Abstract
Olfactory deficits are present in numerous neurodegenerative disorders and are accompanied by pathology in related brain regions. In several of these disorders, olfactory disturbances appear early and are considered as prodromal symptoms of the disease. In addition, pathological protein aggregates affect olfactory regions prior to other regions, suggesting that the olfactory system might be particularly vulnerable to neurodegenerative diseases. Exposed to the external environment, the olfactory epithelium and olfactory bulb allow pathogen and toxin penetration into the brain, a process that has been proposed to play a role in neurodegenerative diseases. Determining whether the olfactory bulb could be a starting point of pathology and of pathology spread is crucial to understanding how neurodegenerative diseases evolve. We argue that pathological changes following environmental insults contribute to the initiation of protein aggregation in the olfactory bulb, which then triggers the spread of the pathology within the brain by a templating mechanism in a prion-like manner.
We review the evidence for the early involvement of olfactory structures in neurodegenerative diseases and the relationship between neuropathology and olfactory function. We discuss the vulnerability and putative underlying mechanisms by which pathology could be initiated in the olfactory bulb, from the entry of pathogens (promoted by increased permeability of the olfactory epithelium with aging or inflammation) to the sensitivity of the olfactory system to oxidative stress and inflammation. Finally, we review changes in protein expression and neural excitability triggered by pathogenic proteins that can promote pathogenesis in the olfactory bulb and beyond.
Keywords: Olfactory system, Alzheimer’s disease, Parkinson’s disease, Synucleinopathies, Tauopathies, Alpha-synuclein, Tau, Beta-amyloid, TDP-43, Neuroinflammation
1. Introduction
Identifying the mechanisms whereby neurodegenerative disorders progress throughout the brain is of critical importance both for the development of early diagnostic methods and for impeding disease progression. The occurrence of olfactory perceptual deficits in neurological disorders has been characterized for decades, and it is clear that in many of these disorders, olfactory deficits appear early, sometimes preceding the classical cognitive and motor symptoms (Alves, 2014; Barresi et al., 2012; Doty, 2012a, 2012b; Hüttenbrink et al., 2013). In some cases, olfactory dysfunction is unique during disease onset while other sensory systems are spared (e.g., Gilbert and Murphy, 2004; Meissner, 2012), suggesting that the olfactory system might, to some extent, be particularly vulnerable to some neurological disorders. Little is known about why these disorders affect the olfactory system early. It is also unclear which of all of the pathogens in each of these disorders are responsible for the olfactory impairments. Lastly, the mechanisms by which disease-associated pathogens exert their influences upon olfactory neurons are unknown.
We and others have predicted that the olfactory bulb (OB)—the first stage of olfactory system processing and in close contact with the external world—serves as an entry point for pathogens or an access point for environmental insults, which can trigger pathological changes that then can spread throughout the brain via olfactory pathways (Dando et al., 2014; Doty, 2008; Hobson, 2012). Since these neurodegenerative disorders involve proteinaceous pathogens, this prediction is in accord with the prion hypothesis, which states that by spreading in the brain and acting as templates for endogenous proteins to form pathological aggregates, misfolded proteins resistant to degradation are responsible for disease (Aguzzi et al., 2008; Griffith, 1967; Pattison and Jones, 1967; Prusiner, 1982). Indeed, OB pathology is prevalent in the early stages of some neurological disorders (e.g., (Braak et al., 2006; Braak and Braak, 1991; Tabaton et al., 2004; Zanusso et al., 2003). We need to determine whether or not the OB is starting point for pathology and whether aberrant molecular changes there are capable of triggering the spread of protein aggregates throughout the brain. Such knowledge will be crucial for resolving the fundamental biology of neurological diseases.
In this review, we summarize what we know of the relationships between olfactory system function and neurological disorders including Parkinson’s disease (PD), Alzheimer’s disease (AD), Huntington’s disease (HD), frontotemporal dementia (FTD), amyotrophic lateral sclerosis (ALS), Creutzfeldt-Jakob disease (CJD), and others. We also review evidence for the roles of specific pathogenic features in these relationships. We discuss possible ways that olfactory pathology might be triggered in the olfactory bulb and spread throughout the brain. Finally, we synthesize concepts to support the theory that the OB is a starting point for prion-like pathogenic spread throughout the brain.
2. Olfactory dysfunction is common in many neurodegenerative diseases
Olfaction is an overarching term which implies several distinct abilities, i.e. odor detection, odor discrimination, odor memory, and odor identification (with or without a language component). Olfactory dysfunction develops with normal aging (Boyce, 2006; Landis et al., 2004; Rouby et al., 2011), and 6–16% of presumed healthy elderly are affected (Rouby et al., 2011). In neurodegenerative diseases, the percentage of people exhibiting olfactory deficits frequently exceeds 90%, depending on the disease (Godoy et al., 2015; Hawkes, 2003; Kim, 2014). The difficulty in interpreting data on olfactory dysfunction in healthy aging is that elderly people who are assumed to be healthy but have olfactory deficits could be in the early stage of an undiagnosed neurodegenerative disease. Tests of olfactory function have been included as part of the battery of diagnostic tools for PD and AD (Doty, 2012a; Miller and O’Callaghan, 2015; Yoon et al., 2015). In the following sections, we describe olfactory dysfunction in neurodegenerative diseases, with a focus on PD and AD and brief descriptions of other important neurodegenerative conditions in which olfactory deficits are prevalent.
2.1. Parkinson’s disease and other related synucleinopathies
While olfactory dysfunction has been well studied in PD, data from other synucleinopathies are limited. Here we cull the most important observations in PD and review literature on other synucleinopathies.
2.1.1. Parkinson’s disease
Olfactory deficits in PD, first identified by Ansari and Johnson (1975), are considered as one of the earliest neurological signs of PD, preceding the appearance of the classical motor deficits by at least 4 years (Alves, 2014; Attems et al., 2014; Doty, 2012a, 2012b; Doty et al., 1992b; Haehner et al., 2007; Herting et al., 2008; Ponsen et al., 2004; Ross et al., 2006, 2008). Only rarely are PD patients normosmic (Rossi et al., 2016). Olfactory dysfunction is present both in familial and sporadic PD (Doty, 2012a) and occurs in more than 90% of cases (Doty, 2012a; Doty et al., 1988; Hawkes et al., 1997b). These observations are consistent despite the use of different olfactory tests across more than 100 studies published since 1980 (Doty, 2012b). These tests mainly probed olfactory discrimination and odor identification (Doty et al., 1996, 1984a, 1984b), but odor detection and odor memory were also assessed. Olfaction was proposed as a diagnostic criterion for prodromal PD by the International Parkinson and Movement Disorder Society (MDS) (Postuma et al., 2015).
Anosmia (total loss of the sense of smell) occurs only in a small minority of PD patients, while hyposmia (reduced sense of smell) is more common (Doty et al., 1988). PD patients also have deficits in odor detection, discrimination, and identification (Doty, 2012a; Doty et al., 1988, 2014; Hudry et al., 2003; Kranick and Duda, 2008; Zucco et al., 2001), as well as in odor hedonicity (perception of pleasantness) (Hudry et al., 2003; Mrochen et al., 2016). Mild deficits in odor recognition have been described in PD, but it is unclear if they instead were really due to defective detection (Boesveldt et al., 2009). Odor identification deficits affect 82–90% of PD patients (Cavaco et al., 2015). These deficits are summarized in Table 1.
Table 1.
Olfactory perception includes odor detection (lowest perceptible concentration),[ odor discrimination (distinction of two different odorants), odor identification (naming odors), odor retention memory, and judgment of odor hedonicity (pleasantness). The University of Pennsylvania Smell Identification Test (UPSIT) and cross-cultural UPSIT-derived tests are the most common methods used to assess olfaction in neurodegenerative diseases
{Doty et al., 1996, 1984b, 1984a}, and can be used to compare the severity of olfactory deficits in different diseases. Abbreviations: PD: Parkinson’s disease; iLBD: incidental Lewy body disorder; DLB: dementia with Lewy bodies; AD: Alzheimer’s disease; MSA: multiple system atrophy; PSP: progressive supranuclear palsy; FTD: frontotemporal dementia; ALS: amyotrophic lateral sclerosis; PAF: pure autonomic failure; HD: Huntington’s disease; CJD: Creutzfeldt-Jakob disease.
Limited number of patients studied. — Data not reported/not studied.
The origin of olfactory loss in PD remains poorly understood, but it is believed to relate both to low- and high-order olfactory impairments. About 80% of PD patients have abnormal olfactory evoked responses (Barz et al., 1997; Hawkes, 2003; Hawkes et al., 1997a). Indeed, even detection threshold, which largely requires a lower level of perceptual processing, is impaired in PD. This is supported by observations that increased sniffing volume improves olfactory scores in PD (Sobel et al., 2001). Further, odor identification, which is considered by some dependent on central processing, is severely affected in PD (Hedner et al., 2010; Larsson et al., 2004; Rahayel et al., 2012).
Protein inclusions, inflammation in olfactory regions, and alterations in neurotransmitter systems probably play important roles in PD-related olfactory dysfunction. The great number of Lewy bodies (LBs) and Lewy neurites (LNs) in olfactory structures (described further below) could be partly responsible for the alteration of olfactory processing. People without PD who exhibit LBs in the entorhinal cortex and substantia nigra show olfactory identification deficits prior to death (Wilson et al., 2011). This supports the idea that LBs outside motor brain regions negatively affect central olfactory processing. However, there is evidence that LBs are found in the olfactory bulb and olfactory tract of virtually all PD subjects (Beach et al., 2009b). The effects of peripheral olfactory pathology (e.g., in the olfactory epithelium, OE) on olfactory loss have not yet been defined (Wilson et al., 2011). In addition, tau pathology observed in the anterior olfactory nucleus (AON) of PD patients could also contribute to olfactory deficits (Doty, 2012a; Tsuboi et al., 2003). Olfaction is also mildly impaired In incidental Lewy body disease (iLBD; deceased individuals who have LBs in the brain but had no PD symptoms), which is thought to represent prodromal PD or a forerunner of other synucleinopathies (Adler et al., 2010; Driver-Dunckley et al., 2014; Ross et al., 2006).
Numerous studies have investigated whether olfactory loss could be a biomarker for motor dysfunction or cognitive impairments. The earliest cross-sectional studies suggested that olfactory dysfunction in PD is stable over time (Doty et al., 1988; Quinn et al., 1987); thus, it does not clearly correlate with severity of the motor dysfunction or disease stage. It is also not influenced by standard anti-parkinsonian treatments (Doty et al., 1992b), although a recent study suggested that the monoamine oxidase B inhibitor rasagiline might improve odor discrimination in early stage PD (Haehner et al., 2015). Recent investigations suggest a slight worsening of olfactory function over time (Berendse et al., 2011; Tissingh et al., 2001), and marked changes in olfactory threshold and odor discrimination alterations correlate with more rapid disease progression (Ansari and Johnson, 1975; Cavaco et al., 2015; Hawkes, 2003; Tissingh et al., 2001). Other studies have suggested that poor olfactory scores in PD correlate with dementia scores (Alves, 2014; Baba et al., 2012; Damholdt et al., 2011; Lee et al., 2014; Ross et al., 2008). In one study, only PD patients with severe hyposmia and mild cognitive impairments developed severe dementia after 3 years, suggesting that severe hyposmia is associated with increased dementia risk (Alves, 2014; Baba et al., 2012). A recent study demonstrated that in a large cohort of newly diagnosed people with PD, aside from high age, hyposmia and sleep disorder were the two strongest predictors, of a range of measures, that cognitive decline would develop two years later (Schrag et al., 2016). A different longitudinal study described unpredictable variations in olfactory function, possibly coupled to fluctuations in dopaminergic activity in the OB (Herting et al., 2008). Progressive degeneration of the cholinergic, noradrenergic, and serotoninergic neuromodulatory systems innervating olfactory structures has been suggested to correlate with olfactory loss in PD (for review, (Doty, 2012a)).
Olfactory dysfunction has not been studied extensively in familial PD, and there appears to be great heterogeneity depending on the mutation (Doty, 2012a). Two patients of eight carrying the A53T mutation of the α-synuclein (α-syn) gene (G209 on exon 4) exhibited anosmia (Bostantjopoulou et al., 2001), while no signs of olfactory deficits were detected in carriers of the α-syn E46K mutation (Krüger et al., 1998; Tijero et al., 2010). Several reports suggest that cases of autosomal recessive PD due to parkin mutations are normosmic (Alcalay et al., 2011; Khan et al., 2004; Malek et al., 2016). By contrast, patients with pink1 mutations have changes in odor identification, detection, and discrimination (Ferraris et al., 2009). Patients with the most common monogenetic form of PD, i.e., those with mutations of the LRRK2 gene (Doty, 2012a; Ferreira et al., 2007; Kertelge et al., 2010; Saunders-Pullman et al., 2011; Silveira-Moriyama et al., 2010b; Valldeoriola et al., 2011) have olfactory deficits similar to or more severe than idiopathic cases (Saunders-Pullman et al., 2011; Silveira-Moriyama et al., 2010b). Two of three studies describe altered olfaction in LRRK2 mutation carriers prior to signs of PD (Johansen et al., 2014; Kertelge et al., 2010; Saunders-Pullman et al., 2011). Finally, individuals with heterozygous GBA mutations (Doty, 2012b; Goker-Alpan et al., 2008; Saunders-Pullman et al., 2010), who have a six-fold greater risk of PD (Setó-Salvia et al., 2011), exhibit impaired olfaction after the appearance of motor deficits. In one case with a GBA mutation, it was reported that loss of olfaction occurred 30 years before the onset of PD motor symptoms (Saunders-Pullman et al., 2010). It is notable that olfactory dysfunction is not highly common in some atypical Parkinsonian disorders (Hawkes, 2006; Johansen et al., 2014; Malek et al., 2016).
2.1.2. Dementia with Lewy bodies
In dementia with Lewy bodies (DLB), where dementia is the presenting symptom and motor deficits typically appear within a year (Yoon et al., 2015), severe olfactory deficits are seen, akin to PD (Gilbert et al., 2004; Liberini et al., 2000; McShane et al., 2001; Olichney, 2005; Wilson et al., 2011) DLB patients exhibit higher prevalence of and more severe olfactory deficits than Alzheimer’s disease (AD) patients (described in a later section) (Chiba et al., 2012; Olichney, 2005; Sato et al., 2011; Westervelt et al., 2003; Williams et al., 2009). Therefore, in individuals presenting with minimal cognitive impairment, more severe olfactory deficits are predictive of conversion to DLB versus to AD (Yoon et al., 2015). The olfactory deficits are often apparent in early DLB, and might also be considered part of the emerging concept of prodromal DLB (McKeith et al., 2016).
2.2. Alzheimer’s disease
Typically, AD is (though not invariably (Westervelt et al., 2007)) characterized by a progressive worsening of olfactory function. Plentiful reports describe the types of olfactory impairments and how they correspond to AD stages. Here we summarize a selection of those reports to highlight the basic aspects of olfactory dysfunction in AD and their possible origins.
Deficits in odor detection, recognition, discrimination, and long-term odor recognition memory have been reported (e.g., (Doty, 1991; Morgan et al., 1995; Murphy et al., 1999; Serby et al., 1991)) with a prevalence of 90–100% (Attems et al., 2014; Duff et al., 2002), and they appear before cognitive impairment (Devanand et al., 2008). A meta-analysis of studies on olfactory function in AD reported that, in comparison to age-matched controls, AD patients displayed greater deficits in odor detection thresholds, identification ability, and recognition (Mesholam et al., 1998). Olfactory dysfunction appears in early stages of AD (as in PD) and precedes the clinical manifestations of minimal cognitive impairment and AD (R. S. Wilson et al., 2009). Impairments of odor discrimination and identification are more severe than those of odor detection (Fusetti et al., 2010). The overall severity of olfactory deficits in AD is equivalent to those in PD, but PD patients exhibit more severe impairment of detection threshold. This suggests that AD patients are more affected in higher-order olfactory tasks (Rahayel et al., 2012).
Some genetic factors possibly contributing to the olfactory deficits have begun to emerge, one being the presence of one or two copies of the e4 allele of apolipoprotein E, an established risk factor for spontaneous AD (Gilbert and Murphy, 2004). Other factors, found in preclinical mouse models, are the presence of mutations in the amyloid precursor protein (APP) gene (N. Cheng et al., 2011; Guérin et al., 2009; Wesson et al., 2010) or the overexpression of the human tau protein (Macknin et al., 2004).
Most evidence to date suggests that olfactory abnormalities in AD are due to central sensorineural dysfunction more than to the inability of odor information to enter the brain (“conductive” dysfunction). First, as detailed below, central olfactory structures can be greatly burdened with AD pathology during later disease stages (e.g., (Braak and Braak, 1991)). Lower odor identification test scores are associated with higher levels of AD pathology in central olfactory structures (R. S. Wilson et al., 2009), although AD pathology is also observed in the OE (Arnold et al., 1998). These data suggest that the neuroanatomical underpinnings of olfactory impairments in AD versus PD are to some extent different. Second, the hallmark perceptual deficits of reduced discrimination and recognition of odors implies that odor information at least enters the brain in AD. Third, measures of central olfactory physiology, including event-related olfactory potentials measured by scalp EEG (Gilbert and Murphy, 2004; Morgan and Murphy, 2012), functional MRI (Li et al., 2010), and local field potentials (Wesson et al., 2011), reveal a variety of central processing deficits in both humans and mice, some of which can be attributed to genetic variations associated with AD risk (Gilbert and Murphy, 2004; Morgan and Murphy, 2012; Wesson et al., 2011) Finally, the early loss of noradrenergic neurons in the locus coeruleus and cholinergic neurons in the nucleus basalis of Meynert in AD (Marien et al., 2004), which modulate olfactory activity (D’Souza and Vijayaraghavan, 2014; Doucette et al., 2007; Guérin et al., 2008; Mandairon et al., 2008; Veyrac et al., 2007; Wilson et al., 2004), could also severely impact olfaction (Daulatzai, 2015; Rey et al., 2012).
In light of the progressive worsening of olfactory dysfunction in AD, numerous studies have proposed olfactory dysfunction as a predictive biomarker for the disease. In one study, combining olfactory function scores with other biomarkers for AD (e.g., entorhinal cortex volume, amyloid-beta cerebral spinal fluid levels, verbal memory) enhanced the diagnostic accuracy for predicting persons who would progress from mild cognitive impairment (MCI) to AD (Devanand et al., 2008) Further, in aged individuals without MCI or AD, evidence suggests that olfactory dysfunction is related to the level of AD pathology and the risk for developing further prodromal AD symptoms (Wilson et al., 2009). Finally, event-related olfactory potentials measured by EEG are aberrant in non-demented individuals who are positive for the apolipoprotein E E4 allele (Morgan and Murphy, 2012). Thus, olfactory dysfunction might serve as an early biomarker of AD even prior to detectable dementia or MCI.
Interestingly, similar impairments in odor detection thresholds, identification, and recognition were found between the AD and PD groups in meta-analysis (Mesholam et al., 1998). While this presents clear difficulty in differentiating between these disorders based upon olfaction alone, these commonalities may illuminate core principles of dysfunction in the two disorders.
2.3. Examples of other neurodegenerative diseases with olfactory deficits
Several diseases with parkinsonian syndrome that can be mistaken for PD have either no or slight olfactory deficits (Doty, 2012b). This is the case for essential tremor (Applegate and Louis, 2005; Busenbark et al., 1992; Hawkes et al., 2003; Louis et al., 2008; Quagliato et al., 2009; Štenc Bradvica et al., 2015) and for the early stages of the X-linked recessive dystonia-parkinsonism (Evidente et al., 2004). Only mild deficits in odor identification are described in corticobasal degeneration (CBD) (Luzzi et al., 2007; Wenning et al., 1995b) and multiple system atrophy (MSA; for both the subtypes MSA-P and MSA-C) (Abele et al., 2003; Garland et al., 2011; Goldstein et al., 2008; Kikuchi et al., 2011; Müller et al., 2002; Nee et al., 1993; Suzuki et al., 2011; Wenning et al., 1995a). Consequently, olfactory testing is helpful for differential diagnosis between PD and other diseases with Parkinsonism (Busenbark et al., 1992; Doty et al., 1993, 1992a, 1995; Wenning et al., 1995b).
Numerous non-parkinsonian neurodegenerative diseases exhibit mild to severe olfactory dysfunction. Odor identification is impaired in the frontal variant of FTD (prevalence 96%) (Heyanka et al., 2014; Luzzi et al., 2007; McLaughlin and Westervelt, 2008; Pardini et al., 2009), in ALS (prevalence 75%) (Ahlskog et al., 1998; Elian, 1991; Hawkes et al., 1998; Takeda et al., 2015), in some patients with multiple sclerosis (Lucassen et al., 2016), and in Huntington’s disease (Bylsma et al., 1997; Hamilton et al., 1999; Lazic et al., 2007). Other olfactory abilities have not been systematically assessed, but deficits in odor recognition and memory odor detection are described in HD (Barrios et al., 2007; Hamilton et al., 1999; Nordin et al., 1995; Pirogovsky et al., 2007), in a new variant of CJD (Reuber et al., 2001), and in multiple sclerosis (depending on the location of the demyelinating lesions) (Bartosik-Psujek et al., 2004; Constantinescu et al., 1994; Doty et al., 1998; L.-M. Li et al., 2016; Lucassen et al., 2016; Lutterotti et al., 2011). Further, in HD, altered perception of odor hedonicity (Mitchell et al., 2005) and other olfactory deficits precede cognitive deficits and involuntary movements (Larsson et al., 2006; Moberg et al., 1987). It is interesting that all non-parkinsonian diseases are associated with olfactory deficits, while only PD and DLB of the parkinsonian diseases have such deficits (a non-exhaustive summary is presented in Table 1).
3. The olfactory system
In this section, we briefly describe the anatomy and physiology of the olfactory rostral structures, the olfactory mucosa and the OB. Given the extensive anatomical literature arising from rodent-based investigations, most of the descriptions herein are from rat and mouse studies.
3.1. Neuronal organization of the olfactory mucosa and olfactory bulb
The OB is a six-layer structure in which the sequential stages of odor information processing take place. In all terrestrial vertebrates, nasal airflow (e.g., sniffing) carries odorants into contact with olfactory receptors (ORs) located on the cilia of olfactory receptor neurons (ORNs) in the nasal olfactory mucosa (Fig. 1). Odorant binding with an OR triggers a G-coupled protein–mediated intracellular signaling cascade, ultimately producing an action potential (Yoshikawa and Touhara, 2015). The ORs possess unique tuning profiles (Araneda et al., 2000; Bhandawat et al., 2005) that provide a first step at which the olfactory system can sort the essentially limitless number of odorants it may encounter.
Fig. 1.
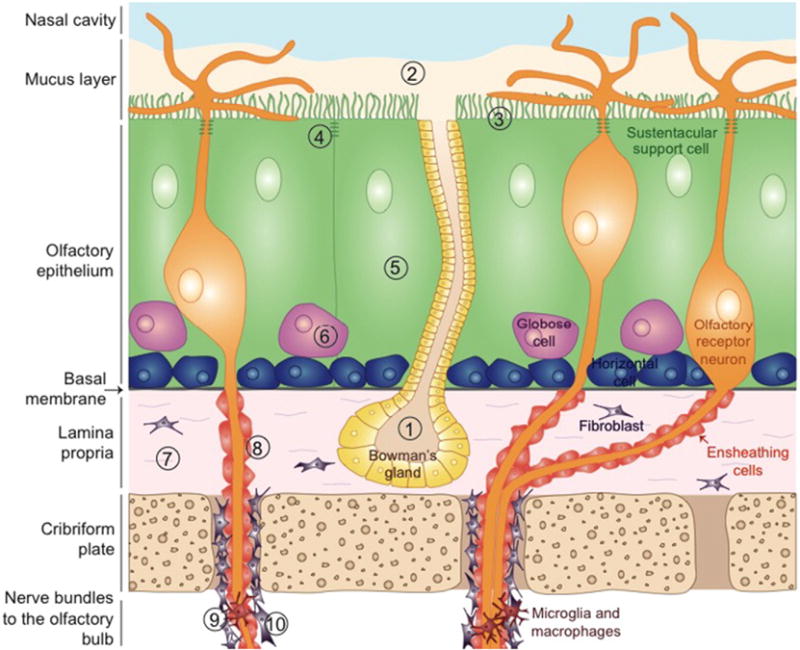
Schematic of the olfactory mucosa and different defense mechanisms against pathogens entry and cellular damages by environmental exposure. 1) Bowman’s glands secrete the olfactory mucus that contains immune factors, lysozymes, enzymes, antioxidants, and possibly xenobiotic-metabolizing enzymes capable of viral inactivation, detoxification, bacterial degradation, and destruction of pro-inflammatory molecules. 2) The mucus layer in which cilia of olfactory receptor neurons (ORNs) are immersed provides electrical insulation to the neurons and catches particles and odorants in suspension in the air. 3) Sustentacular cells maintaining water and salt balance in the mucus and metabolize xenobiotics. 4) Dense network of tight junctions between sustentacular cells and ORNs, and between the serous cells of Bowman’s glands, prevent pathogens from infiltrating the olfactory epithelium (OE). 5) Sustentacular cells might phagocytose debris and dying cells. 6) Damaged ORNs are constantly replaced by newborn ORNs, differentiating from the globose cells that derive from the horizontal cells. 7) In case of damage to the OE or contamination, neutrophils and macrophages can invade the olfactory mucosa though the lamina propria. 8) Ensheathing cells protect and electrically isolate the axons of ORNs. 9) Nerve bundles are protected by fibroblasts enveloping them. 10) Microglial population localized in nerve bundles and in external layers of the olfactory bulb is believed to be in a contact state alert.
The soma of ORNs is located in the OE, and their axons fasciculate and pass through the cribriform plate (Fig. 1), where they then form the olfactory nerve layer of the OB. In mammals, ORNs express only one OR type (Chess et al., 1994), and ORNs expressing the same OR innervate two glomeruli per OB (Mombaerts et al., 1996). Thus, in this arrangement, action potentials in the ORNs relay odorant information into discrete zones in the OB whose activation is dictated by nasal airflow (Buonviso et al., 2006; Mozell, 1964; Verhagen et al., 2007). The spatio-temporal zones formed across the OB can be plotted to form a “spatial map” of odorant information (Johnson et al., 2004; Sharp et al., 1975; Spors et al., 2006; Uchida et al., 2000) and are modulated by local glomerular layer neurons (including periglomerular, short axon, and external tufted cells) a process thought important for the most basic aspects of olfactory perception, including odor recognition and discrimination (Aungst et al., 2003; Hayar et al., 2004; Wachowiak and Shipley, 2006).
Secondary olfactory neurons, called mitral cells (MCs) and tufted cells (TCs), innervate glomeruli, where they receive postsynaptic glutamatergic input from ORNs. The cell body of MCs are located in the mitral cell layer of the OB, whereas the cell body of TCs reside in the external plexiform layer. While both cell types are similar in their reception of monosynaptic odor information from ORNs and their lateral dendrite arbors, they have different physiological responses to odors, including odor intensity coding (Nagayama et al., 2004; Schneider and Scott, 1983). The MCs and TCs provide bisynaptic information into downstream secondary olfactory (cortical) structures, as described below.
An additional major cell type in the OB is the granule cell. Granule cells are found in the most central OB cell layer and can be identified as small axon-less cells organized in patchy aggregated rows. The apical dendrites of granule cells synapse upon, and are synapsed upon by, MTs and TCs. Granule cells also receive centrifugal input from some secondary olfactory structures. Granule cells display broader odor-tuning characteristics than the upstream MCs and TCs (Tan et al., 2010).
Granule cells (GABAergic and glutamatergic) are constantly renewed by neurogenesis during adulthood in many mammalian species, and they derive from neuroblasts originating from the subventricular zone of the anterior forebrain that migrate to the OB. There they differentiate and integrate into the granular and glomerular layers of the OB (Alvarez-Buylla et al., 2008; Brill et al., 2009; Imayoshi et al., 2008; Lledo et al., 2006; Lois and Alvarez-Buylla, 1994). The magnitude and importance of olfactory neurogenesis in human remain debated (Bergmann et al., 2015; Curtis et al., 2007; Johansson et al., 1999; Lötsch et al., 2013; Macklis, 2012; Sanai et al., 2004) and could be limited (Bergmann et al., 2012; Huart et al., 2013).
The activity of MCs, TCs, and interneurons in the OB is subject to neuromodulation (for a review, see (Linster and Fontanini, 2014)). The OB receives dense noradrenergic projections from the locus coeruleus, cholinergic input from the horizontal limb of diagonal band of Broca, and serotoninergic afferents from the medial and dorsal raphe nuclei. Of particular interest to PD, dopamine is synthesized locally in the OB by dopaminergic interneurons of the periglomerular layer (Baker et al., 1983). Interestingly, these interneurons are reported to be greater in number in PD patients compared to age-matched controls (Huisman et al., 2004). In addition, a minor input to the OB from dopa-minergic neurons in the substantia nigra was recently described in rats (Höglinger et al., 2015). Additional details on the function of OB circuits can be found elsewhere (Mori et al., 2006; Shepherd et al., 2004; Wilson and Mainen, 2006).
3.2. Neuronal connections within the olfactory system and with the rest of the brain
Axons from MC and TC fasciculate to form the lateral olfactory tract, from which their distal projections branch and innervate a variety of secondary olfactory structures. Indeed, unlike other sensory networks, the olfactory system bypasses the thalamus for cortical integration of olfactory information; instead, MCs and TCs directly innervate these secondary olfactory structures. Secondary olfactory structures include the anterior olfactory nucleus (AON), piriform cortex, olfactory tubercle, the lateral entorhinal cortex, and others, which receive monosynaptic input from MCs or TCs. While it is thought these structures, unlike the OB, contribute to more perceptually or behaviorally relevant aspects of olfaction, the unique contributions of these secondary structures to olfaction are mostly undefined (Brunjes et al., 2005; Gottfried, 2010; Wesson and Wilson, 2011; Wilson and Sullivan, 2011). Here we summarize the known aspects of some of these structures.
