Abstract
Inhibition of peripheral inflammatory disease by carbohydrate antigens derived from normal gut microbiota has been demonstrated for the GI tract, brain, peritoneum, and most recently the airway. We have demonstrated that polysaccharide A (PSA) from the commensal organism Bacteroides fragilis activates CD4+ T cells upon presentation by the class II major histocompatibility complex, and that these PSA-experienced T cells prevent the development of lung inflammation in murine models. While the PSA-responding T cells themselves are not canonical FoxP3+ regulatory T cells (Tregs), their ability to prevent inflammation is dependent upon the suppressive cytokine IL-10. Using an adoptive T cell transfer approach, we have discovered that PSA-experienced T cells require IL-10 expression by PSA-naïve recipient animals in order to prevent inflammation. A cooperative relationship was found between PSA-activated effector/memory T cells and tissue-resident FoxP3+ Tregs both in vivo and in vitro, and it is this cooperation that enables the suppressive activity of PSA outside of the gut environment where exposure takes place. These findings suggest that carbohydrate antigens from the normal microbiota communicate with peripheral tissues to maintain homeostasis through T cell-to-T cell cooperation.
Keywords: asthma, IL-10, microbiota, polysaccharide, T cell
Introduction
Over the last several decades, molecules derived from the normal gastrointestinal tract (GI) microbiota have been shown to play key roles in promoting overall health. This broad class of symbiosis factors collectively help to maintain immune homeostasis, strengthen border defenses, and exclude potentially harmful organisms. One such symbiosis factor family, collectively known as glycoantigens, are exemplified by the capsular polysaccharide PSA from the commensal gram-negative bacterium Bacteroides fragilis, which has shown a remarkable ability to quell a number of inflammatory conditions in rodent models (Tzianabos et al. 1995, 1998; Holsti et al. 2004; Mazmanian et al. 2008; Ochoa-Reparaz et al. 2010; Johnson et al. 2015a).
The documented decrease in inflammatory and autoimmune disease susceptibility which results from commensal microbial immunologic responses has largely been attributed to CD25+FoxP3+ regulatory T cells (Tregs) (Rudensky 2011; Sakaguchi et al. 2013). Although gut-associated FoxP3− Tregs are well known (Battaglia et al. 2006), the loss of functional FoxP3, whether through murine genetic manipulation or in the human disease called IPEX, results in severe autoimmune pathology (Bacchetta et al. 2006). Thus, it is fair to say that FoxP3+ Tregs are critical for the establishment and maintenance of immune homeostasis throughout the body downstream of gut exposure to beneficial and commensal microbes. This leads to a widely accepted model in which gut antigens directly induce FoxP3+ Tregs, and that these responding cells are necessary for immune suppression mediated by canonical inhibitory cytokines like IL-10 and TGFβ. However, reports regarding PSA and the downstream gut-localized immune activation complicates this model.
PSA is taken in by antigen presenting cells where it is processed to small fragments and presented at the cell surface via interactions with class II major histocompatibility complex (MHC), making it the first non-proteinaceous antigen to be presented by classical MHCII (Cobb et al. 2004) for activation and subsequent clonal expansion of CD4+ T cells (Johnson et al. 2015b). T cell responses to PSA protect against abscess (Tzianabos et al. 1995, 1998) and surgical adhesion formation (Holsti et al. 2004), several models of inflammatory bowel disease (IBD) (Mazmanian et al. 2008), experimental autoimmune encephalomyelitis (EAE) (Ochoa-Reparaz et al. 2010), and most recently airway inflammation (Johnson et al. 2015a) in an IL-10-dependent fashion. However, PSA-responding T cells have long been known to produce high concentrations of both IFNγ and IL-2 (Tzianabos et al. 1999), characteristic of T helper-type I effector cells. We have also shown that depletion of FoxP3+ T cells in adoptive transfer experiments does not reduce the pulmonary inflammation protection afforded by these PSA-experienced T cells in naïve recipients (Johnson et al. 2015a), which directly supports our human observations where PSA failed to induce any FoxP3 expression in responding T cells (Kreisman and Cobb 2011). These findings demonstrate that PSA expands a FoxP3− T cell population in both murine and human systems, which can protect the lung environment through a mechanism that remains to be fully elucidated.
In this study, we employed an adoptive T cell transfer system using wild type and IL-10-deficient backgrounds to show that the IL-10 necessary for the prevention of pulmonary inflammation is not derived from the PSA-experienced T cells. Instead, we found that upon gut exposure to PSA, the responding donor T cells, characterized as being part of a FoxP3−CD45Rblow population (Rblow) of effector T cells, required IL-10 to be produced by the recipient mice. Using IL-10-GFP reporter mice, we further demonstrate that the Rblow PSA-experienced T cells subset selectively induced IL-10 expression and release by recipient, tissue-resident and PSA-naïve FoxP3+ Tregs in vivo and in vitro, and that these Rblow T cells suppress the inflammatory response. Our findings reveal a model of microbiota control over peripheral immune homeostasis through effector T cell-to-Treg communication resulting in IL-10 release and prevention of unwanted pulmonary inflammation.
Results
IL-10 in PSA-experienced T cells is dispensable for protection against pulmonary inflammation
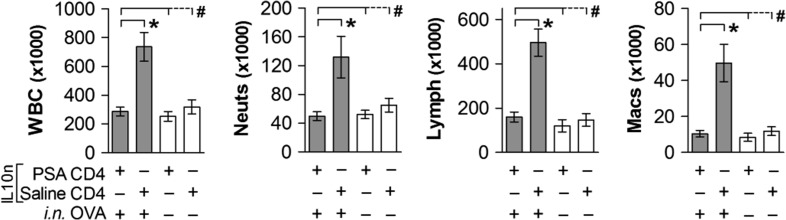
IL-10 has been well established as the key suppressive cytokine downstream of exposure to PSA in humans (Kreisman and Cobb 2011) and in murine lung inflammatory models (Johnson et al. 2015a). In order to determine the source of IL-10 in these systems, we orally gavaged PSA into mice carrying a targeted deletion of the IL-10 locus, harvested their IL-10-null (IL-10n) CD4+ T cells, adoptively transferred them into wild type (WT) PSA-naïve recipients, then induced airway inflammation using a traditional ovalbumin (OVA) model (Johnson et al. 2015a). We reasoned that if PSA-expanded cells were directly responsible for suppression through the production of IL-10, then PSA-experienced IL-10n T cells should be unable to suppress inflammation. We found that despite lacking IL-10, PSA-experienced T cells from IL-10n mice were fully capable of preventing leukocyte infiltration into the airways, as measured by bronchoalveolar lavage fluid (BALF) cell differentials (Figure 1). Total white blood cells (WBC), neutrophils, lymphocytes and monocyte/macrophages were all indistinguishable (P >> 0.05) from the negative non-inflamed controls upon transfer of PSA-experienced but IL-10n CD4+ T cells. Likewise, adoptive transfer of CD4+ T cells from saline-treated mice failed to show protection (Figure 1).
