Abstract
Arsenic exposure has been associated with low birth weight. However, the underlying mechanisms are not well understood. Alterations to metabolites may act as causal mediators of the effect of arsenic exposure on low birth weight. This pilot study aimed to explore the role of metabolites in mediating the association of arsenic exposure on infant birth weight. Study samples were selected from a well-established prospectively enrolled cohort in Bangladesh comprising 35 newborns and a subset of 20 matched mothers. Metabolomics profiling was performed on 35 cord blood samples and 20 maternal peripheral blood samples collected during the second trimester of pregnancy. Inorganic arsenic (iAs) exposure was evaluated via cord blood samples and maternal toenail samples collected during the first trimester. Multiple linear regression and mediation analyses were used to explore the relationship between iAs exposure, metabolite alterations, and low birth weight. Cord blood arsenic level was correlated with elevated levels of 17-methylstearate, laurate (12:0) and 4-vinylphenol sulfate along with lower birth weight. Prenatal maternal toenail iAs level was associated with two peripheral blood metabolites (butyrylqlycine and tartarate), which likely contributed to higher cord blood iAs levels both independently and interactively. Findings of this pilot study indicate that both intrauterine and maternal peripheral blood metabolites appear to influence the toxic effect of inorganic arsenic exposure on low birth weight.
Keywords: Arsenic exposure, environmental health, global health, heavy metals, metabolomics, reproductive health
INTRODUCTION
Arsenic (As) is a well-established toxicant that has been found in drinking water, soil and air from both natural and anthropogenic sources.1 Arsenic exposure occurs primarily through the intake of contaminated food and water in general populations.2 Inorganic forms of arsenic (iAs), especially trivalent arsenic (iAsIII) and oxidized arsenate (iAsV), are easily absorbed and accumulated in tissues and bodily fluids and lead to oxidative stress.3,4 For adult, accumulation of arsenic toxicants is associated with many disorders including cancer, diabetes, hepatotoxicity, nephrotoxicity, dermal toxicity, neurotoxicity, hematotoxicity and so on.5,6 Matenal/fetal arsenic exposure could also result in a wide range of adverse health effects to newborn, including metabolic diseases, carcinogenesis and adverse birth outcomes,1,7–9 as well as impact on children’s neurodevelopment.10 This is of particular concern to public health officials in Bangladesh, where about 46% of the population is exposed to arsenic above the WHO recommended limit of 10 µg/l; indeed, about 27% are exposed to levels above five times the WHO safety threshold.11
Low birth weight is the common adverse birth outcome related to arsenic exposure, defined as a live-born infant weighing <2500 g regardless of gestational age. Low birth weight is a major public health problem in Bangladesh12 and is regarded as the leading cause of the country’s high neonatal morbidity and mortality rates.13 Low birth weight carries numerous long-term health consequences, particularly metabolic-related syndromes and diseases.14 Genomic studies are emerging to explore the underlying causes of low birth weight, and recent metabolomics studies have pointed to a role for metabolites in its determination.15,16
Arsenic exposure is associated with metabolic disruption,17,18 and arsenic can easily pass through the placental barrier via diffusion.19 Therefore, cord blood is a useful source for biomarkers of maternal–fetal transfer of arsenic.20 Maternal arsenic exposure during pregnancy has considerable side effects on intrauterine development21 and arsenic toxicants are known to continue to reside in the infant’s body after delivery, which appears to have long-term consequences.22
Together, these findings support a potential causal relationship between arsenic exposure, metabolite alterations and in turn, low birth weight. We propose that metabolites in umbilical cord serum act as causal mediators of arsenic’s effect on low birth weight. We performed a metabolomics pilot study in a birth cohort in Bangladesh with a high risk of arsenic contamination.21 Our results provide new insights into the underlying mechanisms of arsenic exposure and illuminating possible prevention measures.
MATERIALS AND METHODS
Study Population
Study samples were randomly selected from a well-established birth cohort in the Sirajdikhan and Pabna Sadar upazilas of Bangladesh, as detailed previously.11,21 Thirty-five newborns were recruited to participate in the study. All the mothers were 18 years or older at the time of pregnancy, had an ultrasound-confirmed singleton pregnancy of <28 weeks’ gestation, used the same primary drinking water source for at least six months before pregnancy, planned to live at their current residence during pregnancy, planned to continue prenatal health care with the Sirajdikhan Community Clinic, and agreed to deliver at the Dhaka Community Hospital Trust (DCH) in Bangladesh or at home with a DCH-trained midwife. Women were followed throughout their pregnancies and trained interviewers used structured questionnaires to collect sociodemographic, medical and environmental information. Health care providers also visited participants in their homes once per month to distribute prenatal vitamins, record symptoms, weigh the participants and measure their blood pressure. All births were attended by trained health care workers.
