Abstract
STUDY QUESTION
Is human endometrial leucine-rich repeat-containing G-protein-coupled receptor 5 (LGR5) gene expression limited to the postulated epithelial stem cell niche, stratum basalis glands, and is it hormonally regulated?
SUMMARY ANSWER
LGR5 expressing cells are not limited to the postulated stem cell niche but LGR5 expression is hormonally regulated.
WHAT IS KNOWN ALREADY
The human endometrium is a highly regenerative tissue; however, endometrial epithelial stem cell markers are yet to be confirmed. LGR5 is a marker of stem cells in various epithelia.
STUDY DESIGN, SIZE, DURATION
The study was conducted at a University Research Institute. Endometrial samples from 50 healthy women undergoing benign gynaecological surgery with no endometrial pathology at the Liverpool Women’s hospital were included and analysed in the following six sub-categories; proliferative, secretory phases of menstrual cycle, postmenopausal, those using oral and local progestagens and samples for in vitro explant culture.
PARTICIPANTS/MATERIALS, SETTING, METHODS
In this study, we used the gold standard method, in situ hybridisation (ISH) along with qPCR and a systems biology approach to study the location of LGR5 gene expression in full thickness human endometrium and Fallopian tubes. The progesterone regulation of endometrial LGR5 was examined in vivo and in short-term cultured endometrial tissue explants in vitro. LGR5 expression was correlated with epithelial proliferation (Ki67), and expression of previously reported epithelia progenitor markers (SOX9 and SSEA-1) immunohistochemistry (IHC).
MAIN RESULTS AND THE ROLE OF CHANCE
LGR5 gene expression was significantly higher in the endometrial luminal epithelium than in all other epithelial compartments in the healthy human endometrium, including the endometrial stratum basalis (P < 0.05). The strongest SSEA-1 and SOX9 staining was observed in the stratum basalis glands, but the general trend of SOX9 and SSEA-1 expression followed the same cyclical pattern of expression as LGR5. Stratum functionalis epithelial Ki67-LI and LGR5 expression levels correlated significantly (r = 0.74, P = 0.01), however, they did not correlate in luminal and stratum basalis epithelium (r = 0.5 and 0.13, respectively). Endometrial LGR5 demonstrates a dynamic spatiotemporal expression pattern, suggesting hormonal regulation. Oral and local progestogens significantly reduced endometrial LGR5 mRNA levels compared with women not on hormonal treatment (P < 0.01). Our data were in agreement with in silico analysis of published endometrial microarrays.
LARGE SCALE DATA
We did not generate our own large scale data but interrogated publically available large scale data sets.
LIMITATIONS, REASONS FOR CAUTION
In the absence of reliable antibodies for human LGR5 protein and validated lineage markers for the various epithelial populations that potentially exist within the endometrium, our study does not formally characterise or examine the functional ability of the resident LGR5+ cells as multipotent.
WIDER IMPLICATIONS OF THE FINDINGS
These data will facilitate future lineage tracing studies in the human endometrial epithelium; to identify the location of stem cells and further complement the in vitro functional studies, to confirm if the LGR5 expressing epithelial cells indeed represent the epithelial stem cell population.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by funding from the Wellbeing of Women project grant (RTF510) and Cancer Research UK (A14895). None of the authors have any conflicts of interest to disclose.
Keywords: endometrial epithelial stem cells, leucine-rich repeat-containing G-protein-coupled receptor 5, progesterone regulation, in situ hybridisation, stem cell niche, fallopian tube
Introduction
The human endometrium is a highly regenerative tissue, which undergoes over 400 cycles of menstrual shedding and re-growth in a woman’s life time. It is composed of two functionally distinct layers, the superficial stratum functionalis and the deeper stratum basalis.
The stratum functionalis is completely shed with menstruation and fully regenerated within 2 weeks, up to a thickness of 16 mm (Fleischer, 1999). This impressive regeneration implies that a stem cell population may reside within the endometrial glands. The location of stem/progenitor cells of the endometrium is postulated to be within the stratum basalis, which remains after the menstrual shedding of the stratum functionalis (Prianishnikov, 1978; Gargett et al., 2016).
Of the two main endometrial specific cell types, the mesenchymal stem/progenitor cells that give rise to stromal cells are well described and studied (Gargett et al., 2016). However, the evidence for an endometrial epithelial stem cell population is sparse. Previous work suggests that SSEA-1 and SOX9 expressing epithelial cell subpopulations have some ability to generate gland-like structures in vitro (Valentijn et al., 2013; Turco et al., 2017), but as yet there are no other epithelial markers with the location or functional characterisation suggestive of stem cell specificity described in the endometrium.
Leucine-rich repeat-containing G-protein-coupled receptor 5 (LGR5) is a transmembrane receptor (Barker et al., 2007) which belongs to a family of glycoprotein hormone receptors (Sun et al., 2009). LGR5 is a marker of stem cells in various epithelia such as the intestinal mucosa (Schuijers and Clevers, 2012), gastric mucosa (Barker et al., 2010), hair follicles (Jaks et al., 2008) and kidneys (Barker et al., 2012). In mammary epithelium Lgr5+ cells contribute to both luminal and basal epithelia (de Visser et al., 2012) and are essential to reconstituting mammary glands from single cells (Plaks et al., 2013). In the intestine, LGR5 was shown to be a Wnt target gene, regulating epithelial regeneration with a restricted expression (visualised by in situ hybridisation (ISH)) in the intestinal crypt base (Barker et al., 2007; Schuijers and Clevers, 2012). These basal crypt cells, were previously proposed to be an adult intestinal stem cell population, but their formal functional confirmation awaited the discovery of a specific marker (Leushacke and Barker, 2012). Subsequent work on Lgr5+ cells from the intestine, using in vivo lineage tracing and a heritable-inducible lacZ reporter gene, showed that Lgr5+ cells are long-lived, multipotent stem cells (Gerbe et al., 2011), and a single Lgr5+ stem cell can form organoids with a gut-like architecture containing all epithelial cell types (Schuijers and Clevers, 2012).
LGR5 is expressed in the female reproductive organs. Lgr5 marks stem/progenitor cells of the rodent ovary and the oviduct (Flesken-Nikitin et al., 2013; Ng et al., 2014) where it is critical for the maintenance of a functional corpus luteum and therefore, for successful pregnancy (Sun et al., 2014). In immature and in ovarian hormone deprived mice, Lgr5 is highly expressed in the single layer of epithelia lining the uterine cavity and progesterone treatment down-regulated Lgr5, suggesting an ovarian hormonal regulation (Sun et al., 2009; Boretto et al., 2017). However, mice do not menstruate, their oestrous cycle is characterised by complete reabsorption of the endometrial lining and therefore their epithelial regeneration pattern is proposed to be distinct from women (Gargett et al., 2016). In the human ovary and distal Fallopian tube (fimbriae), LGR5 expression was confirmed by quantitative reverse transcription PCR (qRT-PCR) (Ng et al., 2014), with constitutive LGR5 mRNA expression reported in healthy human endometrial epithelium throughout the menstrual cycle (Schuijers and Clevers, 2012; Krusche et al., 2007).
