Abstract
Rationale & Objective
The KDIGO (Kidney Disease: Improving Global Outcomes) guideline on CKD presented an international classification system that ranks patients' risk for CKD progression. Few data on children informed guideline development
Study Design
Observational cohort study
Settings and participants
Children aged 1-18 years enrolled in the North American Chronic Kidney Disease in Children (CKiD) cohort study and the European Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of CRF in Pediatric Patients (ESCAPE) trial.
Predictor
Level of estimated glomerular filtration rate (eGFR) and proteinuria (urine protein-creatinine ratio (mg/mg)) at study entry
Outcome
A composite event of renal replacement therapy, 50% reduction of estimated GFR (eGFR), or eGFR <15 mL/min/1.73m2. eGFR was estimated using the CKiD-derived “bedside” equation.
Analytical Approach
Accelerated failure time models of the composite outcome using a conventional generalized gamma distribution. Likelihood ratio statistics of nested models were used to amalgamate levels of similar risk.
Results
Among 1232 children, median age was 12 years (IQR, 8-15), median eGFR 47 ml/min/1.73 m2 (IQR, 33-62), 60% were male, 13% had urine protein-creatinine ratio (UPCR; mg/mg) >2.0 at study entry. Six ordered stages with varying combinations of eGFR categories (60-89, 45-59, 30-44 and 15-29 ml/min/1.73m2) and UPCR categories (<0.5, 0.5-2.0, and >2.0) described the risk continuum. Median times to event ranged from >10 years for eGFR 45-90 and UPCR <0.5, to 0.8 years for eGFR 15- 30 and UPCR >2. Children with glomerular disease were estimated to have a 43% shorter time to event than children with nonglomerular disease. Cross-validation demonstrated risk patterns that were consistent across the ten subsample validation models.
Limitations
Observational study, utilized cross validation rather than external validation
Conclusion
CKD staged by level of eGFR and proteinuria characterizes the timeline of progression and can guide management strategies in children.
Index words: pediatric, chronic kidney disease (CKD), disease progression, proteinuria, urinary protein-creatinine ratio (UPCR), estimated glomerular filtration rate (eGFR), children, disease staging, end-stage renal disease (ESRD), risk pattern
Introduction
In adults and children, chronic kidney disease (CKD) is characterized by a progressive decline in kidney function to the point of failure.1-3 Although CKD in children is uncommon4, it represents a higher cost per individual than adult CKD care2. The average life expectancy of pediatric CKD patients initiating renal replacement therapy (RRT) in childhood in the US is only 38 years for those on dialysis and 63 for those with a kidney transplant4. In order to identify patients who are at highest risk for complications of kidney failure and other co-morbid complications of CKD, a classification system has been developed by the KDIGO (Kidney Disease: Improving Global Outcomes) initiative based on three domains – Cause of kidney disease, Glomerular filtration rate (GFR) category and Albuminuria category (CGA)5. The classification system ranks patients' risk for progression based on data derived from adults with CKD. These ordered categories have been promoted for use in clinical practice to predict the risk of adverse outcome and guide management strategies in adults with CKD. However a similar system has not been validated for children with CKD.
We used data collected from two large multi-center study consortia of children with CKD (the Chronic Kidney Disease in Children (CKiD) prospective cohort study6 and the Effect of Strict Blood Pressure Control and ACE Inhibition on Chronic Renal Failure Progression in Pediatric Patients (ESCAPE) clinical trial7) to develop a modified KDIGO classification system for the pediatric CKD community. Classifying individuals by GFR, proteinuria (urine protein-creatinine ratio [uP/C]) and CKD diagnosis, we define unique stages of CKD progression risk. We also estimate progression timelines to help clinicians manage and plan future clinical care needs. We use cross-validation to explore the robustness of the classification system.
Methods
Study participants and design
Data in this analysis were pooled from CKiD and ESCAPE. CKiD is a prospective cohort study of children with CKD from 54 pediatric nephrology centers in North America aged 1-16 years with an estimated GFR of 30-90 ml/min/1.73m2. The study design and objectives have been previously reported6. ESCAPE is a randomized trial at 33 pediatric nephrology centers in Western Europe in which 385 children, 3 to 18 years of age, with chronic kidney disease (glomerular filtration rate of 15 - 80 ml/min/1.73 m2) received fixed-dose ramipril and were randomly assigned to intensified blood-pressure control (with a target 24-hour mean arterial pressure below the 50th percentile) or conventional blood-pressure control. Patients were followed to a 50% decline of GFR or progression to ESRD.7 The research protocols were approved by the institutional review boards at all participating sites and all participants gave written informed consent and/or assent. Analysis was restricted to children with estimated baseline GFR greater than 15 mL/min/1.73m2, measured baseline uP/C and follow-up time greater than zero.
Glomerular filatration rate (GFR) and biomarker measurement
For all participants, GFR was estimated using the CKiD-derived and published “bedside” equation,8 consistent with kidney function assessment in clinical practice.
In CKiD and ESCAPE, serum creatinine was determined enzymatically. Proteinuria was defined by the first-morning urine protein/creatinine ratio (uP/C)9. All creatinine assays used IDMS-traceable standards.
Proteinuria rather than albuminuria was used in our assessment. Except in diabetes, tests for total urine protein are preferred in children. The Up/c is a sensitive screening assay that is considered to be the standard for the measurement of proteinuria in children with CKD.10
CKD Progression Outcome Definition
The primary outcome of CKD progression was a composite endpoint defined as the earliest of either: (i) 50% reduction of baseline GFR, (ii) an estimated GFR less than 15 mL/min/1.73m2, or (iii) initiation of renal replacement therapy (RRT) (dialysis or transplantation).
