Abstract
Background
Individual variability in response to multiple modalities of obesity treatment is well documented yet our understanding of why some individuals respond while others do not is limited. The etiology of this variability is multi-factorial, but at present we lack a comprehensive evidence base to identify which factors or combination of factors influence treatment response.
Objectives
This paper provides an overview and rationale of the Accumulating Data to Optimally Predict obesity Treatment (ADOPT) Core Measures Project, which aims to advance understanding of individual variability in response to adult obesity treatment. We provide an integrated model for how factors in the behavioral, biological, environmental, and psychosocial domains may influence obesity treatment responses and identify a core set of measures to be used consistently across adult weight loss trials. This paper provides the foundation for four companion papers that describe the core measures in detail.
Significance
The accumulation of data on factors across the four ADOPT domains can inform the design and delivery of effective, tailored obesity treatments. ADOPT provides a framework for how obesity researchers collectively can generate this evidence base and is a first step in an ongoing process that can be refined as the science advances.
Keywords: weight loss, weight loss maintenance, individual variability, tailored treatment, personalized medicine
INTRODUCTION
The Problem of Obesity
Over 60% of U.S. adults are now classified as overweight or obese (1) with higher rates of obesity among certain racial and ethnic groups (2). Obesity is a risk factor for several chronic diseases, including cardiovascular disease, diabetes, and cancer (3), and the associated health care burden is over $200 billion annually (4). Obesity also has a detrimental impact on quality of life and general well-being and elicits social stigmatization in many communities (5). The enormous scale and scope of this problem has resulted in considerable public health attention across the world; yet, to date, no country has demonstrated a decline in obesity and overweight prevalence (6). The identification of successful strategies for promoting weight loss and weight maintenance has proven elusive for most overweight and obese individuals (3, 7, 8). Moreover, even those strategies that have shown promise are marked by a high level of variability among individuals in response to treatment (8). The need to develop and disseminate more effective strategies is widely recognized, but the barriers to progress are substantial. Here, we identify these barriers and provide a framework for how obesity researchers, working collectively on one approach to specify the determinants of variability in response to treatment, can generate the evidence base needed to guide the development of effective, tailored strategies for the treatment of obesity.
Barriers to Successful Weight Loss and Weight Loss Maintenance
To reduce the prevalence of obesity, more effective treatments to achieve weight loss and maintain lost weight need to be developed and delivered to those in greatest need. Numerous treatment options exist, including diet regimens, comprehensive lifestyle programs, pharmaceuticals, and surgery/devices (3, 7). Most lifestyle approaches yield only modest weight loss (5–10% of initial weight) and are generally only transiently effective (9, 10, 11). Bariatric surgery yields the greatest weight loss (20–35% of initial weight), but is also associated with weight regain following the procedure (12, 13). In 2015, the National Institutes of Health (NIH) working group on weight loss recidivism identified weight regain after weight loss as the most significant obstacle to successful treatment of obesity (8). This group identified three key barriers to successful weight loss maintenance:
Strong biological resistance to weight loss, including the emergence of maladaptive responses that promote weight regain by increasing appetite and reducing energy expenditure.
Reduced adherence to the behaviors required for weight loss, 3-9 months after initiating lifestyle changes, due in part to the complex psychology of behavior change and in part to increasing biological pressures for weight regain.
Continuous exposure to obesogenic environmental pressures that promote sedentary behavior and the consumption of energy dense and/or sugar-enriched foods.
This complex interplay of factors spanning behavioral, biological, environmental, and psychosocial domains render weight loss and weight loss maintenance difficult to achieve. Efforts to address these barriers and promote successful weight outcomes require a deeper understanding of how these factors operate in an integrated manner to affect body weight.
The Challenge to Developing Better Treatments: Individual Variability
Individual variability in response to obesity treatments is well documented across a wide range of modalities (Figure 1). However, our understanding of why some individuals respond well to a treatment, while others do not, is limited (14, 15, 16). There is a growing understanding that this variability in response is rooted in the inherent variability of behavioral, biological, environmental, and psychosocial factors (8), but at present, we lack datasets that allow a comprehensive examination of how these multiple factors and their interactions influence treatment response. Some explanatory factors have emerged as potential predictors of treatment response and are summarized elsewhere (14, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27), but few have been replicated across trials (15, 28). Several reasons for this have been suggested, including heterogeneous study populations and treatments, imprecisely defined factors and the lack of common measures to assess them, insufficient frequency of longitudinal assessments, insufficient statistical power, and inadequate statistical modelling and analytic approaches (29). Addressing the challenge of individual variability in treatment response requires that we generate the relevant datasets to overcome these limitations, and through systematic reviews and meta-analytic syntheses develop integrative, predictive models that can guide treatment strategies, and apply those models to tailor treatments in clinical settings.
Figure 1. Individual Variability in the Response to Obesity Treatments.
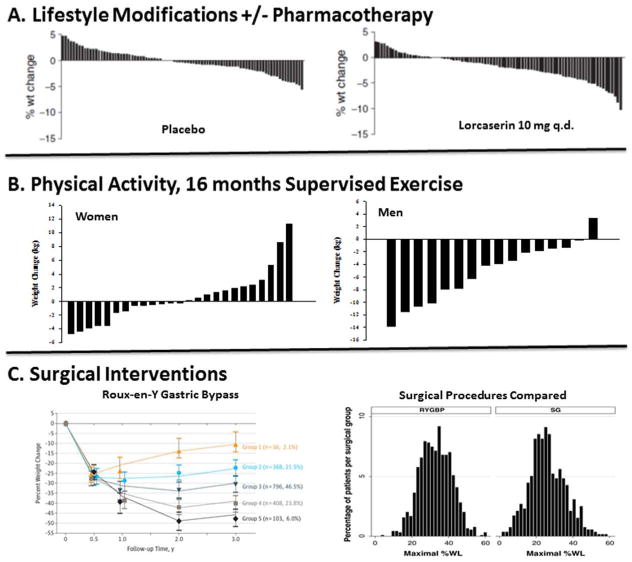
The variable outcomes in weight loss are shown for (A) diet/lifestyle interventions with or without pharmacotherapy (44); (B) supervised exercise in women and men (45, 46); and (C) bariatric procedures, Roux-en-Y Gastric Bypass (left panel) (47) and its comparison with sleeve gastrectomy (SG) (48).
