Abstract
Background
Improving the quality of life is part of the global agenda. The focus is predominantly on prevention of socially significant diseases. Combating dental caries-related diseases is a top priority as it has a huge impact on people’s social lives. Therefore, the purpose of the work was to study the changes in the molecular composition of saliva from subjects with multiple caries lesions using spectroscopic methods of analysis to identify potential tissue markers of caries development for predictive, preventive and personalised medical services.
Objectives and methods
The molecular composition of mixed saliva (oral fluid) from subjects with and without multiple caries was analysed with the use of spectroscopic techniques, FTIR with synchrotron radiation for the excitation. The IR spectra of the oral fluid as well as the calculated mineral-organic, carbon-phosphate, Amide II/Amide I and protein/thiocyanate ratios were compared between subjects with and without multiple caries.
Results
This complex analysis of the obtained experimental data determined that the molecular composition of the oral fluid from those with multiple caries differed from those without caries; the organic-mineral balance in the oral fluid of those with multiple caries shifted towards a reduction in the mineral complexes, accompanied by an increase in the organic component. The thiocyanate content increased more than twofold, accompanied by increased carboxyl groups of esters, lipids and carbohydrates.
Conclusion
The detected features in the IR spectra of mixed saliva as well as the calculated changes in the ratios between organic and inorganic components can be used as biomarkers of cariogenesis in the oral cavity, as a diagnostic criterion in the analysis of the oral fluid samples.
Keywords: Predictive, Preventive, Personalised medical service, FTIR spectroscopy, Molecular composition, Biomarkers, Thiocyanate level
Introduction
Early prevention of diseases using precise monitoring methods is a key paradigm in healthcare [1]. The focus is predominantly on socially significant diseases with dental caries-related diseases being one of the most important ones due to their huge impact on people’s social lives [2]. The analysis of modern publication on the development of cutting-edge medical technologies [3, 4], i.e. on the approaches to early caries prevention [5–7], suggests that the topic is a national top priority.
Therefore, a predictive medicine approach to prevent caries at the stage that is not visually observed is currently one of the leading directions in fundamental medicine and it is directly connected to the use of a wider range of physical methods for monitoring and analysis that are employed in tools for pathology prevention [5, 8, 9].
It has been established that saliva represents one of the most informative fluids of human diseases [10, 11]. Indeed, proteins, lipids, immunoglobulins, enzymes and various metabolites present in saliva can be used as markers for numerous pathologies as a predictive and prognostic tool [10–16]. In comparison with other bodily fluids, namely, urine and blood, saliva has a number of indisputable advantages for clinical diagnostics: saliva sampling is easy and simple, it is non-invasive, and sample preparation involves few operations [4, 16]. Moreover, saliva is less complex than blood serum, which is very important for analysis and interpretation of the results [12, 17, 18]. One should also note that majority of tissue markers present in the blood and urine can be detected in the saliva samples [18–20]. However, some proteins produced and secreted by salivary glands do not have analogues in the blood. Therefore, saliva has a high predictive, preventive and personalised diagnostic potential for the examination of human pathologies [12, 14, 21–23].
Presently, much attention is focused on the use of saliva in the personalised diagnosis of systemic diseases of organs in the oral cavity, such as gums, teeth and salivary glands [12, 19, 21, 24]. Integral markers of saliva including pH, circulation rate, calcium content and microbial profile are often used to estimate the risk of the dental caries development [7, 24, 25]. However, as it was shown in [26], parameters of saliva described above are weakly associated with the dynamics of the caries development process. Consequently, more efficient diagnostic methods should be based on the data concerning the changes in the molecular composition of saliva in the course of the pathological processes [25, 27]. Changes in the molecular composition of saliva associated with cariogenesis can be used as efficient tissue markers of dental caries development [7, 19, 24, 26]. Such an approach requires the precise analysis of the biological fluid composition; in this case, the most suitable technique is Fourier transform infrared spectroscopy (FTIR) [21, 27–31]. FTIR is non-invasive, rapid, precise and highly selective allowing the investigation of erosion in hard dental tissue, its attrition, as well as for the study of different forms of caries [23, 32, 33]. Also, infrared spectroscopy can be employed as a preventive and predictive method to define tooth and gum disease (gingivitis/periodontitis) [31, 34, 35]. IR spectroscopy has been successfully applied for the detection of tissue marker pathologies [21, 27, 29, 36]. With the development of spectroscopic express methods of human saliva analysis, screening of diseases at the molecular level at any early stage is possible [13, 28, 29, 37].
By comparing the changes in the molecular composition of saliva obtained by FTIR at the different stages of pathology in the oral cavity (caries), it is possible to obtain novel data concerning the course of this disease. This information can help not only in specification of the mechanisms responsible for caries development, but also to reveal their relationships with the processes of de-mineralisation/mineralisation of the hard dental tissues [38–41], as well as to specify saliva proteomics of caries development [11, 24] to elucidate potentially significant tissue markers.
Therefore, the purpose of the work was to study the changes in the molecular composition of saliva from subjects with multiple caries lesions using spectroscopic methods of analysis to identify potential tissue markers of caries development.
