Irrespective of the stimulus, acid secretion by the gastric parietal cell ultimately involves H+, K+-ATPase, the enzyme that pumps hydrogen ions (protons) out of the cell and into the gastric lumen in exchange for potassium ions. Proton pump inhibitors (PPIs) bind irreversibly to H+, K+-ATPase, thereby disabling the enzyme and profoundly decreasing gastric acid secretion. It is well established and widely appreciated that PPIs are remarkably effective agents for treating diseases mediated by gastric acid such as gastroesophageal reflux disease (GERD) and peptic ulcer disease. Far less well known are the numerous potential anti-inflammatory effects that have been described for PPIs including their inhibitory influence on inflammatory cells and on pro-inflammatory cytokine production by endothelial and epithelial cells.1,2 These anti-inflammatory PPI effects, which are independent of their effects on gastric acid secretion, might enable PPIs to heal inflammatory disorders of the upper gastrointestinal tract other than GERD and peptic ulceration. Nevertheless, physicians generally have regarded a symptomatic response to PPI therapy as de facto evidence of acid-peptic disease.
Physicians often prescribe PPIs empirically for patients who have symptoms that might be acid related (e.g. heartburn, dyspepsia), withholding diagnostic endoscopy for those whose symptoms persist despite PPI therapy.3 For patients who experience partial symptom relief, the PPIs are not stopped routinely prior to endoscopy, and physicians generally are aware that this practice creates at least two potential problems: 1) PPIs can mask endoscopic evidence of early gastric cancers,4 and 2) PPIs can eliminate endoscopic evidence of reflux esophagitis.5 Although there are well documented cases of PPIs obliterating endoscopic evidence of early gastric cancer by healing associated ulcerations,4 this appears to be a very uncommon phenomenon in Western countries in which the incidence of gastric cancer is low. It is less clear why endoscopists evaluating patients with GERD symptoms so readily accept the strong possibility that PPIs will eliminate evidence of reflux esophagitis at diagnostic endoscopy. The endoscopic demonstration of reflux esophagitis for GERD patients at baseline (off antireflux therapy) has important therapeutic implications. PPI treatment is required indefinitely for patients with severe reflux esophagitis, whereas PPI treatment might be tapered, stopped or not needed at all for patients with no reflux esophagitis at baseline. For patients who have endoscopy while taking PPIs, no meaningful assessment can be made regarding the baseline presence of reflux esophagitis.
Perhaps the practice of not stopping PPIs prior to diagnostic endoscopy evolved in part because, for many GERD patients, the primary indication for endoscopy is to look for Barrett’s esophagus, a condition whose detection can be improved by PPIs healing reflux esophagitis. For patients with GERD-like symptoms not eliminated by PPIs, furthermore, the primary purpose of endoscopy usually is not to establish a diagnosis of GERD, but rather to look for esophageal diseases other than GERD that might be causing the symptoms. The physician’s rationale for not stopping PPI treatment in this setting is likely the widely-held assumption that acid inhibition is the only important effect of PPIs. Since GERD is the only acid-peptic disorder of the esophagus, it would follow that GERD is the only esophageal disease that can respond to PPIs, and therefore PPIs will not interfere with the ability to diagnose non-GERD disorders. These premises, which now appear to be flawed, are the basis for the persistent notion that PPI responsiveness can distinguish GERD from eosinophilic esophagitis (EoE).
EoE, an antigen-mediated disease, and GERD, which is acid-mediated, can have similar symptoms and histologic manifestations including esophageal eosinophilia. The association between GERD and esophageal eosinophilia was first described in 1982,6 and pathologists soon thereafter accepted the concept that esophageal eosinophilia is a manifestation of GERD. The first report describing EoE as a clinico-pathologic syndrome distinct from GERD was not published until 1993,7 and widespread recognition of this new disease by practicing physicians was delayed until well into the new millennium. This delay was due largely to the common clinical practice of attributing esophageal eosinophilia to GERD. In order to establish that EoE was in fact a new disease distinct from GERD, early EoE investigators focused on how to exclude GERD unequivocally, and lack of response to PPIs seemed a good way to accomplish that goal. Accordingly, in 2007, the AGA Institute defined EoE as a primary clinico-pathologic disorder of the esophagus characterized by UGI symptoms, esophageal eosinophilia, and the absence of pathologic GERD as evidenced by a normal esophageal pH monitoring study or by PPI unresponsiveness.8 Although this definition was unrealistic because it implied that GERD and EoE are mutually exclusive disorders, which they clearly are not,9 response to a PPI trial nevertheless seemed a reasonable way to establish a diagnosis of GERD.