Advancing the Science for Obesity Treatment
The Accumulating Data to Optimally Predict obesity Treatment (ADOPT) Core Measures Project represents a first step towards the broader goal of developing and delivering tailored obesity treatments. This paper reviews the rationale for ADOPT and how its products support this goal. We provide an integrated working model for how treatment responses may be influenced by explanatory factors in the behavioral, biological, environmental, and psychosocial domains. We identify a set of factors in each domain that could contribute to the differential response to treatments and present a core list of measures of these factors to use in adult weight loss studies. Accompanying this overview are four companion papers that discuss the identification and selection of these “core” measures for each domain (30, 31, 32, 33). These companion papers also describe critical gaps in knowledge regarding constructs and measures and limitations of available assessment tools.
Operational Definitions for “Construct” and “Measure”
The ADOPT Core Measures Project required an operational synthesis of expertise and terminology from a broad range of disciplines. To facilitate ongoing discussion, it was necessary to develop a common term that represented the various types of explanatory factors that are found across the domains and disciplines. For ADOPT, the term “construct” is used to describe any type of explanatory factor across the four domains, including biological factors such as hormones or aspects of metabolic regulation, behaviors and behavioral traits and tendencies, and features of the environment. In ADOPT, the term “measure” refers to the actual assay, survey instrument, procedure, or data collection tool used to assess the construct.
OBJECTIVES OF THE ADOPT PROJECT
Broader Vision and Short-Term Objectives
The long-term vision of ADOPT is that a better understanding of individual variability in response to obesity treatment can lead to the development of more effective, tailored treatments for adult obesity. The short-term objectives of ADOPT are: 1) to establish an integrated framework for how treatment responses may be influenced by behavioral, biological, environmental, and psychosocial constructs; 2) to develop an initial list of core constructs that are likely to mediate or moderate treatment outcomes; and 3) to identify the current, best measures of these constructs for use in weight loss trials. This framework and the list of core measures are meant to support the scientific community’s ongoing efforts to generate an evidence base that captures the sources of individual variability in treatment response. Consistent use of the ADOPT core measures in adult weight loss trials will enhance opportunities to identify replicable predictors of treatment responses. Through these efforts the research community should be able to advance the design and delivery of effective, tailored obesity treatments.
Specific Products and Outcomes
The ADOPT Core Measures Project sought to generate three specific products:
ADOPT Working Model- An interdisciplinary framework defining how obesity treatments impact weight loss or weight loss maintenance through four ADOPT domains, which explain much of the individual variability in treatment response.
ADOPT Core List of Constructs and Measures- An initial list of measures to assess high priority constructs from the four ADOPT domains based on the current state of science.
Publicly-Available Database of ADOPT Constructs and Measures- A resource that provides the broader scientific community access to the ADOPT measures and supporting information regarding their use to facilitate consistency in data collection and interdisciplinary collaboration across the four ADOPT domains.
Several similar efforts have consolidated lists of measures to facilitate consistency in research across the broader scientific community (PhenX Toolkit, PROMIS®, Cognitive Atlas, NIH Toolbox, the Comet Initiative, etc.). The ADOPT Working Group leveraged these resources in developing the ADOPT Core Measures list. ADOPT is unique in that it has a specific focus on integrating constructs and measures from the four ADOPT domains that are likely to be predictors, mediators, or moderators of adult obesity treatment responses.
THE ADOPT WORKING MODEL
The Pathways Connecting Obesity Treatments to Energy Balance
Obesity treatments are quite heterogeneous and can include a wide variety of dietary or exercise regimens, environmental manipulations, pharmaceuticals, surgical procedures, and devices (3, 7). Yet, to be effective, all treatments must change the energy balance equation to impose a negative energy imbalance for weight loss and to maintain energy balance during weight loss maintenance so that the lost weight is not regained. The pathways that underlie the effect of obesity treatment on weight loss and maintenance rely on aspects of our behavior, biology, psychology, and environment. Figure 2 provides a conceptual model that delineates how possible constructs within these domains are hypothesized to mediate and moderate a treatment’s impact on weight loss and maintenance. A mediator is a construct that explains how a treatment’s effect is due to changes elicited in targeted mechanisms of action, which, in turn, directly or indirectly affect the energy balance equation (34, 35). A moderator is a construct that captures an aspect of people’s behavior, biology, environment, or psychology under which treatment differentially affects energy balance and weight loss (34, 35). In the sections that follow, we provide a brief overview of each of the steps depicted in the model.
Figure 2. ADOPT Working Model: From Treatment to Outcomes.
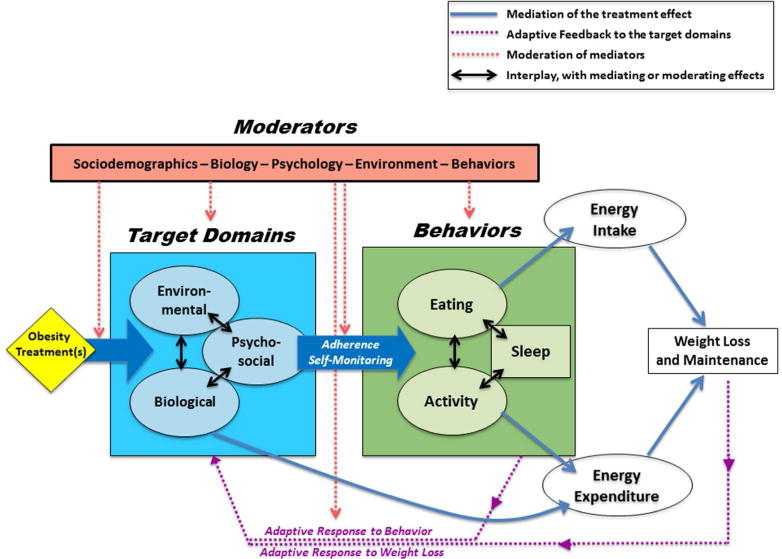
Obesity treatments target specific biological, environmental, and psychosocial constructs. The targeted factors in these domains mediate the effect of the treatment by changing eating and physical activity behaviors or by directly altering metabolic requirements. The changes in behavior and metabolism affect energy balance by reducing energy intake and/or increasing energy expenditure. Compensatory adaptive responses to behavior change and to weight loss feedback in a manner that moderates the target domains and how well they mediate the treatment. Several other factors (age, sex, sociodemographics, etc.) are also likely to moderate the effect of treatment on weight loss or weight loss maintenance. Individual variability is inherent at every stage of the conceptual model connecting the treatment to the outcome.
