Classic clinical and epidemiologic studies have long ago established the presence of an inverse relationship between HDL-C levels and CVD risk, and thus it was assumed that measures that increase HDL-C levels would afford protection against atherosclerosis-based CVD1,2. However, trials of drugs such as cholesterol ester transfer protein (CETP) inhibitors and niacin have failed to provide evidence of cardiovascular benefit in patients on statin therapy, indicating that HDL-C increases from 30% up to 120% are not able to modify risk when LDL is kept very low. Moreover, genetic polymorphisms that associate with increased HDL-C do not predict reduced CVD risk3–5, and isolated low HDL does not predict risk when LDL and triglyceride levels are completely normal6. Therefore, while a causal role for HDL in arterial homeostasis is still widely accepted, current discussions highlight the necessity to identify novel HDL metrics linked to CVD risk and targetable for diagnostic or therapeutic development.
Despite the spectacular collapse of the HDL-C hypothesis and the paucity of alternative strategies in the clinical space, the field as a whole has experienced a groundbreaking shift in the understanding of the basic biology and metabolism of HDL. Advances have been made in the areas of HDL functionality as it relates to particle heterogeneity, biogenesis, and variations in lipid and protein cargo.
The central functions of HDL are believed to be its ability to quell local oxidative and inflammatory events and to accept excess cholesterol from cells via specific efflux mechanisms mediated by trans-membrane transporters such as ABCA1, ABCG1 and SR-B1. The ABCA1 pathway is important in humans, as lipid-laden macrophages–abundant in atherosclerotic plaques–will turn on ABCA1 gene transcription in an effort to maximize sterol losses. Our current understanding of sterol efflux implicates both lipid-poor apoAI and very small HDL particles (HDL3) as main cholesterol acceptors from the ABCA1 transporter 7. Interestingly, the sterol efflux function of HDL were found to correlate with both prevalence and incidence of CVD, independently of HDL-C and apoAI levels8, 9,10. However, these findings were challenged by a case-control study where increased sterol efflux activity was actually associated with increased, not decreased, risk of CVD events11. Moreover, sterol efflux measured from 3H−cholesterol labeled macrophages does not always reflect the functional efficiency of HDL in reverse cholesterol transport in vivo12. In addition, the methodology has not converged yet toward standardization, as the different macrophage lines used (rat J774, murine RAW264, and human THP-1) likely explain the inconsistent results between research groups, since sterol efflux is regulated by multiple cell-specific factors such as activation of transcription factors (i.e., SREBP and LXR), synthesis and secretion of apoE, and expression of other membrane lipid transporters. Even with all these caveats, the sterol efflux method is driving the progress in the field, whereas the anti-inflammatory and antioxidant functions are yet to be tested and validated in clinical studies for CVD risk prediction.
The plasma concentration of HDL particles (HDL-P) and their size distribution can be reliably measured using different approaches to identify subspecies with unique functional and compositional profiles 13. Currently there is no consensus on the relative value of the different methods and on the number, concentration, functional status, and predictive power of the different HDL sub-particles. Further, the classic linear view of HDL evolution from discoidal, lipid-poor nascent particles to spherical, cholesterol- and phospholipid-rich particles packed with a combination of over 100 different proteins has been recently challenged by the finding that HDL is secreted directly from hepatocytes in four distinct sizes, with little interchange between them, and representing all of the plasma HDL sub-particle pools14. Efforts to identify the proteomic, lipidomic, and functional fingerprints of these subspecies are of critical importance and may open paths to novel pharmacologic targets.
However, ongoing attempts to link size and function and to identify the HDL subspecies with the best cardio-protective qualities have yet to provide credible leads. Evidence for a functional specialization of HDL subspecies is not solid, as smaller particles appear to be most efficient at sterol efflux in one study 7 but not in others 12,15.
