Abstract
Objectives
To estimate incidence, mortality, and disability-adjusted life years (DALYs) caused by cancer in the Eastern Mediterranean Region (EMR) between 2005 and 2015.
Methods
Vital registration system and cancer registry data from the EMR region were analyzed for 29 cancer groups in 22 EMR countries using the Global Burden of Disease Study 2015 methodology.
Results
In 2015, cancer was responsible for 9.4% of all deaths and 5.1% of all DALYs. It accounted for 722,646 new cases, 379,093 deaths, and 11.7 million DALYs. Between 2005 and 2015, incident cases increased by 46%, deaths by 33%, and DALYs by 31%. The increase in cancer incidence was largely driven by population growth and population aging. Breast cancer, lung cancer, and leukemia were the most common cancers, while lung, breast, and stomach cancers caused most cancer deaths.
Conclusions
Cancer is responsible for a substantial disease burden in the EMR, which is increasing. There is an urgent need to expand cancer prevention, screening, and awareness programs in EMR countries as well as to improve diagnosis, treatment, and palliative care services.
Electronic supplementary material
The online version of this article (doi:10.1007/s00038-017-0999-9) contains supplementary material, which is available to authorized users.
Keywords: Eastern Mediterranean Region, Cancer, Mortality, Incidence, Disability-adjusted life years
Introduction
With 8.7 million deaths (16% of all deaths), cancer was globally the second-leading cause of death behind cardiovascular diseases in 2015 (GBD 2015 Mortality and Causes of Death Collaborators 2016). There were 17.5 million incident cases globally, and cancer accounted for 209 million DALYs (GBD 2015 DALYs and HALE Collaborators 2016; GBD 2015 Disease and Injury Incidence and Prevalence Collaborators 2016; Global Burden of Disease Cancer Collaboration et al. 2016). In many countries, the epidemiological transition has led to a decrease in communicable, neonatal, maternal, and nutritional diseases, at the expense of an increase in non-communicable diseases over time (GBD 2015 DALYs and HALE Collaborators 2016). Prior studies examining cancer epidemiology in the EMR have either focused on a single year, a single country, or a particular component of cancer treatment (Aljurf et al. 2010; Abdel-Razeq et al. 2015; Kulhánová et al. 2017). What has not been analyzed for the EMR is how the epidemiological and demographical transition through an aging population, urbanization, industrialization, and lifestyle changes, as well political turmoil has affected the cancer burden (GBD 2015 DALYs and HALE Collaborators 2016). This evidence is essential for comprehensive cancer control planning. Given the diverse country profiles in the EMR with large differences in income, age structure, risk factor profile, and political stability, cancer prevention potential and treatment capacity requirements differ substantially between countries. In this study, we therefore present the Global Burden of Disease Study 2015 (GBD 2015) estimates of incidence, mortality, years of life lost (YLLs), years lived with disability (YLDs), and DALYs for 29 cancer groups and 22 EMR countries from 2005 to 2015 by age and sex, which to our knowledge is the most comprehensive assessment of cancer burden in the EMR (GBD 2015 DALYs and HALE Collaborators 2016; GBD 2015 Disease and Injury Incidence and Prevalence Collaborators 2016; GBD 2015 Mortality and Causes of Death Collaborators 2016; GBD 2015 Risk Factors Collaborators 2016). This quantitative assessment is especially important to guide health policy and to measure progress on the third Sustainable Development Goal (SDG) of reducing premature mortality from non-communicable diseases by one third by 2030 (United Nations 2016).
Methods
The GBD 2015 study estimated incidence, prevalence, deaths, YLLs, YLDs, and DALYs for 195 countries and territories from 1990 to 2015. In total, 315 causes of diseases and injuries and 79 risk factors were systematically analyzed. Details of the methodology used in GBD 2015 to estimate general disease burden and cancer burden are described in detail elsewhere (GBD 2015 DALYs and HALE Collaborators 2016; GBD 2015 Disease and Injury Incidence and Prevalence Collaborators 2016; GBD 2015 Mortality and Causes of Death Collaborators 2016; GBD 2015 Risk Factors Collaborators 2016; Global Burden of Disease Cancer Collaboration et al. 2016).
Briefly, to estimate cancer burden, we mapped all neoplasms as defined by the 10th revision of the International Statistical Classification of Diseases (ICD-10) to one of the 29 GBD cancer groups. Input data for cancer mortality estimates came from vital registry mortality and cancer registry incidence data. The latter were transformed to mortality estimates using separately modeled mortality-to-incidence ratios (MIR) (Global Burden of Disease Cancer Collaboration et al. 2016). The raw data were processed to make them comparable and to account for “garbage codes”, which are codes assigned to causes that are not usable from a public health perspective (Naghavi et al. 2010). These causes were redistributed to the most likely underlying cause of death based on a regression model. Data were extracted at the most detailed level, by age group and sex, and mapped to the GBD cause list. Using a cause of death ensemble modeling (CODEm) approach with cause-specific covariates, we computed mortality estimates for each individual cause (Foreman et al. 2012). These estimates were scaled to fit into an independently modeled all-cause mortality estimate using the algorithm CodCorrect (GBD 2015 Mortality and Causes of Death Collaborators 2016). We transformed the final mortality estimates into incidence estimates using modeled MIR. Uncertainty from data sources and processing steps was propagated to the incidence estimates.
Cancer survival was calculated using a MIR-based scaling factor. We calculated 10-year prevalence of each cancer and each incidence cohort using these cancer survival estimates. The total prevalence was divided into four sequelae with variable disability weights: (1) diagnosis and treatment, (2) remission, (3) metastatic, and (4) terminal phase. We assumed a constant duration for sequelae (1), (3), and (4) for all countries over time. Duration of sequela (2) was the remaining prevalence after subtracting the duration of the fixed sequelae. We computed YLLs by multiplying deaths by the normative standard life expectancy at each age of death (GBD 2015 Mortality and Causes of Death Collaborators 2016). For each sequela, YLDs were calculated by multiplying the prevalence of each sequela by its disability weight. Finally, DALYs were calculated by summing YLLs and YLDs.
To analyze the contribution of population aging, population growth, and changes in age-specific incidence rates (ASIR) to the absolute change of cancer incidence, we calculated two scenarios. In the first, the age structure, sex structure, and age-specific rates from 2005 were applied to the 2015 population. The difference between the total number of cases in 2005 and the hypothetical scenario were attributed to population growth. In the second, the age-specific rates from 2005 were applied to the age structure, sex structure, and 2015 population. The differences between the two scenarios were attributed to population aging. Differences between the total number of cases in 2015 and the second hypothetical scenario were attributed to changes in the age-specific rates.
The 22 EMR countries were grouped according to per capita gross national income (GNI) into low-income countries (LICs) (Afghanistan, Djibouti, Somalia, and Yemen); middle-income countries (MICs) (Egypt, Iran, Iraq, Jordan, Lebanon, Libya, Morocco, Pakistan, Palestine, Sudan, Syria, and Tunisia); and high-income countries (HICs) (Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, and the United Arab Emirates). LICs were defined as those having a per capita GNI of $1045 or less, MICs as those with a per capita GNI between $1046 and $12,735, and HICs as countries with per capita GNI of $12,736 or greater.
In this publication, all rates are reported per 100,000 person-years. We report 95% uncertainty intervals (UIs) for all estimates (listed in parentheses after the point estimates).
Results
Regional burden of cancer
Between 2005 and 2015 in the EMR region, incident cancer cases increased by 46.1% (34.5–59.4%) from 495 (457–537) thousand in 2005 to 723 (661–790) thousand cases in 2015 (Table 1). In 2015, cancer caused 379 (350–409) thousand deaths (Table 2) and 11.7 million (10.8–12.7 million) DALYs, of which 3% were attributable to YLDs and 97% to YLLs (eFig. 1). Age-standardized DALY rates (ASDR) remained unchanged between 2005 and 2015: 2663.7 (2486.3–2861.0) in 2005, and 2605.3 (2404.8–2816.0) in 2015 (eTable 4).
Table 1.
