Short abstract
Objective
Positive end-expiratory pressure (PEEP) causes carotid baroreceptor unloading, which leads to thermoregulatory peripheral vasoconstriction. However, the effects of PEEP on intraoperative thermoregulation in the prone position remain unknown.
Methods
Thirty-seven patients undergoing spine surgery in the prone position were assigned at random to receive either 10 cmH2O PEEP (Group P) or no PEEP (Group Z). The primary endpoint was core temperature 180 minutes after intubation. Secondary endpoints were delta core temperature (difference in core temperature between 180 minutes and immediately after tracheal intubation), incidence of intraoperative hypothermia (core temperature of <36°C), and peripheral vasoconstriction-related data.
Results
The median [interquartile range] core temperature 180 minutes after intubation was 36.1°C [35.9°C–36.2°C] and 36.0°C [35.9°C–36.4°C] in Groups Z and P, respectively. The delta core temperature and incidences of intraoperative hypothermia and peripheral vasoconstriction were not significantly different between the two groups. The peripheral vasoconstriction threshold (36.2°C±0.5°C vs. 36.7°C±0.6°C) was lower and the onset of peripheral vasoconstriction (66 [60–129] vs. 38 [28–70] minutes) was slower in Group Z than in Group P.
Conclusions
Intraoperative PEEP did not reduce the core temperature decrease in the prone position, although it resulted in an earlier onset and higher threshold of peripheral vasoconstriction.
Keywords: Positive end-expiratory pressure, prone position, intraoperative body temperature, peripheral vasoconstriction, intubation, spine surgery
Introduction
Intraoperative hypothermia is considered to be associated with postoperative adverse clinical outcomes in various study populations.1–4 The adverse effects of intraoperative hypothermia include impaired platelet function, cold diuresis, increased risk of infection, electrolyte disturbance, insulin resistance, postoperative shivering, and myocardial ischaemia.5–9 Thus, prevention of intraoperative hypothermia is a major concern in anaesthetic management.
The positive end-expiratory pressure (PEEP) is defined as the pressure within the lungs (alveolar pressure) above atmospheric pressure that exists at the end of expiration during mechanical ventilation. Most patients receiving mechanical ventilation under general anaesthesia show atelectasis, especially in dependent regions of both lungs. Therefore, a PEEP of 5 to 10 cmH2O is generally applied to prevent alveolar collapse in such patients, although the amount of PEEP applied is dependent on the patient’s lung condition. However, PEEP can reduce systemic venous return by increasing the intrathoracic pressure. Such a reduction in venous return causes carotid baroreceptor unloading, which leads to peripheral vasoconstriction by increasing the thermoregulatory vasoconstriction threshold, thereby blunting intraoperative hypothermia.10–12 Several studies have shown that in patients undergoing lower abdominal surgery or tympanoplasty, the extent of intraoperative hypothermia can be effectively attenuated by applying PEEP of 5 to 10 cmH2O.10,11,13,14 However, the effect of PEEP on intraoperative thermoregulation in the prone position has not been investigated. The prone position is associated with relative intravascular volume depletion due to a decrease in venous return by compression of the inferior vena cava and increased abdominal pressure,15–17 which may enhance the effect of PEEP on intraoperative thermoregulation.
In the present study, we tested our hypothesis that intraoperative PEEP application would induce thermoregulatory vasoconstriction and reduce the intraoperative core temperature decrease in patients undergoing spine surgery in the prone position.
Methods
This study was approved by the Institutional Review Board of Seoul National University Hospital (registration number: 1503-078-656), and written informed consent for inclusion was obtained from all patients. The study population comprised patients with an American Society of Anesthesiologists physical status of 1 or 2 who underwent spine surgery in the prone position from May 2015 to January 2016. The study protocol was registered at ClinicalTrials.gov (NCT02416557).
Patients with obesity, thyroid disease, peripheral vascular disease, diabetes, uncontrolled hypertension, or chronic obstructive pulmonary disease were excluded. Patients who were planned to undergo an intraoperative position change or induced hypothermia for intraoperative neuroprotection and those with a forehead skin temperature of ≥38.0°C or <35.0°C as determined using a portable infrared thermometer before induction of anaesthesia were also excluded.