Materials and methods
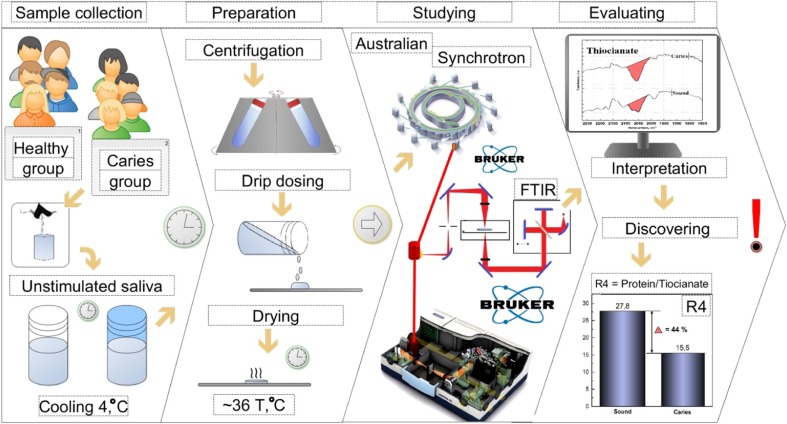
Twenty humans aged between 22 and 28 years of age participated in this study (10 men and 10 women). All participants did not take any medicines or drugs, were non-smokers and did not drink spirits. All of them did not have any records in their medical cards for a year before making examinations and experiments. Participants abstained from food and did not drink for at least 2 h before sampling of their oral fluid.
The first group of participants (5 men and 5 women) was physically healthy without harmful habits (non-smokers with caries-free teeth and without gum disorders). The second group (5 men and 5 women) were conditionally healthy (non-smokers), regularly snacking on easily digestible carbohydrates between meals. On examination, each participant in this group had teeth with lesion focuses related with primary and secondary caries at the stage corresponding to the third degree according to the ICDAS scale [42]. In addition, they repeatedly visited dentists in the 3-year period before their participation in this study for dental caries treatment.
Non-stimulated mixed saliva was sampled during daylight, to minimise circadian rhythm, 5 min after the preliminary rinsing of the oral cavity with pure water. The saliva was placed into 15-ml sterile test tubes for subsequent laboratory investigation according to the standard technique [29] as shown in the inset “а” in Fig. 1. After sampling, the test tubes were cooled to 4 °C, then centrifuged before drying in the oven at 36 °C to remove excess moisture.
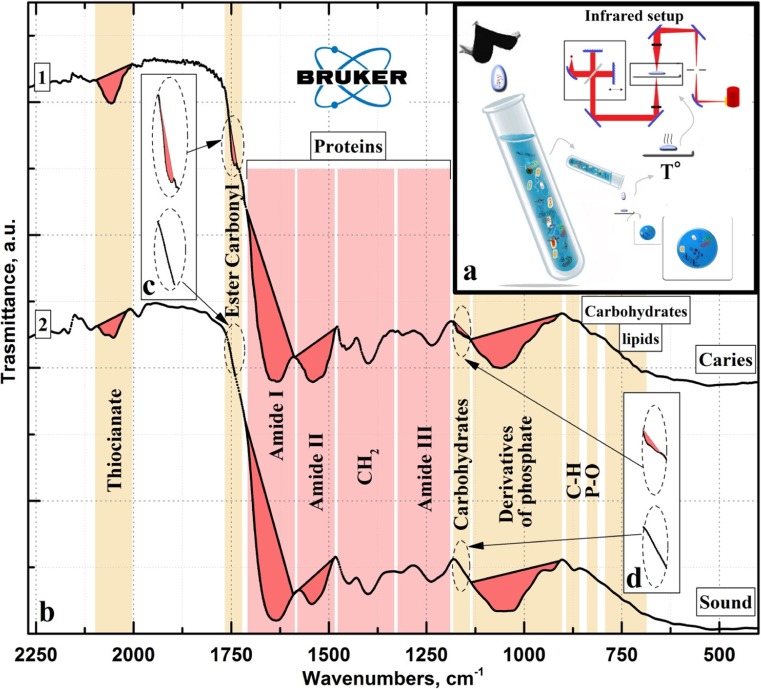
Fig. 1.
IR spectra of oral fluid samples averaged over the study groups: with (curve 1) and without (curve 2) multiple caries
The molecular composition of the mixed saliva samples was analysed by IR spectroscopy. FTIR does not impact on the sample, providing information related to the sample composition rather than changes as a result of being subjected to this technique [17, 31]. Analysis of the mixed saliva samples was performed with the Vertex-70 spectrometer (Bruker, Germany) using an attachment for attenuated total reflection provided with a diamond prism according to the technique described in [27]. In addition, samples were subjected to infrared microspectroscopy (IRM) beamline at the Australian Synchrotron, Victoria, Australia, using a Hyperion 3000 IR microscope (Bruker, Germany) and high-pressure diamond cell for the analysis of microsamples. IR spectra were recorded within the range of 4000–500 cm−1. Spectral data processing (background subtraction, correction for the atmosphere effect, averaging of the spectra and data integration) and analysis were performed using the professional software suite OPUS (version 7.5).
Ethical approval
All participants provided their written consent for participation. The Ethics Committee of Voronezh State University affirmed the performed examination (number of permission 001.018-2017). The examination was made in accordance with the approved principles.
Availability of data and materials
All data collected are available by request.
Experimental results
Preliminary analysis of the data obtained by FTIR demonstrated that the spectra of all samples within a certain experimental group involved one and the same set of vibration modes. The use of FTIR for the analysis qualitatively demonstrated that the molecular composition of the mixed saliva was characterised by a specific set of vibration modes in the IR spectra, in agreement with published data on biological fluids [21, 27–31].
It should be noted that in the present study, the registered vibration modes in the IR spectra of the individuals within each group were similar in intensity; therefore, only IR spectra of the oral fluid averaged over the group are presented. The procedure of spectra averaging over the experimental group also allows the elimination of random experimental errors, as well as the individual features of the participants within each group [43].
The averaged IR spectra of the mixed saliva samples obtained from those with multiple caries (curve 1) and the healthy group (curve 2) are presented in Fig. 1(b). The list of active vibrations in the spectra of the groups and frequencies of these vibration modes as well as their allocation to a certain molecular group are presented in Table 1. Analysis of the obtained data was performed on the basis of the information from the reference literature, where samples of non-stimulated saliva (healthy versus containing pathological tissue markers) were examined by FTIR, in addition to relating phosphate derivatives to the formation of enamel and dentin, as well as to the biological fluids in the oral cavity [21, 27–30, 36, 37, 45, 49, 50].