Soon after publication of the 2007 AGA guidelines, investigators increasingly began to recognize patients who had symptoms, endoscopic findings and esophageal histology typical of EoE, but who responded to PPIs even though they had normal esophageal pH monitoring studies and no signs of reflux esophagitis.10 Since, by the 2007 definition, PPI responsiveness excluded a diagnosis of EoE, this condition was called PPI-responsive esophageal eosinophilia (PPI-REE). In 2011, a working group proposed a new conceptual definition for EoE as an immune/antigen-mediated esophageal disease characterized clinically by symptoms related to esophageal dysfunction and histologically by eosinophil-predominant inflammation.11 Although PPI responsiveness would not violate this conceptual definition, the EoE working group nevertheless recommended in their diagnostic guidelines that PPI-REE should be excluded to establish a diagnosis of EoE.
The mechanisms underlying PPI-REE remain unclear, but might involve an anti-inflammatory effect of PPIs on the secretion of eotaxin-3 (CCL26) by esophageal epithelial cells.12 Eotaxin-3 is a potent eosinophil chemoattractant. Exposure to the Th2 cytokines characteristic of allergic disease causes esophageal epithelial cells to secrete eotaxin-3, an effect that is blocked by PPIs.12 By blocking cytokine-stimulated esophageal secretion of eotaxin-3, PPIs might reduce esophageal eosinophilia. Alternatively, it is possible that patients with PPI-REE have subclinical GERD exacerbating an antigen-mediated esophageal eosinophilia, perhaps through a GERD-induced increase in esophageal permeability that enables food antigens to penetrate the esophageal epithelium.9 In this situation, PPIs might benefit the antigen-mediated eosinophilia through their well-known beneficial effects on GERD.
Irrespective of the mechanism underlying PPI-REE, it is now clear that patients with an antigen-driven esophageal eosinophilia (i.e. EoE) can respond to PPIs. Recent studies have shown that the clinical, endoscopic, histologic and gene expression features of EoE and PPI-REE are virtually identical, and multivariate analyses have not identified any feature (other than PPI responsiveness) that distinguishes EoE from PPI-REE.13,14 Other reports have documented that EoE patients (with GERD excluded by esophageal pH monitoring) who were treated successfully with elimination diets responded to PPIs when those diets were stopped and, conversely, that patients with PPI-REE on unrestricted diets responded to elimination diets in which specific food triggers were identified when the PPIs were stopped.15 In light of all these observations, there is growing consensus that antigen-mediated EoE can respond to PPIs irrespective of the presence of detectable GERD.16 However, U.S. gastroenterology society guidelines have yet to be updated in this regard, and still distinguish EoE from PPI-REE.
One unanticipated consequence of confusion regarding the nature of PPI-REE is lack of awareness among clinicians regarding how PPIs can obscure the diagnosis of EoE. If one accepts the dictum that PPI responsiveness excludes a diagnosis of EoE, then there is no need to stop PPI treatment before an endoscopy performed to look for EoE. How can PPIs obscure a diagnosis that they have already excluded? As discussed above, however, PPI-REE is EoE in many, if not most cases. Although clinicians might be aware of studies documenting that PPIs can improve esophageal eosinophilia, they do not commonly stop PPIs prior to diagnostic endoscopy for patients with symptoms that might be due to EoE. This issue is especially pertinent when endoscopy is performed for patients with GERD-like symptoms that have responded only partially to PPI treatment. Two cases described below illustrate this point.