Target Domains for Obesity Treatments
The majority of obesity treatments attempt to change behaviors with interventions that target specific aspects of an individual’s psychology, biology, or environment (i.e., the three target domains in the BLUE BOX, Figure 2). Not every treatment is designed to affect constructs in every target domain directly, but changes in constructs within one target domain may elicit secondary effects in constructs within the other two domains. Likewise, constructs within the three domains may moderate the magnitude of the treatment’s effect on a construct within a given domain. Therefore, the impact of any one treatment in changing downstream effectors is influenced by the dynamic interplay between psychosocial, biological, and environmental constructs. Measures of constructs in these target domains may predict the treatment outcome if they play a significant role in mediating or moderating the treatment’s impact on key downstream effectors of energy intake and expenditure.
Behaviors as Downstream Mediators
The most prominent downstream effectors of these target domains are the primary behaviors that affect the energy balance equation directly: eating and activity level (GREEN BOX, Figure 2). Eating behaviors encompass the type, amount, and timing of food consumed that ultimately dictate energy intake. In contrast, activity-related behaviors directly influence the non-resting component of daily energy expenditure. A host of other behaviors, such as sleep (36, 37) and self-monitoring practices (14), exert a strong influence on patterns of eating and activity behaviors. As with the target domains, there are likely meaningful interdependencies between these types of behaviors. Exercise, for example, may have secondary influences on food eating behaviors, and vice versa (38). Patterns of behavior may also moderate the magnitude of the treatment’s effect on another behavior. Taken together, measures of key behaviors may predict a treatment’s impact on weight loss and maintenance, because they either mediate its effects or they moderate its impact on energy intake and energy expenditure.
Biology’s Direct Impact on Expenditure
Although obesity treatments primarily affect weight control through behavior change, some treatments target intrinsic biological homeostatic control systems directly to affect expended energy. Such treatments may affect biology to sustain or increase lean mass, increase overall metabolic requirements, reduce metabolic efficiency, or increase thermogenesis in response to food (39). As such, treatments that target factors in the biological domain have the potential to influence energy balance through eating and activity behaviors, as well as by changing energy expenditure directly. Measures of these biological inputs may therefore provide added value for predicting a treatment’s impact on weight loss and maintenance.
Feedback from Behaviors and Weight Change
Thus far, we have described a unidirectional model, whereby treatments affect constructs in the target domains, which, in turn, affect energy balance directly or through behavioral changes. However, an important feature of the model is that both behaviors and weight loss can have feedback effects on constructs in the target domains (PURPLE ARROWS, Figure 2), influencing their ability to mediate or moderate a treatment’s effect over time. One important example is sleep duration and timing, which is known to affect both biological factors (e.g. circadian rhythms, glucose/lipid metabolism, sympathetic activation, neuroendocrine regulation), and psychological aspects of self-monitoring, reward/motivation, mood, and adherence (40). Patterns of physical activity provide another example, as they can alter constructs within the psychological and biological target domains in a manner that affects a treatment’s impact (41). Treatments that target changes in these and other behaviors may, therefore, be mediated through important adaptive responses that reciprocally affect constructs within the target domains. Another important feature of the model is the adaptive (or maladaptive) response to weight change. Weight loss from restricting energy intake, for example, can lead to biological and psychological maladaptive responses that suppress energy expenditure, promote eating behaviors, and increase the rewarding effects of food (42). These maladaptive responses counteract the desired effect of the treatment and their considerable strength may explain, in part, why some treatments are only transiently effective or result in low or no adherence for some individuals.
Moderators of the Pathways Connecting Obesity Treatment to Weight Loss and Maintenance
The pathways that delineate how obesity treatments affect weight loss and maintenance may not operate consistently across populations and their respective environments. Baseline sociodemographic factors (e.g., sex, age, race-ethnicity, cultural norms, socioeconomic status) and baseline behavioral, biological, environmental, and psychosocial constructs, may moderate the effect of treatment on the target domains, the effect of the target domains on behavior, the effect of behavior on energy intake and expenditure, as well as the aforementioned feedback on the target domains (RED BOX, Figure 2). Although some constructs will be stable over time, other constructs will change, and the magnitude of change in these constructs may moderate the effect of treatment on subsequent outcomes. Taken together, these moderating effects have the potential to provide an explanatory framework for the considerable variability that is observed in people’s responses to obesity treatment.
Variability in Treatment Responses
Individual variability is inherent in every aspect of this model. Genetic variability imparts heterogeneity in the biological, behavioral, and psychosocial domains. Similarly, the large range of environmental conditions is evident at multiple levels. Even the baseline moderators are subject to a substantial amount of variability. Individual differences in constructs within the target domains and in baseline moderators are conveyed to the behavioral effectors and to the direct impact that biology has on energy expenditure. Individual variation also extends to the adaptive or maladaptive responses to behavioral change and weight loss. A deeper understanding of the complex interacting constructs within target domains, the interacting behavioral effectors, and variability in the adaptive responses that counter the effects of obesity treatments, will be needed to delineate the causes of large variability in treatment outcomes, and to develop and apply more effective weight loss/maintenance treatments.
THE ADOPT CORE MEASURES WORKING GROUP: PROCESS, CONSTRUCTS, AND MEASURES
Composition of the Working Group
A leadership team comprised of NIH program staff and two extramural scientists was convened to establish project goals and manage the activities of the ADOPT Working Group, a consortium consisting of 43 scientists representing 19 Universities and 5 NIH Institutes and Offices (Supplementary Table S1). The ADOPT Working Group members were selected for their expertise in one or more of the four domains (behavioral, biological, environmental, and psychosocial) affecting body weight regulation and a breadth of knowledge to objectively consider all available measures. The working group attended two in-person meetings (May, 2016; February, 2017) and participated in an extensive series of conference calls organized to generate and refine the ADOPT products.