Table 1.
Active vibration bands in the IR-spectra for the experimental and reference data
| Substance | Vibration modes | Wavenumbers, cm−1 | Normal | Caries | References |
|---|---|---|---|---|---|
| Carbohydrates Lipids |
С–H starch or out-of-plane vibrations, P–O | 700–870 | + | + | [31, 44] |
| Carbohydrates Phosphates |
Oligo, polysaccharides, glycosilated proteins and phosphorus derivatives | 1025–1078 | + | + | [37, 45] |
| Mono and oligosaccharides | 1029 | + | + | [30] | |
| Derivative of phosphate, glycerophosphate and phosphatase. Phospholipids, C–O–P–O–C |
1054 | + | + | [36, 37] | |
| Carbohydrates | C–O/C–C | 1115 | − | + | [29, 45, 46] |
| Proteins | Amide III (CN stretching, NH bending) band components of proteins | 1272 | + | + | [28, 37] |
| CH2/CH3 | 1397–1410, 1452 | + | + | [28, 30, 37] | |
| Amide II (CN stretching, NH bending) band components of proteins | 1548–1553 | + | + | [28, 30, 37] | |
| Amide I (C=O stretching) band components of proteins | 1645–1650 | + | + | [28, 30, 37] | |
| Ester carbonyl | >C=O stretching | 1765–1725 | − | + | [21, 47, 48] |
| Thiocyanate | −N=C=S | 2150–1950 | + | ++ | [29, 30] |
From the obtained experimental data (Fig. 1, Table 1), the main intensive vibration bands in the IR transmission spectra of saliva that are present in all spectra are assigned to the following groups and complexes as follows. The first and most intensive group of vibrations arranged in the interval of 1078–900 cm−1 were assigned to the molecular bonds related to the phosphorus derivatives, such as phosphates, glycerophosphates and phospholipids [27, 49]. The second largest group of vibration bands localised in the range of 1725–1190 cm1 is related to proteins. One can distinguish the bands of secondary amides in this group: Amide I (C=O stretch vibration in the interval 1725–1590 cm−1), Amide II (N–H bend and C–N stretch in the interval 1590–1500 cm−1) and Amide III (C–N stretch, N–H bend in the range of 1350–1190 cm−1), as well as vibrations of CH2/CH3 groups, arranged in the interval 1480–1350 cm−1 [51].
Along with the vibration modes discussed above, several modes with the intensity dependent on the presence of dental caries were observed. The most intensive vibration in this group of bands is a mode in the range of 2150–1950 cm−1. According to the data of [29], this mode is related to the −N=C=S bond of thiocyanate anions that are present in mixed saliva. Visual analysis of the intensity for this vibration in the spectrum of the oral fluid obtained from those with multiple caries demonstrated that it is considerably more intensive than for the healthy subjects. Thiocyanates are well-known local antibacterial agents for anaerobic microorganisms [52], thus protecting against their vital active products, since the antibacterial activity of thiocyanates is an order of magnitude higher than that of hydrogen peroxide. Furthermore, the presence of thiocyanates in saliva can indicate the local immune status of the oral cavity [53].
Interestingly, vibrations in the IR spectra in the three ranges, 1765–1725 cm−1, 1185–1140 cm−1 and 870–700 cm−1, were observed only in the samples of those with multiple caries. The first two regions in the oversized scale are presented in the insets с and d in Fig. 1. Analysis of literature showed that the vibration band in the range of 1185–1140 cm−1 (inset d, Fig. 1) is related to carbohydrates present in the oral fluid. Carbohydrates are involved in the composition of saliva mucins covering and lubricating the mucous tunic surface in the oral cavity [54], prevent adherence of anaerobic bacteria and their colonisation, protecting tissues from physical damage. The IR band in the range of 1765–1725 cm−1 (inset с, Fig. 1) corresponds to the vibration of >C=O and is associated with the carboxylic group of ester (ester carbonyl) [21, 47, 48]. It is of note that the presence of esters in the hard dental tissue of humans suffering from dental caries has been shown [21, 22, 48] and they are more often observed in carious tissue than in intact tissue [55].
As for the third specific region (as it was noted above), a set of low-intensive vibration modes was observed in the range of 870–700 cm−1. These vibrations were characterised by a high relative intensity in comparison with similar bands in the IR spectrum of healthy participants and are associated with С–Н, P–O bonds of phosphodiesters and esters, as well as other lipids and carbohydrates of saliva [56]. The reason for this increased intensity is due to the higher concentration of lipids and esters in the saliva as a result of caries development [57, 58]. It should be noted that the concentration of these substances and their associated molecular complexes is relatively low in comparison to the protein concentration of saliva. Therefore, the change in intensity of vibrations in the IR spectra within the range of 870–700 cm−1 with regard to the changes in the range of vibrations related to proteins is also insignificant. It is necessary to note that all predictive and preventive manipulation can be provided only following spectra calculations and determination of statistically significant FTIR results.