Decomposition Analysis of Cancer Incidence by Country in the Eastern Mediterranean Region, both sexes, 2005–2015 (Global Burden of Disease Study 2015, Eastern Mediterranean Countries, 2005–2015)
| Location | Number of incident cases | Expected number of cases in 2015 | Change in incident cases 2005–2015 in % | Overall change in % | ||||
|---|---|---|---|---|---|---|---|---|
| 2005 | 2015 | Given population growth alone | Given population growth and aging | Due to population growth | Due to population ageing | Due to change in incidence rates | ||
| Eastern Mediterranean Region | 494,690 | 722,646 | 609,771 | 670,386 | 23.3 | 12.3 | 10.6 | 46.1 |
| Afghanistan | 25,015 | 36,809 | 33,400 | 35,375 | 33.5 | 7.9 | 5.7 | 47.2 |
| Bahrain | 663 | 1105 | 1050 | 1218 | 58.5 | 25.3 | −17.1 | 66.7 |
| Djibouti | 772 | 1153 | 879 | 1020 | 13.8 | 18.3 | 17.3 | 49.4 |
| Egypt | 62,489 | 87,853 | 76,106 | 80,680 | 21.8 | 7.3 | 11.5 | 40.6 |
| Iran | 67,019 | 95,011 | 75,054 | 88,510 | 12.0 | 20.1 | 9.7 | 41.8 |
| Iraq | 28,593 | 41,208 | 38,474 | 40,354 | 34.6 | 6.6 | 3.0 | 44.1 |
| Jordan | 4016 | 6188 | 5677 | 6504 | 41.4 | 20.6 | −7.9 | 54.1 |
| Kuwait | 1416 | 2544 | 2430 | 2701 | 71.5 | 19.2 | −11.1 | 79.6 |
| Lebanon | 7446 | 13,272 | 10,867 | 11,933 | 45.9 | 14.3 | 18.0 | 78.2 |
| Libya | 5205 | 7646 | 5638 | 6762 | 8.3 | 21.6 | 17.0 | 46.9 |
| Morocco | 36,743 | 53,370 | 41,475 | 48,931 | 12.9 | 20.3 | 12.1 | 45.3 |
| Oman | 1127 | 2524 | 2016 | 2240 | 78.8 | 19.9 | 25.2 | 123.9 |
| Pakistan | 175,827 | 254,242 | 215,840 | 233,152 | 22.8 | 9.8 | 12.0 | 44.6 |
| Palestine | 2104 | 3479 | 2730 | 3053 | 29.8 | 15.3 | 20.3 | 65.4 |
| Qatar | 529 | 1290 | 1417 | 1281 | 167.7 | −25.7 | 1.7 | 143.7 |
| Saudi Arabia | 9384 | 15,726 | 11,912 | 14,359 | 26.9 | 26.1 | 14.6 | 67.6 |
| Somalia | 7207 | 9862 | 9193 | 8974 | 27.6 | −3.0 | 12.3 | 36.8 |
| Sudan | 20,552 | 29,740 | 25,871 | 27,940 | 25.9 | 10.1 | 8.8 | 44.7 |
| Syria | 7938 | 10,956 | 8134 | 9753 | 2.5 | 20.4 | 15.1 | 38.0 |
| Tunisia | 14,023 | 19,471 | 15,592 | 17,998 | 11.2 | 17.2 | 10.5 | 38.8 |
| United Arab Emirates | 3268 | 9247 | 6663 | 8585 | 103.9 | 58.8 | 20.2 | 182.9 |
| Yemen | 13,352 | 19,950 | 17,472 | 18,375 | 30.9 | 6.8 | 11.8 | 49.4 |
Table 2.
Incidence, deaths and disability-adjusted life years for all cancers and 29 cancer groups in the Eastern Mediterranean Region, both sexes, 2015 (Global Burden of Disease Study 2015, Eastern Mediterranean Region, 2015)
| Cause | Number of incident cases | Number of deaths | Number of DALYs (in thousands) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Males | Females | Both | Males | Females | Both | Males | Females | Both | |
| All cancers groups | 309,240 (282,640–340,657) | 413,406 (361,086–467,300) | 722,646 (660,722–790,102) | 198,164 (181,894–217,561) | 180,929 (160,360–202,560) | 379,093 (350,252–408,580) | 5865 (5354–6474) | 5875 (5191–6608) | 11,740 (10,800–12,742) |
| Lip and oral cavity cancer | 14,068 (10,664–18,701) | 15,358 (11,296–21,357) | 29,426 (23,752–36,473) | 4918 (4009–6069) | 4837 (3841–5943) | 9755 (8315–11,408) | 161 (127–203) | 151 (119–187) | 312 (263–368) |
| Nasopharynx cancer | 3219 (2080–4681) | 1836 (1041–2849) | 5055 (3606–6940) | 1469 (1242–1776) | 825 (680–984) | 2294 (2006–2681) | 52 (43–63) | 30 (25–36) | 82 (71–97) |
| Other pharynx cancer | 4394 (3516–5394) | 3593 (2915–4365) | 7988 (6914–9273) | 1654 (1410–1976) | 1391 (1165–1641) | 3045 (2679–3457) | 46 (40–55) | 40 (34–48) | 87 (76–99) |
| Esophageal cancer | 8795 (7449–10,517) | 7992 (6474–9848) | 16,788 (14,577–19,253) | 9345 (8068–10,879) | 8396 (6823–10,239) | 17,741 (15,743–20,073) | 265 (223–317) | 251 (203–313) | 516 (452–595) |
| Stomach cancer | 27,093 (24,235–30,565) | 17,725 (14,951–20,558) | 44,818 (40,719–49,095) | 17,462 (15,667–19,460) | 11,847 (9954–13,631) | 29,309 (26,728–31,947) | 441 (394–496) | 328 (264–387) | 769 (690–851) |
| Colon and rectum cancer | 18,662 (16,276–21,216) | 17,150 (15,090–19,477) | 35,813 (32,240–39,507) | 13,268 (11,666–14,925) | 13,148 (11,498–14,918) | 26,416 (23,736–29,279) | 392 (335–450) | 373 (321–427) | 764 (678–859) |
| Liver cancer | 14,660 (12,042–16,784) | 9908 (7418–11,788) | 24,568 (20,618–27,385) | 16,617 (13,869–18,735) | 10,747 (8232–12,290) | 27,365 (23,002–30,174) | 448 (347–513) | 292 (211–342) | 740 (588–823) |
| Gallbladder and biliary tract cancer | 2383 (1983–2803) | 4543 (3731–5279) | 6926 (5941–7838) | 1985 (1673–2312) | 3853 (3161–4465) | 5839 (4973–6612) | 50 (41–59) | 98 (79–116) | 148 (124–169) |
| Pancreatic cancer | 6283 (5762–6843) | 4885 (4393–5394) | 11,168 (10,419–11,995) | 7011 (6463–7643) | 5480 (4917–6100) | 12,491 (11,601–13,400) | 179 (164–196) | 129 (116–144) | 308 (285–331) |
| Larynx cancer | 11,975 (10,284–14,128) | 2887 (2458–3493) | 14,862 (13,083–17,018) | 6477 (5676–7380) | 1612 (1345–1917) | 8090 (7222–9049) | 172 (150–199) | 46 (39–54) | 218 (193–245) |
| Tracheal, bronchial and lung cancer | 37,681 (32,768–42,292) | 11,848 (10,591–13,321) | 49,530 (44,083–54,564) | 39,180 (34,316–43,815) | 11,831 (10,442–13,380) | 51,012 (45,430–56,191) | 1013 (879–1144) | 317 (277–364) | 1330 (1171–1475) |
| Malignant skin melanoma | 3021 (2072–3909) | 2733 (2326–3212) | 5755 (4863–6883) | 617 (407–773) | 514 (452–591) | 1131 (947–1322) | 20 (14–27) | 16 (14–19) | 36 (31–44) |
| Non-melanoma skin cancer | 9359 (8443–10,285) | 4697 (4165–5264) | 14,056 (12,711–15,383) | 1015 (921–1117) | 314 (272–361) | 1330 (1223–1449) | 27 (24–30) | 9 (7–10) | 36 (33–39) |
| Breast cancer | 2058 (1810–2359) | 177,389 (148,702–207,371) | 179,447 (150,924–209,304) | 463 (408–530) | 38,117 (32,305–44,251) | 38,581 (32,795–44,698) | 14 (12–16) | 1314 (1101–1546) | 1328 (1115–1561) |
| Cervical cancer | – | 19,634 (14,721–25,505) | 19,634 (14,721–25,505) | – | 7878 (6158–9928) | 7878 (6158–9928) | – | 251 (192–323) | 251 (192–323) |
| Uterine cancer | – | 14,337 (11,576–17,621) | 14,337 (11,576–17,621) | – | 6857 (5641–8076) | 6857 (5641–8076) | – | 193 (157–228) | 193 (157–228) |
| Ovarian cancer | – | 10,946 (9024–13,395) | 10,946 (9024–13,395) | – | 6855 (5953–7833) | 6855 (5953–7833) | – | 235 (201–271) | 235 (201–271) |
| Prostate cancer | 27,533 (20,349–34,378) | – | 27,533 (20,349–34,378) | 13,861 (10,420–17,187) | – | 13,861 (10,420–17,187) | 243 (180–297) | – | 243 (180–297) |
| Testicular cancer | 3143 (2315–4266) | – | 3143 (2315–4266) | 1010 (792–1299) | – | 1010 (792–1299) | 52 (40–68) | – | 52 (40–68) |
| Kidney cancer | 5465 (4635–6345) | 2856 (2463–3279) | 8321 (7364–9305) | 3497 (3046–3942) | 1741 (1505–2039) | 5239 (4699–5834) | 110 (96–125) | 60 (52–71) | 170 (152–190) |
| Bladder cancer | 23,449 (20,144–27,360) | 6404 (5411–7716) | 29,853 (26,404–33,966) | 9452 (8524–10,545) | 3151 (2723–3587) | 12,604 (11,527–13,821) | 217 (194–244) | 73 (63–83) | 289 (263–320) |
| Brain and nervous system | 12,805 (9708–15,710) | 11,045 (9432–12,702) | 23,851 (20,099–27,075) | 10,333 (7909–12,510) | 8395 (7355–9338) | 18,729 (16,185–20,983) | 427 (321–525) | 352 (300–392) | 779 (666–881) |
| Thyroid cancer | 3654 (2966–4357) | 7536 (6026–9477) | 11,191 (9589–13,565) | 565 (484–688) | 1112 (925–1352) | 1678 (1478–2000) | 17 (14–20) | 32 (26–39) | 49 (42–58) |
| Mesothelioma | 839 (724–1015) | 260 (215–339) | 1099 (964–1313) | 794 (717–909) | 299 (245–360) | 1093 (987–1243) | 24 (22–28) | 10 (8–12) | 34 (30–39) |
| Hodgkin lymphoma | 3247 (2539–4383) | 2372 (1488–3502) | 5619 (4436–7142) | 1176 (932–1649) | 811 (547–1245) | 1987 (1655–2658) | 50 (39–70) | 36 (23–53) | 86 (70–114) |
| Non-Hodgkin lymphoma | 16,818 (14,017–20,617) | 14,549 (9501–18,687) | 31,367 (24,638–36,975) | 6745 (5684–8121) | 5999 (4194–7470) | 12,744 (10,225–14,857) | 251 (209–305) | 216 (146–274) | 466 (365–553) |
| Multiple myeloma | 2693 (2323–3268) | 2643 (2205–3157) | 5336 (4694–6150) | 2318 (2038–2761) | 2395 (2028–2834) | 4714 (4196–5411) | 66 (57–80) | 67 (56–80) | 132 (116–154) |
| Leukemia | 26,878 (23,330–31,115) | 20,800 (17,703–24,679) | 47,679 (42,513–53,365) | 14,627 (13,388–16,013) | 11,206 (10,045–12,467) | 25,833 (24,105–27,809) | 637 (579–705) | 498 (445–557) | 1135 (1053–1232) |
| Other neoplasms | 19,054 (15,995–23,621) | 17,468 (14,692–21,110) | 36,523 (32,170–42,695) | 12,292 (10,537–15,191) | 11,306 (9694–13,334) | 23,599 (20,803–27,877) | 494 (418–603) | 459 (389–546) | 953 (840–1103) |
Regional age and sex variations in cancer burden
Females had higher ASIR in 2015 than males, with 199.6 (175.7–224.5) in females and 163.3 (150.2–178.7) in males (Table 3). Age-standardized mortality rate (ASMR) was higher in males compared to females at 113.8 (105.0–124.0) versus 95.8 (85.4–106.7), respectively. In females, breast cancer, leukemia, and cervical cancer were the most common incident cancers with 177 (149–207) thousand, 21 (18–25) thousand, and 20 (15–26) thousand cases, respectively (Table 2). The three cancers responsible for most cancer deaths in females were breast cancer with 38 (32–44) thousand deaths, colon and rectal cancer with 13 (11–15) thousand deaths, and stomach cancer with 12 (10–14) thousand deaths. The top three causes of DALYs in females were breast cancer with 1.3 (1.1–1.5) million DALYs, leukemia with 498 (445–557) thousand DALYs, and other neoplasms with 459 (339–546) thousand DALYs (Table 2).