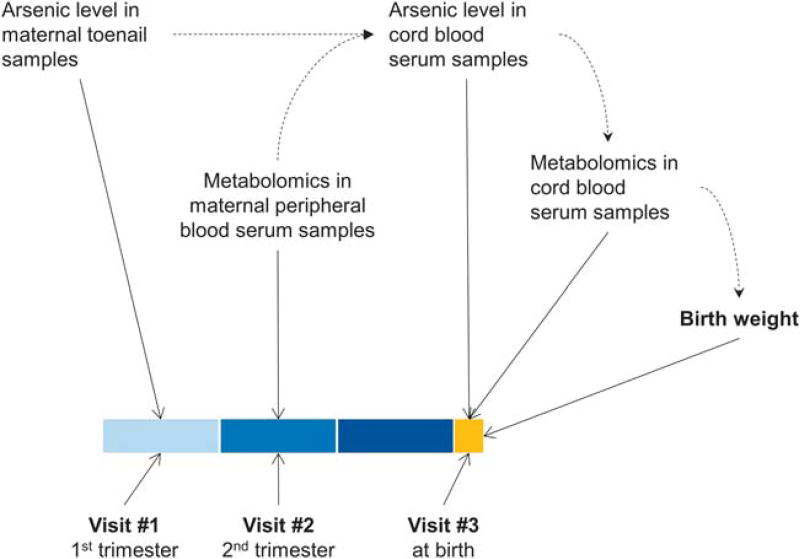
Three assessment visits were scheduled throughout the study for each participant. The first visit (visit #1) was conducted at the time of enrollment into the study, at ≤ 16 weeks of gestation, and was denoted as the first trimester of pregnancy. The second visit (visit #2) was at 28 weeks gestation, during the second trimester, and the third visit (visit #3) was conducted at delivery (Figure 1). Detailed questionnaires were administered to each participant during these visits.
Figure 1.
Study design and work flow.
This study was approved by the Institutional Review Boards of the Harvard T.H. Chan School of Public Health and DCH. All participants provided informed consent before participating in the study.
Inorganic Arsenic Exposure Evaluation
Toenail samples from each subject were collected at visit #1 and placed in individual sealed envelopes to be analyzed at the Harvard School of Public Health (HSPH) by inductively coupled plasma mass spectroscopy as previously described.23 Samples were processed according to the protocol described previously.20 Briefly, digested samples were analyzed for iAs level using a Perkin Elmer Model Elan DRC-II 6100 Inductively Coupled Plasma Mass Spectrometer (ICP-MS) (Perkin Elmer, Shelton, CT, USA). All analytical values were blank-corrected. For sample concentrations that were below the limit of detection (LOD), the value was re-assigned as the LOD divided by square root of 2.
Cord blood was collected at the time of delivery into a trace metal free Vacutainer tube containing K2EDTA (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and stored at 4 °C until it was shipped to HSPH for storage and analysis. As described previously, cord blood was analyzed for iAs level by ICP-MS following acid digestion.20 For sample concentrations below the LOD, the value was re-assigned as the LOD divided by square root of 2.
Metabolite Profiling
Sample preparation
The cord blood samples of all the recruited infants, and peripheral blood samples of a subset of 20 mothers collected at visit #2, were performed the metabolomics profiling. Maternal peripheral blood and cord blood samples collected at birth were stored in EDTA-coated vacutainer tubes (B.D. Scientific, Franklin Lakes, NJ, USA). Frozen samples were sent to Metabolon (Durham, NC, USA), and were processed according to Metabolon’s standard protocols. Briefly, proteins were precipitated with methanol under vigorous shaking for 2 mins (Glen Mills GenoGrinder 2000) and were removed by centrifugation. Each serum sample was divided into five fractions and analyzed by UPLC–MS/MS with positive ion mode electrospray ionization, UPLC–MS/MS with negative ion mode electrospray ionization, liquid chromatography (LC) polar platform, gas chromatography/mass spectrometry (GC–MS), and one reserved for backup. Samples were placed briefly on a TurboVap (Zymark, Westborough, MA, USA) to remove the organic solvent. For LC, the samples were stored overnight in nitrogen before preparation for analysis. For GC, each sample was dried under a vacuum overnight before preparation for analysis.
Data quality evaluation
Instrument variabilities were determined by calculating the median relative standard deviation for the standards that were added to each sample before injection into the mass spectrometers. Overall process variability was determined by calculating the median relative standard deviation for all endogenous metabolites (i.e., non-instrument standards) present in 100% of the pooled matrix samples. In this study, values for instrument (3%) and process variability (6%) met Metabolon’s acceptance criteria.
Compound identification
Compounds were identified by comparison to library entries of purified standards or recurrent unknown entities. More than 3300 commercially available purified standard compounds have been acquired and registered in laboratory information management system for distribution to both the LC–MS and GC–MS platforms for determination of analytical characteristics.
Normalization
All samples were tested within two days. Thus, a data normalization step was performed to correct for variation resulting from instrument tuning variation. Each compound was corrected in run-day blocks by registering the medians to equal one (1.00) and normalizing each data point proportionately. Normalized metabolites were used for initial analysis.