The elaborate and elegant study by Didichenko et al. in the current issue of the Journal describes for the first time a divergence in specific HDL functions according to HDL particle size16. The study was carried out to understand how infusion of CSL112, a discoidal particle made of human apoAI and phospholipids, which is being developed as treatment for acute coronary syndromes, increases both the plasma concentration of small HDL particles and the sterol efflux capacity of plasma. Using plasma samples of healthy subjects enrolled in a phase I clinical trial of CSL112 infusion, the authors demonstrate the spontaneous fusion of CSL112 with native HDL to generate particles of three sizes (lipid poor, small, and large) and with size-specific functions. Lipid poor and small HDL displayed both high capacity for ABCA1-mediated cholesterol efflux and strong anti-inflammatory action, whereas the larger particles showed stronger antioxidant function. This study provides a novel perspective as it suggests that subspecies of HDL may be involved in different functions, and thus the right mix of multiple HDL particle sizes may be most desirable to maximize cardio-protective benefits. Most importantly, the study offers an approach to generate these particles in vivo. One can envision that a possible next step in the development of HDL therapeutics would aim at enriching a specific HDL pool to correct a specific dysfunction, such as reduced sterol efflux or reduced anti-inflammatory capacity, a rather complex task due to the compositional diversity of HDL particles.
The HDL mass is equally distributed between lipid and protein cargo. The HDL lipidome is composed mainly of phospholipids, cholesteryl esters, triglycerides and free cholesterol, for a total of over 200 individual lipid species in normolipidemic subjects. Some lipid components, such as phospholipids, sphingomyelin, and free cholesterol have already been investigated as modulators of HDL functions such as sterol efflux, vasodilation, and control of oxidation and inflammation17. While phospholipids are linked to SR-B1-mediated sterol efflux, less is known about the lipids that modulate ABCA1-mediated sterol efflux. Likely, the protein cargo also associates with HDL function and its composition is affected by factors such as diet, drug treatment, and inflammatory activation. Experimentally, both a high-fat diet and induction of acute inflammation by subcutaneous injection of silver nitrate lead to enrichment of HDL particles in acute phase proteins such as PON1, SAA1, and SAA2 with subsequent reduction in ABCA1-mediated sterol efflux capacity12,18. HDL isolated from statin-treated patients with cardiovascular disease contain increased levels of apoE19 and fenofibrate treatment enriches the HDL proteome of diabetic subjects in apoC proteins and PON120. Altogether, evidence suggests that the HDL proteome is dynamic and responds to metabolic changes, lipid modulating therapy, and disease development.
To define the intricate relationship between HDL protein and lipid cargo, function, and particle size, the compositional signatures of HDL subparticle pools need to be defined. Studies examining the proteomic and lipid remodeling of HDL subspecies in response to drug treatment, diet, and disease are needed to identify the cardio-protective HDL sub-particles hiding among the vast population of HDL particles with no role or even possibly a negative role in cardiovascular health. Bringing this concept back to the subject of this editorial, identification of the proteomic signatures of the HDL subspecies produced by fusion with CSL112 will provide clues on the complex architecture of the HDL compartment and the structural underpinning of novel metrics such as function, particle concentration, lipidome, and proteome.
Plasma lipids and lipoproteins are complex but highly heritable traits. The traditional metric of plasma HDL cholesterol (HDL-C) levels has a heritability estimate between 40% and 60%. The hereditary basis of novel HDL metrics, such as function, particle distribution, and protein or lipid cargo is unknown. Evidence from five inbred mouse strains suggests that sterol efflux function and proteome are genetically regulated, and that the HDL proteome is inherited and predicts the genetic lineage21. Further efforts are needed to identify the heritability of novel HDL metrics independent of HDL-C in humans. Since HDL-C levels are not strongly associated with measures of HDL function, we predict that the gene loci controlling HDL function will be different from those regulating plasma HDL-C levels. Therefore, efforts to map quantitative trait loci for novel HDL metrics are extremely valuable to the field and likely to bring about the anticipated revolution of our diagnostic and management approach to patient with low HDL, the patient with severe combined dyslipidemia, the patient with unexplained family history of CVD, and the patient with early and progressive coronary ischemia.