Table 3.
Age-standardized incidence, mortality and DALY rates per 100,000 for the Eastern Mediterranean Region and its 22 countries, both sexes, 2015 (Global Burden of Disease Study 2015, Eastern Mediterranean Countries, 2015)
| Location | Age-standardized incidence rate | Age-standardized mortality rate | Age-standardized DALY rate | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Males | Females | Both | Males | Females | Both | Males | Females | Both | |
| Eastern Mediterranean Region | 163 (150–179) | 200 (176–225) | 180 (166–195) | 114 (105–124) | 96 (85–107) | 104 (97–112) | 2651 (2435–2915) | 2583 (2287–2897) | 2605 (2404–2816) |
| Afghanistan | 178 (127–237) | 345 (171–642) | 259 (161–411) | 156 (109–205) | 177 (102–276) | 165 (112–222) | 3546 (2355–4889) | 5066 (2618–8449) | 4260 (2634–6153) |
| Bahrain | 134 (105–168) | 156 (122–196) | 138 (117–162) | 88 (69–107) | 70 (56–85) | 77 (66–89) | 1752 (1385–2170) | 1776 (1395–2221) | 1710 (1453–2003) |
| Djibouti | 201 (103–409) | 221 (105–528) | 210 (113–388) | 158 (80–311) | 129 (61–301) | 142 (75–257) | 3972 (1896–8438) | 3634 (1696–8796) | 3783 (1951–7187) |
| Egypt | 146 (135–165) | 137 (126–149) | 139 (131–151) | 100 (94–111) | 65 (62–71) | 81 (77–87) | 2437 (2299–2619) | 1846 (1740–1973) | 2113 (2022–2229) |
| Iran | 208 (166–260) | 135 (104–175) | 173 (146–205) | 123 (98–150) | 69 (52–89) | 97 (81–113) | 2650 (2080–3338) | 1704 (1286–2243) | 2190 (1825–2595) |
| Iraq | 185 (129–243) | 254 (178–344) | 220 (172–280) | 138 (95–176) | 117 (86–156) | 126 (101–155) | 3340 (2303–4462) | 3329 (2385–4498) | 3318 (2625–4180) |
| Jordan | 153 (131–179) | 145 (115–178) | 147 (129–169) | 98 (84–114) | 64 (53–76) | 80 (71–90) | 2193 (1888–2580) | 1708 (1423–2039) | 1939 (1713–2179) |
| Kuwait | 139 (118–167) | 179 (148–214) | 156 (139–176) | 71 (60–84) | 68 (56–82) | 70 (62–79) | 1403 (1181–1664) | 1523 (1256–1815) | 1453 (1292–1644) |
| Lebanon | 284 (192–391) | 246 (165–341) | 262 (195–336) | 158 (104–218) | 110 (73–146) | 133 (99–170) | 3302 (2179–4603) | 2715 (1771–3746) | 2995 (2238–3864) |
| Libya | 226 (187–276) | 160 (133–194) | 189 (166–218) | 157 (128–191) | 91 (74–110) | 121 (104–139) | 3326 (2703–4039) | 2245 (1815–2752) | 2752 (2360–3181) |
| Morocco | 180 (126–258) | 210 (138–296) | 195 (151–252) | 140 (98–201) | 108 (72–148) | 122 (96–159) | 3019 (2053–4385) | 2690 (1776–3789) | 2844 (2205–3728) |
| Oman | 121 (97–143) | 118 (98–144) | 116 (99–132) | 74 (58–87) | 58 (47–69) | 66 (55–75) | 1564 (1237–1867) | 1397 (1115–1711) | 1469 (1220–1688) |
| Pakistan | 153 (127–185) | 278 (213–350) | 214 (180–253) | 109 (91–131) | 126 (97–155) | 117 (100–135) | 2878 (2374–3537) | 3498 (2678–4319) | 3182 (2713–3685) |
| Palestine | 149 (115–192) | 153 (117–205) | 150 (122–182) | 116 (87–146) | 71 (55–92) | 92 (74–110) | 2816 (2094–3653) | 2015 (1546–2663) | 2396 (1925–2898) |
| Qatar | 144 (110–189) | 180 (136–226) | 150 (122–183) | 90 (67–118) | 79 (59–99) | 84 (67–104) | 1667 (1251–2170) | 1932 (1452–2463) | 1700 (1382–2109) |
| Saudi Arabia | 104 (85–128) | 92 (73–113) | 96 (82–111) | 67 (60–77) | 43 (39–49) | 55 (50–60) | 1300 (1154–1490) | 992 (881–1124) | 1134 (1033–1251) |
| Somalia | 144 (62–304) | 257 (76–608) | 202 (69–449) | 130 (53–270) | 166 (44–382) | 149 (49–319) | 3267 (1266–7474) | 4751 (1262–11,703) | 4031 (1274–9504) |
| Sudan | 136 (97–185) | 163 (97–241) | 149 (110–195) | 111 (80–157) | 85 (54–122) | 97 (73–125) | 2485 (1719–3601) | 2216 (1338–3228) | 2338 (1722–3074) |
| Syria | 103 (84–124) | 105 (86–126) | 103 (90–118) | 76 (61–92) | 54 (44–63) | 64 (55–72) | 1653 (1343–1972) | 1345 (1099–1592) | 1487 (1288–1685) |
| Tunisia | 224 (169–289) | 164 (117–213) | 190 (151–231) | 163 (122–210) | 76 (55–100) | 115 (90–141) | 3445 (2596–4545) | 1878 (1356–2479) | 2610 (2057–3170) |
| United Arab Emirates | 213 (155–290) | 226 (158–310) | 207 (157–270) | 106 (81–139) | 85 (65–115) | 97 (76–123) | 2191 (1621–2891) | 2279 (1694–3069) | 2145 (1651–2720) |
| Yemen | 131 (85–198) | 201 (114–353) | 167 (101–268) | 109 (72–168) | 103 (59–164) | 106 (65–164) | 2445 (1529–3909) | 2753 (1558–4552) | 2601 (1527–4180) |
The most common incident cancers in males in 2015 were tracheal, bronchus, and lung cancer (TBL) with 38 (33–42) thousand cases, followed by prostate cancer and stomach cancer, with 28 (20–34) thousand and 27 (24–31) thousand cases, respectively. These cancers accounted for 30% of the incidence of all cancers. The most common causes of cancer deaths in males were TBL, stomach cancer, and liver cancer with 39 (34–44) thousand, 17 (16–19) thousand, and 17 (14–19) thousand deaths, respectively. The top three causes of DALYs in males were TBL with 1.0 (0.8–1.1) million DALYs, leukemia with 637 (579–705) thousand DALYs, and other neoplasms with 494 (418–603) thousand DALYs (Table 2).
In children aged 0–14 years, the most common cancers were leukemia, other neoplasms, and cancer of the brain and nervous system (Fig. 1). These cancers were also the ones responsible for most childhood cancer deaths (Fig. 2). In adolescents and young adults (ages 15–39 years), the most common cancers were breast cancer, followed by leukemia and other neoplasms. These cancers were also main causes of death in this age group.