Fig. 1.
IL-10 in PSA-experienced T cells is dispensable for protection against airway leukocyte infiltration. IL-10-null (IL-10n) mice were exposed to PSA or saline via oral gavage. PSA-experienced CD4+ T cells were harvested, and 2 × 106 cells were adoptively transferred into OVA-sensitized WT recipients. On Day 7 of OVA or saline challenge, mice were sacrificed and lungs lavaged with saline. Recovered cells were analyzed by a Hemavet counter and plotted. PSA-experienced IL-10n CD4+ T cells were able to prevent leukocyte infiltration into the airways. * = P < 0.05; # = P > 0.05. P value calculated from Student's T-test. Error bars show mean with SEM.
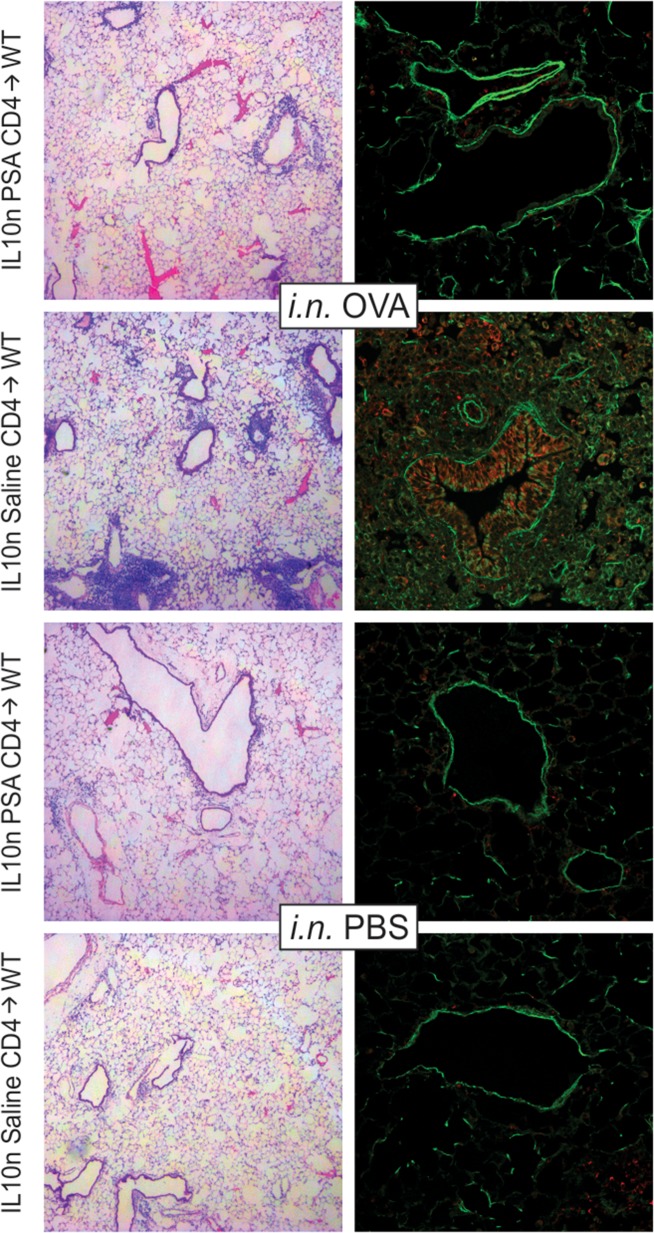
Analysis of tissue pathology by both H&E staining and confocal imaging further revealed that PSA-experienced CD4+ T cells were able to prevent tissue inflammation and airway epithelial cell hyperplasia (Figure 2). Confocal imaging also showed that the airway epithelial cells expressed myeloperoxidase (red; Figure 2), a hallmark of moderate to severe asthma, and that the PSA-experienced but IL-10n T cells robustly prevented this upregulation despite their inability to release IL-10.
Fig. 2.
IL-10 in PSA-experienced T cells is dispensable for protection against tissue inflammation. WT mice receiving 2 × 106 PSA-experienced IL-10n cells as described in Figure 1 were used to harvest lung tissue. Fixed and inflated lungs were sectioned and stained with H&E or EpCAM (green) and myeloperoxidase (red) antibodies. Animals receiving intranasal (i.n.) OVA challenges and saline exposed IL-10n T cells showed myeloperoxidase-positive leukocyte infiltration in and around the major airways, airway epithelial expression of myeloperoxidase and epithelial hyperplasia. PSA-experienced IL-10n T cells, in contrast, prevented these pathologies and returned tissue histology to that seen with saline (PBS) challenged mice. This figure is available in black and white in print and in color at Glycobiology online.
PSA-mediated inflammatory protection requires recipient-derived IL-10
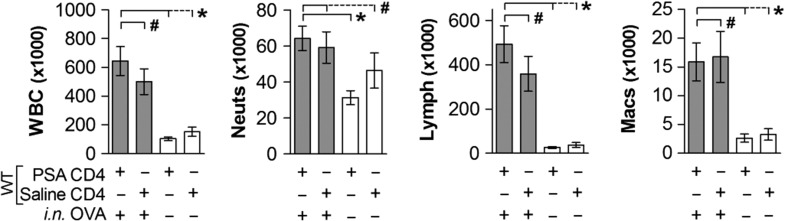
Our previous studies conclusively demonstrated that in the complete absence of IL-10, PSA was incapable of preventing pulmonary inflammation in mice, and was ineffective at suppressive bystander T cell activation in human systems (Kreisman and Cobb 2011; Johnson et al. 2015a); however, the results from our IL-10n transfer studies (Figures 1–2) suggest that either our previous findings were incorrect, or IL-10 is being made by a population other than the PSA-responding CD4+ T cells. In order to distinguish between these possibilities, we performed a reciprocal adoptive transfer experiment in which we gavage PSA into WT mice, and then adoptively transferred their PSA-experienced CD4+ T cell population into PSA-naïve IL-10n recipients. BALF differential analysis of the airways showed that the WT T cells were not capable of suppressing the OVA-induced lung inflammation in IL-10n recipients (Figure 3). In fact, saline and PSA-experienced T cells were indistinguishable from each other (P > 0.05), with both recipient mice showing significantly increased WBCs, neutrophils, lymphocytes and monocyte/macrophages over negative controls (P < 0.05).
Fig. 3.
PSA-mediated inhibition of airway infiltration requires recipient-derived IL-10. WT mice were exposed to PSA or saline via oral gavage. PSA-experienced WT CD4+ T cells were harvested, and 2 × 106 cells were adoptively transferred into OVA-sensitized IL-10n recipients. On Day 7 of OVA or saline challenge, mice were sacrificed and lungs lavaged with saline. Recovered cells were analyzed by a Hemavet counter and plotted. PSA-experienced WT CD4+ T cells failed to prevent leukocyte infiltration into the airways. * = P < 0.05; # = P > 0.05. P value calculated from Student's T-test. Error bars show mean with SEM.
