Abstract
Gonadotropins are pituitary gonadotrope-derived glycoprotein hormones. They act by binding to G-protein coupled receptors on gonads. Gonadotropins play critical roles in reproduction by regulating both gametogenesis and steroidogenesis. Although biochemical and physiological studies provided a wealth of knowledge, gene manipulation techniques using novel mouse models gave new insights into gonadotropin synthesis, secretion and action. Both gain of function and loss of function mouse models for understanding gonadotropin action in a whole animal context have already been generated. Moreover, recent studies on gonadotropin actions in non-gonadal tissues challenged the central dogma of classical gonadotropin actions in gonads and revealed new signaling pathways in these non-gonadal tissues. In this Chapter, we have discussed our current understanding of gonadotropin synthesis, secretion and action using a variety of genetically engineered mouse models.
Keywords: Pituitary, Gonadotrope, Luteinizing hormone, Follicle-Stimulating Hormone, Transgenic mice, Testis, Ovary
Introduction
The anterior pituitary is composed of five cell types- gonadotropes, thyrotropes, somatotropes, lactotropes, and corticotropes. The gonadotropes produce luteinizing hormone (LH) and follicle stimulating hormone (FSH), and the thyrotropes produce thyroid stimulating hormone (TSH). LH, FSH, and TSH belong to the pituitary glycoprotein family of heterodimeric hormones consisting of a common α-subunit non-covalently linked to a hormone-specific β-subunit (1). Human chorionic gonadotropin (hCG) shares the same α-subunit as LH, FSH, and TSH, but the hCG heterodimer is derived from placental syncytiotrophoblast cells (2). While hCG is only found in primates and horses, the pituitary gonadotropins are conserved throughout mammalian evolution and will be the focus of this review (1, 3).
The gonadotropin α– and β-subunits are encoded by distinct single-copy genes located on separate chromosomes. In humans, the α-subunit is mapped to chromosome 6, the LHβ-subunit is mapped to chromosome 19, and the FSHβ-subunit is mapped to chromosome 11 (1, 4). LH and FSH are under transcriptional and translational control by multiple factors including the downstream sex steroids, and the upstream signal from the hypothalamic derived decapeptide, gonadotropin-releasing hormone (GnRH) (5-7). Pituitary specific cell fate and function appears to be under partial control of the GATA family of transcription factors which are present at high levels in the developing pituitary and adult gonadotropes (8). Although LH and FSH are released from the same cell, they are secreted independently of one another, and their secretion is distinctly regulated by GnRH pulsatility and differences in segregation into secretory granules (1, 6, 9).
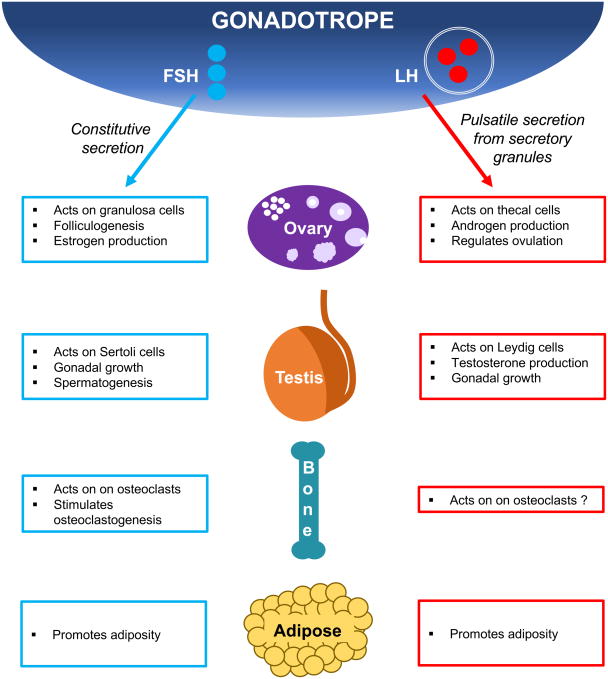
LH acts on target cells by binding to the LH receptor (LHR), and is required for steroidogenesis, gonadal growth, and gametogenesis. LHR is expressed on testicular Leydig cells as well as ovarian granulosa and theca cells. FSH signals via the FSH receptor (FSHR), and is essential for initiation of spermatogenesis and follicle maturation in males and females, respectively. FSHR is expressed on ovarian granulosa cells and on Sertoli cells in the testes. Recent reports have identified expression of FSHR in several extra-gonadal tissues, including uterus, placenta, adipose tissue, bone, and tumor blood vessels (10). In this review, we will focus primarily on mouse models that were developed to study LH and FSH actions at the level of the ovary and testis. We will also review known actions of FSH on bone and adipose tissue, as these tissues in mouse models have recently been highlighted in the field of gonadotropin action (Fig. 1).
Figure 1.
1. Mouse Models of Gonadotropin Secretion and Action
1.1. Transcriptional Models
1.1.1. α-Glycoprotein Subunit Overexpression
The common α-subunit (Cga) mRNA is present as early as embryonic day 11.5 (E11.5) in the mouse pituitary (11). It is the first of the glycoprotein hormone subunits to be expressed during development, and it is synthesized in excess compared to β-subunit transcripts (3). In 1988, using a transgenic mouse model overexpressing human CGA, Fox and Solter demonstrated that the human transgene is only expressed in the pituitary in mice demonstrating that mouse trophoblast cells lack the regulatory factors needed for α-subunit expression in placenta (3). Fox and Solter also observed increased production of the α-subunit in castrated transgenic mice, suggesting a direct role of gonadal steroids as suppressors of α-subunit mRNA expression. Although dimerization of the transgenic α-subunit and the mouse β-subunits was not directly tested in thisstudy, no physiologic abnormalities including impaired dimerization, infertility or thyroid disorders were noted in these mice (3).
Other transgenic mouse models have been useful to better understand the promoter regions involved in α-subunit expression (12), and to identify separate cis-acting promoter regions that regulate α-subunit expression in gonadotropes and thyrotropes (12). Transient transfection studies using a Cre recombinase have identified DNA elements that are important for expression and function of the α-subunit in gonadotropes and thyrotropes. These studies also provided insight into the DNA sequence required for α-subunit expression in mice (13, 14). A placental-specific cAMP response element has also been identified in the α-subunit, and was found to be essential for tissue-specific expression (15).
1.1.2. a-Glycoprotein Subunit Knockout
To study the in vivo consequences of loss of the α -subunit on mouse pituitary and gonad development, the Camper group developed mice lacking the gonadotropin α-subunit (16). Targeted disruption of the α-subunit gene in mice resulted in growth deficiency, severe hypothyroidism, and hypogonadism in male and female mice (16). However, despite hypogonadism, sexual differentiation and genital development were not affected in these mutant mice. Using the same α-subunit knockout model, Gergics and Camper further evaluated thyrotrope hypertrophy and hyperplasia observed in α-subunit knockout mice and found that, although the β-subunit of TSH was abundantly expressed in thyrotropes, it was not secreted without expression of the α-subunit. The impaired TSH heterodimer secretion resulted in endoplasmic reticulum stress, an increased unfolded protein response, and up-regulation of cellproliferation genes, which promoted thyrotrope proliferation and proto-oncogene activation, suggesting a potential role for α-subunit deficiency in thyrotrope adenomas in humans (17).
1.1.3. hCGβ-Subunit Overexpression
The placental gonadotropin, hCG, is expressed at high levels by the placenta during the early stages of pregnancy. The gene encoding hCGβ is located on chromosome 19 (18). The hCGβ-and LHβ-subunits have similar structures and interact with the same receptor, but a carboxyterminal peptide (CTP) sequence found on hCGβ extends its half-life beyond that of LH (2). Several mouse models have been developed to further study and understand the mechanisms of hCG action, as well as the role of hCG in cancer.
Expression of the hCGb-subunit from the mouse metallothionein -1 (mMT-1) or ubiquitin C promoters results in the production of transgenic mice that overexpress hCGβ from multiple tissues, and hCGβ overexpression leads to infertility in both male and female mice (19, 20). These promoters were purposefully chosen to achieve wide-spread expression in multiple tissues. In male MT-hCGβ transgenic mice, no morphological or histological defects were detectable in the testes. However, ovarian defects were observed in female MT-hCGβ mice, including a block in folliculogenesis with no detectable antral follicles or corpora lutea. Some female MT-hCGβ transgenic mice also developed polycystic ovarian syndrome (PCOS)-like ovarian histology with cysts and large corpora lutea, as well as enlarged uterine horns and an increase in testosterone and progesterone (19, 20). The reproductive defects observed in female hCGβ mice are most likely explained by an extreme increase in the progesterone/estrogen ratio (20). Thus, in contrast to hCGα, overexpression of hCGβ impairs fertility in mice, most likely because hCGβ issecreted more efficiently in vivo and interferes with the LH/hCG receptor interaction at the gonads (19, 21, 22).
In addition to reproductive defects, female hCGβ transgenic mice became obese by 6 months of age, weighing as much as twice that of wild type control mice, primarily due to increased accumulation of abdominal adipose tissue (20). These mice also developed progesterone-dependent lactotrope adenomas with elevated serum prolactin and subsequent metabolic disturbances, as well as abnormal lobuloalveolar mammary gland development (20, 23, 24). Metastatic mammary gland adenocarcinomas developed in hCGβ mice by 12 months of age and were likely a result of increased circulating progesterone, which hypersensitizes mammary cells to the action of growth factors (20). The pituitary and mammary phenotypes were prevented by ovariectomy at 6 weeks of age (20), suggesting that elevated gonadotropins are unlikely to produce similar abnormal phenotypes in postmenopausal women. Additionally, the infertility, obesity, and hyperandrogenism observed in hCGβ transgenic females was correctable with short-term administration of a dopamine agonist, if started at a young age, demonstrating the strong effect of hyperprolactinemia on the phenotypes of hCGβ transgenic mice (25). Female hCGβ transgenic mice also displayed an upregulation of genes belonging to the Wnt signaling family, and this was independent of changes in ovarian steroidogenesis. The activation of Wnt signaling promoted beta-catenin-stabilizing mammary tumorigenesis, which may have implications in pregnancy-induced breast cancers (26).
1.1.4. hCGα/β Overexpression
To achieve overexpression of the biologically active hCG dimer, transgenic hCGα/β-expressing mice were generated by cross-breeding transgenic mice expressing the hCGα-subunit with those expressing hCGβ (27). Male mice in this group had a 2000-fold increase in bioactive hCG, and were infertile due to an inability to copulate (27). They were found to have normal spermatogenesis and sperm quality, but tubular degeneration occurred with age and mice displayed progressive detrimental effects to accessory reproductive organs and kidneys (27). Male hCGα/β transgenic mice displayed elevated testosterone levels, but surprisingly, this did not result in precocious puberty (28). Leydig cell adenomas were present in prepubertal mice but not adult mice, providing further insight into the potential tumorigenic effects of elevated gonadotropins (28). Female hCGα/β transgenic mice displayed elevated estrogen, testosterone, and progesterone levels, as well as increased bone mineral density in animals with intact ovaries, and “sclerotic-like” histomorphometric parameters indicative of reduced bone resorption and/or increased osteoblastic activity (29). In contrast, bone mineral density was unchanged in hCGα/β transgenic males (29).