Patient 1: A 29 year-old man experienced heartburn and dysphagia for 8 years. He was treated intermittently with PPIs for suspected GERD, with partial relief. During 6 months prior to evaluation, his symptoms increased and he lost 12 pounds. Endoscopy (performed without stopping PPIs) revealed normal esophageal mucosa, and narrowing in the distal esophagus (Figure 1). The narrowed area was dilated with an 18mm TTS balloon, causing an esophageal tear that raised concern for EoE, but mid-esophageal biopsies showed normal squamous epithelium with no eosinophils (Figure 1). Dilation resulted in incomplete relief of dysphagia, and subsequent esophageal manometry revealed 100% failed peristalsis and an integrated relaxation pressure (IRP) of 12.4 mm Hg, interpreted as suggestive of achalasia (Supplemental Figure 1). Barium swallow showed narrowing of the distal esophagus, which the radiologist interpreted as suggestive of achalasia (Supplemental Figure 2). The patient was referred for Heller myotomy but, because of uncertainty regarding the diagnosis, his surgeons referred him to our Center for Esophageal Diseases. We obtained a history of asthma and seasonal allergies, and considered the possibility that endoscopic and histologic evidence of EoE had been masked by PPI treatment. We stopped PPIs, and 4 weeks later performed an endoscopy that revealed edema, rings, and linear furrows (Figure 2). Passage of the endoscope into the stomach caused an esophageal tear (Supplemental Figure 3). Esophageal biopsies showed typical EoE features including >50 intraepithelial eosinophils per high power field and eosinophil micro-abscesses (Figure 2).
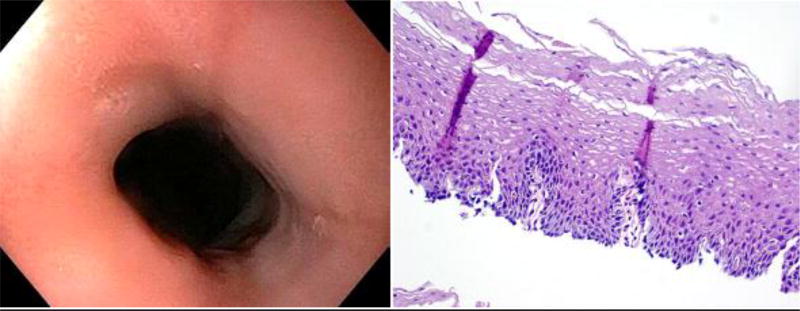
Figure 1.
Endoscopic photograph of the distal esophagus and photomicrograph of an esophageal biopsy from Patient 1’s initial endoscopy (on PPIs) showing no mucosal abnormality endoscopically or histologically.
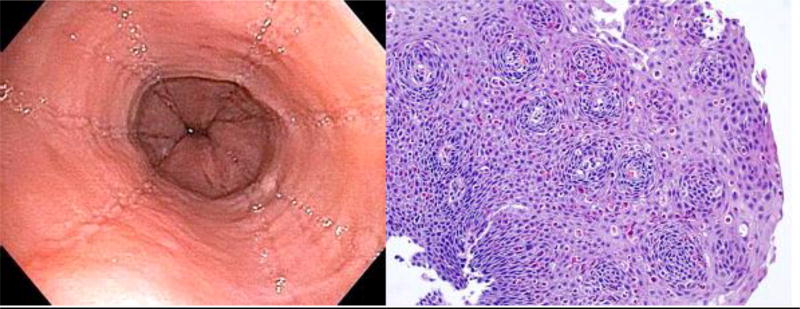
Figure 2.
Endoscopic photograph of the distal esophagus and photomicrograph of an esophageal biopsy from Patient 1’s repeat endoscopy (four weeks off PPIs) showing prominent linear furrows, rings and dense eosinophilia.
Patient 2: A 19 year old man had a one-year history of heartburn, regurgitation and progressive dysphagia. He was treated with PPIs for suspected GERD, with only partial relief. Endoscopy (performed without interrupting PPI therapy) showed a small hiatal hernia, distal esophageal stricture, and mild furrowing in the distal esophagus (Supplemental Figure 4a). EoE was suspected, but esophageal biopsies showed only scattered eosinophils (maximum 9 per high power field). We were consulted, and recommended repeat endoscopy after stopping PPI therapy. Three weeks off PPIs, endoscopy revealed prominent linear furrows, and esophageal biopsies showed up to 30 eosinophils per high power field (Supplemental Figure 4b).