Statistical analysis
Demographic and clinical characteristics of the study population were summarized overall and by CKD diagnosis (glomerular vs. non-glomerular CKD) using median and interquartile range (IQR) for continuous variables and percentage and frequency for categorical variables. Differences by diagnosis were tested using Wilcoxon rank sum tests for continuous variables and Chi-square tests categorical variables. We categorized GFR as delineated by KDIGO5: ≥ 90ml/min/1.73m2 (G1), 60-89ml/min/1.73m2 (G2), 45-59ml/min/1.73m2 (G3a), 30-44 ml/min/1.73m2 (G3b), and 15-29 ml/min/1.73m2 (G4). We also modified the KDIGO guideline classification by using proteinuria rather than albuminuria: uP/C < 0.5mg/mg, 0.5-2mg/mg and > 2mg/mg. As there were few children with baseline GFR ≥ 90 ml/min/1.73m2 and uP/C ≥ 0.5, such children (n=16) were reported in the study population description but excluded from the principal analysis.
Beginning with 13 unique GFR-uP/C levels (4 GFR by 3 uP/C categories, plus a category of GFR ≥ 90 mL/min/1.73m2 and uP/C<0.5), we used a recursive amalgamation algorithm whereby GFR-uP/C levels with similar progression risks were combined iteratively.11-13 Specifically, accelerated failure time (AFT) models using a conventional generalized gamma distribution modeled the time from study baseline to the composite event within baseline GFR-uP/C levels, adjusting for CKD diagnosis and study cohort (CKID vs. ESCAPE). Children who remained event-free at the end of their follow-up were censored at the time of their last study visit. Likelihood ratio statistics of nested models were used to amalgamate GFR-uP/C levels of similar risk for the event. The process was repeated until no further amalgamation was warranted as determined by the a priori statistical cutoff criteria of 0.01. This resulted in six CKD risk stages, labeled A (lowest risk) to F (highest risk). Robustness of the risk order and discriminating ability of the six risk stages for the outcome was evaluated in CKD-specific (glomerular vs. non-glomerular) and cohort specific (CKID vs ESCAPE) sub-strata of the study population using area under the receiver-operator curve (AUC).14,15
These final six risk stages were modeled with and without adjusting for diagnosis and cohort as well as stratified by diagnosis to check for residual risk differences between diagnosis groups or study cohorts; the goodness-of-fit of these models were compared using Akiake information criteria (AIC). Parametrically estimated survival curves were compared to empirical Kaplan-Meier survival curves for the six risk groups and diagnosis-specific event times for the 10th, 25th and 50th percentiles of the study population were estimated.
The final model describing the risk stages was validated using cross validation methods16 with the data divided into ten random samples of 10% of the data 17,18 Excluding each 10% sample in turn, parameters were estimated in the remaining 90% of the data yielding Ŝ as the estimate of the survival function and for the 10% excluded wi = −log Ŝ(ti) should be a variate from the standard exponential distribution (i.e., survival function = e−t). An accurate model fit is indicated by the degree of correspondence to standard exponential distribution, which was assessed graphically by comparing the non-parametric Kaplan-Meier curve of the wi from the 10 cross validations and subjected to the same censoring of the original data against the survival function e−t of the standard exponential.
Analyses were performed using SAS statistical software SAS v9.3 (SAS Institute Inc., Cary, NC, USA), the survival ROC package in R and TIBCO Spotfire S+ 8.2 for Windows.
Results
Data were available for 1269 children, 891 from CKiD and 378 from ESCAPE. Children missing baseline GFR or uP/C (n=26), having a baseline GFR less than 15 mL/min/1.73m2 (n=42), and having no follow-up data (n=32) were excluded from analysis. Thus, analysis was restricted to 1169 children, 857 from CKiD (73%) and 312 from ESCAPE (27%). Six CKiD children in this analysis died during study follow-up. Of those, four experienced an event prior to death; the other two were censored at their last CKID visit. Overall, there were 4698 person-years (median of 3.8 [interquartile range, 2.0-6.0] years per child) and 412 (35%) experienced the outcome of interest (renal replacement therapy, a GFR less than 15 mL/min/1.73m2, or a 50% decline in GFR from baseline) while undergoing follow-up (Table 1). The majority (75%) of children had non-glomerular diagnoses including congenital anomalies of the kidney and urinary tract (CAKUT); the predominant glomerular diagnosis was focal segmental glomerulosclerosis (29% of glomerular). Glomerular CKD children were older and had higher levels of GFR and proteinuria (uP/C).
Table 1. Clinical and Demographic Baseline Characteristics of 1169 Children with CKD, Overall and by CKD Diagnosis.