However, another hCG dimer-expressing transgenic mouse line was created by co-injecting both the MT-hCGα and MT-hCGβ transgenes, and the phenotypes observed were greatly different. Overexpression of hCGa and hCGβ from the mMT-1 promoter results in the production of transgenic mice that overexpress the hCG dimer from multiple tissues. With low-level overexpression of the hCG dimer (both transgenes expressed at low copy number), mice became infertile by 6-7 months of age but did not present with gonadal defects or histological abnormalities (19). With high-level expression of the hCG dimer, both male and female mice were infertile and displayed multiple reproductive defects (19). Male transgenic mice had highserum testosterone as well as low serum LH and FSH, and displayed enlarged seminal vesicles, Leydig cell hyperplasia, and reduced testis size. Some seminiferous tubules from hCG dimer-expressing transgenic mice contained only Sertoli cells, and no spermatogonia or developing spermatocytes, which is indicative of germ cell loss (19). Female hCGα/β transgenic mice had high serum estradiol and enlarged uterine horns, along with ovarian hemorrhage and multiple cysts. These mice also demonstrated abnormal ovarian histology, with follicles that contained enlarged thecal layers and multi-nucleated, proliferating stromal cells (19). High-level hCG dimer-expressing mice also displayed extra-gonadal phenotypes including behavioral aggression in males and urinary tract defects in females. However, the mammary and adrenal glands were not affected by hCG dimer overexpression in this mouse model (19). This study suggests that the extent of overexpression of the hCG dimer is critical for functional activity and phenotypic outcome.
1.1.5. LHβ-Subunit Overexpression
Expression of LHβ-subunit mRNA begins on E13.5 in the mouse pituitary, prior to the expression of FSHβ-subunit mRNA, and becomes significantly increased by E16.5; LHβ protein is detectable shortly after mRNA is expressed (11, 30). Transgenic animal models investigating LHβ-subunit promoter regions have identified DNA elements such as steroidogenic factor-1 (SF-1) (31), NF-Y (32), and Pitx1 (33) as regulators of LHβ-subunit activity, both basally and in response to GnRH (5). In women, overexpression or hypersecretion of LH has been implicated in infertility as well as PCOS. In humans, PCOS is characterized by elevated serum LH, insulin resistance, increased ovarian androgen production, ovarian cysts, anovulation, and infertility (34, 35). To further study the potential association between LH and human disease, and to betterunderstand and characterize properties of the LHβ-subunit, multiple transgenic mouse models have been developed. However, maintaining elevated serum LH in mouse models can be challenging because LH has a short half-life. As mentioned previously, the CTP of hCG functions to prolong the half-life of the hCG by reducing elimination of the heterodimer from serum, and this peptide can be utilized to increase the half-life of LHβ. Therefore, LHβ-CTP-expressing transgenic mice provide a means to study prolonged increases in serum LH without the need for repeated injections or supraphysiologic dosing. Despite this, LHβ-CTP transgenic mice overexpress LHβ at a much lower level than that of hCGβ overexpression in the hCGβ transgenic mouse model discussed previously, as a result of the different promoters used to express the transgenes (20, 36).
Male LHβ-CTP transgenic mice were subfertile with reduced testis size, but had normal circulating testosterone levels (36). Female LHβ-CTP transgenic mice were also subfertile but had more pronounced reproductive defects than males, including chronic anovulation and enlarged, PCOS-like ovaries along with elevated progesterone, an increased life span of corpora lutea, and a diminished primordial follicle pool by three months of age (36, 37). Female LHβ-CTP mice also developed ovarian luteomas and tumors of granulosa/stromal cells, theca/interstitial cells, mammary tissue, and pituitary, in addition to urinary tract and adrenal cortex defects (36, 38-40). The phenotypes observed in LHβ-CTP mice were similar to those seen in patients with ovarian hyperstimulation syndrome (OHSS), and implicate elevated LH as a causative factor for the increased prevalence of ovarian tumors in patients undergoing fertility treatment (32). Expression of LHR was increased in the adrenal glands of LHβ-CTP mice, resulting in elevated production of corticosterone (41). Female LHβ-CTP mice demonstratedincreased serum testosterone as early as 2 weeks of age, accompanied by precocious vaginal opening and enlarged uteri (42). Although female LHβ-CTP mice were infertile, oocytes harvested from these mice formed viable embryos when transferred to nontransgenic pseudopregnant recipients, but oocytes transferred to LHβ-CTP mice from nontransgenic controls failed to implant due to defects in uterine receptivity (43). Transgenic mice expressing LHβ without CTP had equal levels of serum LH and show similar, although less pronounced phenotypes compared to LHβ-CTP transgenic mice, due to the shorter half-life of LHβ (36).
Many of the phenotypes described above with LHβ-CTP transgenic mice are similar to those noted in ERα knockout mice. These ERα null mice also had elevated serum LH, due to a loss of negative feedback by estradiol on the pituitary gland. ERα, but not ERβ, is required for the negative feedback effect of estradiol to maintain serum LH low (44). ERα knockout mice had elevated serum estrogen and testosterone, and exhibited similar ovarian phenotypes as LHβ-CTP transgenic mice, including infertility, chronic anovulation, absence of corpora lutea, ovarian cysts, interstitial and stromal cell hyperplasia, and granulosa cells tumors (44, 45). Although ERβ is not required for negative feedback by estradiol, it is highly expressed by ovarian granulosa cells and is required for formation of cystic ovarian follicles in ERα knockout and LHβ-CTP mice (45).
1.1.6. LHβ-Subunit Knockout
In hpg mutant mice, the GnRH peptide is not expressed, and therefore these mice lack expression of both LH and FSH (46, 47). Cga-null mice also lack both LH and FSH because the common α-subunit is not expressed (16). Both hpg and Cga mutant mice present with a series of reproductive defects but from these mouse models, it is not possible to discern which defects are specific to LH- or FSH- deficiency. However, a null mutation at the Lhb locus resulted in the production of LHβ knockout mice that completely lacked expression of the hormone-specific LHβ-subunit in the pituitary (48, 49). Lhb null mice have normal FSH expression levels, allowing for characterization of physiological parameters that are regulated by LH, independent of FSH. Lhb knockout mice displayed hypogonadism, defective steroidogenesis, and complete infertility (48, 49).
Male LHβ knockout mice displayed decreased testis size, reduced serum testosterone, and elevated serum androstenedione. Leydig cell differentiation was blocked as manifested by diminished Leydig cell number and size, expression of fetal Leydig cell markers in the adult testis, as well as aberrant cell-cycle regulation with increased proliferation and reduced expression of p27, a cell cycle inhibitor (49). Furthermore, male Lhb knockout mice showed reduced expression of steroidogenic enzymes Hsd3b6, Cyp17a1, and Hsd17b3. Mutant male mice also displayed accessory gland abnormalities such as hypoplastic epididymides and seminal vesicles. Spermatogenesis proceeded normally until the production of round spermatids, but was arrested thereafter in the absence of LH (49). Expression of mRNAs encoding inhibinβA and inhibitβB subunits was increased, and serum AMH was also elevated, demonstrating that LH deficiency results in aberrant expression of Sertoli cell markers (49). This Lhb knockout mouse model was also used for further studies to define the role of osteocalcin in male reproductive function (50). Osteocalcin is a bone osteoblast-derived hormone and has been shown to stimulate testosterone production by binding to its receptor GPRC6A expressed on Leydig cells in testis.Interestingly, mice lacking osteocalcin have elevated LH levels. To address whether LH mediates osteocalcin actions on testis, Lhb knockout male mice were treated with recombinant osteocalcin. Serum testosterone levels were elevated compared to those in PBS injected Lhb knockout mice indicating that LH and osteocalcin act in parallel pathways (50).
Female LHβ knockout mice had reduced ovarian size, impaired cyclicity, and defects in ovarian folliculogenesis, including a block at the pre-ovualtory stage with the presence of abnormal antral follicles that contained degenerated and apoptotic oocytes and no corpora lutea (49). Although thecal cell differentiation was not affected in the absence of LH, mutant mice showed reduced expression of steroidogenesis genes including Cyp11a1, Cyp19a1, and Cyp17a1, and expression of Cox2, a marker of ovulation, was suppressed. LHβ-null females had decreased serum estradiol and progesterone, as well as severely hypoplastic uteri (49).
The phenotypes observed in male and female LHβ knockout mice can be rescued by administration of exogenous hCG, which is an LH analog. Successful rescue with hCG demonstrates that the Lhb knockout mice, despite the absence of LHβ expression, remain LH-responsive. Following injection with hCG, male Lhb knockout mice demonstrated increased expression of steroidogenic enzymes, and female Lhb knockout mice displayed activation of ovarian response genes as well as the successful release of oocytes following superovulation (49). Importantly, pharmacological rescue of male LHβ knockout mice with testosterone rescued expression of Hsd3b1 and Hsd3b6, but not Cyp17a1, demonstrating that LH directly regulates expression of Cyp17a1 in a testosterone independent manner (49).
1.1.7. LHR KO
Targeted disruption of the Lhr gene results in infertility in male and female mice, further demonstrating that LH action is required for fertility in both sexes (51). Both male and female LHR knockout mice displayed dramatically elevated circulating LH, moderately elevated circulating FSH, and underdeveloped internal and external genitalia, as well as arrested postnatal sexual development (51, 52). Lhr knockout male mice had reduced circulating testosterone, increased circulating estrogen, Leydig cell hypoplasia, and postmeiotic arrest of spermatogenesis (51). Further analysis showed that FSH action allows for normal spermatogenesis initially, but that LH and testosterone are required for spermatogenesis to proceed beyond the round spermatid stage (53, 54). Pre-pubertal testosterone replacement only partially restored fertility in male Lhr knockout mice, suggesting that testosterone-independent actions of LH may regulate male fertility (55). Female Lhr knockout mice displayed an antral stage block in folliculogenesis in addition to decreased circulating estrogen and progesterone (51). Similar to the arrest in spermatogenesis observed with Lhr knockout males, FSH stimulates normal follicular development from the preantral stage to the early antral stage, but LH is required for development thereafter in females (52). The phenotypes observed in female Lhr knockout mice were not completely reversed by estrogen replacement therapy, suggesting that LH directly regulates reproductive function, independent of sex steroids (56). By 12 months of age, some Lhr knockout mice became obese compared to wild type controls, and all Lhr knockout females eventually developed endometrial tumors. Sex steroids are well known regulators of food intake and thermogenesis and thus they play an important role in energy and body weight homeostasis. Hypogonadism, which results in severely suppressed or absent estrogen is often associated with increased obesity, although emerging evidence indicates that gonadotropins may directlyregulate adiposity. Male and female Lhr knockout and heterozygous mice also displayed decreased bone density by 8 weeks, and this phenotype persisted beyond one year of age (56).
1.1.8. LHR Gain of Function
Two types of transgenic mouse models were generated to study activating Lhr mutations: mice expressing hCG covalently linked to LHR (termed YHR+ mice); and mice expressing an activating LHR mutation (D556H mice) (57). Male YHR+ mice were fertile, and presented with increased serum testosterone and seminal vesicle weight along with decreased serum LH and FSH, but no apparent precocious puberty (58, 59). In contrast, male D556H mice were infertile and had reduced testicular weight, decreased serum testosterone, reduced serum FSH, and normal serum LH (58, 59). Female YHR+ mice displayed a phenotype similar to that of LHβ or hCG overexpressing mice, and presented with precocious puberty, elevated estrogen and progesterone, reduced serum LH and FSH, increased uterine weight, follicular cysts, interstitial cell hypertrophy, and anovulation (58, 59). Female D556H mice were infertile and acyclic, with elevated serum progesterone, ovarian cysts, increased corpora lutea, interstitial cell hypertrophy, and degenerating follicles (58, 59). Importantly, the corresponding D578H mutation in LHR has been identified in humans, but only in males, and results in the formation of Leydig cell tumors (59).