Fig. 1.
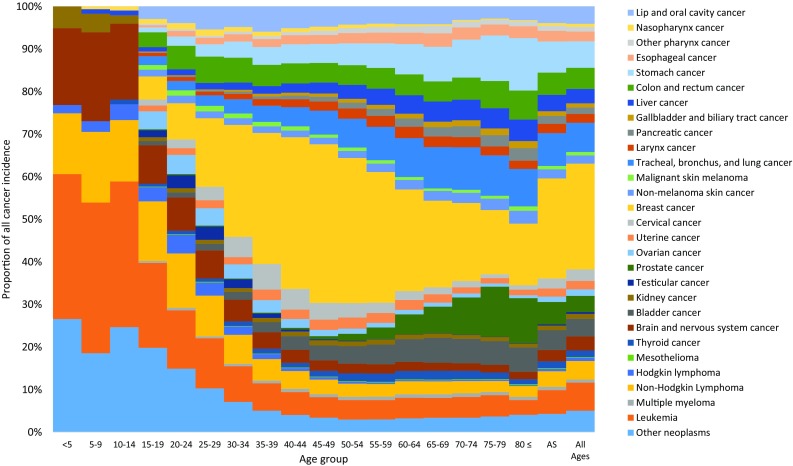
Age-specific contribution of cancer groups to total cancer incidence in the Eastern Mediterranean Region, both sexes, 2015 (Global Burden of Disease Study 2015, Eastern Mediterranean Region, 2015)
Fig. 2.
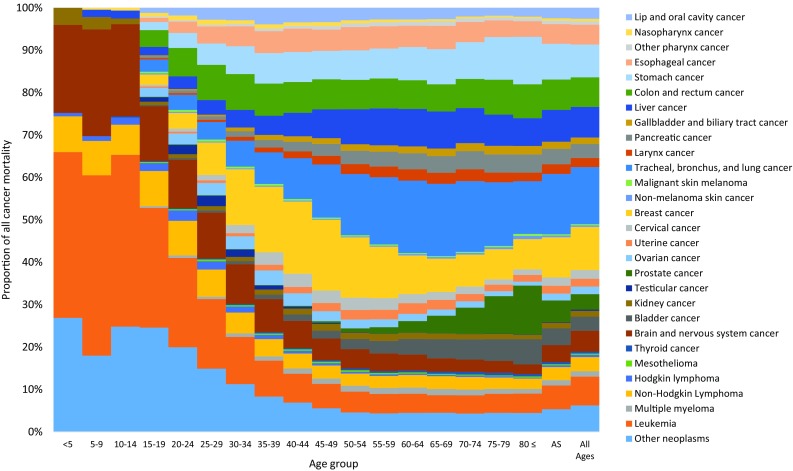
Age-specific contribution of cancer groups to total cancer mortality in the Eastern Mediterranean Region, both sexes, 2015. (Global Burden of Disease Study 2015, Eastern Mediterranean Region, 2015)
National cancer incidence, mortality, and burden
In 2015, Lebanon had the highest ASIR for all cancers at 261.9 (194.6–336.2), followed by Afghanistan at 258.8 (161.3–411.3), and Iraq at 219.9 (172.2–279.8) (Table 3). ASMRs were highest in Afghanistan at 165.0 (111.5–221.7), followed by Somalia at 148.6 (49.1–319.1), and Djibouti at 142.0 (75.4–256.6). Those three countries also had the highest ASDRs in 2015. Saudi Arabia, Syria, and Oman had the lowest ASIRs in 2015 with 95.6 (82.3–111.5), 103.5 (89.9–117.8), and 115.8 (98.5–131.8), respectively. Those countries also had the lowest ASMR with 54.8 (50.1–60.0), 64.0 (55.0–72.4), and 66.0 (55.2–74.7), respectively. Along with Kuwait, these countries had the lowest ASDRs in 2015 as well.
Burden of different cancer groups
Excluding the “other neoplasms” group, five cancers, namely breast cancer, TBL, leukemia, stomach cancer, and colon and rectal cancer ranked highest in terms of incident cases in the region. Breast cancer had the highest ASIR in the EMR in 2015 with 42.3 (35.7–48.9) cases. It also had the second-highest ASMR after TBL in the region with 9.9 (8.5–11.3) deaths (eTable 1). There were 179 (151–209) thousand new cases in 2015, 39 (33–45) thousand deaths, and 1.3 (1.1–1.6) million DALYs caused by breast cancer (Table 2). Only 1% (2058 cases) of breast cancer cases occurred in males (Table 2). Nine percent of all DALYs caused by breast cancer came from YLDs (eFigure 1).
In 2015, TBL had the second highest ASIR the region with 13.9 (12.5–15.2). It was the leading cause of cancer deaths and DALYs in the region with 51 (44–55) thousand incident cases, 51 (45–56) thousand deaths, and 1.3 (1.2–1.5) million DALYs. Seventy-six percent of new cases and deaths occurred in males. Only 1% of DALYs came from YLDs.
There were 48 (43–53) thousand new cases of leukemia in 2015 in the region and 26 (24–28) thousand deaths, making it the third most common cancer in the region. Leukemia caused 1.1 (1.1–1.2) million DALYs, with 97% coming from YLLs.
Stomach cancer had the fourth-highest ASIR the region in 2015 at 13.2 (12.0–14.4), but ranked first in Afghanistan with 40.5 (26.1–55.0) and second in Iran, Yemen, and Sudan with 29.1 (24.0–35.7), 19.0 (11.6–29.4), and 18.1 (13.2–23.6), respectively (Online Appendix Data). There were 45 (41–49) thousand cases in 2015, 29 (27–32) thousand deaths, and 769 (690–851) thousand DALYs, of which only 2% came from YLDs. Sixty percent of incident cases, 60% of deaths, and 57% of DALYs occurred in males.
Colon and rectum cancer was the sixth most frequent cancer in the region in 2015 with 36 (32–40) thousand incident cases, 26 (24–29) thousand deaths, and 764 (678–859) thousand DALYs. It was the second most frequent incident cancer in 2015 in Jordan, Kuwait, Lebanon, Libya, Qatar, and Saudi Arabia.
Drivers of change in cancer incidence
Between 2005 and 2015, the overall change in the number of incident cancer cases ranged between 36.8% in Somalia and 182.9% in the UAE (Table 1). High-income EMR countries in addition to Lebanon experienced the largest increase in cancer incidence, which was mainly driven by population growth in all countries. Population aging was responsible for 12% of the increase in incident cancer cases in the region in total, ranging from −25.7% in Qatar to 58.8% in the UAE. Change in age-specific incident rates ranged between −17.1% in Bahrain and 25.2% in Oman relative to the overall change in incident cases (Table 1).
Discussion
In 2015, cancer was responsible for 9.4% (8.9–9.9%) of all deaths and 5.1% (4.6–5.8%) of all DALYs in the EMR countries compared to 15.7% (15.5–15.9%) of deaths and 8.5% (7.8–9.2%) of all DALYs at the global level (GBD 2015 DALYs and HALE Collaborators 2016; GBD 2015 Mortality and Causes of Death Collaborators 2016). This puts cancer as the third-leading cause of death and the eighth-leading cause of DALYs in the EMR. In EMR countries, cancer deaths between 2005 and 2015 have increased by 32.9%. Females experienced higher cancer incidence in the EMR but lower cancer deaths compared to males, which can be explained by less aggressive cancers (breast, cervical) being among the top cancers in females compared to males (lung, stomach). Age-standardized cancer incidence varied substantially between EMR countries with infection-related cancers playing a more important role in low- and low-middle income countries (e.g., stomach cancer having the highest ASIR in Afghanistan, Iran, Yemen, and Sudan, and cancers related to low physical activity and cancers with strong lifestyle-related risk factors such as colorectal cancer being more common in middle- and high-income EMR countries such as Lebanon, the UAE, and Libya).
Given this alarming trend and the substantial contribution of cancer to the disease burden in EMR countries, cancer control has to be among the top health policy priorities. Compared to other studies (Aljurf et al. 2010; Abdel-Razeq et al. 2015; Kulhánová et al. 2017) analyzing cancer burden in the EMR countries, the GBD study provides analyses of all diseases over time, which means that cancer can be viewed in the context of other health priorities. Kulhánová et al. recently published an analysis using GLOBOCAN data to analyze the cancer burden in the EMR (Kulhánová et al. 2017). Because of different methods to estimate incidence and mortality as well as few high-quality data sources for cancer incidence and mortality in the EMR, GBD estimates for incidence differ between 50% fewer incident cases (for Syria) to 215% more incident cases (for the UAE). For mortality, GBD estimates range from 56% fewer deaths in Syria to 153% more deaths in the UAE (eTable 4). Whereas the GLOBOCAN methodology starts with estimating cancer incidence and then for most EMR countries models survival to estimate mortality (Ferlay et al. 2015), GBD uses cancer registry incidence-based mortality estimates as well as vital registration data to model mortality and then uses these mortality estimates as well as modeled MIR to estimate cancer incidence. An advantage of the GBD study is the ability to compare trends over time, which allows for analysis of the effects of the demographical and epidemiological transition, and also the effectiveness of public health policies. The discrepancies between GLOBOCAN and GBD estimates underscore the need for better data to assess cancer burden in the EMR countries. Few high-quality population-based cancer registries exist in the EMR, with the Global Initiative for Cancer Registry Development (GICR) actively promoting further development of cancer registries (International Agency for Research on Cancer (IARC) 2011). At the same time, strengthening of vital registration systems and integration of surveillance systems for other non-communicable diseases is needed in the region. Until better data become available, model-based estimates have to be used to guide local policy.