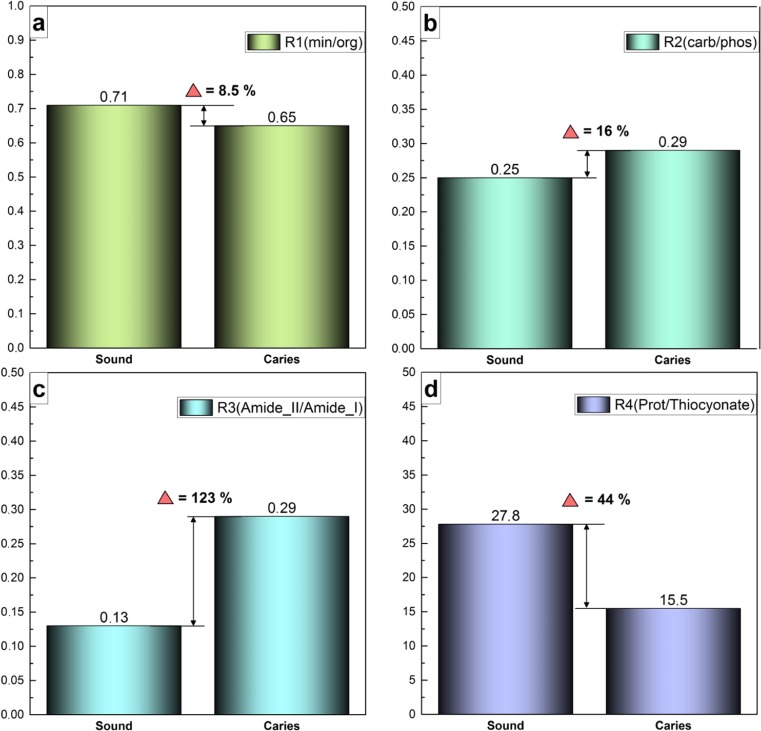
The primary analysis of the experimental IR spectra of the oral fluid samples allows the direct comparison of the presence or absence of vibrations in each group, so if no reference samples and calibration curves are applied, FTIR only provides qualitative estimations. Quantitative estimations based on the FTIR data were used to identify differences in the molecular composition of the oral fluid between the group of healthy participants and those with multiple caries as described previously by our group [27, 59]. The mathematical estimation of the changes in the molecular composition of saliva can be given based on calculations and analysis of four different ratios (coefficients) between the organic and mineral components in the oral fluid sample [60].
First, the R1 mineral-organic ratio is sufficient to calculate the ratio of the integral intensity of the phosphate bands in the IR spectrum (spectral ranges of 1078–900 cm−1) to the integral intensity of vibration band 1700–1590 cm−1 associated with Amide I. Secondly, R2 (carbon-phosphate ratio) can be calculated from the relation of the integral intensity for the vibration bands of C=O and CH2/CH3 bonds localised in the range of 1430–1360 cm−1 to the integral intensity of phosphate bands in the IR-spectrum within the region of 1078–900 cm−1. Thirdly, R3 (Amide II/Amide I) is calculated from the ratio of integral intensity for the band of Amide II (CN stretching, NH bending vibrations) in the range of 1590–1505 cm−1 to the integral intensity of the band of Amide I (C=O stretching) in the interval of 1723–1590 cm−1. The fourth coefficient, R4 protein/thiocyanate, that was proposed in [29], can be calculated from the ratio of the integral intensities of the amide bands (Amide I and Amide II) in the range of 1700–1500 cm−1 to the integral intensity of −N=C=S vibration bands, arranged at 2150–1950 cm−1, associated with thiocyanate.
These ratios were calculated using OPUS 7.5 (Bruker), including a wide set of the functional facilities for processing and estimation of the data obtained by IR-spectroscopy techniques. The results are presented in Fig. 2, as well as the relative changes in all four ratios. It is important to note that the ratios were calculated based on the spectra averaged over the group of participants in this study.
Fig. 2.
Calculated R1-R4 ratios obtained for the two groups of participants at examination. a Mineral-organic ratio (R1). b Carbon-phosphate ratio (R2). c Amide II/Amide I ratio (R3). d Protein/thiocyanate ratio (R4)
Discussion
Integrated assessment of the obtained experimental FTIR results and calculated data presented in Figs. 1 and 2 enabled the comparison of the molecular composition of the oral fluid of subjects with and without multiple caries. The results revealed a decrease of the mineral-organic ratio, R1 (Fig. 2a), indicating a reduction in the number of mineral groups and complexes in the saliva and/or an increase in the organic component in the case of the presence of cariogenic bacteria in the mixed saliva [7, 24]. The considerable increase in the R3 coefficient (ratio of the integral areas of Amide II/Amide I) for the subjects with multiple caries of ~ 120% (Fig. 2c) suggests that the changes in the composition of the organic component in their oral fluid are due to an increase in the number of CN and NH molecular groups relative to the share of C=O bonds (Fig. 1, Table 1). According to published data, these molecular groups are associated with the protein component and changes of its content can occur as a result of the presence of pathologic microflora within the oral cavity, as in the non-stimulated and stimulated saliva [7, 24, 58].
Relative changes in the carbon-phosphate ratio R2 (Fig. 2b) also indicates differences in the molecular composition of the mixed saliva samples obtained from those with and without multiple caries. The value of the R2 coefficient for the participants with multiple caries was ~ 16% higher that the participants without multiple caries (Fig. 2b). This increase is due to the decrease in the phosphate complexes and increase in the bonds associated with C=O and CH2/CH3 in the oral fluid, which is in good agreement with published data [61, 62].
Moreover, significant changes in the composition of the oral fluid occur with regard to the number of −N=C=S groups which is associated with the presence of thiocyanate in saliva (Fig. 1d). According to published data, the level of thiocyanates in saliva having an antibacterial effect can be enhanced during pathological processes in the humans [29]. The R4 coefficient showed that the ratio of protein/thiocyanate was, in fact, decreased twofold, indicating that the chemical bonds inherent to thiocyanate increased relative to the share of proteins in mixed saliva from participants with multiple caries. Considering that relative changes of R1 and R3 ratios imply a noticeable increase in organics (including proteins) in the mixed saliva, a twofold decrease in the R4 ratio is associated with a considerable increase in thiocyanate in the saliva of the subjects with multiple caries. A review of dental literature revealed that similar changes in the molecular composition of saliva (an increase of thiocyanate) were observed for smokers [29, 63].
