REPLY
Strand transfer inhibitors are potent molecules targeting HIV-1 integration, a critical step involved in retroviral replication. To date, three inhibitors, raltegravir, elvitegravir, and dolutegravir (DTG), are available to treat infected patients. Unfortunately, many resistance pathways have been described for raltegravir and elvitegravir, and all mutations leading to strand transfer resistance have been located in the integrase gene. In the case of dolutegravir, the last integrase inhibitor, only a few mutations described from patients or from in vitro selection are reported to confer resistance (1–3).
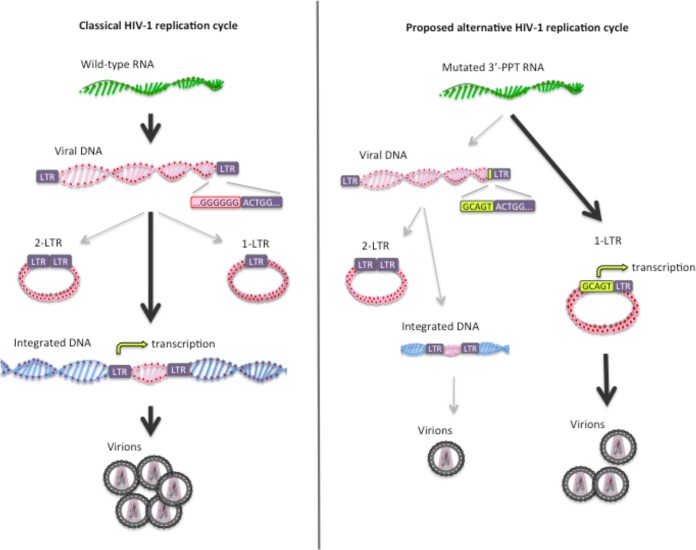
Following the publication of the article describing a virus having selected mutations outside the integrase gene and conferring resistance to HIV-1 integrase inhibitors (4), Das and Berkhout propose a model for the replication of this 3′-polypurine-tract (3′-PPT)-mutated virus (18). Integration of the viral DNA would be possible due to the modification of the 5′ long terminal repeat (LTR) end which makes the virus insensitive to dolutegravir action, allowing it to integrate into the host genome. This hypothesis is plausible since an optimal binding of dolutegravir on the integrase/DNA complex is required for inhibition of the compound and the binding requires the canonical LTR end (5). Since integration is a concerted mechanism occurring in the intasome composed of integrase and both LTR ends, the deficient binding of dolutegravir on the 5′ LTR could allow the unmodified 3′ LTR end to become insensitive to dolutegravir. Indeed, as reported in the literature, mutations at one end could have consequences for the other end (6). Consequently, integration of both 5′ LTR and 3′ LTR could occur despite lower efficiency, explaining the resistance of the 3′-PPT mutant to dolutegravir.
Since 2-LTR circles are formed by ligation of the two LTR ends by the nonhomologous end joining (NHEJ) pathway, the LTR-LTR junction should reflect the integrity of the LTR ends (7). To test this hypothesis, sequencing of the U3-U5 junction of 2-LTR circles was carried out from MT-4 cells infected with the wild-type (WT) and 3′-PPT-mutated viruses with or without DTG (without DTG at day 6 for WT and day 8 for mutated virus and with DTG at day 15 for 3′-PPT-mutated virus). Briefly, after extraction of DNA, a fragment of 307 nucleotides encompassing more than 100 nucleotides from either side of the junction was amplified using primers specific for U3 and U5 sequences, and pyrosequencing on a GS Junior sequencer (Roche 454 Life Sciences) was performed. A total of 1,675 and 1,280 reads per nucleotide position was amplified for WT and mutant viruses, respectively. Interestingly, we found that the LTR-LTR junction was identical to the palindromic sequence found by the predicted ligation of the two unprocessed DNA ends with a total similitude between the two analyzed viruses. Sequence analysis, as described in the literature (17), showed that around 50% (53.7% for WT and 50% for the mutant) of circle junctions were similar to the expected sequence and that the remaining 50% of sequences had multiple deletions ranging from 1 to several tens of nucleotides, located on either side of the junction (U5 or U3) or extending over the U5-U3 junction. These data show that no additional bases were detected in the 3′ PPT compared to the WT virus, highlighting the classical DNA ends of the 2-LTR WT virus. These data sustain the idea that the reverse transcription step does not lead to a modification of LTR ends during the replication of the 3′-PPT-mutated virus (Fig. 1).