Two significant international statements address the threat of non-communicable diseases and propose interventions as well as metrics to measure success. The SDGs, as the successors of the Millennium Development Goals, which shaped public health policy for 15 years, now include non-communicable diseases (NCDs) in the third goal, “by 2030, reduce by one-third premature mortality from non-communicable diseases through prevention and treatment and promote mental health and well-being” (United Nations 2016). Control of NCDs has also been targeted in the WHO Global Action Plan for Prevention and Control of NCDs 2013–2020 (World Health Organization 2013). Our study shows that substantial efforts are required in most EMR countries to meet the SDG targets of reducing cancer mortality. Culprits for the disappointing pace of cancer control to date can be found in all aspects of cancer care, from primary prevention and screening, to early diagnosis, access to cancer treatment, tertiary prevention, and palliative care (World Health Organization 2014).
We have seen exciting advances in our understanding of cancer and resulting treatment approaches in the last decade. However, the increasing cancer burden due to an aging population and the exploding costs associated with complex cancer treatments are leading to unacceptable increases in health care expenditure, which will be impossible to sustain for most countries (Kelly and Smith 2014). For this reason, risk factor reduction has to be a priority for any cancer control effort. The top five risk factors identified in GBD as contributing to cancer mortality in the EMR are tobacco, dietary risks, high body mass index, occupational risks, and air pollution (GBD 2015 Risk Factors Collaborators 2016). With lung cancer being the second-leading cause of cancer death in the region, tobacco control has to be the top priority. Health hazards of cigarette smoking are well established. However, other forms of tobacco consumption such as chewing and shisha (waterpipe) smoking also lead to an increased risk of death, mainly due to cancer (Etemadi et al. 2016). All countries in the EMR with the exception of Somalia and Palestine have signed the WHO Framework Convention on Tobacco Control (WHO FCTC), and all countries except Morocco have ratified it. However, in many EMR countries, smoking rates have not declined, with certain forms of tobacco consumption such as shisha smoking even rising (Maziak et al. 2015). Secondhand smoking is not restricted in most countries in the EMR despite the FCTC recommendations (Heydari et al. 2012).
Obesity and lack of physical activity are risk factors that also follow a dangerous trend, with obesity prevalence rising in many EMR countries to concerning levels (Ng et al. 2014). Obesity has been proven to be a risk factor for esophageal adenocarcinoma, colon, rectal, kidney, and pancreas cancer, gallbladder cancer in females, and postmenopausal breast, ovarian, and uterine cancers (Lauby-Secretan et al. 2016). Physical inactivity has been linked to an increased risk for cancer, especially colon and breast cancer (American Institute for Cancer Research and World Cancer Research Fund 2007). For both sexes combined, breast cancer is the most common incident cancer in every EMR country, and colorectal cancer is among the top four most common cancers in all high-income EMR countries as well as all middle-income EMR countries except for Iran, Pakistan, Sudan, Syria, Egypt, Morocco, and Iraq. This stresses the importance of health intervention programs and environmental policies to increase physical activity and healthy dietary habits.
Other important strategies for primary prevention include vaccination against human papillomavirus (HPV) for cervical cancer prevention, as well as hepatitis B vaccination and treatment of hepatitis B and C, especially in countries with high hepatitis C prevalence such as Egypt, where liver cancer is the leading cause of cancer death (Alavian and Haghbin 2016). In the case of liver cancer, screening of high-risk groups has also been recommended by the National Comprehensive Cancer Network (NCCN) as a core intervention in the resources stratified guidelines (National Comprehensive Cancer Network 2016). However, early detection is dependent on a functioning primary care system as well as universal access to care, the developments of both of which are hampered by fragmented care systems, lack of strategic planning, an unregulated private sector, as well as political turmoil in some EMR countries (Regional Committee for the EM/RC57/Tech.Disc.1 and Eastern Mediterranean 2010).
An emphasis on addressing cancer once it becomes clinically symptomatic rather than on detecting it early is also apparent by the lack of population-wide cancer screening programs. Effective screening is currently available for cervical cancer, colorectal cancer, breast cancer, oral cancer, and stomach cancer (in high-risk populations) (Sankaranarayanan 2014). With breast and cervical cancer being among the most common cancers in females in every EMR country, cancer screening should be among the prioritized prevention efforts (Goldie et al. 2005; Yip et al. 2008). For screening programs to be successful at the population level, strategic implementation should be coordinated at the national level and include educational components, as well monitoring and evaluation to ensure success and sustainability. Opportunistic screening programs in the past have been hampered by low participation rates due to anxiety, misperception of the screening’s purpose, and a general sense that cancer at any stage is a death sentence (Al Mulhim et al. 2015; Al-Zalabani et al. 2016). Civil engagement through education and advocacy is therefore an important pillar of successful and sustainable screening programs.
Cancer treatment programs depend on multidisciplinary approaches that are often unavailable in EMR countries. Laboratory services, pathology, radiology, oncology nursing, surgery, medical and radiation oncology, pharmacology, transfusion services, nutritional and psychosocial support services, as well as palliative care and hospice services are the core disciplines required to provide cancer care. There is a lack of human capital for many, if not of all, of these disciplines, which will require continued and coordinated efforts to train local staff and ensure retention (World Health Organization 2014).
Another important factor contributing to poor health in some EMR countries is war, leading to a large number of displaced people, disruption in care structures and supplies, lack of qualified healthcare personnel, and financial strains on patients and healthcare systems in countries with large refugee populations (Spiegel et al. 2014; Sahloul et al. 2016). Innovative solutions to monitor disease burden in these most vulnerable populations have been proposed and include web-based cancer registries with linkages between countries (Spiegel et al. 2014). Approaches during humanitarian emergencies to prevent and treat cancer and other diseases requiring complex care systems include clear referral guidelines as provided by the United Nations High Commissioner for Refugees (UNHCR), as well as financing systems such as health insurance or social security (United Nations High Commissioner for Refugees 2009, 2012).
Limitations
Our data sources to estimate cancer burden in the EMR are vital registration and cancer registry data. The GBD study tries to identify and utilize all available data sources in the estimation process. However, data sources in low- and middle-income countries are scarce, and causes of death and cancer registration is not a routine procedure in many health care systems. Even when civil registration exits, war or civil unrest can interrupt routine data collection. In the absence of reliable data, our estimates are largely driven by regional trends and the selection of model covariates which lead consequently to wider uncertainty intervals and time trends that are therefore often non-significant. Furthermore, miscoding of causes of death—as the so-called garbage codes—in vital registration data can influence both our mortality estimates and incidence estimates. Misclassifying metastatic sites (e.g., lung, liver, bone) as the primary cancer site or as second cancers is another potential source of bias. This is particularly true in countries with low-quality registration and limited diagnostic sources.