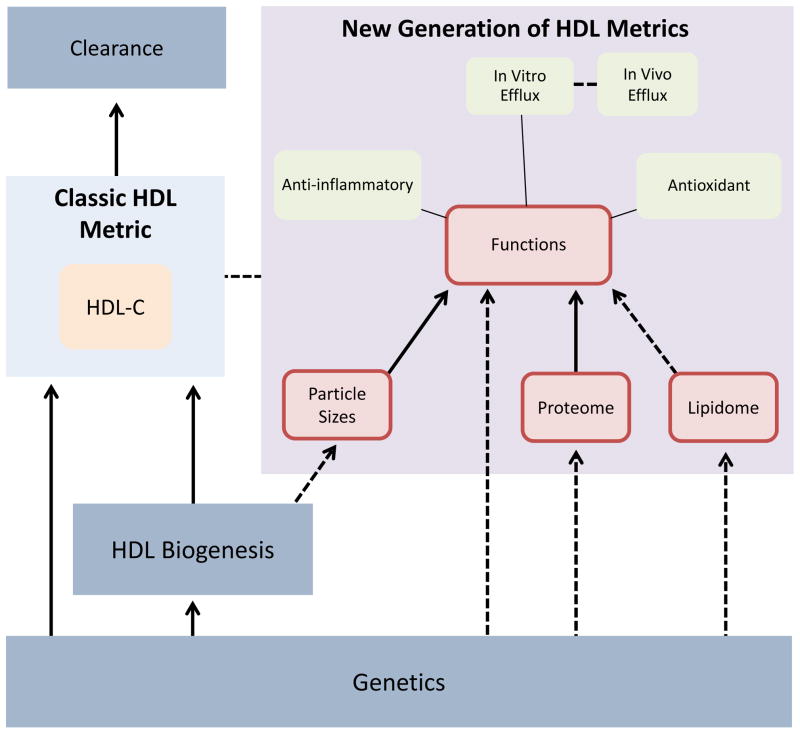
The intriguing complexity and clarity of the results obtained with the infusion of CSL112, a tiny recombinant HDL, opens the path to a better understanding of the structure-function correlates of HDL sub-particles, and encourages the further use of new generation HDL metrics as fertile ground to identify additional diagnostic and therapeutic checkpoints in the HDL pathway. The Figure summarizes the known and suspected interactions between traditional and novel HDL metrics and highlights potential therapeutic targets. The relationship between genetic regulation of HDL protein, HDL biogenesis, and plasma HDL-C levels is well described. For example, mutations in ABCA1, a gene involved in HDL biogenesis, reduce cholesterol efflux from cells and nearly abolish plasma HDL to cause the inherited condition called Tangier disease. The metabolic clearance of HDL occurs primarily by SR-B1-mediated hepatic uptake, the end step of reverse cholesterol transport. On the other hand, the molecular pathways linking HDL biogenesis to function, particle size variation, and cargo signature are not well defined and their genetic control is unknown.
Figure 1.
Overview of the regulation and interaction of traditional and novel HDL measures. Dotted lines: Suspected interactions. Straight lines: Established interactions; Red box: Possible targets for diagnostic or therapeutic development.
It is possible that inheritance controls the production of HDL particles of a certain size and protein and lipid composition and with different sterol efflux capacities. Acquired factors may then rearrange the distribution of these particles and either increase or decrease genetically determined functionality. We lack a model for the assembly of HDL protein and lipid cargo and know very little about its genetic regulation. Characterization of the genetic pathways regulating HDL function, composition, and particle sizes will bring us closer to the next breakthrough in this troubled but still promising field.
Acknowledgments
SOURCES OF FUNDING
The authors were partially supported by National Institutes of Health (NHLBI) R01 Grant HL057986 (to SF) and American Heart Association Scientist Development Grant 13SDG16940064 (to NP).
Footnotes
DISCLOSURES
None.
References
- 1.Gordon DJ, Rifkind BM. High-density lipoprotein--the clinical implications of recent studies. N Engl J Med. 1989;321:1311–1316. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]
- 2.Wilson PW, Abbott RD, Castelli WP. High density lipoprotein cholesterol and mortality. The Framingham Heart Study. Arteriosclerosis. 1988;8:737–741. doi: 10.1161/01.atv.8.6.737. [DOI] [PubMed] [Google Scholar]
- 3.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Hólm H, Ding EL, Johnson T, Schunkert H, Samani NJ, Clarke R, Hopewell JC, Thompson JF, Li M, Thorleifsson G, Newton-Cheh C, Musunuru K, Pirruccello JP, Saleheen D, Chen L, Stewart A, Schillert A, Thorsteinsdottir U, Thorgeirsson G, Anand S, Engert JC, Morgan T, Spertus J, Stoll M, Berger K, Martinelli N, Girelli D, McKeown PP, Patterson CC, Epstein SE, Devaney J, Burnett MS, Mooser V, Ripatti S, Surakka I, Nieminen MS, Sinisalo J, Lokki ML, Perola M, Havulinna A, de Faire U, Gigante B, Ingelsson E, Zeller T, Wild P, de Bakker PI, Klungel OH, Maitland-van der Zee AH, Peters BJ, de Boer A, Grobbee DE, Kamphuisen PW, Deneer VH, Elbers CC, Onland-Moret NC, Hofker MH, Wijmenga C, Verschuren WM, Boer JM, van der Schouw YT, Rasheed A, Frossard P, Demissie S, Willer C, Do R, Ordovas