Grid-Enabled Measures Database (GEM)
After consideration of the available options, the leadership team selected the National Cancer Institute’s Grid-Enabled Measures (GEM) database (Supplementary Table S2) as a collaborative website to develop and maintain the ADOPT list of constructs and measures. GEM is a publicly accessible, easy to use electronic database, developed as a tool for seeking broad input and consensus on common measures in clinical research (www.gem-measures.org).
Crowd-Sourcing Efforts
Feedback and input from the broader scientific community was solicited via email requests to obesity-related NIH funded investigators and members of relevant professional societies. An open forum on the project was held in a joint American Society for Metabolic and Bariatric Surgery/The Obesity Society session at Obesity Week 2016. Ongoing contributions and feedback from the broader scientific community continue through the publicly-available GEM database.
Charge to the Working Group Members
The ADOPT Working Group operated as four subgroups based on the four domains. The subgroups were charged with recommending an initial list of constructs and associated measures within each domain that were most likely to yield predictors, mediators, or moderators of response to obesity treatments. These measures were uploaded to four respective workspaces in GEM. Further, each subgroup was asked to prioritize a set of the constructs to be included in all adult weight loss trials and, in cases where there was more than one measure of a construct, to identify the current “best” measure for those constructs.
Criteria Used in the Selection Process
Each domain subgroup considered the following criteria to guide their selection of high priority constructs and measures: 1) the strength and source of the current evidence related to weight loss outcomes or weight-related behaviors (e.g., randomized clinical trial versus observational data); 2) the quality of the measure (e.g., validity and reliability); 3) the feasibility of using the measure within a clinical trial (e.g., cost, researcher expertise requirements, logistics); and 4) the participant burden posed by the measure (e.g., administration time, invasiveness). Although the entire working group was presented with this list of criteria for consideration, the four domain subgroups were given a considerable amount of flexibility in how they weighed each criterion and the process by which their domain curated the recommended set of core measures (30, 31, 32, 33).
ADOPT Core Constructs and Measures
The ADOPT Core Measures that were selected from the behavioral (30), biological (31), environmental (32), and psychosocial (33) domains are organized by domain in Table 1. More details about the selection of each domain’s constructs and measures are found in the domain-specific papers that accompany this overview. These papers describe domain-specific challenges in selecting constructs and measures, as well as the evidence and perspectives underlying their decisions. Some constructs crossed the predetermined boundaries we set for the ADOPT domains, which made it challenging to classify them within this framework. For example, food intake was discussed by more than one domain subgroup. Instead of grouping all aspects of food intake into one domain, related measures of similar constructs were placed in the most appropriate domain. Specifically, objectively-measured energy intake was included in the biological domain; food eating behaviors were placed in the behavioral domain; and psychological aspects of food intake were included in the psychosocial domain. In addition to identifying core constructs and measures, the subgroups developed supporting materials that provide guidance to investigators regarding best practices for using each of the measures.
Table 1.
ADOPT Core Constructs and Measures Summarized by Domain1.
| Behavioral Domain | Biological Domain | ||
|---|---|---|---|
| Construct | Measure | Construct | Measure |
| Usual Dietary Intake | Multiple Interviewer- Administered 24hr Recalls | Anthropometry | Height, Weight, and BMI |
| Overall Dietary Quality | Healthy Eating Index-2010 | Body Composition and Visceral Fat | Bioelectrical Impedance Spectroscopy (BIS) |
| Eating Away from Home | EARLY Eating Away from Home Questionnaire | Body Fat Distribution | Waist to Hip Ratio, Waist Circumference |
| Sugar-sweetened beverage (SSB) consumption | EARLY SSB Consumption Questionnaire | Expended Energy | REE – Mifflin St.Jeor equation, Total EE, Physical Activity Level (PAL) |
| Objective Physical Activity, Sedentary Behavior, Sleep Duration and Timing | Actigraphy | Energy Intake | Energy Intake - Steady State & Change |
| Self-reported Physical Activity, Sedentary Behavior | Global Physical Activity Questionnaire | Cardiorespiratory Fitness | Resting Heart Rate |
| Self-reported Physical Activity | Paffenbarger Questionnaire | Energy Homeostasis | Adiponectin, Leptin, Amylin, (panel) |
| Self-reported Sedentary Behavior | CARDIA/EARLY Questionnaire | Thyroid Hormones | TSH, T4, fT4I (panel) |
| Self-reported Sleep Duration and Timing | Munich Chronotype Questionnaire (MCTQ) | Hunger/Satiety | Ghrelin, GLP1, GIP, PYY (panel) |
| Sleep Disorders (Apnea) | Berlin Questionnaire for Sleep Apnea | Nutrient Status | Metabolite Panel (Glucose, NEFA, TG) |
| Self-Weighing Behavior | EARLY Self-Weighing Questionnaire | Metabolic Function | Insulin, Glucagon;Insulin Sensitivity - HOMA; Glucose Control - HbA1c |
| Inflammation | IL-6, TNF-alpha, CRP | ||
| Biobanking | Whole Blood | ||
| Environmental Domain | Psychosocial Domain | ||
|---|---|---|---|
| Construct | Measure | Construct | Measure |
| Home address | Home address | State Affect | PANAS (state) |
| Walkability - Objective | Walk Score or EPA Walkability | Perceived Stress | Perceived Stress Scale |
| Land Use Mix - Perceived | Neighborhood Environment Walkability Scale - Land Use Mix Access Subscale | Emotional Eating | Palatable Eating Motives: Coping Subscale |
| Food Outlet Accessibility - Objective | Density of (1) Supermarkets, (2) Fast food restaurants, and (3) Convenience stores | Binge Eating | Questionnaire on Eating and Weight Patterns |
| Food Outlet Accessibility - Perceived | Perceived presence of (1) Supermarkets, (2) Fast Food Restaurants, and (3) Convenience stores, Plus Others (Liese) | Trait Food Craving | Trait Food Craving Questionnaire- Reduced |
| Food Availability - Perceived | MESA Neighborhood Healthy Food Availability | Reward-Related Eating | Reward Based Eating Drive |
| Socioeconomic Deprivation - Objective | Neighborhood Deprivation Index (Diez Roux et al) | Executive Function | Behavior Rating Inventory of Executive Function-Adult |
| Delay Discounting | Kirby Questionnaire | ||
| Personal Safety - Objective | Neighborhood Police-Reported Crime | Behavioral Intention | Behavioral Intention Scale(s) |
| Personal Safety - Perceived | MESA Perceived Neighborhood Safety | Self-Efficacy | Self-Efficacy Scale(s) |
| BMI of Individuals in their Social Network - Objective | BMI of Spouse/Partner | Hedonic Response (‘liking’) and Motivation (‘wanting’) for Food | ‘Liking’ and ‘Wanting’ Visual Analog Scales |
| Weight status of Individuals in their Social Network - Perceived | Weight-related Social Norms Scale | Hunger and Satiety | Hunger and Satiety Visual Analog Scales |
| Support from Social Network | Perceived Autonomy Support Scale; Modified Sallis | Personality: Big Five Factors | Mini-International Personality Item Pool (Short form)/Big Five Inventory-2 (Long form) |
| Perceived Autonomy Support | Important Others’ Questionnaire | ||
CriteriaCriteria, evidence, and rationale for the selection of constructs and measures are described in detailed in the accompanying papers for the behavioral (30), biological (31), environmental (32), and psychosocial (33) domains. This list of high priority, core measures, along with descriptions, references, and relevant resources can be found on the GEM website (www.gem-measures.org).