Conclusions
Cancer is among the leading causes of death and DALYs in most EMR countries. Prioritization of different aspects of the cancer control continuum depends on local health infrastructure as well as disease epidemiology. Given the dramatic increase in cancer cases and deaths over the last decade, all stakeholders including health policymakers, care providers, and the general public need to actively engage to define these priorities and work together on implementation of evidence-based cancer control strategies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
GBD 2015 Eastern Mediterranean Region Cancer Collaborators:
Christina Fitzmaurice, MD (corresponding author), Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Ubai Alsharif, MPH, Charité Universitätsmedizin, Berlin, Germany. Charbel El Bcheraoui, PhD, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Ibrahim Khalil, MD, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Raghid Charara, MD, American University of Beirut, Beirut, Lebanon. Maziar Moradi-Lakeh, MD, Department of Community Medicine, Preventive Medicine and Public Health Research Center, Gastrointestinal and Liver Disease Research Center (GILDRC), Iran University of Medical Sciences, Tehran, Iran. Ashkan Afshin, MD, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Michael Collison, BS, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Adrienne Chew, ND, Institute for Health Metrics and Evaluation, University of Washington. Kristopher J. Krohn, BA, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Farah Daoud, BA/BS, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Daniel Dicker, BS, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Kyle J. Foreman, PhD, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States; Imperial College London, London, United Kingdom. Joseph Frostad, MPH, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Nicholas J. Kassebaum, MD, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States; Department of Anesthesiology & Pain Medicine; Seattle Children's Hospital, Seattle, Washington, United States. Michael Kutz, BS, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Haidong Wang, PhD, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Gebre Yitayih Abyu, MS, Mekelle University, Mekelle, Ethiopia, Ethiopia. Isaac Akinkunmi Adedeji, MS, Olabisi Onabanjo University, Ago-Iwoye, Nigeria. Aliasghar Ahmad Kiadaliri, PhD, Department of Clinical Sciences Lund, Orthopedics, Clinical Epidemiology Unit, Lund University, Lund, Sweden. Muktar Beshir Ahmed, MPH, College of Health Sciences, Department of Epidemiology, ICT and e-Learning Coordinator, Jimma University, Jimma, Ethiopia. Ayman Al-Eyadhy, MD, King Saud University, Riyadh, Saudi Arabia. Khurshid Alam, PhD, Murdoch Childrens Research Institute, The University of Melbourne, Parkville, Victoria, Australia; The University of Melbourne, Melbourne, VIC, Australia; The University of Sydney, Sydney, NSW, Australia. Deena Alasfoor, MSc, Ministry of Health, Al Khuwair, Oman. Raghib Ali, MSc, University of Oxford, Oxford, United Kingdom. Reza Alizadeh-Navaei, PhD, Gastrointestinal Cancer Research Center, Mazandaran University of Medical Sciences, Sari, Iran. Rajaa Al-Raddadi, PhD, Joint Program of Family and Community Medicine, Jeddah, Saudi Arabia. Khalid A. Altirkawi, MD, King Saud University, Riyadh, Saudi Arabia. Nelson Alvis-Guzman, PhD, Universidad de Cartagena, Cartagena de Indias, Colombia. Erfan Amini, MD, Uro-Oncology Research Center, Tehran University of Medical Sciences, Tehran, Iran; Non-communicable Diseases Research Center, Endocrinology and Metabolism Research Institute, Tehran University of Medical Sciences, Tehran, Iran. Nahla Anber, PhD, Mansoura University, Mansoura, Egypt. Palwasha Anwari, MD, Self-employed, Kabul, Afghanistan. Al Artaman, PhD, University of Manitoba, Winnipeg, Manitoba, Canada. Solomon Weldegebreal Asgedom, PhD, Mekelle University, Mekelle, Ethiopia. Tesfay Mehari Atey, MS, Mekelle University, Mekelle, Ethiopia. Ashish Awasthi, PhD, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India. Huda Omer Ba Saleem, PhD, Faculty of Medicine and Health Sciences, Aden University, Aden, Yemen. Umar Bacha, PhD, School of Health Sciences, University of Management and Technology, Lahore, Pakistan. Aleksandra Barac, PhD, Faculty of Medicine, University of Belgrade, Belgrade, Serbia. Neeraj Bedi, MD, College of Public Health and Tropical Medicine, Jazan, Saudi Arabia. Zulfiqar A. Bhutta, PhD, Centre of Excellence in Women and Child Health, Aga Khan University, Karachi, Pakistan; Centre for Global Child Health, The Hospital for Sick Children, Toronto, ON, Canada. Zahid A. Butt, PhD, Al Shifa Trust Eye Hospital, Rawalpindi, Pakistan. Carlos A. Castañeda-Orjuela, MSc, Colombian National Health Observatory, Instituto Nacional de Salud, Bogota, Colombia; Epidemiology and Public Health Evaluation Group, Public Health Department, Universidad Nacional de Colombia, Bogota, Colombia. Abdulaal A. Chitheer, MD, Ministry of Health, Baghdad, Iraq. Hadi Danawi, PhD, Walden University, Minneapolis, Minnesota, United States. José das Neves, PhD, i3S - Instituto de Investigação e Inovação em Saúde, University of Porto, Porto, Portugal; INEB - Instituto de Engenharia Biomédica, University of Porto, Porto, Portugal. Dragos V. Davitoiu, PhD, University of Medicine and Pharmacy Bucharest, Bucharest, Romania. Subhojit Dey, PhD, Indian Institute of Public Health-Delhi, Public Health Foundation of India, Gurgaon, India. Samath D. Dharmaratne, MD, Department of Community Medicine, Faculty of Medicine, University of Peradeniya, Peradeniya, Sri Lanka. Shirin Djalalinia, PhD, Undersecretary for Research & Technology, Ministry of Health & Medical Education, Tehran, Iran. Huyen Phuc Do, MSc, Institute for Global Health Innovations, Duy Tan University, Da Nang, Vietnam. Manisha Dubey, MPhil, International Institute for Population Sciences, Mumbai, India. Hedyeh Ebrahimi, Non-communicable Diseases Research Center, Tehran University of Medical Sciences, Tehran, Iran; Liver and Pancreaticobiliary Diseases Research Center, Digestive Disease Research Institute, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran. Donatus U. Ekwueme, PhD, Centers for Disease Control and Prevention, Atlanta, Georgia, United States. Aman Yesuf Endries, MPH, Arba Minch University, Arba Minch, Ethiopia. Babak Eshrati, PhD, Ministry of Health and Medical Education, Tehran, Iran; Arak University of Medical Sciences, Arak, Iran. Alireza Esteghamati, MD, Endocrinology and Metabolism Research Center, Tehran University of Medical Sciences, Tehran, Iran. Maryam S. Farvid, PhD, Department of Nutrition, Harvard T. H. Chan School of Public Health, Harvard University, Boston, MA, United States; Harvard/MGH Center on Genomics, Vulnerable Populations, and Health Disparities, Mongan Institute for Health Policy, Massachusetts General Hospital, Boston, MA, United States. Seyed-Mohammad Fereshtehnejad, PhD, Department of Neurobiology, Care Sciences and Society (NVS), Karolinska Institutet, Stockholm, Sweden. Florian Fischer, PhD, School of Public Health, Bielefeld University, Bielefeld, Germany. Tsegaye Tewelde Gebrehiwot, MPH, Jimma University, Jimma, Ethiopia. Sameer Vali Gopalani, MPH, Department of Health and Social Affairs, Government of the Federated States of Micronesia, Palikir, Federated States of Micronesia. Nima Hafezi-Nejad, MD, Endocrinology and Metabolism Research Center, Tehran University of Medical Sciences, Tehran, Iran. Randah Ribhi Hamadeh, DPhil, Arabian Gulf University, Manama, Bahrain. Samer Hamidi, DrPH, Hamdan Bin Mohammed Smart University, Dubai, United Arab Emirates. Habtamu Abera Hareri, MS, Addis Ababa University, Addis Ababa, Ethiopia. Roderick J. Hay, DM, International Foundation for Dermatology, London, United Kingdom; King's College London, London, United Kingdom. Nobuyuki Horita MD, Department of Pulmonology, Yokohama City University Graduate School of Medicine, Yokohama, Kanagawa, Japan. Mohamed Hsairi, MD, Department of Epidemiology, Salah Azaiz Institute, Tunis, Tunisia. Mihajlo B. Jakovljevic, PhD, Faculty of Medical Sciences, University of Kragujevac, Kragujevac, Serbia; The Center for Health Trends and Forecasts, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Jost B. Jonas, MD, Department of Ophthalmology, Medical Faculty Mannheim, Ruprecht-Karls-University Heidelberg, Mannheim, Germany. Amir Kasaeian, PhD, Hematology-Oncology and Stem Cell Transplantation Research Center, Tehran University of Medical Sciences, Tehran, Iran; Endocrinology and Metabolism Population Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran. Nigussie Assefa Kassaw, MPH, Addis Ababa University, Addis Ababa, Ethiopia. Yousef Saleh Khader, ScD, Department of Community Medicine, Public Health and Family Medicine, Jordan University of Science and Technology, Irbid, Jordan. Ejaz Ahmad Khan, MD, Health Services Academy, Islamabad, Pakistan. Gulfaraz Khan, PhD, Department of Microbiology and Immunology, College of Medicine & Health Sciences, United Arab Emirates University, Al Ain, United Arab Emirates. Daniel Kim, DrPH, Department of Health Sciences, Northeastern University, Boston, Massachusetts, United States. Yohannes Kinfu, PhD, Centre for Research and Action in Public Health, University of Canberra, Canberra, Australian Capital Territory, Australia. Heidi J. Larson, PhD, Department of Infectious Disease Epidemiology, London School of Hygiene & Tropical Medicine, London, United Kingdom; Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Asma Abdul Latif, PhD, Department of Zoology, Lahore College for Women University, Lahore, Pakistan. Shai Linn MD, University of