Anthropometric Measurement
Birth weight was measured on a pediatric scale that was calibrated before each measurement, and weight was rounded to the nearest 10 g. Infant length, weight and head circumference were measured following standard clinical protocols.24
Statistical Analysis
Characteristics of the study population were described by mean and SD for continuous variables and frequency (%) for categorical variables. Multiple linear regression was performed on the relationship between newborn cord blood iAs levels and cord blood metabolites and on the relationship between cord blood metabolite levels and birth weight. The mediating effects of three metabolites (Mcord_blood), which were nominally significant in both models (P < 0.05), were further evaluated for nature indirect effects (NIE) and nature direct effects (NDE) on the impact of cord blood iAs levels (iAscord_blood) on birth weight by VanderWeele’s mediation approach25,26 as follows:
| (1) |
To evaluate the joint effect of the three metabolites, the combined metabolite score (CMS) was calculated by the linear combination of the three metabolites weighted by the corresponding coefficients:
| (1) |
where the coefficients were estimated by the corresponding multivariate linear regression:
| (3) |
The combined metabolites score was analyzed against the association with cord blood iAs level, birth weight, and its mediating effect accordingly. All models were adjusted for the gender of the newborn, birth gestational age and the mother’s education level. Maternal ages were evaluated against the association with birth weight firstly in quadratic from, which, due to small sample sizes in this pilot study, was not significant, and thus was not adjusted in the following models.
Additionally, to explore the maternal biomarkers for intrauterine iAs exposure, we conducted multiple linear regressions of the maternal toenail iAs exposure level and the maternal peripheral blood metabolite correlation with cord blood iAs level, respectively. Models were compared by log-likelihood ratios.
All analyses were conducted using SAS Version 9.4 (SAS Institute, Cary, NC, USA) or R version 3.2.2 (The R Foundation).
RESULTS
The distribution of demographic characteristics among 35 umbilical cord serum samples, as well as a subset of 20 infants whose maternal peripheral blood was also sampled, is described in Table 1. No significant differences were observed between the entire set and the subset.
Table 1.
Characteristics of study population.
| Variable | Overall samples (N = 35) |
Paired samplesa (N = 20) |
|---|---|---|
| Baby gender, n(%) | ||
| Male | 18 (0.51) | 10 (0.50) |
| Female | 17 (0.49) | 10 (0.50) |
| Birth weight, g, mean ± SD | ||
| Male | 3049.44 ± 286.94 | 3172.00 ± 252.84 |
| Female | 2657.06 ± 358.81 | 2652.00 ± 409.03 |
| Birth length, cm, mean ± SD | ||
| Male | 46.78 ± 1.40 | 47.00 ± 1.56 |
| Female | 45.71 ± 2.39 | 45.80 ± 3.01 |
| Head circumference, cm, mean ± SD | 33.13 ± 1.31 | 33.48 ± 1.12 |
| Birth gestational age, month, mean ± SD | 38 ± 1.86 | 38.05 ± 2.19 |
| N of gestational age <37 weeks | 8 | 5 |
| Mother's pregnancy weight, kg, mean ± SD | 54.29 ± 7.23 | 56.6 ± 7.37 |
| Mother's education level, n(%) | ||
| Primary education or less | 12 (0.34) | 9 (0.45) |
| Secondary education or higher | 23 (0.66) | 11 (0.55) |
| Mother's age at pregnancy, year, mean ± SD | ||
| Male | 22.72 ± 3.68 | 23.8 ± 3.65 |
| Female | 22.35 ± 2.60 | 23.2 ± 2.39 |
A subset of 20 infants whose mothers’ peripheral serum samples were collected at the second trimester.
Among 35 umbilical cord blood serum samples, 638 metabolites were detected (Supplementary Table 1). Only 437 metabolites had a missing rate <5%, which were initially analyzed by linear regression. Twenty-five metabolites showed a nominally significant (P < 0.05) dose–response with newborn cord blood iAs levels (loge scale) (Supplementary Table 2). None reached significance after correction for multiple testing by false discovery rate (FDR-q < 0.05) due to the small sample size in this pilot study. However, interestingly, a significantly higher percentage of fatty acid pathways were found to be disrupted by iAs exposure (10 of 25, 40%) compared to the metabolome-wide background (98 of 638, 15%) (P = 0.003). On the other hand, considering that a large number of metabolites were highly correlated, we further pruned the metabolites by correlation r ≥ 0.5. Ninety-seven relatively independent metabolites were further adjusted for multiple testing. Three biochemical metabolites retained significant correlation with cord blood iAs levels after adjustment for multiple testing, including 4-ethylphenylsulfate, of the benzoate metabolism pathway (increase of metabolite level per SD of iAs level (b) = 1.39, 95% CI 0.69–2.09; P = 0.0003; FDR-q = 0.0308), vanillylmandelate (VMA), of the phenylalanine and tyrosine metabolism pathway (b = 0.40, 95% CI 0.18–0.62; P = 0.0008; FDR-q = 0.0407) and 1,5-anhydroglucitol (1,5-AG), of the glycolysis–gluconeogenesis–pyruvate metabolism pathway (b = 0.26, 95% CI 0.11–0.40; P = 0.0014; FDR-q = 0.0458) (Supplementary Table 3).
