ABSTRACT
Current efforts toward human immunodeficiency virus (HIV) eradication include approaches to augment immune recognition and elimination of persistently infected cells following latency reversal. Natural killer (NK) cells, the main effectors of the innate immune system, recognize and clear targets using different mechanisms than CD8+ T cells, offering an alternative or complementary approach for HIV clearance strategies. We assessed the impact of interleukin 15 (IL-15) treatment on NK cell function and the potential for stimulated NK cells to clear the HIV reservoir. We measured NK cell receptor expression, antibody-dependent cell-mediated cytotoxicity (ADCC), cytotoxicity, interferon gamma (IFN-γ) production, and antiviral activity in autologous HIV replication systems. All NK cell functions were uniformly improved by IL-15, and, more importantly, IL-15-treated NK cells were able to clear latently HIV-infected cells after exposure to vorinostat, a clinically relevant latency-reversing agent. We also demonstrate that NK cells from HIV-infected individuals aviremic on antiretroviral therapy can be efficiently stimulated with IL-15. Our work opens a promising line of investigation leading to future immunotherapies to clear persistent HIV infection using NK cells.
IMPORTANCE In the search for an HIV cure, strategies to enhance immune function to allow recognition and clearance of HIV-infected cells following latency reversal are being evaluated. Natural killer (NK) cells possess characteristics that can be exploited for immunotherapy against persistent HIV infection. We demonstrate that NK cells from HIV-positive donors can be strongly stimulated with IL-15, improving their antiviral and cytotoxic potential and, more importantly, clearing HIV-infected cells after latency reversal with a clinically relevant drug. Our results encourage further investigation to design NK cell-based immunotherapies to achieve HIV eradication.
KEYWORDS: natural killer cells, HIV, latency reversal, HIV eradication, immunotherapy, vorinostat, VOR, SAHA, immune function, IL-15, shock and kill, kick and kill, ADCC, latency reversal, NK cell, human immunodeficiency virus, interleukins
INTRODUCTION
The immune system is not capable of clearing human immunodeficiency virus (HIV) infection, and thus, even in the presence of fully suppressive antiretroviral therapy (ART), infection is chronic. However, in recent years, significant progress has been made in strategies to disrupt the viral latent reservoir, promoting new efforts toward virus clearance or cure (1–3). Awakening the latent reservoir of persistent infection could represent the first step in HIV eradication therapy, but clearance of infection requires a second step involving a potent and efficient immune response. This combined strategy faces challenges, such as insufficient number and function of HIV-specific CD8+ T cells (4–6), dispersed and scarce HIV antigen (Ag) expression in latently infected cells, and selection of virus populations poorly recognized by CD8+ T cells (7–9). Therefore, alternative or complementary immune strategies should be pursued. Here, we model the ability to augment natural killer (NK) cell function in autologous HIV-infected-donor cell systems, demonstrating the potential to fill this unmet need.
NK cells are the main effectors of the innate immune system. They are the first line of defense against viral infections and also play an important role in more advanced stages of infection (10). Although controversial, evidence of viral evolution toward NK cell escape suggests that these cells exert specific immunological pressure on HIV (11, 12). NK cells can eliminate HIV-infected cells by antibody-dependent cell-mediated cytotoxicity (ADCC), by direct lysis via the release of cytotoxic granules, and by facilitating priming of the adaptive immune response. Activation of NK cells is governed by the balance of positive signals via the recognition of cellular stress markers on the surfaces of infected or malignant cells and negative signals received primarily through killer cell immunoglobulin-like receptors (KIRs) engaging the major histocompatibility complex (MHC). It is especially interesting that HIV downregulates MHC class I expression on the surfaces of infected cells, thereby escaping recognition and lysis by CD8+ T cells but, conversely, rendering it more susceptible to NK cell-mediated clearance (13). In addition, lymph nodes are a major anatomic HIV reservoir (14), and CD8+ effectors may be relatively excluded from lymphoid follicles, making them a potential sanctuary for persistent infection (15). Recently, it has been reported that NK cells can accumulate in the follicles of secondary lymphoid organs in simian immunodeficiency virus (SIV)-infected African green monkeys (AGMs), natural controllers of SIV replication (16). These findings encourage investigation of NK cell-based immunotherapies for HIV eradication.
Cytokines play a decisive role in activating NK cells by enhancing their cytotoxic potential (17). Interleukin 15 (IL-15) is a potent enhancer of NK cytotoxic function and is under study in oncology, given its potential to improve the clearance of malignant cells (18). In the present work, we analyzed the potential of cytokine-stimulated NK cells to recognize and clear latently HIV-infected cells after latency reversal and performed a comprehensive characterization of the impact of cytokine treatment on NK cell function. We demonstrate that upon treatment with IL-15, NK cells improve their capacity to target and clear HIV-infected cells.
RESULTS
IL-15 treatment improves antiviral activity of NK cells.
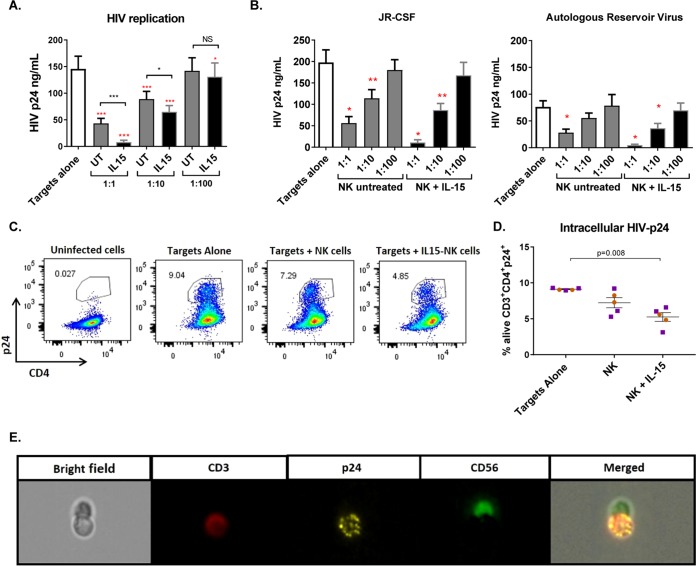
HIV-infected CD4+ T cells derived from 8 donors were superinfected with the laboratory strain JR-CSF, and cells from 6 additional donors were infected with autologous reservoir virus (AR). Infection using AR is relevant, given the diversity of viral isolates within and between donors, as well as the potential for viral isolates that have evolved to escape immune responses. Pooled analysis of all the donors showed that at a 1:1 effector/target cell (E:T) ratio, autologous NK cells significantly reduced viral replication, from 100% under the target-alone condition to 31.2% (standard error of the mean [SEM], 4.3%; P = 0.0002), while IL-15 stimulation of NK cells further decreased viral replication (4.8% [SEM, 1.3%; P = 0.0002]), with significant differences between untreated and IL-15-treated NK cells (P = 0.0005). Virus reduction was also seen at a 1:10 E:T ratio for both untreated NK cells and IL-15-stimulated cells (65% [SEM, 6.3%; P = 0.0004] and 44.3% [SEM, 4,8%; P = 0.0001], respectively), and again, IL-15 significantly improved antiviral activity (P = 0.008). Finally, at a 1:100 ratio, only IL-15-stimulated cells exerted a significant impact on virus production (79.5% [SEM, 5.5%; P = 0.02]) (Fig. 1A). When the experiments were analyzed according to the viral isolate used for infection (JR-CSF or AR), the patterns of inhibition were comparable between the viruses (Fig. 1B).
FIG 1.