Haifa, Israel. Raimundas Lunevicius, PhD, Aintree University Hospital National Health Service Foundation Trust, Liverpool, United Kingdom; School of Medicine, University of Liverpool, Liverpool, United Kingdom. Hassan Magdy Abd El Razek, MBBCH, Mansoura Faculty of Medicine, Mansoura, Egypt. Mohammed Magdy Abd El Razek, MBBCH, Aswan University Hospital, Aswan Faculty of Medicine, Aswan, Egypt. Azeem Majeed, MD, Department of Primary Care & Public Health, Imperial College London, London, England, United Kingdom. Reza Malekzadeh, MD, Digestive Diseases Research Institute, Tehran University of Medical Sciences, Tehran, Iran; Digestive Diseases Research Institute, Tehran University of Medical Sciences, Tehran, Iran. Deborah Carvalho Malta, PhD, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil. Desalegn Markos, MS, Madda Walabu University, Robe, Ethiopia. Peter Memiah, PhD, University of West Florida, Pensacola, FL, United States. Ziad A. Memish, MD, Saudi Ministry of Health, Riyadh, Saudi Arabia; College of Medicine, Alfaisal University, Riyadh, Saudi Arabia. Walter Mendoza, MD, United Nations Population Fund, Lima, Peru. Tuomo J. Meretoja, PhD, Comprehensive Cancer Center, Breast Surgery Unit, Helsinki University Hospital, Helsinki, Finland; University of Helsinki, Helsinki, Finland. Ted R. Miller, PhD, Pacific Institute for Research & Evaluation, Calverton, MD, United States; Centre for Population Health, Curtin University, Perth, WA, Australia. Shafiu Mohammed, PhD, Health Systems and Policy Research Unit, Ahmadu Bello University, Zaria, Nigeria; Institute of Public Health, Heidelberg University, Heidelberg, Germany. Vinay Nangia, MD, Suraj Eye Institute, Nagpur, India. Quyen Le Nguyen, MD, Institute for Global Health Innovations, Duy Tan University, Da Nang, Vietnam. Trang Huyen Nguyen, MSc, Institute for Global Health Innovations, Duy Tan University, Da Nang, Vietnam. Felix Akpojene Ogbo, MPH, Centre for Health Research, Western Sydney University, Sydney, New South Wales, Australia. P. A. Mahesh, DNB, JSS Medical College, JSS University, Mysore, India. Eun-Kee Park, PhD, Department of Medical Humanities and Social Medicine, College of Medicine, Kosin University, Busan, South Korea. Tejas Patel, MD, Mount Sinai Health System, New York, NY, United States. David M. Pereira, PhD, REQUIMTE/LAQV, Laboratório de Farmacognosia, Departamento de Química, Faculdade de Farmácia, Universidade do Porto, Porto, Portugal. Farhad Pishgar, Non-communicable Diseases Research Center, Tehran University of Medical Sciences, Tehran, Iran; Uro-Oncology Research Center, Tehran University of Medical Sciences, Tehran, Iran. Farshad Pourmalek, PhD, University of British Columbia, Vancouver, British Columbia, Canada. Mostafa Qorbani, PhD, Non-communicable Diseases Research Center, Alborz University of Medical Sciences, Karaj, Iran. Amir Radfar, MD, A T Still University, Kirksville, MO, United States. Anwar Rafay, MS, Contech International Health Consultants, Lahore, Pakistan; Contech School of Public Health, Lahore, Pakistan. Vafa Rahimi-Movaghar, MD, Sina Trauma and Surgery Research Center, Tehran University of Medical Sciences, Tehran, Iran. Rajesh Kumar Rai, MPH, Society for Health and Demographic Surveillance, Suri, India. Saleem M. Rana, PhD, Contech School of Public Health, Lahore, Pakistan; Contech International Health Consultants, Lahore, Pakistan. Salman Rawaf, MD, Imperial College London, London, United Kingdom. Andre M. N. Renzaho, PhD, Western Sydney University, Penrith, NSW, Australia. Satar Rezaei, PhD, School of Public Health, Kermanshah University of Medical Sciences, Kermanshah, Iran. Kedir Teji Roba, PhD, Haramaya University, Harar, Ethiopia. Gholamreza Roshandel, PhD, Golestan Research Center of Gastroenterology and Hepatology, Golestan University of Medical Sciences, Gorgan, Iran; Digestive Diseases Research Institute, Tehran University of Medical Sciences, Tehran, Iran. Mahdi Safdarian, MD, Sina Trauma & Surgery Research Center, Tehran University of Medical Sciences, Tehran, Iran. Sare Safi, MS, Ophthalmic Epidemiology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran. Saeid Safiri, PhD, Managerial Epidemiology Research Center, Department of Public Health, School of Nursing and Midwifery, Maragheh University of Medical Sciences, Maragheh, Iran. Payman Salamati, MD, Sina Trauma and Surgery Research Center, Tehran University of Medical Sciences, Tehran, Iran. Abdallah M. Samy, PhD, Ain Shams University, Cairo, Egypt, Lawrence, Kansas, United States. Juan Ramon Sanabria, MD, J Edwards School of Medicine, Marshall Univeristy, Huntington, WV, United States; Case Western Reserve University, Cleveland, OH, United States. Milena M. Santric Milicevic, PhD, Institute of Social Medicine, Faculty of Medicine, University of Belgrade, Belgrade, Serbia; Centre School of Public Health and Health Management, Faculty of Medicine, University of Belgrade, Belgrade, Serbia. Benn Sartorius, PhD, Public Health Medicine, School of Nursing and Public Health, University of KwaZulu-Natal, Durban, South Africa; UKZN Gastrointestinal Cancer Research Centre, South African Medical Research Council (SAMRC), Durban, South Africa. Sadaf G. Sepanlou, PhD, Digestive Diseases Research Institute, Tehran University of Medical Sciences, Tehran, Iran. Masood Ali Shaikh, MD, Independent Consultant, Karachi, Pakistan. Mark G. Shrime, MD, Harvard Medical School, Boston, Massachusetts, United States. Vasiliki Stathopoulou, PhD, Attikon University Hospital, Athens, Greece. Muawiyyah Babale Sufiyan, MBA, Ahmadu Bello University, Zaria, Nigeria. Rizwan Suliankatchi Abdulkader, MD, Ministry of Health, Kingdom of Saudi Arabia, Riyadh, Saudi Arabia. Rafael Tabarés-Seisdedos, PhD, Department of Medicine, University of Valencia, INCLIVA Health Research Institute and CIBERSAM, Valencia, Spain. Arash Tehrani-Banihashemi, PhD, Preventive Medicine and Public Health Research Center, Iran University of Medical Sciences, Tehran, Iran. Tesfalidet Tekelab, MS, Wollega University, Nekemte, Ethiopia; University of Newcastle, Newcastle, New South Wales, Australia. Mohamad-Hani Temsah, MD, King Saud University, Riyadh, Saudi Arabia. Bach Xuan Tran, PhD, Johns Hopkins University, Baltimore, Maryland, United States; Hanoi Medical University, Hanoi, Vietnam. Kingsley Nnanna Ukwaja, MD, Department of Internal Medicine, Federal Teaching Hospital, Abakaliki, Ebonyi State, Nigeria. Olalekan A. Uthman, PhD, Warwick Medical School, University of Warwick, Coventry, United Kingdom. Vasiliy Victorovich Vlassov, MD, National Research University Higher School of Economics, Moscow, Russia. Stein Emil Vollset, DrPH, Center for Disease Burden, Norwegian Institute of Public Health, Bergen, NA, Norway; Department of Global Public Health and Primary Care, University of Bergen, Bergen, Norway; Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Tolassa Wakayo MS, Jimma University, Jimma, Ethiopia. Elisabete Weiderpass, PhD, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden; Department of Research, Cancer Registry of Norway, Institute of Population-Based Cancer Research, Oslo, Norway; Department of Community Medicine, Faculty of Health Sciences, University of Tromsø, The Arctic University of Norway, Tromsø, Norway; Genetic Epidemiology Group, Folkhälsan Research Center, Helsinki, Finland. Andrea Werdecker, PhD, Competence Center Mortality-Follow-Up of the German National Cohort, Federal Institute for Population Research, Wiesbaden, Germany. Mohsen Yaghoubi, MSc, School of Public Health, University of Saskatchewan, Saskatoon, Saskatchewan, Canada. Mehdi Yaseri, PhD, Tehran University of Medical Sciences, Terhan, Iran; Ophthalmic Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran. Hassen Hamid Yimam, MPH, Mizan Tepi University, Mizan Teferi, Ethiopia. Naohiro Yonemoto, MPH, Department of Biostatistics, School of Public Health, Kyoto University, Kyoto, Japan. Maysaa El Sayed Zaki, PhD, Faculty of Medicine, Mansoura University, Mansoura, Egypt. Bassel Zein, MS, Department of Neuroscience, Georgetown University, Washington DC, United States. Aisha O. Jumaan, PhD, Independent Consultant, Seattle, Washington, United States. Theo Vos, PhD, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Simon I. Hay, DSc, Oxford Big Data Institute, Li Ka Shing Centre for Health Information and Discovery, University of Oxford, Oxford, United Kingdom; Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Mohsen Naghavi, PhD, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Christopher J. L. Murray, DPhil, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Ali H. Mokdad, PhD, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States.
Compliance with ethical standards
Conflict of interest
The authors of this paper have complied with all ethical standards and do not have any conflicts of interest to disclose at the time of submission.
Informed consent
The study did not involve human participants and/or animals; therefore, no informed consent was needed.
Funding
The funding source played no role in the design of the study, the analysis and interpretation of data, and the writing of the paper. GBD 2015 is funded by Bill & Melinda Gates Foundation.
Footnotes
This article is part of the supplement “The state of health in the Eastern Mediterranean Region, 1990–2015”.
The members of GBD (Global Burden of Disease) 2015 Eastern Mediterranean Region Cancer Collaborators are listed at the end of the article. Christina Fitzmaurice, on behalf of GBD 2015 Eastern Mediterranean Region Cancer Collaborators, is the corresponding author.
Contributor Information
GBD 2015 Eastern Mediterranean Region Cancer Collaborators, Email: cf11@uw.edu, http://healthdata.org.