1.1.9. FSHβ-Subunit Overexpression
During mouse pituitary development, FSHβ is the last of the gonadotropin subunits to be expressed. FSHβ mRNA is expressed at very low levels at E13.5 (30), and becomes significantlyincreased by E17.5 (11). FSHβ protein expression lags behind FSHβ mRNA expression and is not present in large quantities until birth or as late as postnatal day 14 (30). In an effort to identify essential DNA regulatory elements of the human FSHβ gene, a number of transgenic mouse models have been developed. The regulatory elements on the human FHSβ transgene were analyzed using many of these transgenic models (60). Sequence analyses of the 5′ proximal promoter revealed the presence of several homeodomain binding sites as well as GATA, SMAD, AP-1, NF-1, NF-Y and steroid hormone transcription binding sites within the highly conserved -350 bp promoter region (60).
In 1992, Kumar et al developed a transgenic mouse model of gonadotrope-specific FSHb expression, in which a 10-kb human FSHB transgene was targeted to pituitary gonadotropes (61, 62). Free FSHβ-subunit was not detectable in the serum of these mice, indicating that the human FSHb-subunit assembled with the mouse common α-subunit and was secreted from gonadotropes as a heterodimer. In both male and female transgenic mice, the transgene was found to be highly abundant compared to the endogenous FSHβ message, possibly due to an increase in stability of hFSHβ mRNA, or the presence of multiple copies of the transgene. Serum FSH levels were higher in transgenic males compared to females and interestingly, a sexually dimorphic FSH expression was also observed in wild type mice, with males having higher serum FSH than females (61). Following gonadectomy, female and male HFSHB transgenic mice displayed a 5-fold and 3.5-fold increase in serum FSH, respectively, compared to ovariectomized or castrated wild type mice. Treatment of gonadectomized mice with estrogen or testosterone reduced serum FSH and suppressed HFSHB transgene expression in gonadotropes (61). MaleHFSHB-expressing mice displayed a slight reduction in testis size, but no reproductive defects were evident in female HFSHB-expressing mice (61).
In a subsequent study by our group using the same FSHβ transgenic mouse model, the observed sexual dimorphism and androgen-mediated regulation gene was further elucidated (62). Gonadectomy resulted in similar elevations of serum FSHβ mRNA in both male and female normal and transgenic mice. However, female mice displayed increased FSH levels two weeks after castration, whereas FSH levels decreased in male mice (62). The hFSHB gene was found to be markedly suppressed by testosterone when compared to endogenous mouse Fshb (62). A further study by Kumar et al. investigated the role of GnRH on the function of FSH (63). In this study, the FSHβ transgenic mice described above were given daily injections of GnRH for 14 days to induce hFSHB gene expression. Following GnRH stimulation, hFSHB mRNA levels were increased approximately 4- or 10-fold compared to control males and females, respectively (63). This stimulation of FSH was completely blocked in males with administration of testosterone, and was partially blocked in females with administration of estradiol (63). These findings shed additional light on the species-specific mechanisms regulating human FSHB transgene expression in vivo in a mouse pituitary environment.
To study the consequences of elevated serum FSH, an MT-1 promoter was used to drive high-level expression of human FSHB from multiple tissues in mice. However, FSHB+ female mice were infertile, and therefore male FSHB+ mice were crossed with CGA+ females (also expressed from an MT-1 promoter), to generate mice that overexpressed the FSH dimer. The resulting transgenic mice ectopically overexpressed FSH from multiple tissues and had a more drasticincrease in serum FSH compared to mice expressing gonadotrope-specific HFSHB, with levels that exceeded those observed in postmenopausal women (64). Male mice were infertile and had increased circulating testosterone as well as enlarged seminal vesicles. However, testis size was not reduced, and spermatogenesis appeared qualitatively normal. Therefore, the infertility observed in male MT-hFSH+ mice was not due to a gonadal defect, but instead may have been a result of altered sexual behavioral characteristics secondary to elevated FSH and/or testosterone (64). Female MT-hFSH+ transgenic mice were also infertile and displayed phenotypes comparable to PCOS and OHSS, including disrupted ovarian folliculogenesis, hemorrhagic cysts, elevated estrogen, progesterone, and testosterone, and urinary tract abnormalities; most females did not survive beyond 13 weeks of age. These studies demonstrate that supraphysiologic FSH levels are detrimental to reproduction, but also suggest that FSH does not promote tumorigenesis, as neither male nor female transgenic mice developed gonadal tumors (64).
To study the effects of FSH in the absence of LH, transgenic mice expressing HFSHB from an insulin II promoter (RIP) were crossed with hpg mutant mice, which do not express GnRH and display persistent immature reproductive function as a result of severely suppressed gonadotropin deficiency (65, 66). The RIP was chosen because it efficiently directs heterologous genes to be expressed from pancreas and eliminates the concern of direct GnRH dependence of the native FSH subunit encoding gene promoters. Therefore, the resulting mice have detectable serum FSH, but not LH. Severe hypogonadism was observed in hpg mutant mice, but FSH expression only partially rescued this phenotype; FSH-expressing hpg male mice had increased Sertoli cell number and increased testis weight, but spermiogenesis remained incomplete, similarto the phenotype observed in Lhb-null male mice (49, 65). Treatment of FSH-expressing hpg mice with testosterone resulted in a near complete rescue of the hpg mutant phenotype, demonstrating that the actions of both FSH and LH are required for reproductive function in males (65). Female FSH-expressing hpg mice had increased ovarian preantral follicle recruitment with development to the late antral stage, which was not seen in the hpg mice. Female mice also had an LH-independent inhibin B response when compared to male transgenic mice, indicating a sexual dimorphic expression (65, 67).
Transgenic mice expressing hFSH from the rat insulin II gene promoter on a wild-type background were also analyzed (68). These mice have an intact endogenous reproductive axis with overexpression of hFSH. The overexpression of hFSH had a biphasic effect on female fertility; young transgenic female mice had significantly larger litters when compared to wild type mice, but litter sizes decreased with age and older transgenic females displayed premature infertility when compared to age matched wild type controls (68). Anti-Müllerian hormone (AMH) levels also showed a more drastic decrease in hFSH transgenic females compared to controls. Despite the age-related decrease in litter size, transgenic hFSH females had significantly more corpora lutea at all ages (68). With superovulation, transgenic hFSH females had an increase in total number of oocytes recovered, but had an equivalent number of healthy fertilized embryos at the two-cell stage when compared to wild type controls (68). Although embryo implantation was increased two-fold in hFSH transgenic mice, these mice displayed higher levels of resorption when compared to wild type females. Interestingly, the older hFSH transgenic females exhibited significant parturition failure and a complete failure to deliver term pups, which suggests that elevated FSH impairs uterine function (68). Female hFSH transgenicmice had increased bone mass via an FSH dose- and ovary-dependent mechanism (69). The mechanism by which bone mass was mediated by FSH in this animal model remains unclear, because no FSH receptor mRNA was detectable on bone osteoblasts or osteoclasts. The authors propose that FSH may indirectly stimulate osteoblasts via inhibin-A or testosterone, but further studies are required to investigate this possibility (69).
1.1.10. FSHβ-Subunit Knockout
To study the effects of loss or impaired FSH-mediated signaling on gonad development and fertility, Fshb knockout mice were generated by deleting exons 1, 2, and most of exon 3 of the Fshb subunit gene using embryonic stem cell technology. This resulted in the production of heterozygous mice that were then intercrossed to obtain Fshb knockout mice. Fshb knockout females are infertile, but male mice remain fertile (70). Male Fshb knockout mice had a 60%reduction in testis weight, a 30% reduction in Sertoli cell number, a 75% reduction in epididymal sperm count, and decreased seminiferous tubule volume, but normal spermatogenesis, normal accessory sex glands, and normal serum testosterone compared to wild type control mice (70, 71). Female Fshb knockout mice were acyclic with small ovaries and thin uteri, and displayed a pre-antral stage block in folliculogenesis. Female Fshb knockout mice had undetectable serum estrogen and reduced serum progesterone, and developed increased serum LH, uterine masses, and hypertrophic growth of ovarian tissue with age (70, 72). Female mutant mice displayed altered communication between somatic cells and germ cells, as well as granulosa cells and oocytes (73-75).
In addition to reproductive phenotypes, deletion of FSHβ has physiological consequences in bone and adipose tissues. Ovariectomized Fshb null mice are protected from bone loss, despite dramatically reduced serum estrogen levels (76, 77). Similarly, hypophysectomy diminishes bone loss in mice following ovariectomy (78, 79). Studies have shown that FSH acts on FSHR on osteoclasts, but not osteoblasts, to increase bone resorption (80), contradictory to what was reported by other investigators as described above (50). In addition, in adipose tissue, targeting of FSHβ with a polyclonal antibody in ovariectomized wild type mice resulted in reduced adipose tissue, increased mitochondrial density, and beiging of adipose tissue, as well as activation of brown adipose tissue and increased thermogenesis (81). These data suggest that high serum FSH levels independent of estrogen may have direct implications on bone health and adiposity in post-menopausal women.
1.1.11. Genetic rescue of Fshb Knockout Mice
To determine if the phenotypes observed in FSHβ knockout mice could be rescued, a 10-kilobase HFSHB transgene was expressed specifically from gonadotrope cells (type I rescue) in Fshb null mice (82). The type I rescue restored fertility in both female and male Fshb null mice. Type I rescue females mice displayed normal folliculogenesis and had normal litter sizes. In type I rescue males, testis size, sperm count, and sperm motility were completely restored (82). In a separate genetic rescue approach, MT-CGA/FSHB transgenes were ectopically expressed at low levels from multiple tissues (type II rescue) in Fshb null mice (82). The type II rescue restored fertility in all male mice, but did not completely rescue fertility in female mice. In males, testis size, sperm number, and sperm motility were entirely restored. However, only 3 out of 10 female type II rescue mice became pregnant after mating with control male mice, and the pregnanciesproduced small litters. A pre-antral stage block in folliculogenesis was observed in ovaries from the type II rescue females that did not become pregnant, similar to what was observed in Fshb null mice without genetic rescue (82). Together, these data demonstrate that gonadotrope-specific expression of HFSHB can rescue fertility in male and female Fshb null mice, but ectopic expression of FSH is sufficient to restore fertility only in males, but not in females. Nonetheless, Fshb knockout mice retain responsiveness to exogenous FSH, and the reproductive phenotypes observed in both males and females can be rescued using genetic as well as pharmacologic approaches (9, 82, 83).