Additional Considerations
It is important to note that some potential moderators of treatment response are assumed to be measured in all weight loss trials, including sociodemographic factors (e.g., age, sex, race, and ethnicity). These potential moderators of treatment responses are not listed in the table because they are likely to influence all four domains. Similarly, we did not include measures that may or may not be assessed depending on the population recruited or the obesity treatment provided (e.g. medical and weight history, medication use, and intervention process measures), but assume that these parameters will be measured and used in predictive models, if appropriate. The ADOPT working group endorsed the importance of capturing these additional measures in a uniform manner to facilitate measure harmonization across trials and enable data to be pooled across trials to better understand the effect of these factors on responses to obesity treatment.
APPLICATION OF THE ADOPT PRODUCTS
An Initial Step in an Ongoing Process
The recommended set of ADOPT Core Measures is intended to provide a first step in pursuing the broader project goals. This initial list is comprised of the most promising constructs and measures based on the above criteria and available evidence. For reasons discussed above, they are not always the most accurate but they are, whenever possible, measures that can be incorporated into investigations of various methodologies and sizes with a minimum of additional burden on the participants or researchers. The Working Group recognized that there are many potentially important measures being developed and refined, as well as newer and more promising constructs that will need to be considered as the science advances. To be successful, the research community must adapt to these new developments and discoveries, through periodic reevaluations of ADOPT that will update and refine the recommended list of measures.
Adopting the ADOPT Core Measures
The ADOPT framework is predicated on the assumption that advances in the design of obesity treatment will require a deeper understanding of how constructs across the four ADOPT domains interact with each other. To accomplish this, most investigators will need to expand the scale and scope of the measures they include in their trials. The ideal approach would be to include the entire set of core measures across the four domains in each trial when possible. For this reason, ADOPT is structured to provide guidance and support through GEM for using measures outside of one’s area of expertise in addition to recommending which constructs to assess. Inclusion of such measures is anticipated not only to increase statistical power in combined analyses but also to promote interdisciplinary collaboration.
Feasibility and Participant Burden
The ADOPT Working Group acknowledges that the addition of new measures within a trial is not without costs and considerations. Foremost among them is the added burden placed on study participants, both in terms of the time required for individual assessments and the invasiveness of a measure. Although some constructs need to be assessed only once, others require repeated assessments to capture changes in the construct over time. Certain measures may be more feasible than others to implement, and feasibility may depend on the size of the trial. Although the cost of including some measures is limited, others require specific tools or equipment (e.g., accelerometry) or pose logistical challenges (e.g., fasting blood draw). Identifying genetic predictors of intentional weight loss may not be feasible for most studies, due to participant burden and costs associated with genotyping. One approach to addressing this additional burden is to leverage existing infrastructure, such as core labs, coordinating centers, consortia of weight loss trials, or NIH-funded Obesity Research Centers, to standardize and centralize procedures and analyses associated with the ADOPT Core Measures. Coordination of these measures across existing infrastructure may reduce the overall cost and researcher burden, while establishing the foundation for subsequent proposals of large multi-center trials to test new treatment strategies.
Tissue Banking and Stored Data
A few of the core measures are intended to be banked specimens (e.g., blood) or stored data (e.g., residential address). The specific analyses of these samples or relevant application of the data may not appear on the ADOPT Core Measures list, due to the high cost or technical expertise required. The value of collection and storage of the tissues or data, on the other hand, was judged to be very high since subsequent cross-domain studies that leverage the appropriate resources and expertise may result in substantial strides in understanding the inter-domain interactions and the identification of novel, predictive constructs.
Utility of the GEM Website
To assist with the development of an assessment protocol, the ADOPT workspace within GEM provides investigators with a public, shared resource containing detailed information about best practices for using each measure, including, as appropriate, guidance regarding method of assessment, timing of assessment, resources required, and analysis protocols. This information will help investigators develop a plan for integrating these measures into their study and for collecting the data in a uniform manner. Given the potential value of combining datasets across future studies, it will be important for investigators to obtain the appropriate consent to share data. As investigators begin to work with the ADOPT measures, it would be helpful to the broader community if they share their experiences of applying the ADOPT measures in their research, via the GEM resource.
EVIDENCE GAPS AND NEEDS FOR FUTURE RESEARCH
Throughout the deliberations regarding the four ADOPT domains, several themes emerged concerning the currently available evidence base that guided the selection of specific constructs and measures. We provide a general overview of these issues here; details about specific challenges within each domain can be found in the companion papers (30, 31, 32, 33).
