Abstract
Vitamin D, 25hydroxyvitamin D (25D), and 24,25dihydroxyvitamin D (24,25D) were measured before and after broad spectrum antibiotic (Abx) treatment for 2 wks. Abx treatments increased 25D and 24,25D levels suggesting that the microbiota or Abx were altering vitamin D metabolism. Increased 25D, but not 24,25D, following Abx treatments were found to be dependent on toll like receptor signaling. Conversely, the effects of Abx on 24,25D levels required that the vitamin D receptor (VDR) be expressed in tissues outside of the hematopoietic system (kidney) and not the immune system. Fibroblast growth factor (FGF)23 increased following Abx treatment and the effect of Abx treatment on FGF23 (like the effect on 24,25D) was not present in VDR knockout (KO) mice. The Abx mediated increase in 24,25D was due to changes to the endocrine regulation of vitamin D metabolism. Conversely, 25D levels went up with Abx treatment of the VDR KO mice. Host sensing of microbial signals regulates the levels of 25D in the host.
Keywords: vitamin D metabolism, microbiota, antibiotics, vitamin D receptor
1. Introduction
Vitamin D is a fat-soluble vitamin and a critical regulator of calcium and phosphate homeostasis and bone health. Vitamin D is made in the skin following UVB exposure of the skin or ingested as part of the diet. The vitamin D that is either produced in the skin or obtained through diet is inactive. In the liver vitamin D is hydroxylated primarily by the vitamin D 25-hydroxylase Cyp2R1 and to a lesser extent by Cyp27A1, to produce 25hydryoxyvitamin D (25D) [1]. The amount of 25D in the serum has been used as a measure of vitamin D status since it has a relatively long half-life and is thought to accurately reflect the amount of vitamin D [2]. The active form of vitamin D, 1,25dihydroxyvitamin D (1,25D) is produced largely in the kidney by the 1alpha vitamin D hydroxylase Cyp27B1 [3]. 1,25D is the high affinity ligand that binds to the vitamin D receptor (VDR) at nM concentrations and regulates gene transcription of genes that contain vitamin D response elements [3, 4]. 25D is a low affinity ligand for the VDR and under normal physiological conditions does not bind to the VDR [3].
The amount of 1,25D produced is tightly regulated to prevent hypercalcemia. Important regulators of 1,25D include low calcium and parathyroid hormone (PTH) that induce expression of Cyp27B1 [5] and fibroblast growth factor (FGF)23 that inhibits Cy27B1 [6, 7]. 1,25D induces the 24-hydroxylase Cyp24A1 that inactivates 1,25D by producing 1,24,25D and eliminates the 25D substrate, by producing 24,25D [8, 9]. Conversely, 1,25D increased FGF23 levels, and the FGF23 promoter is directly regulated by 1,25D since it contains a vitamin D response element [10, 11]. In addition, 1,25D inhibits Cyp27B1 and PTH expression in a feedback loop designed to carefully regulate 1,25D levels [12, 13]. The amount of 1,25D is controlled by multiple positive and negative regulators to maintain calcium homeostasis, through 1,25D, PTH, and FGF23 production.
Cyp24A1 and Cyp27B1 have been shown to be expressed in cells of the immune system. Human macrophage, dendritic cells, and T cells express Cyp27B1 and produce 1,25D [14, 15]. The signals that regulate immune production of Cyp27B1 are different than the signals regulating endocrine production of 1,25D in the kidney. In vitro, monocytes and macrophages required stimulation by toll like receptor (TLR) ligands like lipopolysaccharide (LPS) or cytokines like IFN-γ and IL-15 to induce Cyp27B1 [16–18]. Activation of T cells was also required to induce Cyp27B1 expression in human and mouse T cells in vitro [19, 20]. There is less data demonstrating immune production of Cy24A1, however there is evidence that Cyp24A1 may be expressed in monocytes, macrophage, and DC [21–23]. Immune cells may be a local source of 1,25D and production of 1,25D by the immune system is regulated by different signals than endocrine 1,25D production.
The signals that induce immune production of 1,25D are present in the gut. LPS is a microbial antigen, and manipulation of the gut microbiota alters expression of the microbial receptors or TLRs that respond to the microbiota [24, 25]. In addition, mice that lack the ability to make 1,25D (Cyp27B1 KO) or signal through the VDR (VDR KO) have altered microbial populations in the gut compared to normal mice [26]. Dysbiosis in the Cyp27B1 KO and VDR KO mice results in more inflammation in the gut including activated macrophage and T cells [27]. The microbial communities and immune activation in the gut are regulated by the VDR and vitamin D, which in term regulate immune production of 1,25D.
Antibiotics (Abx) alter the community structure of the microbiota in the gut, especially when delivered orally. Experiments were done to determine the effect of Abx disruption of the microbiota on serum vitamin D metabolites. Vitamin D, 25D, and 24,25D were measured before and after Abx treatment. 1,25D levels were below the level of detection in the serum. Abx increased 25D levels, and this was dependent on TLR signaling. 24,25D levels increased following Abx, and were regulated independently from the 25D levels. 24,25D levels reflected Abx-induced changes to FGF23 and required VDR signals since there was no effect of Abx on 24,25D or FGF23 in VDR KO mice. The data show that the Abx mediated effect on 25D and the Abx mediated effect on 24,25D were mediated by distinct mechanisms.
2. MATERIALS AND METHODS
2.1. Mice
C57BL/6 VDR KO, CD45.1, and WT breeders were from Jackson Laboratories (Bar Harbor, ME). C57BL/6 MyD88 KO that have a defect in an adapter protein critical for microbial signaling through TLR were a gift from Dr. Matam Vijay-Kumar (The Pennsylvania State University, University Park, PA). C57BL/6 Cyp27B1 KO were a gift from Dr. Hector DeLuca (University of Wisconsin, Madison, WI). Gnotobiotic mice were bred and maintained by The Pennsylvania State University Gnotobiotic Animal Research Facility. Mice were orally supplemented with 5μg vitamin D3 (+D) in corn oil, or control (Ctl) treated with an equal volume of corn oil, three times weekly throughout the experiment (Fig. 1A). The Abx treatment cocktail contained 4 Abx: ampicillin (1g/L), metronidazole (1g/L), neomycin (1g/L), and vancomycin (0.5g/L) in drinking water, ad libitum, for 2wks (Fig. 1A). Mice were fed purified diets without any added vitamin D (D-) that were made in the lab, contained agar and prevented dehydration due to the Abx treatment [26]. GF mice were fed autoclavable diets in order to maintain the GF status of the mice. GF mice received the Abx cocktail with 5% sucrose to prevent dehydration. All experimental procedures were approved by the Office of Research Protection’s Institutional Animal Care and Use Committee (Pennsylvania State University).
Figure 1. Abx treatments induced vitamin D metabolism.