IL-15 improves the antiviral activity of NK cells from ART-treated HIV-infected donors. (A) Viral replication measured as HIV gag p24 antigen in the supernatants of 7-day cultures with only infected CD4+ T cells (Targets alone) or in the presence of NK cells at different effector/target cell ratios. UT, untreated. The red asterisks indicate statistically significant differences compared to targets alone, and black asterisks indicate differences between untreated and IL-15-stimulated NK cells (n = 14). (B) Viral replication in viral inhibition assays performed with JR-CSF superinfection (n = 8) or autologous reservoir virus (n = 6). Wilcoxon matched-pairs signed-rank test. *, P < 0.05; **, P < 0.01; ***, P < 0.001. The error bars indicate standard error of the mean (SEM). (C) Representative flow cytometry plots of intracellular p24 in cells from one donor gated on the CD3+ population of the live fraction. (D) Proportion of live CD4+ T cells positive for intracellular p24 staining. Coculture of infected CD4 cells with IL-15-treated NK cells significantly reduced the proportion of live CD4+ T cells containing p24 antigen after 5 days in culture. The orange circles correspond to cells from HIV-negative donors (n = 2), and the purple squares correspond to cells from aviremic HIV-positive donors (n = 3). Mann-Whitney U test. (E) Interaction of an NK cell with an infected CD4+ T cell visualized with ImageStreamX.
Intracellular p24 (Fig. 1C) was measured in 5 experiments, 2 of them performed with cells from HIV-negative donors and the other 3 with cells from HIV-infected donors. After 5 days in culture, the percentage of live p24-positive CD4+ T cells was reduced from a mean of 9.12% (SEM, 0.07%) under target-alone conditions to 7.23% (SEM, 0.71%) when target cells were cultured with NK cells, and further, to 5.25% (SEM, 0.60%), when NK cells were treated with IL-15 (Fig. 1D). Finally, we visualized cells from a p24 intracellular-staining experiment using Amnis ImageStreamX and found several interactions between NK cells (marked with CD56-fluorescein isothiocyanate [FITC]) and HIV-infected target cells (CD3-allophycocyanin [APC] to identify targets and p24-phycoerythrin [PE] to detect infection) (Fig. 1E).
Cytotoxicity and IFN-γ production after IL-15 stimulation.
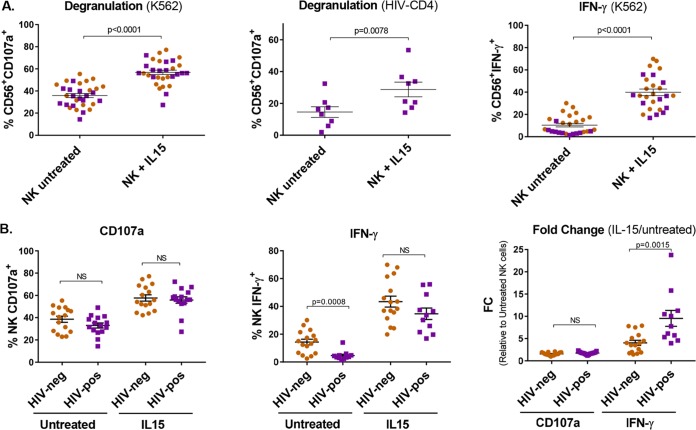
NK cell cytotoxicity was evaluated through the expression of the degranulation marker CD107a. NK cells, with or without IL-15 stimulation, were cultured in the presence of either autologous superinfected CD4 cells or the cell line K562. After coculture with K562 cells, 36.9% (SEM, 2.5%) of NK cells degranulated and became CD107a+ compared to 58.1% (SEM, 2.8%) of the IL-15-stimulated NK cells (P < 0.0001) (Fig. 2A, left). IL-15 stimulation also significantly improved NK cell degranulation in the presence of autologous HIV-superinfected CD4+ T cells, increasing from a mean of 14.5% (SEM, 3.4%) to 28.7% (SEM, 4.6%) (P < 0.01) (Fig. 2A, middle). IL-15 also enhanced NK cell IFN-γ production (P < 0.0001), measured by intracellular staining, from an average of 13.3% (SEM, 2.2%) of NK cells producing IFN-γ to 43% (SEM, 4.2%) (Fig. 2A right). We also compared the performance of NK cells isolated from HIV-negative and HIV-positive donors. NK cells from HIV-negative and HIV-positive individuals degranulated to equal extents, both without treatment and after IL-15 stimulation. However, NK cell baseline IFN-γ production was impaired in aviremic HIV-positive donors (P = 0.0008), but interestingly, IL-15 restored the capacity of NK cells to produce IFN-γ to the same level as HIV-negative individuals (Fig. 2B).
FIG 2.
IL-15 increases the cytotoxic function of NK cells. (A) (Left and middle) Degranulation of NK cells measured by CD107a expression after coculture with the cell line K562 or autologous HIV-superinfected CD4+ T cells (n = 21 and n = 8, respectively). (Right) IFN-γ production after coculture with K562 cells measured by intracellular staining (n = 17). Wilcoxon matched-pairs signed-rank test. (B) (Left and middle) Comparison between degranulation and IFN-γ production in NK cells from HIV-negative or HIV-positive donors. (Right) Fold change (IL-15/untreated) of CD107a expression and IFN-γ production in HIV-negative and HIV-positive donors. Mann-Whitney test. The orange circles correspond to cells from HIV-negative donors, and the purple squares correspond to cells from aviremic HIV-positive donors. The error bars indicate SEM.
Impact of other cytokines on NK cell-mediated cytotoxicity.
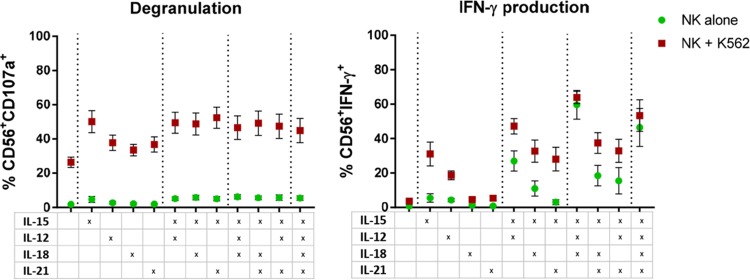
Given that other cytokines may surpass or augment the effect of IL-15 on NK cell immune function, we performed cytotoxicity assays measuring degranulation and IFN-γ production after coculturing K562 cells with NK cells treated with IL-15, IL-12, IL-18, or IL-21 (25 ng/ml) alone or in combination (Fig. 3). Stimulation of NK cells with any of the cytokines alone or in combination provided a significant increase in degranulation against K562 cells compared to untreated NK cells (P = 0.03). However, IL-15 stimulation proved to augment the function more than treatment with IL-12, IL-18, or IL-21 (P = 0.03), and no differences were found between IL-15 alone and IL-15 in combination with any or all of the other cytokines. When degranulation was measured in the absence of a target cell line (NK alone), we observed a modest increase in nonspecific degranulation when NK cells were stimulated with any of the other cytokines in addition to IL-15, reaching statistical significance for IL-15 plus IL-12, IL-15 plus IL-18, IL-15 plus IL-21, and IL-15 plus IL-12 plus IL-21. NK cell production of IFN-γ after coculture with the cell line K562 was increased when the cells were treated with IL-15 or IL-12 alone, as well as with IL-15 in combination with any of the other cytokines (P = 0.03), but no effect was observed upon stimulation with IL-18 or IL-21 alone. Moreover, addition of IL-12 and IL-12 plus IL-18 to IL-15 significantly increased IFN-γ production compared to IL-15 alone (P = 0.03). However, in the absence of target cells, addition of IL-12 or IL-12 plus IL-18 to IL-15 also produced a significant increase (P = 0.03) compared to IL-15 alone. Thus, combination of other cytokines with IL-15, especially IL-12, might improve NK cell capacity to produce IFN-γ, but these cytokine combinations were associated with nonspecific effects, as shown by the significant increase in IFN-γ production in the absence of target cells, supporting further exploration of IL-15 alone.