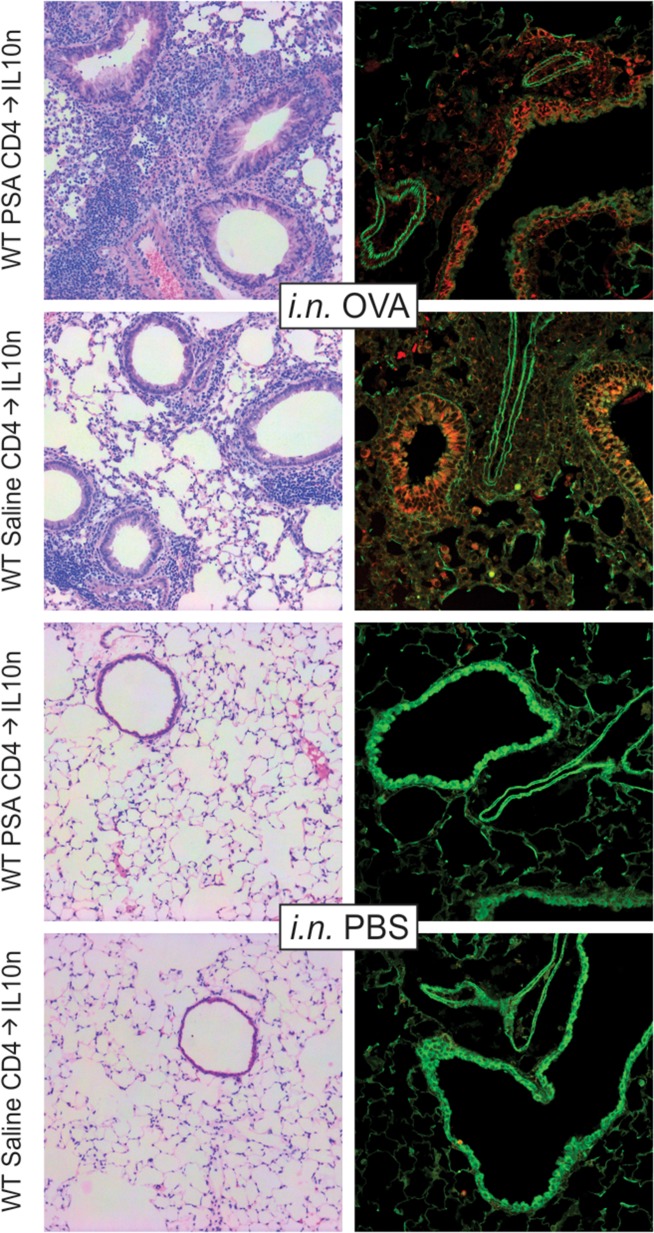
Histological evaluation of these mice by H&E staining and confocal imaging further confirmed the inability of WT PSA-experienced T cells to suppress OVA-induced tissue inflammation, airway epithelial hyperplasia and epithelial myeloperoxidase expression (Figure 4). Indeed, the tissue inflammation is somewhat more severe compared to the reciprocal experiment (Figure 2), likely due to the lack of IL-10 in the recipient animals. These data confirm the requirement for IL-10 in PSA-mediated immune protection of the lung environment shown in our previous studies (Kreisman and Cobb 2011; Johnson et al. 2015a), but also reveal that the IL-10 must be derived from cells in the recipient lung rather than from the PSA-responding CD4+ T cells themselves.
Fig. 4.
PSA-mediated Inhibition of tissue inflammation requires recipient-derived IL-10. IL-10n mice receiving 2 × 106 PSA-experienced WT cells as described in Figure 3 were used to harvest lung tissue. Fixed and inflated lungs were sectioned and stained with H&E or EpCAM (green) and myeloperoxidase (red) antibodies. Animals receiving intranasal (i.n.) OVA challenges and saline exposed WT T cells showed myeloperoxidase-positive leukocyte infiltration in and around the major airways, airway epithelial expression of myeloperoxidase and epithelial hyperplasia. Likewise, PSA-experienced WT T cells failed to prevent these pathologies and did not return tissue histology to that seen with saline (PBS) challenged mice. This figure is available in black and white in print and in color at Glycobiology online.
PSA-experienced T cells induce IL-10 in lung FoxP3+ Tregs
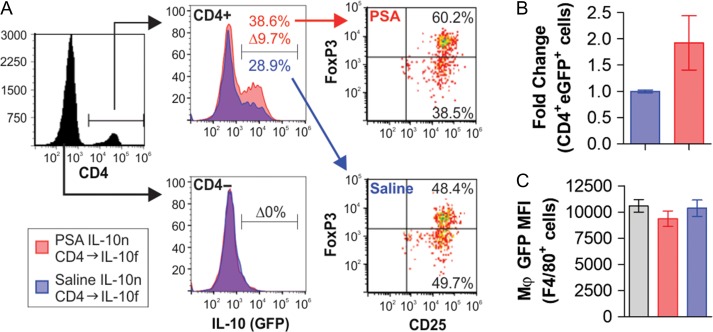
Our findings suggest that PSA-experienced CD4+ T cells induce IL-10 in lung-resident cells of the recipient. Of the candidate cell populations well-documented to produce IL-10 in the lung, alveolar macrophages and FoxP3+ regulatory T cells are the most likely. In order to determine the source of IL-10, we gavaged IL-10n mice with PSA as before (Figure 1), and then adoptively transferred the PSA-experienced CD4+ T cells into IL-10-GFP reporter recipient mice in which GFP expression is controlled by the IL-10 promoter (IL-10f) (Kamanaka et al. 2006). These mice were then subjected to the standard OVA-induced inflammatory model. Upon harvest, lungs were collected for flow cytometric analysis in order identify all IL-10+ (i.e., GFP+) cells. We found that IL-10 expression was increased CD4+ cells, but not CD4– cells (Figure 5A). Adoptive transfer of PSA-experienced cells induced 10% of all CD4+ T cells in the lung to begin expressing IL-10 over background, and that the majority of that increase was accounted for by the FoxP3+ CD25+ population (Figure 5A). The total number of IL-10+CD4+ T cells in the lung increased by a factor of two in mice receiving PSA-experience IL-10n CD4+ T cells (Figure 5B), but no fluorescence over non-reporter WT macrophage fluorescence could be detected (Figure 5C). These data rule out alveolar macrophages and positively identify PSA-naïve FoxP3+ CD25+ Tregs as the IL-10-producing cells which are induced to release IL-10 upon adoptive transfer of PSA-experience CD4+ T cells.