Regarding the qualitative changes in the composition of oral fluid from participants with multiple caries, based on the experimental FTIR data (see Fig. 1), the most significant and representative are the differences in the IR spectra within the range of 1765–1725 cm−1 (Fig. 1, Table 1); vibration bands in this region are related to carbohydrates and ester carbonyls. These two low-intensive bands are observed only in the spectra of the oral fluid samples taken from the second group of participants with multiple caries. The estimations are in agreement with published results [57, 64], where it was shown that the saliva composition of esters, lipids and carbohydrates increased during caries development.
All features of the IR spectra of the oral fluid described above, as well as the changes in the molecular composition as determined on the basis of the ratios, suggest that the organic-mineral balance in the oral fluid of subjects with multiple caries is shifted towards a reduction in the content of the mineral groups and complexes as well as an increases in the organic component. These data are in a good agreement with studies concerned with the development of pathologic processes in the oral cavity including carious process [7, 19, 24, 25, 57, 58, 62, 65].
However, no one of the antimicrobial agents, including thiocyanate, as an integral marker of saliva is indicative of the in vivo diagnosis of future caries development, as they are relatively weakly associated with the dynamics of dental caries [52, 66]. Therefore, in our opinion, the complex analysis related to the quantitative and qualitative data on the changes in the molecular composition of the oral fluid as presented in this report has the potential to increase the accuracy of the detection of future carious processes and to improve preventive diagnostic of this disease.
Conclusion
The use of FTIR, including synchrotron radiation, allowed the comparison of several features in the IR-spectra, as well as the determination of mineral-organic, carbon-phosphate, Amide II/Amide I and protein/thiocyanate ratios, of oral fluid from subjects with and without multiple caries. The complex analysis of the experimental data showed that the organic-mineral balance in the oral fluid of those with multiple caries shifted towards a reduction in the mineral complexes, accompanied by an increase in the organic component. The ratio of Amide II/Amide I was integral to these changes in molecular composition, increasing by ~ 120% in the group with multiple caries compared with those without. The most indicative changes in the composition of the oral fluid of those with multiple caries occurred in relation to the number of −N=C=S groups associated with the presence of thiocyanate observed in the IR-spectrum at 2150–1950 cm−1, which increased twofold.
Moreover, the carboxyl groups of esters, lipids and carbohydrates present in the mixed saliva are typical for the processes of caries development (Fig. 3).
Fig. 3.
Scheme of the experiment and the most indicative changes in the composition of the oral fluid of those with multiple caries occurred in relation to the number of −N=C=S groups associated with the presence of thiocyanate
Expert recommendations
Multiple caries is a systemic disease caused by the state of parodentium tissues that affects the overall homeostasis of the mouth cavity. With a wide range of functions it plays non-stimulated saliva is a great source of information about the condition of the mouth cavity organs. It goes without saying that saliva from an individual person has its own features. This fact gives an opportunity to detect the changes in the saliva molecular composition and predict and to prevent such a disease as multiple caries. The usage of non-stimulated saliva is relatively fast, low-cost method and with the combination of FTIR equipment could give better results in such an application. Furthermore, the complex data analysis presented has the potential for application as both tissue markers and as an effective predictive medical diagnostic approach for the estimation of cariogenesis in mixed saliva samples, and they will also contribute to providing individually tailored dental care. Individual timely prevention considering the data analysis of the condition of saliva microbiota will be beneficial in reducing the risks of caries development and improving caries resistance.
Acknowledgements
The part of this research was undertaken with The Infrared Microspectroscopy (IRM) beamline at the Australian Synchrotron.
Authors’ contributions
P.S: conceived and designed the experiments, analysed the data, performed the experiments, contributed reagents/materials/analysis tools, wrote the manuscript
D.G: contributed reagents/materials/analysis tools, analysed the data, prepared the figures and/or tables, wrote the manuscript
Y.I: contributed reagents/materials/analysis tools, wrote the manuscript
P.V: performed the experiments
Funding
This work was supported by the grant of Russian Science Foundation, grant number 16-15-00003.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All patients whose data were used within the created survey had signed institutional consent for the participation research. All persons who participated in the survey signed written consent. Voronezh State University Ethics Committee approved this study (approval number 001.018-2017). The study was carried out in accordance with the approved guidelines.