FIG 3.
NK cell-mediated cytotoxicity after treatment with different cytokines. The graphs show the proportions of NK cells degranulating or producing IFN-γ in the presence of K562 cells (red squares) or in the absence of target cells (green circles) after stimulation with different cytokines or combinations, as indicated in the table below. Degranulation after culture with the cell line K562 showed a target-specific increase in cytotoxicity after stimulation with any of the cytokines or the studied combinations (P = 0.03). However, IL-15 showed better stimulation than IL-12, IL-18, or IL-21 (P = 0.03), and no difference was found between IL-15 alone and IL-15 in addition to any of the other cytokines (P, NS). IFN-γ production upon culture with K562 cells is increased after stimulation with IL-15 or IL-12 alone or any of the cytokine combinations (P = 0.03). The IL-15 effect was improved when IL-12 or IL-12 and IL-18 were included. However, IFN-γ production in the absence of target cells (NK alone) also increased after IL-12 or IL-12 and IL-18 addition to IL-15, indicating nonspecific activation. Wilcoxon matched-pairs signed-rank test. The error bars indicate SEM.
ADCC activity of NK cells is enhanced after IL-15 stimulation.
We evaluated whether IL-15 stimulation of NK cells had an effect on ADCC activity, using HIV-infected CEM.NKRCCR5 cells as targets in a luciferase assay. NK cells were isolated from 6 HIV-negative and 6 HIV-positive ART-treated aviremic donors. Untreated or IL-15-treated NK cells were tested against subtype C HIV-1 TV-1-infected target cells in the presence of serial dilutions of plasma from an HIV-infected participant. In both groups, we observed very similar statistically significant increases in target-specific killing when NK cells were stimulated with IL-15 (Fig. 4A) (P = 0.001). Comparison of the responses from all the participants was also performed, using antibody titers (Fig. 4B) and the area under the curve (Fig. 4C), which revealed statistically significant differences between the responses obtained with untreated and IL-15-treated effector cells (P = 0.0005). We did not observe statistically significant differences between the ADCC activities of HIV-negative and HIV-positive donors when we compared the results under untreated and IL-15-treated conditions.
FIG 4.
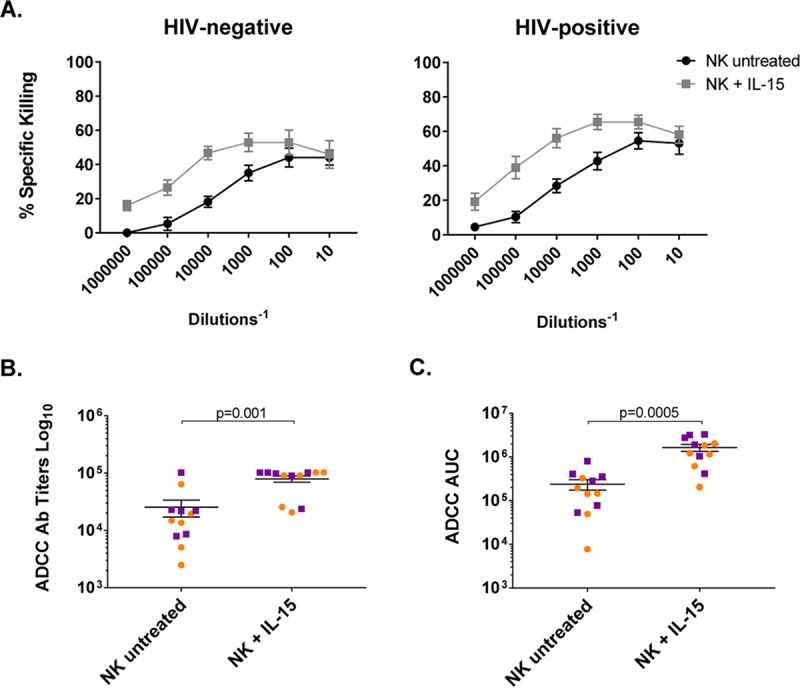
ADCC improves after IL-15 stimulation of NK cells. NK cells were cultured with TV-1-infected CEM.NKR cells in the presence of HIV-negative or A300 (HIV-positive) plasma for 6 h, and specific target killing was recorded using different plasma dilutions. (A) Mean antibody-mediated specific killing with untreated NK cells or after overnight IL-15 stimulation using different plasma dilutions. Cytokine treatment improved ADCC activity in both HIV-negative (n = 6) and HIV-positive (n = 6) donors. Means with SEM are shown. (B) Antibody titers observed with untreated/treated NK cells (n = 12). (C) Areas under the curve at different plasma dilutions with untreated NK and IL-15-stimulated NK cells (n = 12). The orange circles correspond to cells from HIV-negative donors, and the purple squares correspond to cells from ART-treated aviremic HIV-positive donors. Wilcoxon matched-pairs signed-rank test.
IL-15-stimulated NK cells recognize and clear latently HIV-infected cells after reactivation.
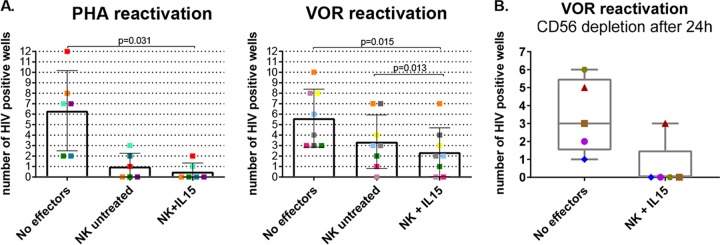
A modified viral outgrowth assay was used to assess the ability of NK cells to recognize and clear latently infected cells once they were induced to emerge from latency and reexpress viral antigen. The goal of this assay is to mimic the conditions that might exist in a future clinical experiment in which latency reversal agents might induce antigen expression without robust viral production or cellular activation. Resting CD4+ T cells were isolated from HIV-positive donors and either activated using phytohemagglutinin (PHA) as a positive control of maximal mitogen stimulation or treated with the histone deacetylase (HDAC) inhibitor vorinostat (VOR) (also known as SAHA) under conditions known to parallel clinical administration of the drug (1). At the end of the culture, the supernatants were harvested, and viral production was measured by p24 enzyme-linked immunosorbent assay (ELISA), recording the number of wells in which virus was recovered, regardless of the quantity of p24 measured. The underlying assumption was that each well would possess one or fewer infected cells and that a decrease in the frequency of wells with detectable p24 reflected clearance of the infected cells.
When resting CD4+ T cells were reactivated with PHA, a decrease in the number of HIV-positive wells was observed when NK cells were added to the culture (P, not significant [NS]) (Fig. 5A, left panel), with a more pronounced reduction when the NK cells were first stimulated with IL-15 (P = 0.031). Of more clinical relevance, when vorinostat was used to reverse latency, IL-15-stimulated NK cells significantly reduced the number of p24 HIV+ wells (P = 0.015), with significant differences between untreated and IL-15-treated NK cells (P = 0.013) (Fig. 5A, right panel).
FIG 5.
Latency clearance assay. (A) Resting CD4+ T cells were incubated with PHA or VOR for 24 h, cocultured with or without autologous NK cells at an E:T ratio of 1:10 for another 24 h, and plated in 12 replicates in the presence of feeders for 19 days, refreshing the medium every 3 or 4 days. The supernatant was assayed for p24 ELISA to assess the presence of HIV replication. The graphs show the numbers of wells in which p24 was detected at day 19, with a different color for each donor. (Left) Reactivation with PHA (n = 6). (Right) Reactivation with 335 nM VOR (n = 8). Wilcoxon matched-pairs signed-rank test. (B) Latency clearance assays in which NK cells were depleted after the initial 24 h of coculture with VOR-reactivated CD4+ T cells (n = 5). IL-15-stimulated NK cells reduced the number of HIV+ wells during the first 24 h of coculture with VOR-reactivated CD4+ T cells. The error bars indicate SEM.