| Baseline Characteristic | Overall (N=1169) | CKD | p-value for differencea | |
|---|---|---|---|---|
|
| ||||
| Non-glomerular (n=872) | Glomerular (n=297) | |||
|
| ||||
| Age, y | 12 [8, 15] | 11 [7, 14] | 14 [10, 16] | <0.001 |
|
| ||||
| Male | 60% (707) | 63% (552) | 52% (155) | <0.001 |
|
| ||||
| GFR, mL/min/1.73m2 | 47 [33, 62] | 45 [31, 58] | 56 [40, 76] | <0.001 |
|
| ||||
| CKD Stage | <0.001 | |||
| 1 | 5% (60) | 3% (27) | 11% (33) | |
| 2 | 23% (265) | 19% (165) | 34% (100) | |
| 3a | 27% (313) | 28% (240) | 25% (73) | |
| 3b | 26% (306) | 29% (249) | 19% (57) | |
| 4 | 19% (225) | 22% (191) | 11% (34) | |
|
| ||||
| UPCR, mg/mg | 0.37 [0.12, 1.06] | 0.32 [0.11, 0.83] | 0.68 [0.21, 2.05] | <0.001 |
|
| ||||
| UPCR category | <0.001 | |||
| <0.5 mg/mg | 56% (656) | 60% (526) | 44% (130) | |
| 0.5-2.0 mg/mg | 31% (358) | 31% (268) | 30% (90) | |
| >2.0 mg | 13% (155) | 9% (78) | 16% (77) | |
|
| ||||
| Scr, mg/dL | 1.20 [0.90, 1.80] | 1.29 [0.90, 1.80] | 1.10 [0.81, 1.60] | <0.001 |
|
| ||||
| CKiD Study Cohort | 73% (857) | 68% (596) | 88% (261) | <0.001 |
|
| ||||
| Years of follow-up | 3.8 [2.0, 6.0] | 4.1 [2.6, 6.2] | 2.3 [1.2, 3.9] | <0.001 |
|
| ||||
| Event Type at last follow-up | <0.001 | |||
| Censored (event free) | 65% (757) | 66% (574) | 62% (183) | |
| RRT (dialysis or transplant) | 6% (65) | 6% (48) | 6% (17) | |
| GFR<15 ml/min/1.73m2 | 12% (137) | 13% (116) | 7% (21) | |
| 50% GFR decline from | 18% (210) | 15% (134) | 26% (76) | |
Values for categorical variables given as count (percentage), for continuous variables, as median [interquartile range]. Abbreviations: GFR, glomerular filtration rate; CKD, Chronic Kidney Disease; UPCR, urine protein-creatinine ratio; Scr, serum creatinine; CKiD, Chronic Kidney Disease in Children cohort study; RRT, renal replacement therapy.
Testing differences by CKD diagnosis and derived from Wilcoxon rank sum tests for continuous variables and Chi-square tests for independence for categorical variables
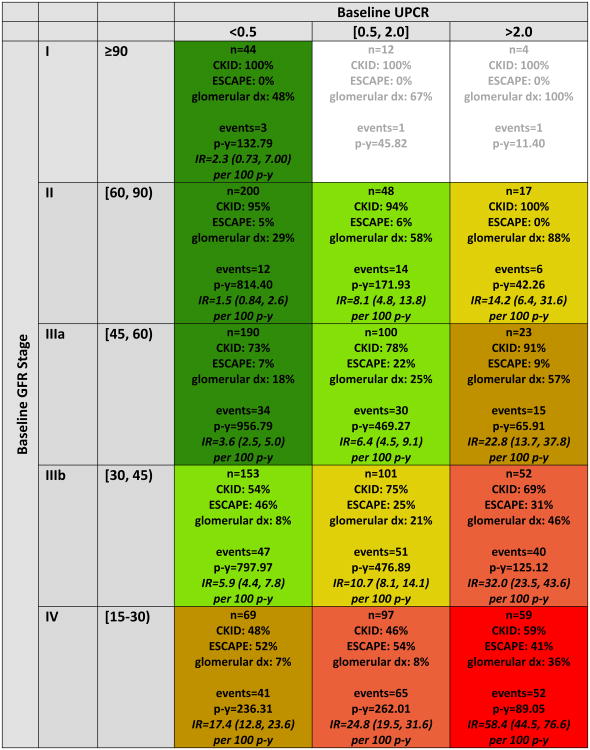
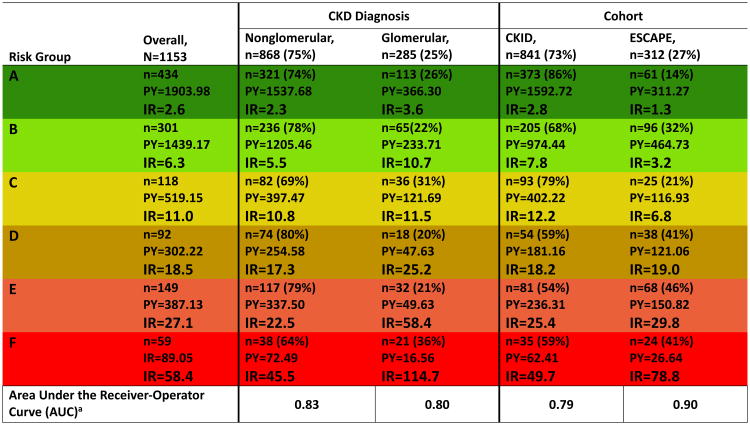
Figure 1 describes the follow-up time, events and empirical incidence rates, in each of the 15 baseline GFR-uP/C levels. Six risk stages described the progression continuum (Figure 1). Specifically, the identified risk stages consist of stage A: GFR category of G1/2/3a and uP/C< 0.5; stage B: GFR category of G2/3a and uP/C 0.5-2.0 or GFR category of G3b and uP/C< 0.5; stage C: GFR category of G3b and Up/c between 0.5 and 2.0 or GFR category of G2 and uP/C>2.0; stage D: GFR category of G4 and Up/c < 0.5 or GFR category of G3a and uP/C>2./0; stage E: GFR category of G4 and Up/c between 0.5 and 2.0 or GFR category of G3b and uP/C>2.0; and stage F: GFR category of G4 and uP/C>2.0. Risk for the composite outcome increased with increasing proteinuria for a given GFR level and was either constant or increased as GFR decreased for a given level of proteinuria. The six risk stages held their rank of risk not only overall but also for CKD-specific sub-strata (glomerular and non-glomerular) as well as for cohort-specific sub-strata (CKiD and ESCAPE) (Figure 2). Similarly, the six-risk-stage model demonstrated effective discrimination for the clinical outcome at 4 years in each of these sub strata. AUC in the non-glomerular and glomerular CKD sub-strata were 0.83 and 0.80, respectively. Stratifying by cohort, the AUC for the CKID and ESCAPE sub-strata were 0.79 and 0.90, respectively. AUC values ≥0.80 are generally considered to reflect “excellent” discrimination by the marker being tested.14,15
Figure 1.