We also explored the relationship between the metabolites in cord serum and birth weight. Fourteen metabolites in cord serum showed a nominally significant association (P < 0.05) with infant birth weight in a dose–response analysis (Supplementary Table 4). None reached the most stringent criteria for multiple testing correction (FDR-q < 0.05). However, interestingly, three metabolites were nominally associated with both cord serum iAs exposure and birth weight, including 17-methylstearate, laurate (12:0) and 4-vinylphenol sulfate (Table 2).
Table 2.
Regression analysis for cord blood arsenic, metabolites and birth weight.
| Biochemical metabolite |
Pathway | Arsenic on metabolite | Metabolite on birth weight | Mediation analysis | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| β (95% CI)a | P(q)b | β (95% CI)a | P(q) | IE (95% CI)a | P-value | ||
| Laurate (12:0) | Medium chain fatty acid | 0.29 (0.05, 0.54) | 0.0211 (0.1283) | −51.57 (−99.26, −3.87) | 0.0350 | −17.46 (−50.99, 7.14) | 0.176 |
| 17-Methylstearate | Fatty acid, branched | 0.22 (0.02, 0.41) | 0.0296 (0.0079) | −61.93 (−114.98, −8.89) | 0.0236 | −17.76 (−39.51, −1.40) | 0.024 |
| 4-Vinylphenol sulfate | Benzoate metabolism | 0.36 (0.10, 0.63) | 0.0095 (0.0382) | −57.56 (−105.70, −9.43) | 0.0207 | −27.43 (−81.17, 3.12) | 0.098 |
Regression coefficient (β), indirect effect (IE) and 95% confidence intervals (95% CI) were rescaled per SD of arsenic with adjustment for birth gestational age, gender, and maternal education level by corresponding regression models.
q represents the adjusted P-value by multiple linear regression involving the cooperation of the three metabolites simultaneously.
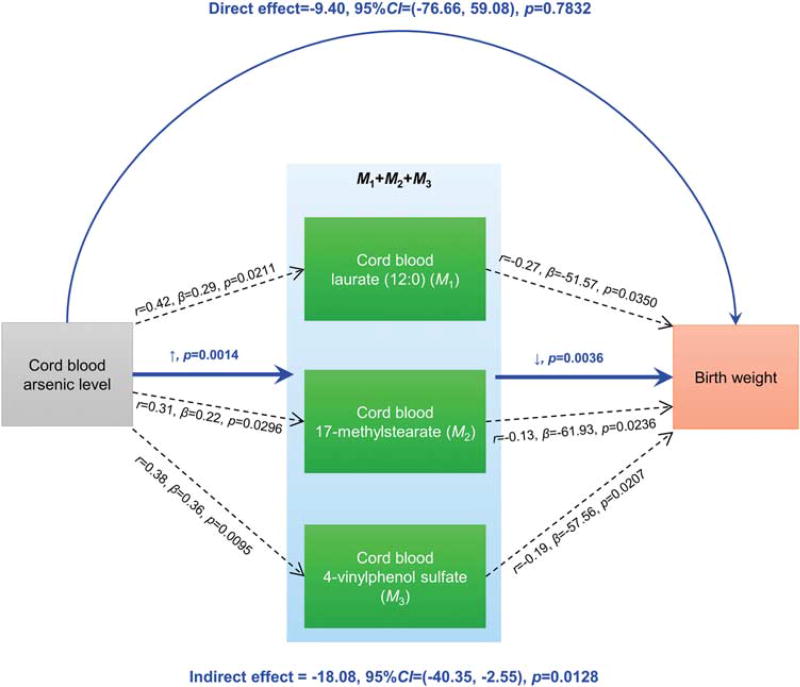
To examine whether these metabolites acted as causal mediators of the iAs side effect on birth weight, we performed a mediation analysis. A significant causal indirect effect was detected for cord serum iAs, which was mediated through 17-methylstearate (IE = −17.76 per SD of loge(iAs); 95% CI 39.51–1.40; P = 0.024; Table 2). The other two metabolites showed a reasonable trend, whereas not significant (Table 2). Additionally, we calculated the combined metabolite score by weighted linear combination of the three metabolites to test joint association. The combined metabolite score demonstrated a higher significant association with cord blood iAs level (P = 0.0014) and a stronger relationship with birth weight (P = 0.0036) (Figure 2) along with a significant mediating effect (IE = −18.08 per SD of loge(iAs); 95% CI −40.35 to −2.55; P = 0.0128), indicating that the three metabolites jointly mediated 64.50% of inorganic arsenic’s impact on birth weight.
Figure 2.