References
- 1.Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, Krapfenbauer K, Mozaffari MS, Costigliola V. Medicine in the early twenty-first century: paradigm and anticipation—EPMA position paper 2016. EPMA J. 2016;7:23. doi: 10.1186/s13167-016-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Golubnitschaja O, Costigliola V, EPMA. General report & recommendations in predictive, preventive and personalised medicine 2012: white paper of the European Association for Predictive, Preventive and Personalised Medicine. EPMA J. 2012;3. 10.1186/1878-5085-3-14. [DOI] [PMC free article] [PubMed]
- 3.Einhorn L, Krapfenbauer K. HTRF: a technology tailored for biomarker determination—novel analytical detection system suitable for detection of specific autoimmune antibodies as biomarkers in nanogram level in different body fluids. EPMA J. 2015;6:23. doi: 10.1186/s13167-015-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lakshmi K, Nelakurthi H, Kumar A, Rudraraju A. Oral fluid-based biosensors: a novel method for rapid and noninvasive diagnosis. Indian J Dent Sci. 2017;9:60. doi: 10.4103/IJDS.IJDS_6_17. [DOI] [Google Scholar]
- 5.Pretty IA, Ellwood RP. The caries continuum: opportunities to detect, treat and monitor the re-mineralization of early caries lesions. J Dent. 2013;41(Supplement 2):S12–S21. doi: 10.1016/j.jdent.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Kunin AA, Belenova IA, Ippolitov YA, Moiseeva NS, Kunin DA. Predictive research methods of enamel and dentine for initial caries detection. EPMA J. 2013;4:19. doi: 10.1186/1878-5085-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao X, Jiang S, Koh D, Hsu C-YS. Salivary biomarkers for dental caries. Periodontol 2000. 2016;70:128–141. doi: 10.1111/prd.12100. [DOI] [PubMed] [Google Scholar]
- 8.Baffi M, Almeida Rodrigues J de, Lussi A. Traditional and novel caries detection methods. In: Li M-Y, editor. Contemp. Approach Dent. Caries. InTech; 2012. http://www.intechopen.com/books/contemporary-approach-to-dental-caries/traditional-and-novel-caries-detection-methods
- 9.Cafiero C, Matarasso S. Predictive, preventive, personalised and participatory periodontology: ‘the 5Ps age’ has already started. EPMA J. 2013;4 10.1186/1878-5085-4-16. [DOI] [PMC free article] [PubMed]
- 10.Cova MAMN, Castagnola M, Messana I, Cabras T, Ferreira RMP, Amado FML, et al. Salivary Omics. In: Streckfus CF, et al., editors. Adv. Salivary Diagn. Springer Berlin Heidelberg. 2015. pp. 63–82. [Google Scholar]
- 11.Yoshizawa JM, Schafer CA, Schafer JJ, Farrell JJ, Paster BJ, Salivary Biomarkers WDTW. Toward future clinical and diagnostic utilities. Clin Microbiol Rev. 2013;26:781–791. doi: 10.1128/CMR.00021-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nunes LAS, Mussavira S, Bindhu OS. Clinical and diagnostic utility of saliva as a non-invasive diagnostic fluid: a systematic review. Biochem Medica. 2015;25:177–192. doi: 10.11613/BM.2015.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bottoni U, Tiriolo R, Pullano SA, Dastoli S, Amoruso GF, Nisticò SP, et al. Infrared saliva analysis of psoriatic and diabetic patients: similarities in protein components. IEEE Trans Biomed Eng. 2016;63:379–384. doi: 10.1109/TBME.2015.2458967. [DOI] [PubMed] [Google Scholar]
- 14.Malamud D, Rodriguez-Chavez IR. Saliva as a diagnostic fluid. Dent Clin N Am. 2011;55:159–178. doi: 10.1016/j.cden.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiappin S, Antonelli G, Gatti R, De Palo EF. Saliva specimen: a new laboratory tool for diagnostic and basic investigation. Clin Chim Acta. 2007;383:30–40. doi: 10.1016/j.cca.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Krapfenbauer K, Drucker E, Thurnher D. Identification of tumour-related proteins as potential screening markers by proteome analysis—protein profiles of human saliva as a predictive and prognostic tool. EPMA J. 2014;5 10.1186/1878-5085-5-20. [DOI] [PMC free article] [PubMed]
- 17.Parker F. Applications of infrared spectroscopy in biochemistry, biology, and medicine [Internet]. Springer Science & Business Media; 2012. http://www.springer.com/gp/book/9781468418743
- 18.Lehtinen J. Spectroscopic studies of human hair, nail, and saliva samples using a cantilever-based photoacoustic detection. Int J Thermophys. 2013;34:1559–1568. doi: 10.1007/s10765-013-1488-x. [DOI] [Google Scholar]
- 19.Dias C de A. Salivary biomarkers of dental caries [Internet] Universidade do Porto: Universidade do Porto; 2013. [Google Scholar]
- 20.Gozes I. Specific protein biomarker patterns for Alzheimer’s disease: improved diagnostics in progress. EPMA J. 2017;8:255–259. doi: 10.1007/s13167-017-0110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujii S, Sato S, Fukuda K, Okinaga T, Ariyoshi W, Usui M, et al. Diagnosis of periodontal disease from saliva samples using Fourier transform infrared microscopy coupled with partial least squares discriminant analysis. Anal Sci Int J Jpn Soc Anal Chem. 2016;32:225–231. doi: 10.2116/analsci.32.225. [DOI] [PubMed] [Google Scholar]
- 22.Almhöjd US, Norén JG, Arvidsson A, Nilsson Å, Lingström P. Analysis of carious dentine using FTIR and ToF-SIMS. Oral Health Dent Manag. 2014;13:735–744. [PubMed] [Google Scholar]
- 23.Armenta S, Garrigues S, de la Guardia M, Brassier J, Alcalà M, Blanco M. Analysis of ecstasy in oral fluid by ion mobility spectrometry and infrared spectroscopy after liquid-liquid extraction. J Chromatogr A 2015;1384:1–8. doi:10.1016/j.chroma.2015.01.036. [DOI] [PubMed]
- 24.Guo L, Shi W. Salivary biomarkers for caries risk assessment. J Calif Dent Assoc. 2013;41:107–118. [PMC free article] [PubMed] [Google Scholar]
- 25.Andrade MRTC, Salazar SLA, de Sá LFR, Portela M, Ferreira-Pereira A, Soares RMA, et al. Role of saliva in the caries experience and calculus formation of young patients undergoing hemodialysis. Clin Oral Investig. 2015;19:1–8. doi: 10.1007/s00784-015-1441-4. [DOI] [PubMed] [Google Scholar]
- 26.Cunha-Cruz DJ, Scott DJ, Rothen MM, Mancl DL, Lawhorn DT, Brossel DK, et al. Salivary characteristics and dental caries: evidence from general dental practices. J Am Dent Assoc 1939. 2013;144:e31–e40. doi: 10.14219/jada.archive.2013.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seredin P, Goloshchapov D, Kashkarov V, Ippolitov Y, Bambery K. The investigations of changes in mineral–organic and carbon–phosphate ratios in the mixed saliva by synchrotron infrared spectroscopy. Results Phys. 2016;6:315–321. doi: 10.1016/j.rinp.2016.06.005. [DOI] [Google Scholar]
- 28.Júnior C, Cesar P, Strixino JF, Raniero L, Júnior C, Cesar P, et al. Analysis of saliva by Fourier transform infrared spectroscopy for diagnosis of physiological stress in athletes. Res Biomed Eng. 2015;31:116–124. doi: 10.1590/2446-4740.0664. [DOI] [Google Scholar]
- 29.Mikkonen JJW, Raittila J, Rieppo L, Lappalainen R, Kullaa AM, Myllymaa S. Fourier transform infrared spectroscopy and photoacoustic spectroscopy for saliva analysis. Appl Spectrosc. 2016;70:1502–1510. doi: 10.1177/0003702816654149. [DOI] [PubMed] [Google Scholar]
- 30.Badea I, Crisan M, Fetea F, Socaciu C. Characterization of resting versus stimulated saliva fingerprints using middle-infrared spectroscopy assisted by principal component analysis. Romanian Biotechnol Lett. 2014;19:9817–9827. [Google Scholar]
- 31.Shaw RA, Mantsch HH. Infrared spectroscopy in clinical and diagnostic analysis: Encycl. Anal. Chem, John Wiley & Sons, Ltd; 2006. 10.1002/9780470027318.a0106.