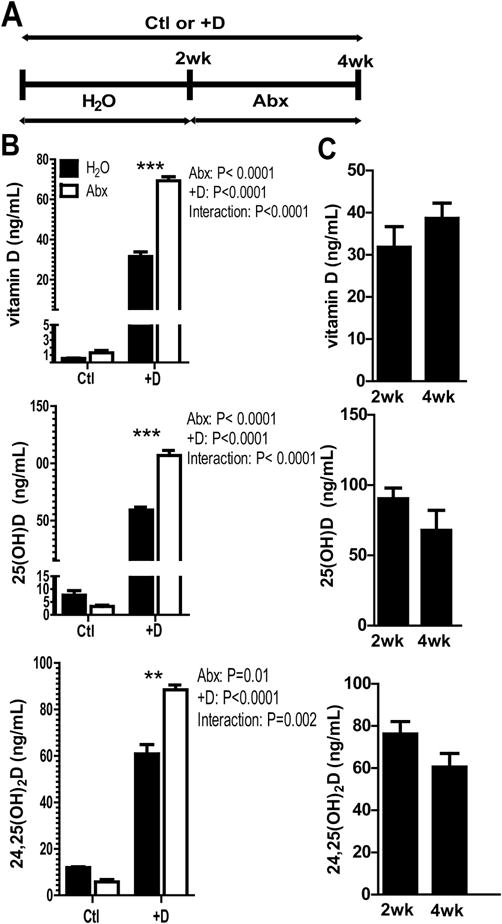
A) Experimental design for Abx treatment and Ctl or +D supplementation. B) Vitamin D, 25D, and 24,25D levels from serum of control (Ctl) and vitamin D supplemented (+D) mice, before and after 2 wks of Abx. The results are from one representative of 3 independent experiments. Values are the mean ± SEM of n=4 mice per group. 2-way ANOVA with Bonferroni post-hoc test was used to test significance. C) Water treated D+ mice after 2 and 4 wks of vitamin D supplementation. Values are the mean ± SEM of n=5 mice per group, mixed sex. Paired t-test was used to test significance. *P<0.05, **P<0.01, ***P<0.001
2.2. Sample preparation for LC-MS/MS
Serum preparation was done as described by Kaufmann et al. [28]. 100μL of pooled 13C3-vitamin D3 (100 ng/mL), d3-25D3 (100 ng/mL), and d6-24,25D3 (50ng/mL) (Isosciences, King of Prussia, PA) was added to 50 μL of each serum sample. Standards were also from Isociences (synthetic vitamin D3, 25D3 and 24,25D3). 50 μL of 0.1M hydrocholoric acid, 50 μL 0.2M zinc sulfate, and 225 μL of 100% methanol were added to precipitate protein. Organic extraction was done by adding 350 μL hexanes, 350 μL MTBE (methyl tertiary butyl ether, Acros Organics, Geel, Belgium) and collecting the upper organic phase. Derivatization was done by dissolving the dried residue in 30 μL DMEQ-TAD (0.1 mg/mL in ethyl acetate, Santa Cruz Biotechnology, Santa Cruz, CA), incubating for 1 h, drying, and the residue was dissolved in 30 μL 50/50 acetonitrile/water. All other LC-MS/MS solvents and reagents were Optima LC-MS grade (Fisher Scientific, Pittsburgh, PA). The limit of detection was 1 ng/mL for vitamin D, 25D, and 24,25D. Concentrations of each vitamin D metabolite were determined by calculating the ratio of the integrated peak areas of the metabolite and the relevant internal standard, compared to the values obtained from a standard curve.
2.3. LC-MS/MS
Samples (5μl) were separated by reverse phase HPLC using a Prominence 20 UFLCXR system (Shimadzu, Columbia MD) with a Waters (Milford, MA) BEH Phenyl column (100mm × 2.1mm 1.7 μm particle size). Solvents used were HPLC grade water with 0.1% formic acid and HPLC grade acetonitrile with 0.1% formic acid. The initial conditions were 70% water and 30% acetonitrile, increasing to 50% acetonitrile at 10 min, 90% acetonitrile at 12 min where it was held at 90% acetonitrile until 13 min before returning to the initial conditions. The eluate was delivered into a 5600 (QTOF) TripleTOF using a Duospray™ ion source (AB Sciex, Framingham, MA). The capillary voltage was set at 5.5 kV in positive ion mode with a declustering potential of 80V. The mass spectrometer was operated with a 250 ms TOF scan from 50 to 950 m/z, and 7 100 ms MS/MS product ion scans (m/z 730.5, 733.5, 746.5, 749.5, 762.5, 765.5, 768.5) from 50 to 950 per duty cycle using a collision energy of 45V with a 30V spread. Chromatograms and ion spectra, and structure and fragmentation of DMEQ-TAD adducts of vitamin D, 25D, and 24,25D used for detection, are shown in Supplementary (S)Figure 1.
2.4. Bone marrow (BM) transplantation
Donor BM cells were from WT CD45.1 donors and were transferred into sub-lethally irradiated CD45.2 WT or VDR KO recipients as described [29]. Mice were allowed to recover for 4 wks and reconstitution was evaluated in the blood by flow cytometry using antibodies to CD45.1 (A20, BD Pharmingen, San Diego CA) and CD45.2 (104, BioLegend, San Diego, CA), and analyzed on an Accuri C6 flow cytometer (BD Bioscience, San Jose, CA).
2.5. Serum calcium measurements
Serum calcium levels were measured by colorimetric assay using QuantiChrom Calcium Assay Kit (BioAssay Systems, Hayward, CA), according to manufacturer’s instructions.
2.6. ELISA
Parathyroid hormone (PTH) (1-84) and intact FGF23 levels in serum were measured by ELISA, according to manufacturer’s instructions (Immutopics, San Clemente, CA). Limits of detection were 32 pg/mL PTH, and 25 pg/mL iFGF23.
2.7. RNA isolation and RT-PCR
RNA from kidney, liver, and colon was extracted using TRIzol reagent using manufacturer’s instructions. 4 μg/sample RNA was reverse transcribed into cDNA using AMV reverse transcriptase (Promega, Madison, WI). Mouse hprt, cyprR1, cyp27a1, cyp24a1, cyp27b1, vdr, tnf-α, il-1β, il-6, and ifn-γ mRNA were quantified by real-time PCR using the StepOnePlus real-time PCR system (Thermo Fisher Scientific, Rockford IL) with StepOnePlus software and BioRad SYBR Green Master Mix (Hercules, CA). Gene expression was determined as relative expression on a linear curve based on a gel-extracted standard and was normalized to hprt amplified from the same cDNA mix. Primer sequences can be found in STable 1.
2.8. Colon histology
Distal colon was fixed in 10% formalin, sectioned, and stained with hematoxylin and eosin (Pennsylvania State University Animal Diagnostic Laboratory). Sections were scored blinded by a board-certified laboratory animal veterinarian with training in pathology (Dr. Mary Kennett) and scored on a scale from 0 none, 1 minimal, 2 mild, 3 moderate, and 4 extreme for inflammation, mucosal damage, and edema. The maximum cumulative histopathology score for each sample would be 15.
2.9. Statistical analysis
All data were assessed for normality and equal variances, and either parametric or nonparametric analyses were used to detect differences between treatment groups. Statistical analyses were performed using GraphPad Prism software (GraphPad, La Jolla, CA). Two-way ANOVA with Bonferroni’s post hoc test was used to compare levels of vitamin D metabolites, PTH, and FGF23 in mice when two experimental factors, such as genotype and Abx, were of interest. One-way ANOVA with Tukey’s multiple comparison was used when one experimental factor, such as changes over multiple time points, were of interest. For the time course data that used n=3 mice per time-point, Kruskal-Wallis test with Dunn’s multiple comparisons was used. Two-tailed Student t test or paired t test was used for some analyses for vitamin D metabolites, FGF23, gene expression, and calcium measurements where only two groups were compared. Paired t test was used when comparing the same cohort of mice before and after treatment, and Student’s t test was used to compare different mice. For all analyses, * indicates P<0.05, ** indicates P<0.01, *** indicates P<0.0001.