The specificity of the available anti-human-LGR5 antibodies is disputed and in general, the antibody based protein expression data do not correlate with RNA data (Munoz et al., 2012). Thus, ISH is considered as the gold standard to detect LGR5 expressing cells in a solid tissue (Munoz et al., 2012). Therefore in the human endometrium, antibody based studies need further validation (Cervello et al., 2017; Gil-Sanchis et al., 2013) to confirm the exact LGR5 expressing cell population and to elucidate the function and regulation of the LGR5 gene in those cells.
We examined the cellular localisation of LGR5 in all epithelial compartments of the human endometrium by ISH. As the human Fallopian tube shares the same embryological origin and exists as a continuum with the endometrium, we compared the expression of LGR5 in the epithelial mucosa of the endometrium with that of the fimbrial end of the Fallopian tube (due to its known stem cell enrichment (Auersperg, 2013)) and Lgr5 expression (Ng et al., 2014). The hormone regulation of LGR5 in the endometrium was also studied in vitro and in vivo. Finally, published microarray datasets were interrogated to confirm LGR5 expression and its progesterone regulation in the endometrium.
Materials and Methods
Human tissue
Human endometrium and tubal fimbriae was obtained from 50 women undergoing benign gynaecological surgery with no endometrial pathology at the Liverpool Women’s hospital (Supplementary Table SI), granted under Local Research Ethics (REC references; 09/H1005/55 and 11/H1005/4). Informed consent was obtained from all patients.
The cycle phase of the endometrium was assigned according to the last menstrual period and histological criteria (Noyes et al., 1975; Dallenbach-Hellweg, 2012). Endometrium and the distal (fimbrial) end of the Fallopian tube samples were divided in to two pieces; one was fixed (⩾24 h in 4% (v/v) buffered formalin) and paraffin-embedded for ISH and immunohistochemical (IHC) staining, and the other immediately placed in to RNAlater (Sigma, Dorset, UK) for RNA extraction and qRT-PCR. A further six endometrial samples from the proliferative phase of the cycle were collected in reduced serum (1%) Dulbecco’s modified Eagle’s medium (DMEM)/F12 media for short-term explant culture. ISH and IHC staining for all antibodies was analysed with specific reference to the three different endometrial epithelial compartments, the luminal epithelium (the single layer of cells that forms the luminal surface or lining of the uterine cavity), the stratum functionalis (glands in the upper two-thirds of the endometrium below the luminal epithelium, surrounded by sparse stroma) and the stratum basalis (glands in the lower one-third of the endometrium adjacent to the endo-myometrial junction, surrounded by densely packed stroma) in full-thickness endometrial tissue sections. Sequential sections were stained with pancytokeratin to confirm the assignment of epithelial compartment.
qRT-PCR
Total RNA from tissue samples was extracted using TRIzol Plus RNA Purification System (Life Technologies, Paisley, UK), and quantified by NanoDrop ND-1000 (Thermo Fisher Scientific, Loughborough, UK). Total RNA was reverse transcribed using AMV First Strand cDNA synthesis kit (New England Bio Labs, Hertfordshire, UK) after DNase treatment (DNase I (#M0303), New England Bio Labs, Hertfordshire, UK), using the manufacturer’s protocol as previously described (Kamal et al., 2016). cDNA was amplified by qPCR using iTaq SYBR Green supermix (Biorad) with the Biorad connect and the following primers: LGR5 forward (5′-CCTGCTTGACTTTGAGGAAGACC), LGR5 reverse (5′-CCAGCCATCAAGCAGGTGTTCA), GAPDH forward (5′-AATCCCATCACCATCTTCCA) and GAPDH reverse (5′-TGGACTCCACGACGTACTCA). Relative transcript expression was calculated using the ΔΔCT method, normalised to the reference gene GAPDH, using Biorad CFX manager.
ISH
ISH for LGR5 expression was performed as previously described (Baker et al., 2015) using the RNAscope 2.5 High Definition Brown assay according to the manufacturer’s instructions (Advanced Cell Diagnostics, Hayward, CA) as detailed in supplementary methods. RNAscope probes used were LGR5 (NM_003667.2, region 560–1589, catalogue number 311021), POLR2A (positive control probe, NM_000937.4, region 2514–3433, catalogue number 310451) and dapB (negative control probe, EF191515, region 414–862, catalogue number 310043) (Supplementary Fig. S1). LGR5 expression was quantified according to the five-grade scoring system recommended by the manufacturer previously described (Baker et al., 2015) (0 = No staining or less than 1 dot to every 10 cells (40× magnification), 1 = 1–3 dots/cell (visible at 20–40× magnification), 2 = 4–10 dots/cell, very few dot clusters (visible at 20–40× magnification), 3 = >10 dots/cell, less than 10% positive cells have dot clusters (visible at 20× magnification), 4 = >10 dots/cell, more than 10% positive cells have dot clusters (visible at 20× magnification)).
IHC
IHC was performed on sequential tissue sections according to standard protocol as previously described (Valentijn et al., 2013). Primary antibodies (mouse pan-monoclonal anti-cytokeratin (C2562, Sigma-Aldrich, Dorset, UK) at 1:4000, goat polyclonal anti-SOX9 (af3075, R&D Systems, Abingdon,UK) at 1:400, mouse monoclonal anti-Ki67 (NCL-Ki67-MM1, Novocastra, Newcastle, UK) at 1:200, mouse monoclonal anti-SSEA-1 (125601/2, Biolegend, San Diego, CA) at 1:800 dilution) were incubated overnight at 4°C in a humidified chamber. All slides were scanned using an Aperio CS2 scanner (http://www.leicabiosystems.com/digital-pathology/aperio-digital-pathology-slide-scanners/products/aperio-cs2/) and analysed using spectrum, ScanScope®.
Analysis of IHC
Percentage of nuclear Ki67 immuno-positive cells of any intensity was evaluated as the Ki67-labelling index (Ki67-LI) (Al Kushi et al., 2002; Kamal et al., 2016) and the three epithelial compartments were scored separately. SOX9 and SSEA-1 immunostaining was assessed as previously described (Valentijn et al., 2013), and detailed in Supplementary methods.
Explant culture
Endometrial explant cultures were prepared from freshly collected endometrial biopsies and treated with 1 μM medroxyprogesterone acetate (MPA) or ethanol (vehicle control) for 24 h as previously described(Valentijn et al., 2015). Harvested tissue after treatment was washed with PBS, immersed in RNAlater and frozen for qRT-PCR (Valentijn et al., 2015).