Randomisation and group assignments
Using a predetermined computer-generated randomisation table, the randomisation was sequenced into four and six blocks using software. The 42 patients were randomly allocated into 2 groups: those who received zero end-expiratory pressure (ZEEP) after tracheal intubation until the end of surgery (Group Z) and those who received PEEP of 10 cmH2O (Group P). The assignments were concealed in opaque envelopes and opened immediately before induction by a nurse who was blinded to the group assignment.
Anaesthesia protocol
None of the patients received premedication. On arriving at the operating room, the patients were monitored with noninvasive blood pressure, electrocardiography, and peripheral oxygen saturation. The skin temperature was measured on the mid-forehead using a portable infrared thermometer (ThermoFlash Lx-26; JXB Co., Ltd., Guangzhou, China). Skin temperature probes (M1024254; GE Healthcare, Helsinki, Finland) were attached to the centre of the anterior aspect of the forearm, which has no intravenous route, and at the ipsilateral second fingertip. General anaesthesia was induced with target-controlled infusion of remifentanil (Minto model, effect site concentration of 4.0 ng/ml) and propofol (Schneider model, effect site concentration of 4.0 µg/ml) using a multi-drug infusion device (Orchestra® Base Primea; Fresenius Kabi, Bad Homburg, Germany). To facilitate tracheal intubation, 0.6 mg/kg of rocuronium was administered 2 minutes before intubation. The tidal volume was set at 8 ml/kg (predictive body weight), and the respiratory rate was adjusted to maintain the end-tidal carbon dioxide concentration at 35 to 40 mmHg. The radial artery was catheterised for invasive arterial blood pressure monitoring after rocuronium administration. After induction of anaesthesia, the patient was moved into the prone position from the supine position. The patient was placed on a Jackson spine table with his or her face resting on a facial pillow (ProneView® Helmet and Mirror system; Mizuho OSI, Union City, CA, USA). A Y-connector was disconnected during supine-to-prone positioning for a short time and then reconnected after prone positioning. PEEP was applied continuously in both the supine and prone positions until the end of surgery.
The depth of anaesthesia was monitored using the bispectral index (A-1050 Monitor; Aspect Medical Systems, Newton, MA, USA) and adjusted to maintain the bispectral index at 40 to 60. The mean arterial blood pressure was maintained within ±20% of the preoperative value during surgery using additional fluid loading of 300 to 500 ml and adjustment of the remifentanil dose.
Study protocol
The core temperature was measured at the mid-oesophageal level using an oesophageal temperature probe (DeRoyal, Powell, TN, USA). After tracheal intubation, the oesophageal temperature probe was inserted to the depth of the maximal heart sounds on auscultation. The core temperature, skin temperature, mean arterial pressure, and heart rate were measured immediately (baseline); 30, 60, 90, 120, 150, and 180 minutes after tracheal intubation; and finally at the end of surgery. Thermoregulatory vasoconstriction was evaluated with the same method used in several previous studies.11,13,14 The difference in skin temperature between the forearm and fingertip was also calculated. A forearm–fingertip skin temperature difference of <0°C was defined as peripheral vasodilation. When the forearm–fingertip skin temperature difference became 0°C, the core temperature at that time was regarded as the thermoregulatory vasoconstriction threshold. The thermoregulatory vasoconstriction threshold and the time taken to reach thermoregulatory vasoconstriction were recorded. The incidence of intraoperative hypothermia, defined as a body temperature of <36°C, was also noted. The delta core temperature was defined as the difference in core temperature from 180 minutes to immediately after tracheal intubation.
During surgery, the ambient temperature was maintained consistently. To prevent excessive loss of the core temperature intraoperatively, the upper and lower parts of the surgical field were covered with blankets to minimise skin exposure. All fluids administered were warmed to 37°C using a fluid warming system (Hotline with L-70 disposable; Level 1 Technologies, Inc., Rockland, MA, USA) and administered at a rate of 2 to 7 ml/kg/h. In addition, a forced-air warmer system (Bair Hugger™; 3M, St. Paul, MN, USA) set to 38°C was applied to all patients’ lower extremities during the entire study period. Postoperative shivering, nausea, and vomiting were checked in the post-anaesthesia care unit. No antiemetic drugs were used in either group.
The primary endpoint measure was the core temperature 180 minutes after intubation, and the secondary endpoint measures were the delta core temperature, the incidence of intraoperative hypothermia, and the threshold and onset of thermoregulatory vasoconstriction.