Distribution of persons, person-years, CKD diagnosis, and events by categories of baseline glomerular filtration rate (GFR) and urine protein-creatinine ratio (UPCR), N=1169. Each cell in the table above describes: the number of subjects, overall and distributed by cohort affiliation; prevalence of glomerular CKD; number of events; number of person-years; and empirical incidence rate (per 100 person-years) of the composite outcome event (50% GFR decline, renal replacement therapy, or GFR < 15 mL/min/1.73m2) with 95% confidence interval. Cell coloring defines the final six GFR/proteinuria risk stages ordered from best prognosis to worst prognosis as follows: A (dark green), B (light green), C (gold), D (tan), E (salmon), F (red). Incidence rates (IR) expressed as events per 100 person years. Cells with <15 subjects do not have an incidence rate calculated (n/a).
Figure 2.
Amalgamated number of subjects, events, person-years and empirical incidence rates in the final six GFR/proteinuria risk stages, A through F, overall, by CKD diagnosis and cohort affiliation. This table shows the preservation of risk order for the final risk stages within diagnosis and cohort substrata. Areas under the Receiver-Operator Curve (AUC) quantify the ability of the six risk stages to discriminate risk of progression in the diagnosis and cohort substrata. Abbreviations: IR, incidence rate expressed in events per 100 person years (PY). a – The area under the ROC curve is calculated at 4 years of follow up.
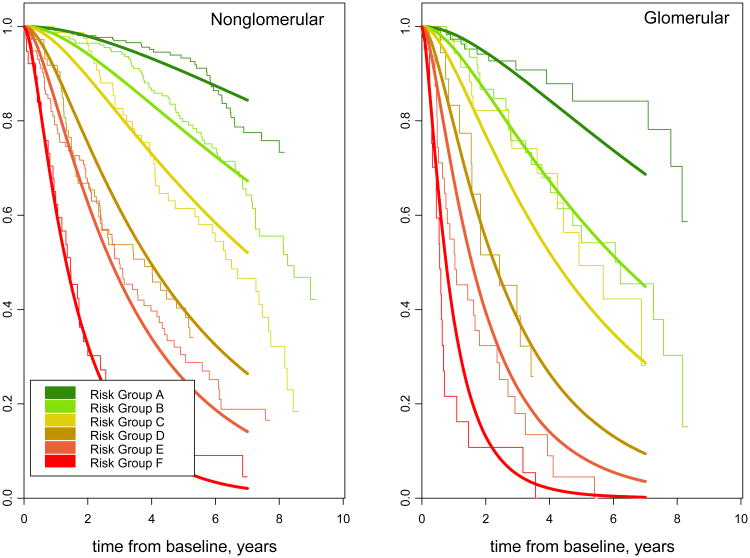
We next compared nested models to see if either glomerular CKD diagnosis or cohort affiliation carried any residual risk unexplained by the six GFR-uP/C risk stage levels. A model that adjusted for glomerular CKD as a covariate provided the best overall fit of the data. Stratifying by CKD diagnosis, specifically dividing the groups into glomerular and non-glomerular disorders, did not offer improvement of fit. Neither adjustment nor stratification by cohort (CKiD or ESCAPE) improved model fit, signifying that the model is generalizable across the two studies. Figure 3 shows the fit of CKD-specific parametric survival curves vs the empirical, Kaplan-Meier derived estimates.
Figure 3.
Parametric and non-parametric survival curves for the six risk stages (A-F) modeling time from study enrollment (baseline) to composite clinical event (50% GFR decline, renal replacement therapy, or GFR less than 15 mL/min/1.73m2), stratified by CKD diagnosis (N=868 non-glomerular and N=285 glomerular CKD children. Parametric survival curves are generated from an accelerated failure time model using a conventional generalized gamma distribution with seven beta indicator parameters: six risk stages (A-F) and glomerular CKD.
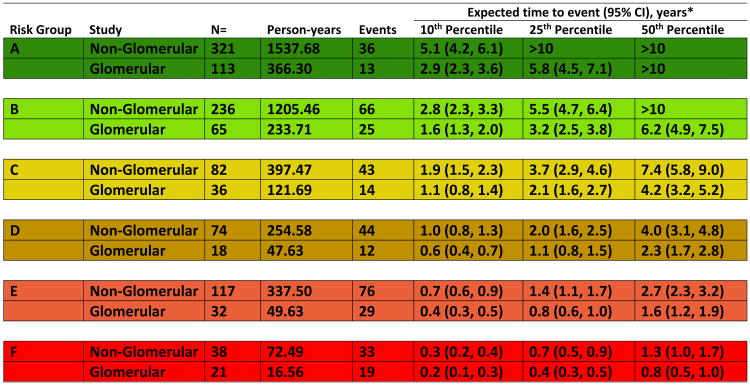
Figure 4 presents the parametrically estimated times from baseline to the event for the 10th, 25th, and 50th percentile of each diagnosis-specific risk stage. These estimates are derived from the model including CKD diagnosis as a “main effect”, which is presented in Figure 2. At any given risk stage, glomerular children were estimated to have a 43% shorter time to event than that of non-glomerular CKD children. By risk stage F, 50% of the non-glomerular CKD children are expected to experience the outcome within 1.3 (95% CI, 1.0-1.7) years; that estimate becomes less than one year for glomerular CKD children in risk stage F.
Figure 4.