Fig. 5.
PSA-experienced T cells induce IL-10 in Lung FoxP3+ Tregs. IL-10n mice were exposed to PSA (red) or saline (blue) via oral gavage. PSA-experienced CD4+ T cells were harvested, and 2 × 106 cells were adoptively transferred into OVA-sensitized IL-10-GFP (IL-10f) reporter recipients. On Day 7 of OVA or saline challenge, mice were sacrificed and lungs dispersed into single cells for flow cytometry. GFP signal was multiplexed by staining recovered cells for CD4, FoxP3 and CD25. (A) Flow analysis of IL-10 expression among CD4+ and CD4– populations, and the breakdown of the IL-10+ cells by FoxP3 and CD25, showing significant skewing towards IL-10 expression in FoxP3+ CD4+ T cells. (B) Accounting for the increase in total CD4+ T cells, the number of IL-10+ T cells was determined and scaled to saline control. (C) Cells were also stained for the macrophage-specific marker F4/80 and compared to lung-isolated cells from non-reporter mice to set a baseline of background fluorescence. No detectable IL-10 was observed. * = P < 0.05; # = P > 0.05. P value calculated from Student's T-test. Error bars show mean with SEM. This figure is available in black and white in print and in color at Glycobiology online.
PSA-experienced T effector cells induce IL-10 in FoxP3+ Tregs in vitro
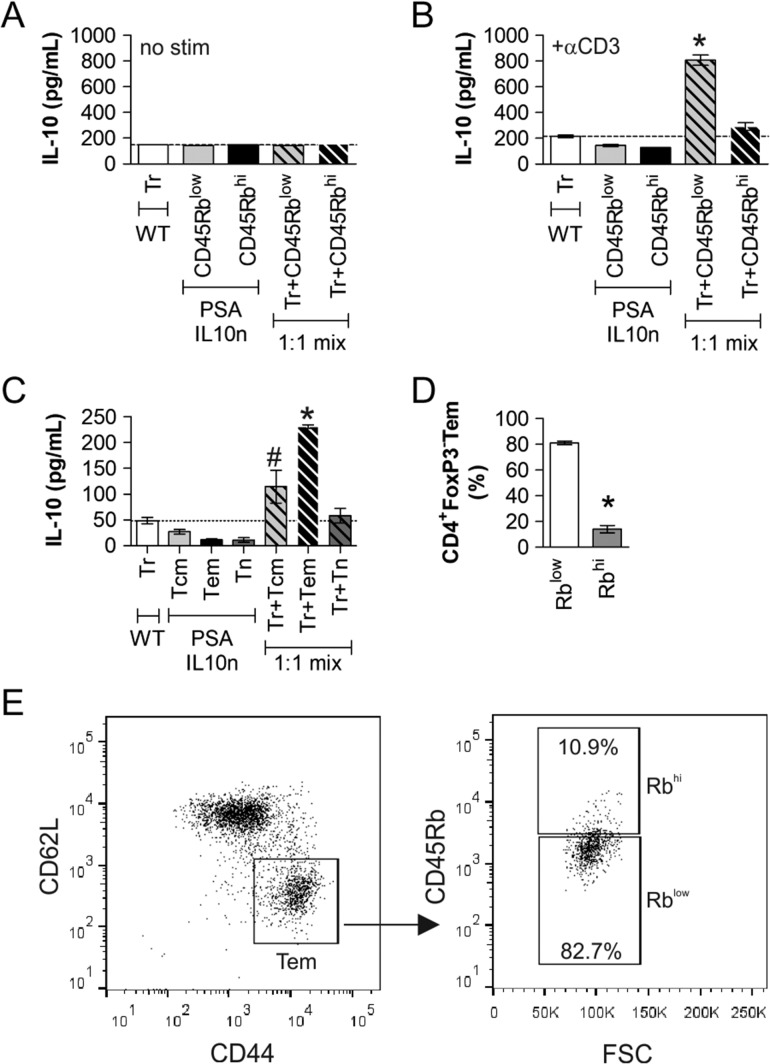
We previously characterized the properties of PSA-responding T cells in both murine and human systems (Kreisman and Cobb 2011; Johnson et al. 2015a). These studies showed these CD4+ T cells lack FoxP3, while expressing IL-10 and IFNγ with a CD45Rblow (Rblow) surface phenotype, suggesting that they share characteristics of both gut-associated Tr1 regulatory T cells and traditional effector T cells. In order to determine whether this subset is primarily responsible for the induction of IL-10 in FoxP3+ Tregs, we devised a 3 day in vitro co-culture assay. We cultured WT PSA-naïve Tregs, IL-10n PSA-experienced CD4+FoxP3−CD45Rblow T cells which are similar to Tr1 cells, and IL-10n PSA-experienced CD4+FoxP3−CD45Rbhi T cells alone or in combination. In the absence of signal through the T cell receptor by plate-bound anti-CD3 antibody, none of these cells produced detectable IL-10 alone or in combination (Figure 6A). Upon stimulation with anti-CD3 antibody, again, none of the cells produced IL-10 alone, but when WT FoxP3+ Tregs were co-cultured at a 1:1 ratio with PSA-experienced CD4+FoxP3−CD45Rblow T cells, robust IL-10 was released (Figure 6B). A similar effect was not seen in co-cultures containing CD4+FoxP3−CD45Rbhi T cells with Tregs, suggesting this activity is a specific property of the CD4+FoxP3−CD45Rblow T cells. In addition, the IL-10 is being produced by the Tregs because these are the only cells in the system with a normal IL-10 allele, demonstrating the communication present between these cell subsets.
Fig. 6.
IL-10 Production by Tregs is enhanced by CD4+FoxP3–CD45Rblow and CD44+CD62L− T cells. FoxP3+ Tregs (Tr) were isolated from WT (IL-10 normal) FoxP3-GFP reporter mice and cultured alone or together with CD4+ CD45Rbhi or CD4+ CD25-CD45Rblow cells from PSA-exposed IL-10n mice in wells without (A) or with (B) anti-CD3ε antibody. ELISA was used to quantify IL-10 production after 72 h of stimulation. IL-10 was found only in co-cultures of Tr and CD45Rblow cells, showing the induction of IL-10 in the FoxP3+ Treg cells. (C) Similar assays were performed using FoxP3+ Tregs (Tr) with CD25−CD62L+CD44+(Tcm), CD25−CD62L−CD44+ (Tem), and CD25−CD62L+CD44− (Tn) cells from PSA-exposed IL-10n mice. Only the combination of Tr and Tem cells produced significant IL-10. (D–E) Flow cytometric analysis of CD4+FoxP3− cells using CD62L and CD44 to differentiate effector/memory T cells (Tem) and their CD45Rb status revealed that over 80% of Tem cells fall into the CD45Rblow gate. Replicates (D) and representative scatter plots (E) are shown. * = P < 0.05; # = P > 0.05. P value calculated from Student's T-test. Error bars show mean with SEM.