Next, we introduced an important modification of the latency clearance assay by removing NK effector cells after the initial 24 h of coculture and prior to the addition of allogeneic uninfected target cells. Using this modification, reduction of wells with detectable p24 could be confidently associated with clearance of reactivated CD4+ T cells rather than with inhibition of viral spread or feeder cell killing. We performed this modified latency clearance assay with IL-15-stimulated NK cells from 5 donors. The efficiency of CD56+ cell depletion was >98% in all cases. In these experiments, we also observed a consistent decline in the number of positive wells when IL-15-treated NK cells were cocultured with resting CD4 cells, confirming that NK cell-mediated clearance takes place before the addition of feeders (Fig. 5B). In addition, annexin V binding was evaluated after 24-h culture of VOR-treated CD4+ T cells in the presence or absence of IL-15-stimulated NK cells to confirm that nonspecific cell killing was not responsible for the observed virus clearance (<5% annexinV-positive CD4+ T cells).
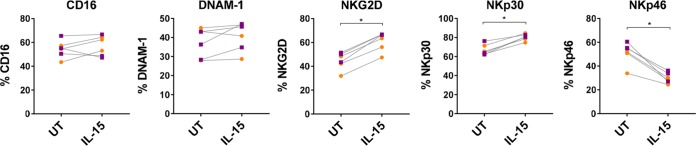
Impact of IL-15 exposure on activating receptor expression in NK cells.
Activating NK cell receptor expression was analyzed to explore the impact of IL-15 exposure. The panel included CD16, DNAM-1, NKG2D, NKp30, and NKp46. IL-15 stimulation did not impact expression of CD16 or DNAM-1 but significantly increased the expression of NKG2D and NKp30 (P = 0.03). Interestingly, IL-15 exposure also produced a significant down-modulation of NKp46 (P = 0.03), as shown in Fig. 6.
FIG 6.
Expression of activating NK cell receptors before and after IL-15 exposure. Cytokine stimulation upregulated the expression of NKG2D and NKp30, while it decreased that of NKp46. The orange circles represent cells from healthy donors (n = 6), and the purple squares represent cells from HIV-infected donors (n = 6). Wilcoxon matched-pairs signed-rank test. *, P < 0.05.
DISCUSSION
The enhancement of the innate antiviral activity of NK cells is a viable adjunctive clinical strategy that may be employed in efforts to clear persistent HIV infection. In the present work, we demonstrate the ability of IL-15 stimulation to enhance NK cell function in the context of HIV. Uniformly, IL-15 augmented NK cell function, improving degranulation, ADCC activity, IFN-γ production, and direct antiviral activity in actively replicating HIV cultures. Of most significance, IL-15-treated NK cells recognized and cleared latently HIV-infected cells following a clinically achievable exposure to the latency-reversing agent vorinostat.
Immunological strategies in the field of HIV eradication have focused on HIV-specific cytotoxic T lymphocytes (CTLs) (19–21), although recently, interest in NK cells has emerged. The capacity of NK cells to produce IFN-γ and upregulate NKp30 and NKp46 has been inversely associated with viral reservoir size (22), and HIV DNA decline was correlated with NK cell frequency after panobinostat treatment in a clinical study (23), suggesting a role for NK cells in the clearance of HIV infection. Moreover, recent data suggest that NK cells might play an important role in viral clearance in the lymph node (16). However, until now, the ability of NK cells to clear latent HIV infection and the effect of cytokine treatment on this NK cell antiviral activity have not been reported.
Soluble recombinant human IL-15 (rhIL-15), the cytokine used in the present work, is sufficient for ex vivo proof-of-concept studies. However, research in oncology has brought into clinical testing several engineered forms of this potent cytokine, including a heterodimeric form of the molecule (24) and a synthetic receptor superagonist (ALT-803) (25), that might have better profiles for clinical use. The latter form of IL-15 has been studied in the simian immunodeficiency virus model of HIV infection, expanding both NK and CD8+ T cells (26). Future directions could also include the incorporation of the IL-15 gene into genetically modified NK cells, so that the cells could provide autocrine cytokine support (27, 28). Moreover, it is expected that additional strategies will be needed to further enhance NK cell function, since in most of our results, despite seeing a robust antiviral effect of the stimulated NK cells, total clearance was not always observed. Thus, NK cell function might be improved by using other priming strategies, such as IFN-α stimulation (29, 30) or therapeutic vaccination (31). In addition, clinical experience gained in oncology may be leveraged to design HIV-specific approaches, given the more advanced stages of NK cell-based therapies that have been developed for cancer (32–36).
The most relevant experiment in this study is undoubtedly the latency clearance assay (19) performed on primary resting CD4+ T cells from HIV-infected individuals on antiretroviral treatment. This assay is the most proximate to the in vivo situation, eliminating artifacts produced by cell models or in vitro infections. As latency reversal was induced by VOR at exposures that have been safely achieved in clinical trials, it is likely that NK cell targeting and clearance can be similarly achieved in vivo.
Here, we proved that NK cells can clear infected cells after VOR exposure. However, a range of novel approaches to achieve HIV latency reversal are under study (37, 38). Thus, in a similar way, future work must address the effect of novel latency-reversing agents (LRAs) on NK-mediated clearance, given that antigen presentation or ligand modulation might be altered by host-targeted LRAs (39). Of interest, there is evidence that HDAC inhibitor exposure upregulates the surface expression of ligands for activating receptors in NK cells (40, 41). Nonetheless, while such upregulation is observed in tumor cells, the effect of LRA on latently HIV-infected cells is unknown. For example, valproic acid, a weak HDAC inhibitor, upregulates NKG2D ligands only in malignant cells, where the ligands are present at baseline (42). However, initial in vitro studies in HIV-infected cell lines suggested that VOR could also upregulate certain NKG2D ligands (43), which in addition may play an important role in facilitating NK-mediated ADCC responses (44). Finally, novel latency-reversing agents, such as Toll-like receptor (TLR) agonists, may simultaneously reverse HIV latency and improve NK cell function (45, 46), although further study is needed to definitively determine the effect of TLR agonists on NK cells, given the multiple and direct effects that these drugs might have on immune cells.
In addition to latency clearance assays, we conducted virus inhibition assays in the setting of active replication using a completely autologous system with NK cells, CD4+ T cells, and reservoir virus from the same donor. The use of patient-derived viral isolates is relevant, given that viruses may differ in antigen presentation. In our studies, we observed similar NK cell-mediated viral replication inhibition after infection with either JR-CSF or autologous reservoir virus. However, in the future, should ADCC be employed to direct NK cell killing, the breadth of antibody recognition of viral species within the latent reservoir may be critical.
In that regard, we also proved that IL-15 enhanced the ADCC activity of NK cells. Ongoing work seeks to identify antibodies or antibody combinations that will broadly recognize infected cells, specifically after latency reversal (47).
Another important finding of our study is the demonstration that NK cells obtained from HIV-positive donors on ART with suppressed viremia were sufficiently functional to respond to IL-15 and exerted antiviral activity in all the assays performed. Interestingly, we observed that IFN-γ production by HIV-positive donors was impaired compared to that by HIV-negative individuals, but IL-15 stimulation restored IFN-γ production in NK cells from HIV-positive donors to the level of HIV-negative donors. In fact, IL-15-mediated improvement in the HIV-positive population was more pronounced than in the HIV-negative group. The functionality of NK cells from HIV+ individuals with virus suppressed has been previously described (48, 49), but our studies further demonstrate their capability to be stimulated by IL-15 and to be functional in an HIV eradication context.
