3202–3210. doi: 10.1161/STROKEAHA.113.002880. [DOI] [PubMed] [Google Scholar]
- 27.Bodhankar S, Chen Y, Lapato A, Dotson AL, Wang J, Vandenbark AA, et al. Pd-l1 monoclonal antibody treats ischemic stroke by controlling central nervous system inflammation. Stroke. 2015;46:2926–2934. doi: 10.1161/STROKEAHA.115.010592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang GY, Pang L, Ge HL, Tan M, Ye W, Liu XH, et al. Attenuation of ischemia-induced mouse brain injury by sag, a redox-inducible antioxidant protein. J Cereb Blood Flow Metab. 2001;21:722–733. doi: 10.1097/00004647-200106000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Zille M, Farr TD, Przesdzing I, Muller J, Sommer C, Dirnagl U, et al. Visualizing cell death in experimental focal cerebral ischemia: Promises, problems, and perspectives. J Cereb Blood Flow Metab. 2012;32:213–231. doi: 10.1038/jcbfm.2011.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu G, Liang Y, Huang Z, Jones DW, Pritchard KA, Jr, Zhang H. Inhibition of myeloperoxidase oxidant production by n-acetyl lysyltyrosylcysteine amide reduces brain damage in a murine model of stroke. J Neuroinflammation. 2016;13:119. doi: 10.1186/s12974-016-0583-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu W, Dotson AL, Libal NL, Lapato AS, Bodhankar S, Offner H, et al. Recombinant t-cell receptor ligand rtl1000 limits inflammation and decreases infarct size after experimental ischemic stroke in middle-aged mice. Neuroscience. 2015;288:112–119. doi: 10.1016/j.neuroscience.2014.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li P, Mao L, Liu X, Gan Y, Zheng J, Thomson AW, et al. Essential role of program death 1-ligand 1 in regulatory t-cell-afforded protection against blood-brain barrier damage after stroke. Stroke. 2014;45:857–864. doi: 10.1161/STROKEAHA.113.004100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nahirney PC, Reeson P, Brown CE. Ultrastructural analysis of blood-brain barrier breakdown in the peri-infarct zone in young adult and aged mice. J Cereb Blood Flow Metab. 2016;36:413–425. doi: 10.1177/0271678X15608396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barreto GE, Sun X, Xu L, Giffard RG. Astrocyte proliferation following stroke in the mouse depends on distance from the infarct. PLoS One. 2011;6:e27881. doi: 10.1371/journal.pone.0027881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidt A, Strecker JK, Hucke S, Bruckmann NM, Herold M, Mack M, et al. Targeting different monocyte/macrophage subsets has no impact on outcome in experimental stroke. Stroke. 2017;48:1061–1069. doi: 10.1161/STROKEAHA.116.015577. [DOI] [PubMed] [Google Scholar]
- 36.Gelderblom M, Melzer N, Schattling B, Gob E, Hicking G, Arunachalam P, et al. Transient receptor potential melastatin subfamily member 2 cation channel regulates detrimental immune cell invasion in ischemic stroke. Stroke. 2014;45:3395–3402. doi: 10.1161/STROKEAHA.114.005836. [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Liu J, Zhao S, Zhang H, Cai W, Cai M, et al. Interleukin-4 is essential for microglia/macrophage m2 polarization and long-term recovery after cerebral ischemia. Stroke. 2016;47:498–504. doi: 10.1161/STROKEAHA.115.012079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan T, Venkat P, Chopp M, Zacharek A, Ning R, Cui Y, et al. Neurorestorative therapy of stroke in type 2 diabetes mellitus rats treated with human umbilical cord blood cells. Stroke. 2015;46:2599–2606. doi: 10.1161/STROKEAHA.115.009870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimada K, Furukawa H, Wada K, Korai M, Wei Y, Tada Y, et al. Protective role of peroxisome proliferator-activated receptor-gamma in the development of intracranial aneurysm rupture. Stroke. 2015;46:1664–1672. doi: 10.1161/STROKEAHA.114.007722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gliem M, Klotz L, van Rooijen N, Hartung HP, Jander S. Hyperglycemia and ppargamma antagonistically influence macrophage polarization and infarct healing after ischemic stroke. Stroke. 2015;46:2935–2942. doi: 10.1161/STROKEAHA.115.010557. [DOI] [PubMed] [Google Scholar]
- 41.Kawano Y, Nakae J, Watanabe N, Kikuchi T, Tateya S, Tamori Y, et al. Colonic pro-inflammatory macrophages cause insulin resistance in an intestinal ccl2/ccr2-dependent manner. Cell Metab. 2016;24:295–310. doi: 10.1016/j.cmet.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 42.Wu Q, Shen Y, Tao Y, Wei J, Wang H, An P, et al. Hemojuvelin regulates the innate immune response to peritoneal bacterial infection in mice. Cell Discov. 2017;3:17028. doi: 10.1038/celldisc.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawano Y, Nakae J, Watanabe N, Fujisaka S, Iskandar K, Sekioka R, et al. Loss of pdk1-foxo1 signaling in myeloid cells predisposes to adipose tissue inflammation and insulin resistance. Diabetes. 2012;61:1935–1948. doi: 10.2337/db11-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sag CM, Schnelle M, Zhang J, Murdoch CE, Kossmann S, Protti A, et al. Distinct regulatory effects of myeloid cell and endothelial cell napdh oxidase 2 on blood pressure. Circulation. 2017;135:2163–2177. doi: 10.1161/CIRCULATIONAHA.116.023877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaked I, Hanna RN, Shaked H, Chodaczek G, Nowyhed HN, Tweet G, et al. Transcription factor nr4a1 couples sympathetic and inflammatory cues in cns-recruited macrophages to limit neuroinflammation. Nat Immunol. 2015;16:1228–1234. doi: 10.1038/ni.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the nalp3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schrijvers DM, De Meyer GR, Martinet W. Autophagy in atherosclerosis: A potential drug target for plaque stabilization. Arterioscler Thromb Vasc Biol. 2011;31:2787–2791. doi: 10.1161/ATVBAHA.111.224899. [DOI] [PubMed] [Google Scholar]