Mediation diagram of cord blood inorganic arsenic (iAs) level, metabolites and birth weight. Three metabolites from cord blood serum, laurate (12:0) (M1), 17-methylstearate (M2) and 4-vinylphenol sulfate (M3), were significantly correlated with both newborn cord blood iAs levels (loge scale) and birth weight (g) (black dash lines). The relationships were evaluated by Pearson correlation coefficient r, regression coefficient β and P-values from linear regression with adjustment for maternal education level, birth gestational age and infant gender. The combined metabolite score demonstrated a higher significant association with cord blood iAs levels (P = 0.0014) and a stronger relationship with birth weight (P = 0.0036) (blue solid lines). Mediation analysis for combined metabolites scores showed a significant mediating effect (P = 0.0164).
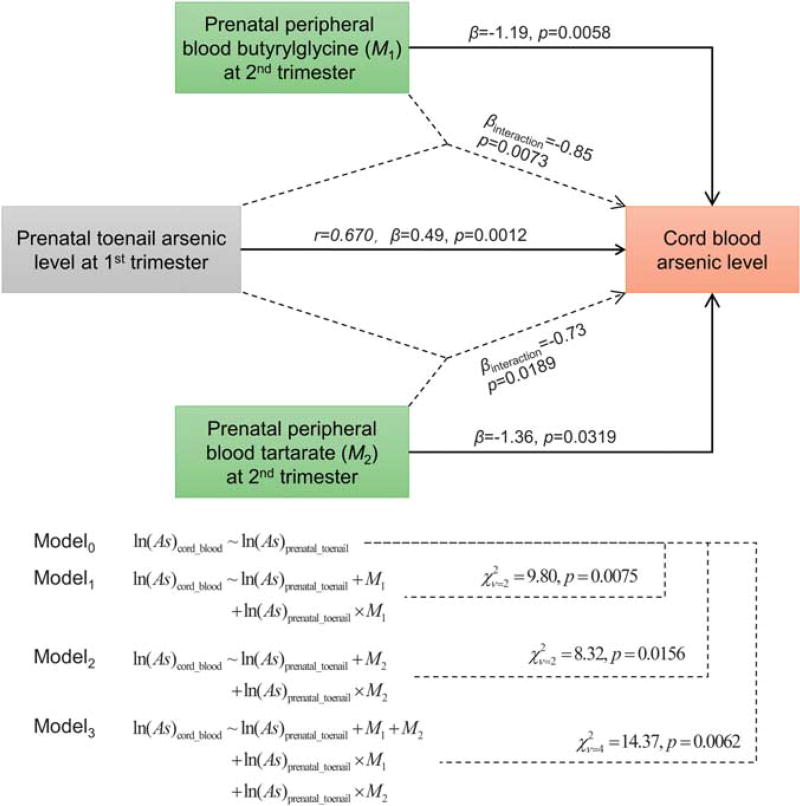
To further explore the maternal biomarkers predicting intrauterine arsenic exposure, we evaluated prenatal toenail inorganic arsenic levels during the first trimester (visit #1), incorporating the metabolomics data from the maternal peripheral samples collected during the second trimester (visit #2). Prenatal toenail iAs exposure (loge scale) was significantly correlated with cord blood iAs levels (r = 0.67, β = 0.49, P = 0.0012; Figure 3). Further, two maternal peripheral blood serum metabolites (butyrylqlycine and tartarate) were associated with lower cord serum iAs (βbutyrylqlycine = −1.19, P = 0.0058; βtartarate = −1.36, P = 0.0319) independent of prenatal toenail iAs. The two metabolites also had significant effects on the impact of maternal toenail iAs levels on birth weight (βbutyrylqlycine_interaction = −0.85, P = 0.0073; βtartarate_interaction = −0.73, P = 0.0189), as well as a stronger joint association (P = 0.0062) (Figure 3).
Figure 3.
Relationships among maternal toenail inorganic arsenic (iAs) levels, prenatal peripheral blood metabolites, and cord blood iAs levels. Prenatal toenail iAs exposure (loge scale) during the first trimester of pregnancy had a significant correlation with the cord blood arsenic level evaluated by Pearson correlation (r), and linear regression (coefficient β and P). Two maternal peripheral blood serum metabolites, butyrylqlycine (M1) and tartarate (M2) at the second trimester were associated with lower cord blood iAs levels evaluated by linear regression with adjustment for prenatal toenail iAs levels, as well as their interaction effect.
DISCUSSION
To our knowledge, this is the first study to explore comprehensively the interplay between inorganic arsenic exposure and metabolites in influencing infant birth weight. The implications of infant inorganic arsenic exposure are far reaching and the results of this study appear to provide insight into the biological mechanisms underlying this life-course epidemiology. Although the mechanisms of arsenic toxicity are not well understood, previous studies indicate that inorganic arsenic exposure may exacerbate the factors that contribute to low birth weight.27 These findings have been confirmed by a study indicating that arsenic’s side effects appear to be reduced by decreasing gestational age.11
Our findings suggest potential roles for fatty acid metabolites as mediators in inorganic arsenic toxicity. A number of studies have indicated that oxidative stress plays a role in arsenic toxicity.4,28 Interestingly, both arsenic exposure and oxidative stress are associated with metabolic disruption.18,29,30 Exposure to inorganic arsenic is tightly connected to the disrupted metabolism pathways, including fatty acid pathways.1,28,31 One of the key elements is probably fatty acid synthase,32,33 which further influence birth weight.