Systems biology
We extended our experimental data by examining all published microarray datasets of normal, premenopausal endometrial samples from women not on hormonal treatments to explore progesterone regulation of the LGR5 gene in the secretory compared with the proliferative menstrual cycle phase (n = 65) (Talbi et al., 2006; Burney et al., 2007; Nguyen et al., 2012; Sigurgeirsson et al., 2017) and in the sorted healthy normal endometrial epithelial side population cells that enrich for the endometrial epithelial stem cell population, against unsorted epithelial cells (Cervello et al., 2010) (n = 8/group). The in silico methodology using oPPOSUM (http://www.cisreg.ca/oPOSSUM/), Con Tra V3, illumina’s BaseSpace Correlation Engine (BSCE; (Kupershmidt et al., 2010) software; https://www.illumina.com/informatics/research/biological-data-interpretation/nextbio.html; Illumina, San Diego, CA, USA) and Ingenuity (IPA) software programmes is detailed in supplementary methods (Supplementary Table II–IV). (Mathew et al., 2016; Broos et al., 2011; Kupershmidt et al., 2010; Cervello et al., 2010; Burney et al., 2007; Talbi et al., 2006; Nguyen et al., 2012; Sigurgeirsson et al., 2017)
Statistical methods
All statistical analyses were performed using GraphPad Prism software (Mann–Whitney U and one-way ANOVA was used to assess differences between groups). Spearman rank correlation was used to determine the association between pairs of variables. The criterion for significance was P ≤ 0.05.
Results
Healthy human premenopausal endometrium demonstrated dynamic spatiotemporal regulation of LGR5 expression with high LGR5 expressing cells in the luminal and in the stratum basalis epithelium
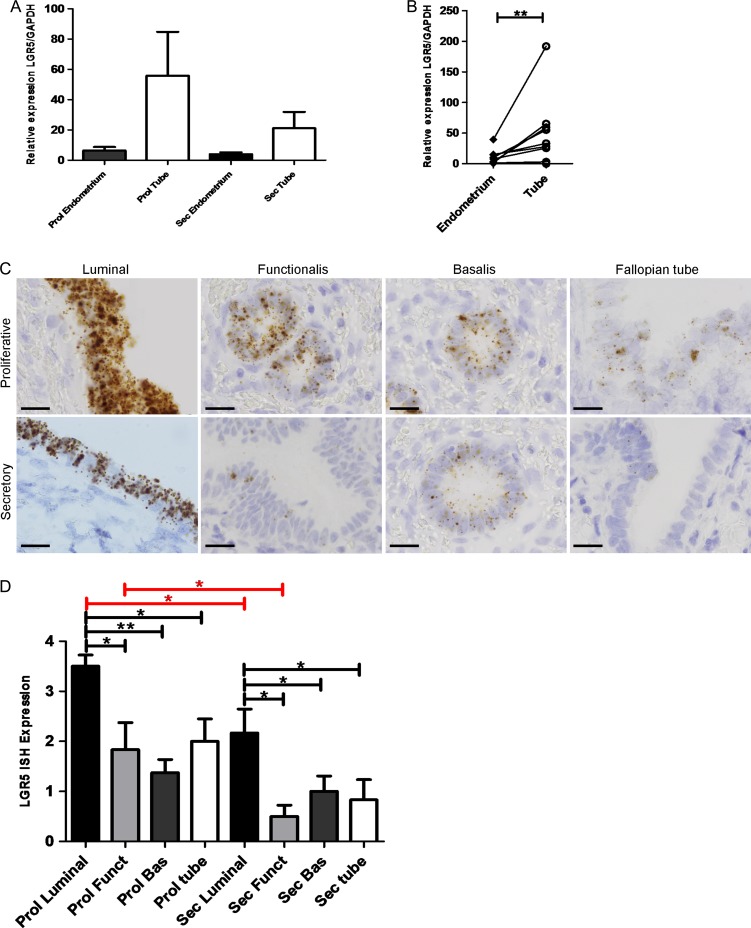
Full thickness whole endometrial tissue samples containing all endometrial layers and cell types from the oestrogen dominant, proliferative phase of the cycle showed a trend of higher LGR5 mRNA expression levels (as measured by qRT-PCR) compared with the samples from the progesterone dominant secretory phase of the menstrual cycle (P = 0.5, Fig. 1A). LGR5 mRNA levels were significantly higher in the stem cell rich distal Fallopian tubes than in the corresponding eutopic endometrium (P < 0.01, Fig. 1B). The cell type expressing LGR5 was identified with ISH, demonstrating that LGR5 expression was limited to the epithelial compartment in both endometrium and tube. Semi-quantitative scoring of LGR5 expression revealed that the luminal epithelial cells expressed significantly higher levels of LGR5 than all other epithelial compartments in the endometrium (P < 0.05, Fig. 1C and D) including the endometrial stratum basalis. The reduction in LGR5 expression in the secretory phase was confirmed with ISH in the luminal (P = 0.03) and functionalis epithelium (P = 0.04) respectively (Fig. 1C and D).
Figure 1.
LGR5 gene expression appears to decrease in the eutopic endometrium and Fallopian tube in the secretory phase of the menstrual cycle. (A) The eutopic endometrial samples and fallopian tube express apparently decreased levels of LGR5 mRNA in the secretory phase of the menstrual cycle when compared with the proliferative phase (n = 21). (B) Fallopian tube (at any stage of the cycle) demonstrate significantly higher levels of LGR5 mRNA than matched eutopic endometrium (P < 0.01) (n = 20). (C) Representative LGR5 ISH images of Fallopian tube and luminal, stratum functionalis and stratum basalis eutopic endometrium in the proliferative and secretory stages of the menstrual cycle (All images ×1000, scale bar = 20 μm, (n = 15)). (D) Graphical representation of semi-quantitative scoring of LGR5 ISH In the proliferative stage of the cycle, the luminal epithelium demonstrated significantly higher LGR5 ISH staining scores than the functionalis (P < 0.05), basalis epithelium (P < 0.01) and Fallopian tube (P < 0.02) as well as the luminal epithelium of the secretory phase (P < 0.03); the proliferative functionalis had significantly higher LGR5 scores than the secretory functionalis (P < 0.04); the secretory luminal epithelium showed significantly higher LGR5 scores than the epithelia of secretory stratum functionalis (P < 0.03), secretory stratum basalis (P < 0.05) and Fallopian tube (P < 0.02) (n = 7 per group) (Prol = Proliferative, Sec = Secretory Funct = stratum Functionalis, Bas = stratum Basalis).