Statistical analysis
The core temperature 180 minutes after intubation, the delta core temperature, and the onset of peripheral vasoconstriction were compared using the Mann–Whitney U-test because of the skewed distribution. The peripheral vasoconstriction thresholds were compared using Student’s t test. Categorical variables were compared using the chi square test or Fisher’s exact test. Core temperature, the forearm–fingertip skin temperature difference, and haemodynamic variables between the two groups were analysed using repeated-measures, followed by the Mann–Whitney U-test to compare the data at each time point. A P value of <0.05 was considered to indicate statistical significance.
In a previous study, when no PEEP was applied, the core temperature 180 minutes after intubation was 35.4°C ± 0.5°C in patients undergoing spine surgery.18 In the present study, we anticipated that the core temperature 180 minutes after intubation would be increased by 0.5°C in patients receiving PEEP of 10 cmH2O. Assuming a type 1 error of 0.05 (two-tailed) and a power of 0.8, 17 patients would be necessary in each group. Considering a possible dropout rate of 20%, 21 patients were enrolled per group.
Results
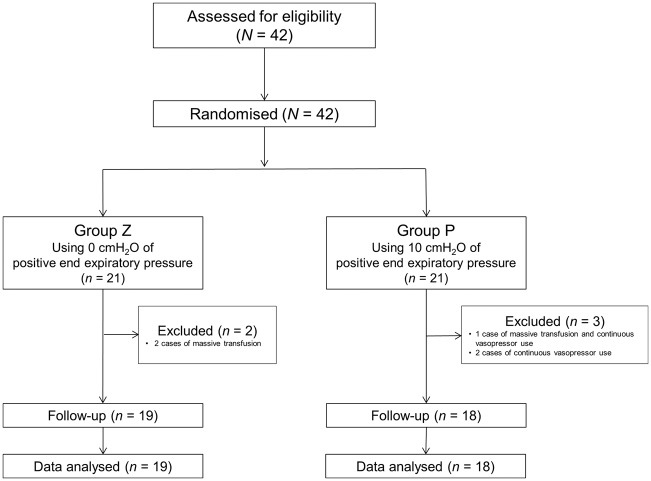
In total, 42 patients aged 20 to 80 years were enrolled in this study; 21 patients were randomised to Group P and 21 to Group Z. Five patients were excluded from the data analysis because of continuous use of vasopressors (2 patients), massive transfusion (2 patients), and continuous use of vasopressors plus massive transfusion (1 patient), all of which can affect peripheral thermoregulatory vasoconstriction (Figure 1). The patients’ demographic and intraoperative data were not significantly different between the two groups (Table 1).
Figure 1.
CONSORT flowchart.
Table 1.
Demographic and intraoperative data
| Variables | Group Z (n = 19) | Group P (n = 18) | P value |
|---|---|---|---|
| Age (y) | 46.9 ± 14.5 | 50.9 ± 16.6 | 0.439 |
| Sex (female:male) | 12:7 | 8:10 | 0.260 |
| Weight (kg) | 64.7 ± 10.3 | 63.5 ± 14.0 | 0.773 |
| Height (cm) | 161.9 ± 10.6 | 162.6 ± 10.0 | 0.822 |
| Body mass index (kg/m2) | 24.7 ± 2.9 | 23.8 ± 3.5 | 0.423 |
| Hypertension | 3 (15.8) | 3 (16.7) | 1.000 |
| Diabetes | 0 (0.0) | 1 (5.0) | 0.305 |
| Diagnosis | 0.213 | ||
| Tumour | 12 (63.2) | 9 (50.0) | |
| Myelopathy | 2 (10.5) | 5 (27.8) | |
| Fracture | 1 (5.3) | 3 (16.7) | |
| Others | 4 (21.1) | 1 (5.6) | |
| Operation site | 0.338 | ||
| Cervical | 3 (15.8) | 6 (28.6) | |
| Thoracic | 8 (42.1) | 9 (50.0) | |
| Lumbar | 6 (31.6) | 2 (11.1) | |
| Sacral | 2 (10.5) | 1 (5.6) | |
| Ambient temperature (°C) | 19.0 [17.5–21.5] | 18.5 [17.5–19.8] | 0.537 |
| Preoperative forehead skin temperature (°C) | 36.5 [36.4–36.6] | 36.6 [36.5–36.7] | 0.161 |
| Preoperative skin temperature difference between forearm and fingertip (°C) | 3.6 [0.8–4.9] | 3.8 [1.1–4.9] | 0.927 |
| Operation time (min) | 208 [166–244] | 194 [158–249] | 0.506 |
| Anaesthesia time (min) | 260 [221–309] | 250 [224–316] | 0.862 |
| Amount of administered propofol (mg) | 2000 [1603–2293] | 1705 [1300–2185] | 0.222 |
| Amount of administered remifentanil (µg) | 2000 [1600–2386] | 1900 [1175–2350] | 0.437 |
| Amount of administered crystalloids (ml) | 1600 [1200–1975] | 2000 [1438–2438] | 0.084 |
| Estimated blood loss (ml) | 400 [262–488] | 550 [300–775] | 0.319 |
| Transfusion | 2 (10.5) | 1 (5.6) | 0.585 |
| Urine output (ml) | 787 ± 564 | 674 ± 496 | 0.491 |
Data are presented as mean ± standard deviation, median [interquartile range], or number (%).