FIG 1 .
Comparison between the classical HIV-1 replication cycle and the proposed alternative replication cycle of the 3′-PPT mutant. (Left) The classical HIV-1 replication cycle leading to the integration of viral DNA at the level of genomic DNA. (Right) The proposed alternative replication cycle leading mainly to the formation of 1-LTR circles during the reverse transcription steps. The CAGT sequence inside the mutated 3′ PPT, upstream of U3, could be an enhancer of transcription initiation, allowing viral production from 1-LTR circles and explaining the resistance to DTG.
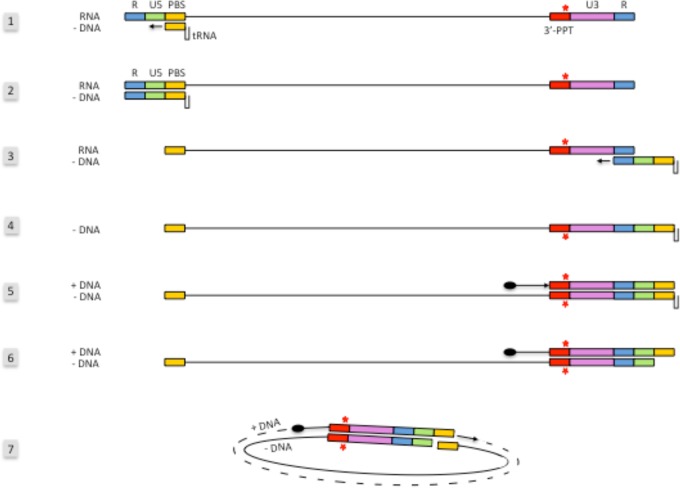
We agree with Das and Berkhout in suggesting that the mutation in the 3′ PPT leads to a modification of the reverse transcription, but instead of modifying LTR ends, we predict for ourselves that the presence of the mutation, disrupting the 3′ PPT, would lead to a total degradation of the RNA by the RNase H activity and an impairment of the synthesis of the U3-R-U5-PBS (primer binding site) +DNA from the 3′ PPT (Fig. 2). The initiation of the +DNA could be then started from sequences located upstream of the 3′ PPT as reported in the literature (8). We can speculate, in this case, that synthesis of a longer +DNA fragment could prevent the translocation of the DNA that would lead to an intramolecular circularization of the viral genome using the complementary PBS regions. After circularization, complementation of the DNA could occur and lead to the circularized genome becoming a 1-LTR circle. It can be noted that Kantor and colleagues have previously shown that mutations in the 3′ PPT lead to an accumulation of 1-LTR circles and a significant decrease of the linear viral DNA from reverse transcription. This observation, made in the context of viral vector, reinforces our hypothesis (9), an assumption that we can test by quantification of 1-LTR circles during infection with the 3′ PPT mutant.
FIG 2 .
Schematic representation of the reverse transcription process of the 3′-PPT mutant leading to the 1-LTR circle formation. The first reverse transcription steps of the 3′-PPT mutant (steps 1 to 3) are similar to the WT. In step 4, we hypothesize that the entire RNA sequence, including the mutated PPT, is degraded by RNase H and that the initiation of the +DNA synthesis (step 5) occurs from sequences located upstream of the 3′ PPT. Once the partial +DNA is synthesized and the RNase H has cleaved the tRNALys (step 6), the partially double-stranded DNA can be circularized by pairing the PBS sequences located on both sides of the DNA, which leads to the formation of 1-LTR circles.
The role of unintegrated viral DNA in HIV-1 expression has always been a topic of debate. First, it has been clearly described that unintegrated viral DNA could be involved in expression of viral accessory proteins such as Nef (10). Second, even if unintegrated viral DNA could hardly be at the origin of the production of infectious viral particles, recent reports describe a role in HIV-1 replication under specific conditions (11).