LGR5 expression correlated with endometrial epithelial cell proliferation in the stratum functionalis epithelial compartment
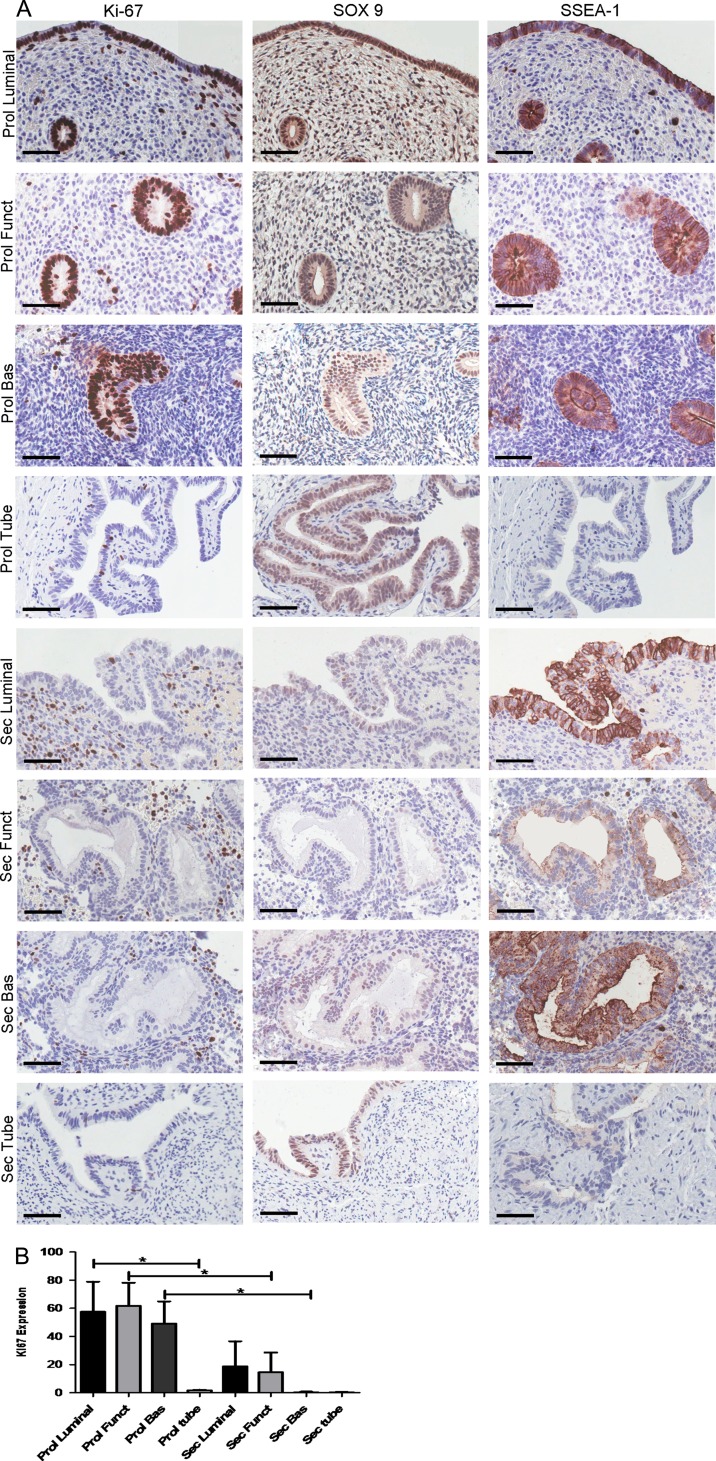
The differences in the cellular proliferative activity in the three endometrial epithelial compartments across the menstrual cycle were demonstrated by the dynamic changes in the expression of the proliferative marker Ki67 (Fig. 2A and B) in sequential tissue sections. Epithelial Ki67-LI was highest in the proliferative phase with the maximum Ki67-LI observed in the cells of stratum functionalis glands (median 70%, range 10–100%) and the lowest Ki67-LI seen in the stratum basalis epithelium (median 30%, range 0–85%). Ki67-LI was higher in the stratum functionalis and in luminal epithelium compared with the stratum basalis glands in all phases of the cycle. In the secretory phase, Ki67-LI in all epithelial compartments decreased, with the luminal epithelial compartment demonstrating the highest Ki67-LI (median 1%, range 0–90%) and Ki67-LI was absent in the stratum basalis glands. Ki67-LI and LGR5 expression levels only correlated significantly (r = 0.74, P = 0.01) in the stratum functionalis epithelium. The stratum basalis LGR5 expression persisted in the secretory phase (Fig. 1D) while the corresponding Ki67-LI reactivity decreased significantly (P = 0.03) (Fig. 2B). The quiescent (absent Ki67-LI) atrophic postmenopausal endometrial epithelium also expressed LGR5 (particularly the luminal epithelium) (Supplementary Fig. S2).
Figure 2.
Ki67-Labelling Index (Ki67-LI) correlated with endometrial epithelial cell proliferation only in the epithelial compartment of the stratum functionalis. Epithelial LGR5 expression scores also correlated with the expression of the previously known progenitor markers SOX9 and SSEA-1 in sequential tissue sections across the cycle. (A) Representative images of Ki67, SOX9 and SSEA-1 IHC in luminal, stratum functionalis, stratum basalis epithelial compartments of the eutopic endometrium and Fallopian tubes in the proliferative and secretory stages of the menstrual cycle (all images ×400, scale bar 10 μm). (B) Quantification of percentage Ki67-positive cells (Ki-67 LI) throughout the cycle. A minimum of 25 fields of cells were counted at ×400 magnification (n = 7 per group). The stratum functionalis in the proliferative phase has the highest Ki67-LI and is statistically higher than the stratum functionalis epithelium in the secretory phase (P < 0.05). Ki67-LI decreased dramatically in the secretory phase of the cycle in all epithelial compartments (Prol = Proliferative, Sec = Secretory Funct = stratum Functionalis, Bas = stratum Basalis).
Epithelial proliferation (Ki67-LI) in the Fallopian tube was consistently very low throughout the cycle, contrasting with the dynamic tubal LGR5 expression pattern (r = 0.23, P = 0.55).
In the human endometrium, luminal and basalis epithelia share distinct patterns of co-expression of LGR5 and the previously known progenitor markers SSEA-1 and SOX9
Sequential tissue sections were employed to examine if the cellular location of LGR5 mRNA (by ISH) was consistent with the expression of previously described endometrial basalis progenitor markers SSEA-1 and nuclear SOX9 (by IHC, Fig. 2A). In general, SOX9 and SSEA-1 expression followed the same cyclical pattern of expression as LGR5: levels decreased in all three endometrial epithelial compartments and also in the tubal epithelium in the secretory phase when compared with the samples from the proliferative phase (Fig. 2A). However, out of all three endometrial epithelial compartments, the strongest LGR5 expression was seen in the luminal epithelium (Fig. 1C and D) whereas the strongest SSEA-1 and SOX9 staining was observed in the stratum basalis glands agreeing with previous reports (Valentijn et al., 2013) (Fig. 2A). It is noteworthy that the luminal staining for both SSEA-1 and SOX9 was consistently high throughout the cycle, even when their expression decreased in the stratum functionalis epithelium in the secretory phase (Fig. 2A).
In the Fallopian tube, SOX9 staining scores and LGR5 ISH scores were high throughout the menstrual cycle, similar to the stratum basalis glands of the endometrium with only an apparent reduction in the intensity during the secretory phase (Fig. 1D). In contrast, SSEA-1 scores were very low in the tubal epithelium in all phases of the cycle. The co-expression of SSEA-1 protein and LGR5 mRNA by ISH was further confirmed with immunofluorescence staining (Supplementary Fig. S3).