Patients in Group Z were mechanically ventilated with zero end-expiratory pressure, while those in Group P were mechanically ventilated with 10 cmH2O positive end-expiratory pressure.
The median [interquartile range] core temperature 180 minutes after intubation was 36.1°C [35.9°C–36.2°C] in Group Z and 36.0°C [35.9°C–36.4°C] in Group P. The median [interquartile range] delta core temperature was −0.9°C [−1.3°C to −0.7°C] and −0.8°C [−1.0°C to −0.8°C] in Groups Z and P, respectively. The overall incidence rate of intraoperative hypothermia was 68.4% in Group Z and 61.1% in Group P. The intraoperative core temperature was not significantly different between the two groups at each time point (Figure 2).
Figure 2.
Changes in core body temperature. There is no statistically significant difference between Group Z (zero end-expiratory pressure, blue box) and Group P (10 cmH2O of positive end-expiratory pressure, red stripe box) at each time point. In the vertical box plot, the median value is the horizontal line in the box and the upper/lower ends of the box indicate interquartile ranges.
T1: 30 minutes after tracheal intubation. T2: 60 minutes after tracheal intubation. T3: 90 minutes after tracheal intubation. T4: 120 minutes after tracheal intubation. T5: 150 minutes after tracheal intubation. T6: 180 minutes after tracheal intubation.
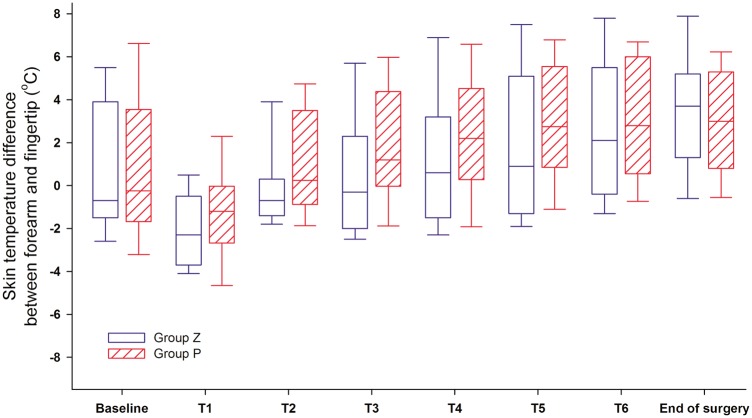
The incidence rate of peripheral vasoconstriction was 68.4% in Group Z and 88.9% in Group P (Table 2). Among patients showing peripheral vasoconstriction, the peripheral vasoconstriction threshold was 36.2°C ± 0.5°C in Group Z and 36.7°C ± 0.6°C in Group P (P = 0.046). The onset of peripheral vasoconstriction was 66 [60–129] minutes in Group Z and 38 [28–70] minutes in Group P (P = 0.025). The forearm–fingertip skin temperature difference was not significantly different between the two groups at each time point (Figure 3).
Table 2.