Finally, we observed that NKG2D and NKp30 were upregulated after IL-15 treatment, which may have contributed to the enhancement of NK cell function. We also observed a downmodulation of NKp46 upon IL-15 exposure. This is in accordance with previous reports (50) and is troublesome, given the importance of this receptor in NK cell antiviral activity (22, 51, 52). Therefore, and even if we observed an important improvement in NK cell function despite NKp46 downmodulation, it might be of interest to consider a complementary strategy to boost NKp46 expression, thus further increasing NK cell antiviral function.
In summary, the present study provides robust evidence that IL-15-stimulated NK cells from virus-suppressed HIV-positive individuals on ART can recognize and clear latently HIV-infected resting CD4+ T cells after relevant exposure to the HDAC inhibitor vorinostat. This finding encourages further exploration of approaches for immunotherapies based on priming NK cells to eradicate latent HIV infection.
MATERIALS AND METHODS
Study samples.
Samples were derived either from HIV-negative donors or from HIV-infected donors on ART with sustained plasma viremia suppression (<50 copies/ml) for at least 6 months. In summary, the mean age of donors was 42.5 (SEM, 3.1) years, with suppressed viremia for an average of 3.4 (SEM, 0.48) years, a mean total of 743 (SEM, 62) CD4 cells/ml, and a mean of 39.8% (SEM, 2.1%) CD4 cells. Peripheral blood mononuclear cells (PBMC) from HIV-negative donors were obtained from buffy coats from the New York Blood Center (Long Island City, NY, USA). PBMC from HIV-infected donors were obtained by Ficoll gradient from buffy coats obtained by leukapheresis. K562 cells were obtained from the ATCC (Manassas, VA).
Ethics statement.
All donors provided written informed consent, and studies were approved by the University of North Carolina (UNC) Institutional Review Board. Samples used in the study were anonymized.
Virus inhibition assays.
NK cells and CD4+ T cells were isolated from the PBMC by negative selection (StemCell Technologies, Vancouver, Canada). The NK cell enrichment antibody cocktail included CD3, CD4, CD14, CD19, CD20, CD36, CD66b, CD123, HLA-DR, and glycophorin. CD4+ T cells were isolated by negative selection in parallel with NK cell isolation from each donor. The CD4+ T cell enrichment antibody cocktail included CD8, CD14, CD16, CD19, CD20, CD36, CD56, CD66b, CD123, T cell receptor γ/δ (TCR-γ/δ), and glycophorin A. After isolation, NK cells were cultured in Iscove's modified Dulbecco's medium (IMDM) supplemented with 10% heat-inactivated bovine serum and 5% penicillin plus streptomycin, with or without the appropriate cytokines, for 24 h, after which the cells were washed and assays were performed. Isolated CD4+ T cells were activated for 24 h with 2 μg/ml PHA (Sigma-Aldrich, St. Louis, MO) and 60 U/ml IL-2 (Peprotech, Rocky Hill, CT). The cells were then infected by spinoculation for 90 min at 2,500 rpm with 50 ng HIV p24 per 10 million cells of the viral strain JR-CSF or with AR, which is virus isolated from a previous viral outgrowth assay performed with cells from the same donor (19). After spinoculation, the cells were extensively washed to remove free virions, and 50,000 infected CD4+ T cells were plated in triplicate for each condition in a 96-well plate. NK cells, previously exposed to cytokines when appropriate, were added to the wells at E:T ratios of 1:1, 1:10, and 1:100 and left in culture for 7 days in complete IMDM with 5 U/ml IL-2; the media were refreshed at day 4. Viral production was assessed in the supernatant by HIV p24 ELISA (ABL, Inc., Rockville, MD), and HIV replication under the different NK cell conditions was compared to replication with only target cells. Viral inhibition was also assessed by measuring intracellular p24 in cells harvested after 5 days of culture. For intracellular quantification of p24, cultures included a total of 800,000 cells, plated in eight replicates, which were pooled to perform the intracellular staining. The cells were first stained with the viability dye Zombie NIR Fixable Viability (Biolegend, San Diego, CA), fixed with 4% paraformaldehyde/lysolecithin, and then resuspended in cold 50% methanol, permeabilized with 0.1% Nonidet P-40, and stained with antibody for p24 Ag (KC-57, Beckman Coulter, Fullerton, CA), followed by surface staining with anti-CD3, -CD4, and -CD56. Infection was recorded as the percentage of viable CD3+ CD4+ cells expressing p24. Finally, we visualized the cells from one of these intracellular p24 experiments using the Amnis ImageStreamX Mark II (Millipore Sigma, St. Louis, MO), and analyzed the images with Ideas V6.2.187.0 software.
Cytotoxicity.
NK cell cytotoxicity and IFN-γ production were analyzed in cocultures of primary NK cells and K562 cells (an NK-sensitive target cell line, due to their lack of MHC) with and without prior exposure of the NK cells to cytokines. Cytotoxicity was assessed by measurement of the degranulation marker CD107a, a reliable marker of NK cell functional activity (53). A total of 200,000 NK cells were cultured with the same number of K562 target cells, along with PE/Cy7-CD107a antibody (Biolegend, San Diego, CA), for 2 to 4 h in a 96-well plate, adding 1 μl of GolgiStop (Becton Dickinson, Franklin Lakes, NJ) after 1 h. Cells were then harvested, washed, and stained with CD56−-FITC and CD3-APC/Fire750 (BioLegend) in staining buffer for 20 min on ice in the dark. The cells were then fixed with fixation buffer for 20 min at room temperature in the dark, permeabilized, and washed with Perm/Wash buffer (Biolegend, San Diego, CA) twice and stained with IFN-γ–PE (BioLegend) for 20 min. After washing, the cells were resuspended in staining buffer and analyzed in an Attune focusing cytometer (Applied Biosystems, Foster City, CA). To evaluate HIV-specific recognition, autologous CD4+ T cells superinfected with the viral strain JR-CSF were used as targets, instead of the cell line K562. Additionally, NK cell degranulation was assessed in the absence of any target to examine nonspecific activation by cytokines. The activation conditions consisted of 25 ng/ml IL-15, but in some experiments, 25 ng/ml IL-12, 25 ng/ml IL-18, and/or 25 ng/ml IL-21 (Peprotech, Rocky Hill, NJ) was also used.
ADCC assay.
We utilized a modified version of a previously published ADCC luciferase procedure (54). Briefly, CEM.NKRCCR5 cells (from A. Trkola, NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH [55]) were used as targets for ADCC luciferase assays after infection with subtype C HIV-1 IMCTV1 (GenBank accession no. HM215437). Purified NK cells were obtained from six HIV-seronegative donors and six ART-treated HIV-seropositive donors. The NK cells were isolated from cryopreserved PBMC by negative selection with magnetic beads (Miltenyi Biotec GmbH, Germany) after overnight incubation with or without 10 ng/ml IL-15 (Miltenyi Biotec GmbH, Germany). The NK cells were used as effector cells at an effector-to-target cell ratio of 5:1. Target and effector cells were plated in opaque 96-well half-area plates and cocultured with serial dilutions of plasma (A300) from an HIV-infected donor starting at 1:100. The cocultures were incubated for 6 h at 37°C in 5% CO2. The final readout was the luminescence intensity generated by the presence of residual intact target cells that had not been lysed by the effector population in the presence of ADCC-mediating plasma Abs. The percentage of killing was calculated using the following formula: percent specific killing = [(RLU of target and effector well − RLU of test well)/(RLU of target and effector well)] × 100, where RLU is relative light units.
In this analysis, the RLU of the target plus effector wells represent spontaneous lysis in the absence of any source of Ab. Plasma from an HIV-seronegative donor (CAVD002) was used as a negative control using the same dilution scheme. The data are reported as percent specific killing observed with the A300 plasma after subtracting the background observed in the presence of the negative control, and the areas under the curve were calculated using Prism 7 (version 7.0b; GraphPad Software).