Need to Identify “Better” and “Best” Measures
Most subgroups identified high priority constructs for which data regarding the reliability and validity of specific measures were limited. These included constructs like food intake in the free-living environment, nutritional content of foods, food eating patterns, and emotional stress eating. In some cases, there was limited evidence regarding the discriminant validity of measures, which would help to clarify whether measures were assessing distinct constructs. In some domains, multiple measures are being used across studies to assess the same construct, but little comparative data are available within a trial to judge the most rigorous or appropriate measure for weight loss trials. These included constructs like neighborhood-level indicators of socioeconomic deprivation, indicators of sleep disorders, and measures of psychological constructs such as self-efficacy, behavioral intentions, or emotional eating. Thus, systematic work focused on the validity of current and new measures will provide valuable evidence to guide future decisions and selections.
Need for Measures that are More Feasible for Trials
In several cases, high priority constructs can be assessed with rigorous, reliable and valid measures, but these measures required expensive instrumentation or were judged too costly, burdensome to participants, or not logistically feasible for large weight loss trials. Examples of these types of measures include doubly-labeled water measures of expended energy, dual energy x-ray absorptiometry, and neuroimaging. Technological advancements that reduce the cost of acquiring these measures in large clinical trials are sorely needed.
Need to Better Specify Measurement Schedules
Many of the constructs identified across the four domains may change over time, and the degree of change has critical implications for treatment responses. Yet, there is limited information available regarding ideal timing of repeated assessments. For example, the initial trajectory of treatment response for some constructs may be of critical importance in predictive models, indicating that it should be measured more frequently during the first few months of treatment. The timing of measurements should include both weight loss and weight maintenance phases of treatment given the paucity of data thus far. Weight outcomes themselves may need to be measured more frequently to better predict critical inflections in weight.
Need to Build a Stronger Evidence Base in Trials
Across the four domains, there was considerable variability in the degree to which measures are traditionally included in weight loss trials. A critical challenge was the paucity or absence of data on purported psychosocial and environmental constructs that influence outcomes in adult weight loss trials. In the biological domain, research is needed to identify additional genetic predictors specific to weight loss and weight maintenance versus those predicting the development of obesity. This lack of evidence presents a clear challenge for these domains and systematic efforts to address this issue will provide data that can inform future decisions regarding the selection of the most appropriate constructs and measures. Multivariate models that include most or all the ADOPT constructs and measures may identify variables that are interdependent and/or serve as a proxy for each other. The engagement of and collaboration with statisticians to use appropriate methods to generate more parsimonious models should in turn reduce redundancy in future revisions of the ADOPT core measures list.
Need to Focus on Variability in Treatment Response Related to Health Disparities
One area—obesity disparities—posed significant challenges to the ADOPT Working Group due to considerable gaps in the available evidence base. Disparities in obesity treatment response across racial and ethnic groups are widely recognized (2, 43). For example, Wingo et al report that African-American adults typically lose 2-3 kg less than non-Hispanic white adults over 6-12 months within the same study (43). Efforts by the ADOPT Working Group to identify constructs with reliable and valid measures that may underlie these disparities were hampered by the lack of data within adult weight loss trials. Too often, sample sizes are insufficient for these subgroups in individual weight loss trials, highlighting a critical need for conducting more trials with harmonized data to examine subgroup differences in meta-analyses. It is also likely that new constructs and measures may be needed to understand the differential response to treatment across these subgroups. In fact, most domain subgroups struggled with issues regarding the validity of measures across different samples due to insufficient testing of instruments among demographic subpopulations. The lack of an evidence base regarding disparities in response to obesity treatment is a critical gap that needs to be a major focus for future research.
LOOKING AHEAD
Opportunities for Collaboration
The ADOPT constructs and measures are housed in GEM to encourage and enable investigators to share information and guidance regarding measures (Supplemental Table 2). There are two ways investigators can engage with ADOPT through GEM. First, investigators can provide comments and additional information regarding the ADOPT core constructs and measures. Second, investigators can upload other constructs and measures into GEM through the four ADOPT domain workspaces. This provides investigators with the opportunity to share information about emerging constructs and measures that can, in turn, inform future recommendations regarding core constructs and measures. The ADOPT workspaces in GEM also have the potential to facilitate other forms of information sharing across a community of investigators. For example, investigators could share which measures are included in their current and past clinical trials to facilitate opportunities for data pooling. Investigators could also identify themselves as experts within specific content areas who are available to provide guidance regarding best practices for specific constructs and measures.
Realizing the Long-Term Vision
As we work collectively toward this goal, it will be important to track the impact of this project on the field. ADOPT presents an opportunity to build a community of investigators committed to advancing the state of the science needed to tailor adult obesity treatment. With the consistent use of the best measurement practices, investigators can enhance the quality of the data collected and the collective value of the entire evidence base. The evidence base generated by the scientific community can then be more effectively utilized through systematic reviews, meta-analytic syntheses, and predictive modeling to identify the mediating and moderating processes that underlie response to obesity treatment. Ultimately, our community of scientists and clinicians will leverage this evidence base to advance our understanding of how to predict optimal responses to obesity treatment and to develop more tailored and effective treatments.
Supplementary Material
What is already known on this subject?
A significant challenge to developing better obesity treatment strategies is the large amount of Individual variability in treatment responses.
Few explanatory variables have been found to reliably predict the response to treatments.
We lack an evidence base with relevant behavioral, biological, environmental, and psychosocial factors that could inform approaches for tailored treatments.
What does our study add?
The ADOPT Core Measures project establishes a long-term vision for developing tailored treatments for obesity in adults based upon a better understanding of individual variability in response to treatments.
ADOPT products are designed to provide investigators with the tools to collect data to map the complex processes that mediate or moderate the effect of treatment on weight loss and weight maintenance in adults.
ADOPT has developed an initial list of core constructs and measures that, if used consistently in weight loss trials, could help to identify key predictors of treatment responses.
Acknowledgments
This overview reflects the culmination of the efforts of the entire ADOPT Core Measures Working Group. A complete list of working group members can be found in supplemental information (Table S1). The authors acknowledge particular members, Drs. Deborah Young-Hyman, Michael Rosenbaum, Aaron Laposky, and Angelina Sutin, who provided a critical review of this manuscript. The authors also acknowledge Dr. Katrina Serrano for her role in developing and conducting this project during the early phases.