1.1.12. FSHR KO
To further study the effects of FSH action on target cells, Fshr knockout mice were generated by creating a targeted mutation by homologous recombination in embryonic stem cells (84). Female Fshr knockout mice were infertile, but male knockout mice displayed normal to sub-fertility. Female Fshr knockout mice were phenotypically similar to Fshb knockout mice with small ovaries, thin uteri, absence of corpora lutea, and a pre-antral stage block in folliculogenesis, as well as increased serum LH and FSH (84, 85). Male Fshr mutant mice had reduced fertility, but produced offspring when mated to wild type females despite markedly reduced serum testosterone levels, elevated serum LH and FSH, and small testes (72, 84, 85). Serum FSH levels were increased 15-fold in females, but only 4-fold in males, and pituitary FSH was elevated in females only. An increase in anterior pituitary lobe size was also observed in females, but not in males. Female Fshr knockout females developed uterine masses by 1 year of age, and this was inversely correlated with plasma LH concentrations, which increased with age (72). Althoughfemale FSHR knockout mice displayed severe hypogonadism, these mice did not have reduced bone mass (77).
1.1.13. FSHR Gain of Function
To better understand the role of aberrant FSH-mediated signaling, several models have been developed. Overexpression of HFSHR in Sertoli cells did not result in increased FSH-mediated signaling (86). However, expression of a gain-of-function D576G FSHR activated mutant in Sertoli cells of hpg mutant mice resulted in increased testis weight, as well as development of mature Sertoli cells and postmeiotic germ cells, which are not present in hpg control mice due to the lack of serum FSH (87). Sertoli cells from mutant FSHR-expressing mice displayed higher basal cAMP activity compared to wild type FSHR-expressing cells, and the mutant receptor could also be activated by hCG and TSH, demonstrating that the mutation allows for constitutive activation of signaling in the absence of FSH ligand, as well as decreased hormone specificity (86). Male transgenic mice expressing mutant FSHR also had elevated serum testosterone and increased expression of Cyp11a and Star, enzymes required for testicular androgen synthesis, despite the lack of serum LH and FSH in hpg mutant mice (86, 87). A separate study identified activating mutations in mouse Fshr, based on the known activating mutations in human LHR, and then targeted the mutant D580H and D580Y Fshr transgenes to granulosa cells in mice. Constitutive activation of FSHR by the D580H mutation resulted in premature follicle depletion, increased estrogen, and irregular estrous cycles, as well as infertility in a subset of animals (88). The D580Y mutation produced a less severe phenotype, which was mainly manifest by hemorrhagic cysts (88). Together these studies demonstrate that aberrant FSH-mediated signaling via FSH receptors is disruptive to reproductive physiology in both males and females.
Post-Transcriptional Models
1.1.14. DICER/miRNA
Given the temporal regulation of gonadotropin α- and β-subunit gene expression discussed previously, models utilizing Cre, DICER, and miRNA have been developed to further study post-transcriptional regulation of the gonadotropin subunits. These include models of Cre deleter strains Cga-Cre, Lhb-Cre, and Fshb-Cre, which are specific to the gonadotrope lineage (30, 89-91). Cga-iCre transgenic mice expressed Cre at E12.5, as expected (89), and Lhb-Cre transgenic mice expressed Cre at E16.5-17.5 (90). One limitation of the Cga-Cre and Lhb-Cre deleter strains is that Cre recombination can also occur in non-gonadotrope cells (thyrotropes and gonadal tissue), which may result in infertility and recombination in undesirable tissues/cells. In an effort to determine the role of microRNAs (miRNAs) in gonadotropin regulation, our laboratory developed a Cre-lox mouse model to delete Dicer-dependent miRNAs in gonadotropes (91). Dicer is an enzyme that is important for synthesis of mature miRNAs, which are involved in post-transcriptional gene regulation. Dicer-dependent miRNAs were found to be involved in transcriptional and post-transcriptional regulation of the gonadotropin β-subunits (91). Using this knowledge, we utilized an Fshb-Cre deleter strain to inactivate Dicer specifically in gonadotrope cells (30). Similar to endogenous FSH expression, Fshb-Cre was expressed in low levels at E14.5, with widespread expression by E16.5, and Cre expression in this model was limited to the gonadotrope lineage, with no ectopic expression observed in the gonads (30). Loss of Dicer-dependent miRNAs in gonadotropes leads to gonadotropin suppression, resulting in infertility in both male and female mice (30).
To further investigate the roles of miRNAs in reproduction, miR-200b and miR-429 were targeted based on their abundant expression in testes. However, miR-200b and miR-429 double-knockout mice (miR-DKO) did not display any testis abnormalities and no fertility defects were noted in males (92). However, female miR-DKO mice were sub-fertile and had altered estrous cyclicity as a result of impaired LH regulation (92). Another recent study also identified that specific deletion of Dicer in GnRH neurons results in hypogonadism and infertility (93), and a set of critical miRNAs including miR-200 and miR-155 acts as epigenetic switches to regulate the initiation of puberty (93).
1.1.15. Transforming Growth Factor-β Superfamily
GnRH and gonadal steroids regulate gonadotropin expression in a coordinated fashion. GnRH activates the GnRH receptor on gonadotrope cells, and regulates synthesis and secretion of gonadotropin hormones, in part by signaling to members of the transforming growth factor (TGF)-β superfamily, including activins and inhibins (94, 95).
1.2.2.1. Activins
Activins stimulate FSH synthesis by binding to type I and II serine/threonine kinase receptors. Matzuk et al. targeted the activin type IIA receptor to disrupt activin action, and showed that type II activin receptor-deficient mice were fertile and displayed normal spermatogenesis, but had decreased testicular size (96). The downstream action of activins have been further characterized using activin receptor 1B (ALK4) knockout mice, which suggest that ALK4 may be the preferred type I receptor for stimulation of Fshb transcription (97). Furthermore, using a type I activin A receptor (ALK7) knockout mouse model, ALK7 was shown to be an important regulator of female reproductive function (98).
Activins have been described to regulate Fshbtranscription via SMAD4- and FOXL2-dependent pathways (99). The available in vitro evidence indicates that activins stimulate Fshb gene expression by activating a canonical type I/II receptor-SMAD3/4-mediated signaling cascade (100). Conditional gene-targeting techniques demonstrated that SMAD4 is required for FSH synthesis in both male and female mice. Deletion of Smad4 and forkhead box L2 (Foxl2), a DNA binding cofactor of Smad4, in gonadotrope cells resulted in infertility in male and female mice with phenotypes similar to FSHβ-knockout mice (99). The same group later showed that Foxl2 is an important transcription factor involved in activin/SMAD signaling (100). Foxl2 knockdown experiments have been completed in Lβ T2 cells to further study regulation of murine Fshb (101). A high-affinity binding element, termed forkhead-binding element, is conserved in the promoter of Fshb in mice and humans (100). Mutations in the FOXL2 gene in humans cause blepharophimosis-ptosis-epicanthus-inversus syndrome (BPES), and can be associated with premature ovarian failure and the need for gonadotropin stimulation to achieve pregnancy (102-104).
1.2.2.2. Inhibin
Inhibin suppresses the release of FSH from the pituitary (94), and is highly expressed by Sertoli cells and granulosa cells (105). To further characterize the role of inhibin in vivo, an inhibin knockout mouse model was created using targeted deletion of the alpha-inhibin gene (106, 107). Inhibin-deficient mice developed hemorrhagic gonadal stromal tumors, suggesting that inhibin may function as a tumor-suppressor (106). Mice lacking inhibin also developed severe cachexia with hepatocellular necrosis, displayed elevated serum activin levels, and if castrated, developed adrenocortical tumors (107).
To evaluate the influence of gonadotropins on the development of gonadal and adrenal tumors in inhibin-deficient mice, Kumar et al. developed an inhibin/gonadotropin double knockout mouse (Inha/hpg double mutant) (108). Heterozygous inhibin mutant mice were crossed with mutant hpg mice to generate double mutant mice lacking both inhibin and GnRH, and thus FSH and LH (108). Unlike inhibin-deficient mice, Inha/hpg double mutant mice did not develop cachexia and failed to exhibit gonadal or adrenal tumors, suggesting that gonadotropins are required for tumor development with loss of inhibin expression (108). To further investigate the specific roles of LH and FSH in tumor development in inhibin-deficient mice, inhibit knockout mice were crossed with either Fshb-knockout or Lhb-knockout mice (64, 109). The Inha/Fshb knockout mice developed minimal cachexia, and although gonadal tumors were observed, they were much slower growing than those seen in inhibin knockout mice (64). Inha/Lhb double mutant mice also displayed delayed tumorigenesis and the mice had increased survival, indicating that LH, unlike FSH is not required for tumor formation with the loss of inhibin (109).
1.2.2.3. Follistatin
Follistatin is a binding protein that is locally produced in the gonads and pituitary, as well as other tissues, and functions to bind and neutralize members of the TGFβ superfamily. It negatively regulates Fshb transcription by binding activin and blocking its action on gonadotropes (94, 105). Follistatin knockout mice are born with multiple defects and die shortly after birth, and therefore gonadal development could not be assessed (110). Therefore, in order to further study the role of follistatin, subsequent models have been designed utilizing a Cre/loxP conditional knockout mouse, as well as transplantation of follistatin-null mouse testes into immunocompromised mice (111, 112). In the Cre/loxP conditional knockout, the follistatin genewas deleted specifically in the granulosa cells of the ovary. In female mice, follistatin deletion resulted in varying degrees of infertility, reduced litter size and number, reduced ovarian follicles and ovulations, and elevated FSH and LH, similar to a premature ovarian insufficiency in humans (111). In a separate study, fetal testes from follistatin-null mice were transplanted onto the external ear of castrated immunocompromised male mice (112). After 7-8 weeks, spermatogenesis appeared normal and was comparable to spermatogenesis in mice that received transplantation of WT testes, suggesting that, in the absence of local follistatin production, circulating follistatin is sufficient to support spermatogenesis (112).
To further evaluate the role of follistatin on reproductive development, Guo et al. created a gain-of-function follistatin mutant mouse model (113). Follistatin was overexpressed in multiple tissues using an mMT-1 promoter, which resulted in a viable MT-FS transgenic model (113). Overexpression of follistatin resulted in live births, and fertile mice that survived to adulthood. Male MT-FS mice, however, displayed testis defects including decreased testis size, Leydig cell hyperplasia, and these mice eventually became infertile as a result of spermatogenic arrest (113). Female MT-FS mice also became infertile overtime due to a block in folliculogenesis at varying stages, and developed thin uteri as well as small ovaries (113). This study suggests follistatin likely acts locally, at the level of the gonad or the pituitary, by regulating activin signaling and/or other TGF-β family members.
1.1.16. FSH Rerouting
In many vertebrates, FSH is secreted in a constitutive manner, whereas LH is secreted in pulses from gonadotropes. The presence of a cabroxyterminal heptapeptide on LHβ directs secretion ofLH via the regulated pathway in gonadotrope cells (9, 114). To determine the physiological role of constitutive versus pulsatile secretion, mice expressing mutant HFSHB containing the carboxyterminal heptapeptide from LH, or wild type HFSHB, were generated on an Fshb null background. Therefore, the resulting transgenic mice expressed either HFSHB, or mutant HFSHB-LHB, in the absence of endogenous mouse Fshb. In HFSHB-LHB transgenic mice, FSH protein containing the heptapeptide was rerouted into the regulated pathway in gonadotropes, stored in dense core granules, and, like LH, was secreted in pulses in response to GnRH (9). Rerouted FSH functionally rescued the ovarian defects observed in Fshb null mice, resulting in restoration of normal estrous cycles and folliculogenesis, and was equally as effective as a genetic rescue with wild type FSH. However, rerouted FSH produced a six-fold increase in the number of eggs per cycle as a result of suppressed atresia. This phenomenon persisted beyond one year of age, demonstrating that increased ovulation efficiency was not due to accelerated follicle growth. These studies indicated that FSH released via the LH secretory pathway prolongs reproductive life span in FSH-rerouted mice (9).