3. RESULTS
3.1 Abx treatments induced vitamin D metabolism
Serum samples were collected before (2 wks) and after (4wks) Abx treatment in the Ctl or +D treated mice (Fig. 1A). Vitamin D levels in the blood of Ctl mice were very low, and did not change significantly after Abx (Fig. 1B). +D mice had significantly higher serum vitamin D than Ctl mice and vitamin D went up in the +D mice after Abx (P<0.0001, Fig. 1B). 25D levels in the Ctl group were less than 12 ng/mL and therefore vitamin D deficient. 25D was significantly higher in +D mice compared to Ctl mice (P<0.0001), and increased significantly in +D mice after Abx (P<0.0001, Fig. 1B). +D mice had significantly higher 24,25D than Ctl mice (P<0.0001), which also increased significantly after Abx (P<0.01, Fig. 1B). There were significant interaction effects between +D and Abx treatments on the vitamin D, 25D and 24,25D levels (Fig. 1). In the absence of Abx treatment, +D supplementation of WT mice for 4wks did not further raise vitamin D, 25D or 24,25D levels over the 2wk +D levels ruling out simple accumulation of vitamin D and vitamin D metabolites in the +D groups (Fig. 1C). +D mice were sacrificed before and after Abx treatments for mRNA analysis of vdr, cyp2r1, cyp27a1, cyp24a1, and cyp27b1. Expression of mRNA for the vdr was not affected by Abx treatment in the kidney (SFig. 2A) or colon (SFig. 2B). There was no effect of Abx treatments on cyp2r1, and cyp27a1 expression in the liver (SFig. 2C), and no effect of Abx on cyp24a1 in the kidney and cyp27b1 in either the kidney or colon (SFig. 2A and 2B). Abx treatment significantly induced cyp24a1 in the colon (SFig. 2B). Abx treatments increased 25D and 24,25D levels in the serum that was not reflected in changes for cyp2r1, cyp27a1, or cyp27b1. However, increased 24,25D levels did correspond with increased expression of cyp24a1 in the colon but not kidney following Abx treatment.
The data in Fig. 1 used mice of either sex. In order to determine if there was a sex effect on the vitamin D measurements, +D males and females were evaluated separately (Fig. 2). Females had higher levels of vitamin D (P=0.0008) and 25D (P=0.0004) compared to males. There was also a significant interaction between sex and Abx treatment for vitamin D (P=0.01) and 25D (P=0.002, Fig. 2A). Sex was not a factor on 24,25D levels (P=0.3) and there was no interaction between sex and Abx treatment for this metabolite (P=0.1, Fig. 2C). Abx treatment of +D mice resulted in increased vitamin D, 25D and 24,25D levels. In addition, there was an effect of sex on vitamin D and 25D levels and females had higher vitamin D and 25D levels than males.
Figure 2. Sex effects of +D supplementation and Abx.
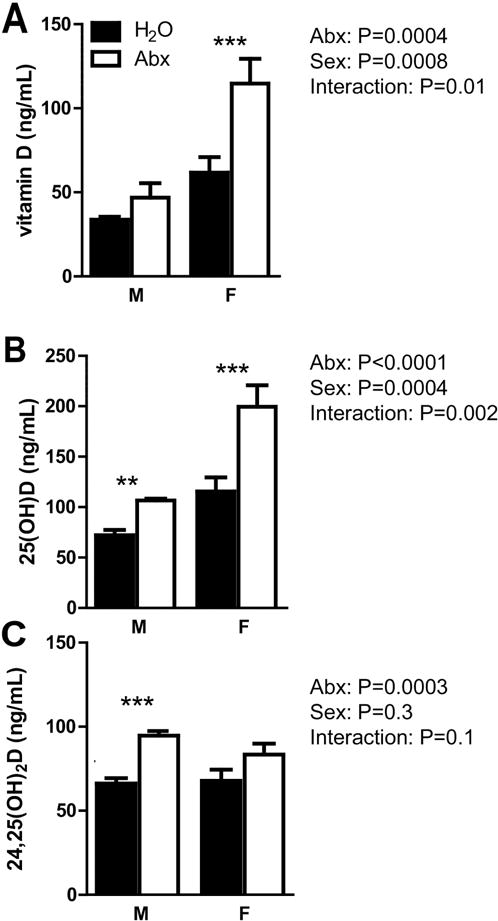
A) Vitamin D, B) 25D, and C) 24,25D levels from serum of +D male and female mice, before and after Abx treatment. Values are the mean ± SEM of a total of n= 8 males or n=5 females, and data from 3 independent experiments. 2-way ANOVA with Bonferroni post-hoc test was used to test significance. *P<0.05, **P<0.01, ***P<0.001
3.2 The effects of single Abx and direct effects of Abx treatment of GF mice on vitamin D metabolites
The effect of metronidazole (M) and vancomycin (V) alone, two of the four individual cocktail of Abx, were tested. Mice were all +D since the effects of Abx was only seen following +D supplementation (Fig. 1). Vitamin D levels were significantly increased following treatment with either M or V alone suggesting an increase in vitamin D absorption with the single Abx treatments (Fig. 3A and 3B). 25D levels were increased following M treatment (P=0.03, Fig. 3A) and following V treatment (P=0.004, Fig. 3B). Neither the M nor V treatment alone had an effect on serum 24,25D levels (Fig. 3A and 3B). The increased production of 24,25D with broad-spectrum Abx was not due to either M or V treatment alone. Interestingly even though vitamin D and 25D were increased in both M and V treated mice there was no subsequent increase in 24,25D. The data suggest that the increased substrate (25D) for Cyp24A1 following Abx treatment may not be the cause of the spike in 24,25D levels.
Figure 3. The effects of single Abx and direct effects of Abx treatment of GF mice on vitamin D metabolites.