Expected times – 10th, 25th and 50th (percentile) - from GFR/proteinuria risk characterization (study baseline) to clinical composite event (50% GFR decline, renal replacement therapy, or GFR less than 15 mL/min/1.73m2), for six GFR/proteinuria risk stages, by CKD diagnosis. Estimated event times are generated from an accelerated failure time model using a conventional generalized gamma distribution with seven beta indicator parameters: six risk stages (A-F) and glomerular CKD. Time to event is from baseline (time at which risk level was defined). Event is defined as RRT, GFR<15mL/min/173m2 or 50% GFR decline.
The ten validation risk models derived from 90%-subsamples of the data are presented in Figure S1. Risk patterns across the dimensions of GFR and proteinuria were consistent between the ten subsample validation models. Figure S2 shows the standardized times, wt, derived from the cross-validation superimposed on the survival function of the standard lognormal LN[0,1]
Discussion
The current KDIGO guideline on CKD classification is based almost entirely on adult data. To our knowledge, our study is the first to apply the principles of this classification to a large cohort of pediatric CKD patients, combining two large multicenter studies via a multi-national collaborative effort. To provide clinically useful measures of risk, we estimated the times by which 10%, 25%, and 50% of the children in a given risk group will reach a clinically defined event (50% decline in GFR, GFR <15 mL/min/1.73m2, or initiation of RRT) using laboratory data that are obtained routinely in children with CKD. We developed a classification system practical for pediatric CKD clinical care, which can be used to guide pediatric CKD management. We presented the results on risk classification in a two-dimensional graphic to facilitate a system analogous to the clinical use of the KDIGO classification system5 as applied to children. Our goal with this analysis was to develop a clinically useful tool that would help the clinician at the bedside anticipate the timing of needed interventions such as a pre-transplantation evaluation or placement of dialysis access and communicate these to the families of children with CKD.
Our results indicate that the combination of GFR, proteinuria, and CKD diagnosis is more informative for assessing the risk of disease progression in pediatric CKD patients than GFR alone. Qualitatively, the ranking of risk was similar across both source cohorts and across disease categories of glomerular and non-glomerular diagnoses. Despite the differences in the CKiD and the ESCAPE cohorts, the stratified analysis presented in Figure 2 shows the strikingly similar risk ordering even though the absolute risks are lower in the ESCAPE group in whom blood pressure was strictly controlled.
Collectively, children with a glomerular etiology of disease showed faster progression after controlling for risk stage than children with disease of nonglomerular cause. On average, glomerular disease children had estimated progression times to the outcomes of interest that were less than half those of non-glomerular disease children in the same risk stage. Thus, as with the adult KDIGO guideline, pediatric CKD progression risk can be well defined by three domains: GFR, proteinuria, and CKD diagnosis.
The ability to combine data from the two cohorts is a strength of this study, as it helps assure a classification system that applies to a broad and heterogeneous clinical population. Children from the two cohorts were complementary regarding their distribution of GFR and proteinuria: CKiD participants disproportionately had high baseline GFR and/or high proteinuria while ESCAPE participants had low baseline GFR and/or low proteinuria. Pooling the two cohorts provided adequate data to estimate risks across the full range of the bivariate distribution of GFR and proteinuria.
By providing diagnosis-specific estimates of the 10th, 25th and 50th percentile of time to an event for a patient in a given risk group, our results can speak to the necessary frequency of monitoring, ranging from annual visits among those with low levels of proteinuria and relatively preserved GFR to more frequent visits among those with heavier proteinuria and eGFR < 30ml/min/1.73m2. Similarly, clinicians can use the quantitative information in our analysis to plan the timing of referral for transplantation, or the need for catheter placement and dialysis initiation.
Our risk staging definitions differed from the KDIGO guideline in that we used levels of proteinuria, instead of albuminuria, as the classification criteria. This is consistent with a CKD staging system tailored for a pediatric context, as causes of CKD in children are often underlying dysplasias or congenital abnormalities (with a preponderance of tubular protein losses), making albuminuria measurement less sensitive. In addition, since as many as 11% of males and 19% of females in the 6-19 year age group in the US were found to have a urine albumin concentration ≥30mg/L19, the specificity of modest increases in albuminuria levels as an independent risk of progression of CKD in children of this age with renal disease is weaker than in adults. Total urine protein loss has been shown to be an important determinant of GFR decline in children with CKD9,20,21, and higher degrees of proteinuria are associated with lower GFR9. Further, the use of UPCR is not inconsistent with the adult KDIGO system, which specified UPCR as the second preferred method for initial protein testing (guideline recommendation 1.4.4.1)5 as this is highly correlated with albuminuria.22,23 In children, uP/C and urinary dipstick for protein remain useful and inexpensive tools for CKD classification.24 A recently published study from CKiD has shown that the utility of urine protein- and albumin-creatinine ratios in characterizing progression is similar.25 In that study, when categories of urine albumin-creatinine ratio were created based on clinically meaningful cutoff values of uP/C, the relative times to the composite end-point were almost identical.
Our study has several limitations. In our classification, the lowest tertile of proteinuria was less than 0.5 mg/mg, this is higher than the traditional definition of significant proteinuria (0.2mg/mg)18 in children. In the CKiD and ESCAPE cohorts, participants with proteinuria < 0.2 mg/mg are rare, limiting our ability to statistically assess risk below this level. Of note, 2 categories of proteinuria used in this analysis are >0.5, corresponding roughly to an ACR of >300 mg/g26, the very high and nephrotic range of albuminuria in the KDIGO guideline. This highlights the severity of disease in this group of children affected with CKD, and the need for more specific guidance on CKD staging in this vulnerable population.