1.1.17. FSH Glycosylation
The FSH heterodimer is post-translationally modified by glycosylation on Asn residues 52 and 78 of the α-subunit, and on Asn residues 7 and 24 of the β-subunit. On the α-subunit of FSH, the Asn52 glycosylation site regulates receptor binding and signal transduction, whereas glycosylation at Asn78 is important for protein folding and subunit stability (115-117). Alterations in the level of glycosylation on the β-subunit of FSH have been shown to impact FSH/FSHR interaction and physiological action of each glycoform, likely as a result of biased signaling (118-120). Although glycosylation of FSHb does not impact signal transduction invitro (121, 122), loss of glycosylation at one or both sites on the β-subunit increases metabolic clearance rate, and therefore FSH dimer containing deglycosylated FSHβ elicits reduced biopotency in vivo (122, 123). The Asn24 glycosylation site on the β-subunit has been specifically shown to be important for hormone/receptor binding as well as signal transduction (115, 119). Although several glycoforms of LH also exist, variation in sialic acid content on the LHβ-subunit does not alter hormone/receptor interaction (120).
Macroheterogeneity in the hormone-specific β-subunit gives rise to different FSH glycoforms, which differ in the number of N-linked glycosylations, and are named based on the molecular weight of the β-subunit: FSH24 (glycosylated at both Asn7 and Asn24), FSH21 (glycosylated at Asn7 only), FSH18 (glycosylated at Asn24 only), and FSH15 (not glycosylated on the β-subunit) (119, 121, 124, 125). In humans, the relative abundance of each glycoform changes with age; FSH21/18 predominates in younger women and FSH24 is most abundant in peri/post-menopausal women (124, 126). In vitro assays showed that, compared to the fully glycosylated FSH24 glycoform, hypoglycosylated FSH21/18 bound more rapidly to FSHR, occupied more ligand binding sites, and elicited a stronger activation of cAMP/PKA signaling and steroidogenesis specifically in granulosa cells (127-130). The in vivo bioactivities of each FSH glycoform were evaluated using a pharmacological rescue approach, in which recombinant FSH glycoforms were injected into Fshb null mice. In females, pharmacological rescue with FSH21/18 resulted in increased ovarian weight and induction of ovarian response genes similar to that observed with FSH24 (83). In males, pharmacological rescue with FSH21/18 resulted in stronger activation of FSH response genes and further increased Sertoli cell proliferation when compared with FSH24 (83). Therefore, in male mice, hypoglycosylated FSH21/18 has increased in vivo bioactivity whencompared to hypoglycosylated FSH24, but the glycoforms elicit comparable in vivo bioactivities in female mice. Double N-glycosylation mutant FSH15 did not rescue Fshb null mice, primarily due to impaired dimerization resulting in inefficient secretion of FSH15 from gonadotrope cells (131). Further studies using this in vivo genetic rescue approach will be useful to characterize the physiological actions of each of these FSH glycoforms in gonadal and non-gonadal cells.
2. Summary
Both gain of function and loss of function mouse models were generated to understand gonadotropin action in a physiological context. In many cases, the mouse models closely phenocopy human mutations (Table 1) and thus these models provide useful tools to developmentally track the phenotypes over longer periods of time. In some instances, genetic rescue of mutant mice lacking a single gonadotropin has also been achieved by combining the loss of function and gain of function mouse models. Loss of function gonadotropin models also provide valuable tools for pharmacological and genetic rescue experiments by providing back the missing gonadotropin ligand or gonadotropin analogs. The genetic rescue approach proved useful for establishing the gonadotropin re-routing and functional significance of N-glycosylation. Gonadotropin loss of function mouse models also unexpectedly revealed extra-gonadal actions of gonadotropins (Figure 1). Whether these new sites of gonadotropin actions are species-specific or have clinical implications remains to be rigorously tested in the future.
Table 1.
| Mouse model | Phenotypes | Relevant
Human Mutations |
Ref. | |||
|---|---|---|---|---|---|---|
| Gene | Mutation | Ovary | Testis | Other | ||
| CGA | Overexpression |
|
|
|
|
(3) |
| Cga | KO |
|
|
|
|
(16, 17) |
| CGB | Overexpression from mMT-1 or ubiquitin-C promoter |
|
|
|
|
(19, 20, 131) |
| CGA/CGB | Overexpression from ubiquitin C promotor |
|
|
|
|
(27-29) |
| Overexpression by co-injection of MT-hCGα and MT-hCGβ transgenes |
|
|
|
(19) | ||
| Lhb | Overexpression of LHβ-CTP |
|
|
|
|
(36-39, 41, 43) |
| KO |
|
|
|
|
(48, 49) | |
| LHCGR | GOF (YHR+) |
|
|
|
(58, 59) | |
| GOF (D556H) |
|
|
(57, 133-135) | |||
| KO |
|
|
|
|
(51, 52, 54, 136) | |
| FSHB | Overexpression of hFSHβ from MT-1 promoter |
|
|
|
|
(63, 64) |
| Overexpression of hFSHβ from rat insulin II promoter |
|
|
|
(68, 69) | ||
| KO |
|
|
|
|
(70-72, 76, 77, 79, 81) | |
| Fshr | Overexpression of HFSHR in Sertoli cells (D576G) or granulosa cells (D580H/D580Y) |
|
|
|
(86-88) | |
| KO |
|
|
|
|
(72, 77, 84) | |
| FSHB Rerouted | Redirecting intracellular trafficking of FSH |
|
|
|
|
(9) |
| FSHB Glycosylation | FSH glycoforms injected into Fshb-/-mice |
|
|
|
(83, 131) | |
KO, Knockout; PCOS, Polycystic ovarian syndrome; OHSS, Ovarian hyperstimulation syndrome; CTP, C-terminal peptide; TMD, Transmembrane domain; GOF, Gain of function; Ovx, ovariectomized
Practice Points.
Mutations in genes encoding gonadotropins and their cognate receptors are relatively rare.
Inactivating mutations in gonadotropin beta subunit genes and gonadotropins receptors usually manifest in various degrees of impaired fertility.
Absence of ligand in patients is usually treated by providing recombinant exogenous hormones to initiate halted gonad development and to restore fertility.
Research Agenda.
Mutant mouse models for gonadotropins phenocopy the corresponding human mutations.
Mouse models allow developmental and age-dependent studies in the future.
Different mouse models for gonadotropins allow identification of in vivo genetic interactions.
Mouse models allow further delineating extra-gonadal actions of gonadotropins in the future.
Acknowledgments
Financial support from the NIH Loan Repayment program (to S.B.G.) and in part from the NIH grants CA166557, AG029531, AG056046, HD081162 and The Makowski Endowment (to T.R.K.) are acknowledged.
Footnotes
Disclosure statement: The Authors have nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bousfield GR, Jia L, Ward DN. Knobil and Neill's Physiology of Reproduction. Third. Academic Press; St Louis: 2006. CHAPTER 30 - Gonadotropins: Chemistry and Biosynthesis A2 - Neill, Jimmy D; pp. 1581–1634. [Google Scholar]
- 2.Boime I, Ben-Menahem D. Glycoprotein hormone structure-function and analog design. Recent progress in hormone research. 1999;54:271–288. discussion 288-279. [PubMed] [Google Scholar]
- 3.Fox N, Solter D. Expression and regulation of the pituitary- and placenta-specific human glycoprotein hormone alpha-subunit gene is restricted to the pituitary in transgenic mice. Molecular and cellular biology. 1988;8(12):5470–5476. doi: 10.1128/mcb.8.12.5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naylor SL, et al. Chromosome assignment of genes encoding the alpha and beta subunits of glycoprotein hormones in man and mouse. Somatic cell genetics. 1983;9(6):757–770. doi: 10.1007/BF01539478. [DOI] [PubMed] [Google Scholar]
- 5.Jorgensen JS, Quirk CC, Nilson JH. Multiple and Overlapping Combinatorial Codes Orchestrate Hormonal Responsiveness and Dictate Cell-Specific Expression of the Genes Encoding Luteinizing Hormone. Endocrine Reviews. 2004;25(4):521–542. doi: 10.1210/er.2003-0029. [DOI] [PubMed] [Google Scholar]
- 6.Kim T, Do MHT, Lawson MA. Translational control of gene expression in the gonadotrope. Molecular and Cellular Endocrinology. 2014;385(1):78–87. doi: 10.1016/j.mce.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernard DJ, Fortin J, Wang Y, Lamba P. Mechanisms of FSH synthesis: what we know, what we don't, and why you should care. Fertility and Sterility. 2010;93(8):2465–2485. doi: 10.1016/j.fertnstert.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 8.Charles MA, et al. Pituitary-specific Gata2 knockout: effects on gonadotrope and thyrotrope function. Molecular endocrinology (Baltimore, Md) 2006;20(6):1366–1377. doi: 10.1210/me.2005-0378. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, et al. Redirecting intracellular trafficking and the secretion pattern of FSH dramatically enhances ovarian function in mice. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(15):5735–5740. doi: 10.1073/pnas.1321404111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar TR. Extragonadal FSH Receptor: Is It Real? Biology of Reproduction. 2014;91(4):99–99. doi: 10.1095/biolreprod.114.124222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Japon MA, Rubinstein M, Low MJ. In situ hybridization analysis of anterior pituitary hormone gene expression during fetal mouse development. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 1994;42(8):1117–1125. doi: 10.1177/42.8.8027530. [DOI] [PubMed] [Google Scholar]
- 12.Kendall SK, et al. Enhancer-mediated high level expression of mouse pituitary glycoprotein hormone alpha-subunit transgene in thyrotropes, gonadotropes, and developing pituitary gland. Molecular endocrinology (Baltimore, Md) 1994;8(10):1420–1433. doi: 10.1210/mend.8.10.7531821. [DOI] [PubMed] [Google Scholar]
- 13.Schoderbek WE, Kim KE, Ridgway EC, Mellon PL, Maurer RA. Analysis of DNA sequences required for pituitary-specific expression of the glycoprotein hormone alpha-subunit gene. Molecular endocrinology (Baltimore, Md) 1992;6(6):893–903. doi: 10.1210/mend.6.6.1379672. [DOI] [PubMed] [Google Scholar]
- 14.Cushman LJ, et al. Cre-mediated recombination in the pituitary gland. Genesis (New York, NY 2000) 2000;28(3-4):167–174. doi: 10.1002/1526-968x(200011/12)28:3/4<167::aid-gene120>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 15.Delegeane AM, Ferland LH, Mellon PL. Tissue-specific enhancer of the human glycoprotein hormone alpha-subunit gene: dependence on cyclic AMP-inducible elements. Molecular and cellular biology. 1987;7(11):3994–4002. doi: 10.1128/mcb.7.11.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kendall SK, Samuelson LC, Saunders TL, Wood RI, Camper SA. Targeted disruption of the pituitary glycoprotein hormone alpha-subunit produces hypogonadal and hypothyroid mice. Genes & development. 1995;9(16):2007–2019. doi: 10.1101/gad.9.16.2007. [DOI] [PubMed] [Google Scholar]