Intraoperative thermoregulatory responses
| Variables | Group Z (n = 19) | Group P (n = 18) | P value |
|---|---|---|---|
| Incidence of peripheral vasoconstriction | 13 (68.4) | 16 (88.9) | 0.136 |
| Threshold of peripheral vasoconstriction (°C) | 36.2 ± 0.5 | 36.7 ± 0.6 | 0.046 |
| Onset of peripheral vasoconstriction (min) | 66 [60–129] | 38 [28–70] | 0.025 |
Data are presented as mean ± standard deviation, median [interquartile range], or number (%).
Patients in Group Z were mechanically ventilated with zero end-expiratory pressure, while those in Group P were mechanically ventilated with 10 cmH2O positive end-expiratory pressure.
Figure 3.
Change in skin temperature difference between forearm and fingertip. There is no statistically significant difference between Group Z (zero end-expiratory pressure, blue box) and Group P (10 cmH2O of positive end-expiratory pressure, red stripe box) at each time point. In the vertical box plot, the median value is the horizontal line in the box and the upper/lower ends of the box indicate interquartile ranges.
T1: 30 minutes after tracheal intubation. T2: 60 minutes after tracheal intubation. T3: 90 minutes after tracheal intubation. T4: 120 minutes after tracheal intubation. T5: 150 minutes after tracheal intubation. T6: 180 minutes after tracheal intubation.
Intraoperative haemodynamic data are shown in Table 3. There were no significant differences in the mean blood pressure or heart rate between the two groups at each time point. No patients developed nausea or vomiting in the post-anaesthesia care unit. Shivering was seen in one (5.3%) patient in Group Z and three (16.7%) in Group P.
Table 3.
Intraoperative haemodynamic and respiratory data
| Variables | Group Z (n = 19) | Group P (n = 18) | P value |
|---|---|---|---|
| Mean arterial pressure (mmHg) | |||
| Before anaesthetic induction | 105 [85–118] | 104 [96–108] | 0.990 |
| After tracheal intubation | 85 [74–94] | 84 [72–97] | 0.980 |
| 30 minutes after tracheal intubation | 74 [71–85] | 71 [68–85] | 0.521 |
| 60 minutes after tracheal intubation | 82 [72–93] | 84 [74–94] | 0.725 |
| 90 minutes after tracheal intubation | 83 [76–94] | 82 [75–87] | 0.399 |
| 120 minutes after tracheal intubation | 81 [77–92] | 80 [71–85] | 0.191 |
| 150 minutes after tracheal intubation | 77 [75–84] | 79 [75–89] | 0.563 |
| 180 minutes after tracheal intubation | 81 [75–86] | 82 [77–91] | 0.513 |
| End of surgery | 82 [76–103] | 89 [80–97] | 0.687 |
| Heart rate (beats/minute) | |||
| Before anaesthetic induction | 75 [67–79] | 82 [72–89] | 0.094 |
| After tracheal intubation | 70 [60–81] | 72 [66–82] | 0.406 |
| 30 minutes after tracheal intubation | 64 [55–70] | 65 [64–77] | 0.078 |
| 60 minutes after tracheal intubation | 61 [51–70] | 65 [62–77] | 0.029* |
| 90 minutes after tracheal intubation | 62 [55–66] | 65 [60–75] | 0.107 |
| 120 minutes after tracheal intubation | 62 [52–70] | 69 [60–80] | 0.070 |
| 150 minutes after tracheal intubation | 63 [58–73] | 71 [60–77] | 0.092 |
| 180 minutes after tracheal intubation | 64 [59–72] | 72 [60–83] | 0.092 |
| End of surgery | 62 [57–83] | 73 [66–79] | 0.102 |
| Peak inspiratory pressure (cmH2O) | |||
| After tracheal intubation | 13 [12–18] | 21 [19–23] | <0.001 |
| 30 minutes after tracheal intubation | 15 [13–18] | 24 [22–25] | <0.001 |
| 60 minutes after tracheal intubation | 16 [13–19] | 23 [21–25] | <0.001 |
| 90 minutes after tracheal intubation | 15 [14–19] | 23 [22–25] | <0.001 |
| 120 minutes after tracheal intubation | 14 [13–20] | 23 [22–25] | <0.001 |
| 150 minutes after tracheal intubation | 15 [13–19] | 23 [22–25] | <0.001 |
| 180 minutes after tracheal intubation | 15 [13–18] | 24 [22–26] | <0.001 |
| End of surgery | 14 [14–20] | 23 [22–25] | <0.001 |
| Plateau airway pressure (cmH2O) | |||
| After tracheal intubation | 12 [11–15] | 20 [18–21] | <0.001 |
| 30 minutes after tracheal intubation | 14 [12–16] | 23 [21–24] | <0.001 |
| 60 minutes after tracheal intubation | 15 [13–18] | 22 [20–24] | <0.001 |
| 90 minutes after tracheal intubation | 14 [13–17] | 22 [21–24] | <0.001 |
| 120 minutes after tracheal intubation | 13 [12–17] | 23 [21–25] | <0.001 |
| 150 minutes after tracheal intubation | 13 [12–16] | 23 [22–24] | <0.001 |
| 180 minutes after tracheal intubation | 14 [13–17] | 23 [21–25] | <0.001 |
| End of surgery | 14 [13–18] | 22 [21–25] | <0.001 |
Data are presented as median [interquartile range].