Located in the basal forebrain, the AON is a layered structure composed of several subdivisions which receive dense MC and TC innervation (Meyer et al., 2006) and whose cells display odor responsivity (Kay et al., 2011). A distinct feature of the AON is its contralateral projection system wherein principal neurons of the AON span the midline of the brain and innervate the contralateral AON (Illig and Eudy, 2009). This contralateral projection system is thought to aid in intra-nostril odor localization (Kikuta et al., 2010). The AON also provides centrifugal modulation of sensory processing in the OB and itself is subject to neuromodulation (Oettl et al., 2016; Rothermel, 2014).
The piriform cortex is well established for its roles in modifying the processing of odors based upon experience and learning (Barkai and Saar, 2001; Gottfried, 2010; Schoenbaum and Eichenbaum, 1995; Wilson and Sullivan, 2011). Pyramidal cells in the piriform cortex receive odor input via MCs and TCs, and pyramidal cell association fibers modulate odor representations both among the piriform cortex neural ensembles and in connected structures (Carriero et al., 2009; Cauthron and Stripling, 2014; Haberly and Price, 1978a; Linster et al., 2009). In rats, piriform cortex neurons display profound adaptation to prolonged or repeated odor exposure (Wilson, 1998). Further, the piriform cortex possesses anatomically defined zones which differentially represent odor components versus synthetic odor images such as those which evolve after learning or those perceived when smelling complex odorant mixtures (Kadohisa and Wilson, 2006).
While the olfactory tubercle receives dense TC input from the OB, making it a secondary olfactory structure, the olfactory tubercle is unique among olfactory structures in that it is a component of the ventral striatum (de Olmos and Heimer, 1999; Wesson and Wilson, 2011). This provides the olfactory tubercle with privileged connection with basal ganglia. Also unlike other secondary olfactory structures, the olfactory tubercle does not possess an association-fiber system (Haberly and Price, 1978b). Similar to neurons in the AON and piriform cortex, olfactory tubercle neurons represent odor information (Carlson et al., 2014; Payton et al., 2012; Wesson et al., 2010; Xia et al., 2015) and can do so in manners dependent upon the behavioral state of animals. Thus, olfactory tubercle neurons robustly reflect odor valence, the occurrence of motivated behaviors, and even the acquisition of rewards, suggesting important roles for the olfactory tubercle in guiding hedonic and valence-dependent responses to odors (Gadziola et al., 2015; Gadziola and Wesson, 2016).
Neurons within secondary olfactory structures project into tertiary olfactory structures, including the orbitofrontal cortex, the insular cortex, and the dorsal hippocampus (Doty, 2003). Of particular relevance to AD, the entorhinal cortex innervates the hippocampus via the perforant pathway (Gomez-Isla et al., 1996). Additionally, thalamic regions receive olfactory information from several of the secondary olfactory structures, including the AON, piriform cortex, and olfactory tubercle. Olfactory information is also transmitted to the hypothalamus via the amygdaloid complex (Doty, 2003).
Neural activity in secondary olfactory structures is subject to neuromodulation, like such activity in the OB (for review see (Linster and Fontanini, 2014)). For instance, AON activity in mice is modulated by oxytocin (Oettl et al., 2016). The activity of the piriform cortex is shaped by cholinergic input from the horizontal diagonal band (Linster et al., 1999). In rats, the activity of the olfactory tubercle is further shaped by the activation of neurons in the ventral tegmentum (Mooney et al., 1987), which release dopamine among other neurotransmitters.
In summary, the olfactory system exhibits a highly complex network of reciprocal, centripetal, centrifugal, and associative connections, and it bridges numerous brain regions involved in many neurodegenerative diseases. Since all of the secondary olfactory structures are densely interconnected with not only each other but also other non-olfactory brain regions, they may provide a conduit for pathogens from the OB into more central brain structures needed for cognition, movement, and other crucial functions.
3.3. Glial and support cells organization in the olfactory mucosa and bulb
Glial and support cells of the olfactory mucosa and OB play an essential role in protecting brain structures against external insults and in sustaining neurons for proper transduction of odorant stimuli into olfactory input.
The olfactory mucosa (OE and lamina propria) includes supporting cells and horizontal and globose basal cells, which are located within the OE in a columnar organization. The supporting or sustentacular cells display fine cellular extensions that are in contact with ORNs. The apex of such cells extends dense, long microvilli into the nasal lumen, where they are in direct contact with mucus and can interact with the cilia of ORNs (Doty, 2003) (Fig. 1). These sustentacular supporting cells have multiple roles: they maintain water and salt balance in the mucus and provide electrical insulation to ORNs. Additionally, the supporting cells are able to metabolize (detoxify) inhaled toxicants and may be involved in removing debris of dying cells, acting as phagocytes (Doty, 2003). With age and with intranasal viral infections, the number of ORNs, cilia, and supporting cell microvilli decreases dramatically. Similar structural alterations of the OE have been observed in AD and PD (Brouillard et al., 1994).
The horizontal basal cells in the OE sit on the lamina propria and slowly divide into globose cells, which are located above them, and give rise to new ORNs. Microvillous cells are present in low numbers and their function is still unclear (therefore not shown in Fig. 1), but they can modulate the activity of ORNs and of supporting cells (Ogura et al., 2011; Rowley et al., 1989). The lamina propria of the olfactory mucosa includes Bowman’s glands, composed of serous and stem cells that produce the mucus in which the olfactory cilia of the ORNs are immersed (Doty, 2003). This mucus contains odorant-binding proteins that aid in odorant absorption (Pelosi, 1994). It also contains immune factors (IgA, M, G), lysozymes, enzymes, and antioxidants, and it might contain xenobiotic-metabolizing enzymes that play an important role in viral inactivation, detoxification, bacterial degradation, and destruction of pro-inflammatory proteins (Ding and Dahl, 2003). Thus, the mucus might protect the OE from external insults.
Finally, the axon bundles from the ORNs that traverse the cribriform plate are surrounded by ensheathing cells that have properties similar to those of Schwann cells and that are enveloped by fibroblasts. In the olfactory nerve fascicles, a specific population of microglia and macrophages halts the passage of viruses and xenobiotics to the brain (Smithson and Kawaja, 2010). A dense population of glial cells, including a unique and abundant microglial population, is also present in the OB (Lawson et al., 1990). Some have concluded that the microglia in the OB are in a constant “alert” state. They can sense an inflammatory response due to an injury taking place far from the OB and become activated as a consequence (Lalancette-Hebert et al., 2008).
4. Neuropathology of the olfactory system in neurodegenerative diseases
The presence of protein inclusions in olfactory structures likely contributes significantly to the development of olfactory deficits in both normal aging and in age-related neurological conditions. Here, we review the neuropathological lesions in the olfactory system in the various neurodegenerative diseases which have been studied to date.
Considering the recent idea that misfolded proteins associated with neurodegenerative diseases can exhibit prion-like properties and propagate protein aggregation between brain regions from one neuron to another, it is crucial to understand where pathological protein inclusions appear first and which neural pathways they preferentially spread along. We review here the main neurodegenerative diseases that 1) involve α-syn, tau, Aβ, TDP-43, and prion inclusions and 2) where pathology and neurodegeneration are present in the olfactory system.
4.1. Lewy body diseases
Lewy body disease progression is classified based on the localization of α-syn inclusions in the postmortem brain. A unified staging system proposes that the OB is the starting point of pathology in DLB, PD, and iLBD (Beach et al., 2009a). From the OB, the pathology then progresses either to the brainstem or the limbic system at stage 2, to then converge to concomitant pathology in brainstem and limbic system at stage 3, and evolve finally to a neocortical stage (Beach et al., 2009a). It is unclear whether or how the OE is involved in this timeline. The OE could be involved prior to the OB in PD, but it could also develop inclusions after the OB, which then would suggest retrograde propagation of the pathology from the OB to the OE.
4.1.1. Parkinson’s disease
Braak and colleagues defined six neuropathological stages for PD, based on α-syn inclusion (both LBs and LNs) localization. According to them, PD pathology progresses via neural connections from the OB to connected structures via the olfactory system, and from the dorsal motor nucleus of the vagal nerve (DMX) via the brainstem to cortical areas (Braak et al., 2006, 2002, 2003a, 2004, 2003b; Del Tredici and H. Braak, 2014). LBs and Lewy neurites (LNs) composed mainly of misfolded α-syn, are detected first (Braak stage 1) in the OB, the AON (Braak et al., 2003b, 2003a; Daniel and Hawkes, 1992; Del Tredici et al., 2002; Hubbard et al., 2007; Sengoku et al., 2008; Ubeda-Bañon et al., 2010) and the vagal and glossopharyngeal nerves and nuclei (Braak et al., 2006; Del Tredici et al., 2002). During stage 1, the inclusions are first found within non-AON areas of the human OB. They then appear in the AON and are present in the OB of patients displaying no α-syn inclusions in the DMX and any other brain region, indicating an earlier involvement of the OB than the DMX or AON (Braak and del Tredici, 2016; Del Tredici and Braak, 2016; Sengoku et al., 2008). In the OB, α-syn inclusions are found in interneurons, in the internal plexiform layer (Doty, 2012a), and less frequently in MCs and TCs, which raises the possibility that these relay neurons might be more resistant to developing α-syn aggregates than other neurons. A role of MCs and TCs in the propagation of pathology is, however, not excluded. MCs and TCs might better resist the development large α-syn aggregates (recognized in the microscope as Lewy pathology), but might allow more transfer of small seeds to connected regions.
In their original publications, Hawkes et al. (2007, 2009a) suggested that the agent causing Lewy pathology might be a neurotropic virus and that the OE was involved even earlier than the OB. At least four studies to date have investigated the presence of LBs and LNs in the olfactory mucosa (Duda et al., 1999; Funabe et al., 2012; Saito et al., 2016; Witt et al., 2009). In one study, asymptomatic LBD patients who had Lewy pathology in the OB rarely displayed inclusions in the olfactory mucosa. Symptomatic patients presented LBs in their olfactory mucosa, but not in their OE (Funabe et al., 2012), suggesting that the olfactory mucosa would be involved later than the OB. A more recent study from the same group, using a different dissection method that damages the OE less, describes Lewy pathology in ORNs of the OE and in the lamina propria of patients with PD, but it is unclear at which disease stage this occurs (Saito et al., 2016). Importantly, ORNs are renewed and have a high turnover (their median survival is 1–2 months); therefore, they might not have time to develop LBs and LNs, but they might still act as conduits for small aggregation-prone species of α-syn.
During stage 2, α-syn inclusions reach the locus coeruleus. In PD at Braak stages 3 and later, α-synuclein aggregates in the OB and AON become denser. At these stages, inclusions are also found in other secondary olfactory brain regions, principally the piriform cortex and cortical amygdala (Braak et al., 2004, 2003a, 2002, 1994; Harding et al., 2002; Silveira-Moriyama et al., 2009a), and to a lesser extent in the olfactory tubercle and entorhinal cortex (Del Tredici and Braak, 2014; Hubbard et al., 2007; Ubeda-Bañon et al., 2010). While the pathology is progressing to piriform, periamygdaloid, and entorhinal cortices, non-olfactory cortical regions remain intact (Braak et al., 2003a; Del Tredici and Braak, 2012; Hawkes et al., 2009a). The olfactory tubercle and the nucleus of the lateral olfactory tract seem to develop α-syn aggregates later than other olfactory structures nearby (Del Tredici and Braak, 2014). This might be interpreted as those structures being more resistant to aggregate pathology; an alternative interpretation is that the absence of projections from these structures to the OB reduces the risk of aggregate propagation, if retrograde axonal transport is crucial.
Regrettably, possible cell loss in the piriform cortex and in the OB (MCs and TCs) has never been investigated in PD brains. Severe cell loss occurs in the AON and correlates with severity of olfactory deficits (Pearce et al., 1995). Significant neuronal loss also occurs in regions connected to the olfactory structures, namely, in the locus coeruleus (70%) (Bertrand et al., 1997; German et al., 1992) and to a lesser extent (30% loss) in the amygdala (Harding et al., 2002). As a result, the volume of the amygdala is reduced by 20% (Harding et al., 2002). The loss of volume in the amygdala and the piriform cortex inversely correlates with olfactory deficits (Wattendorf et al., 2009), suggesting that cell loss in these regions could contribute to the functional deficits. The volume of the OB, the orbitofrontal cortex, and the piriform cortex is also decreased (Lee et al., 2014; J. Li et al., 2016; Pearce et al., 1995; Tanik et al., 2016; Wattendorf et al., 2009), suggesting possible cell loss in these brain regions as well. In addition, olfactory nerves in the olfactory tract undergo atrophy and lose their cellular architecture (Pearce et al., 1995). Volumetric measurement of olfactory tracts can discriminate PD patients from controls (Rolheiser et al., 2011; Scherfler et al., 2006).
Our recent work in mice using microinjections of preformed α-syn fibrils into the OB demonstrate that the olfactory route can be a vector of pathology spreading into the substantia nigra and other regions involved in later stages of PD (Rey et al., 2016b), and this was confirmed by a second study (Mason et al., 2016). Specifically, we found that α-syn aggregates progressively spread from the OB to a total of over 40 different brain sub-regions bilaterally over the course of 12 months, and that the progressive development of synucleinopathy was coupled to the emergence of specific olfactory deficits (Rey et al., 2016b). At the one year time point, there was evidence of very occasional α-syn aggregates in the substantia nigra in a few animals, inconsistent with a level believed necessary for nigral neurodegeneration to occur. Taken together, this mouse model is therefore considered to represent prodromal PD. Understanding that the pathology in the olfactory system precedes that in the substantia nigra opens new and important therapeutic avenues. Essentially, it should be possible to initiate future disease-modifying treatments in PD before the first motor symptoms occur. The time course of α-syn pathology propagation was protracted in the mice, and in view of the short normal lifespan of mice it can be viewed analogous to that suggested to occur in humans. In our study mice were injected at 3 months of age which is considered equivalent to around 20 years in humans, and at the final time point they were 15 months old, which is viewed to represent 50 years of age in humans (slightly younger than the median age of onset of PD). That said, we do not actually know how long it takes for α-syn aggregates to spread after they first develop in the OB in humans, as has been postulated to occur in PD. It is possible that the dynamics of α-syn aggregates spread observed in the mice is not dissimilar to humans, but that the size of the human brain (greater distances between nuclei and larger structures) require longer lag periods before significant levels of pathology are detected in downstream structures.
The notion that the propagation of α-syn pathology involves prion-like mechanisms has gained increased acceptance in recent years, and because it has been reviewed extensively in other articles we will not present all the details here (see e.g. (Dehay and Fernagut, 2016; George et al., 2013; Goedert et al., 2016; Kaufman and Diamond, 2013; Luk and Lee, 2013; Rey et al., 2016a)). Briefly, this hypothesis posits that once α-syn aggregates have formed in a cell, some of them are excreted to the extracellular space. Once there, they are taken up by neighboring cells, including neurons, where they can seed the aggregation of endogenous α-syn through “permissive templating” by the misfolded α-syn species. Because misfolded α-syn can undergo axonal transport between brain regions, this hypothesis also accounts for how α-syn pathology spreads along, e.g., neural connections in the olfactory system. The realization that α-syn aggregates are present in the extracellular space during part of this pathogenic process, has led to suggestions that it might be possible to therapeutically target extracellular α-syn or, e.g., the molecular machinery involved in its cellular uptake (Dehay and Fernagut, 2016). The possible existence of specific “strains” of α-syn fibrils has led to the proposal that pathobiological differences between the family of synucleinopathies (e.g. PD, DLB and MSA) are due to the properties of the α-syn fibrils that propagate between cells (Melki, 2015; Peelaerts et al., 2015). It has been suggested that α-syn can directly cross seed tau (Guo et al., 2013), which could explain why tau aggregates also occur in synucleinopathies, but these observations of possible cross-seeding in mice remain debated.
4.1.2. Incidental Lewy body disease
LB pathology does not appear to follow Braak staging in incidental Lewy body disease (iLBD). The OB exhibits Lewy pathology in 83% of cases (Sengoku et al., 2008), but only 1 patient out of 11 showed inclusions in the OE (Saito et al., 2016). In 77% of cases, LBs and LNs are also found in the AON, along with a minimal amount of neurofibrillary tangles (NFTs) (Tsuboi et al., 2003). It has been proposed that iLBD constitutes prodromal PD, however, as mentioned above only some iLBD cases appear to be consistent with Braak staging, while others show pathology mainly in cortical regions, which could correspond to preclinical DLB ((Frigerio et al., 2011); importantly the OB was not investigated in this study).
4.1.3. Dementia with Lewy bodies
Similar to those with iLBD, patients with DLB frequently exhibit severe pathology in the OB. However, study of the olfactory mucosa in two recent cases found α-syn pathology only in the cribriform plate (Funabe et al., 2012; Saito et al., 2016). Currently, the staging of PD and DLB relies on the presence of LBs or LNs. Novel methods allow the detection of oligomeric α-syn (Roberts et al., 2015) and suggest that accumulation of oligomeric α-syn precedes the appearance of LBs and LNs. Investigating other forms of pathology might help us understand better the clinical variability in the pathology of synucleinopathies (Halliday et al., 2011).
In addition to α-syn pathology, PD, DLB, and Parkinson’s disease dementia (PDD) patients also display tau (neurofibrillary tangles) and Aβ pathology (senile plaques) (Hepp et al., 2016; Horvath et al., 2013; Lei et al., 2010). A limited overlap in pathology is thus observed between diseases. Tau pathology is observed notably in the OB/AON in cases of PD, AD, DLB, and FTD, which are diseases accompanied with severe olfactory alterations, but tau pathology is absent from PSP and CBD, two disorders with no or minor olfactory loss (Mundiñano et al., 2011; Tsuboi et al., 2003), suggesting a role for aggregated tau in the olfactory dysfunction of synucleinopathies.
4.2. Alzheimer’s disease
In AD, brain regions critical to olfactory function are burdened with pathology, including the classic hallmark features of plaques and neurofibrillary tangles (NFTs). NFTs are made up of hyperphosphorylated forms of the microtubule-associated protein tau. Plaques are composed mainly of the amyloid precursor protein-derived amyloid-beta (Aβ). Similar to that for PD, a staging system in six neuropathological stages has been proposed by Braak and colleagues based on the localization of NFTs (H. Braak and E. Braak, 1991). According to this model, pathology is believed to start in the entorhinal cortex and spread to the hippocampus and then to the basal forebrain. While more recent models have proposed the locus coeruleus as the initial site of tau pathology in AD (Braak and del Tredici, 2016, 2012, 2011) the collective evidence for the entorhinal cortex and associated olfactory structures being affected early in the disease process remains compelling. Thus, the olfactory structures are affected early in AD (as in PD), prior to the appearance of cognitive symptoms (Attems et al., 2005; Jellinger and Attems, 2005; Price et al., 1991). NFTs appear first in transentorhinal region and entorhinal cortex at stage 1, and progress to CA1 of the hippocampus at stage 2. Some studies describe tau pathology in all the layers of OB (Kovács et al., 1999; Ohm and Braak, 1987) and in the olfactory tract as early as the appearance of NFTs in the entorhinal cortex (stage 1) (Christen-Zaech et al., 2003) or at stage 0 (Kovács et al., 2001); some others at stage 2 only (Attems et al., 2005; Tsuboi et al., 2003). Tau pathology is also present in the AON at early stages ((Esiri and Wilcock, 1984; Kovács et al., 2001; Ohm and Braak, 1987; Price et al., 1991) (stages 0–1), (Tsuboi et al., 2003) (stage 2), (Jellinger and Attems, 2005) (stage 2)). The periamygdaloid cortex and anterior amygdala are involved later, at or after stage 2 (Kovács et al., 2001). The piriform cortex is also affected early by NFTs, possibly in early stages, but only one study has investigated it and no assessment of Braak stages were done (Reyes et al., 1987). At Braak stage 3, NFTs develop in limbic regions (subiculum) and they progress to the amygdala and thalamus at stage 4. They later reach the isocortex (associative area) at stage 5 and ultimately affect the primary sensory, motor, and visual cortex at stage 6 (Braak and Braak, 1991). Tau aggregates, that make up the NFTs in AD and numerous other tauopathies, have repeatedly been shown to be capable of undergoing cell-to-cell transfer and propagate aggregate pathology in a prion-like fashion in experimental models of disease (Goedert et al., 2016; Walker et al., 2013). Nonetheless, in contrast to α-syn fibrils in PD which we described above, AD and other tauopathies do not exhibit the distinct preference for olfactory pathways (Braak and del Tredici, 2016). Why there are differences between α-syn and tau aggregates regarding preferred anatomical pathways for propagation, despite both types of pathology affecting the olfactory pathways, is presently unclear. One possibility is that the site of initial misfolding events differs between the disorders, with the OB being a stronger candidate in PD than in AD. Another option is that elements of the underlying cell biology of the neuron-to-neuron transfer differ between α-syn and tau aggregates in ways that are not yet understood. Furthermore, some tauopathies primarily affect glial cells (Clavaguera et al., 2013), which can explain that the spreading pattern does not follow neural pathways. However, this still does not account for why the pattern of NFT propagation in AD does not strictly follow axonal pathways in the olfactory system (Braak and del Tredici, 2016).
The progression of Aβ plaques in the brain is less predictable than that of NFTs, and data regarding the involvement of the olfactory system are subject to a lot of variance. Braak et al. described the progression of senile plaques in three stages (A, B, and C), mainly observed in cortical regions. Amyloid plaques are first present in the basal frontal, temporal, and occipital cortices during stage A. Plaques then progress to isocortical areas (sensory, motor, and visual cortices excluded) at stage B, and finally reach the primary isocortical areas and the striatum, thalamus, and hypothalamus notably (H. Braak and E. Braak, 1991). The Thal classification system of amyloid plaques comprises 5 stages. Stage 1 involves isocortical regions, progressing then to allocortical regions in stage 2 (entorhinal cortex, amygdala, insular and cingulate cortex, and hippocampal formation). Stage 3 involves subcortical nuclei (striatum, thalamus, hypothalamus notably). Ultimately, amyloid plaques develop in brainstem structures (substantia nigra, medulla oblongata, colliculi, red nucleus) at Thal stage 4 and the pons (LC, raphe nucleus) at stage 5 (Thal et al., 2002).
Amyloid plaques are rarely detected in early stages (tau Braak staging) of AD, because patients exhibiting tau inclusions did not all show amyloid plaques (Hardy and Higgins, 1992; Kovács et al., 1999; Schönheit et al., 2004; Struble and Clark, 1992), leading to the idea that tau pathology precedes Aβ pathology, an idea still debated (Price and Morris, 2004). Neuritic plaques have been found in the OB and AON in some studies (Christen-Zaech et al., 2003; Kovács et al., 1999), while others never detected them in the OB (Attems et al., 2005; Tsuboi et al., 2003). Finally, central olfactory regions are involved in confirmed cases of AD (Reyes et al., 1993).
Aβ and tau pathology progress differently during the course of AD (Price and Morris, 2004). As AD develop, the density of these pathologies increases. Although a peripheral brain structure, the OE also is impacted pathologically during the progression of AD. In one study (Arnold et al., 1998), Aβ was present in the OE of 71% of AD cases but only in 22% of normal control cases. Paired helical filament tau was also elevated in AD versus control cases.
There are a limited number of studies in humans on the cellular and molecular mechanisms underlying olfactory dysfunction. For example (and as mentioned above), in aged individuals without MCI or AD, olfactory dysfunction is related to the level of AD pathology, including Aβ plaques and NFTs (Wilson et al., 2009).
Severe cell loss occurs in many brain regions in AD. Patients exhibit severe MC loss in the OB before the clinical symptoms emerge (Struble and Clark, 1992) and in the entorhinal cortex when patients exhibit the mildest clinically detectable dementia (Gomez-Isla et al., 1996). However, it is unclear whether the cell loss is a consequence of the accumulating pathology. Neuromodulatory systems are also severely affected in AD, with 30–90% cholinergic cell loss in the nucleus of Meynert (Whitehouse et al., 1983) and severe noradrenergic cell loss in the LC ((Marien et al., 2004) for review), structures both implicated in Minimal Cognitive Impairment (MCI) (Arendt et al., 2015). Neuronal death has also been described in the hippocampus (Kril et al., 2002). In brief, neuronal death in olfactory regions and neuromodulatory systems could, and are likely to, contribute significantly to olfactory deficits in AD.
4.3. Other neurodegenerative diseases
Among the diseases that have mild or no olfactory deficits, MSA is the only one with pathological inclusions in olfactory regions (Katzenschlager and Lees, 2004; Tsuboi et al., 2003). Although neuronal α-syn inclusions are present in MSA (Costa and Duyckaerts, 1993), the main pathological hallmark is the presence of glial cytoplasmic inclusions (GCIs), which are mainly composed of aggregated α-syn, and are found in oligodendrocytes of the putamen, the substantia nigra, the pontine base, inferior olive, and globus pallidus. Consistently, the substantia nigra, caudate nucleus, and the frontal and parietal cortices exhibit neuronal loss, and the putamen and globus pallidus exhibit in addition a loss of oligodendrocytes (Salvesen et al., 2015). A few studies have shown that GCIs are also present in the OB (100% cases), the olfactory tract (Daniel and Hawkes, 1992; Fujishiro et al., 2008; Kovács et al., 2003), and the olfactory tubercle (correlating with the number of GCIs in the OB), but are rarely found in the AON despite severe cell loss in this structure in 57% of cases (Kovács et al., 2003). The presence GCIs and the severe cell loss in the peripheral olfactory system could play a role in the development of mild olfactory disturbances by altering local neuronal activity.
Other diseases that have more-severe olfactory deficits all exhibit pathological inclusions in the olfactory system. The prion disorder CJD severely affects the olfactory structures, with accumulation of misfolded prion protein PrPSc in the olfactory tracts and cortices (olfactory uncal cortex, prepiriform, periamygdaloid, entorhinal cortices). Regrettably, cell loss has never been investigated specifically in the olfactory system. In two reports, PrPSc was detected to a lesser extent in cilia of ORNs and in basal cells of the OE (Tabaton et al., 2004; Zanusso et al., 2003, 2009). Smaller amounts of inclusions have also been found in the thalamus, basal ganglia, and cerebellum, and severe neurodegeneration has been observed in widespread brain regions (Faucheux et al., 2009; Ferrer, 2002; Liberski and Ironside, 2004; Tabaka et al., 2003; Tabaton et al., 2004), It is unclear where pathology starts in CJD, but inclusions have been observed in olfactory regions in patients with short disease duration, suggesting that the olfactory system is affected early (Tabaton et al., 2004; Zanusso et al., 2003). Biopsies of the OE have been proposed for diagnosis of CJD (Zanusso et al., 2009). The DMX is also affected in two subtypes of CJDs (MV2 and VV2), which raised the question of a dual-hit hypothesis of propagation in CJDs (Zanusso et al., 2009), akin to what has been proposed for PD (Hawkes et al., 2007).
In ALS, intracytoplasmic inclusions of the protein TDP-43 accumulate in the frontotemporal cortex and subcortical regions (Neumann et al., 2006), brainstem, and spinal cord (Fatima et al., 2015). These inclusions are associated to severe loss of motor neurons (Brettschneider et al., 2014; Ilieva et al., 2009) and the degeneration and atrophy of many central brain regions (Bede et al., 2013; Brettschneider et al., 2014; Fatima et al., 2015; Oyanagi et al., 2015; Smith, 1960; Wakabayashi et al., 2001). A recent study investigated olfactory regions and showed that TDP-43 inclusions affect the secondary olfactory centers (hippocampus and orbitofrontal cortex), the primary olfactory cortex (AON, entorhinal and piriform cortex), and the OB (Takeda et al., 2015). However, the OB seems to be preserved from cell loss in a small study on 3 patients (Oyanagi et al., 2015). Reductions in the volume of the amygdala have also been reported (Machts et al., 2015; Pinkhardt et al., 2006). The severity of TDP-43 pathology follows a rostro-caudal gradient, and it was proposed that the pathology would start in the motor cortex, brainstem, and spinal cord and spread to the hippocampus (Brettschneider et al., 2013; Fatima et al., 2015). Thus, olfactory regions could also be involved in the latest stages of ALS, meaning that the progression would follow a direction opposite to the direction of pathology progression proposed for PD.
In FTD, Pick’s bodies (tau inclusions) are predominantly found in the cerebral cortex (Goedert et al., 2012). Pick’s bodies are also found in many cells of the AON and in a lesser extent in the OB (MCs and TCs) and olfactory tubercle (Yoshimura, 1989). NFTs (8 cases out of 11) and amyloid deposits (3 cases out of 11) were observed in the OB (Mundiñano et al., 2011) and might be associated with its atrophy (Mundiñano et al., 2011; Yoshimura, 1988), but they were reported to be absent from one FTD case in another study (Yoshimura, 1989). Finally, FTD is associated with severe cell loss in the neocortex, hippocampal region and brainstem, akin to HD (Dayalu and Albin, 2015; Kersaitis et al., 2004; Radanovic et al., 2003; Santillo et al., 2013; Seeley et al., 2006).
Studies of HD have never investigated pathology and cell loss in anterior olfactory structures, despite olfactory deficits occurring early in the course of the disease. Inclusions of mutated huntingtin protein develop markedly in the midbrain and the cortex during early stages of the disease (Aronin et al., 1999; Vonsattel et al., 1985). “Neuropathological alterations” then appear in the brainstem, amygdala, and cerebellum (Vonsattel et al., 1985). Curiously, NFTs are described in atrophied entorhinal cortex, as well as in the hippocampus, frontal and parietal lobe, amygdala, thalamus, midbrain, and locus coeruleus of HD patients, and senile plaques are present in the frontal temporal and piriform cortex (reviewed in (Gratuze et al., 2016)). The presence of pathology in the piriform cortex and the amygdala could be responsible for the severe olfactory dysfunction in HD.