- 17.Gergics P, Christian HC, Choo MS, Ajmal A, Camper SA. Gene Expression in Mouse Thyrotrope Adenoma: Transcription Elongation Factor Stimulates Proliferation. Endocrinology. 2016;157(9):3631–3646. doi: 10.1210/en.2016-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albanese C, et al. The gonadotropin genes: evolution of distinct mechanisms for hormonal control. Recent progress in hormone research. 1996;51:23–58. discussion 59-61. [PubMed] [Google Scholar]
- 19.Matzuk MM, DeMayo FJ, Hadsell LA, Kumar TR. Overexpression of Human Chorionic Gonadotropin Causes Multiple Reproductive Defects in Transgenic Mice1. Biology of Reproduction. 2003;69(1):338–346. doi: 10.1095/biolreprod.102.013953. [DOI] [PubMed] [Google Scholar]
- 20.Rulli SB, et al. Reproductive disturbances, pituitary lactotrope adenomas, and mammary gland tumors in transgenic female mice producing high levels of human chorionic gonadotropin. Endocrinology. 2002;143(10):4084–4095. doi: 10.1210/en.2002-220490. [DOI] [PubMed] [Google Scholar]
- 21.Corless CL, Matzuk MM, Ramabhadran TV, Krichevsky A, Boime I. Gonadotropin beta subunits determine the rate of assembly and the oligosaccharide processing of hormone dimmer in transfected cells. Journal of Cell Biology. 1987;104(5):1173–1181. doi: 10.1083/jcb.104.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keene JL, et al. Expression of biologically active human follitropin in Chinese hamster ovary cells. The Journal of biological chemistryournal of biological chemistry. 1989;264(9):4769–4775. [PubMed] [Google Scholar]
- 23.Ahtiainen P, et al. Enhanced LH action in transgenic female mice expressing hCG -subunit induces pituitary prolactinomas; the role of high progesterone levels. Endocrine Related Cancer. 2010;17(3):611–621. doi: 10.1677/ERC-10-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ratner LD, et al. Hyperprolactinemia induced by hCG leads to metabolic disturbances in female mice. The Journal of endocrinology. 2016;230(1):157–169. doi: 10.1530/JOE-15-0528. [DOI] [PubMed] [Google Scholar]
- 25.Ratner LD, et al. Short-term pharmacological suppression of the hyperprolactinemia of infertile hCG-overproducing female mice persistently restores their fertility. Endocrinology. 2012;153(12):5980–5992. doi: 10.1210/en.2012-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuorelahti A, Rulli S, Huhtaniemi I, Poutanen M. Human chorionic gonadotropin (hCG) up-regulates wnt5b and wnt7b in the mammary gland, and hCGbeta transgenic female mice present with mammary Gland tumors exhibiting characteristics of the Wnt/beta-catenin pathway activation. Endocrinology. 2007;148(8):3694–3703. doi: 10.1210/en.2007-0249. [DOI] [PubMed] [Google Scholar]
- 27.Rulli SB, et al. Elevated Steroidogenesis, Defective Reproductive Organs, and Infertility in Transgenic Male Mice Overexpressing Human Chorionic Gonadotropin. Endocrinology. 2003;144(11):4980–4990. doi: 10.1210/en.2003-0403. [DOI] [PubMed] [Google Scholar]
- 28.Ahtiainen P, et al. Fetal but not adult Leydig cells are susceptible to adenoma formation in response to persistently high hCG level: a study on hCG overexpressing transgenic mice. Oncogene. 2005;24(49):7301–7309. doi: 10.1038/sj.onc.1208893. [DOI] [PubMed] [Google Scholar]
- 29.Yarram SJ, et al. Luteinizing hormone receptor knockout (LuRKO) mice and transgenic human chorionic gonadotropin (hCG)-overexpressing mice (hCG alphabeta+) have bone phenotypes. Endocrinology. 2003;144(8):3555–3564. doi: 10.1210/en.2003-0036. [DOI] [PubMed] [Google Scholar]
- 30.Wang H, Hastings R, Miller WL, Kumar TR. Fshb -i Cre mice are ef fi cient and speci fi c Cre deleters for the gonadotrope lineage. Molecular and Cellular Endocrinology. 2016;419:124–138. doi: 10.1016/j.mce.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keri RA, Nilson JH. A steroidogenic factor-1 binding site is required for activity of the luteinizing hormone beta subunit promoter in gonadotropes of transgenic mice. The Journal of biological chemistry. 1996;271(18):10782–10785. doi: 10.1074/jbc.271.18.10782. [DOI] [PubMed] [Google Scholar]
- 32.Keri RA, Lozada KL, Abdul-Karim FW, Nadeau JH, Nilson JH. Luteinizing hormone induction of ovarian tumors: oligogenic differences between mouse strains dictates tumor disposition. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(1):383–387. doi: 10.1073/pnas.97.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quirk CC, Lozada KL, Keri RA, Nilson JH. A single Pitx1 binding site is essential for activity of the LHbeta promoter in transgenic mice. Molecular endocrinology (Baltimore, Md) 2001;15(5):734–746. doi: 10.1210/mend.15.5.0628. [DOI] [PubMed] [Google Scholar]
- 34.De Koning J, Tijssen AMI, Van Dieten JAMJ, Koppenaal DW. Hypersecretion of luteinising hormone, infertility, and miscarriage. The Lancet. 1991;337(8733):119–120. doi: 10.1016/0140-6736(91)90783-l. [DOI] [PubMed] [Google Scholar]
- 35.Hull MG. Epidemiology of infertility and polycystic ovarian disease: endocrinological and demographic studies. Gynecological endocrinology: the official journal of the International Society of Gynecological Endocrinology. 1987;1(3):235–245. doi: 10.3109/09513598709023610. [DOI] [PubMed] [Google Scholar]
- 36.Risma KA, et al. Targeted overexpression of luteinizing hormone in transgenic mice leads to infertility, polycystic ovaries, and ovarian tumors. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(5):1322–1326. doi: 10.1073/pnas.92.5.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flaws JA, Abbud R, Mann RJ, Nilson JH, Hirshfield AN. Chronically elevated luteinizing hormone depletes primordial follicles in the mouse ovary. Biology of reproduction. 1997;57(5):1233–1237. doi: 10.1095/biolreprod57.5.1233. [DOI] [PubMed] [Google Scholar]
- 38.Mann RJ, Keri RA, Nilson JH. Consequences of elevated luteinizing hormone on diverse physiological systems: use of the LHbetaCTP transgenic mouse as a model of ovarian hyperstimulation-induced pathophysiology. Recent progress in hormone research. 2003;58:343–375. doi: 10.1210/rp.58.1.343. [DOI] [PubMed] [Google Scholar]
- 39.Mohammad HP, Abbud RA, Parlow AF, Lewin JS, Nilson JH. Targeted overexpression of luteinizing hormone causes ovary-dependent functional adenomas restricted to cells of the Pit-1 lineage. Endocrinology. 2003;144(10):4626–4636. doi: 10.1210/en.2003-0357. [DOI] [PubMed] [Google Scholar]
- 40.Nilson JH, Abbud RA, Keri RA, Quirk CC. Chronic hypersecretion of luteinizing hormone in transgenic mice disrupts both ovarian and pituitary function, with some effects modified by the genetic background. Recent progress in hormone research. 2000;55:69–89. discussion 89-91. [PubMed] [Google Scholar]
- 41.Kero J, et al. Elevated luteinizing hormone induces expression of its receptor and promotes steroidogenesis in the adrenal cortex. 2000;105(5):633–641. doi: 10.1172/JCI7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Risma KA, Hirshfield AN, Nilson JH. Elevated Luteinizing Hormone in Prepubertal Transgenic Mice Causes Hyperandrogenemia, Precocious Puberty, and Substantial Ovarian Pathology 1. Endocrinology. 1997;138(8):3540–3547. doi: 10.1210/endo.138.8.5313. [DOI] [PubMed] [Google Scholar]
- 43.Mann RJ, Keri RA, Nilson JH. Transgenic mice with chronically elevated luteinizing hormone are infertile due to anovulation, defects in uterine receptivity, and midgestation pregnancy failure. Endocrinology. 1999;140(6):2592–2601. doi: 10.1210/endo.140.6.6927. [DOI] [PubMed] [Google Scholar]
- 44.Couse JF, Yates MM, Walker VR, Korach KS. Characterization of the Hypothalamic-Pituitary-Gonadal Axis in Estrogen Receptor (ER) Null Mice Reveals Hypergonadism and Endocrine Sex Reversal in Females Lacking ERα But Not ERβ. Molecular Endocrinology. 2003;17(6):1039–1053. doi: 10.1210/me.2002-0398. [DOI] [PubMed] [Google Scholar]
- 45.Couse JF, et al. Formation of cystic ovarian follicles associated with elevated luteinizing hormone requires estrogen receptor-beta. Endocrinology. 2004;145(10):4693–4702. doi: 10.1210/en.2004-0548. [DOI] [PubMed] [Google Scholar]
- 46.Mason AJ, et al. A deletion truncating the gonadotropin-releasing hormone gene is responsible for hypogonadism in the hpg mouse. Science (New York, NY) 1986;234(4782):1366–1371. doi: 10.1126/science.3024317. [DOI] [PubMed] [Google Scholar]
- 47.Mason AJ, et al. The hypogonadal mouse: reproductive functions restored by gene therapy. Science (New York, NY) 1986;234(4782):1372–1378. doi: 10.1126/science.3097822. [DOI] [PubMed] [Google Scholar]
- 48.Kumar TR. Functional analysis of LHbeta knockout mice. Molecular and Cellular Endocrinology. 2007;269:81–84. doi: 10.1016/j.mce.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 49.Ma X, Dong Y, Matzuk MM, Kumar TR. Targeted disruption of luteinizing hormone beta-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(49):17294–17299. doi: 10.1073/pnas.0404743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oury F, et al. Osteocalcin regulates murine and human fertility through a pancreas-bone-testis axis. Journal of Clinical Investigation. 2013;123(6):2421–2433. doi: 10.1172/JCI65952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lei ZM, et al. Targeted disruption of luteinizing hormone/human chorionic gonadotropin receptor gene. Molecular endocrinology (Baltimore, Md) 2001;15(1):184–200. doi: 10.1210/mend.15.1.0586. [DOI] [PubMed] [Google Scholar]
- 52.Zhang FP, Poutanen M, Wilbertz J, Huhtaniemi I. Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Molecular endocrinology. 2001;15(1):172–183. doi: 10.1210/mend.15.1.0582. [DOI] [PubMed] [Google Scholar]
- 53.Zhang FP, Pakarainen T, Poutanen M, Toppari J, Huhtaniemi I. The low gonadotropin-independent constitutive production of testicular testosterone is sufficient to maintain spermatogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(23):13692–13697. doi: 10.1073/pnas.2232815100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lei ZM, et al. Testicular Phenotype in Luteinizing Hormone Receptor Knockout Animals and the Effect of Testosterone Replacement Therapy1. Biology of Reproduction. 2004;71(5):1605–1613. doi: 10.1095/biolreprod.104.031161. [DOI] [PubMed] [Google Scholar]