JM, Abecasis GR, Boehnke M, Mohlke KL, Daly MJ, Guiducci C, Burtt NP, Surti A, Gonzalez E, Purcell S, Gabriel S, Marrugat J, Peden J, Erdmann J, Diemert P, Willenborg C, König IR, Fischer M, Hengstenberg C, Ziegler A, Buysschaert I, Lambrechts D, Van de Werf F, Fox KA, El Mokhtari NE, Rubin D, Schrezenmeir J, Schreiber S, Schäfer A, Danesh J, Blankenberg S, Roberts R, McPherson R, Watkins H, Hall AS, Overvad K, Rimm E, Boerwinkle E, Tybjaerg-Hansen A, Cupples LA, Reilly MP, Melander O, Mannucci PM, Ardissino D, Siscovick D, Elosua R, Stefansson K, O’Donnell CJ, Salomaa V, Rader DJ, Peltonen L, Schwartz SM, Altshuler D, Kathiresan S. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frikke-Schmidt R, Nordestgaard BG, Stene MCA, Sethi AA, Remaley AT, Schnohr P, Grande P, Tybjaerg-Hansen A. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA. 2008;299:2524–2532. doi: 10.1001/jama.299.21.2524. [DOI] [PubMed] [Google Scholar]
- 5.Zanoni P, Khetarpal SA, Larach DB, Hancock-Cerutti WF, Millar JS, Cuchel M, DerOhannessian S, Kontush A, Surendran P, Saleheen D, Trompet S, Jukema JW, De Craen A, Sattar N, Ford I, Packard C, Shafi Majumder al A, Alam DS, Di Angelantonio E, Abecasis G, Chowdhury R, Erdmann J, Nordestgaard BG, Nielsen SF, Tybjaerg-Hansen A, Schmidt RF, Kuulasmaa K, Liu DJ, Perola M, Salomaa V, Männistö S, Amouyel P, Arveiler D, Ferrières J, Müller-Nurasyid M, Ferrario M, Kee F, Willer CJ, Samani N, Schunkert H, Butterworth AS, Howson JMM, Peloso GM, Stitziel NO, Danesh J, Kathiresan S, Rader DJ, Watson S, Schmidt EM, Sengupta S, Gustafsson S, Kanoni S, Ganna A, Chen J, Buchkovich ML, Mora S, Beckmann JS, Bragg-Gresham JL, Chang H-Y, Demirkan A, Hertog Den HM, Do R, Donnelly LA, Ehret GB, Esko T, Feitosa MF, Ferreira T, Fischer K, Fontanillas P, Fraser RM, Freitag DF, Gurdasani D, Heikkilä K, Hyppönen E, Isaacs A, Jackson AU, Johansson Å, Johnson T, Kaakinen M, Kettunen J, Kleber ME, Li X, Luan J, Lyytikäinen L-P, Magnusson PKE, Mangino M, Mihailov E, Montasser ME, Nolte IM, O’Connell JR, Palmer CD, Petersen A-K, Sanna S, Saxena R, Service SK, et al. Consortium CE, Consortium CE, Consortium GLG. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science. 2016;351:1166–1171. doi: 10.1126/science.aad3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartlett J, Predazzi IM, Williams SM, Bush WS, Kim Y, Havas S, Toth PP, Fazio S, Miller M. Is Isolated Low High-Density Lipoprotein Cholesterol a Cardiovascular Disease Risk Factor? New Insights From the Framingham Offspring Study. Circ Cardiovasc Qual Outcomes. 2016;9:206–212. doi: 10.1161/CIRCOUTCOMES.115.002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du X-M, Kim M-J, Hou L, Le Goff W, Chapman MJ, Van Eck M, Curtiss LK, Burnett JR, Cartland SP, Quinn CM, Kockx M, Kontush A, Rye K-A, Kritharides L, Jessup W. HDL Particle Size Is a Critical Determinant of ABCA1-Mediated Macrophage Cellular Cholesterol Export. Circulation Research. 2015;116:1133–1142. doi: 10.1161/CIRCRESAHA.116.305485. [DOI] [PubMed] [Google Scholar]