Many neurodegenerative diseases show pathological hallmarks characteristic of another disease: NFTs in PD (Hepp et al., 2016; Horvath et al., 2013; Iseki et al., 2003; Lei et al., 2010), DLB, and FTD (Mundiñano et al., 2011; Tsuboi et al., 2003), and α-syn inclusions in AD (R. L. Hamilton, 2000; Morales et al., 2013). These occurrences led to the idea that a misfolded protein could be a template for the aggregation of a different protein (cross-seeding) (Morales et al., 2013). The first evidence of direct cross-seeding of tau by Aβ trimers was recently published (Sherman et al., 2016) and, as mentioned above, it has been suggested that that α-syn fibrils can cross-seed tau (Guo et al., 2013). However, whether such cross-seeding occurs in vivo for other proteins is still controversial, and it is unclear if a third protein (or more) is required or if direct cross-seeding occurs.
In summary, alteration of olfactory perception occurs as part of neurodegenerative diseases, accompanied by pathology and cell loss in the primary, secondary, and tertiary olfactory structures. We propose that the OE, OB, and AON are particularly vulnerable to injuries and dysfunction. Unfortunately, these regions have only rarely been investigated in diseases other than AD and PD.
5. Why are the olfactory epithelium and olfactory bulb so vulnerable?
Directly accessible from the external environment, the OE is exposed to a variety of environmental insults. While the OE has several barriers to such insults, ranging from physical to enzymatic and immune defenses (Fig. 1), these barriers deteriorate with age, rendering them more permeable to xenobiotics and toxins. We hypothesize that external agents (bacteria, viruses, toxins, airborne pollutants, micro- and nanoparticles) might trigger disease via the OE. Either these agents gain access to the brain through the OE and spread along its associated connections, or they trigger protein misfolding locally in the OE and OB that, in turn, leads to prion-like propagation of protein aggregates via olfactory pathways. Beyond an anatomically vulnerable location, the OE and the OB have certain characteristics that could make them vulnerable to disease. Our hypothesis does not exclude the existence of other neuronal systems that exhibit inherent properties that could render them vulnerable (e.g. long axons affected early in ALS). Nor does our hypothesis exclude that other neuronal system also are exposed to external agents that potentially can trigger pathological conversion of proteins (e.g. enteric nerves in PD). However, in this review we have chosen to focus on the potential role of the olfactory system in neurodegenerative diseases. Therefore, in the following section, we will review evidence that the OE and OB are sensitive to inflammation and to oxidative stress; express high levels of proteins involved in neurodegenerative diseases; and exhibit intrinsic neural activity that could make them sensitive to cell stress and promote protein accumulation.
5.1. Increased permeability of olfactory epithelium due to infection, mutation, or normal aging
Among external factors that deteriorate the OE are respiratory pathogens and external insults that impair tight junctions between the epithelial cells of the nasal epithelium and the OE, increasing epithelial permeability to external substances (Dando et al., 2014). The loss of detoxifying enzymes in nasal mucus can also affect the permeability of the OE (Lafreniere and Mann, 2009). People with mutations of the cytochrome P450-CYP2D6-debrisoquine hydroxylase, involved in detoxification of xenobiotics, show increased risk of developing PD (Elbaz et al., 2004; Smith and Jones, 1992), possibly due to a compromised barrier in the OE (Doty, 2012a; Hawkes and Doty, 2009b).
Also, the OE dramatically changes with age; the ORNs can undergo necrosis and the OE becomes thinner (Attems et al., 2015). Although not demonstrated in the OE, misfolded α-syn can perturb the expression of tight junction proteins (Kuan et al., 2016) and could contribute to increased permeability of the OE. Other changes in the OE with aging, include a decrease in calbindin-D28k expression, which potentially makes neurons more vulnerable to calcium overload and excitotoxicity (Yamagishi et al., 2016). Furthermore, monoaminergic innervation of the OE decreases (Chen et al., 1993), which affects mucosal secretions. Nasal mucociliary clearance is also less effective in the elderly (Sakakura et al., 1983). Thus, as a consequence of infection, environmental stresses, and aging, the OE becomes more vulnerable to pathogen entry.
5.2. The olfactory mucosa and bulb, a gateway to the brain for viruses
Numerous studies have tentatively linked the exposure to pathogens, particles, or pesticides with increased risk of developing PD. Hawkes et al. proposed a dual-hit hypothesis: PD could be triggered by the entry and the propagation of a viral agent through both the OE and the enteric mucosa. Essentially, the viral agent would be inhaled and then access the gut via the swallowing of saliva and nasal mucus, and could cause nervous system pathology while spreading along olfactory pathways and enteric nerves that access the central nervous system (Braak et al., 2003b; Doty, 2008; Hawkes et al., 2007). As an alternate model, it was suggested later that this neuropathological agent could trigger pathology at the entry site where assemblies of misfolded α-syn could accumulate in neurons in the OB and in enteric nerves of the gut wall, and misfolded α-syn itself would spread in a prion-like manner to connected brain regions (Brundin et al., 2008; George et al., 2013; Li et al., 2008). These two hypotheses are consistent with the appearance of olfactory deficits and of gastrointestinal dysfunction in people before they develop PD or DLB, i.e., during the prodromal stage (McKeith et al., 2016; Meissner, 2012).
Viruses (and toxins, see further below) can reach the brain through the ORNs (Baltazar et al., 2014; Dando et al., 2014; Doty, 2008; Prediger et al., 2011; Tjälve and Henriksson, 1999; van Riel et al., 2015) (reviews; (Hobson, 2012; van Riel et al., 2015)). By penetrating ORNs, they can travel via axonal transport, within the olfactory ensheathing cells, or within the perineural space, and pass through the cribriform plate to access the subarachnoid space (Dando et al., 2014). Viruses, for example, can migrate from the OB to other brain regions (for a review, see (Dando et al., 2014). The trigeminal nerve can also be a route of entrance to the central nervous system from the oral, nasal cavity and from the cornea (Dando et al., 2014). PD risk is increased following exposure to certain viruses (Wu et al., 2015; Zhou et al., 2013), and viral infections can also affect α-syn. Infection of mice with H5N1 virus triggers the phosphorylation and aggregation of α-syn (Jang et al., 2012, 2009). Interestingly, though, α-syn expression in neurons inhibits viral infection (Beatman et al., 2016), so an increase in α-syn expression could be a defense against viral infection that inadvertently increases PD risk. It has also been shown that antibodies to Epstein Barr Virus can cross-react with α-syn (Woulfe et al., 2016), which has been hypothesized to promote its aggregation in the olfactory system and gut in people who later develop PD (Woulfe et al., 2014).
5.3. Xenobiotics and airborne pollutants affect protein aggregation
PD risk is also higher after exposure to several agents that can be inhaled, e.g. pesticides (Baltazar et al., 2014; T. P. Brown et al., 2005; Chin-Chan et al., 2015; Saeedi Saravi and Dehpour, 2016; Tanner et al., 2014), pollutants (Block and Calderón-Garcidueñas, 2009), micro- or nanoparticles (Chin-Chan et al., 2015), organic solvents (R. C. Brown et al., 2005), and metals (R. C. Brown et al., 2005; Chin-Chan et al., 2015; Finkelstein and Jerrett, 2007). In addition, exposure to the bacterial amyloid protein curli promotes α-syn aggregation (Chen et al., 2016). Exposure to environmental toxins, some of which can be inhaled and therefore can gain access to the olfactory system, has been associated with increased PD risk (Goldman, 2014). Laboratory studies have provided insight into mechanisms that might contribute to the triggering of PD following toxin exposure. For example, in vitro experiments have shown that, under certain conditions, metals (e.g. copper) directly induce conformational changes in α-syn and can promote α-syn fibrillization (similar to pesticides (Paik et al., 1999; Uversky et al., 2002; Villar-Piqué et al., 2016)) or increase in α-syn expression (Wu and Xie, 2014). Recent work in mice has shown that systemic exposure to the fungicide maneb and the herbicide paraquat can increase formation of α-syn radicals in the brain, which can promote aggregation of the protein (Kumar et al., 2015). Furthermore, systemic injection in rats with the mitochondrial complex I inhibitor rotenone, which is used as a pesticide, insecticide and fish killer, was recently found to lead to increased levels of phosphorylated and aggregated α-syn (Di Maio et al., 2016) These recent reports on effects of environmental toxins are in agreement with earlier findings (Ischiropoulos and Beckman, 2003) that oxidative damage to (Paxinou et al., 2001) and mitochondrial dysfunction (Przedborski et al., 2001) can promote a-syn aggregation.
Beyond direct effects on α-syn, airborne pollutants, environmental agents and xenobiotics that can penetrate the central nervous system through the olfactory route, producing undesirable effects in the brain such as inflammation, oxidative stress (Block and Calderón-Garcidueñas, 2009; Cheng et al., 2016), increased apoptosis (Chin-Chan et al., 2015), and dysfunction of mitochondria and proteasome (Saeedi Saravi and Dehpour, 2016). All of these effects could affect neuronal function and protein aggregation.
The risk of developing AD is increased by exposure to pesticides (Baltazar et al., 2014; T. P. Brown et al., 2005; Chin-Chan et al., 2015; Saeedi Saravi and Dehpour, 2016; Tanner et al., 2014), viruses (Ball et al., 2013; S. A. Harris and E. A. Harris, 2015; Licastro and Porcellini, 2016; Zhou et al., 2013), pollutants (Block and Calderón-Garcidueñas, 2009), and metals that are able to stimulate Aβ production (R. C. Brown et al., 2005; Chin-Chan et al., 2015). Few studies have investigated DLB, but exposure to metals has been implicated as a risk (McAllum and Finkelstein, 2016). MSA has been linked to metal and organic solvent exposure (Vanacore et al., 2001), environmental toxins, and pollutants (Hanna et al., 1999), but a link to pesticides is controversial (Chrysostome et al., 2004; Vanacore et al., 2001). ALS is also associated with pesticides ((Baltazar et al., 2014), metals (Bozzoni et al., 2016; Johnson and Atchison, 2009; Su et al., 2016; Tanner et al., 2014), solvents, viruses (Zhou et al., 2013), and possibly to pollutants (Malek et al., 2015). CJD is obviously related to infection by the misfolded protein PrPsc itself. Animal models of PrPSc spread show that the nasal route is more efficient for prion infection than the gastric route (Kincaid and Bartz, 2007) and that in infected hamsters, prions can be released in nasal fluid (Bessen et al., 2010). Further, OE damage favors the release of prions in the nose (Bessen et al., 2012).
5.4. Myelination and energy expenditure
Another possible vulnerability to neurodegenerative disease is the myelination level of neuronal populations in the brain. The absence of myelination requires a higher energy expenditure to convey neural impulses (Braak and del Tredici, 2004; Nieuwenhuys et al., 1998; Van der Knaap et al., 1991) and make the cells more susceptible to oxidative stress. Myelination, on the other hand, might protect the cells better against pathogens (Braak et al., 2003b) and against aberrant axonal sprouting (Cafferty et al., 2008; Caroni and Schwab, 1988; Vanek et al., 1998). Braak et al. have proposed that neurons that are unmyelinated or sparsely myelinated, have long, thin axons, and have high energy turnover are more susceptible to disease (Braak et al., 2006; Braak and del Tredici, 2004). The ORNs could thus be particularly sensitive, because they fit these three criteria (Doty, 2003; Nawroth et al., 2007). The MCs and TCs also possess long axons but are often myelinated (M. Tigges and J. Tigges, 1980). It is conceivable that MCs and TCs are thus more resistant to developing inclusions, which would be consistent with the observation of more severe pathology in AON cells than in the mitral cells of OB (Ubeda-Bañon et al., 2010). It is also notable that this lack of myelin coincides with a reduced integrity of the blood brain barrier at the level of the olfactory bulb (Minn et al., 2002).
5.5. Sensitivity of the OE and OB to inflammation and oxidative stress
Many xenobiotics and particles that can penetrate the brain through the OE are known to trigger reactive oxygen species (ROS) production and inflammatory reactions, similar to the effects of misfolded proteins. As an interface between the external environment and the brain, the OE and OB are particularly subject to inflammatory reactions. As mentioned earlier, the OB and olfactory nerve hosts selective dense populations of microglia that act to prevent particles and pathogens from penetrating the brain. The microglia of the OB show more rapid and higher levels of activation for longer periods (lasting for months) relative to microglia in other regions of the brain (Lalancette-Hebert et al., 2008). OB microglia can also be activated by injury or infections occurring in distant sites and might play a role of sentinel for the whole brain (Lalancette-Hebert et al., 2008; Smithson and Kawaja, 2010). Inflammation in the OB is affected by changes in neurotransmitters (a decrease in noradrenaline release by the locus coeruleus increase inflammation level, for example) (Feinstein et al., 2002; Heneka et al., 2010a; Scanzano and Cosentino, 2015). Also, activated microglia release proinflammatory cytokines and ROS that can damage cells (Surace and Block, 2012). These proinflammatory cytokines and ROS (Dvorska et al., 1992; Rhodin et al., 1999), as well as changes in neurotransmitters release (Kalinin et al., 2006) can disrupt the blood–brain barrier, thus facilitating the penetration of xenobiotics and particles into the brain.
While aggregated proteins (α-syn, Aβ, PrP, SOD1, and tau) induce microglial activation (Eikelenboom et al., 2002; Heneka et al., 2010a, 2010b; Y. Li et al., 2014; Zhao et al., 2013), inflammation can in return promote the aggregation of some prion-like proteins in the brain and peripheral cells (Correia et al., 2015; He et al., 2013; Kitazawa et al., 2005; Lee et al., 2008; Sy et al., 2011; Vieira et al., 2015). Similarly, excessive ROS production increases inflammation and favors prion-like protein aggregation (Alavi Naini and Soussi-Yanicostas, 2015; Borza, 2014; Cohen et al., 2011; Milhavet and Lehmann, 2002; Quilty et al., 2006; Rcom-H’cheo-Gauthier et al., 2014; Shodai et al., 2013; Singh et al., 2010), which in return increases oxidative stress (Alavi Naini and Soussi-Yanicostas, 2015; Borza, 2014; Quilty et al., 2006; Rcom-H’cheo-Gauthier et al., 2014).
Hence, a vicious feed-forward loop of inflammation, involving a complex interplay of ROS production and protein aggregation, could play an important role in the pathogenesis of prion-like diseases (Lema Tomé et al., 2012; Rcom-H’cheo-Gauthier et al., 2014), and anterior olfactory regions could be particularly vulnerable due to the presence of highly sensitive microglia and a blood–brain barrier that is easily compromised.
5.6. Level of expression of misfolding-prone proteins in the olfactory epithelium and bulb
5.6.1. α-syn
Genetic forms of PD have taught us that the simple elevation of α-syn expression by gene duplication can cause disease (Singleton et al., 2003). One can imagine that a transient increase in α-syn expression could also contribute to the initiation of disease and that brain regions that naturally express higher levels of α-syn might be more prone to developing α-syn pathology triggered by stochastic events. Another possibility is that an increase in α-syn expression could be a physiological response of an injured cell trying to maintain correct synaptic function. As mentioned above, a local increase in α-syn expression has been suggested as a defense mechanism against viral infection, because α-syn expression was shown to restrict RNA viral infections in the brain (Beatman et al., 2016).
In humans, only a few studies have defined the normal spatial pattern of expression of α-syn, and those focused only on few brain regions (olfactory brain structures were not investigated). More α-syn was reported in the cell body of neurons in the temporal and frontal cortex, CA2 and CA3. In healthy humans, high levels of α-syn, exacerbated by aging, and increased mRNA for proteins that are associated to LBs were detected in the substantia nigra relative to other brain regions (Chu and Kordower, 2007; Freer et al., 2016; F. Mori et al., 2002). When comparing different diseases (PD, DLB, and AD versus healthy controls), no difference in α-syn mRNA levels was found, but protein levels were lower in DLB and PD relative to controls in some brain regions (Quinn et al., 2012). Those studies investigated the brains of patients with severe disease, so the results might not reflect the α-syn levels in early phases of the disease. One study investigated the presence of α-syn in the olfactory mucosa in humans and found that α-syn is most abundant in basal cells and ORNs but is also expressed in sustentacular cells of the OE and in the Bowman’s glands (Duda et al., 1999). In mouse, the pattern of α-syn in the brain was studied in depth by Taguchi et al. (2015). In this study, brain regions that typically are affected early in PD contain high amounts of α-syn. The OB (GL), DMX, and substantia nigra pars compacta display high amounts of α-syn in the neuronal soma. Other layers of the OB (external plexiform and granule cell layers), the AON, the LC, the central and basolateral amygdala, piriform, entorhinal cortices, and the hippocampus display moderate to high amounts of α-syn within presynaptic terminals (Taguchi et al., 2015).
5.6.2. APP
APP is a large membrane-bound glycoprotein that forms the Aβ peptide when metabolized. The APP transcript is highly expressed in neurons of the associative neocortex and hippocampus (Neve et al., 1988), and APP protein is dense in the cortex, particularly in the entorhinal, occipital cortex, and superior temporal gyrus (Caušević et al., 2010). No data are available regarding this protein’s expression in olfactory structures in human. In rats, APP mRNA is found in the cortex, the hippocampus, the piriform cortex, olfactory tubercle, and cerebellum (Mita et al., 1989). The APP protein is highly expressed in the OB, in the nerve layer, EPl and GL of adult rats, and damages to the OE exacerbate the expression of APP in the OB (Struble et al., 1997). Unfortunately, the expression of APP in the OE and AON was not investigated.
5.6.3. Tau
Tau expression has not been investigated in olfactory structures in humans. The only studies exploring olfactory regions were conducted in rats, and showed that tau is highly expressed in the ORNs and in the axons of the ORNs in the OB (Schoenfeld and Obar, 1994), and is also present in mitral cells and interneurons of the OB (Viereck et al., 1989). Tau mRNA has been found in cortical areas in humans (Neve et al., 1988), and the protein is highly expressed in the cortex, particularly the entorhinal, occipital cortex, the frontal cortex, and the superior temporal gyrus; it is moderately expressed in the hippocampus (Caušević et al., 2010; Trabzuni et al., 2012; Trojanowski et al., 1989). The recent transcriptome-wide microarray study by Freer et al. revealed elevated levels of tau and Aβ in the brain regions that are affected early in AD (olfactory regions were not investigated). In addition, Freer found that proteins involved in homeostasis are differentially expressed in AD: aggregation protectors are downregulated while aggregation promoter proteins are upregulated (Freer et al., 2016). This suggests that changes in the pattern of expression of proteins involved in homeostasis within brain regions that express more of those proteins might make those regions more vulnerable to developing disease.
5.6.4. PrP
The regional location of PrPc in human brain has not been studied in detail. Kuczius et al. describe its expression in the frontal neocortex, thalamus, hippocampus, and cerebellum (Kuczius et al., 2007), but olfactory structures were not assessed. In the mouse, a strong immunoreactivity for PrPc is observed in the cerebral cortex (piriform and cingulate), striatum, ventral pallidum, OB, and hippocampus (Beringue, 2003). PrPc is also present in the mouse substantia nigra (Beringue, 2003).
In conclusion, the spatial patterns of expression of α-syn, APP, tau, and PrP remain unclear, especially in humans. No data are available for TDP-43, and for other proteins, many brain structures have not been investigated. For brain regions that express high levels of a prion-like protein, any event that disturbs the balance between protein level and protein clearance might contribute to the development of pathology. Another key element of protein expression is the intrinsic neural activity of brain regions.
5.7. Intrinsic neural activity and prion-like proteins
Here we discuss the observations of changes in neural activity in the OE and OB during disease and their consequences. The level of protein expression can be modulated by neuronal activity, exaggerating the pathological accumulation of protein. As a consequence, high levels of pathological proteins might also disturb cellular function and lead to aberrant neuronal activity. This is particularly interesting in the context of prion-like propagation, where an increase of neuronal activity in one brain region could lead to increase release of prion-like protein at the synaptic level into downstream structures, thus contributing to the propagation of pathological proteins within the brain.
Neuronal hyperactivity increases Aβ production via endocytic pathways (see (Stargardt et al., 2015) for a review) and favors the release of Aβ in interstitial fluid, which is dependent on synaptic activity (Cirrito et al., 2005; Kamenetz et al., 2003). Such events take place at early stages of AD, when the degradation of Aβ is decreasing (Stargardt et al., 2015). On the other hand, high levels of Aβ promote neuronal hyperactivity, due to a decrease in synaptic inhibition and increased incidence of action potential bursts (Busche and Konnerth, 2015). Thus, a vicious cycle between the production of Aβ, its release, and changes in neuronal activity of neurons probably contributes to AD pathogenesis. Because AD is associated with increased risk for epileptic seizures (Busche and Konnerth, 2015; Stargardt et al., 2015), particularly in people with early-onset dementia (Amatniek et al., 2006), the interaction between neuronal hyperactivity and Aβ in AD has been well studied, mainly in hippocampal regions. In AD, neuronal hyperactivity occurs both in large brain networks leading to epileptiform activity and in localized foci. Neuronal hyperactivity occurs at early stages of AD, when epileptiform activity is associated with high level of Aβ (Busche and Konnerth, 2015). In healthy elderly individuals, high levels of amyloid deposits have been linked to aberrant neuronal activity by fMRI (Sperling et al., 2009). The presence of Aβ and tau pathology in central and peripheral brain regions suggests that the neural basis for olfactory dysfunction in AD is not simply dysfunction in one structure at one stage of processing, but dysfunction at numerous stages of processing, from the initial binding of the odorant to the OR to the more central cortical processing of perceptually relevant aspects of olfaction. ORNs of the OE display spontaneous firing in the absence of any odor stimulus in wild-type mice (Joseph et al., 2012). Some findings support the notion that the olfactory system, including the OB and piriform cortex, engages in sparse coding of odor information wherein only a minimal number of cells are needed to be active and to function with the optimal level of excitation and inhibition (Davison and Katz, 2007; Poo and Isaacson, 2009; Rinberg et al., 2006). Modeling evidence suggests that the spare coding of odors in the OB uses low energy and leads to high representational capacity (Yu et al., 2014). This type of activity might be particularly perturbed by hyperactive neurons (Busche and Konnerth, 2015).
Studies in mice have provided some evidence for possible mechanisms of olfactory dysfunction and their influence on neural circuits (Cao et al., 2012; Cheng et al., 2011, 2013; Cramer et al., 2012; Guérin et al., 2009; Morales-Corraliza et al., 2013; Rey et al., 2012; Wesson et al., 2011; Yang et al., 2010). These studies suggest a crucial role of soluble Aβ for neuronal hyperexitability in the hippocampus and OE (Busche et al., 2015; Cao et al., 2012). In some cases, alterations in normal neural activity have been reported, including early-life hyperactivity in the OB (Wesson et al., 2011) that may result in enhanced post-synaptic release of Aβ and other APP metabolites into down-stream structures. Some of these studies have reported aberrations in intrinsic neural activity, including hyper- and hypoactive neural systems in mouse models of AD. The end result of all the proposed mechanisms suggests that AD-associated pathologies and factors associated with them (e.g., cytokines) impair neural function within key olfactory structures, which thereby result in deficits in olfaction.
In synucleinopathies, hyperactivity of neuronal circuits had not been studied extensively, and olfactory circuits have not been explored. One single study investigated network activity in mice overexpressing α-syn and reported changes of network activity in the hippocampus (M. Morris et al., 2015). A high occurrence of myoclonus has been observed in DLB patients, a sign of disturbed neuronal activity; α-syn is suggested to be partially responsible of the hyperactivity of the network (Morris et al., 2015). Phantosmia (olfactory hallucinations) could reflect also hypersensitivity of the olfactory network. Phantosmia is believed to be a clinical manifestation of circuit disinhibition, notably following deafferentation due to OE damage and resulting loss of sensory input (Henkin et al., 2013). Phantosmia has been reported in PD and could also be a symptom of AD, although it is rarely evaluated by physicians (Bannier et al., 2012; Landis and Burkhard, 2008; Tousi and Frankel, 2004). Although not investigated in olfactory structures, neural hyperactivity in PD could be due to an interplay between pathological α-syn and Ca2+ flux, potentiating each other (Hüls et al., 2011; Lowe et al., 2004; Rcom-H’cheo-Gauthier et al., 2014).
TDP-43 aggregation and its effect on neuronal activity have never been studied. However, in a model of frontotemporal dementia with Parkinsonism overexpressing human mutated tau, spontaneous epileptic activity and seizures were observed by EEG, as well as hypersensitivity to a GABAa receptor antagonist (García-Cabrero et al., 2013).
Finally, the loss of PrPc function also contributes to the hyperexcitability of networks in the neocortex and hippocampus, and it can lead to epileptic seizures (Walz et al., 2002). Thus, hyperexcitability of neuronal networks might also occur in CJD.
The molecular mechanisms underlying neuronal hyperactivity induced by pathological protein are unclear. Dysfunction in glutamatergic transmission, which leads to excitotoxicity, is involved in many neurodegenerative diseases (for review: (Ambrosi et al., 2014; Dong et al., 2009). Although not studied specifically in the olfactory system, alteration of glutamatergic transmission by pathological proteins, mutations, or inflammatory mechanisms (Barger et al., 2007; Helton et al., 2008; Parameshwaran et al., 2008; Plowey et al., 2014) could occur in the OE/OB, where glutamatergic cells are particularly vulnerable (Ubeda-Bañon et al., 2010), and could underlie hyperactivity and excitotoxicity.
6. Conclusion
Neuropathological data and olfactory functional measurements support the early involvement of olfactory structures in PD, AD, DLB, HD, and, to some extent, CJD. Pathology and functional alterations are also involved in other neurodegenerative diseases such as MSA and ALS, where the timing of olfactory disturbances is not yet defined. By contrast, in FTD the olfactory network is involved late in the course of the disease, suggesting a spreading mechanism inverted relative to that in PD or DLB. Further work is needed to better understand the involvement of olfactory regions in disease development. Comparisons of propagation patterns between diseases could help determine the spatial origin and etiology of the pathology. For example, in PD, monitoring impairments in olfaction will also allow for better prediction of clinical course (e.g. if a patient is likely to exhibit cognitive decline soon), stratification of patients entering clinical trials, and the possibility to start future disease-modifying therapies before the onset of motor symptoms. Together, the literature reviewed herein support our argument that pathological changes following environmental insults might contribute to the initiation of protein aggregation in the olfactory bulb, which then triggers the spread of the pathology within the brain by a templating mechanism in a prion-like manner.
Acknowledgments
We thank David Nadziejka of Van Andel Research Institute for language editing. We acknowledge Van Andel Research Institute and the many individuals and corporations that financially support the neurodegenerative research at the Institute. N.L. Rey is supported by the Peter C. and Emajean Cook Foundation. D. Wesson reports funding from the National Institutes of Health (NIDCD R01 DC 014443). P. Brundin reports grants from The Michael J. Fox Foundation, National Institutes of Health, Cure Parkinson’s Trust, TEVA Neuroscience, East Tennessee Foundation, KiMe Fund, and Campbell Foundation.
Dr. Brundin has received commercial support as a consultant from Renovo Neural, Inc., Roche, Teva Inc., Lundbeck A/S, AbbVie Inc., Neuroderm, IOS Press Partners. Additionally, he has received commercial support for grants/research from Teva/Lundbeck. Dr. Brundin has ownership interests in Acousort AB and Parkcell AB.
Abbreviations
- α-Syn
α-synuclein
- Aβ
beta-amyloid
- AD
Alzheimer’s disease
- AON
anterior olfactory nucleus
- ALS
amyotrophic lateral sclerosis
- APP
amyloid precursor protein
- CBD
corticobasal degeneration
- CJD
Creutzfeldt-Jakob disease
- DLB
dementia with Lewy bodies
- FTD
frontotemporal dementia
- HD
Huntington’s disease
- iLBD
incidental Lewy body disorder
- LBs
Lewy bodies
- MCs
mitral cells
- MCI
mild cognitive impairment
- MSA
multiple system atrophy
- OB
olfactory bulb
- OE
olfactory epithelium
- ORNs
olfactory receptor neurons
- ORs
olfactory receptors
- PAF
pure autonomic failure
- PD
Parkinson’s disease
- PSP
progressive supranuclear palsy
- ROS
reactive oxygen species
- TCs
tufted cells
Footnotes
Conflict of interest
The authors declare no other conflicts of financial interests.
Available online on ScienceDirect (www.sciencedirect.com).