Since it is also established that PSA induces IFNγ in responding cells, placing them into an effector T cell category, we further determined whether PSA-experienced effector/memory T cells (Tem) cells could also induce Tregs to secret IL-10. Using the same approach as before, we found that when in co-culture with Tregs, PSA-experienced Tem cells (CD44+CD62L−) were also capable of communication with Tregs that resulted in IL-10 release (Figure 6C), whereas naïve (Tn; CD44−CD62L+) and central memory (Tcm; CD44+CD62L+) did not have the same effect.
Finally, we used flow cytometry to determine whether these are distinct populations of PSA-experienced T cells, or if the Tem cells were also FoxP3−CD45Rblow. We found that at least 80% of all Tem cells (CD4+FoxP3−CD44+CD62L−) were also CD45Rblow (Figure 6D–E), supporting the notion that PSA exposure activates a CD4+FoxP3−CD45RblowCD44+CD62L− effector/memory T cell population (RblowTem) with regulatory activity in which they communicate with Tregs to release IL-10 and suppress lung inflammation.
PSA-experienced RblowTem cells protect mice from pulmonary inflammation
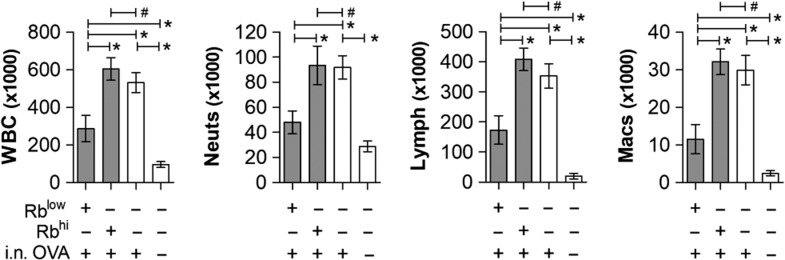
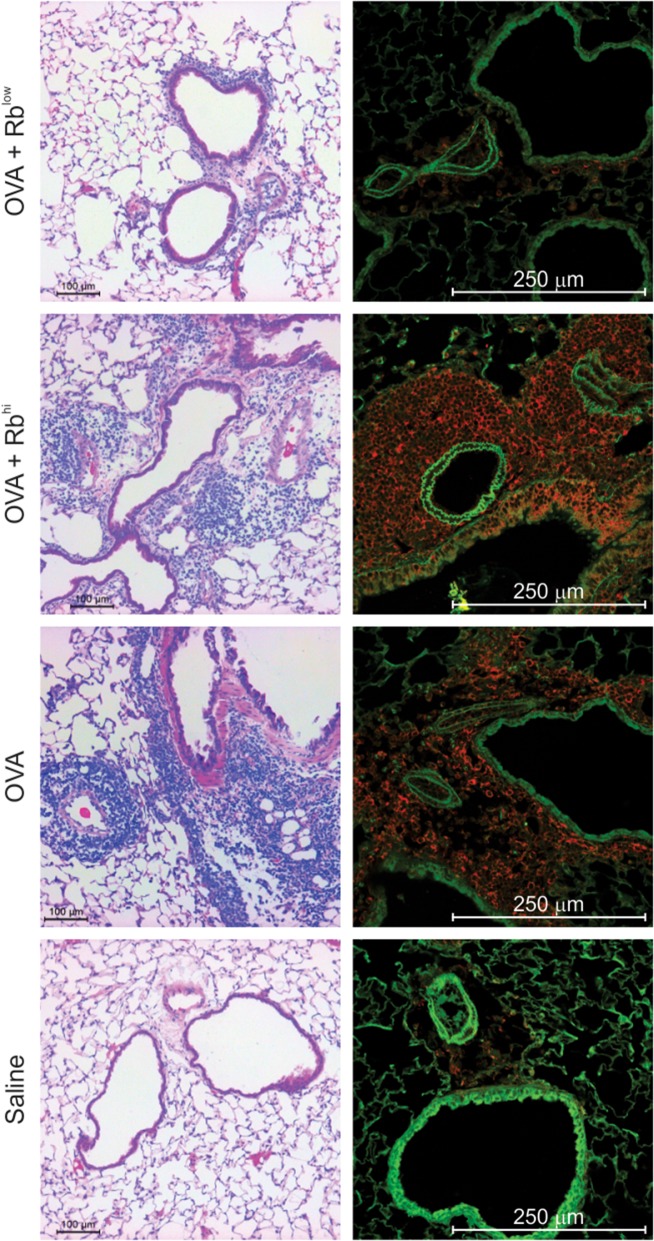
Consistent with our previous findings (Kreisman and Cobb 2011; Johnson et al. 2015a), the co-culture results implicated PSA-experienced RblowTem cells as the key population required for suppression of pulmonary inflammation due to their ability to induce IL-10 in FoxP3+ Tregs. To test this hypothesis, we exposed WT mice to PSA as before, isolated the RblowTem cells by sterile flow cytometric sorting, and adoptively transferred them into WT recipients for OVA-induced inflammation. Upon harvest, BALF cellular differentials showed that the RblowTem cells, but not their Rbhi counterparts, robustly suppressed the infiltration of WBCs, neutrophils, lymphocytes, and monocyte/macrophages (Figure 7). Moreover, tissue pathology by H&E and confocal microscopy confirmed the ability of Rblow but not Rbhi T cells to reduce tissue infiltration, epithelial hyperplasia and myeloperoxidase expression (Figure 8). This inhibitory activity was achieved using 6% of the total number of transferred cells (60,000) compared to the non-differentiated CD4+ T cell transfers used in Figures 1–5 (2 × 106 cells), indicating a 40-fold enrichment of the suppressive activity while confirming the phenotype of the protective PSA-experienced T cells in vivo.
Fig. 7.
PSA-experienced RblowTem cells protect mice from leukocyte airway infiltration. WT mice were exposed to PSA or saline via oral gavage. PSA-experienced CD4+ T cells were harvested, sorted into RblowTem and Rbhi populations, and 6 × 104 cells were adoptively transferred into OVA-sensitized WT recipients. On Day 7 of OVA or saline challenge, mice were sacrificed and lungs lavaged with saline. Recovered cells were analyzed by a Hemavet counter and plotted. PSA-experienced Rblow but not Rbhi T cells were able to prevent leukocyte infiltration into the airways. * = P < 0.05; # = P > 0.05. P value calculated from Student's T-test. Error bars show mean with SEM.
Fig. 8.