FUNDING: The ADOPT Core Measures Working Group was supported by intramural funding from the National Institutes of Health: the National Heart, Lung, and Blood Institute; the National Cancer Institute; and the Office of Disease Prevention. The Grid-Enabled Measures Database (GEM) is supported and administered by the National Cancer Institute.
PSM and APC report grants and/or pending grant applications from NIH and PCORI that are directly relevant to this work.
Footnotes
DISCLOSURE:All other authors declared no conflicts of interest that are directly relevant to the work under consideration. The views expressed in this paper are those of the authors, and do not necessarily represent the positions of the NIH, the DHHS, or the Federal Government.
References
- 1.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of Obesity Among Adults and Youth: United States, 2011–2014. NCHS Data Brief. 2015:1–8. [PubMed] [Google Scholar]
- 2.Whitt-Glover MC, Kumanyika SK, Haire-Joshu D. Introduction to the special issue on achieving healthy weight in black American communities. Obes Rev. 2014;15(Suppl 4):1–4. doi: 10.1111/obr.12210. [DOI] [PubMed] [Google Scholar]
- 3.Heymsfield SB, Wadden TA. Mechanisms, Pathophysiology, and Management of Obesity. N Engl J Med. 2017;376:254–266. doi: 10.1056/NEJMra1514009. [DOI] [PubMed] [Google Scholar]
- 4.Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J Health Econ. 2012;31:219–230. doi: 10.1016/j.jhealeco.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Sikorski C, Luppa M, Kaiser M, Glaesmer H, Schomerus G, Konig HH, et al. The stigma of obesity in the general public and its implications for public health - a systematic review. BMC Public Health. 2011;11:661. doi: 10.1186/1471-2458-11-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bray GA, Fruhbeck G, Ryan DH, Wilding JP. Management of obesity. Lancet. 2016;387:1947–1956. [Google Scholar]
- 8.MacLean PS, Wing RR, Davidson T, Epstein L, Goodpaster B, Hall KD, et al. NIH working group report: Innovative research to improve maintenance of weight loss. Obesity (Silver Spring) 2015;23:7–15. doi: 10.1002/oby.20967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kraschnewski JL, Boan J, Esposito J, Sherwood NE, Lehman EB, Kephart DK, et al. Long-term weight loss maintenance in the United States. International journal of obesity (2005) 2010;34:1644–1654. doi: 10.1038/ijo.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr. 2001;74:579–584. doi: 10.1093/ajcn/74.5.579. [DOI] [PubMed] [Google Scholar]
- 11.Weiss EC, Galuska DA, Kettel Khan L, Gillespie C, Serdula MK. Weight regain in U.S. adults who experienced substantial weight loss, 1999–2002. American journal of preventive medicine. 2007;33:34–40. doi: 10.1016/j.amepre.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 12.Wood GC, Benotti PN, Lee CJ, Mirshahi T, Still CD, Gerhard GS, et al. Evaluation of the Association Between Preoperative Clinical Factors and Long-term Weight Loss After Roux-en-Y Gastric Bypass. JAMA Surg. 2016;151:1056–1062. doi: 10.1001/jamasurg.2016.2334. [DOI] [PubMed] [Google Scholar]
- 13.Adams TD, Davidson LE, Litwin SE, Kolotkin RL, LaMonte MJ, Pendleton RC, et al. Health benefits of gastric bypass surgery after 6 years. Jama. 2012;308:1122–1131. doi: 10.1001/2012.jama.11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elfhag K, Rossner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2005;6:67–85. doi: 10.1111/j.1467-789X.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- 15.Stubbs J, Whybrow S, Teixeira P, Blundell J, Lawton C, Westenhoefer J, et al. Problems in identifying predictors and correlates of weight loss and maintenance: implications for weight control therapies based on behaviour change. Obes Rev. 2011;12:688–708. doi: 10.1111/j.1467-789X.2011.00883.x. [DOI] [PubMed] [Google Scholar]
- 16.Teixeira PJ, Carraca EV, Marques MM, Rutter H, Oppert JM, De Bourdeaudhuij I, et al. Successful behavior change in obesity interventions in adults: a systematic review of self-regulation mediators. BMC Med. 2015;13:84. doi: 10.1186/s12916-015-0323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawamoto R, Nozaki T, Nishihara T, Furukawa T, Hata T, Komaki G, et al. Predictors of successful long-term weight loss maintenance: a two-year follow-up. Biopsychosoc Med. 2017;11:14. doi: 10.1186/s13030-017-0099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross KM, Graham Thomas J, Wing RR. Successful weight loss maintenance associated with morning chronotype and better sleep quality. J Behav Med. 2016;39:465–471. doi: 10.1007/s10865-015-9704-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bachar A, Hermoni D, Livshits G, Birk R. Late successful weight reduction and maintenance among overweight and obese adults–a two-year retrospective study. Diabetes Res Clin Pract. 2014;106:511–521. doi: 10.1016/j.diabres.2014.09.055. [DOI] [PubMed] [Google Scholar]
- 20.Svetkey LP, Ard JD, Stevens VJ, Loria CM, Young DY, Hollis JF, et al. Predictors of long-term weight loss in adults with modest initial weight loss, by sex and race. Obesity (Silver Spring) 2012;20:1820–1828. doi: 10.1038/oby.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Hout GC, Verschure SK, van Heck GL. Psychosocial predictors of success following bariatric surgery. Obes Surg. 2005;15:552–560. doi: 10.1381/0960892053723484. [DOI] [PubMed] [Google Scholar]
- 22.Lazzeretti L, Rotella F, Pala L, Rotella CM. Assessment of psychological predictors of weight loss: How and what for? World J Psychiatry. 2015;5:56–67. doi: 10.5498/wjp.v5.i1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livhits M, Mercado C, Yermilov I, Parikh JA, Dutson E, Mehran A, et al. Preoperative predictors of weight loss following bariatric surgery: systematic review. Obes Surg. 2012;22:70–89. doi: 10.1007/s11695-011-0472-4. [DOI] [PubMed] [Google Scholar]