References
- Abele M, Riet A, Hummel T, Klockgether T, Wüllner U. Olfactory dysfunction in cerebellar ataxia and multiple system atrophy. J Neurol. 2003;250:1453–1455. doi: 10.1007/s00415-003-0248-4. http://dx.doi.org/10.1007/s00415-003-0248-4. [DOI] [PubMed] [Google Scholar]
- Adler CH, Connor DJ, Hentz JG, Sabbagh MN, Caviness JN, Shill HA, Noble B, Beach TG. Incidental Lewy body disease: clinical comparison to a control cohort. Mov Disord. 2010;25:642–646. doi: 10.1002/mds.22971. http://dx.doi.org/10.1002/mds.22971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguzzi A, Baumann F, Bremer J. The Prion’s elusive reason for being. Annu Rev Neurosci. 2008;31:439–477. doi: 10.1146/annurev.neuro.31.060407.125620. http://dx.doi.org/10.1146/annurev.neuro.31.060407.125620. [DOI] [PubMed] [Google Scholar]
- Ahlskog JE, Waring SC, Petersen RC, Esteban-Santillan C, Craig UK, O’Brien PC, Plevak MF, Kurland LT. Olfactory dysfunction in Guamanian ALS, parkinsonism, and dementia. Neurology. 1998;51:1672–1677. doi: 10.1212/wnl.51.6.1672. [DOI] [PubMed] [Google Scholar]
- Alavi Naini SM, Soussi-Yanicostas N. Tau Hyperphosphorylation and Oxidative Stress, a Critical Vicious Circle in Neurodegenerative Tauopathies? Oxidative Med Cell Longev. 2015;2015:151979. doi: 10.1155/2015/151979. http://dx.doi.org/10.1155/2015/151979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcalay RN, Siderowf A, Ottman R, Caccappolo E, Mejia-Santana H, Tang MX, Rosado L, Louis E, Ruiz D, Waters C, Fahn S, Cote L, Frucht S, Ford B, Orbe-Reilly M, Ross B, Verbitsky M, Kisselev S, Comella C, Colcher A, Jennings D, Nance M, Bressman S, Scott WK, Tanner C, Mickel S, Rezak M, Novak KE, Friedman JH, Pfeiffer R, Marsh L, Hiner B, Clark LN, Marder K. Olfaction in Parkin heterozygotes and compound heterozygotes: the CORE-PD study. Neurology. 2011;76:319–326. doi: 10.1212/WNL.0b013e31820882aa. http://dx.doi.org/10.1212/WNL.0b013e31820882aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Kohwi M, Nguyen TM, Merkle FT. The heterogeneity of adult neural stem cells and the emerging complexity of their niche. Cold Spring Harb Symp Quant Biol. 2008;73:357–365. doi: 10.1101/sqb.2008.73.019. http://dx.doi.org/10.1101/sqb.2008.73.019. [DOI] [PubMed] [Google Scholar]
- Alves J. Olfactory dysfunction in dementia. World J Clin Case. 2014;2:661. doi: 10.12998/wjcc.v2.i11.661. http://dx.doi.org/10.12998/wjcc.v2.i11.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatniek JC, Hauser WA, DelCastillo-Castaneda C, Jacobs DM, Marder K, Bell K, Albert M, Brandt J, Stern Y. Incidence and predictors of seizures in patients with Alzheimer’s disease. Epilepsia. 2006;47:867–872. doi: 10.1111/j.1528-1167.2006.00554.x. http://dx.doi.org/10.1111/j.1528-1167.2006.00554.x. [DOI] [PubMed] [Google Scholar]
- Ambrosi G, Cerri S, Blandini F. A further update on the role of excitotoxicity in the pathogenesis of Parkinson’s disease. J Neural Transm. 2014;121:849–859. doi: 10.1007/s00702-013-1149-z. http://dx.doi.org/10.1007/s00702-013-1149-z. [DOI] [PubMed] [Google Scholar]
- Ansari KA, Johnson A. Olfactory function in patients with Parkinson’s disease. J Chronic Dis. 1975;28:493–497. doi: 10.1016/0021-9681(75)90058-2. [DOI] [PubMed] [Google Scholar]
- Applegate LM, Louis ED. Essential tremor: Mild olfactory dysfunction in a cerebellar disorder. Parkinsonism Relat Disord. 2005;11:399–402. doi: 10.1016/j.parkreldis.2005.03.003. http://dx.doi.org/10.1016/j.parkreldis.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Araneda RC, Kini AD, Firestein S. The molecular receptive range of an odorant receptor. Nat Neurosci. 2000;3:1248–1255. doi: 10.1038/81774. http://dx.doi.org/10.1038/81774. [DOI] [PubMed] [Google Scholar]
- Arendt T, Brückner MK, Morawski M, Jäger C, Gertz HJ. Early neurone loss in Alzheimer’s disease: cortical or subcortical? Acta Neuropathol Commun. 2015;3:10. doi: 10.1186/s40478-015-0187-1. http://dx.doi.org/10.1186/s40478-015-0187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SE, Smutzer GS, Trojanowski JQ, Moberg PJ. Cellular and molecular neuropathology of the olfactory epithelium and central olfactory pathways in Alzheimer’s disease and schizophrenia. Ann N Y Acad Sci. 1998;855:762–775. doi: 10.1111/j.1749-6632.1998.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Aronin N, Kim M, Laforet G, DiFiglia M. Are there multiple pathways in the pathogenesis of Huntington’s disease? Philos Trans R Soc Lond Ser B Biol Sci. 1999;354:995–1003. doi: 10.1098/rstb.1999.0451. http://dx.doi.org/10.1098/rstb.1999.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attems J, Lintner F, Jellinger KA. Olfactory involvement in aging and Alzheimer’s disease: an autopsy study. J Alzheimers Dis. 2005;7(149–57):173–180. doi: 10.3233/jad-2005-7208. (discussion) [DOI] [PubMed] [Google Scholar]
- Attems J, Walker L, Jellinger KA. Olfaction and aging: a mini-review. Gerontology. 2015;61:485–490. doi: 10.1159/000381619. http://dx.doi.org/10.1159/000381619. [DOI] [PubMed] [Google Scholar]
- Attems J, Walker L, Jellinger KA. Olfactory bulb involvement in neurodegenerative diseases. Acta Neuropathol. 2014 doi: 10.1007/s00401-014-1261-7. http://dx.doi.org/10.1007/s00401-014-1261-7. [DOI] [PubMed]
- Aungst JL, Heyward PM, Puche AC, Karnup SV, Hayar A, Szabo G, Shipley MT. Centre-surround inhibition among olfactory bulb glomeruli. Nature. 2003;426:623–629. doi: 10.1038/nature02185. http://dx.doi.org/10.1038/nature02185. [DOI] [PubMed] [Google Scholar]
- Baba T, Kikuchi A, Hirayama K, Nishio Y, Hosokai Y, Kanno S, Hasegawa T, Sugeno N, Konno M, Suzuki K, Takahashi S, Fukuda H, Aoki M, Itoyama Y, Mori E, Takeda A. Severe olfactory dysfunction is a prodromal symptom of dementia associated with Parkinson’s disease: a 3 year longitudinal study. Brain. 2012;135:161–169. doi: 10.1093/brain/awr321. http://dx.doi.org/10.1093/brain/awr321. [DOI] [PubMed] [Google Scholar]
- Bacon Moore AS, Paulsen JS, Murphy C. A test of odor fluency in patients with Alzheimer’s and Huntington’s disease. J Clin Exp Neuropsychol. 1999;21:341–351. doi: 10.1076/jcen.21.3.341.918. http://dx.doi.org/10.1076/jcen.21.3.341.918. [DOI] [PubMed] [Google Scholar]
- Baker H, Kawano T, Margolis FL, Joh TH. Transneuronal regulation of tyrosine hydroxylase expression in olfactory bulb of mouse and rat. J Neurosci. 1983;3:69–78. doi: 10.1523/JNEUROSCI.03-01-00069.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball MJ, Lukiw WJ, Kammerman EM, Hill JM. Intracerebral propagation of Alzheimer’s disease: strengthening evidence of a herpes simplex virus etiology. Alzheimers Dement. 2013;9:169–175. doi: 10.1016/j.jalz.2012.07.005. http://dx.doi.org/10.1016/j.jalz.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltazar MT, Dinis-Oliveira RJ, de Lourdes Bastos M, Tsatsakis AM, Duarte JA, Carvalho F. Pesticides exposure as etiological factors of Parkinson’s disease and other neurodegenerative diseases–a mechanistic approach. Toxicol Lett. 2014;230:85–103. doi: 10.1016/j.toxlet.2014.01.039. http://dx.doi.org/10.1016/j.toxlet.2014.01.039. [DOI] [PubMed] [Google Scholar]
- Bannier S, Berdague JL, Rieu I, de Chazeron I, Marques A, Derost P, Ulla M, Llorca PM, Durif F. Prevalence and phenomenology of olfactory hallucinations in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2012;83:1019–1021. doi: 10.1136/jnnp-2012-302414. http://dx.doi.org/10.1136/jnnp-2012-302414. [DOI] [PubMed] [Google Scholar]
- Barger SW, Goodwin ME, Porter MM, Beggs ML. Glutamate release from activated microglia requires the oxidative burst and lipid peroxidation. J Neurochem. 2007;101:1205–1213. doi: 10.1111/j.1471-4159.2007.04487.x. http://dx.doi.org/10.1111/j.1471-4159.2007.04487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkai E, Saar D. Cellular correlates of olfactory learning in the rat piriform cortex. Rev Neurosci. 2001;12:111–120. doi: 10.1515/revneuro.2001.12.2.111. http://dx.doi.org/10.1515/REVNEURO.2001.12.2.111. [DOI] [PubMed] [Google Scholar]
- Barresi M, Ciurleo R, Giacoppo S, Foti Cuzzola V, Celi D, Bramanti P, Marino S. Evaluation of olfactory dysfunction in neurodegenerative diseases. J Neurol Sci. 2012;323:16–24. doi: 10.1016/j.jns.2012.08.028. http://dx.doi.org/10.1016/j.jns.2012.08.028. [DOI] [PubMed] [Google Scholar]
- Barrios FA, Gonzalez L, Favila R, Alonso ME, Salgado PM, Díaz R, Fernandez-Ruiz J. Olfaction and neurodegeneration in HD. Neuroreport. 2007;18:73–76. doi: 10.1097/WNR.0b013e3280102302. http://dx.doi.org/10.1097/WNR.0b013e3280102302. [DOI] [PubMed] [Google Scholar]
- Bartosik-Psujek H, Psujek M, Stelmasiak Z. Rare first symptoms of multiple sclerosis. Ann Univ Mariae Curie Sklodowska Med. 2004;59:242–244. [PubMed] [Google Scholar]
- Barz S, Hummel T, Pauli E, Majer M, Lang CJ, Kobal G. Chemosensory event-related potentials in response to trigeminal and olfactory stimulation in idiopathic Parkinson’s disease. Neurology. 1997;49:1424–1431. doi: 10.1212/wnl.49.5.1424. [DOI] [PubMed] [Google Scholar]
- Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, Sasse J, Boyer S, Shirohi S, Brooks R, Eschbacher J, White CL, Akiyama H, Caviness J, Shill HA, Connor DJ, Sabbagh MN, Walker DG. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009a;117:613–634. doi: 10.1007/s00401-009-0538-8. http://dx.doi.org/10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, White CL, Hladik CL, Sabbagh MN, Connor DJ, Shill HA, Sue LI, Sasse J, Bachalakuri J, Henry-Watson J, Akiyama H, Adler CH, Arizona Parkinson’s Disease Consortium Olfactory bulb alpha-synucleinopathy has high specificity and sensitivity for Lewy body disorders. Acta Neuropathol. 2009b;117:169–174. doi: 10.1007/s00401-008-0450-7. http://dx.doi.org/10.1007/s00401-008-0450-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatman EL, Massey A, Shives KD, Burrack KS, Chamanian M, Morrison TE, Beckham JD. Alpha-synuclein expression restricts RNA viral infections in the brain. J Virol. 2016;90:2767–2782. doi: 10.1128/JVI.02949-15. http://dx.doi.org/10.1128/JVI.02949-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bede P, Elamin M, Byrne S, McLaughlin RL, Kenna K, Vajda A, Pender N, Bradley DG, Hardiman O. Basal ganglia involvement in amyotrophic lateral sclerosis. Neurology. 2013;81:2107–2115. doi: 10.1212/01.wnl.0000437313.80913.2c. http://dx.doi.org/10.1212/01.wnl.0000437313.80913.2c. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Roos DS, Raijmakers P, Doty RL. J Neurol Sci. 2011;310:21–24. doi: 10.1016/j.jns.2011.06.020. http://dx.doi.org/10.1016/j.jns.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Bergmann O, Liebl J, Bernard S, Alkass K, Yeung MSY, Steier P, Kutschera W, Johnson L, Landén M, Druid H, Spalding KL, Frisén J. The age of olfactory bulb neurons in humans. Neuron. 2012;74:634–639. doi: 10.1016/j.neuron.2012.03.030. http://dx.doi.org/10.1016/j.neuron.2012.03.030. [DOI] [PubMed] [Google Scholar]
- Bergmann O, Spalding KL, Frisén J. Adult neurogenesis in humans. Cold Spring Harb Perspect Biol. 2015;7:a018994. doi: 10.1101/cshperspect.a018994. http://dx.doi.org/10.1101/cshperspect.a018994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beringue V. Regional heterogeneity of cellular prion protein isoforms in the mouse brain. Brain. 2003;126:2065–2073. doi: 10.1093/brain/awg205. http://dx.doi.org/10.1093/brain/awg205. [DOI] [PubMed] [Google Scholar]
- Bertrand E, Lechowicz W, Szpak GM, Dymecki J. Qualitative and quantitative analysis of locus coeruleus neurons in Parkinson’s disease. Folia Neuropathol. 1997;35:80–86. [PubMed] [Google Scholar]
- Bessen RA, Shearin H, Martinka S, Boharski R, Lowe D, Wilham JM, Caughey B, Wiley JA. Prion shedding from olfactory neurons into nasal secretions. PLoS Pathog. 2010;6:e1000837. doi: 10.1371/journal.ppat.1000837. http://dx.doi.org/10.1371/journal.ppat.1000837.s006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessen RA, Wilham JM, Lowe D, Watschke CP, Shearin H, Martinka S, Caughey B, Wiley JA. Accelerated shedding of prions following damage to the olfactory epithelium. J Virol. 2012;86:1777–1788. doi: 10.1128/JVI.06626-11. http://dx.doi.org/10.1128/JVI.06626-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandawat V, Reisert J, Yau KW. Elementary response of olfactory receptor neurons to odorants. Science. 2005;308:1931–1934. doi: 10.1126/science.1109886. http://dx.doi.org/10.1126/science.1109886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Calderón-Garcidueñas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009;32:506–516. doi: 10.1016/j.tins.2009.05.009. http://dx.doi.org/10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesveldt S, de Muinck Keizer RJO, Wolters EC, Berendse HW. Odor recognition memory is not independently impaired in Parkinson’s disease. J Neural Transm. 2009;116:575–578. doi: 10.1007/s00702-009-0208-y. http://dx.doi.org/10.1007/s00702-009-0208-y. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Gedela S, Kuwabara H, Constantine GM, Mathis CA, Studenski SA, Moore RY. Selective hyposmia and nigrostriatal dopaminergic denervation in Parkinson’s disease. J Neurol. 2007;254:84–90. doi: 10.1007/s00415-006-0284-y. http://dx.doi.org/10.1007/s00415-006-0284-y. [DOI] [PubMed] [Google Scholar]
- Borza LR. A review on the cause-effect relationship between oxidative stress and toxic proteins in the pathogenesis of neurodegenerative diseases. Rev Med Chir Soc Med Nat Iasi. 2014;118:19–27. [PubMed] [Google Scholar]
- Bostantjopoulou S, Katsarou Z, Papadimitriou A, Veletza V, Hatzigeorgiou G, Lees A. Clinical features of parkinsonian patients with the alpha-synuclein (G209A) mutation. Mov Disord. 2001;16:1007–1013. doi: 10.1002/mds.1221. http://dx.doi.org/10.1002/mds.1221. [DOI] [PubMed] [Google Scholar]
- Boyce JM. Effects of ageing on smell and taste. Postgrad Med J. 2006;82:239–241. doi: 10.1136/pgmj.2005.039453. http://dx.doi.org/10.1136/pgmj.2005.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzoni V, Pansarasa O, Diamanti L, Nosari G, Cereda C, Ceroni M. Amyotrophic lateral sclerosis and environmental factors. Funct Neurol. 2016;31:7–19. doi: 10.11138/FNeur/2016.31.1.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Bohl JRR, Müller CM, Rüb U, de Vos RAI, del Tredici K. Stanley Fahn lecture 2005: the staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov Disord. 2006;21:2042–2051. doi: 10.1002/mds.21065. http://dx.doi.org/10.1002/mds.21065. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Yilmazer D, De Vos RA, Jansen EN, Bohl J, Jellinger KA. Amygdala pathology in parkinson’s disease. Acta Neuropathol. 1994;88:493–500. doi: 10.1007/BF00296485. [DOI] [PubMed] [Google Scholar]
- Braak H, del Tredici K. Potential Pathways of Abnormal Tau and α-Synuclein Dissemination in Sporadic Alzheimer’s and Parkinson’s Diseases. Cold Spring Harb Perspect Biol. 2016 doi: 10.1101/cshperspect.a023630. http://dx.doi.org/10.1101/cshperspect.a023630. [DOI] [PMC free article] [PubMed]
- Braak H, del Tredici K. Where, when, and in what form does sporadic Alzheimer’s disease begin? Curr Opin Neurol. 2012;25:708–714. doi: 10.1097/WCO.0b013e32835a3432. http://dx.doi.org/10.1097/WCO.0b013e32835a3432. [DOI] [PubMed] [Google Scholar]
- Braak H, del Tredici K. Alzheimer’s pathogenesis: is there neuron-to-neuron propagation? Acta Neuropathol. 2011;121:589–595. doi: 10.1007/s00401-011-0825-z. http://dx.doi.org/10.1007/s00401-011-0825-z. [DOI] [PubMed] [Google Scholar]
- Braak H, del Tredici K. Poor and protracted myelination as a contributory factor to neurodegenerative disorders. Neurobiol Aging. 2004;25:19–23. doi: 10.1016/j.neurobiolaging.2003.04.001. http://dx.doi.org/10.1016/j.neurobiolaging.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Braak H, del Tredici K, Bratzke HXR, Hamm-Clement J, Sandmann-Keil D, Rüb U. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages) J Neurol. 2002;249:1. doi: 10.1007/s00415-002-1301-4. http://dx.doi.org/10.1007/s00415-002-1301-4. [DOI] [PubMed] [Google Scholar]
- Braak H, del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003a;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rüb U, Bratzke H, del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. http://dx.doi.org/10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Braak H, Rüb U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm. 2003b;110:517–536. doi: 10.1007/s00702-002-0808-2. http://dx.doi.org/10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- Brettschneider J, Arai K, del Tredici K, Toledo JB, Robinson JL, Lee EB, Kuwabara S, Shibuya K, Irwin DJ, Fang L, Van Deerlin VM, Elman L, McCluskey L, Ludolph AC, Lee VMY, Braak H, Trojanowski JQ. TDP-43 pathology and neuronal loss in amyotrophic lateral sclerosis spinal cord. Acta Neuropathol. 2014;128:423–437. doi: 10.1007/s00401-014-1299-6. http://dx.doi.org/10.1007/s00401-014-1299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brettschneider J, del Tredici K, Toledo JB, Robinson JL, Irwin DJ, Grossman M, Suh E, Van Deerlin VM, Wood EM, Baek Y, Kwong L, Lee EB, Elman L, McCluskey L, Fang L, Feldengut S, Ludolph AC, Lee VMY, Braak H, Trojanowski JQ. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol. 2013;74:20–38. doi: 10.1002/ana.23937. http://dx.doi.org/10.1002/ana.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill MS, Ninkovic J, Winpenny E, Hodge RD, Ozen I, Yang R, Lepier A, Gascón S, Erdelyi F, Szabo G, Parras C, Guillemot F, Frotscher M, Berninger B, Hevner RF, Raineteau O, Götz M. Adult generation of glutamatergic olfactory bulb interneurons. Nat Neurosci. 2009;12:1524–1533. doi: 10.1038/nn.2416. http://dx.doi.org/10.1038/nn.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouillard M, Laccourreye L, Jabbour W, Emile J, Pouplard-Barthelaix A. Ultrastructural and immunohistochemical study of the olfactory mucosa in Alzheimer’s disease. Bull Assoc Anat. 1994;78:25–28. [PubMed] [Google Scholar]
- Brown RC, Lockwood AH, Sonawane BR. Neurodegenerative diseases: an overview of environmental risk factors. Environ Health Perspect. 2005a;113:1250–1256. doi: 10.1289/ehp.7567. http://dx.doi.org/10.1289/ehp.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TP, Rumsby PC, Capleton AC, Rushton L, Levy LS. Pesticides and Parkinson’s disease—is there a link? Environ Health Perspect. 2005b;114:156–164. doi: 10.1289/ehp.8095. http://dx.doi.org/10.1289/ehp.8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundin P, Li JY, Holton JL, Lindvall O, Revesz T. Research in motion: the enigma of Parkinson’s disease pathology spread. Nat Rev Neurosci. 2008;9:741–745. doi: 10.1038/nrn2477. http://dx.doi.org/10.1038/nrn2477. [DOI] [PubMed] [Google Scholar]
- Brunjes PC, Illig KR, Meyer EA. A field guide to the anterior olfactory nucleus (cortex) Brain Res Rev. 2005;50:305–335. doi: 10.1016/j.brainresrev.2005.08.005. http://dx.doi.org/10.1016/j.brainresrev.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Buonviso N, Amat C, Litaudon P. Respiratory modulation of olfactory neurons in the rodent brain. Chem Senses. 2006;31:145–154. doi: 10.1093/chemse/bjj010. http://dx.doi.org/10.1093/chemse/bjj010. [DOI] [PubMed] [Google Scholar]
- Busche MA, Grienberger C, Keskin AD, Song B, Neumann U, Staufenbiel M, Förstl H, Konnerth A. Decreased amyloid-β and increased neuronal hyperactivity by immunotherapy in Alzheimer’s models. Nat Neurosci. 2015;18:1725–1727. doi: 10.1038/nn.4163. http://dx.doi.org/10.1038/nn.4163. [DOI] [PubMed] [Google Scholar]
- Busche MA, Konnerth A. Neuronal hyperactivity – a key defect in Alzheimer’s disease? BioEssays. 2015;37:624–632. doi: 10.1002/bies.201500004. http://dx.doi.org/10.1002/bies.201500004. [DOI] [PubMed] [Google Scholar]
- Busenbark KL, Huber SJ, Greer G, Pahwa R, Koller WC. Olfactory function in essential tremor. Neurology. 1992;42:1631–1632. doi: 10.1212/wnl.42.8.1631. [DOI] [PubMed] [Google Scholar]
- Bylsma FW, Moberg PJ, Doty RL, Brandt J. Odor identification in Huntington’s disease patients and asymptomatic gene carriers. J Neuropsychiatr Clin Neurosci. 1997;9:598–600. doi: 10.1176/jnp.9.4.598. http://dx.doi.org/10.1176/jnp.9.4.598. [DOI] [PubMed] [Google Scholar]
- Cafferty WBJ, McGee AW, Strittmatter SM. Axonal growth therapeutics: regeneration or sprouting or plasticity? Trends Neurosci. 2008;31:215–220. doi: 10.1016/j.tins.2008.02.004. http://dx.doi.org/10.1016/j.tins.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Schrank BR, Rodriguez S, Benz EG, Moulia TW, Rickenbacher GT, Gomez AC, Levites Y, Edwards SR, Golde TE, Hyman BT, Barnea G, Albers MW. Aβ alters the connectivity of olfactory neurons in the absence of amyloid plaques in vivo. Nat Commun. 2012;3:1009. doi: 10.1038/ncomms2013. http://dx.doi.org/10.1038/ncomms2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson KS, Dillione MR, Wesson DW. Odor- and state-dependent olfactory tubercle local field potential dynamics in awake rats. J Neurophysiol. 2014;111:2109–2123. doi: 10.1152/jn.00829.2013. http://dx.doi.org/10.1152/jn.00829.2013. [DOI] [PubMed] [Google Scholar]
- Caroni P, Schwab ME. Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J Cell Biol. 1988;106:1281–1288. doi: 10.1083/jcb.106.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriero G, Uva L, Gnatkovsky V, de Curtis M. Distribution of the olfactory fiber input into the olfactory tubercle of the in vitro isolated Guinea pig brain. J Neurophysiol. 2009;101:1613–1619. doi: 10.1152/jn.90792.2008. http://dx.doi.org/10.1152/jn.90792.2008. [DOI] [PubMed] [Google Scholar]
- Caušević M, Farooq U, Lovestone S, Killick R. β-Amyloid precursor protein and tau protein levels are differently regulated in human cerebellum compared to brain regions vulnerable to Alzheimer’s type neurodegeneration. Neurosci Lett. 2010;485:162–166. doi: 10.1016/j.neulet.2010.08.088. http://dx.doi.org/10.1016/j.neulet.2010.08.088. [DOI] [PubMed] [Google Scholar]
- Cauthron JL, Stripling JS. Long-term plasticity in the regulation of olfactory bulb activity by centrifugal fibers from piriform cortex. J Neurosci. 2014;34:9677–9687. doi: 10.1523/JNEUROSCI.1314-14.2014. http://dx.doi.org/10.1523/JNEUROSCI.1314-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaco S, Goncalves A, Mendes A, Vila-Cha N, Moreira IXS, Fernandes J, Damasio J, Teixeira-Pinto A, Bastos Lima AXN. Research article. Behav Neurol. 2015:1–5. doi: 10.1155/2015/976589. http://dx.doi.org/10.1155/2015/976589. [DOI] [PMC free article] [PubMed]
- Chen SG, Stribinskis V, Rane MJ, Demuth DR, Gozal E, Roberts AM, Jagadapillai R, Liu R, Choe K, Shivakumar B, Son F, Jin S, Kerber R, Adame A, Masliah E, Friedland RP. Exposure to the functional bacterial amyloid protein curli enhances alpha-synuclein aggregation in aged Fischer 344Rats and Caenorhabditis elegans. Sci Rep. 2016:1–10. doi: 10.1038/srep34477. http://dx.doi.org/10.1038/srep34477. [DOI] [PMC free article] [PubMed]
- Chen Y, Getchell TV, Sparks DL, Getchell ML. Patterns of adrenergic and peptidergic innervation in human olfactory mucosa: age-related trends. J Comp Neurol. 1993;334:104–116. doi: 10.1002/cne.903340109. http://dx.doi.org/10.1002/cne.903340109. [DOI] [PubMed] [Google Scholar]
- Cheng H, Davis DA, Hasheminassab S, Sioutas C, Morgan TE, Finch CE. Urban traffic-derived nanoparticulate matter reduces neurite outgrowth via TNFαin vitro. J Neuroinflammation. 2016:1–11. doi: 10.1186/s12974-016-0480-3. http://dx.doi.org/10.1186/s12974-016-0480-3. [DOI] [PMC free article] [PubMed]
- Cheng N, Bai L, Steuer E, Belluscio L. Olfactory functions scale with circuit restoration in a rapidly reversible Alzheimer’s disease model. J Neurosci. 2013;33:12208–12217. doi: 10.1523/JNEUROSCI.0291-13.2013. http://dx.doi.org/10.1523/JNEUROSCI.0291-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N, Cai H, Belluscio L. In vivo olfactory model of APP-induced neurodegeneration reveals a reversible cell-autonomous function. J Neurosci. 2011;31:13699–13704. doi: 10.1523/JNEUROSCI.1714-11.2011. http://dx.doi.org/10.1523/JNEUROSCI.1714-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess A, Simon I, Cedar H, Axel R. Allelic inactivation regulates olfactory receptor gene expression. Cell. 1994;78:823–834. doi: 10.1016/s0092-8674(94)90562-2. [DOI] [PubMed] [Google Scholar]
- Chiba Y, Fujishiro H, Iseki E, Ota K, Kasanuki K, Hirayasu Y, Satoa K. Retrospective survey of prodromal symptoms in dementia with lewy bodies: comparison with Alzheimer’s disease. Dement Geriatr Cogn Disord. 2012;33:273–281. doi: 10.1159/000339363. http://dx.doi.org/10.1159/000339363. [DOI] [PubMed] [Google Scholar]
- Chin-Chan M, Navarro-Yepes J, Quintanilla-Vega B. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front Cell Neurosci. 2015;9:914. doi: 10.3389/fncel.2015.00124. http://dx.doi.org/10.1111/j.1471-4159.2010.07002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou KL, Bohnen NI. Performance on an Alzheimer-selective odor identification test in patients with Parkinson’s disease and its relationship with cerebral dopamine transporter activity. Parkinsonism Relat Disord. 2009;15:640–643. doi: 10.1016/j.parkreldis.2009.03.004. http://dx.doi.org/10.1016/j.parkreldis.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen-Zaech S, Kraftsik R, Pillevuit O, Kiraly M, Martins R, Khalili K, Miklossy J. Early olfactory involvement in Alzheimer’s disease. Can J Neurol Sci. 2003;30:20–25. doi: 10.1017/s0317167100002389. [DOI] [PubMed] [Google Scholar]
- Chrysostome V, Tison F, Yekhlef F, Sourgen C, Baldi I, Dartigues JF. Epidemiology of multiple system atrophy: a prevalence and pilot risk factor study in Aquitaine, France. Neuroepidemiology. 2004;23:201–208. doi: 10.1159/000078506. http://dx.doi.org/10.1159/000078506. [DOI] [PubMed] [Google Scholar]
- Chu Y, Kordower JH. Age-associated increases of α-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: is this the target for Parkinson’s disease? Neurobiol Dis. 2007;25:134–149. doi: 10.1016/j.nbd.2006.08.021. http://dx.doi.org/10.1016/j.nbd.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-β levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. http://dx.doi.org/10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Clavaguera F, Akatsu H, Fraser G, Crowther RA, Frank S, Hench J, Probst A, Winkler DT, Reichwald J, Staufenbiel M, Ghetti B, Goedert M, Tolnay M. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc Natl Acad Sci. 2013;110:9535–9540. doi: 10.1073/pnas.1301175110. http://dx.doi.org/10.1073/pnas.1301175110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen TJ, Hwang AW, Unger T, Trojanowski JQ, Lee VMY. Redox signalling directly regulates TDP-43 via cysteine oxidation and disulphide cross-linking. EMBO J. 2011;31:1241–1252. doi: 10.1038/emboj.2011.471. http://dx.doi.org/10.1038/emboj.2011.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinescu CS, Raps EC, Cohen JA, West SE, Doty RL. Olfactory disturbances as the initial or most prominent symptom of multiple sclerosis. J Neurol Neurosurg Psychiatry. 1994;57:1011–1012. doi: 10.1136/jnnp.57.8.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia AS, Patel P, Dutta K, Julien JP. Inflammation induces TDP-43 Mislocalization and aggregation. PLoS One. 2015;10:e0140248. doi: 10.1371/journal.pone.0140248. http://dx.doi.org/10.1371/journal.pone.0140248.s001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa C, Duyckaerts C. Oligodendroglial and neuronal inclusions in multiple system atrophy. Curr Opin Neurol. 1993;6:865–871. doi: 10.1097/00019052-199312000-00007. [DOI] [PubMed] [Google Scholar]