Latency clearance assay.
A modified viral outgrowth assay (56) was optimized to assess the ability of cytokine-treated NK cells to clear latently infected cells after reactivation. Resting CD4+ T cells were negatively isolated (Stemcell Technologies, Vancouver, Canada) from HIV-infected, ART-treated individuals with virus suppressed and were cultured for 24 h with antiretroviral drugs (10 nM raltegravir and 20 nM efavirenz) to avoid new cycles of infection. The cells were then washed from the antiretrovirals and reactivated with maximal mitogen stimulation (2 μg/ml PHA and 60 U/ml IL-2) or with vorinostat (335 nM). After 24 h, the cells were washed and plated in 12-well plates at 1 million to 2 million cells per well (more cells were used if the donor was known to have a low frequency of latent infection). NK cells were added to the culture at an E:T ratio of 1:10, and 24 h later, allogeneic stimulated CD8-depleted PBMC (feeders) were added to the culture to propagate the infection. To confirm that virus recovery reduction in the presence of NK cells was due to recognition of reactivated CD4+ T cells and not to nonspecific killing or inhibition of viral spread, we conducted experiments in which we depleted the cultures of CD56+ cells (CD56 positive-selection kit; StemCell Technologies) before adding the feeder cells. In either case, the cells were cultured for 19 days, changing the medium every 3 days and adding feeder cells on day 8. The numbers of positive wells were noted based on p24 ELISAs performed in the supernatants of the cultures at day 15 and day 19, and conditions with and without effectors were compared. Additionally, in some experiments, after the first 24 h of culture of the reactivated CD4+ T cells with or without NK cells, cell death was analyzed by flow cytometry using annexin V (BioLegend) staining.
Activating receptor expression.
A panel of NK cell activating receptors was analyzed by flow cytometry comparing untreated NK cells and cells exposed to IL-15. The following surface monoclonal antibodies were used, after staining with the viability dye Zombie Aqua: CD3-peridinin chlorophyll protein (PerCP), dsCD56-APC/Cy7, CD16-AF700, NKG2D-APC, NKp30-PE, NKp46-PE/Cy7, and DNAM-1–FITC (Biolegend). Samples were analyzed on the Attune focusing cytometer (Applied Biosystems). The CD3− CD56+ population was gated in the viable gate, and the expression of each of the receptors was analyzed using FlowJo (Ashland, OR) X software. Fluorescence minus one (FMO) gating controls for each antibody were used to define the gates.
Statistical analysis.
Analysis was performed with GraphPad Prism v7. Comparisons between matched groups were analyzed using the nonparametric Wilcoxon signed-rank test. Statistical significance was assigned when the P value was <0.05. Results are expressed as means and SEM.
ACKNOWLEDGMENTS
We acknowledge the contributions of Nancie Archin, Brigitte Allard, Erin Stuelke, Katherine Sholtis, and Jennifer Kirrchher to the isolation and processing of cell samples and Cynthia Gay, Joann Kuruc, and Caroline Baker to donor recruitment and clinical management. We also thank the HIV-positive participants for their essential assistance with this study.
The research reported here was supported, in part, by CARE, a Martin Delaney Collaboratory program, and the National Institute of Allergy and Infectious Diseases, National Institute of Neurological Disorders and Stroke (NINDS), National Institute on Drug Abuse (NIDA), and National Institute of Mental Health (NIMH) of the National Institutes of Health (grant number 1UM1AI126619-01). The research was also supported by Qura Therapeutics, RR024383 to the UNC TraCS Institute, AI50410 to the UNC Center for AIDS Research, North Carolina Biotech Center Institutional Support Grant 2017-IDG-1025, and the National Institutes of Health (1UM2AI30836-01 and 1 S10 OD017984 to the Flow Core Facility of UNC). The content is solely the responsibility of the authors. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, Parker DC, Anderson EM, Kearney MF, Strain MC, Richman DD, Hudgens MG, Bosch RJ, Coffin JM, Eron JJ, Hazuda DJ, Margolis DM. 2012. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 487:482–485. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sogaard OS, Graversen ME, Leth S, Olesen R, Brinkmann CR, Nissen SK, Kjaer AS, Schleimann MH, Denton PW, Hey-Cunningham WJ, Koelsch KK, Pantaleo G, Krogsgaard K, Sommerfelt M, Fromentin R, Chomont N, Rasmussen TA, Ostergaard L, Tolstrup M. 2015. The depsipeptide romidepsin reverses HIV-1 latency in vivo. PLoS Pathog 11:e1005142. doi: 10.1371/journal.ppat.1005142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasmussen TA, Tolstrup M, Brinkmann CR, Olesen R, Erikstrup C, Solomon A, Winckelmann A, Palmer S, Dinarello C, Buzon M, Lichterfeld M, Lewin SR, Ostergaard L, Sogaard OS. 2014. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. Lancet HIV 1:e13–e21. doi: 10.1016/S2352-3018(14)70014-1. [DOI] [PubMed] [Google Scholar]
- 4.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 5.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. 2006. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med 203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP. 2006. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med 12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 7.Deng K, Pertea M, Rongvaux A, Wang L, Durand CM, Ghiaur G, Lai J, McHugh HL, Hao H, Zhang H, Margolick JB, Gurer C, Murphy AJ, Valenzuela DM, Yancopoulos GD, Deeks SG, Strowig T, Kumar P, Siliciano JD, Salzberg SL, Flavell RA, Shan L, Siliciano RF. 2015. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature 517:381–385. doi: 10.1038/nature14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shan L, Deng K, Shroff NS, Durand CM, Rabi SA, Yang HC, Zhang H, Margolick JB, Blankson JN, Siliciano RF. 2012. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity 36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borrow P, Lewicki H, Wei X, Horwitz MS, Peffer N, Meyers H, Nelson JA, Gairin JE, Hahn BH, Oldstone MB, Shaw GM. 1997. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med 3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- 10.Lanier LL. 2008. Evolutionary struggles between NK cells and viruses. Nat Rev Immunol 8:259–268. doi: 10.1038/nri2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alter G, Heckerman D, Schneidewind A, Fadda L, Kadie CM, Carlson JM, Oniangue-Ndza C, Martin M, Li B, Khakoo SI, Carrington M, Allen TM, Altfeld M. 2011. HIV-1 adaptation to NK-cell-mediated immune pressure. Nature 476:96–100. doi: 10.1038/nature10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elemans M, Boelen L, Rasmussen M, Buus S, Asquith B. 2017. HIV-1 adaptation to NK cell-mediated immune pressure. PLoS Pathog 13:e1006361. doi: 10.1371/journal.ppat.1006361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonaparte MI, Barker E. 2004. Killing of human immunodeficiency virus-infected primary T-cell blasts by autologous natural killer cells is dependent on the ability of the virus to alter the expression of major histocompatibility complex class I molecules. Blood 104:2087–2094. doi: 10.1182/blood-2004-02-0696. [DOI] [PubMed] [Google Scholar]