- 48.Gao L, Jiang T, Guo J, Liu Y, Cui G, Gu L, et al. Inhibition of autophagy contributes to ischemic postconditioning-induced neuroprotection against focal cerebral ischemia in rats. PLoS One. 2012;7:e46092. doi: 10.1371/journal.pone.0046092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carloni S, Buonocore G, Balduini W. Protective role of autophagy in neonatal hypoxia-ischemia induced brain injury. Neurobiol Dis. 2008;32:329–339. doi: 10.1016/j.nbd.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 50.Puyal J, Vaslin A, Mottier V, Clarke PG. Postischemic treatment of neonatal cerebral ischemia should target autophagy. Ann Neurol. 2009;66:378–389. doi: 10.1002/ana.21714. [DOI] [PubMed] [Google Scholar]
- 51.Carmichael ST. Rodent models of focal stroke: Size, mechanism, and purpose. NeuroRx. 2005;2:396–409. doi: 10.1602/neurorx.2.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hermann DM, Kilic E, Hata R, Hossmann KA, Mies G. Relationship between metabolic dysfunctions, gene responses and delayed cell death after mild focal cerebral ischemia in mice. Neuroscience. 2001;104:947–955. doi: 10.1016/s0306-4522(01)00125-7. [DOI] [PubMed] [Google Scholar]
- 53.Liu F, McCullough LD. Middle cerebral artery occlusion model in rodents: Methods and potential pitfalls. J Biomed Biotechnol. 2011;2011:464701. doi: 10.1155/2011/464701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pang L, Ye W, Che XM, Roessler BJ, Betz AL, Yang GY. Reduction of inflammatory response in the mouse brain with adenoviral-mediated transforming growth factor-ss1 expression. Stroke. 2001;32:544–552. doi: 10.1161/01.str.32.2.544. [DOI] [PubMed] [Google Scholar]
- 55.Ito D, Tanaka K, Suzuki S, Dembo T, Fukuuchi Y. Enhanced expression of iba1, ionized calcium-binding adapter molecule 1, after transient focal cerebral ischemia in rat brain. Stroke. 2001;32:1208–1215. doi: 10.1161/01.str.32.5.1208. [DOI] [PubMed] [Google Scholar]
- 56.Zhao X, Wang H, Sun G, Zhang J, Edwards NJ, Aronowski J. Neuronal interleukin-4 as a modulator of microglial pathways and ischemic brain damage. J Neurosci. 2015;35:11281–11291. doi: 10.1523/JNEUROSCI.1685-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smirkin A, Matsumoto H, Takahashi H, Inoue A, Tagawa M, Ohue S, et al. Iba1(+)/ng2(+) macrophage-like cells expressing a variety of neuroprotective factors ameliorate ischemic damage of the brain. J Cereb Blood Flow Metab. 2010;30:603–615. doi: 10.1038/jcbfm.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rawji KS, Mishra MK, Michaels NJ, Rivest S, Stys PK, Yong VW. Immunosenescence of microglia and macrophages: Impact on the ageing central nervous system. Brain. 2016;139:653–661. doi: 10.1093/brain/awv395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB, et al. New tools for studying microglia in the mouse and human cns. Proc Natl Acad Sci U S A. 2016;113:E1738–1746. doi: 10.1073/pnas.1525528113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Milidonis X, Marshall I, Macleod MR, Sena ES. Magnetic resonance imaging in experimental stroke and comparison with histology: Systematic review and meta-analysis. Stroke. 2015;46:843–851. doi: 10.1161/STROKEAHA.114.007560. [DOI] [PubMed] [Google Scholar]
- 61.West GA, Golshani KJ, Doyle KP, Lessov NS, Hobbs TR, Kohama SG, et al. A new model of cortical stroke in the rhesus macaque. J Cereb Blood Flow Metab. 2009;29:1175–1186. doi: 10.1038/jcbfm.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li J, Siegel M, Yuan M, Zeng Z, Finnucan L, Persky R, et al. Estrogen enhances neurogenesis and behavioral recovery after stroke. J Cereb Blood Flow Metab. 2011;31:413–425. doi: 10.1038/jcbfm.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alkayed NJ, Goto S, Sugo N, Joh HD, Klaus J, Crain BJ, et al. Estrogen and bcl-2: Gene induction and effect of transgene in experimental stroke. J Neurosci. 2001;21:7543–7550. doi: 10.1523/JNEUROSCI.21-19-07543.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tureyen K, Vemuganti R, Sailor KA, Dempsey RJ. Infarct volume quantification in mouse focal cerebral ischemia: A comparison of triphenyltetrazolium chloride and cresyl violet staining techniques. J Neurosci Methods. 2004;139:203–207. doi: 10.1016/j.jneumeth.2004.04.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.