Progestogens regulate LGR5 expression In vitro and in vivo
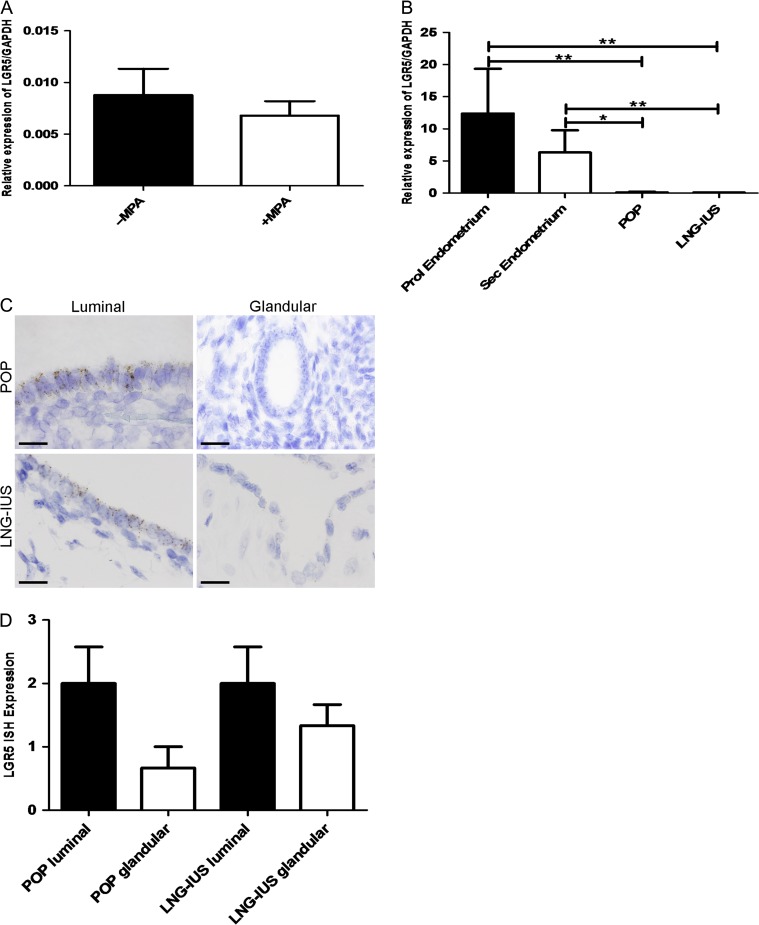
The progestogenic regulation of LGR5 expression was examined by treating endometrial explants in vitro with the synthetic progestogen MPA in short-term culture and MPA treatment decreased LGR5 levels by 1.5-fold (Fig. 3A).
Figure 3.
Progestagens regulate LGR5 expression In vitro and In vivo. (A) LGR5 mRNA expression levels analysed by qRT-PCR. Explants treated with MPA (‘+MPA’) expressed lower LGR5 levels relative to GAPDH when compared with the same of vehicle-treated explants (‘−MPA’) (n = 6 per group). (B) LGR5 mRNA expression by qRT-PCR. Patients taking the oral progesterone only pill (POP) or having the levonorgestrel-releasing intrauterine system (LNG-IUS) have significantly less LGR5 mRNA expression relative to GAPDH when compared with normal eutopic proliferative and secretory endometrium (P < 0.01 and P < 0.04, respectively). Untreated and progesterone treated (POP/LNG-IUS) (n = 6 per group). (C) Representative LGR5 ISH images of POP treated and LNG-IUS treated luminal and glandular eutopic endometrium (All images ×1000, scale bar = 20 μm). (D) Graphical representation of semi-quantitative scoring of LGR5 ISH. The luminal epithelium has more LGR5 when compared with the glandular epithelium in the POP and LNG-IUS treated samples (n = 6 per group).
The in vivo effect of progestogens on the endometrial expression of LGR5, was tested in endometrial samples from women taking synthetic progestogen treatment (progesterone only pill, ‘POP’, or levonorgestrel-releasing intrauterine system, ‘LNG-IUS’) and a significant reduction of LGR5 mRNA levels was observed with progestogen treatment compared with the normal eutopic endometrium of women not on hormonal treatments (P < 0.01, Fig. 3B). Even with these very low levels, the luminal epithelium continued to retain higher LGR5 expression than the glands following progestogen treatment (Fig. 3C and D).
LGR5 expression did not correlate with epithelial cell proliferation in progesterone treated human endometrial samples
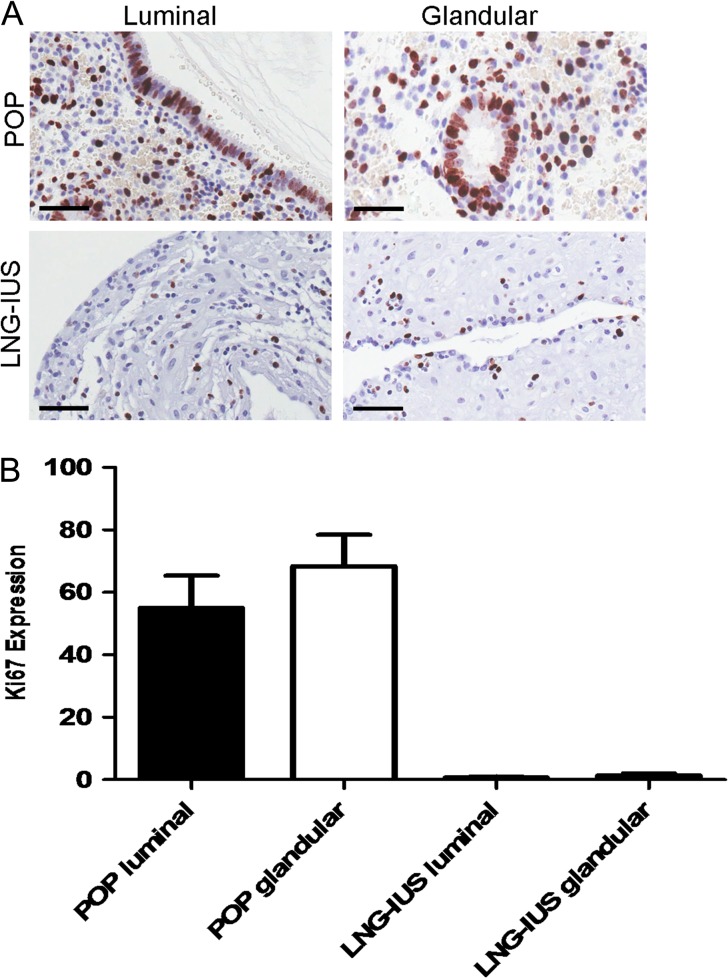
The luminal and glandular epithelial Ki67-LI was much higher in the POP samples when compared to the LNG-IUS treated endometrium (POP luminal median 50%, range 40–75%, glands median 70%, range 50–85%, LNG-IUS luminal median 1%, range 0–1%, glands median 2% range 0–2%, Fig. 4A and B). Therefore, the Ki67-LI levels in the POP treated samples did not correlate with the levels of LGR5 expression, whereas in the atrophic glandular and luminal epithelial cells of the LNG-IUS samples there was low levels of both LGR5 and Ki67-LI.
Figure 4.