GBD 2015 Eastern Mediterranean Region Cancer Collaborators:
Ubai Alsharif, Charbel El Bcheraoui, Ibrahim Khalil, Raghid Charara, Maziar Moradi-Lakeh, Ashkan Afshin, Michael Collison, Adrienne Chew, Kristopher J. Krohn, Farah Daoud, Daniel Dicker, Kyle J. Foreman, Joseph Frostad, Nicholas J. Kassebaum, Michael Kutz, Haidong Wang, Gebre Yitayih Abyu, Isaac Akinkunmi Adedeji, Aliasghar Ahmad Kiadaliri, Muktar Beshir Ahmed, Ayman Al-Eyadhy, Khurshid Alam, Deena Alasfoor, Raghib Ali, Reza Alizadeh-Navaei, Rajaa Al-Raddadi, Khalid A. Altirkawi, Nelson Alvis-Guzman, Erfan Amini, Nahla Anber, Palwasha Anwari, Al Artaman, Solomon Weldegebreal Asgedom, Tesfay Mehari Atey, Ashish Awasthi, Huda Omer Ba Saleem, Umar Bacha, Aleksandra Barac, Neeraj Bedi, Zulfiqar A. Bhutta, Zahid A. Butt, Carlos A. Castañeda-Orjuela, Abdulaal A. Chitheer, Hadi Danawi, José das Neves, Dragos V. Davitoiu, Subhojit Dey, Samath D. Dharmaratne, Shirin Djalalinia, Huyen Phuc Do, Manisha Dubey, Hedyeh Ebrahimi, Donatus U. Ekwueme, Aman Yesuf Endries, Babak Eshrati, Alireza Esteghamati, Maryam S. Farvid, Seyed-Mohammad Fereshtehnejad, Florian Fischer, Tsegaye Tewelde Gebrehiwot, Sameer Vali Gopalani, Nima Hafezi-Nejad, Randah Ribhi Hamadeh, Samer Hamidi, Habtamu Abera Hareri, Roderick J. Hay, Nobuyuki Horita, Mohamed Hsairi, Mihajlo B. Jakovljevic, Jost B. Jonas, Amir Kasaeian, Nigussie Assefa Kassaw, Yousef Saleh Khader, Ejaz Ahmad Khan, Gulfaraz Khan, Daniel Kim, Yohannes Kinfu, Heidi J. Larson, Asma Abdul Latif, Shai Linn, Raimundas Lunevicius, Hassan Magdy Abd El Razek, Mohammed Magdy Abd El Razek, Azeem Majeed, Reza Malekzadeh, Deborah Carvalho Malta, Desalegn Markos, Peter Memiah, Ziad A. Memish, Walter Mendoza, Tuomo J. Meretoja, Ted R. Miller, Shafiu Mohammed, Vinay Nangia, Quyen Le Nguyen, Trang Huyen Nguyen, Felix Akpojene Ogbo, P. A. Mahesh, Eun-Kee Park, Tejas Patel, David M. Pereira, Farhad Pishgar, Farshad Pourmalek, Mostafa Qorbani, Amir Radfar, Anwar Rafay, Vafa Rahimi-Movaghar, Rajesh Kumar Rai, Saleem M. Rana, Salman Rawaf, Andre M. N. Renzaho, Satar Rezaei, Kedir Teji Roba, Gholamreza Roshandel, Mahdi Safdarian, Sare Safi, Saeid Safiri, Payman Salamati, Abdallah M. Samy, Juan Ramon Sanabria, Milena M. Santric Milicevic, Benn Sartorius, Sadaf G. Sepanlou, Masood Ali Shaikh, Mark G. Shrime, Vasiliki Stathopoulou, Muawiyyah Babale Sufiyan, Rizwan Suliankatchi Abdulkader, Rafael Tabarés-Seisdedos, Arash Tehrani-Banihashemi, Tesfalidet Tekelab, Mohamad-Hani Temsah, Bach Xuan Tran, Kingsley Nnanna Ukwaja, Olalekan A. Uthman, Vasiliy Victorovich Vlassov, Stein Emil Vollset, Tolassa Wakayo, Elisabete Weiderpass, Andrea Werdecker, Mohsen Yaghoubi, Mehdi Yaseri, Hassen Hamid Yimam, Naohiro Yonemoto, Maysaa El Sayed Zaki, Bassel Zein, Aisha O. Jumaan, Theo Vos, Simon I. Hay, Mohsen Naghavi, Christopher J. L. Murray, Ali H. Mokdad, and Christina Fitzmaurice
References
- Abdel-Razeq H, Attiga F, Mansour A. Cancer care in Jordan. Hematol Oncol Stem Cell Ther. 2015;8:64–70. doi: 10.1016/j.hemonc.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Al Mulhim FA, Syed A, Bagatadah WA, Al Muhanna AF. Breast cancer screening programme: experience from Eastern province, Saudi Arabia. East Mediterr Health J Rev Sante Mediterr Orient Al-Majallah Al-Sihhiyah Li-Sharq Al-Mutawassit. 2015;21:111–119. doi: 10.26719/2015.21.2.111. [DOI] [PubMed] [Google Scholar]
- Alavian SM, Haghbin H. Relative importance of hepatitis B and C viruses in hepatocellular carcinoma in EMRO countries and the middle east: a systematic review. Hepat Mon. 2016 doi: 10.5812/hepatmon.35106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aljurf M, Zaidi SZ, Hussain F, et al. Status of hematopoietic stem cell transplantation in the WHO Eastern Mediterranean Region (EMRO) Transfus Apher Sci. 2010;42:169–175. doi: 10.1016/j.transci.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Al-Zalabani AH, Alharbi KD, Fallatah NI, et al. Breast Cancer Knowledge and Screening Practice and Barriers Among Women in Madinah, Saudi Arabia. J Cancer Educ. 2016 doi: 10.1007/s13187-016-1057-7. [DOI] [PubMed] [Google Scholar]
- American Institute for Cancer Research, World Cancer Research Fund, editor. Food, nutrition, physical activity and the prevention of cancer: a global perspective: a project of World Cancer Research Fund International. Washington, DC: American Institute for Cancer Research; 2007. [Google Scholar]
- Etemadi A, Khademi H, Kamangar F, et al. Hazards of cigarettes, smokeless tobacco and waterpipe in a Middle Eastern Population: a Cohort Study of 50 000 individuals from Iran. Tob Control. 2016 doi: 10.1136/tobaccocontrol-2016-053245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- Foreman KJ, Lozano R, Lopez AD, Murray CJ. Modeling causes of death: an integrated approach using CODEm. Popul Health Metr. 2012;10:1. doi: 10.1186/1478-7954-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2015 DALYs and HALE Collaborators Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1603–1658. doi: 10.1016/S0140-6736(16)31460-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2015 Mortality and Causes of Death Collaborators Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2015 Risk Factors Collaborators Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1659–1724. doi: 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, et al. Cost-effectiveness of cervical-cancer screening in five developing countries. N Engl J Med. 2005;353:2158–2168. doi: 10.1056/NEJMsa044278. [DOI] [PubMed] [Google Scholar]
- Heydari G, Talischi F, Masjedi MR, et al. Comparison of tobacco control policies in the Eastern Mediterranean countries based on Tobacco Control Scale scores. East Mediterr Health J Rev Sante Mediterr Orient Al-Majallah Al-Sihhiyah Li-Sharq Al-Mutawassit. 2012;18:803–810. doi: 10.26719/2012.18.8.803. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC) (2011) Global initiative for cancer registry development (GICR). http://gicr.iarc.fr/. Accessed 13 May 2017
- Kelly RJ, Smith TJ. Delivering maximum clinical benefit at an affordable price: engaging stakeholders in cancer care. Lancet Oncol. 2014;15:e112–e118. doi: 10.1016/S1470-2045(13)70578-3. [DOI] [PubMed] [Google Scholar]
- Kulhánová I, Bray F, Fadhil I, et al. Profile of cancer in the Eastern Mediterranean region: the need for action. Cancer Epidemiol. 2017;47:125–132. doi: 10.1016/j.canep.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med. 2016;375:794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maziak W, Taleb ZB, Bahelah R, et al. The global epidemiology of waterpipe smoking. Tob Control. 2015;24:i3–i12. doi: 10.1136/tobaccocontrol-2014-051903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghavi M, Makela S, Foreman K, O’Brien J. Algorithms for enhancing public health utility of national causes-of-death data. Popul Heal Metr. 2010;8:9. doi: 10.1186/1478-7954-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network (2016) NCCN guidelines hepatocellular carcinoma. Version 2.2016
- Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014 doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regional Committee for the EM/RC57/Tech.Disc.1, Eastern Mediterranean (2010) Technical discussion on Strategic directions to improve health care financing in the Eastern Mediterranean Region: moving towards universal coverage 2011–2015
- Sahloul E, Salem R, Alrez W, et al. Cancer care at times of crisis and war: the syrian example. J Glob Oncol JGO006189. 2016 doi: 10.1200/JGO.2016.006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan R. Screening for Cancer in low- and middle-income countries. Ann Glob Health. 2014;80:412–417. doi: 10.1016/j.aogh.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Spiegel P, Khalifa A, Mateen FJ. Cancer in refugees in Jordan and Syria between 2009 and 2012: challenges and the way forward in humanitarian emergencies. Lancet Oncol. 2014;15:e290–e297. doi: 10.1016/S1470-2045(14)70067-1. [DOI] [PubMed] [Google Scholar]
- United Nations (2016) Sustainable development goals. https://sustainabledevelopment.un.org/. Accessed 1 Sept 2016
- United Nations High Commissioner for Refugees (2009) Refworld | UNHCR’s principles and guidance for referral health care for refugees and other persons of concern. In: Refworld. http://www.refworld.org/docid/4c1726e82.html. Accessed 16 May 2017
- United Nations High Commissioner for Refugees (2012) A guidance note on health insurance schemes for refugees and other persons of concern to UNHCR. http://www.unhcr.org/4f7d4cb1342.pdf. Accessed 16 May 2017
- World Health Organization (2013) Global action plan for the prevention and control of NCDs 2013–2020. Available at: http://www.who.int/nmh/events/ncd_action_plan/en/. Accessed 16 Sep 2016
- World Health Organization (2014) Report on the expert meeting on scaling-up cancer care in the Eastern Mediterranean Region. Document WHO-EM/NCD/094/E/11.14. http://applications.emro.who.int/docs/IC_Meet_Rep_2014_EN_15570.pdf. Accessed 11 May 2017
- Yip C-H, Smith RA, Anderson BO, et al. Guideline implementation for breast healthcare in low- and middle-income countries: early detection resource allocation. Cancer. 2008;113:2244–2256. doi: 10.1002/cncr.23842. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