Lack of awareness of how PPIs can obscure the endoscopic and histologic diagnosis of EoE resulted in considerable delay in establishing the correct diagnosis for both of the above-described patients. Patient 1 also raises interesting issues regarding the potential effects of PPIs on EoE-associated motility disorders, and of the particular diagnostic difficulties this situation creates. Patient 1 mistakenly had been assumed to have GERD for years. The narrowing of his distal esophagus found at endoscopy performed during PPI treatment was thought to represent either a peptic esophageal stricture due to GERD, or the persistently contracted lower esophageal sphincter of achalasia. The esophageal tear that accompanied dilation of the area raised concern for EoE, but that diagnosis was dismissed when esophageal biopsies were entirely normal. The patient was subsequently found to have an esophageal motility disorder with absent peristalsis, but with a “normal” IRP, which occasionally can occur in achalasia.17 It now appears that his distal esophageal narrowing and dysphagia were due primarily to a stricture caused by a fibrostenotic form of EoE, but he might well have had invasive treatment for achalasia had the correct diagnosis not become clear when PPI discontinuation resulted in the return of esophageal epithelial eosinophilia. Nevertheless, it remains unclear whether EoE and the motility disorder were unrelated, whether EoE contributed to the motility disorder, or whether the motility disorder contributed to the EoE.
There is an incompletely understood association between esophageal eosinophilia and achalasia. Esophageal mucosal irritation from stasis in achalasia appears to be capable of causing mucosal eosinophilia.18 This mechanism seems unlikely in our patient because he had no esophageal dilation endoscopically or radiographically, no retained esophageal material seen at endoscopy, and no evidence of mucosal irritation during the endoscopy performed while on PPIs (which are unlikely to affect irritation due to stasis of ingested material). Conversely, eosinophils infiltrating the esophageal muscularis propria in EoE might release eosinophil products that cause motor dysfunction mimicking achalasia,19 or conceivably, might release cytotoxic eosinophil products (e.g. eosinophil cationic protein, eosinophil derived neurotoxin) that destroy esophageal intramural neurons and hence cause achalasia.20 Since endoscopic esophageal biopsies sample only mucosa, it is not clear how often EoE is associated with eosinophilic infiltration of the muscularis propria. The few cases in which esophagectomy specimens from EoE patients have been examined have revealed full thickness eosinophil infiltration,21,22 and conversely, patients with achalasia (without EoE) have been found unexpectedly to have eosinophils infiltrating the esophageal muscularis propria.20,23 As discussed above, PPI inhibition of Th2 cytokine-stimulated release of eotaxin-3 by esophageal epithelial cells might explain the beneficial effect of PPIs on the esophageal epithelium in EoE,11 but PPIs do not block Th2 cytokine-stimulated eotaxin-3 secretion by subepithelial esophageal fibroblasts.24 Eotaxin-3 also is present in esophageal muscle,25 and the effects of PPIs on eotaxin-3 secretion by that muscle are not known. Thus, PPIs that eliminate eosinophils from the epithelium might have little effect on eosinophilic infiltration of the muscularis propria and, therefore, esophageal motility abnormalities might persist during PPI therapy despite healing of EoE epithelial disease.
In conclusion, there is now compelling evidence that PPI treatment can totally obliterate endoscopic and histologic evidence of EoE. Consequently, an endoscopy performed with a patient on PPIs cannot exclude EoE. This report should not be misconstrued as an indictment of the practice of empiric PPI therapy, which can be an appropriate management strategy in selected patients. For patients scheduled for diagnostic endoscopy for GERD symptoms responding incompletely to PPI treatment, however, the data discussed above suggest that PPIs should be discontinued for three to four weeks (if possible) prior to the procedure if EoE is a diagnostic consideration. Not only will this practice minimize potential diagnostic errors concerning the presence of EoE, it will also provide information regarding the presence and severity of erosive esophagitis that otherwise might be obscured by PPI therapy, and that might have clinical importance regarding the requirement for chronic PPI treatment.
Supplementary Material
Acknowledgments
Stuart Jon Spechler has served as a consultant for Takeda Pharmaceuticals and Ironwood Pharmaceuticals, and receives royalties as an author for UpToDate. Rhonda F. Souza has served as a consultant for and receives grant support from Ironwood Pharmaceuticals.
Funding: This work was supported by the National Institutes of Health (R01-DK63621, R01-DK103598 and R21-DK111369 to R.F.S. and S.J.S)
Footnotes
Conflict of Interest:
No conflicts of interest exist for Eunice Odiase, Armond Schwartz, Jason Martin and Vani Konda.