- 8.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khera AV, Cuchel M, la Llera-Moya de M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, Lukmanova D, Mucksavage ML, Luben R, Billheimer J, Kastelein JJP, Boekholdt SM, Khaw K-T, Wareham N, Rader DJ. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol. 2015;3:507–513. doi: 10.1016/S2213-8587(15)00126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arteriosclerosis, Thrombosis, and Vascular Biology. 2013;33:1696–1705. doi: 10.1161/ATVBAHA.113.301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Reilly M, Dillon E, Guo W, Finucane O, McMorrow A, Murphy A, Lyons C, Jones D, Ryan M, Gibney M, Gibney E, Brennan L, la Llera-Moya de M, Reilly MP, Roche HM, McGillicuddy FC. High-Density Lipoprotein Proteomic Composition, and not Efflux Capacity, Reflects Differential Modulation of Reverse Cholesterol Transport by Saturated and Monounsaturated Fat DietsCLINICAL PERSPECTIVE. Circulation. 2016;133:1838–1850. doi: 10.1161/CIRCULATIONAHA.115.020278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenson RS, Brewer HB, Chapman MJ, Fazio S, Hussain MM, Kontush A, Krauss RM, Otvos JD, Remaley AT, Schaefer EJ. HDL Measures, Particle Heterogeneity, Proposed Nomenclature, and Relation to Atherosclerotic Cardiovascular Events. Clinical Chemistry. 2011;57:392–410. doi: 10.1373/clinchem.2010.155333. [DOI] [PubMed] [Google Scholar]
- 14.Mendivil CO, Furtado J, Morton AM, Wang L, Sacks FM. Novel Pathways of Apolipoprotein A-I Metabolism in High-Density Lipoprotein of Different Sizes in Humans. Arteriosclerosis, Thrombosis, and Vascular Biology. 2016;36:156–165. doi: 10.1161/ATVBAHA.115.306138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ronsein GE, Hutchins PM, Isquith D, Vaisar T, Zhao X-Q, Heinecke JW. Niacin Therapy Increases High-Density Lipoprotein Particles and Total Cholesterol Efflux Capacity But Not ABCA1-Specific Cholesterol Efflux in Statin-Treated Subjects. Arteriosclerosis, Thrombosis, and Vascular Biology. 2016;36:404–411. doi: 10.1161/ATVBAHA.115.306268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enhanced HDL functionality in small HDL species produced upon remodeling of HDL by reconstituted HDL, CSL112: effects on cholesterol efflux, anti-inflammatory and antioxidative activity. Didichenko SA, Navdaev AV, Cukier AMO, Gille A, Schuetz P, Spycher MO, Thérond P, Chapman MJ, Kontush A, Wright SD. Circ Res. 2016 doi: 10.1161/CIRCRESAHA.116.3086. first published on July 19 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kontush A, Lhomme M, Chapman MJ. Unraveling the complexities of the HDL lipidome. J Lipid Res. 2013;54:2950–2963. doi: 10.1194/jlr.R036095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaisar T, Tang C, Babenko I, Hutchins P, Wimberger J, Suffredini AF, Heinecke JW. Inflammatory remodeling of the HDL proteome impairs cholesterol efflux capacity. J Lipid Res. 2015;56:1519–1530. doi: 10.1194/jlr.M059089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green PS, Vaisar T, Pennathur S, Kulstad JJ, Moore AB, Marcovina S, Brunzell J, Knopp RH, Zhao X-Q, Heinecke JW. Combined statin and niacin therapy remodels the high-density lipoprotein proteome. Circulation. 2008;118:1259–1267. doi: 10.1161/CIRCULATIONAHA.108.770669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ronsein GE, Reyes-Soffer G, He Y, Oda M, Ginsberg H, Heinecke JW. Targeted Proteomics Identifies Paraoxonase/Arylesterase 1 (PON1) and Apolipoprotein Cs as Potential Risk Factors for Hypoalphalipoproteinemia in Diabetic Subjects Treated with Fenofibrate and Rosiglitazone. Molecular & Cellular Proteomics. 2016;15:1083–1093. doi: 10.1074/mcp.M115.054528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pamir N, Hutchins P, Ronsein G, Vaisar T, Reardon CA, Getz GS, Lusis AJ, Heinecke JW. Proteomic analysis of HDL from inbred mouse strains implicates APOE associated with HDL in reduced cholesterol efflux capacity via the ABCA1 pathway. J Lipid Res. 2016;57:246–257. doi: 10.1194/jlr.M063701. [DOI] [PMC free article] [PubMed] [Google Scholar]