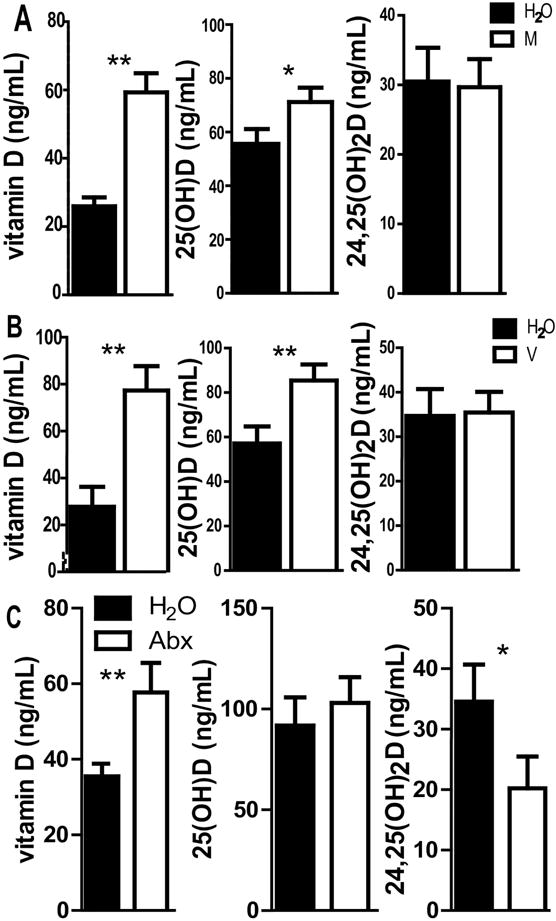
Vitamin D, 25D, 24,25D levels from serum of +D mice, before and after 2 wks of A) metronidazole (M) or B) vancomycin (V) or C) +D WT GF mice before and after Abx treatment. Values are the mean ± SEM of n=8 mice per group, 4 males and 4 females (A and B) or (C) n=12 mice per group, n=9 males and n=3 females. C is the pooled data from two independent experiments. Paired t-test was used to test significance. *P<0.05, **P<0.01, ***P<0.001
Abx treatments have been shown to have direct effects on host metabolism [30]. Vitamin D levels increased significantly in GF mice after Abx treatments (P=0.007, Fig. 3C). Unlike the effect of Abx in conventional WT mice (Fig. 1), there was no effect of Abx treatment on 25D levels in GF mice (P=0.2, Fig. 3C). Abx treatment in GF mice resulted in a significant decrease in 24,25D levels (P=0.02, Fig. 3C). The effect of Abx on 24,25D levels in conventional mice (Fig. 1) was the opposite of the effect of Abx in GF mice (Fig. 3C). Even though the amount of vitamin D was higher in the Abx treated +D GF mice there was no effect of Abx on 25D levels. Overall the direct effects of Abx on the host are to increase vitamin D levels and to decrease 24,25D levels.
3.3 The VDR is necessary for Abx-induced production of 24,25D
Endocrine production of 24,25D is regulated by the VDR [31, 32]. +D WT and VDR KO mice were treated as described in Fig. 1A. The serum vitamin D levels did not change with Abx treatment in +D WT mice in this experiment (Fig. 4A). Overall the effect of Abx on vitamin D levels in the +D WT mice was variable, and vitamin D levels increased significantly in half of our experiments (Fig. 1 and 2) but not in the WT controls for this experiment (Fig. 4A). +D VDR KO mice in two different experiments had increased vitamin D levels following Abx treatment (P=0.0002, Fig. 4A). There were significant interactions between genotype and Abx on serum vitamin D levels (P=0.04, Fig. 4A). There was a significant increase in serum 25D levels in +D WT mice treated with Abx even though the serum vitamin D levels were not higher in these mice following Abx treatment (Fig. 4B), suggesting 25D levels are not simply increasing because of increased vitamin D absorption. The effect of Abx treatment to increase 25D levels occurred in +D VDR KO mice (Fig. 4B). There was no effect of genotype on 25D levels and no interaction between genotype and Abx on 25D levels (Fig. 4B). As in Fig. 1D, there was a significant effect of Abx on serum 24,25D levels in +D WT (P=0.0009, Fig. 4C) mice. Very little 24,25D was detectable in VDR KO mice regardless of whether they received Abx (Fig. 4C). Genotype was a significant factor in determining the 24,25D levels (P<0.0001) and there was a significant interaction between genotype and Abx on serum 24,25D levels (P<0.0001, Fig. 4C). Abx treatment had no effect on 24,25D in VDR KO mice suggesting that the effect of Abx on 24,25D levels required the VDR. The VDR is required for the production of 24,25D (Fig. 4C). Rag KO mice that lack an acquired T and B cell mediated immune system were treated with Abx. Abx treatment effectively induced 24,25D production in both the +D Rag KO and +D WT mice (Fig. 4D). In order to determine whether immune cells were induced by Abx treatment to produce the 1,25D and/or 24,25D, BM chimeras were done into VDR KO mice. The immune cells in the blood of the BM chimeras were 90% of donor origin (WT, CD45.1) in both the WT and VDR KO recipients. There was a significant effect of Abx on serum 24,25D levels in WT-WT (P=0.02, Fig. 4E). 24,25D production in WT-VDR KO was very low and was not affected by Abx treatment, similar to VDR KO (Fig. 4C). Recipient genotype was a significant factor in determining the 24,25D levels (P<0.0001) and there was a significant interaction between recipient genotype and Abx on serum 24,25D levels (P<0.04, Fig. 4E). 24,25D were low in WT-VDR KO mice, indicating that the transplanted WT BM was not able to induce Cyp24A1 and not responsible for the Abx induced production of 24,25D.
Figure 4. The VDR is necessary for Abx-induced production of 24,25D.
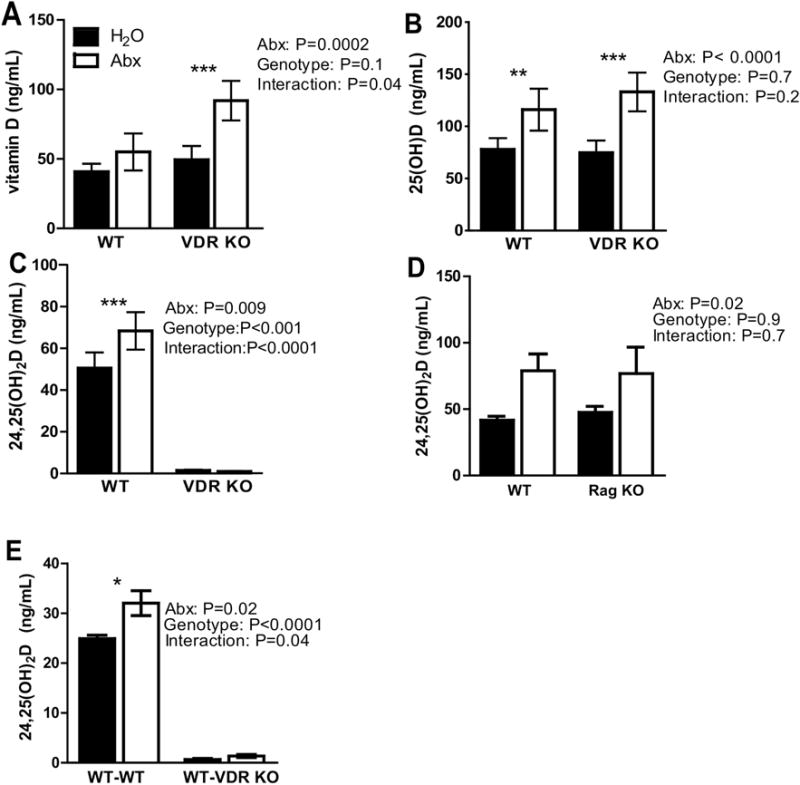
A) Vitamin D, B) 25D and C) 24,25D levels from serum of WT and VDR KO mice. Values are the mean ± SEM of n=11–14 mice per group, 15 males and 10 females, and 2 pooled independent experiments. D) 24,25D levels from serum of WT and Rag KO. Values are the mean ± SEM of n=5 mice per group, 4 males and 6 females. E) 24,25D levels from serum of WT/WT and WT/VDR KO. Values are the mean ± SEM of n=4 recipient male mice per group. 2-way ANOVA with Bonferroni post-hoc test was used to test significance. *P<0.05, **P<0.01, ***P<0.001
3.4 WT levels of 24,25D in Cyp27B1 KO mice following Abx treatment
Cyp27B1 KO mice cannot produce the high affinity 1,25D ligand. However, when Cyp27B1 KO mice are fed +D diets, 25D accumulates in the serum and in the absence of 1,25D binds the VDR [3, 33]. The effect of Abx treatment on 25D levels in WT mice was, as expected, a significant increase (Fig. 5A). The +D treatment of Cyp27B1 KO mice resulted in extremely high levels of 25D in the mice demonstrating accumulation of this metabolite in the Cyp27B1 KO mice similar to what has been reported previously [3, 33]. Abx treatment suppressed the production of 25D in the Cyp27B1 KO mice significantly (Fig. 5A) and resulted in 25D levels similar to those in the WT mice after Abx treatment. The 24,25D levels were higher in WT mice following Abx but unlike all other experiments in WT mice there was not a significant effect of Abx on WT levels of 24,25D in these experiments (Fig. 5A). The levels of 24,25D in the +D Cyp27B1 KO mice matched those in the WT mice and there was no effect of Abx on 24,25D levels in Cyp27B1 KO mice (Fig. 5A). The effect of Abx on 25D in Cyp27B1 KO mice was opposite the effect of Abx in WT mice perhaps because of the extremely high levels of 25D present in the Cyp27B1 KO mice (Fig. 5A).