LGR5 expression did not correlate with epithelial cell proliferation in progesterone treated human endometrial samples. (A) Ki67-LI IHC data, in the progesterone only pill (POP) group, the glandular and luminal epithelium showed high Ki67-LI but the levonorgestrel-releasing intrauterine system (LNG-IUS) group endometrial Ki67-LI was very low. (B) Representative Ki67 images of luminal and glandular eutopic endometrium from patients treated with either POP or LNG-IUS (all images ×400, scale bar = 10 μm, n = 6 per group).
In silico analysis of published microarray data revealed potential LGR5 regulating genes confirming progestagenic control
One hundred and thirty-three out of the 331 potential LGR5 regulating transcription factors (TFs) were differentially regulated in the progesterone dominant secretory endometrium when compared with the proliferative endometrium (Supplementary Tables SII and III), supporting a role for progesterone in the regulation of LGR5 expression. The LGR5 gene promoter has high-affinity binding sites for the progesterone receptor, suggesting a direct regulation. The sorted human endometrial epithelial side population cells (enriched for stem cells) showed differential expression of 48 TFs that potentially regulate LGR5 compared with the unsorted differentiated epithelial cells (Supplementary Table SIV). The analysis of the upstream regulating drugs and chemicals in IPA core analysis of the LGR5 regulating genes that are differentially expressed in both side population epithelial cells and in the secretory phase endometrium identified progesterone, confirming our in vitro and in vivo wet-lab data (Supplementary Fig. S4) suggesting a role for progesterone in LGR5 gene expression.
Discussion
This is the first comprehensive study employing the gold standard method; ISH, in order to examine the cellular location of LGR5 expression in full thickness normal human endometrium. High LGR5 expressing cells were seen in the endometrial luminal epithelium and in the stratum basalis. Healthy human endometrium shows a dynamic spatiotemporal pattern of LGR5 expression, suggesting hormonal regulation. Endogenous and exogenous progestogens appear to inhibit LGR5 expression in the endometrium both in vitro and in vivo, and these data and in silico analysis of published endometrial microarray datasets were in agreement.
Previous evidence from other epithelial tissues proposes LGR5 expression to be limited to stem cells and thus for LGR5 to be an epithelial stem cell marker (Kumar et al., 2014). We have shown that LGR5 was not localised to a small number of cells in the adult endometrial basalis epithelium; the proposed location of the stem cell niche (Valentijn et al., 2013; Gargett et al., 2016). The LGR5 expression we have seen in the human adult endometrium, unlike in the small intestine, mimics the Lgr5 expression pattern seen in mouse uteri (Sun et al., 2009). A uniform expression of Lgr5 was seen in the ovariectomised uterine epithelium and it is suggested that most of these remaining epithelial cells have the potential to proliferate when necessary for uterine glandular growth (Sun et al., 2009). A mouse endometrial epithelial organoid system, which allowed long-term expansion of epithelium, also showed Lgr5 gene expression (Boretto et al., 2017). In contrast, in humans, the whole of the endometrial functional layer is regularly shed with menstruation, a phenomenon not relevant to most mammals including rodents. The initial step of the embryo attachment and implantation occurs at the luminal epithelium, which exists at a relatively distant location in cellular terms from the stratum basalis (up to 16 mm in the mid-secretory phase). Due to external assaults such as mechanical friction or infection, cells are continually lost and replaced from the surface of any epithelial tissue including the skin and intestine (Barker, 2014); therefore a similar daily cellular loss is likely to happen at the endometrial luminal epithelium which is exposed to the uterine cavity and external environment. The daily maintenance of this luminal epithelium may require locally positioned cells with progenitor ability. Supporting this hypothesis, rapid Lgr5+ epithelial cell proliferation can be observed in many other organs upon tissue damage (Beumer and Clevers, 2016; Ng et al., 2014).
We therefore hypothesise that it is possible for the human endometrium to have more than one epithelial stem/progenitor cell pool; one residing in the basalis (SSEA-1++SOX9++LGR5+) supporting the massive regeneration of the functionalis after menstrual shedding or parturition; while the other (LGR5++SSEA-1+SOX9+) supports the embryo-implantation process, and maintains the luminal epithelial cells that are likely to be lost on a daily basis (Fig. 5). This is in agreement with the scanning electron microscopy studies of human endometrium, the endometrial injury model of the rabbit (Ferenczy and Richart, 1974) and neo-natal endometrial glandular development in humans (Cooke et al., 2013). The persistent expression of the progenitor cell markers SOX9 and SSEA-1 in the luminal epithelia, with concomitant high LGR5 expression, corroborate further with the above hypothesis (Barker and Clevers, 2010; Valentijn et al., 2013). Future work examining the functional properties of endometrial epithelial cell subpopulations that are isolated from the two anatomical regions within the human endometrium, which express either high or low LGR5, SOX9 and SSEA-1, is warranted.
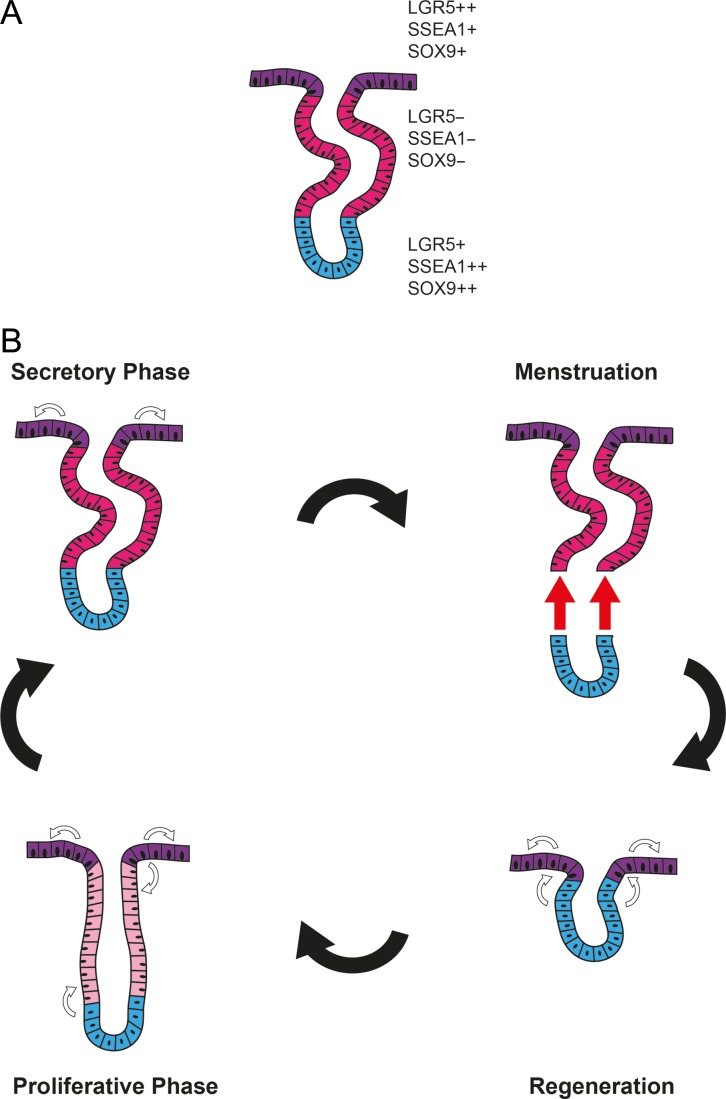
Figure 5.