- Cramer PE, Cirrito JR, Wesson DW, Lee CYD, Karlo JC, Zinn AE, Casali BT, Restivo JL, Goebel WD, James MJ, Brunden KR, Wilson DA, Landreth GE. ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models. Science. 2012;335:1503–1506. doi: 10.1126/science.1217697. http://dx.doi.org/10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MA, Faull RLM, Eriksson PS. The effect of neurodegenerative diseases on the subventricular zone. Nat Rev Neurosci. 2007;8:712–723. doi: 10.1038/nrn2216. http://dx.doi.org/10.1038/nrn2216. [DOI] [PubMed] [Google Scholar]
- D’Souza RD, Vijayaraghavan S. Paying attention to smell: cholinergic signaling in the olfactory bulb. Front Synaptic Neurosci. 2014;6:1–11. doi: 10.3389/fnsyn.2014.00021. http://dx.doi.org/10.3389/fnsyn.2014.00021/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damholdt MF, Borghammer P, Larsen L, Østergaard K. Odor identification deficits identify Parkinson’s disease patients with poor cognitive performance. Mov Disord. 2011;26:2045–2050. doi: 10.1002/mds.23782. http://dx.doi.org/10.1002/mds.23782. [DOI] [PubMed] [Google Scholar]
- Dando SJ, Mackay-Sim A, Norton R, Currie BJ, St John JA, Ekberg JAK, Batzloff M, Ulett GC, Beacham IR. Pathogens penetrating the central nervous system: infection pathways and the cellular and molecular mechanisms of invasion. Clin Microbiol Rev. 2014;27:691–726. doi: 10.1128/CMR.00118-13. http://dx.doi.org/10.1128/CMR.00118-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel SE, Hawkes CH. Preliminary diagnosis of Parkinson’s disease by olfactory bulb pathology. Lancet. 1992;340:186. doi: 10.1016/0140-6736(92)93275-r. [DOI] [PubMed] [Google Scholar]
- Daulatzai MA. Olfactory dysfunction: its early temporal relationship and neural correlates in the pathogenesis of Alzheimer’s diseaseMak Adam Daulatzai. J Neural Transm. 2015;122:1475–1497. doi: 10.1007/s00702-015-1404-6. http://dx.doi.org/10.1007/s00702-015-1404-6. [DOI] [PubMed] [Google Scholar]
- Davison IG, Katz LC. Sparse and selective odor coding by mitral/tufted neurons in the main olfactory bulb. J Neurosci. 2007;27:2091–2101. doi: 10.1523/JNEUROSCI.3779-06.2007. http://dx.doi.org/10.1523/JNEUROSCI.3779-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayalu P, Albin RL. Huntington disease: pathogenesis and treatment. Neurol Clin. 2015;33:101–114. doi: 10.1016/j.ncl.2014.09.003. http://dx.doi.org/10.1016/j.ncl.2014.09.003. [DOI] [PubMed] [Google Scholar]
- de Olmos JS, Heimer L. The concepts of the ventral striatopallidal system and extended amygdala. Ann N Y Acad Sci. 1999;877:1–32. doi: 10.1111/j.1749-6632.1999.tb09258.x. [DOI] [PubMed] [Google Scholar]
- Dehay B, Fernagut PO. Alpha-synuclein-based models of Parkinson’s disease. Rev Neurol. 2016 doi: 10.1016/j.neurol.2016.04.003. http://dx.doi.org/10.1016/j.neurol.2016.04.003. [DOI] [PubMed]
- Del Tredici K, Braak H. Review: sporadic Parkinson’s disease: development and distribution of α-synuclein pathology. Neuropathol Appl Neurobiol. 2016;42:33–50. doi: 10.1111/nan.12298. http://dx.doi.org/10.1111/nan.12298. [DOI] [PubMed] [Google Scholar]
- Del Tredici K, Braak H. Idiopathic Parkinson’s Disease: Staging an α-Synucleinopathy with a Predictable Pathoanatomy [WWW Document] NCBI Bookshelf. 2014 (URL http://www.ncbi.nlm.nih.gov/books/NBK6077/ (accessed 10.21.14))
- Del Tredici K, Braak H. Lewy pathology and neurodegeneration in premotor Parkinson’s disease. Mov Disord. 2012;27:597–607. doi: 10.1002/mds.24921. http://dx.doi.org/10.1002/mds.24921. [DOI] [PubMed] [Google Scholar]
- del Tredici K, Rüb U, de Vos RAI, Bohl JRE, Braak H. Where does parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol. 2002;61:413–426. doi: 10.1093/jnen/61.5.413. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Liu X, Tabert MH, Pradhaban G, Cuasay K, Bell K, de Leon MJ, Doty RL, Stern Y, Pelton GH. Combining early markers strongly predicts conversion from mild cognitive impairment to Alzheimer’s disease. Biol Psychiatry. 2008;64:871–879. doi: 10.1016/j.biopsych.2008.06.020. http://dx.doi.org/10.1016/j.biopsych.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Maio R, Barrett PJ, Hoffman EK, Barrett CW, Zharikov A, Borah A, Hu X, McCoy J, Chu CT, Burton EA, Hastings TG, Greenamyre JT. α-Synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson’s disease. Sci Transl Med. 2016;8:342ra78. doi: 10.1126/scitranslmed.aaf3634. http://dx.doi.org/10.1126/scitranslmed.aaf3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Dahl AR. Olfactory mucosa: composition, enzymatic localization and metabolism. In: Doty RL, editor. Handbook of Olfaction and Gustation. Marcel Dekker, Inc.; New York, Basel: 2003. pp. 51–74. [Google Scholar]
- Djordjevic J, Jones-Gotman M, De Sousa K, Chertkow H. Olfaction in patients with mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2008;29:693–706. doi: 10.1016/j.neurobiolaging.2006.11.014. http://dx.doi.org/10.1016/j.neurobiolaging.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Dong XX, Wang Y, Qin ZH. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin. 2009;30:379–387. doi: 10.1038/aps.2009.24. http://dx.doi.org/10.1038/aps.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL. Olfaction in Parkinson’s disease and related disorders. Neurobiol Dis. 2012a;46:527–552. doi: 10.1016/j.nbd.2011.10.026. http://dx.doi.org/10.1016/j.nbd.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL. Olfactory dysfunction in Parkinson disease. Nat Rev Neurol. 2012b;8:329–339. doi: 10.1038/nrneurol.2012.80. http://dx.doi.org/10.1038/nrneurol.2012.80. [DOI] [PubMed] [Google Scholar]
- Doty RL. The olfactory vector hypothesis of neurodegenerative disease: is it viable? Ann Neurol. 2008;63:7–15. doi: 10.1002/ana.21327. http://dx.doi.org/10.1002/ana.21327. [DOI] [PubMed] [Google Scholar]
- Doty RL. Olfactory capacities in aging and Alzheimer’s disease. Psychophysical and anatomic considerations Ann N Y Acad Sci. 1991;640:20–27. doi: 10.1111/j.1749-6632.1991.tb00185.x. [DOI] [PubMed] [Google Scholar]
- Doty RL, editor. Handbook of Olfaction and Gustation, 200. 3rd. Marcel Dekker, Inc; 2003. [Google Scholar]
- Doty RL, Beals E, Osman A, Dubroff J, Chung I, Leon-Sarmiento FE, Hurtig H, Ying GS. Suprathreshold odor intensity perception in early-stage Parkinson’s disease. Mov Disord. 2014;29:1208–1212. doi: 10.1002/mds.25946. http://dx.doi.org/10.1002/mds.25946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL, Bromley SM, Stern MB. Olfactory testing as an aid in the diagnosis of Parkinson’s disease: development of optimal discrimination criteria. Neurodegeneration. 1995;4:93–97. doi: 10.1006/neur.1995.0011. [DOI] [PubMed] [Google Scholar]
- Doty RL, Deems DA, Stellar S. Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology. 1988;38:1237–1244. doi: 10.1212/wnl.38.8.1237. [DOI] [PubMed] [Google Scholar]
- Doty RL, Golbe LI, McKeown DA, Stern MB, Lehrach CM, Crawford D. Olfactory testing differentiates between progressive supranuclear palsy and idiopathic Parkinson’s disease. Neurology. 1993;43:962–965. doi: 10.1212/wnl.43.5.962. [DOI] [PubMed] [Google Scholar]
- Doty RL, Li C, Mannon LJ, Yousem DM. Olfactory dysfunction in multiple sclerosis. Relation to plaque load in inferior frontal and temporal lobes. Ann N Y Acad Sci. 1998;855:781–786. doi: 10.1111/j.1749-6632.1998.tb10658.x. [DOI] [PubMed] [Google Scholar]
- Doty RL, Marcus A, Lee WW. Development of the 12-item cross-cultural smell identification test (CC-SIT) Laryngoscope. 1996;106:353–356. doi: 10.1097/00005537-199603000-00021. [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984a;32:489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope. 1984b;94:176–178. doi: 10.1288/00005537-198402000-00004. [DOI] [PubMed] [Google Scholar]
- Doty RL, Singh A, Tetrud J, Langston JW. Lack of major olfactory dysfunction in MPTP-induced parkinsonism. Ann Neurol. 1992a;32:97–100. doi: 10.1002/ana.410320116. http://dx.doi.org/10.1002/ana.410320116. [DOI] [PubMed] [Google Scholar]
- Doty RL, Stern MB, Pfeiffer C, Gollomp SM, Hurtig HI. Bilateral olfactory dysfunction in early stage treated and untreated idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1992b;55:138–142. doi: 10.1136/jnnp.55.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucette W, Milder J, Restrepo D. Adrenergic modulation of olfactory bulb circuitry affects odor discrimination. Learn Mem. 2007;14:539–547. doi: 10.1101/lm.606407. http://dx.doi.org/10.1101/lm.606407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver-Dunckley E, Adler CH, Hentz JG, Dugger BN, Shill HA, Caviness JN, Sabbagh MN, Beach TG, Arizona Parkinson Disease Consortium Olfactory dysfunction in incidental Lewy body disease and Parkinson’s disease. Parkinsonism Relat Disord. 2014;20:1260–1262. doi: 10.1016/j.parkreldis.2014.08.006. http://dx.doi.org/10.1016/j.parkreldis.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda JE, Shah U, Arnold SE, Lee VM, Trojanowski JQ. The expression of alpha-, beta-, and gamma-synucleins in olfactory mucosa from patients with and without neurodegenerative diseases. Exp Neurol. 1999;160:515–522. doi: 10.1006/exnr.1999.7228. http://dx.doi.org/10.1006/exnr.1999.7228. [DOI] [PubMed] [Google Scholar]
- Duff K, McCaffrey RJ, Solomon GS. The pocket smell test: successfully discriminating probable Alzheimer’s dementia from vascular dementia and major depression. J Neuropsychiatr Clin Neurosci. 2002;14:197–201. doi: 10.1176/jnp.14.2.197. http://dx.doi.org/10.1176/jnp.14.2.197. [DOI] [PubMed] [Google Scholar]
- Dvorska I, Brust P, Hrbas P, Ruhle HJ, Barth T, Ermisch A. On the blood-brain barrier to peptides: effects of immobilization stress on regional blood supply and accumulation of labelled peptides in the rat brain. Endocr Regul. 1992;26:77–82. [PubMed] [Google Scholar]
- Eikelenboom P, Bate C, Van Gool WA, Hoozemans JJM, Rozemuller JM, Veerhuis R, Williams A. Neuroinflammation in Alzheimer’s disease and prion disease. Glia. 2002;40:232–239. doi: 10.1002/glia.10146. http://dx.doi.org/10.1002/glia.10146. [DOI] [PubMed] [Google Scholar]
- Elbaz A, Levecque C, Clavel J, Vidal JS, Richard F, Amouyel P, Alpérovitch A, Chartier-Harlin MC, Tzourio C. CYP2D6 polymorphism, pesticide exposure, and Parkinson’s disease. Ann Neurol. 2004;55:430–434. doi: 10.1002/ana.20051. http://dx.doi.org/10.1002/ana.20051. [DOI] [PubMed] [Google Scholar]
- Elian M. Olfactory impairment in motor neuron disease: a pilot study. J Neurol Neurosurg Psychiatry. 1991;54:927–928. doi: 10.1136/jnnp.54.10.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esiri MM, Wilcock GK. The olfactory bulbs in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1984;47:56–60. doi: 10.1136/jnnp.47.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evidente VGH, Esteban RP, Hernandez JL, Natividad FF, Advincula J, Gwinn-Hardy K, Hardy J, Singleton A, Singleton A. Smell testing is abnormal in “lubag” or X-linked dystonia-parkinsonism: a pilot study. Parkinsonism Relat Disord. 2004;10:407–410. doi: 10.1016/j.parkreldis.2004.04.011. http://dx.doi.org/10.1016/j.parkreldis.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Fatima M, Tan R, Halliday GM, Kril JJ. Spread of pathology in amyotrophic lateral sclerosis: assessment of phosphorylated TDP-43 along axonal pathways. Acta Neuropathol Commun. 2015:1–9. doi: 10.1186/s40478-015-0226-y. http://dx.doi.org/10.1186/s40478-015-0226-y. [DOI] [PMC free article] [PubMed]
- Faucheux BA, Privat N, Brandel JP, Sazdovitch V, Laplanche JL, Maurage CA, Hauw JJ, Haïk S. Loss of cerebellar granule neurons is associated with punctate but not with large focal deposits of prion protein in Creutzfeldt-Jakob disease. J Neuropathol Exp Neurol. 2009;68:892–901. doi: 10.1097/NEN.0b013e3181af7f23. http://dx.doi.org/10.1097/NEN.0b013e3181af7f23. [DOI] [PubMed] [Google Scholar]
- Feinstein DL, Heneka MT, Gavrilyuk V, Russo DC, Weinberg G, Galea E. Noradrenergic regulation of inflammatory gene expression in brain. Neurochem Int. 2002;41:357–365. doi: 10.1016/s0197-0186(02)00049-9. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Díaz R, Hall-Haro C, Vergara P, Fiorentini A, Nunñez L, Drucker-Colin R, Ochoa A, Yescas P, Rasmussen A, Alonso ME. Olfactory dysfunction in hereditary ataxia and basal ganglia disorders. Neuroreport. 2003;14:1339–1341. doi: 10.1097/01.wnr.0000077551.91466.d3. http://dx.doi.org/10.1097/01.wnr.0000077551.91466.d3. [DOI] [PubMed] [Google Scholar]
- Ferraris A, Ialongo T, Passali GC, Pellecchia MT, Brusa L, Laruffa M, Guidubaldi A, Paludetti G, Albanese A, Barone P, Dallapiccola B, Valente EM, Bentivoglio AR. Olfactory dysfunction in Parkinsonism caused by PINK1mutations. Mov Disord. 2009 doi: 10.1002/mds.22816. (NA–NA) [DOI] [PubMed] [Google Scholar]
- Ferreira JJ, Guedes LC, Rosa MM, Coelho M, van Doeselaar M, Schweiger D, Di Fonzo A, Oostra BA, Sampaio C, Bonifati V. High prevalence of LRRK2 mutations in familial and sporadic Parkinson’s disease in Portugal. Mov Disord. 2007;22:1194–1201. doi: 10.1002/mds.21525. http://dx.doi.org/10.1002/mds.21525. [DOI] [PubMed] [Google Scholar]
- Ferrer I. Synaptic pathology and cell death in the cerebellum in Creutzfeldt-Jakob disease. Cerebellum. 2002;1:213–222. doi: 10.1080/14734220260418448. http://dx.doi.org/10.1080/14734220260418448. [DOI] [PubMed] [Google Scholar]
- Finkelstein MM, Jerrett M. A study of the relationships between Parkinson’s disease and markers of traffic-derived and environmental manganese air pollution in two Canadian cities. Environ Res. 2007;104:420–432. doi: 10.1016/j.envres.2007.03.002. http://dx.doi.org/10.1016/j.envres.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Freer R, Sormanni P, Vecchi G, Ciryam P, Dobson CM, Vendruscolo M. A protein homeostasis signature in healthy brains recapitulates tissue vulnerability to Alzheimer’s disease. Sci Adv. 2016;2:e1600947. doi: 10.1126/sciadv.1600947. http://dx.doi.org/10.1126/sciadv.1600947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio R, Fujishiro H, Ahn TB, Josephs KA, Maraganore DM, DelleDonne A, Parisi JE, Klos KJ, Boeve BF, Dickson DW, Ahlskog JE. Incidental Lewy body disease: do some cases represent a preclinical stage of dementia with Lewy bodies? Neurobiol Aging. 2011;32:857–863. doi: 10.1016/j.neurobiolaging.2009.05.019. http://dx.doi.org/10.1016/j.neurobiolaging.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishiro H, Ahn TB, Frigerio R, DelleDonne A, Josephs KA, Parisi JE, Eric Ahlskog J, Dickson DW. Glial cytoplasmic inclusions in neurologically normal elderly: prodromal multiple system atrophy? Acta Neuropathol. 2008;116:269–275. doi: 10.1007/s00401-008-0398-7. http://dx.doi.org/10.1007/s00401-008-0398-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabe S, Takao M, Saito Y, Hatsuta H, Sugiyama M, Ito S, Kanemaru K, Sawabe M, Arai T, Mochizuki H, Hattori N, Murayama S. Neuropathologic analysis of Lewy-related α-synucleinopathy in olfactory mucosa. Neuropathology. 2012;33:47–58. doi: 10.1111/j.1440-1789.2012.01329.x. http://dx.doi.org/10.1111/j.1440-1789.2012.01329.x. [DOI] [PubMed] [Google Scholar]
- Fusetti M, Fioretti AB, Silvagni F, Sucapane P, Necozione S, Eibenstein A. Smell and preclinical Alzheimer disease: study of 29 patients with amnesic mild cognitive impairment. J Otolaryngol Head Neck Surg. 2010;39:175–181. [PubMed] [Google Scholar]
- Gadziola MA, Tylicki KA, Christian DL, Wesson DW. The olfactory tubercle encodes odor valence in behaving mice. J Neurosci. 2015;35:4515–4527. doi: 10.1523/JNEUROSCI.4750-14.2015. http://dx.doi.org/10.1523/JNEUROSCI.4750-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadziola MA, Wesson DW. The neural representation of goal-directed actions and outcomes in the ventral Striatum’s olfactory tubercle. J Neurosci. 2016;36:548–560. doi: 10.1523/JNEUROSCI.3328-15.2016. http://dx.doi.org/10.1523/JNEUROSCI.3328-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cabrero AM, Guerrero-López R, Giráldez BG, Llorens-Martín M, Ávila J, Serratosa JM, Sánchez MP. Neurobiology of disease. Neurobiol Dis. 2013;58:200–208. doi: 10.1016/j.nbd.2013.06.005. http://dx.doi.org/10.1016/j.nbd.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Garland EM, Raj SR, Peltier AC, Robertson D, Biaggioni I. A cross-sectional study contrasting olfactory function in autonomic disorders. Am Acad Neurol. 2011:1–5. doi: 10.1212/WNL.0b013e31820a0caf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S, Rey NL, Reichenbach N, Steiner JA, Brundin P. α-Synuclein: the long distance runner. Brain Pathol. 2013;23:350–357. doi: 10.1111/bpa.12046. http://dx.doi.org/10.1111/bpa.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German DC, Manaye KF, White CL, Woodward DJ, McIntire DD, Smith WK, Kalaria RN, Mann DM. Disease-specific patterns of locus coeruleus cell loss. Ann Neurol. 1992;32:667–676. doi: 10.1002/ana.410320510. http://dx.doi.org/10.1002/ana.410320510. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Barr PJ, Murphy C. Differences in olfactory and visual memory in patients with pathologically confirmed Alzheimer’s disease and the Lewy body variant of Alzheimer’s disease. J Int Neuropsychol Soc. 2004;10:835–842. doi: 10.1017/s1355617704106024. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Murphy C. The effect of the ApoE ε4 allele on recognition memory for olfactory and visual stimuli in patients with pathologically confirmed Alzheimer’s disease, probable Alzheimer’s disease, and healthy elderly controls. J Clin Exp Neuropsychol. 2004;26:779–794. doi: 10.1080/13803390490509439. http://dx.doi.org/10.1080/13803390490509439. [DOI] [PubMed] [Google Scholar]
- Godoy M, Voegels R, Pinna F, Imamura R, Farfel J. Olfaction in neurologic and neurodegenerative diseases: a literature review. Int Arch Otorhinolaryngol. 2015;19:176–179. doi: 10.1055/s-0034-1390136. http://dx.doi.org/10.1055/s-0034-1390136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Ghetti B, Spillantini MG. Frontotemporal dementia: implications for understanding Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006254. doi: 10.1101/cshperspect.a006254. http://dx.doi.org/10.1101/cshperspect.a006254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Masuda-Suzukake M, Falcon B. Like prions: the propagation of aggregated tau and α-synuclein in neurodegeneration. Brain (aww 230-13) 2016 doi: 10.1093/brain/aww230. [DOI] [PubMed] [Google Scholar]
- Goker-Alpan O, Lopez G, Vithayathil J, Davis J, Hallett M, Sidransky E. The Spectrum of parkinsonian manifestations associated with glucocerebrosidase mutations. Arch Neurol. 2008;65 doi: 10.1001/archneur.65.10.1353. http://dx.doi.org/10.1001/archneur.65.10.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SM. Environmental toxins and Parkinson’s disease. Annu Rev Pharmacol Toxicol. 2014;54:141–164. doi: 10.1146/annurev-pharmtox-011613-135937. http://dx.doi.org/10.1146/annurev-pharmtox-011613-135937. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Holmes C, Bentho O, Sato T, Moak J, Sharabi Y, Imrich R, Conant S, Eldadah BA. Biomarkers to detect central dopamine deficiency and distinguish Parkinson disease from multiple system atrophy. Parkinsonism Relat Disord. 2008;14:600–607. doi: 10.1016/j.parkreldis.2008.01.010. http://dx.doi.org/10.1016/j.parkreldis.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Sewell L. Olfactory dysfunction in pure autonomic failure: Implications for the pathogenesis of Lewy body diseases. Parkinsonism Relat Disord. 2009;15:516–520. doi: 10.1016/j.parkreldis.2008.12.009. http://dx.doi.org/10.1016/j.parkreldis.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Isla T, Price JL, McKeel DW, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA. Central mechanisms of odour object perception. Nat Rev Neurosci. 2010;11:628–641. doi: 10.1038/nrn2883. http://dx.doi.org/10.1038/nrn2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratuze M, Cisbani G, Cicchetti F, Planel E. Is Huntington’s disease a tauopathy? Brain. 2016;139:1014–1025. doi: 10.1093/brain/aww021. http://dx.doi.org/10.1093/brain/aww021. [DOI] [PubMed] [Google Scholar]
- Griffith JS. Self-replication and scrapie. Nature. 1967;215:1043–1044. doi: 10.1038/2151043a0. [DOI] [PubMed] [Google Scholar]
- Guérin D, Peace ST, Didier A, Linster C, Cleland TA. Noradrenergic neuromodulation in the olfactory bulb modulates odor habituation and spontaneous discrimination. Behav Neurosci. 2008;122:816–826. doi: 10.1037/a0012522. http://dx.doi.org/10.1037/a0012522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guérin D, Sacquet J, Mandairon N, Jourdan F, Didier A. Early locus coeruleus degeneration and olfactory dysfunctions in Tg2576 mice. Neurobiol Aging. 2009;30:272–283. doi: 10.1016/j.neurobiolaging.2007.05.020. http://dx.doi.org/10.1016/j.neurobiolaging.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Guo JL, Covell DJ, Daniels JP, Iba M, Stieber A, Zhang B, Riddle DM, Kwong LK, Xu Y, Trojanowski JQ, Lee VMY. Distinct α-synuclein strains differentially promote tau inclusions in neurons. Cell. 2013;154:103–117. doi: 10.1016/j.cell.2013.05.057. http://dx.doi.org/10.1016/j.cell.2013.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberly LB, Price JL. Association and commissural fiber systems of the olfactory cortex of the rat. J Comp Neurol. 1978a;178:711–740. doi: 10.1002/cne.901780408. http://dx.doi.org/10.1002/cne.901780408. [DOI] [PubMed] [Google Scholar]
- Haberly LB, Price JL. Association and commissural fiber systems of the olfactory cortex of the rat. II. Systems originating in the olfactory peduncle. J Comp Neurol. 1978b;181:781–807. doi: 10.1002/cne.901810407. http://dx.doi.org/10.1002/cne.901810407. [DOI] [PubMed] [Google Scholar]
- Haehner A, Habersack A, Wienecke M, Storch A, Reichmann H, Hummel T. Early Parkinson’s disease patients on rasagiline present with better odor discrimination. J Neural Transm. 2015:1–6. doi: 10.1007/s00702-015-1433-1. http://dx.doi.org/10.1007/s00702-015-1433-1. [DOI] [PubMed]
- Haehner A, Hummel T, Hummel C, Sommer U, Junghanns S, Reichmann H. Olfactory loss may be a first sign of idiopathic Parkinson’s disease. Mov Disord. 2007;22:839–842. doi: 10.1002/mds.21413. http://dx.doi.org/10.1002/mds.21413. [DOI] [PubMed] [Google Scholar]
- Halliday GM, Holton JL, Revesz T, Dickson DW. Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol. 2011;122:187–204. doi: 10.1007/s00401-011-0852-9. http://dx.doi.org/10.1007/s00401-011-0852-9. [DOI] [PubMed] [Google Scholar]
- Hamilton JM, Murphy C, Paulsen JS. Odor detection, learning, and memory in Huntington’s disease. J Int Neuropsychol Soc. 1999;5:609–615. doi: 10.1017/s1355617799577035. [DOI] [PubMed] [Google Scholar]
- Hamilton RL. Lewy bodies in Alzheimer’s disease: a neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol. 2000;10:378–384. doi: 10.1111/j.1750-3639.2000.tb00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna PA, Jankovic J, Kirkpatrick JB. Multiple system atrophy: the putative causative role of environmental toxins. Arch Neurol. 1999;56:90–94. doi: 10.1001/archneur.56.1.90. [DOI] [PubMed] [Google Scholar]
- Harding AJ, Stimson E, Henderson JM, Halliday GM. Clinical correlates of selective pathology in the amygdala of patients with Parkinson’s disease. Brain. 2002;125:2431–2445. doi: 10.1093/brain/awf251. [DOI] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Harris SA, Harris EA. Herpes simplex virus type 1 and other pathogens are key causative factors in sporadic Alzheimer’s disease. J Alzheimers Dis. 2015;48:319–353. doi: 10.3233/JAD-142853. http://dx.doi.org/10.3233/JAD-142853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes C. Olfaction in neurodegenerative disorder. Adv Otorhinolaryngol. 2006;63:133–151. doi: 10.1159/000093759. http://dx.doi.org/10.1159/000093759. [DOI] [PubMed] [Google Scholar]
- Hawkes C. Olfaction in neurodegenerative disorder. Mov Disord. 2003;18:364–372. doi: 10.1002/mds.10379. http://dx.doi.org/10.1002/mds.10379. [DOI] [PubMed] [Google Scholar]
- Hawkes C, Shah M, Findley L. Olfactory function in essential tremor: a deficit unrelated to disease duration or severity. Neurology. 2003;61:871–872. doi: 10.1212/wnl.61.6.871-a. (author reply 872) [DOI] [PubMed] [Google Scholar]
- Hawkes CH, Del Tredici K, Braak H. Parkinson’s disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol. 2007;33:599–614. doi: 10.1111/j.1365-2990.2007.00874.x. http://dx.doi.org/10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes CH, del Tredici K, Braak H. Parkinson’s disease: the dual hit theory revisited. Ann N Y Acad Sci. 2009a;1170:615–622. doi: 10.1111/j.1749-6632.2009.04365.x. http://dx.doi.org/10.1111/j.1749-6632.2009.04365.x. [DOI] [PubMed] [Google Scholar]
- Hawkes CH, Doty RL. The Neurology of Olfaction. Cambridge University Press; Cambridge: 2009b. [Google Scholar]
- Hawkes CH, Shephard BC, Daniel SE. Olfactory dysfunction in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1997a;62:436–446. doi: 10.1136/jnnp.62.5.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes CH, Shephard BC, Geddes JF, Body GD, Martin JE. Olfactory disorder in motor neuron disease. Exp Neurol. 1998;150:248–253. doi: 10.1006/exnr.1997.6773. http://dx.doi.org/10.1006/exnr.1997.6773. [DOI] [PubMed] [Google Scholar]
- Hawkes CH, Shephard BC, Kobal G. Assessment of olfaction in multiple sclerosis: evidence of dysfunction by olfactory evoked response and identification tests. J Neurol Neurosurg Psychiatry. 1997b;63:145–151. doi: 10.1136/jnnp.63.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Karnup S, Ennis M, Shipley MT. External tufted cells: a major excitatory element that coordinates glomerular activity. J Neurosci. 2004;24:6676–6685. doi: 10.1523/JNEUROSCI.1367-04.2004. http://dx.doi.org/10.1523/JNEUROSCI.1367-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Yu W, Wu J, Chen C, Lou Z, Zhang Q, Zhao J, Wang J, Xiao B. Intranasal LPS-mediated Parkinson’s model challenges the pathogenesis of nasal cavity and environmental toxins. PLoS ONE. 2013;8:e78418. doi: 10.1371/journal.pone.0078418. http://dx.doi.org/10.1371/journal.pone.0078418.g008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedner M, Larsson M, Arnold N, Zucco GM, Hummel T. Cognitive factors in odor detection, odor discrimination, and odor identification tasks. J Clin Exp Neuropsychol. 2010;32:1062–1067. doi: 10.1080/13803391003683070. http://dx.doi.org/10.1080/13803391003683070. [DOI] [PubMed] [Google Scholar]