- 14.Lederman MM, Margolis L. 2008. The lymph node in HIV pathogenesis. Semin Immunol 20:187–195. doi: 10.1016/j.smim.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leong YA, Chen Y, Ong HS, Wu D, Man K, Deleage C, Minnich M, Meckiff BJ, Wei Y, Hou Z, Zotos D, Fenix KA, Atnerkar A, Preston S, Chipman JG, Beilman GJ, Allison CC, Sun L, Wang P, Xu J, Toe JG, Lu HK, Tao Y, Palendira U, Dent AL, Landay AL, Pellegrini M, Comerford I, McColl SR, Schacker TW, Long HM, Estes JD, Busslinger M, Belz GT, Lewin SR, Kallies A, Yu D. 2016. CXCR5(+) follicular cytotoxic T cells control viral infection in B cell follicles. Nat Immunol 17:1187–1196. doi: 10.1038/ni.3543. [DOI] [PubMed] [Google Scholar]
- 16.Huot N, Jacquelin B, Garcia-Tellez T, Rascle P, Ploquin MJ, Madec Y, Reeves RK, Derreudre-Bosquet N, Muller-Trutwin M. 2017. Natural killer cells migrate into and control simian immunodeficiency virus replication in lymph node follicles in African green monkeys. Nat Med 23:1277–1286. doi: 10.1038/nm.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terme M, Ullrich E, Delahaye NF, Chaput N, Zitvogel L. 2008. Natural killer cell-directed therapies: moving from unexpected results to successful strategies. Nat Immunol 9:486–494. doi: 10.1038/ni1580. [DOI] [PubMed] [Google Scholar]
- 18.Guillerey C, Huntington ND, Smyth MJ. 2016. Targeting natural killer cells in cancer immunotherapy. Nat Immunol 17:1025–1036. doi: 10.1038/ni.3518. [DOI] [PubMed] [Google Scholar]
- 19.Sung JA, Lam S, Garrido C, Archin N, Rooney CM, Bollard CM, Margolis DM. 2015. Expanded cytotoxic t-cell lymphocytes target the latent HIV reservoir. J Infect Dis 212:258–263. doi: 10.1093/infdis/jiv022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mujib S, Saiyed A, Fadel S, Bozorgzad A, Aidarus N, Yue FY, Benko E, Kovacs C, Emert-Sedlak LA, Smithgall TE, Ostrowski MA. 2017. Pharmacologic HIV-1 Nef blockade promotes CD8 T cell-mediated elimination of latently HIV-1-infected cells in vitro. JCI Insight 2:93684. doi: 10.1172/jci.insight.93684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu B, Zou F, Lu L, Chen C, He D, Zhang X, Tang X, Liu C, Li L, Zhang H. 2016. Chimeric antigen receptor T cells guided by the single-chain Fv of a broadly neutralizing antibody specifically and effectively eradicate virus reactivated from latency in CD4+ T lymphocytes isolated from HIV-1-infected individuals receiving suppressive combined antiretroviral therapy. J Virol 90:9712–9724. doi: 10.1128/JVI.00852-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marras F, Casabianca A, Bozzano F, Ascierto ML, Orlandi C, Di Biagio A, Pontali E, Dentone C, Orofino G, Nicolini L, Taramasso L, Magnani M, Marincola FM, Wang E, Moretta L, De Maria A. 2017. Control of the HIV-1 DNA reservoir is associated in vivo and in vitro with NKp46/NKp30 (CD335 CD337) inducibility and interferon gamma production by transcriptionally unique NK cells. J Virol 91:e00647-17. doi: 10.1128/JVI.00647-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olesen R, Vigano S, Rasmussen TA, Sogaard OS, Ouyang Z, Buzon M, Bashirova A, Carrington M, Palmer S, Brinkmann CR, Yu XG, Ostergaard L, Tolstrup M, Lichterfeld M. 2015. Innate immune activity correlates with CD4 T cell-associated HIV-1 DNA decline during latency-reversing treatment with panobinostat. J Virol 89:10176–10189. doi: 10.1128/JVI.01484-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thaysen-Andersen M, Chertova E, Bergamaschi C, Moh ES, Chertov O, Roser J, Sowder R, Bear J, Lifson J, Packer NH, Felber BK, Pavlakis GN. 2016. Recombinant human heterodimeric IL-15 complex displays extensive and reproducible N- and O-linked glycosylation. Glycoconj J 33:417–433. doi: 10.1007/s10719-015-9627-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhode PR, Egan JO, Xu W, Hong H, Webb GM, Chen X, Liu B, Zhu X, Wen J, You L, Kong L, Edwards AC, Han K, Shi S, Alter S, Sacha JB, Jeng EK, Cai W, Wong HC. 2016. Comparison of the superagonist complex, ALT-803, to IL15 as cancer immunotherapeutics in animal models. Cancer Immunol Res 4:49–60. doi: 10.1158/2326-6066.CIR-15-0093-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellis-Connell AL, Balgeman AJ, Zarbock KR, Barry G, Weiler A, Egan JO, Jeng EK, Friedrich T, Miller JS, Haase AT, Schacker TW, Wong HC, Rakasz E, O'Connor SL. 2018. ALT-803 transiently reduces SIV replication in the absence of antiretroviral treatment. J Virol 92:e01748-17. doi: 10.1128/JVI.01748-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu E, Tong Y, Dotti G, Shaim H, Savoldo B, Mukherjee M, Orange J, Wan X, Lu X, Reynolds A, Gagea M, Banerjee P, Cai R, Bdaiwi MH, Basar R, Muftuoglu M, Li L, Marin D, Wierda W, Keating M, Champlin R, Shpall E, Rezvani K. 2018. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 32:520–531. doi: 10.1038/leu.2017.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoyos V, Savoldo B, Quintarelli C, Mahendravada A, Zhang M, Vera J, Heslop HE, Rooney CM, Brenner MK, Dotti G. 2010. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia 24:1160–1170. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomescu C, Tebas P, Montaner LJ. 2017. IFN-alpha augments natural killer-mediated antibody-dependent cellular cytotoxicity of HIV-1-infected autologous CD4+ T cells regardless of major histocompatibility complex class 1 downregulation. AIDS 31:613–622. doi: 10.1097/QAD.0000000000001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomescu C, Chehimi J, Maino VC, Montaner LJ. 2007. NK cell lysis of HIV-1-infected autologous CD4 primary T cells: requirement for IFN-mediated NK activation by plasmacytoid dendritic cells. J Immunol 179:2097–2104. doi: 10.4049/jimmunol.179.4.2097. [DOI] [PubMed] [Google Scholar]
- 31.Cummings JS, Moreno-Nieves UY, Arnold V, Gilbert A, Yarbrough K, Didier C, Levy Y, Barre-Sinoussi F, Scott-Algara D. 2014. Natural killer cell responses to dendritic cells infected by the ANRS HIV-1 vaccine candidate, MVAHIV. Vaccine 32:5577–5584. doi: 10.1016/j.vaccine.2014.07.094. [DOI] [PubMed] [Google Scholar]
- 32.Childs RW, Carlsten M. 2015. Therapeutic approaches to enhance natural killer cell cytotoxicity against cancer: the force awakens. Nat Rev Drug Discov 14:487–498. doi: 10.1038/nrd4506. [DOI] [PubMed] [Google Scholar]
- 33.Robinson TO, Schluns KS. 2017. The potential and promise of IL-15 in immuno-oncogenic therapies. Immunol Lett 190:159–168. doi: 10.1016/j.imlet.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wagner JA, Rosario M, Romee R, Berrien-Elliott MM, Schneider SE, Leong JW, Sullivan RP, Jewell BA, Becker-Hapak M, Schappe T, Abdel-Latif S, Ireland AR, Jaishankar D, King JA, Vij R, Clement D, Goodridge J, Malmberg KJ, Wong HC, Fehniger TA. 2017. CD56bright NK cells exhibit potent antitumor responses following IL-15 priming. J Clin Invest 127:4042–4058. doi: 10.1172/JCI90387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ewen EM, Pahl JHW, Miller M, Watzl C, Cerwenka A. 2018. KIR downregulation by IL-12/15/18 unleashes human NK cells from KIR/HLA-I inhibition and enhances killing of tumor cells. Eur J Immunol 48:355–365. doi: 10.1002/eji.201747128. [DOI] [PubMed] [Google Scholar]