- 32.Fried D, Featherstone JDB, Darling CL, Jones RS, Ngaotheppitak P, Bühler CM. Early caries imaging and monitoring with near-infrared light. Dent Clin N Am. 2005;49:771–793. doi: 10.1016/j.cden.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Seredin P, Kashkarov V, Lukin A, Ippolitov Y, Julian R, Doyle S. Local study of fissure caries by Fourier transform infrared microscopy and X-ray diffraction using synchrotron radiation. J Synchrotron Radiat. 2013;20:705–710. doi: 10.1107/S0909049513019444. [DOI] [PubMed] [Google Scholar]
- 34.Liu KZ, Xiang XM, Man A, Sowa MG, Cholakis A, Ghiabi E, Singer DL, Scott DA. In vivo determination of multiple indices of periodontal inflammation by optical spectroscopy. J Periodontal Res. 2009;44:117–124. doi: 10.1111/j.1600-0765.2008.01112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiang X, Duarte PM, Lima JA, Santos VR, Gonçalves TD, Miranda TS, Liu KZ. Diabetes-associated periodontitis molecular features in infrared spectra of gingival crevicular fluid. J Periodontol. 2013;84:1792–1800. doi: 10.1902/jop.2013.120665. [DOI] [PubMed] [Google Scholar]
- 36.Rodrigues LM, Magrini TD, Lima CF, Scholz J, da Silva Martinho H, Almeida JD. Effect of smoking cessation in saliva compounds by FTIR spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc. 2017;174:124–129. doi: 10.1016/j.saa.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Orphanou C-M. The detection and discrimination of human body fluids using ATR FT-IR spectroscopy. Forensic Sci Int. 2015;252:e10–e16. doi: 10.1016/j.forsciint.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 38.Baker MJ, Hussain SR, Lovergne L, Untereiner V, Hughes C, Lukaszewski RA, Thiéfin G, Sockalingum GD. Developing and understanding biofluid vibrational spectroscopy: a critical review. Chem Soc Rev. 2016;45:1803–1818. doi: 10.1039/C5CS00585J. [DOI] [PubMed] [Google Scholar]
- 39.Rehman IU, Movasaghi Z, Rehman S. Vibrational spectroscopy for tissue analysis [Internet] 1. Boca Raton: CRC Press; 2012. [Google Scholar]
- 40.Jeon RJ, Matvienko A, Mandelis A, Abrams SH, Amaechi BT, Kulkarni G. Detection of interproximal demineralized lesions on human teeth in vitro using frequency-domain infrared photothermal radiometry and modulated luminescence. J Biomed Opt. 2007;12:034028. doi: 10.1117/1.2750289. [DOI] [PubMed] [Google Scholar]
- 41.Goloshchapov D, Seredin P, Minakov D, Domashevskaya E. Study of the nanoporous CHAP photoluminiscence for developing the precise methods of early caries detection. IOP Conf Ser Mater Sci Eng. 2018;307:012027. doi: 10.1088/1757-899X/307/1/012027. [DOI] [Google Scholar]
- 42.Ismail AI, Sohn W, Tellez M, Amaya A, Sen A, Hasson H, Pitts NB. The international caries detection and assessment system (ICDAS): an integrated system for measuring dental caries. Community Dent Oral Epidemiol. 2007;35:170–178. doi: 10.1111/j.1600-0528.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 43.Seredin P, Goloshchapov D, Prutskij T, Ippolitov Y. Phase transformations in a human tooth tissue at the initial stage of caries. PLoS One. 2015;10:1–11. doi: 10.1371/journal.pone.0124008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pretsch E, Bühlmann P, Badertscher M. IR spectroscopy. Struct. Determ. Org. Compd. Springer, Berlin, Heidelberg; 2009. p. 1–67. doi:10.1007/978-3-540-93810-1_7, IR Spectroscopy.