- Helton TD, Otsuka T, Lee MC, Mu Y, Ehlers MD. Pruning and loss of excitatory synapses by the parkin ubiquitin ligase. Proc Natl Acad Sci. 2008;105:19492–19497. doi: 10.1073/pnas.0802280105. http://dx.doi.org/10.1073/pnas.0802280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, O’Banion MK, Terwel D, Kummer MP. Neuroinflammatory processes in Alzheimer’s disease. J Neural Transm. 2010a;117:919–947. doi: 10.1007/s00702-010-0438-z. http://dx.doi.org/10.1007/s00702-010-0438-z. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Rodríguez JJ, Verkhratsky A. Neuroglia in neurodegeneration. Brain Res Rev. 2010b;63:189–211. doi: 10.1016/j.brainresrev.2009.11.004. http://dx.doi.org/10.1016/j.brainresrev.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Henkin R, Potolicchio S, Levy L. Olfactory hallucinations without clinical motor activity: a comparison of unirhinal with birhinal phantosmia. Brain Sci. 2013;3:1483–1553. doi: 10.3390/brainsci3041483. http://dx.doi.org/10.3390/brainsci3041483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepp DH, Vergoossen DLE, Huisman E, Lemstra AW, Netherlands Brain Bank. Berendse HW, Rozemuller AJ, Foncke EMJ, van de Berg WDJ. Distribution and load of amyloid-β pathology in Parkinson disease and dementia with Lewy bodies. J Neuropathol Exp Neurol. 2016 doi: 10.1093/jnen/nlw070. http://dx.doi.org/10.1093/jnen/nlw070. [DOI] [PubMed]
- Herting B, Schulze S, Reichmann H, Haehner A, Hummel T. A longitudinal study of olfactory function in patients with idiopathic Parkinson’s disease. J Neurol. 2008;255:367–370. doi: 10.1007/s00415-008-0665-5. http://dx.doi.org/10.1007/s00415-008-0665-5. [DOI] [PubMed] [Google Scholar]
- Heyanka DJ, Golden CJ, McCue RB, Scarisbrick DM, Linck JF, Zlatkin NI. Olfactory deficits in frontotemporal dementia as measured by the Alberta Smell Test. Appl Neuropsychol Adult. 2014;21:176–182. doi: 10.1080/09084282.2013.782031. http://dx.doi.org/10.1080/09084282.2013.782031. [DOI] [PubMed] [Google Scholar]
- Hobson DE. Asymmetry in parkinsonism, spreading pathogens and the nose. Parkinsonism Relat Disord. 2012;18:1–9. doi: 10.1016/j.parkreldis.2011.06.011. http://dx.doi.org/10.1016/j.parkreldis.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Horvath J, Herrmann FR, Burkhard PR, Bouras C, Kövari E. Parkinsonism Relat. Disord. 2013;19:864–868. doi: 10.1016/j.parkreldis.2013.05.010. http://dx.doi.org/10.1016/j.parkreldis.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Höglinger GU, Alvarez-Fischer D, Arias-Carrión O, Djufri M, Windolph A, Keber U, Borta A, Ries V, Schwarting RKW, Scheller D, Oertel WH. A new dopaminergic nigro-olfactory projection. Acta Neuropathol. 2015:1–16. doi: 10.1007/s00401-015-1451-y. http://dx.doi.org/10.1007/s00401-015-1451-y. [DOI] [PubMed]
- Huart C, Rombaux P, Hummel T. Plasticity of the human olfactory system: the olfactory bulb. Molecules. 2013;18:11586–11600. doi: 10.3390/molecules180911586. http://dx.doi.org/10.3390/molecules180911586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard PS, Esiri MM, Reading M, McShane R, Nagy Z. Alpha-synuclein pathology in the olfactory pathways of dementia patients. J Anat. 2007;211:117–124. doi: 10.1111/j.1469-7580.2007.00748.x. http://dx.doi.org/10.1111/j.1469-7580.2007.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudry J, Thobois S, Broussolle E, Adeleine P, Royet JP. Evidence for deficiencies in perceptual and semantic olfactory processes in Parkinson’s disease. Chem Senses. 2003;28:537–543. doi: 10.1093/chemse/28.6.537. [DOI] [PubMed] [Google Scholar]
- Huisman E, Uylings HBM, Hoogland PV. A 100% increase of dopaminergic cells in the olfactory bulb may explain hyposmia in Parkinson’s disease. Mov Disord. 2004;19:687–692. doi: 10.1002/mds.10713. http://dx.doi.org/10.1002/mds.10713. [DOI] [PubMed] [Google Scholar]
- Hüls S, Högen T, Vassallo N, Danzer KM, Hengerer B, Giese A, Herms J. AMPA-receptor-mediated excitatory synaptic transmission is enhanced by iron-induced α-synuclein oligomers. J Neurochem. 2011;117:868–878. doi: 10.1111/j.1471-4159.2011.07254.x. http://dx.doi.org/10.1111/j.1471-4159.2011.07254.x. [DOI] [PubMed] [Google Scholar]
- Hüttenbrink KB, Hummel T, Berg D, Gasser T, Hähner A. Olfactory dysfunction: common in later life and early warning of neurodegenerative disease. Dtsch Arztebl Int. 2013;110:1–7, e1. doi: 10.3238/arztebl.2013.0001. http://dx.doi.org/10.3238/arztebl.2013.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilieva H, Polymenidou M, Cleveland DW. Non–cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009;187:761–772. doi: 10.1083/jcb.200908164. http://dx.doi.org/10.1146/annurev.neuro.23.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illig KR, Eudy JD. Contralateral projections of the rat anterior olfactory nucleus. J Comp Neurol. 2009;512:115–123. doi: 10.1002/cne.21900. http://dx.doi.org/10.1002/cne.21900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. http://dx.doi.org/10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H, Beckman JS. Oxidative stress and nitration in neurodegeneration: cause, effect, or association? J Clin Invest. 2003;111:163–169. doi: 10.1172/JCI17638. http://dx.doi.org/10.1172/JCI200317638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseki E, Togo T, Suzuki K, Katsuse O, Marui W, de Silva R, Lees A, Yamamoto T, Kosaka K. Dementia with Lewy bodies from the perspective of tauopathy. Acta Neuropathol. 2003;105:265–270. doi: 10.1007/s00401-002-0644-3. http://dx.doi.org/10.1007/s00401-002-0644-3. [DOI] [PubMed] [Google Scholar]
- Jang H, Boltz D, McClaren J, Pani AK, Smeyne M, Korff A, Webster R, Smeyne RJ. Inflammatory effects of highly pathogenic H5N1 influenza virus infection in the CNS of mice. J Neurosci. 2012;32:1545–1559. doi: 10.1523/JNEUROSCI.5123-11.2012. http://dx.doi.org/10.1523/JNEUROSCI.5123-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang H, Boltz DA, Webster RG, Smeyne RJ. Viral parkinsonism. Biochim Biophys Acta (BBA) – Mol Basis Dis. 2009;1792:714–721. doi: 10.1016/j.bbadis.2008.08.001. http://dx.doi.org/10.1016/j.bbadis.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA, Attems J. Alzheimer pathology in the olfactory bulb. Neuropathol Appl Neurobiol. 2005;31:203. doi: 10.1111/j.1365-2990.2004.00619.x. http://dx.doi.org/10.1111/j.1365-2990.2004.00619.x. [DOI] [PubMed] [Google Scholar]
- Johansen KK, Warø BJ, Aasly JO. Olfactory dysfunction in sporadic Parkinson’s disease and LRRK2 carriers. Acta Neurol Scand. 2014;129:300–306. doi: 10.1111/ane.12172. http://dx.doi.org/10.1111/ane.12172. [DOI] [PubMed] [Google Scholar]
- Johansson CB, Svensson M, Wallstedt L, Janson AM, Frisen J. Neural stem cells in the adult human brain. Exp Cell Res. 1999;253:733–736. doi: 10.1006/excr.1999.4678. http://dx.doi.org/10.1006/excr.1999.4678. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Farahbod H, Xu Z, Saber S, Leon M. Local and global chemotopic organization: general features of the glomerular representations of aliphatic odorants differing in carbon number. J Comp Neurol. 2004;480:234–249. doi: 10.1002/cne.20335. http://dx.doi.org/10.1002/cne.20335. [DOI] [PubMed] [Google Scholar]
- Johnson FO, Atchison WD. The role of environmental mercury, lead and pesticide exposure in development of amyotrophic lateral sclerosis. Neurotoxicology. 2009;30:761–765. doi: 10.1016/j.neuro.2009.07.010. http://dx.doi.org/10.1016/j.neuro.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J, Dunn FA, Stopfer M. Spontaneous olfactory receptor neuron activity determines follower cell response properties. J Neurosci. 2012;32:2900–2910. doi: 10.1523/JNEUROSCI.4207-11.2012. http://dx.doi.org/10.1523/JNEUROSCI.4207-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joussain P, Bessy M, Faure F, Bellil D, Landis BN, Hugentobler M, Tuorila H, Mustonen S, Vento SI, Delphin-Combe F, Krolak-Salmon P, Rouby C, Bensafi M. Application of the European test of olfactory capabilities in patients with olfactory impairment. Eur Arch Otorhinolaryngol. 2015;273:381–390. doi: 10.1007/s00405-015-3536-6. http://dx.doi.org/10.1007/s00405-015-3536-6. [DOI] [PubMed] [Google Scholar]
- Kadohisa M, Wilson DA. Separate encoding of identity and similarity of complex familiar odors in piriform cortex. Proc Natl Acad Sci U S A. 2006;103:15206–15211. doi: 10.1073/pnas.0604313103. http://dx.doi.org/10.1073/pnas.0604313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinin S, Feinstein DL, Xu HL, Huesa G, Pelligrino DA, Galea E. Degeneration of noradrenergic fibres from the locus coeruleus causes tight-junction disorganisation in the rat brain. Eur J Neurosci. 2006;24:3393–3400. doi: 10.1111/j.1460-9568.2006.05223.x. http://dx.doi.org/10.1111/j.1460-9568.2006.05223.x. [DOI] [PubMed] [Google Scholar]
- Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- Katzenschlager R, Lees AJ. Olfaction and Parkinson’s syndromes: its role in differential diagnosis. Curr Opin Neurol. 2004;17:417–423. doi: 10.1097/01.wco.0000137531.76491.c2. http://dx.doi.org/10.1097/01.wco.0000137531.76491.c2. [DOI] [PubMed] [Google Scholar]
- Kaufman SK, Diamond MI. Prion-like propagation of protein aggregation and related therapeutic strategies. Neurotherapeutics. 2013;10:371–382. doi: 10.1007/s13311-013-0196-3. http://dx.doi.org/10.1007/s13311-013-0196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay RB, Meyer EA, Illig KR, Brunjes PC. Spatial distribution of neural activity in the anterior olfactory nucleus evoked by odor and electrical stimulation. J Comp Neurol. 2011;519:277–289. doi: 10.1002/cne.22519. http://dx.doi.org/10.1002/cne.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersaitis C, Halliday GM, Kril JJ. Regional and cellular pathology in frontotemporal dementia: relationship to stage of disease in cases with and without Pick bodies. Acta Neuropathol. 2004;108:515–523. doi: 10.1007/s00401-004-0917-0. http://dx.doi.org/10.1007/s00401-004-0917-0. [DOI] [PubMed] [Google Scholar]
- Kertelge L, Brüggemann N, Schmidt A, Tadic V, Wisse C, Dankert S, Drude L, van der Vegt J, Siebner H, Pawlack H, Pramstaller PP, Behrens MI, Ramirez A, Reichel D, Buhmann C, Hagenah J, Klein C, Lohmann K, Kasten M. Impaired sense of smell and color discrimination in monogenic and idiopathic Parkinson’s disease. Mov Disord. 2010;25:2665–2669. doi: 10.1002/mds.23272. http://dx.doi.org/10.1002/mds.23272. [DOI] [PubMed] [Google Scholar]
- Khan NL, Katzenschlager R, Watt H, Bhatia KP, Wood NW, Quinn N, Lees AJ. Olfaction differentiates parkin disease from early-onset parkinsonism and Parkinson disease. Neurology. 2004;62:1224–1226. doi: 10.1212/01.wnl.0000118281.66802.81. [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Baba T, Hasegawa T, Sugeno N, Konno M, Takeda A. Differentiating Parkinson’s disease from multiple system atrophy by [123I] meta-iodobenzylguanidine myocardial scintigraphy and olfactory test. Parkinsonism Relat Disord. 2011;17:698–700. doi: 10.1016/j.parkreldis.2011.07.011. http://dx.doi.org/10.1016/j.parkreldis.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Kikuta S, Sato K, Kashiwadani H, Tsunoda K, Yamasoba T, Mori K. From the cover: neurons in the anterior olfactory nucleus pars externa detect right or left localization of odor sources. Proc Natl Acad Sci. 2010;107:12363–12368. doi: 10.1073/pnas.1003999107. http://dx.doi.org/10.1073/pnas.1003999107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY. Olfaction and neurodegenerative disease. Hanyang Med Rev. 2014;34:116. http://dx.doi.org/10.7599/hmr.2014.34.3.116. [Google Scholar]
- Kincaid AE, Bartz JC. The nasal cavity is a route for prion infection in hamsters. J Virol. 2007;81:4482–4491. doi: 10.1128/JVI.02649-06. http://dx.doi.org/10.1128/JVI.02649-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa M, Oddo S, Yamasaki TR, Green KN, LaFerla FM. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:8843–8853. doi: 10.1523/JNEUROSCI.2868-05.2005. http://dx.doi.org/10.1523/JNEUROSCI.2868-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács T, Cairns NJ, Lantos PL. Olfactory centres in Alzheimer’s disease: olfactory bulb is involved in early Braak’s stages. Neuroreport. 2001;12:285–288. doi: 10.1097/00001756-200102120-00021. [DOI] [PubMed] [Google Scholar]
- Kovács T, Cairns NJ, Lantos PL. Beta-amyloid deposition and neurofibrillary tangle formation in the olfactory bulb in ageing and Alzheimer’s disease. Neuropathol Appl Neurobiol. 1999;25:481–491. doi: 10.1046/j.1365-2990.1999.00208.x. [DOI] [PubMed] [Google Scholar]
- Kovács T, Papp MI, Cairns NJ, Khan MN, Lantos PL. Olfactory bulb in multiple system atrophy. Mov Disord. 2003;18:938–942. doi: 10.1002/mds.10466. http://dx.doi.org/10.1002/mds.10466. [DOI] [PubMed] [Google Scholar]
- Kranick SM, Duda JE. Olfactory dysfunction in Parkinson’s disease. Neurosignals. 2008;16:35–40. doi: 10.1159/000109757. http://dx.doi.org/10.1159/000109757. [DOI] [PubMed] [Google Scholar]
- Kril J, Patel S, Harding A, Halliday G. Neuron loss from the hippocampus of Alzheimer’s disease exceeds extracellular neurofibrillary tangle formation. Acta Neuropathol. 2002;103:370–376. doi: 10.1007/s00401-001-0477-5. http://dx.doi.org/10.1007/s00401-001-0477-5. [DOI] [PubMed] [Google Scholar]
- Krüger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S, Przuntek H, Epplen JT, Schöls L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. http://dx.doi.org/10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Kuan WL, Bennett N, He X, Skepper JN, Martynyuk N, Wijeyekoon R, Moghe PV, Williams-Gray CH, Barker RA. Alpha-synuclein pre-formed fibrils impair tight junction protein expression without affecting cerebral endothelial cell function. Exp Neurol. 2016 doi: 10.1016/j.expneurol.2016.09.003. http://dx.doi.org/10.1016/j.expneurol.2016.09.003. [DOI] [PubMed]
- Kuczius T, Koch R, Keyvani K, Karch H, Grassi J, Groschup MH. Regional and phenotype heterogeneity of cellular prion proteins in the human brain. Eur J Neurosci. 2007;25:2649–2655. doi: 10.1111/j.1460-9568.2007.05518.x. http://dx.doi.org/10.1111/j.1460-9568.2007.05518.x. [DOI] [PubMed] [Google Scholar]
- Kumar A, Leinisch F, Kadiiska MB, Corbett J, Mason RP. Formation and implications of alpha-synuclein radical in maneb- and paraquat-induced models of Parkinson’s disease. Mol Neurobiol. 2015;53:2983–2994. doi: 10.1007/s12035-015-9179-1. http://dx.doi.org/10.1007/s12035-015-9179-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafreniere D, Mann N. Anosmia: Loss of Smell in the Elderly. Otolaryngol Clin N Am. 2009;42:123–131. doi: 10.1016/j.otc.2008.09.001. http://dx.doi.org/10.1016/j.otc.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Lalancette-Hebert M, Phaneuf D, Soucy G, Weng YC, Kriz J. Live imaging of Toll-like receptor 2 response in cerebral ischaemia reveals a role of olfactory bulb microglia as modulators of inflammation. Brain. 2008;132:940–954. doi: 10.1093/brain/awn345. http://dx.doi.org/10.1093/brain/awn345. [DOI] [PubMed] [Google Scholar]
- Landis BN, Burkhard PR. Phantosmias and Parkinson disease. Arch Neurol. 2008;65:1237–1239. doi: 10.1001/archneur.65.9.1237. http://dx.doi.org/10.1001/archneur.65.9.1237. [DOI] [PubMed] [Google Scholar]
- Landis BN, Konnerth CG, Hummel T. A study on the frequency of olfactory dysfunction. Laryngoscope. 2004;114:1764–1769. doi: 10.1097/00005537-200410000-00017. http://dx.doi.org/10.1097/00005537-200410000-00017. [DOI] [PubMed] [Google Scholar]
- Larsson M, Lundin A, Robins Wahlin TB. Olfactory functions in asymptomatic carriers of the Huntington disease mutation. J Clin Exp Neuropsychol. 2006;28:1373–1380. doi: 10.1080/13803390500473746. http://dx.doi.org/10.1080/13803390500473746. [DOI] [PubMed] [Google Scholar]
- Larsson M, Nilsson LG, Olofsson JK, Nordin S. Demographic and cognitive predictors of cued odor identification: evidence from a population-based study. Chem Senses. 2004;29:547–554. doi: 10.1093/chemse/bjh059. http://dx.doi.org/10.1093/chemse/bjh059. [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Lazic SE, Goodman AOG, Grote HE, Blakemore C, Morton AJ, Hannan AJ, van Dellen A, Barker RA. Olfactory abnormalities in Huntington’s disease: decreased plasticity in the primary olfactory cortex of R6/1 transgenic mice and reduced olfactory discrimination in patients. Brain Res. 2007;1151:219–226. doi: 10.1016/j.brainres.2007.03.018. http://dx.doi.org/10.1016/j.brainres.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Lee J, Lee Y, Yuk D, Choi D, Ban S, Oh K, Hong J. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation. 2008;5:37. doi: 10.1186/1742-2094-5-37. http://dx.doi.org/10.1186/1742-2094-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Cho KH, Ham JH, Song SK, Sohn YH, Lee PH. Olfactory performance acts as a cognitive reserve in non-demented patients with Parkinson’s disease. Parkinsonism Relat Disord. 2014;20:186–191. doi: 10.1016/j.parkreldis.2013.10.024. http://dx.doi.org/10.1016/j.parkreldis.2013.10.024. [DOI] [PubMed] [Google Scholar]
- Lei P, Ayton S, Finkelstein DI, Adlard PA, Masters CL, Bush AI. Tau protein: Relevance to Parkinson’s disease. Int J Biochem Cell Biol. 2010;42:1775–1778. doi: 10.1016/j.biocel.2010.07.016. http://dx.doi.org/10.1016/j.biocel.2010.07.016. [DOI] [PubMed] [Google Scholar]
- Lema Tomé CM, Tyson T, Rey NL, Grathwohl S, Britschgi M, Brundin P. Inflammation and α-Synuclein’s prion-like behavior in Parkinson’s disease—is there a link? Mol Neurobiol. 2012;47:561–574. doi: 10.1007/s12035-012-8267-8. http://dx.doi.org/10.1007/s12035-012-8267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Gu CZ, Su JB, Zhu LH, Zhou Y, Huang HY, Liu CF. Changes in olfactory bulb volume in Parkinson’s disease: a systematic review and meta-analysis. PLoS ONE. 2016a;11:e0149286. doi: 10.1371/journal.pone.0149286. http://dx.doi.org/10.1371/journal.pone.0149286.s002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Björklund A, Widner H, Revesz T, Lindvall O, Brundin P. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. doi: 10.1038/nm1746. http://dx.doi.org/10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- Li LM, Yang LN, Zhang LJ, Fu Y, Li T, Qi Y, Wang J, Zhang DQ, Zhang N, Liu J, Yang L. Olfactory dysfunction in patients with multiple sclerosis. J Neurol Sci. 2016b;365:34–39. doi: 10.1016/j.jns.2016.03.045. http://dx.doi.org/10.1016/j.jns.2016.03.045. [DOI] [PubMed] [Google Scholar]
- Li W, Howard JD, Gottfried JA. Disruption of odour quality coding in piriform cortex mediates olfactory deficits in Alzheimer’s disease. Brain. 2010;133:2714–2726. doi: 10.1093/brain/awq209. http://dx.doi.org/10.1093/brain/awq209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Tan MS, Jiang T, Tan L. Review article. Biomed Res Int. 2014:1–7. http://dx.doi.org/10.1155/2014/437483.
- Liberini P, Parola S, Spano PF, Antonini L. Olfaction in Parkinson’s disease: methods of assessment and clinical relevance. J Neurol. 2000;247:88–96. doi: 10.1007/pl00007803. [DOI] [PubMed] [Google Scholar]
- Liberski PP, Ironside JW. An outline of the neuropathology of transmissible spongiform encephalopathies (prion diseases) Folia Neuropathol. 2004;42(Suppl B):39–58. [PubMed] [Google Scholar]
- Licastro F, Porcellini E. Persistent infections, immune-senescence and Alzheimer’s disease. Oncoscience. 2016;3:135–142. doi: 10.18632/oncoscience.309. http://dx.doi.org/10.18632/oncoscience.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster C, Fontanini A. Functional neuromodulation of chemosensation in vertebrates. Curr Opin Neurobiol. 2014;29:82–87. doi: 10.1016/j.conb.2014.05.010. http://dx.doi.org/10.1016/j.conb.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster C, Menon AV, Singh CY, Wilson DA. Odor-specific habituation arises from interaction of afferent synaptic adaptation and intrinsic synaptic potentiation in olfactory cortex. Learn Mem. 2009;16:452–459. doi: 10.1101/lm.1403509. http://dx.doi.org/10.1101/lm.1403509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster C, Wyble BP, Hasselmo ME. Electrical stimulation of the horizontal limb of the diagonal band of broca modulates population EPSPs in piriform cortex. J Neurophysiol. 1999;81:2737–2742. doi: 10.1152/jn.1999.81.6.2737. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. http://dx.doi.org/10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Louis ED, Rios E, Pellegrino KM, Jiang W, Factor-Litvak P, Zheng W. Higher blood harmane (1-methyl-9H-pyrido[3,4-b]indole) concentrations correlate with lower olfactory scores in essential tremor. Neurotoxicology. 2008;29:460–465. doi: 10.1016/j.neuro.2008.02.013. http://dx.doi.org/10.1016/j.neuro.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe R, Pountney DL, Jensen PH, Gai WP, Voelcker NH. Calcium(II) selectively induces alpha-synuclein annular oligomers via interaction with the C-terminal domain. Protein Sci. 2004;13:3245–3252. doi: 10.1110/ps.04879704. http://dx.doi.org/10.1110/ps.04879704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lötsch J, Schaeffeler E, Mittelbronn M, Winter S, Gudziol V, Schwarzacher SW, Hummel T, Doehring A, Schwab M, Ultsch A. Functional genomics suggest neurogenesis in the adult human olfactory bulb. Brain Struct Funct. 2013;219:1991–2000. doi: 10.1007/s00429-013-0618-3. http://dx.doi.org/10.1007/s00429-013-0618-3. [DOI] [PubMed] [Google Scholar]
- Lucassen EB, Turel A, Knehans A, Huang X, Eslinger P. Olfactory dysfunction in multiple sclerosis: a scoping review of the literature. Mult Scler Relat Disord. 2016;6:1–9. doi: 10.1016/j.msard.2015.12.002. http://dx.doi.org/10.1016/j.msard.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Luk KC, Lee VMY. Modeling Lewy pathology in Parkinson’s disease. Parkinsonism Relat Disord. 2013;20:S85–S87. doi: 10.1016/S1353-8020(13)70022-1. http://dx.doi.org/10.1016/S1353-8020(13)70022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutterotti A, Vedovello M, Reindl M, Ehling R, DiPauli F, Kuenz B, Gneiss C, Deisenhammer F, Berger T. Olfactory threshold is impaired in early, active multiple sclerosis. Mult Scler J. 2011;17:964–969. doi: 10.1177/1352458511399798. http://dx.doi.org/10.1177/1352458511399798. [DOI] [PubMed] [Google Scholar]
- Luzzi S, Snowden JS, Neary D, Coccia M, Provinciali L, Lambon Ralph MA. Distinct patterns of olfactory impairment in Alzheimer’s disease, semantic dementia, frontotemporal dementia, and corticobasal degeneration. Neuropsychologia. 2007;45:1823–1831. doi: 10.1016/j.neuropsychologia.2006.12.008. http://dx.doi.org/10.1016/j.neuropsychologia.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Machts J, Loewe K, Kaufmann J, Jakubiczka S, Abdulla S, Petri S, Dengler R, Heinze HJ, Vielhaber S, Schoenfeld MA, Bede P. Basal ganglia pathology in ALS is associated with neuropsychological deficits. Neurology. 2015;85:1301–1309. doi: 10.1212/WNL.0000000000002017. http://dx.doi.org/10.1212/WNL.0000000000002017. [DOI] [PubMed] [Google Scholar]
- Macklis JD. Human adult olfactory bulb neurogenesis? Novelty is the best policy Neuron. 2012;74:595–596. doi: 10.1016/j.neuron.2012.05.005. http://dx.doi.org/10.1016/j.neuron.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Macknin JB, Higuchi M, Lee VMY, Trojanowski JQ, Doty RL. Olfactory dysfunction occurs in transgenic mice overexpressing human τ protein. Brain Res. 2004;1000:174–178. doi: 10.1016/j.brainres.2004.01.047. http://dx.doi.org/10.1016/j.brainres.2004.01.047. [DOI] [PubMed] [Google Scholar]
- Malek AM, Barchowsky A, Bowser R, Heiman-Patterson T, Lacomis D, Rana S, Youk A, Talbott EO. Exposure to hazardous air pollutants and the risk of amyotrophic lateral sclerosis. Environ Pollut. 2015;197:181–186. doi: 10.1016/j.envpol.2014.12.010. http://dx.doi.org/10.1016/j.envpol.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Malek N, Swallow DMA, Grosset KA, Lawton MA, Smith CR, Bajaj NP, Barker RA, Ben-Shlomo Y, Bresner C, Burn DJ, Foltynie T, Morris HR, Williams N, Wood NW, Grosset DG, PRoBaND Investigators Olfaction in Parkin single and compound heterozygotes in a cohort of young onset Parkinson’s disease patients. Acta Neurol Scand. 2016;134:271–276. doi: 10.1111/ane.12538. http://dx.doi.org/10.1111/ane.12538. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Peace S, Karnow A, Kim J, Ennis M, Linster C. Noradrenergic modulation in the olfactory bulb influences spontaneous and reward-motivated discrimination, but not the formation of habituation memory. Eur J Neurosci. 2008;27:1210–1219. doi: 10.1111/j.1460-9568.2008.06101.x. http://dx.doi.org/10.1111/j.1460-9568.2008.06101.x. [DOI] [PubMed] [Google Scholar]
- Marien MR, Colpaert FC, Rosenquist AC. Noradrenergic mechanisms in neurodegenerative diseases: a theory. Brain Res Rev. 2004;45:38–78. doi: 10.1016/j.brainresrev.2004.02.002. http://dx.doi.org/10.1016/j.brainresrev.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Mason DM, Nouraei N, Pant DB, Miner KM, Hutchison DF, Luk KC, Stolz JF, Leak RK. Transmission of α-synucleinopathy from olfactory structures deep into the temporal lobe. Mol Neurodegener. 2016;11:459. doi: 10.1186/s13024-016-0113-4. http://dx.doi.org/10.1186/s13024-016-0113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllum EJ, Finkelstein DI. Metals in Alzheimer. J Mol Neurosci. 2016:1–10. doi: 10.1007/s12031-016-0809-5. http://dx.doi.org/10.1007/s12031-016-0809-5. [DOI] [PubMed]
- McKeith I, Taylor JP, Thomas T, Donaghy P, Kane J. Revisiting DLB diagnosis: a consideration of prodromal DLB and of the diagnostic overlap with Alzheimer disease. J Geriatr Psychiatry Neurol. 2016;29:249–253. doi: 10.1177/0891988716656083. http://dx.doi.org/10.1177/0891988716656083. [DOI] [PubMed] [Google Scholar]
- McLaughlin NCR, Westervelt HJ. Odor identification deficits in frontotemporal dementia: a preliminary study. Arch Clin Neuropsychol. 2008;23:119–123. doi: 10.1016/j.acn.2007.07.008. http://dx.doi.org/10.1016/j.acn.2007.07.008. [DOI] [PubMed] [Google Scholar]