Putative model of endometrial epithelial regeneration. (A) The secretory phase endometrial epithelial configuration; luminal epithelia contains LGR5++SOX9+SSEA-1+ cells, functional glands contain LGR5-SOX9-SSEA-1- epithelial cells and basalis glandular cells with SOX9++SSEA-1++LGR5+ phenotype. (B) Luminal and functional layers are shed at menstruation, luminal epithelium regenerated from the stratum basalis epithelium (SOX9++SSEA-1++LGR5+) after menstrual shedding, subsequently, the luminal epithelium (LGR5++SOX9+SSEA-1+) throughout the cycle regenerates itself and possibly also contributes to the regeneration of functional glands in the proliferative phase (LGR5+SOX9+SSEA-1+) whilst stratum basalis glands are responsible for the regeneration of all/most of the epithelia of the stratum functionalis in the proliferative phase.
The two antibody based studies examining LGR5 in the human endometrium (Gil-Sanchis et al., 2013; Cervello et al., 2017) are in contrast to our work, in that we do not detect LGR5 expression in the stroma but only in the pancytokeratin expressing epithelial cells and the observed proportion of epithelial cells expressing LGR5 exceeded 1%. It should be noted that mRNA and protein levels may not necessarily correlate, and in our hands, the endometrial LGR5 protein expression using two commercially available antibodies, demonstrated non-specific staining (Supplementary Fig. S5). Therefore, agreeing with the general consensus, we concluded that the reliability of antibodies against LGR5 remains in considerable doubt.
Suppression of glandular regeneration and progenitor activity is postulated to occur within the progesterone-dominant, non-proliferative, secretory functional epithelium, where the lowest LGR5 expression levels were observed. This is in agreement with a possible high LGR5-related stem/progenitor cell function and concurs with the in silico study demonstrating the differential expression of many potential regulators of LGR5 gene in the stem cell enriched endometrial epithelial side population cells. Our interrogation of the published microarray datasets, also sought further information on the effect of progesterone on endometrial LGR5 gene expression. We identified binding sites for progesterone, oestrogen and androgen receptors in the LGR5 gene promoter and potential other LGR5 gene regulators were also differentially expressed in the secretory phase endometrium. IPA core analysis re-confirmed the direct influence of progesterone on many of the identified differentially expressed LGR5 regulators in the secretory endometrium. Considering the intricate relationship between steroid hormone receptors and their function, our experimental and in silico analysis data thus suggest that progesterone may directly and also indirectly regulate LGR5 via downstream target genes. Endometrial epithelial differentiation, proliferative quiescence and inhibition of the canonical Wnt pathway in the stratum functionalis layer are known functions of progesterone (Wang et al., 2009) and they were also identified as significant canonical pathways involving the differentially expressed (progesterone regulated), LGR5 regulators in the secretory phase endometrium. This suggests a possible functional involvement of LGR5 in the secretory endometrium that requires exploration in future studies.
In the absence of validated lineage markers for the various epithelial populations that are likely to exist within the endometrium, we cannot formally characterise the resident LGR5+ cells as multipotent. Lineage tracing studies need to be completed in the human endometrial epithelium to identify the location of stem cells, this will further complement the in vitro functional studies to confirm if LGR5 expressing epithelial cells indeed represent the epithelial stem cell population.
Supplementary Material
Acknowledgements
We are grateful for Calypso Dennis, Sarah Northey and Josephine Drury for assistance with qPCR; Amelia Acha-Sagredo and Lakis Liloglou for assistance with ISH; Sofia Makrydima for endometrial explant cultures and John Woodward for assistance with Figure 5.
Authors’ roles
N.T. and D.K.H. conceived the study, designed and performed the experiments, interpreted data, and wrote the first draft of the manuscript. N.A.W. and A.M.B. assisted with ISH experiments and revised the manuscript critically for important intellectual content. All authors revised and read the manuscript and approved the submitted final version.
Funding
This work was supported by funding from the Wellbeing of Women fellowship grant (RTF510 N.T., N.A.W. and D.K.H.) and Cancer Research UK (A14895, A.M.B. and N.A.W.).
Conflict of interest
No conflicts of interest.
References
- Al Kushi A, Lim P, Aquino-Parsons C, Gilks CB. Markers of proliferative activity are predictors of patient outcome for low-grade endometrioid adenocarcinoma but not papillary serous carcinoma of endometrium. Mod Pathol 2002;15:365–371. [DOI] [PubMed] [Google Scholar]
- Auersperg N. The stem-cell profile of ovarian surface epithelium is reproduced in the oviductal fimbriae, with increased stem-cell marker density in distal parts of the fimbriae. Int J Gynecol Pathol 2013;32:444–453. [DOI] [PubMed] [Google Scholar]
- Baker AM, Graham TA, Elia G, Wright NA, Rodriguez-Justo M. Characterization of LGR5 stem cells in colorectal adenomas and carcinomas. Sci Rep 2015;5:8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol 2014;15:19–33. [DOI] [PubMed] [Google Scholar]
- Barker N, Clevers H. Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology 2010;138:1681–1696. [DOI] [PubMed] [Google Scholar]
- Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 2010;6:25–36. [DOI] [PubMed] [Google Scholar]
- Barker N, Rookmaaker MB, Kujala P, Ng A, Leushacke M, Snippert H, van de Wetering M, Tan S, Van Es JH, Huch M et al. Lgr5(+ve) stem/progenitor cells contribute to nephron formation during kidney development. Cell Rep 2012;2:540–552. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007;449:1003–1007. [DOI] [PubMed] [Google Scholar]
- Beumer J, Clevers H. Regulation and plasticity of intestinal stem cells during homeostasis and regeneration. Development 2016;143:3639–3649. [DOI] [PubMed] [Google Scholar]
- Boretto M, Cox B, Noben M, Hendriks N, Fassbender A, Roose H, Amant F, Timmerman D, Tomassetti C, Vanhie A et al. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development 2017;144:1775–1786. [DOI] [PubMed] [Google Scholar]
- Broos S, Hulpiau P, Galle J, Hooghe B, Van Roy F, De Bleser P. ConTra v2: a tool to identify transcription factor binding sites across species, update 2011. Nucleic Acids Res 2011;39:W74–W78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 2007;148:3814–3826. [DOI] [PubMed] [Google Scholar]