*Not statistically significant after multiple comparisons because P < 0.00625 rather than P < 0.05 indicates statistical significance.
Patients in Group Z were mechanically ventilated with zero end-expiratory pressure, while those in Group P were mechanically ventilated with 10 cmH2O PEEP.
Discussion
In the present study, there were no significant differences in the core temperature 180 minutes after intubation or the delta core temperature between PEEP of 10 cmH2O and ZEEP used intraoperatively in patients undergoing spine surgery in the prone position. However, patients receiving PEEP of 10 cmH2O showed a higher peripheral vasoconstriction threshold and an earlier vasoconstriction onset than those receiving ZEEP.
In general, the core body temperature during general anaesthesia decreases rapidly via core-to-peripheral heat redistribution caused by the vasodilatory effect of anaesthetics,12 particularly within 1 hour after induction of anaesthesia, and slowly decreases over the following 3 to 4 hours because of heat loss to the environment via convection and radiation.19,20 In additional to anaesthetic-induced core hypothermia, our study population may have been vulnerable to intraoperative hypothermia because the use of some techniques, such as application of a heating pad/mattress and water-heating mattress, which are routinely used in patients in the supine position, can be limited in patients undergoing spine surgery in the prone position. Therefore, we examined other ways to reduce intraoperative core hypothermia and conducted the present study to determine the beneficial effect of PEEP on the intraoperative core temperature.
Baroreceptor unloading can result from plasma volume reduction due to the use of diuretics, upright posture, and application of PEEP.10,21–23 Baroreceptor unloading is known to modify thermoregulatory responses to body temperature changes. That is, baroreceptor unloading attenuates core hypothermia in cold environments by increasing the body temperature threshold for peripheral vasoconstriction, while it aggravates core hyperthermia in hot environments by increasing the body temperature threshold for peripheral vasodilation.10,11,21 Intraoperative thermoregulatory peripheral vasoconstriction plays a pivotal role in the thermoregulatory response to decreased body temperature. Peripheral vasoconstriction is known to be mediated by norepinephrine released from sympathetic nerves.11,24
Numerous previous reports have indicated that peripheral vasoconstriction by PEEP-induced baroreceptor unloading effectively prevents intraoperative hypothermia.10,11,13,14 Although the present study revealed the beneficial effect of PEEP on thermoregulatory reactions (a high threshold and rapid onset of peripheral vasoconstriction), there were no significant differences in the core temperature changes over time between the two groups. This discrepancy regarding the effects of PEEP on intraoperative core temperature may be explained in part by the cold environment and prone positioning in our experimental setting. First, the ambient temperature was quite low in this study. Therefore, a substantial decrease in the core temperature during surgery was fully expected. We believe that the beneficial effect of PEEP-induced thermoregulatory vasoconstriction on body temperature preservation was overwhelmed by significant heat loss to the environment via convection and radiation. Second, prone positioning itself may affect PEEP-induced thermoregulatory vasoconstriction via haemodynamic changes. Placing patients in the prone position can reduce the cardiac index and increase intrathoracic pressure,25 which can result in baroreceptor unloading and subsequent peripheral vasoconstriction. Indeed, the present showed a higher incidence of peripheral vasoconstriction in patients receiving ZEEP than did previous studies.10,13,14 We believe that prone positioning itself attenuated the beneficial effect of PEEP-induced thermoregulatory vasoconstriction because prone positioning and PEEP have similar effects on baroreceptor unloading. Finally, our intraoperative heat-retaining care including the use of a fluid warming device and forced-air warmer system may also induce peripheral vasodilation, thereby reducing the extent of PEEP-induced peripheral vasoconstriction.