Additionally, we had relatively few children in our study sample that were followed beyond 7 years. The smaller number of children contributing information about renal survival between 7 and 10 years after baseline limits the precision of our estimates of risk of ESRD or halving of GFR at that length of time from initial presentation. Further, there are a number of differences between the CKiD cohort and the ESCAPE cohort, including the fact that CKiD is a longitudinal cohort study and ESCAPE is a clinical trial. However, in cohort stratified analyses, the ordering of risk groups was maintained suggesting that differences due not alter the nature of the relationship of GFR and proteinuria on progression risk.
Finally, our estimates of risk are based on the bedside eGFR equation, which uses only serum creatinine. Although it is true that estimates of GFR which include both creatinine and cystatin C are more accurate and demonstrate less bias than those using only creatinine27, currently, at most centers only creatinine measures are readily available to clinicians, hence we chose to use the more widely and rapidly available formula for developing this clinical tool. Even with the use of the widely available measurement of creatinine to estimate kidney function, we recognize that the estimates of risk presented here are only applicable to developed nations with a population predominantly comprising those of European ancestry. Further assessment of the applicability of this staging system in more diverse populations is warranted.
In our primary analysis, we chose to use a conservative threshold of 50% loss of GFR in the composite outcome to maintain a high specificity for progression. A threshold of 40% decline in GFR has been more recently suggested28; using this more liberal threshold would increase the number of events by only 4% and therefore would be unlikely to change our inferences.
We conclude that classification using a six-risk-stage model of eGFR category, levels of proteinuria, and CKD diagnosis yields valuable prognostic information, with excellent discrimination for outcomes for children with CKD. Our study on data from two large studies in children with CKD adds to existing CKD classification guidelines and provides evidence that this classification scheme can be applied across pediatric CKD practice to inform follow up and clinical decision making. This classification system can be used as an adjunct to clinical judgement in planning for timing of transplant evaluation or dialysis access placement. Further study to externally validate this classification system in a separate cohort of children with CKD would provide stronger evidence of its usefulness in clinical care.
Supplementary Material
Supplementary Figure S1 (PDF). Risk stage models generated from 90% subsamples of 1,169 children for model cross-validation.
Supplementary Figure S2 (PDF). Results of cross-validation analysis.
Acknowledgments
We would like to acknowledge the contributions of Dr. Alvaro Munoz to the cross-validation methods utilized in this manuscript.
Support: The CKiD Study is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01-DK-66143, U01-DK-66174, U01DK-082194, U01-DK-66116). ESCAPE was supported by grants from the Boehringer Ingelheim Stiftung; the European Commission (Fifth Framework Programme, QLRT-2001-00908); Kuratorium für Dialyse und Nierentransplantation, Neu-Isenburg; and the Baxter Extramural Grant Program. The funders of this study did not have any role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Article Information
CKiD Investigators: Joshua Samuels, MD (University of Texas, Houston); Susan Furth, MD, PhD (Children's Hospital of Philadelphia); Meredith Atkinson, MD (Johns Hopkins Children's Center); Amy Wilson, MD (Riley Hospital for Children at Indiana Univ. Health); Alejandro Quiroga, MD (DeVos Children's Hospital at Spectrum); Susan Massengill, MD (Carolinas Medical Center); Dave Selewski, MD (University of Michigan, Mott Hospital); Maria Ferris, MD (University of North Carolina, Chapel Hill); Amy Kogon, MD (Nationwide Children's Hospital, Ohio State Univ.); Frederick Kaskel, MD, PhD (Children's Hospital at Montefiore); Marc Lande, MD, George Schwartz, MD (University of Rochester Medical Center, Golisano Children's Hospital at Strong); Jeffrey Saland, MD (Icahn School of Medicine at Mount Sinai); Victoria Norwood, MD (University of Virginia); Tej Matoo, MD (Children's Hospital of Michigan); Guillermo Hidalgo, MD (East Carolina University); Poyyapakkam Srivaths, MD (Texas Children's Hospital, Baylor); Joann Carlson, MD (Rutgers - Robert Wood Johnson Medical School); Craig Langman, MD (Ann & Robert H. Lurie Children's Hospital of Chicago); Susan Mendley (University of Maryland); Eunice John, MD (University of Illinois, Chicago); Kiran Upadhyay, MD (University of Florida); Patricia Seo-Mayer, MD (INOVA Fairfax Hospital for