Specifically, we found that three arsenic-disrupted metabolites, laurate, 17-methylstearate and 4-vinylphenol sulfate, were connected with low birth weight, and jointly mediated about two-thirds of the side effects of inorganic arsenic exposure. A previous study reported that laurate in mitochondria was associated with the carnitine palmitoyltransferase (CPT) I/CPT II34 which could be inhibited by malonyl CoA—the first committed step in fatty acids synthesis.35 Considering that fatty acid is positively associated with birth weight,36 inorganic arsenic exposure may lead to low birth weight partially by affecting laurate level and, in turn, fatty acids.21 Interestingly, 4-vinylphenol sulfate, which is part of the benzoate metabolism sub-pathway, was recently found to be significantly reduced among populations with early childhood obesity.37 In our study, higher level of 4-vinylphenol sulfate was correlated with lower birth weight, which is consistent with the previous finding. 4-Vinylphenol sulfate may also be regulated by DNA methylation, and likely contributes to arsenic toxic mechanisms as well.38 Both laurate and 17-methylstearate are medium chain saturated fatty acids; however, to date, little is known about functions of 17-methylstearate, warranting further investigation.
Additionally, we identified two metabolites, butyrylglycine and tartarate, from maternal peripheral blood at the second trimester that were inversely associated with cord blood inorganic arsenic exposure levels. Our results indicate that the higher levels of maternal peripheral butyrylglycine and tartarate may attenuate the impact of maternal inorganic arsenic exposure on cord blood arsenic level, implying that butyrylglycine and tartarate could have a preventative role.
We acknowledge several limitations in our study. The sample size of this pilot study is insufficient to guarantee statistical power and further investigations are needed to evaluate the mechanisms that underlie the mediation of metabolites in the association between inorganic arsenic exposure and low birth weight. The attribution of causality is solely based on the statistical analysis, which requires well-designed functional studies. In addition, although the associations between the metabolites and outcome were adjusted for gestational age, due to insufficient preterm samples included in this pilot study, whether the associations are partially explained by preterm warrants further investigation.
In conclusion, this pilot study indicated potential roles for metabolites, originating from both intrauterine and maternal peripheral blood, in mediating the toxic pathway of inorganic arsenic exposure on low birth weight. The finding is of considerable public health importance as arsenic exposure is a growing concern among industrial regions. Future studies will continue to investigate the impact of metabolites on arsenic toxicity and investigate possible preventative measures.
Supplementary Material
Acknowledgments
We would like to acknowledge Nicola Lupoli, Ivan Pantic, Shangzhi Gao, Jongeun Rhee, Pi-I (Debby) Lin, Sakila (Joya) Afroz and Hafiza (Suchanda) Sultana for laboratory support. This study was supported by the National Institute of Environmental Health Sciences (NIEHS) (P30ES000002 and ES0015533 to DCC); the National Natural Science Foundation of China (81402764 to YW, 81402763 to RZ, 81473070 and 81530088 to FC); and the Natural Science Foundation of Jiangsu, China (No. BK20140907 to YW). The work was also partially supported by a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and the Outstanding Young Teachers Training Program of Nanjing Medical University.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
YW, FC and DCC were responsible for the study’s conception; YW and QS performed the study design, data analyses and wrote the manuscript; QQ, MR, ZW and LS collected the samples, processed the samples for analysis and performed the arsenic level evaluation; RZ contributed to the discussion and revised the manuscript. All authors approved the final version of the manuscript.