In accordance with previous reports,10,11,13,14 this study showed that PEEP of 10 cmH2O had a higher peripheral vasoconstriction threshold than ZEEP. This study and two previous studies showed that the mean peripheral vasoconstriction threshold was 36.2°C to 36.7°C when PEEP of 10 cmH2O was applied.10,11 Two other studies using PEEP of 5 cmH2O showed a mean peripheral vasoconstriction threshold of 35.7°C.13,14 These findings suggest that the PEEP level may be positively correlated with the peripheral vasoconstriction threshold. In addition, a previous study demonstrated that the thermoregulatory peripheral vasoconstriction threshold induced by PEEP was dependent on the anaesthetics used.14 Total intravenous anaesthesia with propofol and remifentanil showed a higher peripheral vasoconstriction threshold than inhalation anaesthesia with desflurane.
There were several limitations in the present study. First, the transmural pressure of the right atrium to assess baroreceptor unloading and haemodynamic parameters that reflect the patient’s volume status, such as cardiac index and stroke volume variation, were not directly measured in this study. In addition, the serum norepinephrine level was not measured. A previous study indicated that PEEP-induced baroreceptor unloading augmented the catecholamine response to core hypothermia.11 Second, the use of a high PEEP of >15 cmH2O might facilitate baroreceptor unloading during surgery. However, because such a high PEEP can result in haemodynamic instability, intraoperative PEEP of 10 cmH2O was applied in this study. Finally, the intra-abdominal pressure was not measured in this study. Increased intra-abdominal pressure leads to increased intrathoracic pressure and decreased venous return,26 which can affect thermoregulatory vasoconstriction. In this study, the Jackson table was used for prone positioning in all patients. A recent study showed that the Jackson table minimally increased the intra-abdominal pressure even in the prone position with PEEP of 9 cmH2O.27
Conclusions
In the present study, intraoperative PEEP of 10 cmH2O did not reduce the intraoperative core temperature decrease in patients undergoing spine surgery in the prone position, although it resulted in an earlier onset and higher threshold of peripheral vasoconstriction compared with ZEEP. Such results suggest that when intraoperative heat-retaining care is routinely provided to prevent intraoperative hypothermia in clinical practice, PEEP does not have additional benefits in diminishing intraoperative hypothermia.
Acknowledgements
None.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Yamasaki H, Tanaka K, Funai Y, et al. The impact of intraoperative hypothermia on early postoperative adverse events after radical esophagectomy for cancer: a retrospective cohort study. J Cardiothorac Vasc Anesth 2014; 28: 955–959. [DOI] [PubMed] [Google Scholar]
- 2.Moslemi-Kebria M, El-Nashar SA, Aletti GD, et al. Intraoperative hypothermia during cytoreductive surgery for ovarian cancer and perioperative morbidity. Obstet Gynecol 2012; 119: 590–596. [DOI] [PubMed] [Google Scholar]
- 3.Sumer BD, Myers LL, Leach J, et al. Correlation between intraoperative hypothermia and perioperative morbidity in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg 2009; 135: 682–686. [DOI] [PubMed] [Google Scholar]
- 4.Tekgul ZT, Pektas S, Yildirim U, et al. A prospective randomized double-blind study on the effects of the temperature of irrigation solutions on thermoregulation and postoperative complications in percutaneous nephrolithotomy. J Anesth 2014; 29:165–169. [DOI] [PubMed] [Google Scholar]
- 5.Lenhardt R, Marker E, Goll V, et al. Mild intraoperative hypothermia prolongs postanesthetic recovery. Anesthesiology 1997; 87: 1318–1323. [DOI] [PubMed] [Google Scholar]
- 6.Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med 2009; 37: S186–S202. [DOI] [PubMed] [Google Scholar]
- 7.Rajagopalan S, Mascha E, Na J, et al. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology 2008; 108: 71–77. [DOI] [PubMed] [Google Scholar]