Children); Larry Patterson, MD (Children's National Medical Center); Rulan Parekh, MD, Lisa Robinson, MD (Hospital for Sick Children (Sick Kids)); Adam Weinstein, MD (Children's Hospital at Dartmouth); Dmitry Samsonov, MD (Maria Fareri Children's Hospital at Westchester); Juan Kupferman, MD (Maimonides Medical Center); Jason Misurac, MD (University of Iowa); Anil Mongia, MD (State University of New York, Downstate Med Center); Steffan Kiessling, MD (University of Kentucky); Cheryl Sanchez-Kazi, MD (Loma Linda University); Allison Dart, MD, MSc, FRCPC (Children's Hospital of Winnipeg); Sahar Fathallah, MD (Children's Hospital of Alabama); Donna Claes, MD, Mark Mitsnefes, MD (Cincinnati Children's Hospital and Medical Center); Tom Blydt-Hansen, MD, FRCPC (British Columbia Children's Hospital); Bradley Warady, MD (Children's Mercy Kansas City); Larry Greenbaum, MD, PhD (Egleston Children's Hospital, Emory University); Joseph Flynn, MD (Seattle Children's Hospital); Craig Wong, MD, MPH (University of New Mexico Children's Hospital); Isidro Salusky, MD, Ora Yadin, MD (University of California, Los Angeles); Katherine Dell, MD (Case Western Reserve University); Randall Jenkins, MD (Northwest Pediatric Kidney Specialist); Cynthia Pan, MD (Medical College of Wisconsin); Elaine Ku, MD, MAS (UCSF Children's Hospital); Amira Al-Uzri, MD, Randall Jenkins, MD (Oregon Health and Science University); Nancy Rodig, MD (Boston Children's Hospital); Cynthia Wong, MD (Stanford University Medical Center); Keefe Davis, MD (St. Louis Children's Hospital); Martin Turman, MD, PhD (Phoenix Children's Hospital); Sharon Bartosh, MD (University of Wisconsin); Colleen Hastings, MD (LeBonheur Children's Medical Center); Anjali Nayak, MD (University of Oklahoma Health Sciences Center); Mouin Seikaly, MD (University of Texas Southwestern Medical Center); Nadine Benador, MD, Robert Mak, MD, PhD (University of California, San Diego); Ellen Wood, MD (Cardinal Glennon Hospital); Randall Jenkins, MD (Children's Kidney Specialists, Idaho); Gary Lerner, MD (Children's Hospital of Los Angeles); Gina Marie Barletta, MD (Arizona Kidney Disease and Hypertension Center)
ESCAPE Trial Investigators: A. Anarat (Cukurova University School of Medicine, Balcali, Adana, Turkey); A. Bakkaloglu, F. Ozaltin (Hacettepe University Faculty of Medicine, Sihhiye, Ankara, Turkey); A. Peco-Antic (University Children's Hospital, Belgrade, Serbia); U. Querfeld, J. Gellermann (Charité Children's Hospital, Berlin, Germany); P. Sallay (Semmelweis University Budapest, 1st Dept. of Pediatrics, Budapest, Hungary); D. Drożdż (Polish-American Children's Hospital, Jagiellonian University Collegium Medicum, Krakow, Poland); K.-E. Bonzel, A.-M. Wingen (University Hospital Essen, Essen, Germany); A. Żurowska, I. Balasz (Dpt. of Paediatric and Adolescent Nephrology and Hypertension, Medical University of Gdansk, Gdansk, Poland); A. Trivelli, F. Perfumo (G. Gaslini Institute, Genova, Italy); D.E. Müller-Wiefel, K. Möller (University Children's Hospital Hamburg-Eppendorf, Hamburg, Germany); G. Offner, B. Enke (Hannover Medical School, Children's Hospital, Hannover, Germany); E. Wühl, C. Hadtstein, O. Mehls, F. Schaefer (Center for Pediatric and Adolescent Medicine, Heidelberg, Germany); S. Emre (University of Istanbul, Istanbul Medical Faculty, Capa, Istanbul, Turkey); S. Caliskan (Istanbul University, Cerrahpasa Medical Faculty, Istanbul, Turkey); S. Mir (Ege University Medical Faculty, Dept. of Pediatrics Bornova, Izmir, Turkey); S. Wygoda (Urban Hospital St. Georg, Leipzig, Germany); K. Hohbach-Hohenfellner (University Children's Hospital, Mainz, Germany); N. Jeck, G. Klaus (Philipps University Marburg, Dept. of Pediatrics, Marburg, Germany); G. Ardissino, S. Testa (IRCCS Ospedale Maggiore, Policlinico-Mangiagalli, Milano, Italy); G. Montini (Azienda Ospedaliera-Università di Padova, Padova, Italy); M. Charbit, P. Niaudet (Hopital Necker, Paris, France); A. Caldas-Afonso, A. Fernandes-Teixeira (Hospital S. Joao, Porto, Portugal); J. Dušek (University Hospital Motol, Dpt. of Pediatrics, Prague, Czech Republic); M.C. Matteucci, S. Picca, A. Mastrostefano (Ospedale Pediatrico Bambino Gesù, Rome, Italy); M. Wigger (University Children's Hospital, Rostock, Germany); U.B. Berg, G. Celsi (Karolinska Institute, Huddinge University Hospital, Stockholm, Sweden); M. Fischbach, J. Terzic (Hopitaux Universitaires de Strasbourg, Pediatrie 1, Strasbourg, France); J. Fydryk, T. Urasinski (Pomeranian Academy of Medicine, Szczecin, Poland); R. Coppo, L. Peruzzi (Ospedale Infantile Regina Margherita, Torino, Italy); K. Arbeiter (University Children's Hospital, Vienna, Austria); A. Jankauskiené (Vilnius University Children's Hospital, Vilnius University, Vilnius, Lithuania); R. Grenda, M. Litwin, R. Janas (Children's Memorial Health Hospital, Warsaw, Poland); T.J. Neuhaus (University Children's Hospital, Zürich, Switzerland).