PSA-experienced RblowTem cells protect mice from tissue inflammation. WT mice receiving 6 × 104 PSA-experienced Rblow or Rbhi cells as described in Figure 7 were used to harvest lung tissue. Fixed and inflated lungs were sectioned and stained with H&E or EpCAM (green) and myeloperoxidase (red) antibodies. Animals receiving intranasal (i.n.) OVA challenges and saline exposed Rbhi T cells showed myeloperoxidase-positive leukocyte infiltration in and around the major airways, airway epithelial expression of myeloperoxidase, and epithelial hyperplasia. PSA-experienced Rblow T cells, in contrast, prevented these pathologies and returned tissue histology to that seen with saline (PBS) challenged mice. This figure is available in black and white in print and in color at Glycobiology online.
Discussion
IL-10 has become established as the primary mediator of suppression following exposure to PSA and/or its bacterial source B. fragilis. We have shown that IL-10 is necessary for PSA-activated T cell-mediated suppression in both human ex vivo assays (Kreisman and Cobb 2011) and in models of murine lung inflammation (Johnson et al. 2015a), while others reported that IL-10 was critical in blocking inflammatory bowel disease (Mazmanian et al. 2008) and EAE in mice (Ochoa-Reparaz et al. 2010). PSA and its bacterial source B. fragilis have also been associated with both FoxP3− (Kreisman and Cobb 2011; Johnson et al. 2015a) and FoxP3+ (Ochoa-Reparaz et al. 2010; Round and Mazmanian 2010) CD4+ T cell activation in the context of inflammatory disease, but controversy remains as to the nature of the PSA-specific T cells.
The controversy in the field is not whether IL-10 is necessary for suppression, but the identity of the cells which produce it. Focusing upon the PSA-specific responding T cells, it is clear that PSA directly induces IL-10 production, but that the clonally expanded T cells do not express FoxP3 (Johnson et al. 2015a, 2015b). We have established this in murine (Johnson et al. 2015a) and human T cells (Kreisman and Cobb 2011), and it is supported by earlier findings which identified CD45Rblow cells as being the key population stimulated by polysaccharides (Ruiz-Perez et al. 2005), thereby leading to the model that PSA activates FoxP3− Tr1 cells commonly associated with the gut (Kreisman and Cobb 2011). Conversely, two other groups identified canonical FoxP3+ Tregs as being the PSA or B. fragilis-responding population that leads to immune protection upon oral exposure or colonization, respectively (Ochoa-Reparaz et al. 2010; Round and Mazmanian 2010). Although the reported changes in the number of FoxP3+ Tregs was much lower than the changes seen in CD45Rblow cells after PSA exposure, there was a statistically significant change seen.
In this present study, we have discovered a novel T cell-to-T cell communication pathway which not only reconciles the FoxP3 controversy in the field, but also bridges the gut environment and exposure to microbial polysaccharide antigens with peripheral immune homeostasis. First, this emerging model combines all of the previously reported murine and human data together into a single cohesive pathway which relies upon communication between FoxP3− PSA-experienced Rblow T cells and tissue-resident and PSA-naïve FoxP3+ Tregs. This explains why FoxP3+ Tregs were incorrectly identified as the PSA-responding and suppressive cells in various inflammatory models (Ochoa-Reparaz et al. 2010; Round and Mazmanian 2010), since these Tregs are required for immune suppression. Likewise, these findings confirm the identity of the PSA-responding cells as being FoxP3− (Johnson et al. 2015a, 2015b). The observation that these cell subsets cooperate to generate protection through IL-10 is therefore fully consistent with all of the previously reported findings, including the requirement for IL-10.
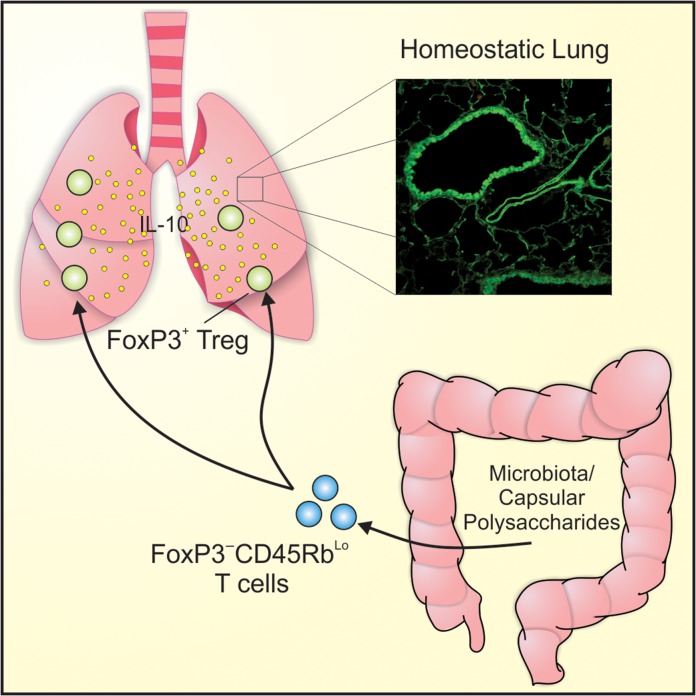
Perhaps more importantly, our results shed light upon how polysaccharide antigens, B. fragilis, and possibly other commensal organisms in the gut can influence distant tissues, including the lungs (Figure 9). It has always been assumed that T cells activated by commensal antigens simply travel to various tissues within the body and suppress unwanted inflammation. Yet, the data herein suggests that immune suppression is driven not only by the commensal antigens and their T cells, but also by the presence of FoxP3+ Tregs in the inflamed tissue and their activation state. Indeed, we found that the Tregs must be activated (Figure 6A and B) in order to be influenced by the PSA-experienced T cells. This raises the possibility that polysaccharide/commensal-driven immune suppression would be the most robust in the tissue(s) with the most activated Tregs, thereby focusing immune suppression where needed.
Fig. 9.
Schematic of the PSA-induced T cell-to-T cell communication leading to lung protection. The resulting model suggests that PSA and potentially other commensal organisms induce CD4+FoxP3−Rblow T cells upon exposure in the GI tract, and that these cells are able to communicate with FoxP3+ Tregs in peripheral tissues to induce IL-10 and suppress the inflammatory cascade. This figure is available in black and white in print and in color at Glycobiology online.
Finally, our findings suggest a possible explanation for why the loss of FoxP3 in IPEX patients leads to such severe autoimmunity. Although FoxP3+ Tregs represent the main Treg phenotype, FoxP3− T cells with potent suppressive activity, such as the IL-27-dependent (Awasthi et al. 2007) CD4+FoxP3− Tr1 subset (Groux et al. 1997), are also present and capable of controlling some inflammatory responses (Battaglia et al. 2006). However, these cells lack the capacity to overcome the systemic autoimmunity resulting from the loss of FoxP3. It has been reported that some CD4+FoxP3− Tregs once expressed FoxP3 (Sujino et al. 2016), implying that suppressive CD4+FoxP3− T cells simply fail to develop in FoxP3− mice. However, suppressive CD4+FoxP3− Tr1 cells have been reported in FoxP3− mice and IPEX patients (Passerini et al. 2011). Although these and other possibilities are not mutually exclusive, it is clear that CD4+FoxP3+ Tregs are critical for optimal maintenance of immune homeostasis throughout the body, and that CD4+FoxP3− Tregs, which are often gut-associated, fail to compensate for their loss. Our findings reveal that CD4+FoxP3+ Tregs synergistically amplify the suppressive capacity of CD4+FoxP3− T cells through the induction of IL-10 within inflamed tissues, suggesting that Tr1 cells are functionally optimal only in the presence of FoxP3+ Tregs. This notion provides a rationale for the inability of CD4+FoxP3− Tregs to overcome the autoimmune response resulting from the loss of FoxP3 in IPEX.