- 24.Wadden TA, Neiberg RH, Wing RR, Clark JM, Delahanty LM, Hill JO, et al. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity. 2011;19:1987–1998. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westover SA, Lanyon RI. The maintenance of weight loss after behavioral treatment. A review. Behav Modif. 1990;14:123–137. doi: 10.1177/01454455900142001. [DOI] [PubMed] [Google Scholar]
- 26.McCaffery JM, Papandonatos GD, Huggins GS, Peter I, Erar B, Kahn SE, et al. Human cardiovascular disease IBC chip-wide association with weight loss and weight regain in the look AHEAD trial. Hum Hered. 2013;75:160–174. doi: 10.1159/000353181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsai AG, Fabricatore AN, Wadden TA, Higginbotham AJ, Anderson A, Foreyt J, et al. Readiness redefined: a behavioral task during screening predicted 1-year weight loss in the look AHEAD study. Obesity. 2014;22:1016–1023. doi: 10.1002/oby.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strohacker K, McCaffery JM, MacLean PS, Wing RR. Adaptations of leptin, ghrelin or insulin during weight loss as predictors of weight regain: a review of current literature. International journal of obesity (2005) 2014;38:388–396. doi: 10.1038/ijo.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teixeira PJ, Going SB, Sardinha LB, Lohman TG. A review of psychosocial pre-treatment predictors of weight control. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2005;6:43–65. doi: 10.1111/j.1467-789X.2005.00166.x. [DOI] [PubMed] [Google Scholar]
- 30.L LA, N HL, R S, E M, J JM, L AD, et al. Accumulating Data to Optimally Predict obesity Treatment (ADOPT) Core measures: Behavioral Domain. Obesity (Silver Spring) 2018 doi: 10.1002/oby.22157. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.R M, A-C T, B MS, H KD, H M, L M, et al. Accumulating Data to Optimally Predict obesity Treatment (ADOPT) Core measures: Biological Domain. 2018 In Press. [Google Scholar]
- 32.S BE, A SS, B D, B RM, G A, P-W T, et al. Accumulating Data to Optimally Predict obesity Treatment (ADOPT) Core Measures: Environmental Domain. Obesity (Silver Spring) 2018 doi: 10.1002/oby.22159. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.S AR, B K, C SM, E ES, G PA, H CM, et al. Accumulating Data to Optimally Predict obesity Treatment (ADOPT) Core Measures: Psychosocial Domain. Obesity (Silver Spring) 2018 doi: 10.1002/oby.22160. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kraemer HC, Kiernan M, Essex M, Kupfer DJ. How and why criteria defining moderators and mediators differ between the Baron & Kenny and MacArthur approaches. Health Psychol. 2008;27:S101–108. doi: 10.1037/0278-6133.27.2(Suppl.).S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fairchild AJ, MacKinnon DP. A general model for testing mediation and moderation effects. Prev Sci. 2009;10:87–99. doi: 10.1007/s11121-008-0109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci U S A. 2013;110:5695–5700. doi: 10.1073/pnas.1216951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leger D, Bayon V, de Sanctis A. The role of sleep in the regulation of body weight. Mol Cell Endocrinol. 2015;418(Pt 2):101–107. doi: 10.1016/j.mce.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 38.King NA, Horner K, Hills AP, Byrne NM, Wood RE, Bryant E, et al. Exercise, appetite and weight management: understanding the compensatory responses in eating behaviour and how they contribute to variability in exercise-induced weight loss. Br J Sports Med. 2012;46:315–322. doi: 10.1136/bjsm.2010.082495. [DOI] [PubMed] [Google Scholar]
- 39.Westerterp-Plantenga MS, Nieuwenhuizen A, Tome D, Soenen S, Westerterp KR. Dietary protein, weight loss, and weight maintenance. Annu Rev Nutr. 2009;29:21–41. doi: 10.1146/annurev-nutr-080508-141056. [DOI] [PubMed] [Google Scholar]
- 40.St-Onge MP. Sleep-obesity relation: underlying mechanisms and consequences for treatment. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2017;18(Suppl 1):34–39. doi: 10.1111/obr.12499. [DOI] [PubMed] [Google Scholar]
- 41.Hopkins M, Gibbons C, Caudwell P, Hellstrom PM, Naslund E, King NA, et al. The adaptive metabolic response to exercise-induced weight loss influences both energy expenditure and energy intake. Eur J Clin Nutr. 2014;68:581–586. doi: 10.1038/ejcn.2013.277. [DOI] [PubMed] [Google Scholar]
- 42.Maclean PS, Bergouignan A, Cornier MA, Jackman MR. Biology’s response to dieting: the impetus for weight regain. Am J Physiol Regul Integr Comp Physiol. 2011;301:R581–600. doi: 10.1152/ajpregu.00755.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wingo BC, Carson TL, Ard J. Differences in weight loss and health outcomes among African Americans and whites in multicentre trials. Obes Rev. 2014;15(Suppl 4):46–61. doi: 10.1111/obr.12212. [DOI] [PubMed] [Google Scholar]
- 44.Smith SR, Prosser WA, Donahue DJ, Morgan ME, Anderson CM, Shanahan WR. Lorcaserin (APD356), a selective 5-HT(2C) agonist, reduces body weight in obese men and women. Obesity (Silver Spring) 2009;17:494–503. doi: 10.1038/oby.2008.537. [DOI] [PubMed] [Google Scholar]
- 45.Donnelly JE, Smith BK. Is exercise effective for weight loss with ad libitum diet? Energy balance, compensation, and gender differences. Exerc Sport Sci Rev. 2005;33:169–174. doi: 10.1097/00003677-200510000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Donnelly JE, Hill JO, Jacobsen DJ, Potteiger J, Sullivan DK, Johnson SL, et al. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women: the Midwest Exercise Trial. Arch Intern Med. 2003;163:1343–1350. doi: 10.1001/archinte.163.11.1343. [DOI] [PubMed] [Google Scholar]
- 47.Courcoulas AP, Christian NJ, Belle SH, Berk PD, Flum DR, Garcia L, et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310:2416–2425. doi: 10.1001/jama.2013.280928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manning S, Pucci A, Carter NC, Elkalaawy M, Querci G, Magno S, et al. Early postoperative weight loss predicts maximal weight loss after sleeve gastrectomy and Roux-en-Y gastric bypass. Surg Endosc. 2015;29:1484–1491. doi: 10.1007/s00464-014-3829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