Figure 5. WT levels of 24,25D in Cyp27B1 KO mice following Abx treatment.
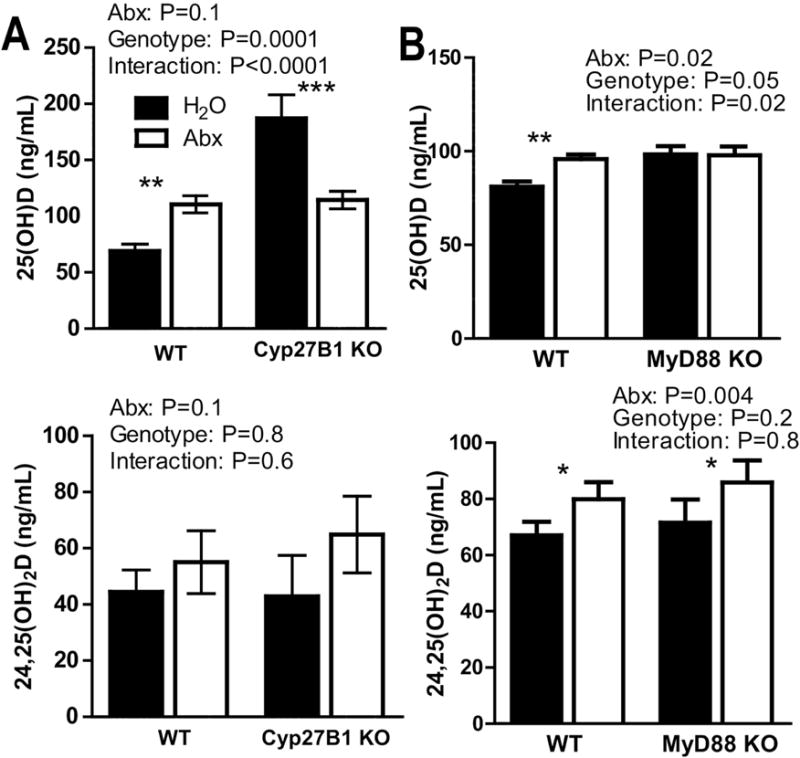
A) 25D and 24,25D levels from Cyp27B1 KO and WT mice. Values are the mean ± SEM of n=12–14 mice per group, 16 males and 10 females combined from 3 independent experiments. B) 25D and 24,25D levels from +D WT and MyD88 KO mice. Values are the mean ± SEM of n=5–6 mice per group, 5 males and 6 females. 2-way ANOVA with Bonferroni post-hoc test was used to test significance. *P<0.05, **P<0.01, ***P<0.001
3.5 Microbial regulation of 25D but not 24,25D following Abx treatment
MyD88 is an adaptor protein necessary for signaling in most TLRs [34, 35]. Treatment of +D MyD88 KO with Abx had no effect on 25D levels (Fig. 5B). There was a significant effect of MyD88 genotype (P=0.05) on 25D levels and a significant interaction between genotype and Abx on 25D levels (P=0.02, Fig. 5B). Abx treatment of WT and MyD88 KO mice resulted in a significant increase in 24,25D levels for a significant effect of Abx on 24,25D levels (P=0.004, Fig. 5B). There was no effect of MyD88 KO genotype on 24,25D levels and no interaction between Abx and genotype on 24,25D levels in the mice (Fig. 5B). The data demonstrate that the increase in 24,25D following Abx treatment does not require MyD88 signals. Conversely the increase in 25D levels following Abx did require MyD88.
3.6 Abx treatments affect PTH, FGF23 and TNF-α but not serum calcium
There was no change in serum Ca in WT mice as a result of Abx treatments (Fig. 6A). Abx treatment resulted in an insignificant (P=0.08) decrease in PTH and a significant increase in FGF23 in WT mice (Fig. 6B). PTH levels in the Cyp27B1 KO and VDR KO mice were significantly elevated compared to WT PTH levels (Fig. 6B). The effect of Abx treatment on PTH in Cyp27B1 KO mice was to decrease PTH and that corresponded with the reduction in 25D levels in the Cyp27B1 KO mice (Fig. 6B, Table 1). VDR KO mice increased PTH with Abx treatment (Fig. 6B). Abx treatments of Cyp27B1 KO mice induced FGF23 significantly, while FGF23 was low in VDR KO mice and did not change with Abx treatment (Fig. 6B). VDR signaling was required for the changes in FGF23 and 24,25D following Abx treatment but not for the increase in 25D (Table 1). mRNA for tnf-α, il-1β and il-6 was measured in the colon before and after 2wk Abx treatment (Fig. 6C). Expression of tnf-α (significant) and IL-1β (trend, P=0.07) decreased, while il-6 had a trend towards an increase (P=0.06) with Abx treatment (Fig. 6C). There was no change in ifn-γ expression with Abx treatment (Fig. 6C). There was less tnf-α in the colon and that decrease was associated with the increases in 25D and 24,25D that occurred following 2wk Abx treatments. The mechanism underlying the increase in 24,25D levels following Abx treatment is different than the mechanism underlying the increase in 25D following Abx treatment.
Figure 6. Abx treatments affect PTH, FGF23, and TNF-α but not serum calcium.