- 8.Reynolds L Beckmann J andKurz A.. Perioperative complications of hypothermia. Best Pract Res Clin Anaesthesiol 2008; 22: 645–657. [DOI] [PubMed] [Google Scholar]
- 9.Rohrer MJ andNatale AM.. Effect of hypothermia on the coagulation cascade. Crit Care Med 1992; 20: 1402–1405. [DOI] [PubMed] [Google Scholar]
- 10.Mizobe T, Nakajima Y, Sunaguchi M, et al. Clonidine produces a dose-dependent impairment of baroreflex-mediated thermoregulatory responses to positive end-expiratory pressure in anaesthetized humans. Br J Anaesth 2005; 94: 536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakajima Y, Mizobe T, Takamata A, et al. Baroreflex modulation of peripheral vasoconstriction during progressive hypothermia in anesthetized humans. Am J Physiol Regul Integr Comp Physiol 2000; 279: R1430–R1436. [DOI] [PubMed] [Google Scholar]
- 12.Sessler DI. Temperature monitoring and perioperative thermoregulation. Anesthesiology 2008; 109: 318–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.An TH andYang JW.. Effects of PEEP on the thermoregulatory responses during TIVA in patients undergoing tympanoplasty. Korean J Anesthesiol 2011; 61: 302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung KT, Kim SH, Lee HY, et al. Effect on thermoregulatory responses in patients undergoing a tympanoplasty in accordance to the anesthetic techniques during PEEP: a comparison between inhalation anesthesia with desflurane and TIVA. Korean J Anesthesiol 2014; 67: 32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yokoyama M, Ueda W, Hirakawa M, et al. Hemodynamic effect of the prone position during anesthesia. Acta Anaesthesiol Scand 1991; 35: 741–744. [DOI] [PubMed] [Google Scholar]
- 16.Wadsworth R Anderton JM andVohra A.. The effect of four different surgical prone positions on cardiovascular parameters in healthy volunteers. Anaesthesia 1996; 51: 819–822. [DOI] [PubMed] [Google Scholar]
- 17.Hatada T, Kusunoki M, Sakiyama T, et al. Hemodynamics in the prone jackknife position during surgery. Am J Surg 1991; 162: 55–58. [DOI] [PubMed] [Google Scholar]
- 18.Lee HK, Jang YH, Choi KW, et al. The effect of electrically heated humidifier on the body temperature and blood loss in spinal surgery under general anesthesia. Korean J Anesthesiol 2011; 61: 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz M andBecker DE.. Thermoregulation: physiological and clinical considerations during sedation and general anesthesia. Anesth Prog 2010; 57: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurz A, Sessler DI, Christensen R, et al. Heat balance and distribution during the core-temperature plateau in anesthetized humans. Anesthesiology 1995; 83: 491–499. [DOI] [PubMed] [Google Scholar]
- 21.Brozmanova A, Jochem J, Javorka K, et al. Effects of diuretic-induced hypovolemia/isosmotic dehydration on cardiorespiratory responses to hyperthermia and its physical treatment in rabbits. Int J Hyperthermia 2006; 22: 135–147. [DOI] [PubMed] [Google Scholar]
- 22.Nakajima Y, Takamata A, Ito T, et al. Upright posture reduces thermogenesis and augments core hypothermia. Anesthesia and analgesia 2002; 94: 1646–1651. [DOI] [PubMed] [Google Scholar]
- 23.Nakajima Y, Mizobe T, Matsukawa T, et al. Thermoregulatory response to intraoperative head-down tilt. Anesth Analg 2002; 94: 221–226. [DOI] [PubMed] [Google Scholar]
- 24.Charkoudian N. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin Proc 2003; 78: 603–612. [DOI] [PubMed] [Google Scholar]
- 25.Edgcombe H Carter K andYarrow S.. Anaesthesia in the prone position. Br J Anaesth 2008; 100: 165–183. [DOI] [PubMed] [Google Scholar]
- 26.Hedenstierna G andLarsson A.. Influence of abdominal pressure on respiratory and abdominal organ function. Curr Opin Crit Care 2012; 18: 80–85. [DOI] [PubMed] [Google Scholar]
- 27.Kim E, Kim HC, Lim YJ, et al. Comparison of intra-abdominal pressure among 3 prone positional apparatuses after changing from the supine to the prone position and applying positive end-expiratory pressure in healthy euvolemic patients: a prospective observational study. J Neurosurg Anesthesiol 2017; 29: 14–20. [DOI] [PubMed] [Google Scholar]