Supplementary Information accompanies the paper on the Journal of Exposure Science and Environmental Epidemiology website (http://www.nature.com/jes)
References
- 1.Garcia-Sevillano MA, Contreras-Acuna M, Garcia-Barrera T, Navarro F, Gomez-Ariza JL. Metabolomic study in plasma, liver and kidney of mice exposed to inorganic arsenic based on mass spectrometry. Anal Bioanal Chem. 2014;406:1455–1469. doi: 10.1007/s00216-013-7564-z. [DOI] [PubMed] [Google Scholar]
- 2.Kile ML, Baccarelli A, Hoffman E, Tarantini L, Quamruzzaman Q, Rahman M, et al. Prenatal arsenic exposure and DNA methylation in maternal and umbilical cord blood leukocytes. Environ Health Persp. 2012;120:1061–1066. doi: 10.1289/ehp.1104173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding W, Hudson LG, Liu KJ. Inorganic arsenic compounds cause oxidative damage to DNA and protein by inducing ROS and RNS generation in human keratinocytes. Mol Cell Biochem. 2005;279:105–112. doi: 10.1007/s11010-005-8227-y. [DOI] [PubMed] [Google Scholar]
- 4.Sepand MR, Razavi-Azarkhiavi K, Omidi A, Zirak MR, Sabzevari S, Kazemi AR, et al. Effect of acetyl-L-carnitine on antioxidant status, lipid peroxidation, and oxidative damage of arsenic in rat. Biol Trace Elem Res. 2015;171:107–115. doi: 10.1007/s12011-015-0436-y. [DOI] [PubMed] [Google Scholar]
- 5.Shen J, Wanibuchi H, Waalkes MP, Salim EI, Kinoshita A, Yoshida K, et al. A comparative study of the sub-chronic toxic effects of three organic arsenical compounds on the urothelium in F344 rats; gender-based differences in response. Toxicol Appl Pharmacol. 2006;210:171–180. doi: 10.1016/j.taap.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 6.Kitchin KT. Recent advances in arsenic carcinogenesis: modes of action, animal model systems, and methylated arsenic metabolites. Toxicol Appl Pharmacol. 2001;172:249–261. doi: 10.1006/taap.2001.9157. [DOI] [PubMed] [Google Scholar]
- 7.Rossman TG. Mechanism of arsenic carcinogenesis: an integrated approach. Mutat Res. 2003;533:37–65. doi: 10.1016/j.mrfmmm.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Laine JE, Bailey KA, Rubio-Andrade M, Olshan AF, Smeester L, Drobna Z, et al. Maternal arsenic exposure, arsenic methylation efficiency, and birth outcomes in the Biomarkers of Exposure to ARsenic (BEAR) pregnancy cohort in Mexico. Environ Health Perspect. 2015;123:186–192. doi: 10.1289/ehp.1307476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan WC, Seow WJ, Kile ML, Hoffman EB, Quamruzzaman Q, Rahman M, et al. Association of low to moderate levels of arsenic exposure with risk of type 2 diabetes in Bangladesh. Am J Epidemiol. 2013;178:1563–1570. doi: 10.1093/aje/kwt195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai SY, Chou HY, The HW, Chen CM, Chen CJ. The effects of chronic arsenic exposure from drinking water on the neurobehavioral development in adolescence. Neurotoxicology. 2003;24:747–753. doi: 10.1016/S0161-813X(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 11.Kile ML, Cardenas A, Rodrigues E, Mazumdar M, Dobson C, Golam M, et al. Estimating effects of arsenic exposure during pregnancy on perinatal outcomes in a Bangladeshi cohort. Epidemiology. 2016;27:173–181. doi: 10.1097/EDE.0000000000000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosain GM, Chatterjee N, Begum A, Saha SC. Factors associated with low birth-weight in rural Bangladesh. J Trop Pediatr. 2006;52:87–91. doi: 10.1093/tropej/fmi066. [DOI] [PubMed] [Google Scholar]
- 13.Cifuentes J, Bronstein J, Phibbs CS, Phibbs RH, Schmitt SK, Carlo WA. Mortality in low birth weight infants according to level of neonatal care at hospital of birth. Pediatrics. 2002;109:745–751. doi: 10.1542/peds.109.5.745. [DOI] [PubMed] [Google Scholar]
- 14.Simeoni U, Boubred F, Buffat C, Grandvuillemin I, Ligi I. Risk for long term disease in low birth weight infants. Arch Pediatr. 2010;17:669–670. doi: 10.1016/S0929-693X(10)70052-X. [DOI] [PubMed] [Google Scholar]
- 15.Ivorra C, Garcia-Vicent C, Chaves FJ, Monleon D, Morales JM, Lurbe E. Metabolomic profiling in blood from umbilical cords of low birth weight newborns. J Transl Med. 2012;10:142. doi: 10.1186/1479-5876-10-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciborowski M, Zbucka-Kretowska M, Bomba-Opon D, Wielgos M, Brawura-Biskupski-Samaha R, Pierzynski P, et al. Potential first trimester metabolomic biomarkers of abnormal birth weight in healthy pregnancies. Prenat Diagn. 2014;34:870–877. doi: 10.1002/pd.4386. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Shen H, Xu W, Xia Y, Barr DB, Mu X, et al. Urinary metabolomics revealed arsenic internal dose-related metabolic alterations: a proof-of-concept study in a Chinese male cohort. Environ Sci Technol. 2014;48:12265–12274. doi: 10.1021/es503659w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei Y, Wang Z, Su L, Chen F, Tejera P, Bajwa EK, et al. Platelet count mediates the contribution of a genetic variant in LRRC16A to ARDS risk. Chest. 2015;147:607–617. doi: 10.1378/chest.14-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saha KK, Engstrom A, Hamadani JD, Tofail F, Rasmussen KM, Vahter M. Pre- and postnatal arsenic exposure and body size to 2 years of age: a cohort study in rural Bangladesh. Environ Health Persp. 