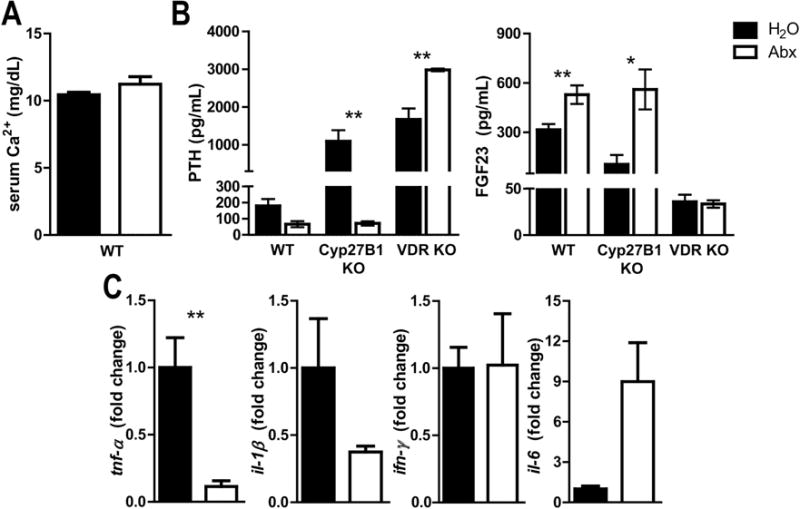
A) Ca levels in WT mice after Abx treatment B) PTH and FGF23 levels from WT, Cyp27B1 KO and VDR KO mice. Values are the mean ± SEM of n=5–10 mice per group and data from 2 independent experiments. C) Expression of tnf-α, il-1β, il-6, and ifn-γ in the colon, n=5–8 per group, and data from 2 independent experiments. Student’s t-test was used to test significance. *P<0.05, **P<0.01, ***P<0.001
Table 1.
The effects of Abx on 24,25D and 25D occur via different pathways.
| Genotype | Metabolite/Hormone | Effect of Abx |
|---|---|---|
| WT | Vitamin D | variable |
| 25D | ↑ | |
| 24,25D | ↑ | |
| PTH | − | |
| FGF23 | ↑ | |
| Cyp27B1 KO | Vitamin D | − |
| 25D | ↓ | |
| 24,25D | − | |
| PTH | ↓ | |
| FGF23 | ↑ | |
| VDR KO | Vitamin D | ↑ |
| 25D | ↑ | |
| 24,25D | − | |
| PTH | ↑ | |
| FGF23 | − |
Change in vitamin D; 25D; 24,25D; FGF23, and PTH in response to Abx. ↑ indicates increased, ↓ indicates decreased, − indicates no significant change from water.
3.7 Abx mediated effects on 25D and 24,25D occur rapidly
The kinetics of the changes in serum 25D and 24,25D were determined following Abx treatments. The increase in 25D, and 24,25D following Abx treatment occurred within 3d of the Abx treatment (Fig. 7A). FGF23 levels initially were significantly lower after 3d of Abx treatment (Fig. 7A) and then went up to the final levels observed after the full 2 wk Abx treatment (Fig. 7A). Evaluation of histopathology from the murine colon showed no significant changes over time post-antibiotic use in the inflammation scores (P=0.5) or the cumulative scores (P=0.2, Fig. 7B). The effects of Abx on 25D and 24,25D occurred within 3d of Abx treatment. The effect of Abx on FGF23 was to first decrease the levels at 3d post-Abx treatment and then to go up by 14d (2wk) Abx treatment.
Figure 7. Abx mediated effects on 25D and 24,25D occur rapidly.
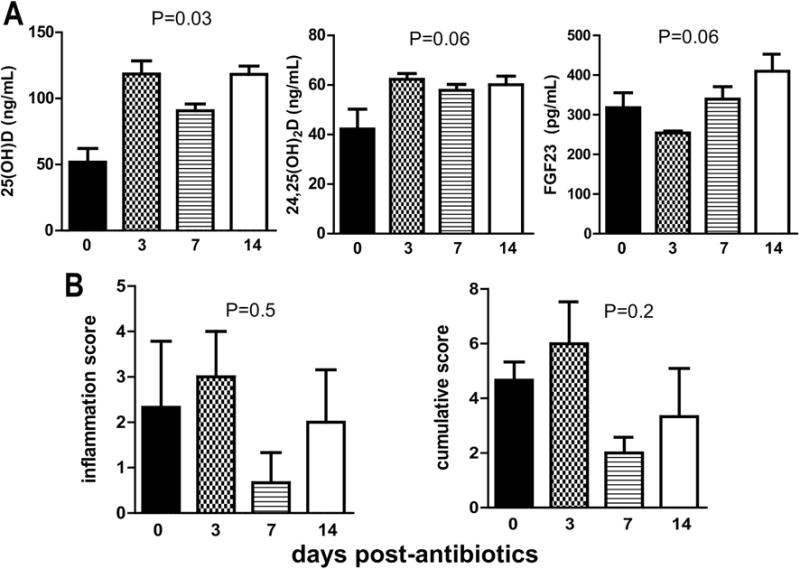
A) 25D, 24,25D and FGF23 levels from +D mice after d0, d3, d7, and d14 of Abx treatment. B) Inflammation and cumulative histology scores of distal colons from mice d0, d3, d7, and d14 after Abx treatment. Values are the mean ± SEM of n=3 females per time point. Kruskal-Wallis test with Dunn’s multiple comparisons test was used to test significance.
4. DISCUSSION
MyD88 is required for the effects of Abx on 25D levels. The MyD88 mediated effect could be due to alterations in inflammation as described by Du and colleagues [36]. Du et. al showed Cyp27B1 induction in the colon of mice with colitis [36]. There was no effect of the Abx treatment on Cyp27B1 in the absence of colitis suggesting that inflammation was regulating Cyp27B1 [36]. Others have shown that toll like receptor signals regulate Cyp27B1 and Cyp24A1 but there is no evidence to support control of the 25-hydroxylase enzymes by toll like receptor signals or inflammation [17]. The requirement of MyD88 signaling for the Abx mediated effect on 25D levels suggest the host sensing of microbial signals through toll like receptors regulates 25D levels.
The production of 25D was thought to be unregulated and controlled by vitamin D intake [37]. The effect of Abx on 25D levels was not due to increased vitamin D absorption following Abx treatment, since there were several experiments where 25D levels increased even when vitamin D levels were unaffected. Cyp2R1 is the major producer of 25D in vivo [38]. Mice with targeted deletions and humans with mutations in Cyp2R1 had reduced 25D levels but circulating 1,25D levels were not affected [1, 39]. A second 25 hydroxylase (Cyp27A1) is primarily involved in bile acid synthesis but has also been shown to contribute to 25D levels in vivo [38]. Together Cyp2R1 and Cyp27A1 account for most of the 25D produced in vitro and in vivo [38]. The Abx treatment did not increase mRNA for either Cyp2R1 or Cyp27A1 in the liver (SFig. 2C). Three wk treatment with a different class of Abx (rifampicin) induced 25D and 24,25D levels in mice, which was attributed to the direct host effects of rifampicin on cytochrome P450 (Cyp) enzymes. VDR KO mice showed an increase in 25D levels following Abx treatment (Table 1). PTH was also increased following Abx treatment of VDR KO mice (Table 1). It seems likely that the changes in 25D and PTH are regulated by a common factor. In support of this observation, a human study showed positive correlations between 25D and PTH levels [40]. Direct regulation of PTH by neomycin has been shown in vitro, and antibiotics like neomycin that affect extracellular calcium levels might regulate PTH in vivo. The data suggest that there is some regulation of 25D levels following disruption of the microbiota with Abx that seems to coincide with a shift in PTH.