- 45.Lopes J, Correia M, Martins I, Henriques AG, Delgadillo I, da Cruz e Silva O, et al. FTIR and Raman spectroscopy applied to dementia diagnosis through analysis of biological fluids. J Alzheimers Dis. 2016;52:801–812. doi: 10.3233/JAD-151163. [DOI] [PubMed] [Google Scholar]
- 46.Wiercigroch E, Szafraniec E, Czamara K, Pacia MZ, Majzner K, Kochan K, Kaczor A, Baranska M, Malek K. Raman and infrared spectroscopy of carbohydrates: a review. Spectrochim Acta A Mol Biomol Spectrosc. 2017;185:317–335. doi: 10.1016/j.saa.2017.05.045. [DOI] [PubMed] [Google Scholar]
- 47.Silverstein RM, Bassler GC, Morrill TC. Wiley. 1991. Spectrometric identification of organic compounds [Internet]. 5th ed. [Google Scholar]
- 48.Scherdin-Almhöjd U. Identification of esters in carious dentine staining and chemo-mechanical excavation [Internet]. 2017. https://gupea.ub.gu.se/handle/2077/51781
- 49.Elkins KM. Rapid presumptive “fingerprinting” of body fluids and materials by ATR FT-IR spectroscopy*,†. J Forensic Sci. 2011;56:1580–1587. doi: 10.1111/j.1556-4029.2011.01870.x. [DOI] [PubMed] [Google Scholar]
- 50.Seredin PV, Goloshchapov DL, Gushchin MS, Ippolitov YA, Prutskij T. The importance of the biomimetic composites components for recreating the optical properties and molecular composition of intact dental tissues. J Phys Conf Ser. 2017;917:042019. doi: 10.1088/1742-6596/917/4/042019. [DOI] [Google Scholar]
- 51.Kong J, Yu S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim Biophys Sin. 2007;39:549–559. doi: 10.1111/j.1745-7270.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- 52.Ihalin R, Loimaranta V, Tenovuo J. Origin, structure, and biological activities of peroxidases in human saliva. Arch Biochem Biophys. 2006;445:261–268. doi: 10.1016/j.abb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 53.Tenovuo J, Jentsch H, Soukka T, Karhuvaara L. Antimicrobial factors of saliva in relation to dental caries and salivary levels of mutans streptococci. J Biol Buccale. 1992;20:85–90. [PubMed] [Google Scholar]
- 54.Baughan LW, Robertello FJ, Sarrett DC, Denny PA, Denny PC. Salivary mucin as related to oral Streptococcus mutans in elderly people. Oral Microbiol Immunol. 2000;15:10–14. doi: 10.1034/j.1399-302x.2000.150102.x. [DOI] [PubMed] [Google Scholar]
- 55.Larmas M. A chromatographic and histochemical study of nonspecific esterases in human carious dentine. Arch Oral Biol. 1972;17:1121–1132. doi: 10.1016/0003-9969(72)90083-0. [DOI] [PubMed] [Google Scholar]
- 56.Gregor Cevc, Theresa M Allen, Saul L Neidleman. Phospholipids handbook [Internet]. CRC press; 1993. https://books.google.ru/books?id=2ZxaucDNWHcC
- 57.Tomita Y, Miyake N, Yamanaka S. Lipids in human parotid saliva with regard to caries experience. J Oleo Sci. 2008;57:115–121. doi: 10.5650/jos.57.115. [DOI] [PubMed] [Google Scholar]
- 58.Belstrøm D, Jersie-Christensen RR, Lyon D, Damgaard C, Jensen LJ, Holmstrup P, Olsen JV. Metaproteomics of saliva identifies human protein markers specific for individuals with periodontitis and dental caries compared to orally healthy controls. PeerJ. 2016;4:e2433. doi: 10.7717/peerj.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Avraamova OG, Ippolitov YA, Plotnikova YA, Seredin PV, Goloshapov DV, Aloshina EO. Increased oral fluid remineraling function by endogenous and exogenous saturation methods of its mineral complexes. Stomatologiia (Sofiia). 2017;96:6–11. [DOI] [PubMed]
- 60.Seredin PV, Goloshchapov DL, Ippolitov YA, Kalivradzhiyan ES. Does dentifrice provide the necessary saturation of ions in oral fluids to favour remineralisation? Russ Open Med J. 2018;7:e0106. 10.15275/rusomj.2018.0106.
- 61.Fiyaz M, Ramesh A, Ramalingam K, Thomas B, Shetty S, Prakash P. Association of salivary calcium, phosphate, pH and flow rate on oral health: a study on 90 subjects. J Indian Soc Periodontol. 2013;17:454–460. doi: 10.4103/0972-124X.118316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rajesh KS, Zareena, Hegde S, Arun Kumar MS. Assessment of salivary calcium, phosphate, magnesium, pH, and flow rate in healthy subjects, periodontitis, and dental caries. Contemp Clin Dent. 2015;6:461–465. doi: 10.4103/0976-237X.169846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schultz CP, Ahmed MK, Dawes C, Mantsch HH. Thiocyanate levels in human saliva: quantitation by Fourier transform infrared spectroscopy. Anal Biochem. 1996;240:7–12. doi: 10.1006/abio.1996.0323. [DOI] [PubMed] [Google Scholar]
- 64.Larsson B, Olivecrona G, Ericson T. Lipids in human saliva. Arch Oral Biol. 1996;41:105–110. doi: 10.1016/0003-9969(95)00077-1. [DOI] [PubMed] [Google Scholar]
- 65.Hicks J, Garcia-Godoy F, Flaitz C. Biological factors in dental caries: role of saliva and dental plaque in the dynamic process of demineralization and remineralization (part 1) J Clin Pediatr Dent. 2004;28:47–52. doi: 10.17796/jcpd.28.1.yg6m443046k50u20. [DOI] [PubMed] [Google Scholar]
- 66.Kirstilä V, Häkkinen P, Jentsch H, Vilja P, Tenovuo J. Longitudinal analysis of the association of human salivary antimicrobial agents with caries increment and cariogenic micro-organisms: a two-year cohort study. J Dent Res. 1998;77:73–80. doi: 10.1177/00220345980770011101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data collected are available by request.