Although much more investigation of this pathway, including details about the involved cells and the identification of the mediators of communication, is necessary to generalize our observations to non-PSA responses, our findings open a new door in how the influence of the normal microbiota may be amplified and circulated throughout the body in times of inflammatory stress.
Materials and methods
Mice
C57BL/6 (Stock #000,664), IL-10-eGFP (B6.129S6-Il10tm1Flv/J, Stock #008,379), OT-II (B6.Cg-Tg(TcraTcrb)425Cbn/J, Stock #004,194) and IL-10-null (B6.129P2-Il10tm1Cgn/J, Stock #002,251) mice, all on the C57B6 background, were purchased from the Jackson Laboratory (Bar Harbor, ME), C57B6. FoxP3-eGFP animals were a kind gift of Dr. Rudensky and Dr. Letterio. Mice were fed standard chow (Purina 5010) on a 12 h light/dark cycle in a specific pathogen free facility. Privacy provided in mating cages by “love shacks” (Bio Serv 53,352–400). Mouse studies, and all animal housing at Case Western Reserve University were approved by and performed according to the guidelines established by the Institutional Animal Care and Use Committee of CWRU.
Primary splenic T cells
Primary splenocytes were isolated from freshly harvested spleens, and reduced to a single cell suspension by passing them through a sterile 100 μM nylon mesh cell strainer (Fisher Scientific, Hampton, NH). For splenic T cell sorting, single cell suspension were labeled with anti-mouse CD4 magnetic microbeads, or alternatively negatively selected for CD4 and positively selected for CD25 by a mouse regulatory T cell isolation kit (Miltenyi Biotec, San Diego, CA), and separated with an autoMACS Pro Separator (Miltenyi Biotec, San Diego, CA), per manufacturer's instructions.
Pulmonary inflammation and adoptive transfer
Mice were sensitized to ovalbumin as previously reported (Johnson et al. 2015a). Briefly, animals were challenged with intranasal ovalbumin (40 μg/dose) for 6 days before sacrifice on Day 7. For those animals adoptively receiving T cells, 2 × 106 CD4+ or 6 × 104 Rblow donor animal cells were recovered as described for primary splenic T cells and intravenously injected into recipients along with 2 × 106 CD4+ OT-II T cells 24 h prior to the start of challenge. Animals receiving spent media were treated with 25 μL of media with each daily dose of OVA on Days 0–6. For murine PSA exposure, animals were orally gavaged with PSA as previously reported (Johnson et al. 2015a). Animals were anesthetized with 3% isoflurane (Baxter) with an anesthesia system (VetEquip, Livermore, CA). Euthanasia, BALF recovery, and lung tissue preparation was performed as previously reported (Johnson et al. 2015a). BALF automated differentials were acquired by a HemaVet 950 Hematology Analyzer.
Bacterial culture
B acteroides fragilis expressing PSA only was a kind gift from Laurie Comstock (Harvard Medical School) and grown in batch culture for 24 h under anaerobic conditions. Anaerobiosis was maintained by nitrogen gas, and neutral pH was maintained by automated titration with 10 N NaOH. The basal medium contained 5 g yeast extract, 20 g proteose peptone, 5 g NaCl, 60 g glucose, 5 mg hemin and 0.5 mg vitamin K1 per liter.
PSA purification
The capsular complex from B. fragilis was isolated as previously described (Pantosti et al. 1991; Cobb et al. 2004). Briefly, bacterial cell pellets were resuspended in a mixture of 1:1 water:phenol solution. The cells were then continuously mixed at 70°C with glass beads before decanting to remove beads and centrifugation to pellet debris. The aqueous phase was collected after centrifugation and then extracted with ethyl ether to remove residual phenol phase. The ethyl ether was removed from aqueous phase on a RotoVap (Buchi, New Castle, DE), and dialyzed against water overnight. The resulting sample was then digested with DNAse and RNAase (Worthington Biochemical, Lakewood, NJ) twice and then with Pronase (Calbiochem/EMD Millipore, Billerica, MA) twice, overnight for each. The digested sample was precipitated overnight in 80% ice cold ethanol. The solid was dried and resuspended in 3% deoxycholate. The sample was separated on a Sephacryl S-300 (GE Healthcare, Chicago, IL) column and 20 mL fractions collected. The presence of PSA and LPS was performed by SDS-PAGE separation followed by Pro-Q Emerald 300 staining according to the manufacturer's instructions (Thermo/Fisher, Waltham, MA). Fractions containing only PSA were collected, extensively dialyzed against distilled water, lyophilized, sealed in glass vials and stored at −80°C until use.
Flow cytometry and cell sorting
For bronchoalveolar lavage fluid (BALF) cell analysis, pelleted cells were stained with anti-CD25-PE and anti-CD4-APC (eBioscience) or alternatively fixed/permeabilized by manufacturer's instructions (eBioscience, San Diego, CA) and stained with anti-CD25-APC and anti-FoxP3-PE (eBioscience, San Diego, CA) before analysis on a BD Accuri C6 flow cytometer (BD Biosciences, San Jose, CA).
For splenic T cell sorting, positively selected CD4+ cells were stained with combinations of antibodies (0.5 μg/mL per) to CD62L-PE (BioLegend, San Diego, CA) or CD62L-BB515 (BD Bioscience), CD44-APC (BioLegend, San Diego, CA), CD45Rb-APC/Cy7 (BioLegend, San Diego, CA), and CD25-BV421 (BD Biosciences, San Jose, CA). Cells were washed twice in MACS buffer (Miltenyi Biotec, San Diego, CA) before sterile cell sorting using a FACSAria (BD Biosciences, San Jose, CA) with the support of the Cytometry & Imaging Microscopy Core Facility of the Case Comprehensive Cancer Center. Analysis of all FACS data was performed using FlowJo vX (Tree Star, Inc., Ashland, OR) or FCS Express (De Novo Software, Glendale, CA).