Author Contributions to the Manuscript:
Eunice Odiase, MD – acquisition of data, analysis and interpretation of data, and drafting of the manuscript
Armond Schwartz, MD - acquisition of data, analysis and interpretation of data, and critical revision of the manuscript for important intellectual content
Rhonda F. Souza, MD – analysis and interpretation of data, and critical revision of the manuscript for important intellectual content
Jason Martin, MD - acquisition of data
Vani Konda, MD – acquisition of data
Stuart Jon Spechler, MD – topic conception and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and responsible for final approval
References
- 1.Kedika RR, Souza RF, Spechler SJ. Potential anti-inflammatory effects of the proton pump inhibitors: a review and discussion of the clinical implications. Dig Dis Sci. 2009;54:2312–7. doi: 10.1007/s10620-009-0951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huo X, Zhang X, Yu C, Zhang Q, Cheng E, Wang DH, Pham TH, Spechler SJ, Souza RF. In oesophageal squamous cells exposed to acidic bile salt medium, omeprazole inhibits IL-8 expression through effects on nuclear factor-κB and activator protein-1. Gut. 2014;63:1042–52. doi: 10.1136/gutjnl-2013-305533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308–28. doi: 10.1038/ajg.2012.444. [DOI] [PubMed] [Google Scholar]
- 4.Griffin SM, Raimes SA. Proton pump inhibitors may mask early gastric cancer. Dyspeptic patients over 45 should undergo endoscopy before these drugs are started. BMJ. 1998;317:1606–7. doi: 10.1136/bmj.317.7173.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunbar KB, Agoston AT, Odze RD, Huo X, Pham TH, Cipher DJ, Castell DO, Genta RM, Souza RF, Spechler SJ. Association of acute gastroesophageal reflux disease with esophageal histologic changes. JAMA. 2016;315:2104–12. doi: 10.1001/jama.2016.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winter HS, Madara JL, Stafford RJ, Grand RJ, Quinlan JE, Goldman H. Intraepithelial eosinophils: a new diagnostic criterion for reflux esophagitis. Gastroenterology. 1982;83:818–23. [PubMed] [Google Scholar]
- 7.Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci. 1993;38:109–16. doi: 10.1007/BF01296781. [DOI] [PubMed] [Google Scholar]
- 8.Furuta GT, Liacouras CA, Collins MH, Gupta SK, Justinich C, Putnam PE, Bonis P, Hassall E, Straumann A, Rothenberg ME. First International Gastrointestinal Eosinophil Research Symposium (FIGERS) Subcommittees. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342–63. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Spechler SJ, Genta RM, Souza RF. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol. 2007;102:1301–6. doi: 10.1111/j.1572-0241.2007.01179.x. [DOI] [PubMed] [Google Scholar]
- 10.Molina-Infante J, Ferrando-Lamana L, Ripoll C, Hernandez-Alonso M, Mateos JM, Fernandez-Bermejo M, Dueñas C, Fernandez-Gonzalez N, Quintana EM, Gonzalez-Nuñez MA. Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin Gastroenterol Hepatol. 2011;9:110–7. doi: 10.1016/j.cgh.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood S, Bonis PA, Burks AW, Chehade M, Collins MH, Dellon ES, Dohil R, Falk GW, Gonsalves N, Gupta SK, Katzka DA, Lucendo AJ, Markowitz JE, Noel RJ, Odze RD, Putnam PE, Richter JE, Romero Y, Ruchelli E, Sampson HA, Schoepfer A, Shaheen NJ, Sicherer SH, Spechler S, Spergel JM, Straumann A, Wershil BK, Rothenberg ME, Aceves S. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20. doi: 10.1016/j.jaci.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 12.Cheng E, Zhang X, Huo X, Yu C, Zhang Q, Wang DH, Spechler SJ, Souza RF. Omeprazole blocks eotaxin-3 expression by oesophageal squamous cells from patients with eosinophilic oesophagitis and GORD. Gut. 2013;62:824–32. doi: 10.1136/gutjnl-2012-302250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dellon ES, Speck O, Woodward K, Gebhart JH, Madanick RD, Levinson S, Fritchie KJ, Woosley JT, Shaheen NJ. Clinical and endoscopic characteristics do not reliably differentiate PPI-responsive esophageal eosinophilia and eosinophilic esophagitis in patients undergoing upper endoscopy: a prospective cohort study. Am J Gastroenterol. 