The Abx mediated increase in 24,25D was not mediated by TLR signaling but was likely due to alterations in endocrine regulation of 1,25D. Functional VDR signaling in the endocrine system was required for the Abx-induced increases in 24,25D. The increased 24,25D is the result of endocrine metabolism of vitamin D, as transfer of WT bone marrow into VDR KO recipients did not have increased 24,25D levels following Abx treatment. 24,25D levels and Cyp24A1 are directly induced by 1,25D/VDR, and regulated by PTH and FGF23 [41]. FGF23 induces Cyp24A1, possibly by inhibiting PTH, while PTH inhibits Cyp24A1 to increase 1,25D [11, 42, 43]. Increased 24,25D was not due to more of the 25D substrate, as there was no direct relationship between 25D and 24,25D levels in multiple experiments. 24,25D only increased in mice that did not have defects in 1,25D or VDR signals (Table 1). In the Cyp27B1 KO mice, Abx treatment reduced 25D levels but there was no effect on 24,25D. This could be due to increased FGF23 and decreased PTH, which increased Cyp24A1 to catabolize the excessively high 25D present in the +D Cyp27B1 KO mice. Abx treatment of Cyp27B1 KO mice restored PTH and FGF23 to WT levels, which provides evidence that endocrine regulation of vitamin D metabolism is regulated by the microbiota. Interestingly the effect of Abx treatment of GF mice was to decrease 24,25D levels and so perhaps the microbiota prevents the direct effects of Abx to decrease host 24,25D or 1,25D levels. The effects of Abx on FGF23 and 24,25D required the VDR and in VDR KO mice FGF23 and 24,25D levels were unaffected by Abx treatments suggesting that endocrine control of 24,25D and FGF23 levels is occurring following Abx treatment to disrupt the microbiota.
The microbiome is important for digestion and nutrient absorption, and it is possible Abx treatments improved absorption of vitamin D. The increase in vitamin D levels following Abx treatment did not occur in all experiments. However, vitamin D levels did increase in GF mice after Abx treatment, suggesting that there was a direct effect of Abx to increase vitamin D levels. The best evidence that the increase in vitamin D was not the cause of the increase in 25D levels was that 25D levels in GF mice treated with Abx did not increase even though vitamin D levels were significantly higher. The Abx treatment effect on vitamin D levels does not require the microbiota and suggest direct regulation of signals in the gut to increase absorption of the vitamin D in GF mice.
In chronic kidney disease vitamin D metabolism is dysregulated. Patients with chronic kidney disease have poor renal function, elevated FGF23 and an impaired ability to produce 1,25D that leads to hyperparathryoidism [44]. The use of Abx that increase FGF23 could exacerbate the imbalance in 1,25D and FGF23, leading to worsening kidney function. Conversely, increased 25D as a result of Abx in diseases in which vitamin D absorption is poor, such as inflammatory bowel disease, may be useful in raising vitamin D status. It is uncommon for people to take four Abx at once, but the data shows M or V alone increased vitamin D and 25D, which is an unexpected benefit of these two Abx treatments. These results demonstrate that the Abx disruption of the gut microbiota results in a shift in the homeostatic control of endocrine vitamin D metabolism. Furthermore, microbial signals altered following Abx treatments regulate the amount of 25D levels available to the host.
Supplementary Material
Acknowledgments
This work was supported by the United States Department of Agriculture (2914-38420-21822) and the National Institutes of Health (RO1AT005378S-1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: none to declare.
References
- 1.Zhu JG, Ochalek JT, Kaufmann M, Jones G, Deluca HF. CYP2R1 is a major, but not exclusive, contributor to 25-hydroxyvitamin D production in vivo. Proc Natl Acad Sci U S A. 2013;110:15650–5. doi: 10.1073/pnas.1315006110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88:582S–6S. doi: 10.1093/ajcn/88.2.582S. [DOI] [PubMed] [Google Scholar]
- 3.Rowling MJ, Gliniak C, Welsh J, Fleet JC. High dietary vitamin D prevents hypocalcemia and osteomalacia in CYP27B1 knockout mice. J Nutr. 2007;137:2608–15. doi: 10.1093/jn/137.12.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol Rev. 2016;96:365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenza HL, Kimmel-Jehan C, Jehan F, Shinki T, Wakino S, Anazawa H, et al. Parathyroid hormone activation of the 25-hydroxyvitamin D3-1alpha-hydroxylase gene promoter. Proc Natl Acad Sci U S A. 1998;95:1387–91. doi: 10.1073/pnas.95.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113:561–8. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chanakul A, Zhang MY, Louw A, Armbrecht HJ, Miller WL, Portale AA, et al. FGF-23 regulates CYP27B1 transcription in the kidney and in extra-renal tissues. PLoS One. 2013;8:e72816. doi: 10.1371/journal.pone.0072816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holick MF, Kleiner-Bossaller A, Schnoes HK, Kasten PM, Boyle IT, DeLuca HF. 1,24,25-Trihydroxyvitamin D3. A metabolite of vitamin D3 effective on intestine. J Biol Chem. 1973;248:6691–6. [PubMed] [Google Scholar]
- 9.Jones G, Prosser DE, Kaufmann M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): its important role in the degradation of vitamin D. Arch Biochem Biophys. 2012;523:9–18. doi: 10.1016/j.abb.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006;17:1305–15. doi: 10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- 11.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–35. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 12.Delmez JA, Tindira C, Grooms P, Dusso A, Windus DW, Slatopolsky E. Parathyroid hormone suppression by intravenous 1,25-dihydroxyvitamin D. A role for increased sensitivity to calcium. J Clin Invest. 1989;83:1349–55. doi: 10.1172/JCI114022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murayama A, Takeyama K, Kitanaka S, Kodera Y, Kawaguchi Y, Hosoya T, et al. Positive and negative regulations of the renal 25-hydroxyvitamin D3 1alpha-hydroxylase gene by parathyroid hormone, calcitonin, and 1alpha,25(OH)2D3 in intact animals. Endocrinology. 1999;140:2224–31. doi: 10.1210/endo.140.5.6691. [DOI] [PubMed] [Google Scholar]
- 14.Gyetko MR, Hsu CH, Wilkinson CC, Patel S, Young E. Monocyte 1 alpha-hydroxylase regulation: induction by inflammatory cytokines and suppression by dexamethasone and uremia toxin. J Leukoc Biol. 1993;54:17–22. doi: 10.1002/jlb.54.1.17. [DOI] [PubMed] [Google Scholar]
- 15.Kundu R, Chain BM, Coussens AK, Khoo B, Noursadeghi M. Regulation of CYP27B1 and CYP24A1 hydroxylases limits cell-autonomous activation of vitamin D in dendritic cells. Eur J Immunol. 2014;44:1781–90. doi: 10.1002/eji.201344157. [DOI] [PubMed] [Google Scholar]
- 16.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 17.Stoffels K, Overbergh L, Giulietti A, Verlinden L, Bouillon R, Mathieu C. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J Bone Miner Res. 2006;21:37–47. doi: 10.1359/JBMR.050908. [DOI] [PubMed] [Google Scholar]