- 55.Pakarainen T, Zhang FP, Mäkelä S, Poutanen M, Huhtaniemi I. Testosterone replacement therapy induces spermatogenesis and partially restores fertility in luteinizing hormone receptor knockout mice. Endocrinology. 2005;146(2):596–606. doi: 10.1210/en.2004-0913. [DOI] [PubMed] [Google Scholar]
- 56.Rao CV, Lei ZM. Consequences of targeted inactivation of LH receptors. Molecular and cellular endocrinology. 2002;187(1-2):57–67. doi: 10.1016/s0303-7207(01)00694-3. [DOI] [PubMed] [Google Scholar]
- 57.Narayan P. Genetic Models for the Study of Luteinizing Hormone Receptor Function. Frontiers in endocrinology. 2015 Sep;6:152–152. doi: 10.3389/fendo.2015.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meehan TP, et al. Gonadal defects and hormonal alterations in transgenic mice expressing a single chain human chorionic gonadotropin-lutropin receptor complex. Journal of molecular endocrinology. 2005;34(2):489–503. doi: 10.1677/jme.1.01669. [DOI] [PubMed] [Google Scholar]
- 59.Meehan TP, Narayan P. Constitutively active luteinizing hormone receptors: Consequences of in vivo expression. Molecular and Cellular Endocrinology. 2007;(1):260–262. 294–300. doi: 10.1016/j.mce.2006.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar TR, Schuff KG, Nusser KD, Low MJ. Gonadotroph-specific expression of the human follicle stimulating hormone β gene in transgenic mice. Molecular and Cellular Endocrinology. 2006;247:103–115. doi: 10.1016/j.mce.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 61.Kumar TR, Fairchild-Huntress V, Low MJ. Gonadotrope-specific expression of the human follicle-stimulating hormone beta-subunit gene in pituitaries of transgenic mice. Molecular endocrinology (Baltimore, Md) 1992;6(1):81–90. doi: 10.1210/mend.6.1.1738375. [DOI] [PubMed] [Google Scholar]
- 62.Kumar TR, Low MJ. Gonadal steroid hormone regulation of human and mouse follicle stimulating hormone beta-subunit gene expression in vivo. Molecular endocrinology (Baltimore, Md) 1993;7(7):898–906. doi: 10.1210/mend.7.7.8413314. [DOI] [PubMed] [Google Scholar]
- 63.Kumar TR, Low MJ. Hormonal regulation of human follicle-stimulating hormone-beta subunit gene expression: GnRH stimulation and GnRH-independent androgen inhibition. Neuroendocrinology. 1995;61(6):628–637. doi: 10.1159/000126889. [DOI] [PubMed] [Google Scholar]
- 64.Kumar TR, et al. Transgenic models to study gonadotropin function: the role of follicle-stimulating hormone in gonadal growth and tumorigenesis. Molecular endocrinology (Baltimore, Md) 1999;13(6):851–865. doi: 10.1210/mend.13.6.0297. [DOI] [PubMed] [Google Scholar]
- 65.Allan CM, et al. A Novel Transgenic Model to Characterize the Specific Effects of Follicle-Stimulating Hormone on Gonadal Physiology in the Absence of Luteinizing Hormone Actions <sup>1</sup>. Endocrinology. 2001;142(6):2213–2220. doi: 10.1210/endo.142.6.8092. [DOI] [PubMed] [Google Scholar]
- 66.Allan CM, Handelsman DJ. Transgenic models for exploring gonadotropin biology in the male. Endocrine. 2005;26(3):235–239. doi: 10.1385/ENDO:26:3:235. [DOI] [PubMed] [Google Scholar]
- 67.Allan CM, et al. Follicle-stimulating hormone increases primordial follicle reserve in mature female hypogonadal mice. The Journal of endocrinology. 2006;188(3):549–557. doi: 10.1677/joe.1.06614. [DOI] [PubMed] [Google Scholar]
- 68.McTavish KJ, et al. Rising follicle-stimulating hormone levels with age accelerate female reproductive failure. Endocrinology. 2007;148(9):4432–4439. doi: 10.1210/en.2007-0046. [DOI] [PubMed] [Google Scholar]
- 69.Allan CM, et al. Follicle-stimulating hormone increases bone mass in female mice. Proc Natl Acad Sci U S A. 2010;107(52):22629–22634. doi: 10.1073/pnas.1012141108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nature genetics. 1997;15(2):201–204. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- 71.Wreford NG, Rajendra Kumar T, Matzuk MM, de Kretser DM. Analysis of the testicular phenotype of the follicle-stimulating hormone beta-subunit knockout and the activin type II receptor knockout mice by stereological analysis. Endocrinology. 2001;142(7):2916–2920. doi: 10.1210/endo.142.7.8230. [DOI] [PubMed] [Google Scholar]
- 72.Abel MH, Huhtaniemi I, Pakarinen P, Kumar TR, Charlton HM. Age-related uterine and ovarian hypertrophy in FSH receptor knockout and FSHβ subunit knockout mice. Reproduction (Cambridge, England) 2003;125(2):165–173. doi: 10.1530/rep.0.1250165. [DOI] [PubMed] [Google Scholar]
- 73.Combelles CM, Carabatsos MJ, Kumar TR, Matzuk MM, Albertini DF. Hormonal control of somatic cell oocyte interactions during ovarian follicle development. Molecular reproduction and development. 2004;69(3):347–355. doi: 10.1002/mrd.20128. [DOI] [PubMed] [Google Scholar]
- 74.El-Hayek S, Demeestere I, Clarke HJ. Follicle-stimulating hormone regulates expression and activity of epidermal growth factor receptor in the murine ovarian follicle. Proc Natl Acad Sci U S A. 2014;111(47):16778–16783. doi: 10.1073/pnas.1414648111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.El-Hayek S, Clarke HJ. Follicle-Stimulating Hormone Increases Gap Junctional Communication Between Somatic and Germ-Line Follicular Compartments During Murine Oogenesis. Biol Reprod. 2015;93(2):47. doi: 10.1095/biolreprod.115.129569. [DOI] [PubMed] [Google Scholar]
- 76.Iqbal J, Sun L, Kumar TR, Blair HC, Zaidi M. Follicle-stimulating hormone stimulates TNF production from immune cells to enhance osteoblast and osteoclast formation. Proceedings of the National Academy of Sciences. 2006;103(40):14925–14930. doi: 10.1073/pnas.0606805103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun L, et al. FSH Directly Regulates Bone Mass. Cell. 2006;125:247–260. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 78.Yeh JK, Chen MM, Aloia JF. Ovariectomy-induced high turnover in cortical bone is dependent on pituitary hormone in rats. Bone. 1996;18(5):443–450. doi: 10.1016/8756-3282(96)00045-2. [DOI] [PubMed] [Google Scholar]
- 79.Yeh JK, Chen MM, Aloia JF. Effects of 17 beta-estradiol administration on cortical and cancellous bone of ovariectomized rats with and without hypophysectomy. Bone. 1997;20(5):413–420. doi: 10.1016/s8756-3282(97)00027-6. [DOI] [PubMed] [Google Scholar]
- 80.Zaidi M, et al. Proresorptive actions of FSH and bone loss. Annals of the New York Academy of Sciences. 2007;1116:376–382. doi: 10.1196/annals.1402.056. [DOI] [PubMed] [Google Scholar]
- 81.Liu P, et al. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature. 2017;546(7656):107–112. doi: 10.1038/nature22342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kumar TR, Low MJ, Matzuk MM. Genetic rescue of follicle-stimulating hormone beta-deficient mice. Endocrinology. 1998;139(7):3289–3295. doi: 10.1210/endo.139.7.6111. [DOI] [PubMed] [Google Scholar]
- 83.Wang H, et al. Evaluation of in vivo bioactivities of recombinant hypo- (FSH(21/18)) and fully- (FSH(24)) glycosylated human FSH glycoforms in Fshb null mice. Molecular and cellular endocrinology. 2016;437:224–236. doi: 10.1016/j.mce.2016.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dierich A, et al. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(23):13612–13617. doi: 10.1073/pnas.95.23.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abel MH, et al. The Effect of a Null Mutation in the Follicle-Stimulating Hormone Receptor Gene on Mouse Reproduction <sup>1</sup>. Endocrinology. 2000;141(5):1795–1803. doi: 10.1210/endo.141.5.7456. [DOI] [PubMed] [Google Scholar]
- 86.Allan CM, Lim P, Robson M, Spaliviero J, Handelsman DJ. Transgenic mutant D567G but not wild-type human FSH receptor overexpression provides FSH-independent and promiscuous glycoprotein hormone Sertoli cell signaling. American journal of physiology Endocrinology and metabolism. 2009;296(5):E1022–1028. doi: 10.1152/ajpendo.90941.2008. [DOI] [PubMed] [Google Scholar]
- 87.Haywood M, et al. An Activated Human Follicle-Stimulating Hormone (FSH) Receptor Stimulates FSH-Like Activity in Gonadotropin-Deficient Transgenic Mice. Molecular Endocrinology. 2002;16(11):2582–2591. doi: 10.1210/me.2002-0032. [DOI] [PubMed] [Google Scholar]
- 88.Peltoketo H, et al. Female Mice Expressing Constitutively Active Mutants of FSH Receptor Present with a Phenotype of Premature Follicle Depletion and Estrogen Excess. Endocrinology. 2010;151(4):1872–1883. doi: 10.1210/en.2009-0966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Perez-Millan MI, Zeidler MG, Saunders TL, Camper SA, Davis SW. Efficient, specific, developmentally appropriate cre-mediated recombination in anterior pituitary gonadotropes and thyrotropes. Genesis (New York, NY: 2000) 2013;51(11):785–792. doi: 10.1002/dvg.22425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Charles MA, Mortensen AH, Potok MA, Camper SA. Pitx2 deletion in pituitary gonadotropes is compatible with gonadal development, puberty, and fertility. Genesis (New York, NY: 2000) 2008;46(10):507–514. doi: 10.1002/dvg.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang H, et al. Gonadotrope-specific deletion of Dicer results in severely suppressed gonadotropins and fertility defects. The Journal of biological chemistry. 2015;290(5):2699–2714. doi: 10.1074/jbc.M114.621565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hasuwa H, Ueda J, Ikawa M, Okabe M. miR-200b and miR-429 function in mouse ovulation and are essential for female fertility. Science (New York, NY) 2013;341(6141):71–73. doi: 10.1126/science.1237999. [DOI] [PubMed] [Google Scholar]
- 93.Messina A, et al. A microRNA switch regulates the rise in hypothalamic GnRH production before puberty. Nature neuroscience. 2016;19(6):835–844. doi: 10.1038/nn.4298. [DOI] [PubMed] [Google Scholar]
- 94.Bilezikjian LM, Justice NJ, Blackler AN, Wiater E, Vale WW. Cell-type specific modulation of pituitary cells by activin, inhibin and follistatin. Mol Cell Endocrinol. 2012;359(1-2):43–52. doi: 10.1016/j.mce.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thompson IR, Kaiser UB. GnRH pulse frequency-dependent differential regulation of LH and FSH gene expression. Mol Cell Endocrinol. 2014;385(1-2):28–35. doi: 10.1016/j.mce.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Matzuk MM, Kumar TR, Bradley A. Different phenotypes for mice deficient in either activins or activin receptor type II. Nature. 1995;374(6520):356–360. doi: 10.1038/374356a0. [DOI] [PubMed] [Google Scholar]