2013;108:1854–60. doi: 10.1038/ajg.2013.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen T, Dellon ES, Moawad FJ, Furuta GT, Aceves SS, Rothenberg ME. Transcriptome analysis of proton pump inhibitor-responsive esophageal eosinophilia reveals proton pump inhibitor-reversible allergic inflammation. J Allergy Clin Immunol. 2015;135:187–97. doi: 10.1016/j.jaci.2014.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucendo AJ, Arias Á, González-Cervera J, Olalla JM, Molina-Infante J. Dual response to dietary/topical steroid and proton pump inhibitor therapy in adult patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2016;137:931–4.e2. doi: 10.1016/j.jaci.2015.07.033. [DOI] [PubMed] [Google Scholar]
- 16.Lucendo AJ, Molina-Infante J, Arias Á, von Arnim U, Bredenoord AJ, Bussmann C, Amil Dias J, Bove M, González-Cervera J, Larsson H, Miehlke S, Papadopoulou A, Rodríguez-Sánchez J, Ravelli A, Ronkainen J, Santander C, Schoepfer AM, Storr MA, Terreehorst I, Straumann A, Attwood SE. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United European Gastroenterol J. 2017;5:335–358. doi: 10.1177/2050640616689525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ponds FA, Bredenoord AJ, Kessing BF, Smout AJ. Esophagogastric junction distensibility identifies achalasia subgroup with manometrically normal esophagogastric junction relaxation. Neurogastroenterol Motil. 2017;29(1) doi: 10.1111/nmo.12908. [DOI] [PubMed] [Google Scholar]
- 18.Cools-Lartigue J, Chang SY, Mckendy K, Mayrand S, Marcus V, Fried GM, Ferri LE. Pattern of esophageal eosinophilic infiltration in patients with achalasia and response to Heller myotomy and Dor fundoplication. Dis Esophagus. 2013;26:766–75. doi: 10.1111/j.1442-2050.2012.01385.x. [DOI] [PubMed] [Google Scholar]
- 19.Savarino E, Gemignani L, Zentilin P, De Bortoli N, Malesci A, Mastracci L, Fiocca R, Savarino V. Achalasia with dense eosinophilic infiltrate responds to steroid therapy. Clin Gastroenterol Hepatol. 2011;9:1104–6. doi: 10.1016/j.cgh.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Tøttrup A, Fredens K, Funch-Jensen P, Aggestrup S, Dahl R. Eosinophil infiltration in primary esophageal achalasia. A possible pathogenic role. Dig Dis Sci. 1989;34:1894–9. doi: 10.1007/BF01536708. [DOI] [PubMed] [Google Scholar]
- 21.Nicholson AG, Li D, Pastorino U, Goldstraw P, Jeffery PK. Full thickness eosinophilia in oesophageal leiomyomatosis and idiopathic eosinophilic oesophagitis. A common allergic inflammatory profile? J Pathol. 1997;183:233–6. doi: 10.1002/(SICI)1096-9896(199710)183:2<233::AID-PATH936>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 22.Rieder F, Nonevski I, Ma J, Ouyang Z, West G, Protheroe C, DePetris G, Schirbel A, Lapinski J, Goldblum J, Bonfield T, Lopez R, Harnett K, Lee J, Hirano I, Falk G, Biancani P, Fiocchi C. T-helper 2 cytokines, transforming growth factor β1, and eosinophil products induce fibrogenesis and alter muscle motility in patients with eosinophilic esophagitis. Gastroenterology. 2014;146:1266–77.e1. 9. doi: 10.1053/j.gastro.2014.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldblum JR, Whyte RI, Orringer MB, Appelman HD. Achalasia. A morphologic study of 42 resected specimens. Am J Surg Pathol. 1994;18:327–37. [PubMed] [Google Scholar]
- 24.Cheng E, Zhang X, Wilson KS, Wang DH, Park JY, Huo X, Yu C, Zhang Q, Spechler SJ, Souza RF. JAK-STAT6 pathway inhibitors block eotaxin-3 secretion by epithelial cells and fibroblasts from esophageal eosinophilia patients: promising agents to improve inflammation and prevent fibrosis in EoE. PLoS One. 2016 Jun 16;11(6):e0157376. doi: 10.1371/journal.pone.0157376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato H, Nakajima N, Takahashi K, Hasegawa G, Mizuno KI, Hashimoto S, Ikarashi S, Hayashi K, Honda Y, Yokoyama J, Sato Y, Terai S. Proposed criteria to differentiate heterogeneous eosinophilic gastrointestinal disorders of the esophagus, including eosinophilic esophageal myositis. World J Gastroenterol. 2017;23:2414–2423. doi: 10.3748/wjg.v23.i13.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.