Footnotes
Authors' Contributions: research idea and study design: SLF, CP, WFH, CAW, AA; data acquisition: SLF, CSW, FS, EW, BAW; data analysis/interpretation: SLF, CP, FS, EW, AA, BAW; statistical analysis: CP, AA; supervision or mentorship: SLF, FS, EW, BAW. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eckardt KU, Coresh J, Devuyst O, Johnson RJ, Köttgen A, Levey AS, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382:158–69. doi: 10.1016/S0140-6736(13)60439-0. [DOI] [PubMed] [Google Scholar]
- 2.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2016 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2017;69(suppl 1)(3):S1–S688. doi: 10.1053/j.ajkd.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levey AS, Stevens LA, Coresh J. Conceptual model of CKD: applications and implications. Am J Kidney Dis. 2009;53(S3):S4–16. doi: 10.1053/j.ajkd.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 4.Harambat J, van Stralen KJ, Kim JJ, Tizard EJ. Epidemiology of chronic kidney disease in children. Pediatr Nephrol. 2012;27:363–73. doi: 10.1007/s00467-011-1939-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney International Suppl. 2013;3:1–150. [Google Scholar]
- 6.Furth SL, Cole SR, Moxey-Mims M, Kaskel, et al. Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol. 2006;1(5):1006–15. doi: 10.2215/CJN.01941205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ESCAPE Trial Group: Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639–50. doi: 10.1056/NEJMoa0902066. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka VR, et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney International. 2012;82(4):445–53. doi: 10.1038/ki.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong CS, Pierce CB, Cole SR, Warady BA, et al. Association of proteinuria with race, cause of chronic kidney disease, and glomerular filtration rate in the chronic kidney disease in children study. Clin J Am Soc Nephrol. 2009;4:812–9. doi: 10.2215/CJN.01780408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vassalotti JA, Stevens LA, Levey AS. Testing for chronic kidney disease: a position statement from the National Kidney Foundation. Am J Kidney Dis. 2007;50:169–180. doi: 10.1053/j.ajkd.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Recursive partitioning with survival data. Ciampi A, Lawless JF, McKinney SM, Singhal K. Regression and recursive partition strategies in the analysis of medical survival data. J Clin Epidemiol. 1988;41(8):737–748. doi: 10.1016/0895-4356(88)90160-6. [DOI] [PubMed] [Google Scholar]
- 12.LeBlanc M, Crowley J. Survival trees by goodness of split. Journal of the American Statistical Association. 1993;88(422):457–467. [Google Scholar]
- 13.Mellors JW, Munoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, Kingsley LA, Todd JA, Sah AJ, Detels R, Phir JP, Rinaldo CR. Plasma viral load and CD4+ lymphocites as prognostic markers of HIV-1 Infection. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 14.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115(7):928–935. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 15.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, et al. Evaluating the added predictive ability of a new biomarker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 16.Altman DG, Royston P. What do we mean by validating a prognostic model? Statist Med. 2000;19:453. doi: 10.1002/(sici)1097-0258(20000229)19:4<453::aid-sim350>3.0.co;2-5. {473. [DOI] [PubMed] [Google Scholar]
- 17.Steyerberga Ewout W, Harrell Jrb Frank E, Borsbooma Gerard JJM, Eijkemansa M J C (René), Vergouwea Yvonne, Habbemaa J Dik F. Internal validation of predictive models: Efficiency of some procedures for logistic regression analysis. Journal of Clinical Epidemiology. 2001;54:774–781. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 18.Warady Bradley A, MD,1, Abraham Alison G, MD,2, Schwartz George J, MD,3, Wong Craig S, MD,4, Muñoz Alvaro, PhD,2, Betoko Aisha, PhD,2, Mitsnefes Mark, MD, MS,5, Kaskel Frederick, MD, PhD,6, Greenbaum Larry A, MD, PhD,7, Mak Robert H, MD, PhD,8, Flynn Joseph, MD,9, Moxey-Mims Marva M, MD,10, Furth Susan., MD, PhD 11 Predictors of Rapid Progression of Glomerular and Non-Glomerular Kidney Disease in Children: The CKiD Cohort. Am J Kidney Dis. 2015 Jun;65(6):878–888. doi: 10.1053/j.ajkd.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones CA, Francis ME, Eberhardt MS, Chavers B, et al. Microalbuminuria in the US population: third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2002;39(3):445–59. doi: 10.1053/ajkd.2002.31388. [DOI] [PubMed] [Google Scholar]
- 20.Furth SL, Abraham AG, Jerry-Fluker J, Schwartz GJ, et al. Metabolic abnormalities, cardiovascular disease risk factors and GFR decline in children with chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(9):2132–40. doi: 10.2215/CJN.07100810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ardissino G, Testa S, Daccò V, Viganò S, et al. Proteinuria as a predictor of a disease progression in children with hypodysplastic nephropathy. Pediatr nephrol. 2004;19:172–77. doi: 10.1007/s00467-003-1268-0. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, DeJong PE, Coresh J, Nahas ME, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney International. 2001;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto K, Yamamoto H, Yoshida K, Miwa K, Nishi Y, Mizuno A, et al. The total urine protein-to-creatinine ration can predict the presence of microalbuminuria. PLoS ONE. 2014;9(3):391067.21. doi: 10.1371/journal.pone.0091067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hogg RJ, Furth S, Lemley KV, Portman R, et al. National Kidney Foundation's Kidney Disease Outcomes Quality Initiative Clinical Practice Guidelines for chronic kidney disease in children and adolescents: evaluation, classification, and stratification. Pediatrics. 2003;111(6):1416–21. doi: 10.1542/peds.111.6.1416. [DOI] [PubMed] [Google Scholar]
- 25.Fuhrman DY, Schneider MF, Dell KM, Blydt-Hansen TD, et al. Albuminuria, Proteinuria and Renal Disease Progression in Children with CKD. CJASN. 2017;12(6):912–920. doi: 10.2215/CJN.11971116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Level AS, Becker C, Inker LA. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: a systematic review. JAMA. 2015 Feb 24;313(3):8378–46. doi: 10.1001/jama.2015.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng DKS, Schwartz GJ, Jacobson LP, et al. Universal GFR determination based on two time points during plasma iohexol disappearance. Kidney Int. 2011;80:423–430. doi: 10.1038/ki.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, Arima H, Chadban SJ, Cirillo M, Djurdjev O, Green JA, Heine GH, Inker LA, Irie F, Ishani A, Ix JH, Kovesdy CP, Marks A, Ohkubo T, Shalev V, Shankar A, Wen CP, de Jong PE, Iseki K, Stengel B, Gansevoort RT, Levey AS CKD Prognosis Consortium. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311:2518–2531. doi: 10.1001/jama.2014.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1 (PDF). Risk stage models generated from 90% subsamples of 1,169 children for model cross-validation.
Supplementary Figure S2 (PDF). Results of cross-validation analysis.