2012;120:1208–1214. doi: 10.1289/ehp.1003378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodrigues EG, Kile M, Dobson C, Amarasiriwardena C, Quamruzzaman Q, Rahman M, et al. Maternal-infant biomarkers of prenatal exposure to arsenic and manganese. J Expo Sci Environ Epidemiol. 2015;25:639–648. doi: 10.1038/jes.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huyck KL, Kile ML, Mahiuddin G, Quamruzzaman Q, Rahman M, Breton CV, et al. Maternal arsenic exposure associated with low birth weight in Bangladesh. J Occup Environ Med. 2007;49:1097–1104. doi: 10.1097/JOM.0b013e3181566ba0. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez-Barranco M, Lacasana M, Aguilar-Garduno C, Alguacil J, Gil F, Gonzalez-Alzaga B, et al. Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: a systematic review and meta-analysis. Sci Total Environ. 2013;454–455:562–577. doi: 10.1016/j.scitotenv.2013.03.047. [DOI] [PubMed] [Google Scholar]
- 23.Chen KL, Amarasiriwardena CJ, Christiani DC. Determination of total arsenic concentrations in nails by inductively coupled plasma mass spectrometry. Biol Trace Elem Res. 1999;67:109–125. doi: 10.1007/BF02784067. [DOI] [PubMed] [Google Scholar]
- 24.Cheikh Ismail L, Knight HE, Bhutta Z, Chumlea WC. International F, Newborn Growth Consortium for the 21st C. Anthropometric protocols for the construction of new international fetal and newborn growth standards: the INTERGROWTH-21st Project. BJOG. 2013;120:42–47. doi: 10.1111/1471-0528.12125. v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bohnke JR. Explanation in causal inference: methods for mediation and interaction. Q J Exp Psychol. 2016;69:1243–1244. doi: 10.1080/17470218.2015.1115884. [DOI] [PubMed] [Google Scholar]
- 26.Valeri L, VanderWeele TJ. SAS macro for causal mediation analysis with survival data. Epidemiology. 2015;26:e23–e24. doi: 10.1097/EDE.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 27.Kile ML, Rodrigues EG, Mazumdar M, Dobson CB, Diao N, Golam M, et al. A prospective cohort study of the association between drinking water arsenic exposure and self-reported maternal health symptoms during pregnancy in Bangladesh. Environ Health. 2014;13:29. doi: 10.1186/1476-069X-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang XX, Mu XL, Zhang J, Huang QY, Alamdar AV, Tian MP, et al. Serum metabolomics reveals that arsenic exposure disrupted lipid and amino acid metabolism in rats: a step forward in understanding chronic arsenic toxicity. Metallomics. 2015;7:544–552. doi: 10.1039/c5mt00002e. [DOI] [PubMed] [Google Scholar]
- 29.Kim YJ, Hong YC, Lee KH, Park HJ, Park EA, Moon HS, et al. Oxidative stress in pregnant women and birth weight reduction. Reprod Toxicol. 2005;19:487–492. doi: 10.1016/j.reprotox.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 30.James AM, Collins Y, Logan A, Murphy MP. Mitochondrial oxidative stress and the metabolic syndrome. Trends Endocrinol Metab. 2012;23:429–434. doi: 10.1016/j.tem.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Li C, Li P, Tan YM, Lam SH, Chan EC, Gong Z. Metabolomic characterizations of liver injury caused by acute arsenic toxicity in zebrafish. PLoS One. 2016;11:e0151225. doi: 10.1371/journal.pone.0151225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carreras-Badosa G, Prats-Puig A, Puig T, Vazquez-Ruiz M, Bruel M, Mendoza E, et al. Circulating fatty acid synthase in pregnant women: relationship to blood pressure, maternal metabolism and newborn parameters. Sci Rep. 2016;6:24167. doi: 10.1038/srep24167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alberts AW, Strauss AW, Hennessy S, Vagelos PR. Regulation of synthesis of hepatic fatty acid synthetase: binding of fatty acid synthetase antibodies to polysomes. Proc Natl Acad Sci USA. 1975;72:3956–3960. doi: 10.1073/pnas.72.10.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sleboda J, Bremer J, Horn RS. Palmitate oxidation in rat hepatocytes is inhibited by foetal calf serum. Acta Physiol Scand. 2001;173:267–274. doi: 10.1046/j.1365-201X.2001.00896.x. [DOI] [PubMed] [Google Scholar]
- 35.Sasaki Y, Kozaki A, Hatano M. Link between light and fatty acid synthesis: thioredoxin-linked reductive activation of plastidic acetyl-CoA carboxylase. Proc Natl Acad Sci USA. 1997;94:11096–11101. doi: 10.1073/pnas.94.20.11096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogers I, Emmett P, Ness A, Golding J. Maternal fish intake in late pregnancy and the frequency of low birth weight and intrauterine growth retardation in a cohort of British infants. J Epidemiol Community Health. 2004;58:486–492. doi: 10.1136/jech.2003.013565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Isganaitis E, Rifas-Shiman SL, Oken E, Dreyfuss JM, Gall W, Gillman MW, et al. Associations of cord blood metabolites with early childhood obesity risk. Int J Obes. 2015;39:1041–1048. doi: 10.1038/ijo.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petersen AK, Zeilinger S, Kastenmuller G, Romisch-Margl W, Brugger M, Peters A, et al. Epigenetics meets metabolomics: an epigenome-wide association study with blood serum metabolic traits. Hum Mol Genet. 2014;23:534–545. doi: 10.1093/hmg/ddt430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.