- 18.Krutzik SR, Hewison M, Liu PT, Robles JA, Stenger S, Adams JS, et al. IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. J Immunol. 2008;181:7115–20. doi: 10.4049/jimmunol.181.10.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ooi JH, McDaniel KL, Weaver V, Cantorna MT. Murine CD8+ T cells but not macrophages express the vitamin D 1alpha-hydroxylase. J Nutr Biochem. 2014;25:58–65. doi: 10.1016/j.jnutbio.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kongsbak M, von Essen MR, Boding L, Levring TB, Schjerling P, Lauritsen JP, et al. Vitamin D up-regulates the vitamin D receptor by protecting it from proteasomal degradation in human CD4+ T cells. PLoS One. 2014;9:e96695. doi: 10.1371/journal.pone.0096695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dusso AS, Kamimura S, Gallieni M, Zhong M, Negrea L, Shapiro S, et al. gamma-Interferon-induced resistance to 1,25-(OH)2 D3 in human monocytes and macrophages: a mechanism for the hypercalcemia of various granulomatoses. J Clin Endocrinol Metab. 1997;82:2222–32. doi: 10.1210/jcem.82.7.4074. [DOI] [PubMed] [Google Scholar]
- 22.Gottfried E, Rehli M, Hahn J, Holler E, Andreesen R, Kreutz M. Monocyte-derived cells express CYP27A1 and convert vitamin D3 into its active metabolite. Biochem Biophys Res Commun. 2006;349:209–13. doi: 10.1016/j.bbrc.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 23.Han X, Li L, Yang J, King G, Xiao Z, Quarles LD. Counter-regulatory paracrine actions of FGF-23 and 1,25(OH)2 D in macrophages. FEBS Lett. 2016;590:53–67. doi: 10.1002/1873-3468.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 25.Hormann N, Brandao I, Jackel S, Ens N, Lillich M, Walter U, et al. Gut microbial colonization orchestrates TLR2 expression, signaling and epithelial proliferation in the small intestinal mucosa. PLoS One. 2014;9:e113080. doi: 10.1371/journal.pone.0113080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ooi JH, Li Y, Rogers CJ, Cantorna MT. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate-induced colitis. J Nutr. 2013;143:1679–86. doi: 10.3945/jn.113.180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bora S, Cantorna MT. The role of UVR and vitamin D on T cells and inflammatory bowel disease. Photochem Photobiol Sci. 2017;16:347–53. doi: 10.1039/c6pp00266h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaufmann M, Gallagher JC, Peacock M, Schlingmann KP, Konrad M, DeLuca HF, et al. Clinical utility of simultaneous quantitation of 25-hydroxyvitamin D and 24,25-dihydroxyvitamin D by LC-MS/MS involving derivatization with DMEQ-TAD. J Clin Endocrinol Metab. 2014;99:2567–74. doi: 10.1210/jc.2013-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harandi OF, Hedge S, Wu DC, McKeone D, Paulson RF. Murine erythroid short-term radioprotection requires a BMP4-dependent, self-renewing population of stress erythroid progenitors. J Clin Invest. 2010;120:4507–19. doi: 10.1172/JCI41291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Lin YS, Zheng XE, Senn T, Hashizume T, Scian M, et al. An inducible cytochrome P450 3A4-dependent vitamin D catabolic pathway. Mol Pharmacol. 2012;81:498–509. doi: 10.1124/mol.111.076356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zierold C, Darwish HM, DeLuca HF. Identification of a vitamin D-response element in the rat calcidiol (25-hydroxyvitamin D3) 24-hydroxylase gene. Proc Natl Acad Sci U S A. 1994;91:900–2. doi: 10.1073/pnas.91.3.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozono K, Yamagata M, Ohyama Y, Nakajima S. Direct repeat 3-type element lacking the ability to bind to the vitamin D receptor enhances the function of a vitamin D-responsive element. J Steroid Biochem Mol Biol. 1998;66:263–9. doi: 10.1016/s0960-0760(98)00055-7. [DOI] [PubMed] [Google Scholar]
- 33.Deluca HF, Prahl JM, Plum LA. 1,25-Dihydroxyvitamin D is not responsible for toxicity caused by vitamin D or 25-hydroxyvitamin D. Arch Biochem Biophys. 2011;505:226–30. doi: 10.1016/j.abb.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Wang S, Charbonnier LM, Noval Rivas M, Georgiev P, Li N, Gerber G, et al. MyD88 Adaptor-Dependent Microbial Sensing by Regulatory T Cells Promotes Mucosal Tolerance and Enforces Commensalism. Immunity. 2015;43:289–303. doi: 10.1016/j.immuni.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duparc T, Plovier H, Marrachelli VG, Van Hul M, Essaghir A, Stahlman M, et al. Hepatocyte MyD88 affects bile acids, gut microbiota and metabolome contributing to regulate glucose and lipid metabolism. Gut. 2017;66:620–32. doi: 10.1136/gutjnl-2015-310904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du J, Wei X, Ge X, Chen Y, Li YC. Microbiota-Dependent Induction of Colonic Cyp27b1 Is Associated With Colonic Inflammation: Implications of Locally Produced 1,25-Dihydroxyvitamin D3 in Inflammatory Regulation in the Colon. Endocrinology. 2017;158:4064–75. doi: 10.1210/en.2017-00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hollis BW, Wagner CL, Drezner MK, Binkley NC. Circulating vitamin D3 and 25-hydroxyvitamin D in humans: An important tool to define adequate nutritional vitamin D status. J Steroid Biochem Mol Biol. 2007;103:631–4. doi: 10.1016/j.jsbmb.2006.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu J, DeLuca HF. Vitamin D 25-hydroxylase - Four decades of searching, are we there yet? Arch Biochem Biophys. 2012;523:30–6. doi: 10.1016/j.abb.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 39.Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci U S A. 2004;101:7711–5. doi: 10.1073/pnas.0402490101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pekkinen M, Saarnio E, Viljakainen HT, Kokkonen E, Jakobsen J, Cashman K, et al. Vitamin D binding protein genotype is associated with serum 25-hydroxyvitamin D and PTH concentrations, as well as bone health in children and adolescents in Finland. PLoS One. 2014;9:e87292. doi: 10.1371/journal.pone.0087292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohyama Y, Ozono K, Uchida M, Shinki T, Kato S, Suda T, et al. Identification of a vitamin D-responsive element in the 5′-flanking region of the rat 25-hydroxyvitamin D3 24-hydroxylase gene. J Biol Chem. 1994;269:10545–50. [PubMed] [Google Scholar]
- 42.Shimada T, Yamazaki Y, Takahashi M, Hasegawa H, Urakawa I, Oshima T, et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol. 2005;289:F1088–95. doi: 10.1152/ajprenal.00474.2004. [DOI] [PubMed] [Google Scholar]
- 43.Zierold C, Mings JA, DeLuca HF. Regulation of 25-hydroxyvitamin D3-24-hydroxylase mRNA by 1,25-dihydroxyvitamin D3 and parathyroid hormone. J Cell Biochem. 2003;88:234–7. doi: 10.1002/jcb.10341. [DOI] [PubMed] [Google Scholar]
- 44.Al-Badr W, Martin KJ. Vitamin D and kidney disease. Clin J Am Soc Nephrol. 2008;3:1555–60. doi: 10.2215/CJN.01150308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