- Cervello I, Gil-Sanchis C, Mas A, Delgado-Rosas F, Martinez-Conejero JA, Galan A, Martinez-Romero A, Martinez S, Navarro I, Ferro J et al. Human endometrial side population cells exhibit genotypic, phenotypic and functional features of somatic stem cells. PLoS One 2010;5:e10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervello I, Gil-Sanchis C, Santamaria X, Faus A, Vallve-Juanico J, Diaz-Gimeno P, Genolet O, Pellicer A, Simon C. Leucine-rich repeat-containing G-protein-coupled receptor 5-positive cells in the endometrial stem cell niche. Fertil Steril 2017;107:510–519 e513. [DOI] [PubMed] [Google Scholar]
- Cooke PS, Spencer TE, Bartol FF, Hayashi K. Uterine glands: development, function and experimental model systems. Mol Hum Reprod 2013;19:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallenbach-Hellweg G. Histopathology of the endometrium. New York: Springer, 2012. [Google Scholar]
- de Visser KE, Ciampricotti M, Michalak EM, Tan DW, Speksnijder EN, Hau CS, Clevers H, Barker N, Jonkers J. Developmental stage-specific contribution of LGR5(+) cells to basal and luminal epithelial lineages in the postnatal mammary gland. J Pathol 2012;228:300–309. [DOI] [PubMed] [Google Scholar]
- Ferenczy A, Richart RM. Scanning electron microscopy of human female genital tract. NY State J Med 1974;74:794–802. [PubMed] [Google Scholar]
- Fleischer AC. Sonographic assessment of endometrial disorders. Semin Ultrasound CT MR 1999;20:259–266. [DOI] [PubMed] [Google Scholar]
- Flesken-Nikitin A, Hwang CI, Cheng CY, Michurina TV, Enikolopov G, Nikitin AY. Ovarian surface epithelium at the junction area contains a cancer-prone stem cell niche. Nature 2013;495:241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargett CE, Schwab KE, Deane JA. Endometrial stem/progenitor cells: the first 10 years. Hum Reprod Update 2016;22:137–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbe F, van Es JH, Makrini L, Brulin B, Mellitzer G, Robine S, Romagnolo B, Shroyer NF, Bourgaux JF, Pignodel C et al. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol 2011;192:767–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Sanchis C, Cervello I, Mas A, Faus A, Pellicer A, Simon C. Leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5) as a putative human endometrial stem cell marker. Mol Hum Reprod 2013;19:407–414. [DOI] [PubMed] [Google Scholar]
- Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgard R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet 2008;40:1291–1299. [DOI] [PubMed] [Google Scholar]
- Kamal AM, Bulmer JN, DeCruze SB, Stringfellow HF, Martin-Hirsch P, Hapangama DK. Androgen receptors are acquired by healthy postmenopausal endometrial epithelium and their subsequent loss in endometrial cancer is associated with poor survival. Br J Cancer 2016;114:688–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusche CA, Kroll T, Beier HM, Classen-Linke I. Expression of leucine-rich repeat-containing G-protein-coupled receptors in the human cyclic endometrium. Fertil Steril 2007;87:1428–1437. [DOI] [PubMed] [Google Scholar]
- Kumar KK, Burgess AW, Gulbis JM. Structure and function of LGR5: an enigmatic G-protein coupled receptor marking stem cells. Protein Sci 2014;23:551–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupershmidt I, Su QJ, Grewal A, Sundaresh S, Halperin I, Flynn J, Shekar M, Wang H, Park J, Cui W et al. Ontology-based meta-analysis of global collections of high-throughput public data. PLoS One 2010;5:e13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leushacke M, Barker N. Lgr5 and Lgr6 as markers to study adult stem cell roles in self-renewal and cancer. Oncogene 2012;31:3009–3022. [DOI] [PubMed] [Google Scholar]
- Mathew D, Drury JA, Valentijn AJ, Vasieva O, Hapangama DK. In silico, in vitro and in vivo analysis identifies a potential role for steroid hormone regulation of FOXD3 in endometriosis-associated genes. Hum Reprod 2016;31:345–354. [DOI] [PubMed] [Google Scholar]
- Munoz J, Stange DE, Schepers AG, van de Wetering M, Koo BK, Itzkovitz S, Volckmann R, Kung KS, Koster J, Radulescu S et al. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent ‘+4’ cell markers. EMBO J 2012;31:3079–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng A, Tan S, Singh G, Rizk P, Swathi Y, Tan TZ, Huang RY, Leushacke M, Barker N. Lgr5 marks stem/progenitor cells in ovary and tubal epithelia. Nat Cell Biol 2014;16:745–757. [DOI] [PubMed] [Google Scholar]
- Nguyen HP, Sprung CN, Gargett CE. Differential expression of Wnt signaling molecules between pre- and postmenopausal endometrial epithelial cells suggests a population of putative epithelial stem/progenitor cells reside in the basalis layer. Endocrinology 2012;153:2870–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol 1975;122:262–263. [DOI] [PubMed] [Google Scholar]
- Plaks V, Brenot A, Lawson DA, Linnemann JR, Van Kappel EC, Wong KC, de Sauvage F, Klein OD, Werb Z. Lgr5-expressing cells are sufficient and necessary for postnatal mammary gland organogenesis. Cell Rep 2013;3:70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prianishnikov VA. A functional model of the structure of the epithelium of normal, hyperplastic and malignant human endometrium: a review. Gynecol Oncol 1978;6:420–428. [DOI] [PubMed] [Google Scholar]
- Schuijers J, Clevers H. Adult mammalian stem cells: the role of Wnt, Lgr5 and R-spondins. EMBO J 2012;31:2685–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurgeirsson B, Amark H, Jemt A, Ujvari D, Westgren M, Lundeberg J, Gidlof S. Comprehensive RNA sequencing of healthy human endometrium at two time points of the menstrual cycle. Biol Reprod 2017;96:24–33. [DOI] [PubMed] [Google Scholar]
- Sun X, Jackson L, Dey SK, Daikoku T. In pursuit of leucine-rich repeat-containing G protein-coupled receptor-5 regulation and function in the uterus. Endocrinology 2009;150:5065–5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Terakawa J, Clevers H, Barker N, Daikoku T, Dey SK. Ovarian LGR5 is critical for successful pregnancy. FASEB J 2014;28:2380–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology 2006;147:1097–1121. [DOI] [PubMed] [Google Scholar]
- Turco MY, Gardner L, Hughes J, Cindrova-Davies T, Gomez MJ, Farrell L, Hollinshead M, Marsh SGE, Brosens JJ, Critchley HO et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat Cell Biol 2017;19:568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentijn AJ, Palial K, Al-Lamee H, Tempest N, Drury J, Von Zglinicki T, Saretzki G, Murray P, Gargett CE, Hapangama DK. SSEA-1 isolates human endometrial basal glandular epithelial cells: phenotypic and functional characterization and implications in the pathogenesis of endometriosis. Hum Reprod 2013;28:2695–2708. [DOI] [PubMed] [Google Scholar]
- Valentijn AJ, Saretzki G, Tempest N, Critchley HO, Hapangama DK. Human endometrial epithelial telomerase is important for epithelial proliferation and glandular formation with potential implications in endometriosis. Hum Reprod 2015;30:2816–2828. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hanifi-Moghaddam P, Hanekamp EE, Kloosterboer HJ, Franken P, Veldscholte J, van Doorn HC, Ewing PC, Kim JJ, Grootegoed JA et al. Progesterone inhibition of Wnt/β-catenin signaling in normal endometrium and endometrial cancer. Clin Cancer Res 2009;15:5784–5793. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.