- McShane RH, Nagy Z, Esiri MM, King E, Joachim C, Sullivan N, Smith AD. Anosmia in dementia is associated with lewy bodies rather than Alzheimer’s pathology. J Neurol Neurosurg Psychiatry. 2001;70:739–743. doi: 10.1136/jnnp.70.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner WG. When does Parkinson’s disease begin? From prodromal disease to motor signs Rev Neurol. 2012;168:809–814. doi: 10.1016/j.neurol.2012.07.004. http://dx.doi.org/10.1016/j.neurol.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Melki R. Role of different alpha-synuclein strains in synucleinopathies, similarities with other neurodegenerative diseases. J Park Dis. 2015;5:217–227. doi: 10.3233/JPD-150543. http://dx.doi.org/10.3233/JPD-150543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesholam RI, Moberg PJ, Mahr RN, Doty RL. Olfaction in neurodegenerative disease: a meta-analysis of olfactory functioning in Alzheimer“s and Parkinson”s diseases. Arch Neurol. 1998;55:84–90. doi: 10.1001/archneur.55.1.84. [DOI] [PubMed] [Google Scholar]
- Meyer EA, Illig KR, Brunjes PC. Differences in chemo- and cytoarchitectural features within pars principalis of the rat anterior olfactory nucleus suggest functional specialization. J Comp Neurol. 2006;498:786–795. doi: 10.1002/cne.21077. http://dx.doi.org/10.1002/cne.21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milhavet O, Lehmann S. Oxidative stress and the prion protein in transmissible spongiform encephalopathies. Brain Res Brain Res Rev. 2002;38:328–339. doi: 10.1016/s0165-0173(01)00150-3. [DOI] [PubMed] [Google Scholar]
- Miller DB, O’Callaghan JP. Biomarkers of Parkinson’s disease: present and future. Metabolism. 2015;64:S40–S46. doi: 10.1016/j.metabol.2014.10.030. http://dx.doi.org/10.1016/j.metabol.2014.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn A, Leclerc S, Heydel JM, Minn AL, Denizot C, Cattarelli M, Netter P, Gradinaru D. Drug transport into the mammalian brain: the nasal pathway and its specific metabolic barrier. J Drug Target. 2002;10:285–296. doi: 10.1080/713714452. http://dx.doi.org/10.1080/10611860290031912. [DOI] [PubMed] [Google Scholar]
- Mita S, Schon EA, Herbert J. Widespread expression of amyloid beta-protein precursor gene in rat brain. Am J Pathol. 1989;134:1253–1261. [PMC free article] [PubMed] [Google Scholar]
- Mitchell IJ, Heims H, Neville EA, Rickards H. Huntington’s disease patients show impaired perception of disgust in the gustatory and olfactory modalities. J Neuropsychiatr Clin Neurosci. 2005;17:119–121. doi: 10.1176/jnp.17.1.119. http://dx.doi.org/10.1176/jnp.17.1.119. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Pearlson GD, Speedie LJ, Lipsey JR, Strauss ME, Folstein SE. Olfactory recognition: differential impairments in early and late Huntington“s and Alzheimer”s diseases. J Clin Exp Neuropsychol. 1987;9:650–664. doi: 10.1080/01688638708405208. http://dx.doi.org/10.1080/01688638708405208. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Mooney KE, Inokuchi A, Snow JB, Kimmelman CP. Projections from the ventral tegmental area to the olfactory tubercle in the rat. Otolaryngol Head Neck Surg. 1987;96:151–157. doi: 10.1177/019459988709600207. [DOI] [PubMed] [Google Scholar]
- Morales R, Moreno-Gonzalez I, Soto C. Cross-seeding of misfolded proteins: implications for etiology and pathogenesis of protein misfolding diseases. PLoS Pathog. 2013;9:e1003537. doi: 10.1371/journal.ppat.1003537. http://dx.doi.org/10.1371/journal.ppat.1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Corraliza J, Schmidt SD, Mazzella MJ, Berger JD, Wilson DA, Wesson DW, Jucker M, Levy E, Nixon RA, Mathews PM. Immunization targeting a minor plaque constituent clears β-amyloid and rescues behavioral deficits in an Alzheimer’s disease mouse model. Neurobiol Aging. 2013;34:137–145. doi: 10.1016/j.neurobiolaging.2012.04.007. http://dx.doi.org/10.1016/j.neurobiolaging.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CD, Murphy C. Individuals at risk for Alzheimer’s disease show differential patterns of ERP brain activation during odor identification. Behav Brain Funct. 2012;8:37. doi: 10.1186/1744-9081-8-37. http://dx.doi.org/10.1093/brain/awl054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CD, Nordin S, Murphy C. Odor identification as an early marker for Alzheimer’s disease: impact of lexical functioning and detection sensitivity. J Clin Exp Neuropsychol. 1995;17:793–803. doi: 10.1080/01688639508405168. http://dx.doi.org/10.1080/01688639508405168. [DOI] [PubMed] [Google Scholar]
- Mori F, Tanji K, Yoshimoto M, Takahashi H, Wakabayashi K. Demonstration of α-synuclein immunoreactivity in neuronal and glial cytoplasm in normal human brain tissue using proteinase K and formic acid pretreatment. Exp Neurol. 2002;176:98–104. doi: 10.1006/exnr.2002.7929. http://dx.doi.org/10.1006/exnr.2002.7929. [DOI] [PubMed] [Google Scholar]
- Mori K, Takahashi YK, Igarashi KM, Yamaguchi M. Maps of odorant molecular features in the mammalian olfactory bulb. Physiol Rev. 2006;86:409–433. doi: 10.1152/physrev.00021.2005. http://dx.doi.org/10.1152/physrev.00021.2005. [DOI] [PubMed] [Google Scholar]
- Morris M, Sanchez PE, Verret L, Beagle AJ, Guo W, Dubal D, Ranasinghe KG, Koyama A, Ho K, Yu GQ, Vossel KA, Mucke L. Network dysfunction in α-synuclein transgenic mice and human Lewy body dementia. Ann Clin Transl Neurol. 2015;2:1012–1028. doi: 10.1002/acn3.257. http://dx.doi.org/10.1002/acn3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozell MM. Evidence for sorption as mechanism of olfactory analysis of vapours. Nature. 1964;203:1181–1182. doi: 10.1038/2031181a0. [DOI] [PubMed] [Google Scholar]
- Mrochen A, Marxreiter F, Kohl Z, Schlachetzki J, Renner B, Schenk T, Winkler J, Klucken J. Parkinsonism and Related Disorders. Parkinsonism Relat Disord. 2016;22:9–14. doi: 10.1016/j.parkreldis.2015.09.035. http://dx.doi.org/10.1016/j.parkreldis.2015.09.035. [DOI] [PubMed] [Google Scholar]
- Mundiñano IC, Caballero MC, Ordóñez C, Hernandez M, DiCaudo C, Marcilla I, Erro ME, Tuñon MT, Luquin MR. Increased dopaminergic cells and protein aggregates in the olfactory bulb of patients with neurodegenerative disorders. Acta Neuropathol. 2011;122:61–74. doi: 10.1007/s00401-011-0830-2. http://dx.doi.org/10.1007/s00401-011-0830-2. [DOI] [PubMed] [Google Scholar]
- Murphy C, Nordin S, Jinich S. Very early decline in recognition memory for odors in Alzheimer’ disease. Aging Neuropsychol Cognit. 1999;6:229–240. [Google Scholar]
- Müller A, Müngersdorf M, Reichmann H, Strehle G, Hummel T. Olfactory function in parkinsonian syndromes. J Clin Neurosci. 2002;9:521–524. doi: 10.1054/jocn.2001.1071. http://dx.doi.org/10.1054/jocn.2001.1071. [DOI] [PubMed] [Google Scholar]
- Nagayama S, Takahashi YK, Yoshihara Y, Mori K. Mitral and tufted cells differ in the decoding manner of odor maps in the rat olfactory bulb. J Neurophysiol. 2004;91:2532–2540. doi: 10.1152/jn.01266.2003. http://dx.doi.org/10.1152/jn.01266.2003. [DOI] [PubMed] [Google Scholar]
- Nawroth JC, Greer CA, Chen WR, Laughlin SB, Shepherd GM. An energy budget for the olfactory glomerulus. J Neurosci. 2007;27:9790–9800. doi: 10.1523/JNEUROSCI.1415-07.2007. http://dx.doi.org/10.1523/JNEUROSCI.1415-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee LE, Scott J, Polinsky RJ. Olfactory dysfunction in the shy-Drager syndrome. Clin Auton Res. 1993;3:281–282. doi: 10.1007/BF01829019. [DOI] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VMY. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. http://dx.doi.org/10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Neve RL, Finch EA, Dawes LR. Expression of the Alzheimer amyloid precursor gene transcripts in the human brain. Neuron. 1988;1:669–677. doi: 10.1016/0896-6273(88)90166-3. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Donkelaar HJ, Nicholson C. The central nervous system of vertebrates. Springer-Verlag; Berlin Heidelberg: 1998. http://dx.doi.org/10.1007/978-3-642-18262-4. [Google Scholar]
- Nordin S, Murphy C. Odor memory in normal aging and Alzheimer’s disease. Ann N Y Acad Sci. 1998;855:686–693. doi: 10.1111/j.1749-6632.1998.tb10646.x. [DOI] [PubMed] [Google Scholar]
- Nordin S, Paulsen JS, Murphy C. Sensory- and memory-mediated olfactory dysfunction in Huntington’s disease. J Int Neuropsychol Soc. 1995;1:281–290. doi: 10.1017/s1355617700000278. [DOI] [PubMed] [Google Scholar]
- Oettl LL, Ravi N, Schneider M, Scheller MF, Schneider P, Mitre M, da Silva Gouveia M, Froemke RC, Chao MV, Young WS, Meyer-Lindenberg A, Grinevich V, Shusterman R, Kelsch W. Oxytocin enhances social recognition by modulating cortical control of early olfactory processing. Neuron. 2016:1–14. doi: 10.1016/j.neuron.2016.03.033. http://dx.doi.org/10.1016/j.neuron.2016.03.033. [DOI] [PMC free article] [PubMed]
- Ogura T, Szebenyi SA, Krosnowski K, Sathyanesan A, Jackson J, Lin W. Cholinergic microvillous cells in the mouse main olfactory epithelium and effect of acetylcholine on olfactory sensory neurons and supporting cells. J Neurophysiol. 2011;106:1274–1287. doi: 10.1152/jn.00186.2011. http://dx.doi.org/10.1152/jn.00186.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohm TG, Braak H. Olfactory bulb changes in Alzheimer’s disease. Acta Neuropathol. 1987;73:365–369. doi: 10.1007/BF00688261. [DOI] [PubMed] [Google Scholar]
- Olichney JM. Anosmia is very common in the Lewy body variant of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2005;76:1342–1347. doi: 10.1136/jnnp.2003.032003. http://dx.doi.org/10.1136/jnnp.2003.032003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyanagi K, Mochizuki Y, Nakayama Y, Hayashi K, Shimizu T, Nagao M, Hashimoto T, Yamazaki M, Matsubara S, Komori T. Marked preservation of the visual and olfactory pathways in ALS patients in a totally locked-in state. Clin Neuropathol. 2015;34:267–274. doi: 10.5414/NP300859. http://dx.doi.org/10.5414/NP300859. [DOI] [PubMed] [Google Scholar]
- Paik SR, Shin HJ, Lee JH, Chang CS, Kim J. Copper(II)-induced self-oligomerization of alpha-synuclein. Biochem J. 1999;340(Pt 3):821–828. [PMC free article] [PubMed] [Google Scholar]
- Parameshwaran K, Dhanasekaran M, Suppiramaniam V. Amyloid beta peptides and glutamatergic synaptic dysregulation. Exp Neurol. 2008;210:7–13. doi: 10.1016/j.expneurol.2007.10.008. http://dx.doi.org/10.1016/j.expneurol.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Pardini M, Huey ED, Cavanagh AL, Grafman J. Olfactory function in corticobasal syndrome and frontotemporal dementia. Arch Neurol. 2009;66 doi: 10.1001/archneurol.2008.521. http://dx.doi.org/10.1001/archneurol.2008.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattison IH, Jones KM. The possible nature of the transmissible agent of scrapie. Vet Rec. 1967;80:2–9. doi: 10.1136/vr.80.1.2. [DOI] [PubMed] [Google Scholar]
- Paxinou E, Chen Q, Weisse M, Giasson BI, Norris EH, Rueter SM, Trojanowski JQ, Lee VM, Ischiropoulos H. Induction of alpha-synuclein aggregation by intracellular nitrative insult. J Neurosci. 2001;21:8053–8061. doi: 10.1523/JNEUROSCI.21-20-08053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payton CA, Wilson DA, Wesson DW. Parallel odor processing by two anatomically distinct olfactory bulb target structures. PLoS One. 2012;7:e34926. doi: 10.1371/journal.pone.0034926. http://dx.doi.org/10.1371/journal.pone.0034926.g009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce RK, Hawkes CH, Daniel SE. The anterior olfactory nucleus in Parkinson’s disease. Mov Disord. 1995;10:283–287. doi: 10.1002/mds.870100309. http://dx.doi.org/10.1002/mds.870100309. [DOI] [PubMed] [Google Scholar]
- Peelaerts W, Bousset L, Van der Perren A, Moskalyuk A, Pulizzi R, Giugliano M, Van den Haute C, Melki R, Baekelandt V. α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature. 2015;522:340–344. doi: 10.1038/nature14547. http://dx.doi.org/10.1038/nature14547. [DOI] [PubMed] [Google Scholar]
- Pelosi P. Odorant-binding proteins. Crit Rev Biochem Mol Biol. 1994;29:199–228. doi: 10.3109/10409239409086801. http://dx.doi.org/10.3109/10409239409086801. [DOI] [PubMed] [Google Scholar]
- Picillo M, Pellecchia MT, Erro R, Amboni M, Vitale C, Iavarone A, Moccia M, Allocca R, Orefice G, Barone P. The use of University of Pennsylvania Smell Identification Test in the diagnosis of Parkinson’s disease in Italy. Neurol Sci. 2013;35:379–383. doi: 10.1007/s10072-013-1522-6. http://dx.doi.org/10.1007/s10072-013-1522-6. [DOI] [PubMed] [Google Scholar]
- Pinkhardt EH, van Elst LT, Ludolph AC, Kassubek J. Amygdala size in amyotrophic lateral sclerosis without dementia: an in vivo study using MRI volumetry. BMC Neurol. 2006;6:81. doi: 10.1186/1471-2377-6-48. http://dx.doi.org/10.1001/archpsyc.63.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirogovsky E, Gilbert PE, Jacobson M, Peavy G, Wetter S, Goldstein J, Corey-Bloom J, Murphy C. Impairments in source memory for olfactory and visual stimuli in preclinical and clinical stages of Huntington’s disease. J Clin Exp Neuropsychol. 2007;29:395–404. doi: 10.1080/13803390600726829. http://dx.doi.org/10.1080/13803390600726829. [DOI] [PubMed] [Google Scholar]
- Plowey ED, Johnson JW, Steer E, Zhu W, Eisenberg DA, Valentino NM, Liu YJ, Chu CT. Biochim Biophys Acta (BBA) – Mol Basis Dis. 2014;1842:1596–1603. doi: 10.1016/j.bbadis.2014.05.016. http://dx.doi.org/10.1016/j.bbadis.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponsen MM, Stoffers D, Booij J, van Eck-Smit BLF, Wolters EC, Berendse HW. Idiopathic hyposmia as a preclinical sign of Parkinson’s disease. Ann Neurol. 2004;56:173–181. doi: 10.1002/ana.20160. http://dx.doi.org/10.1002/ana.20160. [DOI] [PubMed] [Google Scholar]
- Poo C, Isaacson JS. Odor representations in olfactory cortex: “sparse” coding, global inhibition, and oscillations. Neuron. 2009;62:850–861. doi: 10.1016/j.neuron.2009.05.022. http://dx.doi.org/10.1016/j.neuron.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, Deuschl G. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30:1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- Prediger RDS, Aguiar AS, Matheus FC, Walz R, Antoury L, Raisman-Vozari R, Doty RL. Intranasal Administration of Neurotoxicants in animals: support for the olfactory vector hypothesis of Parkinson’s disease. Neurotox Res. 2011;21:90–116. doi: 10.1007/s12640-011-9281-8. http://dx.doi.org/10.1007/s12640-011-9281-8. [DOI] [PubMed] [Google Scholar]
- Price JL, Davis PB, Morris JC, White DL. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer’s disease. Neurobiol Aging. 1991;12:295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- Price JL, Morris JC. So what if tangles precede plaques? Neurobiol Aging. 2004;25(721–3):743–746. doi: 10.1016/j.neurobiolaging.2003.12.017. (discussion) [DOI] [PubMed] [Google Scholar]
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Chen Q, Vila M, Giasson BI, Djaldatti R, Vukosavic S, Souza JM, Jackson-Lewis V, Lee VM, Ischiropoulos H. Oxidative post-translational modifications of alpha-synuclein in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease. J Neurochem. 2001;76:637–640. doi: 10.1046/j.1471-4159.2001.00174.x. [DOI] [PubMed] [Google Scholar]
- Quagliato LB, Viana MA, Quagliato EMAB, Simis S. Olfaction and essential tremor. Arq Neuropsiquiatr. 2009;67:21–24. doi: 10.1590/s0004-282x2009000100006. [DOI] [PubMed] [Google Scholar]
- Quilty MC, King AE, Gai WP, Pountney DL, West AK, Vickers JC, Dickson TC. Alpha-synuclein is upregulated in neurones in response to chronic oxidative stress and is associated with neuroprotection. Exp Neurol. 2006;199:249–256. doi: 10.1016/j.expneurol.2005.10.018. http://dx.doi.org/10.1016/j.expneurol.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Quinn JG, Coulson DTR, Brockbank S, Beyer N, Ravid R, Hellemans J, Irvine GB, Johnston JA. Synuclein mRNA and soluble. Brain Res. 2012;1459:71–80. doi: 10.1016/j.brainres.2012.04.018. http://dx.doi.org/10.1016/j.brainres.2012.04.018. [DOI] [PubMed] [Google Scholar]
- Quinn NP, Rossor MN, Marsden CD. Olfactory threshold in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1987;50:88–89. doi: 10.1136/jnnp.50.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radanovic M, Rosemberg S, Adas R, Miranda SC, Caramelli P, Caixeta L, Nitrini R. Frontotemporal dementia with severe thalamic involvement: a clinical and neuropathological study. Arq Neuropsiquiatr. 2003;61:930–935. doi: 10.1590/s0004-282x2003000600008. [DOI] [PubMed] [Google Scholar]
- Rahayel S, Frasnelli J, Joubert S. The effect of Alzheimer’s disease and Parkinson’s disease on olfaction: a meta-analysis. Behav Brain Res. 2012;231:60–74. doi: 10.1016/j.bbr.2012.02.047. http://dx.doi.org/10.1016/j.bbr.2012.02.047. [DOI] [PubMed] [Google Scholar]
- Razani J, Chan A, Nordin S, Murphy C. Semantic networks for odors and colors in Alzheimer’s disease. Neuropsychology. 2010;24:291–299. doi: 10.1037/a0018269. http://dx.doi.org/10.1037/a0018269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rcom-H’cheo-Gauthier A, Goodwin J, Pountney D. Interactions between calcium and alpha-synuclein in neurodegeneration. Biomolecules. 2014;4:795–811. doi: 10.3390/biom4030795. http://dx.doi.org/10.3390/biom4030795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuber M, Al-Din AS, Baborie A, Chakrabarty A. New variant Creutzfeldt-Jakob disease presenting with loss of taste and smell. J Neurol Neurosurg Psychiatry. 2001;71:412–413. doi: 10.1136/jnnp.71.3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey NL, George S, Brundin P. Review: spreading the word: precise animal models and validated methods are vital when evaluating prion-like behaviour of alpha-synuclein. Neuropathol Appl Neurobiol. 2016a;42:51–76. doi: 10.1111/nan.12299. http://dx.doi.org/10.1111/nan.12299. [DOI] [PubMed] [Google Scholar]
- Rey NL, Jardanhazi-Kurutz D, Terwel D, Kummer MP, Jourdan F, Didier A, Heneka MT. Locus coeruleus degeneration exacerbates olfactory deficits in APP/PS1 transgenic mice. Neurobiol Aging. 2012;33:426.e1–426.e11. doi: 10.1016/j.neurobiolaging.2010.10.009. http://dx.doi.org/10.1016/j.neurobiolaging.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Rey NL, Steiner JA, Maroof N, Luk KC, Madaj Z, Trojanowski JQ, Lee VMY, Brundin P. Widespread transneuronal propagation of α-synucleinopathy triggered in olfactory bulb mimics prodromal Parkinson’s disease. J Exp Med. 2016b;8 doi: 10.1016/0006-8993(87)90196-X. (jem.20160368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes PF, Deems DA, Suarez MG. Olfactory-related changes in Alzheimer’s disease: a quantitative neuropathologic study. Brain Res Bull. 1993;32:1–5. doi: 10.1016/0361-9230(93)90310-8. [DOI] [PubMed] [Google Scholar]
- Reyes PF, Golden GT, Fagel PL, Fariello RG, Katz L, Carner E. The prepiriform cortex in dementia of the Alzheimer type. Arch Neurol. 1987;44:644–645. doi: 10.1001/archneur.1987.00520180062017. [DOI] [PubMed] [Google Scholar]
- Rhodin J, Thomas T, Bryant M, Clark L, Sutton ET. Animal model of vascular inflammation. J Submicrosc Cytol Pathol. 1999;31:305–311. [PubMed] [Google Scholar]
- Rinberg D, Koulakov A, Gelperin A. Sparse odor coding in awake behaving mice. J Neurosci. 2006;26:8857–8865. doi: 10.1523/JNEUROSCI.0884-06.2006. http://dx.doi.org/10.1523/JNEUROSCI.0884-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RF, Wade-Martins R, Alegre-Abarrategui J. Direct visualization of alpha-synuclein oligomers reveals previously undetected pathology in Parkinson’s disease brain. Brain. 2015 doi: 10.1093/brain/awv040. http://dx.doi.org/10.1093/brain/awv040. [DOI] [PMC free article] [PubMed]
- Rolheiser TM, Fulton HG, Good KP, Fisk JD, McKelvey JR, Scherfler C, Khan NM, Leslie RA, Robertson HA. Diffusion tensor imaging and olfactory identification testing in early-stage Parkinson’s disease. J Neurol. 2011;258:1254–1260. doi: 10.1007/s00415-011-5915-2. http://dx.doi.org/10.1007/s00415-011-5915-2. [DOI] [PubMed] [Google Scholar]
- Ross GW, Abbott RD, Petrovitch H, Tanner CM, Davis DG, Nelson J, Markesbery WR, Hardman J, Masaki K, Launer L, White LR. Association of olfactory dysfunction with incidental lewy bodies. Mov Disord. 2006;21:2062–2067. doi: 10.1002/mds.21076. http://dx.doi.org/10.1002/mds.21076. [DOI] [PubMed] [Google Scholar]
- Ross GW, Petrovitch H, Abbott RD, Tanner CM, Popper J, Masaki K, Launer L, White LR. Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann Neurol. 2008;63:167–173. doi: 10.1002/ana.21291. http://dx.doi.org/10.1002/ana.21291. [DOI] [PubMed] [Google Scholar]
- Rossi M, Escobar AM, Bril A, Millar Vernetti P, De Palo JI, Cerquetti D, Merello M. Motor features in Parkinson’s disease with normal olfactory function. Mov Disord. 2016 doi: 10.1002/mds.26687. http://dx.doi.org/10.1002/mds.26687. [DOI] [PubMed]
- Rothermel M. Functional Imaging of Cortical Feedback Projections to the Olfactory Bulb. 2014:1–14. doi: 10.3389/fncir.2014.00073. http://dx.doi.org/10.3389/fncir.2014.00073/abstract. [DOI] [PMC free article] [PubMed]
- Rouby C, Thomas-Danguin T, Vigouroux M, Ciuperca G, Jiang T, Alexanian J, Barges M, Gallice I, Degraix JL, Sicard G. The Lyon Clinical Olfactory Test: Validation and Measurement of Hyposmia and Anosmia in Healthy and Diseased Populations. Int J Otolaryngol. 2011;2011:1–9. doi: 10.1155/2011/203805. http://dx.doi.org/10.1097/00004872-200310000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley JC, Moran DT, Jafek BW. Peroxidase backfills suggest the mammalian olfactory epithelium contains a second morphologically distinct class of bipolar sensory neuron: the microvillar cell. Brain Res. 1989;502:387–400. doi: 10.1016/0006-8993(89)90635-5. [DOI] [PubMed] [Google Scholar]
- Royet JP, Croisile B, Williamson-Vasta R, Hibert O, Serclerat D, Guerin J. Rating of different olfactory judgements in Alzheimer’s disease. Chem Senses. 2001;26:409–417. doi: 10.1093/chemse/26.4.409. [DOI] [PubMed] [Google Scholar]
- Saeedi Saravi SS, Dehpour AR. Potential role of organochlorine pesticides in the pathogenesis of neurodevelopmental, neurodegenerative, and neurobehavioral disorders: a review. Life Sci. 2016;145:255–264. doi: 10.1016/j.lfs.2015.11.006. http://dx.doi.org/10.1016/j.lfs.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Saito Y, Shioya A, Sano T, Sumikura H, Murata M, Murayama S. Lewy body pathology involves the olfactory cells in Parkinson’s disease and related disorders. Mov Disord. 2016;31:135–138. doi: 10.1002/mds.26463. http://dx.doi.org/10.1002/mds.26463. [DOI] [PubMed] [Google Scholar]
- Sakakura Y, Ukai K, Majima Y, Murai S, Harada T, Miyoshi Y. Nasal mucociliary clearance under various conditions. Acta Otolaryngol. 1983;96:167–173. doi: 10.3109/00016488309132888. http://dx.doi.org/10.3109/00016488309132888. [DOI] [PubMed] [Google Scholar]
- Salvesen L, Winge K, Brudek T, Agander TK, Løkkegaard A, Pakkenberg B. Neocortical neuronal loss in patients with multiple system atrophy: a stereological study. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv228. (bhv228) [DOI] [PubMed] [Google Scholar]
- Sanai N, Tramontin AD, Quiñones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel García-Verdugo J, Berger MS, Alvarez-Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. http://dx.doi.org/10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- Santillo AF, Nilsson C, Englund E. Von Economo neurones are selectively targeted in frontotemporal dementia. Neuropathol Appl Neurobiol. 2013;39:572–579. doi: 10.1111/nan.12021. http://dx.doi.org/10.1111/nan.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Hanyu H, Kume K, Takada Y, Onuma T, Iwamoto T. Difference in olfactory dysfunction with dementia with lewy bodies and Alzheimer’s disease. J Am Geriatr Soc. 2011;59:947–948. doi: 10.1111/j.1532-5415.2011.03380.x. http://dx.doi.org/10.1111/j.1532-5415.2011.03380.x. [DOI] [PubMed] [Google Scholar]
- Saunders-Pullman R, Hagenah J, Dhawan V, Stanley K, Pastores G, Sathe S, Tagliati M, Condefer K, Palmese C, Brüggemann N, Klein C, Roe AM, Kornreich R, Ozelius L, Bressman S. Gaucher disease ascertained through a Parkinson’s center: imaging and clinical characterization. Mov Disord. 2010;25:1364–1372. doi: 10.1002/mds.23046. http://dx.doi.org/10.1002/mds.23046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders-Pullman R, Stanley K, Wang C, San Luciano M, Shanker V, Hunt A, Severt L, Raymond D, Ozelius LJ, Lipton RB, Bressman SB. Olfactory dysfunction in LRRK2 G2019S mutation carriers. Neurology. 2011;77:319–324. doi: 10.1212/WNL.0b013e318227041c. http://dx.doi.org/10.1212/WNL.0b013e318227041c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanzano A, Cosentino M. Adrenergic regulation of innate immunity: a review. Front Pharmacol. 2015;6:171. doi: 10.3389/fphar.2015.00171. http://dx.doi.org/10.3389/fphar.2015.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherfler C, Schocke MF, Seppi K, Esterhammer R, Brenneis C, Jaschke W, Wenning GK, Poewe W. Voxel-wise analysis of diffusion weighted imaging reveals disruption of the olfactory tract in Parkinson’s disease. Brain. 2006;129:538–542. doi: 10.1093/brain/awh674. http://dx.doi.org/10.1093/brain/awh674. [DOI] [PubMed] [Google Scholar]
- Schneider SP, Scott JW. Orthodromic response properties of rat olfactory bulb mitral and tufted cells correlates with their projection patterns. J Neurophysiol. 1983;50:358–378. doi: 10.1152/jn.1983.50.2.358. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Eichenbaum H. Information coding in the rodent prefrontal cortex. I. Single-neuron activity in orbitofrontal cortex compared with that in pyriform cortex. J Neurophysiol. 1995;74:733–750. doi: 10.1152/jn.1995.74.2.733. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TA, Obar RA. International Review of Cytology. Elsevier; 1994. Diverse distribution and function of fibrous microtubule-associated proteins in the nervous system; pp. 67–137. http://dx.doi.org/10.1016/S0074-7696(08)62631-5. [DOI] [PubMed] [Google Scholar]
- Schönheit B, Zarski R, Ohm TG. Spatial and temporal relationships between plaques and tangles in Alzheimer-pathology. Neurobiol Aging. 2004;25:697–711. doi: 10.1016/j.neurobiolaging.2003.09.009. http://dx.doi.org/10.1016/j.neurobiolaging.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Schrag A, Siddiqui UF, Anastasiou Z, Weintraub D, Schott JM. Clinical variables and biomarkers in prediction of cognitive impairment in patients with newly diagnosed Parkinson’s disease: a cohort study. Lancet Neurol. 2016 doi: 10.1016/S1474-4422(16)30328-3. http://dx.doi.org/10.1016/S1474-4422(16)30328-3. [DOI] [PMC free article] [PubMed]
- Seeley WW, Carlin DA, Allman JM, Macedo MN, Bush C, Miller BL, DeArmond SJ. Early frontotemporal dementia targets neurons unique to apes and humans. Ann Neurol. 2006;60:660–667. doi: 10.1002/ana.21055. http://dx.doi.org/10.1002/ana.21055. [DOI] [PubMed] [Google Scholar]
- Sengoku R, Saito Y, Ikemura M, Hatsuta H, Sakiyama Y, Kanemaru K, Arai T, Sawabe M, Tanaka N, Mochizuki H, Inoue K, Murayama S. Incidence and extent of lewy body-related alpha-synucleinopathy in aging human olfactory bulb. J Neuropathol Exp Neurol. 2008;67:1072–1083. doi: 10.1097/NEN.0b013e31818b4126. http://dx.doi.org/10.1097/NEN.0b013e31818b4126. [DOI] [PubMed] [Google Scholar]