We hypothesize that, in the case of an HIV-1 genome exclusively present in unintegrated forms, as suggested for the 3′-PPT mutant, the LTR-mediated transcription of the unintegrated viral DNA of this virus should then be stronger than in the case of the wild-type unintegrated viral DNA under DTG.
Interestingly, we can notice in the 3′-PPT mutant the presence of the CAGT pattern just upstream of the 3′ LTR which has been described to be an initiator element for transcription in some viruses, such as baculovirus (12). This sequence needs to be studied for its ability to improve the transcription of the nonintegrated viral DNA of the 3′-PPT mutant, which could then support the hypothesis that the 3′-PPT mutant virus is able to replicate without going through the integration but at a lower level than the wild-type virus.
It is well known that a significant proportion of patients failing on integrase inhibitors and even more when failing on dolutegravir do not select any mutations in the integrase gene (13–15). It is difficult to know precisely why these patients failed on treatment, but depending on the context, it could be explained by the presence of unknown integrase resistance mutations, problems of adherence, and so on. The presence of selected mutations in the 3′ PPT could be also considered, as it has not been explored yet in these patients. In addition, Wijting et al. recently reported (16) the case of a patient who failed a monotherapy treatment with dolutegravir in the DOMONO study without selecting a mutation in the integrase gene but who showed mutations in the 3′ PPT similar to those reported by Malet and colleagues (4).
Taken together, these data could suggest a role of unintegrated viral DNA that would allow a significant replication of the virus, in a way independent of integration. To study this hypothesis, quantifications of integrated and nonintegrated viral DNA will be performed from cells infected with the 3′-PPT virus, and also, the CAGT pattern will be studied for its ability to increase viral transcription of the 3′-PPT mutant.
ACKNOWLEDGMENTS
We thank Nathalie Désiré and Thuy Nguyen for their help regarding the analysis of pyrosequencing on the GS Junior sequencer.
Footnotes
This is a response to a letter by Das and Berkhout (https://doi.org/10.1128/mBio.00006-18).
Citation Malet I, Subra F, Richetta C, Charpentier C, Collin G, Descamps D, Calvez V, Marcelin A-G, Delelis O. 2018. Reply to Das and Berkhout, “How polypurine tract changes in the HIV-1 RNA genome can cause resistance against the integrase inhibitor dolutegravir.” mBio 9:e00623-18. https://doi.org/10.1128/mBio.00623-18.
REFERENCES
- 1.Yoshinaga T, Seki T, Miki S, Miyamoto T, Suyama-Kagitani A, Kawauchi-Miki S, Kobayashi M, Sato A, Stewart E, Underwood M, Fujiwara T. 2018. Novel secondary mutations C56S and G149A confer resistance to HIV-1 integrase strand transfer inhibitors. Antiviral Res 152:1–9. doi: 10.1016/j.antiviral.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Malet I, Ambrosio FA, Subra F, Herrmann B, Leh H, Bouger MC, Artese A, Katlama C, Talarico C, Romeo I, Alcaro S, Costa G, Deprez E, Calvez V, Marcelin AG, Delelis O. 2018. Pathway involving the N155H mutation in HIV-1 integrase leads to dolutegravir resistance. J Antimicrob Chemother 73:1158–1166. doi: 10.1093/jac/dkx529. [DOI] [PubMed] [Google Scholar]
- 3.Malet I, Thierry E, Wirden M, Lebourgeois S, Subra F, Katlama C, Deprez E, Calvez V, Marcelin AG, Delelis O. 2015. Combination of two pathways involved in raltegravir resistance confers dolutegravir resistance. J Antimicrob Chemother 70:2870–2880. doi: 10.1093/jac/dkv197. [DOI] [PubMed] [Google Scholar]