- 36.Davis ZB, Vallera DA, Miller JS, Felices M. 2017. Natural killer cells unleashed: checkpoint receptor blockade and BiKE/TriKE utilization in NK-mediated anti-tumor immunotherapy. Semin Immunol 31:64–75. doi: 10.1016/j.smim.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rasmussen TA, Lewin SR. 2016. Shocking HIV out of hiding: where are we with clinical trials of latency reversing agents? Curr Opin HIV AIDS 11:394–401. doi: 10.1097/COH.0000000000000279. [DOI] [PubMed] [Google Scholar]
- 38.Darcis G, Van Driessche B, Van Lint C. 2016. Preclinical shock strategies to reactivate latent HIV-1: an update. Curr Opin HIV AIDS 11:388–393. doi: 10.1097/COH.0000000000000288. [DOI] [PubMed] [Google Scholar]
- 39.Garrido C, Spivak AM, Soriano-Sarabia N, Checkley MA, Barker E, Karn J, Planelles V, Margolis DM. 2016. HIV latency-reversing agents have diverse effects on natural killer cell function. Front Immunol 7:356. doi: 10.3389/fimmu.2016.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skov S, Pedersen MT, Andresen L, Straten PT, Woetmann A, Odum N. 2005. Cancer cells become susceptible to natural killer cell killing after exposure to histone deacetylase inhibitors due to glycogen synthase kinase-3-dependent expression of MHC class I-related chain A and B. Cancer Res 65:11136–11145. doi: 10.1158/0008-5472.CAN-05-0599. [DOI] [PubMed] [Google Scholar]
- 41.Diermayr S, Himmelreich H, Durovic B, Mathys-Schneeberger A, Siegler U, Langenkamp U, Hofsteenge J, Gratwohl A, Tichelli A, Paluszewska M, Wiktor-Jedrzejczak W, Kalberer CP, Wodnar-Filipowicz A. 2008. NKG2D ligand expression in AML increases in response to HDAC inhibitor valproic acid and contributes to allorecognition by NK-cell lines with single KIR-HLA class I specificities. Blood 111:1428–1436. doi: 10.1182/blood-2007-07-101311. [DOI] [PubMed] [Google Scholar]
- 42.Armeanu S, Bitzer M, Lauer UM, Venturelli S, Pathil A, Krusch M, Kaiser S, Jobst J, Smirnow I, Wagner A, Steinle A, Salih HR. 2005. Natural killer cell-mediated lysis of hepatoma cells via specific induction of NKG2D ligands by the histone deacetylase inhibitor sodium valproate. Cancer Res 65:6321–6329. doi: 10.1158/0008-5472.CAN-04-4252. [DOI] [PubMed] [Google Scholar]
- 43.Desimio MG, Giuliani E, Doria M. 2017. The histone deacetylase inhibitor SAHA simultaneously reactivates HIV-1 from latency and up-regulates NKG2D ligands sensitizing for natural killer cell cytotoxicity. Virology 510:9–21. doi: 10.1016/j.virol.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 44.Parsons MS, Richard J, Lee WS, Vanderven H, Grant MD, Finzi A, Kent SJ. 2016. NKG2D acts as a co-receptor for natural killer cell-mediated anti-HIV-1 antibody-dependent cellular cytotoxicity. AIDS Res Hum Retroviruses 32:1089–1096. doi: 10.1089/aid.2016.0099. [DOI] [PubMed] [Google Scholar]
- 45.Offersen R, Nissen SK, Rasmussen TA, Ostergaard L, Denton PW, Sogaard OS, Tolstrup M. 2016. A novel Toll-like receptor 9 agonist, MGN1703, enhances HIV-1 transcription and NK cell-mediated inhibition of HIV-1-infected autologous CD4+ T cells. J Virol 90:4441–4453. doi: 10.1128/JVI.00222-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vibholm L, Schleimann MH, Hojen JF, Benfield T, Offersen R, Rasmussen K, Olesen R, Dige A, Agnholt J, Grau J, Buzon M, Wittig B, Lichterfeld M, Petersen AM, Deng X, Abdel-Mohsen M, Pillai SK, Rutsaert S, Trypsteen W, De Spiegelaere W, Vandekerchove L, Ostergaard L, Rasmussen TA, Denton PW, Tolstrup M, Sogaard OS. 2017. Short-course Toll-like receptor 9 agonist treatment impacts innate immunity and plasma viremia in individuals with human immunodeficiency virus infection. Clin Infect Dis 64:1686–1695. doi: 10.1093/cid/cix201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bruel T, Guivel-Benhassine F, Amraoui S, Malbec M, Richard L, Bourdic K, Donahue DA, Lorin V, Casartelli N, Noel N, Lambotte O, Mouquet H, Schwartz O. 2016. Elimination of HIV-1-infected cells by broadly neutralizing antibodies. Nat Commun 7:10844. doi: 10.1038/ncomms10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fogli M, Mavilio D, Brunetta E, Varchetta S, Ata K, Roby G, Kovacs C, Follmann D, Pende D, Ward J, Barker E, Marcenaro E, Moretta A, Fauci AS. 2008. Lysis of endogenously infected CD4+ T cell blasts by rIL-2 activated autologous natural killer cells from HIV-infected viremic individuals. PLoS Pathog 4:e1000101. doi: 10.1371/journal.ppat.1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parato KG, Kumar A, Badley AD, Sanchez-Dardon JL, Chambers KA, Young CD, Lim WT, Kravcik S, Cameron DW, Angel JB. 2002. Normalization of natural killer cell function and phenotype with effective anti-HIV therapy and the role of IL-10. AIDS 16:1251–1256. doi: 10.1097/00002030-200206140-00007. [DOI] [PubMed] [Google Scholar]
- 50.Hromadnikova I, Pirkova P, Sedlackova L. 2013. Influence of in vitro IL-2 or IL-15 alone or in combination with Hsp-70-derived 14-mer peptide (TKD) on the expression of NK cell activatory and inhibitory receptors. Mediators Inflamm 2013:405295. doi: 10.1155/2013/405295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tomescu C, Mavilio D, Montaner LJ. 2015. Lysis of HIV-1-infected autologous CD4+ primary T cells by interferon-alpha-activated NK cells requires NKp46 and NKG2D. AIDS 29:1767–1773. doi: 10.1097/QAD.0000000000000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glasner A, Isaacson B, Viukov S, Neuman T, Friedman N, Mandelboim M, Sexl V, Hanna JH, Mandelboim O. 2017. Increased NK cell immunity in a transgenic mouse model of NKp46 overexpression. Sci Rep 7:13090. doi: 10.1038/s41598-017-12998-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alter G, Malenfant JM, Altfeld M. 2004. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods 294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 54.Pollara J, Bonsignori M, Moody MA, Liu P, Alam SM, Hwang KK, Gurley TC, Kozink DM, Armand LC, Marshall DJ, Whitesides JF, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Robb ML, O'Connell RJ, Kim JH, Michael NL, Montefiori DC, Tomaras GD, Liao HX, Haynes BF, Ferrari G. 2014. HIV-1 vaccine-induced C1 and V2 Env-specific antibodies synergize for increased antiviral activities. J Virol 88:7715–7726. doi: 10.1128/JVI.00156-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trkola A, Matthews J, Gordon C, Ketas T, Moore JP. 1999. A cell line-based neutralization assay for primary human immunodeficiency virus type 1 isolates that use either the CCR5 or the CXCR4 coreceptor. J Virol 73:8966–8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siliciano JD, Siliciano RF. 2005. Enhanced culture assay for detection and quantitation of latently infected, resting CD4+ T-cells carrying replication-competent virus in HIV-1-infected individuals. Methods Mol Biol 304:3–15. [DOI] [PubMed] [Google Scholar]