- 97.Bernard DJ, Lee KB, Santos MM. Activin B can signal through both ALK4 and ALK7 in gonadotrope cells. Reproductive biology and endocrinology: RB&E. 2006;4:52. doi: 10.1186/1477-7827-4-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sandoval-Guzman T, et al. Neuroendocrine control of female reproductive function by the activin receptor ALK7. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2012;26(12):4966–4976. doi: 10.1096/fj.11-199059. [DOI] [PubMed] [Google Scholar]
- 99.Fortin J, Boehm U, Deng CX, Treier M, Bernard DJ. Follicle-stimulating hormone synthesis and fertility depend on SMAD4 and FOXL2. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2014;28(8):3396–3410. doi: 10.1096/fj.14-249532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fortin J, et al. Minireview: Activin Signaling in Gonadotropes: What Does theFOX say… to the SMAD? Molecular endocrinology (Baltimore, Md) 2015;29(7):963–977. doi: 10.1210/me.2015-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lamba P, Fortin J, Tran S, Wang Y, Bernard DJ. A novel role for the forkhead transcription factor FOXL2 in activin A-regulated follicle-stimulating hormone beta subunit transcription. Molecular endocrinology (Baltimore, Md) 2009;23(7):1001–1013. doi: 10.1210/me.2008-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stromme P, Sandboe F. Blepharophimosis-ptosis-epicanthus inversus syndrome (BPES) Acta ophthalmologica Scandinavica. 1996;74(1):45–47. doi: 10.1111/j.1600-0420.1996.tb00680.x. [DOI] [PubMed] [Google Scholar]
- 103.Correa FJ, Tavares AB, Pereira RW, Abrao MS. A new FOXL2 gene mutation in a woman with premature ovarian failure and sporadic blepharophimosis-ptosis-epicanthus inversus syndrome. Fertil Steril. 2010;93(3):1006.e1003–1006. doi: 10.1016/j.fertnstert.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 104.Roth LW, Alvero R. Pregnancy in a woman with premature ovarian insufficiency associated with blepharophimosis, ptosis, epicanthus inversus syndrome type I. A case report. The Journal of reproductive medicine. 2014;59(1-2):87–89. [PubMed] [Google Scholar]
- 105.Matzuk MM, et al. Transgenic models to study the roles of inhibins and activins in reproduction, oncogenesis, and development. Recent progress in hormone research. 1996;51:123–154. discussion 155-127. [PubMed] [Google Scholar]
- 106.Matzuk MM, Finegold MJ, Su JG, Hsueh AJ, Bradley A. Alpha-inhibin is a tumour-suppressor gene with gonadal specificity in mice. Nature. 1992;360(6402):313–319. doi: 10.1038/360313a0. [DOI] [PubMed] [Google Scholar]
- 107.Matzuk MM, et al. Development of cancer cachexia-like syndrome and adrenal tumors in inhibin-deficient mice. Proc Natl Acad Sci U S A. 1994;91(19):8817–8821. doi: 10.1073/pnas.91.19.8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kumar TR, Wang Y, Matzuk MM. Gonadotropins are essential modifier factors for gonadal tumor development in inhibin-deficient mice. Endocrinology. 1996;137(10):4210–4216. doi: 10.1210/endo.137.10.8828479. [DOI] [PubMed] [Google Scholar]
- 109.Nagaraja AK, Agno JE, Kumar TR, Matzuk MM. Luteinizing hormone promotes gonadal tumorigenesis in inhibin-deficient mice. Molecular and Cellular Endocrinology. 2008;294(1):19–28. doi: 10.1016/j.mce.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Matzuk MM, et al. Multiple defects and perinatal death in mice deficient in follistatin. Nature. 1995;374(6520):360–363. doi: 10.1038/374360a0. [DOI] [PubMed] [Google Scholar]
- 111.Jorgez CJ, Klysik M, Jamin SP, Behringer RR, Matzuk MM. Granulosa cell-specific inactivation of follistatin causes female fertility defects. Molecular endocrinology (Baltimore, Md) 2004;18(4):953–967. doi: 10.1210/me.2003-0301. [DOI] [PubMed] [Google Scholar]
- 112.Lin SY, Morrison JR, Matzuk MM, de Kretser DM. Spermatogenesis does not require the local production of follistatin. Reproduction (Cambridge, England) 2006;132(4):601–605. doi: 10.1530/rep.1.01172. [DOI] [PubMed] [Google Scholar]
- 113.Guo Q, et al. Overexpression of mouse follistatin causes reproductive defects in transgenic mice. Molecular endocrinology (Baltimore, Md) 1998;12(1):96–106. doi: 10.1210/mend.12.1.0053. [DOI] [PubMed] [Google Scholar]
- 114.Pearl CA, Jablonka-Shariff A, Boime I. Rerouting of a follicle-stimulating hormone analog to the regulated secretory pathway. Endocrinology. 2010;151(1):388–393. doi: 10.1210/en.2009-0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Flack MR, Froehlich J, Bennet AP, Anasti J, Nisula BC. Site-directed mutagenesis defines the individual roles of the glycosylation sites on follicle-stimulating hormone. The Journal of biological chemistry. 1994;269(19):14015–14020. [PubMed] [Google Scholar]
- 116.Matzuk MM, Boime I. The role of the asparagine-linked oligosaccharides of the alpha subunit in the secretion and assembly of human chorionic gonadotrophin. The Journal of cell biology. 1988;106(4):1049–1059. doi: 10.1083/jcb.106.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ulloa-Aguirre A, Timossi C, Damián-Matsumura P, Dias JA. Role of Glycosylation in Function of Follicle-Stimulating Hormone. Endocrine. 1999;11(3):205–216. doi: 10.1385/ENDO:11:3:205. [DOI] [PubMed] [Google Scholar]
- 118.Arey BJ, López FJ. Are circulating gonadotropin isoforms naturally occurring biased agonists? Basic and therapeutic implications. Reviews in Endocrine and Metabolic Disorders. 2011;12(4):275–288. doi: 10.1007/s11154-011-9188-y. [DOI] [PubMed] [Google Scholar]
- 119.Lambert A, Talbot JA, Anobile CJ, Robertson WR. Gonadotrophin heterogeneity and biopotency: Implications for assisted reproduction. Molecular Human Reproduction. 1998;4(7):619–629. doi: 10.1093/molehr/4.7.619. [DOI] [PubMed] [Google Scholar]
- 120.Stanton PG, et al. Application of a sensitive HPLC-based fluorometric assay to determine the sialic acid content of human gonadotropin isoforms. Journal of biochemical and biophysical methods. 1995;30(1):37–48. doi: 10.1016/0165-022x(94)00063-j. [DOI] [PubMed] [Google Scholar]
- 121.Fares F. The role of O-linked and N-linked oligosaccharides on the structure-function of glycoprotein hormones: Development of agonists and antagonists. Biochimica et Biophysica Acta - General Subjects. 2006;1760(4):560–567. doi: 10.1016/j.bbagen.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 122.Galway AB, et al. In Vitro and in Vivo Bioactivity of Recombinant Human Follicle-Stimulating Hormone and Partially Deglycosylated Variants Secreted by Transfected Eukaryotic Cell Lines*. Endocrinology. 1990;127(1):93–100. doi: 10.1210/endo-127-1-93. [DOI] [PubMed] [Google Scholar]
- 123.Bishop LA, Nguyen TV, Schofield PR. Both of the beta-subunit carbohydrate residues of follicle-stimulating hormone determine the metabolic clearance rate and in vivo potency. Endocrinology. 1995;136(6):2635–2640. doi: 10.1210/endo.136.6.7750487. [DOI] [PubMed] [Google Scholar]
- 124.Bousfield GR, et al. All-or-none N-glycosylation in primate follicle-stimulating hormone ??-subunits. Molecular and Cellular Endocrinology. 2007;(1):260–262. 40–48. doi: 10.1016/j.mce.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 125.Walton WJ, et al. Characterization of human FSH isoforms reveals a nonglycosylated β-subunit in addition to the conventional glycosylated β-subunit. Journal of Clinical Endocrinology and Metabolism. 2001;86(8):3675–3685. doi: 10.1210/jcem.86.8.7712. [DOI] [PubMed] [Google Scholar]
- 126.Bousfield GR, et al. Macro- and Micro-heterogeneity in Pituitary and Urinary Follicle-Stimulating Hormone Glycosylation. Journal of glycomics & lipidomics. 2014;4(04) doi: 10.4172/2153-0637.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bousfield GR, Butnev VY, White WK, Hall AS, Harvey DJ. Comparison of Follicle-Stimulating Hormone Glycosylation Microheterogenity by Quantitative Negative Mode Nano-Electrospray Mass Spectrometry of Peptide-N Glycanase-Released Oligosaccharides. Journal of glycomics & lipidomics. 2015;5(1) doi: 10.4172/2153-0637.1000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bousfield GR, et al. Hypo-glycosylated human follicle-stimulating hormone (hFSH21/18) is much more active in vitro than fully-glycosylated hFSH (hFSH24) Molecular and Cellular Endocrinology. 2014;382(2):989–997. doi: 10.1016/j.mce.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Butnev VY, et al. Production, purification, and characterization of recombinant hFSH glycoforms for functional studies. Molecular and Cellular Endocrinology. 2015;405:42–51. doi: 10.1016/j.mce.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jiang C, et al. Hypoglycosylated hFSH has greater bioactivity than fully glycosylated recombinant hFSH in human granulosa cells. Journal of Clinical Endocrinology and Metabolism. 2015;100(6):E852–E860. doi: 10.1210/jc.2015-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang H, Butnev V, Bous GR, Kumar TR. A human FSHB transgene encoding the double N-glycosylation mutant (Asn 7 D Asn 24 D) FSH b subunit fails to rescue Fshb null mice. Molecular and cellular endocrinology. 2016;426:113–124. doi: 10.1016/j.mce.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cole LA. Familial HCG syndrome. Journal of reproductive immunology. 2012;93(1):52–57. doi: 10.1016/j.jri.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 133.Hai L, McGee SR, Rabideau AC, Paquet M, Narayan P. Infertility in Female Mice with a Gain-of-Function Mutation in the Luteinizing Hormone Receptor Is Due to Irregular Estrous Cyclicity, Anovulation, Hormonal Alterations, and Polycystic Ovaries. Biology of reproduction. 2015;93(1):16–16. doi: 10.1095/biolreprod.115.129072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shenker A. Activating mutations of the lutropin choriogonadotropin receptor in precocious puberty. Receptors & channels. 2002;8(1):3–18. [PubMed] [Google Scholar]
- 135.Themmen APN, Huhtaniemi IT. Mutations of Gonadotropins and Gonadotropin Receptors: Elucidating the Physiology and Pathophysiology of Pituitary-Gonadal Function. Endocrine Reviews. 2000;21(5):551–583. doi: 10.1210/edrv.21.5.0409. [DOI] [PubMed] [Google Scholar]
- 136.Kremer H, et al. Male pseudohermaphroditism due to a homozygous missense mutation of the luteinizing hormone receptor gene. Nature genetics. 1995;10:196–201. doi: 10.1038/ng0295-160. [DOI] [PubMed] [Google Scholar]