- 4.Malet I, Subra F, Charpentier C, Collin G, Descamps D, Calvez V, Marcelin A-G, Delelis O. 2017. Mutations located outside the integrase gene can confer resistance to HIV-1 integrase strand transfer inhibitors. mBio 8:e00922-17. doi: 10.1128/mBio.00922-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thierry E, Lebourgeois S, Simon F, Delelis O, Deprez E. 2017. Probing resistance mutations in retroviral integrases by direct measurement of dolutegravir fluorescence. Sci Rep 7:14067. doi: 10.1038/s41598-017-14564-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy JE, Goff SP. 1992. A mutation at one end of Moloney murine leukemia virus DNA blocks cleavage of both ends by the viral integrase in vivo. J Virol 66:5092–5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones FD, Hughes SH. 2007. In vitro analysis of the effects of mutations in the G-tract of the human immunodeficiency virus type 1 polypurine tract on RNase H cleavage specificity. Virology 360:341–349. doi: 10.1016/j.virol.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Miles LR, Agresta BE, Khan MB, Tang S, Levin JG, Powell MD. 2005. Effect of polypurine tract (PPT) mutations on human immunodeficiency virus type 1 replication: a virus with a completely randomized PPT retains low infectivity. J Virol 79:6859–6867. doi: 10.1128/JVI.79.11.6859-6867.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kantor B, Bayer M, Ma H, Samulski J, Li C, McCown T, Kafri T. 2011. Notable reduction in illegitimate integration mediated by a PPT-deleted, nonintegrating lentiviral vector. Mol Ther 19:547–556. doi: 10.1038/mt.2010.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poon B, Chang MA, Chen ISY. 2007. Vpr is required for efficient Nef expression from unintegrated human immunodeficiency virus type 1 DNA. J Virol 81:10515–10523. doi: 10.1128/JVI.00947-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan CN, Trinité B, Lee CS, Mahajan S, Anand A, Wodarz D, Sabbaj S, Bansal A, Goepfert PA, Levy DN. 2016. HIV-1 latency and virus production from unintegrated genomes following direct infection of resting CD4 T cells. Retrovirology 13:1. doi: 10.1186/s12977-015-0234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pullen SS, Friesen PD. 1995. The CAGT motif functions as an initiator element during early transcription of the baculovirus transregulator ie-1. J Virol 69:3575–3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fourati S, Charpentier C, Amiel C, Morand-Joubert L, Reigadas S, Trabaud MA, Delaugerre C, Nicot F, Rodallec A, Maillard A, Mirand A, Jeulin H, Montès B, Barin F, Bettinger D, Le Guillou-Guillemette H, Vallet S, Signori-Schmuck A, Descamps D, Calvez V, Flandre P, Marcelin AG, ANRS AC11 Resistance Study Group . 2015. Cross-resistance to elvitegravir and dolutegravir in 502 patients failing on raltegravir: a French national study of raltegravir-experienced HIV-1-infected patients. J Antimicrob Chemother 70:1507–1512. doi: 10.1093/jac/dku535. [DOI] [PubMed] [Google Scholar]
- 14.White K, Kulkarni R, Miller MD. 2015. Analysis of early resistance development at the first failure timepoint in elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate-treated patients. J Antimicrob Chemother 70:2632–2638. doi: 10.1093/jac/dkv149. [DOI] [PubMed] [Google Scholar]
- 15.Moreno S, Berenguer J. 2015. Efficacy of dolutegravir in treatment-experienced patients: the SAILING and VIKING trials. Enferm Infecc Microbiol Clin 33(Suppl 1):26–30. doi: 10.1016/S0213-005X(15)30006-9. [DOI] [PubMed] [Google Scholar]
- 16.Wijting IEA, Lungu C, Rijnders BJA, van der Ende ME, Pham HT, Mesplede T, Pas SD, Voermans JJC, Schuurman R, van de Vijver DAMC, Boers PHM, Gruters RA, Boucher CAB, van Kampen JJA. 2018. HIV-1 resistance dynamics in patients with virologic failure to dolutegravir maintenance monotherapy. J Infect Dis doi: 10.1093/infdis/jiy176. [DOI] [PubMed] [Google Scholar]
- 17.Mandal D, Prasad VR. 2009. Analysis of 2-LTR circle junctions of viral DNA in infected cells. Methods Mol Biol 485:73–85. doi: 10.1007/978-1-59745-170-3_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das AT, Berkhout B. 2018. How polypurine tract changes in the HIV-1 RNA genome can cause resistance against the integrase inhibitor dolutegravir. mBio 9:e00006-18. doi: 10.1128/mBio.00006-18. [DOI] [PMC free article] [PubMed] [Google Scholar]