Cell culture
After flow sorting, cells were cultured in 96-well plates (Corning, Corning, NY) at 50,000 cells per type per well in advanced RPMI (Gibco/Fisher Scientific, Waltham, MA) supplemented with 5% Australian-produced heat-inactivated fetal bovine serum, 55 μM β-mercaptoethanol, 100 U/mL and 100 μg/mL Penicillin/Streptomycin, and 0.2 mM l-glutamine (Gibco/Fisher Scientific, Waltham, MA) at 5% CO2, 37°C. Under activating conditions, wells were functionalized by coating αCD3ε (eBioscience, San Diego, CA) at 2.5 μg/mL in PBS then incubating at 37°C for 4 h before being washed twice with PBS before receiving cells.
ELISA
Cytokine levels were analyzed by standard sandwich ELISA performed by manufacturer's instructions (BioLegend, San Diego, CA), modified to utilize europium-conjugated streptavidin (Perkin-Elmer) detected with a Victor V3 plate reader (Perkin Elmer, San Jose, CA).
Histology and microscopy
Tissues were blocked, sectioned, and stained with H&E by the Case Western Reserve University Tissue Procurement and Histology Core Facility. Unstained sections were stained with rat-anti-EpCAM-AF488 (eBioscience, San Diego, CA) or rat-anti-EpCAM-AF594 (BioLegend, San Diego, CA) at 6μg/mL, and rabbit-anti-myeloperoxidase (1:100, Abcam, Cambridge, MA) then either anti-rabbit-APC (Thermo/Fisher, Waltham, MA) or anti-rabbit-AF488 (Jackson ImmunoResearch, West Grove, PA) at 1:1000. Confocal analysis and imaging was performed on a Leica SP5 confocal microscope; H&E images were acquired with a Leica DM IL LED.
Data analyses
All data are represented by mean ± SEM. Pulmonary inflammation data sets include a minimum of four, but more commonly six animals per group per experiment. Data and statistical measurements were generated with GraphPad Prism (v5.0). For comparisons between two groups, Student's t-test was used; comparisons between multiple groups utilized analysis of variance.
Acknowledgments
We thank Janice C. Jun and Doug M. Oswald for technical support during dissections, and Jenifer Mikulan for histological technical support. We are also grateful for the support of the Cytometry and Imaging Microscopy Core Facility of the Case Comprehensive Cancer Center, and Alex Huang, MD, PhD for non-confocal histological imaging.
Funding
This work was supported from grants from: The National Institutes of Health (DP2-OD004225, R01-GM082916), the American Asthma Foundation (#10-0187), and the Hartwell Foundation to B.A.C., and the National Institutes of Health (F32-AI114109, T32-AI007024) to M.B.J.
Conflict of interest statement
The authors declare no financial competing interests related to the research herein.
References
- Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. 2007. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 8:1380–1389. [DOI] [PubMed] [Google Scholar]
- Bacchetta R, Passerini L, Gambineri E, Dai M, Allan SE, Perroni L, gna-Bricarelli F, Sartirana C, Matthes-Martin S, Lawitschka A et al. . 2006. Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J Clin Invest. 116:1713–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia M, Gregori S, Bacchetta R, Roncarolo MG. 2006. Tr1 cells: From discovery to their clinical application. Semin Immunol. 18:120–127. [DOI] [PubMed] [Google Scholar]
- Cobb BA, Wang Q, Tzianabos AO, Kasper DL. 2004. Polysaccharide processing and presentation by the MHCII pathway. Cell. 117:677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. 1997. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 389:737–742. [DOI] [PubMed] [Google Scholar]
- Holsti MA, Chitnis T, Panzo RJ, Bronson RT, Yagita H, Sayegh MH, Tzianabos AO. 2004. Regulation of postsurgical fibrosis by the programmed death-1 inhibitory pathway. J Immunol. 172:5774–5781. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Jones MB, Cobb BA. 2015. a. Bacterial capsular polysaccharide prevents the onset of asthma through T-cell activation. Glycobiology. 25:368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL, Jones MB, Cobb BA. 2015. b. Polysaccharide A from the capsule of Bacteroides fragilis induces clonal CD4+ T cell expansion. J Biol Chem. 290:5007–5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamanaka M, Kim ST, Wan YY, Sutterwala FS, Lara-Tejero M, Galan JE, Harhaj E, Flavell RA. 2006. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter Knockin tiger mouse. Immunity. 25:941–952. [DOI] [PubMed] [Google Scholar]
- Kreisman LS, Cobb BA. 2011. Glycoantigens induce human peripheral Tr1 cell differentiation with gut-homing specialization. J Biol Chem. 286:8810–8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian SK, Round JL, Kasper DL. 2008. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 453:620–625. [DOI] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. 2010. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 3:487–495. [DOI] [PubMed] [Google Scholar]
- Pantosti A, Tzianabos AO, Onderdonk AB, Kasper DL. 1991. Immunochemical characterization of two surface polysaccharides of Bacteroides fragilis. Infect Immun. 59:2075–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passerini L, Di NS, Gregori S, Gambineri E, Cecconi M, Seidel MG, Cazzola G, Perroni L, Tommasini A, Vignola S et al. . 2011. Functional type 1 regulatory T cells develop regardless of FOXP3 mutations in patients with IPEX syndrome. Eur J Immunol. 41:1120–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. 2010. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci USA. 107:12204–12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudensky AY. 2011. Regulatory T cells and Foxp3. Immunol Rev. 241:260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Perez B, Chung DR, Sharpe AH, Yagita H, Kalka-Moll WM, Sayegh MH, Kasper DL, Tzianabos AO. 2005. Modulation of surgical fibrosis by microbial zwitterionic polysaccharides. Proc Natl Acad Sci USA. 102:16753–16758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Vignali DA, Rudensky AY, Niec RE, Waldmann H. 2013. The plasticity and stability of regulatory T cells. Nat Rev Immunol. 13:461–467. [DOI] [PubMed] [Google Scholar]
- Sujino T, London M, Hoytema van Konijnenburg DP, Rendon T, Buch T, Silva HM, Lafaille JJ, Reis BS, Mucida D. 2016. Tissue adaptation of regulatory and intraepithelial CD4(+) T cells controls gut inflammation. Science. 352:1581–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzianabos AO, Gibson FC III, Cisneros RL, Kasper DL. 1998. Protection against experimental intraabdominal sepsis by two polysaccharide immunomodulators. J Infect Dis. 178:200–206. [DOI] [PubMed] [Google Scholar]
- Tzianabos AO, Kasper DL, Cisneros RL, Smith RS, Onderdonk AB. 1995. Polysaccharide-mediated protection against abscess formation in experimental intra-abdominal sepsis. J Clin Invest. 96:2727–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzianabos AO, Russell PR, Onderdonk AB, Gibson FC III, Cywes C, Chan M, Finberg RW, Kasper DL. 1999. IL-2 mediates protection against abscess formation in an experimental model of sepsis. J Immunol. 163:893–897. [PubMed] [Google Scholar]