- Serby M, Larson P, Kalkstein D. The nature and course of olfactory deficits in Alzheimer’s disease. Am J Psychiatry. 1991;148:357–360. doi: 10.1176/ajp.148.3.357. http://dx.doi.org/10.1176/ajp.148.3.357. [DOI] [PubMed] [Google Scholar]
- Setó-Salvia N, Pagonabarraga J, Houlden H, Pascual-Sedano B, Dols-Icardo O, Tucci A, Paisán-Ruiz C, Campolongo A, Antón-Aguirre S, Martín I, Muñoz L, Bufill E, Vilageliu L, Grinberg D, Cozar M, Blesa R, Lleó A, Hardy J, Kulisevsky J, Clarimón J. Glucocerebrosidase mutations confer a greater risk of dementia during Parkinson’s disease course. Mov Disord. 2011;27:393–399. doi: 10.1002/mds.24045. http://dx.doi.org/10.1002/mds.24045. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Kauer JS, Shepherd GM. Local sites of activity-related glucose metabolism in rat olfactory bulb during olfactory stimulation. Brain Res. 1975;98:596–600. doi: 10.1016/0006-8993(75)90377-7. [DOI] [PubMed] [Google Scholar]
- Shepherd GM, Chen WR, Greer CA. Olfactory bulb. In: Shepherd GM, editor. The Synaptic Organization of the Brain. fifth. Oxford Univ Press; 2004. pp. 165–216. [Google Scholar]
- Sherman MA, LaCroix M, Amar F, Larson ME, Forster C, Aguzzi A, Bennett DA, Ramsden M, Lesne SE. Soluble conformers of a and tau Alter selective proteins governing axonal transport. J Neurosci. 2016;36:9647–9658. doi: 10.1523/JNEUROSCI.1899-16.2016. http://dx.doi.org/10.1523/JNEUROSCI.1899-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shodai A, Morimura T, Ido A, Uchida T, Ayaki T, TAKAHASHI R, Kitazawa S, Suzuki S, Shirouzu M, Kigawa T, Muto Y, Yokoyama S, Kitahara R, Ito H, Fujiwara N, Urushitani M. Aberrant assembly of RNA recognition motif 1 links to pathogenic conversion of TAR DNA-binding protein of 43 kDa (TDP-43) J Biol Chem. 2013;288:14886–14905. doi: 10.1074/jbc.M113.451849. http://dx.doi.org/10.1074/jbc.M113.451849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira-Moriyama L, Holton JL, Kingsbury A, Ayling H, Petrie A, Sterlacci W, Poewe W, Maier H, Lees AJ, Revesz T. Regional differences in the severity of lewy body pathology across the olfactory cortex. Neurosci Lett. 2009a;453:77–80. doi: 10.1016/j.neulet.2009.02.006. http://dx.doi.org/10.1016/j.neulet.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Silveira-Moriyama L, Hughes G, Church A, Ayling H, Williams DR, Petrie A, Holton J, Revesz T, Kingsbury A, Morris HR, Burn DJ, Lees AJ. Hyposmia in progressive supranuclear palsy. Mov Disord. 2010a;25:570–577. doi: 10.1002/mds.22688. http://dx.doi.org/10.1002/mds.22688. [DOI] [PubMed] [Google Scholar]
- Silveira-Moriyama L, Mathias C, Mason L, Best C, Quinn NP, Lees AJ. Hyposmia in pure autonomic failure. Neurology. 2009b;72:1677–1681. doi: 10.1212/WNL.0b013e3181a55fd2. http://dx.doi.org/10.1212/WNL.0b013e3181a55fd2. [DOI] [PubMed] [Google Scholar]
- Silveira-Moriyama L, Munhoz RP, de J Carvalho M, Raskin S, Rogaeva E, de C Aguiar P, Bressan RA, Felicio AC, Barsottini OGP, Andrade LAF, Chien HF, Bonifati V, Barbosa ER, Teive HA, Lees AJ. Olfactory heterogeneity in LRRK2 related Parkinsonism. Mov Disord. 2010b;25:2879–2883. doi: 10.1002/mds.23325. http://dx.doi.org/10.1002/mds.23325. [DOI] [PubMed] [Google Scholar]
- Singh N, Singh A, Das D, Mohan ML. Redox control of prion and disease pathogenesis. Antioxid Redox Signal. 2010;12:1271–1294. doi: 10.1089/ars.2009.2628. http://dx.doi.org/10.1089/ars.2009.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. Alpha-synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. http://dx.doi.org/10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Smith DA, Jones BC. Speculations on the substrate structure-activity relationship (SSAR) of cytochrome P450 enzymes. Biochem Pharmacol. 1992;44:2089–2098. doi: 10.1016/0006-2952(92)90333-e. [DOI] [PubMed] [Google Scholar]
- Smith MC. Nerve fiber degeneration in the brain on amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 1960;23:269–282. doi: 10.1136/jnnp.23.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithson LJ, Kawaja MD. Microglial/macrophage cells in mammalian olfactory nerve fascicles. J Neurosci Res. 2010;88:858–865. doi: 10.1002/jnr.22254. http://dx.doi.org/10.1002/jnr.22254. [DOI] [PubMed] [Google Scholar]
- Sobel N, Thomason ME, Stappen I, Tanner CM, Tetrud JW, Bower JM, Sullivan EV, Gabrieli JD. An impairment in sniffing contributes to the olfactory impairment in Parkinson’s disease. Proc Natl Acad Sci U S A. 2001;98:4154–4159. doi: 10.1073/pnas.071061598. http://dx.doi.org/10.1073/pnas.071061598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, LaViolette PS, Keefe KO, Brien JO, Rentz DM, Pihlajamaki M, Marshall G, Hyman BT, Selkoe DJ, Hedden T, Buckner RL, Becker JA, Johnson KA. Amyloid deposition is Associatedwith impaired default network function in older persons without dementia. Neuron. 2009;63:178–188. doi: 10.1016/j.neuron.2009.07.003. http://dx.doi.org/10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spors H, Wachowiak M, Cohen LB, Friedrich RW. Temporal dynamics and latency patterns of receptor neuron input to the olfactory bulb. J Neurosci. 2006;26:1247–1259. doi: 10.1523/JNEUROSCI.3100-05.2006. http://dx.doi.org/10.1523/JNEUROSCI.3100-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stargardt A, Swaab DF, Bossers K. Storm before the quiet: neuronal hyperactivity and Aβ in the presymptomatic stages of Alzheimer’s disease. Neurobiol Aging. 2015;36:1–11. doi: 10.1016/j.neurobiolaging.2014.08.014. http://dx.doi.org/10.1016/j.neurobiolaging.2014.08.014. [DOI] [PubMed] [Google Scholar]
- Struble RG, Clark HB. Olfactory bulb lesions in Alzheimer’s disease. Neurobiol Aging. 1992;13:469–473. doi: 10.1016/0197-4580(92)90074-8. [DOI] [PubMed] [Google Scholar]
- Struble RG, Dhanraj DN, Mei Y, Wilson M, Wang R, Ramkumar V. Beta-amyloid precursor protein-like immunoreactivity is upregulated during olfactory nerve regeneration in adult rats. Brain Res. 1997;780:129–137. doi: 10.1016/s0006-8993(97)01187-6. [DOI] [PubMed] [Google Scholar]
- Su FC, Goutman SA, Chernyak S, Mukherjee B, Callaghan BC, Batterman S, Feldman EL. Association of environmental toxins with amyotrophic lateral sclerosis. JAMA Neurol. 2016;73:803. doi: 10.1001/jamaneurol.2016.0594. http://dx.doi.org/10.1001/jamaneurol.2016.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surace MJ, Block ML. Targeting microglia-mediated neurotoxicity: the potential of NOX2 inhibitors. Cell Mol Life Sci. 2012;69:2409–2427. doi: 10.1007/s00018-012-1015-4. http://dx.doi.org/10.1007/s00018-012-1015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Hashimoto M, Yoshioka M, Murakami M, Kawasaki K, Urashima M. The odor stick identification test for Japanese differentiates Parkinson’s disease from multiple system atrophy and progressive supra nuclear palsy. BMC Neurol. 2011;11:157. doi: 10.1186/1471-2377-11-157. http://dx.doi.org/10.1186/1471-2377-11-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sy M, Kitazawa M, Medeiros R, Whitman L, Cheng D, Lane TE, LaFerla FM. Inflammation induced by infection potentiates tau pathological features in transgenic mice. Am J Pathol. 2011;178:2811–2822. doi: 10.1016/j.ajpath.2011.02.012. http://dx.doi.org/10.1016/j.ajpath.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Štenc Bradvica I, Mihaljević I, Butković-Soldo S, Kadojić D, Titlić M, Bradvica M, Kralik K. Transcranial sonography and the pocket smell test in the differential diagnosis between parkinson’s disease and essential tremor. Neurol Sci. 2015;36:1403–1410. doi: 10.1007/s10072-015-2152-y. http://dx.doi.org/10.1007/s10072-015-2152-y. [DOI] [PubMed] [Google Scholar]
- Tabaka J, Nowacki P, Stankiewicz J, Wierzba-Bobrowicz T. Extreme loss of neurons in sporadic Creutzfeldt-Jakob disease with 14-3-3 protein in cerebrospinal fluid. Folia Neuropathol. 2003;41:47–50. [PubMed] [Google Scholar]
- Tabaton M, Monaco S, Cordone MP, Colucci M, Giaccone G, Tagliavini F, Zanusso G. Prion deposition in olfactory biopsy of sporadic Creutzfeldt-Jakob disease. Ann Neurol. 2004;55:294–296. doi: 10.1002/ana.20038. http://dx.doi.org/10.1002/ana.20038. [DOI] [PubMed] [Google Scholar]
- Taguchi K, Watanabe Y, Tsujimura A, Tanaka M. Brain region-dependent differential expression of alpha-synuclein. J Comp Neurol. 2015;524:1236–1258. doi: 10.1002/cne.23901. http://dx.doi.org/10.1002/cne.23901. [DOI] [PubMed] [Google Scholar]
- Takeda T, Iijima M, Uchihara T, Ohashi T, Seilhean D, Duyckaerts C, Uchiyama S. TDP-43 pathology progression along the olfactory pathway as a possible substrate for olfactory impairment in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 2015;74:547–556. doi: 10.1097/NEN.0000000000000198. http://dx.doi.org/10.1097/NEN.0000000000000198. [DOI] [PubMed] [Google Scholar]
- Tan J, Savigner A, Ma M, Luo M. Odor information processing by the olfactory bulb analyzed in gene-targeted mice. Neuron. 2010;65:912–926. doi: 10.1016/j.neuron.2010.02.011. http://dx.doi.org/10.1016/j.neuron.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanik N, Serin HI, Celikbilek A, Inan LE, Gundogdu F. Associations of olfactory bulb and depth of olfactory sulcus with basal ganglia and hippocampus in patients with Parkinson’s disease. Neurosci Lett. 2016;620:111–114. doi: 10.1016/j.neulet.2016.03.050. http://dx.doi.org/10.1016/j.neulet.2016.03.050. [DOI] [PubMed] [Google Scholar]
- Tanner CM, Goldman SM, Ross GW, Grate SJ. The disease intersection of susceptibility and exposure: Chemical exposures and neurodegenerative disease risk. Alzheimers Dement. 2014;10:S213–S225. doi: 10.1016/j.jalz.2014.04.014. http://dx.doi.org/10.1016/j.jalz.2014.04.014. [DOI] [PubMed] [Google Scholar]
- Thal DR, Rüb U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- Tigges M, Tigges J. Distribution and morphology of myelinated perikarya and dendrites in the olfactory bulb of primates. J Neurocytol. 1980;9:825–834. doi: 10.1007/BF01205021. [DOI] [PubMed] [Google Scholar]
- Tijero B, Gomez-Esteban JC, Llorens V, Lezcano E, Gonzalez-Fernández MC, de Pancorbo MM, Ruiz-Martinez J, Cembellin JC, Zarranz JJ. Cardiac sympathetic denervation precedes nigrostriatal loss in the E46K mutation of the α-synuclein gene (SNCA) Clin Auton Res. 2010;20:267–269. doi: 10.1007/s10286-010-0068-4. http://dx.doi.org/10.1007/s10286-010-0068-4. [DOI] [PubMed] [Google Scholar]
- Tissingh G, Berendse HW, Bergmans P, DeWaard R, Drukarch B, Stoof JC, Wolters EC. Loss of olfaction in de novo and treated Parkinson’s disease: possible implications for early diagnosis. Mov Disord. 2001;16:41–46. doi: 10.1002/1531-8257(200101)16:1<41::aid-mds1017>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Tjälve H, Henriksson J. Uptake of metals in the brain via olfactory pathways. Neurotoxicology. 1999;20:181–195. [PubMed] [Google Scholar]
- Tousi B, Frankel M. Olfactory and visual hallucinations in Parkinson’s disease. Parkinsonism Relat Disord. 2004;10:253–254. doi: 10.1016/j.parkreldis.2004.01.003. http://dx.doi.org/10.1016/j.parkreldis.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Trabzuni D, Wray S, Vandrovcova J, Ramasamy A, Walker R, Smith C, Luk C, Gibbs JR, Dillman A, Hernandez DG, Arepalli S, Singleton AB, Cookson MR, Pittman AM, de Silva R, Weale ME, Hardy J, Ryten M. MAPT expression and splicing is differentially regulated by brain region: relation to genotype and implication for tauopathies. Hum Mol Genet. 2012;21:4094–4103. doi: 10.1093/hmg/dds238. http://dx.doi.org/10.1093/hmg/dds238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanowski JQ, Schuck T, Schmidt ML, Lee VM. Distribution of tau proteins in the normal human central and peripheral nervous system. J Histochem Cytochem. 1989;37:209–215. doi: 10.1177/37.2.2492045. [DOI] [PubMed] [Google Scholar]
- Tsuboi Y, Wszolek ZK, Graff-Radford NR, Cookson N, Dickson DW. Tau pathology in the olfactory bulb correlates with Braak stage, Lewy body pathology and apolipoprotein epsilon4. Neuropathol Appl Neurobiol. 2003;29:503–510. doi: 10.1046/j.1365-2990.2003.00453.x. http://dx.doi.org/10.1046/j.0305-1846.2003.00453.x. [DOI] [PubMed] [Google Scholar]
- Ubeda-Bañon I, Saiz-Sanchez D, Rosa-Prieto C, Argandoña-Palacios L, Garcia-Muñozguren S, Martinez-Marcos A. α-synucleinopathy in the human olfactory system in Parkinson’s disease: involvement of calcium-binding protein- and substance P-positive cells. Acta Neuropathol. 2010;119:723–735. doi: 10.1007/s00401-010-0687-9. http://dx.doi.org/10.1007/s00401-010-0687-9. [DOI] [PubMed] [Google Scholar]
- Uchida N, Takahashi YK, Tanifuji M, Mori K. Odor maps in the mammalian olfactory bulb: domain organization and odorant structural features. Nat Neurosci. 2000;3:1035–1043. doi: 10.1038/79857. http://dx.doi.org/10.1038/79857. [DOI] [PubMed] [Google Scholar]
- Uversky VN, Li J, Bower K, Fink AL. Synergistic effects of pesticides and metals on the fibrillation of α-synuclein: implications for Parkinson’s disease. Neurotoxicology. 2002;23:527–536. doi: 10.1016/s0161-813x(02)00067-0. http://dx.doi.org/10.1016/S0161-813X(02)00067-0. [DOI] [PubMed] [Google Scholar]
- Valldeoriola F, Gaig C, Muxí A, Navales I, Paredes P, Lomeña F, la Cerda DA, Buongiorno M, Ezquerra M, Santacruz P, Marti M-J, Tolosa E. 123I-MIBG cardiac uptake and smell identification in parkinsonian patients with LRRK2 mutations. J Neurol. 2011;258:1126–1132. doi: 10.1007/s00415-010-5896-6. http://dx.doi.org/10.1007/s00415-010-5896-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap MS, Valk J, Bakker CJ, Schooneveld M, Faber JA, Willemse J. Myelination as an expression of the functional maturity of the brain. Dev Med Child Neurol. 1991;33:849–857. doi: 10.1111/j.1469-8749.1991.tb14793.x. [DOI] [PubMed] [Google Scholar]
- van Riel D, Verdijk R, Kuiken T. The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J Pathol. 2015;235:277–287. doi: 10.1002/path.4461. http://dx.doi.org/10.1002/path.4461. [DOI] [PubMed] [Google Scholar]
- Vanacore N, Bonifati V, Fabbrini G, Colosimo C, De Michele G, Marconi R, Nicholl D, Locuratolo N, Talarico G, Romano S, Stocchi F, Bonuccelli U, De Mari M, Vieregge P, Meco G. Epidemiology of multiple system atrophy. Neurol Sci. 2001;22:97–99. doi: 10.1007/s100720170064. http://dx.doi.org/10.1007/s100720170064. [DOI] [PubMed] [Google Scholar]
- Vanek P, Thallmair M, Schwab ME, Kapfhammer JP. Increased lesion-induced sprouting of corticospinal fibres in the myelin-free rat spinal cord. Eur J Neurosci. 1998;10:45–56. doi: 10.1046/j.1460-9568.1998.00018.x. [DOI] [PubMed] [Google Scholar]
- Velayudhan L, Gasper A, Pritchard M, Baillon S, Messer C, Proitsi P. Pattern of smell identification impairment in Alzheimer’s disease. J Alzheimers Dis. 2015;46:381–387. doi: 10.3233/JAD-142838. http://dx.doi.org/10.3233/JAD-142838. [DOI] [PubMed] [Google Scholar]
- Verhagen JV, Wesson DW, Netoff TI, White JA, Wachowiak M. Sniffing controls an adaptive filter of sensory input to the olfactory bulb. Nat Neurosci. 2007;10:631–639. doi: 10.1038/nn1892. http://dx.doi.org/10.1038/nn1892. [DOI] [PubMed] [Google Scholar]
- Veyrac A, Nguyen V, Marien M, Didier A, Jourdan F. Noradrenergic control of odor recognition in a nonassociative olfactory learning task in the mouse. Learn Mem. 2007;14:847–854. doi: 10.1101/lm.708807. http://dx.doi.org/10.1101/lm.708807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira BDM, Radford RA, Chung RS, Guillemin GJ, Pountney DL. Neuroin-flammation in multiple system atrophy: response to and cause of α-synuclein aggregation. Front Cell Neurosci. 2015;9:437. doi: 10.3389/fncel.2015.00437. http://dx.doi.org/10.3389/fncel.2015.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viereck C, Tucker RP, Matus A. The adult rat olfactory system expresses microtubule-associated proteins found in the developing brain. J Neurosci. 1989;9:3547–3557. doi: 10.1523/JNEUROSCI.09-10-03547.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar-Piqué A, Lopes da Fonseca T, Sant’Anna R, Szegö ÉM, Fonseca-Ornelas L, Pinho R, Carija A, Gerhardt E, Masaracchia C, Abad Gonzalez E, Rossetti G, Carloni P, Fernandez CO, Foguel D, Milosevic I, Zweckstetter M, Ventura S, Outeiro TF. Environmental and genetic factors support the dissociation between α-synuclein aggregation and toxicity. Proc Natl Acad Sci. 2016:201606791. doi: 10.1073/pnas.1606791113. http://dx.doi.org/10.1073/pnas.1606791113. [DOI] [PMC free article] [PubMed]
- Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP. Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol. 1985;44:559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Shipley MT. Coding and synaptic processing of sensory information in the glomerular layer of the olfactory bulb. Semin Cell Dev Biol. 2006;17:411–423. doi: 10.1016/j.semcdb.2006.04.007. http://dx.doi.org/10.1016/j.semcdb.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Piao YS, Hayashi S, Kakita A, Yamada M, Takahashi H. Ubiquitinated neuronal inclusions in the neostriatum in patients with amyotrophic lateral sclerosis with and without dementia–a study of 60 patients 31 to 87 years of age. Clin Neuropathol. 2001;20:47–52. [PubMed] [Google Scholar]
- Walker LC, Diamond MI, Duff KE, Hyman BT. Mechanisms of protein seeding in neurodegenerative diseases. JAMA Neurol. 2013;70:304–312. doi: 10.1001/jamaneurol.2013.1453. http://dx.doi.org/10.1001/jamaneurol.2013.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz R, Castro RMRPS, Velasco TR, Carlotti CG, Sakamoto AC, Brentani RR, Martins VR. Cellular prion protein: implications in seizures and epilepsy. Cell Mol Neurobiol. 2002;22:249–257. doi: 10.1023/a:1020711700048. [DOI] [PubMed] [Google Scholar]
- Wattendorf E, Welge-Lussen A, Fiedler K, Bilecen D, Wolfensberger M, Fuhr P, Hummel T, Westermann B. Olfactory impairment predicts brain atrophy in Parkinson’s disease. J Neurosci. 2009;29:15410–15413. doi: 10.1523/JNEUROSCI.1909-09.2009. http://dx.doi.org/10.1523/JNEUROSCI.1909-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenning GK, Ben-Shlomo Y, Magalhães M, Daniel SE, Quinn NP. Clinicopathological study of 35 cases of multiple system atrophy. J Neurol Neurosurg Psychiatry. 1995a;58:160–166. doi: 10.1136/jnnp.58.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenning GK, Shephard B, Hawkes C, Petruckevitch A, Lees A, Quinn N. Olfactory function in atypical parkinsonian syndromes. Acta Neurol Scand. 1995b;91:247–250. doi: 10.1111/j.1600-0404.1995.tb06998.x. [DOI] [PubMed] [Google Scholar]
- Wesson DW, Borkowski AH, Landreth GE, Nixon RA, Levy E, Wilson DA. Sensory network dysfunction, behavioral impairments, and their reversibility in an Alzheimer’s -Amyloidosis mouse model. J Neurosci. 2011;31:15962–15971. doi: 10.1523/JNEUROSCI.2085-11.2011. http://dx.doi.org/10.1523/JNEUROSCI.2085-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DW, Levy E, Nixon RA, Wilson DA. Olfactory dysfunction correlates with amyloid-beta burden in an Alzheimer’s disease mouse model. J Neurosci. 2010;30:505–514. doi: 10.1523/JNEUROSCI.4622-09.2010. http://dx.doi.org/10.1523/JNEUROSCI.4622-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DW, Wilson DA. Sniffing out the contributions of the olfactory tubercle to the sense of smell: Hedonics, sensory integration, and more? Neurosci Biobehav Rev. 2011;35:655–668. doi: 10.1016/j.neubiorev.2010.08.004. http://dx.doi.org/10.1016/j.neubiorev.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westervelt HJ, Carvalho J, Duff K. Presentation of Alzheimer’s disease in patients with and without olfactory deficits. Arch Clin Neuropsychol. 2007;22:117–122. doi: 10.1016/j.acn.2006.11.005. http://dx.doi.org/10.1016/j.acn.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Westervelt HJ, Stern RA, Tremont G. Odor identification deficits in diffuse lewy body disease. Cogn Behav Neurol. 2003;16:93–99. doi: 10.1097/00146965-200306000-00002. [DOI] [PubMed] [Google Scholar]
- Whitehouse PJ, Struble RG, Hedreen JC, Clark AW, White CL, Parhad IM, Price DL. Neuroanatomical evidence for a cholinergic deficit in Alzheimer’s disease. Psychopharmacol Bull. 1983;19:437–440. [PubMed] [Google Scholar]
- Williams SS, Williams J, Combrinck M, Christie S, Smith AD, McShane R. Olfactory impairment is more marked in patients with mild dementia with Lewy bodies than those with mild Alzheimer disease. J Neurol Neurosurg Psychiatry. 2009;80:667–670. doi: 10.1136/jnnp.2008.155895. http://dx.doi.org/10.1136/jnnp.2008.155895. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Habituation of odor responses in the rat anterior piriform cortex. J Neurophysiol. 1998;79:1425–1440. doi: 10.1152/jn.1998.79.3.1425. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Fletcher ML, Sullivan RM. Acetylcholine and olfactory perceptual learning. Learn Mem. 2004;11:28–34. doi: 10.1101/lm.66404. http://dx.doi.org/10.1101/lm.66404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. Cortical processing of odor objects. Neuron. 2011;72:506–519. doi: 10.1016/j.neuron.2011.10.027. http://dx.doi.org/10.1016/j.neuron.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Mainen ZF. Early events in olfactory processing. Annu Rev Neurosci. 2006;29:163–201. doi: 10.1146/annurev.neuro.29.051605.112950. http://dx.doi.org/10.1146/annurev.neuro.29.051605.112950. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Schneider JA, Boyle PA, Buchman AS, Bennett DA. Olfactory impairment in presymptomatic Alzheimer’s disease. Ann N Y Acad Sci. 2009;1170:730–735. doi: 10.1111/j.1749-6632.2009.04013.x. http://dx.doi.org/10.1111/j.1749-6632.2009.04013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Yu L, Schneider JA, Arnold SE, Buchman AS, Bennett DA. Lewy bodies and olfactory dysfunction in old age. Chem Senses. 2011;36:367–373. doi: 10.1093/chemse/bjq139. http://dx.doi.org/10.1093/chemse/bjq139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt M, Bormann K, Gudziol V, Pehlke K, Barth K, Minovi A, Hähner A, Reichmann H, Hummel T. Biopsies of olfactory epithelium in patients with Parkinson’s disease. Mov Disord. 2009;24:906–914. doi: 10.1002/mds.22464. http://dx.doi.org/10.1002/mds.22464. [DOI] [PubMed] [Google Scholar]
- Woulfe J, Gray MT, Ganesh MS, Middeldorp JM. Human serum antibodies against EBV latent membrane protein 1 cross-react with α-synuclein. Neurol Neuroimmunol Neuroinflamm. 2016;3:e239–10. doi: 10.1212/NXI.0000000000000239. http://dx.doi.org/10.1212/NXI.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woulfe JM, Gray MT, Gray DA, Munoz DG, Middeldorp JM. Hypothesis: a role for EBV-induced molecular mimicry in Parkinson’s disease. Parkinsonism Relat Disord. 2014;20:685–694. doi: 10.1016/j.parkreldis.2014.02.031. http://dx.doi.org/10.1016/j.parkreldis.2014.02.031. [DOI] [PubMed] [Google Scholar]
- Wu J, Xie H. Effects of titanium dioxide nanoparticles on. Artif Cells Nanomed Biotechnol. 2014:1–5. doi: 10.3109/21691401.2014.980507. http://dx.doi.org/10.3109/21691401.2014.980507. [DOI] [PubMed]
- Wu WYY, Kang KH, Chen SLS, Chiu SYH, Yen AMF, Fann JCY, Su CW, Liu HC, Lee CZ, Fu WM, Chen HH, Liou HH. Hepatitis C virus infection: a risk factor for Parkinson’s disease. J Viral Hepat. 2015;22:784–791. doi: 10.1111/jvh.12392. http://dx.doi.org/10.1111/jvh.12392. [DOI] [PubMed] [Google Scholar]
- Xia CZ, Adjei S, Wesson DW. Coding of odor stimulus features among secondary olfactory structures. J Neurophysiol. 2015;114:736–745. doi: 10.1152/jn.00902.2014. http://dx.doi.org/10.1152/jn.00902.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi M, Takami S, Getchell TV. Ontogenetic expression of spot 35 protein (calbindin-D28k) in human olfactory receptor neurons and its decrease in Alzheimer’s disease patients. Ann Otol Rhinol Laryngol. 2016;105:132–139. doi: 10.1177/000348949610500208. [DOI] [PubMed] [Google Scholar]
- Yang DS, Stavrides P, Mohan PS, Kaushik S, Kumar A, Ohno M, Schmidt SD, Wesson D, Bandyopadhyay U, Jiang Y, Pawlik M, Peterhoff CM, Yang AJ, Wilson DA, St George-Hyslop P, Westaway D, Mathews PM, Levy E, Cuervo AM, Nixon RA. Reversal of autophagy dysfunction in the TgCRND8 mouse model of Alzheimer’s disease ameliorates amyloid pathologies and memory deficits. Brain. 2010;134:258–277. doi: 10.1093/brain/awq341. http://dx.doi.org/10.1093/brain/awq341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Kim M, Moon SY, Yong SW, Hong JM. J Neurol Sci. 2015;355:174–179. doi: 10.1016/j.jns.2015.06.013. http://dx.doi.org/10.1016/j.jns.2015.06.013. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K, Touhara K. Olfactory receptor function. In: Doty RL, editor. Handbook of Olfaction and Gustation. third. John Wiley & Sons, Inc; 2015. pp. 109–121. [Google Scholar]
- Yoshimura N. Topography of Pick body distribution in Pick’s disease: a contribution to understanding the relationship between Pick’s and Alzheimer’s diseases. Clin Neuropathol. 1989;8:1–6. [PubMed] [Google Scholar]
- Yoshimura N. Olfactory bulb involvement in Pick’s disease. Acta Neuropathol. 1988;77:202–205. doi: 10.1007/BF00687432. [DOI] [PubMed] [Google Scholar]
- Yu Y, Migliore M, Hines ML, Shepherd GM. Sparse coding and lateral inhibition arising from balanced and unbalanced dendrodendritic excitation and inhibition. J Neurosci. 2014;34:13701–13713. doi: 10.1523/JNEUROSCI.1834-14.2014. http://dx.doi.org/10.1523/JNEUROSCI.1834-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanusso G, Ferrari S, Benedetti D, Sbriccoli M, Rizzuto N, Monaco S. Different prion conformers target the olfactory pathway in sporadic Creutzfeldt-Jakob disease. Ann N Y Acad Sci. 2009;1170:637–643. doi: 10.1111/j.1749-6632.2009.03905.x. http://dx.doi.org/10.1111/j.1749-6632.2009.03905.x. [DOI] [PubMed] [Google Scholar]
- Zanusso G, Ferrari S, Cardone F, Zampieri P, Gelati M, Fiorini M, Farinazzo A, Gardiman M, Cavallaro T, Bentivoglio M, Righetti PG, Pocchiari M, Rizzuto N, Monaco S. Detection of pathologic prion protein in the olfactory epithelium in sporadic Creutzfeldt-Jakob disease. N Engl J Med. 2003;348:711–719. doi: 10.1056/NEJMoa022043. http://dx.doi.org/10.1056/NEJMoa022043. [DOI] [PubMed] [Google Scholar]
- Zhao W, Beers DR, Appel SH. Immune-mediated mechanisms in the Pathoprogression of amyotrophic lateral sclerosis. J NeuroImmune Pharmacol. 2013;8:888–899. doi: 10.1007/s11481-013-9489-x. http://dx.doi.org/10.1007/s11481-013-9489-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Miranda-Saksena M, Saksena NK. Viruses and neurodegeneration. Virol J. 2013;10:1. doi: 10.1186/1743-422X-10-172. http://dx.doi.org/10.1186/1743-422X-10-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucco G, Zeni MT, Perrone A, Piccolo I. Olfactory sensitivity in early-stage Parkinson patients affected by more marked unilateral disorder. Percept Mot Skills. 2001;92:894–898. doi: 10.2466/pms.2001.92.3.894. http://dx.doi.org/10.2466/pms.2001.92.3.894. [DOI] [PubMed] [Google Scholar]


